Abstract
Introduction:
The COVID-19 pandemic highlighted the need for a nationwide health information technology solution that could improve upon manual case reporting and decrease the clinical and administrative burden on the US health care system. We describe the development, implementation, and nationwide expansion of electronic case reporting (eCR), including its effect on public health surveillance and pandemic readiness.
Methods:
Multidisciplinary teams developed and implemented a standards-based, shared, scalable, and interoperable eCR infrastructure during 2014-2020. From January 27, 2020, to January 7, 2023, the team conducted a nationwide scale-up effort and determined the number of eCR-capable electronic health record (EHR) products, the number of reportable conditions available within the infrastructure, and technical connections of health care organizations (HCOs) and jurisdictional public health agencies (PHAs) to the eCR infrastructure. The team also conducted data quality studies to determine whether HCOs were discontinuing manual case reporting and early results of eCR timeliness.
Results:
During the study period, the number of eCR-capable EHR products developed or in development increased 11-fold (from 3 to 33), the number of reportable conditions available increased 28-fold (from 6 to 173), the number of HCOs connected to the eCR infrastructure increased 143-fold (from 153 to 22 000), and the number of jurisdictional PHAs connected to the eCR infrastructure increased 2.75-fold (from 24 to 66). Data quality reviews with PHAs resulted in select HCOs discontinuing manual case reporting and using eCR-exclusive case reporting in 13 PHA jurisdictions. The timeliness of eCR was <1 minute.
Practice Implications:
The growth of eCR can revolutionize public health case surveillance by producing data that are more timely and complete than manual case reporting while reducing reporting burden.
Keywords: electronic case reporting, electronic health record, case surveillance, data standards, infrastructure
Public health surveillance is defined as the continuous and systematic collection, analysis, and interpretation of data. 1 Although public health reporting laws vary by state, tribal, local, and territorial jurisdiction, laws exist that give public health agencies (PHAs) broad authority to collect data, including case reports from health care providers, to prevent and control disease. 2 Historically, case reporting has been done manually, which is often slow, results in incomplete data, and places a substantial burden of work on health care providers and PHAs.3 -6 For example, manual reporting resulted in only 1 in 10 identified cases of Lyme disease being reported by health care providers to PHAs from 2010 to 2018. 7
An innovation in case reporting is electronic case reporting (eCR), the automated generation and transmission of case reports from electronic health records (EHRs) to PHAs for review and action. 8 eCR operates behind the scenes within EHRs to identify potential reportable events and create case reports, confirms the reportability of conditions through the application of rules, and securely transmits information to the appropriate PHA(s). 9 By automating these tasks, eCR can decrease the clinical and administrative burden associated with manual reporting.10,11 For example, a 1-year internal time–cost study showed that using eCR saved more than $4 million in health care provider time when compared with manual case reporting. 12 Additionally, eCR and its Health Level Seven (HL7) standards, 13 including the electronic initial case report (eICR), have shown the ability to substantially improve data completeness.14 -16
eCR can play an important role in pandemic readiness. As the COVID-19 pandemic unfolded, the Council of State and Territorial Epidemiologists (CSTE) developed a national surveillance case definition and added COVID-19 to the list of national notifiable conditions.17,18 Around that time, the Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL) developed and launched the eCR Now initiative for health care organizations to implement eCR.19,20 With COVID-19 added to the list of reportable conditions and the rapid expansion of eCR from pilot sites,21 -25 health care organizations could send COVID-19 case data electronically, and PHAs could conduct contact tracing and disease monitoring activities. 26
As part of pandemic readiness, eCR may also identify trends in clinical presentations and diagnoses associated with novel conditions. COVID-19 eCR data contained information about associated conditions that could only be identified through clinical diagnosis, such as multisystem inflammatory syndrome in children. 27 For example, the Idaho Department of Health and Welfare used eCR data to identify cases of multisystem inflammatory syndrome in children who had a recent history of COVID-19 (K. Turner, Idaho Department of Health and Welfare, email communication, October 28, 2021).
In this article, we present (1) the development and implementation of the eCR infrastructure during 2014-2020, (2) early results of eCR implementation and nationwide expansion across health care and public health during 2020-2023, and (3) the impact of eCR on the quality of public health surveillance and pandemic readiness for a novel condition such as COVID-19.
Background and Methods
The nationwide development, implementation, and expansion of eCR in the United States have involved a collaboration among CDC, CSTE, APHL, PHAs, health care organizations and their EHR/health information technology industry partners. The shared and scalable components of eCR were developed by numerous multidisciplinary teams, which are hereinafter referred to collectively as the eCR team.
Description of eCR and eCR Data and Context
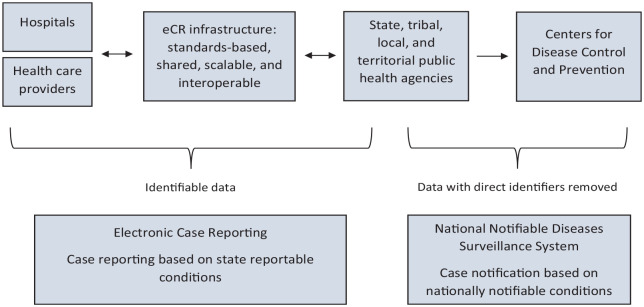
Electronic case reports include data that are reportable to states by virtue of state laws and considered necessary according to CSTE position statements. 28 Some of these data are used for reporting to CDC through the National Notifiable Diseases Surveillance System, 29 after direct identifiers have been removed. eCR is a component of the larger public health case surveillance process (P. Yoon, CDC, email communication, May 24, 2022) (Figure 1). Direct identifiers are data that contain personally identifiable information and are removed by jurisdictional PHAs before the notifiable disease data are sent to CDC. 30
Figure 1.
Description of jurisdictional case reporting and national case notification. Person-based case reports flow electronically from health care organizations to state, tribal, local, and territorial (STLT) public health agencies (PHAs). Derived data, with direct patient identifiers removed, are then shared by STLT PHAs with the Centers for Disease Control and Prevention (CDC) through the National Notifiable Diseases Surveillance System (NNDSS). Electronic case reporting (eCR) supports bidirectional data exchange between health care organizations and STLT PHAs. NNDSS supports receipt of notifiable disease information from STLT PHAs to CDC.
The eCR team developed and implemented a standards-based, shared, scalable, and interoperable infrastructure. The eCR team created a trust framework, which addresses the policy and legal practices, to help enable a “hub-and-spoke” architecture for public health reporting from many health care organizations to many PHAs. 31 In a hub-and-spoke architecture, many data connections or “spokes,” such as from many health care organizations, connect to a single connection point or “hub.” In eCR, many PHAs are also connected to a single hub. This architecture makes for a single, consistent method of connection. Multiple steps were required for health care organizations to report cases electronically to PHAs using eCR (Box).
Box.
Steps health care organizations can take to perform electronic case reporting (eCR) a
| 1. Use of eCR HL7 data exchange standards: (1) electronic initial case report (eICR), (2) reportability response, and (3) electronic reporting and surveillance distribution (eRSD). 2. Use of an electronic health record (EHR) industry partner that has developed an eCR-capable EHR or is using the eCR Now FHIR app in conjunction with its EHR. 3. Leverage of an appropriate chain of trust for data exchange using the eCR trust framework. 4. Connection to the “hub and spoke” eCR infrastructure using direct secure messaging or the XDR standards. Public health agencies are also connected to that eCR infrastructure. 5. Use of eCR shared services: the eRSD helps trigger eICRs, and the Reportable Conditions Knowledge Management System confirms reportability to public health agencies in accordance with jurisdictional laws and practices. 6. Performance of data quality and validation processes. |
Abbreviations: app, application; FHIR, Fast Healthcare Interoperability Resources; HL7, Health Level Seven; XDR, Cross-Enterprise Document Reliable Interchange.
After completing the steps, health care organizations can send case reports electronically using eCR to state, tribal, local, and territorial public health agencies.
Components of eCR Development, Implementation, and Expansion
The eCR architecture comprises multiple components (Figure 2) that were involved in achieving eCR prototyping, rapid scaling of COVID-19 eCR implementation during the pandemic, and expansion of eCR to include 173 reportable conditions.
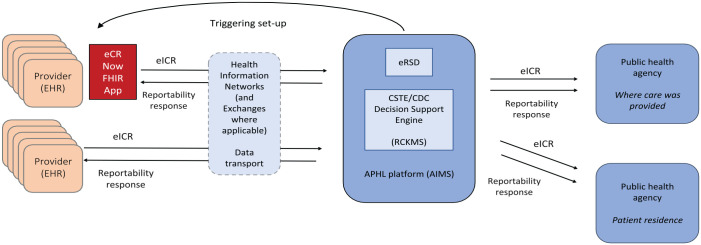
Figure 2.
Electronic case reporting (eCR) architecture. Using eCR-capable electronic health reporting (EHR) products or the eCR Now FHIR app, electronic initial case reports (eICRs) are triggered, created, and transmitted before, during, or after patient encounters, and then EHRs and health care providers receive and process the related reportability responses. Created in collaboration with health information networks, a trust framework provides authorities for the appropriate sharing of identifiable patient information with the Association of Public Health Laboratories (APHL) and public health disclosures to public health authorities. The “hub” of the eCR infrastructure is the APHL Informatics Messaging Services (AIMS) platform and includes an electronic reporting and surveillance distribution (eRSD) system and Reportable Conditions Knowledge Management System (RCKMS), which help EHRs/health care organizations report in accordance with jurisdictional laws and practices. eICRs are then delivered to public health agencies. Abbreviations: app, application; CDC, Centers for Disease Control and Prevention; CSTE, Council of State and Territorial Epidemiologists; FHIR, Fast Healthcare Interoperability Resources.
Electronic initial case report (eICR) standard
Efforts to develop the HL7 eICR standard began in 2015. A CSTE eCR taskforce convened to recommend the necessary data elements for an all-conditions, all-jurisdictions case report. 32 These data elements were used to develop the HL7 eICR standard.33,34 The eICR data elements capture patient and clinical data on demographic characteristics, comorbidities, immunizations, medications, and treatments. PHAs require use of the eICR standard by health care organizations that are connecting to the eCR infrastructure. The eICR standard implementation guide R1.1 was first published in 2017. 35 Wherever possible, the eICR data elements were based on, and continuously harmonized with, HL7 Consolidated Clinical Document Architecture templates, the US Core Implementation Guide, and the United States Core Data for Interoperability.36 -38 The eCR team collaborated with the HL7 Public Health Workgroup to ballot (ie, vote on) and publish the eICR standard and subsequent updates.39,40
Reportability response standard
For every eICR that is sent, the health care organization receives a reportability response with information from the PHA and the reporting process. The eCR team developed and published the HL7 reportability response standard in 2018. 41 The reportability response includes the status of reporting, which conditions were reported, information about the condition(s) reported, and to which PHA(s) it was reported. 42 The reportability response may also contain information from the PHA, including treatment guidelines or fact sheets about the condition. This process enables the bidirectional exchange of information between health care organizations and PHAs.
Electronic reporting and surveillance distribution (eRSD) standard
The HL7 Fast Healthcare Interoperability Resources (FHIR) eCR standard includes FHIR eICR, reportability response, and specifications for the eRSD. 43 The eCR team developed the eRSD standard through HL7 to convey to EHR industry partners and health care organizations the eCR trigger codes and reporting guidance used to trigger and report eICRs. The eRSD standard was released in 2020 40 and the latest version of the eRSD specification is eRSDR2. 44 The eRSD is intended to be electronically consumable by EHRs, and this specification is a core part of the eRSD shared service described hereinafter.
eCR Now FHIR Application (App)
The eCR team developed the eCR Now FHIR App using the HL7 FHIR application programming interface version R4 to connect to any EHR.45,46 The app uses timed queries based on health care organization patient encounters to retrieve specific data in the EHR. When those data match trigger codes, the app automatically generates and transmits eICRs to APHL and, when reportable, on to PHAs. The app was built on the HL7 FHIR application programming interface that is required of EHR industry partners to meet regulations included in the 21st Century Cures Act. 47 The initial version of the eCR Now FHIR App and its source code were released on May 1, 2020.
eCR Trust Framework
Health care organizations need to use an appropriate chain of trust to provide data-sharing authorities for users that are exchanging electronic health information.48 -51 The eCR team established a trust framework to share identifiable patient information that complied with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and relevant jurisdictional laws.52 -54
The trust framework provides an efficient chain of trust for APHL to act on behalf of health care providers to carry out required reporting using existing agreements with health care organizations. 55 The framework works with 2 secure data transport standards: (1) the Direct Simple Mail Transfer Protocol for encrypted health information through DirectTrust and (2) the Nationwide Health Information Network Cross Enterprise Document Reliable Interchange using the eHealth Exchange hub.56,57 EHR industry partners are expected to support these transport standards as part of the EHR certification process. 58 When appropriate standards are in use, the trust framework can apply to numerous technical data exchange networks even when data are not physically exchanged through the participating health information networks.
Hub-and-Spoke eCR Infrastructure
The objective of the eCR infrastructure is to enable the automated, secure transport of patient case reports from health care providers to PHAs. eCR is constructed using a hub-and-spoke architecture that allows multiple health care organizations to connect to multiple PHAs through a single interface and shared infrastructure. The hub of the eCR infrastructure is hosted on the APHL Informatics Messaging Services (AIMS) platform, a Federal Information Security Management Act Moderate Impact cloud-based platform. 59 Electronic case reports automated through eCR are securely sent from health care organizations to PHAs. The single interface allows for consistency of the connection that health care organizations and their EHR industry partners need to connect to. The shared infrastructure supports eCR shared services including confirming reportability and the routing and distribution of eICRs and reportability responses to appropriate PHAs.
Shared Services
The hub-and-spoke eCR architecture also allows for the provision of other shared services to support health care organizations and PHAs.
eRSD shared service
The eCR team developed the eRSD shared, web-based distribution service to provide trigger codes and trigger code guidance to health care organizations. The eRSD shared service distributes the eRSD specification to facilitate and guide the triggering and reporting of eICRs from EHRs and contains reportable conditions trigger codes—a list of codes related to reportable conditions that can match against patient data in the EHR. When health care providers record or update data (eg, diagnoses, laboratory data) in the EHR, the data are checked against a series of codes. If the data match a code, then an eICR is triggered to be sent. The triggers identify diseases and conditions with 5 triggering EHR data categories (Table).44,60 -64 Once the eICR is triggered and generated, it is then transmitted to the shared eCR infrastructure.
Table.
Triggering of an electronic initial case report (eICR) by the electronic reporting and surveillance distribution (eRSD) shared service a
| EHR data category (coding standard b ) | Primary triggering scenario |
|---|---|
| Laboratory orders (LOINC) | An eICR will be triggered when a laboratory order is placed in the EHR that matches a value within the eRSD “Lab_Order_Test_Name” c value set. Because this trigger handles reporting on suspicion of a condition to meet related laws, this triggering should occur before laboratory results are available. |
| Diagnoses and suspected diagnoses (SNOMED, ICD-10 CM) | An eICR will be triggered when a diagnosis is recorded in the EHR (in the problem list or diagnosis fields) that matches a value within the eRSD “Diagnosis_Problem” value set. An eICR will be triggered when a suspected diagnosis is recorded in the EHR that matches a value in the eRSD “Suspected_Disorder”c,d value set. The suspected diagnosis triggering may be used to replace laboratory order triggering for EHRs that cannot do laboratory order triggering. |
| Laboratory results (SNOMED) | An eICR will be triggered when a laboratory result is received by the EHR that matches a value within the eRSD “Organism_Substance” value set. |
| Laboratory result test names (LOINC) | An eICR will be triggered when a laboratory result is received by the EHR that matches a value within the eRSD “Lab_Observation_Test_Name” value set. |
| Medications (CVX, RxNorm, SNOMED) | An eICR will be triggered when a medication is administered or prescribed in the EHR that matches a value within the eRSD “Medication” value set. |
Abbreviations: EHR, electronic health record; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; LOINC, Logical Observation Identifiers Names and Codes; SNOMED, Systematized Nomenclature of Medicine.
The trigger codes contained in the eRSD specification are related to reportable diseases and can be matched against patient data in EHRs. If the data in an EHR match 1 or more of these codes, then an eICR is “triggered” to be sent to the Association of Public Health Laboratories (APHL) Informatics Messaging Services (AIMS) platform for confirmation of reportability in the Reportable Conditions Knowledge Management System. 59 The triggers identify diseases and conditions based on 5 EHR data categories that are related to 6 primary triggering scenarios.
The trigger codes in the eRSD shared service tool are based on the following coding standards: LOINC, 60 SNOMED, 61 ICD-10 CM, 62 Vaccine Administered Code Set (CVX), 63 and RxNorm. 64
The “Lab_Order_Test_Name” and “Suspected_Disorder” value sets are both intended to meet public health needs for the reporting of a certain set of conditions when they are only suspected.
The “Suspected_Disorder” trigger codes are all SNOMED “suspicion of” diagnosis codes and are recorded in the eICR diagnosis template.
Trigger codes are created and managed by the Reportable Conditions Knowledge Management System (RCKMS) content team. When an update for the trigger code appears, the value sets are added to an eRSD loader application and joined with a template that includes guidance for trigger and report timing. The resultant eRSD specification is posted to the eRSD distribution system, and users of the system are notified that an updated version is available.
RCKMS shared service
The eCR team created a shared decision support tool, RCKMS,65 -68 to apply jurisdictional reportability laws. It confirms reportability and routes the case report to the appropriate jurisdiction(s).
The default rules are refined or “authored” to align with reporting laws for each jurisdiction relevant for the PHA. The rules can then be applied for the PHA where care was provided and, if different, the PHA of the patient’s residence. The authored rules are then published in the Open Clinical Decision Support (OpenCDS) engine so they can be used to process incoming eICRs. PHAs can choose jurisdiction-specific reporting criteria. PHAs update reporting criteria in the centralized authoring tool regularly.
Health Care Organizations Leveraging Trust Agreements
The method of health care organizations using the eCR trust framework is either using (1) health information network partners, eHealth Exchange, or Carequality for trust or (2) the APHL participation agreement. The eCR team tracked this information using Smartsheets version 11.6.1 (Smartsheet, Inc) and compared data over time.
Number of Health Care Facilities and PHAs Connected to the eCR Infrastructure
When health care organizations connect to the eCR infrastructure, they are required to provide a list of all their facilities. Facilities that do not generate an eICR, such as laboratories and physical therapy clinics, are not included. Facilities are then deduplicated so that only 1 facility is listed at each address. The eCR team used the final list of facilities to determine the number of health care facilities connected to the eCR infrastructure. Similarly, after a PHA has connected to the eCR infrastructure, has successfully received an eICR, and has a reportability response, the name and location of the PHA are added to the platform tracker, which is then used to determine the number of eCR-connected PHAs.
Scalability: Determining the Number of Reportable Conditions in RCKMS
The eCR team compared the number of reportable conditions available in RCKMS 69 over time to track progress.
Timeliness
Timeliness of eCR is defined as the time it takes from the trigger code match in the EHR until the time the eICR is made available to the PHA. Most PHAs receive eICRs immediately from AIMS. On January 24, 2023, the eCR team assessed the timeliness of eCR by analyzing 13 eICR transit times from 13 health care organizations captured within AIMS from the trigger code match in the EHR until the eICRs were available to PHAs. The eCR team obtained the following information from the 13 eICRs accumulated during this period: (1) eICR unique identification number, (2) health care organization name, (3) EHR/health information technology industry partner, (4) data transport method, (5) trigger date/time, and (6) PHA delivery date/time. Additional methods are available in the supplement (eSupplement).
Institutional Review Board Review
This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (45 CFR part 46, 21 CFR part 56; 42 USC §241[d]; 5 USC §552a; 44 USC §3501 et seq). Institutional review board determination was not required because the study did not involve research among human subjects and was conducted as part of public health surveillance.
Results
eCR-capable EHR Products
In January 2020, there were 3 EHR/health information technology industry partners, each with a single eCR-capable product developed or in development. As of January 7, 2023, a total of 18 EHR/health information technology industry partners were in the process of developing or had developed eCR capability in 33 EHR and/or health information technology products. Of the 33 EHR/health information technology products, 23 were using the eCR FHIR Now App, which they hosted and connected to their EHR/health information technology product using the FHIR application programming interface, and 10 were developing or using a custom-developed eCR solution in their products. In 2021, approximately 60% of hospitals were using an EHR industry partner with an eCR-capable product or the eCR FHIR Now App, including Epic and Oracle Cerner. 70 In addition, 33% of hospitals were using an EHR industry partner that was actively developing eCR capabilities in its products.
Health Care Organizations Leveraging Trust Agreements
In January 2020, 2 health care organizations representing 153 health care facilities were using the eCR trust framework. As of January 7, 2023, the trust framework supported 223 health care organizations representing more than 22 000 health care facilities. Of these 223 health care organizations that implemented eCR, 217 (97%) were using health information network partners, eHealth Exchange, or Carequality for trust and 6 (3%) were using the APHL participation agreement.
Health Care Organizations and PHAs Connected to the Hub-and-Spoke eCR Infrastructure
As of January 7, 2023, a total of 223 health care organizations were representing more than 22 000 health care facilities that were technically connected to the hub-and-spoke eCR infrastructure. The number of health care facilities using eCR for COVID-19 case reporting increased 143-fold, from 153 in January 2020 to >22 000 in January 2023 (Figure 3A). As of January 7, 2023, 57 of 64 cooperative agreement–funded jurisdictional PHAs and 9 additional nonfunded jurisdictions were connected to the eCR infrastructure, resulting in a total of 66 eCR-connected jurisdictions (Figure 3B).
Figure 3.
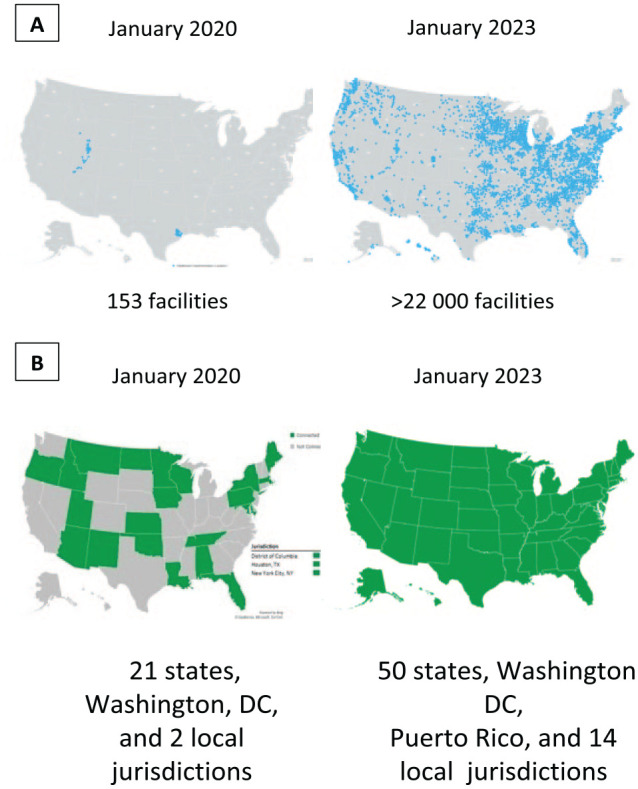
Health care facilities and state, tribal, local, and territorial public health agencies (PHAs) connected to the electronic case reporting (eCR) infrastructure. (A) The dots on the maps indicate the location of health care facilities connected to the eCR infrastructure. On January 27, 2020, 153 facilities representing 2 health care organizations were using eCR to send COVID-19 case reports to PHAs. On January 7, 2023, more than 22 000 facilities representing 223 health care organizations were using eCR to send COVID-19 case reports electronically. (B) Shaded PHAs were connected to the eCR infrastructure and unshaded PHAs were not connected. In January 2020, only 24 PHAs were connected to the eCR infrastructure. As of January 7, 2023, 66 PHAs were connected to the eCR infrastructure, including all 50 states, Washington DC, Puerto Rico, and 14 large local jurisdictions (Chicago, Illinois; Contra Costa County, California; Dallas County, Texas; Houston, Texas; Los Angeles County, California; New York, New York; Philadelphia, Pennsylvania; Rockland County, New York; St. Louis County, Missouri; San Diego County, California; Solano County, California; Southern Nevada Health District, Nevada; Santa Clara County, California; and Tarrant County, Texas).
Scalability of RCKMS
RCKMS content was available for reporting 6 conditions, including COVID-19, in January 2020, and increased to 173 conditions, including opioid overdose, sexually transmitted infections, and Parkinson’s disease, in January 2023. This total represented a 28-fold increase in the number of conditions that could be reported from health care organizations to PHAs electronically using eCR. Moreover, a CSTE RCKMS community of practice is now available for all PHAs to encourage interaction and share knowledge about reportable conditions.
Electronic Data Timeliness, Quality, and Validation Outcome Measures
The eCR team reviewed 13 eICR transit times from the trigger code match in the EHR until the eICRs were processed and made available for PHAs. The eICR transit times varied by data transport methods: (1) direct secure transport times ranged from 45 to 55 seconds and (2) nationwide health information network cross-enterprise document reliable interchange transport times ranged from 8 to 10 seconds. Among the 13 records evaluated on a single day from 13 health care organizations, all were <1 minute.
In a review of eICRs versus manual case reports sent by health care organizations for quality, which included validation of eCR data completeness, 13 PHAs (Alabama, Florida, Hawaii, Idaho, Kansas, Massachusetts, Minnesota, North Carolina, Rhode Island, Tennessee, Vermont, Virginia, and Wisconsin) informed health care organizations in their jurisdictions that clinicians could stop sending manual case reports for COVID-19 as of January 7, 2023, based on eICRs meeting data quality requirements. Also as of January 7, 2023, a total of 2725 health care facilities had turned off COVID-19 manual case reporting and were exclusively using eCR for COVID-19 reporting.
Discussion
From January 2020 to January 2023, a rapid scale-up occurred in the development, implementation, and expansion of eCR in the United States. It involved 2.75- to 143-fold increases in eCR-capable EHR development, health care organization leveraging of trust agreements, reportable conditions in RCKMS, and connection to the eCR infrastructure by health care facilities and PHAs. Work is also underway to connect tribal health care and tribal public health authorities to eCR. 71
The timeliness of eCR was <1 minute in our study, whereas the median timeliness of health care provider report submissions to PHAs using manual case reporting was 5 days in 2017. 5 Pandemic readiness depends upon timely and complete information flowing from health care organizations to PHAs that is scalable during an emergency response. 72 Once COVID-19 became a reportable condition in January 2020, the eCR team worked with health information technology and EHR industry partners, health care organizations, and jurisdictional PHAs to enable electronic reporting of COVID-19 case reports through eCR within 6 days. Furthermore, mpox was updated during June 2022 in RCKMS, enabling eCR to support the CDC mpox response. 73 In just 4 days, health care facilities were able to send mpox patient case reports electronically using eCR. The utility of eCR for reporting emergent conditions in 4-6 days demonstrated substantial advancement in automation, interoperability, scalability, and support for disease outbreak management.
The assessment of timeliness was limited to 1 day and 13 health care facilities. A more robust assessment would involve using a random sample of eICRs across multiple days and health care facilities to ensure representativeness. Despite this limitation, the findings support evidence that eCR is improving the quality of public health surveillance for reportable conditions and pandemic readiness for a novel reportable condition such as COVID-19.
Practice Implications
As eCR expands nationwide, it is delivering on its promise 8 to improve the quality of public health surveillance through (1) reporting quality case data in near-real time for public health action, (2) supporting earlier detection of cases for earlier intervention, (3) diminishing the clinical and administrative reporting burden for both health care and public health, (4) offsetting the costs and resources associated with manual reporting, and (5) providing an interoperable and scalable infrastructure that supports rapid reporting to improve outbreak detection and response.
Additional work remains to be done to connect more health care organizations and PHAs to the eCR infrastructure, evaluate and address data quality issues, and provide ongoing technical assistance to PHAs to ingest and integrate eCR data into their surveillance systems. A need also exists to increase the number of reportable conditions from 173 to the approximately 339 possible reportable conditions to which eCR is capable of scaling.
This core data modernization program demonstrated rapid and promising growth. It has the potential to revolutionize public health case surveillance by producing data that are timelier, richer, and more complete than manual case reporting, while also reducing the reporting burden on health care and jurisdictional PHAs.
Supplemental Material
Supplemental material, sj-docx-1-phr-10.1177_00333549241227160 for Electronic Case Reporting Development, Implementation, and Expansion in the United States by Kimberly Knicely, John W. Loonsk, Janet J. Hamilton, Annie Fine and Laura A. Conn in Public Health Reports
Acknowledgments
The authors thank our core team for their daily and foundational work, without which the eCR program could not have been implemented: Meredith Lichtenstein Cone, MPH, Alexis Darden, MSHI, Jordyn Easterling, BS, Gillian Haney, MPH, Shaily Krishan, MPH, Rebecca Lampkins, MPH, Charisse LaVell, MPH, and Shahidah Williams, MPH (Council of State and Territorial Epidemiologists); Imari Genias, MPH, Kashaine Gray, MPH, Brian Gugerty, MS, DNS, Grace Mandel, MPH, Terynn Mingo, MPH, Barbara Nichols, BS, Jill Raudabaugh, MPH, Preeti Ravindhran, MPH, Sarah Sobonya, PhD, and Melinda Thomas, MPH (Centers for Disease Control and Prevention [CDC]); Robert Brown, BS, Andrew Gabbeitt, BS, Analisa Lamair-Orosco, MBA, Midhun Mathew, BS, and Maureen Metcalfe, MS (Deloitte Consulting, LLP); and colleagues at the Association of Public Health Laboratories: Nagesh Bashyam, BE (Drajer, LLC); Patrick Caneer, MPH (Dataflow Informatics, LLC); Mike Flanigan, MS (Carradorra); Sarah Gaunt, BS (Lantana Consulting Group); Chuck Hagan, BS (Deloitte Consulting); Joel Hartsell, PhD, MPH (Epi-Vant Consulting); Travis Kushner, MPH (Informatics Turbine LLC); Julie Lipstein, MPH (Inductive Health); Timothy Morris, BS (ScioInformatics LLC); and Crystal Snare, DVM, MPH (University of Washington).
We acknowledge that eCR has been an ongoing multiyear effort that has involved more than 120 people with expertise in public health practice; interoperability standards electronic reporting and surveillance distribution (eRSD) experts; eCR Now Fast Healthcare Interoperability Resources (FHIR) application experts; Reportable Condition Knowledge Management System (RCKMS) teams; electronic health record industry partner and health care onboarding teams; state, tribal, local, and territorial public health agency team members; Health Equity & Data Analysis & Visualization team members; Geospatial Research, Analysis, and Service Program (GRASP) Geographic Information Systems (GIS) team members; communications team members and liaisons; policy liaisons; and liaisons in surveillance and data modernization
The authors also acknowledge Thomas Lupo, MEd (ViTek Consulting through Chenega Services & Federal Solutions), for his work on the citations. We acknowledge our current and previous leaders for their ongoing support. Most importantly, we acknowledge our jurisdictional public health agency partners who work tirelessly every day to protect the health of all US citizens
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC. Additionally, the eCR process described in this article uses a standards-based, shared, and interoperable infrastructure that is scalable nationwide for all reportable conditions, including sexually transmitted infections. Despite a similar name and focus, it is different and should not be confused with an initiative called eCR of exclusively sexually transmitted infections.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: APHL and CSTE authors received the following financial support for the research, authorship, and/or publication of this article: This work was supported by CDC to APHL (cooperative agreement no. OE20-2001) and to CSTE (cooperative agreement no. OT18-1802). CDC provided funding through a cooperative agreement with the National Indian Health Board and the National Council of Urban Indian Health (cooperative agreement no. OT18-1802). Contractual funding was provided by CDC to the Agency for Toxic Substances and Disease Registry, Geospatial Research, Analysis, and Services Program (GRASP) (contract no. 75D30118D01974). Onboarding, go live, and surge eCR support was provided by Deloitte Consulting, LLP, through contract no. 75D30120F09978, 75D30121F11838, and 75D30122F13737, respectively. Communications, technical writing, and business analytical support was provided by Chenega Professional & Technical Services, LLC (contract no. 75D30120C07723, HCPN1-2020-44296, and 200-2017-94367). Health informatics support was provided by 4ES Corporation (contract no. 75D301-20-C-08450). Program software engineering support was provided by Kapili Services, LLC (contract no. 75D30119D0535200007 on task order 200-2019-05352-0007). Health information systems support was provided by Booz Allen Hamilton as part of a contract with CDC’s National Center for Emerging and Zoonotic Infectious Diseases (contract no. 75D301-20-F-07161).
ORCID iDs: Kimberly Knicely, PhD  https://orcid.org/0000-0001-7794-3996
https://orcid.org/0000-0001-7794-3996
Laura A. Conn, MPH  https://orcid.org/0000-0002-3851-8265
https://orcid.org/0000-0002-3851-8265
Supplemental Material: Supplemental material for this article is available online. The authors have provided these supplemental materials to give readers additional information about their work. These materials have not been edited or formatted by Public Health Reports’s scientific editors and, thus, may not conform to the guidelines of the AMA Manual of Style, 11th Edition.
References
- 1. Barros JM, Duggan J, Rebholz-Schuhmann D. The application of internet-based sources for public health surveillance (infoveillance): systematic review. J Med Internet Res. 2020;22(3):e13680. doi: 10.2196/13680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foldy S. National public health informatics, United States. In: Magnuson JA, Dixon DE, eds. Public Health Informatics and Information Systems. Springer; 2020:439-458. doi: 10.1007/978-3-030-41215-9_24 [DOI] [Google Scholar]
- 3. Lee LM, Teutsch SM, Thacker SB, St Louis ME, eds. Principles and Practice of Public Health Surveillance. Oxford University Press; 2010. doi: 10.1093/acprof:oso/9780195372922.001.0001 [DOI] [Google Scholar]
- 4. Effer P, Ching-Lee M, Bogard A, Leong MC, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA. 1999;282(19):1845-1850. doi: 10.1001/jama.282.19.1845 [DOI] [PubMed] [Google Scholar]
- 5. Dixon BE, Zhang Z, Lai PTS, et al. Completeness and timeliness of notifiable disease reporting: a comparison of laboratory and provider reports submitted to a large county health department. BMC Med Inform Decis Mak. 2017;17(1):87. doi: 10.1186/s12911-017-0491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bender J. A Catalog and Review of Public Health Reporting Burden in the U.S. Office of the Chief Technology Officer, US Department of Health and Human Services; July 6, 2018. Accessed November 1, 2023. https://www.healthit.gov/sites/default/files/page/2020-03/ACatalogandReviewofPublicHealthReportingBurdenintheU.S.e.pdf [Google Scholar]
- 7. Centers for Disease Control and Prevention. Lyme disease: data and surveillance. Updated April 29, 2021. Accessed October 20, 2021. https://www.cdc.gov/lyme/datasurveillance/index.html
- 8. Mac Kenzie WR, Davidson AJ, Wiesenthal A, et al. The promise of electronic case reporting. Public Health Rep. 2016;131(6):742-746. doi: 10.1177/0033354916670871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. COVID-19 electronic case reporting. Updated November 12, 2021. Accessed December 15, 2021. https://www.cdc.gov/coronavirus/2019-ncov/php/electronic-case-reporting.html
- 10. Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36:345-359. doi: 10.1146/annurev-publhealth-031914-122747 [DOI] [PubMed] [Google Scholar]
- 11. Black J, Hulkower R, Suarez W, Patel S, Elliott B. Public health surveillance: electronic reporting as a point of reference. J Law Med Ethics. 2019;47(2 suppl):19-22. doi: 10.1177/1073110519857309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews P. The provider cost of manual reporting for COVID. Oral presentation at: Office of the National Coordinator for Health Information Technology Annual Meeting; (virtual); March 29-30, 2021. [Google Scholar]
- 13. Health Level Seven International. HL7 standards. Accessed March 23, 2023. https://www.hl7.org
- 14. Hoferka S. Evaluation of first electronic case reports received in Illinois. Online J Public Health Inform. 2019;11(1):e283. doi: 10.5210/ojphi.v11i1.9709 [DOI] [Google Scholar]
- 15. Illinois Department of Public Health. Infectious disease reporting. Updated 2022. Accessed February 11, 2022. https://dph.illinois.gov/topics-services/diseases-and-conditions/infectious-diseases/infectious-disease-reporting.htm
- 16. Rajamani S, Kayser A, Ruprecht A, et al. Electronic case reporting (eCR) of COVID-19 to public health: implementation perspectives from the Minnesota Department of Health. J Am Med Inform Assoc. 2022;29(11):1958-1966. doi: 10.1093/jamia/ocac133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamilton JJ, Turner K, Lichtenstein Cone M. Responding to the pandemic: challenges with public health surveillance systems and development of a COVID-19 national surveillance case definition to support case-based morbidity surveillance during the early response. J Public Health Manag Pract. 2021;27(suppl 1):S80-S86. doi: 10.1097/PHH.0000000000001299 [DOI] [PubMed] [Google Scholar]
- 18. Council of State and Territorial Epidemiologists. Update to the standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Updated January 2022. Accessed March 2, 2022. https://cdn.ymaws.com/www.cste.org/resource/resmgr/ps/ps2021/21-ID-01_COVID-19_updated_Au.pdf
- 19. Conn LA, Lane SR, Loonsk JW, Staes C. eCR Now: A Webinar Describing Efforts to Scale eCase Reporting Nationwide. American Medical Informatics Association; April 28, 2020. Accessed November 18, 2021. https://amia.org/news-publications/amia-covid-19-resource-center/amia-covid-19-webinar-series/ecr-now-webinar [Google Scholar]
- 20. eCR. AIMS Platform: eCR general information: congratulations to the winners of the eCR Now COVID-19 FHIR App Challenge! Accessed January 5, 2023. https://ecr.aimsplatform.org/general/ecr-now-covid-19-fhir-app-challenge.php
- 21. Digital Bridge. Multisite evaluation report: digital bridge electronic case reporting (eCR) implementations. 2019. Accessed November 15, 2021. https://ecr.aimsplatform.org/cms/resources/blocks/digital-bridge-ecr-evaluation-report-12-32019.pdf
- 22. Digital Bridge. Electronic case reporting conceptual architecture. February 2017. Accessed November 15, 2021. https://digitalbridge.us/wp-content/uploads/2017/08/DB_ConceptualArchitecture_022817.pdf
- 23. Rennick M, Gordon S, Huang M, Sum M, Soper P. The public health community platform: implementing electronic case reporting. Online J Public Health Inform. 2016;8(1):e155. doi: 10.5210/ojphi.v8i1.6572 [DOI] [Google Scholar]
- 24. Arzt NH, Minami M, Chertcoff D, Hui J. Strategies for clinical decision support for electronic case reporting. Online J Public Health Inform. 2019;11(1):e291. doi: 10.5210/ojphi.v11i1.9719 [DOI] [Google Scholar]
- 25. Cooney MA, Iademarco MF, Huang M, MacKenzie WR, Davidson AJ. The public health community platform, electronic case reporting, and the digital bridge. J Public Health Manag Pract. 2018;24(2):185-189. doi: 10.1097/PHH.0000000000000775 [DOI] [PubMed] [Google Scholar]
- 26. David J Sencer CDC Museum Public Health Academy. Contact tracing. Accessed October 1, 2023. https://www.cdc.gov/museum/pdf/cdcm-pha-stem-lesson-contact-tracing-lesson.pdf
- 27. Radia T, Williams N, Agrawal P, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51-57. doi: 10.1016/j.prrv.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Council of State and Territorial Epidemiologists. Position statement archive. Accessed January 5, 2023. https://www.cste.org/page/PositionStatements?&hhsearchterms=%22position+and+statement%22
- 29. Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Updated February 15, 2022. Accessed February 15, 2022. https://www.cdc.gov/nndss/index.html
- 30. Centers for Disease Control and Prevention. What is personally identifiable information? Accessed January 2, 2023. https://www.cdc.gov/nchs/training/confidentiality/training/page581.html
- 31. The Geography of Transport Systems. Point-to-point versus hub-and-spoke networks. 2020. Accessed January 8, 2023. https://transportgeography.org/contents/chapter2/geography-of-transportation-networks/point-to-point-versus-hub-and-spoke-network
- 32. Council of State and Territorial Epidemiologists. CSTE electronic case reporting update. SlideShare. September 14, 2015. Accessed November 12, 2021. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fcdn.ymaws.com%2Fwww.cste.org%2Fresource%2Fresmgr%2Fsurveillanceinformatics%2FeCR_Summary_Turner_revised_.pptx&wdOrigin=BROWSELINK
- 33. Association of Public Health Laboratories. EHR implementers—eICR creation, validation, and standards: what is an eICR document? What does it include? 2022. Accessed January 10, 2022. https://ecr.aimsplatform.org/ehr-implementers/eicr-creation-validation-standards
- 34. Staes C. RCKMS: strengths and limitations of the electronic initial case report (eICR) as a data source for reportable condition decision support. Paper presented at: 2018 CSTE Annual Conference; June 10-14, 2018; West Palm Beach, FL. Accessed November 11, 2021. https://cste.confex.com/cste/2018/meetingapp.cgi/Paper/10149 [Google Scholar]
- 35. Health Level Seven International. HL7 FHIR implementation guide: electronic case reporting (eCR)—US realm. Electronic initial case report (eICR) transaction and profiles. January 29, 2017. Accessed February 28, 2023. http://www.hl7.org/implement/standards/product_brief.cfm?product_id=436 [Google Scholar]
- 36. Health Level 7 International. HL7 CDA® R2 implementation guide: public health case report—the electronic initial case report (eICR) release 2, STU release 2.1, FHIR version 4.0.1—US realm. 2022. Accessed February 28, 2023. http://www.hl7.org/implement/standards/product_brief.cfm?product_id=436
- 37. Health Level Seven International. US core implementation guide 6.1.0—STU6 release. Updated June 28, 2021. Accessed January 25, 2022. https://www.hl7.org/fhir/us/core
- 38. Office of the National Coordinator for Health Information Technology. United States core data for interoperability (USCDI). 2022. Accessed January 25, 2022. https://www.healthit.gov/isa/united-states-core-data-interoperability-uscdi
- 39. McKenzie L. (modified by Peters M). HL7 balloting. Updated March 3, 2021. Accessed January 25, 2022. https://confluence.hl7.org/display/HL7/HL7+Balloting
- 40. Health Level Seven International. FHIR electronic case reporting (eCR) (FHIR IG) publication (version) history. 2022. Accessed January 25, 2022. http://hl7.org/fhir/us/ecr/history.html
- 41. Health Level Seven International. HL7 CDA® R2 implementation guide: reportability response, release 1, STU release 1.0—US realm. 2018. Accessed January 25, 2022. http://www.hl7.org/implement/standards/product_brief.cfm?product_id=470
- 42. Association of Public Health Laboratories. EHR implementers—reportability response receipt and use. What is a reportability response? What is in it? 2022. Accessed January 8, 2022. https://ecr.aimsplatform.org/ehr-implementers/reportability-response-receipt-and-use
- 43. Health Level Seven International. HL7 FHIR® implementation guide: electronic case reporting (eCR)—US realm. R3.1—STU 2. Electronic reporting and surveillance distribution (eRSD) transaction and profiles. January 2020. Accessed February 28, 2023. http://build.fhir.org/ig/HL7/case-reporting/electronic_reporting_and_surveillance_distribution_ersd_transaction_and_profiles.html [Google Scholar]
- 44. Association of Public Health Laboratories. EHR implementers—EHR triggering: triggering of eICRs. 2022. Accessed February 5, 2022. https://ecr.aimsplatform.org/ehr-implementers/triggering
- 45. Association of Public Health Laboratories. eCR Now FHIR app. 2021. Accessed November 24, 2021. https://ecr.aimsplatform.org/ecr-now-fhir-app
- 46. Health Level Seven International. FHIR release 4. Updated November 1, 2019. Accessed January 15, 2022. https://hl7.org/fhir/R4
- 47. 21st Century Cures Act, Pub L No 114-255 (2016). [Google Scholar]
- 48. Association of Public Health Laboratories. AIMS platform. 2023. Accessed August 25, 2023. https://www.aphl.org/programs/informatics/pages/aims_platform.aspx
- 49. Lynn T, van der Werff L, Fox G. Understanding trust and cloud computing: an integrated framework for assurance and accountability in the cloud. In: Lynn T, Mooney JG, van der Werff L, Fox G. eds. Data Privacy and Trust in Cloud Computing: Building Trust in the Cloud Through Assurance and Accountability. Palgrave MacMillan; 2021:1-20. [Google Scholar]
- 50. DirectTrust. Trust framework. 2021. Accessed September 30, 2021. https://directtrust.org/what-we-do/trust-framework
- 51. Office of the National Coordinator for Health Information Technology. Governance framework for trusted electronic health information exchange. 2013. Accessed November 25, 2021. https://www.healthit.gov/sites/default/files/GovernanceFrameworkTrustedEHIE_Final.pdf
- 52. Centers for Disease Control and Prevention. Health Insurance Portability and Accountability Act of 1996 (HIPAA). Updated September 14, 2018. Accessed October 20, 2021. https://www.cdc.gov/phlp/publications/topic/hipaa.html
- 53. Health Insurance Portability and Accountability Act, Pub L No 104-191 (1996). [Google Scholar]
- 54. Office of the National Coordinator for Health Information Technology. State health IT privacy and consent laws and policies. Updated August 21, 2019. Accessed October 20, 2021. https://www.healthit.gov/topic/state-health-it-privacy-and-consent-laws-and-policies
- 55. Association of Public Health Laboratories. Healthcare—establish AIMS connectivity for testing. 2022. Accessed January 15, 2022. https://ecr.aimsplatform.org/healthcare/establish-aims-connectivity-for-testing
- 56. DirectTrust. Trust communities. Accessed November 1, 2023. https://directtrust.org/what-we-do/trust-framework#trust-framework-concepts
- 57. eHealth Exchange. eHealth Exchange launches electronic case reports (eCR) nationwide. August 17, 2020. Accessed December 3, 2021. https://ehealthexchange.org/ehealth-exchange-launches-electronic-case-reports-ecr-nationwide
- 58. Office of the National Coordinator for Health Information Technology. Certification process. 2023. Accessed January 5, 2023. https://www.healthit.gov/topic/certification-ehrs/certification-process
- 59. US Department of Commerce, National Institute of Standards and Technology. Federal Information Security Management Act (FISMA) implementation project. March 19, 2018. Accessed February 28, 2022. https://www.nist.gov/programs-projects/federal-information-security-management-act-fisma-implementation-project
- 60. LOINC. Universal laboratory order codes from LOINC. Updated August 23, 2021. Accessed January 26, 2022. https://loinc.org/usage/orders
- 61. SNOMED International. SNOMED. Updated 2022. Accessed January 25, 2022. https://www.snomed.org
- 62. National Center for Health Statistics. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Last updated June 29, 2023. Accessed November 30, 2023. https://www.cdc.gov/nchs/icd/icd-10-cm.htm [PubMed]
- 63. Centers for Disease Control and Prevention. Immunization Information Systems (IIS): IIS current HL7 standard code set CVX—vaccines administered. January 25, 2022. Accessed February 6, 2022. https://www2a.cdc.gov/vaccines/iis/iisstandards/vaccines.asp?rpt=cvx
- 64. National Institutes of Health, National Library of Medicine. Unified Medical Language System: RxNorm. July 9, 2021. Accessed January 26, 2022. https://www.nlm.nih.gov/research/umls/rxnorm/index.html
- 65. Council of State and Territorial Epidemiologists. Surveillance/informatics: Reportable Conditions Knowledge Management System. 2022. Accessed January 5, 2022. https://www.cste.org/members/group.aspx?code=RCKMS
- 66. Hamilton JJ, Turner K, Altamore R, et al. Electronic case reporting (eCR). Updated 2016. Accessed January 5, 2022. https://cdn.ymaws.com/www.cste.org/resource/resmgr/2016PS/16_SI_02.pdf
- 67. LaVell C, Krishan S, Lichtenstein M, Minami M. Reportable Conditions Knowledge Management System (RCKMS): real-life implementation progress and our future. Paper presented at: 2019 CSTE Annual Conference; June 2-6, 2019; Raleigh, NC. Accessed January 5, 2022. https://cste.confex.com/cste/2019/meetingapp.cgi/Paper/11888 [Google Scholar]
- 68. Altamore R. It’s not the mirror making it look closer: progress toward the Reportable Conditions Knowledge Management System. Paper presented at: 2014 CSTE Annual Conference; June 22-26, 2014; Nashville, TN. Accessed January 5, 2022. https://cste.confex.com/cste/2014/webprogram/Paper3771.html [Google Scholar]
- 69. Reportable Conditions Knowledge Management System. RCKMS content and RCTC release plan. Updated January 2023. Accessed August 18, 2023. https://www.rckms.org/wp-content/uploads/2023/01/RCKMS-Release-Plan-January-2023.pdf
- 70. Definitive Healthcare. 10 most common inpatient EHR systems by 2021 market share. Updated April 2023. Accessed June 12, 2023. https://www.definitivehc.com/blog/most-common-inpatient-ehr-systems
- 71. Centers for Disease Control and Prevention. Electronic case reporting: two tribal public health agencies to receive eCR data. Last reviewed October 11, 2023. Accessed November 1, 2023. https://www.cdc.gov/ecr/stories/tribal-public-health-agencies.html
- 72. Centers for Disease Control and Prevention. Public health surveillance and data: DMI and COVID-19. Updated January 9, 2023. Accessed January 5, 2023. https://www.cdc.gov/surveillance/data-modernization/dmi-covid-19-data-improvement.html
- 73. Centers for Disease Control and Prevention. eCR team provides rapid support for mpox virus reporting. Reviewed August 15, 2023. Accessed November 28, 2023. https://www.cdc.gov/ecr/action.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-phr-10.1177_00333549241227160 for Electronic Case Reporting Development, Implementation, and Expansion in the United States by Kimberly Knicely, John W. Loonsk, Janet J. Hamilton, Annie Fine and Laura A. Conn in Public Health Reports