Abstract
A second autologous stem‐cell transplantation (ASCT2) is considered for relapsed multiple myeloma (RMM) patients showing prolonged response after a first ASCT. However, given breakthrough treatments like anti‐CD38 and immunotherapy, its role remains debated. We conducted a real‐life study in 10 French centers (1996–2017) involving 267 RMM patients receiving ASCT2. The median age was 61 years, with 49% females. Most patients received melphalan 200 mg/m² before ASCT2, with low early mortality (1%). Very good partial response or better (VGPR+) rate post ASCT2 was 78%. Post ASCT2, 48% received consolidation therapy and 40% maintenance therapy. Median event‐free survival (EFS) after ASCT2 was 2.6 years (95% confidence interval [CI]: 2.3–2.8), and 2‐year EFS estimate was 63% (95% CI: 57–70). Median overall survival (OS) was 8.1 years (95% CI: 5.9–NA), and 2‐year OS estimate was 92% (95% CI: 88–95). Multivariate analysis revealed that VGPR+ status and maintenance therapy post ASCT2 were associated with better EFS (hazard ratio [HR]: 0.6; 95% CI: 0.3–0.9, p = 0.012 and HR: 0.4; 95% CI: 0.3–0.6, p < 0.001, respectively) and OS (HR: 0.4; 95% CI: 0.2–0.9, p = 0.017 and HR: 0.2; 95% CI: 0.1–0.4, p < 0.001, respectively), while male sex correlated with poorer outcomes for EFS (HR: 2.5; 95% CI: 1.7–3.7, p < 0.001) and OS (HR: 2.7; 95% CI: 1.4–4.9, p = 0.002). Overall, ASCT2 appeared efficient with low toxicity in RMM. Maintenance therapy was associated with extended EFS and OS, particularly in patients with VGPR+ status post ASCT2. These findings underscore ASCT2's potential in RMM when coupled with maintenance therapy in selected patients.
INTRODUCTION
Induction therapy, followed by intensification with high‐dose chemotherapy (HDC) and autologous stem‐cell transplantation (ASCT), has become the standard of care for newly diagnosed multiple myeloma (NDMM) in young and fit patients (age <70 years) in the 1990s. 1 Until recent approval of the anti‐CD38 monoclonal antibody (mAb) daratumumab as part of frontline treatment, the most widely used induction regimens consisted of triplet combinations based on proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and steroids. 2 , 3 , 4 , 5 Frontline ASCT improved both overall survival (OS) and progression‐free survival (PFS) compared with prolonged induction therapy alone without intensification. 4 , 6
Nevertheless, despite new drugs and continuous improvement of prognostics, multiple myeloma is still considered incurable, and almost all patients will ultimately relapse. As such, an effective second‐line treatment is needed. Based on retrospective data and case‐control studies, a second intensification with HDC and ASCT presents an appealing option for relapsed multiple myeloma (RMM) over chemotherapy alone, due to its potential to provide prolonged OS and PFS. 7 , 8 , 9
Several retrospective studies have reported that prolonged remission after frontline ASCT is strongly associated with prolonged PFS and OS after a second ASCT. 7 , 8 , 9 , 10 , 11 , 12 , 13 Based on these observations, the International Myeloma Working Group (IMWG) and the European Society of Medical Oncology (ESMO) recommend this strategy for patients with relapsed myeloma who experienced prolonged remission upper than 24–36 months after the first ASCT. 14 , 15
Most of the randomized controlled trials evaluating this strategy were conducted before the widespread use of combination regimens of PIs and IMiDs. They were therefore difficult to interpret, especially in the current therapeutic landscape. Indeed, at relapse, treatment regimens combining PIs, IMiDs, and/or anti‐CD38 mAb are currently used in daily practice. 16 , 17 , 18 In summary, real‐life data are needed about the sequence therapeutic of a second ASCT (HDC type, consolidation, maintenance) and true prognosis value of duration of response after frontline ASCT. Therefore, we reported here survival data and survival analysis of a multicentric real‐life cohort of RMM patients treated with a second ASCT.
MATERIALS AND METHODS
Population
We included retrospectively RMM patients, diagnosed with MM between 1996 and 2017, who received at least two HDC treatments followed by ASCT in 10 French tertiary centers. Patients were identified from local cell therapy unit registers among those for which cell therapy products had been thawed twice: patients who underwent a second HDC followed by ASCT at first relapse or later. Patients who received allogeneic stem cell transplantation after the first ASCT or frontline tandem ASCT were secondarily excluded. Patients with plasma cell leukemia were excluded. The study was performed in respect of the Declaration of Helsinki and was registered by an ethics committee (n° PI2021_843_0171).
Definitions and data collection
Hereafter, we will refer to ASCT performed frontline as “ASCT1” and ASCT performed in relapse setting as “ASCT2.” Patients were diagnosed according to the IMWG. 19 Treatment was initiated frontline and at relapse according to contemporaneous local guidelines. 19 Nature of drugs administered and treatment modalities, including the use of consolidation and/or maintenance therapy, were at the treating physician's discretion. Response rates were retrospectively evaluated according to the latest IMWG guidelines based on available data from medical records. 20 Because bone marrow evaluation was only performed in a minority of cases, we choose not to distinguish patients in complete response from patients in very good partial response according to IMWG: all these patients will subsequently be considered in very good partial or better (VGPR+). Time without new treatment after ASCT1 (TNT1) was defined as the time from ASCT1 realization to start of a new line of treatment. Neutrophil recovery time after ASCT2 infusion was defined as the time between ASCT infusion and absolute neutrophils count >0.5 G/L for three consecutive days. Adverse events (AEs) were divided into two categories: early toxicity (during the first 3 months following ASCT2) and late toxicity (after the first 3 months). Also, only grade 3–4 adverse events of special interest (AEIs) were collected, excluding transfusion requirement, febrile neutropenia of unknown origin, and sepsis without need for intensive care stay during the aplasia period. Event‐free survival (EFS) was defined as the time from ASCT2 to start of a new line of treatment, death, or last follow‐up, whichever occurred first. Any consolidation and/or maintenance therapy given after ASCT2 was considered to belong to the same line of treatment as ASCT2. Overall survival after ASCT2 (OS) was calculated from the ASCT2 realization until death or last follow‐up, whichever appeared first. For landmark analysis, EFS and OS were calculated based on landmark time as defined below. The cut‐off for survival analysis was June 2021.
Statistical analysis
Descriptive statistics were used to characterize the data: the median and interquartile range have been calculated for continuous variables; absolute frequencies and percentages have been provided for each qualitative variable. The chi‐squared test, Kruskal–Wallis test, and Student test were used to compare the distribution between subgroups of nominal, ordinal, and quantitative variables, respectively.
Survival functions were estimated using the Kaplan–Meier method, and a direct comparison between groups was made using the log‐rank test for EFS and OS. Univariate and multivariate analysis used a time‐dependent, semi‐parametric, Cox proportional hazard regression model to identify prognostic factors influencing EFS and OS. Variables with a p < 0.1 in univariate models were included in multivariate models for OS and EFS. “Baseline variable” referred to a variable whose value was known at the time of ASCT2, before the start of survival analysis. “Time‐dependent variable” was a variable whose value wasn't known at the time of ASCT2 but was reported during follow‐up. To consolidate the results of the multivariate time‐dependent analysis regarding the impact of time‐dependent covariates, univariate, and multivariate landmark approach for survival analysis was performed: landmark time was defined as 90 days after ASCT2 corresponding to the start of maintenance therapy if effectively introduced. Start of follow‐up and covariates' status were defined at the time of this landmark time.
To avoid any confounding bias attributable to any of these parameters, multivariate analysis was stratified on the center and time period of ASCT2 (arbitrarily divided between before vs. after 2015) for both EFS and OS. The final Cox model was inspected for interactions and collinearity with variance inflation factors, while the proportional hazards assumption was verified using statistical tests and graphical diagnostics based on the scaled Schoenfeld Residuals.
The optimal cut‐point for continuous variables like age at the time of ASCT2 and time to next treatment after ASCT1 was determined using the maximally selected rank statistics, providing a value of a cut‐point corresponding to the most significant relationship with OS and/or EFS. 21 To simplify, we chose to use a single value to therefore categorize these continuous variables into binary ones for survival analysis for both EFS and OS and kept cut‐point values identified as most relevant for OS.
Continuous variables were therefore categorized into binary variables for survival analysis for EFS and OS and, reported p values were two‐sided. Only p < 0.05 were considered significant.
Statistical analysis was performed using the free software R version 3.6.3 and the integrated development environment for R, RStudio. Survival analysis, survival curves, and graphics were all performed using “survival,” “survminer,” and “ggplot2” packages for R (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/). Time‐dependent analysis was done using the “tmerge” package for R as described by Therneau et al. 22
RESULTS
Patient characteristics
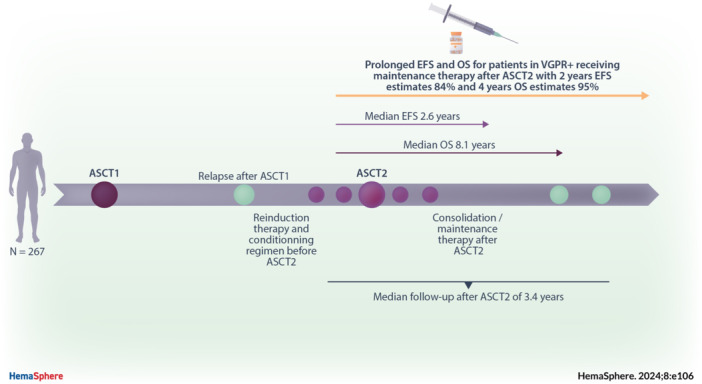
We included 267 patients diagnosed with MM between 1996 and 2017 and treated twice, both at the frontline and at relapse, with HDC followed by ASCT (Table 1). A total of 131 patients (49%) were female, with a median age of 55 years at diagnosis (interquartile range [IQR]: 49–59). Among the 159 patients with available international staging system (ISS) classification at diagnosis, 45% (n = 72) had a favorable score. Among the 165 (62%) patients with available cytogenetic, 30 patients (18%) showed high‐risk cytogenetic abnormalities: del(17p) (n = 3, 2%) or translocation involving IGH locus on chromosome 14 (except t(11;14)) (n = 27, 16%). As frontline induction therapy before ASCT1, 190 (71%) patients received a PI, 113 (42%) patients received an IMiD, and 102 (38%) received a combination of both IMiD and PI. The overall response rate (ORR) after ASCT1 was 96%, with 69% achieving very good partial response or better (VGPR+). Eleven percent received maintenance therapy after ASCT1. The median TNT1 was 3.3 years (IQR: 2.4–4.8).
Table 1.
Patient characteristics.
| Characteristics | All, N = 267 |
|---|---|
| Sex | |
| Female | 131 (49.1%) |
| Male | 136 (50.9%) |
| Age at time of second ASCT (years) | |
| Median (Q1; Q3) | 61.0 (55.0–64.0) |
| ISS score at diagnosis of MM | |
| I | 72 (27.0%) |
| II | 46 (17.2%) |
| III | 41 (15.4%) |
| Missing | 108 (40.4%) |
| Cytogenetic risk at diagnosis of MM | |
| Higha | 30 (11.2%) |
| Intermediate/low | 135 (50.6%) |
| Missing | 102 (38.2%) |
| Time without new treatment after first ASCT (months) | |
| Median (Q1; Q3) | 40.0 (28.5–58.0) |
| Number of prior line(s) of therapy before second ASCT | |
| 1 (i.e., second ASCT at first relapse) | 220 (82.4%) |
| >1 (i.e., second ASCT at second relapse or later) | 47 (17.6%) |
| Induction regimen prior to second ASCT | |
| IMiD & PI‐based | 134 (50.2%) |
| PI‐based | 60 (22.5%) |
| IMiD‐based | 46 (17.2%) |
| Anti‐CD38 mAb‐based | 10 (3.7%) |
| Conventional chemotherapy and others (including no treatment or radiotherapy alone) | 17 (6.4%) |
| Conditioning regimen of second ASCT | |
| Intensive (melphalan + busulfan or melphalan + TBI) | 33 (12.4%) |
| Standard (melphalan 200 mg/m²) | 183 (68.5%) |
| Reduce (melphalan 140 mg/m² or melphalan 100 mg/m²) | 51 (19.1%) |
| Consolidation therapy after second ASCT | |
| Yes | 128 (47.9%) |
| No | 139 (52.1%) |
| Maintenance therapy after second ASCT | |
| Yes | 107 (40.1%) |
| No | 160 (59.9%) |
Abbreviations: ASCT, autologous stem‐cell transplantation; IMiD, immunomodulatory drug; ISS, International Staging System; MM, multiple myeloma; mAb, monoclonal antibody; PI, proteasome inhibitor; TBI, total body irradiation.
t(4;14), t(14;16), t(14;20), del(17p), del(1p32) and 1q amplification.
Eighty‐three percent (n = 220) of patients received ASCT2 at first relapse and 44 (16%) received it at second relapse or later, including 28 (10%) at second relapse, nine (3%) at third relapse, and seven (3%) at fourth relapse or later. The number of lines of therapy received between ASCT1 and ASCT2 was unknown in 3 (1%) patients. Patients who received ASCT2 at second relapse or later were treated with less intensive HDC (melphalan 140 mg/m² or less in 34% vs. 16%; p = 0.010) and had lower response rate after ASCT2 (ORR 75% vs. 87% and VGPR+ 41% vs. 59%; p < 0.001) when compared to those treated at the first relapse. No significant difference for median age at the time of ASCT2 (62 vs. 61 years; p = 0.540) was observed between both groups. The most common induction therapy before ASCT2 was a triplet combination of steroids, IMiD, and PI in 134 patients (50%). Otherwise, 60 patients (23%) received an IMiD‐based doublet, 46 (11%) received a PI‐based doublet, and 17 (6%) received conventional chemotherapy or radiotherapy alone. Ten patients (4%) received daratumumab and 18 (7%) received carfilzomib‐based regimens. Currently, used MM therapies (PI, IMiD and/or anti‐CD38 based regimen) were associated with increased ORR (92% vs. 69%; p = 0.009) and VGPR+ rates (62% vs. 25%; p = 0.009) before ASCT2 compared to conventional chemotherapy or radiotherapy alone.
Among all patients, 170 (64%) received ASCT2 after 2015 and 97 (36%) received ASCT2 before 2015, including 26 before 2010 and 71 between 2010 and 2015. When compared to patient treaded with ASCT2 before 2015, patients treated with ASCT2 after 2015 were older (median age at the time of ASCT2 62 vs. 60 years, p = 0.013), had prolonged response after ASCT1 (median TNT1 of 43 vs. 32 months, p = 0.001), and were more likely to be treated with triplet combination of steroids, IMiD, and PI or anti‐CD38 based regimen (n = 115 vs. 29, 68% vs. 30%, p < 0.001). Both groups were comparable for female proportion (n = 85 vs. 46, 50% vs. 47%), high ISS score when available (n = 27/90 vs. 14/49, 25% vs. 29%), presence of high‐risk cytogenetic abnormalities when available (n = 19/114 vs. 11/51, 17% vs. 22%), and timing of ASCT2 (ASCT2 at first relapse in n = 139 vs. 81, 83% vs. 84%).
ASCT2: Procedures, response, whole cohort EFS, and OS
The median age at the time of ASCT2 was 61 years (IQR: 55–64). The most used conditioning regimen before ASCT2 was melphalan 200 mg/m² (Mel 200) in 183 patients (69%). A reduced‐intensity conditioning regimen was used in 51 patients (19%), including melphalan 140 mg/m² (Mel 140) in 38 patients (14%) and melphalan 100 (Mel 100) mg/m² in 13 patients (5%). A more intensive regimen was used in 33 (12%) patients, including melphalan + busulfan in 25 patients (9%) or melphalan + total body irradiation in eight patients (3%). When compared to patients who received reduced‐intensity conditioning regimen, patients treated with Mel 200 or more intensive regimen were younger (median age at the time of ASCT2 60 years vs. 64 years, p < 0.001) and received fewer lines of therapy before ASCT2 (ASCT2 at first relapse in n = 184 vs. 36, 86% vs. 71%, p = 0.012). No significant difference was observed between the two groups about gender, ISS score and cytogenetic at diagnosis, and response depth before ASCT2.
Among patients with available data (n = 231), the best ORR before ASCT2 was 91%, including 61% VGPR+. After ASCT2, response depth improved in 47 patients (20%) and worsened in 12 patients (5%) with an ORR of 96% and a VGPR+ rate of 78% (Figure 1). Currently, used MM therapies led to a better VGPR+ rate after ASCT2 when compared to conventional chemotherapy or radiotherapy alone (82% vs. 29%; p < 0.001). A reduced intensity regimen was associated with a significantly poorer response depth (Kruskal–Wallis test; p = 0.012) and a poorer VGPR+ rate after ASCT2 when compared to Mel 200 or more intensive conditioning (69% vs. 82%; p = 0.057). On the other hand, a more intensive regimen did not seem to lead to a better VGPR+ rate when compared to Mel 200 (81% vs. 80%; p = 0.140).
Figure 1.
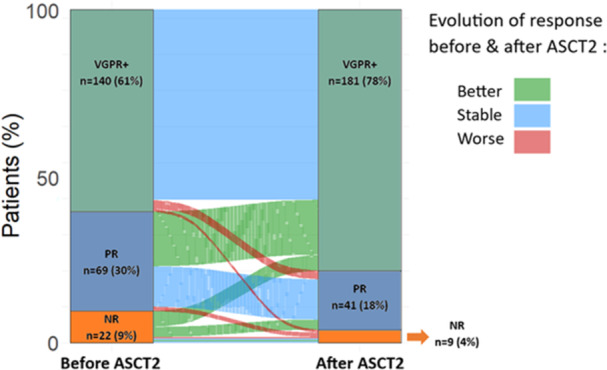
Response rate (evaluated according to IMWG) before and after the autologous stem‐cell transplantation (ASCT2). NR, nonresponder; PR, partial response; VGPR+, very good partial response or complete response, that is, stable disease or progressive disease.
After ASCT2, 128 patients (48%) received consolidation therapy and 107 (40%) received maintenance therapy. Seventy‐two patients (27%) received both consolidation and maintenance therapy. The most used consolidation therapy combined IMiD and PI in 76/128 patients (59%). The most used maintenance therapy after ASCT2 was IMiD alone in 87/107 patients (81%), and most used IMiD in this setting was lenalidomide in 80/107 patients (75%). Median duration of exposure to maintenance therapy was 15 months (IQR: 8.5–24.5). No difference for gender, age at the time of ASCT2, ISS score and cytogenetic at diagnosis, number of lines of therapy before ASCT2, and response depth after ASCT2 was observed between patients treated with consolidation therapy and those who did not or between patients treated with maintenance therapy and those who did not. When compared to those who did not, patients who received consolidation and/or maintenance therapy were more likely to have been treated with triplet combination of steroids, IMiD, and PI or anti‐CD38‐based regimen. Patient's characteristics according to whether they received or not consolidation and/or maintenance therapy are summarized in Supporting Information S1: Table 1.
When compared to patient treated with ASCT2 before 2015, patients treated with ASCT2 after 2015 were significantly more likely to receive consolidation therapy (n = 95/170 vs. 33/97, 56% vs. 34%; p < 0.001) and significantly more likely to receive maintenance therapy (n = 87/170 vs. 20/97, 51% vs. 21%; p < 0.001). There was no difference between the two groups for conditioning used before ASCT2 (Mel 200 or more intensive in regimen in n = 140/170 vs. 76/97, 74% vs. 79%).
Median follow‐up after ASCT2 was 3.4 years (IQR: 1.6–5). For all patients, 2‐year EFS and OS estimates were 63% (95% CI: 57–70) and 92% (95% CI: 88–95), respectively, and 4‐year EFS and OS estimates were 28% (95% CI: 22–36) and 79% (95% CI: 74–85), respectively. Median calculated EFS and OS were 2.6 years (95% CI: 2.3–2.8) and 8.1 years (95% CI: 5.9–not reached [NR]), respectively (Figure 2). Survival appeared to be better in patients who received ASCT2 after than before 2015 for both EFS (2‐year estimates 71% [95% CI: 64–79] vs. 51% [95% CI: 42–62]) and OS (2‐year estimates 93% [95% CI: 89–97] vs. 88% [95% CI: 82–95]).
Figure 2.
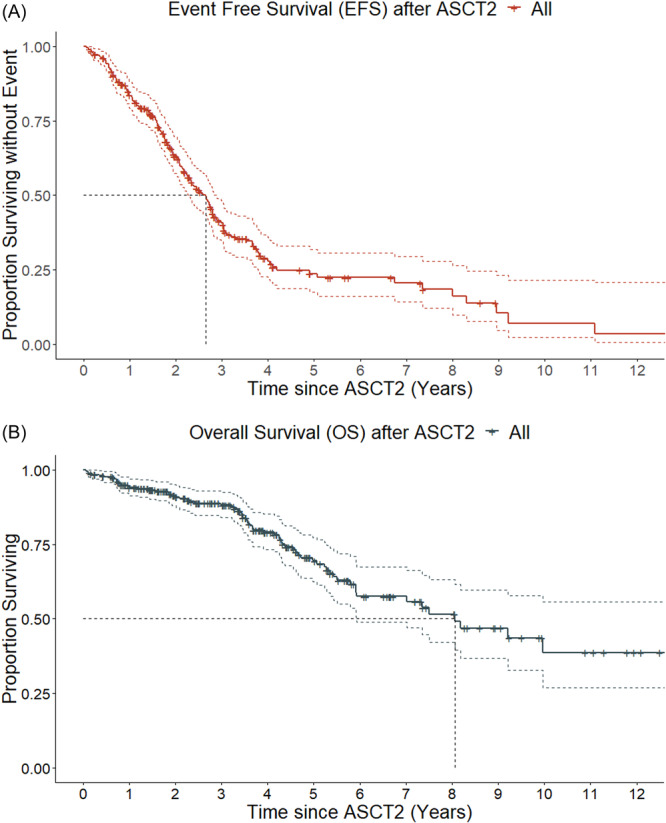
Survival outcome after the second autologous stem‐cell transplantation (ASCT2) for all patients; (A) For Kaplan–Meier estimates for event‐free survival (EFS), median EFS was 2.6 years. (B) For Kaplan–Meier estimates for overall survival (OS), median OS was 8.1 years.
Toxicity and mortality after ASCT2
The median time for neutrophil recovery was 12 days (IQR: 10–14, ranging from 5 to 24 days). Regarding early toxicity, seven patients (3%) required an intensive care unit stay and four (1%) had died, two from sepsis and two from an unknown cause, within 3 months of ASCT2. Among those four patients, two received Mel 200, one received Mel 140, and one received melphalan + busulfan. During follow‐up, 25 patients (9%) experienced late AESIs, including seven deaths unrelated to myeloma (partial response or better at death). The most frequently reported were infections in 11 patients, hematological malignancies in nine patients (seven myelodysplastic syndrome, one patient with acute myeloid leukemia, and one patient with acute lymphoid leukemia), solid neoplasia in four patients, and severe pulmonary embolism in one patient.
At the last follow‐up, 75 patients (28%) died. The principal death cause was MM progression (n = 58, 77%). For the others, five (6%) died from infectious disease, one (1%) from pulmonary embolism, one (1%) from ovarian adenocarcinoma, and 10 (15%) from an unknown cause.
ASCT2: Univariate and multivariate analysis for EFS and OS
Most discriminating cut‐points regarding EFS and OS for age at the time of ASCT2 were 55.0 and 52.5 years, respectively. Most discriminating cut‐points regarding EFS and OS for TNT1 were 26 and 33 months, respectively. We kept the value corresponding to the best cut‐point for OS and rounded the 33 months to 36, that is, 3 years. Distribution and probability density for age at the time of ASCT2 and TNT1 are represented in Supporting Information S1: Figure 1.
As described above, multivariate analyses were stratified by center and time period of ASCT2.
In a univariate analysis for EFS, including time‐dependent analysis for relevant variables, gender, TNT1 > 3 years, age at the time of ASCT2 > 52.5 years, response before ASCT2, conditioning regimen before ASCT2, response after ASCT2, and maintenance therapy after ASCT2 were all significantly associated with TNT1 whereas ISS at diagnosis, cytogenetic, and number of prior lines of therapy before ASCT2 (i.e., ASCT2 at first relapse vs. ASCT2 at second relapse or later) were not significantly associated with EFS (Table 2).
Table 2.
Univariate and time‐dependent multivariate survival analysis for event‐free survival (EFS) after second autologous stem‐cell transplantation (ASCT2).
| Univariate analysis (Log‐Rank) | Multivariate analysis (Cox) | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | p value* | HR | 95% CI | p value** |
| Sex | ||||||
| Female | Ref. | – | – | Ref. | – | – |
| Male | 1.50 | 1.10−2.00 | 0.020 | 2.47 | 1.66−3.66 | <0.001 |
| Age at time of second ASCT2 | ||||||
| <52.5 years | Ref. | – | – | Ref. | – | – |
| >52.5 years | 1.60 | 1.10−2.50 | 0.023 | 0.80 | 0.49−1.31 | 0.376 |
| ISS score at diagnosis of MM | ||||||
| I | Ref. | – | – | |||
| II–III | 1.20 | 0.81−1.90 | 0.310 | |||
| Cytogenetic risk at diagnosis of MM | ||||||
| Intermediate/low | Ref. | – | – | |||
| Higha | 0.98 | 0.59–1.60 | 0.940 | |||
| Number of prior line(s) of therapy before ASCT2 | ||||||
| 1 | Ref. | – | – | |||
| >1 | 1.20 | 0.78–1.80 | 0.420 | |||
| Time without new treatment after first ASCT (months) | ||||||
| <36 months | Ref. | – | – | Ref. | – | – |
| >36 months | 0.57 | 0.41–0.78 | <0.001 | 0.79 | 0.53–1.16 | 0.232 |
| Response depth before ASCT2 | ||||||
| VGPR+ | Ref. | – | – | Ref. | – | – |
| PR, SD, or PD | 1.50 | 1.10–2.00 | 0.026 | 0.82 | 0.52–1.30 | 0.404 |
| Conditioning regimen of second ASCT | ||||||
| Standard or Intensive | Ref. | – | – | Ref. | – | – |
| Reduce | 1.60 | 1.10–2.30 | 0.019 | 1.00 | 0.64−1.58 | 0.974 |
| Response depth after ASCT2b | ||||||
| VGPR+ | Ref. | – | – | Ref. | – | – |
| PR, SD, or PD | 1.76 | 1.35–2.28 | <0.001 | 1.83 | 1.14–2.93 | 0.012 |
| Consolidation therapy after ASCT2b | 1.25 | 0.89–1.75 | 0.200 | |||
| Maintenance therapy after ASCT2b | 0.61 | 0.43–0.88 | 0.007 | 0.43 | 0.28–0.65 | <0.001 |
Abbreviations: CI 95%, 95% confidence interval; HR, hazard ratio; MM, multiple myeloma; Ref., reference for HR calculus and interpretation; PD, progressive disease; PR, partial response; SD, stable disease; VGPR+, very good partial response or complete response. All according to IMWG.
t(4;14), t(14;16), t(14;20), del(17p), del(1p32) and 1q amplification.
time‐dependent variable.
p Value for Log‐Rank test
p Value for Wald test.
Multivariate analysis including only baseline variables showed that EFS was significantly decreased in male patients (HR: 1.8; 95% CI: 1.2–2.5, p = 0.002) and older patients (HR: 1.9; 95% CI: 1.2–3.1, p = 0.008). Conversely, VGPR+ before ASCT2 (HR: 0.6; 95% CI: 0.4–0.8, p < 0.001) and TNT1 > 3 years (HR: 0.6; 95% CI: 0.4–0.8, p = 0.003) were significantly associated with better EFS (data not shown). When significant time‐dependent variables (response depth after ASCT2 and maintenance therapy after ASCT2) were included in the multivariate model, only VGPR+ after ASCT2 (HR: 0.6; 95% CI: 0.3–0.9, p = 0.012) and maintenance therapy after ASCT2 (HR: 0.4; 95% CI: 0.3–0.6, p < 0.001) were associated with prolonged EFS, whereas male sex was associated with shorter EFS (HR: 2.5; 95% CI: 1.7–3.7, p < 0.001) (Table 2).
In a univariate analysis for OS, including time‐dependent analysis for relevant variables, gender, TNT1 > 3 years, age at the time of ASCT2 > 52.5 years, response after ASCT2, and maintenance therapy after ASCT2 were all significantly associated with OS, whereas ISS at diagnosis, cytogenetic, number of prior lines of therapy before ASCT2 (i.e., ASCT2 at first relapse vs. ASCT2 at second relapse or later), response before ASCT2, and conditioning regimen before ASCT2 were not significantly associated with OS (Table 3).
Table 3.
Univariate and time‐dependent multivariate survival analysis for overall survival (OS) after second autologous stem‐cell transplantation (ASCT2).
| Univariate analysis (Log‐Rank) | Multivariate analysis (Cox) | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | p value* | HR | 95% CI | p value** |
| Sex | ||||||
| Female | Ref. | – | – | Ref. | – | – |
| Male | 1.50 | 0.96–2.50 | 0.074 | 2.66 | 1.42–4.95 | 0.002 |
| Age at time of second ASCT2 | ||||||
| <52.5 years | Ref. | – | – | Ref. | – | – |
| >52.5 years | 2.20 | 1.10–4.60 | 0.036 | 1.72 | 0.71–4.14 | 0.227 |
| ISS score at diagnosis of MM | ||||||
| I | Ref. | – | – | |||
| II–III | 1.50 | 0.81–2.80 | 0.190 | |||
| Cytogenetic risk at diagnosis of MM | ||||||
| Intermediate/low | Ref. | – | – | |||
| Higha | 1.00 | 0.49–2.20 | 0.940 | |||
| Number of prior line(s) of therapy before ASCT2 | ||||||
| 1 | Ref. | – | – | |||
| >1 | 1.10 | 0.56–2.00 | 0.870 | |||
| Time without new treatment after first ASCT (months) | ||||||
| <36 months | Ref. | – | – | Ref. | – | – |
| >36 months | 0.53 | 0.33–0.85 | 0.009 | 0.63 | 0.35–1.15 | 0.133 |
| Response depth before ASCT2 | ||||||
| VGPR+ | Ref. | – | – | |||
| PR, SD, or PD | 0.89 | 0.54–1.50 | 0.660 | |||
| Conditioning regimen of second ASCT | ||||||
| Standard or intensive | Ref. | – | – | |||
| Reduce | 1.40 | 0.80–2.50 | 0.230 | |||
| Response depth after ASCT2b | ||||||
| VGPR+ | Ref. | – | – | Ref. | – | – |
| PR, SD, or PD | 1.90 | 1.08–3.34 | 0.020 | 2.30 | 1.16–4.55 | 0.017 |
| Consolidation therapy after ASCT2b | 1.07 | 0.61–1.90 | 0.800 | |||
| Maintenance therapy after ASCT2b | 0.22 | 0.11–0.46 | <0.001 | 0.20 | 0.09–0.42 | <0.001 |
Abbreviations: CI 95%: 95% confidence interval; HR, hazard ratio; MM, multiple myeloma; PD, progressive disease; PR, partial response; Ref., reference for HR calculus and interpretation; SD, stable disease; VGPR+: very good partial response or complete response, all according to IMWG.
t(4;14), t(14;16), t(14;20), del(17p), del(1p32) and 1q amplification.
time‐dependent variable.
p value for log‐rank test
p value for Wald test.
Multivariate analysis including only baseline variables showed that OS was significantly decreased in male patients (HR: 1.6; 95% CI: 1.1–2.6, p = 0.047) and older patients (HR: 2.3; 95% CI: 1.1–4.9, p = 0.025). Conversely, TNT1 > 3 years was significantly associated with better OS (HR: 0.6; 95% CI: 0.3–0.9, p = 0.020) (data not shown). When significant time‐dependent variables (response depth after ASCT2 and maintenance therapy after ASCT2) were included in the multivariate model, only VGPR+ after ASCT2 (HR: 0.4; 95% CI: 0.2–0.9, p = 0.017) and maintenance therapy after ASCT2 (HR: 0.2; 95% CI: 0.1–0.4, p < 0.001) were significantly associated with prolonged OS, whereas male sex was associated with shorter OS (HR: 2.7; 95% CI: 1.4–4.9, p = 0.002) (Table 3).
Overall, in time‐dependent multivariate analysis, patients in VGPR+ and maintenance therapy after ASCT2 were associated with significantly prolonged survival, whereas male sex was associated with poorer outcome for both EFS and OS (Table 3 and Figure 3). Thus, for good responders (VGPR+) treated with maintenance therapy (n = 80), median EFS was NR (95% CI: 3.7 years–NR), median OS was NR (95% CI: 5.9 years–NR), 2‐year EFS estimate was 84% (95% CI: 75–94), and 4‐year OS estimate was 95% (95% CI: 87–100). On the other hand, for bad responders (partial response of worse) who did not receive maintenance therapy (n = 28), median EFS was 0.9 years (95% CI: 0.6–1.8), median OS was 4.3 years (95% CI: 3.6–NR), 2‐year EFS estimate was 24% (95% CI: 12–47), and 4‐year OS estimate was 57% (95% CI: 40–82).
Figure 3.
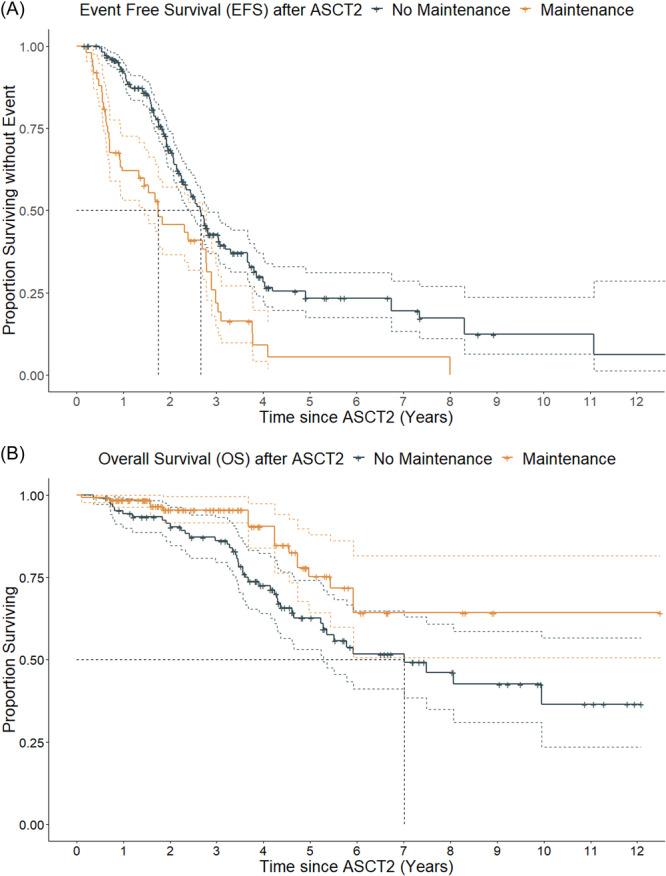
Comparative outcomes between patients receiving maintenance therapy or not after the second autologous stem‐cell transplantation (ASCT2); (A) Kaplan–Meier estimates for event‐free survival (EFS); according to time‐dependant multivariate analysis, the hazard ratio (HR) for death or need of new treatment was 0.4 for patients receiving maintenance therapy. (B) Kaplan–Meier estimates for overall survival (OS); according to time‐dependant multivariate analysis, the HR for death was 0.2 in patients receiving maintenance therapy.
Landmark analysis carried out in parallel produced consistent results with VGPR+ and maintenance after ASCT2 significantly associated with prolonged EFS and OS, whereas male sex was associated with shorter EFS and OS. However, the two analyses differed on a few points: multivariate landmark analysis showed significant benefit of prolonged TNT1 on both EFS and OS but a negative impact of older age at the time of ASCT2 and consolidation therapy after ASCT2 on OS without any significant effect on EFS (Supporting Information S1: Tables 2 and 3).
DISCUSSION
Here, we reported the real‐life outcomes of MM patients treated at relapse with ASCT2. ASCT at relapse was associated with a good response rate, low toxicity profile (1% of early death), and extended survival (median EFS 2.6 years and OS 8.1 years).
First, our results are consistent with previous reports of ASCT2 in RMM. 8 , 9 , 10 , 11 , 12 , 13 , 23 It should be noted that most patients in our study were treated after 2015, which is closest to the current therapeutic situation when compared to older studies. In this setting, Cook et al. 7 and Goldschmidt et al. 18 reported a median PFS of 1.6 and 1.8 years, respectively. Previous retrospective studies reported median PFS or EFS ranging between 0.8 and 2.5 years. More recently, the EBMT chronic malignancies working party and Galligan et al. published the results of retrospective studies conducted in a similar setting, showing median PFS after ASCT2 of 1.4 and 1.8 years, respectively. 24 , 25 One factor that could explain such survival is also a selective bias (inherent in ASCT2 studies): overall, all patients in our cohort received two ASCTs; as such, only patients who lived long enough to receive a second ASCT were selected, accounting for a low‐risk profile of our cohort consistent with a good response to ASCT1, low‐risk cytogenetic, ISS score, and prolonged TNT1 (median 3.3 years) when compared with recent studies reporting median PFS after ASCT1 around 2.5 years. 24 , 25 More than half of the patients studied here obtained VGPR+ before the start of the conditioning regimen and up to 78% reached this state after ASCT2. This good response rate could be explained by the wide use of a combination of IMiD and PI over doublet therapies of IMiD or PI or conventional chemotherapy in our cohort, consistent with the latest reports in the same setting of relapsed MM and ASCT2. 26 Continuous combined treatments such as DKd 27 , KRd, 26 and DRd 28 , 29 have shown prolonged PFS, ranging from 2.3 to 3.0 years across trials, but these trials included high‐risk patients who represent only a minority of the patients studied in our study. Furthermore, only 10 patients (4%) in our cohort were treated with anti‐CD38 making comparison of our results with these trials difficult.
Overall, ASCT2 improved response depth, but it remained lower in patients treated with reduced‐intensity conditioning regimen (Mel 100 or Mel 140) than in those who received Mel 200 or more intensive conditioning regimen. Unfortunately, true reason for using reduced‐dose conditioning was only available in a very limited number of cases and could not be reported in our study. Hence, we can only speculate that the use of reduced‐dose conditioning prior to ASCT2 may have been motivated by poor renal function at this time and, perhaps, by the will to spare toxicity in older and more heavily pretreated subjects. Even so, Mel 200 seems to be superior to less intensive conditioning regimen, and an additional cohort would be necessary to compare Mel 200 to more intensive conditioning.
Several retrospective studies identified different cut‐off values for TNT1, ranging from 12 to 24 months. 7 , 9 , 10 , 11 , 12 , 13 Based on our data, it would appear that a longer duration TNT1 (>36 months) could be of better clinical relevance, in line with ESMO guidelines, supporting upward reassessment of this cut‐off value in the era of the wide use of IMiD and PI. 15 Although TNT1 > 36 months was here associated with prolonged EFS and OS in univariate analysis, significance of this association was discordant between time‐dependent and landmark multivariate analysis. In the same way, older age (>52.5 years), good response with VGPR+ before ASCT2, and use of Mel 200 instead of reduced‐intensity conditioning regimen before ASCT2 were significantly associated with better EFS in univariate analysis but not in time‐dependent multivariate analysis. Overall, in time‐dependent multivariate analysis, effects of baseline variables seemed to be outweighed by the highly significant effect of time‐dependent variables, such as response depth after ASCT2 and use of maintenance therapy after ASCT2. Thus, better response after ASCT2 was significantly associated with prolonged EFS (HR 0.6; 95% CI: 0.3–0.9, p = 0.012 for VGPR+ vs. PR/SD/PD) as previously described. 11 , 12 This benefit in EFS appears to be reflected here in prolonged OS (HR: 0.4; 95% CI: 0.2–0.9, p = 0.017 for VGPR+ vs. PR/SD/PD). To our knowledge, only two retrospective studies reported a significant improvement in survival with lenalidomide as maintenance therapy after a second ASCT in the setting of RMM. 9 , 12 The survival benefit of maintenance therapy was here observed in a larger cohort with a time‐dependent analysis considering the immortality bias: in time‐dependent multivariate analysis patients who received maintenance therapy after ASCT2 had significantly better EFS (HR: 0.4; 95% CI: 0.3–0.6, p < 0.001) and prolonged OS (HR: 0.2; 95% CI: 0.1–0.4, p < 0.001). These observations are consistent with previous reports in newly diagnosed MM. 30 , 31 Thus, our study confirmed the importance of maintenance therapy after ASCT2 and highlights the need of continuous treatment even after second intensification. Conversely, no significant benefit from consolidation treatment could be observed here, either in terms of EFS or OS. Therefore, consolidation therapy after ASCT2 might be avoided to spare patients from unnecessary toxicity. In this study, male sex was significantly associated with poorer outcome in multivariate time‐dependent and landmark analysis, with shorter EFS (HR: 2.5; 95% CI: 1.7–3.7, p < 0.001) and OS (HR: 2.7; 95% CI: 1.4–4.9, p = 0.002). This result is surprising, especially as the male and female individuals had similar characteristics, and such a difference has not been reported in the literature.
Our study has several limitations, and main ones are related to its retrospective and noncomparative nature. Indeed, we present here the results of a therapeutic strategy based on the real‐life experience of centers using ASCT2, without comparing it with a strategy that does not. Our study is therefore not designed to assess the superiority of an ASCT2 strategy over a nonautograft strategy at relapse for MM. This question would have required a prospective comparative analysis or, failing that, a retrospective case‐control analysis, which would have been difficult to implement given the disparities in treatment over time and the need for particularly exhaustive data collection over a very long period. Another limitation concerns the retrospective design of a study spanning over two decades, although this was considered by stratifying the survival analysis based on time period of ASCT2. Moreover, only a small number of patients have been treated with anti‐CD38. It is therefore difficult to generalize these results at a time when most patients are receiving this treatment as first‐ or second‐line therapy. A study with sufficient hindsight on these new treatments is needed to answer these questions in a field with many recent paradigm shifts in the standard of care. 19 , 32 Also, some results cannot be totally explained, such as the poor prognosis of the male group and low cut‐off for age (<52.5 years) identified as the most relevant. We found no confounding factor that could explain such a difference. We must assume that nonreported factors, unbalanced between genders, influenced survival outcomes. Additional studies are required to understand these points.
Nevertheless, our cohort is one of the largest reported to date and shows promising results: ASCT2 for RMM is feasible and effective without major toxicity. It seems to improve response rate after reinduction therapy, translating into prolonged survival, especially in younger patients (<52.5 years) who already experienced prolonged response (>3 years) after frontline ASCT. However, the significant benefit of maintenance therapy observed here should prompt physicians who choose this strategy to maintain continuous treatment after a second intensification followed by ASCT. Further studies are required to clarify the role of this therapeutic approach in the anti‐CD38/immunotherapy era and age/sex impact on outcome after a second ASCT.
AUTHOR CONTRIBUTIONS
Stéphanie Harel, Pierre Morel, Axel André, and Lydia Montes designed the study. Axel André and Pierre Morel performed data analyses. Axel André, Stéphanie Harel, Lydia Montes, Pierre‐Edouard Debureaux, Pierre Morel, and Bertrand Arnulf wrote the manuscript. Axel André and Lydia Montes collected data. Axel André, Lydia Montes, Damien Roos‐Weil, Laurent Frenzel, Marguerite Vignon, Thomas Chalopin, Agathe Farge, Alexis Talbot, Fabrice Jardin, Karim Belhadj, Bruno Royer, Jean‐Pierre Marolleau, Bertrand Arnulf, Pierre Morel, and Stéphanie Harel identified and treated eligible patients. All authors reviewed the manuscript. Stéphanie Harel and Pierre Morel supervised the study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This research received no funding.
Supporting information
Supporting information.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Attal M, Harousseau J‐L, Stoppa A‐M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335(2):91‐97. 10.1056/NEJM199607113350204 [DOI] [PubMed] [Google Scholar]
- 2. Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet (London, England). 2010;376(9758):2075‐2085. 10.1016/S0140-6736(10)61424-9 [DOI] [PubMed] [Google Scholar]
- 3. Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589‐1596. 10.1182/blood-2012-02-408922 [DOI] [PubMed] [Google Scholar]
- 4. Attal M, Lauwers‐Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311‐1320. 10.1056/NEJMoa1611750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem‐cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open‐label, phase 3 study. Lancet. 2019;394(10192):29‐38. 10.1016/S0140-6736(19)31240-1 [DOI] [PubMed] [Google Scholar]
- 6. Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem‐cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open‐label, phase 3 study. Lancet Haematol. 2020;7(6):456. 10.1016/S2352-3026(20)30099-5 [DOI] [PubMed] [Google Scholar]
- 7. Cook G, Liakopoulou E, Pearce R, et al. Factors influencing the outcome of a second autologous stem cell transplant (ASCT) in relapsed multiple myeloma: a study from the british society of blood and marrow transplantation registry. Biol Blood Marrow Transplant. 2011;17(11):1638‐1645. 10.1016/j.bbmt.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 8. Grövdal M, Nahi H, Gahrton G, et al. Autologous stem cell transplantation versus novel drugs or conventional chemotherapy for patients with relapsed multiple myeloma after previous ASCT. Bone Marrow Transplant. 2015;50(6):808‐812. 10.1038/bmt.2015.39 [DOI] [PubMed] [Google Scholar]
- 9. Gössi U, Jeker B, Mansouri Taleghani B, et al. Prolonged survival after second autologous transplantation and lenalidomide maintenance for salvage treatment of myeloma patients at first relapse after prior autograft. Hematol Oncol. 2018;36(2):436‐444. 10.1002/hon.2490 [DOI] [PubMed] [Google Scholar]
- 10. Fenk R, Liese V, Neubauer F, et al. Predictive factors for successful salvage high‐dose therapy in patients with multiple myeloma relapsing after autologous blood stem cell transplantation. Leuk Lymphoma. 2011;52(8):1455‐1462. 10.3109/10428194.2011.575967 [DOI] [PubMed] [Google Scholar]
- 11. Jimenez‐Zepeda VH, Mikhael J, Winter A, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression‐free and overall survival. Biol Blood Marrow Transplant. 2012;18(5):773‐779. 10.1016/j.bbmt.2011.10.044 [DOI] [PubMed] [Google Scholar]
- 12. Lemieux E, Hulin C, Caillot D, et al. Autologous stem cell transplantation: an effective salvage therapy in multiple myeloma. Biol Blood Marrow Transplant. 2013;19(3):445‐449. 10.1016/j.bbmt.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 13. Zannetti BA, Tacchetti P, Pantani L, et al. Novel agent‐based salvage autologous stem cell transplantation for relapsed multiple myeloma. Ann Hematol. 2017;96(12):2071‐2078. 10.1007/s00277-017-3140-5 [DOI] [PubMed] [Google Scholar]
- 14. Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, blood and marrow transplant clinical trials network, and international myeloma working group consensus conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015;21(12):2039‐2051. 10.1016/j.bbmt.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA‐ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2021;32(3):309‐322. 10.1016/j.annonc.2020.11.014 [DOI] [PubMed] [Google Scholar]
- 16. Cook G, Williams C, Brown JM, et al. High‐dose chemotherapy plus autologous stem‐cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem‐cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2014;15(8):874‐885. 10.1016/S1470-2045(14)70245-1 [DOI] [PubMed] [Google Scholar]
- 17. Cook G, Ashcroft AJ, Cairns DA, et al. The effect of salvage autologous stem‐cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open‐label, phase 3 trial. Lancet Haematol. 2016;3(7):e340‐e351. 10.1016/S2352-3026(16)30049-7 [DOI] [PubMed] [Google Scholar]
- 18. Goldschmidt H, Baertsch M‐A, Schlenzka J, et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia. 2021;35(4):1134‐1144. 10.1038/s41375-020-0948-0 [DOI] [PubMed] [Google Scholar]
- 19. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328‐e346. 10.1016/S1470-2045(16)30206-6 [DOI] [PubMed] [Google Scholar]
- 20. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538‐e548. 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 21. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43(2):121‐137. 10.1016/S0167-9473(02)00225-6 [DOI] [Google Scholar]
- 22. Therneau T, Crowson C, Atkinson E. Using time‐dependent covariates and time‐dependent coefficients in the cox model. Survival Vignettes. 2017;2(3):1‐25. https://cran.rproject.org/web/packages/survival/vignettes/timedep.pdf [Google Scholar]
- 23. Auner HW, Szydlo R, Rone A, et al. Salvage autologous stem cell transplantation for multiple myeloma relapsing or progressing after up‐front autologous transplantation. Leuk Lymphoma. 2013;54(10):2200‐2204. 10.3109/10428194.2013.773998 [DOI] [PubMed] [Google Scholar]
- 24. Drozd‐Sokołowska J, Gras L, Zinger N, et al. Autologous hematopoietic cell transplantation for relapsed multiple myeloma performed with cells procured after previous transplantation–study on behalf of CMWP of the EBMT. Bone Marrow Transplant. 2022;57(4):633‐640. 10.1038/s41409-022-01592-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galligan D, Williamson S, Myers J, et al. Second autologous stem cell transplant as salvage in multiple myeloma—The Oregon Health and Science University Experience. Clin Lymphoma Myeloma Leuk. 2022;22(2):105‐112. 10.1016/j.clml.2021.08.008 [DOI] [PubMed] [Google Scholar]
- 26. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142‐152. 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]
- 27. Dimopoulos M, Quach H, Mateos M‐V, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open‐label, phase 3 study. Lancet. 2020;396(10245):186‐197. 10.1016/S0140-6736(20)30734-0 [DOI] [PubMed] [Google Scholar]
- 28. Kaufman JL, Usmani SZ, San‐Miguel J, et al. Four‐year follow‐up of the phase 3 pollux study of daratumumab plus lenalidomide and dexamethasone (D‐Rd) versus lenalidomide and dexamethasone (Rd) alone in relapsed or refractory multiple myeloma (RRMM). Blood. 2019;134:1866. 10.1182/blood-2019-123483 [DOI] [Google Scholar]
- 29. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319‐1331. 10.1056/NEJMoa1607751 [DOI] [PubMed] [Google Scholar]
- 30. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a meta‐analysis. J Clin Oncol. 2017;35(29):3279‐3289. 10.1200/JCO.2017.72.6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Attal M, Lauwers‐Cances V, Marit G, et al. Lenalidomide maintenance after stem‐cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782‐1791. 10.1056/NEJMoa1114138 [DOI] [PubMed] [Google Scholar]
- 32. Group TIMW. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749‐757. 10.1046/j.1365-2141.2003.04355.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


