Abstract
The UL15 gene of herpes simplex virus type 1 (HSV-1), like UL6, UL17, UL28, UL32, and UL33, is required for cleavage of concatameric DNA into genomic lengths and for packaging of cleaved genomes into preformed capsids. A previous study indicated that the UL15 gene encodes minor capsid proteins. In the present study, we have shown that the amino-terminal 509 amino acids of the UL15-encoded protein are sufficient to confer capsid association inasmuch as a carboxyl-terminally truncated form of the UL15-encoded protein with an Mr of approximately 55,000 readily associated with capsids. This and previous studies have shown that, whereas three UL15-encoded proteins with apparent Mrs of 83,000, 80,000, and 79,000 associated with wild-type B capsids, only the full-length 83,000-Mr protein associated with B capsids purified from cells infected with viruses lacking functional UL6, UL17, UL28, UL32, and UL33 genes (B. Salmon and J. D. Baines, J. Virol. 72:3045–3050, 1998). Thus, all viral mutants that fail to cleave viral DNA into genomic-length molecules also fail to produce capsid-associated UL15 80,000- and 79,000-Mr proteins. In contrast, the 80,000- and 79,000-Mr proteins were readily detected in capsids purified from cells infected with a UL25 null virus that cleaves, but does not package, DNA. The conclusion that the amino terminus of the 83,000-Mr protein is truncated to produce the 80,000- and/or 79,000-Mr protein was supported by the following observations. (i) Whereas the C termini of the 83,000-, 80,000-, and 79,000-Mr proteins are identical, immunoreactivity dependent on the first 35 amino acids of the UL15 83,000-Mr protein was absent from the 80,000- and 79,000-Mr proteins. (ii) The 79,000- and 80,000-Mr proteins were detected in capsids from cells infected with HSV-1(UL15M36V), an engineered virus encoding valine rather than methionine at codon 36. Thus, initiation at codon 36 is unlikely to account for production of the 80,000- and/or 79,000-Mr protein. Taken together, these data strongly suggest that capsid-associated UL15-encoded protein is proteolytically cleaved near the N terminus and indicate that this modification is tightly linked to maturation of genomic DNA.
Herpesvirus assembly has been reviewed recently (16, 29). At least three types of capsids, designated A, B, and C, accumulate in the nuclei of cells infected with herpes simplex virus type 1 (HSV-1). All three capsid forms have an external shell of approximately 120 nm in diameter, consisting of hexons and pentons formed from VP5, the major capsid protein. The hexons and pentons are linked by triplexes composed of VP19c and VP23, encoded by UL38 and UL18, respectively (21, 25). In the absence of intact VP23, triplexes are nonfunctional and capsids are not detected (13). VP22a, which forms an internal shell or scaffold, and a viral protease, VP24, are located within the cores of B capsids (12, 15).
Procapsids likely resemble B capsids in protein content but contain an internal core of larger diameter and a highly porous external shell (20). In one model of capsid assembly, the procapsid is the precursor of all other capsid types. The conversion from procapsid to small-cored B capsid is coupled with cleavage of the internal shell from the outer shell by the packaged viral protease and conversion of the outer shell into a stable icosahedral structure (33, 34, 39). Thus, A capsids, lacking core proteins and DNA, are believed to arise from an abortive packaging mechanism in which the scaffold proteins are lost and in which DNA is not inserted; B capsids result when the inner shell is locked within the outer shell; and C capsids, the precursors of virions, are the consequence of scaffold expulsion or degradation and packaging of genomic viral DNA. This model implies that the events of scaffold cleavage and expulsion, outer shell conformational changes, and DNA packaging are tightly coordinated to ensure efficient production of C capsids.
Replicated viral DNA accumulates as head-to-tail concatamers that are cleaved by viral machinery into unit-length molecules; unit-length genomes are then packaged into preformed capsids. Mutations in at least UL6, UL15, UL17, UL28, UL32, and UL33 prevent generation of unit-length molecules as well as production of C capsids but do not significantly affect assembly of B-like capsids (1, 2, 17, 22, 24, 27, 28, 32, 38, 40). Unlike the UL6, UL15, UL17, UL28, UL32, and UL33 genes, the UL25 gene is dispensable for cleavage of replicated viral DNA (19). The observation that A capsids but not C capsids are generated in cells infected with a virus lacking UL25 suggests that the DNA cleavage-packaging reaction initiates in the absence of UL25 but that cleaved DNA is not retained in the capsid.
The UL15 gene of HSV contains two exons separated by genes UL16 and UL17 (10, 18). Previous studies showed that UL15 encodes several proteins detectable in infected-cell lysates and purified B-type capsids (4, 26, 41). Experiments performed in our laboratory demonstrated that UL15-encoded proteins with apparent Mrs of 83,000, 80,000, and 79,000 accumulated in B capsids and remained detectable in capsids treated with 1.0 M guanidine hydrochloride. It was also observed that only the 83,000-Mr protein associated with B capsids purified from cells infected with viral mutants lacking the UL6, UL17, or UL28 gene, suggesting that capsid association of the 80,000- and 79,000-Mr proteins requires an intact DNA cleavage and packaging machinery (26). The primary goals of this study were to further characterize the 80,000- and 79,000-Mr proteins and to determine the conditions under which these proteins become capsid associated.
MATERIALS AND METHODS
Cells and viruses.
Wild-type viruses HSV-1(F) and HSV-1(17) were previously described, and their titers were determined on Vero cell monolayers (8, 14). G5 transformed cells were derived from Vero cells and contained HSV-1 DNA from UL16 to UL21 (13). Clone 17 cells were derived from rabbit skin cells and contain a cDNA copy of the UL15 gene (3). The G33 cell line was derived from Vero cells and contains HSV-1 DNA from UL6 to UL8 (22). The 158 cell line was derived from Vero cells and contains the UL32 gene (19). The 81 cell line was derived from Vero cells and contains the UL25 gene (19). The C1 cell line was derived from Vero cells and contains the entire UL28 gene and the UL27 gene minus a 969-bp BstEII fragment at the 5′ end of UL27 (32).
The UL33-expressing cell line was made by first cloning the UL33 gene into pGEM 3Z (Promega) from the SmaI site at position 69145 to the EcoRI site at position 69697, generating the construct pJB94 (18). The UL33 gene was subsequently cloned into pcDNA 3 (Invitrogen) by using the HindIII site in pGEM 3Z and the EcoRI site in UL33, thus placing the UL33 gene under the control of the cytomegalovirus promoter in a construct that also contained a gene encoding neomycin resistance. The plasmid was designated pJB95. The D4 cell line was made by transfection of rabbit skin cells with pJB95 followed by selection for growth in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and 500 μg of G418 per ml. Individual cell lines were cloned by limiting dilution and were screened for the ability to support growth of the UL33 null virus Cos-UL33− (11).
Vero, rabbit skin cells, HEp-2, clone 17, G5, G33, 158, 81, C1, and D4 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% newborn calf serum, penicillin, and streptomycin as previously described (3, 4, 6, 13, 17, 19, 22). Viruses pertinent to these studies are listed in Table 1. S648 contains stop codons in all three open reading frames of exon I of the UL15 gene and was grown on clone 17 cells (3). HSV-1(ΔUL15) contains a lacZ expression cassette in place of 226 codons of exon II of UL15 and was also grown on clone 17 cells (3). HSV-1(ΔUL17) contains a lacZ expression cassette inserted between the NotI site 105 bp from the 5′ end of UL17 and an XhoI site 516 bp from the 3′ end of UL17 (27); it was grown and titrated on G5 cells. K23Z contains a lacZ cassette fused in frame with the first 10 codons of VP23 and was grown and titrated on G5 cells (13). Cos-UL6− was derived from the HSV-1(17) strain and contains a 4-bp insertion at a site corresponding to amino acid residue 381; it was grown and titrated on G33 cells (11, 22). The mutant gCB contains a 1,881-bp deletion in the UL28 gene and was grown and titrated on C1 cells (32). The hr64 null virus contains an ICP6::lacZ cassette in UL32 at the codon corresponding to amino acid 274 and was grown and titrated on 158 cells (17). The KUL25NS virus contains an SpeI linker with stop codons in all three open reading frames in the NotI site located at codon 104 of the UL25 open reading frame and was grown and titrated on 81 cells (19). The Cos-UL33− virus was derived from HSV-1(17) and was grown and titrated on D4 cells. Cos-UL33− contains stop codons in all three open reading frames at codon 30 of the UL33 gene (11).
TABLE 1.
Genotypes of recombinant viruses used in this study
| Virus | Relevant characteristic | Reference or source |
|---|---|---|
| HSV-1(F) | Wild-type strain | 14 |
| HSV-1(17) | Wild-type strain | 8 |
| HSV-1(ΔUL15) | lacZ cassette in place of 3′ sequences in exon II of UL15 | 3 |
| S648 | Stop codons in exon I of UL15 | 3 |
| HSV-1(ΔUL17) | lacZ cassette in place of UL17 sequences | 27 |
| Cos-UL6− | Stop codons in UL6 | 11, 22 |
| gCB | Deletion of the UL28 gene | 32 |
| Cos-UL33− | Stop codons in UL33 | 11 |
| hr64 | lacZ cassette in UL32 | 17 |
| KUL25NS | Stop codons in UL25 | 19 |
| K23Z | lacZ cassette in UL18 | 13 |
| HSV-1(UL15M36V) | The second ATG (methionine) of UL15 changed to GTG (valine)in the tk gene | This study |
Plasmids.
In the following description, nucleotide numbers are indicated according to the data of McGeoch et al. (18). PCR-based mutagenesis was used to generate a point mutation at position 29125 by changing the second methionine (ATG) at the codon corresponding to amino acid 36 in the UL15-encoded protein to a valine (GTG). During the first round of PCR, a 305-bp fragment was amplified with the primer 5′-CCT CGA GAT CTG CAG GGT CTG-3′ (starting at position 28827) and the primer 5′-CAT CGC CGC CCA CGG TGA GGC-3′, into which a point mutation was incorporated (underlined); a 1,192-bp product was amplified with the primer 5′-CCT CAC CGT GGG CGG CGA TG-3′, which contains a point mutation, and the primer 5′-TAT AAC AAG AAC AGG CCG TG-3′ (starting at position 33905). The product DNAs were mixed and heated at 95°C to dissociate double-stranded DNAs and were subsequently cooled to promote annealing. The annealed DNA was used as a template in a second round of PCR primed with the outermost primers, thus generating a 1,491-bp product with single point mutations incorporated in both strands of the amplicon. This product was gel purified, cloned into PCR II.1 (Invitrogen), and sequenced to confirm the presence of the mutation (not shown); the resulting plasmid was designated pJB163. A full-length UL15 cDNA containing the mutation changing codon 36 from methionine to valine was constructed by replacing identical sequences in pJB125 containing the wild-type UL15 cDNA cloned into pcDNA 3 (Invitrogen) with the mutant amplicon. The fragment was cloned with HindIII sites in both vectors and a unique BstEII site in the UL15 cDNA at position 33832, generating pJB164. The reconstituted UL15 cDNA within pJB164 was then cloned into the tk gene as a BglII (incorporated in the primer starting at position 28827) and SacI (in pcDNA 3) fragment into the BglII and SacI restriction sites in the tk gene at positions 47855 and 47358, respectively. The construct was designated pJB165. For verification of the viral genotype, viral sequences were amplified with one primer within tk gene sequences (5′-TCT TGT CAT TGG CGA ATT CGA-3′) starting at position 48004 and with the second primer within UL15 sequences (5′-AGG AAT TCC AGC TTG GCC GTG-3′) starting at position 29345, thus generating an amplicon of 667 bp. The amplicon was subsequently sequenced with the primer 5′-CCT CGA GAT CTG CAG GGT CTG-3′.
A glutathione S-transferase (GST) fusion protein was generated by amplification of 420 bp of the 5′ end of UL15 with a sense-strand primer containing an EcoRI site. The amplicon was cloned into the EcoRI site of pCRII (Invitrogen), and the resultant plasmid was designated pJB54. A 312-bp EcoRI fragment from pJB54 was then cloned into pGEX-4T1 (Pharmacia) by using the EcoRI site from the sense-strand primer and the EcoRI site in exon I of UL15, thereby placing the 5′ end of the UL15 gene in frame with the gene encoding GST. DNAs encoding the junctions of the respective genes were sequenced to ensure that the UL15 and GST open reading frames were maintained.
The maltose binding protein (MBP) was fused to the first 35 amino acids of the UL15-encoded protein by amplification of 102 bp of the 5′ end of UL15 with the sense-strand primer 5′-TGA ATT CTT TGG TCA GCA GCT GGC GT-3′ (beginning at position 29022), which contains an EcoRI site, and the reverse primer 5′-CAA GCT TAT GGT GAG GCC CGC CGA CG-3′ (beginning at position 29124), which contains a HindIII site. An MBP fusion protein containing amino acids 37 to 103 of the UL15-encoded protein was generated by amplification of 210 bp of UL15. The sense-strand primer contained an EcoRI site (5′-TGA ATT CGG CGG CGA TGC CCT ACG A-3′ [beginning at position 29127]), and the antisense primer contained a HindIII site (5′-CAA GCT TAG CTT GGC CGT GTG GTC G-3′ [beginning at position 29334]). Amplicons from the two PCRs were cloned separately into PCR II.1 (Invitrogen) and designated pJB174 and pJB175, respectively. The first 102 bp of UL15 from JB174 and the next 210 bp of UL15 from pJB175 were subsequently cloned as EcoRI/HindIII fragments in frame with the MBP gene in pMAL-C (New England Biolabs) and were designated pJB176 and pJB177, respectively. The plasmids pJB176 and pJB177 were sequenced to confirm that the fusion proteins were maintained in frame (not shown).
In vitro expression of the UL15 protein.
pRB4503 contains a cDNA of the UL15 gene inserted into the pGEM 3Z vector (Promega) and has been described previously (26). pJB185 contains a 20-amino-acid linear epitope from the human cytomegalovirus glycoprotein B gene incorporated into the carboxyl terminus of the UL15 gene (7) and was derived by replacing the sequence in pRB4503 from the BstEII site to the Bsu361 site at the 3′ end of UL15 with a sequence from pRB4203, a construct described previously (4) containing an epitopically tagged UL15 cDNA. pRB4503, pJB185, and pJB164 (see above) were transcribed and translated for 1 h at 30°C with the TNT T7/SP6 coupled reticulocyte system (Promega) according to the manufacturer’s protocol.
Capsid purification and analysis.
Capsids were purified as described previously with some modifications (23, 26). In a typical purification, Vero or rabbit skin cell monolayers from three to six 850-cm2 roller bottles were infected at a multiplicity of infection of 5.0 PFU per cell and incubated at 34°C for 18 h. Nuclear lysates were prepared as described previously (23) and were separated on a 20 to 50% continuous sucrose gradient. Light-scattering bands near the center of the tube were collected with a Pasteur pipette and subsequently pelleted at 20,000 rpm for 2 h and repurified on a second continuous sucrose gradient or were collected by a fractionating device (Haake Buchler) beginning at the top of the gradient. Capsid proteins were acetone precipitated, pelleted for 1 h at 4°C, and resuspended in a buffer containing sodium dodecyl sulfate, followed by separation on denaturing 10% polyacrylamide gels (30).
Production of UL15–GST(1-104) antiserum and immunoblotting.
Immunoblotting was performed as described previously (5) except that nitrocellulose sheets containing electrophoretically separated proteins were probed either with a previously characterized UL15-MBP antiserum directed against the carboxy terminus of UL15 at a dilution of 1:1,000 or with ICP5-specific polyclonal antiserum (NC1) at a dilution of 1:5,000 (9). The UL15-MBP antiserum was generated by immunization with an affinity-purified bacterial protein containing MBP fused to the protein encoded by the majority of UL15 exon II (4). For production of antiserum directed against the amino terminus of the UL15-encoded protein, the first 312 base pairs of UL15 were cloned in frame with the gene for GST as described above. Fusion proteins were purified on GST–cross-linked Sepharose beads (Pharmacia) and used to immunize two Flemish Giant/Chinchilla rabbits with approximately 100 μg of purified fusion protein suspended in complete Freund’s adjuvant. The rabbits were given booster injections four subsequent times with 100 μg of fusion protein emulsified in incomplete Freund’s adjuvant. For immunoblotting, the antiserum was diluted 1:500 in phosphate-buffered saline with 1.0% bovine serum albumin–1.0% Tween 20. Bound antibody was visualized (i) by reaction with goat anti-rabbit alkaline phosphatase (Jackson Immunoresearch) followed by fixation of the colored substrate as described by the manufacturer (Bio-Rad) or (ii) by the enhanced-chemiluminescence (ECL) detection method (Amersham). Where applicable, blots were stripped in a solution containing 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris-HCl (pH 6.7) and incubated at 50°C for 30 min as suggested in the ECL product information manual.
RESULTS
Capsid association of the UL15-encoded 80,000- and 79,000-Mr proteins correlates with viral DNA cleavage but not DNA packaging.
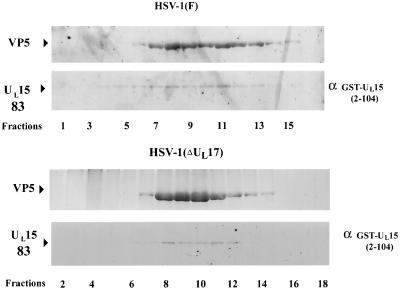
Previous findings demonstrated that association of UL15-encoded proteins with apparent Mrs of 79,000 and 80,000 with B capsids required at least UL6, UL17, and UL28. To determine the roles of other packaging proteins in capsid association of UL15-encoded proteins, capsids were purified from cells infected with the wild-type strain HSV-1(F) or a cleavage and packaging mutant defective in UL17 [HSV-1(ΔUL17)], UL32 (virus hr64, a kind gift from Sandra Weller) (17), or UL33 (Cos-UL33−) (a kind gift from Andrew Davison) (11). The gradients were fractionated into 24 0.5-ml fractions, and proteins within the fractions were acetone precipitated overnight, electrophoretically separated on a denaturing polyacrylamide gel, and transferred to nitrocellulose. The nitrocellulose was reacted with a previously described antiserum directed against UL15 exon II-encoded protein sequences, MBP (UL15-MBP) (4), and NC1, a polyclonal antibody directed against the major capsid protein ICP5 (9). Bound antibody was visualized by reaction with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin followed by the addition of a chromogenic substrate. Only the portion of the gradient containing fractions 1 to 17 or 2 to 18 are shown in Fig. 1; fractions 19 to 24 did not contain immunoreactivity with either VP5- or UL15-specific antiserum (not shown).
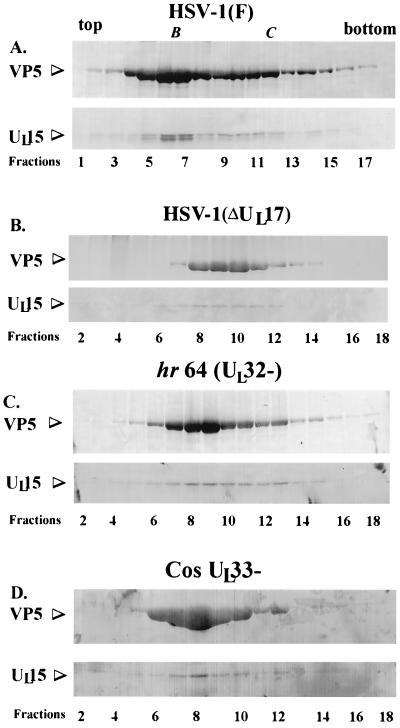
FIG. 1.
Scanned digital images of immunoblots probed with UL15-MBP antibody and the NC1 antibody. Fractions (0.5 ml) of a 14-ml continuous sucrose gradient containing purified B capsids from Vero cells infected with HSV-1(F), HSV-1(ΔUL17), hr64 (UL32−), and Cos-UL33− (panels A, B, C, and D, respectively) were collected starting at the top of the tube. Acetone-precipitated material was electrophoretically separated on a denaturing gel, transferred to nitrocellulose, and reacted with an antibody specific for the UL15-encoded protein, UL15-MBP antiserum, and with an antibody specific for VP5, NC1. Bound immunoglobulin was visualized by addition of alkaline phosphatase-conjugated anti-rabbit antibody followed by fixation of the colored substrate. The upper part of each panel shows regions of the immunoblot containing VP5, and the lower part shows UL15-encoded proteins. Fractions 1 to 18 or 2 to 18 are shown, as indicated. B and C indicate the presence of B- and C-type capsids, respectively.
As shown previously (26), the 83,000- and 80,000-apparent-Mr proteins are components of wild-type B capsids. Capsid association of UL15-encoded proteins was confirmed by the fact that fractions 6 and 7 of the sucrose gradient contained peak levels of both the 83,000- and the 80,000-Mr protein when they were probed with the UL15-MBP antiserum (Fig. 1A). The presence of B-type capsids in fractions 6 and 7 (Fig. 1A) was supported by two lines of evidence: (i) levels of the major capsid protein VP5, as assessed on the immunoblot by reaction with the antiserum NC1, were maximal in fractions 6 and 7 and (ii) fractions 6 and 7 were taken from a region of the sucrose gradient that contained a light-scattering band consistent with the presence of B-type capsids. The presence of C-type capsids (Fig. 1A) in fractions 10 to 12 was also supported by two lines of evidence: (i) levels of VP5 remained high in fractions 10 to 12, and (ii) fractions 10 to 12 were taken from a region of the sucrose gradient that contained a second light-scattering band migrating lower in the sucrose gradient (i.e., containing material of higher density) than the band containing B-type capsids. As is apparent in Fig. 1A, and as described previously, the 83,000-Mr protein is the predominant form of the UL15-encoded protein detected in fractions 10 to 12 containing C-type capsids and represents the full-length UL15 protein (26). The band corresponding to the 80,000-Mr protein was slightly broader than the band containing the 83,000-Mr protein, and in many experiments (e.g., see Fig. 3) could be resolved into two bands containing proteins with apparent Mrs of 79,000 and 80,000.
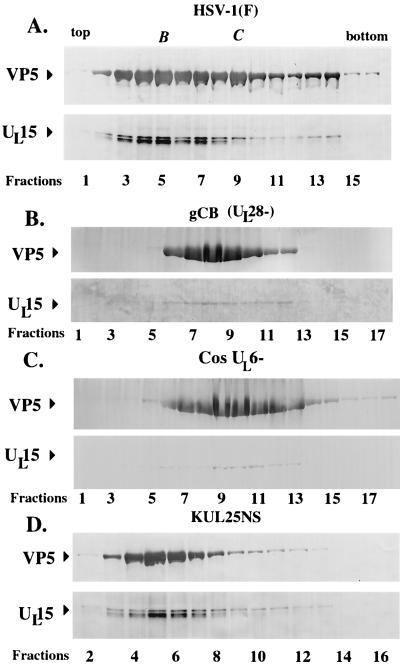
FIG. 3.
Scanned digital images of immunoblots probed with UL15-MBP antiserum and NC1. Fractions (0.5 ml) of 14-ml continuous sucrose gradients containing capsids from Vero cells infected with HSV-1(F), gCB (UL28−), Cos-UL6−, and KUL25NS (UL25−) were collected starting at the top of the tube. Acetone-precipitated material from the fractions was electrophoretically separated and probed with an antibody specific for the UL15-encoded protein, UL15-MBP antiserum, and with an antibody specific for VP5, NC1. Arrows delineate regions of the immunoblot containing VP5 and UL15-encoded proteins. Fraction numbers are indicated on the figure.
Also consistent with previous findings, the 83,000-Mr protein was the predominant form detected in capsid-containing fractions from cells infected with HSV-1(ΔUL17), a virus which lacks the UL17 gene and produces only B-like capsids (Fig. 1B, fractions 8 to 11). The conclusion that fractions 8 to 11 contained capsids was supported by the observation that these fractions exhibited high levels of immunoreactivity with the VP5-specific antibody NC1 and corresponded to a region of the sucrose gradient containing a single light-scattering band. Strikingly, there was virtually no 80,000-Mr protein detected in any of the sucrose gradient fractions. We therefore conclude that capsid association of the 80,000-Mr UL15 protein requires UL17.
Also in contrast to the results of analyses of wild-type capsids, in fractions containing capsids from cells infected with the UL32 mutant hr64, the 83,000-Mr protein was readily apparent in fractions 8 to 13 (Fig. 1C) but virtually no 80,000- or 79,000-Mr protein was detectable, as ascertained by reaction with the UL15-specific antibody. Lightly staining bands corresponding to the 83,000-Mr protein were also visible in fractions 6 and 14 of the sucrose gradient; however, fractions 6 and 14 contained immunoreactivity with the VP5-specific antibody NC1, suggesting that fractions 6 and 14 also contained small amounts of capsids. Similarly, fractions from cells infected with the UL33 mutant Cos-UL33− primarily contained the UL15 83,000-Mr protein, as shown by reaction with the UL15-specific antibody (see fractions 6 to 8). These same fractions reacted with the VP5-specific antibody NC1 and were obtained from a region of the sucrose gradient containing a light-scattering band. We conclude that the UL32 and UL33 genes are necessary for capsid association of the UL15 80,000-Mr protein.
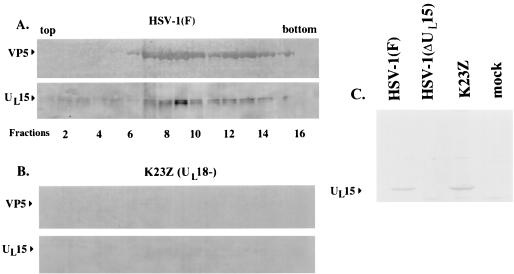
As described previously (26), the 83,000-, 80,000-, and 79,000-Mr proteins (when the last is resolved by electrophoresis) remain detectable in wild-type B capsids in the presence of 1.0 M guanidine hydrochloride, suggesting that the UL15-encoded proteins are tightly associated with wild-type B capsids. To further demonstrate that sedimentation of UL15-encoded proteins in sucrose gradients is a consequence of association with capsids, we used a recombinant virus, designated K23Z, containing a deletion in UL18 (a kind gift of Stan Person), which does not assemble capsids due to the failure of the virus to produce VP23, a triplex precursor (13). Thus, if the sedimentation of UL15 proteins in the sucrose gradient is dependent upon capsid association, then a UL15-encoded protein should not be detectable in a sucrose gradient from lysates of cells infected with K23Z. As a first step in addressing this prediction, efforts were made to ensure that wild-type levels of UL15 accumulated in K23Z-infected cells. Proteins from lysates of HEp-2 cells that were mock infected or infected with HSV-1(F), HSV-1(ΔUL15), or K23Z were separated on a denaturing polyacrylamide gel, transferred to nitrocellulose, and probed with the UL15-MBP antiserum. As shown in Fig. 2C, cells infected with K23Z contained readily detectable UL15-encoded protein, indicating that UL15 expression was not altered by the absence of UL18. Vero cells were then infected separately with K23Z and wild-type viruses and were treated identically according to the capsid purification protocol described in Materials and Methods. Briefly, infected-cell lysates were layered onto a 35% sucrose cushion and pelleted material was separated by rate zonal centrifugation on sucrose gradients. The gradients were then collected as 24 0.5-ml fractions, and proteins in the fractions were acetone precipitated, electrophoretically separated on a denaturing polyacrylamide gel, transferred to nitrocellulose, and probed with the UL15-MBP antiserum and NC1. As shown in Fig. 2A, one or more of the 83,000-, 80,000-, and 79,000-Mr proteins were detected in fractions 7 to 13 of the gradient containing wild-type infected-cell lysates. These fractions were taken from a region of the gradient that contained a light-scattering band indicative of B capsids and peak levels of VP5, as was assessed from the immunoblot probed with the antiserum NC1. In contrast to these results, both UL15 and VP5 immunoreactivities were virtually undetectable in the sucrose gradient containing lysates of K23Z-infected cells, likely due to the removal of UL15 proteins during sedimentation through the 35% sucrose cushion in preliminary phases of the capsid purification procedure. These data, therefore, indicate that sedimentation of UL15-encoded proteins in capsid-containing fractions is largely dependent upon association with capsids rather than sedimentation of capsid-free UL15 proteins across the sucrose gradient.
FIG. 2.
Scanned digital images of immunoblots probed with anti-UL15-MBP antibody and the NC1 antibody. Fractions (0.5 ml) of 14-ml continuous sucrose gradients containing nuclear lysates from Vero cells infected with HSV-1(F) (A) and K23Z (UL18−) (B) were collected starting at the top of the tubes. Material was electrophoretically separated, transferred to nitrocellulose, and reacted with an antibody specific for the UL15-encoded protein, UL15-MBP antiserum, and with an antibody specific for VP5, NC1. Bound immunoglobulin was visualized by addition of alkaline phosphatase-conjugated anti-rabbit antibody followed by fixation of the colored substrate. Fractions 1 to 16 are shown. (C) As a control, proteins in lysates of cells that were mock infected or infected with the indicated viruses were electrophoretically separated and reacted with UL15-MBP antiserum as indicated in panels A and B.
To determine if the association of the 80,000-Mr protein with capsids was dependent on viral DNA cleavage or DNA packaging, analysis of capsids obtained from KUL25NS (a kind gift from Fred Homa) was performed. KUL25NS lacks a functional UL25 gene and cleaves viral DNA but does not produce DNA-containing C capsids (19). To determine if UL15-encoded proteins associate with capsids in the absence of the UL25 gene, capsids from KUL25NS-infected cells were purified in parallel with capsids purified from cells infected with HSV-1(F) and from cells infected with gCB (UL28−) on identical continuous sucrose gradients. Gradients were fractionated into 24 0.5-ml fractions, and proteins were acetone precipitated, electrophoretically separated on a denaturing polyacrylamide gel, transferred to nitrocellulose, and reacted with anti-UL15-MBP and NC1 antisera (Fig. 3). As expected, three proteins with Mrs of 83,000, 80,000, and 79,000 which reacted strongly with the UL15-MBP-specific antiserum were detected in fractions 3 to 8 of the sucrose gradient from HSV-1(F)-infected cells; these fractions also contained peak levels of VP5 immunoreactivity. In lanes where the 79,000- and 80,000-Mr proteins were particularly prominent (e.g., fractions 4 and 5), the proteins migrated as a single broad band as shown in Fig. 1A. Reactivity with the UL15-MBP antiserum was also detected in fractions 9 to 14 of the gradient (Fig. 3A). Fractions 9 to 11 were derived from a second light-scattering band (C-type capsids) within the sucrose gradient. Consistent with previous findings, the UL15-MBP antiserum did not react as strongly with the 80,000- and 79,000-Mr proteins in C-type capsids as it did with those proteins in B-type capsids (26). As described previously, only the UL15-encoded protein with the Mr of 83,000 was present in detectable levels in capsids purified from cells infected with the UL28 deletion virus gCB and from cells infected with the UL6 null virus Cos-UL6−.
In contrast to the appearance in immunoblots of capsid proteins from lysates of cells infected with HSV-1(ΔUL17), hr64, UL33−, gCB, and Cos-UL6−, as shown in Fig. 1B to D and 3B and C, all three UL15-encoded proteins with Mrs of 83,000, 80,000, and 79,000 were detected in fractions 3 to 8 of KUL25NS-infected cells. These fractions also contained peak levels of VP5. From these data, we conclude that capsid association of the 80,000- and 79,000-Mr proteins correlates with cleavage of viral DNA (which occurs in cells infected with the UL25 deletion virus) but not necessarily with DNA packaging.
It also appeared that total UL15-specific immunoreactivity was reduced in all packaging mutants examined (i.e., mutants lacking UL6, UL17, UL28, UL32, and UL33), in comparison to levels detected in wild-type and UL25− capsids (Fig. 1 and 3). At least some of the difference was attributable to the absence of the 80,000- and 79,000-Mr proteins from capsids purified from cells infected with UL6, UL17, UL28, UL32, and UL33 mutants.
The amino-terminal 509 amino acids of UL15 protein is sufficient to mediate capsid association.
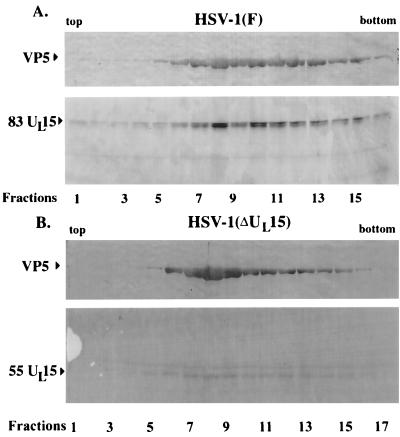
To begin to identify domains of UL15 protein which mediate capsid association, we took advantage of an available recombinant virus, HSV-1(ΔUL15), containing a lacZ cassette inserted into UL15 exon II, extending from the BamHI site at position 34129 to an MluI site at position 34803. The position of the lacZ cassette in exon II of UL15 is inserted into codon 509 of the UL15 open reading frame and therefore should truncate the UL15 protein from an 83,000-Mr protein to one with a predicted Mr of approximately 55,000 (3, 18). To determine if a C-terminally truncated form of UL15 was detectable in capsids purified from HSV-1(ΔUL15)-infected cells, Vero cell monolayers were infected with HSV-1(F) or HSV-1(ΔUL15) and capsids from these cells were purified on a single continuous sucrose gradient. Both gradients were fractionated into 24 0.5-ml fractions, and proteins were acetone precipitated, electrophoretically separated on a denaturing polyacrylamide gel, transferred to nitrocellulose, and reacted with UL15-MBP antiserum and NC1 antiserum (Fig. 4). Consistent with previous findings, at least two proteins with apparent Mrs of 83,000, and 80,000 were most readily detected in fractions 7 to 11 of the gradient from HSV-1(F)-infected cells (indicative of B-type capsids), whereas one protein with an Mr of 83,000 was the predominant form in fractions 12 to 15 of the gradient (containing C-type capsids) (26). As shown previously, the 83,000- and 80,000-Mr proteins were absent from capsids purified from HSV-1(ΔUL15)-infected cells. In the gradient containing mutant capsids, peak levels of a protein with an Mr of approximately 55,000 were detected in fractions 6 to 11. The 55,000-Mr protein was absent from capsids purified from HSV-1(F)-infected cells. It is noteworthy that a 55,000-Mr protein has been detected only in samples highly enriched in B-type capsids and has not been detected in immunoblots of HSV-1(ΔUL15)-infected-cell lysates probed with the UL15-MBP antiserum (data not shown), suggesting that the 55,000-Mr protein is highly enriched in capsids or is unstable in infected-cell lysates. The observation that levels of the 55,000-Mr protein peaked in capsid-containing fractions purified from HSV-1(ΔUL15) suggests that a truncated form of UL15-encoded protein retains the ability to associate with capsids and, furthermore, that the last 227 amino acids of UL15 protein (absent in the UL15 deletion mutant) are dispensable for capsid association of UL15 protein. While peak levels of the 55,000-Mr protein had a sedimentation profile similar to that of VP5, small amounts of the protein were also detected as a broad peak in the gradient in fractions not containing large amounts of VP5 or capsids. Peak levels in capsid-containing fractions and the observation that the 55,000-Mr protein was also detected in capsids purified on two successive sucrose gradients (see Fig. 7) lend further support to the notion that the truncated UL15-encoded protein maintains a specific interaction with B-type capsids.
FIG. 4.
Scanned digital images of immunoblots probed with UL15-MBP antiserum and NC1. Fractions (0.5 ml) of 14-ml continuous sucrose gradients containing capsids from Vero cells infected with HSV-1(ΔUL15), which lacks most of UL15 exon II, and HSV-1(F) were collected starting at the top of the tube. Acetone-precipitated material from the fractions was electrophoretically separated and probed with an antibody specific for the UL15-encoded protein, UL15-MBP antiserum, and with an antibody specific for VP5, NC1. The positions of the bands corresponding to the 83,000-Mr and the truncated 55,000-Mr proteins encoded by UL15 are indicated. Fractions 1 to 17 are shown.
FIG. 7.
Scanned digital images of immunoblots of capsid proteins probed with antibodies directed against the N and C termini of the UL15-encoded protein. B capsids from cells infected with wild-type HSV-1(F) or HSV-1(ΔUL15), which lacks most of UL15 exon II, were purified on two successive sucrose gradients. Capsids were pelleted, and electrophoretically separated proteins were transferred to nitrocellulose. The proteins were reacted with antiserum directed against a fusion protein containing the C terminus of UL15 fused to MBP (UL15-MBP) (A) or antiserum directed against a fusion protein containing the first 104 amino acids of UL15 fused to GST [GST–UL15(2-104)] (B). The positions of the bands corresponding to UL15 proteins are indicated.
Production of an antiserum directed against the N terminus of UL15 and mapping of immunoreactive epitopes.
Previous experiments demonstrated that the UL15-encoded proteins with Mrs of 79,000 and 80,000 did not result from carboxyl-terminal cleavage of the 83,000-Mr protein inasmuch as an epitopic tag inserted at the carboxy terminus was retained in all three UL15-encoded proteins (26). To address the possibility that UL15 proteins are modified at their N termini, DNA encoding the first 104 codons of UL15 was cloned in frame with the gene encoding GST. The induced fusion protein [designated GST–UL15(2-104)] was affinity purified on GST-cross-linked Sepharose beads (Pharmacia) and used to immunize rabbits for the production of polyclonal antisera (see Materials and Methods).
Previous experiments indicated that the UL15-encoded protein with an Mr of 83,000 detected in vivo comigrated with the translational product of the full-length UL15 cDNA but that the protein with an Mr of 79,000 comigrated with an additional protein produced in in vitro reticulocyte lysates. It was surmised that the 79,000-Mr protein was derived from initiation at a methionine codon at position 36 (26). To map epitopes recognized by the GST–UL15(2-104) antiserum, a UL15 cDNA was constructed such that codon 36 was changed from methionine to valine (see Materials and Methods). The UL15 cDNA bearing this mutation (designated M36V [Fig. 5]), a wild-type UL15 cDNA (designated UL15cDNA), and a UL15 cDNA with an epitopic tag from the human cytomegalovirus glycoprotein B gene inserted at the 3′ end (designated UL15cDNA-tag), were transcribed and translated in separate rabbit reticulocyte lysates (see Materials and Methods). The UL15-encoded products and a control lysate lacking such proteins were divided into two equal samples, electrophoretically separated on a denaturing polyacrylamide gel, transferred to separate sheets of nitrocellulose, and reacted with the UL15-MBP or the GST–UL15(2-104) antiserum. As shown in Fig. 5A, the UL15-MBP antiserum recognized only the 83,000-Mr protein expressed from M36V DNA (lane 1) whereas the antiserum reacted with two proteins with Mrs of approximately 83,000 and 79,000 derived from translation of the wild-type UL15 cDNA (lane 2). The antiserum also recognized proteins with Mrs of 85,000 and 81,000 translated from UL15cDNA-tag (lane 3). These data, therefore, indicate that (i) the 79,000-Mr protein arises from initiation at the ATG at codon 36, inasmuch as the 79,000-Mr protein was not produced upon translation of the UL15 cDNA in which codon 36 was changed to valine, and (ii) both the 83,000- and 79,000-Mr proteins contain the carboxyl terminus of UL15, inasmuch as they were decreased in electrophoretic mobility due to the presence of DNA encoding an epitopic tag inserted at the 3′ end of UL15cDNA.
FIG. 5.
Scanned digital image of immunoblots probed with UL15-MBP and GST–UL15(2-104) antisera. Constructs containing UL15 cDNAs were transcribed and translated in vitro, electrophoretically separated, transferred to nitrocellulose, and reacted with antiserum directed against a fusion protein containing the C terminus of UL15 fused to MBP (UL15-MBP) (A) or with antiserum directed against a fusion protein containing the first 104 amino acids of UL15 fused to GST [GST–UL15(2-104)] (B). The positions of the bands corresponding to the UL15-encoded proteins are indicated (e.g., the 83,000-Mr protein is designated 83). A protein in the lysate that cross-reacts with the GST–UL15(2-104) antiserum is labeled X in the figure.
As shown in Fig. 5B, lanes 2 and 3, only the 83,000-Mr protein from UL15cDNA and the 85,000-Mr protein from UL15cDNA-tag were recognized by the GST–UL15(2-104) antiserum. Neither the 79,000-Mr protein nor the 81,000-Mr protein, from the translation of UL15cDNA and UL15cDNA-tag, respectively, were recognized by the antiserum directed against GST–UL15(2-104). An additional protein with an Mr of approximately 77,000 (Fig. 5) was also recognized in the control lysate lacking input DNA, indicating that this was not a product of UL15. These data suggest that the GST–UL15(2-104) antiserum recognized epitopes located upstream of the second methionine codon, i.e., within the first 35 amino acids of the full-length UL15 protein.
To confirm the possibility that the GST–UL15(2-104) antiserum recognized epitopes between codons 2 and 35 of UL15, UL15 codons 2 to 35 and 37 to 103 were cloned in frame with the malI gene, which encodes MBP, yielding the fusion proteins MBP–UL15(2-35), and MBP–UL15(37-103), respectively. The fusion proteins were affinity purified by virtue of their maltose binding activity and were electrophoretically separated on a denaturing polyacrylamide gel. Equal amounts of separated proteins were stained with Coomassie blue or transferred to nitrocellulose and reacted with the GST–UL15(2-104) antiserum. As shown in Fig. 6, top panel, both fusion proteins were decreased in electrophoretic mobility compared to that of MBP. When the purified proteins were probed with the GST–UL15(2-104) antiserum, neither MBP nor fusion protein MBP–UL15(37-103) was recognized, whereas fusion protein MBP–UL15(2-35) was strongly recognized by the antiserum. We therefore conclude that GST–UL15(2-104) antiserum recognizes epitopes contained within the first 35 amino acids of UL15 protein. Amino acids 37 to 103 do not comprise such epitopes.
FIG. 6.
Scanned digital image of Coomassie blue-stained denaturing polyacrylamide gel and corresponding immunoblot. (Top) Fusion proteins containing the first 35 codons [MBP–UL15(2-35)] or codons 37 to 103 [MBP–UL15(37-103)] were affinity purified, separated on a denaturing polyacrylamide gel, and stained with Coomassie blue. (Bottom) Equal amounts of fusion protein were separated on a denaturing polyacrylamide gel, transferred to nitrocellulose, and reacted with the antiserum directed against GST–UL15(2-104). α, antiserum.
The UL15-encoded proteins with Mrs of 79,000 and 80,000 are derived from N-terminal truncation of the 83,000-Mr protein.
To characterize the 80,000- and 79,000-Mr proteins detected in HSV-1(F) capsids, Vero cell monolayers were infected with HSV-1(F) or HSV-1(ΔUL15) and capsids were purified on two successive sucrose gradients, as described above. Pelleted capsids from both HSV-1(F)- and HSV-1(ΔUL15)-infected cells were divided into equal aliquots and were electrophoretically separated in different lanes of the same denaturing polyacrylamide gel. Proteins were transferred to separate sheets of nitrocellulose and were probed with the UL15-MBP antiserum or the antiserum directed against GST–UL15(2-104). The results (shown in Fig. 7) were as follows.
(i) Consistent with previous experiments, lanes containing HSV-1(F) capsid proteins probed with the UL15-MBP antiserum contained three UL15-encoded proteins with Mrs of 83,000, 80,000, and 79,000 (Fig. 7, lane 2). In contrast, only the protein of HSV-1(F) capsids with the apparent Mr of 83,000 was recognized by the antiserum directed against GST–UL15(2-104) (Fig. 7, lane 4). These data, taken together with epitope mapping data from Fig. 5 and 6, indicate that epitopes comprised within the first 35 amino acids of UL15 protein are absent from the 80,000-Mr protein seen in wild-type capsids. Thus, the UL15-encoded proteins with the Mrs of 79,000 and 80,000 that associate with wild-type capsids are amino-terminally truncated forms of the 83,000-Mr protein.
(ii) In lysates of capsids purified from HSV-1(ΔUL15)-infected cells, a product with an Mr of approximately 55,000 was detected upon reaction with the UL15-MBP antiserum (directed against codons 384 to 736) (Fig. 7, lane 1). A protein with an electrophoretic mobility indistinguishable from that of the 55,000-Mr protein was also recognized by the antiserum directed against GST–UL15(2-104) (lane 3). These observations therefore indicate that the C-terminally truncated UL15 product in capsids from HSV-1(ΔUL15)-infected cells retains epitopes derived from the first 35 codons of UL15.
To confirm that the single UL15 protein found in DNA cleavage mutants represents the full-length UL15-encoded protein with a Mr of 83,000, immunoreactivities associated with capsids from both HSV-1(F)- and HSV-1(ΔUL17)-infected cells (Fig. 4A and 1B, respectively) were removed as suggested in the ECL product information manual (see Materials and Methods) and the immunoblots were reprobed with the GST–UL15(2-104) antiserum. As shown in Fig. 8, the GST–UL15(2-104) antiserum, unlike the UL15-MBP antiserum, recognized only the full-length UL15 protein with an apparent Mr of 83,000 in capsids from HSV-1(F)-infected cells and the protein with the apparent Mr of 83,000 in capsids from HSV-1(ΔUL17)-infected cells. We conclude that full-length UL15 protein associates with wild-type and mutant capsids.
FIG. 8.
Scanned digital images of immunoblots probed with GST–UL15(2-104) antiserum and NC1. Immunoreactivities in immunoblots shown in Fig. 4A and 1B [associated with capsids from HSV-1(F) and HSV-1(ΔUL17), respectively] were removed, and the nitrocellulose sheets were reacted with anti-GST–UL15(2-104) and NC1 antisera. The bound antibody was visualized by the ECL detection method. Fractions 1 to 16 or 2 to 18 are indicated. α, antiserum.
The UL15-encoded proteins with Mrs of 79,000 and 80,000 do not arise from initiation at the second methionine codon in vivo.
To exclude the possibility that the apparent truncation of the 83,000-Mr protein arises in vivo by initiation of translation at a second methionine encoded by UL15 codon 36, a recombinant virus containing the mutant UL15 gene (UL15M36V) within the viral tk gene was generated and analysis of the mutant protein in viral capsids was performed. The virus was constructed as follows. The UL15 cDNA, bearing a mutation from an ATG (Met) to a GTG (Val), was inserted into the viral thymidine kinase gene (tk) under the control of the tk promoter. The resultant plasmid (designated pJB165) was cotransfected with S648 viral DNA into rabbit skin cells containing a UL15 cDNA (clone 17 cells). S648 has been described previously and contains a DNA oligomer bearing stop codons in all three open reading frames of UL15 exon I (3). The stop codons should preclude expression of UL15 protein from UL15 located at the native position; thus, the mutant copy of UL15 inserted into the tk gene should be the sole source of UL15-encoded protein in HSV-1(UL15M36V)-infected cells.
Thymidine kinase-negative viruses were selected among the progeny of the cotransfection by growth on rabbit skin cells in M199 medium supplemented with anti-HSV antibody, 1% newborn calf serum, and 100 μg of bromodeoxyuridine per ml. After four rounds of plaque purification under a bromodeoxyuridine overlay, viruses were screened by PCR for the presence of UL15 within the viral tk. The PCR was driven by primers that hybridized to tk sequences and sequences within the UL15 gene (see Materials and Methods). The 667-bp amplicons from five putative viral recombinants were sequenced with the primer that hybridized with tk sequences. Of these five recombinants, all maintained the point mutation within codon 36 (not shown). One virus was chosen for further studies and was designated HSV-1(UL15M36V). A schematic representation of HSV-1(UL15M36V) genomic DNA is depicted in Fig. 9. Viral stocks of HSV-1(UL15M36V) were grown and titrated on clone 17 cells containing a UL15 gene.
FIG. 9.
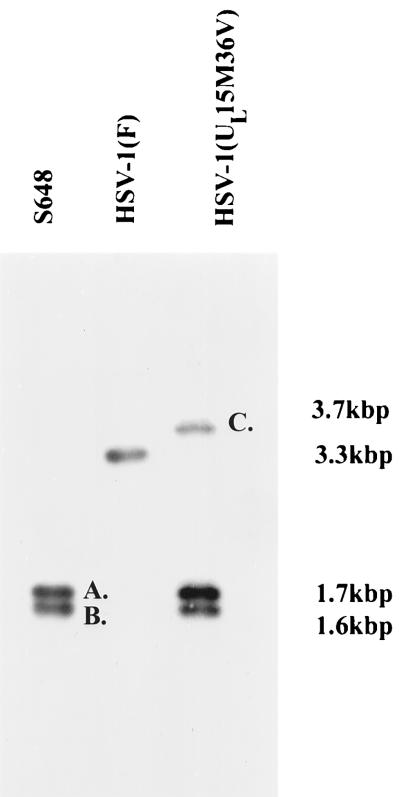
Schematic representation of collinear HSV sequences relevant to the production and documentation of the HSV-1(UL15M36V) mutant. (Line 1) Schematic collinear diagram of the exon I probe used to produce the results shown in Fig. 10. The probe contained sequence from the HindIII site downstream of the UL14 gene to a BglII site in the UL16 gene. (Line 2) Schematic representation of the HSV-1(S648)-specific SacI/XbaI fragments shown in Fig. 10 (labeled A and B). (Line 3) Schematic representation of the 3.3-kbp SacI fragment containing exon I of the UL15 gene of HSV-1(17) in its native position. (Line 4) Representation of the HSV-1 genome. Open rectangles represent inverted repeat regions flanking the UL and US components. (Line 5) Schematic representation of the SacI fragments that include the tk gene in its native position. (Line 6) Schematic representation of insertion sites of UL15M36V DNA into the tk locus. The UL15M36V DNA contains a UL15 cDNA with a point mutation changing methionine to valine (designated by an asterisk). (Line 7) Schematic representation of the resulting HSV-1(UL15M36V) viral DNA containing stop codons (designated by an × in line 2) in all three open reading frames in UL15 exon I. (Line 8) The probe used to produce Fig. 10 was derived from UL15 exon I sequences and hybridizes to the novel 3.7-kbp fragment C (Fig. 10).
To verify the genotype of UL15M36V, viral DNAs were purified from lysates of Vero cells infected with HSV-1(F), S648, and HSV-1(UL15M36V), digested with SacI and XbaI, transferred to nitrocellulose, and probed with radiolabeled DNA delimited by a HindIII site downstream of UL14 coding sequences and a BglII site within UL16. The DNAs were digested with XbaI because UL15 exon I of the parent virus S648 bears a unique XbaI site incorporated into stop codons in all three open reading frames (3). As shown in Fig. 10, the radiolabeled probe hybridized to a band of approximately 3.3 kbp in the lane containing digested HSV-1(F) viral DNA, corresponding to UL15 in its native position. The probe also hybridized with fragments with apparent sizes of 1.7 and 1.6 kbp in lanes containing S648 and HSV-1(UL15M36V) viral DNAs, confirming that the stop codons present in UL15 exon I at the native position were retained. The probe also hybridized with a novel fragment of approximately 3.7 kbp in HSV-1(UL15M36V) viral DNA. The size of the fragment corresponded to the predicted size of a SacI fragment containing the UL15 gene within the truncated tk gene (Fig. 9). Of the fragments recognized by the UL15-specific probe, only the 3.7-kbp fragment from HSV-1(UL15M36V) DNA hybridized with radiolabeled tk sequences (data not shown).
FIG. 10.
Scanned digital images of autoradiographs of electrophoretically separated viral DNAs probed with UL15 sequences. Viral DNA was purified from cells infected with the indicated viruses, digested with SacI and XbaI, transferred to nitrocellulose, and probed with radiolabeled UL15 sequences. A schematic representation of DNAs within fragments designated A and B is shown in Fig. 9, line 2. A diagram of sequences in the fragment designated C is shown in Fig. 9, line 8. The sizes of the DNA fragments are indicated to the right of the figure.
To determine if either of the UL15-encoded proteins with Mrs of 79,000 and 80,000 was able to associate with capsids from cells infected with HSV-1(UL15M36V), Vero cells were infected with HSV-1(UL15M36V) or HSV-1(F), capsids were purified on separate sucrose gradients, the gradients were fractionated, and proteins were separated on a denaturing polyacrylamide gel and transferred to nitrocellulose. The nitrocellulose was reacted with the UL15-MBP antibody and the polyclonal antibody to VP5. Results are shown in Fig. 11. As expected, one or more of the 83,000-, 80,000-, and 79,000-Mr proteins were present in capsids infected with HSV-1(F). Similarly, capsids purified from cells infected with HSV-1(UL15M36V) contained the 83,000-, 80,000-, and/or 79,000-Mr protein (Fig. 11, lower rightmost panel). These data therefore indicate that initiation at the second methionine within the UL15 gene cannot account for the presence of any of the 83,000-, 80,000-, and 79,000-Mr capsid-associated proteins. It is noteworthy that the ratio of level of the 83,000-Mr protein to the level of the 80,000- or 79,000-Mr protein was higher in capsids purified from HSV-1(UL15M36V)-infected cells than in wild-type capsids. Increased amounts of the 83,000-Mr protein compared to the amounts of the 80,000- and 79,000-Mr proteins in HSV-1(UL15M36V) capsids suggest that the mutation at amino acid 36 reduced the efficiency of truncation of the full-length UL15-encoded protein to the 80,000- and 79,000-Mr products. Parenthetically, we cannot determine whether the 79,000-Mr product is derived by proteolytic cleavage of the 83,000-Mr protein or by proteolytic cleavage of the 80,000-Mr protein. The conversion from the 83,000-Mr protein to at least the 80,000-Mr protein, however, does coincide with an intact DNA cleavage reaction.
FIG. 11.
Scanned image of an immunoblot probed with the UL15-MBP-specific antiserum. B capsids were purified from HSV-1(F)- and HSV-1(UL15M36V)-infected cells, and associated proteins were electrophoretically separated and reacted with antibodies against UL15-MBP and VP5 (NC1). An enlarged image at the right illustrates the positions of the 83,000-, 80,000-, and 79,000-Mr proteins.
An unexpected observation was made during experiments to characterize HSV-1(UL15M36V) replication. Whereas HSV-1(UL15M36V) produced titers of approximately 5.0 × 109 PFU/ml upon infection of rabbit skin cells and clone 17 cells (derived from rabbit skin cells but containing the UL15 gene), peak titers of HSV-1(UL15M36V) reached only 3.0 × 108 PFU/ml on Vero cells. Identical titers of approximately 5.0 × 109 PFU/ml were obtained upon infection of Vero, rabbit skin, and clone 17 cells with wild-type virus HSV-1(F). Furthermore, light-scattering bands containing C-type capsids were consistently smaller in sucrose gradients containing lysates of HSV-1(UL15M36V)-infected Vero cells than the corresponding bands in sucrose gradients containing lysates of HSV-1(F)-infected cells (not shown). These data suggest that an incomplete block in viral DNA packaging, as seen in Vero cells infected with HSV-1(UL15M36V), is largely alleviated upon propagation of the recombinant virus in rabbit skin cells.
DISCUSSION
Taken together, these results indicate that the UL15-encoded proteins with Mrs of 79,000 and 80,000 are derived by truncation near the amino terminus of full-length UL15 protein and that association of the truncated proteins with capsids is tightly coupled with maturation of concatameric DNA into unit-length genomes. Data leading to this conclusion include the following. (i) The UL15-encoded proteins with Mrs of 79,000 and 80,000 did not associate with capsids in the absence of the UL6, UL17, UL28, UL32, and UL33 genes. Thus, all mutations known to prevent cleavage of viral DNA also prevent capsid association of the UL15 80,000- and 79,000-Mr proteins. (ii) All three forms of the UL15-encoded protein can associate with capsids in cells infected with a UL25 null virus that cleaves but does not package viral DNA. (iii) The conclusion that the UL15-encoded proteins with Mrs of 79,000 and 80,000 are amino-terminally truncated forms of the UL15-encoded protein with an Mr of 83,000 is supported by the observation that epitopes within the first 35 amino acids of full-length UL15 protein are absent from the 79,000- and 80,000-Mr proteins.
The origin of the 79,000- and 80,000-Mr proteins may be a consequence of proteolytic cleavage of the full-length protein or initiation at a second methionine at codon 36. We favor the proteolytic cleavage model because at least the 80,000-Mr protein associated with capsids upon mutation of codon 36 to valine, as seen in capsids from HSV-1(UL15M35V), albeit at reduced levels. Also arguing against the use of an internal methionine is the observation that initiation at the second methionine in reticulocyte lysates does not produce an 80,000-Mr protein (Fig. 5). Thus, initiation at the second methionine, without invocation of additional protein modification steps, cannot entirely explain the origin of the 80,000-Mr protein in capsids. Nevertheless, the proteolytic cleavage event has not been shown directly, and further studies will be required to rule out the alternative model of initiation at the second methionine.
Our experiments also demonstrated that HSV-1(UL15M36V) produces titers in Vero cells that are at least 10-fold lower than titers in rabbit skin cells. Amounts of truncated products were reduced in HSV-1(UL15M36V) capsids compared to amounts in wild-type capsids, suggesting that, in terms of the favored model, the mutation at codon 36 reduces the efficiency of proteolytic cleavage. It is possible that reduced efficiency of proteolytic cleavage is responsible for the reduced infectious titers of HSV-1(UL15M36V) in Vero cells, but the current data do not exclude the possibility that the mutation partially disrupts UL15 functionality, thereby reducing DNA maturation and coupled proteolytic cleavage. The observation that the replication defect imposed by the mutation was complemented in rabbit skin cells suggests that host proteins may be involved in the DNA cleavage-packaging reaction, as has been suggested in another study (35).
The putative proteolytic cleavage site(s) within the UL15 N terminus is unknown. As noted above, truncation destroys epitopes contained entirely within amino acids 2 through 36 of the UL15 protein. The observation that the electrophoretic mobilities of the 79,000- and 80,000-Mr proteins are similar to that of a product made in vitro from initiation at codon 36 suggests that the putative cleavage site is near codon 36. Analysis of the primary amino acid sequence of UL15 predicts the presence of a highly charged alpha helix composed of UL15 amino acids 2 to 37 followed by a turn. Both the alpha helix and the turn are predicted by the sequences of UL15 homologs of all members of the family Herpesviridae for which sequence data are available (not shown). If the motif confers the ability to bind to capsid proteins through ionic interactions, removal of this alpha helix should reduce the affinity of UL15 proteins for capsids. Thus, the reduction of the 79,000- and 80,000-Mr proteins in DNA-containing C capsids may be a consequence of decreased binding affinity and displacement by packaged DNA (Fig. 1A and 3A) (26). Other data presented herein indicate that the first 509 codons of UL15 are sufficient to confer association with capsids, further supporting the hypothesis that the N terminus is involved in capsid association.
An additional question arising from this study is the identity of the putative protease responsible for cleavage of the UL15 protein. Attempts to demonstrate trans cleavage of UL15 protein by coexpression of UL15 protein with the HSV-1(UL26−)-encoded viral protease have been unsuccessful (data not shown). The UL15 protein containing valine at amino acid 36 does not produce the 80,000- or 79,000-Mr protein in rabbit reticulocyte lysates (Fig. 5), suggesting that, at least in this expression system, UL15 protein does not exhibit self-cleavage. The hypothesis that proteolytic cleavage of UL15 protein is important to the functioning of this highly conserved protein predicts that homologs in other herpesviruses will also undergo N-terminal cleavage. Inasmuch as the primary amino acid sequences within the proposed cleavage site (i.e., immediately following the highly charged N terminus) are not highly conserved, the cleavage may be mediated by a protease like the signal peptidases which demonstrate flexibility in their respective recognition sequences (36). Alternatively, different virus- or host-encoded proteases may mediate cleavage in different herpesvirus systems.
Although quantitation from immunoblots is not precise, amounts of UL15 immunoreactivity seemed to be decreased in mutants defective in DNA cleavage. This decrease is consistent with the results of a published study demonstrating that association of normal levels of UL15 proteins with capsids requires at least UL6 and UL28 (41). In the experiments reported herein, reduced levels of UL15 proteins in capsids purified from cells infected with packaging-deficient viruses is especially noticeable because of the absence of the 80,000- and 79,000-Mr proteins.
Two explanations may account for the observation that levels of UL15 proteins in capsids of viruses that do not cleave DNA appear to be reduced. One possibility is that delivery of the 80,000- and/or 79,000-Mr protein to procapsids occurs during the DNA cleavage reaction. A failure to deliver UL15-encoded proteins to the capsid may occur for different reasons; e.g., proper docking of UL15 proteins might require minor capsid proteins encoded by UL6 and UL28, which comprise docking sites within capsids (30, 41), whereas a dependence on UL17 might reflect the fact that procapsids are sequestered from intranuclear sites containing UL15 proteins (31, 37).
An alternative model is that incorporation of normal levels of UL15 proteins into procapsids, and subsequent truncation of procapsid-associated UL15 proteins, requires functions encoded by UL6, UL17, UL28, UL32, and UL33. In this model, large amounts of full-length UL15 protein are expected to associate with wild-type procapsids and serve as the substrate for proteolytic cleavage. Thus, UL15 might be incorporated into the core of the procapsid and during DNA cleavage become proteolytically cleaved and expelled from C capsids, much like the scaffold protein ICP35. This model would therefore explain why there are reduced levels of the truncated UL15 products in DNA-containing capsids lacking other core proteins.
ACKNOWLEDGMENTS
We thank Gary Cohen and Roselyn J. Eisenberg for providing the NC1 antibody. We thank Fred Homa, Arvind Patel, Stan Person, Andrew Davison, and Sandra Weller for recombinant viruses and the cell lines necessary for their propagation. We also thank Jarek Okulicz-Kozaryn for excellent technical assistance.
This study was supported by NIH grant R01 GM50740.
REFERENCES
- 1.Addison C, Rixon F J, Preston V G. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990;71:2377–2384. doi: 10.1099/0022-1317-71-10-2377. [DOI] [PubMed] [Google Scholar]
- 2.Al-Kobashi M F, Rixon F J, McDougall I, Preston V G. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180:380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- 3.Baines J D, Cunningham C, Nalwanga D, Davison A J. The UL15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the UL15 gene product. J Virol. 1997;71:2666–2673. doi: 10.1128/jvi.71.4.2666-2673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines J D, Poon A P W, Rovnak J, Roizman B. The UL15 gene of herpes simplex virus encodes two proteins and is required for cleavage of viral DNA. J Virol. 1994;68:8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines J D, Roizman B. The UL21 gene of herpes simplex virus 1 is dispensable for replication in cell culture. J Virol. 1994;68:2929–2936. doi: 10.1128/jvi.68.5.2929-2936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basgoz N, Qadri I, Navarro D, Sears A, Lennette E, Youngbloom J, Pereira L. The amino terminus of human cytomegalovirus glycoprotein B contains epitopes that vary among strains. J Gen Virol. 1992;73:983–988. doi: 10.1099/0022-1317-73-4-983. [DOI] [PubMed] [Google Scholar]
- 8.Brown S M, Ritchie D A, Subak-Sharpe J H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973;18:329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G H, Ponce de Leon M, Diggelmann H, Lawrence W C, Vernon S K, Eisenberg R. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa R H, Draper K G, Kelly T J, Wagner E K. An unusual spliced herpes simplex virus type 1 transcript with sequence homology to Epstein-Barr virus DNA. J Virol. 1985;54:317–328. doi: 10.1128/jvi.54.2.317-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham C, Davison A J. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 12.Davison M D, Rixon F J, Davison A J. Identification of genes encoding two capsid proteins (VP24 and VP26) of herpes simplex type 1. J Gen Virol. 1992;73:2709–2713. doi: 10.1099/0022-1317-73-10-2709. [DOI] [PubMed] [Google Scholar]
- 13.Desai P, DeLuca N A, Glorioso J C, Person S. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol. 1993;67:1357–1364. doi: 10.1128/jvi.67.3.1357-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 15.Gibson W, Roizman B. Proteins specified by herpes simplex virus. VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10:1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homa F L, Brown J C. Capsid assembly and DNA packaging in herpes simplex virus. Rev Med Virol. 1997;7:107–122. doi: 10.1002/(sici)1099-1654(199707)7:2<107::aid-rmv191>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Lamberti C, Weller S K. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J Virol. 1998;72:2463–2473. doi: 10.1128/jvi.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 19.McNab A R, Desai P, Person S, Roof L L, Thomsen D R, Newcomb W W, Brown J C, Homa F L. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newcomb W W, Homa F L, Thomsen D R, Booy F P, Trus B L, Steven A C, Spencer J V, Brown J C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 21.Newcomb W W, Trus B L, Booy F P, Steven A C, Wall J S, Brown S C. Structure of the herpes simplex virus capsid molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 22.Patel A H, Rixon F J, Cunningham C, Davison A J. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology. 1996;217:111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- 23.Perdue M L, Kemp M C, Randall C C, O’Callaghan D J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974;59:201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- 24.Poon A P W, Roizman B. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J Virol. 1993;67:4497–4503. doi: 10.1128/jvi.67.8.4497-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rixon F J, Davison M D, Davison A J. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990;71:1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- 26.Salmon B, Baines J D. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of UL15-encoded proteins with B capsids requires at least the UL6, UL17, and UL28 genes. J Virol. 1998;72:3045–3050. doi: 10.1128/jvi.72.4.3045-3050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon B, Cunningham C, Davison A J, Harris W J, Baines J D. The herpes simplex virus 1 UL17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J Virol. 1998;72:3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman G, Bachenheimer S L. DNA processing in temperature-sensitive morphogenetic mutants of HSV-1. Virology. 1987;158:427–430. doi: 10.1016/0042-6822(87)90214-5. [DOI] [PubMed] [Google Scholar]
- 29.Steven A C, Spear P G. Herpesvirus capsid assembly and envelopment. In: Chiu W, Burnett R M, Garcea R L, editors. Structural biology of viruses. New York, N.Y: Oxford Press; 1998. pp. 312–351. [Google Scholar]
- 30.Taus N S, Baines J D. Herpes simplex virus DNA cleavage and packaging: the UL28 gene product is a minor component of B capsids. Virology. 1998;252:443–449. doi: 10.1006/viro.1998.9475. [DOI] [PubMed] [Google Scholar]
- 31.Taus N S, Salmon B, Baines J D. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology. 1998;252:115–125. doi: 10.1006/viro.1998.9439. [DOI] [PubMed] [Google Scholar]
- 32.Tengelsen L A, Pedersen N E, Shaver P R, Wathen M W, Homa F L. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993;67:3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomsen D R, Newcomb W W, Brown J C, Homa F L. Assembly of the herpes simplex virus capsid: requirement for the carboxyl-terminal twenty-five amino acids of the proteins encoded by the UL26 and UL26.5 genes. J Virol. 1995;69:3690–3703. doi: 10.1128/jvi.69.6.3690-3703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19C and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 35.Umene K, Nishimoto T. Inhibition of generation of authentic genomic termini of herpes simplex virus type 1 DNA in temperature-sensitive mutant BHK-21 cells with a mutated CCG1/TAFII250 gene. J Virol. 1996;70:9008–9012. doi: 10.1128/jvi.70.12.9008-9012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Heijne G. Patterns of amino acids near signal sequence cleavage sites. Eur J Biochem. 1993;113:17–22. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- 37.Ward P L, Ogle W O, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weller S K, Carmichael E P, Aschman D P, Goldstein D J, Schaffer P A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987;161:198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- 39.Wilson D W, Church G A. Study of herpes simplex virus maturation during a synchronous wave of assembly. J Virol. 1997;71:3603–3612. doi: 10.1128/jvi.71.5.3603-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D, Shaeffer A K, Tenny D J, Weller S K. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J Virol. 1997;71:2656–2665. doi: 10.1128/jvi.71.4.2656-2665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu D, Weller S K. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J Virol. 1998;72:7428–7439. doi: 10.1128/jvi.72.9.7428-7439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]