Abstract
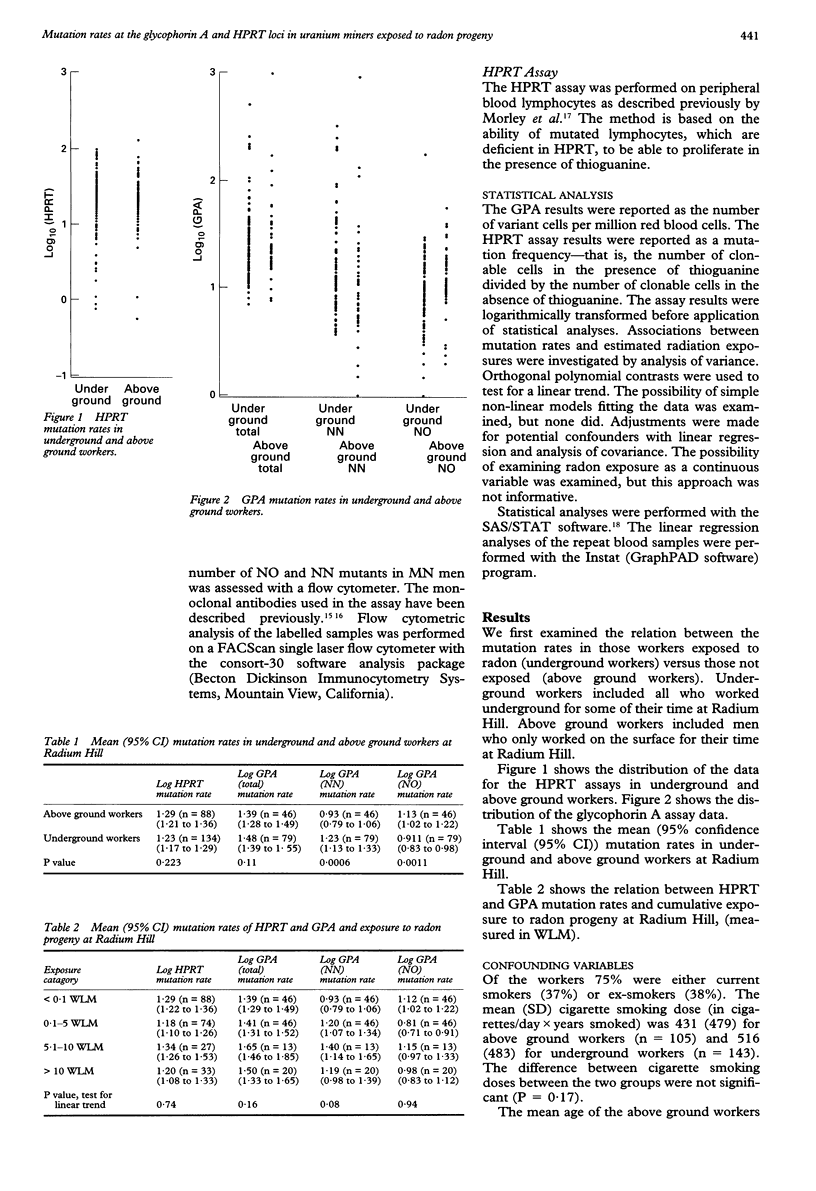
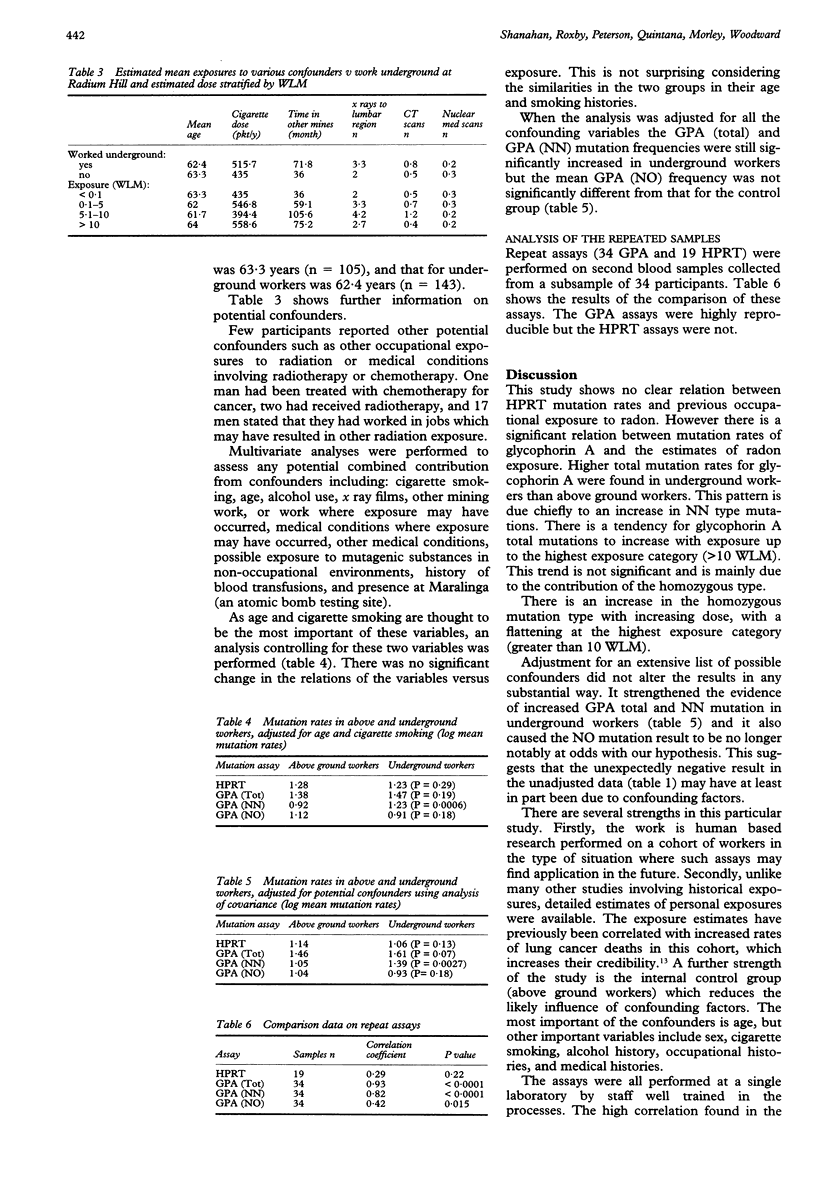
OBJECTIVES--To find whether a relation exists between estimated levels of exposure to radon and its progeny and mutations in hypoxanthine phosphoribosyl transferase (HPRT) and glycophorin A in a cohort of former uranium miners. METHODS--A cohort study involving a sample of miners from the Radium Hill uranium mine in South Australia, which operated from 1952 to 1961. Radiation exposures underground at Radium Hill were estimated from historical radon gas measures with a job exposure matrix. Workers from the mine who worked exclusively above ground according to mine records were selected as controls. In 1991-2 miners were interviewed and blood taken for measurement of somatic mutations. Mutation rates for HPRT and glycophorin A were estimated with standard assay techniques. RESULTS--Homozygous mutations of glycophorin A were increased in underground miners (P = 0.0027) and the mutation rate tended to rise with increasing exposure with the exception of the highest exposure (> 10 working level months). However, there was no association between place of work and either the hemizygous mutations of glycophorin A or the HPRT mutation. CONCLUSIONS--There may be an association between glycophorin A mutations and previous occupational exposure to ionising radiation. However, not enough is known at present to use these assays as biomarkers for historical exposure in underground mining cohorts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Castle K. L., Borcherding W. R. T-cell cloning to detect the mutant 6-thioguanine-resistant lymphocytes present in human peripheral blood. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6617–6621. doi: 10.1073/pnas.79.21.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigbee W. L., Vanderlaan M., Fong S. S., Jensen R. H. Monoclonal antibodies specific for the M- and N-forms of human glycophorin A. Mol Immunol. 1983 Dec;20(12):1353–1362. doi: 10.1016/0161-5890(83)90166-9. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Cole J., Arlett C. F., Green M. H., Waugh A. P., Beare D., Henshaw D. L., Last R. D. Possible association between mutant frequency in peripheral lymphocytes and domestic radon concentrations. Lancet. 1991 May 18;337(8751):1187–1189. doi: 10.1016/0140-6736(91)92859-z. [DOI] [PubMed] [Google Scholar]
- Dempsey J. L., Morley A. A., Seshadri R. S., Emmerson B. T., Gordon R., Bhagat C. I. Detection of the carrier state for an X-linked disorder, the Lesch-Nyhan syndrome, by the use of lymphocyte cloning. Hum Genet. 1983;64(3):288–290. doi: 10.1007/BF00279414. [DOI] [PubMed] [Google Scholar]
- Hakoda M., Akiyama M., Kyoizumi S., Awa A. A., Yamakido M., Otake M. Increased somatic cell mutant frequency in atomic bomb survivors. Mutat Res. 1988 Sep;201(1):39–48. doi: 10.1016/0027-5107(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Henshaw D. L., Eatough J. P., Richardson R. B. Radon as a causative factor in induction of myeloid leukaemia and other cancers. Lancet. 1990 Apr 28;335(8696):1008–1012. doi: 10.1016/0140-6736(90)91071-h. [DOI] [PubMed] [Google Scholar]
- Kyoizumi S., Nakamura N., Hakoda M., Awa A. A., Bean M. A., Jensen R. H., Akiyama M. Detection of somatic mutations at the glycophorin A locus in erythrocytes of atomic bomb survivors using a single beam flow sorter. Cancer Res. 1989 Feb 1;49(3):581–588. [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Flow cytometric characterization of normal and variant cells with monoclonal antibodies specific for glycophorin A. J Immunol. 1985 Jun;134(6):4009–4017. [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Kyoizumi S., Nakamura N., Bean M. A., Akiyama M., Jensen R. H. Evidence for increased somatic cell mutations at the glycophorin A locus in atomic bomb survivors. Science. 1987 Apr 24;236(4800):445–448. doi: 10.1126/science.3563520. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Nisbet B. A., Bigbee W. L., Ridinger D. N., Jensen R. H. An improved flow cytometric assay for somatic mutations at the glycophorin A locus in humans. Cytometry. 1990;11(4):513–521. doi: 10.1002/cyto.990110410. [DOI] [PubMed] [Google Scholar]
- Mendelsohn M. L. New approaches for biological monitoring of radiation workers. Health Phys. 1990 Jul;59(1):23–28. doi: 10.1097/00004032-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Dempsey J. L., Seshadri R. S. Methods for study of mutations and mutagenesis in human lymphocytes. Mutat Res. 1985 Dec;147(6):363–367. doi: 10.1016/0165-1161(85)90005-6. [DOI] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R., Ryall R. G. Measurement of in vivo mutations in human lymphocytes. Nature. 1983 Mar 10;302(5904):155–156. doi: 10.1038/302155a0. [DOI] [PubMed] [Google Scholar]
- Perera F. P. Molecular cancer epidemiology: a new tool in cancer prevention. J Natl Cancer Inst. 1987 May;78(5):887–898. [PubMed] [Google Scholar]
- Richardson R. B., Eatough J. P., Henshaw D. L. Dose to red bone marrow from natural radon and thoron exposure. Br J Radiol. 1991 Jul;64(763):608–624. doi: 10.1259/0007-1285-64-763-608. [DOI] [PubMed] [Google Scholar]
- Woodward A., Roder D., McMichael A. J., Crouch P., Mylvaganam A. Radon daughter exposures at the Radium Hill uranium mine and lung cancer rates among former workers, 1952-87. Cancer Causes Control. 1991 Jul;2(4):213–220. doi: 10.1007/BF00052136. [DOI] [PubMed] [Google Scholar]


