Abstract
Ecosystem engineers modify their environment and influence the availability of resources for other organisms. Burrowing species, a subset of allogenic engineers, are gaining recognition as ecological facilitators. Burrows created by these species provide habitat for a diverse array of other organisms. Following disturbances, burrows could also serve as ecological refuges, thereby enhancing ecological resistance to disturbance events. We explored the ecological role of Common Wombat (Vombatus ursinus) burrows using camera traps in forests of southeastern Australia. We compared animal activity at paired sites with and without burrows, from the same fire severity class and habitat. We examined how animal activity at Common Wombat burrows was affected by the 2019–20 Black Summer bushfires in Australia. We predicted that burrows would serve as hotspots for animal activity and as refuges in burned areas. The activity of several species including Bush Rat (Rattus fuscipes), Agile Antechinus (Antechinus agilis), Lace Monitor (Varanus varius), Painted Button-quail (Turnix varius), and Grey Shrike-thrush (Colluricincla harmonica) increased at sites where Common Wombat burrows were present, while other species avoided burrows. Species that were more active at burrows tended to be smaller mammal and bird species that are vulnerable to predation, whereas species that avoided burrows tended to be larger mammals that might compete with Common Wombat for resources. Species composition differed between sites with and without burrows, and burrow sites had higher native mammal species richness. The association of several species with burrows persisted or strengthened in areas that burned during the 2019–20 Black Summer bushfires, suggesting that Common Wombat burrows may act as ecological refuges for animals following severe wildfire. Our findings have relevance for understanding how animals survive, persist, and recover following extreme wildfire events.
Keywords: allogenic engineer, Black Summer bushfires, ecological facilitation, eucalypt forests, fire refuge, fire severity
Burrows created by burrowing species, like the Common Wombat, serve as hotspots for animal activity and ecological refuges for small mammal, bird, and reptile species following bushfires. Burrows alter local communities and provide refuge for other species, increasing species richness and potentially aiding in the survival, persistence, and recovery of animal populations following severe wildfire events.
Ecosystem engineers modify their environment by influencing resources available to other organisms (Jones et al. 1997; Coggan et al. 2018). Modification by ecosystem engineers occurs via 2 pathways: autogenic engineers, which alter the environment by modifying themselves (e.g., trees and coral; Jones et al. 1994); and allogenic engineers, which alter the environment by mechanically changing the form of biotic and abiotic materials (e.g., dam-building beavers; Jones et al. 1994). Ecosystem engineers are distributed across terrestrial, freshwater, and marine environments (Jones et al. 1994), and include species from an array of taxonomic groups, including invertebrates, reptiles, birds, and mammals.
Species that displace soil through burrowing, digging, and foraging—known as bioturbators—play an important role as ecosystem engineers and often provide resources for co-occurring species (Whitford and Kay 1999; Coggan et al. 2018). Bioturbation modifies soil by changing water run-off and erosion (Halstead et al. 2020) and increasing water infiltration (Fleming et al. 2014; Davies et al. 2019), which alters the chemical properties of soils (Guy and Kirkpatrick 2021) and can enhance seed germination (Eldridge and James 2009; Fleming et al. 2014). For example, in Australia, the bioturbation activity of Quenda (Isoodon fusciventer) increases soil nutrients and microbial activity, promoting seedling growth (Valentine et al. 2018). In addition, well-digging by feral equids in North American deserts increases water availability and vertebrate richness (Lundgren et al. 2021). Such engineering activities can provide important resources and enhance local biodiversity.
Burrow engineers are a subset of bioturbators that combine bioturbation with the creation of often large and complex burrow systems. Burrow creation is a facilitative interaction whereby commensal species exploit resources offered from burrows, including the provision of shelter from predators and extreme conditions, as well as foraging and breeding opportunities. For instance, American Badger (Taxidea taxus) burrows are exploited by 31 species, including numerous mammal species that use the burrows for shelter (Andersen et al. 2021). Similarly, Gopher Tortoise (Gopherus polyphemus) burrows are used by over 60 vertebrate species (Dziadzio and Smith 2016), and Giant Armadillo (Priodontes maximus) burrows are visited by over 50 vertebrate species (Desbiez and Kluyber 2013). In northern Australia, deep nesting warrens created by Sand Goanna (Varanus gouldii) provide shelter for at least 28 vertebrate species (Doody et al. 2021). Hence, the habitat created by burrow engineers can result in hotspots of animal activity through ecological facilitation.
Refuges are features that facilitate survival or persistence of species during and following disturbance (Keppel et al. 2012; Reside et al. 2019). There is some evidence that burrow engineers can create refuges for other species: for instance, burrows of Southern Hairy-nosed Wombat (Lasiorhinus latifrons) and Desert Tortoise (Gepherus agassizzi) are used by birds to escape extreme heat (Attwood 1982; Walde et al. 2009). The increased use of burrows by commensal species during disturbance events is consistent with the stress-gradient hypothesis, which predicts that facilitative interactions between species are most important in harsh ecological conditions (Bertness and Callaway 1994; Lowney and Thomson 2021).
Fire is a global driver of environmental change (Pausas and Keeley 2021) and has shaped the evolution of species over millions of years (Pausas and Parr 2018; Nimmo et al. 2021). Retreating to “fire refuges”—features that allow for the survival, persistence, and reestablishment of populations during and following fire (Robinson et al. 2013)—is 1 strategy that animals deploy during and after fire (Nimmo et al. 2019; Jolly et al. 2022). The occurrence of refuges created by ecosystem engineers has rarely been considered within this context (Dawson et al. 2019; Reside et al. 2019), but could theoretically enhance the resistance of wildlife to a range of disturbances, while resources provided by these engineers could hasten recovery. Knapp et al. (2018) provides some support for the importance of burrow engineers in fire-prone landscapes, showing that vertebrate use of Gopher Tortoise (G. polyphemus) burrows increased >8-fold at sites that experienced prescribed burns compared to unburned sites. Given that the frequency of large, severe wildfires is predicted to increase as a result of climatic change (Wu et al. 2021), fire refuges created by ecosystem engineers could play a particularly critical role in the future.
The 2019–20 Australian Black Summer bushfires were a series of megafires and gigafires (fires >10,000 and 100,000 ha, respectively; Linley et al. 2022) that were unprecedented in their scale (Nolan et al. 2020) and severity (Collins et al. 2021). These fires burnt >10 million ha of southeastern Australian forests (Wintle et al. 2020), including a record amount (1.8 million ha) burnt at high severity (Collins et al. 2021), impacting nearly 3 billion native vertebrate animals (van Eeden et al. 2020). During these bushfires, viral stories emerged on social media of wombats herding native wildlife into their burrows to protect them from fire. While these stories were ultimately dismissed (Nimmo 2020), an element of truth could still be gleaned from them.
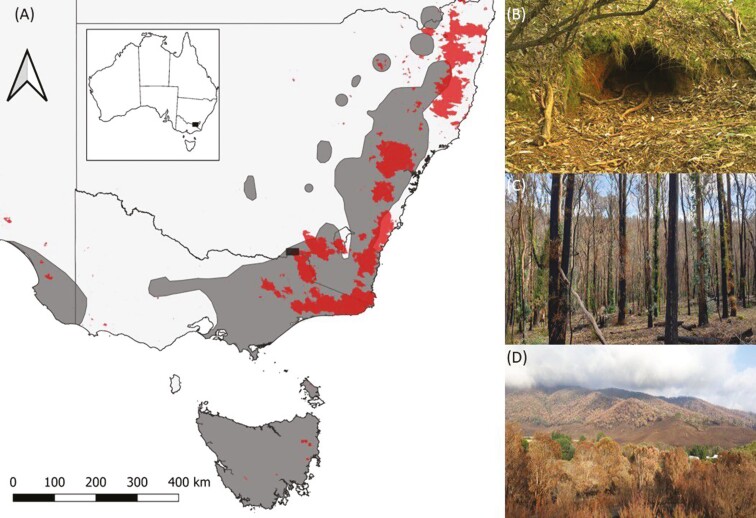
Wombats (family Vombatidae) are the largest burrowing marsupials on earth (Evans et al. 2006). There are 3 extant wombat species, all of which occur in Australia. The Common Wombat (Vombatus ursinus; Fig. 1), also known as a Bare-nosed Wombat, has the largest distribution of the 3 species (Taylor 1993; Evans 2008; Baker and Gynther 2023), which coincides with several of the 2019–20 bushfires (Fig. 2). Despite being listed as Least Concern by the IUCN (2008), the species has undergone substantial decline across its range since European colonization (Roger et al. 2007), with climate change predicted to cause further range contractions (Graham et al. 2019). Common Wombat are active in forests after fire (Lunney and O’Connell 1988), thus having an ability to maintain burrows in burnt habitat. Wombats excavate and use multiple burrows (Thornett et al. 2017), which consist of a large network of underground tunnels (Swinbourne et al. 2016b). Common Wombat burrows can be upwards of 15 m long, with multiple entrances—and can be deep, complex, and multichambered (Triggs 2009; Browne et al. 2021). Soil turnover from burrow construction can vary from 2.8 to 9.8 t/ha (Triggs 2009) and the soil in mounds contain increased nitrogen, which supports higher herb cover (Guy and Kirkpatrick 2021).
Fig. 1.
An adult and juvenile Common Wombat (Vombatus ursinus).
Fig. 2.
(A) The Australian distribution of the Common Wombat (Vombatus ursinus; dark gray; IUCN 2008) in relation to the areas burned in the 2019–20 Black Summer bushfires (red; Department of Agriculture 2020), and the study location (black); (B) a Common Wombat burrow; (C) the early stages of postfire regrowth; and (D) landscape impacts of the 2019–20 Black Summer bushfires at Woomargama National Park, New South Wales, Australia (images in C and D courtesy of Kylie Durant).
Wombat burrows are exploited by a range of species: a study showed that Common Wombat burrows were visited by 11 other species including possums, rodents, and birds (Old et al. 2018). Similarly, Thornett et al. (2017) recorded 11 vertebrate species that used burrows of Southern Hairy-nosed Wombat. The internal temperatures of wombat burrows are typically cooler and more stable than aboveground temperatures (Finlayson et al. 2003; Evans 2008; Swinbourne et al. 2016b), which can allow them to act as thermal refuges during heatwaves (Attwood 1982). Fossorial South-eastern Slider (Lerista bougainvillii) have also been observed exploiting the soil mounds around burrows, suggesting that burrows might offer increased thermoregulatory opportunities (Hodgson and Ritchie 2023). This prompts the question: could Common Wombat burrows also provide refuge to animals from the impacts of fire?
In this study, we monitored the activity (i.e., the number of detection events separated by >30 min) of a range of native vertebrates around Common Wombat burrows in habitats that burned at varying severity during the 2019–20 bushfires, and at nearby unburned sites. We hypothesized that: (i) if Common Wombat are ecological facilitators via their bioturbation, then animal activity will be higher around burrows compared to nearby sites without burrows—the affinity of species with burrows will differ depending on their shelter and resource requirements, and hence community composition will differ between burrows and ecologically similar areas lacking burrows; and (ii) if Common Wombat burrows are acting as fire refuges, then the negative impacts of fire on animal activity—which we expect to be widespread given the severity of the 2019–20 bushfires—will be reduced at burrows located in burned habitat, and these buffering effects should be most apparent at sites that burned at high severity, consistent with the stress-gradient hypothesis.
Materials and methods
Study area
We conducted this study at Woomargama National Park (24,185 ha) and Woomargama State Forest (7,120 ha), New South Wales, Australia. Geology consists predominantly of steeply sloping Silurian Koetong granite, with soils consisting of yellow to red podsolics (NSW National Parks and Wildlife Service 2009). The average annual rainfall is 886 mm, with the majority of rainfall occurring in winter and spring (Bureau of Meteorology 2023). The area consists of continuous eucalypt forest and woodland communities. Dominant canopy species consists of a mix of Eucalyptus spp., primarily Broad-leaved Peppermint (E. dives), Narrow-leaved Peppermint (E. radiata), and Brittle Gum (E. mannifera; NSW National Parks and Wildlife Service 2009). Both mid and ground stories consist of a mix of shrubs and grasses (NSW National Parks and Wildlife Service 2009). The area has undergone historical land use changes including mining, forestry, and agricultural activities (NSW National Parks and Wildlife Service 2009). During the 2019–20 bushfires, the Green Valley/Tunnel Road fire complex was part of a larger gigafire (Linley et al. 2022) in which 6 fires merged and burned 632,315 ha. The fire burned ~18,119 ha of Woomargama National Park and Woomargama State Forest at mixed severities, occurred predominantly on the eastern side of the park, started by lightning strike on 29 December 2019, and was extinguished by 18 February 2020. Fires in these environments can have long-lasting impacts on ecosystems (Bradstock 2008; Williams et al. 2008).
Site selection
We used a paired design to assess the use of Common Wombat burrows in differing fire severity treatments, in comparison to nearby areas with the same fire severity but without a burrow. Common Wombat burrows were selected from ground searching the study area in conjunction with fire severity maps. Ground-truthing revealed the extreme impact of the fires on vegetation throughout the study area, with many large trees killed and understory regrowth and canopy resprouting occurring postfire (Fig. 2). Using the Australian Google Earth Engine Burnt Area Map of the 2019–20 bushfires (Aus GEEBAM; Department of Agriculture 2020) fire severity maps were used to classify the change in vegetation. Aus GEEBAM fire classes were derived from changes in a vegetation index following fire, in comparison to nearby ecologically similar areas that escaped the fire (Department of Agriculture 2020). Aus GEEBAM fire classes are: unburnt, where little or no change in the vegetation index was observed; low and moderate severity, where moderate change to reference unburnt area was detected; high severity, where the vegetation was mostly scorched; and very high severity, where vegetation was consumed (Department of Agriculture 2020).
In total, 28 site pairs (i.e., 56 sites in total) were selected. Site pairs consisted of a Common Wombat burrow (Fig. 2) and a paired site approximately 50 m away in the same fire severity class and in similar habitat (i.e., vegetation type, topography), to act as an experimental control (Coggan et al. 2018). Fire severity classes were ground-truthed to ensure the classification from maps matched the burn severities on the ground and canopy layers. We reclassified the Aus GEEBAM fire severity classes listed above to include: unburnt areas outside of the fire scar; unburnt areas within the fire scar; low and moderate severities; and high severities that encompasses both high and very high burned areas, as differences between these 2 classes were not obvious on the ground. Seven sites were established within each of the fire severity classes. Preliminary analysis suggested few differences in animal activity in unburned areas outside of and within the fire scar, and so all unburned sites were classified simply as unburned. All sites were located within Eucalypt woodlands with shrubby or grassy understory. Vegetation at sites within the fire scar exhibited significant regrowth, manifesting at various stages depending on the fire severity at each location.
Animal activity
We surveyed the activity of terrestrial mammals at each site using wildlife cameras (Reconyx HC600 Hyperfire, Reconyx Inc., Holmen, Wisconsin). Each of the 28 site pairs consisted of 2 cameras (56 sites), 1 placed at a Common Wombat burrow and the other at the paired control site. To capture smaller mammals, cameras were fastened to a stake at a 20° downward angle. At burrows, cameras were placed approximately 2 m from the burrow entrance, facing the entrance. Camera orientation was consistent between burrows and control sites, with the only difference being that control sites were oriented toward bare ground. Cameras operated day and night, recording 5 images per burst, with no time delay between bursts, and sensitivity set to high. Cameras were deployed 16 months after the 2019–20 bushfires and operated continuously between June 2021 and April 2022, totaling 16,645 trap-days. Our study followed guidelines (Sikes et al. 2016) and all applicable international, national, and/or institutional guidelines for the use of animals. Research was conducted in accordance with the Charles Sturt University Animal Ethics Committee (permit number A21049) and permissions from relevant management authorities.
Image tagging and data extraction
Images from 56 cameras were tagged and processed using Wildlife Insights (Ahumada et al. 2020). When present, animals were identified to species level, if species-level identification was not feasible, identification was made to the highest possible taxonomic resolution (typically genus level). This was achieved using Wildlife Insights’ artificial intelligence, which automatically detects and identifies species in images (Ahumada et al. 2020), although every image and species identification was independently reviewed by wildlife experts. Behaviors evident in images were manually recorded where possible, including number of individuals and the occurrence of specific behaviors around burrows including: bathing, referring to an animal using water in the burrow to clean themselves; drinking, referring to an animal consuming water from the burrow; entering or emerging, referring to an animal entering or exiting the burrow entirely; inspecting, referring to an animal investigating the burrow entrance but not entering the burrow completely; foraging, referring to animals feeding in or directly around the lip of the burrow; and geophagy, referring to animals consuming soil from the burrow. Classifying behaviors was not always possible due to the small size of some species—e.g., House Mouse (Mus musculus) and Agile Antechinus (Antechinus agilis), making it difficult to assign a specific behavior to some images. We also recorded dates when burrows were filled with water to assess how often Common Wombat burrows provide a water source to other wildlife. We extracted the tagged image metadata, including site information, date, and time using camtrapRdelux package (https://github.com/carlopacioni/camtrapRdeluxe, an extension of camtrapR (Niedballa et al. 2016) and the exiftool add on (Harvey 2020). We treated all detections as independent events when more than 30 min separated detections of the same species (Cunningham et al. 2018).
Data analysis
To test our hypothesis that animal activity will be higher around burrows, we fit Bayesian mixed-effects models using the package brms (Bürkner 2017), which fits Bayesian models in stan which compiles models to sample from posterior distributions (Stan Development Team 2022). We included site pairs as random effects and used 2,000 iterations and 4 chains, with a 2,000-iteration warm-up. We used default priors, which are weakly informative, to improve convergence and sampling efficiency, with minimal impact on estimations. Model parameters are regarded as showing evidence of an effect when the 89% credible interval did not overlap zero (McElreath 2020). We used 89% credible intervals as they are commonly used in Bayesian statistics (Samaš et al. 2021), and due to their relative stability compared to 95% credible intervals (Kruschke 2014). Our response variables were: the number of independent events for each species; species richness (i.e., count of all species detected at a site) of all species (total richness); native mammal species only; and native bird species only. Individual species were modeled if recorded by at least 10 camera traps.
We modeled our response variables as a function of whether the site was a burrow or a control (“site type”). Control sites were used as the reference category. If the 89% credible intervals of the mixed models did not overlap zero, it was considered evidence that the response variable at burrows was substantially different from the control sites (McElreath 2020). To test for compositional differences between species assemblages at burrows and control sites, we used a permutational multivariate analysis of variance (PerMANOVA). We used the Bray–Curtis index to calculate dissimilarities based on a square root transformation of our activity data. PerMANOVA was fit using the adonis2 function from the vegan package (Oksanen et al. 2013). We permuted dissimilarities 999 times to assess significance. Community composition was visualized using nonmetric multidimensional scaling (nMDS: Legendre and Legendre 1998). To determine which species most influenced dissimilarities at burrows and control sites, we performed indicator species analysis using the multipatt function from the indicspecies package (De Cáceres et al. 2016).
Finally, to examine if associations were most pronounced in areas that experienced high-severity fires, we again used Bayesian models in brms (Bürkner 2017) to model response variables as a function of site type and fire severity class. We combined site type and fire severity to create a 6-level categorical variable (unburned burrow, unburned control, low–moderate burned burrow, low–moderate burned control, high severity burrow, high severity control) and used unburned controls as the reference category. Because some categories had few detections for some species, we used weakly informative priors with a Student’s t-distribution to aid in model convergence. Specifying unburned controls as the reference allows both a comparison between burrows and control sites, which reveal whether burrows experience increased or decreased activity relative to controls—as well as a comparison between unburned and burned controls, which reveal the impacts of fire severity on each response variable. This allows the impact of burrows to be contextualized in terms of the background influence of fire.
Results
Camera traps recorded 746,674 images containing 370,845 wildlife images. Images were compressed into 14,580 events that represented 15,723 individual animals. Fifty-six species were identified (47 native species, 9 introduced), consisting of 19 mammal, 33 bird, and 4 reptile species. Common Wombat were present at all sampled burrows, which had an average burrow opening height of 19.18 cm (±2.77 SE) and width of 22.66 cm (±3.21 SE). Excluding Common Wombat, 48 species were identified at burrows and 43 at control sites (Supplementary Data SD1). Species found only at burrows included Common Ringtail Possum (Pseudocheirus peregrinus), Grey Fantail (Rhipidura albiscapa), Spotted Pardalote (Pardalotus punctatus), and White-throated Treecreeper (Cormobates leucophaea). Species only observed at control sites included Australian Raven (Corvus coronoides), Common Bronzewing (Phaps chalcoptera), and Eastern Rosella (Platycercus eximius). A total of 31 species were recorded interacting with burrows (Supplementary Data SD2), including several species inspecting (30 of 31 species), foraging (11/31), and entering and emerging (10/31) from burrows—while less common behaviors included drinking (4/31) and bathing (1/31) in flooded burrows, and geophagy (1/31; Supplementary Data SD2; Fig. 3). From 16,645 trap-days, images containing animals occurred over 8,384 trap-days, of which burrows contained pooled water for 207 days. From 28 burrows, 19 filled with water at least once for an average total time of 10.89 days (±3.74 SE). From 207 days where burrows were filled with water, 109 days occurred in spring, 54 in winter, and 44 in summer.
Fig. 3.
Examples of animals and behaviors observed at Common Wombat burrows: (A) a Lace Monitor foraging in the burrow entrance; (B) a Short-beaked Echidna inspecting the lip of the burrow; (C) a Swamp Wallaby drinking from a burrow full of water; (D) a Red-necked Wallaby inspecting the burrow entrance; (E) a Grey Shrike-thrush foraging at the burrow entrance; and (F) a Pied Currawong drinking from a partially filled burrow.
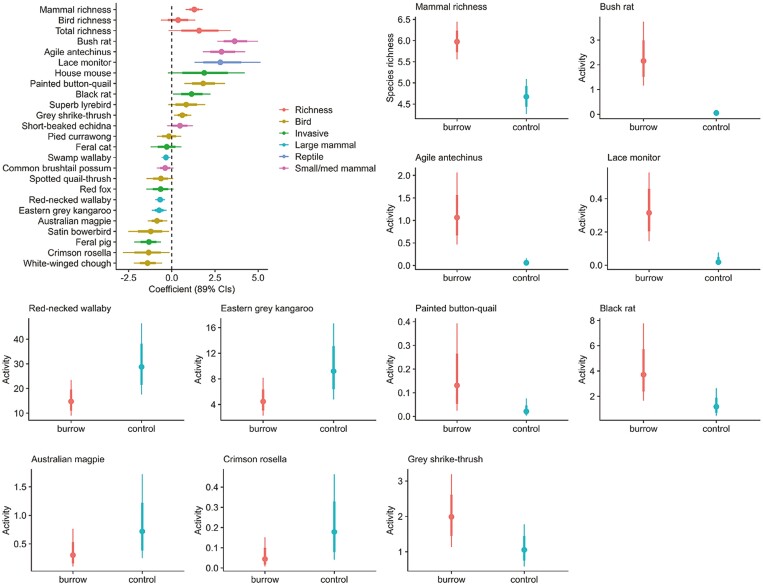
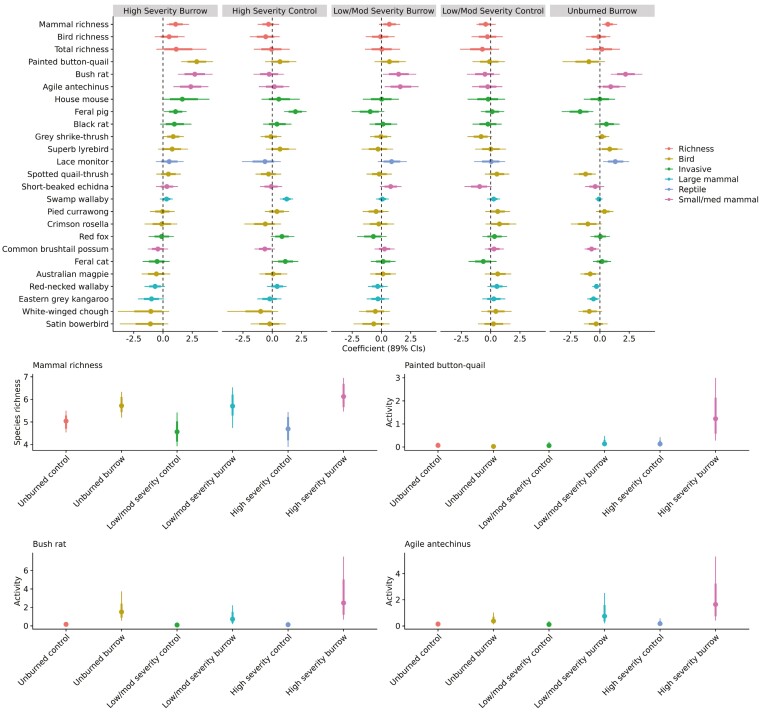
Several species showed increased activity around burrows when compared to control sites (i.e., 89% CIs did not overlap zero; Fig. 4; Supplementary Data SD3), including several native species such as Bush Rat (Rattus fuscipes), Agile Antechinus, Lace Monitor (Varanus varius), Painted Button-quail (Turnix varius), Grey Shrike-thrush (Colluricincla harmonica), and introduced Black Rat (Rattus rattus; Fig. 4; Supplementary Data SD3). Several native species including Swamp Wallaby, Red-necked Wallaby, Eastern Grey Kangaroo, Australian Magpie (Gymnorhina tibicen), Satin Bowerbird (Ptilonorhynchus violaceus), Crimson Rosella (Platycercus elegans), White-winged Chough (Corcorax melanorhamphos), and introduced Feral Pig (Sus scrofa) were more active at control sites (Fig. 4; Supplementary Data SD3). The activity of all other species did not differ substantially between burrows and control sites. Native mammal richness was greater at burrows than control sites (Fig. 4; Supplementary Data SD3), whereas total richness and bird richness did not substantially differ (i.e., 89% CIs overlapped zero). For mammals, there was a tendency for smaller species to be positively associated with burrows and for larger species to be negatively associated with burrows (Fig. 5). A linear regression comparing the effect size from Bayesian mixed-effects models to the natural logarithm of body mass revealed a strong, negative relationship for mammals (Coefficient ± 89% CI = −0.494 [−0.638 to −0.350], t = −6.026, R2 = 0.784). This relationship was far weaker when considering birds and reptiles in addition to mammals (Coefficient ± 89% CI = −0.295 [−0.502 to −0.089], t = −0.621, R2 = 0.052), suggesting that this size-dependent relationship with burrows was specific to mammals.
Fig. 4.
Coefficients and credible intervals from Bayesian models comparing species activity in burrows versus control sites (controls specified as the reference category), and examples of species activity predicted from those models. In activity plots: colored dot = point estimate; thick bars = 66% CIs; and thin bars = 89% CIs.
Fig. 5.
Effect size from Bayesian mixed-effects models of treatment (burrow vs. control) against the natural logarithm of body mass for mammals at Woomargama National Park: black line = fitted relationship from linear regression model; black dots = coefficients ± 89% credible intervals; effect sizes > 0 indicate a positive association with burrows, and effect sizes < 0 indicate negative associations.
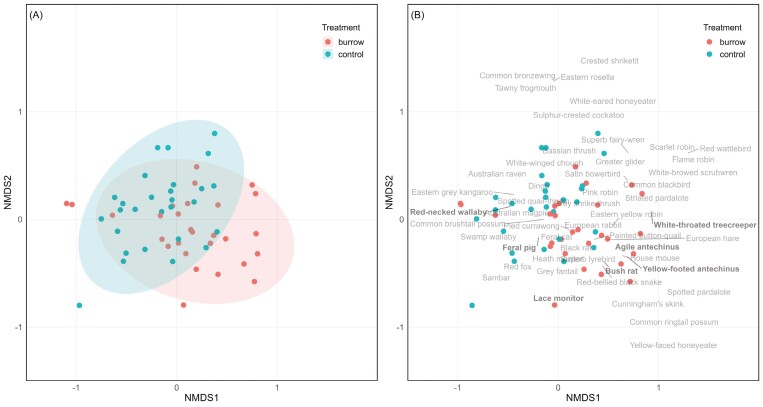
Species composition varied between burrow and control sites (PerMANOVA, F1,55 = 2.188, P = 0.003; Fig. 6). Indicator species analysis identified several species associated with burrows: Bush Rat (stat = 0.849, P = 0.001); Agile Antechinus (stat = 0.773, P = 0.001); Lace Monitor (stat = 0.602, P = 0.003); White-throated Treecreeper (stat = 0.463, P = 0.023); and Yellow-footed Antechinus (Antechinus flavipes; stat = 0.463, P = 0.026). Indicator species for control sites were the Red-necked Wallaby (stat = 0.799, P = 0.022) and Feral Pig (stat = 0.639, P = 0.037; Fig. 6).
Fig. 6.
The difference in species composition at burrows and control sites in Woomargama National Park. Ordinations show nMDS plots of square root transformed activity indices: (A) sites (points) colored by treatment (burrow vs. control)—distances between sites indicate community dissimilarity, and shaded areas are ellipses based on a multivariate t-distribution; and (B) the location of species in ordination space with indicator species highlighted in bold.
The association between Agile Antechinus and burrows was most apparent at burrows burned at high severity, remained evident at burrows burned at low–moderate severity, and was least evident (89% CIs overlapping zero) at unburned burrows (Fig. 7; Supplementary Data SD4). Bush Rat were more active at burrows burned at high severity, low–moderate severity, and unburned burrows. Painted Button-quail were more active around burrows that burned at high severity compared to unburned control sites (Fig. 7; Supplementary Data SD4). The association of Lace Monitor with burrows was only evident in unburned sites (Fig. 7; Supplementary Data SD4). Feral Pig, Spotted Quail-thrush, Common Brushtail Possum, and Australian Magpie were all less active around unburnt burrows (Fig. 7; Supplementary Data SD4). Native mammal richness was higher at burrows that were either burned at high severity or remained unburnt (Fig. 7; Supplementary Data SD4). Swamp Wallaby, Feral Pig, and feral Domestic Cat (Felis catus) were the only species for which activity varied depending on fire severity, all being more active in areas that burned at high severity during the 2019–20 bushfires (Fig. 7; Supplementary Data SD4).
Fig. 7.
Coefficients and credible intervals from Bayesian models comparing species activity in burrows versus control sites in sites that vary in fire severity (controls specified as the reference category), and examples of species activity predicted from those models. In activity plots: colored dot = point estimate; thick bars = 66% CIs; and thin bars = 89% CIs.
Discussion
Ecological facilitation by Common Wombat increases the activity of numerous native species, alters community composition, and boosts native mammal richness. Body size determined whether mammals were positively or negatively associated with burrows, with smaller species being more active and larger species being less active around burrows. The association of several species with burrows persisted—and in some instances was strengthened—after severe wildfire, consistent with the stress-gradient hypothesis. Our results add to a growing literature underscoring the significance of burrowing engineers as ecological facilitators.
Wombat burrows affect animal community composition
The presence of Common Wombat burrows alters the local environment sufficiently to elicit clear changes in local animal communities. Of particular interest is an apparent size dependency in species interactions with burrows, with smaller mammals being more active and larger mammals less active at burrows. The capacity of a species to enter Common Wombat burrows appears to be linked to the size of the species, with larger animals being excluded from utilizing the available shelter and foraging potential due to the size of the burrow openings. We detected numerous examples of smaller species using Common Wombat burrows, including Bush Rat, Black Rat, Short-beaked Echidna, Grey Shrike-thrush, White-throated Treecreeper, and Lace Monitor. Our survey method may have even underestimated the activity of some of these species, as camera traps are often less effective at capturing smaller species of mammals, birds, and reptiles (Jumeau et al. 2017). Other researchers have documented small mammals entering and exiting burrows: Triggs (2009:33) mentions antechinus and bush rats seen “scurrying away from burrow entrances,” that antechinus “emerge from small crevices in the walls of Bare-nosed Wombat burrows,” and that goannas “have also been found in wombat burrows,” while McIlroy (1973) found Bush Rat in Common Wombat burrows. One of our authors (DRM) has observed antechinus and bush rats escaping to Common Wombat burrows not far from our study region. Even relatively larger species, like the Swamp Wallaby and Red-necked Wallaby, were observed entering burrows, although these were rare events (relative to other behaviors). Common Wombat are large-bodied and stocky mammals with occasional displays of aggression, and they are known to compete with macropods for resources (Tamura et al. 2021). Thus, it is plausible that Common Wombat actively deter larger competitors away from their burrows, or that macropods avoid burrows to reduce the risk of a negative encounter, as Common Wombat vigorously defend their territories against unwanted intruders (Triggs 2009).
Aside from providing shelter, Common Wombat burrows could affect animal communities via changes in soils, vegetation, and topography around the burrow. Soil around Common Wombat burrows has greater nitrogen than surrounding soil due to the deposition of wombat feces, while vegetation in the vicinity of burrows typically includes more bare ground and herbaceous plants, with less shrub cover (Guy and Kirkpatrick 2021). These variables could affect the foraging opportunities for small herbivores and carnivores alike. Common Wombat burrows may increase the activity of ground-dwelling insectivores and omnivores such as Bush Rat, Agile Antechinus, Grey Shrike-thrush, and Painted Button-quail, by providing increased foraging opportunities. While little is known of invertebrate communities present at Common Wombat burrows (and was not a specific subject of our research here), high diversities of invertebrates have been recorded at the burrows of other species. For instance, Gopher Tortoise burrows support hundreds of arthropod species (Jackson and Milstrey 1989), while burrows and mounds from Gunnison’s Prairie Dog (Cynomys gunnisoni), Black-tailed Prairie Dog (C. ludovicianus), and Banner-tailed Kangaroo Rat (Dipodomys spectabilis) increase arthropod abundance and richness compared to surrounding areas without mounds and burrows (Davidson and Lightfoot 2007). Additionally, native Australian predators including Western Quoll (Dasyurus geoffroii), Sand Goanna (V. gouldii), and Mulga Snake (Pseudechis australis) use burrows from European Rabbit (Oryctolagus cuniculus) that concentrated prey species (Dean et al. 2023). The increase in activity of small vertebrates around Common Wombat burrows may cascade through the system, as these species attract and are exploited by larger native predators such as Lace Monitor. These large, generalist, ectothermic carnivores may also derive additional benefits from Common Wombat burrows, because burrows provide a thermal refuge, buffering against extreme environmental conditions (Pike and Mitchell 2013).
Finally, we observed several instances of Common Wombat burrows filling with water including during summer, signaling that water provision may be another ecological role that they provide. Macropods including Eastern Grey Kangaroo, Red-necked Wallaby, and Swamp Wallaby were all captured drinking from burrows, and Pied Currawong were observed bathing. These observations suggest that burrows could provide an important water resource to animals, although they may only provide critical services during periods of low water availability. For example, well-digging by feral equids in North American deserts increases water availability and, probably due to the scarcity of water in this system, vertebrate richness (Lundgren et al. 2021). While water was not scarce during our study period, or compared to other drier ecosystems, it does play a vital role in regulating and maintaining ecosystem services, especially in the drier months (Pepper et al. 2008). As such, water provision via Common Wombat burrows, especially during warmer periods, presents an interesting observation that warrants further investigation.
Wombat burrows as fire refuges
We found that associations between species and burrows persisted, and often strengthened in fire-impacted habitat, particularly in areas that burned at high severity. Smaller-sized animals including Agile Antechinus, Bush Rat, and Painted Button-quail all retained positive associations with burrows in areas that were subject to high-severity fire, and all 3 were most active at burrows subject to high-severity fire. By contrast, the association of Lace Monitor with burrows was only evident at unburned burrows. These findings suggest that the association of species with Common Wombat burrows is highly context-dependent, and that fire plays a significant role in determining the nature of this relationship in Australian forests.
For small vertebrates that are vulnerable to predators, many flammable refuges such as logs are consumed by severe fires (Bassett et al. 2015). Wombat burrows—which survive fire and can persist for decades (Taggart and Temple-Smith 2008)—potentially provide important postfire shelter. Our study suggested that the 2019–20 bushfires had relatively negligible impacts on the activity of species that were relatively similar between burnt and unburnt habitat. However, it did indicate that the impacts of fire might concentrate the activity of small vertebrates within the vicinity of burrows, providing a locus from which foraging and intraspecific interactions can occur in relative proximity to predictable shelter and protection from predators. Disturbances in other regions likely shape associations between other ecosystem engineers and the co-occurring species. Allospecific use of Gopher Tortoise burrows dramatically increases during and after a fire (Knapp et al. 2018), and European Rabbit warrens support greater densities of Mediterranean lizards in unfavorable habitat (Gálvez Bravo et al. 2009), further highlighting the context-dependent importance of burrows in disturbed landscapes.
Our results have important implications for conservation. The geographic range of Common Wombat has declined since European colonization of Australia (Buchan and Goldney 1998; Triggs 2009; Baker and Gynther 2023), and is predicted to substantially contract from climate change (Graham et al. 2019). The 2 other species of wombat are vulnerable to extinction (Swinbourne et al. 2016a), and numerous other burrowing Australian marsupials have been driven to extinction since European colonization (Woinarski et al. 2019). Throughout the forests of eastern Australia, Common Wombat are the only extant species of native mammal capable of excavating wide, deep, and elaborate burrows, and our research suggests that they play a critical role for a host of other species. The importance of ecosystem engineers for providing critical shelter for other species highlights the often-overlooked risk of co-extinctions due to the loss of burrowers. For example, endangered pygmy Blue-tongued Skink (Tiliqua adelaidensis)—presumed extinct for 33 years (Armstrong and Reid 1992; Armstrong et al. 1993)—are entirely reliant on burrows constructed by lycosid and mygalomorph spiders (Hutchinson et al. 1994). Changed land use has caused declines in these ecosystem engineering spiders and the near extinction of the lizards (Souter et al. 2007). The value of burrowers as providers of shelter and refuge likely increases after disturbance across a range of ecosystems. The occurrence of larger and more severe wildfires around the world has placed a premium on understanding how animals survive, persist, and recover following extreme wildfire events. Our research has revealed that Common Wombat burrows play a valuable and underappreciated role in the fire-prone forests of Australia.
Supplementary data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Species which were identified at burrows and controls.
Supplementary Data SD2.—Number of images per behavior observed from species interacting with wombat burrows.
Supplementary Data SD3.—Results of coefficients, with 89% confidence intervals, from Bayesian models comparing species activity in burrows versus controls.
Supplementary Data SD4.—Results of coefficients, with 89% confidence intervals, from Bayesian models comparing species activity in burrows versus controls in sites that vary in fire severity.
Acknowledgments
We acknowledge the Wiradjuri People, the Traditional Owners of the land upon which this study took place, and we pay respects to Elders both past, present, and emerging. Dave Pearce from NSW National Parks and Wildlife Service provided access and insights into Woomargama National Park. Kylie Durant from Holbrook Landcare Group provided information and insights into the regional area. Kylee Imlach provided administrative and logistical support. Caroline Maurel and Mitch Cowan assisted with fieldwork. Alana de Laive helped with image identification. The Gulbali Institute’s GAPS funding was instrumental in the completion of this manuscript.
Contributor Information
Grant D Linley, Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Thurgoona, NSW 2640, Australia; School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Thurgoona, NSW 2640, Australia.
William L Geary, Biodiversity Strategy and Planning Branch, Biodiversity Division, Department of Environment, Land, Water and Planning, East Melbourne, VIC 3002, Australia; School of Life and Environmental Sciences, Deakin University, Burwood, VIC 3125, Australia; School of Agriculture, Food and Ecosystem Sciences, University of Melbourne, Parkville, VIC 3010, Australia.
Chris J Jolly, Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Thurgoona, NSW 2640, Australia; School of Natural Sciences, Macquarie University, Macquarie Park, NSW 2109, Australia.
Emma E Spencer, WWF-Australia, Suite 3.01, Level 3/45 Clarence Street, Sydney, NSW 2000, Australia.
Kita R Ashman, Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Thurgoona, NSW 2640, Australia; WWF-Australia, Suite 3.01, Level 3/45 Clarence Street, Sydney, NSW 2000, Australia.
Damian R Michael, Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Thurgoona, NSW 2640, Australia.
Dylan M Westaway, Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Thurgoona, NSW 2640, Australia; School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Thurgoona, NSW 2640, Australia.
Dale G Nimmo, Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Thurgoona, NSW 2640, Australia; School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Thurgoona, NSW 2640, Australia.
Author contributions
GDL helped conceptualize the study, collect data, curate the data set, wrote the original draft, and led the analysis; DGN conceptualized the study, provided analysis assistance, and assisted on the original draft; WLG played a pivotal role in the analysis of the data and editing and revising the manuscript; CJJ assisted with the analysis and played a significant role in editing and revising the manuscript; EES, KA, DRM, and DMW helped edit and revise the manuscript.
Funding
Funding for this project was provided by the WWF-Australia’s Regenerate Australia scheme and Charles Sturt University’s Gulbali Institute.
Conflict of interest
None declared.
Data availability
Data are available upon reasonable request.
References
- Ahumada JA, Fegraus E, Birch T, Flores N, Kays R, O’Brien TG, Palmer J, Schuttler S, Zhao JY, Jetz W, et al. 2020. Wildlife insights: a platform to maximize the potential of camera trap and other passive sensor wildlife data for the planet. Environmental Conservation 47(1):1–6. 10.1017/s0376892919000298 [DOI] [Google Scholar]
- Andersen ML, Bennett DE, Holbrook JD.. 2021. Burrow webs: clawing the surface of interactions with burrows excavated by American badgers. Ecology and Evolution 11(17):11559–11568. 10.1002/ece3.7962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G, Reid J.. 1992. The rediscovery of the Adelaide Pygmy Bluetongue Tiliqua adelaidensis (Peters, 1863). Herpetofauna 22(2):3–6. [Google Scholar]
- Armstrong G, Reid J, Hutchinson M.. 1993. Discovery of a population of the rare scincid lizard Tiliqua adelaidensis (Peters). Records of the South Australian Museum 36:153–155. [Google Scholar]
- Attwood R. 1982. Birds sheltering in the burrows of mammals during a heat wave. South Australian Ornithologist 29:7–9. [Google Scholar]
- Baker A, Gynther I.. 2023. Strahan’s Mammals of Australia. 4th ed. Sydney (NSW, Australia): New Holland Publishers. [Google Scholar]
- Bassett M, Chia EK, Leonard SW, Nimmo DG, Holland GJ, Ritchie EG, Clarke MF, Bennett AF.. 2015. The effects of topographic variation and the fire regime on coarse woody debris: insights from a large wildfire. Forest Ecology and Management 340:126–134. 10.1016/j.foreco.2014.12.028 [DOI] [Google Scholar]
- Bertness MD, Callaway R.. 1994. Positive interactions in communities. Trends in Ecology & Evolution 9(5):191–193. 10.1016/0169-5347(94)90088-4 [DOI] [PubMed] [Google Scholar]
- Bradstock RA. 2008. Effects of large fires on biodiversity in south-eastern Australia: disaster or template for diversity? International Journal of Wildland Fire 17(6):809–822. 10.1071/wf07153 [DOI] [Google Scholar]
- Browne E, Driessen MM, Ross R, Roach M, Carver S.. 2021. Environmental suitability of bare-nosed wombat burrows for Sarcoptes scabiei. International Journal for Parasitology: Parasites and Wildlife 16:37–47. 10.1016/j.ijppaw.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan A, Goldney DC.. 1998. The common wombat Vombatus ursinus in a fragmented landscape. In: Wells RT, Pridmore PA, editors. Wombats. Chipping Norton (NSW, Australia): Surrey Beatty and Sons; p. 251–261. [Google Scholar]
- Bureau of Meteorology. 2023. Rainfall data for Woomargama. [accessed 18 Jan 2023]. http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=072171
- Bürkner P-C. 2017. brms: an R package for Bayesian multilevel models using Stan. Journal of Statistical Software 80(1):1–28. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- Coggan NV, Hayward MW, Gibb H.. 2018. A global database and “state of the field” review of research into ecosystem engineering by land animals. Journal of Animal Ecology 87(4):974–994. 10.1111/1365-2656.12819 [DOI] [PubMed] [Google Scholar]
- Collins L, Bradstock RA, Clarke H, Clarke MF, Nolan RH, Penman TD.. 2021. The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environmental Research Letters 16(4):044029. 10.1088/1748-9326/abeb9e [DOI] [Google Scholar]
- Cunningham CX, Johnson CN, Barmuta LA, Hollings T, Woehler EJ, Jones ME.. 2018. Top carnivore decline has cascading effects on scavengers and carrion persistence. Proceedings of the Royal Society of London, B: Biological Sciences 285(1892):20181582. 10.1098/rspb.2018.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AD, Lightfoot DC.. 2007. Interactive effects of keystone rodents on the structure of desert grassland arthropod communities. Ecography 30(4):515–525. 10.1111/j.0906-7590.2007.05032.x [DOI] [Google Scholar]
- Davies GTO, Kirkpatrick JB, Cameron EZ, Carver S, Johnson CN.. 2019. Ecosystem engineering by digging mammals: effects on soil fertility and condition in Tasmanian temperate woodland. Royal Society Open Science 6(1):180621. 10.1098/rsos.180621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S, Broussard L, Adams P, Moseby K, Waddington K, Kobryn H, Bateman P, Fleming P.. 2019. An outback oasis: the ecological importance of bilby burrows. Journal of Zoology 308(3):149–163. 10.1111/jzo.12663 [DOI] [Google Scholar]
- De Cáceres M, Jansen F, Dell N.. 2016. Version 1.7. Indicspecies: relationship between species and groups of sites. R package. https://cran.r-project.org/web/packages/indicspecies/index.html
- Dean AT, Brandle R, Barmuta LA, Jones ME, Jansen J.. 2023. Rabbit warrens: an important resource for invasive alien species in semi-arid Australia. Wildlife Research 51(1):WR22154. 10.1071/WR22154 [DOI] [Google Scholar]
- Department of Agriculture, Water and the Environment. 2020. Australian Google Earth Engine Burnt Area Map: a rapid, national approach to fire severity mapping. Canberra (Australia): Department of Agriculture, Water and the Environment. [Google Scholar]
- Desbiez ALJ, Kluyber D.. 2013. The role of giant armadillos (Priodontes maximus) as physical ecosystem engineers. Biotropica 45(5):537–540. 10.1111/btp.12052 [DOI] [Google Scholar]
- Doody JS, Soennichsen KF, James H, McHenry C, Clulow S.. 2021. Ecosystem engineering by deep‐nesting monitor lizards. Ecology 102(4):e03271. 10.1002/ecy.3271 [DOI] [PubMed] [Google Scholar]
- Dziadzio MC, Smith LL.. 2016. Vertebrate use of Gopher Tortoise burrows and aprons. Southeastern Naturalist 15(4):586–594. 10.1656/058.015.0403 [DOI] [Google Scholar]
- Eldridge DJ, James AI.. 2009. Soil-disturbance by native animals plays a critical role in maintaining healthy Australian landscapes. Ecological Management & Restoration 10(s1):S27–S34. 10.1111/j.1442-8903.2009.00452.x [DOI] [Google Scholar]
- Evans MC. 2008. Home range, burrow-use and activity patterns in common wombats (Vombatus ursinus). Wildlife Research 35(5):455–462. 10.1071/wr07067 [DOI] [Google Scholar]
- Evans MC, Macgregor C, Jarman PJ.. 2006. Diet and feeding selectivity of common wombats. Wildlife Research 33(4):321–330. 10.1071/wr05047 [DOI] [Google Scholar]
- Finlayson G, Shimmin G, Temple-Smith P, Handasyde K, Taggart D.. 2003. Monitoring the activity of a southern hairy-nosed wombat, Lasiorhinus latifrons, using temperature dataloggers. Australian Mammalogy 25(2):205–208. 10.1071/am03205 [DOI] [Google Scholar]
- Fleming PA, Anderson H, Prendergast AS, Bretz MR, Valentine LE, Hardy GES.. 2014. Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function? Mammal Review 44(2):94–108. 10.1111/mam.12014 [DOI] [Google Scholar]
- Gálvez Bravo L, Belliure J, Rebollo S.. 2009. European rabbits as ecosystem engineers: warrens increase lizard density and diversity. Biodiversity and Conservation 18(4):869–885. 10.1007/s10531-008-9438-9 [DOI] [Google Scholar]
- Graham EM, Reside AE, Atkinson I, Baird D, Hodgson L, James CS, VanDerWal JJ.. 2019. Climate change and biodiversity in Australia: a systematic modelling approach to nationwide species distributions. Australasian Journal of Environmental Management 26(2):112–123. 10.1080/14486563.2019.1599742 [DOI] [Google Scholar]
- Guy TR, Kirkpatrick JB.. 2021. Environmental associations and effects of disturbances by common wombats in alpine Tasmania. Austral Ecology 46(8):1392–1403. 10.1111/aec.13093 [DOI] [Google Scholar]
- Halstead LM, Sutherland DR, Valentine LE, Rendall AR, Coetsee AL, Ritchie EG.. 2020. Digging up the dirt: quantifying the effects on soil of a translocated ecosystem engineer. Austral Ecology 45(1):97–108. 10.1111/aec.12833 [DOI] [Google Scholar]
- Harvey P. 2020. Version 12. ExifTool. https://exiftool.org/
- Hodgson MJ, Ritchie D.. 2023. Strange bedfellows: mammal burrow disturbances may provide thermoregulatory microsites for fossorial reptiles in densely vegetated dunes. Austral Ecology 48(7):1473–1478. 10.1111/aec.13408 [DOI] [Google Scholar]
- Hutchinson M, Milne T, Croft T.. 1994. Redescription and ecological notes on the Pygmy Bluetongue, Tiliqua adelaidensis (Squamata: Scincidae). Transactions of the Royal Society of South Australia 118(4):217–226. [Google Scholar]
- IUCN. 2008. Vombatus ursinus. [accessed 24 Dec 2022].
- Jackson D, Milstrey E.. 1989. The fauna of Gopher Tortoise burrows. In: Diemer JE, Jackson DR, Landers JL, Layne JN, Wood DA, editors. Gopher Tortoise Relocation Symposium Proceedings. Gainesville (FL, USA): Florida Game and Fresh Water Fish Commission. p. 86–98. [Google Scholar]
- Jolly CJ, Dickman CR, Doherty TS, van Eeden LM, Geary WL, Legge SM, Woinarski JCZ, Nimmo DG.. 2022. Animal mortality during fire. Global Change Biology 28(6):2053–2065. 10.1111/gcb.16044 [DOI] [PubMed] [Google Scholar]
- Jones CG, Lawton JH, Shachak M.. 1994. Organisms as ecosystem engineers. Oikos 69(3):373–386. 10.2307/3545850 [DOI] [Google Scholar]
- Jones CG, Lawton JH, Shachak M.. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78(7):1946–1957. 10.2307/2265935 [DOI] [Google Scholar]
- Jumeau J, Petrod L, Handrich Y.. 2017. A comparison of camera trap and permanent recording video camera efficiency in wildlife underpasses. Ecology and Evolution 7(18):7399–7407. 10.1002/ece3.3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Van Niel KP, Wardell‐Johnson GW, Yates CJ, Byrne M, Mucina L, Schut AG, Hopper SD, Franklin SE.. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21(4):393–404. 10.1111/j.1466-8238.2011.00686.x [DOI] [Google Scholar]
- Knapp DD, Howze JM, Murphy CM, Dziadzio MC, Smith LL.. 2018. Prescribed fire affects diurnal vertebrate use of Gopher Tortoise (Gopherus polyphemus) burrows in a Longleaf Pine (Pinus palustris) forest. Herpetological Conservation and Biology 13(3):551–557. [Google Scholar]
- Kruschke J. 2014. Doing Bayesian data analysis: a tutorial with R, JAGS, and Stan. London (UK): Academic Press. [Google Scholar]
- Legendre P, Legendre L.. 1998. Numerical ecology: developments in environmental modelling. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Linley GD, Jolly CJ, Doherty TS, Geary WL, Armenteras D, Belcher CM, Bliege Bird R, Duane A, Fletcher M-S, Giorgis MA, et al. 2022. What do you mean, ‘megafire’? Global Ecology and Biogeography 31(10):1906–1922. 10.1111/geb.13499 [DOI] [Google Scholar]
- Lowney AM, Thomson RL.. 2021. Ecological engineering across a temporal gradient: sociable weaver colonies create year-round animal biodiversity hotspots. The Journal of Animal Ecology 90(10):2362–2376. 10.1111/1365-2656.13544 [DOI] [PubMed] [Google Scholar]
- Lundgren EJ, Ramp D, Stromberg JC, Wu J, Nieto NC, Sluk M, Moeller KT, Wallach AD.. 2021. Equids engineer desert water availability. Science 372(6541):491–495. 10.1126/science.abd6775 [DOI] [PubMed] [Google Scholar]
- Lunney D, O’Connell M.. 1988. Habitat selection by the swamp wallaby, Wallabia bicolor, the Red-necked Wallaby, Macropus rufogriseus, and the Common Wombat, Vombatus ursinus, in logged, burnt forest near Bega, New-South-Wales. Wildlife Research 15(6):695–706. [Google Scholar]
- McElreath R. 2020. Statistical rethinking: a Bayesian course with examples in R and Stan. New York (NY, USA): Chapman and Hall/CRC. [Google Scholar]
- McIlroy JC. 1973. Aspects of the ecology of the common wombat, Vombatus ursinus (Shaw, 1800) [Doctor of Philosophy]. Canberra (ACT, Australia): The Australian National University. [Google Scholar]
- Niedballa J, Sollmann R, Courtiol A, Wilting A.. 2016. camtrapR: an R package for efficient camera trap data management. Methods in Ecology and Evolution 7(12):1457–1462. 10.1111/2041-210x.12600 [DOI] [Google Scholar]
- Nimmo D. 2020. Tales of wombat ‘heroes’ have gone viral. Unfortunately, they’re not true. Australia: The Conversation. [Google Scholar]
- Nimmo DG, Avitabile S, Banks SC, Bliege Bird R, Callister K, Clarke MF, Dickman CR, Doherty TS, Driscoll DA, Greenville AC, et al. 2019. Animal movements in fire-prone landscapes. Biological Reviews of the Cambridge Philosophical Society 94(3):981–998. 10.1111/brv.12486 [DOI] [PubMed] [Google Scholar]
- Nimmo DG, Carthey AJR, Jolly CJ, Blumstein DT.. 2021. Welcome to the Pyrocene: animal survival in the age of megafire. Global Change Biology 27(22):5684–5693. 10.1111/gcb.15834 [DOI] [PubMed] [Google Scholar]
- Nolan RH, Boer MM, Collins L, Resco de Dios V, Clarke HG, Jenkins M, Kenny B, Bradstock RA.. 2020. Causes and consequences of eastern Australia’s 2019–20 season of mega-fires. Global Change Biology 26(3):1039–1041. 10.1111/gcb.14987 [DOI] [PubMed] [Google Scholar]
- NSW National Parks and Wildlife Service. 2009. Woomargama National Park, Woomargama State Conservation Area, Mullengandra Nature Reserve and Mullengandra State Conservation Area plan of management. (NSW, Australia): Department of Environment, Climate Change and Water. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H.. 2013. Package ‘vegan’. Community ecology package. [Google Scholar]
- Old JM, Hunter NE, Wolfenden J.. 2018. Who utilises bare-nosed wombat burrows? Australian Zoologist 39(3):409–413. 10.7882/az.2018.006 [DOI] [Google Scholar]
- Pausas JG, Keeley JE.. 2021. Wildfires and global change. Frontiers in Ecology and the Environment 19(7):387–395. 10.1002/fee.2359 [DOI] [Google Scholar]
- Pausas JG, Parr CL.. 2018. Towards an understanding of the evolutionary role of fire in animals. Evolutionary Ecology 32(2–3):113–125. 10.1007/s10682-018-9927-6 [DOI] [Google Scholar]
- Pepper DA, McMurtrie RE, Medlyn BE, Keith H, Eamus D.. 2008. Mechanisms linking plant productivity and water status for a temperate Eucalyptus forest flux site: analysis over wet and dry years with a simple model. Functional Plant Biology 35(6):493–508. 10.1071/FP08125 [DOI] [PubMed] [Google Scholar]
- Pike D, Mitchell J.. 2013. Burrow‐dwelling ecosystem engineers provide thermal refugia throughout the landscape. Animal Conservation 16(6):694–703. 10.1111/acv.12049 [DOI] [Google Scholar]
- Reside AE, Briscoe NJ, Dickman CR, Greenville AC, Hradsky BA, Kark S, Kearney MR, Kutt AS, Nimmo DG, Pavey CR, et al. 2019. Persistence through tough times: fixed and shifting refuges in threatened species conservation. Biodiversity and Conservation 28(6):1303–1330. 10.1007/s10531-019-01734-7 [DOI] [Google Scholar]
- Robinson NM, Leonard SW, Ritchie EG, Bassett M, Chia EK, Buckingham S, Gibb H, Bennett AF, Clarke MF.. 2013. Refuges for fauna in fire‐prone landscapes: their ecological function and importance. Journal of Applied Ecology 50(6):1321–1329. 10.1111/1365-2664.12153 [DOI] [Google Scholar]
- Roger E, Laffan SW, Ramp D.. 2007. Habitat selection by the common wombat (Vombatus ursinus) in disturbed environments: implications for the conservation of a ‘common’ species. Biological Conservation 137(3):437–449. 10.1016/j.biocon.2007.03.001 [DOI] [Google Scholar]
- Samaš P, Hauber ME, Honza M.. 2021. A meta-analysis of avian egg traits cueing egg-rejection defenses against brood parasitism. Frontiers in Ecology and Evolution 9:703208. 10.3389/fevo.2021.703208 [DOI] [Google Scholar]
- Sikes RS, The Animal Care and Use Committee of the American Society of Mammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97(3):663–688. 10.1093/jmammal/gyw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter NJ, Bull CM, Lethbridge MR, Hutchinson MN.. 2007. Habitat requirements of the endangered Pygmy Bluetongue Lizard, Tiliqua adelaidensis. Biological Conservation 135(1):33–45. 10.1016/j.biocon.2006.09.014 [DOI] [Google Scholar]
- Stan Development Team. 2022. Version 2.26. Stan modeling language user’s guide and reference manual. https://mc-stan.org [Google Scholar]
- Swinbourne MJ, Taggart DA, Peacock D, Ostendorf B.. 2016a. Historical changes in the distribution of hairy-nosed wombats (Lasiorhinus spp.): a review. Australian Mammalogy 39(1):1–16. 10.1071/am15046 [DOI] [Google Scholar]
- Swinbourne MJ, Taggart DA, Sparrow E, Hatch M, Ostendorf B.. 2016b. Ground penetrating radar as a non-invasive tool to better understand the population dynamics of a fossorial species: mapping the warrens of southern hairy-nosed wombats (Lasiorhinus latifrons). Wildlife Research 42(8):678–688. 10.1071/wr15068 [DOI] [Google Scholar]
- Taggart DA, Temple-Smith PD.. 2008. Southern Hairy-nosed Wombat. In: Van Dyck S, Strahan R, editors. The mammals of Australia. Sydney (NSW, Australia): New Holland Publishing Pty Ltd; p. 204–206. [Google Scholar]
- Tamura J, Ingram J, Martin AM, Burridge CP, Carver S.. 2021. Contrasting population manipulations reveal resource competition between two large marsupials: bare-nosed wombats and eastern grey kangaroos. Oecologia 197(2):313–325. 10.1007/s00442-021-04959-y [DOI] [PubMed] [Google Scholar]
- Taylor RJ. 1993. Observations on the behaviour and ecology of the common wombat Vombatus ursinus in northeast Tasmania. Australian Mammalogy 16(1):1–7. 10.1071/am93001 [DOI] [Google Scholar]
- Thornett E, Ostendorf B, Taggart DA.. 2017. Interspecies co-use of southern hairy-nosed wombat (Lasiorhinus latifrons) burrows. Australian Mammalogy 39(2):205–212. 10.1071/am15052 [DOI] [Google Scholar]
- Triggs B. 2009. Wombats. Collingwood (VIC, Australia): CSIRO Publishing. [Google Scholar]
- Valentine LE, Ruthrof KX, Fisher R, Hardy GESJ, Hobbs RJ, Fleming PA.. 2018. Bioturbation by bandicoots facilitates seedling growth by altering soil properties. Functional Ecology 32(9):2138–2148. 10.1111/1365-2435.13179 [DOI] [Google Scholar]
- van Eeden L, Nimmo D, Mahony M, Herman K, Ehmke G, Driessen J, O’Connor J, Bino G, Taylor M, Dickman C.. 2020. Impacts of the unprecedented 2019–2020 bushfires on Australian animals. Ultimo (NSW, Australia): WWF-Australia. [Google Scholar]
- Walde AD, Walde AM, Delaney DK, Pater LL.. 2009. Burrows of desert tortoises (Gopherus agassizii) as thermal refugia for horned larks (Eremophila alpestris) in the Mojave Desert. The Southwestern Naturalist 54(4):375–381. 10.1894/mh-41.1 [DOI] [Google Scholar]
- Whitford WG, Kay FR.. 1999. Biopedturbation by mammals in deserts: a review. Journal of Arid Environments 41(2):203–230. 10.1006/jare.1998.0482 [DOI] [Google Scholar]
- Williams RJ, Wahren C-H, Tolsma AD, Sanecki GM, Papst WA, Myers BA, McDougall KL, Heinze DA, Green K.. 2008. Large fires in Australian alpine landscapes: their part in the historical fire regime and their impacts on alpine biodiversity. International Journal of Wildland Fire 17(6):793–808. 10.1071/wf07154 [DOI] [Google Scholar]
- Wintle BA, Legge S, Woinarski JCZ.. 2020. After the megafires: what next for Australian wildlife? Trends in Ecology & Evolution 35(9):753–757. 10.1016/j.tree.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woinarski JC, Braby M, Burbidge AA, Coates D, Garnett ST, Fensham RJ, Legge S, McKenzie NL, Silcock J, Murphy BP.. 2019. Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239:108261. 10.1016/j.biocon.2019.108261 [DOI] [Google Scholar]
- Wu C, Venevsky S, Sitch S, Mercado LM, Huntingford C, Staver AC.. 2021. Historical and future global burned area with changing climate and human demography. One Earth 4(4):517–530. 10.1016/j.oneear.2021.03.002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.