Abstract
Background and Aims
Orchid seeds are reputed to be short lived in dry, cold storage conditions, potentially limiting the use of conventional seed banks for long-term ex situ conservation. This work explores whether Cattleya seeds are long lived or not during conventional storage (predried to ~12 % relative humidity, then stored at −18 °C).
Methods
We explored the possible interaction of factors influencing seed lifespan in eight species of the genus Cattleya using physiological (germination and vigour), biochemical (gas chromatography), biophysical (differential scanning calorimetry) and morphometric methods. Seeds were desiccated to ~3 % moisture content and stored at −18 °C for more than a decade, and seed quality was measured via three in vitro germination techniques. Tetrazolium staining was also used to monitor seed viability during storage. The morphometric and germination data were subjected to ANOVA and cluster analysis, and seed lifespan was subjected to probit analysis.
Key Results
Seeds of all Cattleya species were found to be desiccation tolerant, with predicted storage lifespans (P50y) of ~30 years for six species and much longer for two species. Cluster analysis showed that the three species with the longest-lived seeds had smaller (9–11 %) airspaces around the embryo. The post-storage germination method impacted the quality assessment; seeds equilibrated at room temperature for 24 h or in 10 % sucrose solution had improved germination, particularly for the seeds with the smallest embryos. Chromatography revealed that the seeds of all eight species were rich in linoleic acid, and differential scanning calorimetry identified a peak that might be auxiliary to selecting long-lived seeds.
Conclusions
These findings show that not all orchids produce seeds that are short lived, and our trait analyses might help to strengthen prediction of seed longevity in diverse orchid species.
Keywords: Cryobiotechnology, differential scanning calorimetry, fatty acid composition, orchid conservation, seed lifespan, seed longevity
INTRODUCTION
Seed banking is a very effective methodology for storing and conserving plant genetic resources (Walters and Pence, 2021). Commonly used for crop species and their relatives, the techniques are well documented and established for many species, including wild species (Nagel and Börner, 2010; Castañeda-Álvarez et al., 2016; Colville and Pritchard, 2019). The suitability of orchid species for ex situ seed storage and conservation has been debated for many years, because storability is known to vary with species (Shoushtari et al., 1994). Contrasting findings during storage span refridgerator conditions (Machado-Neto and Custódio, 2005), −10 °C for Cattleya loddigessi and C. coccinea (syn. Sophronitis coccinea) (Bowling and Thompson, 1972), −18 °C or lower temperatures for Disa uniflora (Thornhill and Koopowitz, 1992), and down to liquid nitrogen at −196 °C (Pritchard, 1984; Popova et al., 2016; Fileti et al., 2021). (Note that abbreviations of scientific names follow the rules of the Royal Horticultural Society for orchid genera and hybrids.) Moreover, seeds have been predried by different methods and to varying moisture contents, e.g. over calcium chloride, silica gel and lithium chloride, making the development of reliable predictive tools for orchid seed storage difficult. The problem is compounded by the use of various in vitro germination tests, necessary for most orchids (Prasongsom et al., 2022), that can impact the assessment of seed quality. Because the small seeds of orchids have a large surface area-to-volume ratio and must be disinfected before sowing on an in vitro germination medium, there is a risk of imbibition damage to the dry seeds (Pritchard et al., 1999). Additionally, the medium used for germination might be suboptimal for the stored seed, which might be evident only over a relatively long period of storage as seed vigour and viability fall and within the individual germination test that can vary from <1 month (Hosomi et al., 2011, 2012) to >1 year (Rasmussen et al., 2015), depending on the species and culture conditions (Diantina et al., 2020). Media must also be devised to support the conversion of seeds into seedlings post-storage. Alternatively, post-storage seed survival can be assessed in ~1 day using the vital stain triphenyl tetrazolium chloride (Hosomi et al., 2011, 2012). Different sensitivities to these potential confounding factors could have contributed to the regular perception that orchids produce short-lived seeds.
Seeds tend to fit three broad classes of storage responses: orthodox seeds tolerate drying to <0.12 g H2O g−1 dry matter (DM) and storage at −20 °C; intermediate seeds tolerate partial drying to 0.1–0.15 g H2O g−1 DM and tend to be short lived, particularly at −20 °C; and recalcitrant seeds are sensitive to drying in the region l–0.25 g H2O g−1 DM, requiring tissue cryopreservation for long-term storage (Walters, 2015; Nadarajan et al., 2023). Furthermore, fully desiccation-tolerant, apparently orthodox seeds have vastly different lifespans (Walters et al., 2005; Colville and Pritchard, 2019; Mira et al., 2019). Longevity can be compromised by a negative interaction between lipid transformation, particularly crystallization, and sub-zero storage temperature. This risk has been inferred from differential scanning calorimetry (DSC) thermograms, for orchids (Pritchard and Seaton, 1993), Cuphea sp. (Crane et al., 2003, 2006) and brassicas (Mira et al., 2019), supporting the notion that there might be species-specific sub-zero storage temperature optima for oilseeds (Pritchard, 2004). A determinant of such behaviour could be the seed fatty acid (FA) composition, which drives lipid thermal transformation (Crane et al., 2003, 2006; Mira et al., 2019). Variability in lipid composition is evident amongst seeds of Cattleya species; for instance, C. kautskyana, C. tigrina and C. amethystoglossa contain four to seven main FAs (Fileti et al., 2021) and C. crispata, C. rupestris, C. tenebrosa, C. loddigesii, C. intermedia, C. schilleriana and C. warneri contain five to seven main FAs (Hengling et al., 2020). Similar variation has been observed in seeds of five other species: Dendrobium strebloceras, Den. cunninghamii, Gastrodia cunninghamii, Pterostylis banksii and Thelymitra nervosa (Diantina et al., 2022). Although ageing can trigger decreases in orchid seed saturated, polyunsaturated, mono-unsaturated and total FA content, composition alone appears not to be an adequate marker for seed longevity in orchids (Diantina et al., 2022).
The known variability in dry seed storage lifespan in orchids remains a concern for conservationists. The seeds of a wide range of species tend to be relatively short lived in various ageing conditions [Guarianthe aurantiaca (Seaton and Pritchard, 1999; Pritchard and Dickie, 2003); Caladenia arenicola, Calda. flava, Calda. huegelii, Diuris laxiflora, Microtis media ssp. media, Pterostylis recurva, Ptst. sanguinea, Thelymitra crinita, Thel. macrophylla and Diuris fragrantissima; (Hay et al., 2010); Calda. flava, Diuris laxiflora, Microtis media, Ptst. recurva and Thel. crinita (Merritt et al., 2014a); Cattleya schilleriana, C. tigrina, C. crispate and C. labiata (Fileti et al., 2015); Cattleya amethystoglossa, C. brevicaulis, C. briegeri, C. crispata, C. duvenii, C. endsfeldzii, C. milleri, C. nobilior, C. purpurata, C. rupestris, C. sanguiloba, C. tenebrosa, C. tigrina, C. forbesii, Constatia cristinae, Oncidium isthmi, Onc. hastatum, Phalaenopsis sp., Trichocentrum pumilum, Vanda curvifolia, V. tesselata and V. testacea (Machado-Neto and Custódio, 2005); Bifrenaria inodora, Cattleya bicolor, C. intermedia, C. purpurata, Encyclia pygmaea, Enc. odorantissima, Epidendrum fulgens, Grobya sp., Maxillaria picta, Gomesa enderianum, Gom. flexsuosum, Trt. Pumilum and Acianthera glumacea (Alvarez-Pardo and Ferreira, 2006); Coelogyne asperata, Coel. foerstermannii, Coel. pandurata, Coel. rumphii, Dendrobium macrophyllum, Den. mirbelianum, Den. Stratiotes and Xylobium undulatum (Seaton et al., 2013)]. Should all orchids be assigned to exceptionality factor 3 of Exceptional Species sensu Pence et al. (2022)? That is, the seeds may be desiccation tolerant but cannot survive ‘long term’ at −20 °C. Moreover, how do orchids fit into a new conceptual framework for longevity that has evolved over the last few decades (Nadarajan et al., 2023)? For example, seeds of Cattleya trianaei have been described as orthodox (Pritchard and Seaton, 1993) or intermediate (Shoushtari et al., 1994). Cattleya crispata, formerly classified as Laelia flava, is reputed not to survive in storage (Hengling et al., 2020) or to be orthodox (Machado-Neto and Custódio, 2005). Cattleya brevicaulis, C. forbesii, C. tigrina, C. rupestris and C. sanguiloba (syn. C. cinnabarina) were all considered to be short lived in the same work (Machado-Neto and Custódio, 2005).
Multi-species comparisons in seed longevity are relatively rare in orchids. Seed storage lifespan in Cattleya species has previously been assessed for C. tenebrosa, C. warneri, C. intermedia, C. schilleriana, C. loddigessi, C. crispata and C. rupestris by Hengling et al. (2020) and C. amethystoglossa, C. tigrina and C. brevicaulis by Fileti et al. (2021). Moreover, we have recently observed that interspecies variability in Cattleya species seed survival can be associated with differences in seed morphology, even in such small seeds (Fileti et al., 2021). Thus, Cattleya as a genus appears to be a good model to explore both the mechanistic drivers for seed ageing in orchids and to consider practical conservation interventions. In this study, we determined the long-term (i.e. >10 years) dry seed storage potential at −18 °C for eight species of Cattleya and explored the potential interactions with various seed traits (as determined through morphometry, biochemistry and biophysics). Our main hypothesis was that orchid seeds are always short lived.
MATERIALS AND METHODS
Seed storage
Seeds of eight Cattleya species (C. alvaroana, C. briegeri, C. crispata, C. harrisoniana, C. hegeriana, C. granulosa, C. rupestris and C. sanguiloba; Table 1) were obtained from the Aurora Orchid House in Taciba, São Paulo estate, from controlled cross-pollination with pollen from plants of different origins. Fruits were harvested at the first signs of maturity and stored in fine paper envelopes at 45 % relative humidity (RH) and 22 ± 3 °C until complete dehiscence. The dispersed seeds were cleaned, and the seeds from different capsules of the same species were pooled and split into subsamples, placed in open 2 mL cryogenic microtubes and equilibrated over a saturated lithium chloride solution at 20 °C at ~12 % RH (Hay et al., 2008). To check the moisture content (MC), three samples from each seed lot were weighed in aluminium pans and placed in an oven at 105 ± 3 °C for 24 h. Subsequently, the pans were placed over silica gel, left to cool, and reweighed (BRASIL, 2009). The MC was calculated on a wet basis (MCwb). Seeds reached 3–4 % MCwb, except for C. hegeriana (7 %), and were placed into separate 2 mL cryovials, sealed with a silicon ringed cap and stored at −18 °C. Samples were maintained in tight vials inside large vials containing silica gel orange sachets equilibrated to the same eRH (equilibrium Relative Humidity). These vials were placed in a box containing white silica gel mixed with orange indicator silica gel to enable monitoring of hermetic sealing over the storage evaluation period. Seed longevity was determined as in the studies by Newton et al. (2014) and Hengling et al.(2020), applying probit analysis to the seed survival curves based on at least four sampling times (i.e. at the establishment of the UNOESTE Orchid Seed Bank more than a decade ago and in 2017, 2020 and 2022).
Table 1.
Species, origin, growth habit and conservation status of the used Cattleya species.
| Species | Biome | Places of origin | Growth habit | Conservation status |
|---|---|---|---|---|
| C. alvaroana | Atlantic Rainforest over rocks | RJ | Rupicolous | NE |
| C. briegeri | Savannah, Atlantic Rainforest | MG | Rupicolous | EN |
| C. crispata | Savanah | MG | Rupiculous | NT |
| C. granulosa | Seasonal semi-deciduous forest, Restinga | AL, BA, PB, PE, RN, ES | Epiphytic, terrestrial | VU |
| C. harrisoniana | Riparian Forest, Atlantic Rainforest | ES, MG, RJ and SP | Epiphytic | VU |
| C. hegeriana | Atlantic Rainforest | MG, RJ | Rupiculous | LC |
| C. rupestris | Savannah | MG | Rupicolous | NE |
| C. sanguiloba | Savannah | MG | Rupicolous | LC |
Brazilian estates: AL, Alagoas; BA, Bahia; ES, Espirito Santo; MG, Minas Gerais; PB, Paraíba; PE, Pernambuco RJ, Rio de Janeiro; RN, Rio Grande do Norte; SP, São Paulo. Conservation status: EN, Endangered; LC, Least concern; NE, not evaluated; NT, Near Threatened; VU, vulnerable (CNCFlora2020).
Germination and viability testing
Seed disinfection was performed according to Hosomi et al. (2011), and sowing in Petri dishes was on a half-strength Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) with 30 g L−1 sucrose and 6 g L−1 agar. Before autoclaving, the pH was adjusted to 5.6 (with 0.1 m NaOH). Seed germination dishes were kept in a growth room at 25 ± 3 °C with a 16 h light photoperiod (511 μE m−2 s−1).
Over the storage experiment, seeds were quality assessed using three different methods: (1) at the beginning, the original technique (OT) of germination was to remove the seed container from storage and keep it at room temperature for ≥1 h; after we made two modifications, seed containers were removed from storage, left for 24 h at room temperature (25 ± 2 °C) and then either handled (2) without sucrose pretreatment (WoS) or (3) given a 24 h sucrose pretreatment (coded as WS), plus washing twice with distilled water as part of the preparation for the germination test. After these handling treatments (1, 2 and 3), the seeds were disinfected and sown in vitro.
The seeds were considered to have germinated when the embryonic mass was green and expanded to the point at which the testa was clearly breached, i.e. stage 1, spherical protocorm (Seaton and Ramsay, 2005; Prasongsom et al., 2022). The germinated seeds were counted weekly until germination was stable. The seeds were evaluated in three marked fields to include ≥100 full seeds (embryo present and excluding empty testae) per field. Images were captured with a Sony DSC-P10 (Sony Electronics Inc., Japan) digital camera manually coupled to a stereomicroscope eyepiece lens. Germination (G) was expressed as a percentage of the number of filled seeds per marked field, with three determinations for each of the three methods at each sampling time.
Viability was evaluated by a tetrazolium (Tz; 2,3,5-triphenyl tetrazolium chloride) test as described by Hosomi et al. (2011, 2012). Three repetitions were made for each species and at each sampling time.
Transesterification of fatty acids and gas chromatography
Fatty acid extraction and transesterification followed the method of Colville et al. (2016). The FA methyl esters were analysed by gas chromatography (Shimadzu GC QP2010, Shimadzu Corporation Global, Kyoto, Japan) with a flame ionization detector in an NST23 capillary column (Nano Separation Technologies, São Carlos, Brazil) with a length of 60 m, an internal diameter of 0.25 µm and film thickness of 0.20 µm. Nitrogen was used as the carrier gas at a flow rate of 0.6 mL min−1. The temperature programme followed that of Colville et al. (2016). Two samples of each species were made. Fatty acids were separated into saturated FA (SFA), monounsaturated FA (MUFA) and polyunsaturated FA (PUFA). The MUFAs and PUFAs were used to calculate the double bond index (DBI) and peroxidation index (PI) according to Pamplona et al. (1998).
Thermal analysis
Seeds were placed in 34 µL aluminium pans, crimp-sealed and weighed on a UMX2 Ultra-Microbalance (Mettler Toledo; ±0.1 µg) to determine the precise quantity of the sample and submitted to thermal analysis using a differential scanning calorimeter (Shimadzu DSC-60 analyser). The cooling and warming cycle sequence was as follows: cooling to −80 °C at 10 °C min−1; holding for 1 min at −80 °C; warming from −80 °C to 75 °C at 10 °C min−1; holding for 1 min; then cooling down to room temperature. Warming thermograms and melting peaks were measured in ORIGINPro© 2019b software (OriginLab Corporation, Northampton, MA, USA). Representative thermograms are presented based on three independent samples.
Morphometric analysis
Morphometric analysis was performed according to Fileti et al. (2021), measuring 40 seeds in each seed lot.
Statistical analysis
The percentages of germination (G) and viability (Tz) were transformed with for normalization. The data were expressed as the mean values, and significant differences in means were compared with Tukey’s test (P ≤ 0.05). The experiment was a factorial 8 × 3 (eight species × three conditions of sowing) and conducted as completely randomized with three repetitions each, with ≥200 seeds. These data were used to calculate seed conservation parameters for each species and for ANOVA. Probit regressions of the seed-ageing data (σ and P50y) were calculated with GLM (General Linear Model) and fitted with ORIGINPro© 2019b software (Origin LAB Corporation, Northampton, MA, USA). All analyses were performed with SISVAR software (Ferreira, 2011).
Morphometric, principal FAs volumetric relationships between seed and embryo and P50y data were submitted to correlation analysis and cluster analysis using PAST software v.4 (Hammer et al., 2001).
Results
Morphometric analyses
Based on their dimensions and volumes, all Cattleya species studied produced micro-seeds (Table 2). In comparison to the six rupiculous species, C. granulosa and C. harrisoniana produced smaller seeds (~350 µm × 170 µm) and embryos (~76 µm in diameter) (Table 2). Overall, C. rupestris had the significantly longest and widest seed. Embryo diameters separated the species into two groups of four, with diameters of 76–97 and 108–120 µm. Embryo volumes ranged 17-fold, from 0.17 to 2.97 × 10−3 mm3 for C. harrisoniana and C. briegeri, respectively (Table 2). All seeds had relatively large embryos in comparison to the whole seed, with air spaces varying from only 9 % (C. granulosa) to 27 % (C. crispata) of seed volume.
Table 2.
Seed morphovolumetric characteristics of eight Cattleya species. Non-rupicolous species are shown in bold text.
| Dimensions (µm; mean ± s.e.m.) | Volume (×10−3 mm3; mean ± s.e.m.) | Air space | |||||
|---|---|---|---|---|---|---|---|
| Species | Seed length | Seed width | Embryo diameter | Embryo (prolated spheroid) | Seed | Air | (% of seed) |
| C. alvaroana | 602 ± 2.0c | 248 ± 0.9c | 108 ± 0.4ab | 1.54 ± 0.14bc | 1.90 ± 0.15bc | 0.32 ± 0.05bc | 17.7 ± 2.2bc |
| C. briegeri | 779 ± 2.8b | 326 ± 1.6a | 120 ± 0.5a | 2.52 ± 0.5a | 2.97 ± 0.25a | 0.45 ± 0.07ab | 15.7 ± 0.1bc |
| C. crispata | 637 ± 2.2c | 244 ± 1.1c | 90 ± 0.3cd | 0.99 ± 0.08cd | 1.39 ± 0.11c | 0.39 ± 0.06ab | 27.3 ± 2.4a |
| C. granulosa | 357 ± 1.1d | 172 ± 0.5d | 76 ± 0.3d | 0.53 ± 0.05d | 0.58 ± 0.06d | 0.06 ± 0.01d | 9.4 ± 1.5c |
| C. harrisoniana | 348 ± 0.9d | 164 ± 0.5d | 77 ± 0.3d | 0.17 ± 0.04d | 0.49 ± 0.04d | 0.07 ± 0.01d | 10.4 ± 2.0c |
| C. hegeriana | 642 ± 2.8c | 264 ± 0.9bc | 118 ± 0.4a | 1.94 ± 0.15ab | 2.38 ± 0.21ab | 0.44 ± 0.09ab | 18.9 ± 2.6abc |
| C. rupestris | 874 ± 2.9a | 340 ± 1.3a | 110 ± 0.4ab | 2.18 ± 0.22ab | 2.82 ± 0.23a | 0.61 ± 0.01a | 21.5 ± 2.9ab |
| C. sanguiloba | 667 ± 1.9c | 297 ± 0.9ab | 97 ± 0.5bc | 1.59 ± 0.14bc | 1.75 ± 0.16bc | 0.22 ± 0.06bc | 10.9 ± 2.5c |
| MSD (minimun significant difference) (5 %) | 9.28 | 4.19 | 0.389 | 0.063 | 0.016 | 0.006 | 2.22 |
Germination, viability and seed longevity parameters
Seed storage moisture contents were ~0.03–0.04 g H2O g−1 DW, with only C. hegeriana seed being higher (0.06 g H2O g−1 DW). The germination level was influenced, in most cases, by the post-storage treatment, e.g. being kept for 24 h at room temperature or this first step followed by sucrose imbibition for another 24 h (Table 3). For three of the species, the resulting germination levels were statistically different. Although for the other five species the results of such treatments were not different, there was a tendency for increment. For C. briegeri, C. granulosa, C. harrisoniana and C. sanguiloba, the treatment with sucrose was the most effective at increasing germination post-storage. Similar letters, in the collumns, are not statistically different by the Tukey’s test (P < 0.05).
Table 3.
Longevity parameters (σ and P50y) of seeds from eight Cattleya species stored for ≥13 years at −18 °C and low moisture content (MC). Viability (as a percentage) is for the tetrazolium (Tz) test. Initial germination is shown as determined (as a percentage) and estimated (Ki in probits). Final germinations (as a percentage and probit value) obtained with three germination methods are also shown. Total storage times varied from 4963 days (13.6 years) to 5222 days (14.3 years). Four time points were included in the probit analysis of the longevity parameters. Non-rupicolous species are shown in bold text.
| Species (days in storage) |
Germination treatmenta | MC (g H2O) g−1 FW) |
Initial germination (%) | Estimated Ki |
Final Tz (%) | Final germination (%) | Final probit | σ (years) | P 50y (years) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS | 92.2 | aA | 6.43 | aCD | 9.6 | aBC | 38.9 | aBC | |||||
| C. alvaroana | WoS | 0.026 ± 0.005 | 100 | 8.09 | 82.6 ± 0.97 | 91.1 | aAB | 6.47 | aC | 9.0 | aA | 36.5 | aA |
| (5187) | OT | 88.0 | aAB | 6.20 | aBC | 8.5 | aA | 34.4 | aA | ||||
| WS | 93.3 | aA | 6.57 | aBC | 6.4 | aD | 25.7 | aD | |||||
| C. briegeri | WoS | 0.033 ± 0.005 | 100 | 8.09 | 80.1 ± 2.39 | 87.1 | aB | 6.15 | aCD | 6.8 | aA | 27.3 | aA |
| (5187) | OT | 65.0 | bD | 5.40 | bDE | 6.5 | aA | 26.3 | aA | ||||
| WS | 91.0 | aA | 6.43 | aCD | 8.1 | aD | 29.5 | aD | |||||
| C. crispata | WoS | 0.027 ± 0.001 | 99 | 7.33 | 85.0 ± 0.98 | 94.0 | aAB | 6.47 | aBC | 7.1 | aA | 26.0 | aA |
| (4963) | OT | 87.0 | aAB | 6.20 | aC | 7.1 | aA | 26.1 | aA | ||||
| WS | 96.3 | aA | 6.83 | aABC | 22.4 | aAB | 82.1 | aAB | |||||
| C. granulosa | WoS | 0.041 ± 0.002 | 99 | 7.33 | 90.6 ± 0.61 | 70.2 | bC | 5.53 | bE | 8.4 | bA | 30.9 | bA |
| (5199) | OT | 47.8 | cC | 4.94 | cE | 5.9 | bA | 21.7 | bA | ||||
| WS | 98.2 | aA | 7.05 | aAB | 30.1 | aA | 110.3 | aA | |||||
| C. harrisoniana | WoS | 0.035 ± 0.001 | 99 | 7.33 | 93.8 ± 0.25 | 89.8 | bAB | 6.29 | bC | 16.6 | bA | 60.9 | bA |
| (5185) | OT | 80.0 | cBC | 6.03 | bC | 9.8 | bA | 36.0 | bA | ||||
| WS | 99.2 | aA | 7.33 | aA | 7.4 | aD | 29.8 | aD | |||||
| C. hegeriana | WoS | 0.068 ± 0.002 | 100 | 8.09 | 90.0 ± 0.44 | 97.6 | aA | 7.09 | abAB | 7.3 | aA | 29.6 | aA |
| (5222) | OT | 96.0 | aA | 6.85 | bA | 6.3 | aA | 25.3 | aA | ||||
| WS | 98.6 | aA | 7.14 | aAB | 6.5 | aD | 26.3 | aD | |||||
| C. rupestris | WoS | 0.030 ± 0.003 | 100 | 8.09 | 93.7 ± 0.13 | 99.0 | aA | 7.33 | abA | 5.5 | aA | 22.3 | aA |
| (4963) | OT | 95.0 | aA | 6.73 | bAB | 4.8 | aA | 19.3 | aA | ||||
| WS | 79.7 | aB | 5.87 | aD | 13.2 | aBC | 48.4 | aBC | |||||
| C. sanguiloba | WoS | 0.028 ± 0.002 | 99 | 7.33 | 73.2 ± 0.45 | 71.9 | bC | 5.57 | aDE | 9.2 | aA | 33.8 | aA |
| (5222) | OT | 75.7 | abC | 5.70 | aCD | 8.9 | aA | 32.7 | aA | ||||
aGermination method: OT, original technique; WoS, without sucrose pretreatment; WS, with 24 h sucrose pretreatment.
Significant differences between species are indicated by: uppercase italics in the columns for WS, uppercase bold in the columns for WoS, and normal uppercase in the columns for OW. Significant differences amongst treatments for each species are denoted by lowercase letters.
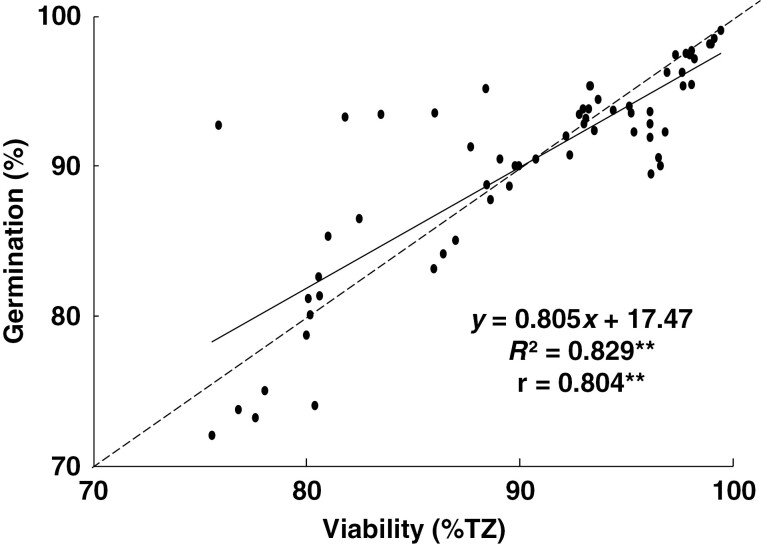
Amongst the species, germination after various storage times was strongly correlated (R2 = 0.829) with Tz viability (Fig. 1), meaning that the Tz test can be a reliable predictor of germination for the studied species.
Fig. 1.
Correlation between germination with sucrose pretreatment and tetrazolium test of eight stored Cattleya seeds. The null line is dashed. ** P < 0.01.
Thermal and fatty acid analyses
Thermal fingerprints for the seeds of the eight species were similar based on the DSC warming thermograms, with pronounced melting events in seven of the eight species (Fig. 2). In contrast, C. granulosa seeds generated a weaker signal in the DSC (Fig. 2A). For the seven species, four main peaks were evident: centred on −45, −30 and −18 °C and finally at 5–15 °C. For C. granulosa, no peak was evident at −45 °C, but peaks were observed at −25, −10 and 25 °C. These complex melting transitions suggest a variability in FA acid composition in the seeds (Fig. 2; Supplementary Data Table S1).
Fig. 2.
Warming differential scanning calorimetry curves of stored seeds from eight Cattleya species. (A) Cattleya briegeri, C. crispata, C. granulosa and C. rupestris. (B) Cattleya alvaroana, C. harrisoniana, C. hegeriana and C. sanguiloba. Each coloured band is a signal of lipid melting. The scanning baseline is shown as a dashed line for C. crispata in A and C. harrisoniana in B.
The gas chromatography analysis of the seeds revealed large differences in FAs amongst the species (Supplementary Data Table S2). The major FA components were palmitic acid (16C:0) and linoleic acid (18C:2; Supplementary Data Table S2), which varied among species from 14 to 32 % and from 51 to 79 %, respectively, of the total amount of FAs. Two species, C. crispata and C. rupestris, had a similar amount of stearic acid (18C:0). Linolenic acid (18C:3) in high amounts (>3 %) was found only in C. granulosa. Oleic acid (18C:1) was 4–8 % in all species, being highest in C. crispata and lowest in C. hegeriana. The seed UFA:SFA ratio among species varied from 1.7 (C. granulosa) to 5.7 (C. harrisoniana). Consequently, PI, DBI and PUFA parameters were the lowest in C. granulosa and the highest in C. harrisoniana (Supplementary Data Table S2).
The amounts of FA also varied among species from 12 to 34 % of the seed weight. When these values were normalized to 100 % filled seed, the values increased to 28–51 % (Table 4). Cattleya granulosa exhibited the lowest level of filled seeds (41 %), which might be responsible for the weak signal in the DSC thermograms (Fig. 2).
Table 4.
Total of filled seeds in each sample and their total fatty acid content of eight Cattleya species. Non-rupicolous species are shown in bold text.
| Species | Full-filled seeds (% ± s.d.) | Fatty acid content (% ± s.d.) | Adjusted lipid content (100% filled seeds)a |
|---|---|---|---|
| C. alvaroana | 76.36 ± 0.20 | 33.85 ± 2.11 | 44.33 |
| C. briegeri | 83.63 ± 0.49 | 26.205 ± 1.56 | 31.33 |
| C. crispata | 83.47 ± 0.86 | 33.96 ± 2.91 | 40.68 |
| C. granulosa | 41.00 ± 0.08 | 11.65 ± 0.60 | 28.41 |
| C. harrisoniana | 69.36 ± 1.32 | 33.2 ± 0.92 | 47.87 |
| C. hegeriana | 54.85 ± 4.15 | 27.92 ± 0.01 | 50.90 |
| C. rupestris | 80.34 ± 0.24 | 31.80 ± 5.99 | 39.58 |
| C. sanguiloba | 77.97 ± 1.48 | 34.0 ± 2.25 | 43.61 |
aAdjusted to account for the proportionate presence of the embryo by weight and then for fatty acids.
Most species had 70–80 % filled seeds, with C. granulosa and C. hegeriana in the range ~41–55 %. Seed oil contents were 28 to ~51 %, except for C. granulosa at 28 %, contributing to low DSC signal trace of this species (Fig. 2).
Co-correlants of seed biochemistry, morphology and storage physiology
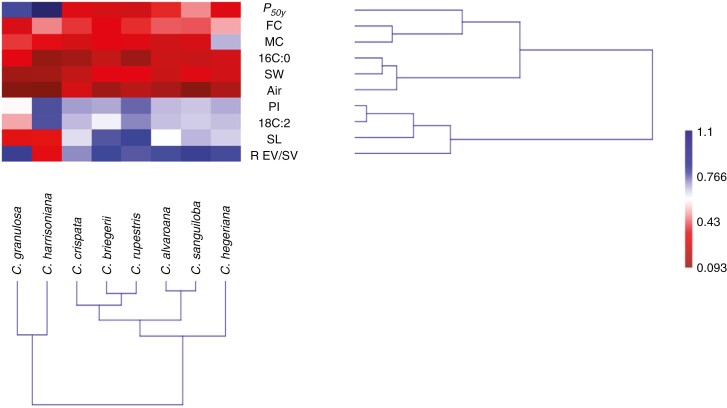
The grouping analysis of the morphovolumetric variables, some longevity parameters and principal FAs (Fig. 3) showed that the species were grouped according to taxonomic affiliation by the seed dimensions, linoleic acid content, which also influences PI, or lifespan (P50y; Table 3). Palmitic acid or moisture content also influenced the grouping of species, but with less strength. The bifoliate Cattleya of the subgenus Intermediae (Berg, 2014), i.e. C. harrisoniana and C. granulosa, are together in one cluster and the other six species, from series Parviflorae (Berg, 2014), in another cluster.
Fig. 3.
Species grouping according to morphometric, best longevity parameters and principal fatty acids. Abbreviations: P50y, half live of the seeds in years; FC, fatty acid content; MC, moisture content; 16C:0, palmitic acid; SW, seed width; Air, air content; PI, peroxidation index; 18C:2, linoleic acid; SL, seed length; R EV/SC, relation embryo volume/seed volume.
Seed length and width have many correlations with the other variables and a significant strong positive correlation between them and embryo diameter. This means that longer seeds are wider seeds and have bigger embryos. These three variables had a significant strong negative correlation with the conservation parameter P50y, e.g. the bigger the seed, the worse their conservation for these species. Palmitic acid (C16:0) content had significant negative correlations with linoleic acid (C18:2) content and PI and DBI (Table 5). Oleic acid (C18:1) content exhibited a significant negative correlation with P50y, meaning that seeds with higher contents of oleic acid potentially preserve comparatively less well. The linoleic acid (C18:2) content was strongly correlated significantly with PI and DBI. The PI and DBI had a significant strong negative correlation with palmitic acid (C16:0) and a significant stronger one with linoleic acid (C18:2).
Table 5.
Correlation analysis between moisture content, morphovolumetric variables, principal fatty acids and conservation parameter (P50y).
| SL | SW | 16C:0 | 18C:1 | 18C:2 | P 50y | PI# | DBI## | |
|---|---|---|---|---|---|---|---|---|
| SL | – | – | – | – | −0.880** | – | – | |
| SW | 0.972*** | – | – | – | −0.805* | – | – | |
| ED | 0.827* | 0.852** | – | – | – | −0.832* | – | – |
| EV/SV | – | – | 0.733* | – | −0.782* | – | −0.837* | −0.871** |
| Air | – | – | – | 0.837** | – | −0.745** | – | – |
| 16C:0 | – | – | – | – | – | – | −0.908*** | |
| 18C:1 | – | – | – | – | −0.736* | – | – | |
| 18C:2 | – | – | −0.848** | – | – | – | 0.967*** | |
| PI** | – | – | −0.900** | – | 0.946*** | – | 0.991*** |
Abbreviations: Air, percentage of air inside the testae; C16:0, palmitic acid; 18C:1 oleic acid; C18:2, linoleic acid; ED, embryo diameter; EV/SV, ratio of embryo volume/seed volume; P50y, half-life of a seed lot; PI#, peroxidation index; DBI##, double bond index; SL, seed length; SW, seed width.
Significance: *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
A stochastic model for the loss of cell viability over time means that small orchid seeds with relatively undifferentiated and few cells in the embryo are potentially at a disadvantage for lifespan. Amongst Dendrobium and other orchid species, seed and embryo dimensions are known to vary, but this might not translate into differences in the comparative rate of decay of the seeds, with all being fairly short lived (Diantina et al., 2020, 2022). In contrast, across seeds of three Cattleya species, the one with the biggest seeds, C. kautskyana, another species belonging to the series Parviflorae (Berg, 2014), had the greater longevity (Fileti et al., 2021). Also, amongst orchid species there was a weak correlation between relative air space and storability (Diantina et al., 2022). These earlier studies used ageing in natural or accelerated conditions, whereas our study compared longevity over the long term (>10 years). We found a significant negative correlation (r = −0.745, P < 0.01) between relative air space (as a percentage) around the embryo and longevity, such that seeds of C. granulosa and C. harrisoniana with less air space stored relatively well. However, C. rupestris and C. hegeriana with higher air space also had good preservation potential, indicating that air space alone is not the determinant of orchid seed longevity. One possibility is that differences in gas permeability into the embryo could also be a modulator of the seed longevity response (Groot et al., 2015).
The seed longevity parameters determined here for eight species of Cattleya indicate that the seeds are not short lived, and these species are not likely to be exceptional, as defined as having a seed P50y of <20 years in conventional seed bank conditions (Walters, 2015; Pence et al., 2022). Such longevity achieved here for Cattleya seeds might reflect the optimization of steps in post-harvest handling. Nonetheless, it is difficult to predict the full longevity potential of the stored seeds because 70 % of the germination estimates (i.e. 17 of 24) yielded values of >85 % after 13 years. In seed population ageing studies, this level of viability is considered to be a critical node (Zhou et al., 2022), below which the loss of viability is relatively faster (as a percentage per year). Also, when most of the data analysed in longevity studies are above this value, it is not easy to derive reliable estimates of longevity, or to forecast regeneration requirements (Hay et al., 2021). Thus, regular monitoring of orchid seed longevity is recommended at intervals shorter than the 10-year assessment that is often used in seed bank accession monitoring (Hay et al., 2021).
Both temperature and moisture content are recognized as the main drivers of seed longevity (Roberts and Ellis, 1977; Ellis and Hong, 2007), yet our understanding of what is dry for orchid seeds is incomplete. The relationship between seed moisture content and relative humidity is such that the isotherm shifts to lower equilibrium moisture content (eMC) as oil content increases (Hay et al., 2022). Orthodox (desiccation-tolerant) seed storage guidelines recommend drying in conditions that equilibrate the seeds to 3–7 % moisture content (FAO, 2013). For seven of the species studied here, eMC values were ~3–4 %, which could be expected because the determined seed oil contents are in the range of 28–51 % once empty seeds are accounted for (Tables 3 and 4). The exception is C. hegeriana with ~7 % eMC, although the seed oil content is similar to the other species studied. Such a high eMC suggests that the oil content in this seed should be much lower, and we do not yet know the reason for this apparent anomaly. The presence of oil in these seeds is also confirmed by the main melting peaks at −30 and −15 °C in the thermal fingerprint (Fig. 3).
For any oil-rich seed there is a risk of falling germination/viability over time from lipid peroxidation as the first step in a cascade of events that can lead to the formation of several by-products (Zinsmeister et al., 2020). Oxidation occurs preferentially from PUFA to MUFA to SFA. Although some Brassicaceae could be stored successfully in the dry, cold state for >40 years, Sinapis arvensis with high oleic acid (19.9 %) stored less well (Mira et al., 2019). In the case of orchids seeds, linoleic acid is often the most common FA present, with low concentrations of linolenic acid and oleic acid (Hengling et al., 2020; Fileti et al., 2021; Diantina et al., 2022). Although seeds with higher levels of unsaturated lipids might be expected to undergo peroxidation the fastest, we found that a relatively high oleic acid content was associated with shorter seed longevity (P50y, r = −0.837, P < 0.05) and positively correlated with seed air space (r = 0.837, P < 0.01), which was also negatively correlated with P50y (R2 = −0.745, P < 0.01). This is not to suggest that peroxidation of unsaturated fats is unimportant for orchid seed survival. Although the specific mix of FAs is not a reliable marker for the relative rate of ageing, peroxidation is a common feature of decreasing seed viability in a range of orchid species (Diantina et al., 2022).
The accumulation of FAs in seeds is a highly efficient means of energy storage, especially in small seeds. Species that have evolved in hot environments in the tropics tend to store relatively less unsaturated FAs than temperate species. Unsaturated fats have lower freezing (crystallization) temperature, and the plants and seeds potentially reduce the risk of freezing stress at any time of their life cycle. In contrast, seeds with more saturated lipids will have higher melting temperatures. This applies to the cell membranes, including the half-unit membrane around the oil bodies, and the storage lipid, mainly triacylglycerol (TAG; Crane et al., 2006; Schwallier et al., 2011). Linoleic acid, which we and others have found to be present in orchid seeds (Hengling et al., 2020; Fileti et al., 2021; Diantina et al., 2022), potentially melts around −12.7 °C as free acid and −50 °C as a TAG (Knothe and Dunn, 2009). Peaks coinciding with these temperatures are evident for all seven species with clear thermal fingerprints (Fig. 2; Supplementary Data Table S1). Lipid transitions at −80 °C, between −40 and −30 °C and around −10 °C have also been noted in seeds of three other orchid species (Calda. flava, Microtis media and Ptst. recurva; Merritt et al., 2014a). For some species of Cuphea, the main storage lipids in the seeds are medium-chain lauric (12C:0) and myristic (14C:0) acids (Crane et al., 2003, 2006; Volk et al., 2007). When present in large quantities, these latter lipids are thought to compromise seed viability during storage in cold conditions and recovery, requiring melting through the application of a dry thermal shock (45 °C) to facilitate germination. Such a thermal shock was ~20 °C beyond the peak temperature for the final lipid melt in Cuphea species, which is ~25 °C. Only for seeds of three of the seven Cattleya species for which we had clear thermal fingerprints is the 25 °C germination treatment used here 20 °C above the final melting peak, viz. C. alvaroana, C. harrisoniana and C. sanguiloba (Fig. 2). Although we did not take specific measures to reduce this potential risk to germination efficiency, the seeds of none of the Cattleya species appeared to show the type of compromised storability and germinability noted for Cuphea species (Crane et al., 2003, 2006). In the case of Cuphea species, it was recommended that heating was applied before the start of the germination test, suggesting a negative interaction with the imbibition process. For orchid seeds, water is likely to enter the embryo in the vapour phase across the air space, suggesting that seed morphology also plays a role in the realization of germination after storage.
But we also found that the method of germination was crucial to our estimate of seed survival after storage, with seeds having the smallest embryos (C. granulosa and C. harrisoniana) and potentially the fewest cells benefitting the most from both sucrose priming after 1 day of equilibration at 25 °C and from 25 °C equilibration alone after removal from storage (Table 3). Germination of C. briegeri seeds post-storage also benefitted from these treatments without influencing estimates of longevity (σ and P50y). Given that these orchid seeds do not possess any thick protective structure, such as a lignified coat, to buffer changes in the environment around the seeds, some time might be needed to stabilize cell structures before the start of germination (De Bont et al., 2019; Nadarajan et al., 2023). Given that we are not sure that lipid crystallization was a feature of the post-storage handling of the Cattleya seeds, we feel that the impact of a 1-day equilibration period at room temperature (≥25 °C) post-storage on the revival of long-term stored orchid seeds should be assessed further. In the case of two of the species studied here (C. sanguiloba and C. rupestris), we have previously considered the seeds to be intermediate, and two (C. briegeri and C. crispata) were considered short-lived orthodox (Machado-Neto and Custódio, 2005). However, with improved handling throughout the storage and germination processes, we have found that medium-term lifespans (P50 > 30 years) in gene bank conditions appear to be attainable. The discrepancy that Hengling et al. (2020) documented between germination and Tz tests was related to how the seeds were treated after being removed from the freezer. In the case of Hosomi et al. (2012), some seeds showed an increment during storage. Furthermore, the differences between germination and Tz, when the seeds were manipulated in a similar manner, were not so discrepant.
In our study, we found several interesting correlations between seed biochemistry, morphology and storage physiology, emphasizing the importance of determining a range of seed traits. For example, the strong negative correlation between oleic acid and potential storage (P50y estimates) in Cattleya species supports the finding of relative poor storage in another orchid, Ptst. banksi, with seeds having a high oleic acid content (~23 %) (Diantina et al., 2022). Given that oleic acid is less dense than linoleic and linolenic acids (Knothe and Steidley, 2014), it might facilitate the production of relatively larger seeds and embryos. Such seeds also tended to contain relatively more air (i.e. larger air space as a proportion of seed volume), which, in turn, affects the storability of the polyunsaturated FAs.
Finally, the long-lived seeds in our long-term conventional seed bank study come from species (C. harrisoniana and C. granulosa) that are either epiphytic or terrestrial (growing in dunes) and grow in environments that are usually hotter and drier than the other six species investigated. This finding is slightly at odds with the finding of Merritt et al. (2014b), using accelerated seed-ageing conditions on 172 species from many families, who observed that plants from warmer, moist environments have seeds that are longer lived than seeds from colder, drier environments. We also found that conventional seed banking longevity in orchid species with smaller seeds appears to be greater than in congeneric species with bigger seeds. Together with the findings that larger seeds of Australian alpine species (Satyanti et al., 2018) and Aegilops seed morphs (Guzzon et al., 2018) are shorter lived, our results on orchids emphasize the importance of exploring further the association between the seed morph and seed longevity phenotypes.
In conclusion, not all orchids produce seeds that are relatively short lived in conventional seed bank conditions, as demonstrated here for several species. The longevity findings combined with our multi-trait analyses might help to strengthen ex situ seed conservation strategies for species in this diverse and highly threatened plant family.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: differential scanning calorimetry peak measurements of seeds of eight Cattleya species. Table S2: lipid composition of eight Cattleya species.
ACKNOWLEDGEMENTS
We thank Philip Seaton [orchid seed stores for sustainable use (OSSSU); Darwin Initiative Project 16-012] for the initial support in the installation of the UNOESTE-Orchid Seed Bank and all collaborators who, during the existence of the UOSB, helped and worked with us to maintain that collection. We also thank the Department of Chemistry and Biochemistry and Chemistry College (DCB—UNESP-Presidente Prudente) for access to facilities.
Contributor Information
Ana Maria Francisqueti, Unoeste Campus II, Agronomy College, Building 2, room 201, Rodovia Raposo Tavares, km 572. Limoeiro, Presidente Prudente SP, 19067-175, Brazil.
Rafael Rubio Marin, Unoeste Campus II, Agronomy College, Building 2, room 201, Rodovia Raposo Tavares, km 572. Limoeiro, Presidente Prudente SP, 19067-175, Brazil.
Mariane Marangoni Hengling, Unoeste Campus II, Agronomy College, Building 2, room 201, Rodovia Raposo Tavares, km 572. Limoeiro, Presidente Prudente SP, 19067-175, Brazil.
Silvério Takao Hosomi, Unoeste Campus II, Agronomy College, Building 2, room 201, Rodovia Raposo Tavares, km 572. Limoeiro, Presidente Prudente SP, 19067-175, Brazil.
Hugh W Pritchard, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650201, PR China; Royal Botanic Gardens, Kew, Wakehurst, Ardingly, West Sussex RH17 6TN, UK.
Ceci Castilho Custódio, Unoeste Campus II, Agronomy College, Building 2, room 201, Rodovia Raposo Tavares, km 572. Limoeiro, Presidente Prudente SP, 19067-175, Brazil.
Nelson Barbosa Machado-Neto, Unoeste Campus II, Agronomy College, Building 2, room 201, Rodovia Raposo Tavares, km 572. Limoeiro, Presidente Prudente SP, 19067-175, Brazil.
FUNDING
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) scholarships: PIBIC to R.R.M., a PhD Scholarship to M.M.H. (401047/2019-9) and a DT2 fellowship of N.B.M.-N. (07924/2020-3). H.W.P. acknowledges funding from the Chinese Academy of Sciences, Kunming Institute of Botany project on ‘Seed biology on important species of Magnoliaceae, Euphorbiaceae, Acanthopanaceae, Orchidaceae and Lauraceae’, and the Government of China, Ministry of Science and Technology (MOST) for ‘MOST’s senior foreign experts introduction plan project’.
CONFLICT OF INTEREST
None declared.
LITERATURE CITED
- Alvarez-Pardo V, Ferreira AG. 2006. Armazenamento de sementes de orquídeas. Revista Brasileira de Sementes 28: 92–98. [Google Scholar]
- Berg C. 2014. Reaching a compromise between conflicting nuclear and plastid phylogenetic trees: a new classification for the genus Cattleya (Epidendreae; Epidendroideae; Orchidaceae). Phytotaxa 186: 75–86. [Google Scholar]
- Bowling JC, Thompson PA. 1972. On storing orchid seed. Orchid Review 80: 120–121. [Google Scholar]
- BRASIL. 2009. Regras para análise de sementes. Brasília: Ministério da Agricultura, Pecuária e Abastecimento. [Google Scholar]
- Castañeda-Álvarez NP, Khoury CK, Achicanoy HA, et al. 2016. Global conservation priorities for crop wild relatives. Nature Plants 2: 16022. [DOI] [PubMed] [Google Scholar]
- CNCFlora. 2020. Centro Nacional de Conservação da Flora (CNCFlora). http://www.cncflora.jbrj.gov.br/portal/pt-br/listavermelha/ORCHIDACEAE (15 December 2023, date last accessed).
- Colville L, Pritchard HW. 2019. Seed life span and food security. The New Phytologist 224: 557–562. [DOI] [PubMed] [Google Scholar]
- Colville L, Marks TR, Pritchard HW, Custódio CC, Machado-Neto NB. 2016. Development of a reliable GC-MS method for fatty acid profiling using direct transesterification of minimal quantities of microscopic orchid seeds. Seed Science Research 26: 84–91. [Google Scholar]
- Crane J, Miller AL, Van Roekel JW, Walters C. 2003. Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 217: 699–708. [DOI] [PubMed] [Google Scholar]
- Crane J, Kovach D, Gardner C, Walters C. 2006. Triacylglycerol phase and ‘intermediate’ seed storage physiology: a study of Cuphea carthagenensis. Planta 223: 1081–1089. [DOI] [PubMed] [Google Scholar]
- De Bont L, Naim E, Arbelet-Bonnin D, et al. 2019. Activation of plasma membrane H+-ATPases participates in dormancy alleviation in sunflower seeds. Plant Science 280: 408–415. [DOI] [PubMed] [Google Scholar]
- Diantina S, McGill C, Millner J, Nadarajan J, Pritchard HW, McCormick AC. 2020. Comparative seed morphology of tropical and temperate orchid species with different growth habits. Plant Cell Tissue and Organ Culture 143: 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diantina S, McGill C, Millner J, et al. 2022. Seed viability and fatty acid profiles of five orchid species before and after ageing. Plant Biology 24: 168–175. [DOI] [PubMed] [Google Scholar]
- Ellis RH, Hong TD. 2007. Seed longevity–moisture content relationships in hermetic and open storage. Seed Science and Technology 35: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO - Food and Agriculture Organization of the United Nations. 2013. Genebank standards: for plant genetic resources for food and agriculture. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Ferreira DF. 2011. Sisvar: um sistema computacional de análise estatística. Ciencia e Agrotecnologia 35: 1039–1042. [Google Scholar]
- Fileti JF, Hosomi ST, Custódio CC, Machado-Neto NB. 2015. Teste de deterioração controlada em sementes de quatro espécies de orquídeas para avaliação do comportamento fisiológico. Colloquium Agrariae 11: 32–37. [Google Scholar]
- Fileti JF, Hengling MM, Gianeti TMR, et al. 2021. Seed longevity and cryobiotechnology in the orchid genus Cattleya. Cryo Letters 42: 356–365. [PubMed] [Google Scholar]
- Groot SP, de Groot L, Kodde J, van Treuren R. 2015. Prolonging the longevity of ex situ conserved seeds by storage under anoxia. Plant Genetic Resources 13: 18–26. [Google Scholar]
- Guzzon F, Orsenigo S, Gianella M, et al. 2018. Seed heteromorphy influences seed longevity in Aegilops. Seed Science Research 28: 277–285. [Google Scholar]
- Hammer O, Harper DAT, Ryan P. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 4. [Google Scholar]
- Hay FR, Adams J, Manger K, Probert R. 2008. The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology 36: 737–746. [Google Scholar]
- Hay FR, Merritt D, Soanes J, Dixon K. 2010. Comparative longevity of Australian orchid (Orchidaceae) seeds under experimental and low temperature storage conditions. Botanical Journal of the Linnean Society 164: 26–41. [Google Scholar]
- Hay FR, Whitehouse KJ, Ellis RH, et al. 2021. CGIAR genebank viability data reveal inconsistencies in seed collection management. Global Food Security 30: 100557. [Google Scholar]
- Hay FR, Rezaei S, Buitink J. 2022. Seed moisture isotherms, sorption models, and longevity. Frontiers in Plant Science 13: 891913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengling MM, Gianeti TMR, Hosomi ST, Machado-Neto NB, Custódio CC. 2020. Storage of Brazilian Cattleya seeds from diverse biomes: lipid composition and effects on germination. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 155: 487–497. [Google Scholar]
- Hosomi ST, Santos RB, Custódio CC, Seaton PT, Marks TR, Machado-Neto NB. 2011. Preconditioning Cattleya seeds to improve the efficacy of the tetrazolium test for viability. Seed Science and Technology 39: 178–189. [Google Scholar]
- Hosomi ST, Custódio CC, Seaton PT, Marks TR, Machado-Neto NB. 2012. Improved assessment of viability and germination of Cattleya (Orchidaceae) seeds following storage. In Vitro Cellular & Developmental Biology - Plant 48: 127–136. [Google Scholar]
- Knothe G, Dunn RO. 2009. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. Journal of the American Oil Chemists’ Society 86: 843–856. [Google Scholar]
- Knothe G, Steidley KR. 2014. A comprehensive evaluation of the density of neat fatty acids and esters. Journal of the American Oil Chemists’ Society 91: 1711–1722. [Google Scholar]
- Machado-Neto NB, Custódio CC. 2005. Orchid conservation through seed banking: ins and outs. Selbyana 26: 229–235. [Google Scholar]
- Merritt DJ, Hay FR, Swarts ND, Sommerville KD, Dixon KW. 2014a. Ex situ conservation and cryopreservation of orchid germplasm. International Journal of Plant Sciences 175: 46–58. [Google Scholar]
- Merritt DJ, Martyn AJ, Ainsley P, et al. 2014b. A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodiversity and Conservation 23: 1081–1104. [Google Scholar]
- Mira S, Nadarajan J, Liu U, González-Benito ME, Pritchard HW. 2019. Lipid thermal fingerprints of long-term stored seeds of Brassicaceae. Plants 8: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Nadarajan J, Walters C, Pritchard HW, Ballesteros D, Colville L. 2023. Seed longevity – the evolution of knowledge and a conceptual framework. Plants 12: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Börner A. 2010. The longevity of crop seeds stored under ambient conditions. Seed Science Research 20: 1–12. [Google Scholar]
- Newton R, Hay FR, Probert RJ. 2014. Protocol for comparative seed longevity testing. West Sussex, UK: Millennium Seed Bank Partnership. [Google Scholar]
- Pamplona R, Portero-Otín M, Riba D, et al. 1998. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. Journal of Lipid Research 39: 1989–1994. [PubMed] [Google Scholar]
- Pence VC, Meyer A, Linsky J, et al. 2022. Defining exceptional species—a conceptual framework to expand and advance ex situ conservation of plant diversity beyond conventional seed banking. Biological Conservation 266: 109440. [Google Scholar]
- Popova E, Kim HH, Saxena PK, Engelmann F, Pritchard HW. 2016. Frozen beauty: the cryobiotechnology of orchid diversity. Biotechnology Advances 34: 380–403. [DOI] [PubMed] [Google Scholar]
- Prasongsom S, Thammasiri K, Pritchard HW. 2022. Seed dormancy concepts in orchids: Dendrobium cruentum as a model species. Seed Science Research 32: 175–186. [Google Scholar]
- Pritchard HW. 1984. Liquid nitrogen preservation of terrestrial and epiphytic orchid seed. Cryo Letters 5: 295–300. [Google Scholar]
- Pritchard HW. 2004. Classification of seed storage types for ex situ conservation in relation to temperature and moisture. In: Guerrant EO, Havens-Young K, Maunder M. eds. Ex situ plant conservation: supporting species survival in the wild. Washington, D.C.: Island Press, 139–161. [Google Scholar]
- Pritchard HW, Dickie JB. 2003. Predicting seed longevity: the use and abuse of seed viability equations. In: Smith RD. ed. Seed conservation: turning science into practice. Kew: Royal Botanic Gardens, 653–722. [Google Scholar]
- Pritchard HW, Seaton PT. 1993. Orchid seed storage: historical perspective, current status, and future prospects for long-term conservation. Selbyana 14: 89–104. [Google Scholar]
- Pritchard HW, Steadman KJ, Nash JV, Jones C. 1999. Kinetics of dormancy release and the high temperature germination response in Aesculus hippocastanum seeds. Journal of Experimental Botany 50: 1507–1514. [Google Scholar]
- Rasmussen HN, Dixon KW, Jersáková J, Těšitelová T. 2015. Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany 116: 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Ellis R. 1977. Prediction of seed longevity at sub-zero temperatures and genetic resources conservation. Nature 268: 441–433.895850 [Google Scholar]
- Satyanti A, Nicotra AB, Merkling T, Guja LK. 2018. Seed mass and elevation explain variation in seed longevity of Australian alpine species. Seed Science Research 28: 319–331. [Google Scholar]
- Schwallier R, Bhoopalan V, Blackman S. 2011. The influence of seed maturation on desiccation tolerance in Phalaenopsis amabilis hybrids. Scientia Horticulturae 128: 136–140. [Google Scholar]
- Seaton PT, Pritchard HW. 1999. Recent developments in orchid seed banking. In: Proceedings of the World Orchid Conference, Vancouver, 390–396.
- Seaton P, Ramsay M. 2005. Growing orchids from seed. London: Royal Botanic Garden. [Google Scholar]
- Seaton PT, Kendon J, Pritchard HW, Murti Puspitaningtyas D, Marks TR. 2013. Orchid conservation: the next ten years. Lankesteriana International Journal on Orchidology 13: 93–101. [Google Scholar]
- Shoushtari BD, Heydari R, Johnson GL, Arditti J. 1994. Germination and viability staining of orchid seeds following prolonged storage. Lindleyana 9: 77–84. [Google Scholar]
- Thornhill A, Koopowitz H. 1992. Viability of Disa uniflora Berg (Orchidaceae) seeds under variable storage conditions: is orchid gene-banking possible? Biological Conservation 62: 21–27. [Google Scholar]
- Volk GM, Crane J, Caspersen AM, Kovach D, Gardner C, Walters C. 2007. Hydration of Cuphea seeds containing crystallised triacylglycerols. Functional Plant Biology 34: 360–367. [DOI] [PubMed] [Google Scholar]
- Walters C. 2015. Orthodoxy, recalcitrance and in-between: describing variation in seed storage characteristics using threshold responses to water loss. Planta 242: 397–406. [DOI] [PubMed] [Google Scholar]
- Walters C, Pence VC. 2021. The unique role of seed banking and cryobiotechnologies in plant conservation. Plants, People, Planet 3: 83–91. [Google Scholar]
- Walters C, Hill LM, Wheeler LJ. 2005. Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integrative and Comparative Biology 45: 751–758. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lu L, Chen C, et al. 2022. Comparative changes in sugars and lipids show evidence of a critical node for regeneration in safflower seeds during ageing. Frontiers in Plant Science 13: 1020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsmeister J, Leprince O, Buitink J. 2020. Molecular and environmental factors regulating seed longevity. Biochemical Journal 477: 305–323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.