Abstract
Background
The National Institute of Health and Social Care Research (NIHR) Health Informatics Collaborative (HIC) for Hearing Health has been established in the UK to curate routinely collected hearing health data to address research questions. This study defines priority research areas, outlines its aims, governance structure and demonstrates how hearing health data have been integrated into a common data model using pure tone audiometry (PTA) as a case study.
Methods
After identifying key research aims in hearing health, the governance structure for the NIHR HIC for Hearing Health is described. The Observational Medical Outcomes Partnership (OMOP) was chosen as our common data model to provide a case study example.
Results
The NIHR HIC Hearing Health theme have developed a data architecture outlying the flow of data from all of the various siloed electronic patient record systems to allow the effective linkage of data from electronic patient record systems to research systems. Using PTAs as an example, OMOPification of hearing health data successfully collated a rich breadth of datapoints across multiple centres.
Conclusion
This study identified priority research areas where routinely collected hearing health data could be useful. It demonstrates integration and standardisation of such data into a common data model from multiple centres. By describing the process of data sharing across the HIC, we hope to invite more centres to contribute and utilise data to address research questions in hearing health. This national initiative has the power to transform UK hearing research and hearing care using routinely collected clinical data.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12911-024-02589-x.
Keywords: Database, Data sharing, Hearing health
Background
An estimated 12 million UK adults have hearing loss. This is set to rise to 14.2 million by 2035 [1, 2]. The total cost in the UK of untreated, disabling hearing loss is estimated at £25.5 billion annually [3]. Hearing loss affects functioning, communication [4], social interactions [5] and employment opportunities [6].
Despite hearing loss ranking third for Years Lived with Disability [7] and being the commonest sensory disorder [8], it receives less than 1% of UK research funding [9]. Research funding spent for hearing loss per individual is only £1, compared to £11 for sight loss. Efforts are underway to raise awareness and increase the budget for hearing research [10].
This article highlights how the National Institute of Health and Social Care Research (NIHR) Health Informatics Collaborative (HIC) for Hearing Health [11] has been established. We first list priority research areas where data could be useful, then outline the NIHR HIC Hearing Health’s aims, governance structure and study adoption processes. We demonstrate how hearing health data have been integrated into a common data model, using pure tone audiometry (PTA) as a case study. Finally, we detail how health data from contributing centres are ingested and stored.
Priority areas for data driven hearing research
Most estimates on the prevalence of hearing loss are from the 1980s [12]. There is a need for up-to-date data on the burden of hearing loss, causes, risk factors and predictors of progression so that new and effective treatments can be developed.
Hearing loss is unequally distributed, with people from lower socioeconomic and ethnic minority backgrounds being at higher risk [13, 14]. Since these groups are less likely to seek interventions and participate in research, true risk may be underestimated, calling for novel approaches to include data from these groups.
Hearing loss has recently been linked to other chronic conditions such as dementia [15], diabetes [16] and falls [17]. Data-driven approaches could disentangle these associations and provide possible models of causation.
Hearing devices are the most common treatment for adults with hearing loss. The NHS is the largest purchaser of hearing aids worldwide, procuring 1.2 million annually. However, most people with aidable hearing loss never receive a hearing aid [18] and those that do may not always use them. Identifying patient, disease and device characteristics that predict who is most unlikely to be offered or use hearing devices would help devise strategies to improve uptake and usage.
Recent insights in genetic and molecular mechanisms causing hearing loss [19] have allowed detection of therapeutic targets and development of therapies aimed at protecting or restoring hearing [20]. These highly targeted treatments call for large scale geno- and phenotyping efforts to improve patient selection for upcoming clinical trials.
National institute of health and social care research health informatics collaborative
The UK offers a unique infrastructure for data-driven research because 80% of all healthcare is provided by the National Health Service (NHS). This creates an unparalleled flow of routine health data across diverse ethnic and socio-economic groups [21]. To standardise and combine data across NHS providers, NIHR established its HIC; a collaboration between NHS trusts and their partner universities, hosted by the Biomedical Research Centres (BRCs) [22]. The NIHR HIC brings together clinical, scientific, and informatics expertise to support the establishment and maintenance of catalogued, comparable, and comprehensive flows of patient data at each Trust, and to create a governance framework for data sharing and re-use across the trusts and partner organisations.
NIHR HIC hearing heath theme
Recognising clinical need and opportunities for hearing health, the NIHR HIC Hearing Health theme was established in 2022. It aims to bring together the following routinely collected hearing health data and repurpose them for research. We interviewed NHS England, local commissioning groups, NHS procurement and NHS genomics to better understand hearing data collected through NHS organisations across England. Parameters included number of individuals undergoing hearing tests as part of the newborn hearing screen, volume of routine hearing consultations, number of audiometric assessments, number of hearing devices fitted and amount of requests for hearing panels. Based on responses received, the following was obtained:
New-born hearing screen
This national programme uses automated oto-acoustic emissions (OAE) at birth and auditory brainstem response (ABR) for those who fail or have specific risk factors. Since 2017, the UK has had between 680,000 and 750,000 births annually and a coverage of the new-born hearing screen of > 95%. This equates to hearing data of over three million patients, of which 80,000 failed the initial OAE screen and had ABR, and an estimated 5,000 who had confirmed permanent hearing loss.
Routine hearing loss consultations
Across the UK, 355,000 new consultations by audiologists and ENT surgeons are undertaken for hearing loss annually. These provide data on demographics, hearing symptoms, risk factors and interventions. Data are documented in electronic hospital records.
Audiometric assessments
Audiometric assessments inform the diagnosis of hearing loss and effects of interventions. Each of the 106 UK Clinical Commissioning Groups commissions 10,000–30,000 assessments annually, totalling 10 million hearing tests over five years, stored on NHS hospital or audiology clinic servers in codified format.
Hearing devices
The NHS is the largest procurer of hearing aids in the world, fitting new hearing aids for the first time on 355,000 adults annually. Additionally, 1,000 NHS patients receive cochlear implants annually, with over 12,000 NHS patients with cochlear implants so far. Data on the provision, maintenance, and use of these devices, as well as user, environmental and impedance data are stored on NHS servers.
Genetic testing for hearing loss
Since 2021, all children and adults in the UK with potential inherited causes of bilateral sensorineural hearing loss are eligible for genetic testing. There has been an increase in the number of hearing loss gene panels requested, with nearly 1000 requested in 2022.
NIHR HIC hearing health theme key topics
Between 2nd December 2020 and 16th January 2021, a stakeholder consultation took place online using a newly developed questionnaire on the platform Select Surveys (Appendix 1). It was aimed at clinicians, academic scientists and industry partners. They were asked to list any questions they would like to see formally investigated relating to diagnosis/assessment, treatment/ intervention and follow-up support. A total of 74 stakeholders (34 clinicians, 33 academic scientists, 7 industry partners) responded. The responses were condensed into four key topics:
Exploration of effects of known and novel risk factors such as disease clustering for hearing loss.
Identification of genetic causes of hearing loss.
Definition of hearing loss sub-types.
Optimisation of benefit from individualised treatment strategies.
NIHR HIC hearing health theme governance
The NIHR HIC Hearing Health theme is co-led by the founding BRCs: University College London Hospital Trust (UCLH), Nottingham University Hospitals Trust (NUH) and Manchester Foundation Trust (MFT), and their academic partners. Patients are key to decision-making processes. Specifically, regulation around the national data opt-out was directly informed by patient representatives. Patient public involvement (PPI) support is offered to all researchers submitting study requests to ensure that their research question is in line with patient priorities, and that their research study includes proportionate and meaningful PPI.
Data contribution and management
All NHS hearing health providers are encouraged to join the NIHR HIC Hearing Health theme as contributing centres, and contribute their locally stored, de-identified datasets to the central data repository, which is stored in a secure server at University College London (UCL).
The NIHR HIC Hearing Health theme does not allow any data to be removed once it has arrived in the data repository. All researchers at contributing centres are welcome to submit a study request for approval by the steering committee. Once the request is approved, the study is added to the Hearing Health HIC’s portfolio, and researchers are allowed to undertake research within the secure server at UCL.
Further detail is available here, which describes the framework through which we created this resource (https://github.com/uclh-criu/hic-hearing-health-docs) and specific code that relates to a licensed version of their electronic patient record can be accessed upon request.
The protocol for the collection and management of data was approved by Central Bristol Research Ethics Committee (Reference Number: 21/SW/0139).
NIHR data sharing agreement
All NHS hearing health providers can contribute data to the NIHR HIC Hearing Health theme under the NIHR data sharing framework. This covers a range of data and research collaborations and must be signed by all contributing centres. The NIHR HIC data sharing framework addresses common requirements and considerations regarding data sharing between centres, contractual responsibilities, confidentiality, intellectual property and a publications policy. This general agreement will underpin individual agreements for research collaborations with third party academic, clinical and industry partners. Any collaboration with industry partners requires additional agreements, with additional governance checks by participating sites. Industry partners will only be allowed to participate in research by collaborating with a contributing centre.
The HIC has applications to other international healthcare systems where existing relationships between hospitals and academic institutes exist, however given that it focuses on the UK based on the NIHR data sharing agreement, this is a limitation of this paper that its application is not directly transferrable and inevitably obstacles may be encountered in other counties based on local frameworks.
Study adoption
The process that allows hearing health researchers from contributing centres to undertake research on the NIHR HIC Hearing Health theme’s dataset is summarised in Fig. 1.
Fig. 1.
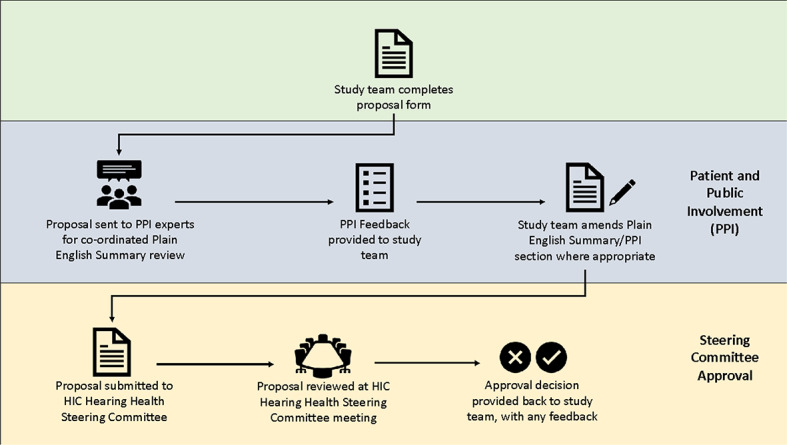
Process of requesting approval to use NIHR HIC Hearing Health data for research
Researchers from contributing centres can request to undertake an analysis on existing data, or can request new data fields to be extracted from contributing centres to be added to the central data repository for the purpose of their study.
The steering committee reviews study requests from researchers at contributing centres. At the quarterly meeting, each proposal is reviewed by the contributing centres and PPI group, and a decision is made, through consensus amongst the steering committee, as to whether the study should be adopted, rejected, or sent back for further refinement. Studies may be rejected if they do not align with the key topics described above or are unachievable.
For studies that are to be adopted by the NIHR HIC Hearing Health theme, each centre is given the opportunity to submit their de-identified hospital data for pooled analysis. This offers each centre continued autonomy over their local data, irrespective of where the data are housed. This policy has been introduced following patient and key stakeholder input into anxieties over loss of autonomy over local data.
Researchers from the approved study centre will be onboarded to the central data repository and given access to the environment to analyse an excerpt of the dataset that is relevant to their study questions. Whilst the results of their analyses can be extracted from the secure environment, no raw data will be sent out.
Development of a common data model
Hearing healthcare data are stored across multiple platforms on local servers. Since 2002, all providers of NHS hearing aids have moved to patient management systems as part of the Department of Health and Social Care Modernising Hearing Aid Services; however, these management systems preceded electronic patient records and are not integrated with Trust electronic care records or with each other. No healthcare recording system currently exists that collects and stores all NHS hearing healthcare information within a single database. Each hospital has its own data flow and software specific databases. This is because a multitude of proprietary audiology-specific hard- and software products are used.
The variety of electronic patient record systems means that assessment and management data are stored across multiple software databases:
Diagnostic hearing tests are undertaken using locally purchased proprietary hardware, each with its own software and data model (assessment).
Hearing aid fitting is undertaken through manufacturer specific hardware, each with its own software and data model (management).
Patient management system stores patient data.
Linkage software communicates between the above software systems that stores its own versions of datasets.
The integration of assessment and management data is undertaken by a third-party software called NOAH, developed by Hearing Instrument Manufacturers’ Software Association (HIMSA). NOAH’s primary function is to provide a unified system managing data collected during a diagnostic hearing test, which can then be used by manufacturer-specific hearing aid fitting systems. NOAH software is built into patient management software, which captures appointment level data and allows a single portal to link assessment and management.
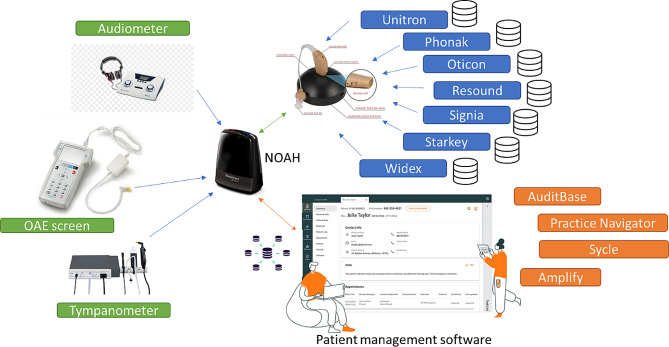
Locally, databases exist for diagnostic hearing tests, hearing aid fitting software, NOAH and clinical management systems, each storing part of the dataset (see Fig. 2). In addition to hearing-related information, general operational data around patient referrals, waiting times and staffing capacity are stored on bespoke databases. Medical information, including surgical interventions is captured on general hospital medical records, whilst imaging data is stored on Picture Archiving and Communication Systems (PACS).
Fig. 2.
Local databases for auditory assessment (green) and hearing aid fitting (blue) and patient management (orange) all need to be combined in a meaningful way
Combining all these different types of data in a meaningful way is challenging. The NIHR HIC Hearing Health theme have developed a data architecture outlying the flow of data from all the various siloed electronic patient record systems to allow the effective linkage of data from electronic patient record to research systems.
Data extraction, linkage and standardisation, within contributing centres
Whilst each software package allows patient by patient data review and occasionally extraction, very few allow wholesale extraction. Using Open Database Connectivity (ODBC), an application programming interface, we have been able to extract all diagnostic hearing tests and hearing device fitting data stored on audiology patient management software, which generally are all based on SQL databases.
The architecture of the database, as well as data structure within each database, varies between software packages. We pursued manufacturers to release internal database architecture and data keys. This information was used to identify and decode key data fields, such as patient identifiers and clinical fields such as hearing test results.
Using probabilistic linkage algorithms, based on national ID, hospital ID, name, date of birth, we linked hearing data to hospital medical records. We prioritised demographic data from patient medical records if they conflicted with patient management software. Patients who signed up for the national data opt-out were removed from the cohort.
The formats in which demographics, diagnoses, and treatments are stored within separate databases within and across hospitals do not always match. Therefore, we opted to convert all data into a model using common data terminologies. We chose the Observational Medical Outcomes Partnership (OMOP) as our common data model. This is an international data model that enables the capture of information (e.g., encounters, patients, providers, diagnoses, drugs, devices, measurements and procedures) in the same way across different institutions. Its usefulness has been demonstrated in multiple health themes [23]. This model is coding language agnostic and maps across multiple vocabularies. Additionally, OMOP does not require a specific technology. It can be realised in any relational database, such as Oracle, SQL Server etc. or as SAS analytical datasets.
The NIHR HIC Hearing Health OMOP data model outlines the structure of the dataset and the associations between the data fields. Local vocabularies are mapped onto standardised OMOP vocabularies and labelled with OMOP domains.
To provide details on how we standardised data with OMOP, we have used hearing test data as an example.
A case study: omopification of hearing test data
The challenge
PTA is the standard test of hearing [24]. It measures the lowest level (in Decibels) that a pure tone can be reliably heard at multiple sound frequencies in each ear (Hertz). The sound can be presented to the ear canal (air conduction), or onto the bone behind the ear (bone conduction). The non-tested ear can be deliberately presented with noise (masking) to prevent it from hearing sound presented to the test ear.
There are already several PTA-related concepts imported from various clinical vocabularies into the OMOP framework (Table 1). However, there is no architecture that inter-relates these codes. As such, new classifications and an inter-relational architecture were required.
Table 1.
Pre-existing OMOP concepts
| Concept | OMOP Concept ID | Domain | |
|---|---|---|---|
| Pure tone audiometry | 4091134 | Procedure | |
| Soundfield audiometry | 4091877 | Procedure | |
| Air conduction | 4236015 | Observation | |
| Bone conduction | 4040628 | Observation | |
| Decibel | 44777591 | Unit | |
| Auditory threshold at 250 Hz |
Left Right |
37208578 37208579 |
Observation |
| Auditory threshold at 500 Hz |
Left Right |
37208584 37208585 |
Observation |
| Auditory threshold at 1000 Hz |
Left Right |
37208572 37208573 |
Observation |
| Auditory threshold at 1500 Hz |
Left Right |
37208574 37208575 |
Observation |
| Auditory threshold at 2000 Hz |
Left Right |
37208576 37208577 |
Observation |
| Auditory threshold at 3000 Hz |
Left Right |
37208580 37208581 |
Observation |
| Auditory threshold at 4000 Hz |
Left Right |
37208582 37208583 |
Observation |
| Auditory threshold at 6000 Hz |
Left Right |
37208586 37208587 |
Observation |
| Auditory threshold at 8000 Hz |
Left Right |
37208588 37208589 |
Observation |
The solution
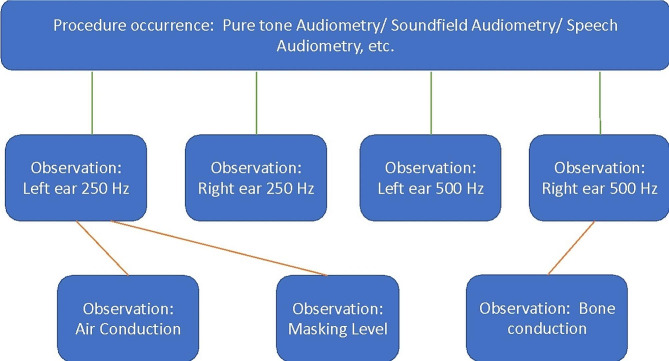
We mapped test outcomes from PTA to OMOP by having a [Procedure_Occurrence] record for each test performed. The most appropriate OMOP procedure concept (from the Systematized Nomenclature of Medicine - SNOMED - vocabulary) was used in each case (e.g. 4091134 = Pure tone audiometry, or 4091877 = Soundfield audiometry). Multiple records from the [Observation] table were then related to the [Procedure_Occurrence], to describe results for each frequency (and ear) tested. Concepts such as masking level, air conduction or bone conduction could then be related to the [Observation], whereas concepts such as headphone (worn over the ear) or inserts (worn in the ear) could be related to the [Procedure Occurrence]. Fact relationship tables were used to communicate these relations [25]. These tables allow relationships to be defined between concepts from the same table or different tables. Figure 3 shows the structure of how codes were linked using fact relationships, using a few common examples. Whilst codes exist for most variables, some local codes were developed when no concept was previously available, for example to assign noise status (see Fig. 3).
Fig. 3.
Examples of linking structure of codes using fact relationships
Data pipeline for collection and integration of data, between contributing centres
Collection
The process of data flow from contributing centres to the central data repository is summarised in Fig. 4. The HIC Data Alchemist platform has been designed to import data from contributing centres within the NIHR HIC Hearing Health theme into a centralised OMOP database. This platform was established to manage data provided in its most raw form, as CSV files compliant with RFC 4180, placing as little burden as possible on individual hospitals. Data is provided in data bundles, each of a different data type, allowing the contributing centre to progressively build their data contribution. Each data bundle focuses on a different goal. This stepwise process facilitates incremental development and feedback. To progress to the next data bundle, the previous data bundle must be completed to a specific standard. Compliance will be measured strictly, as any issues at an early stage may create a risk for further data bundles.
Fig. 4.
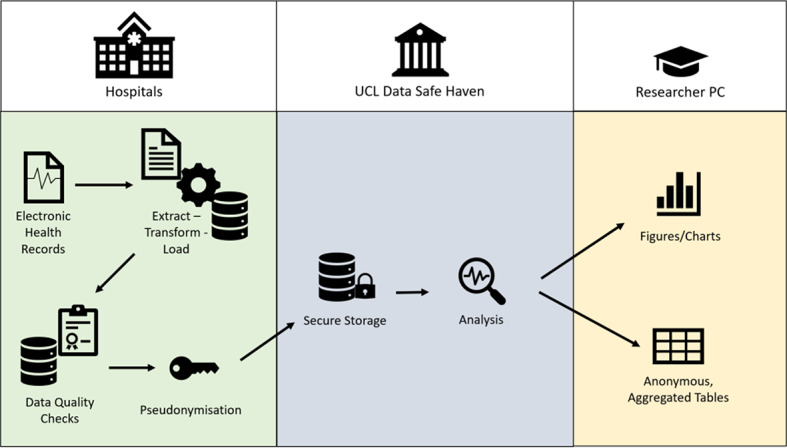
The different stages of data standardisation and integration from different types of data across a variety of platforms and providers within the field of hearing health research. There are two main steps:1. Step 1: generate local view of the uploaded CSV files for the sites to inspect and ensures it has been possible to load data into the database2. Step 2: updates and merges site’s existing data with newly ingested data, ensuring there are valid foreign keys (a system of connecting different tables of data), duplicates have been removed and deletions and updates have been processed to obtain the latest local version of a site’s data
Integration
Each data bundle undergoes a data ingestion process that runs from handling raw CSV files to integration within the multisite database (see Fig. 4).
Further details about the HIC Data Alchemist can be found at https://uclh-criu.github.io/hic-hearing-health-docs/.
Storage
Data is stored in the UCL Data Safe Haven (DSH). This is an ISO27001 certified and NHS Data Security Protection Toolkit compliant Trusted Research Environment (TRE). Processing, analysis and storage capabilities exceed what is available to hospital-based researchers and include a full High Performance Computing cluster, cutting-edge GPU computing nodes for AI research, end-user environments with the latest analytical software and the facility to host any database or application server on enterprise-grade virtual machine infrastructure.
The NIHR HIC Hearing Health theme Research Database has been developed and is in the process of OMOPification. Data ingestion is underway. The data collected fall into the following categories: (a) basic information (e.g., demographics, hospital visits, death, discharge and study sites), (b) laboratory data, (c) treatments, (d) diagnoses, (e) hearing test data, (f) device data and (g) other clinical information.
Conclusion
An NIHR HIC Hearing Health theme has been established, to bring together routinely collected hearing health data, to address urgent research questions in an efficient and cost-effective way. After identifying priority research areas where these data could be useful, the NIHR HIC Hearing Health’s aims, governance structure and study adoption processes were identified. Key hearing health data can be integrated into a common data model. Health data from contributing centres are ingested and stored on a research ready database. Data across a variety of platforms and providers within the field of hearing health research can be standardised and integrated. By detailing the process of sharing data and submitting research proposals we hope to inspire more hearing-health researchers and NHS trusts to contribute to the database and use the wealth of its data to address urgent questions in hearing-health research. This national initiative has the power to transform UK hearing research and hearing care using routinely collected clinical data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
NIHR BRC colleagues at University of Manchester, University College London and University of Nottingham.
Author contributions
Planning – NM, BBR, LD, LJE, CE, AH, NJ, KM, GR, LR, AS, RVS, RN, TR, GTConception and design– NM, BBR, LD, LJE, CE, AH, NJ, KM, GR, LR, AS, RVS, RN, TR, GTAcquisition of data– NM, BBR, LD, LJE, CE, AH, NJ, KM, GR, LR, AS, RVS, RN, TR, GTAnalysis– NM, BBR, LD, LJE, CE, AH, NJ, KM, GR, LR, AS, RVS, RN, TR, GTWrite up– NM, BBR, LD, LJE, CE, AH, NJ, KM, GR, LR, AS, RVS, RN, TR, GT.
Funding
Not applicable.
Data availability
All the raw data (including participants’ voice files and the texts of the interviews) will be confidential and will not be able to share publicly. However, the codes that emerged during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The protocol for the collection and management of data was approved by Central Bristol Research Ethics Committee (Reference Number: 21/SW/0139) and carried out according to the Declaration of Helsinki. The lack of need for informed consent was confirmed by the ethics committee due to the lack of identifiable patient data being included.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original version of this article was revised: Some subheadings were not processed correctly.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/19/2024
A Correction to this paper has been published: 10.1186/s12911-024-02632-x
References
- 1.Royal National Institute for the Deaf. Facts and figures - RNID. https://rnid.org.uk/about-us/research-and-policy/facts-and-figures/ (accessed 12 Apr 2023).
- 2.Office of National Statistics. 2011 censuses UK comparability report. https://www.ons.gov.uk/census/planningforcensus2021/ukcensusdata (accessed 12 Apr 2023).
- 3.Shield B. Hearing Loss-Numbers and Costs EVALUATION OF THE SOCIAL AND ECONOMIC COSTS OF HEARING IMPAIRMENT A report for Hear-It AISBL. 2019.
- 4.Demorest ME, Erdman SA. Relationships among behavioral, environmental, and affective communication variables: a canonical analysis of the CPHI. J Speech Hear Disorders. 1989;54:180–8. 10.1044/jshd.5402.180. 10.1044/jshd.5402.180 [DOI] [PubMed] [Google Scholar]
- 5.Meadow-Orlans KP. Social and psychological effects of hearing loss in adulthood: a literature review. Adjustment Adult Hear loss 1985;:35–57.
- 6.Shan A, Ting JS, Price C, et al. Hearing loss and employment: a systematic review of the association between hearing loss and employment among adults. J Laryngol Otol. 2020;134:387–97. 10.1017/S0022215120001012. 10.1017/S0022215120001012 [DOI] [PubMed] [Google Scholar]
- 7.Haile L, Orji A, Briant P, et al. Updates on hearing from the global burden of Disease Study. Innov Aging. 2020;4:808–808. 10.1093/geroni/igaa057.2934. 10.1093/geroni/igaa057.2934 [DOI] [Google Scholar]
- 8.Raviv D, Dror AA, Avraham KB. Hearing loss: a common disorder caused by many rare alleles. Ann N Y Acad Sci. 2010;1214:168–79. 10.1111/j.1749-6632.2010.05868.x. 10.1111/j.1749-6632.2010.05868.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Lancet. Calling for research into deafness. Lancet. 2007;370:1738. 10.1016/S0140-6736(07)61729-2. 10.1016/S0140-6736(07)61729-2 [DOI] [PubMed] [Google Scholar]
- 10.Wilson BS, Tucci DL, O’Donoghue GM, et al. A Lancet Commission to address the global burden of hearing loss. Lancet. 2019;393:2106–8. 10.1016/S0140-6736(19)30484-2. 10.1016/S0140-6736(19)30484-2 [DOI] [PubMed] [Google Scholar]
- 11.Health Informatics Collaborative – National Institute for Health Research. https://hic.nihr.ac.uk/ (accessed 12 Apr 2023).
- 12.Davis AC. The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. 1389. https://academic.oup.com/ije/article/18/4/911/683421. [DOI] [PubMed]
- 13.Tsimpida D, Kontopantelis E, Ashcroft DM, et al. Regional patterns and trends of hearing loss in England: evidence from the English longitudinal study of ageing (ELSA) and implications for health policy. BMC Geriatr. 2020;20:536. 10.1186/s12877-020-01945-6. 10.1186/s12877-020-01945-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer CS, Armitage CJ, Munro KJ, et al. Correlates of hearing Aid Use in UK adults. Ear Hear. 2019;40:1061–8. 10.1097/AUD.0000000000000695. 10.1097/AUD.0000000000000695 [DOI] [PubMed] [Google Scholar]
- 15.Lin FR, Albert M. Hearing loss and dementia – who is listening? Aging Ment Health. 2014;18:671–3. 10.1080/13607863.2014.915924. 10.1080/13607863.2014.915924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samocha-Bonet D, Wu B, Ryugo DK. Diabetes mellitus and hearing loss: a review. Ageing Res Rev. 2021;71:101423. 10.1016/j.arr.2021.101423. 10.1016/j.arr.2021.101423 [DOI] [PubMed] [Google Scholar]
- 17.Lin FR. Hearing loss and Falls among older adults in the United States. Arch Intern Med. 2012;172:369. 10.1001/archinternmed.2011.728. 10.1001/archinternmed.2011.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien W. Prevalence of hearing Aid Use among older adults in the United States. Arch Intern Med. 2012;172:292. 10.1001/archinternmed.2011.1408. 10.1001/archinternmed.2011.1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melgar-Rojas P, Alvarado JC, Fuentes-Santamaría V et al. Cellular mechanisms of Age-related hearing loss. 2015. 305–33. 10.1007/978-3-319-13473-4_15.
- 20.Schilder AGM, Su MP, Mandavia R, et al. Early phase trials of novel hearing therapeutics: avenues and opportunities. Hear Res. 2019;380:175–86. 10.1016/j.heares.2019.07.003. 10.1016/j.heares.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 21.Rai T, Dixon S, Ziebland S. Shifting research culture to address the mismatch between where trials recruit and where populations with the most disease live: a qualitative study. BMC Med Res Methodol. 2021;21:80. 10.1186/s12874-021-01268-z. 10.1186/s12874-021-01268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Experimental medicine | NIHR. https://www.nihr.ac.uk/explore-nihr/support/experimental-medicine.htm (accessed 12 Apr 2023).
- 23.Reinecke I, Zoch M, Reich C et al. The Usage of OHDSI OMOP – A Scoping Review. 2021. 10.3233/SHTI210546. [DOI] [PubMed]
- 24.ISO 8253-1. 2010(en), Acoustics — Audiometric test methods — Part 1: Pure-tone air and bone conduction audiometry. https://www.iso.org/obp/ui/#iso:std:iso:8253:-1:ed-2:v1:en (accessed 12 Apr 2023).
- 25.documentation:cdm:single. -page [Observational Health Data Sciences and Informatics]. https://www.ohdsi.org/web/wiki/doku.php?id=documentation:cdm:single-page (accessed 12 Apr 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw data (including participants’ voice files and the texts of the interviews) will be confidential and will not be able to share publicly. However, the codes that emerged during the current study are available from the corresponding author upon reasonable request.