Abstract
Polyspora axillaris (Roxb. ex Ker Gawl.) Sweet 1825, is a shrub or tree that is about 9 meters tall in the Theaceae family, mainly distributed in China and Vietnam, and it is widely used as a green tree species in many regions owing to its rapid growth and good adaptability. It is rich in various beneficial extracts for humans, but there are limited studies on it. In this study, we sequenced and annotated the complete plastome of P. axillaris. The chloroplast genome length of P. axillaris is 156,770 bp, with a total of 132 genes, including 37 tRNA genes, 8 rRNA genes and 87 protein-coding genes. The complete chloroplast genome of P. axillaris contains two Inverted Repeats (IRs) of 26,077 bp, a Large Single-Copy (LSC) region of 86,286 bp and a Small Single-Copy (SSC) region of 18,330 bp. The overall G/C content in the chloroplast is 37.3%. Phylogenetic inference shows that P. axillaris formed a sister relationship with P. hainanensis, along with 10 Theaceae species. The research result of P. axillaris will contribute to the genetic preservation of the species and the phylogenetic study of Polyspora.
Keywords: Genomic structure, Polyspora, Theaceae, plastome
Introduction
Polyspora axillaris (Roxb. ex Ker Gawl.) Sweet 1825, is a shrub or tree that is about 9 meters tall in the Theaceae family, mainly distributed in China and Vietnam (Ming and Bartholomew 2007; Nguyet et al. 2020). It grows rapidly and is adaptable, so it is widely used as a greening tree (Qin 1990; Fan et al. 2021; Fan et al. 2023). P. axillaris not only has ecological value, but also has health value. The extracts from its roots, stems, and fruits have antiviral and preventive effects on cardiovascular and cerebrovascular diseases (Li et al. 2017, 2019; Fan et al. 2021). However, although P. axillaris has many valuable values, the research on it is still insufficient. Based on this, we reports the complete plastome of P. axillaris, with the aim of improving the quality of related collection and phylogenetic research on Polyspora Sweet plants.
Materials
The fresh leaves of Polyspora axillaris was sampled from the Jianfengling in Hainan, China (108.79°E, 18.69°N) (Figure 1). The voucher specimen (voucher code: D. J. Chen, X. R. Ke, SK19, HUTB) was deposited at Hainan University, Hainan province, China (contact person Hua-Feng Wang, hfwang@hainanu.edu.cn).
Figure 1.
Photograph of polyspora axillaris (the photograph was taken by the author in jianfengling of Hainan, China). The specimen was stored at herbarium of Hainan university (http://en. hainanu. edu. cn/, contact person Hua-Feng wang, hfwang@hainanu. edu. cn) under the voucher number D. J. Chen, X. R. Ke, SK19, without any copyright issues. The leaves in the axils exhibit an inverted oval to oblanceolate shape, characterized by a hairless appearance at 6–14 cm × 2. 5–4 cm, with a dark green dorsal surface. Flowers are individual or paired.
Methods
Total genomic DNA extraction was performed using the cetyltrimethylammonium bromide (CTAB) protocol (Doyle and Doyle 1987). Subsequently, we constructed a paired-end libraries with insertion fragment sizes of 200–400 bp using genomic DNA of the selected samples. The sequencing work of these libraries was completed on the BGISEQ-500 platform of BGI in Shenzhen, China. The original read data was trimmed using SOAPfilter_v2. 2. Approximately 5 Gbp paired-end sequencing data were used for chloroplast genome assembly through GetOrganelle v1. 7. 5. 0 (Jin et al. 2020). To verify the accuracy of assembly, we compared the sequencing data with the assembled chloroplast genome using HISAT 2. 1. 0 (Kim et al. 2019), and statistically analyzed the sequencing depth of each site using SAMtools 1. 19. 2 (Danecek et al. 2021). In terms of gene annotation, we used Geneious Prime v2022. 1. 1 (Kearse et al. 2012) with reference to the chloroplast of Polyspora axillaris (NC_035645) and manually validated the results to detect potential errors. For gene structures that are difficult to annotate, such as cis and trans splicing genes, identification was carried out using CPGView online website(http://www.1kmpg. cn/cpgview, Liu et al. 2023). The complete chloroplast genome loop diagram was drawn by OGDRAW version 1. 3. 1 (Greiner et al. 2019).
In order to understand the phylogenetic relationship between P. axillaris and related species, we reconstructed a phylogenetic tree using the maximum likelihood (ML) method based on the complete chloroplast genome of P. axillaris, 5 other Polyspora species and 6 Camellia species, with Pyrenaria khasiana (NC_035644) as the outgroup (Table S1). All sequences were aligned by MAFFT (Katoh and Standley 2013)and trimmed by Gblocks (Talavera and Castresana 2007). Maximum likelihood phylogenies were inferred using IQ-TREE (Guindon et al. 2010; Nguyen et al. 2015) in PhyloSuite (Zhang et al. 2020) for 5000 ultrafast (Minh et al. 2013) bootstraps. In ML analysis, IQ-TREE was used with 1,000 bootstrap replicates automatically without setting specific models. The results were visualized by iTOL (https://iTOL. embl. de/) (Letunic and Bork 2016).
Results
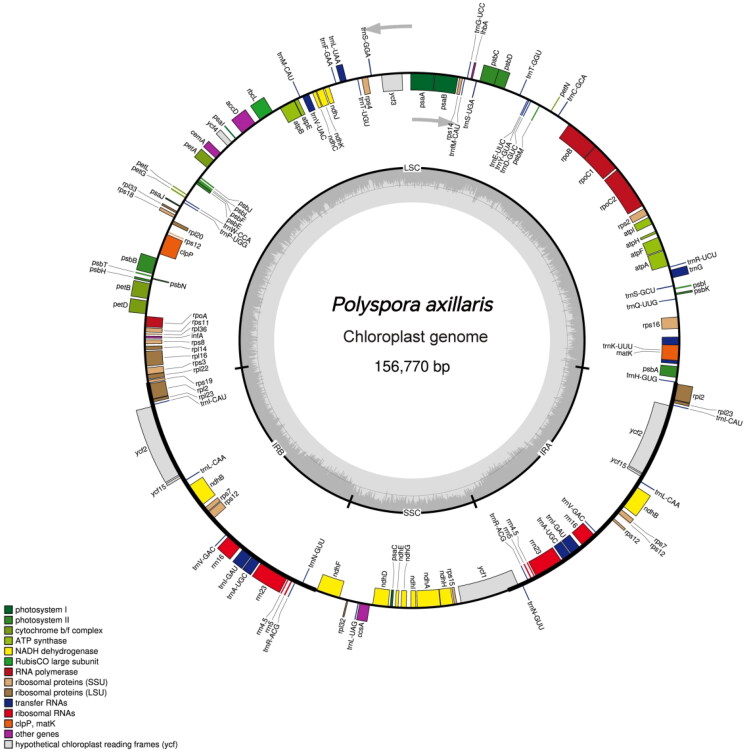
The minimum and average read mapping depths for assembled genomes were ×41, and ×476, respectively(Figure S1)The chloroplast genome length of Polyspora axillaris is 156,770 bp, with a total of 132 genes, including 37 tRNA genes, 8 rRNA genes(4. 5S rRNA, 5S rRNA, 16S rRNA and 23S rRNA) and 87 protein-coding genes. And the complete chloroplast of P. axillaris contains two Inverted Repeats (IRs) of 26,077 bp, a Large Single-Copy (LSC) region of 86,286 bp and a Small Single-Copy (SSC) region of 18,330 bp (Figure 2). Eight protein-coding genes are duplicated in the IR. Seven tRNA genes are duplicated in the IR. Four rRNA genes are duplicated in the IR. Among them, 11 genes (rsp16, atpF, rpoC1, ycf3, clpP, petB, petD, rpl16, rp12, ndhB, and ndhA) are cis-splicing gene, and rps12 is also a trans-splicing gene (Figures S2 and S3). The overall G/C content in the chloroplast is 37. 3%, of which the corresponding value for the LSC, SSC, and IR regions are 35.3%, 30.5%, and 43%, respectively. The annotated complete chloroplast genome of P. axillaris was submitted to GenBank (accession number OR863388). The raw reads were deposited in the NCBI Sequence Read Archive (accession number SRR27011443).
Figure 2.
Gene map of polyspora axillaris chloroplast genome. The circular chloroplast genome map displays 132 genes, including 37 tRNA genes and 8 rRNA genes, as well as 87 protein coding genes. The arrow inside the circle indicates the transcription direction of the gene inside the circle (clockwise). The arrow outside the circle indicates the transcription direction of the gene outside the circle (counterclockwise direction). The light gray line inside the circle represents the at content, and the dark gray area represents the GC content. At the bottom, genes from different functional groups are explained in various colors.
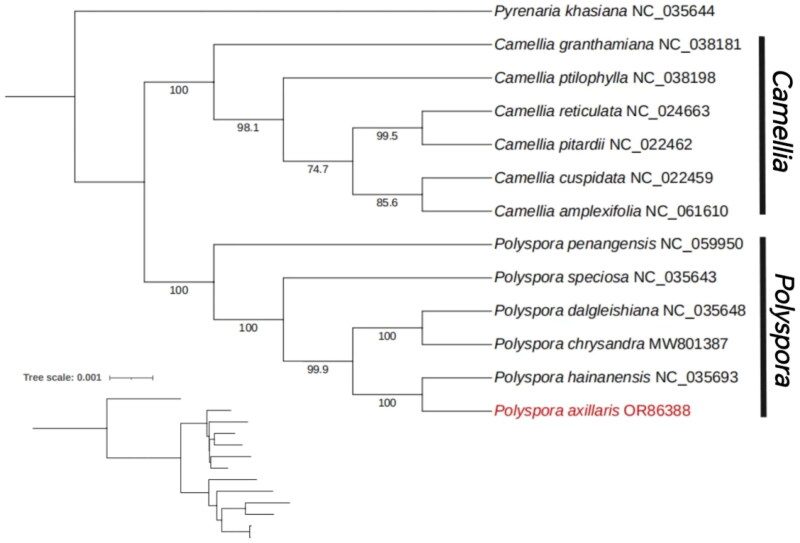
The alignment results of these 13 sequences are displayed in MEGA11 (Tamura et al. 2021). Figure S4 is a partial screenshot of multiple sequence alignment. And the results of phylogenetic research based on ML indicate that P. axillaris belongs to the genus polyspora in the Theaceae family. And Compared with other species, P. axilaris has a closer genetic relationship with P. hainanensis (Figure 3). The phylogenetic tree in a Newick text format is as supplementary file. Most nodes in the maximum likelihood (ML) tree of the Theaceae family plastomes are highly supported. In addition, the plastome sequence of P. axillaris will be beneficial for the phylogenetic research related to the Theacea family, deepening the understanding of the complex relationships among Theaceae plants.
Figure 3.
Maximum likelihood phylogenetic tree based on the complete chloroplast genomes of 12 species of theaceae, with pyrenaria khasiana (NC_035644) as the outgroup. Numbers on the nodes are bootstrap values from 1000 replicates. All sequences were downloaded from NCBI’s GenBank. The sequences used to build the tree is as follows: P. hainanensis (NC_035693, Yu et al. 2017), P. chrysandra (MW801387, Fan and Ma 2022), P. dalgleishiana (NC_035648, Yu et al. 2017), P. speciosa (NC_035643, Yu et al. 2017), P. penangensis (NC_059950), camellia pitardii (NC_022462, Yang et al. 2013), camellia reticulata (NC_024663, Huang et al. 2014), camellia amplexifolia (NC_061610), camellia cuspidata (NC_022459, Yang et al. 2013), camellia ptilophylla (NC_038198), camellia granthamiana (NC_038181), pyrenaria khasiana (NC_035644, Yu et al. 2017). The chloroplast genome of polyspora axillaris is displayed in red and the NCBI GenBank accession numbers are shown in parentheses.
Discussion and conclusions
CP genome analysis has been used to study the genetic diversity of plant species. However, there is limited knowledge about the CP genome of Polyspora, especially about Polyspora axillaris. Therefore, we sequenced the complete plastome of P. axillaris. The genomic structure of P. axillaris consists of a pair of IRs, a SSC region, and a LSC region that is similar to the majority of species in Theaceae, especially it has a stronger similarity with the Polyspora species (Fan and Ma 2022; Fan et al. 2024). We analyzed the cp genomes of P. axillaris and five other Polyspora species, which showed a same number of genes, CDS, tRNA, and rRNA. And the cp genomes of six Chinese Polyspora species varied in length between 156,452 bp (P. chrysandra) and 157,066 bp (P. speciosa), which is Consistent with Fan and Ma (2022) and Fan et al. (2024) research findings. However, compared to the six species in the Camellia genus, we found that the similarity in gene and tRNA numbers between them was not as strong. And basically speaking, the number of genes in the chloroplast genome of Camellia species is 1–2 more than that of Polyspora species (Table S2). Perhaps during the evolutionary process, these 1–2 genes were the reason for the emergence of the genus Polyspora and Camellia.
In this study, we also reconstructed a phylogenetic tree using the sequenced chloroplast genomes of P. axillaris, as well as the chloroplast genome sequences of 5 other Polyspora species and 6 Camellia species (these sequence all downloaded from NCBI), confirming the close genetic relationship between the Polyspora and Camellia genus. P. pentangensis is located at the bottom of the genus Polyspora, while P. speciosa, P. axillaris and P. hainanensis, P. chrysandra and P. dalgleishiana formed three branches within the genus. This is similar to the results of Fan and Ma (2022) using chloroplast and ribosomal 18-26 S rRNA data to construct a phylogenetic tree of the Theaceae family.
In summary, compared with previous research, we focus on analyzing the whole chloroplast genome sequence of P. axillaris and confirming its evolutionary position in the genus Polyspora. The chloroplast genome produced in our study provides additional genomic resources for the evolution research of the chloroplast genomes in the Polyspora genus. We hope our research results can deepen understanding of P. axillaris and promote the phylogenetic study of Theaceae.
Supplementary Material
Funding Statement
No funding was received.
Ethical approval
This study was approved by the Institutional Review Committee of Hainan University in Haikou, China. The collection of plant materials is carried out in accordance with the guidelines provided by Hainan University and the regulations of Hainan Province. The field research comply with the legislation of Hainan Province.
Authors contributions
Hui-Ting Tang designed the project. Hui-Ting Tang, Shi-You Zuo and Pei-Feng Liu analyzed and interpreted the data; Hui-Ting Tang and Chun-Fang Li drafted the manuscript. All authors have made critical revisions to obtain intellectual content and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genomic sequence data supporting this research result is publicly available In NCBI GenBank (https://www.ncbi.nlm.nih.gov/) with registration number OR863388. The relevant BioProject, Bio-Sample, and SRA numbers are PRJNA1043575, SAMN38414101, and SRR27011443, respectively (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1043575). A specimen was deposited at Herbarium of Hainan University (http://en.hainanu.edu.cn/, contact person Hua-Feng Wang, hfwang@hainanu.edu.cn) under the voucher number D J Chen, X R Ke, SK19.
References
- Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, et al. 2021. Twelve years of SAMtools and BCFtools. Gigascience. 10(2):giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL.. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19:11–15. [Google Scholar]
- Fan Z, Gao C, Lin L.. 2024. Phylogeographical and population genetics of Polyspora sweet in China provides insights into its phylogenetic evolution and subtropical dispersal. BMC Plant Biol. 24(1):89. doi: 10.1186/s12870-024-04783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZF, Han L, Ma CL.. 2021. Research advances of Polyspora sweet (Theaceae). Guihaia. 41(10):1755–1766. doi: 10.11931/guihaia.gxzw202010042. [DOI] [Google Scholar]
- Fan ZF, Ma CL.. 2022. Comparative chloroplast genome and phylogenetic analyses of Chinese Polyspora. Sci Rep. 12(1):15984. doi: 10.1038/s41598-022-16290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZF, Qian SJ, Zhang YH, Ma CL.. 2023. Characterization of the complete chloroplast genome of Polyspora tiantangensis (Theaceae), an endemic and endangered species in southwestern China. Mitochondrial DNA B Resour. 6(3):814–815. doi: 10.1080/23802359.2021.1884013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S, Lehwark P, Bock R.. 2019. OrganellarGenomeDRAW (OGDRAW) version 1. 3. 1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64. doi: 10.1093/nar/gkz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O.. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Huang H, Shi C, Liu Y, Mao SY, Gao LZ.. 2014. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: genome structure and phylogenetic relationships. BMC Evol Biol. 14(1):151. doi: 10.1186/1471-2148-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, Li D-Z.. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi: 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL.. 2019. Graph-based genome alignment and genoty** with HISAT2 and HISAT-genotype. Nat Biotechnol. 37(8):907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P.. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44(W1):W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cao SY, Lin SJ, Zhang JR, Gan RY, Li HB.. 2019. Polyphenolic profile and antioxidant capacity of extracts from Gordonia axillaris fruits. Antioxidants. 8(6):150. doi: 10.3390/antiox8060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Lin SJ, Zhang JJ, Zhao CN, Li HB.. 2017. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: optimization and identification of phenolic compounds. Molecules. 22(9):1481. doi: 10.3390/molecules22091481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ni Y, Li J, Zhang X, Yang H, Chen H, Liu C.. 2023. CPGView: a package for visualizing detailed chloroplast genome structures. Mol Ecol Resour. 23(3):694–704. doi: 10.1111/1755-0998.13729. [DOI] [PubMed] [Google Scholar]
- Ming TL, Bartholomew B.. 2007. Theaceae. Flora China. 12:366–478. [Google Scholar]
- Minh BQ, Nguyen MAT, Von Haeseler A.. 2013. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol. 30(5):1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ.. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyet HNL, Luong VD, Nguyen VC, Pham TTD, Luu TT, The PV.. 2020. An updated checklist of Theaceae and a new species of Polyspora from Vietnam. Taiwania. 65(2):216–227. doi: 10.6165/tai.2020.65.216. [DOI] [Google Scholar]
- Qin ZS. 1990. Mountain tree species—Gordonia axillaris. Guangdong For Sci Technol. 1:20–22. [Google Scholar]
- Talavera G, Castresana J.. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 56(4):564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S.. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38(7):3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JB, Yang SX, Li HT, Yang J, Li DZ.. 2013. Comparative chloroplast genomes of Camellia species. PLOS One. 8(8):e73053. doi: 10.1371/journal.pone.0073053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X-Q, Gao L-M, Soltis DE, Soltis PS, Yang J-B, Fang L, Yang S-X, Li D-Z.. 2017. Insights into the historical assembly of East Asian subtropical evergreen broadleaved forests revealed by the temporal history of the tea family. New Phytol. 215(3):1235–1248. doi: 10.1111/nph.14683. [DOI] [PubMed] [Google Scholar]
- Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT.. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomic sequence data supporting this research result is publicly available In NCBI GenBank (https://www.ncbi.nlm.nih.gov/) with registration number OR863388. The relevant BioProject, Bio-Sample, and SRA numbers are PRJNA1043575, SAMN38414101, and SRR27011443, respectively (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1043575). A specimen was deposited at Herbarium of Hainan University (http://en.hainanu.edu.cn/, contact person Hua-Feng Wang, hfwang@hainanu.edu.cn) under the voucher number D J Chen, X R Ke, SK19.