Abstract
Neurons, exhibiting unique polarized structures, rely primarily on the mitochondrial production of ATP to maintain their hypermetabolic energy requirements. To maintain a normal energy supply, mitochondria are transported to the distal end of the axon. When mitochondria within the axon are critically damaged beyond their compensatory capacity, they are cleared via autophagosomal phagocytosis, and the degradation products are recycled to replenish energy. When the mitochondria are dysfunctional or their transport processes are blocked, axons become susceptible to degeneration triggered by energy depletion, resulting in neurodegenerative diseases. As the final checkpoint for mitochondrial quality control, axonal mitophagy is vital for neuronal growth, development, injury, and regeneration. Furthermore, abnormal axonal mitophagy is crucial in the pathogenesis of optic nerve-related diseases such as glaucoma. We review recent studies on axonal mitophagy and summarize the progress of research on axonal mitophagy in optic nerve-related diseases to provide insights into diseases associated with axonal damage in optic ganglion cells.
Keywords: Mitophagy, Axon, Optic nerve, Energy
Introduction
Neurons primarily depend on mitochondrial oxidative phosphorylation to provide ATP for their energy requirements. Retinal ganglion cells (RGCs) with complex dendrites and significantly longer axonal structures have relatively higher energy requirements. Furthermore, mitochondria are involved in various physiological processes in RGCs, such as maintaining metabolic balance, regulating intracellular calcium levels, generating reactive oxygen species (ROS), and mediating apoptotic signaling [1–3]. Hence, preserving mitochondrial quality is critical for sustaining energy balance and ensuring normal physiological functioning in RGCs.
Autophagy is a critical degradative pathway for eukaryotic cells, important for the clearance of aggregated proteins and dysfunctional organelles, and is an essential homeostatic mechanism for neurons [4]. Specifically, mitochondrial autophagy, or mitophagy, is a specialized form of autophagy that targets mitochondria. Mitophagy removes and recycles damaged mitochondria and regulates the biogenesis of new, fully functional ones preserving healthy mitochondrial functions and activities [5]. This process helps to prevent the accumulation of defective mitochondria which can lead to cellular stress and various diseases [6, 7]. Under normal physiological conditions, important macromolecular precursors are produced through mitochondrial autophagy to replenish cells while preventing the accumulation of dead and dysfunctional mitochondria. Due to their polarized structure, mitochondria must be transported in RGCs through long axons and terminals to meet high energy demands and maintain energy homeostasis. The maintenance of axonal mitochondrial quality is primarily accomplished through mitochondrial biosynthesis, fission and fusion, bidirectional transport, and clearance. Mitophagy modulates mitochondrial mass in axons and clears senescent and damaged mitochondria. Autophagosomes in the axons transport engulfed mitochondria to the soma to complete the autophagy process [8, 9]. Ashrafi et al. demonstrated that localized mitophagy in distal axons is mediated by PINK1-Parkin [10]. The process of axonal mitophagy is complex, and its mechanism has not been fully elucidated. Various studies have shown that impaired regulation of neuronal mitophagy leads to axonal degeneration and synaptic instability, which are associated with neurodegenerative diseases, including Alzheimer disease, Huntington disease, Parkinson disease (PD), and amyotrophic lateral sclerosis [11–17]. Regulation of mitophagy is a potential target for the treatment of neurodegenerative diseases. Two crucial genes, Pink1 and Parkin, have been identified in hereditary PD, playing a significant role in maintaining mitochondrial integrity [18], as well as facilitating the process of local mitophagy at the distal axon [10, 19]. Furthermore, diseases associated with axonal damage in RGCs, such as glaucoma, are inextricably linked to axonal mitophagy [20–24]. We review recent studies on axonal mitophagy and summarize the progress of research on axonal mitophagy in optic nerve-related diseases, which are intended to provide insights into diseases associated with axonal damage in RGC.
Mitochondrial biogenesis and transport in RGC axons
In most mammalian species, the axons of RGCs within the retina are unmyelinated. However, they extend centripetally along the lamina cibrosa to the optic nerve head (ONH), where they converge and make a turn at right angles to form the optic nerve; they are myelinated in the retrolaminar region of the ONH, and the morphology is maintained through the rest of the optic nerve [25, 26]. Most mitochondria are located in the axons of RGCs since the axon length is at least three orders of magnitude greater than the soma diameter [26, 27]. A high density of mitochondria is required in the unmyelinated regions of RGC axons, nodes of Ranvier, and synaptic terminals to support the high energy demands of nerve fiber conduction and neurotransmitter release [28–30]. Because RGCs possess longer axons compared to other neurons, the distribution and consumption of intracellular energy are not homogeneous [26]. However, the diffusion capacity of ATP in the cytoplasm is limited, therefore, mitochondria biosynthesize, transport, and fission/fusion in RGC axons in order to provide sufficient ATP to supply energy locally to RGC axons [29, 31, 32].
Mitochondrial biosynthesis in axons
Mitochondrial biosynthesis is a subcellular process by which mitochondria are replenished and modified by the continuous input and integration of new proteins and lipids, the replication of mitochondrial DNA, the transcription of mitochondria-encoded genes, and fusion and division according to the needs and bioenergetic load of the cell. The transcriptional coactivator PPARγ coactivator 1 (PGC-1α), as in other eukaryotic cells, is a regulator of neuronal mitochondrial biosynthesis, consequently resulting in the expression of several mitochondrial genes via the activation of nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2) [33–35], including proteins (mitochondrial transcription factor A [TFAM]) required for mitochondrial DNA transcription and replication [36, 37]. Moreover, the promoter regions of nuclear genes encoding the subunits of the five complexes in the mitochondrial electron transport chain are targeted by NRF1 and NRF2 [38]. This leads to an increased assembly of respiratory organs and regulation of the import of nuclear-encoded mitochondrial proteins. Mitochondrial DNA replication and transcription heavily rely on the essential role of TFAM. TFAM plays a critical role by binding to the upstream region of the transcription start site, facilitating DNA folding, and enabling the recruitment of mitochondrial RNA polymerase.
The mitochondrial biosynthesis can be activated through various signaling cascades using the PGC-1α-NRF-1/2-TFAM pathway, where Ca2+, AMP/ATP and NAD+/NADH ratio are the main triggers. Elevated levels of Ca2+ result in the upregulation of PGC-1α and TFAM. [39]. The p38 mitogen-activated protein kinase (p38 MAPK), a downstream target of Ca2+/calmodulin-dependent protein kinase (CaMK), regulates the activation and expression of PGC-1α. Ca2+ triggers the activation of CaMK, which then phosphorylates p38 MAPK. This phosphorylation event subsequently leads to the activation and expression of PGC-1α, resulting in an enhanced mitochondrial biosynthesis [40]. In addition, CaMK can lead to increased mitochondrial biosynthesis via CREB protein stimulation of PGC-1α [41]. Increased AMP levels caused by physiological stimuli such as exercise and starvation activate AMP-activated kinase (AMPK), which phosphorylates PGC-1α directly [42]. After phosphorylation, PGC-1α is transferred from the cytoplasm to the nucleus, triggering mitochondrial biosynthesis. Phosphorylation of epigenetic factors by AMPK leads to increased expression of PGC-1α and TFAM. The NAD+/NADH ratio is a vital indicator of mitochondrial biosynthesis; an increased NAD+/NADH ratio activates SIRT1, stimulating mitochondrial biosynthesis via the deacetylation of PGC-1α [42, 43]. It is notable that the mechanism by which deacetylation of PGC-1α stimulates mitochondrial biosynthesis is dependent on Ca2+, AMPK, and Sirt1 [44], which implies that all of the major mitochondrial biogenesis stimuli may be interrelated. Furthermore, mitochondrial biosynthesis may be activated by CaMK-CREB, Akt-CREB, PKA-CREB, NO-cGMP, and PPAR-PGC-1α pathways [45–49].
Mitochondrial biosynthesis in neurons occurs predominantly in the soma, with the region around the nucleus generally presumed to be the primary site of mitochondrial production [50]. Mitochondria generated in the soma are transported to dendrites, axons, and other compartments. Notably, the mitochondrial transit speed in neurons is approximately 0.5 μm/s, indicating that newly synthesized mitochondria in the soma may take several days to be transported to the ends of long axons. Additionally, only 10–30% of mitochondria in neuronal axons are motile [51]; therefore, it would be impossible to meet the energy demand to sustain axonal physiological activity if we relied solely on the cytosolic synthesis of mitochondria. Mitochondrial biosynthesis during axonal localization is essential to meet the energy requirements of neuronal axons.
Although the mechanism of mitochondrial synthesis in axons has not been elucidated, studies have revealed localized axonal synthesis [52–54]. Nuclear-encoded mitochondrial mRNA is present in neuronal axons, and mRNA encoding the mitochondrial protein is more enriched in axons than in dendrites [55]. In addition, the translation of mitochondrial proteins was observed in the axons. Jung et al.’s bioinformatics analysis of the transcriptome and translatome of mouse optic nerve axon terminals revealed that mitochondrial proteins are highly translated at the axon terminals [56]. The intermediate filament protein, lamin B2, which binds to mitochondria, is locally synthesized in Xenopus laevis RGC axons [57]. The breakdown of lamin B2 leads to mitochondrial dysfunction and RGC axonal degeneration; however, the soma of the RGC remains intact [57]. Mitochondria-driven neuronal activity induces the translation of proteins within the local environment, crucial for the promotion of synaptic plasticity and memory formation. Kuzniewska et al. reported that the stimulation of neuronal synaptic activity induces the local synthesis of mitochondrial proteins, which are translocated to the mitochondria and admixed into the respiratory chain protein complexes [58].
Axonal mitochondrial transport
The direction of mitochondrial transport depends on the unique axonal arrangement of the microtubules. In axons, microtubules are evenly spaced, with all positive poles pointing toward the end of the axon and negative poles pointing toward the cell body. The prograde movement of mitochondria in axons refers to the movement of mitochondria towards the positive pole of the microtubule, while retrograde movement refers to the movement of mitochondria towards the negative pole of the microtubule. [59]. Furthermore, in axons, mitochondria are transported by energy-dependent motor proteins (motors), including kinesins and dyneins. Kinesins mediate the prograde movement of microtubules; the kinesin-1 family is a major driver of mitochondrial transport [60]. However, mitochondrial retrograde transport is driven by dynein. Mitochondrial translocation along actin microfilaments may be driven by dynein V [61]. It has been shown that in axons of hippocampal neurons grown in vitro, about 20–30% of the mitochondria are active, moving equally in the prograde and retrograde directions, while 70–80% are quiescent [51]. In vivo, moving axonal mitochondria account for approximately 10% of the total and are more inclined to move in the prograde direction. Using “MIMIR” (for “minimally invasive in vivo imaging of mitochondrial axonal transport in the RGC”), Yuji Takihara et al. reported that mitochondria in the axons of the nerve fiber layer of the RGC in mice were significantly dynamic, as observed with both shorter and longer mitochondrial complexes [62].
Milton and Miro are transport regulatory proteins involved in mitochondrial axoplasmic transport. Miro, an essential protein located in the mitochondrial outer membrane, plays a critical role in various mitochondrial processes, including microtubule-mediated transport within the axoplasm, regulation of mitochondrial dynamics encompassing fusion and fission events, as well as maintenance of mitochondrial calcium homeostasis. Milton is a ligand (protein) that recruits the heavy chain of kinesin-1 protein to the mitochondria for axoplasmic transport. Notably, Milton and Miro connect with the microtubule proteins kinesin and dynein to form a transporter complex for bidirectional mitochondrial axoplasmic transport [59].
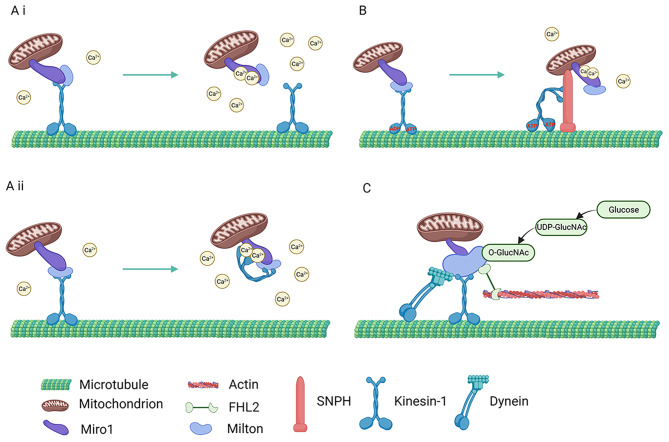
The final localization of mitochondria in neurons is regulated by the microenvironment, including features of synaptic activity such as the ADP: ATP ratio, increasing levels of intracellular Ca2+ and elevated glucose, and the anchoring function of axon-targeted syntaphilin (SNPH). The ADP: ATP ratio is a crucial signal in regulating mitochondrial transport; mitochondrial velocity increased in regions with high ATP levels and decreased when ATP was depleted. Regions with a high ADP: ATP ratio may reduce kinesin activity through the competitive binding of ADP to motor proteins, causing mitochondria to quiesce at higher ADP levels. Another important signal is the neuronal Ca2+ concentration; enhanced synaptic activity elevates intracellular Ca2+ levels, consequently resulting in the immobilization of mitochondria in active synaptic regions, where the requirements for energy and Ca2+ buffering are high. The distribution of mitochondria at the synapses can affect synaptic transmission and strength. Stable mitochondrial localization at the presynapse maintains a stable release of synaptic vesicles, which leads to a stable amplitude of excitatory postsynaptic currents [63]. Ca2+-dependent quiescence of mitochondrial motility is mediated by Miro, which contains two Ca2+-sensitive EF-hand motifs oriented toward the cytoplasmic side of the cell to detect cytoplasmic Ca2+ levels [64]. Additionally, there are currently two available mechanisms that explain how Miro regulates the movement of mitochondria in a Ca2+-dependent manner. As per the “Motor Release Model”, an increase in Ca2+ causes Miro to stay connected to Milton and mitochondria but becomes separated from kinesin-1. This separation ultimately hinders the transport of mitochondria (Fig. 1Ai) [65]. On the other hand, based on the “motor-Miro binding model”, high levels of Ca2+ surrounding the mitochondria result in its binding to the Miro EF-hand motif. This binding induces a change in conformation, leading to a direct interaction between kinesin-1 and Miro, which prohibits spatial binding to the mitochondria and microtubules and therefore impedes mitochondrial movement (Fig. 1Aii) [66]. Additionally, glucose is an essential factor affecting mitochondrial localization at synapses. Glucose is metabolized to uridine diphosphate-N-acetylglucosamine (UDP-GlucNAc), which is used in Milton’s modification via oxygen-linked nitrogen acetylglucosamine (OGT) [67–69]. The modified Milton binds to protein four and a half LIM domain protein 2 (FHL2) in the same complex, which docks the mitochondria to actin (Fig. 1C). Therefore, the mitochondria remain at a higher glucose concentration to maximize their capacity. Furthermore, SNPH is enriched in resting mitochondria in axons, with SNPH knockout mice exhibiting enhanced axonal mitochondrial viability but no effects on dendritic mitochondrial viability [70]. SNPH anchors mitochondria to the cytoskeleton, a process that may be regulated by SNPH ubiquitination, which, instead of triggering SNPH degradation, fortifies the attachment of SNPH to microtubules. Ca2+ promotes SNPH binding to microtubules and kinesin-1, decreasing the ATPase occupancy of kinesin-1 and inhibiting mitochondrial motility; however, this phenomenon occurs only in the axons (Fig. 1B). In addition, the stability of the axonal microtubule structure may affect RGC mitochondrial transport; it has been shown that in a glaucoma model, the massive accumulation of TAU, a microtubule-binding protein, in RGC axons within the retina disrupts axonal prograde transport function [21]. Therefore, disruption of microtubule integrity or function may compromise mitochondrial transport in RGC axons.
Fig. 1.
Axonal mitochondrial transport. (A) Ca2+ affects axonemal mitochondrial transport and localization, where axonemal mitochondria are docked in regions of high Ca2+ concentration. (i) Motor Release Model. (ii) Motor-Miro binding model. (B) Ca2+ promotes binding of SNPH to microtubules and kinesin-1, and SNPH stably anchors axonal mitochondria by inhibiting motor ATPase activity. (C) Mitochondria in axons are anchored in regions of higher glucose concentration; OGT, a glucose metabolite, modifies Milton, and FHL2 anchors mitochondria to actin
Axonal mitochondrial fusion and fission
Axonal mitochondria can undergo morphological changes through fission and fusion, and the balance between fission and fusion enables them to rapidly adapt to environmental changes to meet the energy demands of neurons.
Mitochondrial fusion is a homologous reaction in which two adjacent mitochondria achieve the fusion of their outer and inner membranes, resulting in a fibrillarly extended and networked-structured mitochondrion that allows the exchange of mtDNA, proteins, lipids, and metabolites. Mitochondrial fusion resists environmental stress by buffering the adverse effects of large fluctuations in the mitochondrial membrane potential. In addition, fused mitochondria have a metabolic advantage due to their more elongated morphology than individual mitochondria, allowing them to have higher concentrations of ATP. The mechanism of mitochondrial fusion (the fusion of the outer and inner membranes) is complex and currently unknown. The outer membrane fusion protein mitofusin (MFN) is essentially a GTPase with two structurally similar isoforms, MFN1 and MFN2, both of which have two heptapeptide repeats, with HR1 in the N-terminal region and HR2 in the C-terminal region. Upon hydrolysis of GTP, the two isoforms of the HR1 structure undergo residue rearrangement to form the “G interface” and dimerize through this interface to form the G-HR1 dimer, which undergoes a major conformational change. However, the direction of the HR2 structure changes from the open conformation to the closed conformation, completing the fusion of the outer mitochondrial membrane [71]. Fusion of the inner mitochondrial membrane primarily depends on the lipid components of optic atrophy 1 (OPA1) and IMM [72]. OPA1 is a dynamin-like GTPase that includes an N-terminal transmembrane (TM) structural domain, a G structural domain, and a predicted long helical region, with a minimum G structural domain of MGD, which forms an OPA1-MGD nucleotide-dependent dimer during hydrolysis by GTPase and promotes endosomal fusion. Fusions of inner and outer membranes are reciprocally inter-regulated in a delicate dynamic balance; once the outer membrane is fused, fusion of the inner membrane occurs rapidly, and the rare fusion of the outer membrane that does not result in fusion of the inner membrane leads to defects in the formation of mitochondria [73].
Mitochondrial fission is the process by which one mitochondrion splits into two parts. Mitophagy controls mitochondrial quality by removing damaged organelles, promoting apoptosis under high cellular stress levels, and promoting mitochondrial fission. Like fusion, mitochondrial fission is coordinated by the dynamin-like GTPase, Drp1 [74]. In addition to Recombinant Dynamin 2 (DNM2), also known as DYN2, human chromosome 19p13.2, human mitochondrial dynamics proteins 49 (MID49) and human mitochondrial dynamics proteins 51 (MID51), mitochondrial fission 1 (FIS1), mitochondrial fission factor, and other proteins are involved in mitochondrial fission [75]. Drp1 is a cytosolic protein consisting of four structural domains: the N-terminal GTPase structural domain, the posterior intermediate structural domain, the variable structural domain, and the GED in the C-terminus, which is recruited to the mitochondria via a junction on the outer mitochondrial membrane (OMM). When fission occurs, the mitochondria are first contacted by ER tubules, which mediate mitochondrial contraction and a reduction in diameter to allow for the formation of the Drp1 oligomerization ring. At this point, Drp1 has been recruited to the OMM to form an oligomerization ring around the mitochondria and to enhance contraction of the mitochondria that is already present, and then GTP hydrolyzes, and DNM2 is recruited to the contractile sites of the mitochondria, assembling and completing the process of fission [48]. Furthermore, it has been demonstrated that Drp1 is contractile and severing and that the presence of DNM2 seems dispensable [76].
The relationship between mitochondrial fusion/fission kinetics and mitophagy is complicated. In addition to the fact that mitochondrial fission can facilitate the completion of mitochondrial autophagy, excessive fusion prevents mitochondrial degradation, which can be compensated for by increased fission and inhibition of fusion in mitochondria deficient in mitochondrial autophagy factors. The fusion and fission of mitochondria affect the morphological changes of mitochondria and determine their mobility and localization, which are essential for maintaining the normal physiological activities of RGC axons based on the specificity of mitochondrial functions.
Axonal mitophagy
With a complex dendritic structure, extensive branching of long axons, and axons enriched with ion pumps and ion channels to maintain membrane potential, neurons are the most energy-demanding cell type [77]. The ATP required by normal cells is produced primarily through glycolysis and oxidative phosphorylation of mitochondria; however, neurons rely primarily on oxidative phosphorylation of mitochondria to provide ATP. Mitochondrial transport to the distal end of the axon is essential to ensure a normal energy supply due to the long axons of neurons; when mitochondria are dysfunctional or when the transportation process is blocked, the axon is highly susceptible to degeneration caused by energy exhaustion, leading to the occurrence of neurodegenerative diseases [78]. Common degenerative changes within axons include Wallerian degeneration, which occurs in severe acute injuries, and retrograde death, associated with chronic injuries. During retrograde death, the distal synapse of the axon first degenerates and gradually progresses retrograde to the soma [21]. At the initial stage of injury, neurons can activate protective mechanisms to restore the number of functional mitochondria in the axon; however, when the mitochondria are severely damaged beyond their compensatory capacity, the damaged mitochondria in the axon are phagocytosed and removed by autophagosomes, and the degradation products are recycled to replenish energy, a process known as axonal mitophagy [78, 79]. Mitochondria are the primary sites of intracellular energy production. As major oxygen-consuming organelles, mitochondria are the main source and target of ROS in hypoxia, making them more susceptible to ROS-induced oxidative damage [80]. Mitophagy is the selective degradation of damaged or dysfunctional mitochondria to maintain normal intracellular energy metabolism, and several studies have indicated that it plays a vital role in the differentiation and development of eukaryotes [81–84]. Mitochondrial autophagy is crucial to maintaining neuronal activity and is the last barrier to maintaining mitochondrial quality in neurons. The mechanism by which mitochondrial autophagy occurs in axons is complex, and in recent years, it has become an exciting area of investigation.
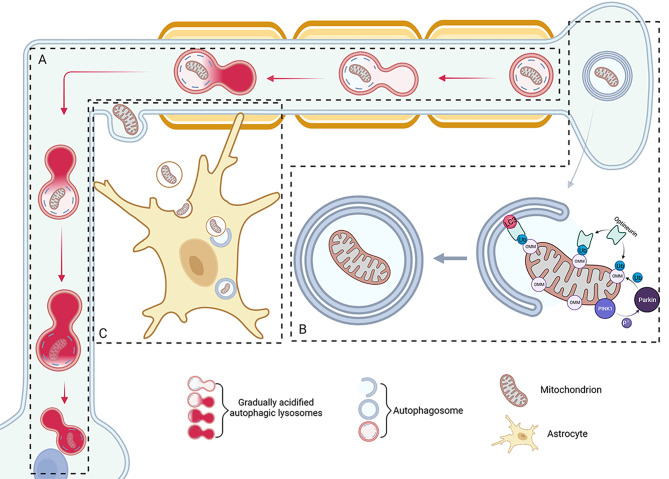
Axonal mitochondria are transported back to the soma by autolysosomes for autophagy, which dominates axonal mitochondrial autophagy. Autophagosomes are formed at the distal end of the axon, phagocytose organelles, such as mitochondria and part of the cytoplasm, and move bidirectionally in the distal ends of the axon [8]. Notably, some autophagosomes escape from localized bidirectional movement and move in the reverse direction along the axon toward the cell body under the action of kinesin [85, 86]. During retrograde transport, autophagosomes gradually acidify and fuse with lysosomes until they reach the cytosol, become fully acidified, and mature [8]. This process allows the degradation products within the autophagosome to be transported directly to the primary sites of synthesis of biomolecules such as proteins and lipids, consequently increasing the efficiency of recycling (Fig. 2A).
Fig. 2.
RGC Axonal mitophagy. (A) Axonal mitochondria can be transported back to the soma by autolysosomes for autophagy. During retrograde transport, the autophagosome gradually acidifies and fuses with the lysosome until it arrives at the soma fully acidified. (B) Mitophagy via the PINK1-Parkin pathway localized in the distal axon. (C) At ONH, mitochondria accumulate in axons near the membrane site of astrocytes, and axonal avulsions are filled with mitochondria internalized by astrocytes
However, Ashrafi et al. suggested that mitophagy can occur locally in the distal axons (Fig. 2B) [10]. Mitophagy is usually activated by two pathways: one is dependent on the receptor, and the other is mediated by ubiquitin [87]. Under stress, proteins such as BNIP3, NIX, and FUNDC1, mammalian mitochondrial autophagy receptors, can be recruited to the OMM and interact with LC3 to mediate autophagosomal phagocytosis of injured mitochondria [88]. Mitochondrial autophagy at the distal ends of axons is primarily mediated by PINK1-Parkin. PINK1 is a mitochondrial protein that rapidly degrades in the normal inner membrane and is maintained at low intracellular levels. Dysfunctional mitochondria undergo a loss of membrane potential that allows PINK1 to accumulate selectively in the outer membrane of dysfunctional mitochondria; therefore, PINK1 can act as a sensor for mitochondrial dysfunction [89, 90]. Parkin is a member of the ubiquitin E3 ligase family and is autoinhibited in normal cells. When mitochondrial function is abnormal, PINK undergoes multisite phosphorylation. Parkin is recruited to the mitochondrial outer membrane by PINK and is further activated by phosphorylation of PINK1, causing extensive and rapid phosphorylation and ubiquitination events on the mitochondrial surface; OMM proteins are rapidly ubiquitinated by Parkin [91–95]. Optineurin is an autophagy receptor with a ubiquitin-binding structural domain and LIR (LC3 interaction region) motifs, which bind to the ubiquitinated OMM in PINK1-Parkin and connect to autophagosomes via LC3 on the autophagosome membrane. Subsequently, damaged mitochondria are surrounded and engulfed by autophagosomes and transported to lysosomes for degradation to remove damaged mitochondria and maintain cellular function stability [4, 92]. During autophagy, the mitochondrion is encapsulated in a double-layered vesicle known as the autophagosome, which is then transported to the lysosome for degradation [96]. Localized axonal mitophagy, which does not require retrograde transport to somatic cells, provides rapid neuroprotection, reduces oxidative stress, and prevents the spread of oxidative damage. Application of acute stress stimuli to axons triggers local PINK1-dependent mitochondrial autophagy; however, the short half-life of PINK1 makes it unlikely to be transported as a protein to the distal regions of neuronal axons. The PINK1-Parkin pathway is dependent on the sustained synthesis of PINK1 and is activated by the inhibition of PINK1 degradation. To induce mitophagy that occurs locally in the axon, the mRNA encoding PINK1 is expressed locally in the axon [10]. It has been observed that the mRNA for PINK1 co-localizes with mitochondria in the axon, and the mitochondrial outer membrane proteins, synaptojanin 2-binding protein (SYNJ2BP) and synaptojanin 2 (SYNJ2), through the RNA-binding domain in SYNJ2, tether PINK1 mRNA to mitochondria [19]. This neuron-specific adaptation to the localized translation of PINK1 provides distal mitochondria with a continuous supply of PINK1 to activate mitophagy. It has been shown that the total number of mitochondria in the mouse RGC soma is 10 times higher than that in the ONH [97]; notably, the total number of mitochondria that undergo degradation in the two regions is indeed comparable, with a unique process of mitophagy in the ONH. Davis et al. observed by transmission electron microscopy that in the mouse ONH, mitochondria accumulate in axons near the membrane site of astrocytes, axonal avulsions fill the mitochondria, astrocytes undergo phagocytosis, and detached avulsions are internalized by astrocytes (Fig. 2C) [97]. Davis et al. further determined that mitochondria-rich torn-offs were degraded in astrocytes because the organelles colocalized with lysosomal markers, were surrounded by the astrocyte membrane, and detected degraded neuronal mitochondrial DNA in astrocytes. This evidence suggests that transcytotic autophagy may occur in the mitochondria of RGC axons [97, 98]. However, it remains uncertain whether the axonal mitochondria eliminated at the ONH are solely those that reside locally within the axon or if other axonal mitochondria destined for degradation are actively transported to the ONH for processing. The reason for the selective degradation of axonal mitochondria in the ONH remains unclear, which was hypothesized by Davis CH et al [97]. One possible explanation could be that this pathway is triggered by a focal axonal injury, which is inadequate to initiate axonal loss. An alternative possibility is that transporting axonal mitochondria back to the soma for degradation through the narrow region of the lamina propria is arduous or energy-demanding. A third hypothesis is that the ONH contains a subset of astrocytes displaying exceptional phagocytic capability, as indicated by the high expression of molecular markers like Mac2. Astrocytes have demonstrated their capacity to phagocytose synapses in the developing visual pathway. Synapses usually encompass large quantities of mitochondria; thus, the molecular mechanisms employed by astrocytes in synapse phagocytosis are likely employed in the phagocytosis of axonal mitochondria in the ONH and other regions.
Optic nerve-related diseases and axonal mitophagy
The role of mitochondrial autophagy in optic nerve-related diseases has recently attracted increasing attention. Several studies have shown that mitochondrial autophagy is crucial in various neurological disorders, including Alzheimer disease, Huntington chorea, PD, and amyotrophic lateral sclerosis. RGCs are highly susceptible to mitochondrial dysfunction due to their intricate dendritic and prolonged axonal structures, as well as their elevated energy demands. Noteworthy, various optic neuropathies, like Leber hereditary optic neuropathy and autosomal dominant optic atrophy, are linked to inherited mitochondrial abnormalities [99, 100]. However, the association of axonal mitochondrial autophagy with these disorders has not yet been elucidated. Glaucoma is the most common optic neuropathy and the leading cause of irreversible blindness. Age-related mitochondrial defects play a central role in glaucoma pathogenesis, and defects in multiple aspects of axonal mitochondrial autophagy may be responsible for glaucomatous optic nerve degeneration.
It has been suggested that axonal degeneration precedes the death of RGC soma [101]. Mitophagy occurs in the soma and axons [10, 102]; however, there is evidence from studies indicating that neighboring astrocytes degrade a large number of mitochondria in the ONH, a nontraditional mitochondrial autophagy process known as transcellular degradation [97, 98]. In a mouse model of glaucoma (DBA/2J), the absence of mitochondrial regions in RGC axons increased, mitochondrial length decreased with age, and mitochondrial transport was impaired [62]. Despite their increased mobility, these short, fragmented mitochondria are functionally defective and cannot efficiently energize the optic nerve, becoming targets for degradation. Despite the increase in autophagosomes in the optic nerves of DBA/2J mice, increased mitochondria and decreased LAMP1 suggest that fragmented mitochondria are not efficiently recycled in axons via mitophagy [23]. Therefore, in the mouse glaucoma model, autophagic degradation of axonal mitochondria occurs primarily in the soma rather than locally in the axon via the PINK1-Parkin pathway. Because of the impaired axonal transport function caused by mitochondrial dysfunction, autophagosomes accumulate in the axon, and broken mitochondria cannot be effectively cleared, further impairing the function of the RGC and aggravating neurodegeneration. Howell et al. concluded that the prominent focal swelling of axons and mitochondria-filled dystrophic neuromasts within the glial layer of the ONH are the earliest indications of glaucomatous pathology. Therefore, we hypothesized that mitochondrial transcellular autophagy occurring at the ONH maintains mitochondrial homeostasis in the unmyelinated axons of RGC and that factors such as mechanical stress influence this process, resulting in an increase in fragmented mitochondria at the ONH that are not efficiently cleared and do not adequately energize the axons in the unmyelinated region.
Shim et al. [103] conducted a study that revealed the role of E50K expression in inducing degradation and subsequent mitophagy through mitochondrial division in the glial layer axons of aged E50K− tg mice. The presence of OPTN mutations has been linked to primary open-angle glaucoma. Among the various types of OPTN protein mutations, the E50K mutation is particularly common. This mutation specifically triggers age-related loss of RGCs in transgenic mice, resulting in axonal degeneration and visual impairment [104, 105]. Furthermore, it was observed that overexpressing E50K led to increased expression of LC3-II protein in RGCs and their axons [106]. The formation of autophagosomes coincided with the division-mediated degradation of mitochondria in the glial layer axons of aged E50K− tg mice. Interestingly, in vitro experiments demonstrated that E50K overexpression did not affect the kinetics of mitochondrial transport or alter their length in RGCs. Considering the mechanisms involved, it is suggested that the E50K mutation may initiate injury to mitochondrial dynamics through Bax-mediated pathways. Additionally, this mutation further contributes to the degradation and dysfunction of mitochondria in RGC axons during RGC degeneration [103, 107]. Kim et al. observed that the inhibition of dynamin-related protein 1 expression in the retina of DBA/2J mice reduced the number of fragmented mitochondria and maintained mitochondrial integrity in the retinal glial layer, protecting RGC axons [32]. It has been shown that in ischemic hypoxic neuronal axons, axonal mitochondria are retrogradely transported to the soma for autophagic degradation. Blockade of mitochondrial movement aggravates an ischemic neuronal injury, and enhanced retrograde mitochondrial movement is neuroprotective. Promoting retrograde mitochondrial transport enhances mitochondrial autophagy and reduces mitochondrial quantity but improves mitochondrial function, further promoting neuronal survival. In contrast, inhibition of mitochondrial movement results in mitochondrial accumulation and impaired mitochondrial quality, exacerbating neuronal injury. These results suggest that mitochondrial quality is more important for neuronal survival than mitochondrial quantity. Therefore, promoting retrograde mitochondrial transport and facilitating mitochondrial autophagy in axonal injury may be an important strategy for improving mitochondrial quality control during ischemia-reperfusion, providing new ideas for the treatment of glaucoma and other ischemic optic nerve diseases.
Summarization and prospect
Structurally, with long axons, the distribution of mitochondria in different RGC compartments is conducive to meeting the energy demands of local metabolic activities. Functionally, as projection neurons in the retina, RGC transmits large amounts of signaling molecules to the synaptic terminal via the axon and then presents and exchanges the signaling molecules with CNS neurons through endocytosis and exocytosis; this phenomenon results in a large amount of energy loss during the transportation and exchange of signaling molecules. Therefore, once mitochondrial quality is impaired, the structure and function of RGC become highly susceptible to damage. The close coordination and cooperation between biosynthesis, fission, fusion, bidirectional transport, and clearance of axonal mitochondria are crucial for regulating the mitochondrial quality of RGCs. An insufficient supply of healthy mitochondria or the accumulation of fragmented mitochondria can lead to an energy crisis in RGC. There is growing evidence that axonal mitophagy is critical for maintaining normal axonal physiological activity and plays an essential role in glaucomatous diseases. The mechanisms of axonal mitochondrial autophagy have not been fully elucidated; however, three pathways have been identified: retrograde mitochondrial transport to the soma autophagy, axonal local mitophagy, and ONH axonal mitophagy across the cell membrane. Transporting mitochondria back into the cytosol for autophagy is more conducive to the recycling and reuse of post-autophagic products, and this approach is now considered the primary pathway by which autophagy occurs in the axonal mitochondria. The rapid mitophagic response that occurs locally in the distal axon provides rapid neuroprotection, reduces oxidative stress, and prevents the spread of oxidative damage during transport. Transcellular degradation by astrocytes in the ONH to assist axonal mitophagy accelerates the rate of mitochondrial renewal and supports the high demand for mitochondria by unmyelinated axons in the nerve fiber layer. Based on various studies, it can be hypothesized that dysregulation of axonal mitochondrial autophagy may result in the accumulation of axonal autophagosomes and mitochondrial debris and a shortage of axonal energy supply, which may lead to impaired axonal function, the inability to recycle fragmented mitochondria and other organelles, and the inability of the soma to transport healthy mitochondria in support of the axon’s physiological activities, which may lead to or exacerbate neuronal damage.
Furthermore, several aspects of axonal mitophagy are not yet clearly understood, and there are many questions have not yet been sufficiently explored. Does axonal mitophagy play different roles in normal and damaged axons [108, 109]? Why do multiple modes of axonal mitophagy exist [8, 10, 97]? What regulatory signals initiate different axonal mitophagy modalities? Could the existence of multiple pathways of mitophagy not be related to the differing structures and functions of different cell types, coupled with the different environmental signals they are exposed to? Is there another method for removing axon-damaged mitochondria [110]? Promoting axonal mitophagy is beneficial for maintaining axonal homeostasis; however, this may have a negative effect. How is axonal mitophagy regulated? How can modulation of axonal mitophagy be utilized to treat glaucoma and other neurodegenerative diseases? With more intensive research, regulating axonal mitophagy through various links may lead to breakthroughs in the future treatment of optic nerve-related diseases and other nervous system diseases.
Acknowledgements
We thank Editage (www.editage.cn) for their assistance during the preparation of this manuscript.
Abbreviations
- RGC
Retinal ganglion cell
- ROS
Reactive oxygen species
- PD
Parkinson disease
- ONH
Optic nerve head
- NRF-1
Nuclear respiratory factors 1
- NRF-2
Nuclear respiratory factors 2
- TFAM
Mitochondrial transcription factor A
- p38 MAPK
p38 mitogen-activated protein kinase
- CaMK
Calmodulin-dependent protein kinase
- AMPK
AMP-activated kinase
- MIMIR
Minimally invasive in vivo imaging of mitochondrial axonal transport in the RGC
- SNPH
Syntaphilin
- UDP-GlucNAc
Uridine diphosphate-N-acetylglucosamine
- OGT
Oxygen-linked nitrogen acetylglucosamine
- MFN
Membrane fusion protein mitofusin
- OPA1
Optic atrophy 1
- TM
Transmembrane
- DNM2, DYN2
Dynamin 2
- MID49
Mitochondrial dynamics proteins 49
- MID51
Mitochondrial dynamics proteins 51
- FIS1
Mitochondrial fission 1
- OMM
Outer mitochondrial membrane
- SYNJ2BP
Synaptojanin 2-binding protein
- SYNJ2
Synaptojanin 2
Author contributions
Y. L. and G.-Y. L. prepared the first draft of the manuscript. Y.L. and Y.-L. L. prepared Figs. 1 and 2. All authors edited the review article. Guangyu Li approved the submission of the manuscript. All authors contributed to the writing and editing and agreed to the submission of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (No.82171053, 81570864).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. 10.3389/fnsyn.2010.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromol Med. 2002;2(2):215–31. 10.1385/NMM:2:2:215 [DOI] [PubMed] [Google Scholar]
- 3.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta. 2010;1797(6–7):1171–7. 10.1016/j.bbabio.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 4.Stavoe AKH, Holzbaur ELF. Autophagy in neurons. Annu Rev Cell Dev Biol. 2019;35:477–500. 10.1146/annurev-cellbio-100818-125242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picca A, Faitg J, Auwerx J, Ferrucci L, D’Amico D. Mitophagy in human health, ageing and disease. Nat Metabolism. 2023;5(12):2047–61. 10.1038/s42255-023-00930-8 [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Mi Y, Meng Q, Liu Y, Wang Y, Zhang Y, et al. A quinolinyl resveratrol derivative alleviates acute ischemic stroke injury by promoting mitophagy for neuroprotection via targeting CK2α’. Int Immunopharmacol. 2024;137:112524. 10.1016/j.intimp.2024.112524 [DOI] [PubMed] [Google Scholar]
- 7.Zhao P, Shi W, Ye Y, Xu K, Hu J, Chao H, et al. Atox1 protects hippocampal neurons after traumatic brain injury via DJ-1 mediated anti-oxidative stress and mitophagy. Redox Biol. 2024;72:103156. 10.1016/j.redox.2024.103156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196(4):407–17. 10.1083/jcb.201106120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neuroscience: Official J Soc Neurosci. 2011;31(21):7817–30. 10.1523/JNEUROSCI.6412-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206(5):655–70. 10.1083/jcb.201401070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong YC, Holzbaur EL. The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J Neuroscience: Official J Soc Neurosci. 2014;34(4):1293–305. 10.1523/JNEUROSCI.1870-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaillant-Beuchot L, Eysert F, Duval B, Kinoshita PF, Pardossi-Piquard R, Bauer C, et al. The amyloid precursor protein and its derived fragments concomitantly contribute to the alterations of mitochondrial transport machinery in Alzheimer’s disease. Cell Death Dis. 2024;15(5):367. 10.1038/s41419-024-06742-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veverová K, Laczó J, Katonová A, Horáková H, Matušková V, Angelucci F et al. Alterations of human CSF and serum-based mitophagy biomarkers in the continuum of Alzheimer disease. Autophagy. 2024:1–11. [DOI] [PMC free article] [PubMed]
- 14.Li L, Zhang Y, Chen Z, Xu C, Xu Z, Pei H, et al. Cytarabine prevents neuronal damage by enhancing AMPK to stimulate PINK1 / parkin-involved mitophagy in Parkinson’s disease model. Eur J Pharmacol. 2024;977:176743. 10.1016/j.ejphar.2024.176743 [DOI] [PubMed] [Google Scholar]
- 15.Hong X, Zheng Y, Hou J, Jiang T, Lu Y, Wang W, et al. Detection of elevated levels of PINK1 in plasma from patients with idiopathic Parkinson’s disease. Front Aging Neurosci. 2024;16:1369014. 10.3389/fnagi.2024.1369014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Zhang Z, Gu J, Ke P, Liu J, Meng Y, et al. FUDNC1-dependent mitophagy ameliorate motor neuron death in an amyotrophic lateral sclerosis mouse model. Neurobiol Dis. 2024;197:106534. 10.1016/j.nbd.2024.106534 [DOI] [PubMed] [Google Scholar]
- 17.Belosludtseva NV, Matveeva LA, Belosludtsev KN. Mitochondrial dyshomeostasis as an early Hallmark and a therapeutic target in amyotrophic lateral sclerosis. Int J Mol Sci. 2023;24(23). [DOI] [PMC free article] [PubMed]
- 18.Dodson MW, Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Curr Opin Neurobiol. 2007;17(3):331–7. 10.1016/j.conb.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Harbauer AB, Hees JT, Wanderoy S, Segura I, Gibbs W, Cheng Y, et al. Neuronal mitochondria transport Pink1 mRNA via synaptojanin 2 to support local mitophagy. Neuron. 2022;110(9):1516–e319. 10.1016/j.neuron.2022.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Z, You M, Fan C, Rong R, Li H, Xia X. Pathologically high intraocular pressure induces mitochondrial dysfunction through Drp1 and leads to retinal ganglion cell PANoptosis in glaucoma. Redox Biol. 2023;62:102687. 10.1016/j.redox.2023.102687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleesattel D, Crish SD, Inman DM. Decreased Energy Capacity and increased autophagic activity in Optic nerve axons with defective Anterograde Transport. Investig Ophthalmol Vis Sci. 2015;56(13):8215–27. 10.1167/iovs.15-17885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jassim AH, Inman DM, Mitchell CH. Crosstalk between Dysfunctional Mitochondria and inflammation in Glaucomatous Neurodegeneration. Front Pharmacol. 2021;12:699623. 10.3389/fphar.2021.699623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coughlin L, Morrison RS, Horner PJ, Inman DM. Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Investig Ophthalmol Vis Sci. 2015;56(3):1437–46. 10.1167/iovs.14-16126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y, Hu X, Sun X. Overexpression of parkin protects retinal ganglion cells in experimental glaucoma. Cell Death Dis. 2018;9(2):88. 10.1038/s41419-017-0146-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb H. Morphology and Circuitry of Ganglion Cells. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. Salt Lake City (UT): University of Utah Health Sciences Center Copyright: © 2024 Webvision. 1995.
- 26.Yu DY, Cringle SJ, Balaratnasingam C, Morgan WH, Yu PK, Su EN. Retinal ganglion cells: energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res. 2013;36:217–46. 10.1016/j.preteyeres.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Andrews RM, Griffiths PG, Johnson MA, Turnbull DM. Histochemical localisation of mitochondrial enzyme activity in human optic nerve and retina. Br J Ophthalmol. 1999;83(2):231–5. 10.1136/bjo.83.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minckler DS, McLean IW, Tso MO. Distribution of axonal and glial elements in the rhesus optic nerve head studied by electron microscopy. Am J Ophthalmol. 1976;82(2):179–87. 10.1016/0002-9394(76)90416-5 [DOI] [PubMed] [Google Scholar]
- 29.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. 10.1016/j.preteyeres.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 30.Wilkison SJ, Bright CL, Vancini R, Song DJ, Bomze HM, Cartoni R. Local Accumulation of Axonal Mitochondria in the Optic nerve glial Lamina precedes myelination. Front Neuroanat. 2021;15:678501. 10.3389/fnana.2021.678501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muench NA, Patel S, Maes ME, Donahue RJ, Ikeda A, Nickells RW. The Influence of Mitochondrial Dynamics and function on retinal ganglion cell susceptibility in Optic nerve disease. Cells. 2021;10(7). [DOI] [PMC free article] [PubMed]
- 32.Kim KY, Perkins GA, Shim MS, Bushong E, Alcasid N, Ju S, et al. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015;6(8):e1839. 10.1038/cddis.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ongwijitwat S, Wong-Riley MT. Is nuclear respiratory factor 2 a master transcriptional coordinator for all ten nuclear-encoded cytochrome c oxidase subunits in neurons? Gene. 2005;360(1):65–77. 10.1016/j.gene.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 34.Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88(Pt B):179–88. 10.1016/j.freeradbiomed.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Li Y, Wang J, Zhang D, Wu H, Li W, et al. Mitophagy receptor FUNDC1 is regulated by PGC-1α/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 2021;22(3):e50629. 10.15252/embr.202050629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. 10.1042/bse0470069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA. 1994;91(4):1309–13. 10.1073/pnas.91.4.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial Biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. 2019;10:435. 10.3389/fgene.2019.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2 + in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB Journal: Official Publication Federation Am Soc Experimental Biology. 2003;17(6):675–81. 10.1096/fj.02-0951com [DOI] [PubMed] [Google Scholar]
- 40.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282(26):18793–9. 10.1074/jbc.M611252200 [DOI] [PubMed] [Google Scholar]
- 41.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA. 2003;100(12):7111–6. 10.1073/pnas.1232352100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, et al. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO Signaling. Cell. 2013;154(2):430–41. 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–22. 10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 44.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313–9. 10.1038/nature08991 [DOI] [PubMed] [Google Scholar]
- 45.Mercy L, Pauw A, Payen L, Tejerina S, Houbion A, Demazy C, et al. Mitochondrial biogenesis in mtDNA-depleted cells involves a Ca2+-dependent pathway and a reduced mitochondrial protein import. FEBS J. 2005;272(19):5031–55. 10.1111/j.1742-4658.2005.04913.x [DOI] [PubMed] [Google Scholar]
- 46.Li Y, He L, Zeng N, Sahu D, Cadenas E, Shearn C, et al. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling regulates mitochondrial biogenesis and respiration via estrogen-related receptor α (ERRα). J Biol Chem. 2013;288(35):25007–24. 10.1074/jbc.M113.450353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Signorile A, Micelli L, De Rasmo D, Santeramo A, Papa F, Ficarella R, et al. Regulation of the biogenesis of OXPHOS complexes in cell transition from replicating to quiescent state: involvement of PKA and effect of hydroxytyrosol. Biochim Biophys Acta. 2014;1843(4):675–84. 10.1016/j.bbamcr.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 48.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Sci (New York NY). 2003;299(5608):896–9. 10.1126/science.1079368 [DOI] [PubMed] [Google Scholar]
- 49.Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB Journal: Official Publication Federation Am Soc Experimental Biology. 2006;20(11):1889–91. 10.1096/fj.05-5189fje [DOI] [PubMed] [Google Scholar]
- 50.Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol. 1996;135(4):883–93. 10.1083/jcb.135.4.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4(3):413–9. 10.1016/j.celrep.2013.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amiri M, Hollenbeck PJ. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol. 2008;68(11):1348–61. 10.1002/dneu.20668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gale JR, Aschrafi A, Gioio AE, Kaplan BB. Nuclear-encoded mitochondrial mRNAs: a powerful force in Axonal Growth and Development. The neuroscientist: a review journal bringing neurobiology. Neurol Psychiatry. 2018;24(2):142–55. [DOI] [PubMed] [Google Scholar]
- 54.Van Laar VS, Arnold B, Howlett EH, Calderon MJ, St Croix CM, Greenamyre JT, et al. Evidence for compartmentalized axonal mitochondrial Biogenesis: mitochondrial DNA replication increases in distal axons as an early response to Parkinson’s disease-relevant stress. J Neuroscience: Official J Soc Neurosci. 2018;38(34):7505–15. 10.1523/JNEUROSCI.0541-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shigeoka T, Jung H, Jung J, Turner-Bridger B, Ohk J, Lin JQ, et al. Dynamic Axonal Translation Developing Mature Visual Circuits Cell. 2016;166(1):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung J, Ohk J, Kim H, Holt CE, Park HJ, Jung H. mRNA transport, translation, and decay in adult mammalian central nervous system axons. Neuron. 2023;111(5):650–e684. 10.1016/j.neuron.2022.12.015 [DOI] [PubMed] [Google Scholar]
- 57.Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148(4):752–64. 10.1016/j.cell.2011.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuzniewska B, Cysewski D, Wasilewski M, Sakowska P, Milek J, Kulinski TM, et al. Mitochondrial protein biogenesis in the synapse is supported by local translation. EMBO Rep. 2020;21(8):e48882. 10.15252/embr.201948882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melkov A, Abdu U. Regulation of long-distance transport of mitochondria along microtubules. Cell Mol Life Sci. 2018;75(2):163–76. 10.1007/s00018-017-2590-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17(4):2057–68. 10.1091/mbc.e05-06-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neuroscience: Official J Soc Neurosci. 2010;30(26):8984–92. 10.1523/JNEUROSCI.1621-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takihara Y, Inatani M, Eto K, Inoue T, Kreymerman A, Miyake S, et al. In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS. Proc Natl Acad Sci USA. 2015;112(33):10515–20. 10.1073/pnas.1509879112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vevea JD, Chapman ER. Mitofusin 2 sustains the Axonal Mitochondrial Network to support presynaptic ca(2+) homeostasis and the synaptic vesicle cycle in rat hippocampal axons. J Neuroscience: Official J Soc Neurosci. 2023;43(19):3421–38. 10.1523/JNEUROSCI.1356-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA. 2008;105(52):20728–33. 10.1073/pnas.0808953105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136(1):163–74. 10.1016/j.cell.2008.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61(4):541–55. 10.1016/j.neuron.2009.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278(7):5399–409. 10.1074/jbc.M209384200 [DOI] [PubMed] [Google Scholar]
- 68.Sermikli BP, Aydogdu G, Yilmaz E. Role of the O-GlcNAc modification on insulin resistance and endoplasmic reticulum stress in 3T3-L1 cells. Mol Biol Rep. 2020;47(8):5927–42. 10.1007/s11033-020-05665-3 [DOI] [PubMed] [Google Scholar]
- 69.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158(1):54–68. 10.1016/j.cell.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214(1):103–19. 10.1083/jcb.201605101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Sci (New York NY). 2004;305(5691):1747–52. 10.1126/science.1100612 [DOI] [PubMed] [Google Scholar]
- 72.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26(2):211–5. 10.1038/79944 [DOI] [PubMed] [Google Scholar]
- 73.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–32. 10.1091/mbc.e09-03-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10(12):4403–17. 10.1091/mbc.10.12.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osellame LD, Singh AP, Stroud DA, Palmer CS, Stojanovski D, Ramachandran R, et al. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J Cell Sci. 2016;129(11):2170–81. 10.1242/jcs.185165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamerkar SC, Kraus F, Sharpe AJ, Pucadyil TJ, Ryan MT. Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat Commun. 2018;9(1):5239. 10.1038/s41467-018-07543-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris JJ, Jolivet R, Attwell D. Synaptic Energy Use and Supply. Neuron. 2012;75(5):762–77. 10.1016/j.neuron.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 78.Cheng XT, Huang N, Sheng ZH. Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron. 2022;110(12):1899–923. 10.1016/j.neuron.2022.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sulkshane P, Ram J, Thakur A, Reis N, Kleifeld O, Glickman MH. Ubiquitination and receptor-mediated mitophagy converge to eliminate oxidation-damaged mitochondria during hypoxia. Redox Biol. 2021;45. [DOI] [PMC free article] [PubMed]
- 80.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Et Biophys Acta-Bioenergetics. 2010;1797(6–7):1171–7. 10.1016/j.bbabio.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 81.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20(9):1013–22. 10.1038/s41556-018-0176-2 [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Liu N, Lu B. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci Ther. 2019;25(7):859–75. 10.1111/cns.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryu S-W, Han EC, Yoon J, Choi C. The mitochondrial Fusion-related proteins Mfn2 and OPA1 are Transcriptionally Induced during differentiation of bone marrow progenitors to immature dendritic cells. Mol Cells. 2015;38(1):89–94. 10.14348/molcells.2015.2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khalil B, El Fissi N, Aouane A, Cabirol-Pol MJ, Rival T, Lievens JC. PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease. Cell Death Dis. 2015;6. [DOI] [PMC free article] [PubMed]
- 85.Goldsmith J, Ordureau A, Harper JW, Holzbaur ELF. Brain-derived autophagosome profiling reveals the engulfment of nucleoid-enriched mitochondrial fragments by basal autophagy in neurons. Neuron. 2024;112(3):520. 10.1016/j.neuron.2024.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maday S, Holzbaur EL. Autophagosome assembly and cargo capture in the distal axon. Autophagy. 2012;8(5):858–60. 10.4161/auto.20055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei HF, Liu L, Chen Q. Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim Et Biophys Acta-Molecular Cell Res. 2015;1853(10):2784–90. 10.1016/j.bbamcr.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 88.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harbauer AB, Schwarz TL. Mitochondrial hitch-hiking of Pink1 mRNA supports axonal mitophagy. Autophagy. 2022;18(12):3048–9. 10.1080/15548627.2022.2070332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6(8):1090–106. 10.4161/auto.6.8.13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–14. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–6. 10.1038/nature12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205(2):143–53. 10.1083/jcb.201402104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. 10.1038/cdd.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci USA. 2014;111(26):9633–8. 10.1073/pnas.1404651111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burdett TC, Freeman MR. Neuroscience. Astrocytes eyeball axonal mitochondria. Sci (New York NY). 2014;345(6195):385–6. 10.1126/science.1258295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danese A, Patergnani S, Maresca A, Peron C, Raimondi A, Caporali L, et al. Pathological mitophagy disrupts mitochondrial homeostasis in Leber’s hereditary optic neuropathy. Cell Rep. 2022;40(3):111124. 10.1016/j.celrep.2022.111124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lopez Sanchez MI, Crowston JG, Mackey DA, Trounce IA. Emerging mitochondrial therapeutic targets in Optic neuropathies. Pharmacol Ther. 2016;165:132–52. 10.1016/j.pharmthera.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 101.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31(6):702–19. 10.1016/j.preteyeres.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biology: CB. 2012;22(6):545–52. 10.1016/j.cub.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shim MS, Takihara Y, Kim KY, Iwata T, Yue BY, Inatani M, et al. Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci Rep. 2016;6:33830. 10.1038/srep33830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chi ZL, Akahori M, Obazawa M, Minami M, Noda T, Nakaya N, et al. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum Mol Genet. 2010;19(13):2606–15. 10.1093/hmg/ddq146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tseng HC, Riday TT, McKee C, Braine CE, Bomze H, Barak I, et al. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol Aging. 2015;36(6):2201–12. 10.1016/j.neurobiolaging.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chalasani ML, Kumari A, Radha V, Swarup G. E50K-OPTN-induced retinal cell death involves the Rab GTPase-activating protein, TBC1D17 mediated block in autophagy. PLoS ONE. 2014;9(4):e95758. 10.1371/journal.pone.0095758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1(1):17–26. 10.1371/journal.pgen.0010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang B, Huang M, Shang D, Yan X, Zhao B, Zhang X. Mitochondrial behavior in Axon Degeneration and Regeneration. Front Aging Neurosci. 2021;13:650038. 10.3389/fnagi.2021.650038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pozo Devoto VM, Onyango IG, Stokin GB. Mitochondrial behavior when things go wrong in the axon. Front Cell Neurosci. 2022;16:959598. 10.3389/fncel.2022.959598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin MY, Cheng XT, Xie Y, Cai Q, Sheng ZH. Removing dysfunctional mitochondria from axons independent of mitophagy under pathophysiological conditions. Autophagy. 2017;13(10):1792–4. 10.1080/15548627.2017.1356552 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.