Abstract
Background
Cardiovascular disease (CVD) is the most prevalent complication of type 2 diabetes with an estimated 65% of people with type 2 diabetes dying from a cause related to atherosclerosis. Adenosine‐diphosphate (ADP) receptor antagonists like clopidogrel, ticlopidine, prasugrel and ticagrelor impair platelet aggregation and fibrinogen‐mediated platelet cross‐linking and may be effective in preventing CVD.
Objectives
To assess the effects of adenosine‐diphosphate (ADP) receptor antagonists for the prevention of cardiovascular disease in type 2 diabetes mellitus.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (issue 2, 2011), MEDLINE (until April 2011) and EMBASE (until May 2011). We also performed a manual search, checking references of original articles and pertinent reviews to identify additional studies.
Selection criteria
Randomised controlled trials comparing an ADP receptor antagonist with another antiplatelet agent or placebo for a minimum of 12 months in patients with diabetes. In particular, we looked for trials assessing clinical cardiovascular outcomes.
Data collection and analysis
Two review authors extracted data for studies which fulfilled the inclusion criteria, using standard data extraction templates. We sought additional unpublished information and data from the principal investigators of all included studies.
Main results
Eight studies with a total of 21,379 patients with diabetes were included. Three included studies investigated ticlopidine compared to aspirin or placebo. Five included studies investigated clopidogrel compared to aspirin or a combination of aspirin and dipyridamole, or compared clopidogrel in combination with aspirin to aspirin alone. All trials included patients with previous CVD except the CHARISMA trial which included patients with multiple risk factors for coronary artery disease. Overall the risk of bias of the trials was low. The mean duration of follow‐up ranged from 365 days to 913 days.
Data for diabetes patients on all‐cause mortality, vascular mortality and myocardial infarction were only available for one trial (355 patients). This trial compared ticlopidine to placebo and did not demonstrate any statistically significant differences for all‐cause mortality, vascular mortality or myocardial infarction. Diabetes outcome data for stroke were available in three trials (31% of total diabetes participants). Overall pooling of two (statistically heterogeneous) studies showed no statistically significant reduction in the combination of fatal and non‐fatal stroke (359/3194 (11.2%) versus 356/3146 (11.3%), random effects odds ratio (OR) 0.81; 95% confidence interval (CI) 0.44 to 1.49) for ADP receptor antagonists versus other antiplatelet drugs. There were no data available from any of the trials on peripheral vascular disease, health‐related quality of life, adverse events specifically for patients with diabetes, or costs.
Authors' conclusions
The available evidence for ADP receptor antagonists in patients with diabetes mellitus is limited and most trials do not report outcomes for patients with diabetes separately. Therefore, recommendations for the use of ADP receptor antagonists for the prevention of CVD in patients with diabetes are based on available evidence from trials including patients with and without diabetes. Trials with diabetes patients and subgroup analyses of patients with diabetes in trials with combined populations are needed to provide a more robust evidence base to guide clinical management in patients with diabetes.
Keywords: Humans; Aspirin; Aspirin/therapeutic use; Cardiovascular Diseases; Cardiovascular Diseases/prevention & control; Clopidogrel; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Dipyridamole; Dipyridamole/therapeutic use; Drug Therapy, Combination; Drug Therapy, Combination/methods; Platelet Aggregation Inhibitors; Platelet Aggregation Inhibitors/therapeutic use; Purinergic P2Y Receptor Antagonists; Purinergic P2Y Receptor Antagonists/therapeutic use; Randomized Controlled Trials as Topic; Ticlopidine; Ticlopidine/analogs & derivatives; Ticlopidine/therapeutic use
Plain language summary
Adenosine‐diphosphate (ADP) receptor antagonists for the prevention of cardiovascular disease in patients with type 2 diabetes mellitus
Patients with type 2 diabetes have a much higher risk of strokes and heart attacks than the general population. Most strokes and heart attacks are caused by blood clots. Adenosine‐diphosphate (ADP) receptor antagonists are drugs which prevent the aggregation ('clumping') of platelets and consequently reduce the formation of blood clots. These medications are used to prevent cardiovascular disease such as heart attacks and strokes in the general population. This review assessed if these medications would be useful in patients with diabetes. We included eight trials with 21,379 patients and a mean duration of follow‐up ranging from 365 to 913 days. Specific data for patients with diabetes were only available in full for one of these trials and partial data were available for two trials. Analysis of the available data demonstrated that adenosine‐diphosphate receptor antagonists (such as clopidogrel, prasugrel, ticagrelor, ticlopidine) were not more effective than other blood thinning drugs or placebo for death from any cause, death related to cardiovascular disease, heart attacks or strokes. There was no available information on the effects of adenosine‐diphosphate receptor antagonists on health‐related quality of life, adverse effects specially for people with diabetes, or costs. The use of adenosine‐diphosphate receptor antagonists in patients with diabetes needs to be guided by the information available from trials which included patients with and without diabetes. All future trials on adenosine‐diphosphate receptor antagonists should include data which relate specifically to patients with diabetes in order to inform evidence‐based clinical guidelines.
Summary of findings
for the main comparison.
| Adenosine‐diphosphate (ADP) receptor antagonists (clopidogrel, ticlopidine, prasugrel, ticagrelor) for the prevention of cardiovascular disease in type 2 diabetes mellitus | ||||||
|
Patient or population: patients with type 2 diabetes mellitus Settings: out‐patients Intervention: ADP receptor antagonists Comparison: Aspirin (/dipyramidole)/placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ADP receptor antagonist | |||||
|
All‐cause mortality (ticlopidine vs placebo) |
14.1% | 17.4% | RR 1.29 (0.71 to 2.32) | 335 (1) | moderate quality | Seea |
| Vascular mortality (ticlopidine vs placebo) | 11% | 10.5% | RR 0.94 (0.47 to 1.88) | 335 (1) | moderate quality | Seea |
| Myocardial infarction (fatal and non‐fatal) (ticlopidine vs placebo) | 11.7% | 9.3% | RR 0.78 (0.39 to 1.57) | 335 (1) | moderate quality | Seea |
| Stroke (fatal and non‐fatal) (a. ticlopidine vs aspirin) (b. clopidogrel vs aspirin & dipyramidole) | a. 0.14% b. 0.11% |
a. 0.9% b. 0.12% |
a. RR 0.56 (0.37 to 0.94) b. RR 1.12 (0.95 ‐ 1.32) |
a. 597 (1) b. 5770 (1) |
a. moderate quality b. moderate quality |
a. Seea b. Seeb |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated for diabetes sub‐population |
| Adverse effects | See comment | See comment | Not estimable | See comment | See comment | Not investigated for diabetes sub‐population |
| Costs | See comment | See comment | Not estimable | See comment | See comment | Not investigated for diabetes sub‐population |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOnly one study with small number of participants providing data
bUnclear random sequence generation and high attrition rates
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About', 'Cochrane Review Groups (CRGs)'). For an explanation of methodological terms, see the main glossary in The Cochrane Library.
Diabetes, cardiovascular disease and the role of platelets
The most prevalent complication of type 2 diabetes mellitus is cardiovascular disease (CVD). It is estimated that 65% of patients with type 2 diabetes die of a cause related to atherosclerosis (Gu 1998). Risk of mortality and morbidity due to myocardial infarction, stroke and peripheral arterial disease are two‐ to four‐fold increased compared with the general population. Once diagnosed with having diabetes, overall survival is comparable to a patient with a prior myocardial infarction (Haffner 1998).
Given the upcoming pandemic of patients with diabetes, world wide prevalence is expected to increase from 2.8% in 2000 to 4.4% in 2030 (Wild 2004), cardiovascular disease will continue to be responsible for a large proportion of global healthcare expenditures. This stresses the need for preventive measures. In the development of a cardiovascular event, platelets play an important role. Platelet adhesion and subsequent activation on dysfunctional endothelium fuel the inflammatory process in the atherosclerotic plaque. After disruption of the plaque, large aggregates of platelets lead to vessel occlusion. In the patient with diabetes, anti‐aggregatory mechanisms in the endothelium are impaired (Ceriello 2004). Platelets are more susceptible to activation and aggregation (Kutti 1986) and antifibrinolytic activity is attenuated (Colwell 2003).
Description of the intervention
Adenosine‐diphosphate (ADP) is a platelet activator that is released from red blood cells, activated platelets and damaged endothelial cells which induces platelet adhesion and aggregation (Kam 2003). ADP receptor antagonists noncompetitively and irreversibly inhibit platelet ADP receptors preventing platelet activation. This prevents the activations of the glycoprotein IIb/IIIa receptor complex and prolongs bleeding time, impairs platelet aggregation and fibrinogen‐mediated platelet cross‐linking (Goodwin 2011). The result is a 50% to 70% inhibition of platelet fibrinogen binding achieved after three to five days (Kam 2003).
Adverse effects of the intervention
Gastrointestinal side effects and skin rashes are common. Neutropenia and thrombotic thrombocytopenic purpura are significant but rarely fatal adverse events. Recovery of platelet function is delayed after discontinuation of the medication for approximately three to seven days (Kam 2003). In terms of cost, ADP receptor antagonists are considerably more expensive than aspirin.
How the intervention might work
There are clues that ADP receptor antagonists are superior to aspirin in preventing cardiovascular disease. In a Cochrane review, clopidogrel and ticlopidine were slightly, but significantly, more effective than aspirin in preventing serious vascular events in high‐risk patients (previous clinical manifestations of cardiovascular disease) (Sudlow 2009). Other evidence was derived from an analysis of the diabetic subgroup in the CAPRIE‐study. In that trial, 15.6% of patients with diabetes experienced a vascular endpoint versus 17.7% in the aspirin group (P = 0.042). In addition, patients in the clopidogrel group had less bleeding events: 1.8% versus 2.8% in the aspirin group (P = 0.031) (Bhatt 2002).
Why it is important to do this review
Despite the evidence for possible advantages, the role of ADP receptor antagonists in the treatment of patients with diabetes seems to be limited. A recent position statement by the American Diabetes Association suggested the use of clopidogrel only in case of allergy for aspirin or in combination therapy with aspirin for one year after acute coronary syndrome (ADA 2011). The high costs of ADP receptor antagonists seem to be the major reason for the modest position these drugs have in treatment strategies. Evidence from systematic reviews and meta‐analyses is lacking for ADP receptor antagonists in patients with diabetes. To value the use of these agents in the treatment of patients with diabetes, a well‐designed systematic review is needed.
Objectives
To assess the effects of adenosine‐diphosphate (ADP) receptor antagonists for the prevention of cardiovascular disease in type 2 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials comparing an adenosine‐diphosphate (ADP) receptor antagonist with another antiplatelet agent or placebo for a minimum of 12 months. Trials with blinded trial participants and investigators were preferred, however, single‐blind and unblinded trials were considered. We included studies published in any language.
Types of participants
Eligible patients were adults (18 years and older) with diabetes mellitus. Trials which used the current standard criteria to establish the diagnosis of diabetes at the time of the trial were preferred, however, we accepted authors' definitions (ADA 1997; ADA 1999; WHO 1980; WHO 1985; WHO 1998).
Types of interventions
Intervention
Any orally administered ADP receptor antagonist given on a continuing basis.
Control
Placebo, another antiplatelet agent or no treatment.
Types of outcome measures
Primary outcomes
Mortality
All‐cause mortality.
Mortality related to cardiovascular events (death from myocardial infarction, stroke, peripheral vascular disease, or sudden death).
Cardiovascular events
Myocardial infarction (fatal and non‐fatal).
Stroke (fatal and non‐fatal).
Peripheral arterial disease.
Secondary outcomes
Adverse effects, such as any major bleeding event, defined as intracranial bleeding or bleeding requiring surgical intervention or transfusion.
Health‐related quality of life, ideally measured with a validated instrument.
Costs.
Search methods for identification of studies
Electronic searches
The following sources were used from inception to specified time for the identification of trials.
The Cochrane Library (issue 2, 2011).
MEDLINE (until April 2011).
EMBASE (until May 2011).
We also searched databases of ongoing trials (http://www.controlled‐trials.com/ with links to several databases and https://www.clinicaltrialsregister.eu/).
For detailed search strategies please see under Appendix 1.
No additional key words of relevance were detected during any of the electronic or other searches. Thus, the electronic search strategies were not modified. Studies published in any language were included.
Searching other resources
We sought additional unpublished information and data from the principal investigators of all included trials. We performed an extensive manual search, checking references of original articles and pertinent reviews to identify additional studies.
Data collection and analysis
Selection of studies
Two review authors (NV and MVD) independently scanned the titles and abstracts from the original search. If inadequate information was provided in the abstract to determine eligibility, the full text of the article was obtained. Any disagreement was resolved through discussion between the reviewers. In studies which did not report detailed subgroup data about patients with diabetes, we contacted the principal investigators for additional information. An adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection (Figure 1) (Liberati 2009) is attached.
1.
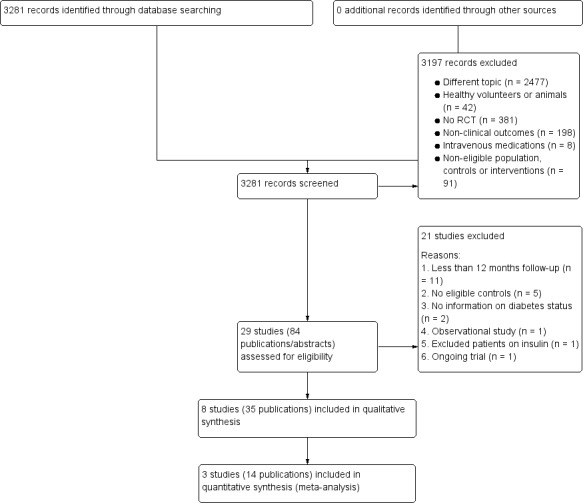
Study flow diagram.
Data extraction and management
For studies that fulfilled the inclusion criteria, we extracted relevant population and intervention characteristics using standard data extraction templates (for details see 'Characteristics of included studies; Table 2; Appendix 2; Appendix 3; Appendix 4).
1. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and control(s) | [n] screened | [n] randomised | [n] ITT | [n] finishing study | [%] of randomised participants finishing study | |
| AAASPS | I(d): Ticlopidine 250 mg bd (diabetes patients) C(d): Aspirin 325 mg bd (diabetes patients) I(t): Ticlopidine 250 mg bd (all patients) C(t): Aspirin 325 mg bd (all patients) |
I‐ | I(d): 359 C(d): 379 I(t): 902 C(t): 907 |
I(d): 359 C(d): 379 I(t): 902 C(t): 907 |
I(d): ‐ C(d): ‐ I(t): 626 C(t): 661 |
I(d): ‐ C(d): ‐ I(t): 69.4 C(t): 72.9 |
|
| CAPRIE | I(d): Clopidogrel 75 mg daily (diabetes patients) C(d): Aspirin 325 mg daily (diabetes patients) I(t): Clopidogrel 75 mg daily (all patients) C(t): Aspirin 325 mg daily (all patients) |
‐ | I(d): 20% C(d): 20% I(t): 9599 C(t): 9586 |
I(d): 20% (1920) C(d): 20% (1917) I(t): 9599 C(t): 9586 |
I(d): ‐ C(d): ‐ I(t): 9577 C(t): 9566 |
I(d): ‐ C(d): ‐ I(t): 99.8 C(t): 99.8 |
|
| CATS | I(d): Ticlopidine 250 mg bd (diabetes patients) C(d): Placebo (diabetes patients) I(t): Ticlopidine 250 mg bd (all patients) C(t): Placebo (all patients) |
‐ | I(d): 172 C(d): 163 I(t): 525 C(t): 528 |
I(d): 172 C(d): 163 I(t): 519 C(t): 515 |
I(d): ‐ C(d): ‐ I(t): 287 C(t): 364 |
I(d): ‐ C(d): ‐ I(t): 54.7 C(t): 68.9 |
|
| CHARISMA | I(d): Clopidogrel 75 mg daily and aspirin 75 to 162 mg daily (diabetes patients) C(d): Aspirin 75 to 162 mg daily (diabetes patients) I(t): Clopidogrel 75 mg daily and aspirin 75 to 162 mg daily (all patients) C(t): Aspirin 75 to 162 mg daily (all patients) |
‐ | I(d): 3304 C(d): 3252 I(t): 7802 C(t): 7801 |
I(d): 3304 C(d): 3252 I(t): 7802 C(t): 7801 |
I(d): ‐ C(d): ‐ I(t): 79.6% C(t): 81.8% |
I(d): ‐ C(d): ‐ I(t): 79.6 C(t): 81.8 |
|
| CURE | I(d): Clopidogrel 75 mg daily and aspirin 75 to 300 mg daily (diabetes patients) C(d): Aspirin 75 to 300 mg daily (diabetes patients) I(t): Clopidogrel 75 mg daily and aspirin 75 to 300 mg daily (all patients) C(t): Aspirin 75 to 300 mg daily (all patients) |
‐ | I(d): 1405 C(d): 1435 I(t): 6259 C(t): 6303 |
I(d): 1405 C(d): 1435 I(t): 6259 C(t): 6303 |
I(d): ‐ C(d): ‐ I(t): 78.9% C(t): 81.2% |
I(d): ‐ C(d): ‐ I(t): 78.9 C(t): 81.2 |
|
| Park | I(d): Clopidogrel 75 mg daily and aspirin 100 to 200 mg daily (diabetes patients) C(d): Aspirin 100 to 200 mg daily (diabetes patients) I(t): Clopidogrel 75 mg daily and aspirin 100 to 200 mg daily (all patients) C(t): Aspirin 100 to 200 mg daily (all patients) |
‐ | I(d): 340 C(d): 364 I(t): 1357 C(t): 1344 |
‐ | I(d): ‐ C(d): ‐ I(t): 327 C(t): 313 |
‐ | |
| PRoFESS | I(d): Clopidogrel 75 mg daily (diabetes patients) C(d): Aspirin/dipyridamole 25 mg/200 mg bd (diabetes patients) I(t): Clopidogrel 75 mg daily (all patients) C(t): Aspirin/dipyridamole 25 mg/200 mg bd (all patients) |
‐ | I(d): 2840 C(d): 2903 I(t): 10.151 C(t): 10.181 |
I(d): 2840 C(d): 2903 I(t): 10.151 C(t): 10.181 |
I(d): ‐ C(d): ‐ I(t): 7736 C(t): 7095 |
I(d): ‐ C(d): ‐ I(t): 76.2 C(t): 69.7 |
|
| TASS | I(d): Ticlopidine 250 mg bd (diabetes patients) C(d): Aspirin 650 mg bd (diabetes patients) I(t): Ticlopidine 250 mg bd (all patients) C(t): Aspirin 650 mg bd (all patients) |
‐ | I(d): 291 C(d): 306 I(t): 1529 C(t): 1540 |
I(d): ‐ C(d): ‐ I(t): 1529 C(t): 1540 |
I(d): ‐ C(d): ‐ I(t): 828 C(t): 941 |
I(d): ‐ C(d): ‐ I(t): 54.2 C(t): 61.1 |
|
| Total | All interventions (diabetes patients) | 8711 (and CAPRIE participants) | |||||
| All controls (diabetes patients) | 8802 (and CAPRIE participants) | ||||||
| All interventions (all patients) | 38,124 | ||||||
| All controls (all patients) | 38,190 | ||||||
| All interventions and controls (diabetes patients) | 21,379 | ||||||
| All interventions and controls (all patients) | 76,314 |
"‐" denotes not reported
Abbreviations:
bd: twice daily; C: control; C(d): intervention (diabetes patients); C(t): intervention (all patients); I: intervention; I(d): intervention (diabetes patients); I(t): intervention (all patients); ITT: intention‐to‐treat
Assessment of risk of bias in included studies
Two authors (NV and MVD) assessed each trial independently. Disagreements were resolved by consensus, or with consultation of a third party.
We assessed risk of bias using The Cochrane Collaboration’s tool (Higgins 2011). We used the following risk of bias criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A 'Risk of bias' figure (Figure 2) and a 'Risk of bias summary' figure (Figure 3) are attached.
2.
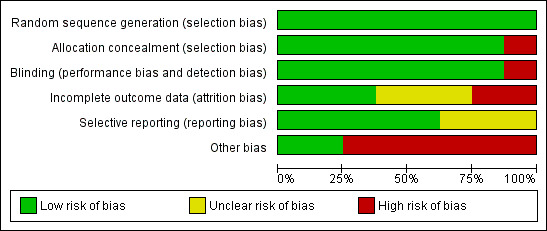
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
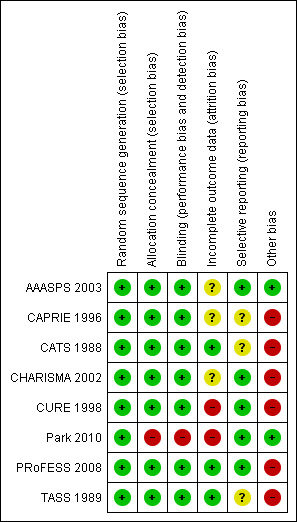
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We assessed the impact of individual bias domains on study results at endpoint and study levels.
Measures of treatment effect
Dichotomous data are expressed as odds ratios (OR) with 95% confidence intervals (CI). We planned to calculate the risk difference (RD) and convert it into the number needed to treat to benefit (NNTB) or the number needed to treat to harm (NNTH) taking into account the time of follow‐up if the overall estimate of effect was statistically significant.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. However, no such trial designs were included in this review.
Dealing with missing data
We attempted to obtain relevant missing data from authors and carefully performed evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) population. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals and critically appraised issues of missing data and imputation methods (for example, last‐observation‐carried‐forward (LOCF)).
Assessment of heterogeneity
As there was substantial clinical heterogeneity, study results were only reported as meta‐analytically pooled effect estimates for stroke (fatal and non‐fatal). We identified heterogeneity by visual inspection of the forest plots, by using a standard Chi2 test and a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity with the I2 statistic quantifying inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% and more indicates a considerable level of inconsistency (Higgins 2011). In the presence of considerable statistical heterogeneity we used a random‐effects model for pooling.
Assessment of reporting biases
If we had identified more than 10 RCTs, we planned to use funnel plots to assess for the potential existence of small study bias. There are a number of explanations for the asymmetry of a funnel plot (Sterne 2001) and we planned to carefully interpret results (Lau 2006).
Data synthesis
We summarised data statistically if they were available, sufficiently similar and of sufficient quality. We performed statistical analyses according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The table of comparisons was divided into all possible outcomes (e.g. all‐cause mortality, disease specific death). It was not possible to subdivide into different dosages within the outcome subgroups as these data were not available.
Subgroup analysis and investigation of heterogeneity
No further subgroup analyses were performed.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on the effect estimates.
Restricting the analysis to published studies.
Restricting the analysis taking account of risk of bias, as specified above.
Restricting the analysis to very long or large studies in order to establish how much they dominated the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We planned to test the robustness of the results by repeating the analysis using different measures of effect estimates (relative risk, odds ratio etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For details see Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
Our search identified a total of 3281 references. We excluded 2477 references because they were on a different topic, 42 were conducted on healthy volunteers or animals and 381 were not randomised controlled trials. A further 198 studies were excluded as they used non‐clinical outcomes, eight used intravenous medications and 91 used non‐eligible populations, controls or interventions. The remaining 84 abstracts were reviewed for duplication and 29 separate trials were identified. We obtained full text papers for these 29 trials. Of these, 20 were excluded (11 because the follow‐up was less than 12 months, five did not have eligible controls, two did not collect information about diabetes status, one was observational and one excluded patients on insulin). One ongoing trial was identified (SPS3 2011) leaving eight included studies. There were 35 references identified in the search referring to these eight included studies. For details please see Figure 1.
Included studies
We identified a total of eight completed randomised trials which met the inclusion criteria (AAASPS 2003; CAPRIE 1996; CATS 1988; CHARISMA 2002; CURE 1998; Park 2010; PRoFESS 2008; TASS 1989). The oldest study was completed in 1988 (CATS 1988) and the most recent study was completed in 2010 (Park 2010). A total of 21,379 patients with diabetes participated in the eight studies. The mean duration of follow‐up was 913 days (range 365 days to 2373 days).
Three of the included studies investigated ticlopidine (AAASPS 2003; CATS 1988; TASS 1989). Ticlopidine was compared to aspirin in two trials: AAASPS 2003 which included African American participants with a recent history of stroke, and TASS 1989 which included patients with a neurological deficit. The third study (CATS 1988) compared ticlopidine to placebo in patients with a thromboembolic stroke.
Five included studies investigated clopidogrel. One study compared clopidogrel to aspirin in 3866 diabetes patients with ischaemic stroke, myocardial infarction or peripheral arterial disease (CAPRIE 1996). The PRoFESS 2008 study compared clopidogrel to aspirin and dipyridamole in 20,332 patients with ischaemic stroke, of which 5473 patients had diabetes. The remaining three studies compared clopidogrel and aspirin to aspirin alone in a total of 10,099 patients with diabetes; of whom 6555 patients had multiple risk factors for coronary artery disease (CHARISMA 2002), 2840 patients had unstable angina (CURE 1998) and 704 patients had undergone insertion of drug eluding stents 12 months prior (Park 2010).
All trials investigating clopidogrel used a dose of 75 mg daily and all ticlopidine trials used a dose of 250 mg twice daily. The dose of aspirin ranged from 75 mg daily to 650 mg twice daily. All trials recorded all‐cause mortality, vascular mortality, myocardial infarction and stroke except two (Park 2010; PRoFESS 2008). Only three trials contributed to the main primary outcome as diabetes data were not available for the other five trials. See Characteristics of included studies for details of each trial.
The CAPRIE 1996 trial classified patients as having diabetes if defined as such by the investigator. No other studies provided information on how the diagnosis of diabetes was made for patients classified with diabetes.
Excluded studies
The full text of 21 potential studies was reviewed and excluded from this study. Further information is available in Characteristics of excluded studies and Characteristics of ongoing studies.
Risk of bias in included studies
The risk of bias assessment is demonstrated in Figure 2 and Figure 3.
Random sequence generation (selection bias)
All included studies used and reported an appropriate method of randomisation, most commonly computer generated randomisation.
Allocation
Seven of the eight trials used and reported appropriate methods of allocation concealment. Park 2010 was an open label trial with study participants and investigators aware of the treatment assignments.
Blinding
All of the included trials were double‐blinded, except for the Park 2010 trial in which patients were asked at every visit which medication they were taking. Outcomes were assessed as per standard criteria; however, the assessors knew which medication patients were taking and thus outcome assessment was not blinded either.
Incomplete outcome data
The number of participants lost to follow‐up in the CHARISMA trial was not reported (CHARISMA 2002). All other trials reported their loss to follow‐up rate. There was a similar withdrawal rate ‐ approximately 20% ‐ in three trials (AAASPS 2003; CAPRIE 1996; CHARISMA 2002). The withdrawal rate of Park 2010 was not clearly reported, although it appeared to be over half of the patients. The withdrawal rate in CURE 1998 was also very high (approximately 45%).
Selective reporting
The five most recent trials have available published protocols or registered trial documents. The oldest three trials (CAPRIE 1996; CATS 1988; TASS 1989), do not have previously published protocols with outcomes and so risk of selective reporting is unknown.
Other potential sources of bias
All trials except Park 2010 received funding from the pharmaceutical companies who developed and sold adenosine‐diphosphate (ADP) receptor antagonists. The role of the pharmaceutical funding for the AAASPS 2003 trial was reported to be limited to supplying medications only.
Effects of interventions
See: Table 1
Unpublished data were sought from all trials, however, additional unpublished information was only provided by the CATS trial. The results presented are derived from data extracted from three trials (CATS 1988; PRoFESS 2008; TASS 1989) including unpublished data provided for the CATS trial. No specific data on patients with diabetes were available for any of the other trials. For details see Appendix 3.
Primary outcomes
Mortality
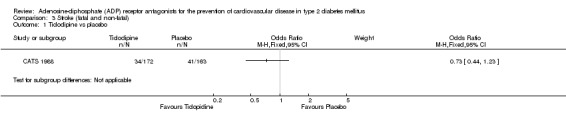
All‐cause mortality and vascular mortality data were only available for one trial (CATS 1988). This trial included 335 patients with diabetes (1.5% of the total diabetes participants included in this review). In this trial, when compared to placebo, ticlopidine was noted to have no significant effect on all‐cause mortality (30/172 (17.4%) versus 23/163 (14.1%); OR 1.29 (95% CI 0.71 to 2.32) (Analysis 1.1) or vascular mortality (18/172 (10.5%) versus 18/163 (11%); OR 0.94 (95% CI 0.47 to 1.88) (Analysis 2.1).
1.1. Analysis.
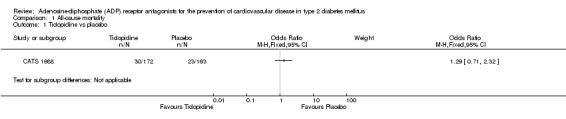
Comparison 1 All‐cause mortality, Outcome 1 Ticlopidine vs placebo.
2.1. Analysis.
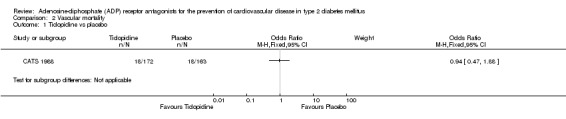
Comparison 2 Vascular mortality, Outcome 1 Ticlopidine vs placebo.
Cardiovascular events
Fatal and non‐fatal myocardial infarction
Data for combined fatal and non‐fatal myocardial infarction were only available for one trial (CATS 1988). Ticlopidine was not noted to have any statistically significant effect on reducing this outcome when compared to placebo (16/172 (9.3%) versus 19/163 (11.7%); OR 0.78 (95% CI 0.39 to 1.57) (Analysis 4.1).
4.1. Analysis.
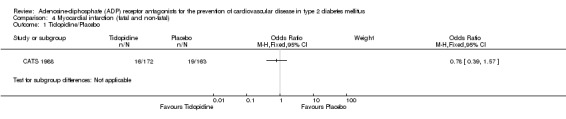
Comparison 4 Myocardial infarction (fatal and non‐ fatal), Outcome 1 Ticlopidine/Placebo.
Fatal and non‐fatal stroke
Data for the outcome fatal or non‐fatal stroke were available from two trials (30% of the total diabetes participants), comparing ADP receptor antagonists with other antiplatelet drugs (PRoFESS 2008; TASS 1989). These two trials were statistically heterogeneous (I2 = 81%) and a random‐effects model was used for pooling. Overall, in the pooled analysis there was no statistically significant reduction in the combination of fatal and non‐fatal stroke ((359/3194 (11.2%) versus 356/3146 (11.3%); OR 0.81 (95% CI 0.44 to 1.49)) for ADP receptor antagonists versus other antiplatelet drugs (Analysis 3.4). In the study comparing ticlopidine with aspirin (TASS 1989) a reduction in fatal and non‐fatal stroke was demonstrated with the use of ticlopidine (25/291 (0.9%) versus 44/306 (0.14%) for aspirin; OR 0.56 (95% CI 0.33 to 0.94) (Analysis 3.2). There was no significant reduction in fatal and non‐fatal strokes in diabetes patients when clopidogrel was compared to aspirin combined with dipyramidole (334/2840 (0.12%) versus 312/2903 (0.11%); OR 1.12 (95% CI 0.05 to 1.32)) (PRoFESS 2008; Analysis 3.3).
3.4. Analysis.
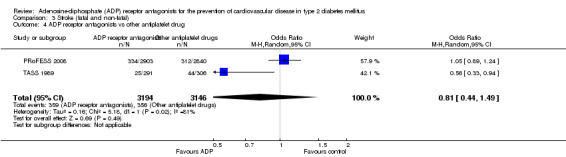
Comparison 3 Stroke (fatal and non‐fatal), Outcome 4 ADP receptor antagonists vs other antiplatelet drug.
3.2. Analysis.
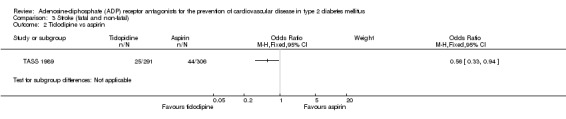
Comparison 3 Stroke (fatal and non‐fatal), Outcome 2 Ticlodipine vs aspirin.
3.3. Analysis.
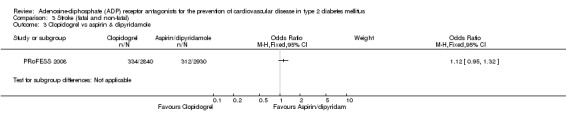
Comparison 3 Stroke (fatal and non‐fatal), Outcome 3 Clopidogrel vs aspirin & dipyridamole.
Peripheral vascular disease
No data were available from any of the trials on the reduction of peripheral vascular disease.
Secondary outcomes
Published or unpublished data on safety and adverse events, health‐related quality of life specifically for patients with diabetes and costs were not available for any of the trials.
Discussion
This systematic review summarises the available data from eight eligible studies on the effect of adenosine‐diphosphate (ADP) receptor antagonists on mortality and cardiovascular disease in patients with diabetes.
Summary of main results
Available data did not demonstrate a significant benefit of ADP receptor antagonists over placebo or other antiplatelet drugs in reducing all‐cause mortality, vascular mortality, stroke or myocardial infarction. Ticlopidine was demonstrated to reduce combined fatal and non‐fatal strokes when compared to aspirin in one trial (Analysis 3.2).
Overall completeness and applicability of evidence
For most studies, data for patients with diabetes were incomplete. Although eight trials comparing ADP receptor antagonists with other antiplatelet drugs or placebo were eligible for inclusion, only three provided data on patients with diabetes (CATS 1988; PRoFESS 2008; TASS 1989) and only one CATS 1988 provided data on more than one outcome for patients with diabetes. Authors of all eight trials were contacted, one author provided information (CATS 1988), the author of one study declined to provide information (AAASPS 2003), one author did not have any further data (PRoFESS 2008) and the authors of the five remaining studies did not respond to two requests (CAPRIE 1996; CHARISMA 2002; CURE 1998; Park 2010; TASS 1989).
All trials reported on safety and adverse outcomes for all included patients; however, none of the trials reported on adverse outcomes for patients with diabetes separately. For all patients, all four trials comparing ADP receptor antagonists with aspirin noted slightly lower bleeding rates for clopidogrel and ticlopidine compared to aspirin or aspirin and dipyridamole in combination (AAASPS 2003; CAPRIE 1996; PRoFESS 2008; TASS 1989). However, these trials used higher doses of aspirin than are commonly prescribed today. The trial comparing ticlopidine to placebo noted one serious bleeding event in the placebo group compared with two serious bleeding events in the ticlopidine group (CATS 1988). The three trials which compared dual aspirin and clopidogrel therapy to aspirin alone all noted increased bleeding risk with dual therapy (CHARISMA 2002; CURE 1998; Park 2010). A Cochrane review comparing dual therapy of clopidogrel and aspirin to aspirin alone noted there would be an additional six major bleeds per 1000 people treated (Squizzato 2011).
None of the included trials reviewed peripheral vascular disease as a cardiovascular endpoint. No trial used standard diagnostic criteria to diagnose diabetes and none of the trials separated patients into type 1 or type 2 diabetes.
There are no trials specifically designed to look at the efficacy and safety of ADP receptor antagonists for patients with type 2 diabetes. Patients with diabetes have different metabolism and platelet function to patients without diabetes which predisposes them to cardiovascular disease (Angiolillo 2009) and this may change the efficacy and safety of ADP receptor antagonists.
The use of ADP receptor antagonists in diabetes patients is based on evidence for their use from the analysis of patients with and without diabetes. Many major guidelines now recommend the use of clopidogrel in certain situations. For example, the National Institute of Health and Clinical Excellence guidelines recommend clopidogrel for 12 months in all patients with unstable angina or non‐ST elevated myocardial infarction with a six month mortality risk of greater than 1.5% (NICE 2010), based on the CURE trial findings. As a result, many patients with diabetes are being prescribed ADP receptor antagonists and newer ADP receptor antagonists are now compared to clopidogrel rather than to other antiplatelet agents despite there being limited evidence for patients with diabetes.
The intention of this review was to assess the use of ADP receptor antagonists in primary and secondary prevention. However, all of the trials except one (CHARISMA 2002) enrolled patients who had previous cardiovascular or cerebrovascular events. ADP receptor antagonists in primary prevention of cardiovascular disease in high risk vascular patients was investigated by the CHARISMA trial, however, outcome data for patients with diabetes were unavailable from this trial.
Quality of the evidence
Overall the methodological quality of the eight included trials was high. All of the trials except one (Park 2010) were double‐blind. The majority of the studies were funded by pharmaceutical companies.
Most studies reported composite end points as well as individual outcome events. A specific analysis of diabetes patients of the CAPRIE 1996 trial only reported a composite end point of vascular death, myocardial infarction, stroke and rehospitalisation for bleeding or ischaemia (Bhatt 2002). From a clinical perspective, reporting of composite endpoints is less useful as clinicians are unable to provide relevant information to patients, for example, the patient's risk of death.
Potential biases in the review process
It is possible that we have missed trials through the search strategy despite three databases being searched and relevant review articles being handsearched. Of the included trials, unpublished diabetes‐specific data were provided by only one trial and this may have introduced bias into the review.
Agreements and disagreements with other studies or reviews
The largest meta‐analysis of antiplatelet therapy versus control was done by the Antithrombotic Trialists Collaboration (Antithrombotic Trialists' Collaboration 2002). This meta‐analysis reviewed all antiplatelet agents including aspirin and failed to show a statistically significant reduction in risk of serious vascular events in patients with diabetes.
There are two Cochrane reviews on ADP receptor antagonists which differ from our review as they are not limited to patients with diabetes. One review included all patients with previous clinical manifestations of atherosclerosis of the cerebral, coronary or peripheral circulation and assessed the composite end point of stroke, myocardial infarction or vascular death. This review noted that ADP receptor antagonists modestly reduced the odds of a serious vascular event when compared to aspirin. The result just reached statistical significance (11.6% versus 12.5%, OR 0.92 (0.85 to 0.99)) and the authors concluded that ADP receptor antagonists were at least as effective as aspirin, possibly more so (Sudlow 2009). A different Cochrane review of clopidogrel and aspirin versus aspirin alone included all patients with known cardiovascular disease or at high risk of atherothrombotic disease. The authors concluded that in patients with acute non‐ST coronary syndromes the benefits of clopidogrel plus aspirin outweighed the harms (Squizzato 2011).
Authors' conclusions
Implications for practice.
The role of adenosine diphosphate (ADP) receptor antagonists in patients with diabetes is unclear. The use of ADP receptors for prevention of cardiovascular disease in patients with diabetes needs to be guided by evidence available from analysis done on all patients, including those with and without diabetes. Current available evidence analysed in previous Cochrane reviews suggests ADP receptor antagonists were at least as effective as aspirin, possibly more so in preventing cardiovascular disease in secondary prevention (Sudlow 2009) and that in acute non‐ST coronary syndromes the benefits of clopidogrel and aspirin outweighed the harms (Squizzato 2011). Another factor to take into account is the cost of ADP receptor antagonists which in many countries is significantly higher than aspirin.
Implications for research.
All future trials should separately report outcome data for patients with diabetes to allow for development of clinical guidelines for this specific patient population. Any previously conducted trial should make diabetes specific data available for analysis regardless of outcomes. Improved identification and thorough analysis of subgroups of diabetes patients in large primary and secondary prevention trials would provide a more solid evidence base for recommendations for primary and secondary prevention in diabetes patients.
History
Protocol first published: Issue 3, 2005 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 24 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Sarah Thorning for her assistance with the search strategy and finding the full text for some of the trials.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Diabetes mellitus, type 2 explode all trees #2 (obes* in All Text near/6 diabet* in All Text) #3 (MODY in All Text or NIDDM in All Text or TDM2 in All Text or TD2 in All Text) #4 ( (non in All Text and insulin* in All Text and depend* in All Text) or (noninsulin* in All Text and depend* in All Text) or (non in All Text and insulindepend* in All Text) or noninsulindepend* in All Text) #5 (typ? in All Text and (2 in All Text near/6 diabet* in All Text) ) #6 (typ? in All Text and (II in All Text near/6 diabet* in All Text) ) #7 (non in All Text and (keto* in All Text near/6 diabet* in All Text) ) #8 (nonketo* in All Text near/6 diabet* in All Text) #9 (adult* in All Text near/6 diabet* in All Text) #10 (matur* in All Text near/6 diabet* in All Text) #11 (late in All Text near/6 diabet* in All Text) #12 (slow in All Text near/6 diabet* in All Text) #13 (stabl* in All Text near/6 diabet* in All Text) #14 (insulin* in All Text and (defic* in All Text near/6 diabet* in All Text) ) #15 (plurimetabolic in All Text and syndrom* in All Text) #16 (pluri in All Text and metabolic in All Text and syndrom* in All Text) #17 (typ?2 in All Text near/3 diabet* in All Text) #18 (keto in All Text and (resist* in All Text near/3 diabet* in All Text) ) #19 (non in All Text and (keto* in All Text near/3 diabet* in All Text) ) #20 (nonketo* in All Text near/3 diabet* in All Text) #21 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20) #22 MeSH descriptor Ticlopidine explode all trees #23 (ticlopidin* in All Text or clopidogrel in All Text or prasugrel in All Text or ticagrelor in All Text) #24 (ticlodone in All Text or ticlodix in All Text or ticlid in All Text) #25 (#22 or #23 or #24) #26 (#21 and #25) |
| Medline |
| 1 exp Diabetes Mellitus, Type 2/ 2 exp Insulin Resistance/ 3 exp Glucose Intolerance/ 4 exp Metabolic Syndrome X/ 5 (impaired glucos$ toleranc$ or glucos$ intoleranc$ or insulin resistan$).tw,ot. 6 (obes$ adj3 diabet$).tw,ot. 7 (MODY or NIDDM or T2DM or T2D).tw,ot. 8 (non insulin$ depend$ or noninsulin$ depend$ or noninsulin?depend$ or non insulin?depend$).tw,ot. 9 ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet$).tw,ot. 10 ((keto?resist$ or non?keto$) adj6 diabet$).tw,ot. 11 (((late or adult$ or matur$ or slow or stabl$) adj3 onset) and diabet$).tw,ot. 12 metabolic syndrom*.tw,ot. 13 or/1‐12 14 exp Diabetes Insipidus/ 15 diabet$ insipidus.tw,ot. 16 14 or 15 17 13 not 16 18 exp Ticlopidine/ 19 (ticlopidin* or clopidogrel or prasugrel).tw,ot. 20 (ADP adj3 receptor antagonist*).tw,ot. 21 (ticlodone or ticlodix or ticlid).tw,ot. 22 or/18‐21 23 17 and 22 24 randomized controlled trial.pt. 25 controlled clinical trial.pt. 26 randomi?ed.ab. 27 placebo.ab. 28 drug therapy.fs. 29 randomly.ab. 30 trial.ab. 31 groups.ab. 32 or/24‐31 33 Meta‐analysis.pt. 34 exp Technology Assessment, Biomedical/ 35 exp Meta‐analysis/ 36 exp Meta‐analysis as topic/ 37 hta.tw,ot. 38 (health technology adj6 assessment$).tw,ot. 39 (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 40 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 41 or/33‐40 42 32 or 41 43 (comment or editorial or historical‐article).pt. 44 42 not 43 45 23 and 44 |
| Embase |
| 1 exp Diabetes Mellitus, Type 2/ 2 exp Insulin Resistance/ 3 (MODY or NIDDM or T2D or T2DM).tw,ot. 4 ((typ? 2 or typ? II or typ?II or typ?2) adj3 diabet*).tw,ot. 5 (obes* adj3 diabet*).tw,ot. 6 (non insulin* depend* or non insulin?depend* or noninsulin* depend* or noninsulin?depend*).tw,ot. 7 ((keto?resist* or non?keto*) adj3 diabet*).tw,ot. 8 ((adult* or matur* or late or slow or stabl*) adj3 diabet*).tw,ot. 9 (insulin* defic* adj3 relativ*).tw,ot. 10 (insulin* resistanc* or impaired glucos* toleranc* or glucos* intoleranc*).tw,ot. 11 or/1‐10 12 exp Diabetes Insipidus/ 13 diabet* insipidus.tw,ot. 14 12 or 13 15 11 not 14 16 exp ticlopidine/ 17 exp clopidogrel/ 18 exp prasugrel/ 19 exp ticagrelor/ 20 (ADP adj3 receptor antagonist*).tw,ot. 21 (ticlopidin* or clopidogrel or prasugrel or ticagrelor).tw,ot. 22 (ticlodone or ticlodix or ticlid).tw,ot. 23 or/16‐22 24 15 and 23 25 exp Randomized Controlled Trial/ 26 exp Controlled Clinical Trial/ 27 exp Clinical Trial/ 28 exp Comparative Study/ 29 exp Drug comparison/ 30 exp Randomization/ 31 exp Crossover procedure/ 32 exp Double blind procedure/ 33 exp Single blind procedure/ 34 exp Placebo/ 35 exp Prospective Study/ 36 ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 37 (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 38 ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 39 (cross over or crossover).ab,ti. 40 or/25‐39 41 exp meta analysis/ 42 (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 43 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 44 exp Literature/ 45 exp Biomedical Technology Assessment/ 46 hta.tw,ot. 47 (health technology adj6 assessment$).tw,ot. 48 or/41‐47 49 40 or 48 50 (comment or editorial or historical‐article).pt. 51 49 not 50 52 24 and 51 53 review.pt. 54 52 not 53 |
Appendix 2. Baseline characteristics
|
Study ID Characteristic |
AAASPS | CAPRIE | CATS | CHARISMA | CURE | Park | PRoFESS | TASS |
| Intervention(s) and control(s) | I: Ticlopidine 250 mg bd C: Aspirin 325 mg bd |
I: Clopidogrel 75 mg daily C: Aspirin 325 mg daily |
I: Ticlopidine 250 mg bd C: Placebo |
I: Clopidogrel 75 mg daily and aspirin 75 to 162 mg daily C: Aspirin 75 to 162 mg daily |
I: Clopidogrel 75 mg daily and aspirin 75 to 300 mg daily C: Aspirin 75 to 300 mg daily |
I: Clopidogrel 75 mg daily and aspirin 100 to 200 mg daily C(d): Aspirin 100 to 200 mg daily |
I: Clopidogrel 75 mg daily C: Aspirin / dipyridamole 25 mg / 200 mg bd |
I: Ticlopidine 250 mg bd C: Aspirin 650 mg bd |
| Participating population | Patients with cerebral infarcts | Patients with vascular disease | Patients with thrombo‐embolic strokes | High‐risk vascular patients | Patients with acute coronary syndrome | Patients with drug eluding stents | Patients with ischaemic strokes | Patients with ischaemic neurological events |
| Country | USA | 16 countries | USA, Canada | 32 countries | 28 countries | South Korea | 35 countries | USA, Canada |
| Setting | Hospital and community | Hospital and community | Hospital inpatients | Hospital | Hospital | Inpatients and community | Hospital and community | Hospital and community |
| Sex [female%] | I: 54.5 C: 52.4 |
I: 28 C: 28 |
I: 40 C: 37 |
I: 29.7 C: 29.8 |
I: 38.7 C: 38.3 |
I: 30.0 C: 30.6 |
I: 36.0 C: 35.9 |
I: 36 C: 35 |
| Age [mean years (SD)] | I: 60.9 (10.7) C: 61.6 (10.4) |
I: 62.5 (11.1) C: 62.5 (11.1) |
I: 66 C: 65 |
Median I: 64.0 C: 64.0 |
I: 64.2 (11.3) C: 64.2 (11.3) |
I: 62 (9.8) C: 61.9 (9.9) |
I: 66.2 (8.5) C: 66.1 (8.6) |
I: 62.7 (9.4) C: 63.2 (9.3) |
| HbA1c [mean % (SD)] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| BMI [mean kg/m2 (SD)] | I: 29.9 (7.1) C: 30.0 (6.8) |
‐ | ‐ | ‐ | ‐ | ‐ | I: 26.8 (5.0) C: 26.8 (5.0) |
‐ |
| Ethnic groups [%] | I: African American C: African American |
‐ | Caucasian I: 73% C: 71% |
White I: 80.4% C: 79.9% |
‐ | ‐ | White / European I: 57.3% C: 57.7% |
White I: 80% C: 81% |
| Duration of follow‐up (mean) | I: 710 days C: 716 days |
I: 1.91 years C: 1.91 years |
I: 17 months C: 19 months |
Median I: 28 months C: 28 months |
I: 12 months C: 12 months |
I: 19.2 months C: 19.2 months |
I: 2.5 years C: 2.5 years |
I: 778 days C: 858 days |
|
Footnotes "‐" denotes not reported Abbreviations: bd: twice daily; BMI: body mass index; C: control; C(d): intervention (diabetes patients); C(t): intervention (all patients); HbA1c: glycosylated haemoglobin A1c; I: intervention; I(d): intervention (diabetes patients); I(t): intervention (all patients); SD: standard deviation | ||||||||
Appendix 3. Study outcomes
|
Study ID Characteristic |
AAASPS | CAPRIE | CATS | CHARISMA | CURE | Park | PRoFESS | TASS |
| Intervention(s) and control(s) | I: Ticlopidine 250 mg bd C: Aspirin 325 mg bd |
I: Clopidogrel 75 mg daily C: Aspirin 325 mg daily |
I: Ticlopidine 250 mg bd C: Placebo |
I: Clopidogrel 75 mg daily and aspirin 75 to 162 mg daily C: Aspirin 75 to 162 mg daily |
I: Clopidogrel 75 mg daily and aspirin 75 to 300 mg daily C: Aspirin 75 to 300 mg daily |
I: Clopidogrel 75 mg daily and aspirin 100 to 200 mg daily C(d): Aspirin 100 to 200 mg daily |
I: Clopidogrel 75 mg daily C: Aspirin / dipyridamole 25 mg / 200 mg bd |
I: Ticlopidine 250 mg bd C: Aspirin 650 mg bd |
| All‐cause mortality | I(d): ‐ C(d): ‐ I(t): 45 C(t): 40 |
I(d): ‐ C(d): ‐ I(t): 560 C(t): 571 |
I(d): 30 C(d): 23 I(t): 66 C(t): 65 |
I(d): ‐ C(d): ‐ I(t): 371 C(t): 374 |
I(d): ‐ C(d): ‐ I(t): 359 C(t): 390 |
I(d): ‐ C(d): ‐ I(t): 20 C(t): 13 |
I(d): ‐ C(d): ‐ I(t): 756 C(t): 739 |
I(d): ‐ C(d): ‐ I(t): 175 C(t): 196 |
| Vascular mortality | I(d): ‐ C(d): ‐ I(t): 18 C(t): 18 |
I(d): ‐ C(d): ‐ I(t): 350 C(t): 378 |
I(d): 18 C(d): 18 I(t): 35 C(t): 43 |
I(d): ‐ C(d): ‐ I(t): 238 C(t): 229 |
I(d): ‐ C(d): ‐ I(t): 318 C(t): 345 |
‐ | ‐ | I(d): ‐ C(d): ‐ I(t): 120 C(t): 116 |
| Myocardial infarction (fatal) | I(d): ‐ C(d): ‐ I(t): 1 C(t): 0 |
I(d): ‐ C(d): ‐ I(t): 53 C(t): 75 |
I(d): 13 C(d): 10 I(t): 21 C(t): 22 |
‐ | ‐ | ‐ | ‐ | I(d): ‐ C(d): ‐ I(t): 21 C(t): 14 |
| Myocardial infarction (non‐fatal) | I(d): ‐ C(d): ‐ I(t): 8 C(t): 8 |
I(d): ‐ C(d): ‐ I(t): 255 C(t): 301 |
I(d): 3 C(d): 9 I(t): 11 C(t): 15 |
I(d): ‐ C(d): ‐ I(t): 146 C(t): 155 |
‐ | ‐ | ‐ | ‐ |
| Myocardial infarction (fatal and non‐fatal) | I(d): ‐ C(d): ‐ I(t): 9 C(t): 8 |
I(d): ‐ C(d): ‐ I(t): 308 C(t): 376 |
I(d): 16 C(d): 19 I(t): 32 C(t): 37 |
‐ | I(d): ‐ C(d): ‐ I(t): 324 C(t): 419 |
I(d): ‐ C(d): ‐ I(t): 10 C(t): 7 |
I(d): ‐ C(d): ‐ I(t): 197 C(t): 178 |
‐ |
| Stroke (fatal) | I(d): ‐ C(d): ‐ I(t): 4 C(t): 2 |
I(d): ‐ C(d): ‐ I(t): 37 C(t): 42 |
I(d): 6 C(d): 9 I(t): 14 C(t): 17 |
‐ | ‐ | ‐ | ‐ | I(d): ‐ C(d): ‐ I(t): 16 C(t): 23 |
| Stroke (non‐fatal) | I(d): ‐ C(d): ‐ I(t): 102 C(t): 84 |
I(d): ‐ C(d): ‐ I(t): 472 C(t): 504 |
I(d): 28 C(d): 32 I(t): 67 C(t): 86 |
I(d): ‐ C(d): ‐ I(t): 150 C(t): 189 |
‐ | ‐ | ‐ | I(d): ‐ C(d): ‐ I(t): 156 C(t): 189 |
| Stroke (fatal and non‐fatal) | I(d): ‐ C(d): ‐ I(t): 106 C(t): 86 |
I(d): ‐ C(d): ‐ I(t): 509 C(t): 546 |
I(d): 34 C(d): 41 I(t): 81 C(t): 103 |
‐ | I(d): ‐ C(d): ‐ I(t): 75 C(t): 87 |
I(d): ‐ C(d): ‐ I(t): 9 C(t): 4 |
I(d): 334 C(d): 312 I(t): 862 C(t): 879 |
I(d): 25 C(d): 44 I(t): 172 C(t): 212 |
|
Footnotes "‐" denotes not reported Abbreviations: bd: twice daily; C: control; C(d): intervention (diabetes patients); C(t): intervention (all patients); I: intervention; I(d): intervention (diabetes patients); I(t): intervention (all patients) | ||||||||
Appendix 4. Adverse events
|
Study ID Characteristic |
AAASPS | CAPRIE | CATS | CHARISMA | CURE | Park | PRoFESS | TASS |
| Intervention(s) and control(s) | I: Ticlopidine 250 mg bd C: Aspirin 325 mg bd |
I: Clopidogrel 75 mg daily C: Aspirin 325 mg daily |
I: Ticlopidine 250 mg bd C: Placebo |
I: Clopidogrel 75 mg daily and aspirin 75 to 162 mg daily C: Aspirin 75 to 162 mg daily |
I: Clopidogrel 75 mg daily and aspirin 75 to 300 mg daily C: Aspirin 75 to 300 mg daily |
I: Clopidogrel 75 mg daily and aspirin 100 to 200 mg daily C(d): Aspirin 100 to 200 mg daily |
I: Clopidogrel 75 mg daily C: Aspirin / dipyridamole 25 mg / 200 mg bd |
I: Ticlopidine 250 mg bd C: Aspirin 650 mg bd |
| Serious adverse events (including bleeding) | I(d): ‐ C(d): ‐ I(t): 270 C(t): 262 |
I(d): ‐ C(d): ‐ I(t): 360 C(t): 409 |
I(d): ‐ C(d): ‐ I(t): 43 C(t): 15 |
‐ | ‐ | ‐ | I(d): ‐ C(d): ‐ I(t): 373 C(t): 426 |
‐ |
| Serious bleeding | I(d): ‐ C(d): ‐ I(t): 10 C(t): 19 |
I(d): ‐ C(d): ‐ I(t): 77 C(t): 109 |
I(d): ‐ C(d): ‐ I(t): 2 C(t): 1 |
I(d): ‐ C(d): ‐ I(t): 130 C(t): 104 |
I(d): ‐ C(d): ‐ I(t): 231 C(t): 169 |
I(d): ‐ C(d): ‐ I(t): 3 C(t): 1 |
I(d): ‐ C(d): ‐ I(t): 116 C(t): 128 |
I(d): ‐ C(d): ‐ I(t): 137 C(t): 152 |
|
Footnotes "‐" denotes not reported Abbreviations: bd: twice daily; C: control; C(d): intervention (diabetes patients); C(t): intervention (all patients); I: intervention; I(d): intervention (diabetes patients); I(t): intervention (all patients) | ||||||||
Data and analyses
Comparison 1. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ticlopidine vs placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. Vascular mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ticlopidine vs placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Stroke (fatal and non‐fatal).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ticlodipine vs placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Ticlodipine vs aspirin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Clopidogrel vs aspirin & dipyridamole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 ADP receptor antagonists vs other antiplatelet drug | 2 | 6340 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.44, 1.49] |
3.1. Analysis.
Comparison 3 Stroke (fatal and non‐fatal), Outcome 1 Ticlodipine vs placebo.
Comparison 4. Myocardial infarction (fatal and non‐ fatal).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ticlopidine/Placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AAASPS 2003.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: African American 29 to 85 years of age Non‐cardioembolic cerebral infarct Cerebral infarct 7 to 90 days prior CT or MRI consistent with occurrence of entry cerebral infarct (shows entry infarct, old infarct, or no infarct) Measurable neurological deficit that correlates with onset of entry cerebral infarct Able to follow an outpatient treatment program EXCLUSION CRITERIA: Transient ischaemic attack Subarachnoid haemorrhage Cardiac embolism Iatrogenic stroke Postoperative stroke within 30 days of operation Carotid endarterectomy as preventive treatment of entry cerebral infarct Mean arterial blood pressure > 130 mm Hg on 3 consecutive days Modified Barthel Index < 10 History of dementia or neurodegenerative disease Severe comorbid condition such as cancer that would limit survival during 2‐year follow‐up period Concurrent enrolment in another clinical trial Sensitivity or allergy to aspirin or ticlopidine Women of childbearing potential Peptic ulcer disease, active bleeding diathesis, lower gastrointestinal bleeding, platelet or other haematologic abnormality clinically active in the past year Haematuria Positive stool guaiac Prolonged prothrombin time or partial thromboplastin time Blood urea nitrogen > 40 mg %, Serum creatinine > 2.0 mg % Thrombocytopenia or neutropenia as defined by the lower limit of normal for the platelet count or white blood cell count ≥ 2 times the upper range of normal on liver function tests PARTICIPANTS: Total participants: 1809 (Aspirin: 907, Ticlodipine: 902) Diabetes participants: 738 (Aspirin: 379, Ticlodipine: 359) Total participants withdrawn or prematurely discontinuing study medication: 454 (Aspirin: 215, Ticlodipine: 239) Total participants lost to follow‐up: 68 (Aspirin: 31, Ticlodipine: 37) |
|
| Interventions |
INTERVENTION: Ticlopidine 250 mg twice daily and placebo CONTROL: Aspirin 325 mg twice daily and placebo NUMBER OF STUDY CENTRES: 62 COUNTRY/ LOCATION: United States of America SETTING: Hospital and community |
|
| Outcomes |
OUTCOMES(as documented in the published protocol): Composite endpoint of myocardial infarction, recurrent stroke and vascular death All cause mortality patients with diabetes: unknown All cause mortality all patients: Aspirin: 40, Ticlopidine: 45 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: Aspirin:18, Ticlopidine: 18 Myocardial infarction patients with diabetes (fatal): unknown Myocardial infarction patients with diabetes (non‐fatal): unknown Myocardial infarction all patients (fatal): Aspirin: 0, Ticlopidine: 1 Myocardial infarction all patients (non‐fatal): Aspirin: 8, Ticlopidine: 8 Stroke patients with diabetes (fatal): unknown Stroke patients with diabetes (non‐fatal): unknown Stroke all patients (fatal): Aspirin: 2, Ticlopidine: 4 Stroke all patients (non‐fatal): Aspirin: 84, Ticlopidine: 102 Serious adverse event patients with diabetes: unknown Serious adverse event all patients: Aspirin: 262, Ticlopidine: 270 |
|
| Study details |
RUN‐IN PERIOD: None STUDY TERMINATED BEFORE REGULAR END: Yes ‐ at 6.5 years |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | "the primary hypothesis of this ... trial ... is that ticlopidine hydrochloride is more effective than aspirin in preventing the composite endpoints of recurrent stroke, myocardial infarction, and vascular death among middle‐aged and elderly African Americans with non‐cardioembolic ischemic stroke" | |
| Notes | Supported by the National Institute of Neurological Disorders and Stroke. Medications and placebos were supplied by Roche Laboratories and Bayer Abbreviations: CT: computed tomography; MRI: magnetic resonance imaging |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation algorithm developed by chief study statistician" |
| Allocation concealment (selection bias) | Low risk | "Local site personnel call the Automated Phone Registration System for AAASPS operated by the Moffitt Cancer Research Institute of the University of South Florida ... for allocation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all study personnel were masked (blinded) from treatment assignment with the exception of 1 study statistician who developed the randomization algorithm" "placebo tablets had identical physical properties" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 276 participants withdrew in the Ticlopidine group and 246 in the Aspirin group. Analysis was done on intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated in the published protocol |
| Other bias | Low risk | "Roche Laboratories and Bayer had no role beyond supplying study medications and placebos." |
CAPRIE 1996.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: Ischaemic stroke: (including retinal and lacunar infarction) ≥ 1 week and ≤ 6 months and neurological signs persisting ≥ 1 week from stroke onset and CT or MRI ruling out haemorrhage or non‐relevant disease MI: Onset ≤ 35 days before randomisation and two of: Characteristic ischaemic pain for ≥ 20 min Elevation of CK, CK‐MB, LDH, or AST to 2 times upper limit of laboratory normal with no other explanation Development of new ≥ 40 Q waves in at least two adjacent ECG leads or new dominant R wave in V1 (R ≥ 1 mm > S in V1) Atherosclerotic peripheral arterial disease: Intermittent claudication (WHO: leg pain on walking, disappearing in < 10 min on standing) of presumed atherosclerotic origin; and ankle/arm systolic BP ratio ≤ 0·85 in either leg at rest (two assessments on separate days); or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty with no persisting complications from intervention EXCLUSION CRITERIA: Age < 21 years Severe cerebral deficit likely to lead to patient being bedridden or demented Carotid endarterectomy after qualifying stroke Qualifying stroke induced by carotid endarterectomy or angiography Patient unlikely to be discharged alive after qualifying event Severe comorbidity likely to limit patient's life expectancy to less than 3 years Uncontrolled hypertension Scheduled for major surgery Contraindications to study drugs Severe renal or hepatic insufficiency Haemostatic disorder or systemic bleeding History of haemostatic disorder or systemic bleeding History of thrombocytopenia or neutropenia History of drug‐induced haematologic or hepatic abnormalities Known to have abnormal WBC, differential, or platelet count Anticipated requirement for long‐term anticoagulants, non‐study antiplatelet drugs or NSAIDs affecting platelet function History of aspirin sensitivity Women of childbearing age not using reliable contraception Currently receiving investigation drug Previously entered in other clopidogrel studies Geographic or other factors making study participation impractical CO‐MEDICATIONS: Antiplatelet or anticoagulation drugs were discontinued before randomisation. Patients with anticipated need for long term anticoagulation or NSAIDs were excluded. Concomitant medications were recorded. PARTICIPANTS: Total participants: 19,185 (Aspirin: 9546, Clopidogrel: 9553) [86 did not receive medication, Aspirin 40, Clopidogrel 46] Diabetes participants: 3866 (Aspirin: 20% of total group, Clopidogrel: 20% of total group) Total participants withdrawn or prematurely discontinuing study medication: 4059 (Aspirin: 21.1%, Clopidogrel: 21.3%) Total participants lost to follow‐up: 42 (Aspirin: 20, Clopidogrel: 22) |
|
| Interventions |
INTERVENTION: Clopidogrel 75 mg daily and placebo CONTROL: Aspirin 325 mg daily and placebo NUMBER OF STUDY CENTRES: 384 centres COUNTRY/ LOCATION: 16 countries SETTING: Hospital and community |
|
| Outcomes |
OUTCOMES (as stated in the publication): Non‐fatal Ischaemic Stroke: acute neurological event with focal signs for ≥ 24 hrs; if new location without evidence of intracranial haemorrhage; if worsening of previous event, must have lasted > 1 week, or more than 24 hrs if accompanied by appropriate CT or MRI findings Non‐fatal MI: same as inclusion criteria Non‐fatal primary intracranial haemorrhage: Intracerebral haemorrhage (including intracranial and subarachnoid), and subdural haematoma documented by appropriate neuroimaging investigations. Non‐fatal leg amputation: Only if above the ankle and not done for trauma or cancer. Fatal events: Ischaemic stroke (within 28 days of onset of symptoms/signs), MI (within 28 days of onset of symptoms signs), haemorrhage, other vascular causes (deaths that were not clearly non‐vascular and did not meet other criteria) or non‐vascular causes All cause mortality patients with diabetes: unknown All cause mortality all patients: Aspirin: 571, Clopidogrel: 560 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: Aspirin: 378, Clopidogrel: 350 Myocardial infarction patients with diabetes (fatal): unknown Myocardial infarction patients with diabetes (non‐fatal): unknown Myocardial infarction all patients (fatal): Aspirin: 75, Clopidogrel: 53 Myocardial infarction all patients (non‐fatal): Aspirin: 301, Clopidogrel: 255 Stroke patients with diabetes (fatal): unknown Stroke patients with diabetes (non‐fatal): unknown Stroke all patients (fatal): Aspirin: 42, Clopidogrel: 37 Stroke all patients (non‐fatal): Aspirin: 504, Clopidogrel: 472 Serious adverse event patients with diabetes: unknown Serious adverse event all patients: Aspirin: 409, Clopidogrel: 360 |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No (recruitment completed early but follow‐up remained at one year post recruitment completion) |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | “to assess the potential benefit of clopidogrel, compared with aspirin, in reducing the risk of ischaemic stroke, myocardial infarction, or vascular death in patients with recent ischaemic stroke, recent myocardial infarction or peripheral arterial disease” | |
| Notes | This study was funded by Sanofi and Bristol‐Myers Squibb Abbreviations: AST aspartate aminotransferase; BP: blood pressure; CK: creatine kinase; CK‐MB: creatine kinase myocardial band; CT: computed tomography; ECG: electrocardiogram; LDH: lactate dehydrogenase; MI: myocardial infarction; MRI: magnetic resonance imaging; WBC: white blood cells; WHO: World Health Organisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The Independent Statistical Centre provided computer‐generated .... with random allocation” |
| Allocation concealment (selection bias) | Low risk | “The Independent Statistical Centre provided computer‐generated .... with random allocation” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "These [Medication] supplies were in the form of blister packs containing either 75 mg tablets of clopidogrel plus aspirin placebo tablets or 325 mg aspirin tablets plus clopidogrel placebo tablets, such blister packs being indistinguishable from one another " |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “19,185 patients ... were randomised”; “46 patients in clopidogrel group and 40 in aspirin group never took any study drug”; “42 patients lost to follow‐up ... included in the analysis” “4059 patients discontinued study drug early...., 21.3% in clopidogrel and 21.1% in the aspirin group. Reasons for stopping study drug early were similar in the two groups”. Comment: equal loss in both groups, included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Registration of trial or prior protocol publication unavailable. Outcomes stated in publication document reported |
| Other bias | High risk | "This study was funded by Sanofi and Bristol‐Myers Squibb" |
CATS 1988.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: Thromboemoblic stroke 1 week to 4 months prior to entry into study Diagnosis based on clinical assessment and history Neurological deficit had to be persistent at time of entry into study CT scan excluded haemorrhagic stroke or other intracerebral pathology EXCLUSION CRITERIA: Cardioembolic strokes (defined as two of AF, sick sinus syndrome, seizure at onset, involvement in more than one vascular territory) Bedridden or dementia Life‐limiting illness Contraindications to ticlopidine (hepatic or renal insufficiency, haemostatic disorder, thrombocytopenia, history of drug‐induced haematologic abnormalities) Significant laboratory test abnormalities Carotid endarterectomy prior to randomisation Stroke due to carotid endarterectomy Long term anticoagulation or antiplatelet therapy required History of drug or alcohol abuse Inclusion in another trial PARTICIPANTS: Total participants: 1053 (Ticlodipine: 525, Placebo: 528) Diabetes participants: 335 (Ticlodipine: 172, Placebo: 163) Total participants withdrawn or prematurely discontinuing study medication: 402 (Ticlodipine: 238, Placebo: 164) Total participants lost to follow‐up: 4 (Ticlodipine: 3, Placebo: 1) |
|
| Interventions |
INTERVENTION: Ticlopidine 250 mg twice daily CONTROL: Placebo NUMBER OF STUDY CENTRES: 25 COUNTRY/ LOCATION: USA and Canada SETTING: Hospital inpatient |
|
| Outcomes |
OUTCOMES (as reported in published protocol): Non‐fatal stroke: Diagnosis based on clinical assessment and history Duration of > 24 hours if in new location or > 1 week if worsening of previous deficit unless accompanied by new CT finding CT scan excluded haemorrhagic stroke or other intracerebral pathology Non‐fatal myocardial infarction: At least two of: a) typical symptoms of pain b) compatible ECG changes or positive pyrophosphate radionuclide scan c) appropriate serum enzyme changes (peak value > twice upper limit of normal) Vascular death: New cerebral infarction Myocardial infarction Sudden death Congestive heart failure All cause mortality patients with diabetes: 30 ticlopidine, 23 placebo All cause mortality all patients: 66 ticlopidine, 65 placebo Vascular mortality patients with diabetes with diabetes: 18 ticlopidine, 18 placebo Vascular mortality all patients: 35 ticlopidine, 43 placebo Myocardial infarction patients with diabetes (fatal): 13 ticlopidine, 10 placebo Myocardial infarction patients with diabetes (non‐fatal): 3 ticlopidine, 9 placebo Myocardial infarction all patients (fatal): 21 ticlopidine, 22 placebo Myocardial infarction all patients (non‐fatal): 11 ticlopidine, 15 placebo Stroke patients with diabetes (fatal): 6 ticlopidine, 9 placebo Stroke patients with diabetes (non‐fatal): 28 ticlopidine, 32 placebo Stroke all patients (fatal): 14 ticlopidine, 17 placebo Stroke all patients (non‐fatal): 67 ticlopidine, 86 placebo Serious adverse event patients with diabetes: unknown Serious adverse event all patients: 43 ticlopidine, 15 placebo |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | “to assess the effect of ticlopidine (250 mg twice daily) in reducing the rate of subsequent occurrence of stroke, myocardial infarction, or vascular death in patients who have had a recent thromboembolic stroke" | |
| Notes | This study was funded by Syntax Research (USA) Inc and Sanofi, France Abbreviations: AF: atrial fibrillation; CT: computed tomography; ECG: electrocardiogram |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prescribed randomisation arrangement generated separately for each clinical centre, 528 patients randomised to placebo, 525 to ticlopidine |
| Allocation concealment (selection bias) | Low risk | Only the safety committee knew the randomisation code for a particular centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Ticlopidine was supplied.... in plastic containers .... placebo tablets were identical in packaging and appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients lost to follow‐up, 3 in ticlopidine group, 1 in placebo group |
| Selective reporting (reporting bias) | Unclear risk | Registration of trial or prior protocol publication unavailable. Outcomes stated in publication document reported |
| Other bias | High risk | Funded by pharmaceutical company |
CHARISMA 2002.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: High risk primary prevention: 2 major risk factors or 3 minor risk factors or 1 major and 2 minor risk factors. Major risk factors: Diabetes mellitus (on drug therapy) Diabetic neuropathy ABI < 0.9 Asymptomatic carotid stenosis ≥ 70% Carotid plaque evidence by intima‐media thickness Minor risk factors: Systolic BP ≥ 150 mm Hg despite therapy for 3 months Primary hypercholesterolaemia Current smoker > 15 cigarettes per day Male ≥ 65 years, female ≥ 70 years Coronary artery disease: 1 of: Stable angina with document multivessel coronary disease History of multivessel CABG History of multivessel PCI Previous MI Cerebrovascular disease: previous ischaemic stroke or TIA Peripheral arterial disease: intermittent claudication with ABI ≤ 0.85 or intermittent claudication with previous intervention EXCLUSION CRITERIA: Chronic oral antithrombotic medications (warfarin, high dose aspirin, NSAIDs) Requiring prolonged clopidogrel therapy CO‐MEDICATIONS: Concurrent oral antithrombotic therapy not permitted. Other standard therapy at the discretion of clinicians. PARTICIPANTS: Total participants: 15,603 (Clopidogrel: 7802, Placebo: 7801) Diabetes participants: 6556 (Clopidogrel: 3304, Placebo: 3252) Total participants withdrawn or prematurely discontinuing study medication: Clopidogrel: 20.4%, Placebo: 18.2% Total participants lost to follow‐up: unknown |
|
| Interventions |
INTERVENTION: Clopidogrel 75 mg daily and aspirin 75 mg to 162 mg daily CONTROL: Placebo and aspirin 75 mg to 162 mg daily NUMBER OF STUDY CENTRES: 768 COUNTRY/ LOCATION: 32 countries SETTING: Hospital |
|
| Outcomes |
OUTCOMES (as stated in the published protocol): Primary outcome: first occurrence of cardiovascular death, MI (according to American College of Cardiology definition) or stroke (acute onset of focal neurological symptoms or signs lasting more than 24 hours, with CT or MRI to exclude other causes). All cause mortality patients with diabetes: unknown All cause mortality all patients: Clopidogrel: 371, Placebo: 374 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: Clopidogrel: 238, Placebo: 229 Myocardial infarction patients with diabetes (fatal): unknown Myocardial infarction patients with diabetes (non‐fatal): unknown Myocardial infarction all patients (fatal): unknown Myocardial infarction all patients (non‐fatal): Clopidogrel: 146, Placebo: 155 Stroke patients with diabetes (fatal): unknown Stroke patients with diabetes (non‐fatal): unknown Stroke all patients (fatal): unknown Stroke all patients (non‐fatal): Clopidogrel: 150, Placebo: 189 Serious adverse event patients with diabetes: unknown Serious adverse event all patients (only bleeding recorded): Clopidogrel: 130, Placebo: 104 |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL & NON‐COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | To test if "clopidogrel in combination with aspirin would be an improvement over aspirin alone across the spectrum of high risk patients with atherothrombosis" | |
| Notes | Sponsored and funded by Sanofi‐Aventis and Bristol‐Myers Squibb and the National Institutes of Health Abbreviations: ABI: ankle brachial index; CABG: coronary artery bypass grafting; CT: computed tomography; MI: myocardial infarction; MRI: magnetic resonance imaging; NSAIDs: non‐steriodal anti‐inflammatory drugs; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "pre‐established randomisation scheme, stratified according to site" |
| Allocation concealment (selection bias) | Low risk | "Study drug assignment was performed centrally by an interactive voice‐response system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Matching placebo drug" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rate 20% for clopidogrel group, 18.2% for placebo group. Number of patients lost to follow‐up unknown. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated |
| Other bias | High risk | Pharmaceutical funding from Sanofi‐Aventis and Bristol‐Myers Squibb |
CURE 1998.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: Admitted to hospital with: Symptoms suggestive of ACS without ST segment elevation > 1 mm Within 24 hours of onset of chest pain/symptoms Either ECG changes compatible with new ischaemia or elevated cardiac enzymes or troponin I or T at least twice upper limit of normal EXCLUSION CRITERIA: Contraindications to antiplatelet/antithrombotic therapy High risk of bleeding Class IV heart failure Ongoing long term need for anticoagulants PTCA/stent or CABG within 3 months prior to randomisation CO‐MEDICATIONS: Aspirin (75 mg to 325 mg) started at randomisation or continued if already taking PARTICIPANTS: Total participants: 12,562 (Clopidogrel: 6259, Placebo: 6303) Diabetes participants: 2840 (Clopidogrel: 1405, Placebo: 1435) Total participants withdrawn or prematurely discontinuing study medication: Clopidogrel: 21.1%, Placebo: 18.8% Total participants lost to follow‐up: 13 (Clopidogrel: 6, Placebo: 7) |
|
| Interventions |
INTERVENTION: 300 mg loading dose of clopidogrel then 75 mg per day and aspirin 75 mg to 325 mg daily CONTROL: Placebo and aspirin 75 mg to 325 mg daily NUMBER OF STUDY CENTRES: 482 COUNTRY/ LOCATION: 28 countries SETTING: Hospital |
|
| Outcomes |
OUTCOMES (as stated in the published protocol): 1. The composite of cardiovascular death, non‐fatal myocardial infarction or stroke 2. The composite of cardiovascular death, non‐fatal myocardial infarction, stroke or refractory ischaemia Death from cardiovascular cause: death for which there was no clearly documented non‐vascular cause Myocardial infarction: presence of at least two of: ischaemic chest pain, elevation of cardiac markers or enzymes to at least twice upper limit of normal within 48 hours after percutaneous coronary intervention (or to a level 20% higher than the previous value if the level had already been elevated because of an early myocardial infarction), ECG changes compatible with infarction Stroke: result of intracranial haemorrhage, ischaemia or uncertain cause Refractory ischaemia in hospital: recurrent chest pain lasting more than five minutes with new ischaemic ECG changes while the patient was receiving optimal medical therapy (two anti‐anginal agents, including V nitrate unless contraindicated) leading to additional intervention by midnight of the next calendar day. Refractory ischaemia on discharge: rehospitalisation lasting at least 24 hours for unstable angina with ischaemic ECG changes Severe ischaemia: Ischaemia similar to refractory ischaemia for which no urgent intervention was performed All cause mortality patients with diabetes: unknown All cause mortality all patients: Clopidogrel: 359, Placebo: 390 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: Clopidogrel: 318, Placebo: 345 Myocardial infarction patients with diabetes (fatal): unknown Myocardial infarction patients with diabetes (non‐fatal): unknown Myocardial infarction all patients (fatal and non‐fatal): Clopidogrel: 324, Placebo 419 Stroke patients with diabetes (fatal): unknown Stroke patients with diabetes (non‐fatal): unknown Stroke all patients (fatal and non‐fatal): Clopidogrel: 75, Placebo: 87 Serious adverse event patients with diabetes: unknown Serious adverse event all patients (only bleeding recorded): Clopidogrel: 231, Placebo: 169 |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No – study initially designed for 9000 patients, however, increased to 12,500 due to lower event rates than expected |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | “To compare the efficacy and safety of the early and long‐term use of clopidogrel plus aspirin with those of aspirin alone in patients with acute coronary syndromes and no ST‐segment elevation” | |
| Notes | Supported by Sanofi‐Synthelabo and Bristol‐Myers Squibb Abbreviations: ACS: acute coronary syndrome; ECG: electrocardiogram; PTCA: percutaneous transluminal coronary angioplasty; CABG: coronary artery bypass grafting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised to either clopidogrel or placebo by telephone call to central computerised randomisation service" “Permuted block randomisation .... is used” |
| Allocation concealment (selection bias) | Low risk | “Telephone call to central computerised randomisation service” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Blinded study drug... matching placebo” “A central committee of clinicians who are blinded to treatment allocation will adjudicate ... outcomes” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Vital status was ascertained for 12,549 of the 12,562 patients who underwent randomization (99.9 percent), with 6 patients in the clopidogrel group and 7 in the placebo group lost to follow‐up” “46.2% of patients in clopidogrel group discontinued study medication ... as compared with 45.4% in the placebo group” |
| Selective reporting (reporting bias) | Low risk | Outcome data reported as stated in published protocol |
| Other bias | High risk | Funded by pharmaceutical company |
Park 2010.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: Undergone implantation of drug eluting stents at least 12 months before enrolment Receiving dual antiplatelet therapy at time of enrolment EXCLUSION CRITERIA: Major adverse cardiovascular event (myocardial infarction, stroke, repeat revascularisation) since implantation Major bleeding since implantation Contraindications to the use of antiplatelet drugs Requirement of long term clopidogrel Non‐cardiac coexisting condition with anticipated life expectancy of less than one year Participitation in another trial CO‐MEDICATIONS: Usual care as per treating physician PARTICIPANTS: Total participants: 2701 (Clopidogrel and aspirin: 1357, Aspirin only: 1344) Diabetes participants: 704 (Clopidogrel and aspirin: 340, Aspirin: 364) Total participants withdrawn or prematurely discontinuing study medication: 2061 (Clopidogrel and aspirin: 1030, Aspirin: 1031) Total participants lost to follow‐up: unknown |
|
| Interventions |
INTERVENTION: Clopidogrel 75 mg daily and aspirin 100 mg to 200 mg daily CONTROL: Aspirin 100 mg to 200 mg per day NUMBER OF STUDY CENTRES: 22 COUNTRY/ LOCATION: South Korea SETTING: Inpatient and community TREATMENT BEFORE STUDY: Dual antiplatelet therapy |
|
| Outcomes |
OUTCOMES (as stated in registered trial documents): Myocardial infarction Death from cardiac causes Death from any cause Composite of myocardial infarction, stoke, death from cardiac causes Major bleeding All cause mortality patients with diabetes: unknown All cause mortality all patients: Aspirin and clopidogrel: 20, Aspirin only 13 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: unknown Myocardial infarction patients with diabetes (fatal and non‐fatal): unknown Myocardial infarction all patients (fatal and non‐fatal): Aspirin and clopidogrel: 10, Aspirin only: 7 [specific numbers for fatal and non‐fatal unknown] Stroke patients with diabetes (fatal and non‐fatal): unknown Stroke all patients (fatal and non‐fatal): Aspirin and clopidogrel: 9, Aspirin only: 4 [specific numbers for fatal and non‐fatal unknown] Major bleeding patients with diabetes: unknown Major bleeding all patients: Aspirin and clopidogrel: 3, Aspirin only: 1 |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: No NON‐COMMERCIAL FUNDING: Unknown PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | "evaluated the effect of the use of dual antiplatelet therapy for more than 12 months on long‐term clinical outcomes in patients who had undergone initial PCI with the placement of a drug‐eluting stent" | |
| Notes | "There was no industry involvement in the design, conduct, financial support, or analysis" Abbrevations: PCI: percutaneous coronary intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assignments were made according to a pre‐established, computer‐generated randomization scheme, with stratification on the basis of site and type of ... drug eluting stent" |
| Allocation concealment (selection bias) | High risk | "the trial was open label study..... subjects and the investigators were aware of the treatment assignments" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and investigators were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than half of the patients discontinued study medication by the end of the study |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated |
| Other bias | Low risk | "no industry involvement in the design, conduct, financial support, or analysis" |
PRoFESS 2008.
| Methods |
RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN FACTORIAL RCT: 2 x 2 factorial design comparing aspirin and dipyridamole with clopidogrel and telmisartan with placebo |
|
| Participants |
INCLUSION CRITERIA: 1. Patients ≥ 55 years AND ischaemic stroke within 90 days before randomisation. After enrolment of 6000 patients the protocol was amended to broaden the inclusion criteria as follows: 2. Patients 50to 54 years AND ischaemic stroke within 120 days AND two additional risk factors (diabetes mellitus, hypertension, smoker at time of qualifying stroke, obesity (body mass index > 30), previous vascular disease (stroke, MI or peripheral arterial disease), end‐organ damage (retinopathy, left ventricular hypertrophy or microalbuminuria) or hyperlipidaemia. 3. Patients ≥ 55 years AND ischaemic stroke within 120 days AND two additional risk factors EXCLUSION CRITERIA: 1. Patients unable to give informed consent 2. Patients presenting with a primary haemorrhagic stroke (intracerebral haemorrhage or subarachnoid haemorrhage) 3. Patients who are unable to take by mouth all required study medication 4. Known brain tumour 5. Pre‐stroke history of dementia requiring institutional care 6. A modified Rankin scale score > 4 at baseline 7. The patient is unlikely to be released from hospital following the qualifying stroke, or the presence of a severe disability after the qualifying stroke likely to lead to the patient being bedridden or demented, or a non‐vascular disease or condition which makes it unlikely that the patient will survive to the end of the trial 8. Patients whose qualifying stroke had been induced by a surgical or cardiovascular procedure such as carotid endarterectomy, angiogram or cardiac surgery 9. Patients with known hypersensitivity to dipyridamole, clopidogrel, aspirin or telmisartan 10. Uncontrolled hypertension which equals or exceeds either a sitting systolic BP greater than 180 mm Hg, or a sitting diastolic BP greater than 110 mm Hg (all hypertensive patients are treated appropriately, and ‘goal’ BPs are much lower than 180/110) 11. Seated systolic BP ≤ 120 mm Hg for patients who are still hospitalised following the qualifying stroke 12. Patients currently being treated with an angiotensin II receptor blocker who are unable or unwilling to discontinue treatment with this type of drug 13. Patients with required or planned continued treatment with antithrombotics or anticoagulants including heparin or warfarin, or non‐study platelet inhibitors 14. Known severe renal insufficiency defined as renal artery stenosis or creatinine clearance < 0.6 ml/s or serum creatinine > 265 μmol/L (> 3.0 mg/dL) 15. Known severe hepatic dysfunction as defined by the following laboratory parameters: SGPT (ALT) or SGOT (AST) > 4 times upper limit of normal, or total bilirubin > 20 μmol/L 16. Hyperkalaemia, defined as potassium > 5.5 mmol/L 17. Uncorrected volume depletion or sodium depletion 18. Known current active peptic ulcer disease 19. Patients with the syndrome of asthma, rhinitis and nasal polyps (all three present) 20. Known severe coronary artery disease including unstable angina pectoris or an MI within the previous 3 months 21. Patients considered unreliable by the investigator concerning the requirements for follow‐up during the study and/or compliance with study drug administration 22. Known presence of or history of a haemostatic disorder or systemic bleeding 23. History of thrombocytopenia (i.e. less than 100 × 109/L for platelets) or neutropenia (1.2 × 109/L for neutrophils) 24. Women who are breast‐feeding, pregnant or of childbearing potential who do not use a medically acceptable form of contraception 25. Patients who have been exposed to an investigational drug or device within the last 30 days, or are currently participating in such a trial 26. Patients scheduled for major surgery, carotid endarterectomy or carotid angioplasty should not enter the study; such patients may enter the trial 4 weeks after such procedures, if they still meet all other entry criteria PARTICIPANTS: Total participants: 20,332 (Clopidogrel: 10,151, Aspirin/dipyridamole: 10,181) Diabetes participants: 5743 (Clopidogrel: 2840, Aspirin/dipyridamole: 2903) Total participants withdrawn or prematurely discontinuing study medication: Clopidogrel: 2290, Aspirin/dipyridamole: 2961) Total participants lost to follow‐up: 250 (Clopidogrel: 125, Aspirin/dipyridamole: 125) |
|
| Interventions |
INTERVENTION: Clopidogrel 75 mg daily and placebo CONTROL: Aspirin/dipyridamole 25 mg/200 mg twice daily and placebo For the first 8 months, 2027 patients in the clopidogrel group were receiving aspirin and clopidogrel however the MATCH trial demonstrated increased bleeding with aspirin and clopidogrel so the protocol was changed to clopidogrel only. NUMBER OF STUDY CENTRES: 695 COUNTRY/ LOCATION: 35 countries SETTING: Hospital and community |
|
| Outcomes |
OUTCOMES (as stated in the published protocol): Primary outcome event: Stroke (non‐fatal or fatal) either ischaemic or haemorrhagic or of uncertain cause Secondary outcome events: Vascular events either stroke or MI or vascular death All cause mortality patients with diabetes: unknown All cause mortality all patients: Clopidogrel: 756, Aspirin/dipyridamole: 739 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: unknown Myocardial infarction patients with diabetes (fatal and non‐fatal): unknown Myocardial infarction all patients (fatal and non‐fatal): Clopidogrel: 197, Aspirin/dipyridamole: 178 [specific numbers for fatal and non‐fatal unknown] Stroke patients with diabetes (fatal and non‐fatal): Clopidogrel: 334, Aspirin/dipyridamole: 312 [specific numbers for fatal and non‐fatal unknown] Stroke all patients (fatal and non‐fatal): Clopidogrel: 862, Aspirin/dipyridamole: 879 [specific numbers for fatal and non‐fatal unknown] Serious adverse event patients with diabetes: unknown Serious adverse event all patients (only bleeding recorded): Clopidogrel: 365, Aspirin/dipyridamole: 419 |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | "to compare the relative efficacy and safety of aspirin plus extended‐release dipyridamole with that of clopidogrel among patients who had a recent ischemic stroke" | |
| Notes | Supported by Boehringer Ingelheim (which manufactures Aggrenox [a combination of extended‐release dipyridamole and aspirin] and Micardis [telmisartan]) Abbreviations: BP: blood pressure; MI: myocardial infarction; SGOT (AST): Serum glutamic oxaloacetic transaminase / aspartate transaminase;.SGPT (ALT): Serum glutamic pyruvate transaminase / alanine transaminase |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization to telmisartanwas stratified based on whether or not individuals were receiving ACE inhibitors, although the analysis will be based on both strata combined. Patients were randomized using a central telephone randomization system." (from protocol of PRoFESS) |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized using a central telephone randomization system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the medication was ‘double dummy’ ... so that all patients received identically appearing medication kits" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 175 patients lost in both groups |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated |
| Other bias | High risk | "the ASA active and placebo component of the clopidogrel arm was discontinued following the presentation of the MATCH results and removed from subsequent treatment kits" Funded by Boehringer Ingelheim |
TASS 1989.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL, RANDOMISATION RATIO: 1:1, EQUIVALENCE DESIGN | |
| Participants |
INCLUSION CRITERIA: One of the following within the past 3 months: Transient Ischaemic attack: focal ischaemic cerebrovascular event lasting < 24 hours followed by complete recovery Amaurosis fugax: unilateral ischaemic retinal episode lasting < 24 hours Reversible ischaemic neurologic deficit: focal ischaemic cerebrovascular event lasting > 24 hours and < 3 weeks followed by complete recovery Minor stroke: focal ischaemic cerebrovascular event results in minimal permanent neurologic deficit and at least 80% recovery of function within three weeks EXCLUSION CRITERIA: Carotid artery surgery within last three months Moderate to major stroke within last three months Women with child bearing potential Cardiogenic embolism Haematological disorders History of peptic ulcer disease or gastrointestinal bleeding Life‐threatening diseases such as cancer Hypersensitivity or intolerance to aspirin Need for continued use of aspirin or anticoagulants CONCURRENT MEDICATIONS: Anticoagulants and antiplatelets prohibited. PARTICIPANTS: Total participants: 3069 (Ticlodipine: 1529, Aspirin: 1540) [12 participants later judged ineligible] Diabetes participants: 597 (Ticlodipine: 291, Aspirin: 306) Total participants withdrawn or prematurely discontinuing study medication: 1216 (Ticlopidine: 655, Aspirin: 561) Total participants lost to follow‐up: 84 (Ticlodipine: 46, Aspirin: 38) |
|
| Interventions |
INTERVENTION: Ticlopidine 250 mg twice daily CONTROL: Aspirin 650 mg twice daily NUMBER OF STUDY CENTRES: 56 COUNTRY/ LOCATION: United States and Canada SETTING: Hospital and community |
|
| Outcomes |
OUTCOMES(as stated in the published report): Stroke: Demonstration of brain infarction at autopsy or rapid onset of focal cerebral dysfunction > 24 hours associated with typical findings on neurologic examination, laboratory studies and radiologic tests Death from all causes All cause mortality patients with diabetes: unknown All cause mortality all patients: Ticlopidine: 175, Aspirin: 196 Vascular mortality patients with diabetes: unknown Vascular mortality all patients: Ticlopidine: 120, Aspirin: 116 Myocardial infarction patients with diabetes (fatal): unknown Myocardial infarction patients with diabetes (non‐fatal): unknown Myocardial infarction all patients (fatal): Ticlopidine: 21, Aspirin: 14 Myocardial infarction all patients (non‐fatal): unknown Stroke patients with diabetes (fatal and non‐fatal): Ticlopidine: 25, Aspirin: 44 [specific numbers for fatal and non‐fatal unknown] Stroke all patients (fatal): Ticlopidine: 16, Aspirin: 23 Stroke all patients (non‐fatal): Ticlopidine: 156, Aspirin:189 Serious adverse event patients with diabetes: unknown Serious adverse event (bleeding only) all patients: Ticlopidine: 137, Aspirin: 152 |
|
| Study details |
RUN‐IN PERIOD: No STUDY TERMINATED BEFORE REGULAR END: No |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING PUBLICATION STATUS: PEER REVIEW JOURNAL |
|
| Stated aim of study | "to test the usefulness of ticlopidine in preventing stroke or death in patients at high risk, we undertook a randomized trial comparing ticlopidine and aspirin" | |
| Notes | "supported by Syntex Research, Palo Alto, Calif." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization within each center stratified on ... history of ischemic cardiovascular disease, the occurrence of a moderate or major stroke more than three months before entry, and the patient’s sex" "Maryland Medical Research Institute ... randomly assigned eligible patients" |
| Allocation concealment (selection bias) | Low risk | "only the Maryland Medical Research Institute and an independent, external safety committee had access to the randomized code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "medications were suppIied in identical capsules and containers" "Blinded adjudicators ... classified alI end‐point events using standardized criteria drawn up before the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "intention to treat analyses were performed" less than 3% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Published protocol or registered trial document not available |
| Other bias | High risk | Pharmaceutical funded |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balsano 1990 | Follow‐up only 6 months |
| CHANCE 2010 | Follow‐up only 3 months |
| CLARITY 2005 | Follow‐up only 30 days |
| COMMIT 2005 | Follow‐up only 30 days |
| CREDO 2002 | Placebo group received clopidogrel for 30 days after PCI |
| Cun 2002 | Follow‐up only 6 months |
| Harrington 2009 | Follow‐up only 30 days |
| Ishikawa 1997 | Observational trial |
| JUMBO TIMI 2005 | Follow‐up only 30 days |
| Li 1999 | Diabetes information not collected |
| Machraoui 2001 | Follow‐up only 3 months |
| MATCH 2004 | ADP‐receptor inhibitor was not compared with alternative treatment or placebo |
| Mueller 2003 | Intervention for only 4 weeks |
| PLATO 2006 | ADP‐receptor inhibitor is not compared with alternative treatment or placebo |
| STAMI 2001 | Follow‐up only 3 months |
| STIMS 1990 | Diabetes patients on insulin excluded |
| TISS 1997 | Follow‐up only 30 days |
| Tohgi 1987 | Diabetes information not collected |
| TOPALS 2003 | ADP‐receptor inhibitor was not compared with alternative treatment or placebo |
| TRITON‐TIMI 38 2008 | ADP‐receptor inhibitor was not compared with alternative treatment or placebo |
Characteristics of ongoing studies [ordered by study ID]
SPS3 2011.
| Trial name or title | Secondary Prevention of Small Subcortical Strokes (SPS3) |
| Methods | Randomised controlled trial |
| Participants | 3000 patients with small subcortical strokes within the last 6 months |
| Interventions | Clopidogrel and aspirin or aspirin and placebo Intensive or usual blood pressure treatment |
| Outcomes | Recurrent stroke, rate of cognitive decline and major vascular events |
| Starting date | February 2003 |
| Contact information | SPS3 Coordinating Centre UBC Department of Medicine | Division of Neurology S169‐2211 Wesbrook Mall, Vancouver, BC, V6T 2B5 Phone: 604‐822‐1789 |
| Notes |
Differences between protocol and review
At the time of protocol publication, it was anticipated there would be more data on patients with diabetes available for this review. Due to the limited data available, we were not able to undertake various statistical analyses. The protocol stated that data would be extracted on outcomes including peripheral artery disease, health‐related quality of life and costs; however, these were not available. Similarly, data were not available on potential patient covariates such as compliance and glycaemic control.
The protocol also intended for an analysis to be done on patients with type 2 diabetes only. None of the included trials separated patients into type 1 and type 2 diabetes so analysis was done on all types of diabetes mellitus.
The search strategy published in the protocol was changed as it was found to be too narrow. The actual search strategy used (Appendix 1) did not include a subheading 'diabetes' as all of the trials on ADP receptor antagonists were conducted on patients both with and without diabetes.
The authors undertaking this review were different to the authors of the protocol due to other commitments. The title of the review has been expanded to include additional oral ADP receptor antagonists which have become available since the publication of the protocol.
Contributions of authors
Nyoli Valentine: Searching for trials, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development.
Floris van de Laar: Protocol development and review development.
Mieke van Driel: Searching for trials, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development.
Sources of support
Internal sources
-
Radboud University Nijmegen Medical Centre, Netherlands.
Support for FvdL
External sources
-
General Practice Education & Training, Australia.
Funding support for NV
Declarations of interest
No declarations are known for Nyoli Valentine, Floris van de Laar or Mieke van Driel.
New
References
References to studies included in this review
AAASPS 2003 {published data only (unpublished sought but not used)}
- Gorelick PB, Leurgans S, Richardson J, Harris Y, Billingsley M and the AAASPS Investigators. African American Antiplatelet Stroke Prevention Study: clinical trial design. Journal of Stroke and Cerebrovascular Diseases 1998;7(6):426‐34. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Richardson D, Kelly M, Ruland S, Hung E, Harris Y, et al. Aspirin and ticlopidine for prevention of recurrent stroke in black patients. JAMA 2003;289(22):2947‐57. [DOI] [PubMed] [Google Scholar]
CAPRIE 1996 {published data only (unpublished sought but not used)}
- Bhatt DL, Marso SP, Hirsch AT, Tingleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. The American Journal of Cardiology 2002;90:625‐8. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). The Lancet 1996;348(9038):1329‐39. [DOI] [PubMed] [Google Scholar]
- Creager MA. Results of the CAPRIE trial: efficacy and safety of clopidogrel. Clopidogrel versus aspirin in patients at risk of ischaemic events. Vascular Medicine 1998;3(3):257‐60. [DOI] [PubMed] [Google Scholar]
- Harker LA, Boissel JP, Pilgrim AJ, Gent M. Comparative safety and tolerability of clopidogrel and aspirin: results from CAPRIE. CAPRIE Steering Committee and Investigators. Clopidogrel versus aspirin in patients at risk of ischaemic events. Drug Safety 1999;21(4):325‐35. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Bhatt DL. Comparative benefits of clopidogrel and aspirin in high‐risk patient populations. Archives of Internal Medicine 2004;164:2106‐10. [DOI] [PubMed] [Google Scholar]
CATS 1988 {published and unpublished data}
- Gent M, Blakely JA, Easton JD, Ellis DJ, Hachinski VC, Harbison JW, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Design, organisation, and baseline results. Stroke 1988;19:1203‐10. [DOI] [PubMed] [Google Scholar]
- Gent M, Easton JD, Hachinski VC, Panak E, Sicurella J, Blakely JA, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. The Lancet 1989;8649:1215‐20. [DOI] [PubMed] [Google Scholar]
- Gent M, Ellis D. Canadian American ticlopidine study (cats) in thromboembolic stroke. Agents and Actions. Supplements 1984;15:283‐96. [PubMed] [Google Scholar]
CHARISMA 2002 {published data only (unpublished sought but not used)}
- Bakhru MR, Bhatt DL. Interpreting the CHARISMA study. What is the role of dual antiplatelet therapy with clopidogrel and aspirin?. Cleveland Clinic Journal of Medicine 2008;75(4):289‐95. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. Journal of the American College of Cardiology 2007;49(19):1982‐8. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KAA, Hacke W, Berger PB, Black HR, for the CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. The New England Journal of Medicine 2006;354(16):1706‐17. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Topol EJ, on behalf of the CHARISMA Executive Committee. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high‐risk primary prevention: Rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. American Heart Journal 2004;148( 2 ):263‐8. [DOI] [PubMed] [Google Scholar]
- Collet JP, Montalescot G, Steg PG, Steinhubl SR, Fox KA, Hu TF, et al. Clinical outcomes according to permanent discontinuation of clopidogrel or placebo in the CHARISMA trial. Archives of Cardiovascular Diseases 2009;102(6‐7):485‐96. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, for the CHARISMA Investigators. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischaemic stabilization, management, and avoidance [CHARISMA] trial). American Journal of Cardiology 2009;103(10):1359‐63. [DOI] [PubMed] [Google Scholar]
- Gresele P, Migliacci R. The peripheral arterial disease subgroup in the CHARISMA trial: does it tell us anything new?. European Heart Journal 2009;30(2):131‐2. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Johnston SC, Easton JD, Hacke W, Mas JL, Brennan D, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. International Journal of Stroke 2011;6(1):3‐9. [DOI] [PubMed] [Google Scholar]
- Wang TH, Bhatt DL, Fox KA, Steinhubl SR, Brennan DM, Hacke W, et al. An analysis of mortality rates with dual‐antiplatelet therapy in the primary prevention population of the CHARISMA trial. European Heart Journal 2007;28(18):2200‐7. [DOI] [PubMed] [Google Scholar]
CURE 1998 {published data only (unpublished sought but not used)}
- Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004;110(10):1202‐8. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Bhatt DL. Comparative benefits of clopidogrel and aspirin in high‐risk patient populations: Lessons from the CAPRIE and CURE studies. Archives of Internal Medicine 2004;164(19):2106‐10. [DOI] [PubMed] [Google Scholar]
- Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study . Circulation 2003;108(12):1682‐7. [DOI] [PubMed] [Google Scholar]
- The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. The New England Journal of Medicine 2001;345(7):494‐502. [DOI] [PubMed] [Google Scholar]
Park 2010 {published data only (unpublished sought but not used)}
- Park SJ, Park DW, Kim YH. Duration of dual antiplatelet therapy after implantation of drug‐eluding stents. The New England Journal of Medicine 2010;362(15):1374‐82. [DOI] [PubMed] [Google Scholar]
PRoFESS 2008 {published data only (unpublished sought but not used)}
- Bath PM, Cotton D, Martin RH, Palesch Y, Yusuf S, Sacco R, et al. Effect of combined aspirin and extended‐release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke 2010;41(4):732‐8. [DOI] [PubMed] [Google Scholar]
- Diener HC. The PRoFESS trial: future impact on secondary stroke prevention. Expert Review of Neurotherapeutics 2007;7(9):1085‐91. [DOI] [PubMed] [Google Scholar]
- Diener HS, Sacco R, Yusuf S, for the Steering Committee and PRoFESS Study Group. Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS). Cerebrovascular Diseases 2007;23(5‐6):368‐80. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, for the PRoFESS Study Group. Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke. The New England Journal of Medicine 2008;359(12):1238‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
TASS 1989 {published data only (unpublished sought but not used)}
- Bellavance A. Efficacy of ticlopidine and aspirin for prevention of reversible cerebrovascular ischemic events. The ticlopidine aspirin stroke study. Stroke 1993;24(10):1452‐7. [DOI] [PubMed] [Google Scholar]
- Grutta JC, Norris JW, Kamin B and the TASS Baseline and Angiographic Data Subgroup. Prevention of stroke with ticlopidine: Who benefits most?. Neurology 1992;42( 1 ):111‐5. [DOI] [PubMed] [Google Scholar]
- Harbison JW. Ticlopidine versus aspirin for the prevention of recurrent stroke. Analysis of patients with minor stroke from the ticlopidine aspirin stroke study. Stroke 1992;23(12):1723‐7. [DOI] [PubMed] [Google Scholar]
- Hass WK, Easton J, Adams HP, Pryse‐Phillips W, Molony BA, for the Ticlopidine Aspirin Stroke Study Group. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. The New England Journal of Medicine 1989;321(8):501‐7. [DOI] [PubMed] [Google Scholar]
- Pryse‐Phillips WAU, for the TASS Group. Ticlopidine aspirin stroke study: outcome by vascular distribution of the qualifying event. Journal of Stroke and Cerebrovascular Diseases 1993;3:49‐56. [DOI] [PubMed] [Google Scholar]
- Ticlopidine Aspirin Stroke Study Group. Ticlopidine versus aspirin for stroke prevention: on‐treatment results from the ticlopidine aspirin stroke study. Journal of Stroke and Cerebrovascular Diseases 1993;3:168‐76. [DOI] [PubMed] [Google Scholar]
- Weisberg LA. The efficacy and safety of ticlopidine and aspirin in non‐whites: analysis of a patient subgroup from the ticlopidine aspirin stroke study. Neurology 1993;43(1):27‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Balsano 1990 {published data only}
- Balsano F, Rizzon P, Violi F, Scrutinio D, Cimminiello C, Aguglia F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. Circulation 1990;82(1):17‐26. [DOI] [PubMed] [Google Scholar]
CHANCE 2010 {published data only}
- Wang Y, Johnston SC, for the CHANCE investigators. Rationale and design of a randomized, double‐blind trial comparing the effects of a 3‐month clopidogrel‐aspirin regimen versus aspirin alone for the treatment of high‐risk patients with acute nondisabling cerebrovascular event. American Heart Journal 2010;160(3):380‐6. [DOI] [PubMed] [Google Scholar]
CLARITY 2005 {published data only}
- Sabatine MS, Cannon CP, Gibson CM, López‐Sendón JL, Montalescot G, Theroux P, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics: the PCI‐CLARITY study. JAMA 2005;294(10):1224‐32. [DOI] [PubMed] [Google Scholar]
COMMIT 2005 {published data only}
- Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial. The Lancet 2005;366(9497):1607‐21. [DOI] [PubMed] [Google Scholar]
CREDO 2002 {published data only}
- Steinhubl SR, Berger PB, Mann JT III, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288(19):2411‐20. [DOI] [PubMed] [Google Scholar]
Cun 2002 {published data only}
- Cun VG, Temuzhan A, Pekdemur H, Toka M, Tartanoglu O. The effects of ticlopidine in acute myocardial infarction as an adjunctive treatment to aspirin in an intermediate term setting. Turkish Journal of Medical Sciences 2002;32(4):329‐34. [Google Scholar]
Harrington 2009 {published data only}
- Harrington RA, Stone GW, McNulty S, White HD, Lincoff AM, Gibson CM, et al. Platelet inhibition with cangrelor in patients undergoing PCI. The New England Journal of Medicine 2009;361(24):2318‐29. [DOI] [PubMed] [Google Scholar]
Ishikawa 1997 {published data only}
- Ishikawa K, Kanamasa K, Hama J, Ogawa I, Takenaka T, Naito T, et al. Aspirin plus either dipyridamole or ticlopidine is effective in preventing recurrent myocardial infarction. Secondary Prevention Group. Japanese Circulation Journal 1997;61(1):38‐45. [DOI] [PubMed] [Google Scholar]
JUMBO TIMI 2005 {published data only}
- Wiviott SD, Antman EM, Winters KJ, Weerakkody G, Murphy SA, Behounek BD, et al. Randomized comparison of prasugrel (CS‐747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)‐TIMI 26 trial. Circulation 2005;111(25):3366‐73. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li YZ, Wang L, Yu PX. A randomized trial comparing ticlopidine with aspirin for the prevention of ischaemic cerebral stroke. Acta Pharmacologica Sinica 1999;20(5):476. [Google Scholar]
Machraoui 2001 {published data only}
- Machraoui A, Germing A, Lindstaedt M, Dryander S, Bojara W, Lawo T, et al. Efficacy and safety of ticlopidine monotherapy versus ticlopidine and aspirin after coronary artery stenting: follow‐up results of a randomized study. Journal of Invasive Cardiology June 2001;13(6):431‐6. [PubMed] [Google Scholar]
MATCH 2004 {published data only}
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial. The Lancet 2004;364(9431):331‐7. [DOI] [PubMed] [Google Scholar]
Mueller 2003 {published data only}
- Mueller C, Roskamm H, Neumann FJ, Hunziker P, Marsch S, Perruchoud A, et al. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary artery stents. Journal of the American College of Cardiology 2003;41(6):969‐73. [DOI] [PubMed] [Google Scholar]
PLATO 2006 {published data only}
- Wallentin L, Becker RC, Budaj A, Cannon C, for the PLATO investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine 2009;361(11):1045‐57. [DOI] [PubMed] [Google Scholar]
STAMI 2001 {published data only}
- Scrutinio D, Cimminiello C, Marubini E, Pitzalis MV, Biase M, Rizzon P. Ticlopidine versus aspirin after myocardial infarction (STAMI) trial. Journal of American College of Cardiology 2001;37(5):1259‐65. [DOI] [PubMed] [Google Scholar]
STIMS 1990 {published data only}
- Janzon L, Berggvist D, Boberg J, Boberg M, Eriksson I, Lindgarde F, et al. Prevention of myocardial infarction and stroke in patients with intermittent claudication; effects of ticlopidine. Results from STIMS, the Swedish Ticlopidine Multicentre Study. Journal of Internal Medicine 1990;227(5):301‐8. [DOI] [PubMed] [Google Scholar]
TISS 1997 {published data only}
- Bergamasco B, Benna P, Carolei A, Rasura M, Rudelli G, Fieschi C. A randomized trial comparing ticlopidine hydrochloride with ibuprofen for the prevention of stroke in high‐risk patients (TISS Study). Ticlopidine Indobufen Stroke Study. Functional Neurology 1997;12(1):33‐43. [PubMed] [Google Scholar]
Tohgi 1987 {published data only}
- Tohgi H, Murakami M. The effect of ticlopidine on TIA compared with aspirin ‐ a double‐blind, twelve‐month and open 24 month follow up study. Japanese Journal of Medicine 1987;26(1):117‐9. [Google Scholar]
TOPALS 2003 {published data only}
- Ito E, Takahashi A, Yamamoto H, Kuzuhara S, Uchiyama S, Nakajima M. Ticlopidine alone versus ticlopidine plus aspirin for preventing recurrent stroke (the Tokai Panaldine Aspirin Long‐term Study (TOPALS)). Internal Medicine 2003;42(9):793‐9. [DOI] [PubMed] [Google Scholar]
TRITON‐TIMI 38 2008 {published data only}
- Wivott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine 2007;357(20):2001‐15. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
SPS3 2011 {published data only}
- Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, and the SPS3 Investigators. The secondary prevention of small subcortical strokes (SPS3) study. International Journal of Stroke April 2011;6(2):164‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ADA 1997
- American Diabetes Association. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 1999
- American Diabetes Association. The Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22(Suppl 1):S1‐114. [PubMed] [Google Scholar]
ADA 2011
- American Diabetes Association. Standards of medical care in diabetes‐2011. Diabetes Care 2011;34(Suppl 1):s11‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angiolillo 2009
- Angiolillo DJ, Suryadevara S. Aspirin and clopidogrel: efficacy and resistance in diabetes mellitus. Best Practice & Research Clinical Endocrinology & Metabolism 2009 Jun;23(3):375‐88. [DOI] [PubMed] [Google Scholar]
Antithrombotic Trialists' Collaboration 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324(7329):71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatt 2002
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. American Journal of Cardiology 2002;90(6):625‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ceriello 2004
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arteriosclerosis, thrombosis, and vascular biology 1: Arteriosclerosis, thrombosis, and vascular biology 2004;24(5):816‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Colwell 2003
- Colwell JA. Aspirin for primary prevention of cardiovascular events in diabetes. Diabetes Care 2003;26(12):3349‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodwin 2011
- Goodwin MM, Desilets AR, Willett KC. Thienopyridines in acute coronary syndrome. The Annals of Pharmacotherapy 2011;45(2):207‐17. [DOI] [PubMed] [Google Scholar]
Gu 1998
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971‐1993. Diabetes Care 1998;21(7):1138‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haffner 1998
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New England Journal of Medicine 1998;339(4):229‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kam 2003
- Kam, CA, Nethery, CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and developments. Anaesthesia 2003;58(1):28‐35. [DOI] [PubMed] [Google Scholar]
Kutti 1986
- Kutti J, Wadenvik H, Henestam B, Stenstrom G. Evaluation of platelet reactivity in diabetes mellitus. Acta Medica Scandinavica 1986;219(2):195‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- National Insitute for Health and Clinical Excellence. Unstable angina and NSTEMI. The early management of unstable angina and non‐ST‐segment‐elevation myocardial infarction. Clinical guideline 94. http://guidance.nice.org.uk/CG94/NICEGuidance/pdf/English (accessed March 2010).
Squizzato 2011
- Squizzato A, Keller T, Romualdi E, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD005158.pub3] [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Sudlow 2009
- Sudlow CLM, Mason G, Maurice JB, Wedderburn CJ, Hankey GJ. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD001246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. World Health Organisation: Technical Report Series 646; 1980. [PubMed]
WHO 1985
- World Health Organisation. Diabetes Mellitus: Report of a WHO Study Group. World Health Organisation: Technical Report Series No. 727; 1985. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539‐53. [DOI] [PubMed] [Google Scholar]
Wild 2004
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


