Abstract
Background
The evidence on whether vitamin D supplementation is effective in decreasing cancers is contradictory.
Objectives
To assess the beneficial and harmful effects of vitamin D supplementation for prevention of cancer in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, Science Citation Index Expanded, and the Conference Proceedings Citation Index‐Science to February 2014. We scanned bibliographies of relevant publications and asked experts and pharmaceutical companies for additional trials.
Selection criteria
We included randomised trials that compared vitamin D at any dose, duration, and route of administration versus placebo or no intervention in adults who were healthy or were recruited among the general population, or diagnosed with a specific disease. Vitamin D could have been administered as supplemental vitamin D (vitamin D₃ (cholecalciferol) or vitamin D₂ (ergocalciferol)), or an active form of vitamin D (1α‐hydroxyvitamin D (alfacalcidol), or 1,25‐dihydroxyvitamin D (calcitriol)).
Data collection and analysis
Two review authors extracted data independently. We conducted random‐effects and fixed‐effect model meta‐analyses. For dichotomous outcomes, we calculated the risk ratios (RRs). We considered risk of bias in order to assess the risk of systematic errors. We conducted trial sequential analyses to assess the risk of random errors.
Main results
Eighteen randomised trials with 50,623 participants provided data for the analyses. All trials came from high‐income countries. Most of the trials had a high risk of bias, mainly for‐profit bias. Most trials included elderly community‐dwelling women (aged 47 to 97 years). Vitamin D was administered for a weighted mean of six years. Fourteen trials tested vitamin D₃, one trial tested vitamin D₂, and three trials tested calcitriol supplementation. Cancer occurrence was observed in 1927/25,275 (7.6%) recipients of vitamin D versus 1943/25,348 (7.7%) recipients of control interventions (RR 1.00 (95% confidence interval (CI) 0.94 to 1.06); P = 0.88; I² = 0%; 18 trials; 50,623 participants; moderate quality evidence according to the GRADE instrument). Trial sequential analysis (TSA) of the 18 vitamin D trials shows that the futility area is reached after the 10th trial, allowing us to conclude that a possible intervention effect, if any, is lower than a 5% relative risk reduction. We did not observe substantial differences in the effect of vitamin D on cancer in subgroup analyses of trials at low risk of bias compared to trials at high risk of bias; of trials with no risk of for‐profit bias compared to trials with risk of for‐profit bias; of trials assessing primary prevention compared to trials assessing secondary prevention; of trials including participants with vitamin D levels below 20 ng/mL at entry compared to trials including participants with vitamin D levels of 20 ng/mL or more at entry; or of trials using concomitant calcium supplementation compared to trials without calcium. Vitamin D decreased all‐cause mortality (1854/24,846 (7.5%) versus 2007/25,020 (8.0%); RR 0.93 (95% CI 0.88 to 0.98); P = 0.009; I² = 0%; 15 trials; 49,866 participants; moderate quality evidence), but TSA indicates that this finding could be due to random errors. Cancer occurrence was observed in 1918/24,908 (7.7%) recipients of vitamin D₃ versus 1933/24,983 (7.7%) in recipients of control interventions (RR 1.00 (95% CI 0.94 to 1.06); P = 0.88; I² = 0%; 14 trials; 49,891 participants; moderate quality evidence). TSA of the vitamin D₃ trials shows that the futility area is reached after the 10th trial, allowing us to conclude that a possible intervention effect, if any, is lower than a 5% relative risk reduction. Vitamin D₃ decreased cancer mortality (558/22,286 (2.5%) versus 634/22,206 (2.8%); RR 0.88 (95% CI 0.78 to 0.98); P = 0.02; I² = 0%; 4 trials; 44,492 participants; low quality evidence), but TSA indicates that this finding could be due to random errors. Vitamin D₃ combined with calcium increased nephrolithiasis (RR 1.17 (95% CI 1.03 to 1.34); P = 0.02; I² = 0%; 3 trials; 42,753 participants; moderate quality evidence). TSA, however, indicates that this finding could be due to random errors. We did not find any data on health‐related quality of life or health economics in the randomised trials included in this review.
Authors' conclusions
There is currently no firm evidence that vitamin D supplementation decreases or increases cancer occurrence in predominantly elderly community‐dwelling women. Vitamin D₃ supplementation decreased cancer mortality and vitamin D supplementation decreased all‐cause mortality, but these estimates are at risk of type I errors due to the fact that too few participants were examined, and to risks of attrition bias originating from substantial dropout of participants. Combined vitamin D₃ and calcium supplements increased nephrolithiasis, whereas it remains unclear from the included trials whether vitamin D₃, calcium, or both were responsible for this effect. We need more trials on vitamin D supplementation, assessing the benefits and harms among younger participants, men, and people with low vitamin D status, and assessing longer duration of treatments as well as higher dosages of vitamin D. Follow‐up of all participants is necessary to reduce attrition bias.
Keywords: Aged, Female, Humans, Male, Middle Aged, Dietary Supplements, Calcitriol, Calcitriol/administration & dosage, Cholecalciferol, Cholecalciferol/administration & dosage, Hydroxycholecalciferols, Hydroxycholecalciferols/administration & dosage, Neoplasms, Neoplasms/epidemiology, Neoplasms/prevention & control, Randomized Controlled Trials as Topic, Vitamin D, Vitamin D/administration & dosage, Vitamin D/analogs & derivatives, Vitamins, Vitamins/administration & dosage
Plain language summary
Vitamin D supplementation for prevention of cancer in adults
Review question
Does vitamin D supplementation prevent cancer?
Background
The available evidence on vitamin D and cancer occurrence is intriguing but inconclusive. Many observational studies as well as randomised trials suggest that high vitamin D levels in the blood are related to reduced cancer occurrence. However, results of randomised trials testing the effect of vitamin D supplementation for cancer prevention are contradictory.
Study characteristics
The aim of this systematic review was to analyse the benefits and harms of the different forms of vitamin D especially on cancer occurrence. A total of 18 trials provided data for this review; 50,623 participants were randomly assigned to either vitamin D or placebo or no treatment. All trials were conducted in high‐income countries.
Key results
The age range of the participants was 47 to 97 years and on average 81% were women. The majority of the included participants did not have vitamin D deficiency. Vitamin D administration lasted on average six years and most trial investigators used vitamin D₃ (cholecalciferol). We did not find firm evidence that vitamin D supplementation decreases or increases cancer occurrence in predominantly elderly community‐dwelling women. We observed decreases in all‐cause mortality and cancer‐related mortality among the vitamin D/D₃ treated participants in comparison with the participants in the control groups. However, using trial sequential analysis, a statistical approach to reconfirm or question these findings, we conclude that these results could be due to random errors (play of chance). We also found evidence that combined vitamin D₃ and calcium supplements increased renal stone occurrence, but it remains unclear from the included trials whether vitamin D₃, calcium, or both were responsible for this effect. Moreover, these results could also be due to random errors (play of chance).
Quality of the evidence
A large number of the study participants left the trials before completion, and this raises concerns regarding the validity of the results. Most of the trials were judged not to be well and fairly conducted so that the results were likely to be biased (that is, possibly an overestimation of benefits and underestimation of harms).
Currentness of evidence
This evidence is up to date as of February 2014.
Summary of findings
Summary of findings for the main comparison. Vitamin D versus placebo or no intervention for prevention of cancer in adults.
| Vitamin D versus placebo or no intervention for prevention of cancer in adults | ||||||
|
Patient or population: healthy participants or recruited among the general population; individuals diagnosed with a specific disease in a stable phase or with vitamin D deficiency Settings: outpatients Intervention: vitamin D versus placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin D versus placebo or no intervention | |||||
|
Cancer occurrence Follow‐up: 0.5 to 7 years |
Study population | RR 1.00 (0.94 to 1.06) | 50623 (18) | ⊕⊕⊕⊝ moderatea |
Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. | |
| 77 per 1000 | 77 per 1000 (72 to 81) | |||||
| Moderate | ||||||
| 28 per 1000 | 28 per 1000 (26 to 30) | |||||
|
Cancer occurrence in trials using vitamin D₃ (cholecalciferol) Follow‐up: 0.5 to 7 years |
Study population | RR 1.00 (0.94 to 1.06) | 49891 (14) | ⊕⊕⊕⊝ moderatea |
Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. | |
| 77 per 1000 | 77 per 1000 (73 to 82) | |||||
| Moderate | ||||||
| 28 per 1000 | 28 per 1000 (26 to 30) | |||||
|
All‐cause mortality Follow‐up: 0.5 to 7 years |
Study population | RR 0.93 (0.88 to 0.98) | 49866 (15) | ⊕⊕⊝⊝ lowb |
Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 80 per 1000 | 75 per 1000 (71 to 79) | |||||
| Moderate | ||||||
| 16 per 1000 | 15 per 1000 (14 to 16) | |||||
|
Cancer mortality in trials using vitamin D₃(cholecalciferol) Follow‐up: 5 to 7 years |
Study population | RR 0.88 (0.78 to 0.98) | 44492 (4) | ⊕⊕⊝⊝ lowb |
Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 29 per 1000 | 25 per 1000 (22 to 28) | |||||
| Moderate | ||||||
| 37 per 1000 | 33 per 1000 (29 to 36) | |||||
|
Adverse events: nephrolithiasis in trials using vitamin D₃(cholecalciferol) combined with calcium Follow‐up: 0.5 to 7 years |
Study population | RR 1.17 (1.03 to 1.34) | 42753 (3) | ⊕⊕⊝⊝ lowb |
Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 18 per 1000 | 21 per 1000 (18 to 24) | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 (1 to 1) | |||||
| Health‐related quality of life | See comment | Not investigated. | ||||
| Health economics | See comment | Not investigated. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level because of risk of attrition bias
bDowngraded by two levels because of risk of attrition bias and imprecision
Background
Vitamin D is a fat‐soluble vitamin, which maintains calcium and phosphorus homeostasis (Holick 2007; Horst 2005; Lips 2006). Vitamin D₃ (cholecalciferol) is synthesised in the skin from 7‐dehydrocholesterol during exposure to sunlight. Alternatively, vitamin D, in the form of either vitamin D₃ or D₂, can be obtained from dietary sources. Vitamin D₃ (cholecalciferol) is the predominant form of vitamin D in humans. Vitamin D₂ (ergocalciferol) is derived mainly from irradiated plants. Vitamin D, as either D₃ or D₂, does not have biological activity. It must be metabolised within the liver to 25‐hydroxycalciferol (calcidiol) and in the kidney to the biologically active form known as 1,25‐dihydroxycalciferol (calcitriol) (Holick 2007; Horst 2005; Lips 2006). Current interest in vitamin D is engendered by the hypothesis that it may prevent cancer and prolong life (Bjelakovic 2014; Davis 2007; Giovannucci 2005). The evidence on whether vitamin D is effective in decreasing cancers is contradictory.
Description of the condition
Vitamin D status is determined by the measurement of the serum 25‐hydroxyvitamin D level (Bischoff‐Ferrar 2009c; Dawson‐Hughes 2005; Lips 2004). There is controversy about the definition of optimal vitamin D status (Bouillon 2013; Hilger 2014). The Institute of Medicine recently recommended a target serum 25‐hydroxyvitamin D level of 20 ng/ml (50 nmol/L) (IOM 2011). Based upon the systematic review prepared by the Institute of Medicine there are insufficient data to determine the safe upper limit of serum 25‐hydroxyvitamin D level (IOM 2011). However, serum 25‐hydroxyvitamin D level concentrations above 50 ng/mL (125 nmol/L) were considered potentially harmful (IOM 2011). The International Osteoporosis Foundation and the Endocrine Society Task Force recommend a target serum 25‐hydroxyvitamin D level of 30 ng/ml (75 nmol/L) (Dawson‐Hughes 2010; Holick 2011). The worldwide prevalence of suboptimal vitamin D status is estimated to be high (Hilger 2014; Holick 2007; Lamberg‐Allardt 2006; Lips 2010: Van Schoor 2011; Zittermann 2003). The major causes of vitamin D deficiency are insufficient exposure to sunlight, decreased dietary intake, skin pigmentation, obesity, and advanced age (Lips 2006). Vitamin D deficiency in childhood results in rickets, while in adults it precipitates or exacerbates osteopenia and osteoporosis, and induces osteomalacia (Holick 2007). It has been speculated that vitamin D insufficiency is related to increased risks of cancer and cardiovascular diseases (Chiang 2013; Freedman 2007; Garland 2007; Giovannucci 2005; Gorham 2007; Michos 2008; Norman 2014; Schwartz 2007; Zittermann 2005; Zittermann 2006), the leading causes of death in middle‐ and high‐income countries (Mathers 2006). Vitamin D insufficiency might also be the consequence of a disease, but not the cause (Marshall 2008).
Description of the intervention
Vitamin D supplementation prevents osteoporosis, osteomalacia, and fractures (Holick 2007; Lamberg‐Allardt 2006). It has been postulated that vitamin D may have additional health effects beyond prevention of bone diseases (Bikle 2009; Sutton 2003). Ecologic studies have found that living at higher altitudes with lower exposure to sunlight is linked to increased cancer risk (Apperly 1941; Garland 1980). Most observational studies have associated increased vitamin D intake with decreased risk of cancer (Garland 2007; Gorham 2007; Schwartz 2007). Although high vitamin D status was connected with increased pancreatic cancer risk (Helzlsouer 2010; Stolzenberg‐Solomon 2006; Stolzenberg‐Solomon 2010), the range of 25‐hydroxyvitamin D levels associated with the cancer risk was below that considered to reflect hypervitaminosis D (Krstic 2011).
How the intervention might work
The active form of vitamin D functions as a steroid‐like hormone (Horst 2005). The effects of vitamin D are mediated by its binding to a vitamin D receptor (Norman 2006; Wesley Pike 2005), which is present in most tissues and cells in the body (Lips 2006). Upon binding to its receptors, vitamin D may enhance cell differentiation and cell apoptosis, and may inhibit cell proliferation in a variety of cell types (Flynn 2006). In addition, vitamin D is required for a functional immune system which is important for an adequate physiological response to infections, inflammatory diseases, immune‐system‐mediated diseases, and cancer (Bikle 2009; Cutolo 2009; Hart 2011; Sutton 2003). Thus, vitamin D supplementation could reduce cancer development.
Adverse effects of the intervention
Vitamin D toxicity is the result of excessive vitamin D intake. There is sparse evidence that ingestion of high quantities of vitamin D is harmful. The majority of the trials that reported hypercalcaemia, hypercalciuria, or nephrocalcinosis were conducted in participants with renal failure (Cranney 2007). We have shown that vitamin D₃ combined with calcium may increase nephrolithiasis, and that alfacalcidol and calcitriol may increase hypercalcaemia (Bjelakovic 2014).
Why it is important to do this review
The available evidence on vitamin D and cancer occurrence is intriguing but inconclusive. Results of recently completed randomised trials testing the influence of vitamin D supplementation for cancer prevention are contradictory. Lappe 2007 found that vitamin D supplementation is associated with significantly decreased cancer incidence. In contrast, another large randomised trial found no effect of vitamin D and calcium supplementation on cancer incidence (Brunner 2011). Although Chung 2011 found inconclusive evidence regarding vitamin D supplementation for the prevention of cancer in a recently updated meta‐analysis, the same review suggests that hazard ratios and risk ratios are correlated better with the cancer mortality when compared to the cancer incidence rates (Krstic 2012). Our aim was to systematically review and statistically analyse the available evidence in order to assess the effects of vitamin D supplementation on cancer prevention in adults.
Objectives
To assess the beneficial and harmful effects of vitamin D supplementation for prevention of cancer in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials, irrespective of blinding, publication status, or language.
Types of participants
Adult participants (aged 18 years or over) who were:
healthy or were recruited from the general population;
diagnosed with a specific disease in a stable phase;
diagnosed with vitamin D deficiency.
We excluded trials including:
people with secondary induced osteoporosis (e.g., glucocorticoid‐induced osteoporosis, thyroidectomy, primary hyperparathyroidism, chronic kidney disease, liver cirrhosis, Crohn's disease, gastrointestinal bypass surgery);
pregnant or lactating women (as they are usually in need of vitamin D);
people with cancer.
Types of interventions
We considered for inclusion randomised trials that compared vitamin D at any dose, duration, and route of administration versus placebo or no intervention.
The vitamin D was administered.
As monotherapy.
In combination with calcium.
Concomitant interventions were allowed if used equally in all intervention groups of the trial.
Types of outcome measures
Primary outcomes
Cancer occurrence.
All‐cause mortality.
Cancer mortality.
Secondary outcomes
Adverse events depending on the availability of data, we attempted to classify adverse events as serious or non‐serious. Serious adverse events were defined according to the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice as any untoward medical occurrence that at any dose resulted in death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was a congenital anomaly/birth defect, or any medical event, which might have jeopardised the patient, or required intervention to prevent it (ICH‐GCP 1997). All other adverse events (that is, any medical occurrence not necessarily having a causal relationship with the treatment, but causing a dose reduction or discontinuation of the treatment) were considered as non‐serious.
Health‐related quality of life.
Health economics.
Covariates, effect modifiers, and confounders
We noted and recorded any possible covariates, effect modifiers, and confounders (for example, compliance or other medications).
Timing of outcome measurement
We calculated the outcome effects at the end of the follow‐up period. We did not apply any restrictions regarding the length of intervention or the length of follow‐up.
Search methods for identification of studies
Electronic searches
We used the following sources from inception to the specified date for identification of the trials.
The Cochrane Central Register of Controlled Trials (CENTRAL) (until February 2014).
MEDLINE (until February 2014).
EMBASE (until February 2014).
LILACS (until February 2014).
Science Citation Index Expanded (until February 2014).
We also searched databases of ongoing trials (www.clinicaltrials.gov/ and www.controlled‐trials.com/ (with links to several databases) and the World Health Organization International Clinical Trials Registry Platform (ICTRP 2011)). For detailed search strategies, see Appendix 1. We included trials published in any language.
Searching other resources
We have contacted the main manufacturers of vitamin D to ask for unpublished randomised trials. We tried to identify additional trials by searching the reference lists of included trials and (systematic) reviews, meta‐analyses, and health technology assessment reports during the review preparation.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two review authors (GB and DN) independently scanned the abstract, title, or both sections of every record retrieved. We investigated all potentially relevant articles as full text. Where differences in opinion existed, we resolved them by recourse to a third party (CG). If resolving disagreement was not possible, we added the article to those 'awaiting assessment' and contacted the authors for clarification. An adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection is attached (Figure 1) (Liberati 2009).
1.
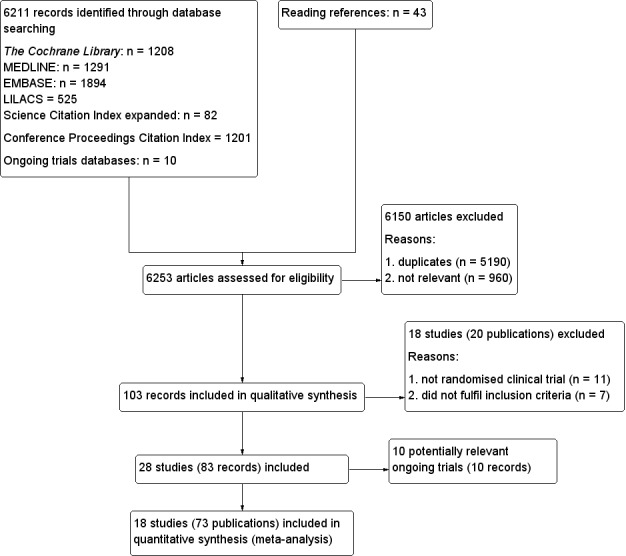
Study flow diagram.
Data extraction and management
For studies that fulfilled our inclusion criteria, two review authors (GB and DN) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details, see 'Characteristics of included studies'; Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6), resolving any disagreements by discussion, or if required by recourse to a third party. We sought any relevant missing information on the trial from the study author(s) of the article, if required.
1. Overview of study populations.
| Characteristic | Intervention(s) and comparator(s) | Screened/eligible [N] | Randomised [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] |
|
(1) Avenell 2012 |
I1: vitamin D₃ | 15,024 | 1343 | 1343 | 1813 | 68 |
| I2: vitamin D₃ plus calcium | 1306 | 1306 | ||||
| C1: calcium | 1311 | 1311 | 1762 | 67 | ||
| C2: matched placebo tablets | 1332 | 1332 | ||||
| total: | 5292 | 5292 | 3575 | 68 | ||
|
(2) Bolton‐Smith 2007 |
I1: vitamin D₃ plus calcium | ‐ | 62 | 62 | 50 | 81 |
| C1: matched placebo | 61 | 61 | 56 | 92 | ||
| total: | 123 | 123 | 106 | 86 | ||
|
(3) Brunner 2011 |
I1: vitamin D₃ plus calcium | 68,132 | 18,176 | 18,176 | 16,936 | 93 |
| C1: matched placebo | 18,106 | 18,106 | 16,815 | 93 | ||
| total: | 36,282 | 36,282 | 33,751 | 93 | ||
|
(4) Daly 2008 |
I1: calcium‐vitamin D₃‐fortified milk plus calcium | 422 | 85 | 85 | 76 | 89 |
| C1: usual diet | 82 | 82 | 73 | 89 | ||
| total: | 167 | 167 | 149 | 89 | ||
|
(5) Gallagher 2001 |
I1: calcitriol | 1905 | 123 | 123 | 101 | 82 |
| C1: matched placebo | 123 | 123 | 112 | 91 | ||
| total: | 246 | 246 | 213 | 87 | ||
|
(6) Glendenning 2012 |
I1: cholecalciferol | 2110 | 353 | 353 | 331 | 94 |
| C1: placebo vitamin D | 333 | 333 | 307 | 92 | ||
| total: | 686 | 686 | 638 | 93 | ||
|
(7) Grady 1991 |
I1: calcitriol | 98 | 50 | 50 | 49 | 98 |
| C1: placebo vitamin D | 48 | 48 | 48 | 100 | ||
| total: | 98 | 50 | 97 | 99 | ||
|
(8) Janssen 2010 |
I1: vitamin D₃ plus calcium | 91 | 36 | 36 | 18 | 50 |
| C1:placebo vitamin D₃ plus calcium | 34 | 34 | 31 | 91 | ||
| total: | 70 | 70 | 49 | 70 | ||
|
(9) Komulainen 1999 |
I1: vitamin D₃ plus calcium | 13,100 | 116 | 116 | 112 | 97 |
| C1: placebo | 116 | 116 | 115 | 99 | ||
| total: | 232 | 232 | 227 | 98 | ||
|
(10) Lappe 2007 |
I1: vitamin D₃ plus calcium | 1180 | 446 | 446 | 403 | 90 |
| C1: vitamin D₃ placebo plus calcium | 445 | 445 | 416 | 93 | ||
| C2: vitamin D₃ placebo plus calcium placebo | 288 | 288 | 266 | 92 | ||
| total: | 1179 | 1179 | 1085 | 92 | ||
| (11) Larsen 2012 | I1: vitamin D₃ | 136 | 65 | 65 | 55 | 85 |
| C1: vitamin D placebo | 65 | 65 | 57 | 88 | ||
| total: | 130 | 130 | 112 | 86 | ||
| (12) Murdoch 2012 | I1: vitamin D₃ | 351 | 161 | 161 | 148 | 92 |
| C1: vitamin D placebo | 161 | 161 | 146 | 91 | ||
| total: | 322 | 322 | 294 | 91 | ||
|
(13) Ott 1989 |
I1: calcitriol plus calcium | ‐ | 43 | 43 | 39 | 91 |
| C1: placebo vitamin D plus calcium | 43 | 43 | 37 | 86 | ||
| total: | 86 | 86 | 76 | 88 | ||
|
(14) Prince 2008 |
I1: vitamin D₂ plus calcium | 827 | 151 | 151 | 144 | 95 |
| C1: placebo vitamin D plus calcium | 151 | 151 | 145 | 96 | ||
| total: | 302 | 302 | 289 | 95 | ||
|
(15) Sanders 2010 |
I1: vitamin D₃ | 7204 | 1131 | 1131 | 1015 | 90 |
| C1: vitamin D placebo | 1127 | 1127 | 1017 | 90 | ||
| total: | 2258 | 2258 | 2032 | 90 | ||
|
(16) Trivedi 2003 |
I1: vitamin D₃ | ‐ | 1345 | 1345 | 1262 | 94 |
| C1:placebo vitamin D | 1341 | 1341 | 1264 | 94 | ||
| total: | 2686 | 2686 | 2526 | 94 | ||
| (17) Witham 2013 | I1: vitamin D₃ | 341 | 80 | 80 | 73 | 91 |
| C1: placebo vitamin D | 79 | 79 | 69 | 87 | ||
| total: | 159 | 159 | 142 | 89 | ||
| (18) Wood 2012 | I1: vitamin D₃ | 424 | 102 | 102 | 84 | 82 |
| I2: vitamin D₃ | 101 | 101 | 90 | 89 | ||
| C1: placebo vitamin D | 102 | 102 | 91 | 89 | ||
| total: | 305 | 305 | 265 | 87 | ||
| Grand total | All interventions | 25,275 | 22,799 | 90 | ||
| All controls | 25,348 | 22,827 | 90 | |||
| All interventions and controls | 50,623 | 45,626 | 90 |
"‐" denotes not reported ITT: intention‐to‐treat
Dealing with duplicate publications and companion papers
In the case of duplicate publications and companion papers of a primary study, we tried to maximise our yield of information by simultaneous evaluation of all available data.
Assessment of risk of bias in included studies
Due to the risk of overestimation of beneficial intervention effects in randomised trials of unclear or inadequate methodological quality (Kjaergard 2001; Lundh 2012; Moher 1998; Savović 2012a; Savović 2012b; Schulz 1995; Wood 2008), we assessed the influence of the risk of bias on our results. We used the following domains: allocation sequence generation, allocation concealment, blinding, incomplete outcome data reporting, selective outcome reporting, and other apparent biases (Higgins 2011). We used the following definitions:
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (for example, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants, personnel, and outcome assessors
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias into the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes were likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias into the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were predefined and reported, or all clinically relevant and reasonably expected outcomes were reported.
Uncertain risk of bias: it is unclear whether all predefined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
For a trial to be assessed with low risk of bias in the selective outcome reporting domain, the trial should have been registered either on the www.clinicaltrials.gov website or a similar register, or there should be a protocol, e.g., published in a paper journal. In the case of a trial being run and published in the years when trial registration was not required, we tried to carefully scrutinise the publication reporting on the trial to identify the trial objectives and outcomes. If usable data on all outcomes specified in the trial objectives were provided in the publications results section, then the trial was considered to be at low risk of bias in the selective outcome reporting domain.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other kind of for‐profit support that may manipulate the trial design, conduct, or results of the trial.
Uncertain risk of bias: the trial may or may not be free of for‐profit bias, as no information on clinical trial support or sponsorship is provided.
High risk of bias: the trial is sponsored by the industry or has received some other kind of for‐profit support.
Other bias
Low risk of bias: the trial appears to be free of other components (for example, academic bias) that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: there are other factors in the trial that could put it at risk of bias (for example, authors have conducted trials on the same topic, etc).
We considered trials assessed as having 'low risk of bias' in all of the specified individual domains as being 'trials with low risk of bias'. We considered trials assessed as having 'uncertain risk of bias' or 'high risk of bias' in one or more of the specified individual domains as being trials with 'high risk of bias' (Gluud 2011).
Dealing with missing data
We tried to obtain relevant missing data from authors whenever we lacked important numerical data such as number of screened or randomised participants, or lack of data regarding the performance of intention‐to‐treat (ITT) analyses, or data on as‐treated or per‐protocol participant analyses in order to perform our analyses as rigorously as possible. We investigated attrition rates, (for example, dropouts, losses to follow‐up, and withdrawals) and critically appraised issues of missing data (for example, last‐observation‐carried‐forward (LOCF)) and imputation methods.
Regarding the primary outcomes, we included participants with incomplete or missing data in sensitivity analyses by imputing them according to the following scenarios (Hollis 1999).
Extreme case analysis favouring the experimental intervention ('best‐worst' case scenario: none of the dropouts/participants lost from the experimental arm, but all of the dropouts/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator.
Extreme case analysis favouring the control ('worst‐best' case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator.
Assessment of heterogeneity
We identified heterogeneity by visual inspection of the forest plots, by using a standard Chi² test and a significance level of α = 0.1, in view of the low power of such tests. We specifically examined heterogeneity with the I² statistic (Higgins 2002), where I² values of 50% or more indicated a substantial level of heterogeneity (Higgins 2003). For the heterogeneity adjustment of the required information size in the trials sequential analyses, we used diversity (D²), as I² used for this purpose consistently underestimates the required information size (Wetterslev 2009).
When we found heterogeneity, we attempted to determine the potential reasons for it by examining the individual trial and subgroup characteristics.
Assessment of reporting biases
We used funnel plots in an exploratory data analysis to assess the potential existence of bias in small trials. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design of small trials, and publication bias. We performed adjusted rank correlation (Begg 1994) and a regression asymmetry test (Egger 1997) for detection of bias. We considered a P value less than 0.10 significant in the latter analyses. We carefully interpreted results of funnel plots (Lau 2006).
Data synthesis
We performed statistical analyses according to the statistical guidelines referenced in the latest version of The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For the statistical analyses, we used Review Manager 5 (RevMan 2012), Trial Sequential Analysis version 0.9 beta (TSA 2011; www.ctu.dk/tsa), STATA 8.2 (STATA Corp, College Station, Texas), and Sigma Stat 3.0 (SPSS Inc, Chicago, Ill). We analysed the data with both fixed‐effect (DeMets 1987) and random‐effects (DerSimonian 1986) model meta‐analyses. In the review text, we present the results of the random‐effects model analyses. For dichotomous outcomes, we calculated the Mantel‐Haenszel risk ratios (RRs). For all association measures, we used 95% confidence intervals (CIs). We conducted the analyses using the intention‐to‐treat principle, including all randomised participants irrespective of completeness of data. We included participants with missing data in the analyses using a carry forward of the last observed response. Accordingly, we counted participants who had been lost to follow‐up as being alive.
We calculated weighted averages for factors related to the trials, such as duration of the intervention and length of the follow‐up period.
For trials using a factorial design, we performed 'at the margins' analysis, combining all participants randomised to vitamin D (McAlister 2003). Due to the risk of interaction between different treatment regimens, we also performed 'inside the table' analysis in which we compared vitamin D only with placebo or no intervention. In the trials with a parallel group design with more than two arms and additional therapy, we compared the vitamin D arm alone with placebo or no intervention.
Trial sequential analysis Meta‐analyses may result in type 1 errors due to sparse data and repeated significance testing when meta‐analyses are updated with new trials (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). In a single trial, interim analysis increases the risk of type 1 errors. To avoid type 1 errors (rejecting the null hypothesis when it is in fact true) group sequential monitoring boundaries (Lan 1983) are applied to decide whether a trial could be terminated early because of a sufficiently small P value, that is, when the cumulative Z‐curve crosses the alpha‐spending monitoring boundary. Sequential monitoring boundaries can be applied to meta‐analyses as well and are called trial sequential monitoring boundaries. In 'trial sequential analysis' (TSA), the addition of each trial in a cumulative meta‐analysis is regarded as an interim meta‐analysis and helps to decide whether additional trials are needed (Wetterslev 2008). So far, several meta‐analyses and reviews have been published, including an increasing number of trial results as new trials have been published. It therefore seems appropriate to adjust new meta‐analyses for sparse accumulating data and multiple testing to control the overall type 1 error risk in a cumulative meta‐analysis (Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2008). The idea behind TSA is that if the cumulative Z‐curve crosses a trial sequential monitoring boundary (TSMB), a sufficient level of evidence is reached and no further trials may be needed. However, there is insufficient evidence to reach a conclusion if the cumulative Z‐curve does not cross the TSMB or does not surpass the futility boundaries before the required information size is reached. To construct the TSMBs, a required information size is needed which is calculated as the least number of participants needed in a well‐powered single trial (Brok 2008; Pogue 1998; Wetterslev 2008). We adjusted the required information size to account for statistical between‐trial heterogeneity with a diversity adjustment factor (Wetterslev 2009).
We performed trial sequential analysis to avoid random errors due to repetitive analyses of accumulated data and to prevent premature statements of superiority of intervention or lack of effect of an intervention (TSA 2011). We used the diversity‐adjusted required information size estimated from a control event proportion of the included trials and an a priori intervention effect of 5% and 10% relative risk reduction (RRR) (Wetterslev 2009), and the diversity which was estimated in the included trials.
Subgroup analysis and investigation of heterogeneity
We mainly performed subgroup analyses if one of the primary outcomes demonstrated statistically significant differences between the intervention groups. In any other case, subgroup analyses were clearly marked as a hypothesis‐generating exercise.
We conducted the following subgroup analyses.
Trials with a low risk of bias compared to trials with a high risk of bias.
Trials without risk of for‐profit bias compared to trials with risk of for‐profit bias
Primary prevention trials compared to secondary prevention trials.
Trials including participants with vitamin D insufficiency compared to trials with vitamin D adequacy.
Vitamin D₃ compared to placebo or no intervention.
Trials that administered vitamin D₃ singly compared to trials that administered vitamin D₃ combined with calcium.
Vitamin D₂ compared to placebo or no intervention.
Calcitriol compared to placebo or no intervention.
Sensitivity analysis
We performed the following sensitivity analyses to explore the influence of these imputations on the intervention effect size.
Best‐worst case scenario analyses.
Worst‐best case scenario analyses.
We also tested the robustness of the results by repeating the analysis using different statistical models (fixed‐ and random‐effects model meta‐analyses).
Summary of findings tables
We used GRADE (tech.cochrane.org/revman/other‐resources/gradepro) to construct a 'Summary of findings' table for vitamin D for all of the review outcomes. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias, the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Andrews 2013a; Andrews 2013b; Balshem 2011; Brunetti 2013; Guyatt 2011a; Guyatt 2011b; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2011i; ; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013). We used results obtained withTSA for the rating for imprecision. If there is insufficient evidence to reach a conclusion, i.e., if the TSA indicates that the required information size had not been reached, we downgraded the quality of the evidence by one level. In addition, we used risk of attrition bias for the rating for imprecision. If there was significant risk of attrition bias, we further downgraded the quality of the evidence by one level.
Results
Description of studies
Results of the search
We identified a total of 6211 references of possible interest through searching The Cochrane Library (n = 1208), MEDLINE (n = 1291), EMBASE (n = 1894), LILACS (n = 525), Science Citation Index Expanded (n = 82), Conference Proceedings Citation Index‐Science (n = 1201), and reference lists (n = 43). We identified an additional 10 ongoing trials through searching databases of ongoing trials. We will include data from the ongoing trials in future updates of this review. We then excluded 5190 duplicates and 960 clearly irrelevant references through reading the abstracts. Accordingly, we retrieved 103 references for further assessment. Of these, we excluded 20 references describing 18 studies because they were not randomised trials or did not fulfil the inclusion criteria of our review. We list reasons for exclusion in the table Characteristics of excluded studies. In total, 18 randomised trials described in 74 references fulfilled the inclusion criteria (Figure 1). The trials included a total of 50,623 participants.
We contacted eight authors for missing information and received answers from all of them.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies and appendices). The following is a succinct overview:
Trial characteristics
Out of the 18 trials reporting cancer occurrence, 14 trials used a parallel‐group design and four trials (Avenell 2012; Bolton‐Smith 2007; Gallagher 2001; Komulainen 1999) used a 2‐by‐2 factorial design (Pocock 2004). The trials were published between 1989 and 2013.
In 16 trials, vitamin D was provided free of charge by pharmaceutical companies. Two trials were not funded by industry (Trivedi 2003; Wood 2012).
The trials were conducted in Europe (n = 8) (Avenell 2012; Bolton‐Smith 2007; Janssen 2010; Komulainen 1999; Larsen 2012; Trivedi 2003; Witham 2013; Wood 2012), North America (n = 5) (Brunner 2011; Gallagher 2001; Grady 1991; Lappe 2007; Ott 1989), and Oceania (n = 5) (Daly 2008; Glendenning 2012; Murdoch 2012; Prince 2008; Sanders 2010). All 18 trials were conducted in high‐income countries.
Participants
A total of 50,623 participants were randomly assigned in the 18 trials reporting cancer occurrence (Table 2). The number of participants in each trial ranged from 70 to 36,282 (median 313). The mean age of participants was 69 years (range 47 to 97 years). The mean proportion of women was 81%.
Sixteen trials were primary prevention trials, that is, included healthy participants, or participants from the general population. Of these, 11 trials included elderly and postmenopausal women (Bolton‐Smith 2007; Brunner 2011; Gallagher 2001; Glendenning 2012; Janssen 2010; Komulainen 1999; Lappe 2007; Ott 1989; Prince 2008; Sanders 2010; Wood 2012), three trials included elderly people (Avenell 2012; Grady 1991; Trivedi 2003), and two trials included healthy volunteers (Daly 2008; Murdoch 2012).
Two trials were secondary prevention trials that included participants with cardiovascular disease (arterial hypertension) (Larsen 2012; Witham 2013).
Of the 18 trials reporting cancer occurrence, 16 (89%) reported the baseline vitamin D status of participants based on serum 25‐hydroxyvitamin D levels. Participants in nine trials (Bolton‐Smith 2007; Daly 2008; Gallagher 2001; Glendenning 2012; Grady 1991; Larsen 2012; Murdoch 2012; Ott 1989; Trivedi 2003) had baseline 25‐hydroxyvitamin D levels at or above vitamin D adequacy (20 ng/ml). Participants in the other seven trials had baseline 25‐hydroxyvitamin D levels considered vitamin D insufficient (< 20 ng/ml) (Avenell 2012; Brunner 2011; Janssen 2010; Prince 2008; Sanders 2010; Witham 2013; Wood 2012). Two trials did not report the baseline vitamin D status of participants (Komulainen 1999; Lappe 2007).
The main outcomes in the trials were cancer occurrence, all‐cause mortality, bone mineral density, and number of falls and fractures.
Experimental interventions
Vitamin D₃ ‐ cholecalciferol
Vitamin D was administered as vitamin D₃ (cholecalciferol) in 14 trials (49,891 participants; 78% women; mean age 67 years). Vitamin D₃ was tested singly in seven trials (Glendenning 2012; Murdoch 2012; Sanders 2010; Trivedi 2003; Witham 2013; Wood 2012) and combined with calcium in six trials. One trial tested vitamin D₃ singly and combined with calcium (Avenell 2012). Vitamin D₃ was administered orally in all trials. Vitamin D₃ was given daily in nine trials and at intervals in five trials (monthly (Murdoch 2012); three‐monthly (Glendenning 2012; Witham 2013); four‐monthly (Trivedi 2003); and yearly (Sanders 2010)). The daily dose of the vitamin D₃ was 300 IU to 3333 IU (mean daily dose 1146 IU; median daily dose 810 IU). The duration of supplementation in trials using vitamin D₃ was five months to seven years (weighted mean 6.0 years), and the duration of the follow‐up period was five months to seven years (weighted mean 6.3 years).
Vitamin D₂ ‐ ergocalciferol
Vitamin D was administered as vitamin D₂ (ergocalciferol) in one trial (302 participants; 100% women; mean age 77.2 years). Vitamin D₂ was tested in a dose of 1000 IU, combined with 1000 mg of calcium, orally, and daily for a one‐year period.
Calcitriol ‐ 1,25‐dihydroxyvitamin D
Vitamin D was administered as calcitriol in three trials (430 participants; 85% women; aged 50 to 97 years). Calcitriol was tested singly in two trials and combined with calcium in one trial (Ott 1989). Calcitriol was tested orally and daily in all trials. The dose of calcitriol was 0.5 μg in two trials (Gallagher 2001; Grady 1991); while two doses of calcitriol (0.5 μg and 2 μg) were tested in another trial (Ott 1989). The duration of supplementation in trials using calcitriol was two to five years (weighted mean 2.5 years) and the duration of the follow‐up period was two to five years (weighted mean 4.0 years).
Comparator interventions
Seventeen trials used placebo, and one trial used no intervention in the control group (Daly 2008).
Co‐interventions
Seven trials used calcium combined with vitamin D in the experimental intervention groups. Two trials tested calcium separately in one of the intervention groups (Avenell 2012; Lappe 2007). Calcium was administered orally and daily in all trials. The dose of calcium was 500 mg to 1500 mg (mean 883 mg; median 1000 mg).
Three trials used calcium in the control group, combined with vitamin D placebo, in a dose of 500 mg to 1000 mg (mean 833 mg; median 1000 mg). These trials used an equal dose of calcium in the experimental intervention groups. One trial with a 2‐by‐2 factorial design tested a combination of vitamin D₃, vitamin K₁, and calcium in one group (Bolton‐Smith 2007). The factorial design of this trial allowed us to compare only the vitamin D₃ plus calcium group with the placebo group of this trial. Two trials with a 2‐by‐2 factorial design tested vitamin D and hormone replacement (Gallagher 2001; Komulainen 1999). We have compared only the vitamin D group with the placebo group of these trials.
Excluded studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of excluded studies and appendices).
Risk of bias in included studies
Two trials (11%) were considered to be at low risk of bias. The remaining 16 trials had unclear bias control in one or more of the components assessed (Figure 2; Figure 3). Inspection of the funnel plot does not suggest potential bias (asymmetry) (Figure 4). The adjusted‐rank correlation test (P = 1.00) found no significant evidence of bias, while the regression asymmetry test found significant evidence of bias (P = 0.007).
2.
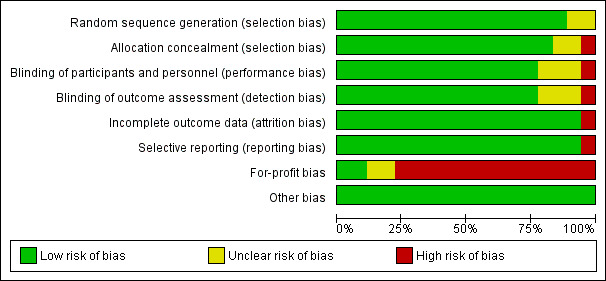
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
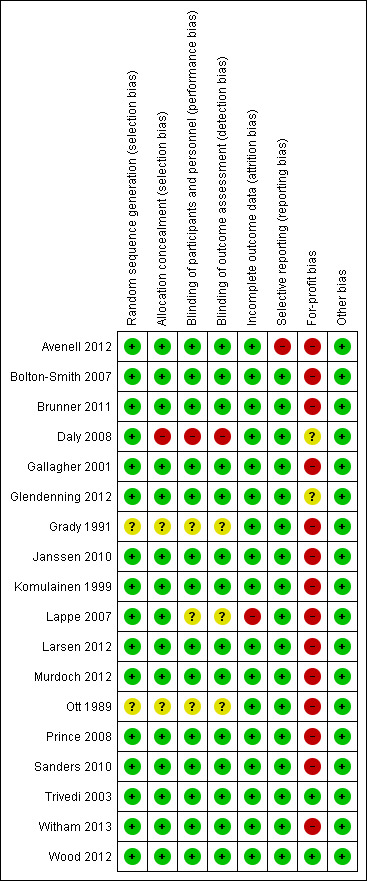
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
4.
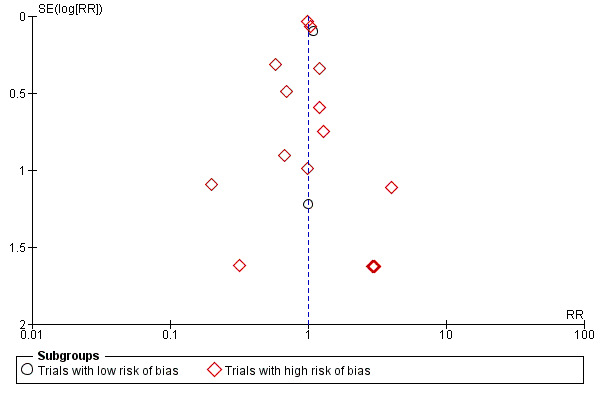
Funnel plot of comparison: 1 Vitamin D versus placebo or no intervention, outcome: 1.1 Cancer occurrence in trials with a low or high risk of bias.
Allocation
The generation of the allocation sequence was adequately described in 16 trials. The remaining two trials (Grady 1991; Ott 1989) were described as randomised, but the method used for sequence generation was not described.
The method used to conceal allocation was adequately described in 15 trials. We judged the method used for allocation concealment to be unclear in two trials (Grady 1991; Ott 1989) and inadequate in one trial (Daly 2008).
Blinding
The method of blinding was adequately described in 14 trials. The method of blinding was unclear in three trials (Grady 1991; Lappe 2007; Ott 1989). One trial was not blinded (Daly 2008).
Incomplete outcome data
Incomplete data were addressed adequately in 17 trials. In one trial, information is insufficient to allow assessment of whether the missing data mechanism in combination with the method used to handle missing data is likely to introduce bias into the estimate of effect (Lappe 2007).
Selective reporting
Predefined primary and secondary outcomes were reported in all trials.
For‐profit bias
Two trials were not funded by industry (Trivedi 2003; Wood 2012). Fifteen trials were funded by industry (Avenell 2012; Bolton‐Smith 2007; Brunner 2011; Daly 2008; Gallagher 2001; Grady 1991; Komulainen 1999; Janssen 2010; Lappe 2007; Larsen 2012; Murdoch 2012; Ott 1989; Prince 2008; Sanders 2010; Witham 2013). The source of funding is not clear for one trial (Glendenning 2012).
Other potential sources of bias
All included trials appear to be free of other components that could put them at risk of bias.
Effects of interventions
See: Table 1
Primary outcomes
Cancer occurrence
Overall, vitamin D had no statistically significant effect on cancer occurrence (risk ratio (RR) 1.00 (95% CI 0.94 to 1.06); P = 0.88; I² = 0%; 18 trials; 50,623 participants; Analysis 1.1). A total of 1927 of 25,275 participants (7.6%) randomised to the vitamin D group and 1943 of 25,348 participants (7.7%) randomised to the placebo or no intervention group had cancer at the end of follow‐up. Trial sequential analysis of all vitamin D trials suggests that we reached the futility area after the 10th trial, allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction (Figure 5).
1.1. Analysis.
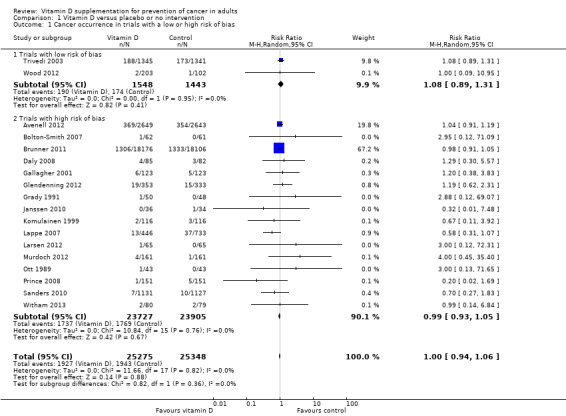
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 1 Cancer occurrence in trials with a low or high risk of bias.
5.
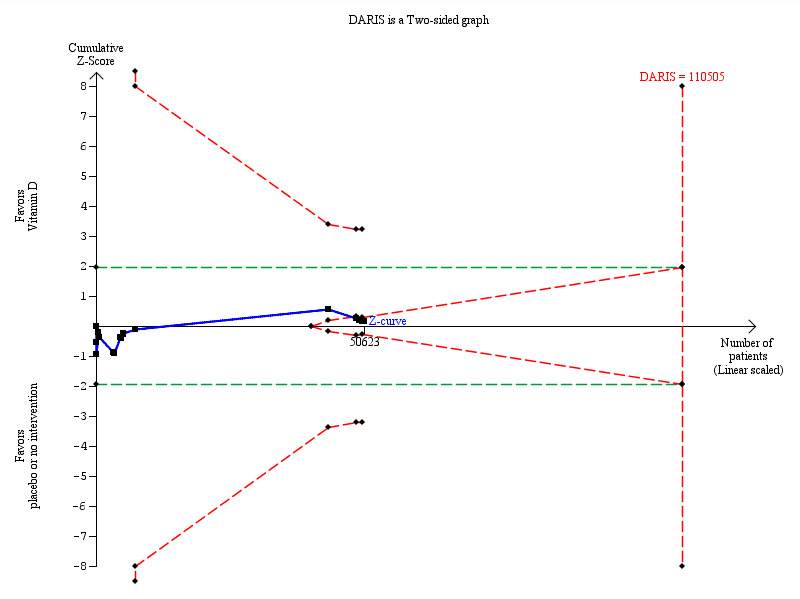
Trial sequential analysis on cancer occurrence in the 18 vitamin D trials was performed based on cancer occurrence of 10% in the control group, a relative risk reduction of 5% with vitamin D supplementation, a type I error of 5%, and a type II error of 20% (80% power). There was no diversity. This resulted in a required information size of 110,505 participants. Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries.
Intervention effects according to bias risk of trials
Vitamin D had no statistically significant effect on cancer occurrence in trials with a low risk of bias (RR 1.08 (95% CI 0.89 to 1.31); P = 0.41; I² = 0%; 2 trials; 2991 participants), nor in trials with a high risk of bias (RR 0.99 (95% CI 0.93 to 1.05); P = 0.67; I² = 0%; 16 trials; 47,632 participants; Analysis 1.1). The difference between the effect estimate of vitamin D on cancer in trials with low risk of bias and trials with a high risk of bias was not statistically significant (P = 0.36); (Analysis 1.1).
Trials without risk of for‐profit bias compared to trials with risk of for‐profit bias
Vitamin D had no significant effect on cancer occurrence in the trials without risk of for‐profit bias (RR 1.08 (95% CI 0.89 to 1.31); P = 0.41; I² = 0%; 2991 participants; 2 trials; Analysis 1.2). Vitamin D had no significant effect on cancer occurrence in the trials with risk of for‐profit bias (RR 0.99 (95% CI 0.93 to 1.05); P = 0.67; I² = 0%; 47,632 participants; 16 trials; Analysis 1.2). The difference between the estimate of the effect of vitamin D on cancer occurrence in the trials without risk of for‐profit bias and the trials with risk of for‐profit bias was not statistically significant by the test of interaction (P = 0.36; Analysis 1.2).
1.2. Analysis.
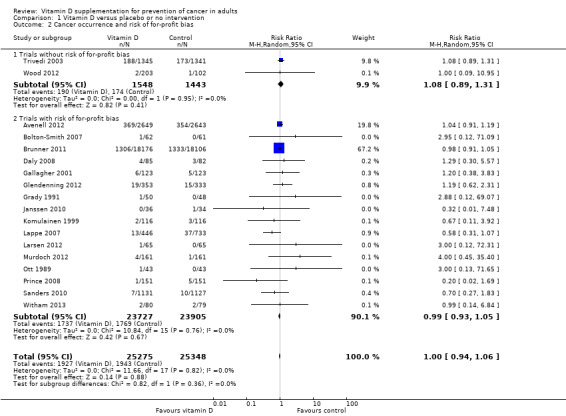
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 2 Cancer occurrence and risk of for‐profit bias.
Primary prevention compared to secondary prevention
Vitamin D had no significant effect on cancer occurrence in the primary prevention trials (RR 1.00 (95% CI 0.94 to 1.06); P = 0.87; I² = 0%; 50,334 participants; 16 trials; Analysis 1.3). Vitamin D had no statistically significant effect on cancer occurrence in the secondary prevention trials (RR 1.33 (95% CI 0.26 to 6.96); P = 0.73; I² = 0%; 289 participants; 2 trials; Analysis 1.3). The difference between the estimates of the effect of vitamin D on cancer occurrence in the primary prevention and the secondary prevention trials was not statistically significant by the test of interaction (P = 0.73; Analysis 1.3).
1.3. Analysis.
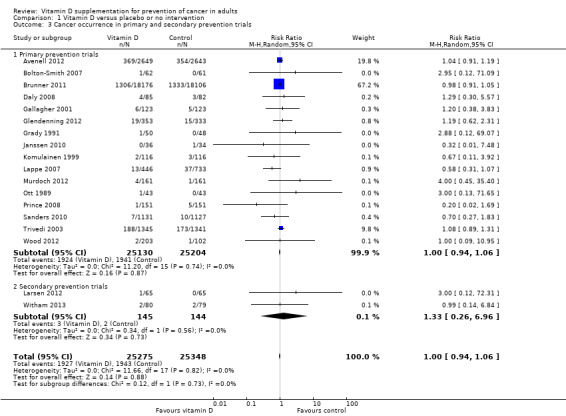
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 3 Cancer occurrence in primary and secondary prevention trials.
Intervention effects according to vitamin D status
Vitamin D had no statistically significant effect on cancer occurrence in participants with vitamin D insufficiency (RR 0.99 (95% CI 0.93 to 1.05); P = 0.68; I² = 0%; 7 trials; 44,668 participants), nor in participants with vitamin D adequacy (RR 1.12 (95% CI 0.94 to 1.34); P = 0.21; I² = 0%; 9 trials; 4544 participants; Analysis 1.4). The difference between the estimates of vitamin D on cancer in trials including participants with vitamin D adequacy and trials including participants with vitamin D insufficiency was not statistically significant (P = 0.19; Analysis 1.4).
1.4. Analysis.
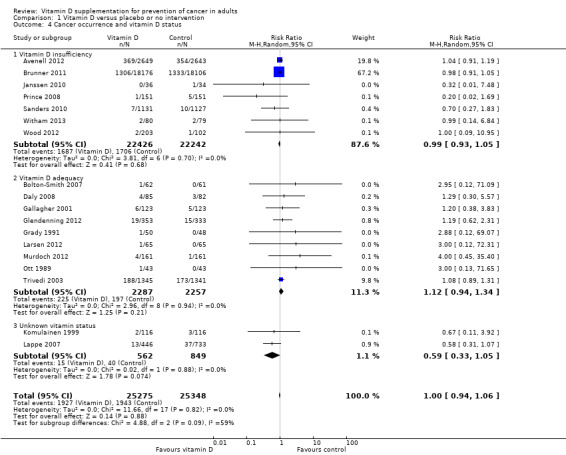
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 4 Cancer occurrence and vitamin D status.
Sensitivity analyses taking attrition into consideration
Of the 18 trials reporting cancer occurrence, 17 trials reported the exact numbers of participants with missing outcomes in the intervention and control groups. One trial did not report losses to follow‐up for the intervention groups separately (Lappe 2007). A total of 849 of 24,829 participants (3.4%) had missing outcomes in the vitamin D group versus 791 of 24,615 participants (3.2%) in the control group.
'Best‐worst case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group had no cancer and all those with missing outcomes in the control intervention group developed cancer, vitamin D supplementation significantly decreased cancer occurrence (RR 0.41 (95% CI 0.31 to 0.54); P < 0.00001; I² = 82%; 49,444 participants; 17 trials; Analysis 1.5).
1.5. Analysis.
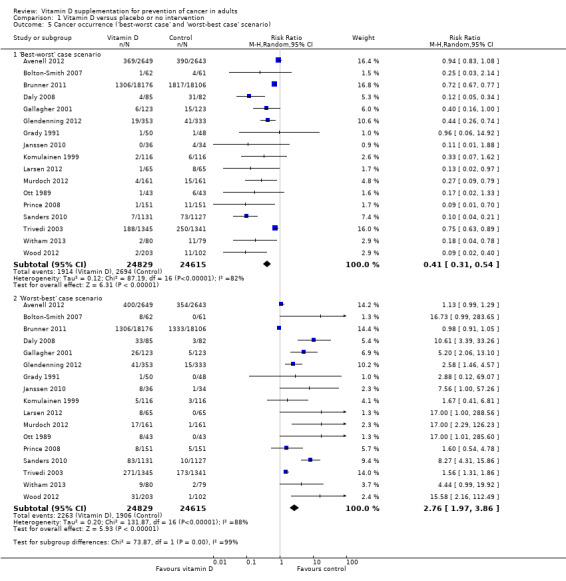
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario).
'Worst‐best case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group developed cancer and all those with missing outcomes in the control intervention group had no cancer, vitamin D supplementation significantly increased cancer occurrence (RR 2.76 (95% CI 1.97 to 3.86); P < 0.00001; I² = 88%; 49,444 participants; 17 trials; Analysis 1.5).
Intervention effects according to administered form of vitamin D
Vitamin D₃ (cholecalciferol)
Vitamin D₃ was tested in 14 trials (49,891 participants). Inspection of the funnel plot does not suggest potential bias (asymmetry). The adjusted‐rank correlation test found no significant evidence of bias (P = 0.59), while a regression asymmetry test found significant evidence of bias (P = 0.01). Overall, vitamin D₃ had no statistically significant effect on cancer (RR 1.00 (95% CI 0.94 to 1.06); P = 0.88; I² = 0%; Analysis 1.6). Vitamin D₃ had no statistically significant effect on cancer in trials with a low risk of bias (RR 1.08 (95% CI 0.89 to 1.31); P = 0.41; I² = 0%; 2 trials; 2991 participants), nor in trials with a high risk of bias (RR 0.99 (95% CI 0.93 to 1.05); P = 0.67; I² = 0%; 12 trials; 46,900 participants; Analysis 1.6). The difference between the estimates of vitamin D₃ on cancer in trials with low risk of bias versus trials with a high risk of bias was not statistically significant (P = 0.36).
1.6. Analysis.
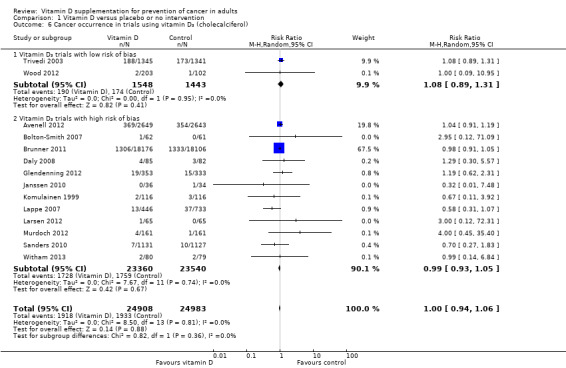
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol).
Vitamin D₃ and calcium
Vitamin D₃ administered singly versus placebo had no statistically significant effect on cancer (RR 1.03 (95% CI 0.90 to 1.17); P = 0.69; I² = 0%; 9200 participants; 8 trials; Analysis 1.7). Vitamin D₃ combined with calcium versus placebo or no intervention had no statistically significant effect on cancer (RR 0.97 (95% CI 0.91 to 1.04); P = 0.36; I² = 0%; 40,670 participants; 7 trials; Analysis 1.7).
1.7. Analysis.
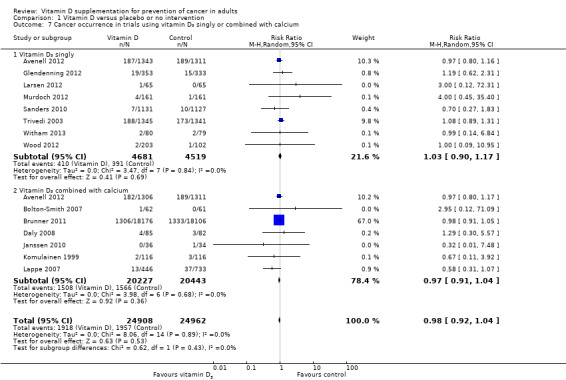
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium.
Cancer site occurrence in trials using vitamin D₃
Vitamin D₃ had no significant effect on lung cancer (RR 0.86 (95% CI 0.69 to 1.07); P = 0.17; I² = 0%; 5 trials; 45,509 participants; Analysis 1.8); breast cancer (RR 0.97 (95% CI 0.86 to 1.09); P = 0.61; I² = 0%; 7 trials; 43,669 participants; Analysis 1.9); colorectal cancer (RR 1.11 (95% CI 0.92 to 1.34); P = 0.26; I² = 0%; 5 trials; 45,598 participants; Analysis 1.10); or pancreatic cancer (RR 0.91 (95% CI 0.57 to 1.46); P = 0.69; I² = 0%; 2 trials; 36,405 participants; Analysis 1.11). Vitamin D₃ had no significant effect on prostate, uterine, ovarian, oesophageal, stomach, or liver cancer (one trial each) (Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15, Analysis 1.16; Analysis 1.17).
1.8. Analysis.
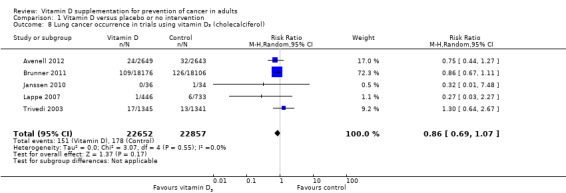
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.9. Analysis.
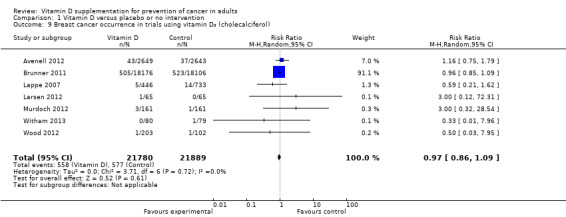
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.10. Analysis.
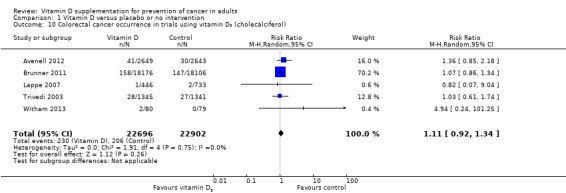
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.11. Analysis.
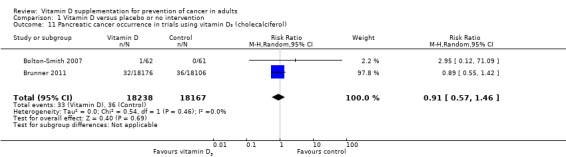
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.12. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.13. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.14. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.15. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.16. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol).
1.17. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol).
Vitamin D₂ (ergocalciferol)
Vitamin D₂ was tested in one trial (302 participants). Vitamin D₂ had no statistically significant effect on cancer (RR 0.20 (95% CI 0.02 to 1.69); P = 0.14; Analysis 1.18).
1.18. Analysis.
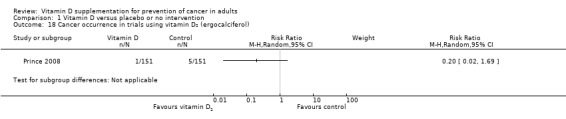
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol).
Calcitriol (1,25‐dihydroxyvitamin D)
Calcitriol was tested in three trials (430 participants). Inspection of the funnel plot does not suggest potential bias (asymmetry). Overall, calcitriol had no statistically significant effect on cancer (RR 1.45 (95% CI 0.52 to 4.06); P = 0.48; I² = 0%; Analysis 1.19).
1.19. Analysis.
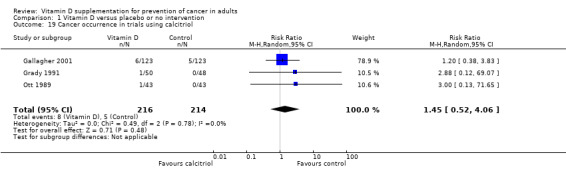
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 19 Cancer occurrence in trials using calcitriol.
Cancer site occurrence in trials using calcitriol
Calcitriol had no significant effect on breast, uterine, or stomach cancer (one trial each) (Analysis 1.20; Analysis 1.21; Analysis 1.22)
1.20. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 20 Breast cancer occurrence in trials using calcitriol.
1.21. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 21 Uterine cancer occurrence in trials using calcitriol.
1.22. Analysis.
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 22 Stomach cancer occurrence in trials using calcitriol.
All‐cause mortality
Overall, vitamin D significantly decreased all‐cause mortality (RR 0.93 (95% CI 0.88 to 0.98); P = 0.009; I² = 0%; 15 trials; 49,866 participants; Analysis 1.23). A total of 1854 of the 24,846 participants (7.5%) randomised to the vitamin D group and 2007 of the 25,020 participants (8.0%) randomised to the placebo or no intervention group died. In a trial with low risk of bias, mortality was not significantly changed (RR 0.90 (95% CI 0.77 to 1.07); P = 0.23; 1 trial; 2686 participants). In trials with a high risk of bias, mortality was significantly decreased in the vitamin D group (RR 0.93 (95% CI 0.88 to 0.99); P = 0.02; I² = 0%; 14 trials; 47,180 participants; Analysis 1.23). The difference between the effect estimate of vitamin D on mortality in trials with low risk of bias and in trials with a high risk of bias was not statistically significant (P = 0.75; Analysis 1.23). Trial sequential analysis on mortality in the 15 vitamin D trials was performed based on a mortality rate in the control group of 10%, a relative risk reduction (RRR) of 5% in the experimental group, a type I error of 5%, and type II error of 20% (80% power). There was no diversity. The required information size was 110,505 participants. The cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit after the 15th trial.
1.23. Analysis.
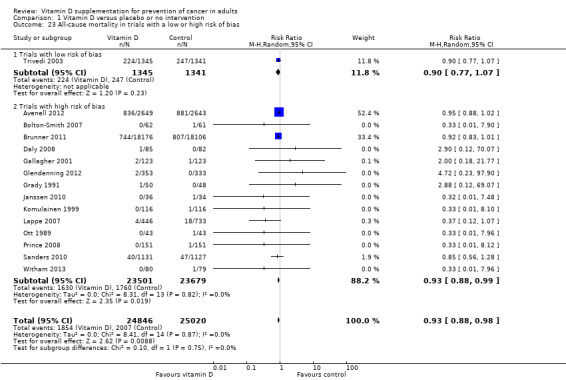
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 23 All‐cause mortality in trials with a low or high risk of bias.
Sensitivity analyses taking attrition into consideration
Of the 15 trials reporting mortality, 14 trials reported the exact numbers of participants with missing outcomes in the intervention and control groups. One trial did not report losses to follow‐up for the intervention groups separately (Lappe 2007). A total of 797 of 24,400 participants (3.3%) had missing outcomes in the vitamin D group versus 757 of 24,287 participants (3.1%) in the control group.
'Best‐worst case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group survived and all those with missing outcomes in the control intervention group died, vitamin D supplementation significantly decreased mortality (RR 0.43 (95% CI 0.31 to 0.60); P < 0.00001; I² = 89%; 48,687 participants; 14 trials; Analysis 1.24).
1.24. Analysis.
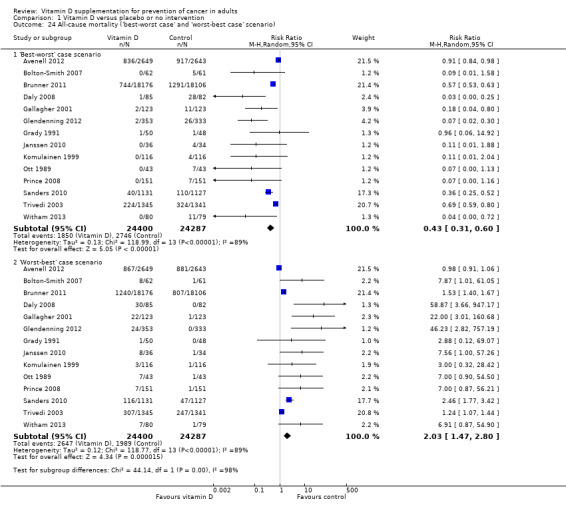
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario).
'Worst‐best case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group died and all those with missing outcomes in the control intervention group survived, vitamin D supplementation significantly increased mortality (RR 2.03 (95% CI 1.47 to 2.80); P < 0.0001; I² = 89%; 48,687 participants; 14 trials; Analysis 1.24).
Cancer mortality
Vitamin D₃ may decrease cancer mortality (RR 0.88 (95% CI 0.78 to 0.98); P = 0.02; I² = 0%; 4 trials; 44,492 participants; Analysis 1.25). However, we lack firm evidence for even a 10% RRR since the required information size of 110,505 participants has not yet been reached for such an effect, and the cumulative Z‐curve did not cross the monitoring boundaries (Figure 6).
1.25. Analysis.
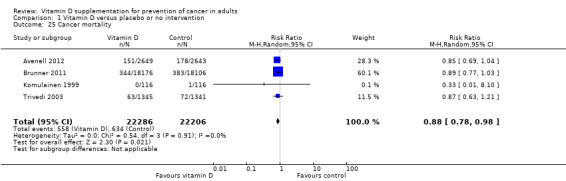
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 25 Cancer mortality.
6.
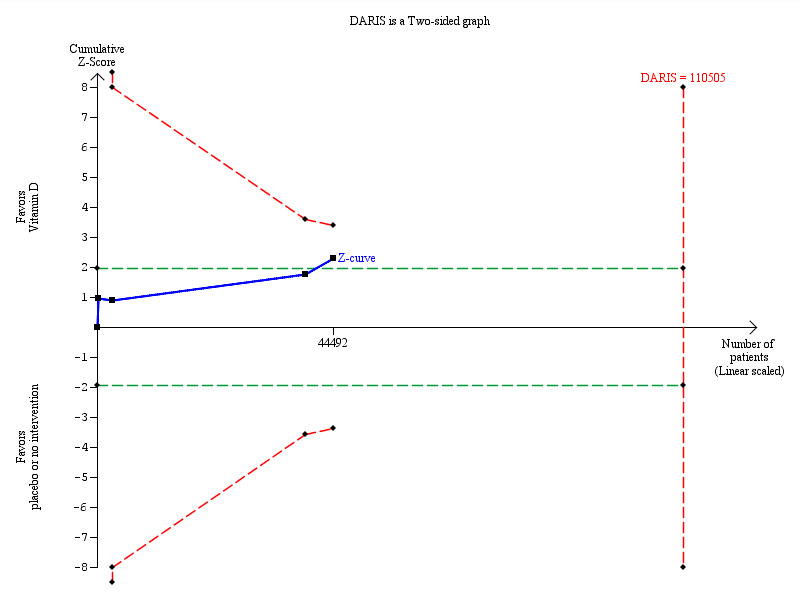
Trial sequential analysis on cancer mortality in the four vitamin D trials was performed based on cancer mortality of 3% in the control group, a relative risk reduction of 10% with vitamin D₃ supplementation, a type I error of 5%, and a type II error of 20% (80% power). There was no diversity. The required information size was 110,505 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundary (red line) after the fourth trial. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries.
Sensitivity analyses taking attrition into consideration
All four trials reporting cancer mortality reported the exact numbers of participants with missing outcomes in the intervention and control groups. A total of 613 of 22,286 participants (2.8%) had missing outcomes in the vitamin D group versus 600 of 22,206 participants (2.7%) in the control group.
'Best‐worst case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group survived and all those with missing outcomes in the control intervention group died from cancer, vitamin D supplementation significantly decreased mortality (RR 0.48 (95% CI 0.33 to 0.70); P < 0.0001; I² = 88%; 44,492 participants; 4 trials; Analysis 1.26).
1.26. Analysis.
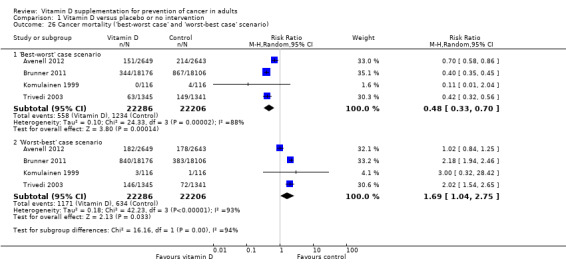
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario).
'Worst‐best case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group died from cancer and all those with missing outcomes in the control intervention group survived, vitamin D supplementation significantly increased mortality (RR 1.69 (95% CI 1.04 to 2.75); P < 0.03; I² = 93%; 44,492 participants; 4 trials; Analysis 1.26).
Secondary outcomes
Adverse events
Several adverse events were reported (for example, hypercalcaemia, nephrolithiasis, hypercalciuria, renal insufficiency, cardiovascular disorders, gastrointestinal disorders, and psychiatric disorders). The supplemental forms of vitamin D (D₃ and D₂) (RR 1.41 (95% CI 0.64 to 3.09); P = 0.39; I² = 0%; 4 trials; 5879 participants), and active form of vitamin D (calcitriol) (RR 4.03 (95% CI 0.56 to 29.22); P = 0.17; I² = 53%; 2 trials; 332 participants) had no statistically significant effect on the risk of hypercalcaemia (Analysis 1.27).
1.27. Analysis.
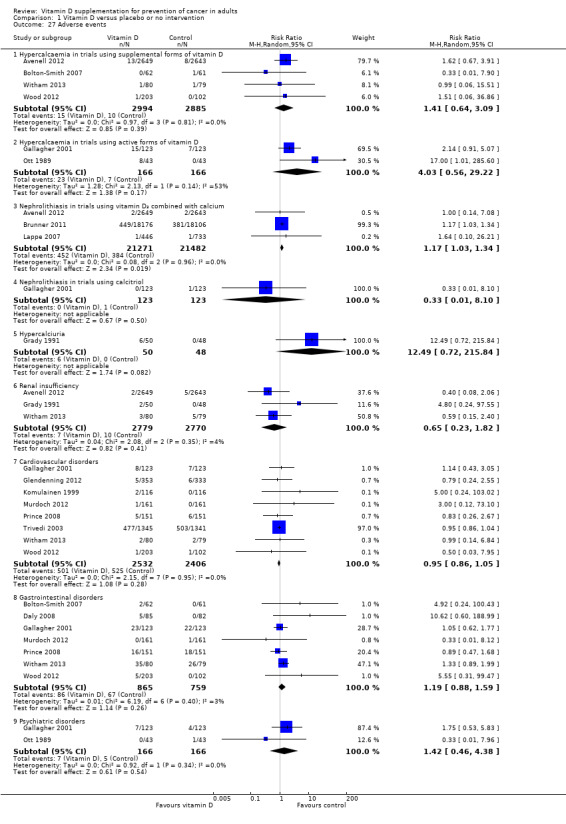
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 27 Adverse events.
Combined vitamin D₃ and calcium supplements significantly increased nephrolithiasis (RR 1.17 (95% CI 1.03 to 1.34); P = 0.02; I² = 0%; 3 trials; 42,753 participants; Analysis 1.27). Calcitriol had no statistically significant effect on nephrolithiasis (RR 0.33 (95% CI 0.01 to 8.10); P = 0.50; 1 trial; 246 participants; Analysis 1.27). The effect of vitamin D on the other adverse events was not statistically significant: hypercalciuria (RR 12.49 (95% CI 0.72 to 215.84); P = 0.08; 1 trial; 98 participants); renal insufficiency (RR 0.65 (95% CI 0.23 to 1.82); P = 0.41; I² = 4%; 3 trials; 5549 participants); cardiovascular disorders (RR 0.95 (95% CI 0.86 to 1.05); P = 0.28; I² = 0%; 8 trials; 4938 participants); gastrointestinal disorders (RR 1.19 (95% CI 0.88 to 1.59); P = 0.26; I² = 3%; 7 trials; 1624 participants); and psychiatric disorders (RR 1.42 (95% CI 0.46 to 4.38); P = 0.54; I² = 0%; 2 trials; 332 participants; Analysis 1.27).
Health‐related quality of life and health economics
We did not find any data on health‐related quality of life or health economics in the randomised trials included in this review.
Discussion
Summary of main results
Our systematic review contains a number of important findings. We have found evidence that vitamin D supplements in the form of vitamin D₃, vitamin D₂, or calcitriol have no clear effect on occurrence of cancer in mainly elderly community‐dwelling women. This effect does not seem to be due to random errors ('play of chance'). Vitamin D supplements have no clear effect on any specific cancer type. Vitamin D supplements decreased cancer mortality and all‐cause mortality, but these estimates are at risk of type I errors due to the fact that too few participants were examined and to substantial attrition bias. Combined vitamin D₃ and calcium supplements increased nephrolithiasis, but it remains unclear from the included trials whether vitamin D₃, calcium, or both were responsible for this effect. Vitamin D supplements have no clear effects on other adverse events.
Overall completeness and applicability of evidence
Our published protocol described our plan to analyse the effect of vitamin D on cancer in primary and secondary prevention randomised trials in adults. We included all eligible randomised trials up to February 2014. All trials were conducted in high‐income countries. Participants of both genders were included. Most of the participants were elderly community‐dwelling women. The vast majority of the participants came from primary prevention trials, and we assume that they were apparently healthy when included in the trials. Few trials of secondary prevention with low participation rates were included, so our ability to say anything about such patients is severely limited. We included randomised trials with both vitamin D–deficient participants and those who seemed to have adequate vitamin D levels at entry. We were unable to detect substantial differences regarding these variables on the estimated intervention effect on cancer. We found surprisingly little statistical heterogeneity in any of our analyses. Most trials assessed vitamin D₃, and our major conclusions relate to this intervention. Most of the trials were considered to be at high risk of bias, mainly for‐profit bias. Our analyses revealed that outcome reporting was missing on more than 3% of participants. This number is too high when cancer occurrence is about 7% in the placebo or no intervention/placebo group. Accordingly, our 'best‐worst case' and 'worst‐best case' analyses revealed that our results were compatible with both a very large beneficial effect and a very large detrimental effect of vitamin D on cancer. Although these extreme sensitivity analyses are unlikely, they reveal how few missing participants would need to have developed cancer to substantially change our findings of modest benefit into a null effect or maybe even harm. We therefore warn against uncritical application of our findings.
Quality of the evidence
Our review follows the overall plan of a published, peer‐reviewed Cochrane protocol (Bjelakovic 2008). It represents a comprehensive review of the topic, including 18 randomised trials with more than 50,000 participants. This increases the precision and power of our analyses (Higgins 2011; Turner 2013). We were not able to find in the literature earlier meta‐analyses of preventive trials of vitamin D on cancer occurrence. We conducted a thorough review in accordance with The Cochrane Collaboration methodology (Higgins 2011) and implementing findings of methodological studies (Kjaergard 2001; Lundh 2012; Moher 1998; Savović 2012a; Savović 2012b; Schulz 1995; Wood 2008). Between‐trial statistical heterogeneity is almost absent from our meta‐analyses, which enhances the consistency of our findings.
We conducted a number of subgroup analyses. We observed no statistically significantly different effects of the intervention of vitamin D supplementation on cancer in subgroup analyses of trials at low risk of bias compared to trials at high risk of bias; of trials at no risk of for‐profit bias compared to trials at risk of for‐profit bias; of trials assessing primary prevention compared to trials assessing secondary prevention; of trials including participants with vitamin D level below 20 mg/mL at entry compared to trials including participants with normal vitamin D levels at entry; and of vitamin D₃ trials using concomitant calcium supplementation compared to vitamin D₃ trials without calcium.
We also performed trial sequential analyses to control for the risk of random errors in a cumulative meta‐analysis and to prevent premature statements of superiority of vitamin D, based on estimation of the diversity‐adjusted required information size (Brok 2008; Brok 2009; Thorlund 2009; Thorlund 2011a; Thorlund 2011b; Wetterslev 2008; Wetterslev 2009).
A major obstacle in many of the included trials is the relatively large proportion of about 3% of participants with missing outcomes in both experimental and control groups. This opens the way for attrition bias, and our 'best‐worst' and 'worst‐best' intention‐to‐treat analyses demonstrate that the intervention effect of vitamin D may be either beneficial or harmful. Although both of the two extreme scenarios are unlikely, they demonstrate that we cannot depend fully on the estimates we arrive at. Our 'best‐worst case' and 'worst‐best case' scenario analyses revealed much more extreme confidence limits for cancer occurrence (95% CI 0.31 to 3.76) compared to our 'complete‐case' scenario analysis (95% CI 0.94 to 1.06); for all‐cause mortality (95% CI 0.31 to 2.80) compared to our 'complete‐case' scenario analysis (95% CI 0.88 to 0.98), and for cancer mortality (0.33 to 2.75) compared to our 'complete‐case' scenario analysis (95% CI 0.78 to 0.98). Those analyses convey a message of a noticeable degree of uncertainty regarding our results. This observation calls for more comprehensive meta‐analyses of individual participant data.
We used GRADE to construct a 'Summary of findings' table for vitamin D for the review outcome measures. Results obtained by use of TSA were applied for the rating for imprecision. If there was insufficient evidence to reach a conclusion, i.e., if the TSA indicates that the required information size had not been reached, we downgraded the quality of the evidence by one level. We also used risk of attrition bias for the rating for imprecision. As the cumulative Z‐curve for the intervention effect on cancer occurrence has reached the futility area for a 5% relative risk reduction (RRR) even though the required information size for such an effect has not been reached, there is precision enough to refute a 5% RRR. However, if, for example, a 2.5% RRR is still a clinically relevant effect, there may still be imprecision for detecting or rejecting such an effect of 2.5% RRR. If we consider a 2.5% RRR, which is equivalent to a number needed to treat for an additional beneficial outcome (NNTB) of 400, a worthwhile effect, then the downgrading for imprecision seems appropriate for the ability to refute a 5% RRR of cancer occurrence.
Potential biases in the review process
Most of the included trials are at high risk of bias, which undermines the validity of our results (Kjaergard 2001; Lundh 2012; Moher 1998; Savović 2012a; Savović 2012b; Schulz 1995; Wood 2008). Our meta‐analyses show a lack of statistical heterogeneity, which may emphasise the consistency of our findings but should also raise concern (Ioannidis 2006). Statistical homogeneity may be due to inappropriate inferences of the asymptotic Q test with sparse data (Ioannidis 2006). We have also performed trial sequential analyses, based on the estimation of the diversity‐adjusted required information size in order to avoid an undue risk of random errors in a cumulative meta‐analysis and to prevent premature statements of superiority of vitamin D or of lack of effect (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009).
Certain potential limitations of this review warrant consideration. As with all systematic reviews, our findings and interpretations are limited by the quality and quantity of available evidence on the effects of vitamin D on cancer. Despite extensive speculations in the literature and a large number of epidemiological studies that claim cancer preventive effects of vitamin D, few randomised trials have been conducted assessing cancer occurrence. The duration of supplementation and duration of follow‐up was short in some of the included trials compared to the long process of carcinogenesis. This may make it difficult to detect any effects, beneficial or harmful. Among the 18 included randomised trials, cancer occurrence was the primary prespecified outcome in few of them. Different forms of vitamin D were used for supplementation. The majority of included trials used vitamin D₃, one trial tested vitamin D₂, and three trials tested calcitriol. Our subgroup analyses found that the effect of vitamin D on cancer was neutral, irrespective of the form of vitamin D used for supplementation. Most trials investigated the effects of vitamin D administered at lower doses than those recently suggested as cancer‐preventive (Garland 2011). All the included trials came from high‐income countries, and the majority of them included participants without overt deficiencies of vitamin D. Accordingly, we are unable to assess how vitamin D affects cancer in populations with specific needs.
Agreements and disagreements with other studies or reviews
We found that vitamin D had neutral effect on cancer occurrence. This finding is in accord with the result of our previous review (Bjelakovic 2014), and contradicts the results of epidemiological studies (Bikle 2014; Garland 2006; Giovannucci 2005; Ordóñez‐Mena 2013; Redaniel 2014; Woloszynska‐Read 2011; Yin 2013). Our results are in accord with the conclusions of the recently published International Agency for Research on Cancer and Institute of Medicine reports stating that vitamin D status is not correlated with cancer occurrence (IARC 2008; IOM 2011). Recently, an updated meta‐analysis prepared for the US Preventive Services Task Force found inconclusive evidence regarding vitamin D supplementation for the prevention of cancer (Chung 2011).
We found no substantive differences regarding the effect of vitamin D on cancer in trials including participants with vitamin D insufficiency (25‐hydroxyvitamin D level less than 20 ng/mL) compared to trials including participants with optimal vitamin D status. Optimal vitamin D status has been linked to decreased incidence of several types of cancer (Garland 2007; Gorham 2007). However, a number of observational studies have also suggested that high vitamin D status might be connected with increased oesophageal (Chen 2007), pancreatic (Stolzenberg‐Solomon 2006), breast (Goodwin 2009), and prostate cancer risks (Ahn 2008). One should consider the possibility of a U‐shaped relationship between vitamin D status and cancer risk (Toner 2010; Tuohimaa 2012). Once again, we may be witnessing the flawed inferences that are sometimes drawn from observational epidemiological data (Jakobsen 2013).
We have examined the influence of different forms of vitamin D on cancer occurrence. We have found neutral effect irrespective of the form of vitamin D used. Our previous systematic review on vitamin D and mortality has found weak evidence that only vitamin D₃ may be of benefit for survival (Bjelakovic 2014). We found no evidence for vitamin D₂, alfacalcidol, and calcitriol affecting mortality, but these estimates are at risk of type II errors (the chance of not rejecting the null hypothesis when it is in fact false) due to the fact that small groups of participants were examined. A number of recently published clinical trials (Armas 2004; Heaney 2011; Lehmann 2013; Leventis 2009; Logan 2013; Romagnoli 2008; Trang 1998), and the systematic review (Tripkovic 2012) found evidence that vitamin D₃ increases serum 25‐hydroxyvitamin D more efficiently than vitamin D₂. Several clinical trials have been conducted to examine the effects of alfacalcidol and calcitriol on different health outcomes. These trials have mostly included women with osteoporosis (Ott 1989; Shiraki 2004) and people with chronic kidney disease (Palmer 2009). Due to the lack of a demonstrable effect, and observed adverse events (hypercalcaemia), the active forms of vitamin D are increasingly being replaced with the supplemental forms of vitamin D (cholecalciferol and ergocalciferol). Three trials included in our present systematic review tested calcitriol. The evidence for an effect on cancer is inconclusive, but the sample size was too small to exclude possible effects. We were not able to identify trials testing alfacalcidol that reported cancer occurrence at the end of the follow‐up period.
The effect of vitamin D₃ on cancer occurrence was not statistically significant in trials using vitamin D₃ singly or trials using vitamin D₃ combined with calcium. Because of the small number of included trials assessing vitamin D₃ alone or combined with calcium, the findings could be due to a type II error. Our finding seems consistent with the result obtained by Bristow 2013, who found that calcium supplements did not affect cancer, but contradict the results of recent meta‐analyses examining the influence of vitamin D on mortality (Rejnmark 2012) or bone health (DIPART 2010). These meta‐analyses concluded that vitamin D is effective in preventing mortality (Rejnmark 2012) and hip fractures (DIPART 2010) only when combined with calcium. A recent meta‐analysis observed that calcium supplementation (with or without co‐administration of vitamin D) is associated with increased risk of cardiovascular events, especially myocardial infarction (Bolland 2010; Bolland 2011). Another review of prospective studies and randomised clinical trials found no evidence for an effect of calcium (Patel 2012). A US Preventive Services Task Force recently recommended against daily supplementation with 400 IU or less of vitamin D₃ and 1000 mg or less of calcium for the primary prevention of fractures in non‐institutionalised postmenopausal women (Moyer 2013).The complex interactions between vitamin D and calcium make it difficult to separate their effects (Lips 2012). More clinical research seems needed.
We have examined the influence of vitamin D supplementation on different cancer types. Vitamin D showed no substantive effect on any specific cancer type. Our results are in accord with the results of the recently published systematic review and meta‐analysis investigating the role of vitamin D supplementation on breast cancer (Sperati 2013). Our results contradict earlier speculations in the literature about the preventive effects of vitamin D on certain cancer types (Fleet 2012; Woloszynska‐Read 2011).
We have found no evidence for an effect of vitamin D supplements on cancer occurrence. However, vitamin D supplements seemed to decrease cancer mortality. Epidemiological studies that examined the relationship between vitamin D status and cancer mortality have shown mixed results. Pilz and coworkers found that optimal vitamin D status seems to be related to decreased cancer mortality in some studies (Pilz 2009; Pilz 2013). On the other hand, Freedman 2010 found no overall relationship between vitamin D status and cancer mortality in the general population. Our finding of no evidence that vitamin D has an effect on cancer occurrence seems to be at odds with the statistically significant beneficial effect of vitamin D on cancer mortality. However, due to low participation rates in the included trials, the decreased cancer mortality could be a random error as indicated by our trial sequential analysis. One should also consider that as yet unknown preventive effects of vitamin D supplementation on premature death, which may not necessarily influence cancer incidence, might play a role (Bjelakovic 2014).
Vitamin D supplements decreased all‐cause mortality. This is in accord with the results of our previous review of the role of vitamin D in mortality prevention (Bjelakovic 2014), in which we disregarded the risks of random error and attrition bias. However, if these risks are considered, we do not yet know whether vitamin D affects mortality.
Combined vitamin D₃ and calcium supplements increased nephrolithiasis. This is in accord with the results of our previous review (Bjelakovic 2014). Other adverse events, including elevated urinary calcium excretion; renal insufficiency; cardiovascular, gastrointestinal, or psychiatric disorders, were not substantively influenced by vitamin D supplementation.
We lack sufficient evidence for the effect of vitamin D supplementation on health‐related quality of life or the cost effectiveness of vitamin D supplementation. However, vitamin D₃ products and calcium are affordable, with multiple producers across the world, so these interventions may be cost‐effective.
Despite biological plausibility for the role of vitamin D in the prevention of cancer, the available evidence does not seem to support this possibility. Several recently published evidence reports have assessed the influence of vitamin D and calcium on different health outcomes (Bolland 2014; Chung 2011; Cranney 2007; Moyer 2013; Rosen 2012). The majority of the findings on different health outcomes including cancer were equivocal. The US Institute of Medicine reported that available evidence supports a role for vitamin D and calcium in skeletal health (IOM 2011). The evidence was, however, considered insufficient and inconclusive for extraskeletal outcomes including cancer (IOM 2011). Most recent systematic review and meta‐analyses concluded that vitamin D supplementation for osteoporosis prevention in free‐living adults without specific risk factors for vitamin D deficiency seems to be inappropriate (Reid 2014). In the same vein, the recently updated Systematic Evidence Review for the US Preventive Service Task Force found no evidence of benefit from vitamin supplementation for the prevention of cancer and cardiovascular disease (Fortmann 2013). Accordingly, these comprehensive reports are compatible with our findings.
It seems that health claims are again ahead of the evidence. It is very likely that low vitamin D status is not the cause, but rather the consequence of disease (Autier 2014; Guallar 2010; Guallar 2013; Harvey 2012; Kupferschmidt 2012). Results of several large ongoing randomised trials that assess vitamin D supplementation for different health outcomes will likely help us to elucidate the role of vitamin D.
Authors' conclusions
Implications for practice.
We found no evidence that vitamin D, irrespective of the form used, has an effect on cancer occurrence in predominantly elderly community‐dwelling women. Vitamin D decreased cancer mortality and all‐cause mortality, but these estimates are at risk of type I errors due to the fact that too few participants were examined. Combined vitamin D₃ and calcium supplements increased nephrolithiasis.
Implications for research.
We need more evidence before drawing firm conclusions on the effect of vitamin D on cancer. More randomised trials are needed on the effects of vitamin D₃ on cancer in younger persons, in men, and in people with low vitamin D status. More randomised trials assessing a longer duration of vitamin D intervention and higher dosages of vitamin D may also be needed. The effects of vitamin D on health‐related quality of life and cost effectiveness deserve further investigation. Future trials should be designed according to the SPIRIT guidelines (Chan 2013) and reported according to the CONSORT guidelines (www.consort‐statement.org). Future trials should reduce attrition bias to a minimum.
Acknowledgements
We extend our gratitude to all participants and investigators who took part in the randomised clinical trials. We are grateful to the many authors who kindly responded to our requests for further information on the trials they were involved in. We thank the individual trial authors in our Characteristics of included studies tables. We thank Sarah Louise Klingenberg, the Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark, for her help with paper copies of publications.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| 1. MeSH descriptor Vitamin D explode all trees 2. MeSH descriptor Cholecalciferol explode all trees 3. MeSH descriptor Ergocalciferols explode all trees 4. MeSH descriptor Dihydrotachysterol explode all trees 5. MeSH descriptor 25‐hydroxyvitamin D 2 explode all trees 6. MeSH descriptor Hydroxycholecalciferols explode all trees 7. ( (vitamin* in All Text and d in All Text and 2 in All Text) or (vitamin* in All Text and d2 in All Text) ) 8. (cholecalciferol* in All Text or calciferol* in All Text or calcitriol* in All Text or dihydrotachysterol* in All Text or (hydroxyvitamin* in All Text and d* in All Text) ) 9. (alfacalcidol* in All Text or alphacalcidol* in All Text or colecalciferol* in All Text) 10. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) 11. MeSH descriptor Mortality explode all trees 12. (mortality in All Text or mortaliti* in All Text) 13. (#11 or #12) 14. MeSH descriptor Primary Prevention explode all trees 15. prevent* in All Text 16. MeSH descriptor Neoplasms explode all trees 17. (cancer* in All Text or neoplasm* in All Text or tumo?r* in All Text) 18. (#14 or #15 or #16 or #17) 19. (#10 and #13) 20. (#10 and #18) 21. (#19 or #20) |
| MEDLINE |
| 1. exp Vitamin D/ 2. exp Cholecalciferol/ 3. exp ergocalciferols/ or exp dihydrotachysterol/ or exp 25‐hydroxyvitamin d 2/ 4. exp Hydroxycholecalciferols/ 5. vitamin D?.tw,ot. 6. (cholecalciferol$ or calcifediol$ or calcitriol$ or dihydrotachysterol$ or hydroxyvitamin$ d?).tw,ot. 7. (alfacalcidol$ or alphacalcidol$ or colecalciferol$).tw,ot. 8. or/1‐7 9. exp Mortality/ 10. mortality.tw,ot. 11. mortaliti$.tw,ot. 12. or/9‐11 13. exp Primary Prevention/ 14. (prevention$ or prevent$).tw,ot. 15. exp Neoplasm/ 16. (cancer$ or neoplasm$ or tumo?r$).tw,ot. 17. or/13‐16 18. exp Randomized Controlled Trials as topic/ 19. Randomized Controlled Trial.pt. 20. exp Controlled Clinical Trials as topic/ 21. Controlled Clinical Trial.pt. 22. exp Random Allocation/ 23. exp Double‐Blind Method/ 24. exp Single‐Blind Method/ 25. or/18‐24 26. exp "Review Literature as topic"/ 27. exp Technology Assessment, Biomedical/ 28. exp Meta‐analysis as topic/ 29. Meta‐analysis.pt. 30. hta.tw,ot. 31. (health technology adj6 assessment$).tw,ot. 32. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 33. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 34. or/26‐33 35. 25 or 34 36. 8 and 17 and 35 37. 8 and 12 and 35 38 36 or 37 39. limit 38 to animals 40. limit 38 to humans 41. 39 not 40 42 38 not 41 |
| EMBASE |
| 1. exp ergocalciferol/ or exp vitamin D/ 2. exp colecalciferol/ 3. exp dihydrotachysterol/ 4. exp 25 hydroxyvitamin D/ 5. exp hydroxycolecalciferol/ 6. (vitamin* D? or vitamin*D?).tw,ot. 7. (cholecalciferol* or colecalciferol* or calcifediol* or calcitriol* or dihydrotachysterol* or hydroxyvitamin* d?).tw,ot. 8. exp alfacalcidol/ 9. (alfacalcidol* or alphacalcidol*).tw,ot. 10. or/1‐9 11. exp mortality/ 12. (mortality or mortaliti*).tw,ot. 13. 11 or 12 14. exp prevention/ 15. prevent*.tw,ot. 16. exp neoplasm/ 17. or/14‐16 18. randomized controlled trial/ 19. double blind procedure/ 20. single blind procedure/ 21. exp randomization/ 22. exp controlled clinical trial/ 23. or/18‐22 24. exp meta analysis/ 25. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 26. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 27. exp Literature/ 28. exp Biomedical Technology Assessment/ 29. hta.tw,ot. 30. (health technology adj6 assessment$).tw,ot. 31. or/24‐30 32. (comment or editorial or historical‐article).pt. 33. 31 not 32 34. 23 or 33 35. 10 and 13 and 34 36. 10 and 17 and 34 37. 35 or 36 38. limit 37 to human |
| LILACS |
| 1. Vitamin D 2. Cholecalciferol 3. Ergocalciferol 4. Alfacalcidol 5. Calcitriol |
| ISI Web of Science |
| 1. TS=(vitamin d2 OR vitamin d 2 OR hydroxyvitamin* OR cholecalciferol* OR calciferol* OR calcitriol* OR calcifediol* OR dihydrotachysterol* OR alfacalcidol* OR alphacalcidol* OR colecalciferol*) 2. TS=(mortalit* OR prevent* OR cancer* OR neoplasm* OR tumor* OR tumour*) 3. #2 AND #1 4. TS=(random* OR blind* OR placebo* OR meta‐analys*) 5. #4 AND #3 |
Appendix 2. Description of interventions
| Characteristic | Intervention(s) [route, frequency, total dose/day] | Comparator(s) [route, frequency, total dose/day] |
| Avenell 2012 | I1: vitamin D₃ (800 IU) orally, daily | C1: calcium (1000 mg) orally, daily |
| I2: vitamin D₃ (800 IU) plus calcium (1000 mg) orally, daily | C2: matched placebo tablet orally, daily | |
| Bolton‐Smith 2007 | I1: vitamin D₃ (400 IU) plus calcium 1000 mg orally, daily | C1: vitamin K₁ 200 μg orally, daily |
| I2: vitamin D₃ (400 IU) plus calcium 1000 mg plus vitamin K₁ 200 μg orally, daily | C2: matched placebo tablet orally, daily | |
| Brunner 2011 | I: vitamin D₃ (400 IU) plus calcium (1000 mg) orally, daily | C: matched placebo tablet orally, daily |
| Daly 2008 | I: calcium‐vitamin D₃‐fortified milk containing vitamin D₃ (800 IU) plus calcium (1000 mg) daily | C: usual diet |
| Gallagher 2001 | I1: calcitriol (0.5 μg) daily | C1: calcitriol (0.5 μg daily) plus conjugated oestrogens 0.625 mg/daily plus medroxyprogesterone acetate 2.5 mg orally, daily |
| I2: conjugated oestrogens 0.625 mg/daily plus medroxyprogesterone acetate 2.5 mg orally, daily | C2: matched placebo tablet orally, daily | |
| Glendenning 2012 | I: cholecalciferol 150,000 IU 3‐monthly | C: placebo vitamin D 3‐monthly |
| Grady 1991 | I: calcitriol (0.5 μg) orally, daily | C: placebo vitamin D orally, daily |
| Janssen 2010 | I: vitamin D₃ (400 IU) plus calcium (500 mg) orally, daily | C: matched placebo vitamin D₃ plus calcium (500 mg) orally, daily |
| Komulainen 1999 | I1: sequential combination of 2 mg estradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) | C1: sequential combination of 2 mg estradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) plus vitamin D₃ (300 IU) and calcium (500 mg) orally, daily |
| I2: vitamin D₃ (300 IU) plus calcium (500 mg) dailya | C2: placebo | |
| Lappe 2007 | I1: vitamin D₃ (1000 IU) plus calcium (1400 to 1500 mg) orally, daily | C1: vitamin D₃ placebo plus calcium placebo, orally, daily |
| I2: vitamin D₃ placebo plus calcium (1400 to 1500 mg) orally, daily | ||
| Larsen 2012 | I: vitamin D₃ 3000 IU orally, daily | C: matched placebo vitamin D orally |
| Murdoch 2012 | I: vitamin D₃ an initial dose of 200,000 IU of vitamin D₃, then 200,000 IU 1 month later, then 100,000 IU monthly orally | C: matched placebo vitamin D orally |
| Ott 1989 | I: calcitriol 0.25 to 2 μg plus calcium 1000 mg, orally, daily | C: matched placebo vitamin D plus calcium 1000 mg orally, daily |
| Prince 2008 | I: vitamin D₂ 1000 IU plus calcium 1000 mg orally, daily | C: matched placebo tablet of vitamin D plus calcium 1000 mg orally, daily |
| Sanders 2010 | I: vitamin D₃ 500,000 IU orally, yearly | C: matched placebo tablet of vitamin D orally, yearly |
| Trivedi 2003 | I: vitamin D₃ 100,000 IU every 4 months orally | C: matched placebo vitamin D every 4 months orally |
| Witham 2013 | I: vitamin D₃ 100,000 IU every 3 months orally | C: matched placebo vitamin D every 3 months orally |
| Wood 2012 | I1: vitamin D₃ 400 IU orally, daily | C: matched placebo vitamin D orally, daily |
| I2: vitamin D₃ 1000 IU orally, daily | ||
| "‐" denotes not reported aNo intake during June to August, the vitamin D₃ dosage was lowered to 100 IU/day after 4 years of treatment C: comparator; I: intervention | ||
Appendix 3. Baseline characteristics (I)
|
Characteristic Study ID |
Design of study | Duration of intervention [years] | Duration of follow‐up [years] | Description of participants | Country | Setting |
| Avenell 2012 | Factorial RCT | 3.75 | 6.2 | Elderly people with low‐trauma, osteoporotic fracture in the previous 10 years | United Kingdom | Outpatients |
| Bolton‐Smith 2007 | Factorial RCT | 2 | 2 | Elderly nonosteoporotic women | United Kingdom | Outpatients |
| Brunner 2011 | RCT | 7 | 7 | Postmenopausal women | United States | Outpatients |
| Daly 2008 | RCT | 2 | 3.5 | Healthy ambulatory men | Australia | Outpatients |
| Gallagher 2001 | Factorial RCT | 3 | 5 | Elderly women | United States | Outpatients |
| Glendenning 2012 | RCT | 0.5 | 0.75 | Elderly women | Australia | Outpatients |
| Grady 1991 | RCT | 0.5 | 0.5 | Elderly people | United States | Outpatients |
| Janssen 2010 | RCT | 1 | 1 | Elderly vitamin D‐insufficient women | Netherlands | Outpatients |
| Komulainen 1999 | Factorial RCT | 5 | 5 | Postmenopausal women | Finland | Outpatients |
| Lappe 2007 | RCT | 4 | 4 | Postmenopausal women | United States | Outpatients |
| Larsen 2012 | RCT | 0.38 | 0.38 | Hypertensive patients | Denmark | Outpatients |
| Murdoch 2012 | RCT | 1 | 1 | Healthy adults | New Zealand | Outpatients |
| Ott 1989 | RCT | 2 | 2 | Postmenopausal women | United States | Outpatients |
| Prince 2008 | RCT | 1 | 1 | Elderly women with vitamin D insufficiency | Australia | Outpatients |
| Sanders 2010 | RCT | 2.96 | 2.96 | Elderly women | Australia | Outpatients |
| Trivedi 2003 | RCT | 5 | 5 | Elderly people | United Kingdom | Outpatients |
| Witham 2013 | RCT | 1 | 1 | Elderly patients with isolated systolic hypertension | United Kingdom | Outpatients |
| Wood 2012 | RCT | 1 | 1 | Healthy postmenopausal white women | United Kingdom | Outpatients |
Appendix 4. Baseline characteristics (II)
|
Characteristic Study ID |
Sex [female %] | Age [mean years (range)] | Ethnic groups [%] | Co‐medications / Co‐interventions | Co‐morbidities |
| Avenell 2012 | 85 | 77 | ‐ | ‐ | Low‐trauma osteoporotic fracture in the previous 10 years |
| Bolton‐Smith 2007 | 100 | 68 | ‐ | Vitamin K1 | ‐ |
| Brunner 2011 | 100 | 62.4 (50 to 79) | ‐ | ‐ | ‐ |
| Daly 2008 | 0 | 61.9 | ‐ | ‐ | ‐ |
| Gallagher 2001 | 100 | 71 (65 to 67) | ‐ | Conjugated oestrogens plus medroxyprogesterone acetate | ‐ |
| Glendenning 2012 | 100 | 76.7 | ‐ | ‐ | ‐ |
| Grady 1991 | 54 | 79.1 (70 to 97) | ‐ | ‐ | ‐ |
| Janssen 2010 | 100 | 80.8 | ‐ | ‐ | ‐ |
| Komulainen 1999 | 100 | 52.7 (47 to 56) | ‐ | Oestradiol valerate and cyproterone acetate | ‐ |
| Lappe 2007 | 100 | 66.7 | White: 100 | ‐ | ‐ |
| Larsen 2012 | 69 | 60 | White: 100 | Arterial hypertension | |
| Murdoch 2012 | 75 | 47 | ‐ | ‐ | ‐ |
| Ott 1989 | 100 | 67.5 (50 to 80) | ‐ | ‐ | ‐ |
| Prince 2008 | 100 | 77.2 (70 to 90) | ‐ | ‐ | ‐ |
| Sanders 2010 | 100 | 76 | ‐ | ‐ | ‐ |
| Trivedi 2003 | 24 | 74.7 (65 to 85) | ‐ | ‐ | ‐ |
| Witham 2013 | 48 | 77 (>70) | ‐ | ‐ | Arterial hypertension |
| Wood 2012 | 100 | 64 (60 to 70) | White: 100 | ‐ | ‐ |
| "‐" denotes not reported | |||||
Appendix 5. Matrix of study endpoints
|
Characteristic Study ID |
Primary endpoint(s) [time of measurement] | Secondary endpoint(s) [time of measurement] | Other endpoint(s) [time of measurement] |
| Avenell 2012 | Fractures | Overall mortality, vascular disease mortality, cancer mortality, and cancer occurrence (3.75, 6.2 y) | ‐ |
| Bolton‐Smith 2007 | Bone mineral density (6, 12, 18, 24 mo) | Markers of bone turnover, and vitamin status (0, 24 mo) | Overall mortality (24 mo) |
| Brunner 2011 | Fractures, cancer occurrence, mortality (3, 7 y) | ‐ | ‐ |
| Daly 2008 | Bone mineral density | ‐ | Overall mortality (24 mo) |
| Gallagher 2001 | Bone mineral density (1.5, 3, 6, 12, 18, 24, 30, 36 mo) | ‐ | Overall mortality (24 mo) |
| Glendenning 2012 | Falls, muscle strength, and mobility (0, 3, 6, 9 mo) | Serum 25‐hidrohyvitamin D levels, and adverse events (0, 3, 6, 9 mo) | Overall mortality (9 mo) |
| Grady 1991 | Muscle strength (1, 2, 4, 8, 12, 18, 24 wk) | ‐ | Overall mortality (24 mo) |
| Janssen 2010 | Muscle strength, power and functional mobility (0, 6 mo) | ‐ | Overall mortality (6 mo) |
| Komulainen 1999 | Bone mineral density (0, 1, 2, 3, 4, 5 y) | ‐ | Adverse events (5 y), overall mortality (5 y) |
| Lappe 2007 | Fractures | Cancer occurrence (0, 6, 12, 18, 24, 30, 36, 42, 48 mo), vitamin D status (0, 12 mo) | Overall mortality (48 mo) |
| Larsen 2012 | Systolic blood pressure | Diastolic blood pressure and heart rate, central blood pressure, central augmentation index, carotid‐femoral pulse wave velocity, urinary calcium‐creatinine ratio, and plasma levels of renin, angiotensin II, aldosterone, brain natriuretic peptide, 25(OH)D, intact parathyroid hormone, ionized calcium, phosphate, and fibroblast growth factor 23 at 20 weeks | Adverse events (20 wk) |
| Murdoch 2012 | Upper respiratory tract infections | Duration and severity of upper respiratory tract infections episodes, and number of days of missed work due to upper respiratory tract infections episodes 1 yr | Adverse events (1 yr) |
| Ott 1989 | Bone mass (0, 6, 12, 18, 24 mo) | Adverse events (24 mo) | Overall mortality (24 mo) |
| Prince 2008 | Falls (12 mo) | Adverse events (12 mo) | Overall mortality (12 mo) |
| Sanders 2010 | Falls and fractures (3, 9, 15, 24, 27, 36 mo) | Adverse events (36 mo) | Overall mortality (36 mo) |
| Trivedi 2003 | Fractures (5 y), cause‐specific mortality (5 y) | Cancer occurrence (5 y), cardiovascular disease (5 y) | Overall mortality (5 y) |
| Witham 2013 | Blood pressure (0, 3, 6, 9, 12 mo) | 24‐hour blood pressure, soluble markers of cardiovascular risk, endothelial function, pulse wave velocity, other biochemical measurements (glucose, total cholesterol, LDL and HDL cholesterol, triglycerides, serum albumin and calcium), exercise capacity and falls (3, 6, 9, 12 mo) | Adverse events (3, 6, 9, 12 mo) |
| Wood 2012 | Markers of cardiovascular disease risk (12 mo) | Adverse events (12 mo) | ‐ |
| Primary or secondary endpoint(s) refer to verbatim statements in the publication, other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the publication "‐" denotes not reported mo: months; wk: weeks; y: years | |||
Appendix 6. Adverse events
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Randomised [N] | Deaths [n/N (%)] | All adverse events [n/N (%)] | Severe/serious adverse events [n/N (%)] | Discontinued study due to adverse events [n/N (%)] |
| Avenell 2012 | I1: vitamin D₃ | 2649 | 836/2649 (31.6) | 363/2649 (13.7) | ‐ | ‐ |
| C1: matched placebo | 2643 | 881/2643 (33.3) | 386/2643 (14.6) | |||
| all: | 5292 | 33 (0.6) | ||||
| Bolton‐Smith 2007 | I1: vitamin D₃ plus calcium | 62 | 0/62 (0) | ‐ | ‐ | ‐ |
| C1: matched placebo | 61 | 1/60 (1.7) | ||||
| all: | 123 | |||||
| Brunner 2011 | I1: vitamin D₃ plus calcium | 18,176 | 744/18,176 (4.1) | 449/18176 (2.5) | 449/18,176 (2.5) | ‐ |
| C1: matched placebo | 18,106 | 807/18,106 (4.5) | 381/18106 (2.1) | 381/18,106 (2.1) | ||
| all: | 36,282 | |||||
| Daly 2008 | I1: calcium‐vitamin D₃‐fortified milk plus calcium | 85 | 1/85 (1.2) | 9/85 (10.6) | 9/85 (10.6) | 9/85 (10.6) |
| C1: no intervention | 82 | 0/82 (0) | 2/82 (2.4) | 2/82 (2.4) | 2/82 (2.4) | |
| all: | 167 | |||||
| Gallagher 2001 | I1: calcitriol | 123 | 2/123 (1.6) | 87/123 (71.0) | 55/123 (45.0) | ‐ |
| C1: matched placebo | 123 | 1/123 (0.8) | (56/123 (45.5) | 46/123 (37.0) | ||
| all: | 246 | |||||
| Glendenning 2012 | I: cholecalciferol 150,000 3‐monthly | 353 | 2/353 (0.6) | 24/353 (6.8) | 19/353 (5.4) | ‐ |
| C: placebo vitamin D 3‐monthly | 333 | 0/333 (0) | 21/333 (6.3) | 15/333 (4.5) | ||
| all: | 686 | |||||
| Grady 1991 | I: calcitriol | 50 | 1/50 (2) | 7/50 (14.0) | 7/50 (14.0) | ‐ |
| C: placebo vitamin D | 48 | 0/48 (0) | 2/48 (4.2) | 2/48 (4.2) | ||
| all: | 98 | |||||
| Janssen 2010 | I: vitamin D₃ plus calcium | 36 | 0/36 (0) | ‐ | ‐ | ‐ |
| C: matched placebo vitamin D₃ plus calcium | 34 | 0/34 (0) | ||||
| all: | 70 | |||||
| Komulainen 1999 | I: vitamin D₃ plus calcium | 116 | 0/116 (0) | ‐ | 5/116 (4.3) | ‐ |
| C: placebo | 116 | 1/116 (0.9) | 4/116 (3.4) | |||
| all: | 232 | |||||
| Lappe 2007 | I1: vitamin D₃ plus calcium | 446 | 4/446 (0.9) | 1/446 (0.2) | 13/446 (2.9) | ‐ |
| I2: calcium plus vitamin D placebo | 733 | 18/733 (2.5) | 4/733 (0.5 | 20/733 (2.7) | ||
| all: | 1179 | |||||
| Larsen 2012 | I1: vitamin D₃ | 65 | 2/65 (3.1) | 0/65 (0.0) | 2/65 (3.1) | 1/65 (1.5) |
| C1: matched placebo vitamin D | 65 | 0/65 (0.0) | 0/65 (0.0) | 0/65 (0.0) | 0/65 (0.0) | |
| all: | 130 | |||||
| Murdoch 2012 | I1: vitamin D₃ | 161 | 0/161 (0) | 700/161 | 21/161 (13) | ‐ |
| C1: matched placebo vitamin D | 161 | 0/161 (0) | 792/161 | 19/161 (11.8) | ||
| all: | 322 | |||||
| Ott 1989 | I1: vitamin D3 plus calcium | 43 | 0/43 (0) | 11/43 (25.6) | ‐ | ‐ |
| C1: matched placebo vitamin D plus calcium | 43 | 1/43 (2.3) | 1/43 (2.3) | |||
| all: | 86 | |||||
| Prince 2008 | I1: vitamin D₂2 plus calcium | 151 | 0/151 (0) | ‐ | ‐ | ‐ |
| C1: matched placebo tablet of vitamin D plus calcium | 151 | 1/151 (0.7) | ||||
| all: | 302 | |||||
| Sanders 2010 | I1: vitamin D₃ | 1131 | 40/1131 (3.5) | 223/1131 (19.7) | 244/1131 (19.7) | ‐ |
| C1: matched placebo tablet | 1127 | 47/1127 (4.2) | 201/1127 (17.8) | 207/1127 (17.8) | ||
| all: | 2258 | |||||
| Trivedi 2003 | I1: vitamin D₃ | 1345 | 224/1345 (16.7) | ‐ | ‐ | ‐ |
| C1: matched placebo vitamin D | 1341 | 247/1341 (18.4) | ||||
| all: | 2686 | |||||
| Witham 2013 | I1: vitamin D₃ | 80 | 0/80 (0) | 18/80 (22.5) | 11/80 (13.7) | 0/80 (0) |
| C1: matched placebo vitamin D | 79 | 1/79 (1.3) | 21/79 (26.6) | 13/79 (16.5) | 0/79 (0) | |
| all: | 159 | |||||
| Wood 2012 | I1: vitamin D₃ | 203 | 0/203 (0) | 32/203 (15.8) | 15/203 (7.4) | 11/203 (5.4) |
| C1: matched placebo vitamin D | 102 | 0/102 (0) | 20/102 (19.6) | 4/102 (3.9) | 2/102 (2.0) | |
| all: | 305 | |||||
| "‐" denotes not reported | ||||||
Data and analyses
Comparison 1. Vitamin D versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cancer occurrence in trials with a low or high risk of bias | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 1.1 Trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 1.2 Trials with high risk of bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 2 Cancer occurrence and risk of for‐profit bias | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 2.1 Trials without risk of for‐profit bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 2.2 Trials with risk of for‐profit bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 3 Cancer occurrence in primary and secondary prevention trials | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.1 Primary prevention trials | 16 | 50334 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.2 Secondary prevention trials | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.26, 6.96] |
| 4 Cancer occurrence and vitamin D status | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Vitamin D insufficiency | 7 | 44668 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 4.2 Vitamin D adequacy | 9 | 4544 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 4.3 Unknown vitamin status | 2 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario) | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 'Best‐worst' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.31, 0.54] |
| 5.2 'Worst‐best' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.97, 3.86] |
| 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 14 | 49891 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 6.1 Vitamin D₃ trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 6.2 Vitamin D₃ trials with high risk of bias | 12 | 46900 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium | 14 | 49870 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 7.1 Vitamin D₃ singly | 8 | 9200 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 7.2 Vitamin D₃ combined with calcium | 7 | 40670 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 5 | 45509 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 7 | 43669 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 5 | 45598 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.92, 1.34] |
| 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 2 | 36405 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19 Cancer occurrence in trials using calcitriol | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.52, 4.06] |
| 20 Breast cancer occurrence in trials using calcitriol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21 Uterine cancer occurrence in trials using calcitriol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22 Stomach cancer occurrence in trials using calcitriol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23 All‐cause mortality in trials with a low or high risk of bias | 15 | 49866 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 23.1 Trials with low risk of bias | 1 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 23.2 Trials with high risk of bias | 14 | 47180 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario) | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 'Best‐worst' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.60] |
| 24.2 'Worst‐best' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.47, 2.80] |
| 25 Cancer mortality | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.98] |
| 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 'Best‐worst' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.70] |
| 26.2 'Worst‐best' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.04, 2.75] |
| 27 Adverse events | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Hypercalcaemia in trials using supplemental forms of vitamin D | 4 | 5879 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.64, 3.09] |
| 27.2 Hypercalcaemia in trials using active forms of vitamin D | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.56, 29.22] |
| 27.3 Nephrolithiasis in trials using vitamin D₃ combined with calcium | 3 | 42753 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.03, 1.34] |
| 27.4 Nephrolithiasis in trials using calcitriol | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.10] |
| 27.5 Hypercalciuria | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 12.49 [0.72, 215.84] |
| 27.6 Renal insufficiency | 3 | 5549 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 27.7 Cardiovascular disorders | 8 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 27.8 Gastrointestinal disorders | 7 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.88, 1.59] |
| 27.9 Psychiatric disorders | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.46, 4.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avenell 2012.
| Methods | Randomised Evaluation of Calcium Or vitamin D (RECORD) Multicentre, randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design |
|
| Participants |
Number of participants randomised: 5292 people (85% women) aged 70 and over (mean 77 years) with low‐trauma, osteoporotic fracture in the previous 10 years. Inclusion criteria: elderly people aged 70 years or older, who were mobile before developing a low‐trauma fracture. Exclusion criteria: bed‐ or chair‐bound before fracture; cognitive impairment indicated by an abbreviated mental test score of < 7; cancer in the past 10 years that was likely to metastasise to bone; fracture associated with pre‐existing local bone abnormality; those known to have hypercalcaemia; renal stone in the past 10 years; life expectancy of < 6 months; individuals known to be leaving the United Kingdom; daily intake of more than 200 IU vitamin D or more than 500 mg calcium supplements; intake in the past 5 years of fluoride, bisphosphonates, calcitonin, tibolone, hormone‐replacement therapy, selective oestrogen‐receptor modulators, or any vitamin D metabolite (e.g., calcitriol); and vitamin D by injection in the past year. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D₃ (800 IU) daily (n = 1343) Intervention group 2: calcium (1000 mg) daily (n = 1311) Intervention group 3: vitamin D₃ (800 IU) plus calcium (1000 mg) daily (n = 1306) Comparator group: matched placebo tablets (n = 1332) for a 45‐month period; participants were followed for a period of 6.2 years; tablets varied in size and taste, and thus each had matching placebos. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: all‐new low‐energy fractures including clinical, radiologically‐confirmed vertebral fractures, but not those of the face or skull. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To assess whether vitamin D₃ and calcium, either alone or in combination, were effective in prevention of secondary fractures." | |
| Notes | "Compliance was measured by a postal questionnaire sent every four months, in which participants were asked how many days of the past seven days they had taken tablets. A randomly selected 10% sample was asked to return unused tablets for pill counting. Based on questionnaire responses at 24 months, 2886 (54,5%) of 5292 were still taking tablets. Throughout the trial about 80% of those taking tablets did so on more than 80% of days, which is consistent with pill counts in the subsample (data not shown). However, the number who were taking any tablets fell over time. At 24 months, 2268 of 4841 (46,8%), who returned questionnaires, had taken pills on more than 80% of days." „The United Kingdom Medical Research Council funded the central organization of the RECORD Trial (Grant G9706483). In the last two years, Roger M. Francis has received lecture fees from Shire Pharmaceuticals Group plc. All other authors have nothing to declare in the last two years. Before this, all authors received research grant support to their institutions from the United Kingdom Medical Research Council, Shire Pharmaceuticals Group plc, and Nycomed AS for the RECORD Trial.“ Additional information received through personal communication with Dr Alison Avenell (02.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was centralized, computer generated, stratified by canter, and minimized by age (under 80 yr or 80 yr and over), gender, time since fracture (previous 3 months or longer), and type of enrolling fracture (proximal femur, distal forearm, clinical vertebral, and other)." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Allocation was controlled by a central and independent randomisation unit. The allocation programme was written by the trial programmer and the allocation remained concealed until the final analyses (other than for confidential reports to the data monitoring committee)." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "All outcomes were reported or verified by people who were masked to the allocation scheme. Tablets varied in size and taste, and so each had matching placebos. Calcium and calcium and vitamin D tablets were large, and those for vitamin D were small. Placebos matched in size were provided for each of these three types of tablets." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "All outcomes were reported or verified by people who were masked to the allocation scheme. Tablets varied in size and taste, and so each had matching placebos. Calcium and calcium and vitamin D tablets were large, and those for vitamin D were small. Placebos matched in size were provided for each of these three types of tablets." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | High risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Shire Pharmaceuticals co‐funded the drugs together with Nycomed who also manufactured the drugs." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Bolton‐Smith 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design | |
| Participants |
Number of participants randomised: 244 healthy, nonosteoporotic women, aged 60 years or over (mean 68). Inclusion criteria: healthy, non‐osteoporotic women, aged 60 years or over. Exclusion criteria: clinical osteoporosis or chronic disease (e.g., diabetes mellitus, cardiovascular disease, cancer, fat malabsorption syndromes), routine medication that interferes with vitamin K, vitamin D, or bone metabolism (notably warfarin and steroids), and consumption of nutrient supplements that provided in excess of 30 µg vitamin K, 400 IU vitamin D, or 500 mg calcium daily. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D₃ (400 IU) plus calcium 1000 mg daily (n = 62) Intervention group 2: vitamin D₃ (400 IU) plus calcium 1000 mg plus vitamin K₁ 200 μg daily (n = 61) Intervention group 3: vitamin K₁ 200 μg daily (n = 60) Comparator group: matched placebo daily (n = 61), for a 2‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication.: Primary outcomes: bone mineral density. Secondary outcomes: possible interaction with vitamin K, of vitamin D and calcium. |
|
| Stated aim of study | Quote from the publication: "The putative beneficial role of high dietary vitamin K₁ (phylloquinone) on bone mineral density and the possibility of interactive benefits with vitamin D were studied." | |
| Notes | "Of the 244 eligible women randomised in the trial, 209 (85.6%) completed the two‐year trial. Compliance with the trial intervention was good based on pill count (median, 99; interquartile range, 97.3 to 99.8%)." This study was supported by a contract (N05001) from the UK Food Standards Agency. Additional information on mortality, adverse events, and risk of bias domains was received through personal communication with Dr Martin J Shearer (03.02.2009; 05.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "An independent statistician at Hoffmann‐La Roche, who had no other connection to the study, provided a computer‐generated randomisation list to the researchers Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "An independent statistician at Hoffmann‐La Roche, who had no other connection to the trial, provided a randomisation list to the researchers." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "A flow chart with the numbers of subjects randomly assigned and retained in each treatment arm at successive 6‐month visits is shown in Fig. 1. Of the 244 eligible women randomised into the study, 209 (85.6%) completed the two‐year study, with good supplement adherence based on pill count (median, 99; interquartile range, 97.3 to 99.8%). Reasons for withdrawal were illness unrelated to the study (n = 17); volunteer preference, noncompliance, or other violations of the inclusion criteria (n = 14); and low BMD necessitating further medical intervention (n = 4)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Hoffmann‐La Roche Vitamins Division (now DSM Nutritional Products), Basel, Switzerland provided the supplementation tablets." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Brunner 2011.
| Methods | Women’s Health Initiative (WHI) Multicentre, randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
|
| Participants |
Number of participants randomised: 36,282 50 to 79 (mean 62) years of age, healthy postmenopausal women Inclusion criteria: postmenopausal women 50 to 79 years of age at the initial screening without evidence of a medical condition associated with a predicted survival of < 3 years and no safety, adherence, or retention risks. Exclusion criteria: hypercalcaemia, renal calculi, corticosteroid use, and calcitriol use. Personal supplemental calcium (up to 1000 mg per day) and vitamin D (up to 600 IU per day) were allowed. In 1999, the upper limit of personal vitamin D intake was raised to 1000 IU; the calcium with vitamin D trial permitted the use of bisphosphonates and calcitonin; use of oestrogen (with or without a progestin) was according to randomisation among women in the Hormone Therapy trial; independent use of hormone therapy or selective oestrogen‐receptor modulators was permitted for women in the Dietary Modification trial. |
|
| Interventions |
Number of study centres: 40 Participants were randomly assigned to receive: Intervention group: vitamin D₃ (400 IU) plus calcium (1000 mg) daily (n = 18,176) Comparator group: matched placebo daily (n = 18,106), for a 7‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: hip fracture. Secondary outcomes: other fractures and colorectal cancer. |
|
| Stated aim of study | Quote from the publication: "To test the primary hypothesis that postmenopausal women randomly assigned to vitamin D supplementation plus calcium would have a lower risk of hip fracture, and, secondarily, of all fractures than women assigned to placebo. Another secondary hypothesis was that women receiving calcium with vitamin D supplementation would have a lower rate of colorectal cancer than those receiving placebo." | |
| Notes | "The Women’s Health Initiative was clinical investigation of strategies for the prevention of some of the most common causes of morbidity and mortality among postmenopausal women. It consisted of two components, the randomised controlled clinical trial and observational study. Randomised controlled trial tested two interventions (hormone therapy and dietary modification). Women who were ineligible or unwilling to enrol in randomised trial were invited to participate in the observational study. One year later participants enrolled in the dietary modification trial, hormone therapy trials, or both were invited to join the Women Health Initiative calcium‐vitamin D trial." "Adherence to the trial medication was established by weighing returned pill bottles during clinic visits. The rate of adherence (defined as use of 80% or more of the assigned trial medication) ranged from 60% to 63% during the first three years of follow‐up, with an additional 13% to 21% of the participants taking at least half of their trial pills. At the end of the trial, 76% were still taking the trial medication, and 59% were taking 80% or more of it." "The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–19, 32122, 42107–26, 42129–32, and 44221. The funding organization had representation on the steering committee, which governed the design and conduct of the study, the interpretation of the data, and approval of the article but did not participate in the preparation of the article. The corresponding author has full access to the data and made the final decision when and where to submit the article for publication. R. T. Chlebowski has received a speaker’s fee and honorarium for advisory boards and consulting from AstraZeneca and Novartis; honorarium for advisory boards and consulting for Lilly, Amgen, and Pfizer; and grant support from Amgen. All of the authors have received grant support from National Institutes of Health; Robert L. Brunner and R. T. Chlebowski additionally have received grant support from the National Cancer Institute of Canada. M. L. S. Gass has received grant support fromWyeth. The remaining authors do not report conflicts of interest." We extracted data about cancer incidence and cancer mortality from the following article: Brunner RL, Wactawski‐Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) calcium plus vitamin D randomised clinical trial. Nutrition an Cancer 2011;63(6):827‐41. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Eligible women were randomly assigned in a double‐blind fashion to receive supplements or placebo (provided by GlaxoSmithKline) in equal proportions with use of a permuted‐block algorithm stratified according to clinical canter and age." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Eligible women were randomly assigned in a double‐blind fashion to receive supplements or placebo." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Blinding of the study was achieved by bottle labeling" Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "Blinding of the study was achieved by bottle labeling" Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Ninety‐seven percent of participants were followed to study completion. At the time the study ended, 352 women assigned to calcium with vitamin D supplements and 332 women assigned to placebo had withdrawn; 144 and 152, respectively, had been lost to follow‐up; and 744 and 807, respectively, had died." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The active trial drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh)." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Daly 2008.
| Methods | Randomised controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 167 ambulatory community‐living men 50 to 87 (mean 61.9) years of age Inclusion criteria: ambulatory community‐living men aged 50 years or over. Exclusion criteria: taking calcium and/or vitamin D supplements in the preceding 12 months, participating in regular high‐intensity resistance training in the previous six months or more, then 150 minutes a week of moderate‐ to high‐impact weight‐bearing exercise, had a body mass index > 35 kg/m², lactose intolerance, consuming more than 4 alcoholic beverages per day, a history of osteoporotic fracture or medical disease, or medication use that is known to affect metabolism of bones. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: calcium‐vitamin D₃‐fortified milk containing vitamin D₃ (800 IU) plus calcium (1000 mg) daily (n = 85) Comparator group: usual diet (n = 82), for a 2‐year period; participants were followed for additional 1½ years. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: bone mineral density. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To assess the effects of calcium and vitamin D₃ fortified milk on bone mineral density in community living men > 50 years of age." | |
| Notes | "To monitor milk compliance, participants were asked to record the number of tetra packs consumed per day on a compliance calendar, which was collected and checked every three months. Compliance proportion (expressed as a percentage) was calculated as the actual number of tetra packs consumed, divided by the expected consumption each month. The overall mean reported milk compliance, calculated as the percentage of the tetra packs consumed and based on daily diaries was 85.1%." "None of the authors had a personal or financial conflict of interest." Additional information on mortality was received through personal communication with Professor Robin Daly (04.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Within each stratum, participants were randomised to either the milk supplementation or control group from computer generated random number lists." Comment: sequence generation was achieved using a computer‐generated random number list. |
| Allocation concealment (selection bias) | High risk | Quote from the publication: "Men randomised to receive the calcium‐vitamin D3–fortified milk were asked to consume 400 ml (2 × 200‐ml tetra packs) of reduced‐fat (∼1%) ultra high temperature (UHT) milk specifically formulated by Murray Goulburn Cooperative Co. (Brunswick, Australia). Participants assigned to the control group continued with their usual diet." Comment: the allocation sequence was known to the investigators who assigned participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "The reasons for not attending the follow‐up visit in the milk supplementation group were that the subject was not interested (n = 12), had been diagnosed with cancer (n = 2), could not be contacted (n = 4), had moved away (n = 2), or had died (n=1). For the control group, the main reasons were that the subject was not interested (n = 13), had been diagnosed with cancer (n = 2), could not be contacted (n = 2), or had moved away (n = 2)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Unclear risk | Quote from the publication: "Milk was specifically formulated by Murray Goulburn Cooperative Co. (Brunswick, Australia). The added milk calcium salt (Natra‐Cal) was prepared by Murray Goulburn Cooperative Co. The vitamin D (Vitamin D₃) used to fortify the milk was obtained from DSM Nutritional Products Pty (NSW, Australia)." |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Gallagher 2001.
| Methods | Sites Testing Osteoporosis Prevention / Intervention Treatment (STOP IT) Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design |
|
| Participants |
Number of participants randomised: 489 healthy elderly women 65 to 77 (mean 71.5) years of age. Inclusion criteria: healthy elderly women 65 to 77 years of age and femoral neck density within the normal range for their age. Exclusion criteria: severe chronic illness, primary hyperparathyroidism or active renal stone disease, and were on certain medications, such as bisphosphonates, anticonvulsants, oestrogen, fluoride, or thiazide diuretics in the previous 6 months. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: calcitriol (0.5 μg) daily (n = 123) Intervention group 2: conjugated oestrogens (Premarin) 0.625 mg/daily plus medroxyprogesterone acetate (Provera) 2.5 mg daily (n = 121) Intervention group 3: calcitriol (0.5 μg) plus conjugated oestrogens daily (Premarin) 0.625 mg/daily plus medroxyprogesterone acetate (Provera) 2.5 mg daily (n = 122) Comparator group: matched placebo daily (n = 123) for a 3‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: change in bone mineral density of the femoral neck and spine. Secondary outcomes: incidence of non‐vertebral fractures. |
|
| Stated aim of study | Quote from the publication: "To examine the effect of oestrogen and 1,25‐dihydroxyvitamin D therapy given individually or in combination on bone loss in elderly women." | |
| Notes | "Compliance to trial medication was evaluated by pill counts. At 36 months, treatment group differences in adherence to assigned therapy were evident, with 78% of those assigned to placebo, 70% of those assigned to calcitriol, 65% of those assigned to HRT/ERT and 62% of those assigned to HRT/ERT calcitriol still adherent to their assigned medication. Among those still on medication the compliance for the groups calculated at six months and compared with 36 months, respectively, was: conjugated oestrogens, 86% and 92%; medroxyprogesterone acetate, 91% and 94%; calcitriol, 87% and 93%; placebos, 94% and 92%." This study was primarily supported by the NIH (Grants UO1‐ AG10373 and RO1‐AG10373). Additional support was provided by Wyeth‐Ayerst Laboratories, Inc. Pharm., Hoffman‐LaRoche Inc. And Pharmacia & Upjohn, Inc.. Additional information on mortality and risk of bias domains was received through personal communication with Dr John Gallagher (09.02.2009; 11.03.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The study assigned subjects to treatment groups using simple randomization stratified on hysterectomy status." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "An independent statistical group performed the blinding and randomization." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. An independent statistical group performed the blinding and randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "All investigators and staff conducting the study remained blinded throughout the treatment period." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "All investigators and staff conducting the study remained blinded throughout the treatment period." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "The major reasons given for discontinuing medication were bleeding problems (21), breast tenderness (13), other significant health problems (21), lost interest in the study (19), cerebrovascular incident, cerebral thrombosis, cerebral haemorrhage, transient Ischaemic attack (15), and gastrointestinal problems (14). Five of the subjects died during study from causes unrelated to study medication. There were four deaths from congestive heart failure, one from each treatment group, and one case of sudden death due to myocardial infarct on the combination treatment." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The active trial drug and placebo were supplied by Wyeth‐Ayerst Laboratories, Inc Pharm, Hoffman‐LaRoche Inc and Pharmacia & Upjohn, Inc." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Glendenning 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 686 community‐dwelling ambulant women aged over 70 years (mean 76.7) Inclusion criteria: age over 70 years, registration with a general practitioner, and likelihood, in the investigators’ opinion, of attending 4 study visits over 9 months. Exclusion criteria: consumption of vitamin D supplementation either in isolation or as part of a combination treatment; e.g. Actonel combi +D or Fosamax plus, cognitive impairment (Mini Mental State Score < 24), and individuals who in the investigators’ opinion were not suitable for the study. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: cholecalciferol 150,000 3‐monthly (n = 353) Comparator group: placebo vitamin D 3‐monthly (n = 333), for a 9‐month period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: falls, muscle strength, and mobility. Secondary outcomes: serum 25‐hydrohyvitamin D levels, and adverse events. |
|
| Stated aim of study | Quote from the publication: "to evaluate the effects of cholecalciferol treatment and lifestyle advice compared to lifestyle advice alone on falls, serum 25OHD levels, physical function, and adverse events in 686 women aged over 70 years." | |
| Notes | "The study was supported by the Department of Health, Western Australia State Health Research Advisory Council Research Translation Project Grant, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and Royal Perth Hospital Medical Research Foundation Grant." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The study used a computer generated randomization sequence with a block size of 10 to assign participants to either cholecalciferol therapy or placebo in a ratio of 1:1." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization sequence was generated by a pharmacist at Captain Stirling Pharmacy, Perth, Western Australia, where participants were assigned to intervention and test capsules were appropriately labeled." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. An independent statistical group performed the blinding and randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "The study participants and researchers at the Sir Charles Gairdner Hospital responsible for recruitment and assessment of outcomes measures remained blinded to group assignment." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "The study participants and researchers at the Sir Charles Gairdner Hospital responsible for recruitment and assessment of outcomes measures remained blinded to group assignment." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Subject disposition is presented in Figure 1." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: pre‐defined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Unclear risk | Quote from the publication: "Captain Stirling Pharmacy formulated the test capsules." Comment: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship is provided. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias . |
Grady 1991.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 98 elderly ambulatory men and women (54% women), aged 70 to 97 (mean 79.1) years of age Inclusion criteria: elderly ambulatory men and women. Exclusion criteria: serum calcium levels of 2.57 mmol/L or more, urinary calcium levels of 7.28 mmol/day or more, creatinine clearance less than 0.42 mmol/s, history of hypercalcaemia, nephrolithiasis, seizure disorder, hyperparathyroidism, treatment with calcium, vitamin D or thiazide diuretics, and average calcium intake greater than 1000 mg/day. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: calcitriol (0.5 μg) daily (n = 50) Comparator group: placebo vitamin D (n = 48), for a 6‐months period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: muscle strength. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To test the hypothesis that the weakness associated with aging is in part due to inadequate serum concentrations of 1,25‐(OH₂) D₃." | |
| Notes | "Participants were evaluated at 1, 2, 4, 8, 12, 18, and 24 weeks of intervention regimen to maintain compliance. Participants in both groups took more than 95% of the assigned medication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from the publication: "We conducted a randomized controlled, double‐blinded trial in 98 men and women volunteers over 69 yr old." Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from the publication: "We conducted a randomized controlled, double‐blinded trial in 98 men and women volunteers over 69 yr old." Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "One subject (assigned to treatment with 1,25‐(OH)₂ 2D₃) died following surgery for gastric cancer during the second month of the study. A second subject (assigned to placebo) suffered a stroke during the third month of the study and was unable to complete the protocol." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes are reported on. |
| For‐profit bias | High risk | Quote from the publication: "Calcitriol and placebo capsules were provided by Hoffman‐LaRoche (Nutley, NJ)." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Janssen 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 70 geriatric women older than 65 years with serum 25 hydroxyvitamin D concentrations between 20 and 50 nmol/L Inclusion criteria: vitamin D‐insufficient geriatric women able to walk and follow simple instructions. Exclusion criteria: treatment with vitamin D or steroids in the previous 6 months, a history of hypercalcaemia or renal stones, liver cirrhosis, serum creatinine > 200 μmol/L, malabsorptive bowel syndrome, primary hyperparathyroidism, uncontrolled thyroid disease, anticonvulsant drug therapy, and/or presence of any other condition that would probably interfere with the participants' compliance (i.e., surgery planned). |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₃ (400 IU) plus calcium (500 mg) daily (n = 36) Comparator group: placebo vitamin D₃ plus calcium (500 mg) daily (n = 34), for a 6‐months period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: muscle strength, power and functional mobility. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To test the hypothesis that vitamin D plus calcium supplementation improves muscle strength and mobility, compared with calcium monotherapy in vitamin D insufficient female geriatric patients." | |
| Notes | "This study was financially supported by the Prevention Program (Project 96070602) of ZonMw, The Netherlands." Additional information on funding of the trial received through personal communication with Dr Henie C.J.P. Janssen (06.02.2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was done in blocks of six, to minimize any seasonal influence between the treatment groups." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Trial medication was provided by an independent hospital pharmacist who also performed the randomization." Comment: allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "No person involved, i.e., subjects, investigators, or physicians who treated the subjects, had access to the randomization procedure." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial.. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "No person involved, i.e., subjects, investigators, or physicians who treated the subjects, had access to the randomization procedure." Comment: the outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Eleven subjects withdrew from the trial: death (1), cognitive decline (4), a malignant lung tumor (1), recurrent upper urinary tract infections with malaise (2), acute emotional distress (1), hip fracture (1) and peritonitis (1)." Comment: the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias . |
Komulainen 1999.
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design | |
| Participants |
Number of participants randomised: 464, recently postmenopausal women without contraindications to hormone replacement therapy, 47 to 56 (mean 52.7) years of age. Inclusion criteria: non‐osteoporotic, early postmenopausal women (6 to 24 months had elapsed since their last menstruation). Exclusion criteria: history of breast or endometrial cancer, thromboembolic diseases, and medication‐resistant hypertension. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: sequential combination of 2 mg estradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) (n = 116) Intervention group 2: vitamin D₃ (300 IU) plus calcium (500 mg) daily, intervention‐free interval June ‐ August, the vitamin D₃ dosage was lowered to 100 IU/day after 4 years of treatment because of adverse lipid changes noticed during the first years of the trial (n = 116) Intervention group 3: sequential combination of 2 mg estradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and an intervention‐free interval (days 22 to 28) plus vitamin D₃ (300 IU) and calcium (500 mg) daily (n = 116) Comparator group: placebo daily (n = 116), for a 5‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: bone mineral density. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To examine the long term effects of a sequential oestrogen‐progestin combination therapy (estradiol valerate and cyproterone acetate) and low dose vitamin D₃ supplementation on bone mineral density in nonosteoporotic, early postmenopausal women and to determine whether vitamin D₃ supplementation can give additional benefit to hormone replacement therapy." | |
| Notes | "Of the 464 women enrolled in the trial, 435 (94%) eligible women completed it. Among the 29 drop‐outs were 20 women who could not be contacted in the end of the trial and 3 who died from unrelated causes during the trial period. In addition, 6 osteoporotic women were withdrawn from the trial after enrolment when participant eligibility data were available (baseline lumbar or femoral BMD above ‐2 SD of the mean of the whole trial population)." "This work was supported by Leiras Oy, Finland and Schering AG, Germany." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The women were randomized to the four different groups through use of a computer program." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The group allocation was masked in data analysis." Comment: allocation was controlled by a central and independent randomisation unit, so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "The personnel involved were unaware of the group allocations." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "The personnel involved were unaware of the group allocations." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Of the 464 women enrolled in the study, 435 (94%) eligible women completed it. Among the 29 drop‐outs were 20 women who could not be contacted in the end of the study and 3 who died from unrelated causes during the study period. In addition, 6 osteoporotic women were withdrawn from the study after enrolment when subject eligibility data were available (baseline lumbar or femoral BMD above ‐2 SD of the mean of the whole study population)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The trial was supported by Leiras Oy, Finland and Schering AG, Germany; Hormone replacement therapy was provided by Climen, Schering AG, Germany; Vitamin D₃ by D‐Calsor, Orion Ltd, Finland, and calcium by Rohto Ltd, Tampere, Finland." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Lappe 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (3 intervention groups) | |
| Participants |
Number of participants randomised: 1179 healthy postmenopausal white women, 55 years of age and older (mean 66.7) Inclusion criteria: age > 55 years, at least 4 years past last menses; in generally good health, living independently in the community, and weighing less than 300 pounds Exclusion criteria: a medical diagnosis of any chronic kidney disease, Paget's or other metabolic bone disease, and history of cancer except for superficial basal or squamous cell carcinoma of the skin and other malignancies treated curatively more than 10 years prior to entry into the trial |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D₃ (1000 IU) plus calcium (1400 to 1500 mg) daily (n = 446) Intervention group 2: vitamin D₃ placebo plus calcium (1400 to 1500 mg) daily (n = 445) Comparator group: placebo, consisting of both vitamin D₃ placebo and a brand‐specific calcium placebo daily (n = 288), for a 4‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: fracture incidence. Secondary outcomes: cancer incidence. |
|
| Stated aim of study | Quote from the publication: "To determine the efficacy of calcium alone and calcium plus vitamin D in reducing incident cancer risk of all types." | |
| Notes | "Compliance with trial medication was assessed at six months intervals by bottle weight. Mean adherence (defined as taking 80% of assigned doses) was 85.7% for the vitamin D component of the combined regimen and 74.4% for the calcium component." „None of the authors was affiliated in any way with an entity involved with the manufacture or marketing of vitamin D. RRR has served on scientific advisory boards for Lilly, P&G, Merck, Roche, and Amgen. RPH has served on scientific advisory boards for the International Dairy Foods Association and ConAgra and on the speaker bureau for Merck and P&G.“ Additional information on mortality was received through personal communication with Professor Joan M Lappe (21.11.2007). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The study statistician generated the randomization sequence with the use of a computer‐generated permuted blocks (n = 5) randomization scheme, and the study nurses enrolled the subjects and assigned them to groups." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The study statistician generated the randomization sequence." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: there was insufficient information to assess whether blinding was likely to introduce bias into the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: there was insufficient information to assess whether blinding was likely to introduce bias into the results. |
| Incomplete outcome data (attrition bias) | High risk | Quote from the publication: "Of 1180 women enrolled, 1024 (86.8%) completed the 4 y of study. Most of the losses (n = 92) occurred within the first year." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were not described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The calcium supplements were provided by Mission Pharmacal (San Antonio, TX) and GlaxoSmithKline (Parsippany, NJ). The vitamin D₃ was obtained from Tishcon Corporation (Westbury, NY) Comment: the trial was sponsored by the industry." |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Larsen 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 130 participants with hypertension, mean age 60 years, 69% women. Inclusion criteria: arterial hypertension, white, and resident in Denmark (56º N). Exclusion criteria: 24‐hour ambulatory blood pressure > 150 mm Hg systolic and/or 95 mm Hg diastolic, malignant disease, atrial flutter or fibrillation, hypercalcaemia, pregnancy or nursing, alcohol abuse (> 24 g of alcohol per day for women and > 36 for men), regular use of nonsteroidal anti‐inflammatory drugs or glucocorticoids, daily vitamin D intake exceeding 10 μg of chole‐ or ergocalciferol, tanning bed usage, and changes in antihypertensive medication during trial period. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: cholecalciferol 3000 IU daily (n = 65) Comparator group: placebo vitamin D daily (n = 65), for a 20‐week period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: 24‐hour systolic blood pressure. Secondary outcomes: 24‐hour diastolic blood pressure and heart rate, central blood pressure, central augmentation index, carotid‐femoral pulse wave velocity, urinary calcium‐creatinine ratio, and plasma levels of renin, angiotensin II, aldosterone, brain natriuretic peptide, 25(OH)D, intact parathyroid hormone, ionized calcium, phosphate, and fibroblast growth factor 23. |
|
| Stated aim of study | Quote from the publication: "to test the hypothesis that daily cholecalciferol supplementation in the winter lowers blood pressure in patients with hypertension." | |
| Notes | Mean compliance by pill count was 99% in both groups. "This study was supported by The Danish Osteoporosis Association; The Local Health Service in Randers, Randers Central Hospital, Aarhus County; The Pharmacy Association of 1991; The Danish Health Foundation; and Nycomed DAK." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Participants were allocated to treatment via permutated block randomization conducted at http://www.randomization.com." |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: The hospital pharmacy generated the randomization sequence and labeled the bottles. The randomization code was kept in a sealed envelope until after the last visit of the last participant." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Investigators, participants, and other study personnel were blinded to treatment assignment for the duration of the study." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "Investigators, participants, and other study personnel were blinded to treatment assignment for the duration of the study." Comment: the assessment of outcomes was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Eighteen patients were excluded due to withdrawal of consent (6), 24‐h systolic blood pressure >150 mm Hg (5), inability to complete 24‐h blood pressure measurement (2), changes in antihypertensive medication (2), ibuprofen treatment (1), cancer (1), and major surgery close to follow‐up (1). Thus, 112 patients completed the trial." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Cholecalciferol and placebo tablets were obtained from Ferrosan A/S, Soeborg, Denmark." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Murdoch 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 322 healthy adults mean age 47 years, 75% women Inclusion criteria: aged 18 years or older, able to give written informed consent, a resident of the Christchurch region for the study period. Exclusion criteria: use of vitamin D supplements other than as part of a daily multivitamin preparation (in which the daily intake was ≤ 400 IU); use of immunosuppressants or medications that interfere with vitamin D metabolism (e.g., thiazide diuretics, phenytoin, carbamazepine, primidone, phenobarbital, doses of prednisone > 10 mg/d, methotrexate, azathioprine, cyclosporin); history of hypercalcaemia or nephrolithiasis; sarcoidosis; kidney disorders requiring dialysis or polycystic kidney disease; cirrhosis; current malignancy diagnosis in which the cancer was aggressive and prognosis was poor; baseline plasma calcium (corrected for plasma albumin concentration) greater than 10.4 mg/dL or less than 8.4 mg/dL; enrolment or planned enrolment in other research that would conflict with full participation in the study or confound the observation or interpretation of the study findings (e.g., in which 25‐OHD levels were tested and results known by the participant; in which the participant was required to take conflicting medications; any investigations of viruses and antiviral treatments); and pregnancy or planned pregnancy during the study period |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: an initial dose of 200,000 IU oral vitamin D₃, then 200,000 IU 1 month later, then 100,000 IU monthly (n = 161) Comparator group: placebo administered in an identical dosing regimen (n = 161), for a 1½‐year period |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: number of upper respiratory tract infections episodes. Secondary outcomes: duration of upper respiratory tract infections episodes, severity of upper respiratory tract infections episodes, and number of days of missed work due to upper respiratory tract infections episodes. |
|
| Stated aim of study | Quote from the publication: "To determine the effect of vitamin D supplementation on incidence and severity of upper respiratory tract infections in healthy adults." | |
| Notes | "The study was funded by the Health Research Council of New Zealand. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Participants were assigned using computer‐generated randomisation to receive either vitamin D₃ or placebo." |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization process and bottling of tablets were performed in Auckland, New Zealand, under the supervision of the study biostatistician (A.W.S.) to ensure that those running the study, including outcome assessors and those administering the intervention, were blinded to allocation." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Those running the study, including outcome assessors and those administering the intervention, were blinded to allocation." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "Those running the study, including outcome assessors and those administering the intervention, were blinded to allocation." Comment: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Two hundred ninety‐four participants (91%) completed the study treatment and follow‐up, 18 (6%) withdrew from the study altogether, and 10 (3%) withdrew from treatment but completed the 18‐month follow‐up." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Both vitamin D₃ and placebo tablets were sourced from Tishcon Corp." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Ott 1989.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 86 postmenopausal women, 50 to 80 (mean 67.5) years of age. Inclusion criteria: postmenopausal women with at least 2 compression fractures (> 15% reduction in anterior height) without history of serious trauma. Exclusion criteria: history of corticosteroid use, malnutrition, sarcoidosis, liver disease, rheumatoid arthritis, nephrolithiasis, renal disease, or recent malignancy. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: calcitriol 0.25 to 2 μg plus calcium 1000 mg (n = 43) Comparator group: placebo vitamin D plus calcium 1000 mg daily (n = 43), for a 2‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication: Primary outcomes: bone mass Secondary outcomes: adverse effects of calcitriol |
|
| Stated aim of study | Quote from the publication: "To determine if calcitriol is an effective treatment in postmenopausal osteoporosis." | |
| Notes | "The study was supported by Hoffmann‐La Roche Inc. Dr. Ott is the recipient of a National Institutes of Health (NIH) Clinical Investigator Award #AR‐01244. The study used the General Clinical Research Center, funded by NIH grant #RR‐00037." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Of the 86 women enrolled in the study, 76 completed at least one year and 72 completed 2 years. Four women on calcitriol discontinued the study within the 12 weeks for personal reasons. Four others discontinued after a year; 1 for personal reasons and 1 for multiple fractures; 1 was placed on corticosteroids for a pulmonary disease, and 1 developed clinical signs of chronic lymphocytic leukemia. Six women receiving placebo discontinued within 6 months: 4 for personal reasons, 1 for severe depression, and 1 woman died of myocardial infarction." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Hoffman‐La Roche (Nutley, New Jersey) supplied the vitamin D supplements." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias |
Prince 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 302 community‐dwelling ambulant older women aged 70 to 90 (mean 77.2) years with a history of falling and vitamin D insufficiency. Inclusion criteria: community‐dwelling ambulant older women with a history of falling in the past 12 months and a plasma 25 hydroxyvitamin D concentration of less than 24.0 ng/mL. Exclusion criteria: current vitamin D consumption; current consumption of bone or mineral active agents apart from calcium; a bone mineral density z score at the total hip site of less than ‐2.0; medical conditions or disorders that influence bone mineral metabolism, including laboratory evidence of renal insufficiency (a creatinine level more than 2‐fold above the reference range); a fracture in the past 6 months; a Mini‐Mental State Examination score of < 24; or the presence of marked neurological conditions likely to substantially impair balance or physical activity, such as stroke and Parkinson's disease. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₂ 1000 IU plus calcium 1000 mg daily (n = 151) Comparator group: matched placebo tablet of vitamin D plus calcium 1000 mg daily (n = 151), for a 1‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: risk of falls in older women at high risk of falling. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To evaluate the effect of vitamin D₂ and calcium supplementation compared with calcium alone on the risk of falls in older women at high risk of falling." | |
| Notes | "Adherence to the trial medications was established by counting tablets returned at the clinic visits at 6 and 12 months. The rate of compliance with trial medication in participants who continued to receive the medication, as determined from tablet counting, was 86% in both groups." "This study was supported by a research grant from the National Health and Medical Research Council of Australia (project grant 353638)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The randomization procedure used a random number generator with a block size of 10 to assign participants to ergocalciferol or placebo in a ratio of 1:1, thus ensuring equal recruitment to the 2 groups during the various seasons." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization schedule to ergocalciferol or placebo was generated by an independent research scientist (I.M.D.) and was kept in the pharmacy department of the Sir Charles Gairdner Hospital, where the bottles were labeled and dispensed to the subjects." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment, |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "The study subjects and the study staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial, |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "The study subjects and the study staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Participant flow through the study is shown in Figure 1." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Vitamin D₂ (ergocalciferol) or identical placebo was provided by Ostelin; Boots Healthcare, North Ryde, Australia. Calcium as calcium citrate was provided by Citracal; Mission Pharmacal, Key Pharmaceutical Pty Ltd, Rhodes, Australia." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias |
Sanders 2010.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups); The Vital D study | |
| Participants |
Number of participants randomised: 2258 community‐dwelling women, 70 years or older (mean age 76 years) considered to be at high risk of fracture. Inclusion criteria: community‐dwelling women at higher risk of hip fracture, defined by criteria such as maternal hip fracture, past fracture, or self‐reported faller. Exclusion criteria: unable to provide informed consent or information about falls or fractures; permanently resident at a high‐level care facility; had an albumin‐corrected calcium level higher than 2.65 mmol/L; or had a creatinine level higher than 150 μmol/L, or currently took vitamin D doses of 400 IU or more, calcitriol, or antifracture therapy. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₃ 500,000 IU yearly (n = 1131) Comparator group: matched placebo tablet of vitamin D yearly (n = 1127), for 3 ‐ 5 years (in autumn or winter), median 2.96 years. "Ten tablets were mailed to participants annually (March‐August, determined by recruitment date) with instructions to take all tablets on a single day. Study staff confirmed by telephone the ingestion of study medication within 2 weeks. Subsequent dosing occurred within 2 weeks of the anniversary of the first dose." |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: falls and fractures. Secondary outcomes: serum 25‐hydroxycholecalciferol and intact parathyroid hormone levels. |
|
| Stated aim of study | Quote from the publication: "To determine whether a single annual dose of 500 000 IU of cholecalciferol administered orally to older women in autumn or winter would improve adherence and reduce the risk of falls and fracture." | |
| Notes | "Study staff confirmed by telephone the ingestion of study medication." "The study was supported by project grant No. 251682 from the National Health and Medical Research Council and by the Australian Government Department of Health and Ageing. Financial Disclosures: none reported" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Allocation was performed by an independent statistician using computer generated randomization of numbers performed in blocks of 500." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Allocation was performed by an independent statistician. Treatment allocation status was e‐mailed directly to the hospital clinical trials pharmacist responsible for dispensing study medication." Comment: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "The participants and study staff were blinded to intervention group." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "The participants and study staff were blinded to intervention group." Comment: the assessment of outcomes was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Enrollment and outcomes are shown in Figure 1. There was no difference between the treatment groups in the proportion who withdrew nor in the reasons for withdrawal." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Study medication was supplied by PSM Healthcare, Auckland, New Zealand." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Trivedi 2003.
| Methods | Randomised double‐blind placebo‐controlled trial with parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 2686 elderly people (24% women) aged 65 to 85 (mean 74) years Inclusion criteria: elderly people living in the general community. Exclusion criteria: already taking vitamin D supplements and conditions that were contraindications to vitamin D supplementation (a history of renal stones, sarcoidosis, or malignancy). |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₃ (100,000 IU) every 4 months orally (n = 1345) Comparator group: matched placebo every 4 months orally (n = 1341), for a 5‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: fracture incidence and total mortality by cause. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "To determine the effect of four monthly vitamin D supplementation on the rate of fractures in men and women aged 65 years and over living in the community." | |
| Notes | "Seventy six percent of participants had at least 80% compliance (12/15 doses). Compliance for the final dose was 66%; excluding participants who had died, compliance was estimated to be 80%." "Funding: Start up grant from the Medical Research Council. Competing interests: None declared." Additional information received through personal communication with Professor Kay‐Tee Khaw (23.05.2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The randomisation was conducted by our computer associate who did this by computer." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Ipswich Pharmacy kept the coding." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Participants and investigators were blinded to the treatment until the end of the trial, when Ipswich Pharmacy revealed the coding." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "Participants and investigators were blinded to the treatment until the study ended, when Ipswich Pharmacy revealed the coding." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "A total of 631 (23.5%) participants, including those who died, did not complete the full five years to March 2002—22.8% (307) in the vitamin D group and 24.2% (324) in the placebo group (P = 0.41). No significant difference existed between the two groups in the number known to be alive but who withdrew (that is, discontinued questionnaire follow up) from the study: 5.7% (77) in the placebo group and 6.2% (83) in the active group (P = 0.64)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Low risk | Quote from the publication: "The 100,000 IU vitamin D supplement or placebo used in this trial was specially prepared by the Ipswich Hospital Pharmacy." Comment: the trial appears to be free of industry sponsorship or other kind of for‐profit support that may manipulate the trial design, conductance, or results of the trial. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Witham 2013.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants |
Number of participants randomised: 159 community‐dwelling people aged 70 years and over, mean age 77 years, with isolated systolic hypertension. Inclusion criteria: age 70 years and over, serum 25‐hydroxyvitamin D level < 75 nmol/L; office systolic blood pressure > 140 mm Hg. Exclusion criteria: diastolic blood pressure > 90 mm Hg, systolic blood pressure > 180 mm Hg, hypertension known to be due to a correctable underlying medical or surgical cause; estimated glomerular filtration rate < 40 ml/min (by 4 variable Modified Diet in Renal Disease equation 1); any liver function test (alanine aminotransferase, bilirubin, alkaline phosphatase) > 3 times upper limit of local normal range; albumin‐adjusted serum calcium > 2.60 mmol/L or < 2.15 mmol/L. We also excluded people with known metastatic malignancy or sarcoidosis, a history of renal calculi, diagnosis of heart failure with left ventricular systolic dysfunction, atrial fibrillation, and those already taking prescription vitamin D supplements. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: 100,000 IU oral vitamin D₃ 3‐monthly (n = 80) Comparator group: matched placebo vitamin D (n = 79), for a 1‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: between‐group difference in office blood pressure at 3 months. Secondary outcomes: 24‐hour blood pressure, soluble markers of cardiovascular risk, endothelial function, pulse wave velocity, other biochemical measurements (glucose, total cholesterol, LDL and HDL cholesterol, triglycerides, serum albumin and calcium), exercise capacity and falls. |
|
| Stated aim of study | Quote from the publication: "To test whether high‐dose, intermittent cholecalciferol supplementation lowers blood pressure in older patients with isolated systolic hypertension." | |
| Notes | This study was supported by CSO grant CZB/4/300, a Chief Scientist Office, the Scottish Government, and NHS Education Scotland/CSO Clinician Scientist Award (to Dr Witham). Dr Witham has received grant funding for vitamin D research from the Scottish Government, Diabetes UK, Chest Heart and Stroke Scotland, Heart Research UK, and the ME Society. Dr Struthers has received grant funding for vitamin D research from the Scottish Government, Diabetes UK, and Chest Heart and Stroke Scotland. Dr McMurdo has received grant funding for vitamin D research from the Scottish Government." Additional information received through personal communication with Dr Miles D. Witham (04.02.2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Stratified randomization was performed using a minimization algorithm, administered by the Robertson Centre for Biostatistics (Glasgow Clinical Trials Unit, University of Glasgow, United Kingdom) using a telephone‐based system to conceal study allocation from investigators and participants." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Stratified randomisation was performed using a telephone‐based system to conceal study allocation from investigators and participants." Comment: allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Stratified randomisation was performed using a telephone‐based system to conceal study allocation from investigators and participants. Identical, masked medication bottles were used." Comment: The allocation sequence was unknown to the investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "All outcome measures were performed by researchers who were masked to treatment allocation." Comment: the outcome assessors were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Tablets containing cholecalciferol (Vigantol oil) or matching placebos (Mygliol oil) were produced by Merck KgAA." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias |
Wood 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (3 intervention groups) | |
| Participants |
Number of participants randomised: 305 healthy postmenopausal women aged 60 to 70 years, mean age 64 years. Inclusion criteria: white postmenopausal women. Exclusion criteria: pre‐existing cardiovascular disease, diabetes, asthma, malabsorption, hypertensive blood pressure measurements of at least 160 mm Hg systolic or 99 mm Hg diastolic, difficulty in swallowing tablets or capsules, or who were taking medications or supplements known to affect any dependent variable, current smokers or participants with abnormal blood biochemistry at screening |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: 400 IU oral vitamin D₃ daily (n = 102) Intervention group 1: 1000 IU oral vitamin D₃ daily (n = 101) Comparator group: matched placebo capsule of vitamin D (n = 102), for a 1‐year period. |
|
| Outcomes |
Outcomes reported in abstract of publication Primary outcomes: serum lipid profile, estimate of insulin resistance, inflammatory biomarkers, and blood pressure. Secondary outcomes: none defined. |
|
| Stated aim of study | Quote from the publication: "to test whether daily doses of vitamin D₃ at 400 or 1000 IU/d for one year affected conventional markers of cardiovascular disease risk." | |
| Notes | "This work was funded by the UK Department of Health. The authors have nothing to disclose." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was computer generated. Research nurses assigned participants to one of three intervention groups using an automated telephone service (Health Services Research Unit, University of Aberdeen, UK)." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Capsules containing vitaminD₃ (400 or 1000 IU) or identical placebo were purchased (Pure Encapsulations, Sudbury, MA), packaged into white plastic coded containers, and sealed in sequentially numbered study packs (Bilcare, Powys, UK)." Comment: the allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Both participants and study investigators were blinded to intervention groupings throughout the study." Comment: The allocation sequence was unknown to the investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "Both participants and study investigators were blinded to intervention groupings throughout the study." Comment: the outcome assessors were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "A total of 305 women were randomly assigned to one of three study interventions. In total, 40 withdrew (six due to personal reasons, 34 due to clinical reasons)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Low risk | Quote from the publication: "Capsules containing vitamin D₃ (400 or 1000 IU) or identical placebo were purchased (Pure Encapsulations, Sudbury, MA)." Comment: the trial appears to be free of industry sponsorship or other kind of for‐profit support that may manipulate the trial design, conductance, or results of the trial. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2010 | Not a randomised trial. |
| Chadha 2010 | Randomised controlled trial that included participants with cancer. |
| Crew 2009 | Not a randomised trial. |
| Diamond 2013 | All participants were supplemented with vitamin D. |
| Fedirko 2010 | Randomised controlled trial without clinical outcomes. |
| Garland 2011 | Not a randomised trial. |
| Glossmann 2011 | Not a randomised trial. |
| Hermann 2013 | All participants were supplemented with vitamin D. |
| Holt 2006 | Not a randomised trial. |
| Jacobs 2011 | Not a randomised trial. |
| Kampman 2000 | Not a randomised trial. |
| Lagari 2012 | All participants were supplemented with vitamin D. |
| Peppone 2011 | Not a randomised trial. |
| Rheem 2010 | Not a randomised trial. |
| Tellioglu 2012 | All participants were supplemented with vitamin D. |
| Vashi 2010 | Not a randomised trial. |
| Vieth 2009 | Not a randomised trial. |
| Zabihiyeganeh 2013 | All participants were supplemented with vitamin D. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000402943.
| Trial name or title | ViDA (Vitamin D Assessment) Trial |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants |
Country: New Zealand Estimated number of participants: 5100 Inclusion criteria: age 50 to 84 years; ability to give informed consent; resident in Auckland at recruitment; anticipated residence in New Zealand for the 4‐year study period Exclusion criteria: current use of vitamin D supplements (> 600 IU per day if aged 50 to70 years; >800 IU per day if aged 71 to 84 years); diagnosis of psychiatric disorders that would limit ability to comply with study protocol i.e., history of regular exacerbation of major psychosis (schizophrenia, bipolar disorder) in last two years; history of hypercalcaemia, nephrolithiasis, sarcoidosis, parathyroid disease or gastric bypass surgery; enrolled in another study which could affect participation in the vitamin D study; serum calcium from baseline blood sample >2.50 mmol/L |
| Interventions | Intervention group: Vitamin D3 200,000 IU oral capsule at baseline, then 100,000 IU oral capsule monthly (aside from 200,000 IU oral capsule in each June) Comparator group: placebo (sunflower lecithin), for four years. |
| Outcomes | Incidence rate of fatal and non‐fatal cardiovascular disease, as assessed by mortality, hospital discharges and family doctors |
| Starting date | April 2011 |
| Contact information | Robert Scragg (r.scragg@auckland.ac.nz) |
| Notes |
ISRCTN17873085.
| Trial name or title | The impact of vitamin D supplementation in chronic heart failure |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants |
Country: United Kingdom Estimated number of participants: 100 Inclusion criteria: participants aged 18 years or over with class II and III heart failure due to left ventricular systolic dysfunction (left ventricular ejection fraction ≤ 40%); stable symptoms for 3 months on maximally tolerated medical therapy with no recent change in medication; able to give informed written consent Exclusion criteria: currently taking (or have taken in the previous 3 months) calcium or other vitamin supplements; currently prescribed amlodipine or other calcium channel antagonists (intake of spironolactone will be recorded); chronic heart failure due to untreated valvular heart disease; history of primary hyperparathyroidism, sarcoidosis, tuberculosis or lymphoma; vitamin D levels greater than 50 nmol/L |
| Interventions | Participants will be randomly assigned to receive: Intervention group: vitamin D₃ (4000 IU) daily Comparator group: placebo daily for a period of 1 year |
| Outcomes | The primary outcome measure will be: left ventricular function assessed at baseline and 12 months, measured by cardiac magnetic resonance. Secondary outcome measures will be: symptom status (New York Heart Association status), measured at baseline, 1, 4, 8, 12 months; exercise tolerance, measured at baseline and 12 months; quality of life (Minnesota living with heart failure questionnaire, European Quality of Life instrument and a 19‐item Likert scale index), measured at baseline, 1, 4, 8, 12 months; flow mediated dilatation, measured at baseline and 12 months; immune status, measured at baseline and 12 months; insulin resistance, measured at baseline and 12 months; autonomic activation (measured by heart rate variability), measured at baseline and 12 months; renal function, measured at baseline, 1, 4, 8, and 12 months; B‐type natriuretic peptide, measured at baseline, 1, 4, 8, and 12 months |
| Starting date | January 2009; expected completion December 2012 |
| Contact information | Klaus Witte Division of Cardiovascular and Diabetes Research LIGHT building University of Leeds, Leeds, United Kingdom, LS2 9JT klauswitte@hotmail.com |
| Notes |
Manson 2009.
| Trial name or title | Vitamin D and Omega‐3 Trial (VITAL) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2‐by‐2 factorial design |
| Participants |
Country: United States Estimated number of participants: 20,000 Inclusion criteria: men aged 50 or more or women aged 55 or more who have at least a high school education Exclusion criteria: history of cancer (except non‐melanoma skin cancer), heart attack, stroke, transient Ischaemic attack, angina pectoris, coronary artery bypass graft, or percutaneous coronary intervention; history of renal failure or dialysis, hypercalcaemia, hypo‐ or hyperparathyroidism, severe liver disease (cirrhosis), or sarcoidosis or other granulomatous diseases such as active chronic tuberculosis or Wegener's granulomatosis; allergy to fish or soy; other serious illness that would preclude participation; consuming no more than 800 IU of vitamin D from all supplemental sources combined (individual vitamin D supplements, calcium+vitamin D supplements, medications with vitamin D [e.g., Fosamax Plus D], and multivitamins), or, if taking, willing to decrease or forego such use during the trial; consuming no more than 1200 mg/d of calcium from all supplemental sources combined, or, if taking, willing to decrease or forego such use during the trial; taking fish oil supplements, or, if taking, willing to forego their use during the trial |
| Interventions | Intervention group 1: vitamin D₃ and omega‐3 Intervention group 2: vitamin D₃ and omega‐3 placebo Intervention group 3: vitamin D placebo and omega‐3 Comparator group: vitamin D placebo and omega‐3 placebo orally, daily for a 2‐year period |
| Outcomes | Cancer and cardiovascular disease |
| Starting date | July 2010 |
| Contact information | Project Manager 1‐800‐388‐3963 vitalstudy@rics.bwh.harvard.edu www.vitalstudy.org |
| Notes |
NCT00585637.
| Trial name or title | Vitamin D for chemoprevention |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (4 intervention groups) |
| Participants |
Country: United States Estimated number of participants: 320 Inclusion criteria: healthy black participants 30 to 80 years of age; comfortable communicating in English; currently has a primary care physician; willing to discontinue vitamin D or calcium supplements; willing to have all protocol‐specific tests run. Exclusion criteria: plans on taking a vacation or travel to a sunny region within 3 months of vitamin supplementation period except for a short period (i.e., 1 weekend); pregnant or breast feeding or planning on becoming pregnant in the following year; pre‐existing calcium (including hypercalcaemia), parathyroid conditions (including hyperparathyroidism), sarcoidosis; no concurrent active malignancies (other than non‐melanoma skin cancer) or previous diagnosis of prostate cancer; cognitively impaired; active thyroid disease (e.g., Graves, Hashimoto's or thyroiditis); history of nephrolithiasis, chronic liver disease, chronic renal disease, or renal dialysis |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (1000 IU) daily Intervention group 2: vitamin D₃ (2000 IU) daily Intervention group 3: vitamin D₃ (4000 IU) daily Comparator group: placebo daily for a period of 3 month; participants will be followed for 6 months |
| Outcomes | The primary outcome measures will be: among Blacks, identify a dose of oral vitamin D supplementation that will result in levels of plasma 25‐hydroxyvitamin D that would be predicted to reduce colorectal cancer occurrence. Secondary outcome measures will be: the influence of oral vitamin D supplementation on inflammatory markers and compare germline polymorphic variation in Vitamin D pathway genes between Blacks and a cohort of Whites |
| Starting date | October 2007; expected completion October 2009 |
| Contact information | Charles Fuchs, MD tel: (617) 632‐5840 Charles_Fuchs@dfci.harvard.edu |
| Notes |
NCT00662844.
| Trial name or title | Vitamin D supplementation in younger women |
| Methods | Randomised, double‐blind, placebo controlled trial using parallel group design (5 intervention groups) |
| Participants |
Country: United States Estimated number of participants: 200 Inclusion criteria: premenopausal white or African American women, aged 25 to 45 years; (women with hysterectomy and/or oophorectomy must have a premenopausal Follicle‐stimulating hormone level); serum 25‐hydroxyvitamin D level: 5 to 20 ng/ml; BMI < 45 kg/m²; willing to discontinue vitamin D supplements after entering the trial; negative pregnancy test before BMD and calcium absorption tests; willing to give signed informed consent form Exclusion criteria: cancer (exceptions: basal cell carcinoma or if cancer occurred more than 10 years ago) or terminal illness; previous hip fracture; hemiplegia; uncontrolled type I diabetes ± significant proteinuria or fasting blood sugar > 140 mg in type II diabetes; kidney stones more than 2 in a lifetime; chronic renal failure (serum creatinine > 1.4 mg/dl); evidence of chronic liver disease, including alcoholism; physical conditions such as severe osteoarthritis, rheumatoid arthritis, heart failure severe enough to prevent reasonable physical activity; previous treatment with bisphosphonates (more than 3 months), parathyroid hormone (PTH) or PTH derivatives, (e.g., teriparatide or fluoride in the last 6 months; previous treatment within the last 6 months with calcitonin or oestrogen (except birth control pills); chronic high dose corticosteroid therapy (> 10 mg/day) for over 6 months and not within the last 6 months; anticonvulsant therapy. (Dilantin, Phenobarbital); high dose thiazide therapy (> 37.5 mg); 24‐hour urine calcium > 290 mg on 2 baseline tests; serum calcium exceeding upper normal limit on 2 baseline tests; bone mineral density. T‐score < ‐3.0 for spine or hip |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (400 IU) daily Intervention group 2: vitamin D₃ (800 IU) daily Intervention group 3: vitamin D₃ (1600 IU) daily Intervention group 4: vitamin D₃ (2400 IU) daily Comparator group: placebo daily for a period of 1 year. |
| Outcomes | The primary outcome measures will be serum 25‐hydroxyvitamin D, and parathyroid hormone. Secondary outcome measures will be serum and urine calcium levels |
| Starting date | October 2007; expected completion January 2012 |
| Contact information | JC Gallagher, MD tel: 402‐280‐4518 bones@creighton.edu |
| Notes |
NCT00784511.
| Trial name or title | Vitamin D, glucose control and insulin sensitivity in African‐Americans |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants |
Country: United States Estimated number of participants: 96 Inclusion criteria: African‐American by self designation aged 40 and older; glucose intolerance; body mass index 25.0 to 39.9. Exclusion criteria: diabetes potentially requiring pharmacotherapy, defined as A1c > 7%; uncontrolled thyroid disease; current parathyroid, liver or kidney disease; renal stone within 5 years; sarcoidosis, current pancreatitis, active tuberculosis, hemiplegia, gout; inflammatory bowel disease, colostomy, malabsorption; cancer other than basal cell skin cancer within 5 years; uncontrolled arrhythmia in past year; albinism or other condition associated with reduced skin pigmentation; pregnancy over the last 1 year; intent to become pregnant; menopause onset within 1 year; any other unstable medical condition laboratory tests; fasting plasma glucose < 100; haemoglobin A1c > 7%; laboratory evidence of liver disease (e.g., AST > 70 U/L or ALT > 72 IU/L); laboratory evidence of kidney disease (e.g., estimated glomerular filtration rate < 60 ml/min/1.73 m²; elevated spot urine calcium to creatinine ratio > 0.38 mg/dl); abnormal serum calcium (serum calcium > 10.5 mg/dl); anaemia (hematocrit < 36% in men, < 33% in women); medications (use in past 3 months; oestrogen or testosterone); prescription vitamin D, lithium; oral corticosteroids; anti‐seizure medications; unstable doses of psychotropics or phenothiazines; cholestyramine supplements (current use ‐ may discontinue after screening); vitamin D supplements, cod liver oil, calcium supplements; body mass index less < 25 or > 39.9; consumption of more than 14 alcoholic drinks per week; inability to attend all 3 trial visits as scheduled; inability to provide written informed consent; age < 40 years; not African‐American (by self designation); participation in another research intervention trial; corresponds to a 24‐hour urinary calcium excretion > 400 mg |
| Interventions | Participants will be randomly assigned to receive: Intervention group: vitamin D₃ (4000 IU) daily Comparator group: placebo daily for a period of 12 weeks |
| Outcomes | The primary outcome measure will be insulin secretion, insulin sensitivity and glucose control |
| Starting date | July 2008; expected completion: February 2011 |
| Contact information | Nancy Palermo, B.S. tel: 617‐556‐3073 nancy.palermo@tufts.edu |
| Notes |
NCT01052051.
| Trial name or title | Clinical trial of vitamin D3 to reduce cancer risk in postmenopausal women (CAPS) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants |
Country: United States Estimated number of participants: 2300 postmenopausal women Inclusion criteria: age: ≥ 55 years, last menstrual period ≥ 4 years, good general health, willingness to participate in this 4‐year long study, able to give informed consent, able to live independently and travel to the Fremont Area Medical Center for study visits Exclusion criteria: history of cancer except superficial basal or squamous cell carcinoma of the skin, other malignancies treated curatively more than 10 years ago, history of renal calculi or chronic kidney disease, history of sarcoidosis, history of tuberculosis, participation in the previous Creighton cancer prevention study, Mini‐Mental Status Exam (MMSE) score of ≤ 23 |
| Interventions | Intervention group: vitamin D₃ and calcium 1200 mg daily Comparator group: calcium 1200 mg daily for a period of 5 years |
| Outcomes | Cancer, hypertension, cardiovascular disease, osteoarthritis, colonic adenomas, diabetes, upper respiratory infections, fractures and falls |
| Starting date | June 2009 expected completion June 2015 |
| Contact information | Joan Lappe, Professor of Medicine, Creighton University |
| Notes |
NCT01176344.
| Trial name or title | VItamin D effect on osteoarthritis study VIDEO |
| Methods | Randomised, double‐blind, placebo controlled trial using parallel group design (2 intervention groups) |
| Participants |
Country: Australia Estimated number of participants: 400 Inclusion criteria: age 50 to 79 years old, men and women with symptomatic knee osteoarthritis for at least 6 months with a pain visual analogue scale of at least 20 mm, meet the America College of Rheumatology (ACR) criteria for symptomatic knee osteoarthritis (OA), have an ACR functional class rating of I, II and III, have relatively good health (0 ‐ 2 according to the investigator’s global assessment of disease status on a 5‐point Likert scale, range 0 [very well] to 4 [very poor]), have serum vitamin D level of > 12.5 nmol/L and < 60 nmol/L, and is able to read, speak and understand English, capable of understanding the study requirements and willing to co‐operate with the study instructions. Exclusion criteria: severe radiographic knee OA (grade 3 according to Altman’s atlas), people with severe knee pain (on standing more than 80 mm on a 100‐mm VAS), any contra‐indication to having an MRI, rheumatoid arthritis, psoriatic arthritis, lupus, or cancer, severe cardiac or renal function impairment, hypersensitivity to vitamin D, any condition possibly affecting oral drug absorption (e.g., gastrectomy or clinically significant diabetic gastroenteropathy), significant trauma to the knees including arthroscopy or significant injury to ligaments or menisci of the knee within 1 year preceding the study, anticipated need for knee or hip surgery in the next 2 years, having taken Vitamin D supplements in last 30 days, having taken an investigational drug in last 30 days. |
| Interventions | Intervention group: vitamin D₃ (50,000 IU) monthly Comparator group: placebo monthly for a period of 2 years. |
| Outcomes | Loss of knee cartilage volume, progression of knee cartilage defects, loss of limb muscle strength, enlargement of tibial bone area, central blood pressure, radial applanation tonometry, aortic stiffness, carotid to femoral pulse wave velocity. |
| Starting date | March 2010 |
| Contact information | A/Prof Changhai Ding, Menzies Research Institute, 17 Liverpool St, Hobart 7000, Tasmania; Department of Epidemiology & Preventive Medicine, 99 Commercial Rd, Melbourne 3004, Victoria, Australia Tel: 61‐3‐62267730 Fax: 61‐3‐62267704 Email: chding@utas.edu.au |
| Notes |
NCT01463813.
| Trial name or title | The Finnish Vitamin D Trial (FIND) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (3 intervention groups) |
| Participants |
Country: Finland Estimated number of participants: 18,000 Inclusion criteria: men 60 years or older, women 65 years or older. Exclusion criteria: Cardiovascular disease (including myocardial infarction, stroke, transient ischaemic attack, angina pectoris, coronary artery bypass grafting, or percutaneous coronary intervention), cancer (except non‐melanoma skin cancer), any disease or state that raises a vitamin D‐related safety concern (such as chronic liver, thyroid or kidney disease, hypercalcaemia, sarcoidosis or other granulomatous diseases such as active chronic tuberculosis or Wegener's granulomatosis), use of supplements yielding vitamin D over 20 µg/day or calcium over 1200 mg/day and unwillingness to discontinue the use |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (1600 IU) daily; Intervention group 1: vitamin D₃ (3000 IU) daily Comparator group: placebo daily for a period of 5 years |
| Outcomes | The primary outcome measure will be cancer and cardiovascular diseases |
| Starting date | January 2012; expected completion December 2019 |
| Contact information | Tomi‐Pekka Tuomainen, MD, PhD 358 40 355 2956 tomi‐pekka.tuomainen@uef.fi Jyrki Virtanen, PhD 358 40 355 2957 jyrki.virtanen@uef.fi |
| Notes |
Rees 2013.
| Trial name or title | Vitamin D/calcium polyp prevention study |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2‐by‐2 factorial design |
| Participants |
Country: United States Estimated number of participants: 2200 Inclusion criteria: aged 45 to 75 years; 1 or more histologically verified neoplastic polyp (adenoma) that is at least 2 mm in size removed from the large bowel with the entire large bowel examined by colonoscopy and documented to be free of further polyps or areas suspicious for neoplasia within 120 days of trial entry; anticipated colonoscopic follow‐up 3 or 5 years after the qualifying colonoscopy; agreement to avoid pregnancy (i.e., use of standard contraception); willingness to forego calcium supplementation (including multivitamins containing calcium) or, for women only, option of taking calcium supplementation of 1200 mg/daily (contained in the trial pills); willingness to forego vitamin D supplementation (including multivitamins containing vitamin D); agreement to daily dietary intake of the equivalent of not more than 1200 mg calcium; agreement to daily dietary intake of the equivalent of not more than 400 IU vitamin D; blood calcium level within normal range; blood creatinine level not to exceed 20% above upper limit of normal; serum 25‐hydroxyvitamin D within lower limit of normal to 70 ng/ml; ability and willingness to follow the trial protocol, as indicated by provision of informed consent to participate; good general health, with no severely debilitating diseases or active malignancy that might compromise the participant's ability to complete the trial. Exclusion criteria: participation in another colorectal (bowel) trial in the past 5 years; current participation in any other clinical trial (intervention trial); pregnancy or lactation; a diagnosis of narcotic or alcohol dependence in the past 5 years; a diagnosis of dementia (e.g., Alzheimer's) in the past 5 years; a diagnosis of a significant psychiatric disability (e.g., schizophrenia, refractory bipolar disorder, current severe depression) in the past 5 years; any diagnosis of kidney stones; a diagnosis of granulomatous diseases, e.g., sarcoidosis, active chronic fungal or mycobacterial infections (tuberculosis, histoplasmosis, coccidioidomycosis, blastomycosis), berylliosis, Wegener's granulomatosis in the past 5 years; hyperparathyroidism or other serious disturbance of calcium metabolism in the past 5 years; a diagnosis of severe kidney disease, e.g., chronic renal failure in the past 5 years; unexplained hypercalcaemia in the past 5 years; osteoporosis with physician recommendation for treatment of low bone mass; 2 or more low trauma fractures in the past 5 years; medical condition requiring treatment with vitamin D (e.g., osteomalacia) in the past 5 years; invasive carcinoma of the large bowel (even if confined to a polyp); familial colorectal cancer syndromes, e.g., Familial Adenomatous Polyposis (FAP) (including Gardner syndrome, Turcot's syndrome), Hereditary Nonpolyposis Colorectal Cancer (HNPCC), Hamartomatous Polyposis syndromes (including Peutz‐Jeghers or Familial Juvenile Polyposis); inflammatory bowel disease, e.g., Crohn's Disease, Ulcerative Colitis; a diagnosis of chronic intestinal malabsorption syndromes, e.g., celiac sprue, bacterial overgrowth, chronic pancreatitis, pancreatic insufficiency in the past 5 years; large bowel resection; a diagnosis of malignancy, other than non‐melanoma skin cancer in the past 5 years; severe lung disease ‐ class 3 or 4 (e.g., COPD or emphysema requiring; oxygen) in the past 5 years; severe heart disease: cardiovascular disease functional class 3 or 4 in the past 5 years; severe liver disease, e.g., cirrhosis; any HIV positive diagnosis; active hepatitis B, defined as : Hep B surface antigen positive; active hepatitis C, defined as: measurable HCV RNA; use of chronic oral corticosteroid therapy in the past 5 years; use of lithium in the past 5 years; use of phenytoins in the past 5 years; use of quinidine in the past 5 years; use of therapeutic vitamin D in the past 5 years. |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (1000 IU) daily Intervention group 2: calcium (1200 mg) daily Intervention group 3: vitamin D₃ (1000 IU) plus calcium (1200 mg) daily Comparator group: placebo daily; for a period of 5 years; women who decline to forego calcium supplementation will be randomised only to calcium alone or to calcium plus vitamin D intervention group |
| Outcomes | The primary outcome measure will be new adenomas detected on follow‐up colonoscopy |
| Starting date | July 2004; expected completion: December 2017 |
| Contact information | John A Baron, MD, Principal Investigator, Dartmouth‐Hitchcock Medical Center |
| Notes |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; BMD: bone mass density; COPD: chronic obstructive pulmonary disease; HCV RNA: hepatitis C virus ribonucleic acid; HIV: human immunodeficiency virus
Differences between protocol and review
1. Methods section. Criteria for considering studies for this review. Types of participants. We have now added the following categories of participants, which were excluded in order to be more precise: people with secondary induced osteoporosis (for example, glucocorticoid‐induced osteoporosis, thyroidectomy, primary hyperparathyroidism, chronic kidney disease, liver cirrhosis, Crohn's disease, and gastrointestinal bypass surgery). Firstly, all of these conditions are accompanied by deranged vitamin D metabolism, and by an increase in bone resorption and by a decrease in bone formation. Secondly, we decided to follow exclusion criteria applied in our previous Cochrane review on vitamin D supplementation for prevention of mortality (Bjelakovic 2014). 2. Methods section. Criteria for considering studies for this review. Types of interventions. We have now deleted the following types of interventions: in combination with other vitamins or trace elements; in combination with calcium and other vitamins and trace elements. Our intention was to eliminate the influence of other co‐interventions on our results. We wanted to obtain results that would reflect the pure influence of vitamin D on the outcome measures. 3. We changed QUORUM (Moher 1999) into PRISMA (Moher 2009) as the guideline was updated. 4. Data collection and analysis. Assessment of risk of bias in included studies. We have now added the following risk of bias domains: incomplete outcome data; selective outcome reporting; for‐profit bias; and risk of other bias as the guidelines for risk of bias were updated. 5. Data collection and analysis. Data synthesis. We also planned to conduct trial sequential analyses with diversity‐adjusted required information size instead of heterogeneity‐adjusted required information size. The reason is that the diversity‐adjusted required information size seems to give less biased estimates of the required information size than the inconsistency‐adjusted required information size (Wetterslev 2009). 6. Data collection and analysis. Dealing with missing data. Regarding the primary outcomes, we included participants with incomplete or missing data in sensitivity analyses by imputing them according to the extreme case analysis favouring the experimental intervention ('best‐worst' case scenario): none of the dropouts/participants lost from the experimental arm, but all of the dropouts/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator, and extreme case analysis favouring the control ('worst‐best' case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator. 7. Data collection and analysis. Subgroup analysis. We have decided against performing the following subgroup analysis: "trials without risk of for‐profit bias compared to trials with risk of for‐profit bias", due to the introduction of this source of bias in the review. 8. Data collection and analysis. Sensitivity analysis. We have now decided against performing the following sensitivity analyses: repeating the analysis excluding unpublished trials; repeating the analysis excluding trials using the following filters: diagnostic criteria, language of publication, country. The reason is that all included trials used the same diagnostic criteria. We did not find unpublished trials. All included trials came from high‐income countries and were published in the English language. 9. Goran Krstic joined the team of authors during the preparation of the review.
Contributions of authors
Goran Bjelakovic (GB): initiated the review, drafted the protocol, performed the literature search, data extraction, and statistical analyses, and drafted the review. Lise Lotte Gluud (LLG): revised the protocol, performed data extraction, and revised the review. Dimitrinka Nikolova (DN): revised the protocol, performed data extraction, and revised the review. Kate Whitfield (KW): developed the search strategy, revised the protocol, performed data extraction, and revised the review. Goran Krstic (GK): joined the team of authors during the preparation of the review, performed data extraction, and revised the review. Jørn Wetterslev (JW): revised the protocol, performed data extraction, and revised the review. Christian Gluud (CG): revised the protocol, acted as arbiter for disagreements, and revised the review.
Sources of support
Internal sources
The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
Ministry of Education, Science and Technological Development of the Republic of Serbia, Serbia.
Declarations of interest
Goran Bjelakovic (GB): None known. Lise Lotte Gluud (LLG): None known. Dimitrinka Nikolova (DN): None known. Kate Whitfield (KW): None known. Goran Krstic (GK): None known. Jørn Wetterslev (JW): None known. Christian Gluud (CG): None known.
New
References
References to studies included in this review
Avenell 2012 {published data only}
- Alexander C. Prevention of low‐trauma fractures in older people. Lancet 2005;366(9485):544. [DOI] [PubMed] [Google Scholar]
- Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub‐study of a randomised placebo‐controlled trial in older people (RECORD trial, ISRCTN 51647438). Age and Ageing 2007;36(5):574‐7. [DOI] [PubMed] [Google Scholar]
- Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long‐term follow‐up for mortality and cancer in a randomized placebo‐controlled trial of vitamin D(3) and/or calcium (RECORD trial). Journal of Clinical Endocrinology and Metabolism 2012;97(2):614‐22. [DOI] [PubMed] [Google Scholar]
- Cameron ID, Kurrle SE. Prevention of low‐trauma fractures in older people. Lancet 2005;366(9485):543. [DOI] [PubMed] [Google Scholar]
- Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet 2005;365(9471):1621‐8. [DOI] [PubMed] [Google Scholar]
- McDonald A, Ross S, Campbell M, the RECORD Study Group. Delivering clinical trial supplies by post to elderly trial participants: a feasibility study. Applied Clinical Trials 2004;Feb:58‐9. [Google Scholar]
- Sambrook P. Vitamin D and fractures: quo vadis. Lancet 2005;365(9471):1599‐600. [DOI] [PubMed] [Google Scholar]
- Scharla S. Prevention of low‐trauma fractures in older people. Lancet 2005;366(9485):543. [DOI] [PubMed] [Google Scholar]
Bolton‐Smith 2007 {published data only}
- Bolton‐Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, et al. Two‐year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. Journal of Bone and Mineral Research 2007;22(4):509‐19. [DOI] [PubMed] [Google Scholar]
Brunner 2011 {published data only}
- Brunner RL, Cochrane B, Jackson RD, Larson J, Lewis C, Limacher M, et al. Calcium, vitamin D supplementation, and physical function in the Women's Health Initiative. Journal of the American Dietetic Association 2008;108(9):1472‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner RL, Wactawski‐Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutrition and Cancer 2011;63(6):827‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Archives of Internal Medicine 2007;167(9):893‐902. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski‐Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. Journal of the National Cancer Institute 2008;100(22):1581‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 2008;31(4):701‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women's Health Initiative randomized trial. International Journal of Cancer 2008;122(8):1690‐4. [DOI] [PubMed] [Google Scholar]
- Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115(7):846‐54. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium‐vitamin D trial: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13(9 Suppl):S98‐106. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. New England Journal of Medicine 2006;354(7):669‐83. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Shidham S. The role of hormone therapy and calcium plus vitamin D for reduction of bone loss and risk for fractures: lessons learned from the Women's Health Initiative. Current Osteoporosis Reports 2007;5(4):153‐9. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Wright NC, Beck TJ, Sherrill D, Cauley JA, Lewis CE, et al. Calcium plus vitamin D supplementation has limited effects on femoral geometric strength in older postmenopausal women: the Women's Health Initiative. Calcified Tissue International 2011;88(3):198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women's Health Initiative. Menopause 2010;17(4):683‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Ray RM, Horn L, Manson JE, Allison MA, Black HR, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women's Health Initiative Randomized Trial. Hypertension 2008;52(5):847‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL, Anderson GL. The women's health initiative: lessons learned. Annual Review of Public Health 2008;29:131‐50. [DOI] [PubMed] [Google Scholar]
- Rajpathak SN, Xue X, Wassertheil‐Smoller S, Horn L, Robinson JG, Liu S, et al. Effect of 5 y of calcium plus vitamin D supplementation on change in circulating lipids: results from the Women's Health Initiative. American Journal of Clinical Nutrition 2010;91(4):894‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan TE, Negassa A, Chlebowski RT, Ceria‐Ulep CD, Cochrane BB, Lane DS, et al. A randomized controlled trial of calcium plus vitamin D supplementation and risk of benign proliferative breast disease. Breast Cancer Research and Treatment 2009;116(2):339‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women's health initiative randomized controlled trial. Journal of Clinical Oncology 2011;29(22):3078‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Twombly R. Negative Women's Health Initiative findings stir consternation, debate among researchers. Journal of the National Cancer Institute 2006;98(8):508‐10. [DOI] [PubMed] [Google Scholar]
- Wallace RB, Wactawski‐Wende J, O'Sullivan MJ, Larson JC, Cochrane B, Gass M, et al. Urinary tract stone occurrence in the Women's Health Initiative (WHI) randomized clinical trial of calcium and vitamin D supplements. American Journal of Clinical Nutrition 2011;94(1):270‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittes J, Barrett‐Connor E, Braunwald E, Chesney M, Cohen HJ, Demets D, et al. Monitoring the randomized trials of the Women's Health Initiative: the experience of the Data and Safety Monitoring Board. Clinical Trials 2007;4(3):218‐34. [DOI] [PubMed] [Google Scholar]
Daly 2008 {published data only}
- Daly RM, Bass S, Nowson C. Long‐term effects of calcium‐vitamin‐D3‐fortified milk on bone geometry and strength in older men. Bone 2006;39(4):946‐53. [DOI] [PubMed] [Google Scholar]
- Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium‐ and vitamin D3‐fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2‐year randomized controlled trial. Journal of Bone and Mineral Research 2006;21(3):397‐405. [DOI] [PubMed] [Google Scholar]
- Daly RM, Petrass N, Bass S, Nowson CA. The skeletal benefits of calcium‐ and vitamin D3‐fortified milk are sustained in older men after withdrawal of supplementation: an 18‐mo follow‐up study. American Journal of Clinical Nutrition 2008;87(3):771‐7. [DOI] [PubMed] [Google Scholar]
Gallagher 2001 {published data only}
- Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. Journal of Steroid Biochemistry and Molecular Biology 2004;89‐90(1‐5):497‐501. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age‐related bone loss. Journal of Clinical Endocrinology and Metabolism 2001;86(8):3618‐28. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. Journal of Clinical Endocrinology and Metabolism 2002;87(11):4914‐23. [DOI] [PubMed] [Google Scholar]
- Rapuri PB, Gallagher JC, Haynatzki G. Effect of vitamins D2 and D3 supplement use on serum 25OHD concentration in elderly women in summer and winter. Calcified Tissue International 2004;74(2):150‐6. [DOI] [PubMed] [Google Scholar]
- Sai AJ, Gallagher JC, Fang X. Effect of hormone therapy and calcitriol on serum lipid profile in postmenopausal older women: association with estrogen receptor‐α genotypes. Menopause 2011;18(10):1101‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glendenning 2012 {published data only}
- Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility and muscle strength in older postmenopausal women: a randomised controlled trial. Journal of Bone and Mineral Research 2012;27(1):170‐6. [DOI] [PubMed] [Google Scholar]
Grady 1991 {published data only}
- Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, et al. 1,25‐Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. Journal of Endocrinology and Metabolism 1991;73(5):1111‐7. [DOI] [PubMed] [Google Scholar]
Janssen 2010 {published data only}
- Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D‐insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clinical an Experimental Research 2010;22(1):78‐84. [DOI] [PubMed] [Google Scholar]
Komulainen 1999 {published data only}
- Heikkinen AM, Niskanen L, Ylä‐Herttuala S, Luoma J, Tuppurainen MT, Komulainen M, et al. Postmenopausal hormone replacement therapy and autoantibodies against oxidized LDL. Maturitas 1998;29(2):155‐61. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Parviainen M, Niskanen L, Komulainen M, Tuppurainen MT, Kröger H, et al. Biochemical bone markers and bone mineral density during postmenopausal hormone replacement therapy with and without vitamin D3: a prospective, controlled, randomized study. Journal of Clinical Endocrinology and Metabolism 1997;82(8):2476‐82. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Tuppurainen MT, Niskanen L, Komulainen M, Penttilä I, Saarikoski S, et al. Long‐term vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. European Journal of Endocrinology / European Federation of Endocrine Societies 1997;137(5):495‐502. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population‐based 5‐year randomized trial. Journal of Clinical Endocrinology and Metabolism 1999;84(2):546‐52. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Honkanen R, Saarikoski S. Identification of early postmenopausal women with no bone response to HRT: results of a five‐year clinical trial. Osteoporosis International 2000;11(3):211‐8. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Tuppurainen MT, Kröger H, Heikkinen AM, Puntila E, Alhava E, et al. Vitamin D and HRT: no benefit additional to that of HRT alone in prevention of bone loss in early postmenopausal women. A 2.5‐year randomized placebo‐controlled study. Osteoporosis International 1997;7(2):126‐32. [DOI] [PubMed] [Google Scholar]
- Komulainen MH, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. HRT and Vit D in prevention of non‐vertebral fractures in postmenopausal women; a 5 year randomized trial. Maturitas 1998;31(1):45‐54. [DOI] [PubMed] [Google Scholar]
- Salmen T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Pallonen H, et al. Relation of aromatase gene polymorphism and hormone replacement therapy to serum estradiol levels, bone mineral density, and fracture risk in early postmenopausal women. Annals of Medicine 2003;35(4):282‐8. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Pallonen H, et al. Relation of androgen receptor gene polymorphism to bone mineral density and fracture risk in early postmenopausal women during a 5‐year randomized hormone replacement therapy trial. Journal of Bone and Mineral Research 2003;18(2):319‐24. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, et al. Early postmenopausal bone loss is associated with PvuII estrogen receptor gene polymorphism in Finnish women: effect of hormone replacement therapy. Journal of Bone and Mineral Research 2000;15(2):315‐21. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, et al. Relation of estrogen receptor‐alpha gene polymorphism and hormone replacement therapy to fall risk and muscle strength in early postmenopausal women. Annals of Medicine 2002;34(1):64‐72. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, et al. The protective effect of hormone‐replacement therapy on fracture risk is modulated by estrogen receptor alpha genotype in early postmenopausal women. Journal of Bone and Mineral Research 2000;15(12):2479‐86. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Heikkinen AM, Penttilä I, Saarikoski S. Does vitamin D3 have negative effects on serum levels of lipids? A follow‐up study with a sequential combination of estradiol valerate and cyproterone acetate and/or vitamin D3. Maturitas 1995;22(1):55‐61. [DOI] [PubMed] [Google Scholar]
- Tuppurainen MT, Komulainen M, Kröger H, Honkanen R, Jurvelin J, Puntila E, et al. Does vitamin D strengthen the increase in femoral neck BMD in osteoporotic women treated with estrogen. Osteoporosis International 1998;8(1):32‐8. [DOI] [PubMed] [Google Scholar]
Lappe 2007 {published data only}
- Bolland MJ, Reid IR. Calcium supplementation and cancer incidence. American Journal of Clinical Nutrition 2008;87(3):792‐3. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Davies KM, Travers‐Gustafson D, Heaney RP. Vitamin D status in a rural postmenopausal female population. Journal of the American College of Nutrition 2006;25(5):395‐402. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers‐Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. American Journal of Clinical Nutrition 2007;85(6):1586‐91. [DOI] [PubMed] [Google Scholar]
- Ojha RP, Felini MJ, Fischbach LA. Vitamin D for cancer prevention: valid assertion or premature anointment. American Journal of Clinical Nutrition 2007;86(6):1804‐5. [DOI] [PubMed] [Google Scholar]
- Schabas R. Artifact in the control group undermines the conclusions of a vitamin D and cancer study. American Journal of Clinical Nutrition 2008;87(3):792. [DOI] [PubMed] [Google Scholar]
- Sood MM, Sood AR. Dietary vitamin D and decreases in cancer rates: Canada as the national experiment. American Journal of Clinical Nutrition 2007;86(5):1549. [DOI] [PubMed] [Google Scholar]
Larsen 2012 {published data only}
- Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo‐controlled trial. American Journal of Hypertension 2012;25(11):1215‐22. [DOI] [PubMed] [Google Scholar]
Murdoch 2012 {published data only}
- Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 2012;308(13):1333‐9. [DOI] [PubMed] [Google Scholar]
Ott 1989 {published data only}
- Ott SM, Chesnut CH 3rd. Calcitriol treatment is not effective in postmenopausal osteoporosis. Annals of Internal Medicine 1989;110(4):267‐74. [DOI] [PubMed] [Google Scholar]
Prince 2008 {published data only}
- Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high‐risk women. Archives of Internal Medicine 2008;168(1):103‐8. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bruce D, Austin N, Devine A, Ebeling PR, Prince RL. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone‐related chemistry in elderly women with vitamin D insufficiency. Journal of Bone and Mineral Research 2008;23(8):1343‐8. [DOI] [PubMed] [Google Scholar]
Sanders 2010 {published data only}
- Sanders KM, Stuart AL, Merriman EN, Read ML, Kotowicz MA, Young D, et al. Trials and tribulations of recruiting 2,000 older women onto a clinical trial investigating falls and fractures: Vital D study. BMC Medical Research Methodology 2009;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, et al. Annual high‐dose vitamin D3 and mental well‐being: randomised controlled trial. British Journal of Psychiatry 2011;198(5):357‐64. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high‐dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303(18):1815‐22. [DOI] [PubMed] [Google Scholar]
Trivedi 2003 {published data only}
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witham 2013 {published data only}
- Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Internal Medicine 2013;173(18):1672‐9. [DOI] [PubMed] [Google Scholar]
Wood 2012 {published data only}
- Macdonald HM, Wood AD, Aucott LS, Black AJ, Fraser WD, Mavroeidi A, et al. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1‐year double‐blind RCT in postmenopausal women. Journal of Bone and Mineral Research 2013;28(10):2202‐13. [DOI] [PubMed] [Google Scholar]
- Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel‐group, double‐blind, placebo‐controlled RCT. Journal of Clinical Endocrinology and Metabolism 2012;98(11):4507‐15. [DOI] [PubMed] [Google Scholar]
- Wood AD, Secombes KR, Thies F, Aucott LS, Black AJ, Reid DM, et al. A parallel group double‐blind RCT of vitamin D3 assessing physical function: is the biochemical response to treatment affected by overweight and obesity. Osteoporosis International 2014;25(1):305‐15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2010 {published data only}
- Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre‐ and postmenopausal women. American Journal of Clinical Nutrition 2010;91(6):1699‐707. [DOI] [PubMed] [Google Scholar]
Chadha 2010 {published data only}
- Chadha MK, Tian L, Mashtare T, Payne V, Silliman C, Levine E, et al. Phase 2 trial of weekly intravenous 1,25 dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration‐resistant prostate cancer. Cancer 2010;116(9):2132‐9. [DOI] [PubMed] [Google Scholar]
Crew 2009 {published data only}
- Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, et al. Association between plasma 25‐hydroxyvitamin D and breast cancer risk. Cancer Prevention Research 2009;2(6):598‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2013 {published data only}
- Diamond T, Wong YK, Golombick T. Effect of oral cholecalciferol 2,000 versus 5,000 IU on serum vitamin D, PTH, bone and muscle strength in patients with vitamin D deficiency. Osteoporosis International 2013;24(3):1101‐5. [DOI] [PubMed] [Google Scholar]
Fedirko 2010 {published data only}
- Ahearn TU, McCullough ML, Flanders WD, Long Q, Sidelnikov E, Fedirko V, et al. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on markers of their metabolism in normal mucosa of colorectal adenoma patients. Cancer Research 2011;71(2):413‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiology, Biomarkers and Prevention 2010;19(1):280‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Bostick RM, Daniel CR, Flanders WD, Shaukat A, Davison J, et al. Vitamin D status and impact of vitamin D3 and/or calcium supplementation in a randomized pilot study in the Southeastern United States. Journal of the American College of Nutrition 2009;28(6):678‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2011 {published data only}
- Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25‐hydroxyvitamin D in the range associated with cancer prevention. Anticancer Research 2011;31(2):607‐11. [PubMed] [Google Scholar]
Glossmann 2011 {published data only}
- Glossmann H. Vitamin D, UV, and skin cancer in the elderly: to expose or not to expose. Gerontology 2011;57(4):350‐3. [DOI] [PubMed] [Google Scholar]
Hermann 2013 {published data only}
- Herrmann W, Kirsch SH, Kruse V, Eckert R, Gräber S, Geisel J, et al. One year B and D vitamins supplementation improves metabolic bone markers. Clinical Chemistry and Laboratory Medicine 2013;51(3):639‐47. [DOI] [PubMed] [Google Scholar]
Holt 2006 {published data only}
- Holt PR, Bresalier RS, Ma CK, Liu KF, Lipkin M, Byrd JC, et al. Calcium plus vitamin D alters preneoplastic features of colorectal adenomas and rectal mucosa. Cancer 2006;106(2):287‐96. [DOI] [PubMed] [Google Scholar]
Jacobs 2011 {published data only}
- Jacobs ET, Thomson CA, Flatt SW, Al‐Delaimy WK, Hibler EA, Jones LA, et al. Vitamin D and breast cancer recurrence in the Women's Healthy Eating and Living (WHEL) Study. American Journal of Clinical Nutrition 2011;93(1):108‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampman 2000 {published data only}
- Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States). Cancer Causes and Control 2000;11(5):459‐66. [DOI] [PubMed] [Google Scholar]
Lagari 2012 {published data only}
- Lagari VS, Gómez‐Marín O, Levis S. Differences in vitamin D3 dosing regimens in a geriatric community‐dwelling population. Endocrine Practice 2012;18(6):847‐54. [DOI] [PubMed] [Google Scholar]
Peppone 2011 {published data only}
- Peppone LJ, Huston AJ, Reid ME, Rosier RN, Zakharia Y, Trump DL, et al. The effect of various vitamin D supplementation regimens in breast cancer patients. Breast Cancer Research and Treatment 2011;127(1):171‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rheem 2010 {published data only}
- Rheem DS, Baylink DJ, Olafsson S, Jackson CS, Walter MH. Prevention of colorectal cancer with vitamin D. Scandinavian Journal of Gastroenterology 2010;45(7‐8):775‐84. [DOI] [PubMed] [Google Scholar]
Tellioglu 2012 {published data only}
- Tellioglu A, Basaran S, Guzel R, Seydaoglu G. Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly. Maturitas 2012;72(4):332‐8. [DOI] [PubMed] [Google Scholar]
Vashi 2010 {published data only}
- Vashi PG, Trukova K, Lammersfeld CA, Braun DP, Gupta D. Impact of oral vitamin D supplementation on serum 25‐hydroxyvitamin D levels in oncology. Nutrition Journal 2010;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieth 2009 {published data only}
- Vieth R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Research 2009;29(9):3675‐84. [PubMed] [Google Scholar]
Zabihiyeganeh 2013 {published data only}
- Zabihiyeganeh M, Jahed A, Nojomi M. Treatment of hypovitaminosis D with pharmacologic doses of cholecalciferol, oral vs intramuscular; an open labeled RCT. Clinical Endocrinology 2013;78(2):210‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12611000402943 {published data only}
- ACTRN12611000402943. Effect of vitamin D supplementation on cardiovascular and respiratory disease event rates in lder adults, and the incidence of non‐vertebral fractures. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336777 (accessed 9th May 2014).
ISRCTN17873085 {published data only}
- ISRCTN17873085. Examining the pleiotropic actions of vitamin D supplementation in patients with chronic heart failure. www.controlled‐trials.com/ISRCTN17873085 (accessed 9th May 2014).
Manson 2009 {published data only}
- Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA‐3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega‐3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemporary Clinical Trials 2012;33(1):159‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00585637 {published data only}
- NCT00585637. Defining Optimal Doses of Vitamin D for Chemoprevention in Blacks. clinicaltrials.gov/show/NCT00585637 (9th May 2014).
NCT00662844 {published data only}
- NCT00662844. Optimum vitamin D nutrition in young women. clinicaltrials.gov/ct2/show/NCT00662844?term=NCT00662844&rank=1 (accessed 9th May 2014).
NCT00784511 {published data only}
- NCT00784511. Vitamin D, glucose control and insulin sensitivity in African‐Americans. clinicaltrials.gov/ct2/show/NCT00784511?term=NCT00784511&rank=1 (accessed 9th May 2014).
NCT01052051 {published data only}
- NCT01052051. Clinical trial of vitamin D3 to reduce cancer risk in postmenopausal women. clinicaltrials.gov/ct2/show/NCT01052051?term=NCT01052051&rank=1 (accessed 9th May 2014).
NCT01176344 {published data only}
- NCT01176344. Does vitamin D supplementation prevent progression of knee osteoarthritis? a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT01176344?term=NCT01176344&rank=1 (accessed 9th May 2014).
NCT01463813 {published data only}
- NCT01463813. Finnish vitamin D trial (FIND). clinicaltrials.gov/ct2/show/NCT01463813?term=NCT01463813&rank=1 (accessed 9th May 2014).
Rees 2013 {published data only}
- NCT00153816. Vitamin D/Calcium Polyp Prevention Study. clinicaltrials.gov/show/NCT00153816 (accessed 9th May 2014).
- Rees JR, Hendricks K, Barry EL, Peacock JL, Mott LA, Sandler RS, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clinical Infectious Diseases 2013;57(10):1384‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahn 2008
- Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, et al. Serum vitamin D concentration and prostate cancer risk: a nested case‐control study. Journal of the National Cancer Institute 2008;100(11):796‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2013a
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI] [PubMed] [Google Scholar]
Andrews 2013b
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation‐determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726‐35. [DOI] [PubMed] [Google Scholar]
Apperly 1941
- Apperly FL. The relation of solar radiation to cancer mortality in North American. Cancer Research 1941;1:191‐5. [DOI] [PubMed] [Google Scholar]
Armas 2004
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. Journal of Clinical Endocrinology and Metabolism 2004;89(11):5387‐91. [DOI] [PubMed] [Google Scholar]
Autier 2014
- Autier P, Boniol M, Mullie P. Vitamin D status and ill health: a systematic review. The Lancet Diabetes & Endocrinology 2014;2(1):76‐89. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Bikle 2009
- Bikle D. Nonclassic actions of vitamin D. Journal of Clinical Endocrinology and Metabolism 2009;94(1):26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bikle 2014
- Bikle DD. Vitamin D and cancer: the promise not yet fulfilled. Endocrine 2014;46:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrar 2009c
- Bischoff‐Ferrari H. Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary. Best Practice & Research. Clinical Rheumatology 2009;23(6):789‐95. [DOI] [PubMed] [Google Scholar]
Bjelakovic 2014
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007470.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2010
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2011
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta‐analysis. BMJ 2011;342:d2040. [DOI: 10.1136/bmj.d2040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2014
- Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta‐analysis. Lancet Diabetes Endocrinology 2014;2(4):307‐20. [DOI] [PubMed] [Google Scholar]
Bouillon 2013
- Bouillon R, Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D, et al. Optimal vitamin D status: a critical analysis on the basis of evidence‐based medicine. The Journal of Clinical Endocrinology and Metabolism 2013;98(8):E1283‐304. [DOI] [PubMed] [Google Scholar]
Bristow 2013
- Bristow SM, Bolland MJ, MacLennan GS, Avenell A, Grey A, Gamble GD, et al. Calcium supplements and cancer risk: a meta‐analysis of randomised controlled trials. The British Journal of Nutrition 2013;110(8):1384‐93. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Brunetti 2013
- Brunetti M1, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology 2013;66(2):140‐50. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2007
- Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. Prospective study of serum 25(OH)‐vitamin D concentration and risk of oesophageal and gastric cancers. British Journal of Cancer 2007;97(1):123‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2013
- Chiang KC, Chen TC. The anti‐cancer actions of vitamin D. Anticancer Agents in Medicinal Chemistry 2013;13(1):126‐39. [PubMed] [Google Scholar]
Chung 2011
- Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta‐analysis for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2011;155(12):827‐38. [DOI] [PubMed] [Google Scholar]
Cranney 2007
- Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health Effectiveness and safety of vitamin D in relation to bone health. Evidence Report/Technology Assessment 2007;158:1‐235. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Cutolo 2009
- Cutolo M. Vitamin D and autoimmune rheumatic diseases. Rheumatology 2009;48(3):210‐2. [DOI] [PubMed] [Google Scholar]
Davis 2007
- Davis CD, Dwyer JT. The "sunshine vitamin": benefits beyond bone?. Journal of the National Cancer Institute 2007;99(21):1563‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 2005
- Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International 2005;16(7):713‐6. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 2010
- Dawson‐Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporosis International 2010;21(7):1151‐4. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DIPART 2010
- DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 2010;340:b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleet 2012
- Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. The Biochemical Journal 2012;441(1):61‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flynn 2006
- Flynn G, Chung I, Yu WD, Romano M, Modzelewski RA, Johnson CS, et al. Calcitriol (1,25‐dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor‐derived endothelial cells and induces apoptosis. Oncology 2006;70(6):447‐57. [DOI] [PubMed] [Google Scholar]
Fortmann 2013
- Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2013;159(12):824‐34. [DOI] [PubMed] [Google Scholar]
Freedman 2007
- Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. Jornal of the National Cancer Institute 2007;99(21):1594‐602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Freedman 2010
- Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25‐hydroxyvitamin D and cancer mortality in the NHANES III study (1988‐2006). Cancr Research 2010;70(21):8587‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 1980
- Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer?. International Journal of Epidemiology 1980;9(3):227‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garland 2006
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. American Journal of Public Health 2006;96(2):252‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2007
- Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, et al. Vitamin D and prevention of breast cancer: pooled analysis. The Journal of Steroid Biochemistry and Molecular Biology 2007;103(3‐5):708‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giovannucci 2005
- Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes & Control 2005;16(2):83‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gluud 2011
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 10. Art. No.: LIVER.
Goodwin 2009
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25‐hydroxyvitamin D levels in early breast cancer. Journal of Clinical Oncology 2009;27(23):3757‐63. [DOI] [PubMed] [Google Scholar]
Gorham 2007
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. American Journal of Preventive Medicine 2007;32(3):210‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guallar 2010
- Guallar E, Miller ER 3rd, Ordovas JM, Stranges S. Vitamin D supplementation in the age of lost innocence. Annals of Internal Medicine 2010;152(5):327‐9. [DOI] [PubMed] [Google Scholar]
Guallar 2013
- Guallar E, Stranges S, Mulrow C, Appel LJ, Miller ER 3rd. Enough is enough: Stop wasting money on vitamin and mineral supplements. Annals of Internal Medicine 2013;159(12):850‐1. [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011i
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles‐continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Hart 2011
- Hart PH, Gorman S, Finlay‐Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D. National Review Immunology 2011;11(9):584‐96. [DOI] [PubMed] [Google Scholar]
Harvey 2012
- Harvey NC, Cooper C. Vitamin D: some perspective please. BMJ 2012;345:e4695. [DOI: 10.1136/bmj.e4695] [DOI] [PubMed] [Google Scholar]
Heaney 2011
- Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. Journal of Clinical Endocrinology and Metabolism 2011;96(3):E447‐52. [DOI] [PubMed] [Google Scholar]
Helzlsouer 2010
- Helzlsouer KJ, VDPP Steering Committee. Overview of the cohort consortium vitamin D pooling of rarer cancers. American Journal of Epidemiology 2010;172(1):4‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilger 2014
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. British Journal of Nutrition 2014;111(1):23‐45. [DOI] [PubMed] [Google Scholar]
Holick 2007
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holick 2011
- Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology 2011;96(7):1911‐30. [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horst 2005
- Horst RL, Reinhardt TA, Satyanarayana Reddy G. Vitamin D metabolism. In: Feldman D, Wesley Pike J, Glorieux FH editor(s). Vitamin D. 2nd Edition. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier Academic Press, 2005:15‐36. [Google Scholar]
IARC 2008
- IARC. Vitamin D and Cancer. IARC Working Group Reports. Lyon, France: International Agency for Research on Cancer, 2008; Vol. 5.
ICH‐GCP 1997
- International Committee on Harmonization. Code of Federal Regulations & Guidelines. Vol. 1, Philadelphia, US: Barnett International/PAREXEL, 1997. [Google Scholar]
ICTRP 2011
- World Health Organization. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ 2011.
Ioannidis 2006
- Ioannidis JP, Trikalinos TA, Zintzaras E. Extreme between‐study homogeneity in meta‐analyses could offer useful insights. Journal of Clinical Epidemiology 2006;59(10):1023‐32. [DOI] [PubMed] [Google Scholar]
IOM 2011
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
Jakobsen 2013
- Jakobsen JC, Gluud C. The necessity of randomized clinical trials. British Journal of Medicine & Medical Research 2013;3(4):1453‐68. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krstic 2011
- Krstic G. Re: "Circulating 25‐hydroxyvitamin D and risk of pancreatic cancer". American Journal of Epidemiology 2011;173(4):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krstic 2012
- Krstic G. Vitamin D status vs. cancer survival/prognosis. Annals of Internal Medicine 2012;155:Comments on Chung et al. 2011 (Link: http://annals.org/article.aspx?articleid=1033222). [Google Scholar]
Kupferschmidt 2012
- Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science 2012;337(6101):1476‐8. [DOI] [PubMed] [Google Scholar]
Lamberg‐Allardt 2006
- Lamberg‐Allardt C. Vitamin D in foods and as supplements. Progress in Biophysics and Molecular Biology 2006;92(1):33‐8. [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KK, DeMets D. Discrete sequential monitoring boundaries for clinical trials. Biometrika 1983;70(3):659‐63. [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333(7568):597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehmann 2013
- Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo‐controlled trial. The Journal of Endocrinology and Metabolism 2013;98(11):4339‐45. [DOI] [PubMed] [Google Scholar]
Leventis 2009
- Leventis P, Kiely PD. The tolerability and biochemical effects of high‐dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scandinavian Journal of Rheumatology 2009;38(2):149‐53. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lips 2004
- Lips P. Which circulating level of 25‐hydroxyvitamin D is appropriate. Journal of Steroid Biochemistry and Molecular Biology 2004;89‐90(1‐5):611‐4. [DOI] [PubMed] [Google Scholar]
Lips 2006
- Lips P. Vitamin D physiology. Progress in Biophysics and Molecular Biology 2006;92(1):4‐8. [DOI] [PubMed] [Google Scholar]
Lips 2010
- Lips P. Worldwide status of vitamin D nutrition. The Journal of Steroid Biochemistry and Molecular Biology 2010;121(1‐2):297‐300. [DOI] [PubMed] [Google Scholar]
Lips 2012
- Lips P. Interaction between vitamin D and calcium. Scandinavian Journal of Clinical and Laboratory Investigation 2012;72(243):60‐4. [DOI] [PubMed] [Google Scholar]
Logan 2013
- Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA. Long‐term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25‐hydroxyvitamin D status over the winter months. British Journal of Nutrition 2013;109(6):1082‐8. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Marshall 2008
- Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays 2008;30(2):173‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006; Vol. 3, issue 11:e442. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
McAlister 2003
- McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials ‐ A systematic review. JAMA 2003;289(19):2545‐53. [DOI] [PubMed] [Google Scholar]
Michos 2008
- Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Current Opinion in Clinical Nutrition and Metabolic Care 2008;11(1):7‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyer 2013
- Moyer VA, U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2013;158(9):691‐6. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Norman 2006
- Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 2006;147(12):5542‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norman 2014
- Norman PE, Powell JT. Vitamin d and cardiovascular disease. Circulation Research 2014;114(2):379‐93. [DOI] [PubMed] [Google Scholar]
Ordóñez‐Mena 2013
- Ordóñez‐Mena JM, Schöttker B, Haug U, Müller H, Köhrle J, Schomburg L, et al. Serum 25‐hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention 2013;22(5):905‐16. [DOI] [PubMed] [Google Scholar]
Palmer 2009
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005633.pub2] [DOI] [PubMed] [Google Scholar]
Patel 2012
- Patel VB, Vacek JL, Graves L, Bhattacharya RK. Calcium effects on vascular endpoints. Nutrition and Metabolism 2012;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilz 2009
- Pilz S, Tomaschitz A, Obermayer‐Pietsch B, Dobnig H, Pieber TR. Epidemiology of vitamin D insufficiency and cancer mortality. Anticancer Research 2009;29(9):3699‐704. [PubMed] [Google Scholar]
Pilz 2013
- Pilz S, Kienreich K, Tomaschitz A, Ritz E, Lerchbaum E, Obermayer‐Pietsch B, et al. Vitamin D and cancer mortality: systematic review of prospective epidemiological studies. Anti‐cancer Agents in Medicinal Chemistry 2013;13(1):107‐17. [PubMed] [Google Scholar]
Pocock 2004
- Pocock SJ. Clinical Trials: A Practical Approach. John Wiley & Sons, 2004. [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [DOI] [PubMed] [Google Scholar]
Redaniel 2014
- Redaniel MT, Gardner MP, Martin RM, Jeffreys M. The association of vitamin D supplementation with the risk of cancer in postmenopausal women. Cancer Causes & Control 2014;25(2):267‐71. [DOI] [PubMed] [Google Scholar]
Reid 2014
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta‐analysis. Lancet 2014;383(9912):146‐55. [DOI] [PubMed] [Google Scholar]
Rejnmark 2012
- Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. Journal of Clinical Endocrinology and Metabolism 2012;97(8):2670‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Romagnoli 2008
- Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long‐term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. Journal of Clinical Endocrinology and Metabolism 2008;93(8):3015‐20. [DOI] [PubMed] [Google Scholar]
Rosen 2012
- Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocrine Reviews 2012;33(3):456‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [7823387] [DOI] [PubMed] [Google Scholar]
Schwartz 2007
- Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Current Opinion in Clinical Nutrition and Metabolic Care 2007;10(1):6‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shiraki 2004
- Shiraki M, Fukuchi M, Kiriyama T, Okamoto S, Ueno T, Sakamoto H, et al. Alfacalcidol reduces accelerated bone turnover in elderly women with osteoporosis. Journal of Bone and Mineral Metabolism 2004;22(4):352‐9. [DOI] [PubMed] [Google Scholar]
Sperati 2013
- Sperati F, Vici P, Maugeri‐Saccà M, Stranges S, Santesso N, Mariani L, et al. Vitamin d supplementation and breast cancer prevention: a systematic review and meta‐analysis of randomized clinical trials. PloS One 2013;8(7):e69269. [10.1371/journal.pone.0069269. Print 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolzenberg‐Solomon 2006
- Stolzenberg‐Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, et al. A prospective nested case‐control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Research 2006;66(20):10213‐9. [DOI] [PubMed] [Google Scholar]
Stolzenberg‐Solomon 2010
- Stolzenberg‐Solomon RZ, Jacobs EJ, Arslan AA, Qi D, Patel AV, Helzlsouer KJ, et al. Circulating 25‐hydroxyvitamin D and risk of pancreatic cancer: Cohort consortium vitamin D pooling project of rarer cancers. American Journal of Epidemiology 2010;172(1):81‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutton 2003
- Sutton ALM, MacDonald PN. Vitamin D: More than just a "Bone‐a‐Fide" hormone. Molecular Endocrynology 2003;17(5):777‐91. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2011a
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis—a simulation study. PloS One 2011;6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011b
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). www.ctu.dk/tsa. (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, 2011) 2011.
Toner 2010
- Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. Journal of the American Dietetic Association 2010;110(10):1492‐500. [DOI] [PubMed] [Google Scholar]
Trang 1998
- Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25‐hydroxyvitamin D more efficiently than does vitamin D2. American Journal of Clinical Nutrition 1998;68(4):854‐8. [DOI] [PubMed] [Google Scholar]
Tripkovic 2012
- Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25‐hydroxyvitamin D status: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2012;95(6):1357‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenahgen Trial Unit. Trial Sequential Analysis, version 0.9. Copenahgen Trial Unit, 2011.
Tuohimaa 2012
- Tuohimaa P, Lou YR. Optimal serum calcidiol concentration for cancer prevention. Anticancer Research 2012;32(1):373‐81. [PubMed] [Google Scholar]
Turner 2013
- Turner RM, Bird SM, Higgins JP. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PloS One 2013;8(3):e59202. [DOI: 10.1371/journal.pone.0059202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Schoor 2011
- Schoor NM, Lips P. Worldwide vitamin D status. Best Practice & Research. Clinical Endocrinology & Metabolism 2011;25(4):671‐80. [DOI] [PubMed] [Google Scholar]
Wesley Pike 2005
- Wesley Pike J, Shevde NK. The vitamin D receptor. In: Feldman D, Wesley Pike J, Glorieux FH editor(s). Vitamin D. 2nd Edition. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier Academic Press, 2005:167‐91. [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woloszynska‐Read 2011
- Woloszynska‐Read A, Johnson CS, Trump DL. Vitamin D and cancer: clinical aspects. Best Practices & Research Clinical Endocrinology and Metabolism 2011;25(4):605‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yin 2013
- Yin L, Ordóñez‐Mena JM, Chen T, Schöttker B, Arndt V, Brenner H. Circulating 25‐hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta‐analysis. Preventive Medicine 2013;57(6):753‐64. [DOI] [PubMed] [Google Scholar]
Zittermann 2003
- Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence. British Journal of Nutrition 2003;89(5):552‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zittermann 2005
- Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. British Journal of Nutrition 2005;94(4):483‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zittermann 2006
- Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Progress in Biophysics and Molecular Biology 2006;92(1):39‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bjelakovic 2008
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007469] [DOI] [PMC free article] [PubMed] [Google Scholar]


