Abstract
Background
Acetylcysteine and carbocysteine are the most commonly prescribed mucolytic drugs in Brazil and many European and African countries. To our knowledge, no systematic review has been published on their efficacy and safety for acute upper and lower respiratory tract infections (RTIs) in children without chronic broncho‐pulmonary disease.
Objectives
The objective was to assess the efficacy and safety and to establish a benefit‐risk ratio of acetylcysteine and carbocysteine as symptomatic treatments for acute upper and lower RTIs in paediatric patients without chronic broncho‐pulmonary disease.
Search methods
We searched CENTRAL (2013, Issue 2), MEDLINE (1966 to February week 3, 2013), EMBASE (1980 to March 2013), Micromedex (2010), Pascal (1987 to 2004) and Science Citation Index (1974 to March 2013).
Selection criteria
To study efficacy, we used randomised controlled trials (RCTs) comparing the use of acetylcysteine or carbocysteine versus placebo, either alone or as an add‐on therapy. To study safety, we used trials comparing acetylcysteine or carbocysteine versus active treatment or no treatment and case reports.
Data collection and analysis
In this review update two review authors (YD, MC), with help from a colleague, extracted data and assessed trial quality. We performed a subgroup analysis of children younger than two years of age.
Main results
We included six trials involving 497 participants to study efficacy. They showed some benefit (e.g. reduction of cough at day seven) from mucolytic agents, although differences were of little clinical relevance. No conclusion was drawn about the subgroup of infants younger than two years because data were unavailable. Thirty‐four studies, including the previous six trials involving 2064 children, were eligible to study safety. Overall safety was good but very few data were available to evaluate safety in infants younger than two years. However, 59 cases of paradoxically increased bronchorrhoea observed in infants were reported to the French pharmacovigilance system.
Authors' conclusions
The results have to be interpreted with caution because they are based on a limited number of participants included in studies whose methodological quality is questionable. Acetylcysteine and carbocysteine seem to have a limited efficacy and appear to be safe in children older than two years. These results should take into consideration the fact that acetylcysteine and carbocysteine are prescribed for self limiting diseases (for example, acute cough, bronchitis). Given strong concerns about safety, these drugs should only be used for acute upper and lower RTIs in the context of a RCT with regards to children younger than two years.
Keywords: Child; Child, Preschool; Humans; Infant; Acetylcysteine; Acetylcysteine/adverse effects; Acetylcysteine/therapeutic use; Acute Disease; Age Factors; Carbocysteine; Carbocysteine/adverse effects; Carbocysteine/therapeutic use; Cough; Cough/drug therapy; Expectorants; Expectorants/adverse effects; Expectorants/therapeutic use; Randomized Controlled Trials as Topic; Respiratory Tract Infections; Respiratory Tract Infections/drug therapy
Plain language summary
Acetylcysteine and carbocysteine to treat acute upper and lower respiratory tract infections in children without chronic broncho‐pulmonary disease
Acetylcysteine and carbocysteine are the most commonly prescribed drugs which aim to change the structure of bronchial secretions. This systematic review assessed their efficacy and safety for treating acute upper and lower respiratory tract infections in children without chronic broncho‐pulmonary disease. We also looked in particular at patients younger than two years of age.
Forty‐nine trials met the inclusion criteria. Six trials involving 497 participants were included to study efficacy, and compared acetylcysteine or carbocysteine to placebo. Thirty‐four trials (including the previous six) were eligible to study safety and involved 2064 paediatric patients.
The results of this review suggest actual but limited efficacy of acetylcysteine and carbocysteine (e.g. reduction of cough at day seven) and good overall safety (except for rare mild gastrointestinal side effects) among children older than two years of age. However, the number of participants included was limited and the methodological quality was questionable. These results should also take into consideration the fact that acetylcysteine and carbocysteine are prescribed for self limiting diseases (for example, acute cough, bronchitis). In children younger than two years, and given strong concerns about safety (increased instead of decreased bronchial secretions), these drugs should only be used for acute upper and lower respiratory tract infections in the context of a randomised controlled trial.
Background
Description of the condition
Acute upper and lower respiratory tract infections (RTIs) are the most frequent infections in children, occurring 7 to 10 times a year in school age children (Chang 2006; Shields 2008). The main symptom is acute cough. Coughing can last more than 10 days in half of the cases and more than three weeks in about 10% of cases. Coughing can be distressing, especially in very young children and it has a major impact on the child's and other family members' sleep, and can cause parental anxiety (Shields 2008). Parents frequently seek drugs (over‐the‐counter drugs or drugs requiring a medical prescription) to treat their children's cough.
Description of the intervention
Of the mucolytic drugs available to treat acute upper and lower RTI, the cysteine derivatives (that is, acetylcysteine and carbocysteine) are the most commonly prescribed in many European (Cano Garcinuño 2013; Chalumeau 2000; Duijvestijn 1997; Sen 2011) and African countries (Mourdi 2010) and in Brazil (Bricks 1996). Various systemic (oral, intramuscular or intravenous) or inhaled forms of the drugs are available. In The Netherlands, one‐third of general practitioners prescribe acetylcysteine for asthmatic bronchitis, acute bronchitis, or for productive or dry cough (Duijvestijn 1997; Sen 2011). In France, three studies have shown that acetylcysteine and carbocysteine are some of the most prescribed drugs for children, especially those younger than two years of age (Chalumeau 2000; Collet 1991; Horen 2002). In one of these studies, cysteine derivatives accounted for 18% to 25% of drug prescriptions for acute rhinopharyngitis, acute cough and acute bronchitis (Chalumeau 2000). The high rate of prescription of cysteine derivatives for acute upper and lower RTI in paediatric patients was shown to be unchanged in the last decade in France (Halna 2005). In Italy, carbocysteine is one of the 20 drugs most prescribed by family paediatricians (Cazzato 2001; Sen 2011). In Spain, expectorants are the drugs most prescribed by paediatricians and general practitioners for the treatment of acute bronchitis, and they are the second commonest pharmacological group prescribed to children under two years of age (Cano Garcinuño 2013; Sanz 1988). In Germany, acetylcysteine is in the top five of drugs prescribed to paediatric patients under one year of age (Bücheler 2002).
How the intervention might work
In vitro, cysteine derivatives act by breaking disulphide bridges between macromolecules, which leads to a reduction in mucus viscosity (Medici 1979). This property led physicians in the 1960s and 1970s to develop mucolytic drugs for clinical situations where sputum modification to reduce cough is sought, including cystic fibrosis and chronic and acute bronchitis. In adult patients, the use of cysteine derivatives may cause a small reduction in acute exacerbations of chronic bronchitis (Poole 2012). In paediatric patients with cystic fibrosis, there is no evidence of effectiveness of either oral or inhaled administration of acetylcysteine (Duijvestijn 1999). In addition to being used for patients with severe chronic pulmonary disease, in some countries cysteine derivatives are also widely used to treat previously healthy paediatric patients with acute broncho‐pulmonary disease.
Why it is important to do this review
To our knowledge, no systematic review has been previously published on the efficacy and safety of acetylcysteine and carbocysteine for acute upper and lower RTIs in paediatric patients without chronic broncho‐pulmonary disease.
Objectives
To assess the efficacy of acetylcysteine and carbocysteine as symptomatic treatments for acute upper and lower RTIs in paediatric patients without chronic broncho‐pulmonary disease.
To evaluate the safety of acetylcysteine and carbocysteine in the symptomatic treatment of acute upper and lower RTIs in paediatric patients without chronic broncho‐pulmonary disease.
To establish a benefit‐risk ratio for the use of acetylcysteine and carbocysteine as symptomatic treatments for acute upper and lower RTIs in paediatric patients without chronic broncho‐pulmonary disease.
Methods
Criteria for considering studies for this review
Types of studies
To study efficacy, we used randomised controlled trials (RCTs) comparing the systemic or inhaled use of acetylcysteine and/or carbocysteine versus placebo, either alone or as an add‐on therapy (see Types of interventions).
To study safety, we also used trials comparing acetylcysteine and/or carbocysteine versus active treatment or no treatment and case reports.
Types of participants
We included trials regardless of gender and study setting (ambulatory or hospital‐based). We included trials if the participants met all of the following criteria.
Younger than 18 years (when studies involved adults and children, a minimum of 50% (arbitrary) of children were retained as a threshold to include the study).
Treated in primary, secondary or tertiary care settings.
Physician diagnosis of respiratory tract infection (RTI): acute pneumonia, acute bronchitis, acute bronchiolitis (secondary to respiratory syncytial virus or to another virus) or acute cough (including pertussis).
Duration of symptoms less than four weeks.
We excluded trials which included patients with any of the following conditions.
Acetaminophen (paracetamol) intoxication.
Bronchiectasis, cystic fibrosis or broncho‐pulmonary dysplasia.
Underlying immunodeficiency or respiratory tract anatomical defect.
Acute respiratory distress requiring mechanical ventilation.
We included trials which involved patients with underlying asthma or tuberculosis (as defined by the investigators).
Types of interventions
We included trials assessing the systemic use (that is, oral, intramuscular or intravenous) or inhaled use of acetylcysteine or carbocysteine, regardless of the dose regimen.
In order to study efficacy, we included trials which allowed concurrent use of other treatments (such as antibiotics, corticosteroids, bronchodilators, antitussives, chest physiotherapy, analgesics or antipyretics), if they allowed equal access to such medications for patients in the intervention and control groups.
We included trials comparing the use of acetylcysteine or carbocysteine in association with other treatments (such as antibiotics, corticosteroids, bronchodilators, antitussives, chest physiotherapy, analgesics or antipyretics) versus placebo, active treatment or no treatment, as well as case reports to study the safety of acetylcysteine or carbocysteine in association with other treatments.
Types of outcome measures
We included trials reporting at least one of the following outcome measures.
Primary outcomes
Time to resolution of clinical symptoms, signs or both (where clinical symptoms and signs may include increased respiratory rate, use of accessory respiratory muscles, abnormal lung examination, cough, sputum production, fever or activity limitations).
Proportions of patients with clinical symptoms, signs or both at a designated time.
Global assessment of improvement by clinicians, patients or their parents at a designated time.
Secondary outcomes
Reduced hospitalisation rates or duration, or both, of hospitalisation stay.
Adverse events reported by the investigators.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 2, part of The Cochrane Library, www.thecochranelibrary.com (accessed 6 March 2013), which contains the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register; MEDLINE (January 2008 to February week 3, 2013); EMBASE (January 2008 to March 2013) and Web of Science (2008 to March 2013). (See Appendix 1 for the MEDLINE and CENTRAL search terms).
We combined the MEDLINE search with a filter based on the work of Boluyt 2008 to identify child studies. We adapted the search terms to search EMBASE (see Appendix 2) and Web of Science (see Appendix 3). For details of previous searches see Appendix 4.
Searching other resources
We handsearched the references of trials obtained to identify other relevant studies. We contacted trial authors for additional information if required, and we requested information on unpublished trials from drug companies that manufacture acetylcysteine or carbocysteine in France, The Netherlands and the United States. We imposed no language or publication restrictions.
Data collection and analysis
Selection of studies
In the first publication of our review (Duijvestijn 2009), three review authors (YD, MC, John Smucny) independently searched titles and abstracts to identify potentially relevant articles. We obtained full‐text versions of these articles and articles with ambiguous titles or abstracts. The same three review authors independently selected articles which fulfilled the inclusion criteria. We resolved discrepancies regarding inclusion criteria by discussion. In this 2012 update, two review authors (YD, MC), with help from a colleague (TA), independently searched titles and abstracts to identify potentially relevant articles.
Data extraction and management
In the first published version of our review (Duijvestijn 2009), three review authors (YD, MC, John Smucny) independently extracted data from each study using electronic data collection forms. We resolved disagreements through discussion. In this 2012 update, two review authors (YD, MC) independently extracted data.
Assessment of risk of bias in included studies
Two review authors (YD, MC), with external help of a colleague (TA), independently graded the quality of each included study using The Cochrane Collaboration's tool for assessing risk of bias (RevMan 2012). We resolved disagreements by discussion and consensus. The support for judgement came from a single published or unpublished study report in all cases. We incorporated the results of the 'Risk of bias' assessment into the results.
Measures of treatment effect
We calculated risk ratios (RR) and risk differences (RDs) for dichotomous outcome variables of each individual study. We calculated the summary weighted RDs and their 95% confidence interval (CI) in RevMan 2012 using a fixed‐effect model. We calculated the number needed to treat to benefit (NNTB) and the number needed to treat to harm (NNTH) using the RD and its CI when RDs were significant (Cates 2003).
We recoded polytomous outcome variables as dichotomous variables to enable assessment. For example, an outcome assessed as 'very good, good, poor, no improvement' was recoded as 'actual improvement versus poor or no improvement'.
Unit of analysis issues
We took into account differences in populations, inclusion and exclusion criteria, interventions and outcome assessment, and we undertook a qualitative comparison of the studies to determine if pooling of the results was reasonable. We chose primary endpoints as the proportion of participants presenting a relevant symptom at a common date.
Dealing with missing data
We were not able to contact the original investigators to request missing data because the studies were performed more than 22 years ago (median = 36.5 years). There were no missing data for two‐thirds of studies. For the remaining third, we analysed only the available data (i.e. ignoring the missing data).
Assessment of heterogeneity
We carried out an assessment of possible heterogeneity (the studies did not evaluate the same effect) for pooled effects using a Breslow‐Day test of heterogeneity. We used the I² statistic to describe the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2003). A value above 50% may be considered substantial heterogeneity. In case of heterogeneity, we performed an analysis using a random‐effects model.
Assessment of reporting biases
Given the number of studies for each comparison (mostly less than four), we did not perform visual and statistical tests for funnel plot asymmetry (RevMan 2012). For the single comparison including the results of four studies, we used visual interpretation (RevMan 2012).
Data synthesis
We used meta‐analysis because the systematic review identified several eligible studies with small sample size but with closed designs. We used a random‐effects model for meta‐analysis when studies were combined to evaluate similar (but not exactly the same) endpoints. Otherwise, we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis of infants younger than two years of age because of different pharmacokinetics and pharmacodynamics in this age group, as recommended by the topic E 11 ("Clinical investigation of medicinal products in the paediatric population") of the International Conference on Harmonisation (EMEA 2001).
Sensitivity analysis
We did not judge sensitivity analysis to be pertinent given the number of studies for each comparison (mostly less than four).
Results
Description of studies
Results of the search
In July 2010 we searched CENTRAL 2010, Issue 2 and identified 165 references, but found only one potentially relevant study (Zuppi 1984). We searched MEDLINE from 1966 to June 2010 and retrieved 294 references, including 18 potentially relevant studies (Baldini 1989; Banovcin 1992; Bellomo 1967a; Bellomo 1967b; Berni 1983; Biscatti 1972; Boner 1984; Castello 1979; Chalumeau 2002; Henocq 1985; Loscialpo Ramundo 1968; Mayaud 1980; Plietz 1976; Ribeiro 1980; Santangelo 1985; Trastotenojo 1984; Varricchio 2008; Volkl 1992). We searched EMBASE from 1980 to 2010 and identified 425 references, including 12 potentially relevant studies (Baldini 1989; Banovcin 1992; Camurri 1990; Gusberti 1985; Henocq 1985; Malka 1990; Michael 1986; Nikolic 1980; Rudnik 1980; Santangelo 1985; Szekely 1980; Volkl 1992). We searched Micromedex for acetylcysteine and carbocysteine in 2010 and identified three potentially relevant studies (Malka 1990; Ramenghi 1984; Santangelo 1985). We searched Pascal from 1987 to 2004 and identified 101 references, including one potentially relevant study (Malka 1990). From 2005 onwards we no longer had access to Pascal. We searched Science Citation Index from 1974 to June 2010 and identified 162 references, including four potentially relevant studies (Nikolic 1980; Rudnik 1980; Santangelo 1985; Szekely 1980).
We updated the electronic database searches in March 2013 but did not identify any new studies.
We identified the other potentially relevant studies using the references of relevant and non‐relevant trials and the request for unpublished trials to drug companies that manufacture acetylcysteine or carbocysteine in France, The Netherlands and the United States, and from authors of relevant and non‐relevant studies (Amir 1985; Anonymous 1987; Banovcin 1990; Bellomo 1972; Bellomo 1973; Berger 1978; Caramia 1984; Careddu 1989; Dano 1971; Egreteau 1992; Fiocchi 1989; Gaudier 1968; Ginocchi 1978; Hofmann 1980; Jean 1982; Leupold 1970; Nakayama 1977; Nigam 1981; Olivieri 1979; Poder 1982; Samsygina 1995; Zanini 1974; Zens 1967).
In summary, we included 50 potentially relevant studies to evaluate the efficacy and safety of acetylcysteine and carbocysteine.
Included studies
Studies included to evaluate efficacy
We excluded most of the 50 potentially relevant studies from the efficacy analysis for one or more reason(s) (see Characteristics of excluded studies table). Six trials fulfilled the inclusion criteria and none of the exclusion criteria (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Malka 1990; Nakayama 1977; Zanini 1974) and were eligible to study efficacy (see Characteristics of included studies table).
Four of these six trials were written in French and two in Italian. All six studies were performed more than 17 years ago (median = 32 years). Mucolytics were administrated orally in the six studies.
Studies included to evaluate safety
We excluded sixteen studies from the safety analysis because they mainly involved participants with chronic diseases (Amir 1985; Berger 1978; Egreteau 1992; Hofmann 1980; Nigam 1981; Olivieri 1979; Plietz 1976; Poder 1982; Ribeiro 1980; Rudnik 1980; Samsygina 1995; Szekely 1980; Volkl 1992; Zens 1967; Zuppi 1984) or received additional antibiotic treatments only in the treatment group (Varricchio 2008) (see Characteristics of excluded studies table).
Twenty‐eight studies, in addition to the six trials included to study efficacy, fulfilled the inclusion criteria and none of the exclusion criteria to study safety (Table 1).
1. Characteristics of studies included to evaluate safety.
| Study | Methods | Participants | Intervention | Outcomes | Notes | Allocation concealment |
| Anonymous 1987 | Case report | 20 children (1 to 14 years) | Oral carbocysteine 300 mg/day above 5 years 200 mg/day under 5 years 2 to 3 times daily for 6 days (except 1: 3 days) | Clinical: cough frequency and intensity, sputum quality and quantity | French | Not used |
| Baldini 1989 | RCT Active treatment (ambroxol); participants received antibiotics if necessary | 28 children older than 2 years old | Oral acetylcysteine 300 mg/day above 5 years 200 mg/day under 5 years For 10 days | Clinical: expectoration, sputum viscosity, dyspnoea, cough, difficulty of expectoration. Biological: blood exam, monitoring of hepatic and renal function | Italian | Unclear |
| Banovcin 1990 | Controlled trial Active treatment (Ipecac syrup); antibiotics and vitamins for all participants | 18 children (6 to 15 years) | Oral carbocysteine 250 mg 3 times a day | PFT | Slovak | Unclear |
| Banovcin 1992 | RCT Active treatment (Ipecac syrup); antibiotics for all participants | 51 children (6 to 24 months) | Oral carbocysteine 50 to 62.5 mg 3 times a day | Clinical score: lung auscultation, fever, cough Biological: blood exam | Slovak | Unclear |
| Bellomo 1967a | Controlled trial Control participants received no treatment | 81 children (< 8 years) | Intramuscular acetylcysteine 25 to 50 mg/kg/day | Clinical: cough, dyspnoea, thoracic semeilogic alteration, fever | Italian | Unclear |
| Bellomo 1967b | Uncontrolled trial Thiamphenicol for all participants | 39 children (16: < 2 years) | Intramuscular acetylcysteine 10 to 18 mg/kg/day | Clinical: cough, dyspnoea, thoracic semeilogic alteration, fever Biological: blood exam; X‐ray | Italian | Unclear |
| Bellomo 1972 | RCT All participants received thiamphenicol with or without acetylcysteine | 59 children (22: < 1 year; 28: 1 to 5 years; 9: > 5 years) | Oral acetylcysteine 13.8 mg/kg/day 2 to 4 times daily for 6 to 7 days average (maximum 14 days) | Clinical: duration of febrile state, dyspnoea, thoracic semeiologic alterations, cough | Italian | Unclear |
| Bellomo 1973 | RCT Oral and intramuscular acetylcysteine; thiamphenicol for all participants | 50 children (35: < 2 years) | Oral and intramuscular acetylcysteine 300 mg/day under 2 years 600 mg/day above | Clinical: cough, dyspnoea, thoracic semeilogic alteration, fever | Italian | Unclear |
| Berni 1983 | RCT Antibiotics if necessary | 30 children (2 to 8 years) | Oral carbocysteine 200 to 300 mg | Clinical: cough, dyspnoea, expectoration | Italian | Unclear |
| Biscatti 1972 | RCT All participants received some kind of antibiotic | 50 children (29: < 2 years; 21: > 2 years) | Oral acetylcysteine 100 mg/day under 2 years 200 mg/day between 2 and 4 years 300 mg/day above 4 years for 6 days | Clinical: cough, dyspnoea, thoracic semeilogic alteration, temperature level Biological: ESR and leucocyte count | Italian Possibly not comparable treatment because various antibiotics were used | Unclear |
| Boner 1984 | Uncontrolled trial Cefuroxime for all participants | 60 children (4 to 12 years) | Intramuscular acetylcysteine 15 to 30 mg/kg/day | Clinical: temperature, cough, chest and abdomen pain, dyspnoea, wheezing Bacteriological: respiratory secretion cultures X‐ray | English | Unclear |
| Camurri 1990 | RCT Active treatment (bromhexine) | 32 children (2 to 11 years) | Oral acetylcysteine 300 mg/day | Clinical: cough, dyspnoea, expectoration (difficulty, quantity and quality) | Italian | Unclear |
| Caramia 1984 | Uncontrolled trial Cefuroxime for all participants | 40 children (4 months to 13 years) | Intramuscular acetylcysteine 20 to 30 mg/kg/day | Clinical: cough, temperature, sputum viscosity, intrinsic sputum characteristics Bacteriological: respiratory secretion cultures X‐ray | English | Unclear |
| Careddu 1989 | RCT Active treatment (nesosteine) | 40 children (5 to12 years) | Oral carbocysteine 900 mg/day | Clinical: cough, dyspnoea, expectoration Biological: blood exam | Italian | Unclear |
| Castello 1979 | Controlled trial Antibiotics if necessary | 13 children (1 of 15 months + 12: 2 to 12 years) | Oral carbocysteine; 3 spoons/day (2% under 4 years; 5% above 4 years) | Clinical: monitoring; viscosimetry | Italian | Unclear |
| Chalumeau 2002 | Targeted pharmacovigilance study | 6 children (< 2 years) | 19.5 to 34.6 mg/kg/day 3 received oral carbocysteine 3 received oral acetylcysteine |
Clinical: cough, sputum viscosity | French | Not used |
| Dano 1971 | Uncontrolled trial | 37 children (5 to 17 years) | Inhaled acetylcysteine 2 ml 10% + 2 ml 20% | Clinical: monitoring; PFT | English | Unclear |
| Fiocchi 1989 | RCT All participants received antibiotics if necessary | 100 children (all > 2 years) | Oral acetylcysteine 20 mg/kg/day 3 times a day for 28 days | Clinical: cough, cough productivity and thoracic semeilogic alteration PFT | Italian Side effects: 2 participants vomiting in acetylcysteine group, with 1 drop‐out | Unclear |
| Gaudier 1968 | Uncontrolled trial | 50 children (< 15 years) | Oral acetylcysteine; 2 spoons 2%/day | Clinical: monitoring (evolution of asthma) | French | Unclear |
| Ginocchi 1978 | Uncontrolled trial Antibiotics in 33 children | 44 children (2 months to 13 years) | Oral carbocysteine 2 spoons/day under 5 years; 3 spoons/day above | Clinical: cough, dyspnoea, expectoration, sputum viscosity Biological: blood exam | Italian | Unclear |
| Gusberti 1985 | RCT Active treatment (acetylsalicylate of acetylcysteine 4%, 5 ml x 3) | 40 children (4 months to 13 years) | Oral acetylcysteine 200 mg 3 times a day | Clinical: cough, dyspnoea, expectoration (difficulty, quantity and quality), temperature Biological: blood exam | Italian | Unclear |
| Henocq 1985 | RCT Control participants received no treatment | 50 children (1 month to 4 years) | Oral carbocysteine 5 ml 2% per 5 kg/day | IgA level | French | Unclear |
| Jean 1982 | Uncontrolled trial Thiamphenicol for all participants | 29 children (< 10 years) | Inhaled acetylcysteine 200 mg/day under 3 months 400 mg/day above | Clinical: cough, dyspnoea, expectoration, sputum viscosity, temperature Bacteriological X‐ray, PFT, bronchoscopy | French | Unclear |
| Leupold 1970 | Uncontrolled trial | 36 children (7 to 16 years) | Inhaled acetylcysteine 15 min: 18 participants at 20% and 18 participants at 10% | Clinical: monitoring PFT | German | Unclear |
| Loscialpo Ramundo 1968 | Uncontrolled trial Thiamphenicol for all participants | 84 children (2 to 12 years) | Inhaled acetylcysteine 1.5 ml 10% above 5 years 3 ml 10 % under (the first 5 days twice a day then once a day) | Clinical: cough, dyspnoea, fever Biological: blood exam X‐ray | Italian | Unclear |
| Malka 1990 | RCT All participants received antibiotics if necessary | 106 children (all > 2 years); all participants received antibiotics if necessary | Oral carbocysteine; 200 mg/day under 5 years 300 mg/day above for 7 days | Clinical: cough, expectoration, bronchial congestion, dyspnoea PFT | French 9 participants with side effects (in the carbocysteine group: 2 nausea, 3 diarrhoea, 1 stomach pain; in the placebo group: 1 nausea, 2 stomach pain) | Unclear |
| Mayaud 1980 | Uncontrolled trial Thiamphenicol for all participants | 112 children (< 8 years; 33: < 1 year) | Oral and intramuscular acetylcysteine 50 mg/kg/day | Clinical: fever, expectoration (quality); Biological: blood exam Bacteriological: quality of sputum X‐ray | French | Unclear |
| Michael 1986 | Uncontrolled trial | 374 children (mean: 5 years +/‐ 3) | Oral carbocysteine; 500 mg/day under 4 years 750 mg/day above | Clinical: cough, sputum viscosity, expectoration, breathing | German | Unclear |
| Nakayama 1977 | RCT Patients received bronchodilators, antibiotics, antihistamine if previously treated | 152 children (0 to 18 years) | Oral carbocysteine; 30 mg/kg/day (in 3 to 4 doses a day for 7 days) | Clinical: overall assessment, cough, stridor, expectoration PFT | French | Adequate |
| Nikolic 1980 | Uncontrolled trial | 20 children (3 to 14 years) | Oral acetylcysteine 100 to 200 mg 3 times a day | Clinical PFT | English | Unclear |
| Ramenghi 1984 | Uncontrolled trial Cefuroxim for all participants | 20 children (10 months to 2 years) | Intramuscular acetylcysteine 15 mg/kg/day | Clinical: cough, rale, vesicular murmur, irritability, hypo‐alimentation Biological: blood exam Bacteriological: on pharyngotracheal aspirate | English | Unclear |
| Santangelo 1985 | Uncontrolled trial Cefuroxim for all participants | 103 children (2 months to 11 years) | Intramuscular acetylcysteine; 20 to 30 mg/kg/day | Clinical: monitoring Bacteriological: secretion cultures; X‐ray | English | Unclear |
| Trastotenojo 1984 | Controlled trial Antibiotics and bronchodilator if necessary | 60 children (2 months to 13 years) | Oral acetylcysteine 100 mg 3 times a day | Clinical: cough, dyspnoea, findings on auscultation; Biological: blood exam X‐ray | English | Unclear |
| Zanini 1974 | RCT In treatment group 15 children received antibiotics + 4 inhaled acetylcysteine; in control group 8 children received antibiotics | 30 children (18 < 1 year; 12 > 1 year) | Oral carbocysteine from 100 to 400 mg/day depending on age for 5 to 9 days | Clinical: cough, dyspnoea, temperature level, appetite, general condition | Italian | Unclear |
RCT: randomised controlled trial PFT: pulmonary function tests ESR: erythrocyte sedimentation rate
Among these 28 studies, 11 were written in Italian (Baldini 1989; Bellomo 1967a; Bellomo 1967b; Bellomo 1973; Berni 1983; Camurri 1990; Careddu 1989; Castello 1979 ; Ginocchi 1978; Gusberti 1985; Loscialpo Ramundo 1968), seven in English (Boner 1984; Caramia 1984; Dano 1971; Nikolic 1980; Ramenghi 1984; Santangelo 1985; Trastotenojo 1984), six in French (Anonymous 1987; Chalumeau 2002; Gaudier 1968; Henocq 1985; Jean 1982; Mayaud 1980), two in German (Leupold 1970; Michael 1986) and two in Slovak (Banovcin 1990; Banovcin 1992). Except for a targeted pharmacovigilance study (Chalumeau 2002), the studies were at least 15 years old (median = 23 years). The route of administration was oral in 16 studies, intramuscular in six, inhaled in four, and both oral and intramuscular in two.
Excluded studies
We excluded 44 studies (see Characteristics of excluded studies) for different reasons: active control treatment, chronic disease, no control group, no placebo, no randomisation and/or type of outcome measures.
Risk of bias in included studies
Because the studies dated mainly from at least 15 to 40 years ago (median = 26 years) we were not successful in obtaining additional trial data.
The risk of bias of the six studies included to evaluate efficacy is mentioned in the 'Risk of bias' table (Figure 1; Figure 2). One study had a 'low risk of bias' for all items except for 'incomplete outcome data' for which we judged the risk of bias as 'high' (Nakayama 1977).
1.
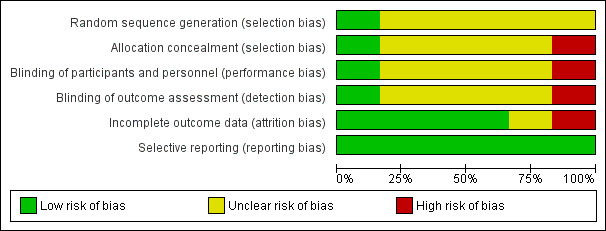
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
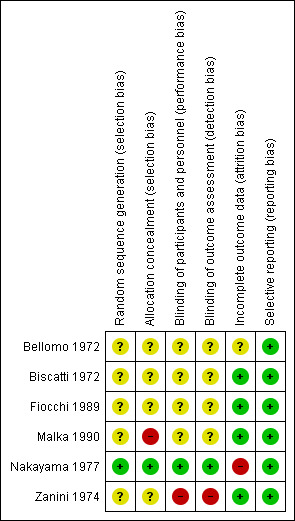
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies did not report sufficient details on randomisation and allocation concealment to make meaningful conclusions (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Zanini 1974). Only one study had low risk of bias for random sequence generation and allocation concealment (Nakayama 1977). For the last study the investigators did not respect the randomisation list, and therefore we classified it as having a high risk of bias (Malka 1990).
Blinding
Four studies did not report sufficient details on blinding to make meaningful conclusions (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Malka 1990). For one study, we assessed a low risk of bias because all the blinding conditions were clearly mentioned and respected (Nakayama 1977). For the last study, authors "had noticed the better efficacy of one of the products in looking clinically at the therapeutic results" and so we assessed this as a high risk of bias (Zanini 1974).
Incomplete outcome data
In four studies, analyses were based on the data of all included patients, and therefore we assessed them as having a low risk of bias (Biscatti 1972; Fiocchi 1989; Malka 1990; Zanini 1974). For one study, it was mentioned that not all the patients were included in the analysis (Nakayama 1977).
Selective reporting
All studies were considered as having a low risk of reporting bias because they all mentioned relevant outcomes.
Other potential sources of bias
None.
Effects of interventions
1. Efficacy
The six included trials involved 497 participants and compared cysteine derivatives with placebo (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Malka 1990; Nakayama 1977; Zanini 1974).
1.a Analysis of included studies with acetylcysteine
Acetylcysteine was tested in three trials (Bellomo 1972; Biscatti 1972; Fiocchi 1989) with a total of 209 participants. The first study included 59 patients with acute bronchitis or broncho‐pulmonary infections and compared oral administration of the antibiotic‐mucolytic combination (thiamphenicol glycinate acetylcysteinate) (n1 = 30) with thiamphenicol alone (n2 = 29) as an active control at the dose of 14 mg/kg/day of acetylcysteine with a duration of treatment of six days (Bellomo 1972). There was total remission of febrile state, dyspnoea, "thoracic semeiologic alterations" (i.e., "wheezing breathing", rattling) and cough in both groups. However, there was a statistically significant more rapid remission in the group treated with the antibiotic‐mucolytic combination. For example, there was total remission of febrile state in the treated group after six days, but it took nine days for the recovery of all the patients in the placebo group (P = 0.03).
The second study included patients with acute upper and lower respiratory tract infections (RTIs) and compared oral administration of acetylcysteine (n1 = 25) and placebo (n2 = 25) at a daily dose of 100 to 300 mg of acetylcysteine, depending on age, for six days (Biscatti 1972). All participants received some kind of antibiotic. The clinical parameters returned to normal in a statistically significant shorter time in the acetylcysteine group. For example, by the end of the treatment (six days), the remission of cough was total in the treated group whereas the cough persisted in 16% of the participants in the placebo group (risk difference (RD) ‐16%, 95% confidence interval (CI) ‐31% to ‐1%), risk ratio (RR) 0.11 (95% CI 0.01 to 1.95).
The third study compared oral acetylcysteine (n1 = 50) with placebo (n2 = 50) at a daily dose of 20 mg/kg, subdivided into three daily administrations for 28 days in children with bronchitis or tracheitis (Fiocchi 1989). Participants received antibiotics if necessary. Clinical improvement was observed in the four groups by the end of the trial. In the subgroup of children with bronchitis, statistically significant differences in the clinical parameters (cough score, cough productivity and thoracic semeiologic alterations) were recorded favourably for the acetylcysteine treatment. For example, the median cough score (range = 0 to 3) decreased faster in the treated group with a difference before/after of 2.1 (2.20 to 0.10), whereas in the placebo group the median score dropped from 2.20 to 0.50 (P < 0.05).
In the subgroup of children with tracheitis, no statistically significant differences were noted on the various clinical parameters, except a statistically significant improvement of the cough score, with a difference before/after of 2.00 (2.10 to 0.10) in the acetylcysteine treatment subgroup and 1.60 (2.10 to 0.50) in the placebo group (P < 0.05). We performed a comparison of the two groups (Analysis 2.3; Analysis 2.4; Analysis 4.4; Analysis 4.5; Analysis 6.1; Analysis 6.2): the treatment reduced the risk of "thoracic semeiologic alterations" by 83% (RR 0.17, 95% CI 0.03 to 0.99) (Analysis 4.5) and the RD at the end of the treatment was statistically significant: RD ‐14% (‐25% to ‐3%). The number needed to treat to benefit (NNTB) was eight (4 to 34). Regarding cough, the RD was ‐3% (‐13% to 7%) and the RR 0.67 (95% CI 0.16 to 2.76) (Analysis 2.4). At the end of the trial, productive cough was still present in three participants treated with acetylcysteine versus seven patients in the placebo group. The RD was not statistically significant though: RD ‐8% (‐20% to 3%) and the RR 0.41 (95% CI 0.11 to 1.56).
2.3. Analysis.
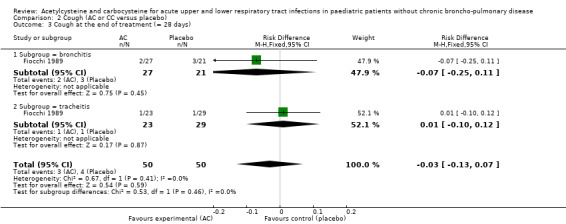
Comparison 2 Cough (AC or CC versus placebo), Outcome 3 Cough at the end of treatment (= 28 days).
2.4. Analysis.
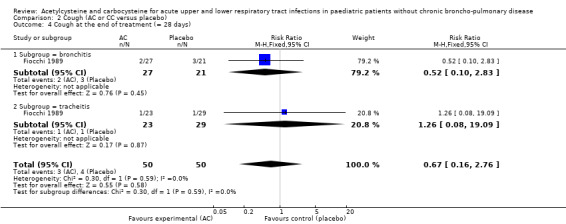
Comparison 2 Cough (AC or CC versus placebo), Outcome 4 Cough at the end of treatment (= 28 days).
4.4. Analysis.
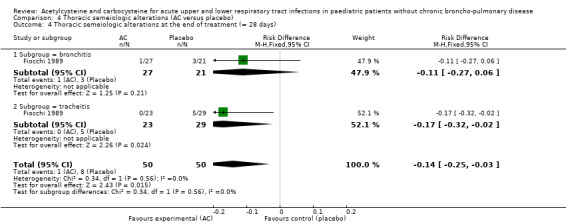
Comparison 4 Thoracic semeiologic alterations (AC versus placebo), Outcome 4 Thoracic semeiologic alterations at the end of treatment (= 28 days).
4.5. Analysis.
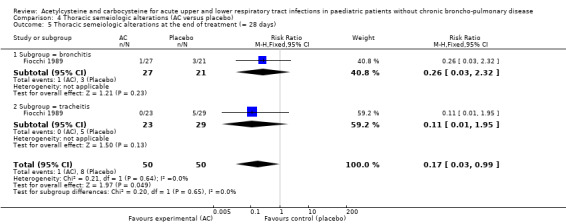
Comparison 4 Thoracic semeiologic alterations (AC versus placebo), Outcome 5 Thoracic semeiologic alterations at the end of treatment (= 28 days).
6.1. Analysis.
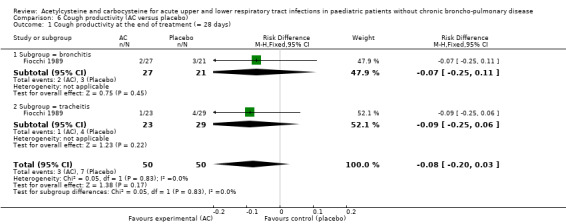
Comparison 6 Cough productivity (AC versus placebo), Outcome 1 Cough productivity at the end of treatment (= 28 days).
6.2. Analysis.
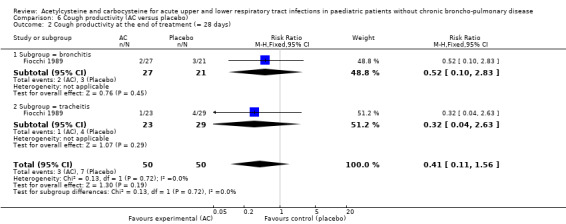
Comparison 6 Cough productivity (AC versus placebo), Outcome 2 Cough productivity at the end of treatment (= 28 days).
1.b Analysis of included studies with carbocysteine
Carbocysteine was tested in three of the six included studies (Malka 1990; Nakayama 1977; Zanini 1974) with a total of 288 participants. The first study included 106 participants with acute bronchitis, and compared oral administration of carbocysteine (n1 = 49) with placebo (n2 = 57) at a dose of 200 to 300 mg/day, depending on age, for seven days (Malka 1990). Both groups received antibiotics if necessary. The clinical symptoms (cough, expectoration, bronchial congestion, dyspnoea) and pulmonary function test abnormalities diminished in both groups. Significant differences between the two groups were observed for expectoration and pulmonary function tests. For example, after four days, expectoration was easier for 69% of the treated participants versus 49% of the participants of the placebo group (RD 23% (1% to 47%)).
The second study included 152 participants with respiratory diseases (bronchial asthma or acute bronchitis) and compared oral administration of carbocysteine (n1 = 74) with placebo (n2 = 78) at a dose of 30 mg/kg/day, three to four times daily for seven days (Nakayama 1977). Both groups received bronchodilators, antibiotics and antihistamines if previously treated. The author reported improvement in clinical symptoms in both groups: global impression, cough, stridor, expectoration. The difference in improvement for overall assessment, stridor and expectoration was statistically significant in favour of the treatment group, but the data were available only for the outcome "overall assessment". The analysis of these data showed a RD of 17% (3% to 31%) and a NNTB of six (4 to 32).
The third study included 30 participants with respiratory infections (such as bronchitis) and compared oral administration of carbocysteine (n1 = 19) with placebo (n2 = 11) at a dose of 100 to 400 mg/day according to age for five to nine days (Zanini 1974) in participants hospitalised for acute respiratory infections. Participants in both groups received antibiotics if necessary. Improvement of clinical signs (cough, vomiting/dyspnoea, temperature, appetite, general condition) was observed in both groups, with significant improvement for cough, vomiting and dyspnoea in both groups, but the actual improvement of general condition was better in the placebo group. Though not significant, the RD was 12% (‐24% to 49%) in favour of the placebo group.
1.c Pooled analysis
Because we could include very few studies, we have pooled the results of trials involving acetylcysteine and/or carbocysteine. We also considered primary endpoints that were similar but not exactly the same, for example, when the endpoint had close but slightly different dates. As a consequence, we used random‐effects models when both acetylcysteine and carbocysteine were involved and/or when the primary endpoints were not exactly the same.
Five major endpoints could be considered for meta‐analysis: febrile state after six days (Analysis 1.1; Analysis 1.2), cough after six to seven days (Analysis 2.1; Analysis 2.2), dyspnoea after six to seven days (Analysis 3.1; Analysis 3.2), thoracic semeiologic alterations after five days (Analysis 4.1; Analysis 4.2; Analysis 4.3) and general condition after six to seven days (Analysis 5.1; Analysis 5.2).
1.1. Analysis.
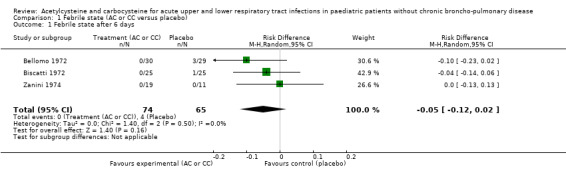
Comparison 1 Febrile state (AC or CC versus placebo), Outcome 1 Febrile state after 6 days.
1.2. Analysis.
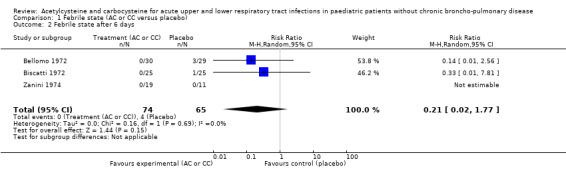
Comparison 1 Febrile state (AC or CC versus placebo), Outcome 2 Febrile state after 6 days.
2.1. Analysis.
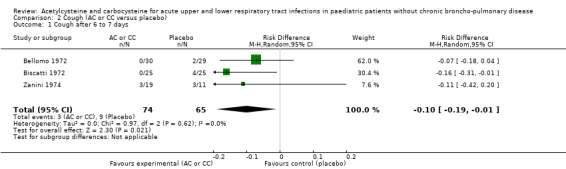
Comparison 2 Cough (AC or CC versus placebo), Outcome 1 Cough after 6 to 7 days.
2.2. Analysis.
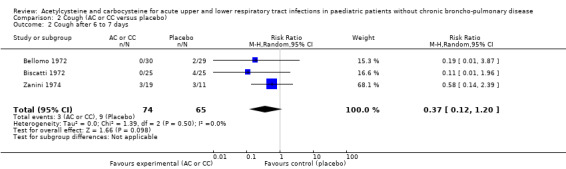
Comparison 2 Cough (AC or CC versus placebo), Outcome 2 Cough after 6 to 7 days.
3.1. Analysis.
Comparison 3 Dyspnoea (AC or CC versus placebo), Outcome 1 Dyspnoea after 6 to 7 days.
3.2. Analysis.
Comparison 3 Dyspnoea (AC or CC versus placebo), Outcome 2 Dyspnoea after 6 to 7 days.
4.1. Analysis.
Comparison 4 Thoracic semeiologic alterations (AC versus placebo), Outcome 1 Thoracic semeiologic alterations after 5 days.
4.2. Analysis.
Comparison 4 Thoracic semeiologic alterations (AC versus placebo), Outcome 2 Thoracic semeiologic alterations after 5 days.
4.3. Analysis.
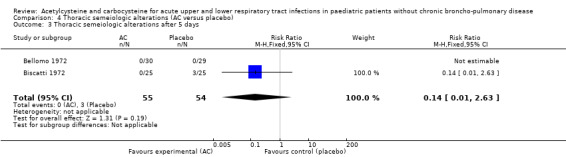
Comparison 4 Thoracic semeiologic alterations (AC versus placebo), Outcome 3 Thoracic semeiologic alterations after 5 days.
5.1. Analysis.
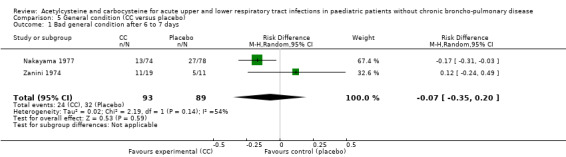
Comparison 5 General condition (CC versus placebo), Outcome 1 Bad general condition after 6 to 7 days.
5.2. Analysis.
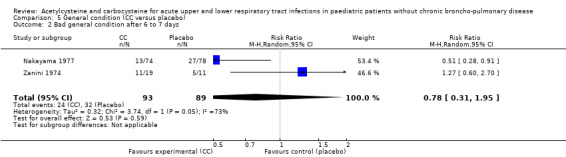
Comparison 5 General condition (CC versus placebo), Outcome 2 Bad general condition after 6 to 7 days.
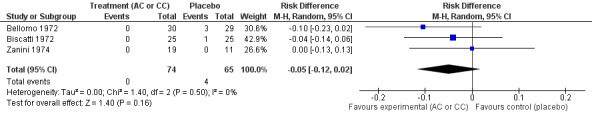
We calculated pooled RDs and when possible, pooled RRs to assess these outcomes. Febrile state after six days and cough after six to seven days were evaluated in three trials (Bellomo 1972; Biscatti 1972; Zanini 1974). The first two studies involved acetylcysteine and the last one, carbocysteine, and the outcome was not considered at exactly the same date (after six days or after an average of six days), so we used a random‐effects model. After six days, three participants were still febrile among the participants from the placebo group, whereas none presented fever in the treated group: the pooled RD was ‐5% (‐12% to 2%) (Figure 3). Then, the RR was 0.21 (95% CI 0.02 to 1.77). Regarding cough, after six to seven days of treatment, the risk difference was ‐10% (‐19% to ‐1%) (Figure 4) and the NNTB was 10 (6 to 101). The RR was 0.37 (95% CI 0.12 to 1.20).
3.
Forest plot of comparison: 1 Febrile state (AC vs placebo), outcome: 1.1 Febrile state after 6 days.
4.
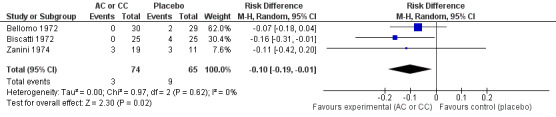
Forest plot of comparison: 4 Cough (AC vs placebo), outcome: 4.1 Cough after 6 to 7 days.
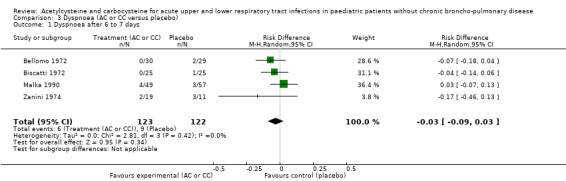
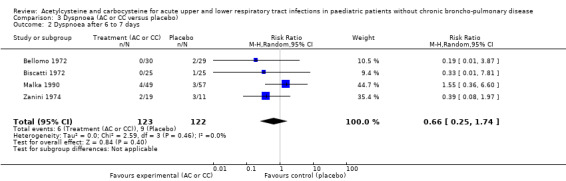
The assessment of the efficacy of acetylcysteine and carbocysteine to treat dyspnoea involved an additional trial (Malka 1990). Six participants treated complained of dyspnoea after six to seven days versus nine in the placebo group. The RD was ‐3% (‐9% to 3%) (Figure 5) and the RR was 0.66 (95% CI 0.25 to 1.74).
5.
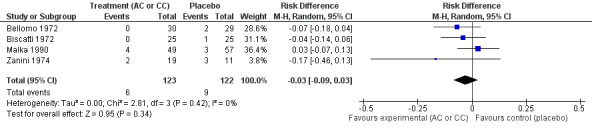
Forest plot of comparison: 3 Dyspnoea (AC or CC versus placebo), outcome: 3.1 Dyspnoea after 6 to 7 days.
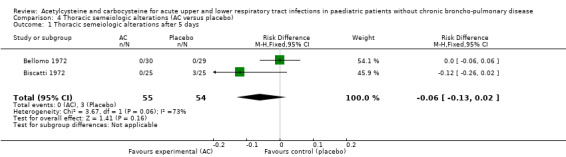
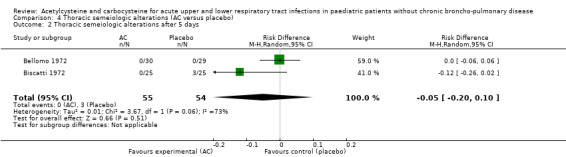
We pooled data from two studies to evaluate the efficacy of acetylcysteine in the treatment of thoracic semeiologic alterations (Bellomo 1972; Biscatti 1972). After three days, no treated participant was presenting that symptom versus three in the placebo group; the RD was 6% (‐13% to 2%) (Figure 6). However, there was heterogeneity between the studies. After using a random‐effects model the RD was ‐5% (‐20% to 10%).
6.
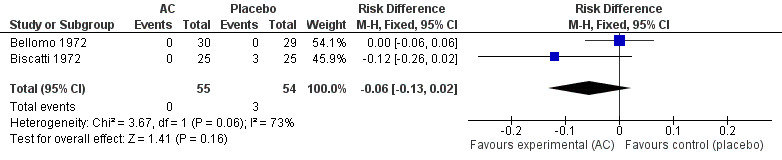
Forest plot of comparison: 3 Thoracic semeiologic alterations (AC vs placebo), outcome: 3.1 Thoracic semeiologic alterations (after 3 days).
We also assessed the efficacy on the general condition using the data from two studies involving carbocysteine (Nakayama 1977; Zanini 1974). After seven days, 24 children were still presenting a deteriorated general condition in the treatment group and 32 in the placebo group. The RD was ‐7% (‐35% to 20%) and the RR was 0.78 (95% CI 0.31 to 1.95).
1.d Subgroup analysis of infants under two years
Among the studies with acetylcysteine, Bellomo 1972 studied 22 children under one year of age and 28 children between one and five years of age, and Biscatti 1972 studied 29 children under two years of age. However, it was not possible to obtain more information about possible differences in results in the different age groups.
Among the studies with carbocysteine, Nakayama 1977 studied children from one to 14 years old and Zanini 1974 studied 18 children under one year of age. However, it was not possible to obtain more information about possible differences in results in the children under two years of age.
2. Safety
Our search strategy identified 28 studies to evaluate safety, in addition to the six trials above (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Malka 1990; Nakayama 1977; Zanini 1974). These 34 studies involved 2064 paediatric participants. Safety was evaluated using clinical, biological, radiographic or pulmonary function test parameters. Among these studies, 17 were controlled trials (including 14 randomised controlled trials (RCTs)), 16 were non‐controlled trials and the last one was a targeted pharmacovigilance study. The studies reporting adverse events were not comparable in terms of participants, interventions or adverse events, and so we judged pooling of the results to be inappropriate.
2.a Analysis of included studies with acetylcysteine
Twenty studies evaluated acetylcysteine, involving 1080 participants among which 831 were treated (Baldini 1989; Bellomo 1967a; Bellomo 1967b; Bellomo 1972; Bellomo 1973; Biscatti 1972; Boner 1984; Camurri 1990; Caramia 1984; Dano 1971; Fiocchi 1989; Gusberti 1985; Jean 1982; Leupold 1970; Loscialpo Ramundo 1968; Mayaud 1980; Nikolic 1980; Ramenghi 1984; Santangelo 1985; Trastotenojo 1984).
Nine of these studies were controlled trials in which the treatment was administered orally except in one study for which the route of administration was intramuscular. Control participants received either placebo (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Trastotenojo 1984), an active control treatment (Baldini 1989; Camurri 1990; Gusberti 1985) or nothing (Bellomo 1967a). If a concomitant treatment was authorised, for example, antibiotics, it was authorised for all participants. The controlled trials studied clinical or biological tolerance, or both. The biological tests consisted usually of full blood tests (haemoglobin (Hb), red blood count (RBC), white blood count (WBC) and platelets) and the monitoring of hepatic (bilirubin, serum glutamate‐oxaloacetate transaminase (SGOT), serum glutamate‐pyruvate transaminase (SGPT), alkaline phosphatase) and renal function (creatinine). In two studies, safety was also evaluated using radiographic (Trastotenojo 1984) or pulmonary function test parameters (Fiocchi 1989). The controlled trials all showed good clinical safety, except mild gastrointestinal tract adverse events (vomiting) in two participants (2%), leading to the withdrawal of one of them (Fiocchi 1989).
In the other trials, the route of administration was oral in one study, intramuscular in five, inhaled in four, and both oral and intramuscular in one. Most of these studies authorised concomitant treatments, mainly antibiotics. All participants had equal access to these treatments, except in one trial (Zanini 1974). Safety was evaluated using clinical and typically biological tests. Some studies also evaluated safety using radiographic (Boner 1984; Caramia 1984; Jean 1982; Loscialpo Ramundo 1968; Mayaud 1980; Santangelo 1985) or pulmonary function test parameters (Dano 1971; Leupold 1970). In one study, the tolerance was not documented (Nikolic 1980). The main potential adverse event observed was broncho‐constriction induced by inhaled N‐acetylcysteine in children older than two years of age (Dano 1971; Leupold 1970). As a consequence, 11 children (31%) were withdrawn from one of these studies (Leupold 1970). This side effect was mainly explained by the trial authors by the high concentration (20%) of acetylcysteine. Whereas in another study, no such effect was reported with a lower concentration (10%) (Loscialpo Ramundo 1968). In the other uncontrolled trials clinical safety was very good except in one study where 12 participants (11%) complained of gastro‐intestinal tract disorders (nausea, vomiting, diarrhoea), but none were withdrawn (Mayaud 1980).
2.b Analysis of included studies with carbocysteine
Thirteen studies evaluated carbocysteine, involving 989 participants, among which 755 were treated (Anonymous 1987; Banovcin 1990; Banovcin 1992; Berni 1983; Careddu 1989; Castello 1979; Gaudier 1968; Ginocchi 1978; Henocq 1985; Malka 1990; Michael 1986; Nakayama 1977; Zanini 1974).
The treatment was administrated orally. Eight trials were controlled. The control participants received either a placebo (Malka 1990; Nakayama 1977; Zanini 1974), or an active treatment (Banovcin 1990; Banovcin 1992; Berni 1983; Careddu 1989) or nothing (Henocq 1985). If a concomitant treatment was authorised, for example, antibiotics, it was authorised for all participants. The controlled trials used clinical and sometimes biological parameters to evaluate safety. The biological tests consisted of blood tests and/or the monitoring of hepatic and renal function. In two studies, safety was also evaluated using pulmonary function test parameters (Malka 1990; Nakayama 1977). Three studies did not document adverse events (Banovcin 1990; Banovcin 1992; Henocq 1985). The overall clinical safety was good in the other trials, with few participants (n = 19; 13%) complaining of gastrointestinal tract disorders (nausea, vomiting, diarrhoea) (Careddu 1989; Malka 1990), leading to the withdrawal or dropping out of two (1.9%) treated children and one (0.9%) among the control patients (Malka 1990). In one of these two studies (Careddu 1989) the dose used was high (900 mg/day for children weighing around 25 kg).
The other studies involved 501 paediatric participants. One study authorised antibiotics for all participants if necessary (Castello 1979) and another one authorised antibiotics in 33 children (75%) (Ginocchi 1978). All trials studied clinical tolerance. Biological parameters (Ginocchi 1978) and sputum viscosimetry (Castello 1979) were also used in two trials. Adverse events were observed in 13 participants (2.6%). These participants experienced gastrointestinal tract disorders (stomach pain, nausea, vomiting, diarrhoea) in two studies (Gaudier 1968; Michael 1986), leading to the withdrawal of five participants (1.3%) in one study (Michael 1986).
Our search identified only one study designed to evaluate safety (Anonymous 1987). This study was an open uncontrolled trial that involved 20 participants with acute upper and lower RTIs, including some infants. Participants received carbocysteine for six days at a dose of 200 mg per day via the oral route. No concomitant treatment was allowed, except antibiotics in one child. The safety was evaluated clinically by questioning parents and children. The only potentially adverse event reported (vomiting) occurred after the end of the treatment and involved only the child on antibiotics.
2.c Subgroup analysis of infants under two years of age
Among the 22 studies that provided clear data on participants' ages, only 10 included participants under two years, involving 262 patients (68%) under this age (Banovcin 1992; Bellomo 1967a; Bellomo 1967b; Bellomo 1973; Biscatti 1972; Castello 1979; Chalumeau 2002; Ginocchi 1978; Jean 1982; Ramenghi 1984). Among the 10 studies, only one was a good‐quality controlled trial (Biscatti 1972) and three were low‐quality controlled trials (Banovcin 1992; Bellomo 1967a; Bellomo 1973). One hundred and seventy‐four participants (75%) under two years of age were included in these four trials; 26 were treated with carbocysteine (Banovcin 1992) and 59 with acetylcysteine, including 14 in the single good‐quality controlled trial (Biscatti 1972). None of them had side effects. No side effect was reported in the other six trials.
Our search strategy also identified a targeted pharmacovigilance study (Chalumeau 2002). This study followed the spontaneous reporting to the French pharmacovigilance system of cases of paradoxically increased bronchorrhoea in infants receiving mucolytic agents. This prospective two‐month study was performed in the emergency departments of two paediatric teaching hospitals in Paris, France. During this period, six participants were diagnosed as having a paradoxically increased bronchorrhoea after having received acetylcysteine (n = 3) or carbocysteine (n = 3) for acute upper and lower respiratory infections. Participants were aged 2.5 to 7.5 months old. The median delay between treatment and the diagnosis of paradoxically increased bronchorrhoea was 4.5 days. The dose was on average 26 mg/kg/day, ranging from 20 to 35 mg/kg/day. Two children were hospitalised because of their respiratory state. Using the Naranjo scale for causality assessment, the causality between the exposure and the adverse reaction was considered possible or plausible (Naranjo 1981).
Discussion
Summary of main results
We found a limited number of studies that evaluated the efficacy of acetylcysteine and carbocysteine as symptomatic treatments for acute upper and lower respiratory tract infections (RTIs) in paediatric patients without chronic broncho‐pulmonary disease (Bellomo 1972; Biscatti 1972; Fiocchi 1989; Malka 1990; Nakayama 1977; Zanini 1974). These trials showed some benefit from mucolytic agents, although differences were sometimes small, not statistically significant and/or of little clinical relevance. Considering the pooling of data, the effect of acetylcysteine or carbocysteine was not statistically significant except for cough after six to seven days. The treatment (acetylcysteine or carbocysteine) reduced the risk of cough by 63%. However, the number needed to treat to benefit (NNTB) was 10 with a large CI (6 to 101), suggesting a questionable effect size. Only one of the six trials was considered to have a low risk of bias (Nakayama 1977; Figure 2). However, in the low risk of bias trial by Nakayama 1977, an overall criterion based on an improvement in global condition was used as an outcome, but this composite criterion has not been validated and may not be clinically relevant. No statistically significant difference was observed regarding most symptoms considered separately, especially expectoration and cough. It was not possible to make any evaluation about the subgroup of infants under two years of age, because none of the six studies that we included to evaluate efficacy mentioned the results for this age group separately.
The overall safety of acetylcysteine and carbocysteine was good, with mainly minor gastrointestinal tract disorders in few participants (n = 46; 2%). These findings should not lead to the conclusion that mucolytic agents are well tolerated in paediatric patients. In fact, (i) the size of the low risk of bias trial was not sufficient to provide enough statistical power to detect any rare potentially severe adverse event, (ii) many high risk of bias trials did not provide detailed descriptions of the severity of adverse events and abnormal laboratory tests or reasons for treatment discontinuation separately for each intervention group, and (iii) very few children under two years of age were involved in the studies. RCTs of adequate sample size offer the only unbiased approach for assessing the frequency and severity of side effects from a given medication, but when that kind of evidence is lacking, other sources, such as pharmacovigilance and pharmaco‐epidemiology can be helpful.
Regarding the safety of mucolytic agents in paediatric patients, attention should be paid to the youngest age group. In a targeted prospective pharmacovigilance study in two paediatric emergency departments, six infants (all younger than eight months old) were diagnosed with paradoxically increased bronchorrhoea during a two‐month period (Chalumeau 2002). An analysis of the French pharmacovigilance system concerning adverse drug reactions to acetylcysteine and carbocysteine showed 59 respiratory adverse drug reactions in children younger than six years from 1989 to 2008 (30 children received carbocysteine, 28 received acetylcysteine and one child received both drugs at the same time). The respiratory adverse drug reactions reported were increased and/or prolonged cough, increased bronchorrhoea, worsening of respiratory distress, mucous vomiting and dyspnoea (Mallet 2011). Fifty‐one children (86%) required hospitalisation or extended hospitalisation because of the adverse drug reactions. Outcome was favourable in all cases except for one patient in whom pleuropneumonia developed and a one‐year‐old girl who died of pulmonary oedema that was considered secondary to mucous vomiting according to the reporting physician. These side effects led to the withdrawal of the licence for carbocysteine and acetylcysteine in paediatric patients younger than two years of age in France and Italy in April 2010 (Mourdi 2010).
This paradoxical reaction was also mentioned but not documented with references in some articles (Harris 1967; Jean 1982) and textbooks (Anonymous 2000a; Anonymous 2000b). The paradoxically increased bronchorrhoea could be explained by the effective action of mucolytic agents which increase bronchial mucous flow. This flow may exceed the capacity of spontaneous drainage of the infant which is limited by the small bronchial diameter and neuromuscular physiologic immaturity (Wohl 1998). This could also be explained by a dose‐related effect. No dose‐response trial has ever been performed for acetylcysteine, leading to a unique dose recommendation of 200 mg per day, whatever the weight of the child. According to the World Health Organization (WHO) standards (WHO 2007), the median weight of a one‐month‐old infant is about 4.5 kg as opposed to 12.2 kg for a two‐year‐old. The recommended dose for the youngest infants is then three times higher than the dose recommended for the oldest (about 45 mg/kg/day versus 16 mg/kg/day). This may lead to dose‐related adverse events in very young infants. In the same way, no clinical research has been implemented to support the recommended dose of the marketing authorisation of carbocysteine (200 to 300 mg/day). The evaluation of drug safety should depend on age, considering that pharmacokinetics and pharmacodynamics in paediatric patients differ greatly from adults, especially in neonates and infants. Paradoxical side effects are not rare in these age groups (Hughes 1994) but a causal relationship is difficult to prove when a paradoxical side effect of a drug is suspected because of protopathic bias. The term protopathic bias is used if the first symptoms of the outcome of interest are the reasons for use of treatment (Salas 1999). This bias leads people to believe that when symptoms worsen during the course of a drug that is prescribed for a specific disease it may be related to the disease itself and not to the drug (Delgado‐Rodriguez 2004; Horwitz 1980). The only way to evaluate paradoxical side effects of drugs avoiding protopathic bias would be theoretically to study them in another unrelated disease. However, this is not possible in the case of carbocysteine because its only indications are RTIs. For acetylcysteine, in a multicentre, post‐marketing safety study, respiratory symptoms were reported in 2.2% of paediatric (n = 1905) patients receiving intravenous acetylcysteine for acetaminophen overdose between 1980 and 2005. Respiratory symptoms included bronchospasm, cough, wheezing, stridor, shortness of breath, chest tightness or respiratory distress (Cumberland Pharmaceuticals 2008).
Overall completeness and applicability of evidence
The external validity of this review is difficult to evaluate. On the one hand, it seems high given the various settings of the studies, the various types of participants, the various type of interventions and outcomes. On the other hand, the meaning of results observed in studies performed 40 years ago in patients with clinical conditions and general management that are very different from the current practices is questionable.
The evaluation of the benefit‐risk ratio of mucolytic agents should take into consideration the fact that these medicines are prescribed for self limiting diseases (for example, acute cough, bronchitis). For example, in the Bellomo 1972 study, remission for all symptoms was complete in the placebo group within nine days at most. Regarding paediatric patients older than two years, no serious adverse events were reported in the available studies included in the present review. These studies suggest that acetylcysteine and carbocysteine may reduce frequency, intensity and duration of symptoms in acute upper and lower RTIs. For the patient group younger than two years, the benefit‐risk ratio is most probably negative according to available evidence for side effects in this age group.
Our review has some implications for drug regulation agencies. Acetylcysteine and carbocysteine are licensed for use for the treatment of acute upper and lower RTIs in paediatric patients in many European countries. According to our review, this license is not supported by strong evidence in children older than two years of age and is not supported by any evidence in children under two years of age, considering some important concerns about safety (Mallet 2011; Mourdi 2010). A re‐evaluation of the benefit‐risk ratio of these drugs by the drug regulation agencies of the countries where they are licensed is necessary, particularly in the age group younger than two years.
Quality of the evidence
The body of evidence identified allows a robust conclusion regarding patients younger than two years old. Indeed no study supports the use of acetylcysteine nor carbocysteine among this age group. For children older than two years, our conclusions are based on a small number of studies and patients, with an overall high risk of bias. Thus, the conclusions should be interpreted cautiously.
Potential biases in the review process
Given the effort that was made to contact all drug companies that manufacture acetylcysteine and carbocysteine, the high response rate obtained during this process (as shown by the numerous unpublished studies included) and the various contacts, we are confident that we analysed all relevant data in this review.
Agreements and disagreements with other studies or reviews
Our conclusions are in complete agreement with clinical practice guidelines for the management of cough in children (Chang 2006; Shields 2008) and another systematic review (Smith 2012).
Authors' conclusions
Implications for practice.
The results of the present review have to be interpreted with caution because (i) it was based on a limited number of patients included in studies whose methodological quality was questionable, and which dated mainly from the 1970s and 1980s, (ii) these results should take into consideration the fact that acetylcysteine and carbocysteine are prescribed for self limiting diseases (for example, acute cough, bronchitis), and (iii) millions of paediatric patients are exposed to these drugs each year in many European countries (Cano Garcinuño 2013; Cazzato 2001; Chalumeau 2000; Collet 1991; Duijvestijn 1997; Horen 2002; Sanz 1988; Sen 2011). These mucolytic agents seem to have some benefits on frequency, intensity and duration of symptoms, and appear to be safe in children older than two years. Regarding children younger than two years old, there are current strong concerns about the safety of acetylcysteine and carbocysteine (Mallet 2011). These concerns led to the withdrawal of their licence in this age group in France and Italy in 2010 (Mourdi 2010). Therefore, these drugs should only be used for acute respiratory tract infections in neonates and infants in the context of a randomised controlled trial.
Implications for research.
An adequately powered, randomised, double‐blind, placebo‐controlled trial evaluating the efficacy and safety of acetylcysteine or carbocysteine should be performed in patients under two years of age. This trial should use a main outcome which is clinically relevant (for example, cough frequency, intensity and duration).
Feedback
Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho‐pulmonary disease, 12 March 2013
Summary
Comment: I did not understand which criteria were selected to dertermine efficacy of acetylcysteine and carbocysteine drugs. Until now I thought they were of no interest in ARTI's and this article doesn't give me any point to change my point of view. Thank you for answer.
I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
We have just finished updating our review which will published soon. The criteria selected to determine efficacy of acetylcysteine and carbocysteine drugs are included in the 'Types of outcome measures'. I have also copied our conclusions:
Types of outcome measures
We included trials reporting at least one of the following outcome measures.
Primary outcomes
1. Time to resolution of clinical symptoms and/or signs (where clinical symptoms and signs may include increased respiratory rate, use of accessory respiratory muscles, abnormal lung examination, cough, sputum production, fever, or activity limitations).
2. Proportions of patients with clinical symptoms and/or signs at a designated time.
3. Global assessment of improvement by clinicians, patients or their parents at a designated time.
Secondary outcomes
1. Reduced hospitalisation rates and/or duration of hospitalisation stay.
Authors' conclusions
The results have to be interpreted with caution because they are based on a limited number of participants included in studies whose methodological quality is questionable. Acetylcysteine and carbocysteine seem to have a limited efficacy and appear to be safe in children older than two years. These results should take into consideration the fact that acetylcysteine and carbocysteine are prescribed for self‐limiting diseases (for example, acute cough, bronchitis). Given strong concerns about safety, these drugs should only be used for acute upper and lower RTIs in the context of a RCT with regards to children younger than two years.
Contributors
Martin Chalumeau Yvonne Duijvestijn
What's new
| Date | Event | Description |
|---|---|---|
| 9 April 2013 | Feedback has been incorporated | Feedback comment and reply added to the review. |
| 6 March 2013 | New citation required but conclusions have not changed | Searches conducted. |
| 6 March 2013 | New search has been performed | In this update, no new trials were identified for inclusion. We excluded one new trial (Varricchio 2008). The conclusions about the efficacy of the treatment remain unchanged. However, we found new literature supporting evidence for side effects of carbocysteine and acetylcysteine, which led to the withdrawal of the licence of carbocysteine and acetylcysteine in paediatric patients younger than two years in France in April 2010, and then in Italy. This has been added to the conclusions of this updated review. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 28 March 2008 | New search has been performed | Searches conducted. |
| 19 February 2008 | Amended | Converted to new review format. |
| 3 October 2006 | New citation required but conclusions have not changed | A subgroup analysis of infants under two years old was added because of reports of paradoxical bronchial congestion and obstruction in infants under two years old receiving carbocysteine and acetylcysteine for acute cough. |
Acknowledgements
To Thibault Andrieu (TA) for valuable help in updating the review. To Nadjette Mourdi and John Smucny for their important contributions to the first version of this review. To Gérard Bréart, INSERM U953, and Gérard Pons, Paris Descartes University, for their valuable advice on the protocol, the analyses and the manuscript (Paris, France). To Liz Dooley, Cochrane Acute Respiratory Infections Group Managing Editor, for her very comprehensive editorial work. To Ruth Foxlee and Sarah Thorning, for their help with the electronic searches. To Bernard and France Drujon‐d'Astros (Aix‐en‐Provence, France), Barbara Duijvestijn (Heiloo, The Netherlands) and Grace Tjebbes (Heiloo, The Netherlands), for their help with translations from German, Italian and Russian. To Dr Françoise Bavoux and Dr Caroline Pecriaux, Regional Center for Pharmacovigilance and Information on Drugs, Paris, France. Finally, the review authors wish to thank the following people for commenting on drafts of this review: Anne Lyddiatt, Linda Hornbeek, Ann Fonfa, Inge Axelsson, Bruce Arroll, Teresa Neeman, Conor Teljeur and Jenny Doust.
Appendices
Appendix 1. CENTRAL and MEDLINE search strategy
MEDLINE (Ovid) 1 exp Respiratory Tract Infections/ 2 respiratory tract infection*.tw. 3 respiratory infection*.tw. 4 exp Rhinitis/ 5 rhinit*.tw. 6 (rhinopharyngit* or nasopharyngit* or rhinosinusit* or nasosinusit*).tw. 7 Common Cold/ 8 common cold*.tw. 9 Influenza, Human/ 10 (influenza* or flu).tw. 11 Pharyngitis/ 12 pharyngit*.tw. 13 exp Sinusitis/ 14 sinusit*.tw. 15 exp Laryngitis/ 16 laryngit*.tw. 17 Tracheitis/ 18 tracheit*.tw. 19 tracheobronchit*.tw. 20 sore throat*.tw. 21 Cough/ 22 cough*.tw. 23 exp Bronchitis/ 24 exp Bronchiolitis/ 25 (bronchiolit* or bronchit*).tw. 26 exp Pneumonia/ 27 (pneumon* or bronchopneumon* or pleuropneumon* or broncho‐pulmon*).tw. 28 or/1‐27 29 Acetylcysteine/ 30 acetylcysteine.tw,nm. 31 (n‐acetylcysteine or n‐acetyl‐l‐cysteine or acetyl‐cysteine or nac).tw,nm. 32 Carbocysteine/ 33 (carbocysteine or carbocisteine).tw,nm. 34 exp Expectorants/ 35 expectorant*.tw. 36 mucolytic*.tw. 37 or/29‐36 38 28 and 37
Appendix 2. EMBASE search strategy
41. #30 AND #40 40. #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 39. 'nursery school':ab,ti OR 'primary school':ab,ti OR 'secondary school':ab,ti OR 'elementary school':ab,ti OR 'high school':ab,ti OR kindergar*:ab,ti OR highschool*:ab,ti 38. pediatric*:ab,ti OR paediatric*:ab,ti 37. 'pediatrics'/exp 36. adoles*:ab,ti OR teen*:ab,ti OR boy*:ab,ti OR girl*:ab,ti 35. 'adolescent'/exp 34. child*:ab,ti OR schoolchild*:ab,ti OR 'school age':ab,ti OR 'school aged':ab,ti OR preschool*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti 33. 'child'/exp 32. infant*:ab,ti OR infancy:ab,ti OR newborn*:ab,ti OR baby:ab,ti OR babies:ab,ti OR neonat*:ab,ti OR preterm*:ab,ti OR prematur*:ab,ti 31. 'infant'/exp 30. #20 AND #29 29. #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 28. expectorant*:ab,ti 27. 'expectorant agent'/de 26. mucolytic*:ab,ti 25. 'mucolytic agent'/exp 24. carbocysteine:ab,ti OR carbocisteine:ab,ti 23. 'carbocisteine'/exp 22. acetylcysteine:ab,ti OR 'n‐acetylcysteine':ab,ti OR 'n‐acetyl‐l‐cysteine':ab,ti OR 'acetyl‐cysteine':ab,ti OR nac:ab,ti 21. 'acetylcysteine'/de 20. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 19. bronchit*:ab,ti OR bronchiolit*:ab,ti 18. 'bronchitis'/exp 17. 'sore throat':ab,ti OR 'sore throats':ab,ti 16. 'sore throat'/de 15. laryngit*:ab,ti 14. 'laryngitis'/exp 13. sinusit*:ab,ti 12. 'sinusitis'/exp 11. pharyngit*:ab,ti 10. 'pharyngitis'/exp 9. influenza*:ab,ti 8. 'influenza'/exp 7. 'common cold':ab,ti OR 'common colds':ab,ti 6. 'common cold'/exp 5. rhinopharyngit*:ab,ti 4. rhinit*:ab,ti 3. 'rhinitis'/exp 2. 'respiratory tract infection':ab,ti OR 'respiratory tract infections':ab,ti OR 'respiratory infection':ab,ti OR 'respiratory infections':ab,ti 1. 'respiratory tract infection'/exp
Appendix 3. Web of Science search strategy
Topic=(respiratory infection* or respiratory tract infection* or rhinit* or rhinopharyngit* or common cold* or influenza* or pharyngit* or sinusit* or laryngit* or sore throat* or bronchit* or bronchiolit*) AND Topic=(acetylcysteine or n‐acetyl‐l‐cysteine or acetyl‐cysteine or nac or carbocysteine or carbocisteine or expectorant* or mucolytic*)
Refined by: Topic=(child* or infant* or infancy or newborn* or baby or babies or prematur* or preterm* or toddler* or preschool* or school age* or schoolchild* or nursery school* or primary school* or elementary school* or highschool* or high school* or kid or kids or adoles* or teen* or boy* or girl* or kindergar* or pediatric* or paediatric*)
Appendix 4. Previous CENTRAL and MEDLINE search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 4) which contains the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register; MEDLINE (1966 to March 2008); EMBASE (1980 to March 2008); Micromedex (April 2008); Pascal (1987 to 2004) and Science Citation Index (1974 to March 2008).
We used the following search terms to search MEDLINE and CENTRAL; we adapted these terms to search the other electronic databases.
MEDLINE (PubMed) #1. Exp Acetylcysteine/ #2. acetylcysteine #3. exp Carbocysteine/ #4. carbocysteine #5. exp Expectorants/ #6. expectorant$ #7. exp Mucolytics/ #8. mucolytics #9. 1‐8 OR #10. Exp Respiratory Tract Infections/ #11. respiratory tract infections #12. respiratory infections #13. exp Rhinitis/ #14. rhinitis #15. exp Common Cold/ #16. common cold #17. exp Influenza/ #18. influenza #19. exp Pharyngitis/ #20. pharyngitis #21. exp Sinusitis/ #22. sinusitis #23. exp Laryngitis/ #24. laryngitis #25. sore throat #26. exp Bronchitis/ #27. bronchitis #28. exp Bronchiolitis/ #29. bronchiolitis #30. 10‐29 OR #31. Exp Child/ #32. child* #33. paediatric* #34. paediatric* #35. 32‐34 OR #36. 9 AND 30 AND 35
Data and analyses
Comparison 1. Febrile state (AC or CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Febrile state after 6 days | 3 | 139 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 2 Febrile state after 6 days | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.02, 1.77] |
Comparison 2. Cough (AC or CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cough after 6 to 7 days | 3 | 139 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 2 Cough after 6 to 7 days | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.12, 1.20] |
| 3 Cough at the end of treatment (= 28 days) | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 3.1 Subgroup = bronchitis | 1 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 3.2 Subgroup = tracheitis | 1 | 52 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.10, 0.12] |
| 4 Cough at the end of treatment (= 28 days) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.16, 2.76] |
| 4.1 Subgroup = bronchitis | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.83] |
| 4.2 Subgroup = tracheitis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.08, 19.09] |
Comparison 3. Dyspnoea (AC or CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dyspnoea after 6 to 7 days | 4 | 245 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 2 Dyspnoea after 6 to 7 days | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.25, 1.74] |
Comparison 4. Thoracic semeiologic alterations (AC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Thoracic semeiologic alterations after 5 days | 2 | 109 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 2 Thoracic semeiologic alterations after 5 days | 2 | 109 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.20, 0.10] |
| 3 Thoracic semeiologic alterations after 5 days | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.63] |
| 4 Thoracic semeiologic alterations at the end of treatment (= 28 days) | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.14 [‐0.25, ‐0.03] |
| 4.1 Subgroup = bronchitis | 1 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.27, 0.06] |
| 4.2 Subgroup = tracheitis | 1 | 52 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.17 [‐0.32, ‐0.02] |
| 5 Thoracic semeiologic alterations at the end of treatment (= 28 days) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.99] |
| 5.1 Subgroup = bronchitis | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.32] |
| 5.2 Subgroup = tracheitis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.95] |
Comparison 5. General condition (CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bad general condition after 6 to 7 days | 2 | 182 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.35, 0.20] |
| 2 Bad general condition after 6 to 7 days | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.95] |
Comparison 6. Cough productivity (AC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cough productivity at the end of treatment (= 28 days) | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.20, 0.03] |
| 1.1 Subgroup = bronchitis | 1 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 1.2 Subgroup = tracheitis | 1 | 52 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.25, 0.06] |
| 2 Cough productivity at the end of treatment (= 28 days) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.11, 1.56] |
| 2.1 Subgroup = bronchitis | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.83] |
| 2.2 Subgroup = tracheitis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04, 2.63] |
Comparison 7. Appetite (CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Appetite trouble at the end of treatment (5 to 9 days) | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.29, 0.11] |
7.1. Analysis.
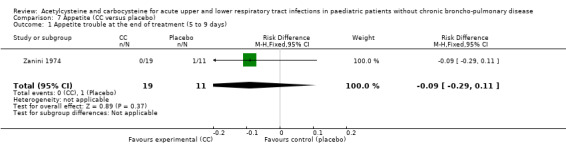
Comparison 7 Appetite (CC versus placebo), Outcome 1 Appetite trouble at the end of treatment (5 to 9 days).
Comparison 8. Expectoration (CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Expectoration at the end of treatment (= 7 days) | 1 | 106 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.15 [‐0.31, 0.01] |
| 2 Expectoration at the end of treatment (= 7 days) | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.27, 1.10] |
8.1. Analysis.
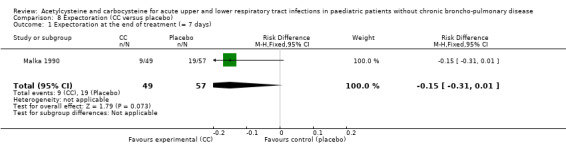
Comparison 8 Expectoration (CC versus placebo), Outcome 1 Expectoration at the end of treatment (= 7 days).
8.2. Analysis.
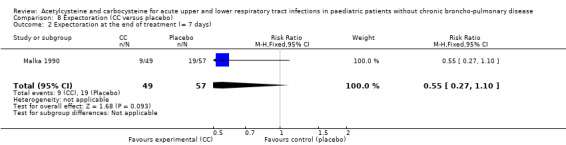
Comparison 8 Expectoration (CC versus placebo), Outcome 2 Expectoration at the end of treatment (= 7 days).
Comparison 9. Pulmonary function tests (CC versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alteration of the pulmonary function after 3 days | 1 | 106 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.14, 0.24] |
| 2 Alteration of the pulmonary function after 3 days | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.75, 1.66] |
9.1. Analysis.
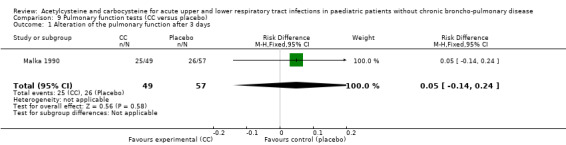
Comparison 9 Pulmonary function tests (CC versus placebo), Outcome 1 Alteration of the pulmonary function after 3 days.
9.2. Analysis.
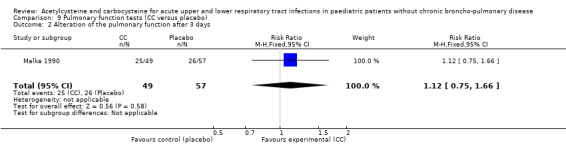
Comparison 9 Pulmonary function tests (CC versus placebo), Outcome 2 Alteration of the pulmonary function after 3 days.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bellomo 1972.
| Methods | RCT All participants received thiamphenicol with or without acetylcysteine | |
| Participants | 59 paediatric outpatients (22 < 1 year; 28 1 to 5 years; 9 > 5 years) seen for acute bronchitis or broncho‐pulmonary infections | |
| Interventions | Oral acetylcysteine 13.8 mg/kg/day 2 to 4 times a day for 6 to 7 days average (maximum 14 days) | |
| Outcomes | Clinical: duration of febrile state, dyspnoea, thoracic semeiologic alterations, cough | |
| Notes | Italian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported that allocation was done randomly |
| Allocation concealment (selection bias) | Unclear risk | Nothing in the text deals with allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only reported that treatment was randomly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing in the text deals with blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Nothing in the results clearly mentioned the number of patients used in the analysis |
| Selective reporting (reporting bias) | Low risk | All the judgement criteria were reported in the analysis |
Biscatti 1972.
| Methods | RCT All participants received some kind of antibiotic | |
| Participants | 50 children (29 < 2 years; 21 > 2 years) hospitalised for acute respiratory infections | |
| Interventions | Oral acetylcysteine 100 mg/day under 2 years 200 mg/day between 2 and 4 years 300 mg/day above 4 years for 6 days | |
| Outcomes | Clinical: cough, dyspnoea, thoracic semeiologic alteration, temperature reading Biological: ESR and leucocyte count | |
| Notes | Italian Possibly not a comparable treatment because various antibiotics were used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is only mentioned that treatments were assigned randomly |
| Allocation concealment (selection bias) | Unclear risk | Nothing is mentioned about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Nothing is mentioned about blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing is mentioned about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are mentioned in the analyses and the tables |
| Selective reporting (reporting bias) | Low risk | All criteria mentioned in 'Methods' were mentioned in the analysis |
Fiocchi 1989.
| Methods | RCT All participants received antibiotics if necessary | |
| Participants | 100 paediatric ambulatory participants (all > 2 years) seen in a paediatric department for acute lower respiratory infections | |
| Interventions | Oral acetylcysteine 20 mg/kg/day 3 times daily for 28 days | |
| Outcomes | Clinical: cough, cough productivity and thoracic semeilogic alteration PFT | |
| Notes | Italian Side effects: 2 participants vomited in the acetylcysteine group, with 1 drop‐out | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is only mentioned that randomisation was based on a list |
| Allocation concealment (selection bias) | Unclear risk | Nothing is mentioned about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Nothing is mentioned about blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing is mentioned about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are mentioned in the analyses and the tables |
| Selective reporting (reporting bias) | Low risk | All the criteria mentioned in 'Methods' were mentioned in the analysis |
Malka 1990.
| Methods | RCT All participants received antibiotics if necessary | |
| Participants | 106 paediatric ambulatory participants (2 to 12 years) seen in general practice for acute bronchitis | |
| Interventions | Oral carbocysteine for 7 days: 200 mg/day under 5 years. 300 mg/day above 5 years | |
| Outcomes | Clinical: cough, expectoration, bronchial congestion, dyspnoea PFT | |
| Notes | French 9 participants with side effects (in the carbocysteine group: 2 nausea, 3 diarrhoea, 1 stomach pain; in the placebo group: 1 nausea, 2 stomach pain) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised multicentre trial" |
| Allocation concealment (selection bias) | High risk | Quote: "The lack of respect in the order of the randomisation list..." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing in the text deals with blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The patients included for wrong reasons or who did not respect the protocol were included in the analysis" |
| Selective reporting (reporting bias) | Low risk | All the judgement criteria were reported in the analysis |
Nakayama 1977.
| Methods | RCT Participants received bronchodilators, antibiotics, antihistamine if previously treated | |
| Participants | 152 paediatric outpatients (0 to 18 years) bronchial asthma or acute bronchitis | |
| Interventions | Oral carbocysteine 30 mg/kg/day (administered in 3 or 4 doses) for 7 days | |
| Outcomes | Clinical: overall assessment, cough, stridor, expectoration PFT | |
| Notes | French Results tables were not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a table of random administration of products" |
| Allocation concealment (selection bias) | Low risk | Quote: "to ensure the impossibility of identifying the products, the random distribution, the secrecy of the key table..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "aspect, characteristics, taste, flavour and packaging were verified by a independent person" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The data statistics analyses were performed by a independent person" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the 'Results' section, it is mentioned that not all the patients were included in the statistical analyses |
| Selective reporting (reporting bias) | Low risk | All the criteria mentioned in the 'Methods' section were mentioned in the 'Results' section |
Zanini 1974.
| Methods | RCT In treatment group: 15 children received antibiotics; 4 received inhaled acetylcysteine In control group: 8 children received antibiotics | |
| Participants | 30 children (18 < 1 year; 12 > 1 year) hospitalised for acute respiratory infections | |
| Interventions | Oral carbocysteine from 100 to 400 mg/day depending on age for 5 to 9 days | |
| Outcomes | Clinical: cough, dyspnoea, temperature level, appetite, general condition | |
| Notes | Italian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were assigned randomly" |
| Allocation concealment (selection bias) | Unclear risk | The text did not mention anything about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "we had noticed the better efficacy of one of the produce in looking clinically at the therapeutic results" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "we had noticed the better efficacy of one of the produce in looking clinically at the therapeutic results" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients included were mentioned in the analysis |
| Selective reporting (reporting bias) | Low risk | All the relevant criteria were mentioned in the analysis |
AC: acetylcysteine ESR: erythrocyte sedimentation rate Gastralgia: localised stomach ache PFT: pulmonary function tests RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amir 1985 | No control group; chronic disease |
| Anonymous 1987 | No control group |
| Baldini 1989 | Active control treatment |
| Banovcin 1990 | Non‐randomised; active control treatment |
| Banovcin 1992 | Non‐randomised; active control treatment |
| Bellomo 1967a | Non‐randomised |
| Bellomo 1967b | No control group |
| Bellomo 1973 | Active control treatment |
| Berger 1978 | No control group; chronic disease |
| Berni 1983 | Active control treatment |
| Boner 1984 | No control group |
| Camurri 1990 | Active control treatment |
| Caramia 1984 | No control group |
| Careddu 1989 | Active control treatment |
| Castello 1979 | No control group |
| Chalumeau 2002 | No control group |
| Dano 1971 | No control group |
| Egreteau 1992 | Chronic disease |
| Gaudier 1968 | No control group |
| Ginocchi 1978 | No control group |
| Gusberti 1985 | Active control treatment |
| Henocq 1985 | No placebo Type of outcome measures |
| Hofmann 1980 | No placebo Chronic disease |
| Jean 1982 | No placebo |
| Leupold 1970 | No placebo |
| Loscialpo Ramundo 1968 | No control group |
| Mayaud 1980 | No placebo |
| Michael 1986 | No placebo |
| Nigam 1981 | No placebo Chronic disease |
| Nikolic 1980 | No placebo |
| Olivieri 1979 | Chronic diseases No control group |
| Plietz 1976 | Chronic diseases No control group |
| Poder 1982 | Chronic diseases No control group |
| Ramenghi 1984 | No control group |
| Ribeiro 1980 | Chronic diseases No control group |
| Rudnik 1980 | Chronic diseases No control group |
| Samsygina 1995 | Chronic disease No randomisation Active control treatment |
| Santangelo 1985 | No control group |
| Szekely 1980 | Chronic diseases No control group |
| Trastotenojo 1984 | No randomisation |
| Varricchio 2008 | Antibiotic treatment only in treatment group Type of disease |
| Volkl 1992 | No control group |
| Zens 1967 | Chronic diseases No control group |
| Zuppi 1984 | Chronic diseases |
Differences between protocol and review
None.
Contributions of authors
Yvonne Duijvestijn (YD) participated in the writing of the protocol, then took the lead of the review, participated in the data collection and analysis, wrote the first draft of the review and participated in the update of the review. Martin Chalumeau (MC) had the original idea for the study, wrote the first draft of the protocol, participated in data collection and analysis, in the writing of the review and wrote the first draft of the update.
Declarations of interest
No financial conflict of interest.
New search for studies and content updated (no change to conclusions), comment added to review
References
References to studies included in this review
Bellomo 1972 {published data only}
- Bellomo G, Giudice S. Controlled study on the efficacy of a combination "thiamphenicol‐acetylcysteine" in oral administration in respiratory infections in pediatrics [Studio controllata sull'efficacia della combinazione "tiamfenicolo‐acetilcisteina" per via orale nel trattamento delle infezioni broncopolmonari dell'infanzia]. Clinical Pediatrics 1972;54:30‐51. [Google Scholar]
Biscatti 1972 {published data only}
- Biscatti G, Bruschelli M, Damonte G, Capozzi F. Controlled studies of the clinical effects of acetylcysteine in oral administration in respiratory infections in pediatrics [Ricerca controllata sugli effetti clinici dell'acetilcisteina per via orale nelle infezioni respiratorie in pediatria]. Minerva Pediatrica 1972;24:1075‐84. [PubMed] [Google Scholar]
Fiocchi 1989 {published data only}
- Fiocchi A, Vignati B, Sala M, Arancio R, Banderali G, Marangione P, et al. A new n‐acetylcysteine formulation in acute bronchopneumopathy in children [La n‐acetilcisteina nel trattamento delle broncopneumopatie acute in pediatria]. Giornale Italiano delle Malattie del Torace 1989;43:327‐34. [Google Scholar]
Malka 1990 {published data only}
- Malka M, Lablache Combier B. Carbocysteine in the treatment of acute bronchitis in children [Interet d'un mucolytique, la carbocistéine, dans la bronchite aiguë de l'enfant]. Medicina Infantil 1990;97:687‐92. [Google Scholar]
Nakayama 1977 {published data only}
- Nakayama Y, Maeda K, Fuanabashi S, Kubagawa T, Simanugi K, Tateno K, et al. Carbocysteine syrup in pediatrics: a clinical trial [Effets cliniques de s‐carboxymethylcysteine sous forme de sirop en pédiatrie (traduction)]. Japanese Journal of Pediatrics 1977;10:1823. [Google Scholar]
Zanini 1974 {published data only}
- Zanini A, Dodesni G. Lisomucil in pediatrics [Il lisomucil in campo pediatrico]. Clinica Europea 1974;13:1‐12. [Google Scholar]
References to studies excluded from this review
Amir 1985 {published data only}
- Amir J, Wilunsky E, Zelikovic I, Reisner SH. Acetylcysteine for severe atelectasis in premature infants. Clinical Pharmacy 1985;4:255. [PubMed] [Google Scholar]
Anonymous 1987 {unpublished data only}
- Anonymous. A study of tolerance of carbocisteine syrup in children [Etude de la tolérance clinique du sirop carbocistéine chez l'enfant]. Dossier d'autorisation de mise sur le marché, Laboratoires Alpharma, Aubervilliers, France 1987.
Baldini 1989 {published data only}
- Baldini G, Gucci M, Taro D, Memmini C. Controlled clinical study of a new Ambroxol formula in the treatment of infantile spastic bronchitis [Studio clinico controllato sull'attivita di una nuova formulazione di Ambroxol nella bronchite asmatiforme del bambino]. Minerva Pediatrica 1989;41:91‐5. [PubMed] [Google Scholar]
Banovcin 1990 {published data only}
- Banovcin P. Efficacy of carbocysteine in the treatment of bronchopneumonias in children. Prakticky Lekar 1990;70:885‐7. [Google Scholar]
Banovcin 1992 {published data only}
- Banovcin P, Jakusova L, Rosslerova V, Miklerova M, Pullman R. Carbocysteine in the treatment of recurrent bronchitis in infants [Karbocystein v liecbe recidivujucich bronchitid u dojciat]. Ceskoslovenska Pediatrie 1992;47:543‐6. [PubMed] [Google Scholar]
Bellomo 1967a {published data only}
- Bellomo G, Frigerio G, Tirantello G. Controlled study on the use of intramuscular acetylcysteine in acute respiratory infections in pediatrics [Ricerche controllate sull'impiego dell'acetilcisteina per via intramuscolare nelle infezioni respiratorie acute in pediatria]. Clinica Terapeutica 1967;43:437‐58. [PubMed] [Google Scholar]
Bellomo 1967b {published data only}
- Bellomo G, Tirantello G, Giudice S. On the use of a combination of acetylcysteine with thiamphenicol glycinate in the systemic treatment of bronchopulmonary infections in children [Sull'uso di una combinazione dell'acetilcysteina con tiamfenicolo glicato nel trattamento sistemico delle infezioni broncopolmonari in eta' pediatrica]. Clinical Pediatrics 1967;49:402‐24. [PubMed] [Google Scholar]
Bellomo 1973 {published data only}
- Bellomo G, Giudice S, Ganci C. Comparative study of the efficacy of acetylcysteine by the oral and intramuscular routes in acute respiratory affections in pediatrics [Studio comparativo dell'efficacia dell'acetilcisteina per via orale ed intramuscolare nelle affezioni respiratorie acute in pediatria]. Minerva Pediatrica 1973;25:844‐9. [PubMed] [Google Scholar]
Berger 1978 {published data only}
- Berger von G, Gottschalk B, Leupold W. Initial experiences with ultrasonic aerosol therapy at home in children with bronchiectasis [Erste erfahrungen mit der ultraschall‐aerosolbehandlung unter heimbedingungen bei kindern mit bronchiektasen]. Kinderärztliche Praxis 1978;46:523‐7. [PubMed] [Google Scholar]
Berni 1983 {published data only}
- Berni M, Collina A, Zavattini G. Ambroxol in bronchopulmonary pathology in children [Ambroxol nella patologia broncopolmonare del bambino]. Clinica Terapeutica 1983;106:351‐5. [PubMed] [Google Scholar]
Boner 1984 {published data only}
- Boner AL, Valletta EA, Stocchero L, Richelli C, Bennati D. Combined antibiotic plus mucolytic treatment of acute or recurrent pneumonia in children. Drugs Under Experimental and Clinical Research 1984;10:627‐30. [Google Scholar]
Camurri 1990 {published data only}
- Camurri S, Marenco G. Clinical evaluation of the safety and efficacy of a new pharmaceutical formulation of bromhexine compared to N‐acetylcysteine in pediatric patients with acute bronchitis [Valutazione clinica dell'efficacia e della tollerabilita della nuova formulazione farmaceutica bromessina granulare nei confronti dell'N‐acetilcisteina granulare in piccoli pazienti affetti da bronchite acuta]. Gazzetta Medica Italiana 1990;149:45‐8. [Google Scholar]
Caramia 1984 {published data only}
- Caramia G, Bizzarri V, Compagnoni L, Gregorini S. Combined antibiotic plus mucolytic treatment in broncho‐pulmonary disease: cefuroxime plus acetylcysteine. Current Therapeutic Research, Clinical and Experimental 1984;36:658‐63. [Google Scholar]
Careddu 1989 {published data only}
- Careddu P, Bernocchi D, Scotti L, Manini G, Mezzopane A. Therapeutic activity and tolerability of a new mucoregulating drug called nesosteine in the treatment of acute diseases of the respiratory apparatus. Controlled pediatric study vs carbocysteine [Attivita terapeutica e tollerabilita di un nuovo farmaco mucoregolatore denominato nesosteina nel trattamento di affezioni acute dell'apparato respiratorio studio pediatrico controllato vs carbocisteina]. Giornale Italiano Delle Malattie Del Torace 1989;43:47‐52. [Google Scholar]
Castello 1979 {published data only}
- Castello D, Costa G, Candussio G. Modifications in catarrhal bronchitis secretion in paediatric age following treatment with S‐carboxymethyl‐cystein (viscosimetric study) [Modificazioni dell'escreato nella bronchite catarrale in eta pediatrica dopo trattamento con S‐carbossimetilcisteina (indagine viscosimetrica)]. Minerva Pediatrica 1979;31:371‐80. [PubMed] [Google Scholar]
Chalumeau 2002 {published data only}
- Chalumeau M, Cheron G, assathiany R, Moulin F, Bavoux F, Breart G, et al. Mucolytic agents for acute respiratory tract infections in infants: a pharmacoepidemiologic problem? [Fluidifiants bronchiques dans les infections respiratoires aiguës du nourrisson: un problème pharmacoépidémiologique?]. Archives de Pédiatrie 2002;9:1128‐36. [DOI] [PubMed] [Google Scholar]
Dano 1971 {published data only}
- Dano G. Bronchospasm caused by acetylcysteine in children with bronchial asthma. Acta Allergologica 1971;26:181‐90. [PubMed] [Google Scholar]
Egreteau 1992 {published data only}
- Egreteau L, Gaillard D, Jouet JB, Plotkowski L, Zahm JM, Pierrot D, et al. Effect of carbocisteine on mucus and respiratory mucosa in children with recurrent bronchitis. European Respiratory Journal 1992;Suppl 5:301. [DOI] [PubMed] [Google Scholar]
Gaudier 1968 {unpublished data only}
- Gaudier B, Lelong M. Carbocysteine to treat asthma in children [L'utilisation de la S‐Carboxy Methyl Cystéine (LJ 206) dans l'asthme de l'enfant]. Laboratoires Joullie, Paris 1968.
Ginocchi 1978 {published data only}
- Ginocchi G. Clinical effects of a mucolytic agent (Pol 65) in a pediatric statistical study [Effeti clinici di un mucoliyico (Pol 65) in una casistica pediatrica]. Settimana Medica 1978;66:391‐408. [Google Scholar]
Gusberti 1985 {published data only}
- Gusberti C, Bellazzi A, Lonati C, Modesti R. Clinical evaluation of a new synthetic derivative of acetylcysteine for treating respiratory inflammations in pediatric patients [Valutazione clinica di uno nuovo derivato sintetico dell'acido acetilsalicilico nella terapia di flogosi respiratorie di pazienti pediatrici]. Archivio di Medicina Interna 1985;37:123‐9. [Google Scholar]
Henocq 1985 {published data only}
- Henocq A, Moreau C, Mallet E, Sauger F, Menibus CH de. Changes in IgA levels in nasal mucus after upper respiratory tract diseases in infants treated with carbocysteine [Modifications du taux IgA du mucus nasal au décours des affections des voies aériennes supérieures du nourrisson traitées par la carbocistéine]. Annales d'Oto‐Laryngologie et de Chirurgie Cervico Faciale 1985;102:373‐5. [PubMed] [Google Scholar]
Hofmann 1980 {published data only}
- Hofmann A. Oral acetylcystein in the treatment of bronchial asthma and chronic respiratory tract diseases [Orale anwendung von acetylcystein bei der behandlung von asthma bronchiale und chronischen erkrankungen der luftwege]. Therapiewoche 1980;30:2040‐6. [Google Scholar]
Jean 1982 {unpublished data only}
- Jean R, Gardair F. Efficacy on expectoration of acetylcysteine nebulization in pediatric patients [Utilisation du Fluimucil antibiotic 750 en aérosols chez le nourisson et l'enfant son efficacité d'expectoration]. Laboratoires Zambon, Paris 1982.
Leupold 1970 {published data only}
- Leupold W von. Lung function in bronchial asthma during treatment with Mucosolvin [Die lungenfunktion bei asthma bronchiale unter der behandlung mit Mucosolvia]. Das Deutsche Gesundheitswesen 1970;25:1400‐2. [PubMed] [Google Scholar]
Loscialpo Ramundo 1968 {published data only}
- Loscialpo Ramundo D. On the use of the association of acetylcysteine and thiamphenicol glycinate in some pediatric pulmonary diseases [Sull'impiego associazone acetilcisteina‐tiamfenicolo glicinato in alcune forme di patologia polmonare pediatrica]. Clinical Pediatrics 1968;50:43‐56. [PubMed] [Google Scholar]
Mayaud 1980 {published data only}
- Mayaud C, Lentschner C, Bouchoucha S, Marsac J. Thiamphenicol glycinate acetylcysteinate in the treatment of acute respiratory infections with mucostasis [L'acetylcysteinate de thiamphenicol glycinate dans le traitement des infections respiratoires aigues avec mucostase]. European Journal of Respiratory Diseases Supplement 1980;61(111):70‐3. [PubMed] [Google Scholar]
Michael 1986 {published data only}
- Michael P, Haase W. Treatment of acute and chronic bronchitis. Therapeutic experience with Pulmoclase [Behandlung akuter und chronischer bronchitis. Therapeutische erfahrungen mit Pulmoclase]. Therapiewoche 1986;36:4440‐5. [Google Scholar]
Nigam 1981 {published data only}
- Nigam BK. S‐Carboxymethylcysteine (S‐CMC) in bronchography technique. Indian Journal of Chest Diseases and Allied Sciences 1981;23:81‐4. [PubMed] [Google Scholar]
Nikolic 1980 {published data only}
- Nikolic P, Korac D. The influence of acetylcysteine on respiratory functions in children with recurrent bronchitis. European Journal of Respiratory Diseases Supplement 1980;61(111):141. [Google Scholar]
Olivieri 1979 {published data only}
- Olivieri D, Pezza A, Polistina D, D'Agostino F. Clinical evaluation of a new pharmacological association in the therapy of acute and chronic bronchitis [Valutazione clinica di una nuova associazione farmacologica nella terapia delle broncopneumopatie acute e croniche]. Archivio Monaldi per la Tisiologia e le Malattie dell'Apparato Respiratorio 1979;34:29‐41. [PubMed] [Google Scholar]
Plietz 1976 {published data only}
- Plietz J. The treatment of bronchitic syndrome using Transbronchin in the practice. Zeitschrift für Allgemeinmedizin (Stuttgart) 1976;52:1832‐4. [PubMed] [Google Scholar]
Poder 1982 {unpublished data only}
- Poder G, Puskas J, Kelemen J, Gegesi Kiss A, Cserhati E. The effect of long term treatment with N‐Acetylcysteine in infants with chronic obstructive bronchitis [Die Wirkung der Langzeitbehandlung mit N‐Acetylcystein bei chronisch‐obstruktiver Bronchitis im Sauglings‐ und Kleinkindalter]. Laboratoires Zambon (Pharmaceutical company), Paris 1982.
Ramenghi 1984 {published data only}
- Ramenghi M, Sabayini G, Mengoni M. Combined antibiotic plus mucolytic treatment for recurrent bronchial diseases in infancy. Current Therapeutic Research 1984;36:664‐7. [Google Scholar]
Ribeiro 1980 {published data only}
- Ribeiro TM, Cunha LG, Santos M, Frenkiel S. Treatment of bronchial diseases in pediatrics. Results of the use of oral acetylcysteine in 80 cases. European Journal of Respiratory Diseases Supplement 1980;61(111):136‐8. [PubMed] [Google Scholar]
Rudnik 1980 {published data only}
- Rudnik J, Gawel J, Haluszka J, Kurzawa R, Mielnicka B, Majewska‐Zalewska H, et al. Oral acetylcysteine treatment in children with chronic lung diseases. European Journal of Respiratory Diseases Supplement 1980;61(111):140. [Google Scholar]
Samsygina 1995 {published data only}
- Samsygina G, Kazyukova T, Vykhristyuk O, Motina A, Tsaregorodtseva L, Dudina T. Acetylcysteine 100 in treatment of children with acute and chronic respiratory diseases: experience of use. Pediatriya (Moscow) 1995;1:76. [Google Scholar]
Santangelo 1985 {published data only}
- Santangelo G, Lombardo S, Giannotti G. A combination of cefuroxime and N‐acetyl‐cysteine for the treatment of lower respiratory tract infections in children. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1985;23:279‐81. [PubMed] [Google Scholar]
Szekely 1980 {published data only}
- Szekely E, Farkas E. Treatment of chronic bronchitis with oral acetylcysteine in children. European Journal of Respiratory Diseases Supplement 1980;61(111):142. [Google Scholar]
Trastotenojo 1984 {published data only}
- Trastotenojo MS, Harsoyo N, Sachro AD, Soemantri AG, Said HW. Use of acetyl cysteine in respiratory tract disease in children. Paediatrica Indonesiana 1984;24:1‐10. [PubMed] [Google Scholar]
Varricchio 2008 {published data only}
- Varricchio A, Capasso M, Giocchino M, Ciprandi G. Inhaled thiamphenicol and acetylcysteine in children with acute bacterial rhinopharyngitis. International Journal of Immunopathology & Pharmacology 2008;21:625‐9. [DOI] [PubMed] [Google Scholar]
Volkl 1992 {published data only}
- Volkl VKP, Schneider B. Treatment of airway diseases with N‐acetylcystein. An open therapeutic observational study involving 2512 patients. Fortschritte der Medizin 1992;110:346‐50. [PubMed] [Google Scholar]
Zens 1967 {published data only}
- Zens VM. Clinical trial with a mucolytic agent, acetylcysteine, in children with bronchitis [Klinische Erfahrungen mit Acetylcystein, einer mukoltytiscen Substanz, bei der Bronchitis im Kindesalter]. Fortschritte der Medizin 1967;85:206‐7. [Google Scholar]
Zuppi 1984 {published data only}
- Zuppi LJ, Croce J. Effect of oral route acetylcysteine on the spirometric parameters in children with asthma or rhinitis [Efeito da acetilcisteina oral sobre os parametros espirometricos em criancas asmaticas ou com rinite]. Folha Médica 1984;89:305‐8. [Google Scholar]
Additional references
Anonymous 2000a
- Anonymous. Acetylcysteine. In: McVoy GK editor(s). American Hospital Formulary System Drug Information. Bethesda, USA: American Society of Health‐System Pharmacists Incorporation, 2000:472‐4. [Google Scholar]
Anonymous 2000b
- Anonymous. Acetylcysteine. Anonymous. Drug Facts and Comparisons 2000. 4th Edition. Saint Louis, USA: Facts and Comparisons, 2000:677‐9. [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatrics and Adolescent Medicine 2008;162(2):111‐6. [DOI] [PubMed] [Google Scholar]
Bricks 1996
- Bricks LF, Leone C. Use of medicines by children attending nursery schools. Revista de Saúde Pública 1996;30:527‐35. [DOI] [PubMed] [Google Scholar]
Bücheler 2002
- Bücheler R, Schwab M, Mörike K, Kalchthaler B, Mohr H, Schröder H, et al. Off label prescribing to children in primary care in Germany: retrospective cohort study. BMJ 2002;324(7349):1311‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cano Garcinuño 2013
- Cano Garcinuño A, Casares Alonso I, Rodríguez Barbero J, Pérez García I, Blanco Quirós A. Prescription of systemic cold and cough drugs to children 0‐13 years old. An unresolved problem. Anales de Pediatría 2013;78(1):43‐50. [DOI] [PubMed] [Google Scholar]
Cates 2003 [Computer program]
- Cates C. Visual Rx. Online NNT Calculator (http://www.nntonline.net/). nntonline, 2003.
Cazzato 2001
- Cazzato T, Pandolfi C, Campi R, Bonati M. Drug prescribing in out‐patient children in Southern Italy. European Journal of Clinical Pharmacology 2001;57:611‐6. [DOI] [PubMed] [Google Scholar]
Chalumeau 2000
- Chalumeau M, Treluyer JM, Salanave B, Assathiany R, Cheron G, Crocheton N, et al. Off label and unlicensed drug use among French office based paediatricians. Archives of Disease in Childhood 2000;83(6):502‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2006
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics. Chest 2006;129(Suppl):260‐83. [DOI] [PubMed] [Google Scholar]
Collet 1991
- Collet JP, Bossard N, Floret D, Gillet J, Honegger D, Boissel JP. Drug prescription in young children: results of a survey in France. European Journal of Clinical Pharmacology 1991;41:489‐91. [DOI] [PubMed] [Google Scholar]
Cumberland Pharmaceuticals 2008
- Cumberland Pharmaceuticals. Acetadote(r) iv injection, acetylcysteine iv injection. Product information 2008.
Delgado‐Rodriguez 2004
- Delgado‐Rodriguez M, Llorca J. Bias. Journal of Epidemiology and Community Health 2004;58:635‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duijvestijn 1997
- Duijvestijn YC, Gerritsen J, Brand PL. Acetylcysteine in children with lung disorders prescribed by one‐third of family physicians: no support in the literature. Nederlands Tijdschrift Voor Geneeskunde 1997;141:826‐30. [PubMed] [Google Scholar]
Duijvestijn 1999
- Duijvestijn YC, Brand PL. Systematic review of N‐acetylcysteine in cystic fibrosis. Acta Paediatrica 1999;88:38‐41. [DOI] [PubMed] [Google Scholar]
EMEA 2001
- European Agency for the Evaluation of Medicinal Products. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Clinical investigation of medicinal products in the paediatric population (ICH Topic E11). http://www.emea.europa.eu/pdfs/human/ich/271199en.pdf 2001 (accessed 15 October 2007).
Halna 2005
- Halna M, Leblond P, Aissi E, Dumonceaux A, Delepoulle F, Kohen R, et al. Impact of the consensus conference on outpatient treatment of infant bronchiolitis. Presse Médicale 2005;34:277‐81. [DOI] [PubMed] [Google Scholar]
Harris 1967
- Harris RL, Riley HD. Reactions to aerosol medication in infants and children. JAMA 1967;201:953‐5. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JD, Altman G. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horen 2002
- Horen B, Montastruc J‐L, Lapeyre‐Mestre M. Adverse drug reactions and off‐label drug use in paediatric outpatients. Journal of Clinical Pharmacology 2002;54:665‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horwitz 1980
- Horwitz RI, Feinstein AR. The problem of "protopathic bias" in case‐control studies. American Journal of Medicine 1980;68:255‐8. [DOI] [PubMed] [Google Scholar]
Hughes 1994
- Hughes J, Gill A, Leach HJ, Nunn AJ, Billingham I, Ratcliffe J, et al. A prospective study of the adverse effects of midazolam on withdrawal in critically ill children. Acta Paediatrica 1994;83:1194‐9. [DOI] [PubMed] [Google Scholar]
Mallet 2011
- Mallet P, Mourdi N, Dubus J‐C, Bavoux F, Boyer‐Gervoise M‐J, Jean‐Pastor M‐J, et al. Respiratory paradoxical adverse drug reactions associated with acetylcysteine and carbocysteine systemic use in paediatric patients: a national survey. PLoS One 2011;6:e22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medici 1979
- Medici TC, Radielovic P. Effects of drugs on mucus glycoproteins and water in bronchial secretion. Journal of International Medical Research 1979;7(5):434‐42. [DOI] [PubMed] [Google Scholar]
Mourdi 2010
- Mourdi N, Dubus JC, Bavoux F, Boyer‐Gervoise M, Jean‐Pastor MJ, Chalumeau M. Mucolytic drugs: towards a contraindication in infants. Archives de Pédiatrie 2010;17:735‐6. [DOI] [PubMed] [Google Scholar]
Naranjo 1981
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clinical Pharmacology and Therapeutics 1981;30:239‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poole 2012
- Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001287.pub4] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Salas 1999
- Salas M, Hofman A, Strieker BHC. Confounding by indication: an example of variation in the use of epidemiologic terminology. American Journal of Epidemiology 1999;149:981‐3. [DOI] [PubMed] [Google Scholar]
Sanz 1988
- Sanz EJ, Boada JN. Drug utilization by children in Tenerife Island. European Journal of Clinical Pharmacology 1988;34:495‐9. [DOI] [PubMed] [Google Scholar]
Sen 2011
- Sen EF, Verhamme KM, Felisi M, 't Jong GW, Giaquinto C, Picelli G, et al. Effects of safety warnings on prescription rates of cough and cold medicines in children below 2 years of age. British Journal of Clinical Pharmacology 2011;71:943‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 2008
- Shields MD, Bush A, Everard ML, McKenzie S, Primhak R, British Thoracic Society Cough Guideline Group. BTS guidelines: recommendations for the assessment and management of cough in children. Thorax 2008;63(Suppl 3):iii1‐15. [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001831.pub4] [DOI] [PubMed] [Google Scholar]
WHO 2007
- WHO Child Growth Standards. Weight‐for‐age. http://www.who.int/childgrowth/standards/weight_for_age/en/ 2013 (accessed 1 April 2013).
Wohl 1998
- Wohl MEB. Developmental physiology of the respiratory system. In: Chernick V, Boat TF editor(s). Kendig’s Disorders of the Respiratory Tract in Children. Vol. 6th, Philadelphia, USA: WB Saunders Company, 1998. [Google Scholar]
References to other published versions of this review
Duijvestijn 2009
- Duijvestijn YCM, Mourdi N, Smucny J, Pons G, Chalumeau M. Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho‐pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003124.pub3] [DOI] [PubMed] [Google Scholar]


