Abstract
Background
Low false negative rates can be achieved with sentinel lymph node biopsy (SLNB) after neoadjuvant chemotherapy (NAC) in breast cancer (BC) patients with clinical N1 (cN1) disease. We examined changes in axillary management and oncologic outcomes in BC patients with cN1 disease receiving NAC.
Methods
BC patients with biopsy proven cN1 disease treated with NAC were selected from our institutional cancer registry (2014–2017). Patients were grouped by axillary management, axillary lymph node dissection (ALND), SLNB followed by ALND, or SLNB alone. Univariable and multivariable survival analysis for recurrence-free survival (RFS) and overall survival (OS) were performed.
Results
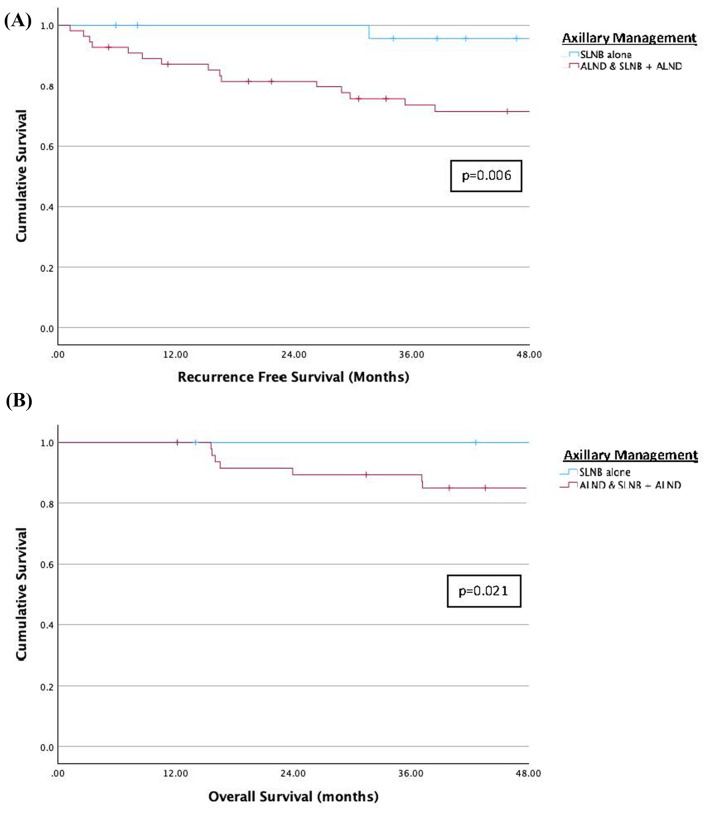
81 patients met inclusion criteria: 31 (38%) underwent ALND, 25 (31%) SLNB + ALND, and 25 (31%) SLNB alone. A SLN was identified in 45/50 (90%) patients who had SLNB. ALND was performed in 25/50 (50%) patients who had SLNB: 18 for a + SLNB, 5 failed SLNB, and 2 insufficient SLNs. 25 patients had SLNB alone, 17 were SLN- and 8 SLN+. In the SLNB alone group, 23/25 (92%) patients received adjuvant radiation (RT). 20 (25%) patients developed BC recurrence: 14 distant (70%), 3 local (15%), 2 regional + distant (10%), and 1 contralateral (5%). In the SLNB alone group, there was 1 axillary recurrence in a patient with a negative SLNB who did not receive RT. Univariable survival analysis showed significant differences in RFS and OS between axillary management groups, ALND/SLNB + ALND vs. SLNB alone (RFS: p = 0.006, OS: p = 0.021). On multivariable survival analysis, worse RFS and OS were observed in patients with TNBC (RFS: HR 3.77, 95% CI 1.70–11.90, p = 0.023; OS: HR 8.10, 95% CI 1.84–35.60, p = 0.006).
Conclusions
SLNB alone and RT after NAC in BC patients with cN1 disease who have negative SLNs at surgery provides long-term regional disease control. This analysis provides support for the practice of axillary downstaging with NAC and SLNB alone.
Keywords: Breast cancer, Neoadjuvant therapy, Axillary surgery, De-escalation of treatment, Sentinel lymph node biopsy
Background
Management of the axilla in patients with breast cancer (BC) has undergone a significant transformation in the last 25 years. Landmark clinical trials support the use of sentinel lymph node biopsy (SLNB) alone instead of axillary lymph node dissection (ALND) in most patients with early-stage breast cancer (ESBC) [1, 2]. Omission of SLNB may also be considered in older women with estrogen receptor (ER) positive, human epidermal growth factor receptor-2 (HER2) negative ESBC [3, 4]. This de-escalation of axillary surgery has reduced the morbidity of BC surgery, particularly the risk of lymphedema, with subsequent improvements in quality of life for BC survivors [5].
Efforts to reduce the extent of axillary surgery in BC patients with clinically positive lymph nodes at diagnosis have been more challenging. However, increasing use of neoadjuvant chemotherapy (NAC) in patients with node positive disease and improvements in systemic therapy resulting in higher pathologic complete response (pCR) rates, have allowed for evaluation of less extensive axillary surgery in these patients. There are multiple clinical trials which show that low false negative rates (FNR) are achieved using SLNB after NAC when dual tracers are utilized for sentinel lymph node (SLN) mapping, 3 or more SLNs are retrieved, and/or the clipped, biopsied lymph node is removed [6–9]. Implementation of this strategy in patients with pre-treatment positive lymph nodes who convert to clinically node-negative disease after NAC has resulted in a significant decrease in ALND rates [10, 11].
A critical factor in the effort to de-escalate axillary surgery using SLNB after NAC, is ensuring oncologic outcomes are not compromised by this approach. Several small studies show low regional recurrence rates in patients undergoing SLNB alone after NAC who convert from node-positive to node-negative disease after treatment [12, 13]. However, the follow-up times in many of these studies are short and in turn, may not adequately capture recurrence or survival rates. The present study seeks to report long-term oncologic outcomes and temporal changes in axillary management after NAC in BC patients with clinical N1 (cN1) disease at diagnosis.
Methods
Study population
Breast cancer patients (female/male) with a primary diagnosis of non-metastatic, clinical T0-4 N1 (cTN) as defined by American Joint Committee on Cancer [AJCC] 7th Edition, disease at diagnosis who received NAC followed by surgery were selected from the institutional tumor registries of a large South Florida academic health system and affiliated safety-net hospital from 2014 to 2017. All patients had biopsy proven axillary lymph node involvement. Patients were excluded if they had surgery outside of the health system or evidence of metastatic disease. The study was approved by the institutional review board as part of a protocol evaluating surgical management, quality measures, and oncologic outcomes for patients treated in the Division of Surgical Oncology.
Data collection
A retrospective chart review collected demographic, clinical, treatment variables, and outcomes for included patients. Data collected included age at diagnosis, race/ethnicity, sex, tumor histology, tumor grade, BC subtype, clinical stage, NAC regimens, HER2-targeted therapy, surgical procedure (mastectomy or lumpectomy), axillary lymph node management, pathological response, and recurrence (local, regional and/or distant). Pathologic data included tumor category (pT), nodal category (pN), SLNB status (if performed), and number of positive and total nodes resected. Patients were considered for SLNB after NAC if they had a clinical complete response (cCR) or clinical partial response (cPR) in the axilla. A cCR in the axilla was considered complete resolution of prior axillary lymphadenopathy with normalization of lymph node morphology. For patients with a cPR in the axilla to be considered for SLNB, a reduction in both nodal size and cortical thickening was required and in most cases near normalization of lymph node morphology was observed. However, the decision to perform SLNB was also based on individual surgeon assessment of axillary tumor burden at diagnosis and response to NAC. A pCR in the breast and axilla was defined by the College of American Pathologists as ypT0/Tis ypN0 (i.e., no invasive residual in breast or nodes; noninvasive breast residual allowed) at the time of definitive surgery. Our institutional guideline for adjuvant radiation therapy (RT) during this time was to recommended RT to the chest wall/breast and regional lymph nodes (RNI) in patients with node positive disease at diagnosis unless patients were enrolled on a clinical trial. Patients were further sub-grouped by nodal management: ALND, SLNB followed by completion ALND (SLNB + ALND), or SLNB alone.
Statistical analysis
Descriptive statistics with chi square and analysis of variance (ANOVA) for categorical variables and t-tests for continuous variables were conducted by nodal management subgroups. We also describe the frequency of nodal management subgroups over the study time period. Kaplan-Meier survival analysis for recurrence-free survival (RFS) and overall survival (OS) were performed comparing those patients who underwent SLNB alone to patients who had ALND (ALND and SLNB + ALND groups). Recurrence and death were both recorded as yes/no and dates of each were recorded and used for survival analysis. RFS was defined as time from surgery to the last assessment or office visit without recurrence. OS was defined as the time from diagnosis to recorded last date of known contact (any visit type where patient was alive). A multivariable cox regression analysis which included age (≤ 50 vs. > 50), BC subtype: estrogen receptor (ER) positive/human epidermal growth factor receptor-2 (HER2) negative (ER+/HER2-), HER2+, and triple negative BC (TNBC), and pCR (Yes vs. No) was performed for RFS and OS. Data were analyzed using SPSS statistics version 28. Statistical significance was based on a two-sided alpha of 0.05.
Results
Patient characteristics
A total of 81 patients met inclusion criteria: 31 (38%) underwent ALND, 25 (31%) SLNB + ALND, and 25 (31%) SLNB alone. (Table 1) In total, 79 (98%) patients were female, 43 (53%) were greater than 50 years of age, 40 (49%) were Hispanic, 20 (25%) were Non-Hispanic Black and 19 (23%) were Non-Hispanic White. Patient characteristics were similar between groups.
Table 1.
Patient and Clinicopathologic Characteristics
| Total | ALND* | SLNB†+ALND | SLNB Alone | p | |
|---|---|---|---|---|---|
| n = 81 | n = 31 (38%) | n = 25 (31%) | n = 25 (31%) | ||
| Patient Factors | |||||
| Age | 0.97 | ||||
| ≤ 50 years | 38 (47%) | 14 (45%) | 12 (48%) | 12 (48%) | |
| > 50 years | 43 (53%) | 17 (55%) | 13 (52%) | 13 (52%) | |
| Race / Ethnicity | 0.143 | ||||
| Non-Hispanic White | 19 (23%) | 6 (19%) | 4 (16%) | 9 (36%) | |
| Non-Hispanic Black | 20 (25%) | 9 (29%) | 8 (32%) | 3 (12%) | |
| Hispanic | 40 (49%) | 16 (52%) | 11 (44%) | 13 (52%) | |
| Other | 2 (2%) | - | 2 (8%) | - | |
| Gender | 0.622 | ||||
| Female | 79 (98%) | 30 (97%) | 25 (100%) | 24 (96%) | |
| Male | 2 (2%) | 1 (3%) | - | 1 (4%) | |
| Tumor Factors | |||||
| Histology | 0.658 | ||||
| Ductal | 75 (93%) | 28 (90%) | 23 (92%) | 24 (96%) | |
| Lobular | 4 (5%) | 2 (7%) | 1 (4%) | 1 (4%) | |
| Unknown | 2 (2%) | 1 (3%) | 1 (4%) | - | |
| Grade | 0.669 | ||||
| 1 | 2 (2%) | 0 (0%) | 1 (4%) | 1 (4%) | |
| 2 | 27 (33%) | 10 (33%) | 10 (42%) | 7 (28%) | |
| 3 | 50 (62%) | 20 (67%) | 13 (54%) | 17 (68%) | |
| Unknown | 2 (2%) | 1 (3%) | 1 (4%) | - | |
| Breast Cancer Subtype ‡ | 0.822 | ||||
| ER+/HER2-§‖ | 30 (37%) | 13 (41%) | 10 (40%) | 7 (28%) | |
| ER+/HER2+ | 27 (33%) | 9 (28%) | 9 (36%) | 9 (36%) | |
| ER-/HER2+ | 11 (14%) | 4 (13%) | 2 (8%) | 5 (20%) | |
| TNBC¶ | 14 (17%) | 6 (19%) | 4 (16%) | 4 (16%) | |
| Clinical T | 0.256 | ||||
| 0–2 | 44 (54%) | 15 (48%) | 12 (48%) | 17 (68%) | |
| 3–4 | 37 (46%) | 16 (52%) | 13 (52%) | 8 (32%) | |
| Treatment Factors | |||||
| Multi-agent Neoadjuvant Chemotherapy | 80 (99%) | 30 (97%) | 25 (100%) | 25 (100%) | 0.442 |
| HER2-targeted Therapy | 38 (46%) | 13 (42%) | 11 (44%) | 14 (56%) | 0.543 |
| Clinical CR # | < 0.001 | ||||
| Yes | 26 (32%) | 9 (29%) | 4 (16%) | 13 (52%) | |
| No | 55 (67%) | 22 (71%) | 21 (84%) | 12 (48%) | |
| Breast cCR only | 2 (2%) | 2 (7%) | - | - | |
| Axillary cCR only | 30 (37%) | 2 (7%) | 17 (68%) | 11 (42%) | |
| Surgery | 0.300 | ||||
| Mastectomy | 58 (72%) | 24 (77%) | 19 (76%) | 15 (60%) | |
| Partial Mastectomy | 23 (28%) | 7 (23%) | 6 (24%) | 10 (40%) | |
| SLN Biopsy Technique | < 0.001 | ||||
| Radiotracer Alone | 25 (31%) | - | 11 (44%) | 14 (56%) | |
| Radiotracer and Blue Dye | 25 (31%) | - | 14 (56%) | 11 (44%) | |
| Clipped Node | 0.011 | ||||
| Localized & Removed | 12 (15%) | - | 7 (28%) | 5 (19%) | |
| Removed (Not Localized) | 5 (6%) | - | 2 (8%) | 3 (12%) | |
| SLN Identified | 45 (90%) | - | 20 (80%) | 25 (100%) | 0.018 |
| SLN Biopsy Results | 0.001 | ||||
| SLNB Negative | 18 (36%) | - | 1 (4%) | 17 (68%) | |
| SLNB Positive | 27 (54%) | - | 19 (76%) | 8 (32%) | |
| Radiation Therapy | 73 (90%) | 25 (80%) | 25 (100%) | 23 (92%) | 0.098 |
| Endocrine Therapy | 56 (67%) | 22 (71%) | 17 (68%) | 17 (65%) | 0.961 |
| Pathologic Response | |||||
| Pathologic T Stage | 0.026 | ||||
| 0-Tis | 29 (36%) | 8 (26%) | 6 (24%) | 15 (60%) | |
| 1–2 | 48 (59%) | 20 (65%) | 18 (72%) | 10 (40%) | |
| 3–4 | 4 (5%) | 3 (10%) | 1 (4%) | - | |
| Pathologic N Stage | < 0.001 | ||||
| 0 | 30 (38%) | 12 (39%) | 1 (4%) | 17 (68%) | |
| 1 | 34 (42%) | 11 (36%) | 15 (60%) | 8 (32%) | |
| 2–3 | 17 (21%) | 8 (26%) | 9 (36%) | - | |
| Pathologic CR | 0.002 | ||||
| Yes | 21 (26%) | 8 (26%) | 1 (4%) | 12 (48%) | |
| No | 60 (74%) | 23 (74%) | 24 (96%) | 13 (52%) | |
| Breast pCR only | 8 (10%) | - | 5 (20%) | 3 (12%) | |
| Axillary pCR only | 9 (11%) | 4 (13%) | - | 5 (20%) | |
| # Lymph Nodes Removed | < 0.001 | ||||
| 1–5 | 17 (21%) | 1 (3%) | 2 (8%) | 14 (56%) | |
| 6–9 | 10 (12%) | 1 (3%) | 3 (12%) | 6 (24%) | |
| 10 or more | 54 (67%) | 29 (94%) | 20 (80%) | 5 (20%) |
*ALND = axillary lymph node dissection, †SLNB = sentinel lymph node biopsy, ‡One patient in the ALND group had 2 tumors in the breast with different biomarkers for a total of 82 tumors in 81 patients §ER = estrogen receptor, ‖HER2 = human epidermal growth factor receptor-2, ¶TNBC = triple negative breast cancer, #CR = complete response
Tumor characteristics
Overall, 75 (93%) tumors were ductal and 50 (62%) were grade 3. (Table 1) Most tumors were ER+/HER2- (37%) or HER2+ (47%). The distribution of breast cancer subtypes was similar between groups. For the ALND and SLNB + ALND groups, 52% of the tumors were cT3-4, while in the SLNB alone group, only 32% were cT3-4 and none of these tumors were T4. Overall, 9 patients had T4 tumors, 5 who underwent ALND and 4 who underwent SLNB + ALND.
Treatment
Multi-agent systemic chemotherapy was utilized in 80 (99%) patients, 1 patient had single-agent chemotherapy, and HER2-targeted therapy was given to all 38 (100%) patients with HER2+ tumors (36/38 patients (95%) received trastuzumab + pertuzumab, 2/38 patients (5%) trastuzumab alone). (Table 1)
SLNB was performed in 50 patients and at least 1 SLN was identified in 45 (90%) of the patients. (Table 1) The utilization of SLNB was 20% in 2014 but increased to ≥ 50% for patients taken to surgery from 2015 to 2018. (Fig. 1) Patients were more likely to undergo SLNB if they had a cCR in the axilla after NAC (45/56 (80%)). In the SLNB + ALND group, 21/25 (80%) patients had a cCR in the axilla after NAC, while in the SLNB alone group, 24/25 (96%) patients had a cCR in the axilla after NAC. During the SLNB procedure, a clipped LN was removed in 17/50 (34%) patients. Completion ALND was performed in 25/50 (50%) patients who had SLNB: 18 (72%) for a positive SLN, 5 (20%) for failed SLN mapping, and 2 (8%) for insufficient SLN sampling. On final pathology, 24/25 (96%) had positive lymph nodes, and of the 19 (76%) patients with positive SLNs on final pathology, additional positive non-SLNs were found in 10/19 (53%) on ALND. In the 25 patients who had SLNB alone, 17 (68%) were SLN negative and 8 were SLN positive on final pathology. Of the 8 patients who were SLN positive, 4 (50%) had > 10 lymph nodes removed with SLNB at the time of surgery and completion ALND was not performed.
Fig. 1.
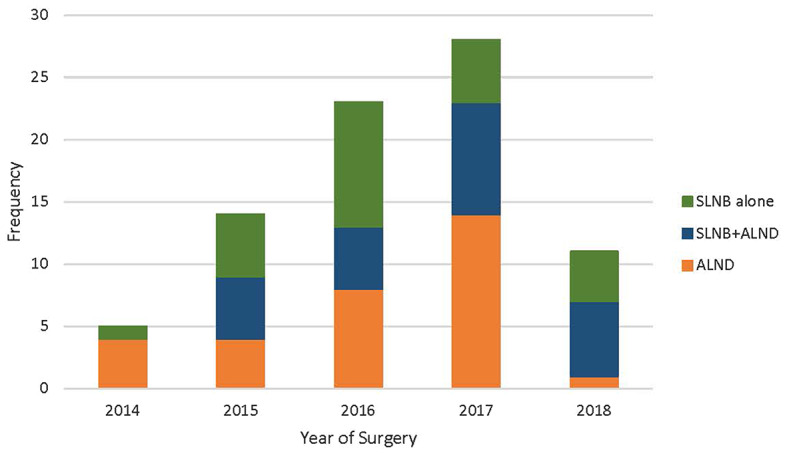
Axillary Management During Study Period
Adjuvant radiation was utilized in 73/81 (90%) patients. In 67/73 (92%) patients, chest wall/breast and RNI was administered while in 6 patients (8%) the radiation fields were unknown. In the SLNB along group, 23/25 (92%) patients received adjuvant RT including the 8 patients with a positive SLNB who did not have ALND.
Pathological response
Overall, 21 (26%) patients had a pCR including 12/25 (48%) patients in the SLNB alone group. (Table 1) As expected, the residual nodal disease burden in the axilla was greater in patients who underwent ALND or SLNB + ALND compared to SLNB alone (Table 1). In the ALND group, 8 (26%) patients had ypN2-3 disease and in the SLNB + ALND group, 9 (36%) patients were ypN2-3. In contrast, in the SLNB alone group, no patients had ypN2-3 disease and 17 (68%) had a pCR in the axilla.
Oncologic outcomes
Median follow-up time from surgery for the entire group was 67.5 months (IQR 36.2–79.5), 70.2 months (IQR 36.2–79.5) for ALND, 51.4 months (IQR 29.5–72.0) for SLNB + ALND, and 74.6 months (IQR 62.5–89.8) for SLNB alone. There were 20 BC recurrence events: 14 distant (70%), 3 local (15%), 2 regional + distant (10%), and 1 contralateral (5%) and 10/81 (12%) deaths from breast cancer. (Table 2) Of the 20 patients who developed BC recurrence, only 4 (20%) had a pCR to NAC, while 14 (70%) had residual disease in the breast and axilla with 11 (55%) having ypN2-3 disease.
Table 2.
Oncologic outcomes
| Total | ALND* | SLNB†+ALND | SLNB Alone | p | |
|---|---|---|---|---|---|
| n = 81 | n = 31 (38%) | n = 25 (31%) | n = 25 (31%) | ||
| BC ‡ Recurrence | 20 (25%) | 11 (36%) | 7 (28%) | 2 (8%) | 0.018 |
| Local Only | 3 (4%) | 2 (7%) | - | 1 (4%) | 0.191 |
| Regional Only | - | - | - | - | - |
| Distant Only | 14 (17%) | 8 (26%) | 6 (24%) | - | 0.023 |
|
Regional + Distant |
2 (2%) | 1 (3%) | - | 1 (4%) | 0.622 |
| Contralateral | 1 (1%) | - | 1 (4%) | - | 0.322 |
| BC Recurrence by Subtype (n = 20) | 0.256 | ||||
| ER+/HER2-§‖ | 7 (35%) | 6 (55%) | 1 (14%) | - | |
| ER+/HER2+ | 6 (30%) | 2 (18%) | 3 (43%) | 1 (50%) | |
| ER-/HER2+ | - | - | - | - | |
| TNBC¶ | 7 (35%) | 3 (27%) | 3 (43%) | 1 (50%) | |
| BC Deaths | 10 (12%) | 4 (13%) | 6 (24%) | - | 0.022 |
| BC Deaths by Subtype (n = 10) | 0.329 | ||||
| ER+/HER2- | 3 (30%) | 2 (50%) | 1 (17%) | - | |
| ER+/HER2+ | 2 (20%) | - | 2 (33%) | - | |
| ER-/HER2+ | - | - | - | - | |
| TNBC | 5 (50%) | 2 (50%) | 3 (50%) | - |
*ALND = axillary lymph node dissection, †SLNB = sentinel lymph node biopsy, ‡BC = breast cancer, §ER = estrogen receptor, ‖HER2 = human epidermal growth factor receptor-2, ¶TNBC = triple negative breast cancer
Only 2 (2%) patients developed regional recurrence. One patient in the SLNB alone group with an ER+/HER2+ BC who had negative SLNs at surgery, a pCR to NAC, and did not receive adjuvant RT developed an axillary recurrence 31 months after initial surgery which was managed with ALND. This patient subsequently developed distant disease and is alive > 100 months after initial BC diagnosis. The other patient with an ER+/HER2- tumor had an upfront ALND with residual disease in the breast and axilla at the time of surgery, ypT1cN1a, and received adjuvant RT. This patient developed simultaneous regional + distant recurrence 34 months after initial surgery and is currently alive > 80 months after initial BC diagnosis.
Univariable survival analysis showed significant differences in RFS and OS between axillary management groups, ALND/SLNB + ALND vs. SLNB alone (RFS: p = 0.006, Fig. 2(A); OS: p = 0.021, Fig. 2(B)) On multivariable survival analysis, worse RFS and OS were observed in patients with TNBC (RFS: HR 3.77, 95% CI 1.70–11.90, p = 0.023; OS: HR 8.10, 95% CI 1.84–35.60, p = 0.006). (Table 3)
Fig. 2.
(A) Univariable Survival Analysis for Recurrence-Free Survival. (B) Univariable Survival Analysis for Overall Survival
Table 3.
Cox proportional hazards analysis for recurrence-free survival and overall survival
| RFS | OS | |||
|---|---|---|---|---|
| HR* (95% CI†) | p | HR (95% CI) | p | |
| Age | ||||
| ≤ 50 years | 1.61 (0.64–4.02) | 0.310 | 3.14 (0.80–12.40) | 0.102 |
| > 50 years | Ref | |||
| BC ‡ Subtype | ||||
| ER+/HER2-§‖ | Ref | |||
| HER2+ | 0.94 (0.30–2.92) | 0.914 | 0.96 (0.16–5.94) | 0.965 |
| TNBC¶ | 3.77 (1.70–11.90) | 0.023 | 8.10 (1.84–35.60) | 0.006 |
| Pathologic CR # | ||||
| Yes | Ref | |||
| No | 2.34 (0.64–8.54) | 0.197 | 3.90 (0.47–32.21) | 0.207 |
*HR = hazard ratio, †CI = confidence interval, ‡BC = breast cancer, §ER = estrogen receptor, ‖HER2 = human epidermal growth factor receptor-2, ¶TNBC = triple negative breast cancer, #CR = complete response
Discussion
The results of this study show that SLNB alone and adjuvant RT after NAC in BC patients with cN1 disease at diagnosis with a negative SLNB at surgery provides durable regional disease control. In the current analysis, there were no axillary recurrences in patients treated in this manner. This provides support for the practice of axillary downstaging with NAC and de-escalation of axillary surgery in appropriately selected patients.
Over the past decade, the approach to management of the axilla for patients with BC with cN1 disease receiving NAC has changed considerably. There has been a de-escalation of axillary surgery in these patients with a shift towards axillary management with SLNB alone and a decrease in ALND rates [10, 11, 14, 15]. While the SENTINA and ACOSOG Z1071 trials raised concerns as to the accuracy of SLNB after NAC due to FNRs > 10%, subsequent review of these trials demonstrated that the FNR is decreased to < 10% by use of dual mapping agents for SLNB and retrieval of more than 2 SLNs [6, 7]. An analysis by Caudle et al. [9]. also showed that retrieval of the clipped, biopsied lymph node could further reduce the FNR. Each of these studies called for changes in approach and selection of patients to support the use of SLN surgery as an appropriate alternative to ALND. Adoption of these findings to clinical practice was evident in our study as reflected by the significant change in axillary management in patients following the publication of these key clinical trials. In 2014, < 20% of patients were considered for SLNB, while after 2014, at least 50% of patients underwent SLNB as their first staging procedure and overall ALND was omitted in 31% of patients. Similar trends in axillary management have been observed across the US in patients with node positive breast cancer after NAC [11, 15, 16].
Equally important when considering SLNB alone following NAC, is the oncologic safety of this approach compared to ALND. There are several studies which examined axillary recurrence rates in patients undergoing SLNB alone with pathologically negative nodes after NAC and showed low axillary recurrence rates with short-term follow-up [13, 15, 17, 18]. In a study by Barrio et al., [17] at a median follow-up of 40 months, there was only 1 axillary recurrence in 234 patients with cN1 ypN0 disease who underwent SLNB alone. This nodal recurrence was in a patient who did not receive adjuvant RT, and there were no nodal recurrences in the 205 patients who received adjuvant RT. A similar study by Wong et al. [13]. found that in patients with cN1-2 disease at diagnosis who were node negative on SLNB after receiving NAC, the 5-year local and regional recurrence rates were 4.1% (95% CI 1.0-15.5) and 0%, respectively. The median follow-up for this group of patients was 36 months.
In the current analysis, 25 patients had SLNB alone after NAC, 17 with negative SLNs and 8 with positive SLNs. With a median follow-up of 74.6 months in this group of patients, there was only 1 axillary recurrence in a patient who did not receive adjuvant RT. This provides long-term follow up and further support for the oncologic safety of axillary downstaging with NAC and SLNB alone in patients who are node negative at surgery. It is important to note that 88% of patients with a negative SLNB received adjuvant RT which may be necessary for regional disease control. The recently reported results of the NSABP B-51/RTOG 1304 trial showed that axillary recurrences with omission of RNI in this group of patients are very low [19]. However, there are likely multiple factors which need to be considered when omitting RNI in these patients including the initial burden of nodal disease in the axilla at diagnosis which may vary even in cN1 patients and breast cancer subtype. The pCR rate in patients included in the NSABP B-51/RTOG 1304 trial was close to 80% which is higher than seen in most clinical trials utilizing NAC. Therefore, the patients in NSABP B-51 were exceptional responders and omission of adjuvant RNI may not be appropriate for all patients who covert to node negative disease after NAC. In addition, the median follow-up time for patients in the NSABP B-51 trial was 59.5 months which may be sufficient time to capture recurrences for patients with HER2 + breast cancer or TNBC but may be too short a time interval for patients with ER+/HER2- tumors where late recurrences are more common. Therefore, as we de-escalate both axillary surgery and RNI in patients with node positive BC at diagnosis who convert to node negative disease after NAC, patients will need to be closely monitored for axillary recurrences and the impact that this may have on breast cancer survival. Our study also included 8 patients with a positive SLNB after NAC who did not undergo formal ALND. All of these patients received adjuvant RT and none experienced an axillary recurrence. Therefore, for select patients with residual SLN disease after NAC, adjuvant RT may be sufficient for regional disease control. However, more information is required to consider routine omission of ALND in these patients, and the results of the Alliance A011202 trial will help guide management.
On univariable survival analysis, we found that patients who underwent SLNB alone had better RFS and OS than patients who underwent ALND and SLNB + ALND. This likely reflects the better response to NAC and higher pCR rates observed in this group. It is also consistent with information obtained from NAC clinical trials and patient-level data which show that response to NAC and residual cancer burden (RCB) provide important prognostic information [20–24]. In a pooled analysis which examined event-free survival (EFS) in over 5,000 patients who received NAC from 1994 to 2019, RCB was prognostic within each BC subtype and increased RCB was associated with worse EFS [24]. A similar association between RCB, EFS, and BC subtype was also recently reported for patients treated on the I-SPY2 trial [23]. This information supports the utilization of standardized quantifiable methods such as RCB to evaluate clinical response to NAC when determining appropriate post-neoadjuvant treatment and follow up. In the current analysis, although pCR was not associated with better RFS and OS on multivariable analysis, this is probably due to the small patient numbers, since only 26% of patients had a pCR. The only factor that was found to be significant on multivariable analysis for both RFS and OS was TNBC and is likely a reflection of the biology of the disease.
The unique value of our study is the longer follow-up time, median 67.5 months for the entire cohort and 74.6 months for the SLNB alone group, when compared to previous studies. This provides information on durable regional disease control with SLNB alone, particularly since most axillary recurrences are seen within the first 5 years. [2, 25] However, this study has limitations given that it is a single institution retrospective analysis with small overall patient numbers and particularly a small number of patients in the SLNB alone group. Nevertheless, the patients who were included in the analysis came from both a large private academic institution and an affiliated safety-net hospital providing socioeconomic and racial/ethnic diversity and resulting in a more representative patient sample which may be more generalizable to other healthcare systems. In addition, although guidelines for consideration for SLNB after NAC have been established at our institution, use of SLNB after NAC was based on both imaging response to NAC and individual surgeon assessment of axillary tumor burden and response to NAC likely resulting in some differences in patient selection for SLNB and omission of ALND. Therefore, additional long-term follow-up from clinical trials or multi-institutional analyses will be necessary to provide further support for the safety of omission of ALND in these patients.
Conclusions
Our study shows that SLNB alone and adjuvant RT provide excellent long-term regional disease control in BC patients with cN1 disease with node negative disease after NAC. This provides additional support for the oncologic safety of axillary downstaging with NAC and de-escalation of axillary surgery. Additionally, in select patients with a positive SLNB after NAC durable regional disease control may be feasible with RT alone. However, given the small number of patients in our study who met these criteria, we await the results of Alliance A011202 and analysis of a larger patient population to confirm the safety of omission of ALND and use of RT alone in this group of patients. As we continue to de-escalate therapy in patients with breast cancer, including both axillary surgery and RNI, and individualize therapy based on breast cancer subtype and response to NAC, close follow-up of patients will be necessary to ensure that these treatment decisions due not have a negative impact on long-term breast cancer outcomes.
Acknowledgements
Not applicable.
Abbreviations
- SLNB
Sentinel Lymph Node Biopsy
- NAC
Neoadjuvant Chemotherapy
- BC
Breast Cancer
- ALND
Axillary Lymph Node Dissection
- RFS
Recurrence Free Survival
- OS
Overall Survival
- RT
Radiation
- ESBC
Early-Stage Breast Cancer
- ER
Estrogen Receptor
- HER2
Human Epidermal Growth Factor Receptor 2
- pCR
Pathologic Complete Response
- FNR
False Negative Rate
- cTN
Clinical Tumor and Nodal Category
- pT
Pathologic Tumor Category
- pN
Pathologic Nodal Category
- SLN
Sentinel Lymph Node
- cCR
Clinical Complete Response
- cPR
Clinical Partial Response
- RNI
Regional Nodal Irradiation
- TNBC
Triple Negative Breast Cancer
- RCB
Residual Cancer Burden
Author contributions
C.C.S: Data Collection, Analysis of Data, Main Manuscript/Figures Preparation and ReviewA.E.H: Analysis and Interpretation of Data, Main Manuscript/Figures Preparation and ReviewK.R.: Data Collection, Interpretation of Data, Manuscript ReviewK.K.: Data Collection, Manuscript ReviewG.G.H: Data Collection, Manuscript ReviewM.M.: Interpretation of Data, Manuscript ReviewE.A.: Interpretation of Data, Manuscript ReviewN.G.: Data Collection, Analysis and Interpretation of Data, Manuscript ReviewJ.C.: Interpretation of Data, Manuscript ReviewS.B.K: Manuscript Concept, Data Collection, Analysis and Interpretation of Data, Main Manuscript/Figures Preparation and Review.All authors read and approved the final manuscript.
Funding
Research Funding, Ruth L. Kirschstein Institutional National Research Service Award, T32CA211034; Financial support for Dr. Alexandra Hernandez, research resident. None of the research funding, honoraria, or consulting fees for Dr. Kristin Rojas and Dr. Neha Goel directly or indirectly supported this analysis and manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the University of Miami Institutional Review Board as part of a protocol in the Division of Surgical Oncology assessing quality and outcomes in cancer patients treated with surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33. 10.1016/S1470-2045(10)70207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of Axillary Dissection vs no Axillary dissection on 10-Year overall survival among women with invasive breast Cancer and Sentinel Node Metastasis: the ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918–26. 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7. 10.1200/JCO.2012.45.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martelli G, Miceli R, Daidone MG, Vetrella G, Cerrotta AM, Piromalli D, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18(1):125–33. 10.1245/s10434-010-1217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–63. 10.1200/JCO.2006.07.4062 [DOI] [PubMed] [Google Scholar]
- 6.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61. 10.1001/jama.2013.278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18. 10.1016/S1470-2045(13)70166-9 [DOI] [PubMed] [Google Scholar]
- 8.Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64. 10.1200/JCO.2014.55.7827 [DOI] [PubMed] [Google Scholar]
- 9.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast Cancer using selective evaluation of clipped nodes: implementation of targeted Axillary Dissection. J Clin Oncol. 2016;34(10):1072–8. 10.1200/JCO.2015.64.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TT, Hoskin TL, Day CN, Degnim AC, Jakub JW, Hieken TJ, et al. Decreasing Use of Axillary dissection in node-positive breast Cancer patients treated with Neoadjuvant Chemotherapy. Ann Surg Oncol. 2018;25(9):2596–602. 10.1245/s10434-018-6637-9 [DOI] [PubMed] [Google Scholar]
- 11.Srour MK, Tseng J, Luu M, Alban RF, Giuliano AE, Chung A. Patterns in the Use of Axillary Operations for patients with node-positive breast Cancer after Neoadjuvant Chemotherapy: a National Cancer Database (NCDB) analysis. Ann Surg Oncol. 2019;26(10):3305–11. 10.1245/s10434-019-07540-3 [DOI] [PubMed] [Google Scholar]
- 12.Ling DC, Iarrobino NA, Champ CE, Soran A, Beriwal S. Regional Recurrence Rates with or without complete axillary dissection for breast Cancer patients with node-positive disease on Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy. Adv Radiat Oncol. 2020;5(2):163–70. 10.1016/j.adro.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong SM, Basik M, Florianova L, Margolese R, Dumitra S, Muanza T, et al. Oncologic safety of Sentinel Lymph Node Biopsy alone after neoadjuvant chemotherapy for breast Cancer. Ann Surg Oncol. 2021;28(5):2621–9. 10.1245/s10434-020-09211-0 [DOI] [PubMed] [Google Scholar]
- 14.Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z, et al. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: an up-to-date meta-analysis of 3,578 patients. Breast. 2021;59:256–69. 10.1016/j.breast.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piltin MA, Hoskin TL, Day CN, Davis J Jr., Boughey JC. Oncologic outcomes of Sentinel Lymph node surgery after neoadjuvant chemotherapy for node-positive breast Cancer. Ann Surg Oncol. 2020;27(12):4795–801. 10.1245/s10434-020-08900-0 [DOI] [PubMed] [Google Scholar]
- 16.Boughey JC, Yu H, Dugan CL, Piltin MA, Postlewait L, Son JD, et al. Changes in Surgical Management of the Axilla over 11 years - report on more than 1500 breast Cancer patients treated with Neoadjuvant Chemotherapy on the prospective I-SPY2 trial. Ann Surg Oncol. 2023;30(11):6401–10. 10.1245/s10434-023-13759-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrio AV, Montagna G, Mamtani A, Sevilimedu V, Edelweiss M, Capko D, et al. Nodal recurrence in patients with node-positive breast Cancer treated with Sentinel Node Biopsy alone after Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol. 2021;7(12):1851–5. 10.1001/jamaoncol.2021.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuemmel S, Heil J, Bruzas S, Breit E, Schindowski D, Harrach H, et al. Safety of targeted Axillary Dissection after Neoadjuvant Therapy in patients with node-positive breast Cancer. JAMA Surg. 2023;158(8):807–15. 10.1001/jamasurg.2023.1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamounas EP et al. Loco-Regional Irradiation in Patients with Biopsy-proven Axillary Node Involvement at Presentation Who Become Pathologically Node-negative After Neoadjuvant Chemotherapy: Primary Outcomes of NRG Oncology/NSABP B-51/RTOG 1304 (GS02-07). San Antonio Breast Cancer Symposium; San Antonio, TX2023.
- 20.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Lv L, Gao H, Cheng M. Pathologic Complete Response and its impact on breast Cancer Recurrence and Patient’s survival after Neoadjuvant Therapy: a Comprehensive Meta-Analysis. Comput Math Methods Med. 2021;2021:7545091. 10.1155/2021/7545091 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-term prognostic risk after Neoadjuvant Chemotherapy Associated with residual Cancer burden and breast Cancer subtype. J Clin Oncol. 2017;35(10):1049–60. 10.1200/JCO.2015.63.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Symmans WF, Yau C, Chen YY, Balassanian R, Klein ME, Pusztai L, et al. Assessment of residual Cancer Burden and Event-Free Survival in Neoadjuvant Treatment for High-risk breast Cancer: an analysis of Data from the I-SPY2 Randomized Clinical Trial. JAMA Oncol. 2021;7(11):1654–63. 10.1001/jamaoncol.2021.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yau C, Osdoit M, van der Noordaa M, Shad S, Wei J, de Croze D, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–60. 10.1016/S1470-2045(21)00589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Maaren MC, de Munck L, Strobbe LJA, Sonke GS, Westenend PJ, Smidt ML, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int J Cancer. 2019;144(2):263–72. 10.1002/ijc.31914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.