ABSTRACT
Q fever/coxiellosis poses a significant threat to both human and animal health, with goats serving as important reservoirs for disease transmission. This study aimed to evaluate the prevalence of coxiellosis and identify associated risk factors within meat goat herds in northeastern Thailand. A total of 39 meat goat herds were examined, with 84.61% of these herds experiencing reproductive disorders suggestive of Coxiella burnetii infection. Serum samples (n = 513) and vaginal swabs (n = 334) were collected from 522 goats for serological and molecular analyses, respectively. Results unveiled an overall herd prevalence of 74.35% (29/39), with a within-herd prevalence of 15.49% (95% CI: 10.86–20.12). Univariate analysis indicated that knowledge about the transmission of coxiellosis in herd owners serves as a protective factor against C. burnetii infection at the herd level (OR: 0.10; 95% CI: 0.01–0.92; p = 0.04). Multivariable analysis identified two significant risk factors associated with C. burnetii infection at the herd level, including herd establishment exceeding 5 years (OR: 7.14; 95% CI: 1.05–48.4; p = 0.04), as well as reproductive failures including abortion, infertility, and weak offspring (OR: 17.65; 95% CI: 1.76–177.45; p = 0.01). Individual-level risk factors included female gender (OR: 8.42; 95% CI: 1.14–62.42; p = 0.03), crossbreeding (OR: 2.52; 95% CI: 1.32–4.82; p = 0.005), and clinical signs of anaemia (OR: 1.63; 95% CI: 1.01–2.64; p = 0.04). These findings underscore the widespread prevalence of Q fever in meat goat herds within the study area and emphasize the necessity of implementing targeted control strategies.
KEYWORDS: Coxiellosis, Q fever, risk factors, meat goat, Northeastern, Thailand, Seroprevalence
1. Introduction
Q fever/coxiellosis, caused by the bacterium Coxiella burnetii, is recognized as a significant zoonotic disease with global distribution, posing substantial challenges to both the veterinary and public health sectors. The disease is found on every continent, including Australia, Europe, America, Africa, and Asia [1–6]. Livestock species, particularly sheep and goats, are known as important reservoirs for C. burnetii, facilitating the transmission of the pathogen to humans through various routes, including direct contact, aerosols, and consumption of contaminated products [7]. The clinical manifestations of Q fever in humans can range from asymptomatic infection to severe illness, with symptoms including fever, headache, muscle aches, fatigue, and respiratory or gastrointestinal complications. Chronic Q fever, characterized by persistent infection affecting various organs, can also occur, leading to long-term health implications [7]. The clinical signs of coxiellosis in livestock can vary depending on the host species. Common clinical signs observed in animals infected with C. burnetii include abortion, reproductive disorders such as repeat breeding, infertility, stillbirths, weak-born kids, and mastitis [8,9]. It is important to note that clinical signs of coxiellosis in animals can be subtle or absent, particularly in chronically infected animals.
Human Q fever outbreaks related to spill-over infections from goats to humans have been documented, and the importance of meat goats in Q fever epidemiology is well recognized [10]. Given the significance of the goat farming industry in northeastern Thailand, understanding the impact and epidemiology of C. burnetii is crucial. Nevertheless, studies on the prevalence of coxiellosis/Q fever and risk factors in this area are lacking. Thus, this study addresses the scarcity of research by determining the prevalence and identifying associated risk factors for C. burnetii infection in meat goat herds in northeastern Thailand. Recognizing the importance of understanding C. burnetii dynamics for effective control, this study employs both serological and molecular techniques to investigate prevalence and identify associated risks at both the herd-level and individual-level factors in order to provide comprehensive insights into C. burnetii epidemiology in the region.
2. Materials and methods
2.1. Ethical statement and study area:
The research procedures detailed in this study were subjected to rigorous ethical scrutiny and approval by the Institutional Animal Care and Use Committee (IACUC) of Khon Kaen University under record number IACUC-KKU-64/66, granted on 19 June 2023, for the use of animals, and by the Center for Ethics in Human Research, with record number HE672063, approved on 3 April 2024, for research involving human subjects.
Stringent measures were implemented to safeguard the privacy and confidentiality of all participants involved. Specifically, all cases were anonymized and aggregated at the village level to prevent the disclosure of personal identifiers. Additionally, the maps utilized in this paper were deliberately designed to conceal respondents’ precise addresses, further safeguarding their anonymity.
A cross-sectional study was conducted from August 2023 to February 2024. Chaiyaphum and Khon Kaen provinces are areas with high densities of meat goat herds in the Northeast of Thailand [11]. In total, 39 meat goat herds from both provinces were investigated. In Chaiyaphum province, investigations were conducted in 3 districts including Ban Thaen (4), Kaset Sombun (2), and Noen Sa-nga (2). In Khon Kaen province, investigations covered 10 districts including Ban Haet (2), Chum Phae (2), Khao Suan Kwang (2), Mancha Khiri (2), Muang Khon Kaen (7), Nam Phong (3), Nong Ruea (2), Phra Yuen (1), Phu Wiang (4), and Si Chomphu (6).
2.2. Animal and sampling procedures
The estimated meat goat populations in Chaiyaphum and Khon Kaen are 64,936 goats [11]. Considering the unknown prevalence of C. burnetii infection within these populations, sample size determination was conducted utilizing an expected frequency of 50%, an acceptable margin of error of 5%, a design effect of 1.0, and a single cluster. Consequently, the calculated sample size required for this study was 382 samples, as determined using EPI INFOTM for Windows version 7.2.5.0.
This study employed a combination of convenient and random sampling methods, as it included only those farmers who voluntarily participated in the research. The smallholder meat goat herds are operated as communal entities. The sampling strategy adhered to the following criteria:
Farms with fewer than 30 mature goats: all animals were sampled.
Farms with 30 to 60 mature goats: a maximum of 35 animals were sampled.
Farms with 60 to 90 mature goats: a maximum of 45 animals were sampled.
Samples were collected from 38 herds with fewer than 30 mature goats, and 1 herd met the criteria for category C. In total, 513 serum samples and 334 vaginal swab samples were collected from 522 goats. Both serum and vaginal swabs samples were collected from 325 does within the first three months postpartum or non-pregnant status. Only serum samples were collected from 40 bucks and 148 pregnant goats, while only vaginal swab samples were collected from 9 does within the first three months postpartum. Serum or vaginal swab samples were collected from the animals with the voluntary consent of the goat owners.
All 513 blood samples were systematically collected from mature goats aged over 6 months to ensure a representative sample. Utilizing jugular puncture, approximately 5 mL of whole blood was obtained under aseptic conditions and carefully transferred into Vacutainer® red tubes. To maintain sample integrity, all blood samples were promptly placed on ice and transported to the laboratory at the Faculty of Veterinary Medicine, Khon Kaen University, within 6 hours of collection. Upon arrival, the samples underwent centrifugation at 2,500 rpm for 10 minutes to separate serum from cellular components. The resulting serum was then stored at −20°C until further analysis.
All 334 vaginal swab samples were systematically collected from does utilizing Puritan 6” sterile Rayon Tipped Applicator dry swabs under aseptic conditions. Swabs were carefully dipped into 1 mL phosphate-buffered saline (PBS) solution (0.01 M, pH 7.4) to maintain sample hydration and integrity. Post-collection, swabs were securely stored in sterile conical plastic tubes to minimize the risk of contamination during transport. Similar to blood samples, all vaginal swab specimens were promptly transported on ice to the designated laboratory at the Faculty of Veterinary Medicine, Khon Kaen University, within 6 hours. In the laboratory, stringent biosafety protocols, consistent with biosafety level 2+ standards, were followed during sample processing. Swabs underwent thorough mixing using a vortex mixer for 15 seconds to ensure homogeneity. Samples with sediment were subjected to centrifugation at 3,000 rpm for 5 minutes to facilitate sedimentation and subsequent collection of approximately 1 mL of the supernatant. The collected supernatant, containing the essential genetic material, was carefully extracted for DNA analysis, a crucial step in revealing insights into the microbial ecology and genetic composition of the sampled population.
2.3. Serological test
IgG antibodies specific to Phase I and Phase II antigens of C. burnetii were identified using the indirect enzyme-linked immunosorbent assay (ELISA) IDEXX Q fever Ab test, sourced from IDEXX Switzerland AG, located at Stationsstrasse 12, CH-3097 Liebefeld-Bern, Switzerland. The experimental procedures strictly adhered to the manufacturer’s instructions to ensure methodological consistency and reliability. Optical densities (OD) of both the samples and controls were measured at 450 nm using a spectrophotometer, a standard practice in immunological assays for quantification purposes. The calculation of the sample-to-positive percentage (S/P%) was conducted based on the measured OD values, utilizing a predetermined equation. This systematic approach facilitates the accurate determination of IgG antibody levels against C. burnetii, contributing to the scientific understanding of host immune responses to this pathogen.
The results were categorized based on manufacturer instructions: S/P% > 40 as positive, 30 < S/P% < 40 as suspect (if a test sample was marked as suspect, its result was considered negative), and S/P% < 30 as negative. According to the IDEXX validation report, the test demonstrated 100% sensitivity and specificity, indicating its high accuracy in identifying positive and negative cases accurately [12].
2.4. DNA extraction
DNA extraction from vaginal swab samples was performed using the DNeasy Blood and Tissue Kit (Qiagen), following the manufacturer’s instructions. In summary, 200 µL of the sample was utilized, and 20 µL of proteinase K was added to initiate lysis in buffer AL. Subsequent washing steps with buffer AW1 and AW2 were conducted to remove impurities. The purified DNA was then eluted in buffer AE, and the resulting DNA extracts were stored at −20°C for further analysis.
2.5. Real-time PCR amplification
The detection of C. burnetii DNA in vaginal swab samples was conducted using a real-time PCR assay targeting the insertion sequence IS1111, as outlined by Mediannikov et al. [13]. Each PCR reaction consisted of 0.4 µM of forward primer (5´CAAGAAAGTATCGCTGTGGC3´), 0.4 µM of reverse primer (5´CACAGAGCCACCGTATGAATC3´), and 0.2 µM of TaqMan probe (FAM-CCGAGTTCGAAACAATGAGGCTG-BHQ-1). Additionally, 10 µL of 2× QuantiNova Probe PCR Master Mix (Qiagen), 3 µL of DNA template, and DNase-RNase free water were added to reach a final volume of 20 µL. Real-time PCR was conducted using a Bio-rad instrument, starting with an initial denaturation step at 95°C for 2 minutes, followed by 45 cycles of denaturation at 95°C for 5 seconds, and annealing at 61°C for 30 seconds. Positive controls containing plasmid DNA with the targeted C. burnetii sequence were included, while negative controls with no template DNA were included in each PCR run for quality assurance.
2.6. Questionnaires
Thirty-nine herd owners were interviewed to assess their management practices concerning risk factors associated with C. burnetii infection within their herds. These risk factors encompassed various aspects of herd management, including herd structure, health management practices, and reproductive history. Specifically, factors such as the history of bucks used in the herd, occurrences of reproductive disorders such as abortion, infertility, orchitis, and weak offspring, were investigated. Additionally, management practices related to parturition, pasture, movement and quarantine, manure, and pest control were examined.
Furthermore, the interviews delved into the farmers’ understanding of C. burnetii transmission dynamics, their self-reported symptoms possibly indicating C. burnetii infection, and their adherence to hygiene practices during animal handling, barn sanitation, and parturition assistance. Furthermore, detailed records of animal signalment and clinical signs were obtained, including gender, breed, age, Body Condition Score (BCS), presence of anaemia, and any signs of nasal or ocular discharge.
2.7. Data analysis
Statistical analysis was conducted using IBM SPSS Statistics version 28.0 (IBM Corp: Armonk, NY) and MedCalc® version 22.021 (MedCalc Software Ltd., 2024). A univariate analysis of risk factors was carried out to identify variables associated with positive herds and individual animals, with significance set at 0.05. Variables demonstrating a significance level of p < 0.1 in the univariate analysis were selected to construct a multivariable logistic regression model. Subsequently, a forward stepwise method (with criteria of 0.05 for entry and 0.10 for removal) was used to get the final logistic model. The iterative process continued until identifying the final model with the lowest −2 Log likelihood. Odds ratios with corresponding 95% confidence intervals were computed to assess the strength of associations. Additionally, Kappa agreement test was performed to evaluate the concordance between the ELISA test and PCR assay results, providing insights into the diagnostic agreement between the two methods.
The utilization of Geographic Information Systems (GIS) for epidemiological analysis, particularly through the implementation of the QGIS, an open-source software platform that provides users with powerful tools for creating, editing, visualizing, analysing, and publishing geospatial data. version (3.36.0), represents a critical advancement in public health research, facilitating the spatial visualization, analysis, and interpretation of epidemiological data to uncover patterns, trends, and associations between health outcomes and geographical factors.
3. Results
3.1. Seroprevalence and molecular prevalence of C. burnetii in meat goat herds
This investigation revealed that 33 out of 39 herds (84.61%) had experienced reproductive disorders, such as abortion, infertility, orchitis, and weak kids, at least once. Interestingly, out of the 33 herds with a history of reproductive disorders, 25 (75.75%) were found to be positive for coxiellosis based on the presence of IgG antibodies specific to C. burnetii antigens and/or DNA. Furthermore, 4 out of 6 herds without a history of reproductive disorders (66.67%) also showed seropositivity and/or molecular positivity to C. burnetii . The overall prevalence (positive in serological and/or molecular tests) reached 74.35% (29 out of 39) of the herds tested. The seroprevalence and molecular detection revealed exposure or infection within herds varied from 4.55% to 57.14%, with an average of 15.49% (95% CI, 10.86–20.12) (shown in Table 1).
Table 1.
Seroprevalence, molecular prevalence and Intra-herd prevalence of coxiellosis among meat goat herds in Northeast Thailand.
| no. | Sero-prevalence % (Numbers of seropositive/total sample) | Molecular-prevalence % (Numbers of PCR-positive/total sample) | Intra-herd prevalence % (Numbers of seropositive and molecularly positive animals/total animals) |
|---|---|---|---|
| 1 | 0.0 (0/10) | 0.0 (0/4) | 0.0 (0/10) |
| 2 | 0.0 (0/2) | 0.0 (0/1) | 0.0 (0/2) |
| 3 | 0.0 (0/6) | 0.0 (0/5) | 0.0 (0/6) |
| 4 | 0.0 (0/7) | 0.0 (0/7) | 0.0 (0/7) |
| 5 | 0.0 (0/4) | 0.0 (0/2) | 0.0 (0/4) |
| 6 | 0.0 (0/14) | 0.0 (0/12) | 0.0 (0/14) |
| 7 | 0.0 (0/8) | 0.0 (0/7) | 0.0 (0/8) |
| 8 | 0.0 (0/4) | 0.0 (0/1) | 0.0 (0/4) |
| 9 | 0.0 (0/13) | 0.0 (0/12) | 0.0 (0/13) |
| 10 | 0.0 (0/20) | 0.0 (0/2) | 0.0 (0/20) |
| 11 | 4.55 (1/22) | 20.00 (1/5) | 4.55 (1/22) |
| 12 | 0.0 (0/22) | 5.0 (1/20) | 4.55 (1/22) |
| 13 | 0.0 (0/44) | 7.69 (3/39) | 6.82 (3/44) |
| 14 | 0.0 (0/13) | 8.33 (1/12) | 7.69 (1/13) |
| 15 | 0.0 (0/10) | 11.11 (1/9) | 10.0 (1/10) |
| 16 | 11.11 (1/9) | Not done | 11.11 (1/9) |
| 17 | 0.0 (0/9) | 11.11 (1/9) | 11.11 (1/9) |
| 18 | 0.0 (0/9) | 14.29 (1/7) | 11.11 (1/9) |
| 19 | 0.0 (0/17) | 11.76 (2/17) | 11.76 (2/17) |
| 20 | 12.5 (1/8) | 0.0 (0/7) | 12.50 (1/8) |
| 21 | 7.14 (2/28) | 50.0 (2/4) | 14.29 (4/28) |
| 22 | 10.00 (2/20) | 18.18 (2/11) | 15.38 (4/26) |
| 23 | 16.67 (1/6) | 0.0 (0/4) | 16.67 (1/6) |
| 24 | 0.0 (0/6) | 20.00 (1/5) | 16.67 (1/6) |
| 25 | 8.33 (1/12) | 100 (1/1) | 16.67 (2/12) |
| 26 | 0.0 (0/11) | 20.00 (2/10) | 18.18 (2/11) |
| 27 | 18.75 (3/16) | 0.0 (0/7) | 18.75 (3/16) |
| 28 | 7.14 (1/14) | 23.08 (3/13) | 21.43 (3/14) |
| 29 | 0.0 (0/13) | 42.86 (3/7) | 23.08 (3/13) |
| 30 | 25.00 (2/8) | 0.0 (0/1) | 25.00 (2/8) |
| 31 | 0.0 (0/11) | 30.00 (3/10) | 27.27 (3/11) |
| 32 | 0.0 (0/7) | 28.57 (2/7) | 28.57 (2/7) |
| 33 | 25.00 (6/24) | 4.35 (1/23) | 29.17 (7/24) |
| 34 | 0.0 (0/16) | 33.33 (5/15) | 31.25 (5/16) |
| 35 | 0.0 (0/9) | 37.50 (3/8) | 33.33 (3/9) |
| 36 | 27.27 (3/11) | 14.29 (1/7) | 36.36 (4/11) |
| 37 | 23.08 (3/13) | 50.00 (3/6) | 38.46 (5/13) |
| 38 | 20.00 (6/30) | 90.91 (10/11) | 45.45 (15/33) |
| 39 | 0.0 (0/7) | 66.67 (4/6) | 57.14 (4/7) |
| Total | 0–27.27 (33/513) | 0–100 (57/334) | 0–57.14 (86/522) |
The herd-level seroprevalence was determined to be 35.89% (14 out of 39), while molecular positivity was observed in 24 herds (61.5%). It is worth noting that 15 herds exhibited shedding of the bacterium via vaginal secretions despite testing negative for antibody detection. Conversely, 4 herds identified as exposed to C. burnetii based on seropositivity were negative for molecular detection. The kappa agreement between the serology test and molecular detection was found to be 0.044 (p > 0.05).
3.2. Spatial distribution of seropositive and/or molecularly positive meat goat herds
Figures 1 and 2 depict the spatial distribution of meat goat herds that are seropositive and/or molecularly positive for C. burnetii in the Chaiyaphum and Khon Kaen provinces, respectively. These visual representations illustrate the prevalence of coxiellosis (positive in serological and/or molecular tests) across both provinces and within the individual districts examined. The research findings reveal varying prevalence rates across the study region, highlighting spatial diversity in the distribution of Q fever.
Figure 1.
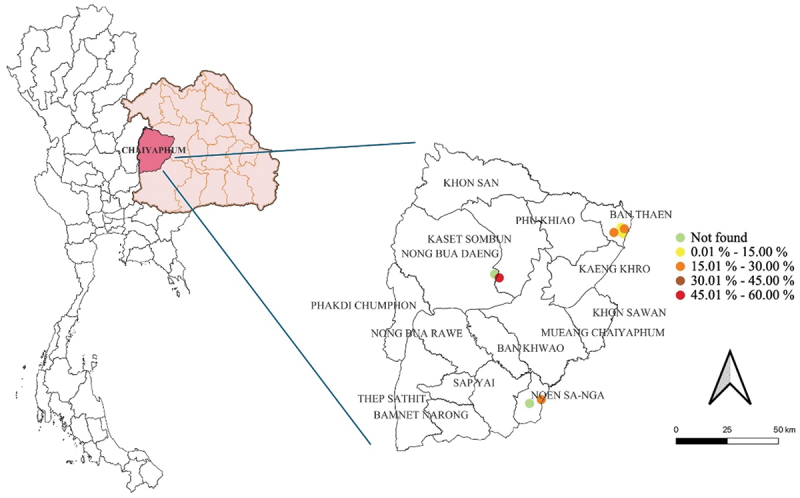
The spatial distribution of C. burnetii seropositive and/or molecularly positive meat goat herds in Chaiyaphum province, Northeast Thailand.
Figure 2.
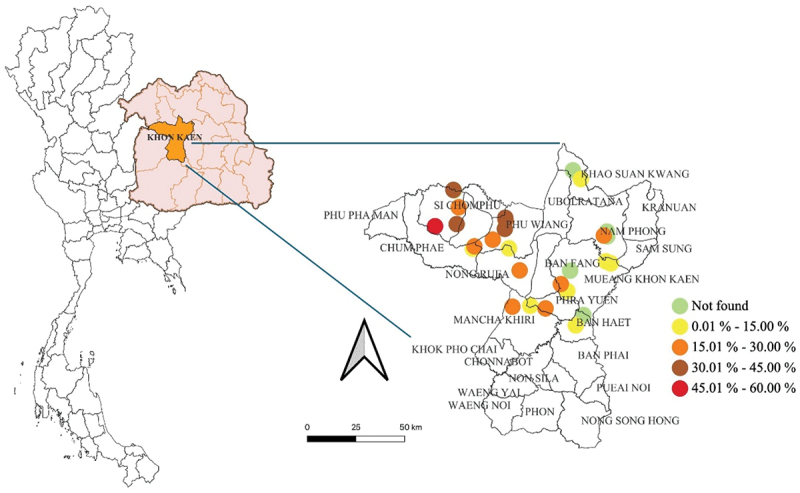
The spatial distribution of C. burnetii seropositive and/or molecularly positive meat goat herds in Khon Kaen province, Northeast Thailand.
3.3. Risk factors associated with coxiellosis in meat goat herds
The analysis investigated the correlation between coxiellosis, indicated by seropositivity against C. burnetii and/or a positive PCR assay, and various independent variables at both the herd and individual animal levels. These variables included herd establishment duration, size, pasture management, reproductive history, buck circulation patterns, parturition management practices, quarantine measures, presence of vectors, manure handling, clinical signs resembling Q fever symptoms, and knowledge of Q fever transmission.
The univariate analysis conducted at the herd level identified several significant factors associated with coxiellosis in meat goat herds (p < 0.05). Notably, herds established for over 5 years showed a higher prevalence of coxiellosis compared to recently established herds (OR: 5.66; 95%CI: 1.01–31.54; p = 0.04). Moreover, herds experiencing multiple instances of reproductive failures, including abortion, infertility, or weak-born kids, exhibited a significantly higher rate of coxiellosis (OR: 14.72; 95%CI: 1.63–132.64; p = 0.01). Additionally, herds where bucks circulated between herds (OR: 6.25; 95%CI: 1.22–31.83; p = 0.02) or were sold to other herds, demonstrated a higher prevalence of coxiellosis (OR: 6.25; 95%CI: 1.22–31.83; p = 0.02). Interestingly, herds whose owners had knowledge of direct contact transmission exhibited a lower infection rate. (OR: 0.10; 95%CI: 0.01–0.92; p = 0.04). All risk factors associated with coxiellosis in meat goat herds are comprehensively presented in Table 2.
Table 2.
Univariate analysis examining herd-level risk factors associated with coxiellosis among meat goat herds in Northeast Thailand.
| Risk factor | Category | Total (%) | Prev. (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Herd established (year) | <5 | 20 (51.3) | 12/39 (30.8) | |||
| ≥5 | 19 (48.7) | 17/39 (43.6) | 5.66 | 1.01–31.54 | 0.048 | |
| Herd size | <12 | 23 (59.0) | 16/39 (41.0) | |||
| ≥13 | 16 (41.0) | 13/39 (33.3) | 1.89 | 0.40–8.82 | 0.415 | |
| Pasture | only meat goat | 21 (53.8) | 16/39 (41.0) | |||
| mixed with other species | 18 (46.2) | 13/39 (33.3) | 0.81 | 0.19–3.42 | 0.777 | |
| Presence of other livestock | No | 20 (51.3) | 16/39 (41.0) | |||
| Yes | 19 (48.7) | 13/39 (33.3) | 0.54 | 0.12–2.33 | 0.411 | |
| Abortion history in the herd | No | 17 (43.6) | 12/39 (30.8) | |||
| Yes | 22 (56.4) | 17/39 (43.6) | 1.42 | 0.33–5.99 | 0.636 | |
| Infertility history in the herd | No | 28 (71.8) | 18/39 (46.2) | |||
| Yes | 11 (28.2) | 11/39 (28.2) | 13.05 | 0.69–244.71 | 0.059 | |
| Orchitis history in the herd | No | 35 (89.7) | 26/39 (66.7) | |||
| Yes | 4 (10.3) | 3/39 (7.7) | 1.03 | 0.09–11.29 | 0.975 | |
| Weak kids’ history in the herd | No | 17 (43.6) | 11/39 (28.2) | |||
| Yes | 20 (56.4) | 18/39 (46.2) | 2.45 | 0.56–10.68 | 0.231 | |
| Abortion or infertility or weak kids’ history in the herd | at least 2 occasional | 19 (48.7) | 18/39 (46.2) | 14.72 | 1.63–132.64 | 0.016 |
| One occasional | 20 (51.3) | 11/39 (28.2) | ||||
| Signs of fever, sweats, or muscle aches like Q fever in human | No | 33 (84.6) | 25/39 (64.1) | |||
| Yes | 6 (15.4) | 4/39 (10.3) | 0.64 | 0.09–4.17 | 0.64 | |
| Knowledge of direct contact transmission | not know | 23 (59.0) | 14/39 (35.9) | |||
| know | 16 (41.0) | 15/39 (38.5) | 0.1 | 0.01–0.92 | 0.043 | |
| Knowledge of ingestion transmission | not know | 36 (92.3) | 26/39 (66.7) | |||
| know | 3 (7.7) | 3/39 (7.7) | 0.36 | 0.01–7.59 | 0.511 | |
| Knowledge of inhalation transmission | not know | 37 (94.9) | 27/39 (69.2) | |||
| know | 2 (5.1) | 2/39 (5.1) | 0.52 | 0.02–11.84 | 0.684 | |
| Buck circulation between herds | not use | 13 (33.3) | 4/39 (10.3) | |||
| use | 26 (66.7) | 25/39 (64.1) | 6.25 | 1.22–31.83 | 0.027 | |
| Duration of Buck in a farm (year) | <3 | 29 (74.4) | 23/39 (59.0) | |||
| ≥3 | 9 (23.1) | 6/39 (15.4) | 0.52 | 0.10–2.72 | 0.44 | |
| Culling of used buck | To slaughterhouse | 9 (23.1) | 4/39 (10.3) | |||
| To another farm to use | 30 (76.9) | 25/39 (64.1) | 6.25 | 1.22–31.83 | 0.027 | |
| Separate parturition area | Yes | 11 (28.2) | 10/39 (25.6) | |||
| No | 28 (71.8) | 19/39 (48.7) | 4.75 | 0.88–25.35 | 0.068 | |
| Mother rearing system | Yes | 39 (100.0) | 29/39 (74.4) | 2.8 | 0.05–150.77 | 0.611 |
| No | 0 (0.0) | 0/39 (0.0) | ||||
| Q fever testing before movement | Yes | 0 (0.0) | 0/39 (0.0) | |||
| No | 39 (100.0) | 29/39 (74.4) | 2.8 | 0.05–150.77 | 0.611 | |
| Introduced a new animal in herds within 6 months | Yes | 25 (64.1) | 20/39 (51.3) | 2.22 | 0.51–9.64 | 0.286 |
| No | 14 (35.9) | 9/39 (23.1) | ||||
| Quarantine a new animal for at least 30 days | Yes | 5 (12.8) | 4/39 (10.3) | |||
| No | 34 (87.2) | 25/39 (64.1) | 0.69 | 0.06–7.06 | 0.758 | |
| Good Farm Management Practice | Approved | 11 (28.2) | 10/39 (25.6) | |||
| Unapproved | 28 (71.8) | 19/39 (48.7) | 0.21 | 0.02–1.91 | 0.166 | |
| Wear gloves during assistance doe when giving birth | Yes | 11 (28.2) | 7/39 (17.9) | |||
| No | 28 (71.8) | 22/39 (56.4) | 2.09 | 0.45–9.62 | 0.341 | |
| Wear a mask during assistance doe when giving birth | Yes | 2 (5.1) | 2/39 (5.1) | |||
| No | 37 (94.9) | 27/39 (69.2) | 0.52 | 0.02–11.84 | 0.688 | |
| Presence of tick | Yes | 4 (10.3) | 4/39 (10.3) | 0.69 | 0.01–41.78 | 0.86 |
| No | 6 (15.4) | 6/39 (15.4) | ||||
| Not know | 29 (74.4) | 19/39 (48.7) | ||||
| Presence of rodent | Yes | 27 (69.2) | 21/39 (53.8) | 1.75 | 0.38–7.87 | 0.466 |
| No | 12 (30.8) | 8/39 (20.5) | ||||
| Presence of dogs or cats | Yes | 32 (82.1) | 22/39 (56.4) | 0.14 | 0.007–2.74 | 0.196 |
| No | 7 (17.9) | 7/39 (17.9) | ||||
| Manure uses | Fertilizer | 35 (89.7) | 26/39 (66.7) | 0.96 | 0.08–10.47 | 0.975 |
| Not used | 4 (10.3) | 3/39 (7.7) |
Prevalence (Prev.), odds ratio (OR), and 95% confidence interval (95%CI) of the variable from 29 positive herds (74.35%) based on a total sample of 39 herds.
The multivariable analysis conducted at the herd level revealed two significant factors associated with coxiellosis in meat goat herds (p < 0.05). Particularly noteworthy, herds established for over 5 years exhibited a higher prevalence of coxiellosis compared to recently established ones (OR: 7.14; 95%CI: 1.05–48.43; p = 0.044). Additionally, herds experiencing multiple instances of reproductive failures, such as abortion, infertility, or weak-born kids, showed a significantly elevated rate of coxiellosis (OR: 17.65; 95%CI: 1.76–177.45; p = 0.015). Table 3 provides a comprehensive overview of all risk factors associated with coxiellosis in meat goat herds.
Table 3.
Multivariable analysis of herd-level risk factors associated with coxiellosis among meat goat herds in Northeast Thailand.
| Risk factor | Category | OR | 95% CI | p-value |
|---|---|---|---|---|
| Herd established (year) | <5 | |||
| ≥5 | 7.14 | 1.052–48.43 | 0.044 | |
| Abortion or infertility or weak kids’ history | at least 2 occasional | 17.65 | 1.76–177.45 | 0.015 |
| One occasional |
Factors such as a history of infertility in the herd, Buck circulation between herds, Culling of used buck, and the presence of a separate parturition area were excluded from the final multivariable regression model (p > 0.05).
Regarding individual factors associated with coxiellosis, notable differences were observed in gender, breed, presence of anaemia, and nasal discharge (p < 0.05). The univariate analysis revealed significantly higher odds of coxiellosis in certain categories: females showed a substantially elevated likelihood of infection (OR: 8.35; 95%CI: 1.13–61.62; p = 0.03), along with cross-bred animals (OR: 2.53; 95%CI: 1.33–4.82; p = 0.004), which demonstrated increased risk compared to purebred counterparts. Moreover, animals displaying signs of anaemia (OR: 1.75; 95%CI: 1.09–2.82; p = 0.01) or nasal discharge (OR: 25.83; 95%CI: 1.22–543.10; p = 0.03) were significantly more associated with coxiellosis. All risk factors associated with coxiellosis in individual animals are comprehensively presented in Table 4.
Table 4.
Univariate analysis of individual-level risk factors associated with coxiellosis among meat goat herds in Northeast Thailand.
| Risk Factor | Category | Total (%) | Prev. (%) | OR | 95%CI | p-value |
|---|---|---|---|---|---|---|
| Gender | Female | 482 (92.3) | 85/522 (16.3) | 8.35 | 1.13–61.62 | 0.037 |
| Male | 40 (7.7) | 1/522 (0.2) | ||||
| Breed | Cross-bred | 383 (73.4) | 74/522 (14.2) | 2.53 | 1.33–4.82 | 0.005 |
| Pure-bred | 139 (26.6) | 12/522 (2.3) | ||||
| Age | >1 year | 498 (95.4) | 84/522 (16.1) | 2.23 | 0.51–9.67 | 0.28 |
| ≤1 year | 24 (4.6) | 2/522 (0.4) | ||||
| BCS | Thin – Emaciated | 309 (59.2) | 57/522 (10.9) | 1.44 | 0.88–2.36 | 0.14 |
| Average – Fat | 207 (39.7) | 28/522 (5.4) | ||||
| Anemia sign | Presence | 168 (32.2) | 37/522 (7.1) | 1.76 | 1.09–2.82 | 0.02 |
| Absence | 354 (67.8) | 49/522 (9.4) | ||||
| Nasal discharge | Presence | 2 (0.4) | 2/522 (0.4) | 25.84 | 1.22–543.10 | 0.036 |
| Absence | 514 (98.5) | 83/522 (15.9) |
Prevalence (Prev.), odds ratio (OR), and 95% confidence interval (95%CI) of the variable from 86 positive samples for ELISA test or PCR assay (16.5%) based on a total sample of 522 animals; seropositivity 33/513 (6.4%) and positive PCR assay 57/334 (17.06%).
The multivariable analysis conducted at the individual level unveiled several significant factors associated with coxiellosis in meat goat herds (p < 0.05). Notably, the study identified significantly higher odds of coxiellosis in specific categories: females showed a substantially elevated likelihood of infection (OR: 8.42; 95%CI: 1.14–62.42; p = 0.037), along with cross-bred animals (OR: 2.52; 95%CI: 1.32–4.82; p = 0.005), which demonstrated increased risk compared to purebred counterparts. Moreover, animals displaying signs of anaemia (OR: 1.63; 95%CI: 1.01–2.64; p = 0.046) were significantly associated with coxiellosis. A comprehensive presentation of all risk factors associated with coxiellosis in individual animals is detailed in Table 5.
Table 5.
Multivariable analysis of individual-level risk factors associated with coxiellosis among meat goat herds in Northeast Thailand.
| Risk Factor | Category | OR | 95%CI | p-value |
|---|---|---|---|---|
| Gender | Female | 8.42 | 1.14–62.42 | 0.037 |
| Male | ||||
| Breed | Cross-bred | 2.52 | 1.32–4.82 | 0.005 |
| Pure-bred | ||||
| Anemia sign | Presence | 1.63 | 1.01–2.64 | 0.046 |
| Absence |
The presence of nasal discharge was omitted from the final multivariable regression model due to its limited sample size.
4. Discussion
The findings of this study reveal a significantly high prevalence of coxiellosis (positive in serological and/or molecular tests) among the meat goat herds investigated. A considerable number of herds examined in this study experienced reproductive failures commonly associated with coxiellosis, such as abortion, infertility, and weak offspring. This highlights the potential impact of C. burnetii on goat populations and the consequent economic implications for farmers. Nevertheless, further investigation is necessary to comprehensively understand the underlying causes of reproductive failures in these farms.
In a previous study conducted in Northeast Thailand, a seropositivity rate of 46.61% was reported for C. burnetii in meat goat herds experiencing clinical reproductive disorders such as abortion, orchitis, repeat breeding, and sterility [14]. In our current study, we observed that three herds previously identified as seropositive for C. burnetii [14] continue to experience reproductive disorders, including abortion, orchitis, and weak offspring. Furthermore, two of these herds tested positive by PCR, confirming the circulation of C. burnetii within their herds. In addition, we observed varying prevalence levels across the study areas, indicating spatial heterogeneity in the distribution of C. burnetii infection in Northeast Thailand.
The results of seroprevalence and molecular prevalence suggest a higher disease burden or exposure compared to the previous study [14], particularly in certain groups. Notably, the molecular prevalence was higher than the seroprevalence, indicating the shedding of C. burnetti, even with a low rate through vaginal secretion during the latent stage of infection, which may occur even in apparently healthy goats [9,15].
This study demonstrates how various risk factors shed light on the complex dynamics that influence disease prevalence within goat herds. It uncovers a multitude of elements contributing to disease transmission and propagation. The association between older herd establishments and higher prevalence suggests the need for sustained vigilance and proactive management practices over time. It also implies that established herds may serve as reservoirs for pathogens, necessitating tailored control strategies to mitigate disease transmission. Moreover, the link between buck circulation and disease prevalence emphasizes the crucial role of animal movement in disease dissemination [14,16]. Implementing stringent biosecurity measures, including thorough health screening of introduced animals and restricted contact between herds, emerges as a critical intervention to curtail disease spread. The impact of participants’ knowledge on disease transmission routes further underscores the importance of education and awareness campaigns. Empowering farmers with accurate information about disease risks and preventive measures can facilitate the adoption of best practices, ultimately reducing disease prevalence and enhancing herd health.
While conducting a risk analysis, it is important to exercise caution when interpreting non-significant associations. In this particular investigation, no significant correlation was found between the presence of other livestock and certain animal species, such as dogs, cats, rodents, and ticks, in coxiellosis- positive herds. However, it is worth noting that C. burnetii can still be transmitted among ruminant species and companion animals [15,17,18]. Previous studies have emphasized that companion animals and rodents can serve as reservoirs of Q fever for humans and livestock animals on farms [19–25]. Additionally, ticks have been identified as vectors for C. burnetii in livestock animals [8,25,26]. Therefore, the presence of these animals should be a concern in goat herds, requiring further attention and surveillance.
Furthermore, in terms of the practice of giving birth, farmers face a high risk of exposure to C. burnetii due to the lack of protective measures such as gloves (56.4%, 22/39) and masks (69.2%, 27/39) during assistance. It is crucial to emphasize the importance of self-protection during birthing assistance to minimize the risk of transmission, as highlighted in a previous study [15].
In the study presented, approximately 90% of farmers reported using manure as fertilizer. This raises concerns as the spreading of the pathogen could occur in other areas. In herds infected with C. burnetii, it is advisable to refrain from applying fresh manure and composted manure to the land for at least 90 days before application. Furthermore, transporting manure and applying it only on damp, low-wind days can help reduce the risk of pathogen dissemination. These practices should be considered to mitigate the spread of C. burnetii and protect both human and animal health on farms [15].
Likewise, at the individual animal level, various potential risk factors are associated with the prevalence of a specific condition, with implications for its connection to C. burnetii exposure and/or current infection. The higher prevalence among female animals suggests a gender-specific susceptibility, consistent with a previous study indicating higher seropositivity for C. burnetii among female goats [27]. Nevertheless, discrepancies in research findings are apparent, as one study revealed no significant difference between genders [28]. This variability might be attributed to the strong tropism of C. burnetii for the trophoblasts of the placenta and mammary lymph nodes [29], impacting disease manifestation and warranting further investigation for a comprehensive understanding of Q fever epidemiology within the herd. However, it is important to note that this study exclusively utilized vaginal swabs from female goats for molecular detection. Furthermore, all samples were obtained from mature goats aged over 6 months, thereby excluding younger animals under 6 months of age. This approach may have led to underrepresentation, and the observed higher prevalence in specific gender and age groups could potentially be attributed to this limitation.
While age and body condition scores did not demonstrate statistically significant associations, they do provide valuable insights into potential trends. This suggests that animals of all ages were equally susceptible to acquiring infection or were exposed to a common source of infection, indicating the potential for disease emergence in the area. However, previous study suggests that older animals and those in poorer body conditions may be more susceptible to C. burnetti [30]. Further investigation is needed to confirm these trends and gain a better understanding of their implications for disease management.
Additionally, the presence of anaemia, significantly associated with higher prevalence, highlights the importance of considering additional health factors within the herd. Given the frequent occurrence of roundworm gastrointestinal parasites in this region [31], heavy infestation of these worms could lead to anaemia. Therefore, the presence of anaemia could serve as an important clinical indicator of coxiellosis within the herd, necessitating further investigation and the implementation of management strategies to mitigate disease spread.
Our study has certain limitations. We cannot definitively confirm active infection of C. burnetii and assess the impacts of reproductive disorders associated with it in the examined herds. These limitations stem from our sampling approach, where we obtained vaginal secretions from apparently healthy does, but not from aborted foetuses, placenta, or vaginal discharges after giving birth or abortion. In addition, all samples were only obtained from mature goats aged over 6 months, thus the infection in younger animals could not be evaluated.
The findings in this study emphasize the interconnected nature of disease transmission dynamics within livestock populations. Factors such as gender, breed type, and clinical indicators not only influence individual susceptibility but also contribute to the broader epidemiology of infectious diseases like coxiellosis within the herd. Hence, implementing a comprehensive strategy encompassing individual risk factors and herd-level management practices is imperative for successful disease control and prevention.
5. Conclusions
In conclusion, our study emphasizes the intricate nature of C. burnetii epidemiology in meat goat herds and emphasizes the significance of comprehensive strategies for disease management and control. An integrated approach that incorporates proactive herd management practices, stringent biosecurity measures, targeted education initiatives, and collaborative research endeavours is indispensable for curtailing disease transmission, safeguarding herd health, and ensuring the long-term viability of goat farming ventures.
Funding Statement
The authors would like to express their gratitude to the farmers who participated in the study for their valuable cooperation. This research on the Prevalence and Risk factors associated with Q fever infection in small ruminant herds of Northeast Thailand, conducted by Khon Kaen University (Fundamental Fund 67) has been financially supported by the National Science Research and Innovation Fund (NSRF) under project [no. 214-2519/2566], as well as the Research and Graduate Studies (Research Program) under project [no. RP66-9-001] by Khon Kaen University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
SR: Conceptualization and Study design, Formal analysis (Serology), Investigation, Data curation, Statistical analysis, Writing – original draft preparation.
SL: Epidemiological analysis
PS: Statistical analysis
PP: Design and analysis of molecular detection, Writing
All authors reviewed the results and approved the final version of the manuscript.
References
- [1].Hamad G, Ranmuthugala G.. Q fever awareness in Australia: a scoping review. Aust N Zeal J Public Health. 2023;47(6):1–17. doi: 10.1016/j.anzjph.2023.100099 [DOI] [PubMed] [Google Scholar]
- [2].Norlander L. Q fever epidemiology and pathogenesis. Microbes Infect. 2000;2(4):417–424. doi: 10.1016/S1286-4579(00)00325-7 [DOI] [PubMed] [Google Scholar]
- [3].Fernandes J, de Lemos ERS. The multifaceted Q fever epidemiology: a call to implement one health approach in Latin America. Lancet Reg Health Am. 2023;20:1–2. doi: 10.1016/j.lana.2023.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Klemmer J, Njeru J, Emam A, et al. Q fever in Egypt: epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLOS ONE. 2018;13(2):e0192188. doi: 10.1371/journal.pone.0192188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Robi DT, Demissie W, Temteme S. Coxiellosis in livestock: epidemiology, public health significance, and prevalence of Coxiella burnetii infection in Ethiopia. Vet Med (Auckl). 2023;14:145–158. doi: 10.2147/VMRR.S418346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang M, Ma J, Jiao J, et al. The epidemic of Q fever in 2018 to 2019 in Zhuhai city of China determined by metagenomic next-generation sequencing. PLOS Negl Trop Dis. 2021;15(7):e0009520. doi: 10.1371/journal.pntd.0009520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ullah Q, Jamil T, Saqib M, et al. Q fever—A neglected zoonosis. Microorganisms. 2022;10(8):1530. doi: 10.3390/microorganisms10081530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Celina Seyma S, Jirí C. Coxiella burnetii in ticks, livestock, pets and wildlife: a mini-review. Front Vet Sci. 2022;9:1068129. doi: 10.3389/fvets.2022.1068129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Agerholm JS. Coxiella burnetii associated reproductive disorders in domestic animals–a critical review. Acta Vet Scand. 2013;55(1):13. doi: 10.1186/1751-0147-55-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anastácio S, de Sousa SR, Saavedra MJ, et al. Role of goats in the epidemiology of Coxiella burnetii. Biology (Basel). 2022;11(12):1703. doi: 10.3390/biology11121703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ict DLD. Goat population in Thailand in 2022. [cited 2023 May 20]. Available from: https://ict.dld.go.th/webnew/images/stories/stat_web/yearly/2565/province/T8-1-Goat.pdf
- [12].IDEXX Validation report . IDEXX Q fever ab test (IDEXX Switzerland AC, stationsstrasse 12, CH-3097 Liebefeld-Bern, Switzerland); 2011.
- [13].Mediannikov O, Fenollar F, Socolovschi C, et al. Coxiella burnetii in humans and ticks in rural Senegal. PLOS Negl Trop Dis. 2010;4(4):e654. doi: 10.1371/journal.pntd.0000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rerkyusuke S, Lerk-U-Suke S, Sirimalaisuwan A, et al. Clinical evidence and risk factors for reproductive disorders caused by bacterial infections in meat goats in Northeastern Thailand. Vet Med Int. 2022;8:1–9. doi: 10.1155/2022/1877317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Plummer PJ, McClure JT, Menzies P, et al. Management of Coxiella burnetii infection in livestock populations and the associated zoonotic risk: a consensus statement. Vet Intern Med. 2018;32(5):1481–1494. doi: 10.1111/jvim.15229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lafi SQ, Talafha AQ, Abu-Dalbouh MA, et al. Seroprevalence and associated risk factors of Coxiella burnetii (Q fever) in goats and sheep in northern Jordan. Trop Anim Health Prod. 2020;52(4):1553–1559. doi: 10.1007/s11250-019-02153-0 [DOI] [PubMed] [Google Scholar]
- [17].Bauer B, Prüfer L, Walter M, et al. Comparison of Coxiella burnetii excretion between sheep and goats naturally infected with one cattle-associated genotype. Pathogens. 2020;9(8):652. doi: 10.3390/pathogens9080652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schimmer B, Luttikholt S, Hautvast JL, et al. Seroprevalence and risk factors of Q fever in goats on commercial dairy goat farms in the Netherlands, 2009-2010. BMC Vet Res. 2011;7(1):81. doi: 10.1186/1746-6148-7-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chitanga S, Simulundu E, Simuunza MC, et al. First molecular detection and genetic characterization of Coxiella burnetii in Zambian dogs and rodents. Parasit Vectors. 2018;11(1):40. doi: 10.1186/s13071-018-2629-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Malo JA, Colbran C, Young M, et al. An outbreak of Q fever associated with parturient cat exposure at an animal refuge and veterinary clinic in southeast Queensland. Aust N Z J Public Health. 2018;42(5):451–455. doi: 10.1111/1753-6405.12784 [DOI] [PubMed] [Google Scholar]
- [21].Pinsky RL, Fishbein DB, Greene CR, et al. An outbreak of cat-associated Q fever in the United States. J Infect Dis. 1991;164(1):202–204. doi: 10.1093/infdis/164.1.202 [DOI] [PubMed] [Google Scholar]
- [22].Ebani VV. Coxiella burnetii infection in cats. Pathogens. 2023;12(12):1415. doi: 10.3390/pathogens12121415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abdel-Moein KA, Hamza DA. Rat as an overlooked reservoir for Coxiella burnetii: a public health implication. Comp Immunol Microbiol Infect Dis. 2018;61:30–33. doi: 10.1016/j.cimid.2018.11.002 [DOI] [PubMed] [Google Scholar]
- [24].Mangombi-Pambou J, Granjon L, Labarrere C, et al. New genotype of Coxiella burnetii causing epizootic Q fever outbreak in rodents, Northern Senegal. Emerg Infect Dis. 2023;29(5):1078–1081. doi: 10.3201/eid2905.221034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cantas L, Muwonge A, Sareyyupoglu B, et al. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Vet Res. 2011;7(13):1–7. doi: 10.1186/1746-6148-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muramatsu Y, Usaki N, Thongchai C, et al. Seroepidemiologic survey in Thailand of Coxiella burnetii infection in cattle and chickens and presence in ticks attached to dairy cattle. Southeast Asian J Trop Med Public Health. 2014;45(5):1167–1172. [PubMed] [Google Scholar]
- [27].Burns RJL, Douangngeun B, Theppangna W, et al. Serosurveillance of coxiellosis (Q-fever) and brucellosis in goats in selected provinces of Lao People’s Democratic Republic. PLOS Negl Trop Dis. 2018;2(4):e0006411. doi: 10.1371/journal.pntd.0006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Asadi J, Khalili M, Kafi M, et al. Risk factors of Q fever in sheep and goat flocks with history of abortion. Comp Clin Pathol. 2014;23(3):625–630. doi: 10.1007/s00580-012-1661-9 [DOI] [Google Scholar]
- [29].Roest HJ, van Gelderen B, Dinkla A, et al. Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. PLOS ONE. 2012;7(11):e48949. doi: 10.1371/journal.pone.0048949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Islam S, Rahman MK, Abedin J, et al. Seroprevalence of and risk factors of rift valley fever and Q fever in domestic ruminants of Bangladesh. Int J Infect Dis. 2023;130:S147. doi: 10.1016/j.ijid.2023.04.361 [DOI] [Google Scholar]
- [31].Rerkyusuke S, Lamul P, Thipphayathon C, et al. Caprine roundworm nematode resistance to macrocyclic lactones in Northeastern Thailand. Vet Integr Sci. 2023;21(2):623–634. doi: 10.12982/VIS.2023.044 [DOI] [Google Scholar]


