Abstract
Background
Data on pyogenic liver abscess (PLA) of children in China have been limited. We aimed to summarize the clinical feather, microbiological characteristics, management, and outcome of PLA in children.
Method
We retrospectively reviewed PLA cases from January 2008 to June 2023 at Beijing Children’s Hospital. Clinical characteristics, pathogens and management were analyzed.
Results
We diagnosed 57 PLA patients in our center. The median onset age was 4.5 years and the male-to-female ratio was 1.6:1. The median diagnostic time was nine days and the median length of stay was 22 days. Twenty-eight patients (49.1%) had predisposing factors, around 71.4% of the patients had malignant hematology and primary immunodeficiency disease. Patients with underlying factors were more likely to have extrahepatic organ involvement (p = 0.024), anemia (p < 0.001), single abscess (p = 0.042), unilateral involvement (p = 0.039), and small size of the abscess (p = 0.008). Twenty-four patients (42.1%) had extrahepatic organ involvement. Pathogens were identified in 17 patients (29.8%), the most common pathogens were Klebsiella pneumoniae and Staphylococcus aureus. The positive rate of metagenomic next-generation sequencing (mNGS) was 87.5% (7/8). On multivariable analysis, the extrahepatic organ involved (p = 0.029) and hepatomegaly (p = 0.025) were two independent factors associated with poor outcomes.
Conclusions
PLA is usually seen in children with predisposing factors. Malignant hematology and primary immunodeficiency disease were the most common underlying diseases. Extrahepatic organ involvement and hepatomegaly are associated with poor prognosis. Increased use of mNGS could be beneficial for identifying pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09634-0.
Keywords: Pyogenic liver abscess, Pathogen, mNGS, Antibiotics, Children
Introduction
Pyogenic liver abscess (PLA) is considered an uncommon infection that comprises the majority of hepatic abscess (HA) [1] According to the limited reports on children, the incidence of PLA is higher in developing countries. For instance, the morbidity was 78.9 per 100,000 admissions in South India [2]. Nevertheless, the incidence of PLA is reported to be lower among children in developed countries, with 25 per 100,000 admissions in the US [3], and 2.9 per 100,000 in Sweden [4]. And in Taiwan, was 8.9 to 20 per 100,000 admissions [5, 6].
Overall, Staphylococcus aureus (S. aureus) is frequently isolated from PLA in children and accounts for nearly 20–50% of cases. Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), and Enterobacter are also common pathogens [7–9]. In recent years, investigators found the prevalence of K. pneumoniae in PLA increased worldwide. Similarly, an upward tendency was shown in China [8]. Patients with inducing factors tend to get PLA. Diabetic mellitus (DM), cirrhosis, and immunocompromised state are well-known predisposing factors [10].
But so far, most studies focused on adults but seldom children, which resulted in limited data in our country. Here, we present a single-center study of PLA and analyze clinical characteristics, pathogens and management in the pediatric population.
Patients and methods
Study population
In this retrospective study, we reviewed data from children (younger than 18 years old) who were admitted to Beijing Children’s Hospital (National Center for Children’s Health, China; A 970-bed tertiary pediatric hospital) and diagnosed PLA from January 2008 to June 2023. We collected information on the risk factors, clinical presentations, and laboratory tests including pathogen results, radiological findings, management, and outcomes of PLA patients.
Inclusion criteria
All of the patients met at least one of the following inclusion criteria: (1) Presence of abscess in the liver on imaging examinations and reduced after anti-bacterial treatment; (2) Bacterial culture positive from aspiration of liver abscess.
The definition of extrahepatic involvement: Except for the liver, infections were found in other tissues or organs through CT, MRI, ultrasound, or cerebrospinal fluid examination.
Statistical analysis
Categorical variables were presented as numbers and percentages, while continuous variables were shown as the median and interquartile range (IQR). Categorical variables were compared using the Chi-square or Fisher’s exact tests as appropriate. Continuous variables within two groups were compared using the Mann–Whitney U test according to their distribution. We used univariable logistic regression to evaluate the risk factors of the poor outcome, when considering factors with p-value < 0.05, multivariable logistic regression was made. The odds ratio (OR) and confidence interval at 95% (CI95%) were presented. P-value < 0.05 was considered significant. All of the statistical analyses were conducted using Statistical Product and Service Solutions (SPSS), version 23.0 (IBM, NY, USA).
Results
Fifty-seven children were diagnosed as PLA from January 2008 to June 2023, including 35 males (61.4%), the median age of onset was 4.5 (0.7, 8.4) years, and four cases were neonates. The median diagnostic time was 9 (4, 15) days, and the median length of stay was 22 (14, 31) days (Table 1).
Table 1.
Clinical characteristics of children with PLA
| Characteristics | Value |
|---|---|
| Male:female | 35:22 |
| Age (years), median | 4.5(0.7–8.4) |
| Time to diagnosis (day), median | 9(4–15) |
| Hospital stay (day), median | 22(14–31) |
| Predisposing factor, n (%) | 28 (49.1) |
| Extrahepatic infection lesion, n (%) | 24 (42.1) |
| Multiple extrahepatic infection lesions, n (%) | 14 (24.6) |
| Symptoms and signs, n (%) | |
| Fever | 51 (89.5) |
| Hepatomegaly | 18 (31.6) |
| Abdominal pain | 14 (24.6) |
| Blood tests, median | |
| WBC (10,000/μL) | 13.6 (11.7–17.4) |
| PCT (ng/mL) | 0.8 (0.2–3.2) |
| CRP (mg/L) | 85 (40–120) |
| ALT (U/L) | 22 (13–56) |
| Imaging findings | |
| Solitary, n (%) | 24 (42.1) |
| Unilateral lobar involved, n (%) | 38 (66.7) |
| Right lobe, n (%) | 31 (54.4) |
| Size (cm), median | 2.5 (1.0–5.6) |
| Defined pathogen, n (%) | 17 (29.8) |
| Ultrasound-guild aspiration, n (%) | 16 (28.1) |
| Favorable outcome, n (%) | 50 (87.7) |
| ICU admission, n (%) | 14 (24.6) |
WBC white blood cell, PCT procalcitonin, CRP C-reactive protein, ESR erythrocyte sedimentation rate, ALT transaminase, ICU intensive care unit
Clinical presentations
Fifty patients (87.7%) initially presented with fever, 13 patients (22.8%) had abdominal pain, four patients (7.0%) experienced cough and four patients (7.0%) had diarrhea. Extrahepatic involvement was observed in 24 patients (42.1%). The splenic abscess was the most common with 15 patients (26.3%), eight patients (14.0%) had kidney abscess, six patients (10.5%) had intra-abdominal infections, and other complications such as meningitis, skin infection, endocarditis, infection of hip joint, soft tissue and spine were also observed (Fig. 1). Significantly, 14 patients (24.6%) had more than two infection foci apart from liver abscess.
Fig. 1.
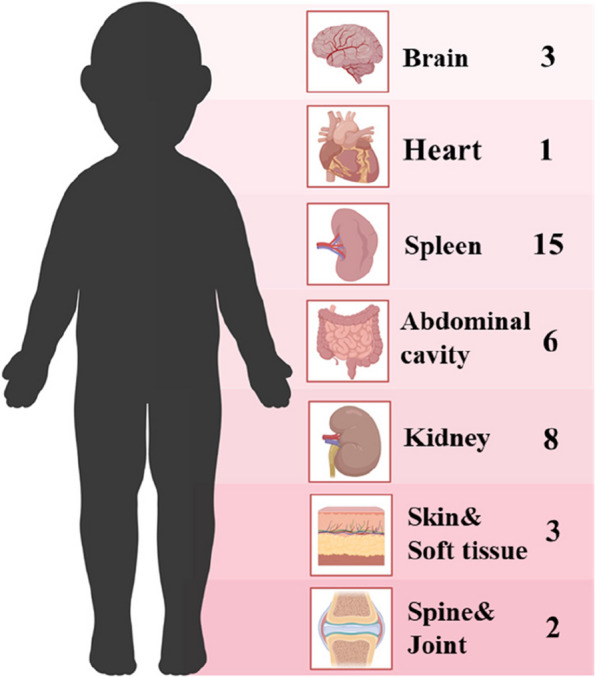
Distribution of extrahepatic organ involved
Among four neonates, case 1 had fever after umbilical vein catheterization, case 2 was asymptomatic with elevated CRP after umbilical vein catheterization, case 3 had fever with abdominal distension, and case 4 only had abdominal distension.
Predisposing factors
Twenty-eight patients (49.1%) had underlying factors, over two-thirds (67.9%, n = 19) were in an immunosuppressed state. Eleven patients (19.3%) received a long-time use of immunosuppressive agents, including nine cases of hematological malignancy (five acute lymphoblastic leukemia, two lymphoma, one hemophagocytic syndrome and one chronic active Epstein-Barr virus infection), the rest were one case of systemic lupus erythematosus and inflammatory bowel disease separately. Eight patients (14.0%) had primary immunodeficiency disease (PID), including five combined immunodeficiency, congenital neutropenia, hyper IgE syndrome and chronic granulomatous disease (CGD) one case each. Appendicitis was found in four patients (7.0%), two of them had perforations. Two neonates were associated with a history of undergoing umbilical vein catheter insertion. Type 1 DM, hypertyrosinemia and glycogen storage disease were observed in the remaining three cases separately.
Patients with predisposing factors were more likely to have extrahepatic involved (p = 0.024), anemia (p < 0.001), single abscess (p = 0.042), unilateral involvement (p = 0.039), and small size of the abscess (p = 0.008)(Table 2).
Table 2.
Comparison of clinical characteristic between the patients with or without predisposing factor
| With predisposing factor (n = 28) | Without predisposing factor (n = 29) | P | |
|---|---|---|---|
| Male, n (%) | 21 (75.0) | 14 (48.3) | 0.038 |
| Age (years), median | 3.8 (0.6–7.0) | 6.0 (1.2–9.0) | 0.508 |
| Time to diagnosis (day), median | 9 (4–13) | 7 (5–18) | 0.754 |
| Hospital stay (day), median | 24 (16–27) | 22 (13–37) | 0.866 |
| Extrahepatic infection lesion, n (%) | 16 (57.1) | 8 (27.6) | 0.024 |
| Multiple extrahepatic infection lesions, n (%) | 6 (21.4) | 8 (27.6) | 0.589 |
| Symptoms and signs | |||
| Fever, n (%) | 22 (78.6) | 28 (96.6) | 0.096 |
| The highest temperature, median | 39.1 (39–39.6) | 39.5 (39–40) | 0.301 |
| Hepatomegaly, n (%) | 10 (35.7) | 9 (31.0) | 0.708 |
| Imaging findings | |||
| Single abscess, n (%) | 8 (28.6) | 16 (55.2) | 0.042 |
| Unilateral lobar involved, n (%) | 15 (53.6) | 23 (79.3) | 0.039 |
| Size(cm), median | 1.8 (0.6–3.5) | 4.2 (1.9–6.4) | 0.008 |
| Blood tests | |||
| WBC (10,000/μL) | 12.7 (7.6–17.9) | 13.7 (12.6–15.7) | 0.278 |
| HGB (g/L) | 92 (81–108) | 116 (107–127) | < 0.001 |
| PCT (ng/mL) | 0.8 (0.2–6.1) | 0.47 (0.05–2.79) | 0.201 |
| CRP (mg/L) | 77.3 (42.1–121.5) | 89 (35.5–120) | 0.842 |
| ALT (U/L) | 19.2 (12.5–94.6) | 29.8 (16.4–46.8) | 0.377 |
| ICU admission, n (%) | 10 (35.7) | 4 (13.8) | 0.055 |
| Poor outcome, n (%) | 6 (21.4) | 1 (3.4) | 0.096 |
WBC white blood cell, HGB hemoglobin, PCT procalcitonin, CRP C-reactive protein, ALT transaminase, ICU intensive care unit
Laboratory data
Blood tests
C-reactive protein (CRP) level was elevated (> 8 mg/L) in 54 patients (94.7%), with a median of 85 (40, 120) mg/L. Increased procalcitonin (PCT) level (> 0.05 ng/mL) was observed in 34 patients (87.2%, n = 39), with a median of 0.8 (0.2, 3.2) ng/mL. Leukocytosis (white blood cell > 10,000/μL) was seen in 46 patients (80.7%), with a median of 13,600 (11,700,17,400) /μL. Seventeen patients (29.8%) had elevated level of alanine transaminase (> 40U/L), with a median of 22 (13, 56) U/L.
Imaging findings
All patients performed ultrasound, 55 patients (96.5%) presented with low hypoechoic lesions in ultrasound, and two patients were normal. Forty-two patients (73.7%) performed the contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) scan. Lesions of low density with edge intensified showed on CECT, hypointensity on T1 and T2 weighted image with edge intensified showed on contrast-enhanced MRI. Thirty-three patients (57.9%) had multiple abscesses. Thirty-eight patients (66.7%) had unilateral involvement and most patients (81.6%, n = 31) occupied the right lobe. The median abscess diameter was 2.5 (1.0, 5.6) cm.
Pathogen findings
In the present study, pathogens were identified in 17 patients (29.8%), and two patients had co-infections (Tables 3 and 4). The positive rate of blood culture was 14.5% (8/55). Sixteen patients (28.1%) finished ultrasound-guild aspiration and pus culture, the positive rate was 25.0% (4/16). Seven (12.3%) abscess samples and one blood sample were sent for mNGS, the positive rate was 87.5% (7/8), mNGS increased the diagnostic rate of pathogen by 10.5% (6/57).
Table 3.
Pathogens defined from enrolled cases. The overall pathogen detection and different method to identify pathgens
| Bacteria | N | Blood culture | Pus culture | mNGS |
|---|---|---|---|---|
| Gram-positive cocci | 7 | 4 | 1 | 3 |
| S.aureusa | 4 | 3 | 1 | 1 |
| Streptococcus agalactiae | 1 | 1 | ||
| Streptococcus intermedius | 1 | 1 | ||
| Bartonella henselae | 1 | 1 | ||
| Gram-negative bacillil | 12 | 5 | 3 | 5 |
| K. pneumoniaeb | 5 | 3 | 3 | |
| P. aeruginosa | 3 | 1 | 2 | |
| E. coli | 2 | 1 | 1 | |
| Fusobacterium nucleatum | 1 | 1 | ||
| Serratia marcescens | 1 | 1 | ||
| Total | 17c | 8d | 4 | 7e |
S. aureus: Staphylococcus aureus, E. coli: Escherichia coli, K. pneumoniae: Klebsiella pneumoniae, P. aeruginosa:Pseudomonas aeruginosa
aOne had growth from blood culture and defined by mNGS of pus.
bOne had growth from pus culture and blood culture.
cTwo patients were mixed infection. One was K. pneumoniae with S.aureus; one was E. coli with P. aeruginosa.
dOne was defined K. pneumoniae with S.aureus by blood culture.
eOne was defined P. aeruginosa and E. coli by mNGS of blood.
Table 4.
The distribution of pathogens in patients with underlying disease and without underlying disease
| Bacteria | n | With predisposing factor (n = 28) | Without predisposing factor (n = 29) |
|---|---|---|---|
| Gram-positive cocci | 7 | 2 | 5 |
| S.aureus | 4 | 2 | 2 |
| Streptococcus agalactiae | 1 | 1 | |
| Streptococcus intermedius | 1 | 1 | |
| Bartonella henselae | 1 | 1 | |
| Gram-negative bacillil | 12 | 8 | 4 |
| K. pneumonia | 5 | 3 | 2 |
| P. aeruginosa | 3 | 3 | |
| E. coli | 2 | 2 | |
| Fusobacterium nucleatum | 1 | 1 | |
| Serratia marcescens | 1 | 1 | |
| Total | 17a | 8a | 9 |
aTwo patients had co-infection. One was K. pneumoniae with S.aureus; one was E. coli with P. aeruginosa
Four patients were K. pneumoniae (one patient was positive for extended-spectrum beta-lactamase and carbapenemases). Three patients were S.aureus (one was methicillin-resistant and others were methicillin-susceptible). Two patients had mixed infections. One was K. pneumoniae (negative of extended-spectrum beta-lactamase and carbapenemases) with S.aureus (methicillin-susceptible) isolated from blood culture, another one was Escherichia coli with Pseudomonas aeruginosa identified by mNGS.
Notably, before 2018, only 18.2% (4/22) of patients performed aspiration and the positive rate of pathogen detection was 9.1% (2/22). After that, with the increased use of mNGS and ultrasound-guided aspiration (34.3%, 12/35), the positive rate of pathogen detection increased to 42.9% (15/35).
Therapy and outcomes
All patients received intravenous antimicrobial therapy (Tables 5 and 6). Twenty-nine patients (50.9%) were prescribed a single agent as initial treatment, and cefoperazone sulbactam/piperacillin/tazobactam (n = 14, 24.6%) was the most common recipe followed by meropenem (n = 10, 17.5%). Meropenem combined with vancomycin or linezolid (n = 14, 24.6%) was the most common combination therapy regimen. Sixteen patients (28.1%) changed antimicrobial treatment, and more than half (n = 9, 56.3%) were correlated with positive pathogen results.
Table 5.
Antibiotic treatment and outcome of children with PLA. The overall antibiotic use and outcome
| Initial anti-infection treatment | N (%) | Changed antibiotics (n, %) | Died (n, %) | ||
|---|---|---|---|---|---|
| Total | Due to pathogen defined | Due to poor prognosis | |||
| SCF based | 22 (38.6) | 4 (18.2) | 3 (13.6) | 1 (4.5) | 2 (9.1) |
| SCF /TZP | 14 (24.6) | 2 (14.3) | 2 (14.3) | 0 (0) | 0 (0) |
| SCF + VA /LZD/MTZ | 8 (14.0) | 2 (25.0) | 1(12.5) | 1(12.5) | 2 (25.0) |
| MEM based | 24 (42.1) | 7 (29.2) | 4 (16.7) | 3 (12.5) | 5 (20.8) |
| MEM | 10 (17.5) | 3 (30.0) | 1 (10.0) | 2 (20.0) | 1 (10.0) |
| MEM + VA /LZD | 14 (24.6) | 4 (28.6) | 3 (21.4) | 1 (7.1) | 4 (28.6) |
| CRO based | 8 (14.0) | 4 (50.0) | 2 (25.0) | 2 (25.0) | 0 (0) |
| CRO | 2 (3.5) | 2 (100.0) | 1 (50.0) | 1 (50.0) | 0 (0) |
| CRO + MTZ | 4 (7.0) | 1 (25.0) | 0 (0) | 1 (25.0) | 0 (0) |
| CRO + VA /LZD | 2 (3.5) | 1 (50.0) | 1 (50.0) | 0 (0) | 0 (0) |
| Others* | 3 (5.3) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) |
| Total | 57 (100.0) | 16 (28.1) | 9 (15.8) | 7 (12.3) | 7 (12.3) |
SCF cefoperazone/sulbactam, TZP piperacillin/tazobactam, VA vancomycin, LZD linezolid, MTZ metronidazole, MEM meropenem, CRO ceftriaxone, RFP rifampicin, AZM azithromycin
* VA = 2; cefamandole = 1
Table 6.
Antibiotic changed due to pathogen defined
| No | Pathogen | Initial anti-infection treatment | Changed antibiotics |
|---|---|---|---|
| 1 | Streptococcus intermedius | SCF | SCF + LZD |
| 2 | Serratia marcescens | SCF | MEM |
| 3 | Fusobacterium nucleatum | SCF + VA + MTZ | SCF + MTZ |
| 4 | K. pneumoniae | MEM + VA | SCF |
| 5 | K. pneumoniae | MEM + VA | SCF |
| 6 | P. aeruginosa + E. coli | MEM + VA | SCF |
| 7 | MRSA | MEM | MEM + VA |
| 8 | Bartonella henselae | CRO | RFP + AZM |
| 9 | Streptococcus agalactiae | CRO + LZD | LZD |
K. pneumoniae: Klebsiella pneumoniae; P. aeruginosa: Pseudomonas aeruginosa; E. coli: Escherichia coli;
MRSA methicillin-resistant staphylococcus aureus
Fourteen (24.6%) patients had ever been treated in the intensive care unit. Finally, 50 patients (87.7%) took a favorable turn. Patients in the death group were more likely to have extrahepatic organ involved and hepatomegaly than the survival group (Table 7). On multivariable analysis, the extrahepatic organ involved [OR 13.134 (1.301–32.438), p = 0.029] and hepatomegaly [OR 8.893 (1.312–13.296), p = 0.025] were two independent factors that lead to poor outcomes.
Table 7.
Comparison of clinical characteristic between the patients in survival and death group
| Survival (n = 50) | Death (n = 7) | P | |
|---|---|---|---|
| Time to diagnosis (day), median | 7 (4–13) | 12 (9–25) | 0.131 |
| Extrahepatic infection lesion, n (%) | 18 (36) | 6 (85.7) | 0.037 |
| Multiple extrahepatic infection lesions, n (%) | 11 (22) | 3 (42.9) | 0.464 |
| Predisposing factors | 22 (44) | 6 (85.7) | 0.096 |
| Symptoms and signs | |||
| The highest temperature, median | 39.3 (39–39.8) | 39.4 (39–40) | 0.479 |
| Hepatomegaly, n (%) | 13 (26) | 5 (71.4) | 0.047 |
| Imaging findings | |||
| Single abscess, n (%) | 23 (46) | 1 (14.3) | 0.237 |
| Unilateral lobar involved, n (%) | 36 (72) | 2 (28.6) | 0.064 |
| Size(cm), median | 2.8 (1.1–5.9) | 1.2 (0.7–3.1) | 0.156 |
| Blood tests | |||
| WBC (10,000/μL) | 13.5 (11.6–17.8) | 13.6 (11.8–17.1) | 0.874 |
| HGB (g/L) | 109 (93.5–122) | 88 (76–104) | 0.053 |
| PCT (ng/mL) | 0.68 (0.11–2.30) | 2.8 (0.2–22.3) | 0.069 |
| CRP (mg/L) | 84 (37–119) | 91.2 (74–160) | 0.215 |
| ALT (U/L) | 23.7 (15–47.3) | 13.5(9.2–126.7) | 0.246 |
WBC white blood cell, HGB hemoglobin, PCT procalcitonin, CRP C-reactive protein ALT transaminase
Discussion
The age at diagnosis was reported 13 years in the USA [11], 10 years in the UK [12], and 9.6 years in Taiwan [13]. Excluding the data on newborns in our study, the median age of pediatricians with PLA was 5.0 years and 79.2% of the total were under 10 years old, which was younger compared with other studies. Possibly it was related to a high portion of patients with hematological malignancy and primary immunodeficiency disease, which had an early onset age.
Predisposing factors play a vital role in the development of PLA and it differs between adults and children. Immunocompromised state (such as PID, malignancy, use of an immunosuppressive agent and chemotherapy), appendicitis with or without perforation, hepatobiliary disease (including congenital abnormalities, liver cirrhosis, liver transplant), DM, trauma and umbilical venous catheterization were showed to be closely related with PLA in several studies [10, 11, 13, 14]. In adults, the main predisposing factors were diabetes, malignancy and hepatobiliary diseases [15, 16]. However, PLA often occurs in children with PID, especially for selective IgA deficiency, T-cell defect, and CGD [17–19]. In a nationwide population-based analysis, it was almost three times increased risk among children with immunodeficiency [11]. Unlike other factors compared with adults, PID is almost unique to children which can be recognized via personal and family history, detection of immune function, and gene sequencing. Although only one case had a history of DM in our study, this factor should not be ignored. DM has been regarded as a potential risk factor for PLA with a hazard risk of 3.6 to ninefold and co-existence rates of 23–36.6% [20–22]. The mechanism was identified to be neutrophil chemotaxis and phagocytosis impaired, which leads to the weakening of the immune system.
PLA in neonates is rare, and the clinical manifestations and predisposing factors were often distinct from older children. Usually present with fever, abdominal distension and even asymptomatic. Diagnosis in newborns relies more on blood tests, imaging examinations and reaction of anti-infective therapy. In our study, two cases did not have LA on admission, developed fever and raised CRP level after umbilical venous catheterization and finally diagnosed PLA. It is worth noting that ascending infection through umbilical venous catheterization can be a potential cause of PLA in a neonatal period [23]. When a neonate suffers progressive sepsis, PLA should be considered, especially with the history of this operation. In addition, omphalitis could be a very rare cause of PLA in neonates [24].
Clinical manifestations of PLA are nonspecific. Fever and abdominal pain are the most common clinical features and basis for the diagnosis of PLA [12]. Four patients in our study had a cough accompanied with pneumonia. Previous studies reported several patients had a cough because of the stimulation of the diaphragm through a liver abscess nearby, which can even be the only symptom besides fever [25]. Six patients were diagnosed by routine test without any symptoms, one patient was neonate and five had hematological malignancy, half of them had normal CRP level and white blood cell counts. Patients are more susceptible to abscess formation due to long-term immunosuppressive therapy and secondary neutropenia, which could be explained as sometimes asymptomatic at onset. Meanwhile, only three cases with hematological malignancy did not show increased CRP. Which are easy to misdiagnose during clinical assessment and could be partially explained the diagnostic time seemed long in our study.
In the past few decades, S.aureus has been the leading casual pathogen isolated from PLA in children all over the world [8, 12]. In recent years, K. pneumoniae has become the main pathogen of PLA whether in adults or children, and is closely related to DM, particularly in Asia [13, 26, 27]. Chung et al. from Korea revealed that 78.2% of PLA patients were caused by K. pneumoniae [28]. A study conducted by Yeh et al. reported that K. pneumoniae was accused of a 36.4% positive blood culture rate and a 64.3% positive pus culture rate in pediatrics [13]. In a meta-analysis of pathogen distribution with PLA in China, Klebsiella spp had an upward tendency during an 11-year period and the highest pooled proportion even reached 71%, meanwhile 66% of patients with DM was related to PLA [8]. One case in our study who suffered from DM was isolated K. pneumoniae from pus culture, therefore K. pneumoniae should be given priority when PLA patients had DM. In addition, in our study we found PLA patients with underlying disease (mainly in hematological malignancy) were more susceptible to gram negative bacterial infection and co-infections, especially for Pseudomonas aeruginosa and E. coli.
Our study showed a relatively low positive rate of blood culture (14.5%) and pus culture (25.0%). Culture-based techniques are the golden standard, the low positive rate of culture in our study could be attributed to sample collection after prior antibiotic treatment of pre-hospitalization. It is worth noting that the broad use of ultrasound-guild aspiration and mNGS leads to increase in the positive rate in the detection of pathogens. Seven patients were confirmed pathogens by mNGS. Significantly, there have been no previous reports of identifying Serratia marcescens, Fusobacterium nucleatum, and Bartonella henselae in PLA patients through mNGS. They were rare pathogens of PLA because of difficulty to identify through conventional methods. Furthermore, co-infection was identified by mNGS in one case. A review indicated that mNGS was proved valuable for the detection of rare and complex pathogens in culture-negative and undiagnosed cases [29]. A study in our center about the evaluation of mNGS for the pathogenic diagnosis showed the ability to identify pathogens from abscess and less affected by prior antibiotics [30, 31]. Zhang et al. reported that compared with the conventional method (57.5%) and culture (45.2%), mNGS (86.3%) showed a better evaluation of diagnostic ability in focal infection [31]. In addition, mNGS could better achieve a rational use of antibiotics (antimicrobial de-escalation and completely antibiotic regimen change). Applying this technology in clinical practice to gain more experience is our direction in the future.
According to our study, 50% of patients had an anti-infection regimen changed based on ceftriaxone and the least change presented when patients received cefoperazone/sulbactam or piperacillin/tazobactam based treatment. Currently, PLA is absent of empirical antibiotic guidelines. Empiric antibiotics should cover Streptococcus, staphylococcus, K. pneumoniae, E.coli and anaerobes, which are commonly seen in pediatric PLA [11].
In our study, almost all dead patients had predisposing factors and extrahepatic organs involved. Four patients died of multiple organ failure caused by serious infection and three patients died of progression of underlying diseases, suggesting that early diagnosis and treatment of disseminated infections and control of underlying diseases were extremely important to improve prognosis. The mortality rate of PLA in pediatrics has decreased from nearly 15% in the 1980s to even null, which was considerably lower than in adults [3, 11, 27]. Research indicated about 15.7% of HA patients develop complications, which can be accused of most death [32]. A study showed that age-related leukocytosis, neutrophilia, elevated aspartate transaminase or alanine transaminase, hypoalbuminemia and abscess size of more than 80 cc at presentation are predictors of poor outcomes in pediatric liver abscess [33].
In conclusion, PLA is more likely to occur in patients with predisposing factors, especially in an immunosuppressed state. Patients with extrahepatic organ involvement and hepatomegaly were relative with poor prognosis. The combined complication of mNGS and culture could increase the positive rate of pathogen detection.
Supplementary Information
Acknowledgements
We would like to thank the Medical Records and Statistics Room, Beijing Children’s Hospital, which provided hospital discharge data. We want to express our gratitude for the drawing materials provided by BioRender.
Abbreviations
- PLA
Pyogenic liver abscess
- HA
Hepatic abscess
- mNGS
Metagenomic next-generation sequencing
- S. aureus
Staphylococcus aureus
- E. coli
Escherichia col i
- K. pneumonia
Klebsiella pneumonia
- DM
Diabetic mellitus
- IQR
Interquartile range
- OR
Odds ratio
- CI95%
Confidence interval at 95%
- WBC
White blood cell
- PCT
Procalcitonin
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- ALT
Transaminase
- ICU
Intensive care unit
- PID
Primary immunodeficiency disease
- CGD
Chronic granulomatous disease
- HGB
Hemoglobin
- P. aeruginosa
Pseudomonas aeruginosa
- CECT
Contrast-enhanced computed tomography
- MRI
Magnetic resonance imaging
- SCF
Cefoperazone/sulbactam
- TZP
Piperacillin/tazobactam
- VA
Vancomycin
- LZD
Linezolid
- MTZ
Metronidazole
- MEM
Meropenem
- CRO
Ceftriaxone
- RFP
Rifampicin
- AZM
Azithromycin
- MRSA
Methicillin-resistant staphylococcus aureus
Authors’ contributions
All the authors had access to the full dataset (including the statistical reports and tables) and take responsibility for the integrity of the data and the accuracy of the data analysis. G.L, L.G and Y.X conceived and designed the study. Y.X, L.G, B.L, H.H, B.H, T.C, S.Q and M.H were involved in the case and sample collection, designed the analysis, and interpreted the data. Y.X wrote the first draft of the paper. Y.X, L.G and G.L reviewed and approved the final report. All authors have read and approved the final manuscript.
Funding
This work was supported by Capital's Funds for Health Improvement and Research (2024–1-2092), 2022 Beijing Major Epidemic Prevention and Control Specially Construction Project (2–1-2–6-15), Beijing Municipal Administration of Hospitals Incubating Program (PX2024042).
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of Beijing Children’s Hospital Affiliated to Capital Medical University (2019-k-374). Informed consent to participate was waived by the Ethics Committee of Beijing Children’s Hospital Affiliated to Capital Medical University because this was a retrospectively study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yue Xie and Ling-yun Guo contributed equally to this work.
References
- 1.Kirtisudha M, Srikanta B, Subhasis R, et al. Liver abscess in children: an overview.[J]. World J Clin Pediatr: WJP. 2010;6(3):210–6. [DOI] [PubMed]
- 2.Kumar A, Srinivasan S, Sharma AK. Pyogenic liver abscess in children–South Indian experiences [J]. J Pediatr Surg. 1998;33(3):417–21. 10.1016/S0022-3468(98)90081-1 [DOI] [PubMed] [Google Scholar]
- 3.Vm P, Jm A. Morbidity and mortality in children with pyogenic liver abscess. Am J Infect Di Child (1960). 1989;143(12):1424–7. [DOI] [PubMed] [Google Scholar]
- 4.Svensson E, Jonsson A, Blackberg A, et al. Increasing incidence of pyogenic liver abscess in Southern Sweden: a population-based study from 2011 to 2020[J]. Infect Dis (Lond). 2023;55(6):375–83. 10.1080/23744235.2023.2190813 [DOI] [PubMed] [Google Scholar]
- 5.Kong MS, Lin JN. Pyogenic liver abscess in children[J]. J Formos Med Assoc. 1994;93(1):45–50. [PubMed] [Google Scholar]
- 6.Tsai C, Chung J, Ko S, et al. Liver abscess in children: a single institutional experience in southern Taiwan. Acta Paediatr Tw. 2003;44(5):282–6. [PubMed] [Google Scholar]
- 7.Ferreira MAB, Pereira FEL, Musso C, et al. Pyogenic liver abscess in children: Some observations in the Espirito Santo State, Brazil[J]. Arq Gastroenterol. 1997;34(1):49–54. [PubMed] [Google Scholar]
- 8.Luo M, Yang XX, Tan B, et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis.[J]. Eur J Clin Microbiol Infect Dis. 2016;35(10):1557–65. [DOI] [PMC free article] [PubMed]
- 9.Muorah M, Hinds R, Verma A, et al. Liver abscesses in children: a single center experience in the developed world.[J]. J Pediatr Gastroenterol Nutri. 2006;42(2):201–6. [DOI] [PubMed]
- 10.Mavilia MG, Molina M, Wu GY. The Evolving Nature of Hepatic Abscess: A Review[J]. J Clin Transl Hepatol. 2016;4(2):158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aravind T, Kishore U K, Jasmine K, et al. Incidence Trends, Comorbidities and Outcomes of Pyogenic Liver Abscess Among Children. A Nationwide Population-Based Analysis.[J]. Journal of pediatric gastroenterology and nutrition, 2020. [DOI] [PubMed]
- 12.Lal SB, Venkatesh V, Kumar A, et al. Liver Abscess in Children-experience From a Single Tertiary Care Center of North India: Etiology, Clinical Profile and Predictors of Complications[J]. Pediatr Infect Dis J. 2021;40(5): e179. 10.1097/INF.0000000000003053 [DOI] [PubMed] [Google Scholar]
- 13.Yeh PJ, Chen CC, Lai MW, et al. Pediatric Liver Abscess: Trends in the Incidence, Etiology, and Outcomes Based on 20-Years of Experience at a Tertiary Center[J]. Front Pediatr. 2020;8:111. 10.3389/fped.2020.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra K, Basu S, Roychoudhury S, et al. Liver abscess in children: an overview[J]. World J Pediatr. 2010;6(3):210–6. 10.1007/s12519-010-0220-1 [DOI] [PubMed] [Google Scholar]
- 15.Yu HX, Lin GS, Zhang JF, et al. Clinical Characteristics of 606 Patients with Community-Acquired Pyogenic Liver Abscess: A Six-Year Research in Yantai[J]. Infect Drug Resist. 2022;15:7067–75. 10.2147/IDR.S372360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo JJ, Lee TK, Kyoung DS, et al. A population-based study of pyogenic liver abscess in Korea: Incidence, mortality and temporal trends during 2007–2017[J]. Liver Int. 2021;41(11):2747–58. 10.1111/liv.15034 [DOI] [PubMed] [Google Scholar]
- 17.S A, L M. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency.[J]. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 2013,11(9):1050–1063. [DOI] [PMC free article] [PubMed]
- 18.Pilania RK, Rawat A, Vignesh P, et al. Liver Abscess in Chronic Granulomatous Disease-Two Decades of Experience from a Tertiary Care Centre in North-West India[J]. J Clin Immunol. 2021;41(3):552–64. 10.1007/s10875-020-00938-9 [DOI] [PubMed] [Google Scholar]
- 19.Pandey A, Rajeshwari K, Kumar D, et al. Assessment of risk factors in pyogenic liver abscesses in children[J]. Afr J Paediatr Surg. 2023;20(3):218–23. 10.4103/ajps.ajps_15_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.W L, H C, S W, et al. A comparison of pyogenic liver abscess in patients with or without diabetes: a retrospective study of 246 cases.[J]. BMC gastroenterology, 2018,18(1):144. [DOI] [PMC free article] [PubMed]
- 21.Du Zhao-Qing, Li-Na Z, Qiang L, et al. Clinical Charateristics and Outcome of Pyogenic Liver Abscess with Different Size: 15-Year Experience from a Single Center.[J]. Scientific reports, 2016,6. [DOI] [PMC free article] [PubMed]
- 22.Cristina S, Chiara E, Christian B, et al. Characteristics and management of pyogenic liver abscess: A European experience.[J]. Medicine. 2018;97(19):e0628. [DOI] [PMC free article] [PubMed]
- 23.Simeunovic E, Arnold M, Sidler D, et al. Liver abscess in neonates[J]. Pediatr Surg Int. 2009;25(2):153–6. 10.1007/s00383-008-2307-5 [DOI] [PubMed] [Google Scholar]
- 24.Dongmo Miaffo DOE, Chafaaoui H, Assan BR, et al. Neonatal pyogenic liver abscess following omphalitis: A case report[J]. Int J Surg Case Rep. 2023;110: 108711. 10.1016/j.ijscr.2023.108711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogasawara K, Ono M, Tamanuki K, et al. Respiratory Symptoms are the First Presentation of Liver Abscess[J]. Pediatr Infect Dis J, 2023. [DOI] [PubMed]
- 26.Li Y, Dong L, Gao W, et al. Hypervirulent Klebsiella pneumoniae Infections in Pediatric Populations in Beijing (2017–2019): Clinical Characteristics, Molecular Epidemiology and Antimicrobial Susceptibility[J]. Pediatr Infect Dis J. 2021;40(12):1059. 10.1097/INF.0000000000003253 [DOI] [PubMed] [Google Scholar]
- 27.Yu-Lung H, Hsiao-Chuan L, Ting-Yu Y, et al. Pyogenic liver abscess among children in a medical center in Central Taiwan.[J]. J Microbiol Immunol Infect. 2015;48(3):302–5. [DOI] [PubMed]
- 28.DR C, SS L, HR L, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea.[J]. The Journal of infection, 2007,54(6):578–583. [DOI] [PubMed]
- 29.Li N, Cai Q, Miao Q, et al. High-Throughput Metagenomics for Identification of Pathogens in the Clinical Settings[J]. Small methods. 2021;5(1):2000792. 10.1002/smtd.202000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Feng W, Guo X, et al. The advantages of next-generation sequencing technology in the detection of different sources of abscess[J]. J Infect. 2019;78(1):75–86. 10.1016/j.jinf.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 31.Zhang HC, Ai JW, Cui P, et al. Incremental value of metagenomic next generation sequencing for the diagnosis of suspected focal infection in adults[J]. J Infect. 2019;79(5):419–25. 10.1016/j.jinf.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 32.Chang-Hua C, Shung-Sheng W, Hung-Chi C, et al. Initial presentations and final outcomes of primary pyogenic liver abscess: a cross-sectional study.[J]. BMC gastroenterology, 2014,14. [DOI] [PMC free article] [PubMed]
- 33.Anand M, Sahi PK, Mandal A. Pediatric Liver Abscess: Outcomes of Protocol-based Management and Predictors of Poor Outcome[J]. Pediatr Infect Dis J. 2023;42(7):549–56. 10.1097/INF.0000000000003923 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.


