Abstract
The more insects there are, the more food there is for insectivores and the higher the likelihood for insect-associated ecosystem services. Yet, we lack insights into the drivers of insect biomass over space and seasons, for both tropical and temperate zones. We used 245 Malaise traps, managed by 191 volunteers and park guards, to characterize year-round flying insect biomass in a temperate (Sweden) and a tropical (Madagascar) country. Surprisingly, we found that local insect biomass was similar across zones. In Sweden, local insect biomass increased with accumulated heat and varied across habitats, while biomass in Madagascar was unrelated to the environmental predictors measured. Drivers behind seasonality partly converged: In both countries, the seasonality of insect biomass differed between warmer and colder sites, and wetter and drier sites. In Sweden, short-term deviations from expected season-specific biomass were explained by week-to-week fluctuations in accumulated heat, rainfall and soil moisture, whereas in Madagascar, weeks with higher soil moisture had higher insect biomass. Overall, our study identifies key drivers of the seasonal distribution of flying insect biomass in a temperate and a tropical climate. This knowledge is key to understanding the spatial and seasonal availability of insects—as well as predicting future scenarios of insect biomass change.
Keywords: arthropods, flying insect biomass, phenology, seasonality, spatial distribution, tropical and temperate climates
1. Introduction
Insects play a key role in natural as well as agricultural systems by providing ecosystem functions such as pollination and decomposition, and by offering a food source for a variety of organisms [1–3]. The quantity of food and ecosystem functions provided varies with the amount of active insect biomass in an ecosystem, which fluctuates through space and time [4,5]. While studies have described trends of insect biomass across years for various regions [6–8], we lack insights into the seasonal dynamics of insect biomass across large spatial scales, as well as into the drivers of these spatial and seasonal distributions. Since all trophic layers directly or indirectly depend on insects, the timing and availability of insects throughout the year affects entire ecosystems, and understanding their drivers is thus a priority [9,10].
Climatic conditions are important drivers of the spatial distribution of insect biomass [11,12]. The temperature of the environment determines the rates of insect development, reproduction and activity and positively contributes to the available energy in the system [13]. Therefore, warmer regions are generally expected to sustain larger insect populations compared with colder regions [14], though temperatures above the thermal maxima of insects will have negative effects [15]. Insect clades with a narrower thermal tolerance—like tropical insects adapted to relatively stable temperature regimes—will be more sensitive to changes in temperature [16,17]. Water availability can also affect insect abundances, either directly by increased risk of desiccation during droughts, or indirectly by reducing the availability of nectar, foliage or pollen or by promoting the spread of fungal pathogens across insects [18–20]. Conversely, extreme rainfall will limit periods of insect flight, and could increase insect mortality [21,22]. In tropical climates, temperatures are more constant throughout the year, and unlikely to fall below the thermal minima of insects [23]. Thus, water availability is likely a more important driver of spatial variation in insect abundances in the tropics [24,25].
Besides climate, different habitat types can cause spatial heterogeneity of flying insect biomass, and human-induced alterations of landscapes could have a large influence on the occurrences and abundances of insects [11,26]. Studies report less flying insect biomass in urban areas [27], but higher biomass in non-irrigated agricultural lands, pastures and orchards compared with more natural landscapes such as forests. Such contrasts may be partly due to increased insect movement in more open landscapes [12]. Studies conducted at larger spatial scales are scarcer in tropical regions [28–30], and mostly focus on specific taxa rather than entire insect faunas [31,32]. Overall, we lack insights into general drivers behind insect biomass distributions in the tropics. Hence, to identify general patterns and drivers of the spatial distribution of insect biomass, we need large-scale sampling efforts across multiple countries, including both temperate and tropical climates [33].
Seasonality (i.e. within-year temporal dynamics) determines the quantity of flying insect biomass through time. The seasonality of insects may vary across climatic gradients; that is, regions may differ in terms of their seasonal amplitude (i.e. difference between highest and lowest insect biomass), and in the timing and speed of increases and decreases in insect abundances. Since insects are ectotherms, accumulated heat may explain spatial differences in their seasonality in temperate zones, with earlier growing seasons and higher peaks in species abundances in warmer compared with colder regions [34,35]. Another driver of specific importance to the temperate zone is snow cover. Since most insects will spend their diapause phase in or close to the soil, snow may determine overwintering conditions. It can isolate the soil from extremely cold temperatures, protect against direct exposure to solar radiation, and contribute to soil moisture once the snow melts [36–38]. Periods of seasonal activity tend to be longer in tropical than in temperate climates, with less pronounced peaks in species abundances [5]. Still, seasonality occurs in the tropics, too, where insect biomass tends to be highest during the rainy season [5]. How local communities respond to the dry and wet season can also vary with the landscape. In dry forests, insect biomass may increase faster at the start of the rainy season than in rainforests [39]. Moreover, insect abundances may decrease more during the dry season in hotter and drier regions than in cooler regions, owing to accentuated desiccation risks [24]. Conversely, hot regions may sustain higher insect abundance during the rainy season, when accumulated heat promotes insect activity without desiccation risks [5]. While seasonality has been studied for certain groups of insects, like butterflies and beetles [40–42], we lack a more comprehensive understanding of trends in insect seasonality in relation to climatic and environmental gradients across temperate and tropical zones [43]. In particular, we need large-scale studies across zones conducted throughout the year.
While seasonality trends might explain much variation in flying insect biomass throughout the year, short-term fluctuations in weather conditions could add variation at a daily or weekly scale. For example, insect activities could increase or decrease with day-specific temperature [44,45] or drop during rainy periods [46]. During periods of drought, lowered water availability could increase insect or host plant desiccation and decrease activity [19]. Identification of such short-term drivers will further refine our understanding of the seasonal availability of insects. Nonetheless, compared with the effects of monthly or yearly climatic conditions [5,12,47,48], the effects of short-term weather fluctuations on flying insect biomass remain poorly explored [40].
We aimed to describe the spatial distribution of overall, yearly flying insect biomass, as well as the seasonality of flying insect biomass. To uncover general drivers behind spatial and seasonal patterns, we combine a massive survey of the entire insect fauna of a temperate and a tropical country, Sweden and Madagascar. Specifically, our research questions targeted both regional patterns within Madagascar and Sweden, and a comparison between the two climatic zones:
-
1. How is flying insect biomass distributed through space?
- a. What are the climatic drivers (accumulated heat, snow depth, rainfall, soil moisture) behind the spatial distribution in biomass?
- b. Is any residual variation in the spatial distribution of flying insect biomass explained by landscape characteristics (habitat type and vegetation cover)?
-
2. How is flying insect biomass distributed through time?
- a. What are the drivers behind spatial variation in insect seasonality, in terms of local climate and landscape? That is, does insect seasonality differ between warmer and colder sites, snowier and less snowy sites, wetter and drier sites, more vegetated and less vegetated sites, and among habitat types?
- b. Can short-term variations in weather conditions, including accumulated heat, rainfall and soil moisture, explain residual variation in flying insect biomass across seasons?
For our a priori expectations related to these questions, see electronic supplementary material, table S1. By intensively sampling a tropical and a temperate country through space and time, our study provides a first and unique insight into the spatiotemporal dynamics of insects in tropical and temperate zones.
2. Material and methods
(a) . Study area and data collection
We sampled insects during one year across a temperate (Sweden; 450 000 km2; latitude 55.3° to 69.1°) and a tropical (Madagascar; 590 000 km2; latitude −25.6° to −12.0°) country (electronic supplementary material, figure S1). The climate in Sweden ranges from oceanic to sub-Arctic, in Madagascar from tropical humid to dry tropical.
Insects were collected with Malaise traps and preserved in 95% ethanol. Site selection in Sweden followed a stratified design based on the major habitat types: forests, grasslands, croplands, alpine and urban areas [49] (electronic supplementary material, figure S1). The proportion of Malaise traps placed in each habitat was decided based on the approximate area covered by each of these habitat types in Sweden. To ensure sufficient replication for each of the habitat types, sampling in rarer habitats was up-weighted (for croplands and wetlands, each covering 8% of area in Sweden, and grasslands and urban areas, each covering 3% of area in Sweden), while sampling in forest sites (covering 60% of area in Sweden) was down-weighted. In Madagascar, traps were placed in both rainforests and dry forests within protected areas (electronic supplementary material, figure S1). Rather than selecting hot-spots of particularly high insect activity, we immersed the traps within the habitats of interest (for details, see supplementary electronic supplementary material, text S1)—an approach that enabled comparisons among sites. In Sweden, we ran 195 Malaise traps from January to December 2019; in Madagascar, we ran 50 Malaise traps from August 2019 to July 2020. Samples were collected and weighed at weekly to biweekly intervals (electronic supplementary material, text S1), with the frequency of sample collection in Sweden adjusted to the season. Within seasons, all samples were collected with the same intervals. By this approach, we avoided any saturation of samples, while keeping sampling times standardized between traps. After collection, none of the samples was filled to more than half of its volume with insects. Methods for wet-weighing of biomass were optimized as described in [50]. Samples were drained of ethanol and weighed to the nearest 0.001 g. For interpolation of missing values, see electronic supplementary material, text S2. Total flying insect biomass per trap—henceforth ‘total biomass’—was obtained by summing the biomass of all samples taken at each trap, then dividing this sum by the total number of sampling days per trap, hence deriving the flying insect biomass per trap per day (electronic supplementary material, table S2 and text S2).
(b) . Climate and landscape variables
(i) . Climate variables
To characterize the local climate, we extracted climate data from the ERA5-Land database (ECMWF, Copernicus) [51]. The data extracted included temperature, precipitation and soil moisture for all sites in Sweden and Madagascar, and snow depth for sites in Sweden (for details, see electronic supplementary material, text S3 and figure S2). From hourly temperature data, we calculated day- and site-specific growing degree days (GDD5; henceforth ‘accumulated heat’) as:
Here, we adopted the base value of 5 for calculating growing degree days, as insect activity and growth have been shown to dramatically slow down below 5°C [52–56].
(ii) . Landscape variables
In Sweden, we distributed Malaise traps among forest, grassland, cropland, wetland, urban areas and alpine areas (as defined by Ståhl et al. [49]) in rough proportion to the national extent of these habitats (see 'Study area and data collection' (§2a)). In Madagascar, traps were placed in the two major forest types: tropical dry forests and tropical rainforests. As further characterizations of habitat, we collected data on vegetation cover from the ERA5-Land database (for details, see electronic supplementary material, text S3). Since all traps in Madagascar were situated in forests, we collected additional data on forest cover (within 1 km2 from the trap) from the ERA5-Land database, and percentage canopy cover above each trap (for details, see electronic supplementary material, text S3).
(c) . Statistical analyses
Statistical analyses were conducted in R v.4.2.0 [57]. We used the package lme4 to fit linear mixed models [58], and the Anova function in the car package to assess significance of the models [59]. Model assumptions were evaluated using the sjPlot package [60].
(i) . Patterns and drivers of spatial distribution of biomass
To relate the spatial distribution of flying insect biomass to regional climate, we modelled total biomass as a function of accumulated heat, rainfall and soil moisture for both Sweden and Madagascar, using linear models (electronic supplementary material, table S2). For Sweden, where all sites receive some snowfall during winter, we added snow depth as an additional predictor. To select a model that best described spatial drivers, we used forward-selection which allowed us to add variables in the following order: accumulated heat, snow depth (only in Sweden), rainfall and soil moisture. The order of addition reflected the a priori perceived importance of these drivers, with accumulated heat capturing the overall constraint of energy available to ectotherms, snow depth reflecting the insulative layer for diapausing insects, as well as moisture available to insects upon snow melt, rainfall reflecting overall water availability, and soil moisture reflecting water availability in the soil (electronic supplementary material, table S1). Since the impact of accumulated heat may differ depending on the amount of rainfall, we also included the interaction between accumulated heat and rainfall. Added variables were retained in the model if the Akaike information criterion (AIC) of the model with the new variable included was markedly lower than the AIC of the previously fitted model . For the terms selected in the final models, see electronic supplementary material, table S2. To investigate whether differences in total flying insect biomass between Sweden and Madagascar were caused by the different habitats sampled in each region, we also fitted the spatial model (as described above) with data from forests only for both Sweden and Madagascar.
To investigate whether unexplained variation in biomass could be due to differences in local landscapes, we modelled the residuals as a function of landscape characteristics. For Sweden, we explored the effect of habitat type, i.e. grassland, cropland, forest, wetland, urban and alpine, and of vegetation cover. For Madagascar, we explored the effect of habitat type (dry versus rain forest), as well as the effect of vegetation cover, percentage forest cover and canopy cover.
(ii) . Patterns and drivers of temporal distribution of biomass
To relate insect seasonality to climatic and landscape predictors, we modelled biomass per day per trap as a function of ‘seasonality’ with the periodic functions and , where d is the Julian day of the year. To explore whether seasonality is affected by environmental predictors, we added interactions between seasonality and all environmental variables to the model. Again, we used forward-selection with the retention criteria above, adding terms in the order of the annual models: accumulated heat and its interaction with seasonality, snow depth and its interaction with seasonality (only in Sweden), rainfall and its interaction with seasonality, soil moisture and its interaction with seasonality, vegetation cover and its interaction with seasonality and habitat type and its interaction with seasonality (electronic supplementary material, table S2). To account for repeated sampling, trap ID was included as a random effect. For the terms selected in the final models, see electronic supplementary material, table S2.
(iii) . Drivers of short-term deviations in biomass
To investigate whether week-to-week fluctuations in weather explain the variation in insect biomass that cannot be attributed to seasonal trends, we modelled the residuals of the temporal models above as a function of weekly weather conditions (i.e. weekly averages of accumulated heat, rainfall and soil moisture, from the week preceding sample collection; electronic supplementary material, table S2), with trap ID added as a random effect.
3. Results
(a) . Spatial variation in climate and flying insect biomass in Sweden and Madagascar
Madagascar was characterized by a higher annual mean of accumulated heat, more annual rainfall, higher annual soil moisture levels and more annual vegetation cover than Sweden (electronic supplementary material, figure S3 and table S3). Accumulated heat and rainfall were more variable in space in Madagascar than in Sweden (as shown by more than double standard deviations), whereas spatial variation in soil moisture and vegetation cover was relatively similar between countries (as shown by less than double standard deviations; electronic supplementary material, figure S3 and table S3).
Flying insect biomass per trap per day was not detectably different between Sweden and Madagascar (figure 1; electronic supplementary material, figures S3a and S4a), either for the across-habitat comparison (Sweden: 0.46 ± 0.25 g, Madagascar: 0.45 ± 0.16 g), or when comparing Swedish forests (0.40 ± 0.18 g) with Malagasy forests (0.45 ± 0.16 g) (electronic supplementary material, table S4). Spatial variation in flying insect biomass was slightly higher across Sweden (s.d.: 0.25) than across Madagascar (s.d.: 0.16) (electronic supplementary material, figures S3b and S4b, and table S4). However, when focusing on forests alone, spatial variation in biomass was similar in Sweden and Madagascar (s.d.: 0.18 and 0.16 respectively; electronic supplementary material, figure S4c and table S4).
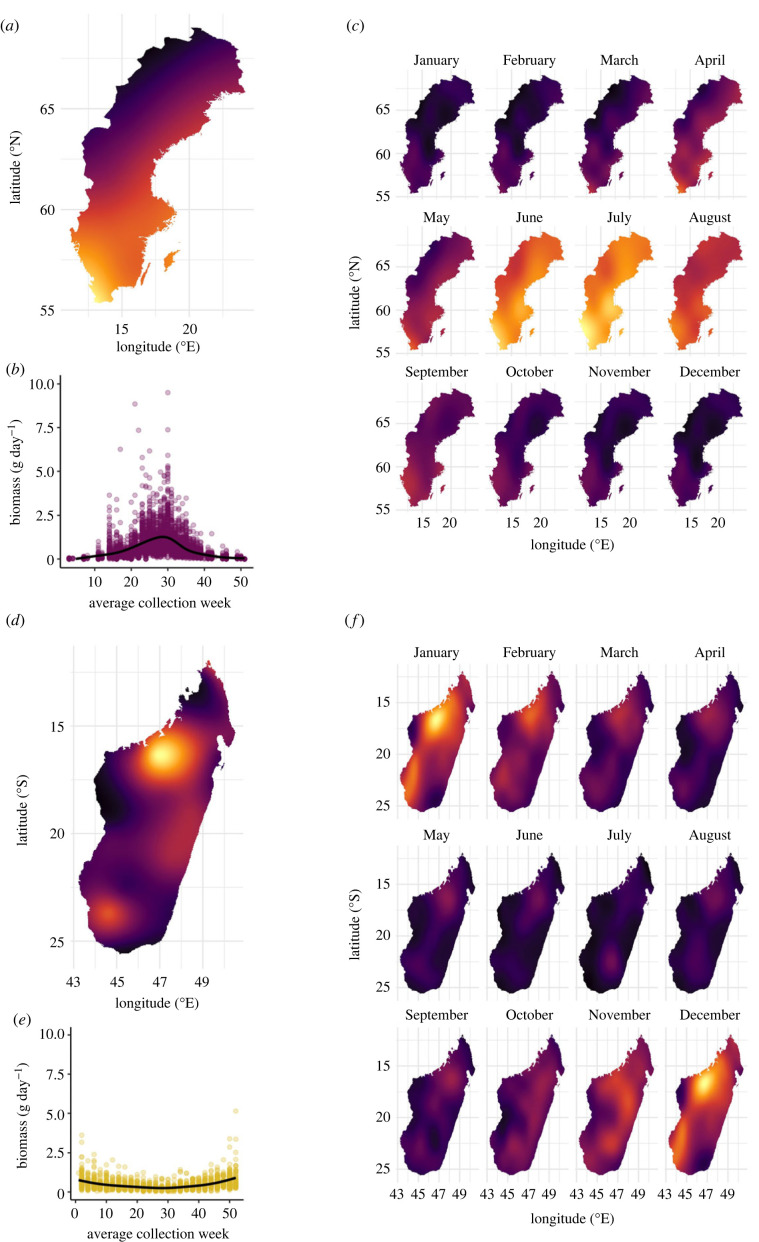
Figure 1.
Patterns in the spatial and seasonal distribution of insect biomass in Sweden and Madagascar. The predicted spatial distribution of total insect biomass is shown in (a) for Sweden and (d) for Madagascar, with warmer colours indicating relatively higher values of flying insect biomass. Seasonal distribution is shown in (b) for Sweden and (e) for Madagascar. Here, purple (Sweden) and yellow (Madagascar) dots present the average biomass per day for each sample, during the average week of sample collection (week number of average Julian day, where average Julian day = . To visualize the seasonal pattern in active insect biomass, we fitted a smooth spline to the scatter. In Sweden, a few large biomass values occurred in spring, which are excluded from (b) to improve clarity of the overall pattern. For a figure including all biomass values, see electronic supplementary material, figure S5. (c,f) Spatiotemporal distribution of insect biomass throughout the year in Sweden and Madagascar respectively, with lighter (yellow) colours indicating higher values of flying insect biomass, and darker (purple) colours indicating lower values of flying insect biomass. Biomass predictions in (a,c,d,f) were based on generalized additive models. In (a,d), daily biomass was modelled as a function of latitude and longitude fitted as an interaction smooth. In (c,f), daily biomass was modelled as a function of latitude and longitude fitted as an interaction smooth, month fitted as a cyclic cubic regression spline, and the three-way interaction smooth between latitude, longitude and month fitted as a tensor product smooth with a cyclic cubic regression spline. For maps with raw data points, see electronic supplementary material, figure S5.
(b) . Drivers of the spatial distribution of flying insect biomass
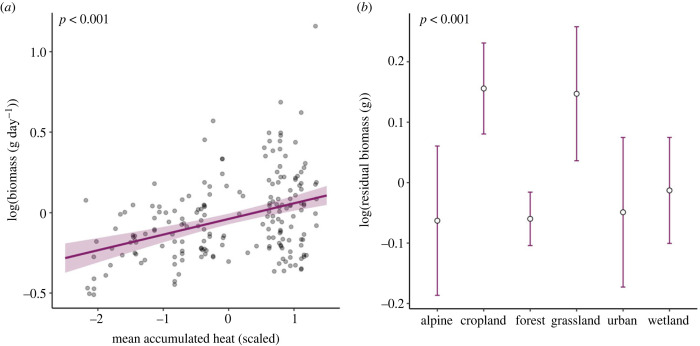
Flying insect biomass per trap per day in Sweden increased with mean annual accumulated heat (figure 2a, F1,189 = 32.49, p < 0.001), while snow depth, rainfall, soil moisture and the interaction between accumulated heat and rainfall were not retained in the model (electronic supplementary material, table S2). Differences in habitat type explained part of the residual variation in flying insect biomass (F5,184 = 6.42, p < 0.001), with the highest biomass of flying insects observed in croplands and grasslands (figure 2b; electronic supplementary material, table S5a). Vegetation cover had no significant effect on residual variation in flying insect biomass (electronic supplementary material, table S5a).
Figure 2.
The effect of climate and habitat on total insect biomass in Sweden during 2019. Effect of (a) mean accumulated heat on the yearly total insect biomass, and (b) habitat types on the residual variation in biomass after accounting for the effect of accumulated heat. In (a), the solid trendline presents the significant relationship predicted by the model. In (b), circles represent estimated means with confidence intervals in purple for each habitat type as based on model predictions. The p-values of predictors are presented in the upper left corners. For model output related to this figure, including p-values, F-values and degrees of freedom, see electronic supplementary material, table S5a.
In Madagascar, mean annual accumulated heat had no detectable effect on flying insect biomass per trap per day (electronic supplementary material, figure S6a, F1,48 = 0.89, p = 0.35). Rainfall, soil moisture and the interaction between accumulated heat and rainfall were not retained in the model (electronic supplementary material, table S2). Residual variation in the spatial distribution of flying insect biomass was not detectably affected by habitat type, vegetation cover, forest cover or canopy cover (electronic supplementary material, figure S6b–d and table S5b).
(c) . Temporal variation in climate and flying insect biomass in Sweden and Madagascar
Temporal variation in accumulated heat and vegetation cover was higher in the temperate zone, while temporal variation in rainfall and soil moisture was higher in the tropics (electronic supplementary material, figure S3 and table S3).
In Sweden, flying insect biomass varied significantly more throughout the year than it did in Madagascar, both across habitats (standard deviation per trap: Sweden 0.65 ± 0.21 g Madagascar 0.30 ± 0.16 g, p < 0.001; figure 1; electronic supplementary material, figure S3b and table S4) and between Swedish and Malagasy forests (standard deviation per trap Swedish forests: 0.58 ± 0.12 g, Malagasy forests 0.30 ± 0.16 g; electronic supplementary material, figure S4d and table S4).
(d) . Drivers of the temporal distribution of flying insect biomass
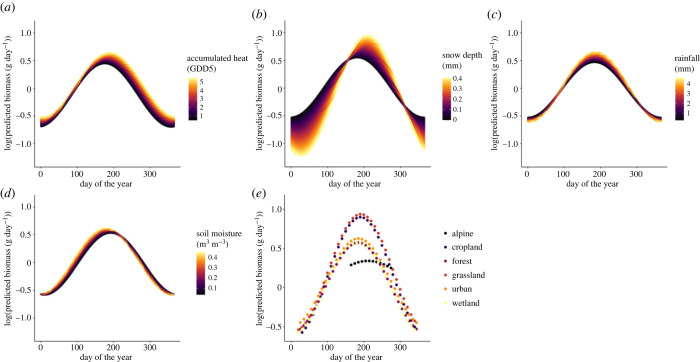
In Sweden, seasonality of insects was significantly influenced by accumulated heat, snow depth, rainfall, soil moisture and habitat type (figure 3a–d; electronic supplementary material, table S6a), while vegetation cover was not retained in this model (electronic supplementary material, table S2). Locations with a higher yearly mean of accumulated heat had a higher peak in flying insect biomass during the growing season. Such locations also showed a later decrease in biomass at the end of the growing season, and sustained slightly higher biomass during the off-season (figure 3a). Locations with higher mean annual snow depth had lower biomass during winter, and started to accumulate biomass later during the growing season (figure 3b). Interestingly, locations with more snow during the winter were also characterized by higher peak biomass during the late summer (figure 3b). Locations with more annual rainfall had higher flying insect biomass during summer, but lower biomass during winter (figure 3c). Locations with higher soil moisture tended to accumulate biomass slightly earlier than drier locations (figure 3d). Seasonality also differed among habitats, where grasslands and croplands had the highest peak biomass, while alpine areas had the lowest (figure 3e). Moreover, biomass in alpine areas peaked slightly later during the growing season than in any other habitat (figure 3e).
Figure 3.
The predicted effects of climatic and landscape variables on insect seasonality in Sweden. Predicted seasonal trends of insect biomass in response to (a) yearly mean accumulated heat, (b) yearly mean snow depth, (c) yearly mean rainfall, (d) yearly mean soil moisture and (e) habitat type. Shown are marginal effects, that is, the impact of the focal factor with all other factors fixed at their mean level. Colour gradients represent the range of values of each of the environmental predictors, with lighter colours indicating higher values. The x-axis displays the day of the year, where day 1 is 1 January. For each of the habitat types (e), we only show predictions for the time-period during which sampling took place in a particular habitat. For model output related to this figure, including p-values, χ2-values and degrees of freedom, see electronic supplementary material, table S6a.
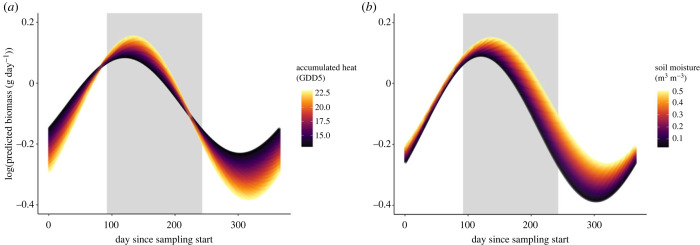
In Madagascar, the seasonality of insects was significantly influenced by accumulated heat and soil moisture (figure 4, electronic supplementary material, table S6b), while rainfall, vegetation cover and habitat type were not retained as predictors (electronic supplementary material, table S2). Locations with a higher annual mean of accumulated heat had higher flying insect biomass during the rainy season, but less biomass during the dry season (figure 4a). Locations with higher annual soil moisture had higher flying insect biomass throughout the year compared to drier locations (figure 4b). The flying insect biomass of both wet and dry locations peaked during the start of the rainy season, but wetter locations sustained a longer period of peak insect activity (figure 4b).
Figure 4.
The predicted effects of climatic and landscape variables on insect seasonality in Madagascar. Predicted seasonal trends of insect biomass in response to (a) accumulated heat and (b) soil moisture. Shown are marginal effects, that is, the impact of the focal factor with all other factors fixed at their mean level. Colour gradients represent the range of values of each of the environmental predictors, with lighter colours indicating higher values. The x-axis displays the day number since the start of sample collection, where day 1 is 1 August. The grey area in the plot indicates the rainy season (November to May). For model output related to this figure, including p-values, χ2-values and degrees of freedom, see electronic supplementary material, table S6b.
(e) . Drivers of short-term deviations in biomass
In Sweden, residual variation in the seasonal distribution of biomass could be explained by weekly fluctuations in weather. Warmer, moister periods had higher flying insect biomass and periods with more rain led to lower flying insect biomass (electronic supplementary material, figure S7 and table S7a).
In Madagascar, weekly fluctuations in soil moisture left the only statistically detectable imprint on residual variation in the seasonal distribution of biomass. Here, weeks with higher soil moisture had higher flying insect biomass than expected from seasonality trends alone (electronic supplementary material, figure S8c and table S7b). Weeks with higher accumulated heat tended to show higher relative biomass, but this effect was not statistically significant (electronic supplementary material, figure S8a and table S7b). Fluctuations in rainfall explained no significant part of the residual variation in flying insect biomass (electronic supplementary material, figure S8b and table S7b).
4. Discussion
By intensively sampling the insect fauna across a tropical and a temperate country, we were able to reveal general as well as region-specific patterns and drivers of flying insect biomass distribution and seasonality (figure 5). While the amount of flying insect biomass is often assumed to be higher in tropical than temperate regions, we found a surprising convergence in daily averages across climatic zones. Nonetheless, temporal variation in biomass was higher for the temperate than for the tropical zone. In terms of the spatial distribution of flying insect biomass, the drivers proved different for the temperate and tropical zones, whereas in terms of insect seasonality, the drivers were partly similar. In particular, accumulated heat and water availability affected insect seasonality in both climate zones. Besides seasonal patterns, weeks with higher soil moisture showed higher flying insect biomass in both the temperate and tropical zone, while warmer weeks with less rain explained increases in biomass only in the temperate zone. While our study provides seminal insights into the patterns and drivers of the spatial and temporal distribution of insect faunas in a tropical and temperate region, future studies across multiple countries are needed to confirm the generality of our findings across a wider range of latitudes.
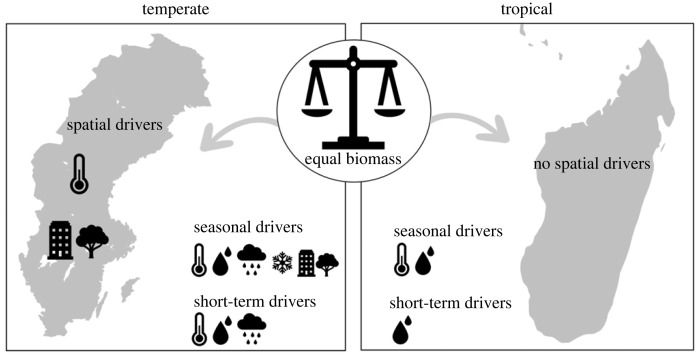
Figure 5.
Visual overview of the main findings of this study. The average weight of insect biomass across the year was similar for the temperate region (Sweden, left) and the tropical region (Madagascar, right). In the temperate region, spatial variation in insect biomass was driven by accumulated heat and habitat type, whereas in the tropical region, none of the candidate drivers measured had a detectable impact on biomass. In the temperate region, seasonal variation in insect biomass was driven by accumulated heat, soil moisture, rainfall, snow depth and habitat type, whereas in the tropical region, seasonal variation was driven by accumulated heat and soil moisture. In the temperate region, short-term variation in insect biomass was driven by accumulated heat, soil moisture and rainfall, whereas in the tropical region, short-term variation was driven by soil moisture.
(a) . Local biomass is similar in Sweden and Madagascar
While a recent meta-analysis suggests the biomass of soil arthropods to be higher in tropical forests than temperate forests or grasslands [7], we found no difference in the biomass of flying insects between Sweden and Madagascar. This observation fills a key knowledge gap left open by Rosenberg et al. [7], as previous studies of aboveground arthropods proved too scarce to compare biomasses across biomes. Indeed, previous studies of flying insect biomass have primarily been focused on the temperate zone [6,11,12]. As these earlier studies sampled insects only during the growing season, they tend to overestimate year-round biomass, which precludes reliable comparisons with the tropical zone. Our year-round sampling campaign allows a direct comparison of total flying insect biomass between a temperate and tropical country. However, since our sampling in Madagascar was explicitly focused on forests, whereas sampling in Sweden included other habitat types too, we should pay special attention to the comparisons among forests alone. This comparison showed similar flying insect biomasses per day in Swedish and Malagasy forests. Notably, equal amounts of year-round biomass in the temperate and tropical region do not translate into similarities in species richness, diversity or abundances, as similar amounts of biomass may represent vastly different numbers of species or individuals. Moreover, Masteller [61] observed that insects were typically smaller in a tropical (Puerto Rico) than a temperate (Pennsylvania) site. Thus, any given mass of insect biomass collected in the tropics could contain more insects than the same mass collected from a temperate region.
In terms of spatial variation in flying insect biomass, we found slightly more variation in Sweden than in Madagascar. This general contrast can be attributed to a larger variety of habitat types being sampled in Sweden, since no differences emerged among Swedish and Malagasy forests. To gain reliable estimates of flying insect biomass across biomes without biases towards seasons, taxa or habitat types [7], extensive sampling campaigns such as ours are urgently needed. Future projects should aim at year-round sampling, while covering biomes across the globe.
(b) . Drivers of spatial variation in biomass differ between Sweden and Madagascar
In Sweden, local flying insect biomass increased with accumulated heat, and was higher in grasslands and croplands than in forests, wetlands, alpine or urban areas. In Madagascar, the spatial distribution of flying insect biomass was unrelated to any of the climate or landscape predictors measured. This difference between zones seems reflective of the additional climatic constraints on insect physiology imposed by temperate conditions. In the temperate zone, low temperatures are likely to limit insect survival and activity [62] (electronic supplementary material, figure S3), and other studies in the temperate zone have reported equally strong impacts of temperature on insect biomass [6,11,12,63]. By comparison, tropical temperatures are typically high enough to sustain insect activities year-round [5] (electronic supplementary material, figure S3). Given that temperature affected the spatial distribution of temperate but not tropical insect biomass, one could predict that insect biomass in the temperate zone will be more sensitive to climate change compared with insect biomass in the tropics. Nevertheless, tropical insects tend to have narrower windows of thermal tolerance than their temperate counterparts [16]. Thus, tropical insects may be disproportionally sensitive to changes in temperature, and future increases in temperature may thus prove harmful to both temperate and tropical insects. As a further alternative, the spatial distribution of flying insect biomass in the tropics may also be determined by biotic rather than abiotic conditions [64]—a hypothesis worth further exploration.
With climatic effects accounted for, the amount of flying insect biomass differed among habitat types in Sweden. We found flying insect biomass to be highest in open, semi-natural and agricultural landscapes (grasslands and croplands), but lower in forests, urban areas, wetlands and alpine areas—a pattern that is fully or partially consistent with other findings from the temperate zone [11,12]. Grasslands and croplands can potentially sustain higher flying insect biomass owing to high plant productivity [65], with added effects of fertilization in croplands [66]. Alternatively, the open landscape of grasslands and croplands might promote insect movement, which could increase local trap catches [67]. Insect communities in forests are more vertically stratified, from the forest floor to the canopy [28], and trap catches may thus underestimate the overall biomass [68,69]. In the tropics, we found no differences in flying insect biomass between dry forest and rainforest, and no effect of vegetation cover, after accounting for climatic conditions. Hence, even though species richness and diversity are generally assumed to be higher in rainforests than dry forests [70], we saw no such pattern in total biomass. All in all, our results illustrate that human land use changes can cause shifts in the amount of insect biomass. Based on our findings, increased urbanization in the future could lead to decreases in local insect biomass, while agricultural expansion may instead increase local insect biomass. Notably, increases in insect biomass due to land-use changes can still go hand-in-hand with impoverishment of insect species richness and diversity [11].
(c) . Drivers of insect seasonality partly converge between countries
Consistent with earlier studies [71,72], we found seasonal fluctuations of flying insect biomass to be more pronounced in the temperate than tropical zone. Nonetheless, the underlying drivers appeared partly similar. In both Sweden and Madagascar, the seasonality of flying insect biomass differed between warmer and colder sites as well as between wetter and drier sites. In Sweden, seasonality also differed between sites with higher or lower snow depth in winter, and among habitat types. In the temperate zone, biomass during the growing season was highest in the warmest locations. Furthermore, flying insect biomass declined later in warmer locations than in colder locations. Both patterns are supported by previous findings regarding phenological responses of insects to temperature [34,35]. In the tropical zone, the effects of accumulated heat on insect seasonality were linked to the timing of the rainy and dry seasons. Warmer locations showed higher biomass during the rainy season, but lower biomass during the dry season than did cooler locations. Thus, while heat promotes insect activity during rainy periods, heat can accelerate desiccation, mortality or inactivity of insects during the dry season [5]. Based on our findings, we predict that insect seasonality could be strongly affected by rises in temperature [73]. In the temperate region, warming may increase the peak insect biomass during the growing season, and the end of the growing season may be extended. Yet, once thermal maxima are reached, this positive effect may well reverse [74]. As tropical insects are adapted to relatively stable temperature regimes, their thermal tolerances may be lower compared with those of temperate insects [16,17]. Hence, even slightly elevated local temperatures may exceed the thermal maxima of tropical insects, and could have negative effects on peak insect biomass—especially so for dryer regions [75]. For Sweden and other temperate regions, insects are moving northwards with climate warming [76,77]. In the tropics, opportunities for large-scale migration to cooler regions are more limited—and even more so for insects in insular regions, like Madagascar.
Water availability affected insect seasonality in both the temperate and tropical zones. In Sweden, rainy locations sustained more biomass during the growing season, but less during the off-season compared with less rainy locations. This pattern suggests accentuated benefits of precipitation during periods of limited water availability [78]. During the off-season, more rain may limit flying time and increase mortality [21,22]. Wet locations also showed an earlier start of the growing season, and a higher peak biomass during the growing season, than did drier locations. Soil moisture may thus be particularly important during the start of the growing season, when overwintering insects in the soil emerge, hatch or eclose [79,80]. Additionally, soil moisture might promote plant growth and vigour at the start of the growing season [81], favouring insects dependent on plants for food or shelter. In the tropics, soil moisture also had a strong effect on insect seasonality. Dry locations had lower flying insect biomass throughout the year, and this difference was most pronounced during the first half of the dry season. Dry and wet locations both peaked in flying insect biomass during the rainy season, but dry locations had a shorter period of insect activity and lower overall biomass. Tropical sites with higher water availability can thus sustain higher abundances of insects for longer periods of time [43]. As climate change is likely to cause prolonged droughts and more extreme episodes of rainfall in many regions [73], our results suggest that the timing and the amount of insect biomass are likely to change across zones.
In Sweden, snow depth emerged as another important driver of latitudinal differences in insect seasonality. Locations with more snow had lower biomass during the off-season, but higher biomass during the growing season. Deeper snow could increase soil moisture during spring and summer, and insulate diapausing insects in winter [36–38], with both mechanisms contributing to higher summer-time insect abundances. Our findings suggest that climate change could alter the seasonality and abundance of insects in temperate regions via changes in snow regimes. A reduction in snow cover is predicted under scenarios of climate change and has been observed empirically in Nordic countries [82]. This may increase insect mortality due to freezing in winter and lower water availability in summer, resulting in lower insect biomass during the growing season [36,37].
(d) . Abiotic factors explain short-term variation in biomass
Short-term deviations from the season-specific expected biomass in Sweden were attributable to week-to-week fluctuations in accumulated heat, rainfall and soil moisture. In Madagascar, only short-term fluctuations in soil moisture contributed to temporal variation in biomass. In line with our a priori expectations (electronic supplementary material, table S1), warmer periods with wetter soils showed more biomass than expected under Swedish conditions, while periods with more rainfall had less biomass than expected. In Madagascar, periods with wetter soils had more biomass than expected. While warmer periods tended to have more biomass, this effect was not significant. The weak or absent effect of temperature on short-term fluctuations in flying insect biomass in Madagascar matches the notion of lower climatic constraints on insect activity in the tropics. In Madagascar, temperatures are generally warm (electronic supplementary material, figure S3c) and relatively stable year-round (electronic supplementary material, figure S3d). By comparison, water availability emerges as a likely limiting factor for insect activity, especially during the dry season. This is supported by our finding of seasonality patterns—where the peak of flying insect biomass was higher for sites with higher soil moisture—and it is consistent with previous findings of higher insect biomass with moister conditions in the tropics [24,75]. Then again, weekly rainfall had no detectable effect on flying insect biomass in Madagascar, possibly owing to some conflicting impacts of rain on insect activity: on the one hand, more rain means more available water, which is expected to promote insect activity [83], but on the other hand, insect flight could be limited during periods of rainfall [46]. Given current predictions of massive changes in precipitation patterns with climate change [73], future studies should explore the trade-offs between positive and negative effects of precipitation on insect performance and seasonality.
5. Conclusion
Our study identified several key patterns and drivers of the spatial distribution and seasonality of temperate and tropical insect faunas (figure 5). Our findings have major implications for the impacts of climate change and land-use on the spatial distribution of insect biomass in temperate and tropical regions. While temperature affected the spatial distribution of insect biomass only in the temperate region, and not in the tropics, insects in both regions can still be affected by rising temperatures in the coming decades. Increased droughts and more extreme periods of rainfall—as predicted under scenarios of climate change—may negatively affect the total biomass of tropical as well as temperate insects. The timing and amplitude of seasonality curves are also likely to shift during the coming decades in response to climate change [84]. The direction of this shift is hard to predict, since shifting climate variables may have opposing effects on seasonality: based on our findings for the temperate zone, the insect growing season may be extended and peak higher in response to rising temperatures, while at the same time, snow melt may cause the growing season of insects to shift earlier and peak lower. Moreover, if rising temperatures exceed the thermal tolerances of temperate insects, impacts on seasonality could reverse. Altered seasonality could have consequences for the temporal availability of functions and food provided by insects. More generally though, such changes could lead to phenological mismatches across trophic levels, with implications for the functioning of the entire food web [85]. As a next step, future studies could aim to describe the cues that determine the emergence and decline, as well as peak abundance, of various taxa throughout seasons across climate zones. Ideally, such studies would focus on multiple taxa from different trophic levels, comparing their phenological changes in response to climate, and identifying or predicting the emergence of potential mismatches [85,86]. Such knowledge will enable us to determine whether the probability for mismatches differs across climatic zones. Uncovering the patterns and drivers of insect distribution and seasonality across geographical regions will allow us to understand the seasonal availability of insects to provide food and ecosystem functions, and inform predictions of future insect biomass declines across the globe.
Acknowledgements
We thank all volunteers, park guards and research assistants for help with the collection of malaise trap samples across Sweden and Madagascar—without them, this study could not have been implemented.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Data are archived in the Dryad repository [87].
Supplementary material is available online [88].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
L.J.A.v.D.: conceptualization, data curation, formal analysis, methodology, validation, visualization, writing—original draft, writing—review and editing; B.L.F.: investigation, resources, writing—original draft, writing—review and editing; A.M.: data curation, investigation, project administration, writing—review and editing; R.M.G.: writing—original draft, writing—review and editing; E.I.-E.: investigation, writing—review and editing; D.R.: investigation, writing—review and editing; E.T.R.: investigation, writing—review and editing; P.Ł.: writing—review and editing; A.F.A.: funding acquisition, writing—review and editing; F.R.: conceptualization, funding acquisition, resources, supervision, writing—original draft, writing—review and editing; T.R.: conceptualization, funding acquisition, resources, supervision, writing—original draft, writing—review and editing; A.J.M.T.: conceptualization, funding acquisition, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was supported by a grant from the Knut and Alice Wallenberg Foundation (KAW2017.088 to F.R., A.F.A., T.R. and A.J.M.T.) and a grant from the Swedish Research Council (2021-03784 to A.J.M.T.).
References
- 1.Crespo-Pérez V, Kazakou E, Roubik DW, Cárdenas RE. 2020. The importance of insects on land and in water: a tropical view. Curr. Opin. Insect Sci. 40, 31-38. ( 10.1016/j.cois.2020.05.016) [DOI] [PubMed] [Google Scholar]
- 2.Kagata H, Ohgushi T. 2006. Bottom-up trophic cascades and material transfer in terrestrial food webs. Ecol. Res. 21, 26-34. ( 10.1007/s11284-005-0124-z) [DOI] [Google Scholar]
- 3.Yang LH, Gratton C. 2014. Insects as drivers of ecosystem processes. Curr. Opin. Insect Sci. 2, 26-32. ( 10.1016/j.cois.2014.06.004) [DOI] [PubMed] [Google Scholar]
- 4.Buckley LB, Arakaki AJ, Cannistra AF, Kharouba HM, Kingsolver JG. 2017. Insect development, thermal plasticity and fitness implications in changing, seasonal environments. Integr. Comp. Biol. 57, 988-998. ( 10.1093/icb/icx032) [DOI] [PubMed] [Google Scholar]
- 5.Wolda H. 1980. Seasonality of tropical insects. J. Anim. Ecol. 49, 277-290. ( 10.2307/4289) [DOI] [Google Scholar]
- 6.Hallmann CA, et al. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12, e0185809. ( 10.1371/journal.pone.0185809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg Y, Bar-On YM, Fromm A, Ostikar M, Shoshany A, Giz O, Milo, R. 2023. The global biomass and number of terrestrial arthropods. Sci. Adv. 9, eabq4049. ( 10.1126/sciadv.abq4049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shortall CR, Moore A, Smith E, Hall MJ, Woiwod IP, Harrington R. 2009. Long-term changes in the abundance of flying insects. Insect Conserv. Divers. 2, 251-260. ( 10.1111/j.1752-4598.2009.00062.x) [DOI] [Google Scholar]
- 9.Roslin T, et al. 2021. Phenological shifts of abiotic events, producers and consumers across a continent. Nat. Clim. Change 11, 241-248. ( 10.1038/s41558-020-00967-7) [DOI] [Google Scholar]
- 10.Visser ME, Gienapp P. 2019. Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 3, 879-885. ( 10.1038/s41559-019-0880-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhler J, et al. 2021. Relationship of insect biomass and richness with land use along a climate gradient. Nat. Commun. 12, 5946. ( 10.1038/s41467-021-26181-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welti EAR, et al. 2022. Temperature drives variation in flying insect biomass across a German malaise trap network. Insect Conserv. Divers. 15, 168-180. ( 10.1111/icad.12555) [DOI] [Google Scholar]
- 13.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105-3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 14.Robinet C, Roques A. 2010. Direct impacts of recent climate warming on insect populations. Integr. Zool. 5, 132-142. ( 10.1111/j.1749-4877.2010.00196.x) [DOI] [PubMed] [Google Scholar]
- 15.Dixon AFG, Honěk A, Keil P, Kotela MAA, Šizling AL, Jarošík V. 2009. Relationship between the minimum and maximum temperature thresholds for development in insects. Funct. Ecol. 23, 257-264. ( 10.1111/j.1365-2435.2008.01489.x) [DOI] [Google Scholar]
- 16.Polato NR, et al. 2018. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proc. Natl Acad. Sci. USA 115, 12 471-12 476. ( 10.1073/pnas.1809326115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668-6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkle LA, Runyon JB. 2016. Drought and leaf herbivory influence floral volatiles and pollinator attraction. Glob. Change Biol. 22, 1644-1654. ( 10.1111/gcb.13149) [DOI] [PubMed] [Google Scholar]
- 19.Chown SL, Sørensen JG, Terblanche JS. 2011. Water loss in insects: an environmental change perspective. J. Insect Physiol. 57, 1070-1084. ( 10.1016/j.jinsphys.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 20.St Leger RJ. 2021. Insects and their pathogens in a changing climate. J. Invertebr. Pathol. 184, 107644. ( 10.1016/j.jip.2021.107644) [DOI] [PubMed] [Google Scholar]
- 21.Lawson DA, Rands SA. 2019. The effects of rainfall on plant–pollinator interactions. Arthropod Plant Interact. 13, 561-569. ( 10.1007/s11829-019-09686-z) [DOI] [Google Scholar]
- 22.Wainwright CE, Volponi SN, Stepanian PM, Reynolds DR, Richter DH. 2022. Using cloud radar to investigate the effect of rainfall on migratory insect flight. Methods Ecol. Evol. 14, 655-668. ( 10.1111/2041-210X.14023) [DOI] [Google Scholar]
- 23.Bigger M. 1976. Oscillations of tropical insect populations. Nature 259, 207-209. ( 10.1038/259207a0) [DOI] [Google Scholar]
- 24.Janzen DH, Schoener TW. 1968. Differences in insect abundance and diversity between wetter and drier sites during a tropical dry season. Ecology 49, 96-110. ( 10.2307/1933565) [DOI] [Google Scholar]
- 25.Levings SC. 1983. Seasonal, annual, and among-site variation in the ground ant community of a deciduous tropical forest: some causes of patchy species distributions. Ecol. Monogr. 53, 435-455. ( 10.2307/1942647) [DOI] [Google Scholar]
- 26.Raven PH, Wagner DL. 2021. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl Acad. Sci. USA 118, e2002548117. ( 10.1073/pnas.2002548117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svenningsen CS, et al. 2022. Flying insect biomass is negatively associated with urban cover in surrounding landscapes. Divers. Distrib. 28, 1242-1254. ( 10.1111/ddi.13532) [DOI] [Google Scholar]
- 28.Basset Y, et al. 2015. Arthropod distribution in a tropical rainforest: tackling a four dimensional puzzle. PLoS ONE 10, e0144110. ( 10.1371/journal.pone.0144110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lourenço GM, Campos RBF, Ribeiro SP. 2015. Spatial distribution of insect guilds in a tropical montane rainforest: effects of canopy structure and numerically dominant ants. Arthropod Plant Interact. 9, 163-174. ( 10.1007/s11829-015-9359-y) [DOI] [Google Scholar]
- 30.Richards LA, Windsor DM. 2007. Seasonal variation of arthropod abundance in gaps and the understorey of a lowland moist forest in Panama. J. Trop. Ecol. 23, 169-176. ( 10.1017/S0266467406003907) [DOI] [Google Scholar]
- 31.Cuevas-Reyes P, Gilberti L, González-Rodríguez A, Fernandes GW. 2013. Patterns of herbivory and fluctuating asymmetry in Solanum lycocarpum St. Hill (Solanaceae) along an urban gradient in Brazil. Ecol. Indic. 24, 557-561. ( 10.1016/j.ecolind.2012.08.011) [DOI] [Google Scholar]
- 32.Jones RW, O'Brien CW, Ruiz-Montoya L, Gómez-Gómez B. 2008. Insect diversity of tropical montane forests: diversity and spatial distribution of weevils (Coleoptera: Curculionidae) inhabiting leaf litter in southern Mexico. Ann. Entomol. Soc. Am, 101, 128-139. ( 10.1603/0013-8746(2008)101[128:IDOTMF]2.0.CO;2) [DOI] [Google Scholar]
- 33.Janzen DH, Hallwachs W. 2019. Perspective: where might be many tropical insects? Biol.Conserv. 233, 102-108. ( 10.1016/j.biocon.2019.02.030) [DOI] [Google Scholar]
- 34.Abarca M, Spahn R. 2021. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 47, 67-74. ( 10.1016/j.cois.2021.04.008) [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez D, Wilson RJ. 2021. Intra- and interspecific variation in the responses of insect phenology to climate. J. Anim. Ecol. 90, 248-259. ( 10.1111/1365-2656.13348) [DOI] [PubMed] [Google Scholar]
- 36.Bale JS, Hayward SAL. 2010. Insect overwintering in a changing climate. J. Exp. Biol. 213, 980-994. ( 10.1242/jeb.037911) [DOI] [PubMed] [Google Scholar]
- 37.Huang J. 2016. Effects of soil temperature and snow cover on the mortality of overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Int. J. Biometeorol. 60, 977-989. ( 10.1007/s00484-015-1090-y) [DOI] [PubMed] [Google Scholar]
- 38.Potopová V, Boroneanţ C, Možný M, Soukup J. 2016. Driving role of snow cover on soil moisture and drought development during the growing season in the Czech Republic. Int. J. Climatol. 36, 3741-3758. ( 10.1002/joc.4588) [DOI] [Google Scholar]
- 39.Wolda H. 1988. Insect seasonality: why? Annu. Rev. Ecol. Syst. 19, 1-18. ( 10.1146/annurev.es.19.110188.000245) [DOI] [Google Scholar]
- 40.Kishimoto-Yamada K, Itioka T. 2013. Seasonality in phytophagous scarabaeid (Melolonthinae and Rutelinae) abundances in an ‘aseasonal’ Bornean rainforest. Insect Conserv. Divers. 6, 179-188. ( 10.1111/j.1752-4598.2012.00201.x) [DOI] [Google Scholar]
- 41.Novotny V, Miller SE, Basset Y, Cizek L, Drozd P, Darrow K, Leps J. 2002. Predictably simple: assemblages of caterpillars (Lepidoptera) feeding on rainforest trees in Papua New Guinea. Proc. R. Soc. Lond. B 269, 2337-2344. ( 10.1098/rspb.2002.2166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spitzer K, Novotny V, Tonner M, Leps J. 1993. Habitat preferences, distribution and seasonality of the butterflies (Lepidoptera, Papilionoidea) in a montane tropical rain forest, Vietnam. J . Biogeogr. 20, 109-121. ( 10.2307/2845744) [DOI] [Google Scholar]
- 43.Kishimoto-Yamada K, Itioka T. 2015. How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? Entomol. Sci. 18, 407-419. ( 10.1111/ens.12134) [DOI] [Google Scholar]
- 44.Mellanby K, Gardiner JS. 1939. Low temperature and insect activity. Proc. R. Soc. Lond. B 127, 473-487. ( 10.1098/rspb.1939.0035) [DOI] [Google Scholar]
- 45.Peng RK, Fletcher CR, Sutton SL. 1992. The effect of microclimate on flying dipterans. Int. J. Biometeorol. 36, 69-76. ( 10.1007/BF01208916) [DOI] [Google Scholar]
- 46.Cormont A, Malinowska AH, Kostenko O, Radchuk V, Hemerik L, WallisDeVries MF, Verboom J. 2011. Effect of local weather on butterfly flight behaviour, movement, and colonization: significance for dispersal under climate change. Biodivers. Conserv. 20, 483-503. ( 10.1007/s10531-010-9960-4) [DOI] [Google Scholar]
- 47.Frith CB, Frith DW. 1985. Seasonality of insect abundance in an Australian upland tropical rainforest. Aust. J. Ecol. 10, 237-248. ( 10.1111/j.1442-9993.1985.tb00886.x) [DOI] [Google Scholar]
- 48.da Silva NAP, Frizzas MR, de Oliveira CM. 2011. Seasonality in insect abundance in the ‘cerrado’ of Goiás State, Brazil. Rev. Bras. Entomol. 55, 79-87. ( 10.1590/S0085-56262011000100013) [DOI] [Google Scholar]
- 49.Ståhl G, et al. 2011. National Inventory of Landscapes in Sweden (NILS)—scope, design, and experiences from establishing a multiscale biodiversity monitoring system. Environ. Monit. Assess. 173, 579-595. ( 10.1007/s10661-010-1406-7) [DOI] [PubMed] [Google Scholar]
- 50.Iwaszkiewicz-Eggebrecht E, et al. 2023. FAVIS: fast and versatile protocol for non-destructive metabarcoding of bulk insect samples. PLOS ONE 18, e0286272. ( 10.1371/journal.pone.0286272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muñoz Sabater J. 2019. ERA5-Land Hourly Data from 1981 to Present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS). ( 10.24381/cds.e2161bac) [DOI]
- 52.Ekholm A, Tack AJM, Bolmgren K, Roslin T. 2019. The forgotten season: the impact of autumn phenology on a specialist insect herbivore community on oak. Ecol. Entomol. 44, 425-435. ( 10.1111/een.12719) [DOI] [Google Scholar]
- 53.Gaytán Á, Gotthard K, Tack AJM. 2022. Strong impact of temperature and resource specialisation on patterns of voltinism within an oak-associated insect community. Ecol. Entomol. 47, 544-552. ( 10.1111/een.13139) [DOI] [Google Scholar]
- 54.Hodgson JA, Thomas CD, Oliver TH, Anderson BJ, Brereton TM, Crone EE. 2011. Predicting insect phenology across space and time. Glob. Change Biol. 17, 1289-1300. ( 10.1111/j.1365-2486.2010.02308.x) [DOI] [Google Scholar]
- 55.Luoto M, Heikkinen RK, Pöyry J, Saarinen K. 2006. Determinants of the biogeographical distribution of butterflies in boreal regions. J. Biogeogr. 33, 1764-1778. ( 10.1111/j.1365-2699.2005.01395.x) [DOI] [Google Scholar]
- 56.Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K. 2009. Species traits explain recent range shifts of Finnish butterflies. Glob. Change Biol. 15, 732-743. ( 10.1111/j.1365-2486.2008.01789.x) [DOI] [Google Scholar]
- 57.R Core Team. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/.
- 58.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 59.Fox J, Weisberg S. 2019. An R companion to applied regression, 3rd edn. Thousand Oaks, CA: Sage. [Google Scholar]
- 60.Lüdecke D. 2020. sjPlot: Data Visualization for Statistics in Social Science.
- 61.Masteller EC. 1993. Comparison of tropical and temperate emergence phenology of aquatic insects from Puerto Rico and Pennsylvania. J. Kans. Entomol. Soc. 66, 192-199. [Google Scholar]
- 62.Régnière J, Powell J, Bentz B, Nealis V. 2012. Effects of temperature on development, survival and reproduction of insects: experimental design, data analysis and modeling. J. Insect Physiol. 58, 634-647. ( 10.1016/j.jinsphys.2012.01.010) [DOI] [PubMed] [Google Scholar]
- 63.Bowler DE, et al. 2017. Cross-realm assessment of climate change impacts on species' abundance trends. Nat. Ecol. Evol. 1, 0067. ( 10.1038/s41559-016-0067) [DOI] [PubMed] [Google Scholar]
- 64.Roslin T, et al. 2017. Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742-744. ( 10.1126/science.aaj1631) [DOI] [PubMed] [Google Scholar]
- 65.Haddad NM, Haarstad J, Tilman D. 2000. The effects of long-term nitrogen loading on grassland insect communities. Oecologia 124, 73-84. ( 10.1007/s004420050026) [DOI] [PubMed] [Google Scholar]
- 66.Altieri MA, Nicholls CI. 2003. Soil fertility management and insect pests: harmonizing soil and plant health in agroecosystems. Soil Till. Res. 72, 203-211. ( 10.1016/S0167-1987(03)00089-8) [DOI] [Google Scholar]
- 67.Cranmer L, McCollin D, Ollerton J. 2012. Landscape structure influences pollinator movements and directly affects plant reproductive success. Oikos 121, 562-568. ( 10.1111/j.1600-0706.2011.19704.x) [DOI] [Google Scholar]
- 68.Sutton SL, Hutson PJ. 1980. The vertical distribution of small flying insects in the lowland rain forest of Zaïre. Zool. J. Linn. Soc. 68, 111-123. ( 10.1111/j.1096-3642.1980.tb01921.x) [DOI] [Google Scholar]
- 69.Knuff AK, Staab M, Frey J, Dormann CF, Asbeck T, Klein A-M. 2020. Insect abundance in managed forests benefits from multi-layered vegetation. Basic Appl. Ecol. 48, 124-135. ( 10.1016/j.baae.2020.09.002) [DOI] [Google Scholar]
- 70.Ødegaard F. 2006. Host specificity, alpha- and beta-diversity of phytophagous beetles in two tropical forests in Panama. Biodivers. Conserv. 15, 83-105. ( 10.1007/s10531-004-3106-5) [DOI] [Google Scholar]
- 71.Hails CJ. 1982. A comparison of tropical and temperate aerial insect abundance. Biotropica 14, 310-313. ( 10.2307/2388092) [DOI] [Google Scholar]
- 72.Janzen DH, Pond CM. 1975. A comparison, by sweep sampling, of the arthropod fauna of secondary vegetation in Michigan, England and Costa Rica. Trans. R. Entomol. Soc. Lond. 127, 33-50. ( 10.1111/j.1365-2311.1975.tb00551.x) [DOI] [Google Scholar]
- 73.IPCC. 2022. Mitigation of climate change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 74.Johansson F, Orizaola G, Nilsson-Örtman V. 2020. Temperate insects with narrow seasonal activity periods can be as vulnerable to climate change as tropical insect species. Scient. Rep. 10, 8822. ( 10.1038/s41598-020-65608-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denlinger DL. 1980. Seasonal and annual variation of insect abundance in the Nairobi National Park, Kenya. Biotropica 12, 100-106. ( 10.2307/2387725) [DOI] [Google Scholar]
- 76.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37-42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 77.Parmesan C, et al. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579-583. ( 10.1038/21181) [DOI] [Google Scholar]
- 78.Staley JT, Hodgson CJ, Mortimer SR, Morecroft MD, Masters GJ, Brown VK, Taylor ME. 2007. Effects of summer rainfall manipulations on the abundance and vertical distribution of herbivorous soil macro-invertebrates. Eur. J. Soil Biol. 43, 189-198. ( 10.1016/j.ejsobi.2007.02.010) [DOI] [Google Scholar]
- 79.Holland JM, Thomas CFG, Birkett T, Southway S. 2007. Spatio-temporal distribution and emergence of beetles in arable fields in relation to soil moisture. Bull. Entomol. Res. 97, 89-100. ( 10.1017/S0007485307004804) [DOI] [PubMed] [Google Scholar]
- 80.Torres-Muros L, Hódar JA, Zamora R. 2017. Effect of habitat type and soil moisture on pupal stage of a Mediterranean forest pest (Thaumetopoea pityocampa). Agric. For. Entomol. 19, 130-138. ( 10.1111/afe.12188) [DOI] [Google Scholar]
- 81.Wang C, Fu B, Zhang L, Xu Z. 2019. Soil moisture–plant interactions: an ecohydrological review. J. Soil Sediments 19, 1-9. ( 10.1007/s11368-018-2167-0) [DOI] [Google Scholar]
- 82.Antão LH, et al. 2022. Climate change reshuffles northern species within their niches. Nat. Clim. Change 12, 587-592. ( 10.1038/s41558-022-01381-x) [DOI] [Google Scholar]
- 83.Tanaka LK, Tanaka SK. 1982. Rainfall and seasonal changes in arthropod abundance on a tropical oceanic island. Biotropica 14, 114-123. ( 10.2307/2387740) [DOI] [Google Scholar]
- 84.Forrest JR. 2016. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 17, 49-54. ( 10.1016/j.cois.2016.07.002) [DOI] [PubMed] [Google Scholar]
- 85.Damien M, Tougeron K. 2019. Prey–predator phenological mismatch under climate change. Curr. Opin. Insect Sci. 35, 60-68. ( 10.1016/j.cois.2019.07.002) [DOI] [PubMed] [Google Scholar]
- 86.Renner SS, Zohner CM. 2018. Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165-182. ( 10.1146/annurev-ecolsys-110617-062535) [DOI] [Google Scholar]
- 87.van Dijk LJA2024. Data from: Temperature and water availability drive insect seasonality across a temperate and a tropical region. Dryad Digital Repository. ( ) [DOI]
- 88.van Dijk LJA2024. Temperature and water availability drive insect seasonality across a temperate and a tropical region. Figshare. ( ) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are archived in the Dryad repository [87].
Supplementary material is available online [88].