Abstract
Although paternal age has been linked to certain psychiatric disorders, the nature of any causal relationship remains elusive. Here, we aimed to comprehensively assess the magnitude of a wide range of offspring’s psychiatric risk conferred by paternal age, leveraging a pedigree inferred from covered-insurance relationship (accuracy >98%) in Taiwan’s single-payer compulsory insurance program. We also examined whether there is an independent role of paternal age and explored the potential effect of parental age difference. A total cohort of 7,264,788 individuals born between 1980–2018 were included; 5,572,232 with sibling(s) were selected for sibling-comparison analyses and 1,368,942 and 1,044,420 children with information of paternal-grandparents and maternal-grandparents, respectively, were selected for multi-generation analyses. Using inpatient/outpatient claims data (1997-2018), we identified schizophrenia, autism, bipolar disorder (BPD), attention deficit-hyperactivity disorder (ADHD), major depressive disorder (MDD), eating disorder (ED), substance use disorder (SUD), mental retardation (MR), tic disorder, obsessive-compulsive disorder (OCD), anxiety, and somatoform disorder. We identified suicides using death certificates. Logistic regression analysis was used to estimate the paternal/maternal/grand-paternal age association with psychiatric risk in the offspring. The total cohort and sibling-comparison cohort resulted in similar estimates. Paternal age had a U-shaped relationship with offspring’s MDD, ED, SUD, and anxiety. A very young maternal age (<20 years) was associated with markedly higher risk in offspring’s SUD, MR, and suicide. Older paternal age (>25 years) was linearly associated with offspring’s schizophrenia, autism, BPD, ADHD, MDD, ED, SUD, MR, OCD, anxiety, and suicide. Older grand-paternal age was linearly associated with offspring’s schizophrenia, autism, ADHD, and MR. Dissimilar parental age was positively associated with offspring’s ADHD, MDD, SUD, MR, anxiety, and suicide, and negatively associated with offspring’s OCD. This comprehensive assessment provides solid evidence for the independent role of paternal age in psychiatric risk in the offspring and clarifies the significance of both early parenthood and delayed paternity.
Introduction
Accumulating evidence suggests that older paternal age could be associated with a variety of psychiatric disorders,1-3 particularly autism, schizophrenia, and bipolar disorder (BPD).1, 4-15 A few studies have proposed an association between paternal age and attention deficit-hyperactivity disorder16 (ADHD) and eating disorders.13, 17 An increased psychiatric risk in the offspring of very young parents has also been reported,9, 10, 18 reflecting a bimodal association of parental age on psychiatric disorders.
The “de novo mutation” hypothesis has been proposed to explain the relationship of older paternal age with psychiatric disorders.19 Sequencing studies have demonstrated a linear relationship between paternal age and the rate of de novo mutations in the offspring,20-27 with approximately two additional de novo mutations per increased year in paternal age. The hypothesis is that this additional mutational load increases the occurrence of severe biological sequelae.28, 29 Alternatively, selection into late fatherhood, which may reflect the fathers’ own increased predisposition to psychiatric disorders, could confound this association with paternal age.30-32
Family-based designs with sibling comparisons18, 33, 34 can help account for unmeasured confounders shared within a family. Siblings within the same family share common environmental factors and the same parental predisposition to psychiatric disorders, hence family-based analyses can help rule out confounding factors by shared familial predispositions. Multi-generation analyses also help in clarifying if there is an independent role of paternal age in the risk for psychiatric disorders. Advanced grand-paternal age at childbirth of the parent was associated with increased risk of autism in the offspring in Sweden35 and Denmark,15 which is consistent with the de novo mutation hypothesis, since paternal-age-related mutations not only result in increased psychiatric risk in the offspring, but could also contribute to the mutation burden in subsequent generations’ psychiatric risk. However, the assessment of schizophrenia from a Swedish group provided inconclusive results: the association with schizophrenia was limited to the maternal grandfather alone and had no association with the age of the paternal grandfather.36 Beyond consideration of paternal and maternal age as independent risk factors, a few studies have also explored the joint effect of both paternal and maternal age by assessing their mean and difference, showing that an increased gap in the parental age was associated with higher risk of developing schizophrenia, autism, and ADHD in their offspring.37-39
To date, large-scale studies on the association between paternal age and the risk of psychiatric disorders have mainly been conducted in the Danish10, 31, 39 and Swedish populations,13, 35, 40, 41 because these countries have comprehensive multi-generation national registries available for scientific research. Insurance claims data has also been used to perform large-scale family-based genetic studies in the US42, 43 and Taiwan.44, 45 To date, however, the existing literature has focused on a limited range of diseases, and the association with paternal age and risk for psychiatric disorders has not been examined in these datasets. Utilizing a nationwide population-based cohort of more than 7 million individuals, with parental information established from covered-insurance data in Taiwan’s single-payer compulsory National Health Insurance (NHI) program, this study aimed: 1) to comprehensively assess the magnitude of a wide range of psychiatric risks in offspring conferred by paternal age effects; 2) to determine if there is an independent role of paternal age using sibling-comparison and multi-generation analyses; and 3) to explore the joint association of paternal and maternal age and the risk of psychiatric disorders in the offspring.
Material and methods
Study design
We conducted a nationwide cohort study of individuals born between January 1, 1980 and December 31, 2018, followed-up from 1997 to 2018 in the National Health Insurance Research Database (NHIRD), which covers approximately 99% of the residents of Taiwan. This study integrated four major nationwide health information databases provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. We inferred pedigree relationships using two databases, the Registry for Beneficiaries and the Maternal and Child Health Database. We identified patients diagnosed with a psychiatric disorder using inpatient and outpatient claim databases. We used data from death certifications to follow-up the vital status of the study population. All entries for each individual were linked by a unique personal identifier number, hence the medical records and the registration files of each enrollee could be linked.
Family ascertainment
The registry for beneficiaries contains information on sex, date of birth, insurance amount, and the identifiers of the relationships between insured person and their dependents. Only spouses and blood relatives are eligible as dependents of an insured person. Based on the Registry for Beneficiaries from 1997 to 2018, family information was ascertained based on the information of the identifiers and unique personal identifier numbers of the parent, child, grandparent, grandchild, and spouse. Indirect identification of the pedigree was further performed based on the above-mentioned direct identification. We selected families wherein the parent and child had an age difference of at least 12 years to maximize the probability to obtain correct familial relatedness. Full siblings were defined as two individuals having the same parents. Twins were defined as two individuals being born on the same day and from the same parents; however, monozygotic and dizygotic twins could not be distinguished.
Nuclear families can be directly identified based on the Maternal and Child Health database, which contains information on the unique personal identifier of infants and both the parents. Among 2,770,736 individuals born between 2004 and 2017 recorded in the database, 2,661,371 individuals had information on the father, 2,769,611 had information on the mother, and 2,660,806 had information on both parents.
We compared the consistency between the established pedigree inferred from the Registry for Beneficiaries and the Maternal and Child Health database. We used the Maternal and Child Health database as the gold standard for retrieving information. Among 2,661,371 father–child relationships identified in the Maternal and Child Health database, fathers of 1,819,313 children could be inferred from the Registry for Beneficiaries, and 1,807,569 (accuracy=99.35%) were identical. Among 2,769,611 mother–child relationships identified in the Maternal and Child Health database, mothers of 2,343,934 children could be inferred from the Registry for Beneficiaries, and 2,300,523 (accuracy=98.15%) were identical.
We then combined the established pedigree relationship inferred from the Registry for Beneficiaries and the Maternal and Child Health database. A total of 7,264,788 eligible individuals from 4,057,701 families born between 1980 and 2018 had information on both parents and were included in this study.
For within-family analyses, we selected families with at least two offspring; one was selected at random for twins or triplets. A total of 5,572,232 persons with sibling(s) from 2,451,930 families were selected for within-family analyses. For multi-generation analyses, individuals with information on paternal-grandparents or maternal-grandparents were selected. A total of 1,368,942 and 1,044,420 persons were enrolled for paternal-grandparent and maternal-grandparent cohorts, respectively. The coverage of linkage to both parents, paternal-grandparents, and maternal-grandparents is shown in Supplementary Figure 1.
Disease definitions
Based on the inpatient and outpatient claims data during 1997 and 2018, we identified twelve major psychiatric disorders, including schizophrenia (ICD9 code: 295; ICD10 code: F20, F25), autism (ICD9 code: 299; ICD10 code: F84), BPD (ICD9 code: 296.0-296.1, 296.4-296.8; ICD10 code:), ADHD (ICD9 code: 314; ICD10 code: F90), major depressive disorder (MDD, ICD9 code: 296.2, 296.3, 300.4, 311; ICD10 code: F32, F33, F34.1), eating disorders (ICD code: 307.1, 307.5; ICD10 code: F50), substance use disorder (SUD, ICD9 code: 291, 292, 303.0, 303.9, 304, 305; ICD10 code: F10-F19), mental retardation (MR, ICD9 code: 317-319; ICD10 code: F70-F79), tic disorder (ICD9 code: 307.2; ICD10 code: F95), obsessive-compulsive disorder (OCD, ICD9 code: 300.3; ICD10 code: F42), anxiety disorder (ICD9 code: 300.0; ICD10 code: F40, F41), and somatoform disorder (ICD9 code: 300.7, 300.8; ICD10: F45). The criterion of at least two outpatient or one inpatient admissions for such disease was chosen to increase diagnostic precision. We identified suicide (ICD-9: E950-E959, ICD-10: X60-X84 or Y87.0) based on information obtained from death certificates.
Statistical analysis
Paternal age was calculated by subtracting the offspring’s birth date from the father’s birth date. Paternal age was categorized as <20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, and ≥50. Maternal age was categorized as <20, 20–24, 25–29, 30–34, 35–39, and ≥ 40. The risk of psychiatric disorder in every paternal or maternal age category was calculated. Attributable risk of psychiatric disorder due to a very old paternal age (≥50 years) was calculated. We estimate the relative risk of paternal age for each psychiatric disorder using the odds ratio (OR) in a multiple logistic regression model, adjusting for maternal age, sex of the offspring, offspring’s age (the length of follow-up), and offspring’s birth cohort as the first adjustment model. Further adjustment for paternal and maternal history of psychiatric disorders was included in a second adjustment model, and a fully adjusted model further included the family’s insurance amount (the highest during the study period) and residential area.
To consider unmeasured confounders shared within a family, we performed sibling-comparison analysis using generalized estimating equations (GEEs) with an exchangeable working correlation structure, which assumes that siblings are equally correlated within a given family. The generalized linear model with binomial distribution and logistic link function was employed to estimate the OR and 95% confidence interval (CI). The first adjustment model included paternal age, maternal age, sex of the offspring, offspring’s age, and offspring’s birth cohort. The fully adjusted model was further adjusted for paternal and maternal history of psychiatric disorders, family’s insurance amount, and residential area.
We performed multi-generation analyses to explore the psychiatric risk across generations by testing the association between the age of paternal or maternal grand-paternal/maternal and the offspring’s psychiatric risk. Grand-paternal age was categorized as <20, 20–24, 25–29, 30–34, 35–39, and ≥ 40. Grand-maternal age was categorized as <20, 20–24, 25–29, 30–34, and ≥ 35. The first model was adjusted for age of the other grandparent, sex of the offspring, offspring’s age, and offspring’s birth cohort. The second model further adjusted for grandparents’ history of psychiatric disorders. The fully adjusted model was further adjusted for paternal age, maternal age, paternal and maternal history of psychiatric disorders, family’s insurance amount, and residential area.
We further examined the joint association of paternal age and maternal age on psychiatric risk in the offspring. Owing to the linear relationship between paternal age, maternal age, and parental age difference, we could not include these three variables in the same model. We included the mean of parental age to capture the effect of ageing, and the difference in the parental age to capture the effects not associated with ageing. We separated dissimilar parental age into: younger fathers reproducing with older mothers and younger mothers reproducing with older fathers. Parental age difference (paternal age subtracted by maternal age) was categorized as ≤ −10 (mother at least 10 years older than father), −5 to −9, −2 to −4, −2 to 2 (mother and father with <2 years age difference), 2 to 4, 5 to 9, 10 to 14, ≥ 15 (father at least 15 years older than father). The first model adjusted for sex of the offspring, offspring’s age, offspring’s birth cohort, and paternal and maternal history of psychiatric disorders. The fully adjusted model was further adjusted for family’s insurance amount and residential area.
Accounting for multiple testing of 13 psychiatric disorders, the significance level was set at a Bonferroni-corrected 0.004. All statistical analyses were performed using the SAS statistical package (version 9.4 for Windows; SAS Institute Inc., Cary, North Carolina).
Results
Demographic distributions
The demographic characteristics of the study participants are presented in Supplementary Table 1. The distribution of paternal and maternal age by birth cohort is presented in Supplementary Figure 2. Generally, both paternal and maternal age were higher in the younger birth cohort.
In the total cohort with 7,264,788 individuals, we identified 25,184 (0.35%) with schizophrenia, 51,210 (0.7%) with autism, 32,959 (0.45%) with BPD, 265,294 (3.65%) with ADHD, 187,615 (2.58%) with MDD, 7,551 (0.10%) with an eating disorder, 89,871 (1.24%) with SUD, 72,247 (0.99%) with MR, 44,763 (0.62%) with tic disorder, 19,623 (0.27%) with OCD, 303,681 (4.18%) with anxiety, 11,310 (0.16%) with somatoform disorder, and 3,603 (0.05%) who committed suicide. The distribution of these psychiatric disorders by paternal and maternal age groups in the total cohort is shown in Supplementary Table 2. The corresponding information on sibling-comparison cohort, paternal grandparent cohort, and maternal grandparent cohort is shown in Supplementary Table 3-5.
The results of total cohort and sibling-comparison cohort
In the total cohort, cumulative incidence of psychiatric disorders in offspring of paternal age groups of 25-29 and of ≥50, and attributable risk of psychiatric disorders in offspring due to advanced paternal age ≥50 is shown in Figure 1. The offspring of very old paternal age of ≥50 have a higher cumulative incidence of every psychiatric disorder compared to paternal age of 25-29, and attributable risk of schizophrenia, autism, MR, and suicide was high (~60%).
Figure 1.
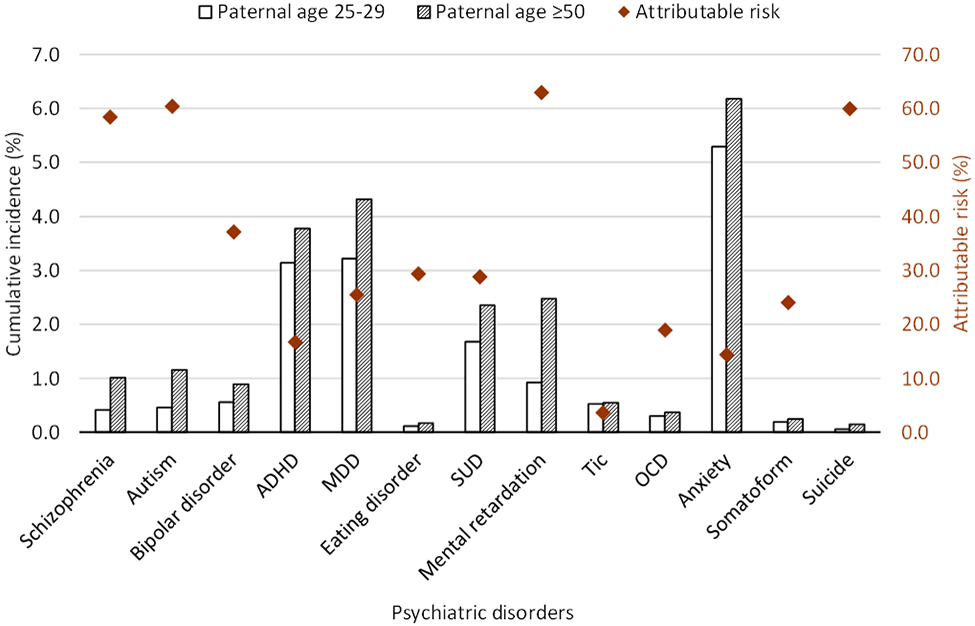
Cumulative incidence of 13 psychiatric disorders in offspring of paternal age groups of 25-29 and of ≥50, and attributable risk of 13 psychiatric disorders in offspring due to advanced paternal age ≥50. Abbreviation: ADHD: attention deficit-hyperactivity disorder; MDD: major depressive disorder; SUD: substance use disorder; OCD: obsessive-compulsive disorder.
The estimated association between paternal and maternal age and the risk of psychiatric disorders in the offspring in the first, second, and fully adjusted models is shown in the Supplementary Table 6-18. After further adjusting for the paternal and maternal history of psychiatric disorders in the second adjustment model, the strength of the association with paternal age attenuated slightly. After further adjusting for family’s insurance amount and residential areas in the fully adjusted model, the strength of the association of paternal age attenuated modestly for schizophrenia, BPD, SUD, MR, and suicide, and the strength of the association of maternal age attenuated for autism; this implies a potential role of the parents’ socioeconomic status on the association with parental age.
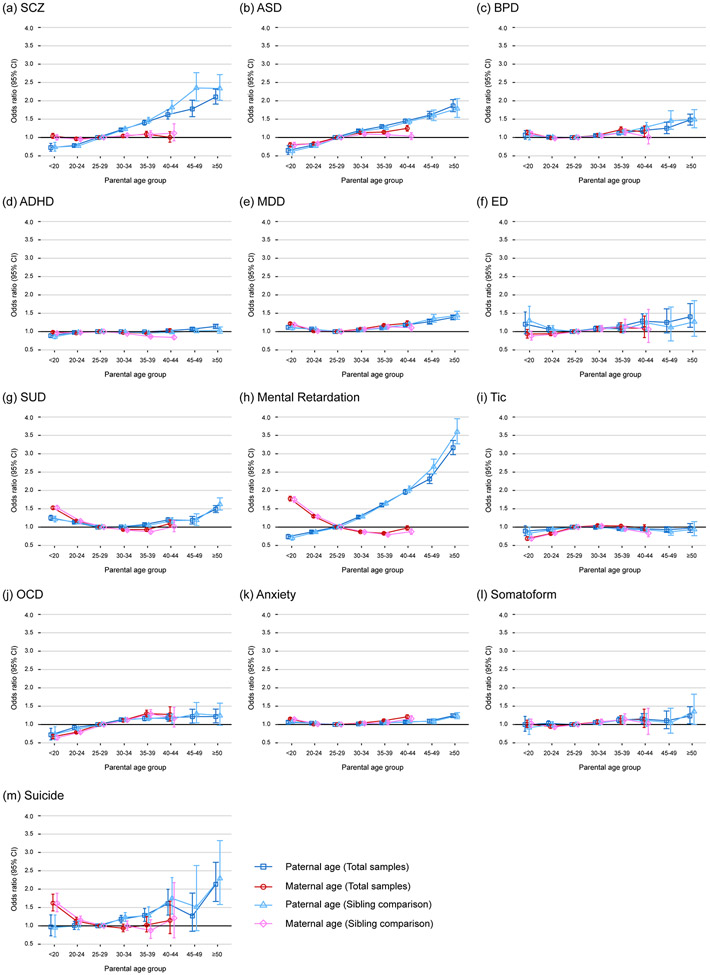
The ORs for each of the offspring psychiatric outcomes across the categories of paternal and maternal age in the fully adjusted models in the total cohort are plotted in Figure 2. The results in the sibling-comparison cohort gave similar estimates (detailed values in Supplementary Table 19-31 with different adjustments). Overall, paternal age categories were significantly (p<0.004) associated with all psychiatric outcomes in the offspring, except for tic and somatoform disorders, and maternal age categories were significantly associated with all psychiatric outcomes in the offspring, except for schizophrenia, somatoform and eating disorders (Table 1). Both paternal and maternal age categories had a U-shaped relationship with the offspring’s risk of developing BPD, MDD, SUD, and anxiety (the lowest offspring risk was observed in parental age of 25–29 years, except for SUD in maternal age of 30–39 years). A U-shaped relationship was also observed between paternal age categories and eating disorder. Notably, a very young maternal age of <20 years was associated with increased risk of BPD (OR=1.14, 95% CI: 1.08-1.20), MDD (OR=1.22, 95% CI: 1.19-1.24), anxiety (OR=1.15, 95% CI: 1.13-1.17), SUD (OR=1.53 , 95% CI: 1.48-1.57), MR (OR=1.78 , 95% CI: 1.71-1.84), and suicide (OR=1.62, 95% CI: 1.41-1.86) in the offspring, compared to maternal age of 25–29 years. In addition, paternal age categories were monotonically associated with the offspring’s risk of developing schizophrenia, autism, ADHD, MR, OCD, and suicide
Figure 2.
Associations between parental age and offspring psychiatric risk in the total cohort and the sibling-comparison cohort. The odds ratio (error bars are 95% CI) with adjustments for the age of the other parent, sex, age, birth cohort, paternal and maternal history of psychiatric disorders, family’s insurance amount tertiles, and residence areas for (a) schizophrenia (SCZ), (b) autism spectrum disorders (ASD), (c) bipolar disorder (BPD), (d) attention deficit-hyperactivity disorder (ADHD), (e) major depressive disorder (MDD), (f) eating disorder (ED), (g) substance use disorder (SUD), (h) mental retardation, (i) tic disorder, (j) obsessive-compulsive disorder (OCD), (k) anxiety disorder, (l) somatoform, and (m) suicide.
Table 1.
Associations of overall effect and per 10-year increase (≥ 25) in paternal age, maternal age, and grand-paternal age with offspring’s psychiatric risk.
| Paternal age | Maternal age | Paternal grandfather age | Maternal grandfather age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Per 10-years increase (≥25) | Overall | Per 10-years increase (≥25) | Overall | Per 10-years increase | Overall | Per 10-years increase | |||||
| p-valuea | OR (95% CI)b | p-value | p-valuea | OR (95% CI)b | p-value | p-valuea | OR (95% CI)c | p-value | p-valuea | OR (95% CI)c | p-value | |
| Schizophrenia | <0.0001 | 1.37 (1.33-1.40) | <0.0001 | 0.0040 | 1.13 (1.07-1.19) | <0.0001 | 0.0015 | 1.23 (1.10-1.38) | 0.0003 | 0.2923 | 1.20 (0.99-1.44) | 0.0642 |
| Autism | <0.0001 | 1.29 (1.27-1.32) | <0.0001 | <0.0001 | 1.24 (1.20-1.27) | <0.0001 | <0.0001 | 1.16 (1.11-1.21) | <0.0001 | <0.0001 | 1.14 (1.09-1.19) | <0.0001 |
| Bipolar disorder | <0.0001 | 1.14 (1.11-1.17) | <0.0001 | <0.0001 | 1.18 (1.13-1.24) | <0.0001 | 0.1397 | 1.10 (1.01-1.21) | 0.0329 | 0.8367 | 1.07 (0.92-1.24) | 0.3686 |
| ADHD | <0.0001 | 1.03 (1.02-1.04) | <0.0001 | <0.0001 | 0.99 (0.98-1.01) | 0.2069 | <0.0001 | 1.11 (1.09-1.13) | <0.0001 | <0.0001 | 1.10 (1.08-1.13) | <0.0001 |
| MDD | <0.0001 | 1.12 (1.11-1.14) | <0.0001 | <0.0001 | 1.16 (1.14-1.19) | <0.0001 | <0.0001 | 1.11 (1.07-1.16) | <0.0001 | 0.3849 | 1.05 (0.99-1.12) | 0.0905 |
| Eating disorder | 0.0010 | 1.16 (1.09-1.23) | <0.0001 | 0.0998 | 1.11 (1.01-1.23) | 0.0312 | 0.0073 | 1.22 (1.03-1.44) | 0.0250 | 0.4415 | 0.84 (0.63-1.10) | 0.2004 |
| SUD | <0.0001 | 1.11 (1.09-1.13) | <0.0001 | <0.0001 | 0.96 (0.93-1.00) | 0.0303 | 0.0021 | 1.08 (1.01-1.15) | 0.0247 | 0.9554 | 1.05 (0.94-1.17) | 0.3633 |
| Mental retardation | <0.0001 | 1.56 (1.54-1.58) | <0.0001 | <0.0001 | 0.85 (0.83-0.88) | <0.0001 | <0.0001 | 1.28 (1.22-1.33) | <0.0001 | <0.0001 | 1.31 (1.25-1.38) | <0.0001 |
| Tic | 0.0438 | 0.97 (0.95-0.99) | 0.0110 | <0.0001 | 1.06 (1.02-1.09) | 0.0017 | 0.0024 | 0.99 (0.91-1.08) | 0.0234 | 0.0010 | 0.92 (0.87-0.98) | 0.0041 |
| OCD | <0.0001 | 1.13 (1.09-1.17) | <0.0001 | <0.0001 | 1.28 (1.21-1.36) | <0.0001 | 0.0456 | 1.09 (0.98-1.21) | 0.1220 | 0.7694 | 0.95 (0.81-1.12) | 0.5644 |
| Anxiety | <0.0001 | 1.06 (1.05-1.07) | <0.0001 | <0.0001 | 1.11 (1.09-1.12) | <0.0001 | <0.0001 | 1.08 (1.05-1.12) | <0.0001 | 0.5950 | 1.02 (0.98-1.06) | 0.3964 |
| Somatoform | 0.0255 | 1.09 (1.04-1.14) | 0.0004 | 0.0132 | 1.16 (1.07-1.25) | 0.0003 | 0.9597 | 1.02 (0.88-1.19) | 0.7780 | 0.4987 | 1.11 (0.90-1.36) | 0.3265 |
| Suicide | 0.0012 | 1.26 (1.17-1.36) | <0.0001 | 0.0002 | 1.09 (0.94-1.27) | 0.2386 | 0.6034 | 0.93 (0.67-1.30) | 0.6850 | 0.9263 | 1.11 (0.63-1.96) | 0.7254 |
Likelihood ratio test for the overall effect of paternal/maternal/grand-paternal age groups.
Estimated from a logistic regression model with adjustments for the age of the other parent, sex, age, birth cohort, paternal and maternal history of psychiatric disorders, family’s insurance amount tertiles, and residence areas.
Estimated from a logistic regression model with adjustments for the grand-maternal age, sex, age, birth cohort, grandparents’ history of psychiatric disorders, paternal age, maternal age, parents’ history of psychiatric disorders, family’s insurance amount tertiles, and residence areas.
Paternal age ≥25 years was linearly associated with several psychiatric disorders in the offspring in the fully adjusted model in the total cohort (Table 1). A strong association was observed between paternal age and schizophrenia (OR=1.37 for per 10-year increase ≥ 25 years, 95% CI: 1.33-1.40), autism (OR=1.29, 95% CI: 1.27-1.32), MR (OR=1.56, 95% CI: 1.54-1.58), and suicide (OR=1.26, 95% CI: 1.17-1.36) in the offspring, and was modest for BPD (OR=1.14, 95% CI: 1.11-1.17), ADHD (OR=1.03, 95% CI: 1.02-1.04), MDD (OR=1.12, 95% CI: 1.11-1.14), eating disorder (OR=1.16, 95% CI: 1.09-1.23), SUD (OR=1.11, 95% CI: 1.09-1.13), OCD (OR=1.13, 95% CI: 1.09-1.17), and anxiety (OR=1.06, 95% CI: 1.05-1.07) in the offspring. An association was observed between older maternal age (over 25 years) and autism (OR=1.24 for per 10-year increase ≥ 25 years, 95% CI: 1.20-1.27) and OCD (OR=1.28, 95% CI: 1.21-1.36).
The sex-stratified results in the total cohort are shown in Supplementary Figure 3. Generally, the paternal age association and maternal age association was similar between the sexes, except for SUD, wherein the increased risk in both younger age and older parental groups was higher in female offspring than in male offspring.
The birth cohort-stratified results are shown in Supplementary Figure 4. Stratifying the total cohort into different birth cohorts did not change the overall trend of the parental age association. The paternal age association with offspring’s autism and MR was markedly strong in offspring of younger birth cohort and was also evidenced in offspring of every birth cohort.
Multi-generation analyses
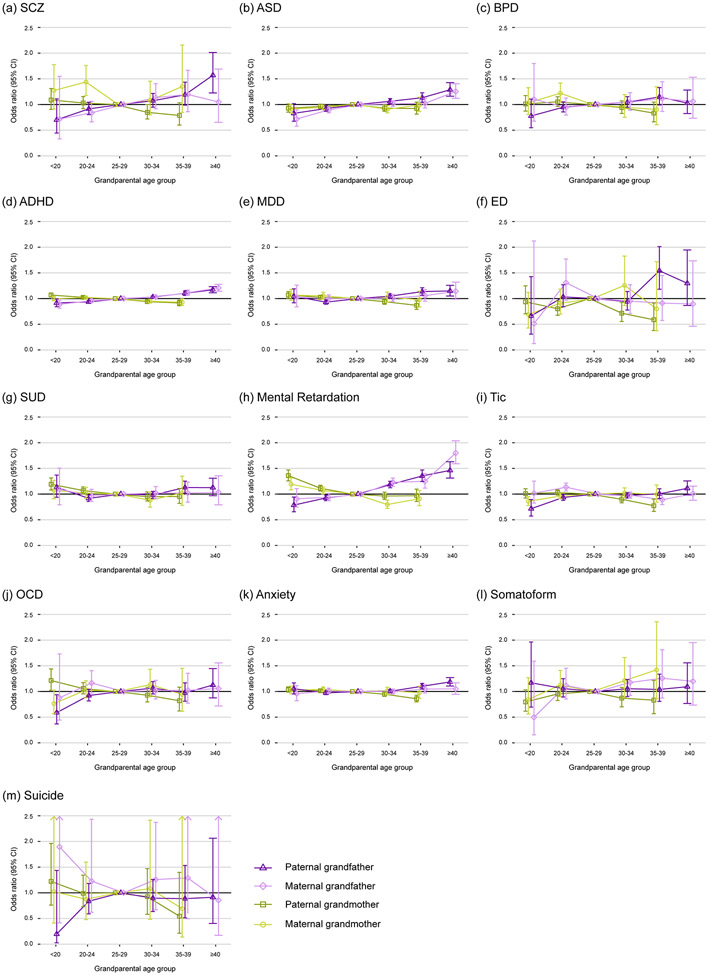
To evaluate the association with paternal age across generations, the results of paternal-grandfather/grandmother age and maternal-grandfather/grandmother age with different modeling adjustments are shown in Supplementary Table 32-44. The ORs for each of the psychiatric outcomes in the offspring across the categories in the fully adjusted models are plotted in Figure 3, and the patterns in paternal- and maternal grandfather age were similar; the confidence interval for maternal grandfather age was wider due to the smaller sample size. A linear relationship with grandfather age was observed for schizophrenia, autism, ADHD, and MR in the offspring. Older age of the grandfather was linearly associated with schizophrenia (OR=1.23 with 95% CI: 1.10-1.38 for per 10-year increase in paternal grandfather age and OR=1.20 with 95% CI: 0.99-1.44 with only birder-line significance for maternal grandfather age, Table 1), autism (OR (95% CI)=1.16 (1.11-1.21) and 1.14 (1.09-1.19)), ADHD (OR (95% CI)=1.11 (1.09-1.13) and 1.10 (1.08-1.13)), and MR (OR (95% CI)=1.28 (1.22-1.33) and 1.31 (1.25-1.38)) in the offspring. Generally, grandmother age was not associated with offspring’s psychiatric risk except for the increased risk of MR in the offspring with grandmaternal age <20 years (OR (95% CI)= 1.36 (1.26-1.47) for paternal-grandmother and 1.19 (1.08-1.31) for maternal-grandmother).
Figure 3.
Associations between paternal- and maternal- grandfather and grandmother age and offspring psychiatric risk in the multi-generational analyses. The odds ratio (error bars are 95% CI) with adjustments for the age of the other grandparent, grandparents’ history of psychiatric disorders, sex, age, birth cohort, paternal age, maternal age, paternal and maternal history of psychiatric disorders, family’s insurance amount tertiles, and residence areas for (a) schizophrenia (SCZ), (b) autism spectrum disorders (ASD), (c) bipolar disorder (BPD), (d) attention deficit-hyperactivity disorder (ADHD), (e) major depressive disorder (MDD), (f) eating disorder (ED), (g) substance use disorder (SUD), (h) mental retardation, (i) tic disorder, (j) obsessive-compulsive disorder (OCD), (k) anxiety disorder, (l) somatoform, and (m) suicide.
Joint association of parental age
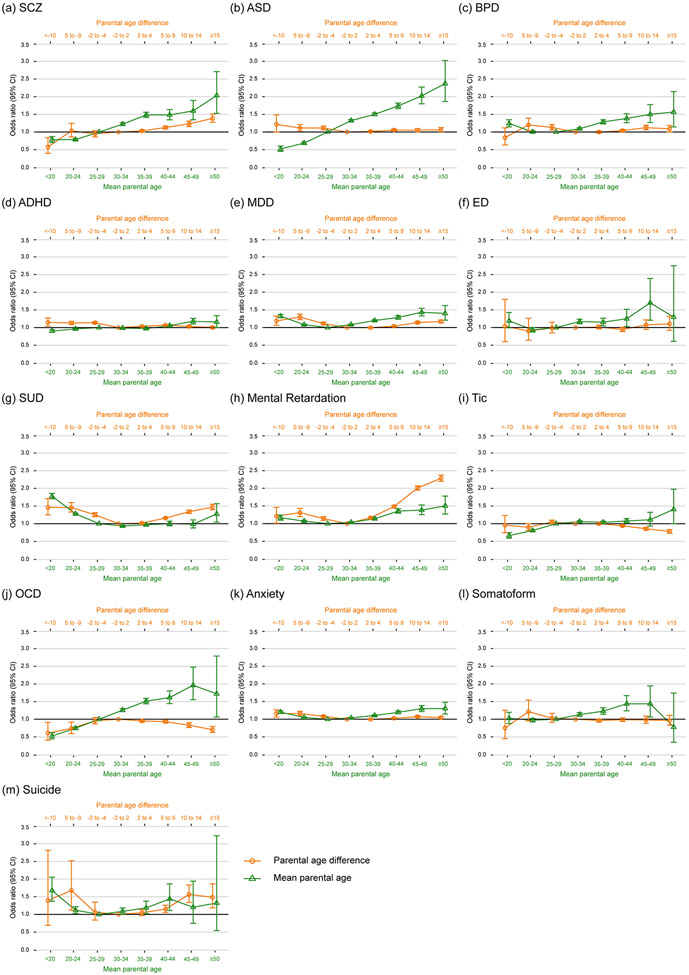
The joint associations of paternal and maternal age (their difference and mean) with psychiatric risks in the offspring are shown in Figure 4 (the distributions and association estimates with different adjustments are shown in Supplementary Table 45-57). A higher mean of parental age was linearly associated with schizophrenia, autism, ADHD, tic, and OCD in the offspring; a U-shaped relationship was observed between mean parental age and BPD, MDD, eating disorder, SUD, MR, anxiety, and suicide in the offspring. Dissimilar parental ages have a U-shaped relationship with the risk of MDD, SUD, MR, anxiety, and suicide in the offspring; an inversed-U-shaped relationship with OCD in the offspring was observed. Offspring of mothers who were older than the father had a higher risk of autism and ADHD. Offspring whose father was older than the mother had a higher risk of schizophrenia and a lower risk of tic disorder.
Figure 4.
The joint association of paternal age and maternal age with offspring psychiatric risk. The odds ratio (error bars are 95% CI) with adjustments for the sex, age, birth cohort, paternal and maternal history of psychiatric disorders, family’s insurance amount tertiles, and residence areas for (a) schizophrenia (SCZ), (b) autism spectrum disorders (ASD), (c) bipolar disorder (BPD), (d) attention deficit-hyperactivity disorder (ADHD), (e) major depressive disorder (MDD), (f) eating disorder (ED), (g) substance use disorder (SUD), (h) mental retardation, (i) tic disorder, (j) obsessive-compulsive disorder (OCD), (k) anxiety disorder, (l) somatoform, and (m) suicide.
Discussion
With the pedigree established from the claims database in Taiwan’s single-payer compulsory insurance program, this nationwide population-based cohort study of over 7 million individuals with nearly 40 years of follow-up comprehensively assessed the magnitude of a wide range of psychiatric risks in the offspring conferred by paternal age. Both paternal and maternal age had a U-shaped relationship with several psychiatric disorders in the offspring. Very young maternal age was associated with a marked increase in the risk of SUD, MR, and suicide in the offspring. Older paternal age than 25 years was associated with a higher risk of several psychiatric disorders. Multi-generation analyses suggested that the effect of paternal age on the risk of schizophrenia, autism, ADHD, and MR in the offspring could accumulate across generations. Dissimilar parental ages also had a relationship with the risk of psychiatric disorder in the offspring. Our findings provide support for an independent role of paternal age in the increased risk of psychiatric disorder in the offspring and the hypothesis that de novo mutations along with paternal age are associated with the risk of psychiatric disorders in the offspring.
Previous assessments of paternal age and a wide range of psychiatric disorders in the offspring have been studied in nationwide cohorts of Sweden12 and Denmark.10, 39 The current Taiwanese nationwide cohort study has a larger sample size, analyzes parental age, and provides more precise estimates. We demonstrated that the increased psychiatric risk was not solely present in the offspring of extremely old fathers, and the psychiatric risk associated with paternal age followed a “dose-response” relationship.
The association of paternal age with psychiatric diagnoses could be confounded by the father’s predisposition to psychiatric disorder and psychosocial factors. Therefore, in our analyses, we adjusted for parental history of psychiatric disorders and the family’s economic status. Although the education level of the father is not adjusted in our analyses, the sibling-comparison analysis exempts this study from confounders by including familial predisposition and socio-economic features as confounding factors, and providing robust paternal age association with the psychiatric risk of the offspring. This is in line with a previous Swedish family-based study12 supporting the independent role of paternal age.
The trans-generational association of paternal age with schizophrenia, autism, ADHD, and MR in the offspring extends previous evidence for autism15, 35 and schizophrenia,36 and not only suggests that advanced grand-paternal age is independently associated with psychiatric risk in the offspring but also provide empirical evidence that paternal-age-related mutations could accumulate across generations.
There are numerous divisions in spermatogonial stem cells throughout life, and delaying fatherhood results in an increase in de novo mutations.46 Certain mutations in the mutant spermatogonial cells are possibly progressively enriched for providing a selective advantage, and these selfish mutations are distinguished from the neutral copy error process that is random and accumulated over time.28, 47 Furthermore, these selfish mutations have been directly identified within individual seminiferous tubules, generating spermatozoa, in human testes.48 Otherwise, family studies in autism and schizophrenia also identified de novo mutation in genes involved in selfish spermatogonial selection.49-51 Other family-based studies implementing sequencing approaches are needed to quantify the importance of de novo mutations in the association of paternal age with the risk of psychiatric disorders in the offspring.
Animal studies have shown that advanced paternal age is associated with the behavioral domains relevant to schizophrenia and autism,52-55 suggesting that paternal age per se plays an independent role in the offspring’s health outcome.
A critical concern about the de novo mutations hypothesis is whether the effect of paternal age is confounded by selection into late fatherhood, such as by a genetic liability for psychiatric disorder in parents. Individuals with a higher genetic liability may choose early or postponed parenthood,56-58 and they will also transmit an increased genetic risk to their children. However, a previous study has shown that even after adjusting for parental genetic liability of schizophrenia, the association of paternal age with early-onset schizophrenia remained;59 this indicated that paternal age per se plays an independent role in the offspring’s psychiatric risk.
Previous population genetic modelling has shown that the de novo mutation hypothesis and selection into late fatherhood hypothesis are not mutually exclusive.60 Furthermore, age-related epigenetic modifications61-63 and cumulative environmental exposure and psychological disadvantages of older fathers3 may altogether contribute to the paternal age association.1, 2, 60, 64
Our finding that offspring of very young fathers have a higher risk of MDD, SUD, and anxiety was similar to a previous Danish nationwide cohort study,10 reflecting a bimodal influence of paternal age on the offspring’s psychiatric risk. Animal studies also suggested that mice born from young postpubescent fathers have a poor behavioral performance than those born from mature fathers.65 The immaturity of spermatids, impulsive characteristics, and disadvantageous home environments in the very young fathers may explain such association. However, in contrast to the higher risk of MR10, ADHD,39, 66, 67 and tic disorder39 in the offspring of young parents, this study, consistent with another previous study,12 did not observe such a pattern.
The increased risk of several psychiatric disorders in the offspring of very young mothers (< 20 years) was noteworthy, i.e., modest risk of BPD, MDD, and anxiety, and marked risk of SUD, MR, and suicide; these findings are in line with a previous Danish nationwide cohort study.10 In contrast, very young fathers had no association with BPD, MR, and suicide in the offspring, and had a slightly increased risk of MDD, SUD, and anxiety in the offspring, as compared with very young mothers. Young mothers have also been associated with an increased risk for childhood behavior and emotional disorders in the offspring.68 However, this study shows no increased risk of autism in the offspring of young mothers, inconsistent with previous studies.10, 37, 39 A possible explanation of the increased psychiatric risk in the offspring of very young mothers may be the shared genetic background and environmental factors. Early motherhood, particularly teenaged motherhood, may be unplanned and might interfere with education and employment,68-70 and perhaps the associations with adverse economic, home environments and parenting behaviors, and subsequent adverse health and social outcomes in both mothers and their children.
We show that older maternal age (over 25 years) was associated with a higher risk of psychiatric disorder in the offspring, and this may be explained by increased genetic alterations, adverse exposures, and perinatal and obstetric complications in delayed motherhood.71 Although the offspring of older mothers (over 40 years) had a higher risk of MR in a Danish cohort (1955–2006),10 the presence of Down syndrome was proposed to be a possible explanation; the present study with a younger cohort (1980–2018) did not show any such findings. This may be due to the screening policy for Down syndrome in pregnant mothers aged > 34 years after 1985 in Taiwan, altogether suggesting that older mothers were not associated with Down syndrome-unrelated MR in the offspring.
In addition to the aging-related effect, the results of dissimilarly aged parents not only provide biological mechanisms but also psychosocial implications. A larger parental age difference, irrespective of which parent was older, have been shown to be associated with schizophrenia, autism and ADHD in the offspring,37-39 yet some potential confounders have not been considered, such as mean parental age,39 parental social economic status, and psychiatric history.37 After adjusting these factors, this study with the largest sample size of a single population to date, suggested that only offspring with mothers older than the father have a higher risk of autism and ADHD, and only offspring with fathers older than the mother have a higher risk of schizophrenia. Otherwise, we provide novel findings that larger parental age difference, either father older than the mother or mother older than the father, was associated with a higher risk of MDD, SUD, MR, anxiety, and suicide in the offspring, and was associated with a lower risk of OCD in the offspring. These findings indicate that the parental age difference play an independent role on the offspring’s psychiatric risk, suggesting that the parents with a larger age difference may represent genetic and psychosocial characteristics that increase the offspring’s psychiatric risk, e.g., women with schizophrenia were more likely to marry older man.72
This study has several limitations. First, we used NHIRD between 1997 and 2018 to establish the pedigree and identify disorders; only individuals, including the study sample and their parents/grandparents, who were alive during the study period could be included in this study. The psychiatric diagnoses would be missed only when they have not used any psychiatric services during the >20 years catchment period (1997-2018). The coverage of linkage to parents, paternal-grandparents, and maternal-grandparents was lower in individuals of older birth cohorts (Supplementary Figure 1) which may be because their parents/grandparents had a lower probability of being captured by insurance registries during the 1997-2018 period, or because that the using of Maternal and Child Health database for identifying parents–child relationship among individuals of the younger birth cohort. To account for this, birth cohort has been controlled for in regression analyses. If the parental/grandparental information is missing-at-random, i.e., no differential loss of coverage related to diagnoses in offspring, the impact of the missing parental/grandparental information is not on the unbiasedness of the parental association estimates but rather on the statistical power. The birth cohort-stratified analyses demonstrated similar trends of the parental age association.
Second, the maternal grandparent cohort had a smaller sample size than the paternal grandparent cohort (especially in the earlier birth cohort of 1980s and 1990s, Supplementary Table 1). This discrepancy may be because mothers in the past were predominantly housewives, and thus the maternal grandparents had a smaller chance of being captured by insurance registries. The proportion of length of follow-up < 10 years was higher in maternal grandparent cohort (61%, Supplementary Table 1) than in the paternal grandparent cohort (46%). Thus, the maternal grandparent cohort identified a smaller proportion of non-childhood onset diseases, e.g., the proportion of schizophrenia was 0.06% compared to 0.14% in paternal grandparent cohort (Supplementary Table 4-5). Taken together, these could result in the inadequate statistical power in the association test in maternal grandparent cohort, e.g., although the estimates for risk of schizophrenia in the offspring were similar between paternal-grandfather age and maternal-grandfather age, the significance was reduced for the association with maternal-grandfather age.
Third, information on parental and grandparental education is not available, hence residual confounding is possible. We used the highest insurance amount as a proxy for a family’s socioeconomic status; generally, the strength of paternal age association was attenuated but remained significant after this adjustment. Socioeconomic status is a complex risk factor and its role in the paternal age association (e.g., mediator or confounder) warrants further investigation. Also, confounding by parental and grandparental genetic propensity for psychiatric disorders is possible. Although we had no genotype data to derive and directly adjust for genetic propensity, we did adjust for parental and grandparental psychiatric history, which partly captures genetic effects.
Fourth, because the onset of psychiatric disorders can have uncertainties in the national insurance claim, as it established the diagnostic time rather than the onset time and can have left-censoring if earlier than the study start year 1997, we decided to perform logistic regression to examine the parental age association. In a future study, if the onset event-time can be accurately ascertained, survival analyses could provide a better estimate of the risk trajectories than logistic regression.73
Paternal age at the birth of offspring is increasing in the developed world,74 potentially resulting in an increase in the number of psychiatric cases and hence becoming an important public health issue.75 This comprehensive assessment of psychiatric disorders conferred by paternal age using a nationwide cohort study with multiple study designs provides solid evidence for the independent role of paternal age on the offspring’s psychiatric risks, highlighting the impact on increased disease burden due to delayed paternity.
Supplementary Material
Acknowledgements
This study was approved by the Central Regional Research Ethics Committee of the China Medical University, Taichung, Taiwan (CRREC-108-28). The requirement for informed consent was waived because the NHIRD consists of de-identified data. The NHIRD used in this study is held by the Taiwan Ministry of Health and Welfare. The Ministry of Health and Welfare approved our application to access these data.
Funding
This study was supported by Taiwan Ministry of Science and Technology (MOST 106-2314-B-039-052-MY2; MOST 108-2314-B-039-030-MY3), and China Medical University (CMU108-MF-57; CMU109-MF-74; CMU110-MF-79). Dr. Fan was supported by grant R01MH122688 and RF1MH120025 funded by the National Institute for Mental Health (NIMH).
Footnotes
Competing interests
The authors declare no conflicts of interest.
Supplementary information is available at MP’s website.
Reference
- 1.de Kluiver H, Buizer-Voskamp JE, Dolan CV, Boomsma DI. Paternal age and psychiatric disorders: A review. Am J Med Genet B Neuropsychiatr Genet 2017; 174B: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janecka M, Mill J, Basson M, Goriely A, Spiers H, Reichenberg A et al. Advanced paternal age effects in neurodevelopmental disorders—review of potential underlying mechanisms. Transl Psychiatry 2017; 7(1): e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couture V, Delisle S, Mercier A, Pennings G. The other face of advanced paternal age: a scoping review of its terminological, social, public health, psychological, ethical and regulatory aspects. Hum Reprod Update 2021; 27(2): 305–323. [DOI] [PubMed] [Google Scholar]
- 4.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Langstrom N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry 2008; 65(9): 1034–1040. [DOI] [PubMed] [Google Scholar]
- 5.Grigoroiu-Serbanescu M, Wickramaratne PJ, Mihailescu R, Prelipceanu D, Sima D, Codreanu M et al. Paternal age effect on age of onset in bipolar I disorder is mediated by sex and family history. Am J Med Genet B Neuropsychiatr Genet 2012; 159B(5): 567–579. [DOI] [PubMed] [Google Scholar]
- 6.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry 2001; 58(4): 361–367. [DOI] [PubMed] [Google Scholar]
- 7.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S et al. Advancing paternal age and autism. Arch Gen Psychiatry 2006; 63(9): 1026–1032. [DOI] [PubMed] [Google Scholar]
- 8.Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingson T, Owen MJ et al. Paternal age and risk for schizophrenia. Br J Psychiatry 2003; 183: 405–408. [DOI] [PubMed] [Google Scholar]
- 9.Miller B, Messias E, Miettunen J, Alaraisanen A, Jarvelin M-R, Koponen H et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull 2011; 37(5): 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry 2014; 71: 301–309. [DOI] [PubMed] [Google Scholar]
- 11.Racinea SE, Culberta KM, Burta SA, Klumpa KL. Advanced paternal age at birth: phenotypic and etiologic associations with eating pathology in offspring. Psychol Med 2014; 44(5): 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjolander A et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry 2014; 71(4): 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javaras K, Rickert ME, Thornton LM, Peat CM, Baker JH, Birgegård A et al. Paternal age at childbirth and eating disorders in offspring. Psychol Med 2017; 47: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan K-C, Chiang H-J, Huang T-L, Chiou Y-J, Hsu T-Y, Ou Y-C et al. Association between paternal age and risk of schizophrenia: a nationwide population–based study. J Assist Reprod Genet 2021; 38: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y, Yu Y, Xiao J, Luo J, Zhang Y, Tian Y et al. Association of grandparental and parental age at childbirth with autism spectrum disorder in children. JAMA Netw Open 2020; 3(4): e202868–e202868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KM, Choi YJ, Lim MH, Ha M, Kwon H-J. Parental age at childbirth and risk for attention-deficit/hyperactivity disorder in offspring. J Psychiatr Res 2020; 131: 180–186. [DOI] [PubMed] [Google Scholar]
- 17.Larsen JT, Bulik CM, Thornton LM, Koch SV, Petersen L. Prenatal and perinatal factors and risk of eating disorders. Psychol Med 2021; 51(5): 870–880. [DOI] [PubMed] [Google Scholar]
- 18.Wang SH, Liu CM, Hwu HG, Hsiao CK, Chen WJ. Association of older paternal age with earlier onset among co-affected schizophrenia sib-pairs. Psychol Med 2015; 45(10): 2205–2213. [DOI] [PubMed] [Google Scholar]
- 19.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull 2001; 27(3): 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 2012; 488(7412): 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner TN, Coe BP, Dickel DE, Hoekzema K, Nelson BJ, Zody MC et al. Genomic patterns of de novo mutation in simplex autism. Cell 2017; 171: 710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature 2017; 542: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goes FS, Pirooznia M, Tehan M, Zandi PP, McGrath J, Wolyniec P et al. De novo variation in bipolar disorder. Mol Psychiatry 2019; 10: 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howrigan DP, Rose SA, Samocha KE, Fromer M, Cerrato F, Chen WJ et al. Exome sequencing in schizophrenia-affected parent–offspring trios reveals risk conferred by protein-coding de novo mutations. Nat Neurosci 2020; 23(2): 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasani TA, Pedersen BS, Gao Z, Baird L, Przeworski M, Jorde LB et al. Large, three-generation human families reveal post-zygotic mosaicism and variability in germline mutation accumulation. Elife 2019; 8: e46922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jónsson H, Sulem P, Arnadottir GA, Pálsson G, Eggertsson HP, Kristmundsdottir S et al. Multiple transmissions of de novo mutations in families. Nat Genet 2018; 50(12): 1674–1680. [DOI] [PubMed] [Google Scholar]
- 27.Jónsson H, Sulem P, Kehr B, Kristmundsdottir S, Zink F, Hjartarson E et al. Parental influence on human germline de novo mutations in 1,548 trios from Iceland. Nature 2017; 549(7673): 519–522. [DOI] [PubMed] [Google Scholar]
- 28.Goriely A, McGrath JJ, Hultman CM, Wilkie AOM, Malaspina D. "Selfish spermatogonial selection": a novel mechanism for the association between advanced paternal age and neurodevelopmental disorders. Am J Psychiatry 2013; 170(6): 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor JL, Debost J-CP, Morton SU, Wigdor EM, Heyne HO, Lal D et al. Paternal-age-related de novo mutations and risk for five disorders. Nat Commun 2019; 10: 3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ek M, Wicks S, Svensson AC, Idring S, Dalman C. Advancing paternal age and schizophrenia: the impact of delayed fatherhood. Schizophr Bull 2015; 41: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry 2011; 168(1): 82–88. [DOI] [PubMed] [Google Scholar]
- 32.Weiser M, Fenchel D, Frenkel O, Fruchter E, Burshtein S, Yehuda AB et al. Understanding the association between advanced paternal age and schizophrenia and bipolar disorder. Psychol Med 2019: 1–7. [DOI] [PubMed] [Google Scholar]
- 33.D’onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health 2013; 103: S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Sjölander A, Song H, Cnattingius S, Fang F, Yang Q et al. Associations of parental and perinatal factors with subsequent risk of stress-related disorders: a nationwide cohort study with sibling comparison. Mol Psychiatry 2022: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frans EM, Sandin S, Reichenberg A, Langstrom N, Lichtenstein P, McGrath JJ et al. Autism risk across generations: a population-based study of advancing grandpaternal and paternal age. JAMA Psychiatry 2013; 70(5): 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frans EM, McGrath JJ, Sandin S, Lichtenstein P, Reichenberg A, Langstrom N et al. Advanced paternal and grandpaternal age and schizophrenia: a three-generation perspective. Schizophr Res 2011; 133(1-3): 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandin S, Schendel D, Magnusson P, Hultman C, Surén P, Susser E et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry 2016; 21: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byars SG, Boomsma JJ. Opposite differential risks for autism and schizophrenia based on maternal age, paternal age, and parental age differences. Evol Med Public Health 2016; 2016: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janecka M, Hansen SN, Modabbernia A, Browne HA, Buxbaum JD, Schendel DE et al. Parental Age and Differential Estimates of Risk for Neuropsychiatric Disorders: Findings From the Danish Birth Cohort. J Am Acad Child Adolesc Psychiatry 2019: 10.1016/j.jaac.2018.1009.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ek M, Wicks S, Svensson AC, Idring S, Dalman C. Advancing Paternal Age and Schizophrenia: The Impact of Delayed Fatherhood. Schizophr Bull 2014: doi: 10.1093/schbul/sbu1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frans E, Lichtenstein P, Hultman C, Kuja-Halkola R. Age at fatherhood: heritability and associations with psychiatric disorders. Psychol Med 2016; 46(14): 2981–2988. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A. Classification of common human diseases derived from shared genetic and environmental determinants. Nature Genet 2017; 49: 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakhani CM, Tierney BT, Manrai AK, Yang J, Visscher PM, Patel CJ. Repurposing large health insurance claims data to estimate genetic and environmental contributions in 560 phenotypes. Nature Genet 2019; 51: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou I-J, Kuo C-F, Huang Y-S, Grainge MJ, Valdes AM, See L-C et al. Familial aggregation and heritability of schizophrenia and co-aggregation of psychiatric illnesses in affected families. Schizophrenia Bull 2017; 43: 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo C-F, Grainge MJ, See L-C, Yu K-H, Luo S-F, Valdes AM et al. Familial aggregation of gout and relative genetic and environmental contributions: a nationwide population study in Taiwan. Ann Rheum Dis 2015; 74: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buwe A, Guttenbach M, Schmid M. Effect of paternal age on the frequency of cytogenetic abnormalities in human spermatozoa. Cytogenet Genome Res 2005; 111(3-4): 213–228. [DOI] [PubMed] [Google Scholar]
- 47.Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet 2012; 90(2): 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maher GJ, McGowan SJ, Giannoulatou E, Verrill C, Goriely A, Wilkie AO. Visualizing the origins of selfish de novo mutations in individual seminiferous tubules of human testes. Proc Natl Acad Sci USA 2016: 201521325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010; 466: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012; 485: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirov G, Pocklington A, Holmans P, Ivanov D, Ikeda M, Ruderfer D et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatr 2012; 17: 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RG, Kember RL, Mill J, Fernandes C, Schalkwyk LC, Buxbaum JD et al. Advancing paternal age is associated with deficits in social and exploratory behaviors in the offspring: a mouse model. PLoS One 2009; 4(12): e8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sampino S, Juszczak G, Zacchini F, Swiergiel A, Modlinski J, Loi P et al. Grand-paternal age and the development of autism-like symptoms in mice progeny. Transl Psychiatry 2014; 4: e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milekic M, Xin Y, O’donnell A, Kumar K, Bradley-Moore M, Malaspina D et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry 2015; 20: 995–1001. [DOI] [PubMed] [Google Scholar]
- 55.Janecka M, Manduca A, Servadio M, Trezza V, Smith R, Mill J et al. Effects of advanced paternal age on trajectories of social behavior in offspring. Genes Brain Behav 2015; 14: 443–453. [DOI] [PubMed] [Google Scholar]
- 56.Mehta D, Tropf FC, Gratten J, Bakshi A, Zhu Z, Bacanu S-A et al. Evidence for genetic overlap between schizophrenia and age at first birth in women. JAMA Psychiatry 2016; 73(5): 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni G, Gratten J, Wray NR, Lee SH. Age at first birth in women is genetically associated with increased risk of schizophrenia. Sci Rep 2018; 8(1): 10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullins N, Ingason A, Porter H, Euesden J, Gillett A, Ólafsson S et al. Reproductive fitness and genetic risk of psychiatric disorders in the general population. Nat Commun 2017; 8(1): 15833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang SH, Hsiao PC, Yeh LL, Liu CM, Liu CC, Hwang TJ et al. Advanced paternal age and early-onset of schizophrenia in sporadic cases: not confounded by parental polygenic risk to schizophrenia. Biol Psychiatry 2019; 86: 56–64. [DOI] [PubMed] [Google Scholar]
- 60.Gratten J, Wray NR, Peyrot WJ, McGrath JJ, Visscher PM, Goddard ME. Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nat Genet 2016; 48: 718–724. [DOI] [PubMed] [Google Scholar]
- 61.Perrin MC, Brown AS, Malaspina D. Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia. Schizophr Bull 2007; 33(6): 1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atsem S, Reichenbach J, Potabattula R, Dittrich M, Nava C, Depienne C et al. Paternal age effects on sperm FOXK1 and KCNA7 methylation and transmission into the next generation. Hum Mol Genet 2016; 25(22): 4996–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denomme MM, Haywood ME, Parks JC, Schoolcraft WB, Katz-Jaffe MG. The inherited methylome landscape is directly altered with paternal aging and associated with offspring neurodevelopmental disorders. Aging Cell 2020; 19(8): e13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khachadourian V, Zaks N, Lin E, Reichenberg A, Janecka M. Advanced paternal age and risk of schizophrenia in offspring–Review of epidemiological findings and potential mechanisms. Schizophr Res 2021; 233: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Auroux M, Nawar NN, Naguib M, Baud M, Lapaquellerie N. Post-pubescent to mature fathers: increase in progeny quality? Hum Reprod 1998; 13(1): 55–59. [DOI] [PubMed] [Google Scholar]
- 66.Chang Z, Lichtenstein P, D’Onofrio BM, Almqvist C, Kuja-Halkola R, Sjölander A et al. Maternal age at childbirth and risk for ADHD in offspring: a population-based cohort study. Int J Epidemiol 2014; 43(6): 1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chudal R, Joelsson P, Gyllenberg D, Lehti V, Leivonen S, Hinkka-Yli-Salomäki S et al. Parental age and the risk of attention-deficit/hyperactivity disorder: a nationwide, population-based cohort study. J Am Acad Child Adolesc Psychiatry 2015; 54(6): 487–494. e481. [DOI] [PubMed] [Google Scholar]
- 68.Saha S, Barnett AG, Buka SL, McGrath JJ. Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr Res 2009; 115(2-3): 130–135. [DOI] [PubMed] [Google Scholar]
- 69.Mills M, Rindfuss RR, McDonald P, Te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Human Reprod Update 2011; 17: 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fergusson DM, Woodward LJ. Maternal age and educational and psychosocial outcomes in early adulthood. J Child Psychol Psychiatry 1999; 40: 479–489. [PubMed] [Google Scholar]
- 71.Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol 2014; 43(1): 107–115. [DOI] [PubMed] [Google Scholar]
- 72.Miller B, Suvisaari J, Miettunen J, Jarvelin MR, Haukka J, Tanskanen A et al. Advanced paternal age and parental history of schizophrenia. Schizophr Res 2011; 133(1-3): 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Annesi I, Moreau T, Lellouch J. Efficiency of the logistic regression and Cox proportional hazards models in longitudinal studies. Stat Med 1989; 8(12): 1515–1521. [DOI] [PubMed] [Google Scholar]
- 74.Khandwala YS, Zhang CA, Lu Y, Eisenberg ML. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod 2017; 32: 2110–2116. [DOI] [PubMed] [Google Scholar]
- 75.Greenberg D, Khandwala Y, Lu Y, Stevenson D, Shaw G, Eisenberg M. Disease burden in offspring is associated with changing paternal demographics in the United States. Andrology 2020; 8(2): 342–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.