Abstract
After mouse mammary tumor virus (MMTV) infection, B lymphocytes present a superantigen (Sag) and receive help from the unlimited number of CD4+ T cells expressing Sag-specific T-cell receptor Vβ elements. The infected B cells divide and differentiate, similarly to what occurs in classical B-cell responses. The amplification of Sag-reactive T cells can be considered a primary immune response. Since B cells are usually not efficient in the activation of naive T cells, we addressed the question of whether professional antigen-presenting cells such as dendritic cells (DCs) are responsible for T-cell priming. We show here, using MMTV(SIM), a viral isolate which requires major histocompatibility complex class II I-E expression to induce a strong Sag response in vivo, that transgenic mice expressing I-E exclusively on DCs (I-EαDC tg) reveal a strong Sag response. This Sag response was dependent on the presence of B cells, as indicated by the absence of stimulation in I-EαDC tg mice lacking B cells (I-EαDC tg μMT−/−), even if these B cells lack I-E expression. Furthermore, the involvement of either residual transgene expression by B cells or transfer of I-E from DCs to B cells was excluded by the use of mixed bone marrow chimeras. Our results indicate that after priming by DCs in the context of I-E, the MMTV(SIM) Sag can be recognized on the surface of B cells in the context of I-A. The most likely physiological relevance of the lowering of the antigen threshold required for T-cell/B-cell collaboration after DC priming is to allow B cells with a low affinity for antigen to receive T-cell help in a primary immune response.
Dendritic cells (DCs) play a crucial role in antigen presentation (3, 4). Immature DCs found mostly in nonlymphoid organs, such as Langerhans cells of the skin, continuously filter the surrounding liquids and carry the antigens or infectious microorganisms to the draining lymph nodes. There they differentiate into professional antigen-presenting cells, called interdigitating DCs, which prime naive T cells to become efficient effector cells (3, 29–31, 36, 51, 52, 55).
B cells are inefficient at inducing immune responses with naive T cells (9, 14, 15, 18, 20, 44, 48). On the other hand, however, they are good presenting cells for antigen-experienced T cells when they express an antigen-specific Ig (11, 37, 54). Most likely, the nature and the density of costimulation molecules required for the long duration of priming observed in the context of DCs in vivo and in vitro is responsible for this difference (29, 40). DCs have also been shown to directly activate B cells when the T cell-DC interaction is replaced by anti-CD40 treatment (13).
T-cell/B-cell collaboration induced by mouse mammary tumor virus (MMTV) infection is indistinguishable from classical antigen responses in lymph nodes (40) and is therefore a valuable model to study T-cell/B-cell collaboration in vivo. MMTV preferentially infects B cells, which then present the superantigen (Sag) in the context of major histocompatibility complex (MHC) class II on their cell surface (2, 5, 25, 26). Sag-presenting B cells receive help from the large number of T cells expressing Sag-specific T-cell receptor Vβ chains. The infected B cells divide and differentiate in both extrafollicular and follicular B-cell compartments, similarly to classical B-cell responses in terms of localization, surface marker phenotypes, antibody secretion, and kinetics (40, 41). After the initial expansion, the Sag-reactive T cells are slowly deleted from the repertoire by peripheral clonal deletion (27, 43, 46, 64).
Although the importance of B cells during MMTV infection has been clearly demonstrated, we were interested to know whether other types of antigen-presenting cells might also be required to mount an efficient Sag-induced immune response. Indeed, since such a response can be considered a primary immune response, and since B cells are usually not efficient in the activation of naive T cells, we addressed the question of whether professional antigen-presenting cells such as DCs could play a cooperative role in the activation of Sag-reactive T cells (32).
We show here, using MMTV(SIM), a viral isolate which requires MHC class II I-E expression to induce a strong Sag response in vivo (42), that transgenic mice expressing I-E exclusively on DCs (I-EαDC tg) reveal a strong Sag response. This Sag response is dependent on the presence of B cells as indicated by the absence of stimulation in I-EαDC tg mice lacking B cells (I-EαDC tg μMT−/−), even if these B cells lack I-E expression. Furthermore, the involvement of either residual transgene expression by B cells or transfer of I-E from DCs to B cells could be excluded by the use of mixed bone marrow chimeras. Our results clearly demonstrate that following professional T-cell priming by DCs, T-cell/B-cell interactions become productive in the immune response induced by the MMTV Sag.
MATERIALS AND METHODS
Mouse strains.
C57BL/6 mice were purchased from Harlan OLAC Ltd. (Bicester, United Kingdom). C57BL/6 I-Eα-transgenic mice (C57BL/6 I-Eα tg) were obtained from D. Mathis (38). Mice transgenic for MHC class II I-E expression restricted to DCs (C57BL/6 I-EαDC tg) were bred in our animal facility (6, 7). B cell-deficient IgM mice (μMT−/−) were obtained from K. Rajewsky (33). C57BL/6 mice deficient in MHC class II expression (I-A−/−) were provided by J. van Meerwijk (63). All the mouse strains were maintained as breeding pairs in our animal facilities, and the C57BL/6 I-EαDC tg μMT−/− mice were obtained by breeding in our animal facilities. For all the experiments described, either nontransgenic littermates or C57BL/6 mice were used as control mice, with no difference in the results obtained.
MMTV injections.
Titered stocks of MMTV(SIM) diluted in phosphate-buffered saline (PBS) were injected subcutaneously into the hind footpads of naïve mice, and the draining popliteal lymph node was isolated 2, 4, or 6 days after injection. Alternatively, mice were tail bled, and leukocytes were recovered from heparinized blood samples by centrifugation through a Ficoll cushion.
AZT treatment.
Three milligrams of 3′-azido-3′-deoxythymidine (AZT; Sigma, St. Louis, Mo.) were injected intravenously, and it was dissolved in the drinking water of the mice at a concentration of 1 mg/ml 36 h after MMTV(SIM) injection.
Antibodies.
The following antibodies were used in this study: fluorescein isothyocyanate (FITC)-labeled anti-Vβ4 (KT4-10) (60), phycoerythrin (PE)-coupled anti-CD4, PE-coupled anti-CD8 and PE- or FITC-coupled anti-B220 (Caltag, San Francisco, Calif.), biotinylated goat anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA (Caltag); anti-Syndecan-1 (Pharmingen, Uppsala, Sweden).
Flow cytometric analysis.
Lymph node cells or peripheral leukocytes were stained in one step, either with a mixture of anti-Vβ4 antibody and anti-CD4 antibody or with a mixture of anti-Syndecan-1 and anti-B220 antibody. Analysis was performed on a FACScan (Becton Dickinson & Co., Mountain View, Calif.) cell analyzer with Lysis II software for data evaluation. Dead cells were excluded by a combination of forward and side scatter characteristics. B and T cells from popliteal lymph nodes were sorted at different times after MMTV(SIM) injection on a FACStar Plus (Becton Dickinson & Co.) flow cytometer after staining with PE-coupled anti-CD4 and anti-CD8 and FITC-labeled anti-B220. After reanalysis, the sorted cell populations had a purity of >98%.
Enzyme-linked immunospot (ELISPOT) assay.
The number of Ig-secreting cells and Ig isotypes were determined. Briefly, microtiter plates (Nunc Maxisorp) were coated with goat anti-mouse IgG and IgM (TAGO, Burlingame, Calif.), and cells isolated from lymph nodes were serially diluted from 105 cells/well. The plate was incubated 4 h at 37°C, and the Ig-secreting cell isotypes were then determined with biotinylated goat anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA. Finally, the plates were developed with 5-bromo-4-chloro-3-indolyl phosphate (Sigma) after incubation with a streptavidin-conjugated alkaline phosphatase (Boehringer Mannheim). The resulting spots were counted and expressed as the number of spot-forming cells (SFC) per 105 B cells.
PCR detection of proviral DNA sequences.
DNA from 25,000 cells (determined by fluorescent-activated cell sorting) was amplified with the 5′ oligonucleotide Ms10 (AGGTGGGTCACAATCAACGGC), which reacts with various Sag sequences, and the 3′ oligonucleotide IM15 (CCCCTCCTTGGTATAATATCT) specific for the MMTV(SIM) Sag or HD57 (CAAACCAAGTCAGGAAACCACTTG) for all Mtvs. The PCR conditions were as follows: 1 cycle consisting of 5 min at 94°C, 1 min at 59°C, and 1 min at 72°C; 25 cycles, with 1 cycle consisting of 30 s at 94°C, 30 s at 59°C, and 30 s at 72°C; and finally, an extension step for 10 min at 72°C in PCR buffer containing 20 mM Tris-HCl (pH 8.55), 16 mM (NH4)2SO4, 2.5 mM MgCl2, 150 μg of bovine serum albumin/ml, and 0.2 mM (each) deoxynucleoside triphosphate; 2.5 U of Taq polymerase (Biotaq; Bioprobe Systems, Les Ulis, France) and each oligonucleotide (10 μM) were added to the PCR mixture. The specific PCR product was detected by liquid hybridization, with 20% of the PCR mixture being hybridized in 150 mM NaCl, 2.5 mM EDTA with 50 fmol of a 32P-labeled internal probe common to various Sag sequences (Ms11 [CAAGGAGGTCTAGCTCTGGCG]). The conditions for the reaction were 5 min at 98°C, 15 min at 60°C, and rapid cooling to 4°C. The reaction products were separated by size on a 2% agarose gel, dried on DE81 paper (Whatman), and autoradiographed at −70°C on X-Omat films (Eastman Kodak Company, Rochester, N.Y.).
Generation of bone marrow chimeras.
The chimeric mice were prepared as previously described (63). C57BL/6 mice were lethally irradiated (1,000 rads) with a 137Cs source and injected the next day intravenously with 107 bone marrow cells depleted of T cells by complement killing with anti-Thy1 antibody (AT83).
RESULTS
T-cell expansion induced by the MMTV(SIM) Sag in mice expressing I-E exclusively on DCs.
The MMTV(SIM) Sag interacts with Vβ4-expressing CD4+ T cells and requires MHC class II I-E expression to induce a strong Sag response in vivo (42). This last property has been demonstrated previously in C57BL/6 mice, which do not express MHC class II I-E molecules, compared with transgenic C57BL/6 mice expressing I-Eα under the control of the MHC class II promoter (I-Eα tg) and thus expressing I-E on all MHC class II positive cells. To further understand the respective roles of different types of antigen-presenting cells, we used transgenic C57BL/6 mice expressing I-Eα specifically on DCs but not on B cells or other MHC class II positive cells (I-EαDC tg). Such a phenotype has been generated through the use of the CD11c promoter to specifically drive I-Eα expression in the DC compartment. I-EαDC tg mice have normal levels of I-E on DCs, with undetectable I-E expression on B cells as shown by flow cytometry (6, 7).
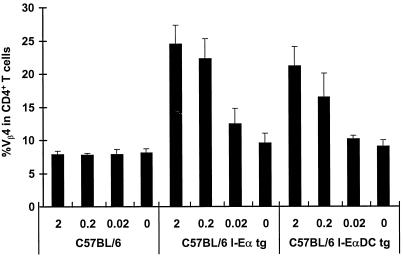
Injection of MMTV(SIM) into the footpads of C57BL/6 mice did not induce an increase in the percentage of the Sag-reactive T cells in the draining popliteal lymph node (Fig. 1, left), whereas in I-Eα tg mice a strong Sag response was detected (Fig. 1, middle). This was true with virus doses contained in 2 to 0.02 μl of milk. Interestingly, transgenic mice expressing I-E exclusively on DCs (I-EαDC tg) showed a strong, albeit slightly lower, Sag response (Fig. 1, right), indicating that expression of I-E in the DC compartment is critical to prime the T-cell response to the MMTV Sag. Indeed, recent immunohistochemical observations have suggested that DCs could be involved in priming of Sag-reactive T cells (41).
FIG. 1.
DCs can present MMTV(SIM) Sags. The T-cell response to decreasing doses of MMTV(SIM), i.e., 2, 0.2, and 0.02 μl of MMTV(SIM) containing purified milk (30) was analyzed 4 days after injection in C57BL/6, C57BL/6 I-Eα tg, or C57BL/6 I-EαDC tg mice. The means of four lymph nodes ± standard deviations are shown. The experiment was repeated three times, with similar results. P < 0.01 (C57BL/6 compared to C57BL/6 I-Eα tg or C57BL/6 I-EαDC tg mice).
B cells contribute to the Sag response in I-EαDC tg mice.
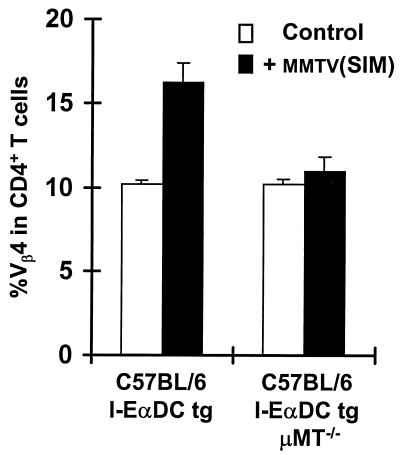
To determine the contribution of B cells in the immune response induced by the MMTV(SIM) Sag in I-EαDC tg mice, the transgenic mice were crossed and backcrossed to C57BL/6 μMT−/− mice, which lack B cells due to disruption of the gene segment encoding the IgM transmembrane region (33). In this way, we generated I-EαDC tg μMT−/− mice, expressing I-E on DCs but lacking B cells. Injection of MMTV(SIM) into the footpads of these mice did not induce an increase in the percentage of Sag-reactive Vβ4+ CD4+ T cells in the draining lymph node (Fig. 2). As a positive control, I-EαDC tg μMT−/− mice were infected with a recombinant vaccinia virus expressing the MMTV(GR) Sag (34). Such an infection induced a large increase in the percentage of Sag-reactive T cells (data not shown), indicating that the T cells are able to respond to Sag in these B cell-deficient mice. Taken together, these results indicated that DCs are efficient presenters in the Sag response induced by MMTV(SIM) but that a measurable response is dependent on the presence of B cells.
FIG. 2.
B cells contribute to the Sag response in I-EαDC tg mice. The T-cell response to 2 μl of MMTV(SIM) containing purified milk (42) was analyzed 4 days after injection in C57BL/6 I-EαDC tg and C57BL/6 I-EαDC μMT−/− tg mice lacking B cells. The means of three lymph nodes ± standard deviations are shown. The experiment was repeated three times, with similar results. P < 0.01 [C57BL/6 I-EαDC compared to C57BL/6 I-EαDC μMT−/− tg mice both injected with MMTV(SIM)].
The MMTV(SIM) Sag is presented to T cells in C57BL/6 mice.
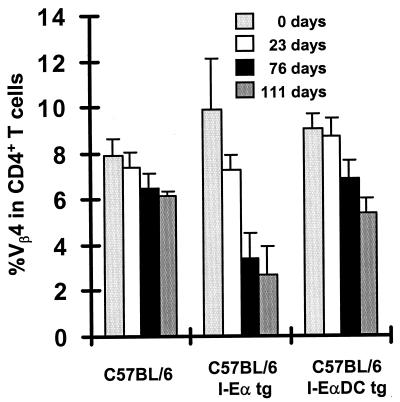
Although C57BL/6 mice failed to show a detectable expansion of Sag-reactive T cells in the draining lymph node, it was important to define whether the MMTV(SIM) Sag can also be presented to a lower extent to T cells in the absence of MHC class II I-E molecules. To address this question, we studied the deletion of Vβ4+ CD4+ T cells after MMTV(SIM) infection. Indeed, systemic deletion of Sag-reactive T cells is a much more sensitive readout for a Sag response than the early localized expansion that was detectable in the draining lymph node (23). Two microliters of MMTV(SIM)-containing milk was injected into the footpads of C57BL/6, C57BL/6 I-Eα tg, or C57BL/6 I-EαDC tg mice. The percentage of Vβ4+ cells among CD4+ peripheral blood lymphocytes was determined at various time points after infection (Fig. 3). The most rapid and complete deletion of Vβ4+ CD4+ T cells was observed in I-Eα tg mice (Fig. 3, middle). Interestingly, a slow and small but significant deletion occurred in C57BL/6 mice in the absence of I-E (Fig. 3, left). C57BL/6 I-EαDC tg mice had an intermediate magnitude and kinetics of deletion (Fig. 3, right). These results indicate a weak but detectable Sag presentation in the absence of I-E. In addition, the slower deletion kinetics in I-EαDC tg mice compared to those in I-Eα tg mice can probably be explained by the weak priming capacity of Sag-presenting I-E-negative B cells. Overall, the presentation of the MMTV(SIM) Sag by I-A is estimated to be at least 100 times weaker than its presentation by I-E [see Fig. 1, compare the absence of response in C57BL/6 mice with 2 μl of MMTV(SIM)-containing milk to the detectable response in I-EαDC tg mice with 0.02 μl of MMTV(SIM)-containing milk].
FIG. 3.
The MMTV(SIM) Sag is presented to T cells in the absence of MHC class II I-E molecules. Vβ4+ T-cell deletion upon injection of 1 μl of MMTV(SIM) containing purified milk was measured in leukocytes recovered from blood. The data represent the means of peripheral blood samples of four mice ± standard deviations. The experiment was repeated twice, with similar results.
MMTV Sag-dependent B-cell differentiation in mice expressing I-E exclusively on DCs.
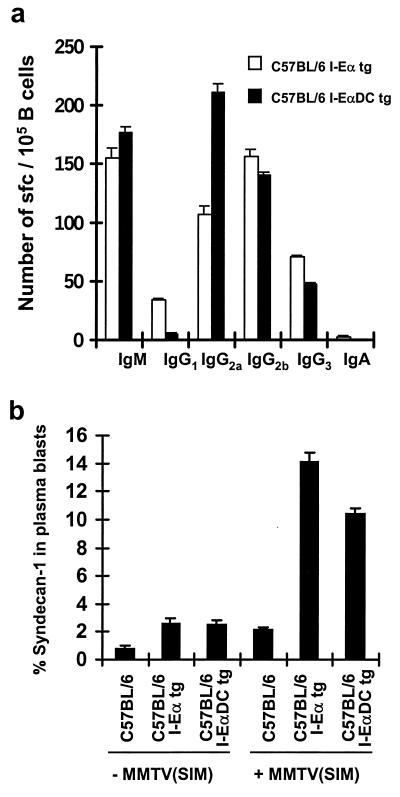
The previous experiments indicated that expression of I-E on DCs in the presence of B cells was required to prime a strong T-cell response to the MMTV(SIM) Sag. Since DCs seemed to be the main antigen-presenting cells in the induction of this primary immune response, we were interested in determining whether B cells would still receive T-cell help and undergo T-dependent B-cell differentiation in I-EαDC tg mice (39). MMTV(SIM) was injected into the footpads of C57BL/6, C57BL/6 I-Eα tg, or C57BL/6 I-EαDC tg mice. Cells from the draining popliteal lymph node were recovered at day 6 after infection and were studied by using an ELISPOT assay for Ig secretion and flow cytometric analysis for the expression of B-cell differentiation markers. B-cell differentiation was comparable in I-Eα tg and I-EαDC tg mice (Fig. 4). Total numbers of IgM- and IgG-secreting B cells were similar in both types of transgenic mice (Fig. 4a). No IgM- and IgG-secreting B cells could be detected in C57BL/6 mice in the same experiment (data not shown). The IgG isotype pattern was somewhat different in I-EαDC tg mice, with a higher number of IgG2a and a lower number of IgG1-producing B cells, perhaps reflecting a different pattern of cytokine secretion in the two transgenic mice. In addition, extrafollicular B-cell differentiation was assessed by flow cytometry through the detection of syndecan-1, a plasma cell differentiation marker (Fig. 4b). Both types of transgenic mice had a similar percentage of syndecan-1 expression among plasma blasts (FSChigh/B220+/MHC class IIlow cells) at day 6 after infection with MMTV(SIM), whereas the expression of this marker in C57BL/6 mice remained at the background level. In summary, these results clearly indicated that B cells could receive adequate T-cell help and could differentiate normally in I-EαDC tg mice, even in the absence of I-E expression in the B-cell compartment.
FIG. 4.
Presentation of Sag by DCs leads to T-cell/B-cell collaboration. (a) Antibody production measured by ELISPOT assay 6 days after MMTV(SIM) injection (42) in C57BL/6 I-Eα and C57BL/6 I-EαDC tg mice. No antibody production could be measured in C57BL/6 mice (data not shown). (b) Syndecan-1 expression 6 days after MMTV(SIM) injection. The data are the means of three independent experiments.
Generation of bone marrow chimeric mice.
The results described so far could be explained (i) by a requirement of I-E expression on DCs for T-cell priming, (ii) by expression of I-E by B cells due to a slight leakiness of the CD11c promoter used for the DC-specific I-E expression, or (iii) by transfer of I-E molecules from transgene-expressing DCs to B cells. To test these possibilities, experiments with bone marrow chimeras were performed. I-EαDC tg mice were crossed and backcrossed with μMT−/− mice to generate I-EαDC tg μMT−/− mice, expressing I-E on DCs but lacking B cells. Mixed bone marrow chimeras with I-EαDC μMT−/− and C57BL/6 bone marrow were generated. In these chimeras, all the B cells originated from the C57BL/6 bone marrow, expressing I-A but not harboring the I-E transgene. In the DC compartment, expression of I-E was shown to correlate with the mixing ratio of the two bone marrows (e.g., 50% of the DCs in the chimera expressed I-E if equal amounts of bone marrow cells were injected, data not shown).
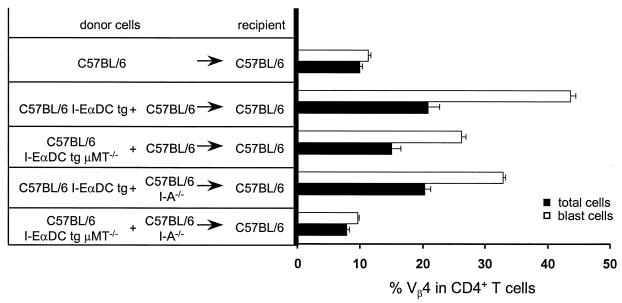
At least 6 weeks after reconstitution, the bone marrow chimeras were infected with MMTV(SIM) by footpad injection, and cells of the draining popliteal lymph node were recovered at day 4 after infection. The percentage of CD4+ T cells or CD4+ T-cell blasts expressing Vβ4 was determined using flow cytometry (Fig. 5). As expected, the negative control, C57BL/6 into C57BL/6 chimeras, had no increase in the percentage of Vβ4+ cells among CD4+ T-cell or T-cell blasts. As the positive control, I-EαDC tg into C57BL/6 mice had a strong Sag response to the MMTV(SIM) Sag. Interestingly, mixed bone marrow chimeras with I-EαDC μMT−/− and C57BL/6 bone marrow also showed a strong response to the MMTV(SIM) Sag. This response was comparable in magnitude to that of the positive control. Minor differences in the degree of response could be correlated to variations in the percentage of reconstitution of the DC compartment with I-E-expressing DCs. Indeed, titration experiments with different ratios of I-EαDC tg and C57BL/6 bone marrow showed that optimal results were obtained when at least 50% of the DC cells in the chimeras were of I-EαDC tg origin (data not shown). Overall, these results indicated that residual transgene expression by B cells was not the explanation for the effects described in Fig. 1 and 3 (if residual expression had been the explanation, no Sag response would have been observed in the bone marrow chimeras).
FIG. 5.
Major contribution of I-E-dependent Sag presentation by B cells in I-EαDC tg mice is excluded. The Vβ4+ T-cell stimulation in radiation bone marrow chimeras was analyzed 4 days after injection of 2 μl of MMTV(SIM) containing purified milk. In mixed bone marrow chimeras, reconstitutions ranged between 40 and 60% as determined by the percentage of DCs expressing I-E (data not shown).
Finally, mixed bone marrow chimeras with I-EαDC μMT−/− and C57BL/6 I-A−/− bone marrow were prepared and challenged with MMTV(SIM). These mice were similar to the other chimeras, except that the B cells expressed neither I-E nor I-A. No Sag response was observed in these mice (Fig. 5, bottom), indicating that transfer of I-E from DCs to B cells could not be the explanation for the Sag response in I-EαDC tg mice [if transfer of I-E had been the explanation, these chimeras would have responded to the MMTV(SIM) Sag]. Therefore, a major contribution of I-E-dependent Sag presentation by B cells in I-EαDC tg mice could be excluded.
Priming of Sag-reactive T cells by DCs led to an increased recognition of Sag molecules presented on B cells by I-A.
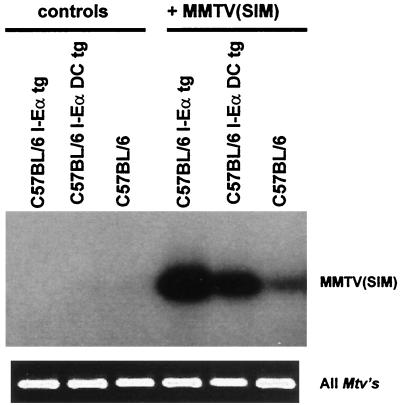
MMTV initially infects only a few B cells, leading to Sag-dependent amplification of the infected cells, with little bystander activation (25, 26, 40). A strong infection at the peak of the response would indicate preferential amplification of the infected B cells and, hence, Sag presentation by I-A. In the case of a polyclonal activation that is not dependent on Sag presentation by I-A, weak infection levels would be maintained at the peak of the response, since there is no preferential amplification of the infected B cells. To address this question, we extracted the cellular DNA from total lymph node cells 4 days after MMTV(SIM) injection and performed a PCR analysis to detect proviral sequences. Figure 6 shows a strong PCR signal at the peak of the Sag response in I-Eα tg mice as well as in I-EαDC tg mice as opposed to a weak PCR signal for the I-E-negative C57BL/6 mice. The endogenous integrated proviral sequences were amplified to control for DNA quantities (Fig. 6, bottom). These results confirm a preferential amplification of the infected B cells.
FIG. 6.
Detection of MMTV(SIM) infection. Proviral MMTV(SIM) DNA sequences were specifically amplified from total lymph node cells by PCR and detected by liquid hybridization 4 days after injection of 2 μl of MMTV(SIM) containing purified milk. All Mtvs are shown as internal controls for the amount of DNA.
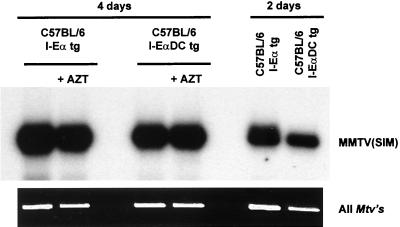
To exclude the possibility that the strong infection is due to preferential virus spread to activated B cells, we blocked viral spread with the reverse transcriptase inhibitor AZT. We have previously shown by administration of AZT that the Sag response does not result from the spread of infectious virus to bystander-activated B cells but is due mostly to the division of the few initially infected cells (24). Chronic application of AZT 36 h after infection blocks viral spread to bystander B cells but does not reduce the overall B-cell amplification. Furthermore, we have shown that the preferential amplification of infected cells under these conditions is due to the preferential division of the infected B cells due to T-cell help (24). If MMTV-infected B cells receive help preferentially, an increase of the PCR signal is expected between days 2 and 4. Therefore, we used fluorescence-activated cell sorted B cells from I-Eα tg and I-EαDC tg mice and determined their infection levels (Fig. 7). A comparably strong PCR signal was observed in both types of mice in the presence or absence of AZT. The relative infection levels increased 7.5-fold between days 2 and 4 as determined by instant imager analysis (data not shown) in both I-EαDC tg and I-Eα tg mice. As a control for the efficiency of AZT treatment, we treated mice with AZT before MMTV infection. This treatment results in a complete absence of a Sag response, absence of a PCR signal, and absence of an increase of lymph node size at day 4 (data not shown [24]). These results confirm that after priming by DCs in the context of I-E, Sag can be recognized under highly limiting conditions in the context of I-A.
FIG. 7.
Proviral MMTV(SIM) DNA sequences specifically amplified from FACS-sorted B cells (>98% pure) by PCR and detected by liquid hybridization 2 and 4 days after injection of 2 μl of MMTV(SIM) containing purified milk with or without AZT treatment 36 h after MMTV injection. All Mtvs are shown as internal controls for the amount of DNA. The results were analyzed by instant imager (see text).
DISCUSSION
The findings reported in this article demonstrate that MMTV uses at least two different types of antigen-presenting cells to trigger a potent immune response to the viral Sag. Indeed, both B cells and DCs were shown to play a crucial role in this immune response, since the Sag response was undetectable or dramatically reduced if one of the two partners was absent or unable to fulfil its function. For example, this was the case in C57BL/6 mice, where B cells were present but DCs did not express the appropriate MHC class II molecules to prime the Sag-reactive T cells. Similarly, in I-EαDC μMT−/− mice, where DCs were present and functional but B cells were absent, no Sag response was detectable. Expression of the Sag-presenting MHC class II I-E molecule exclusively on DCs was sufficient to induce a strong Sag response, provided B cells expressing the weakly Sag-presenting MHC class II I-A molecules were present. Therefore, a response to previously undetectable amounts of antigen on the B-cell surface became possible after DC priming.
The involvement of DCs in the early immune response to the MMTV SAg clarifies several aspects of the interaction between MMTV and the immune system (1). MMTV initially infects fewer than 100 B cells (25, 26). It was thought previously that expression of the viral Sag at the surface of the B cells was able to induce a strong T-cell response, thus providing potent T-cell help to the infected B cells which started to differentiate and to divide, increasing dramatically the amount of proviral DNA. During this process, infected B cells were preferentially stimulated, since they expressed the viral Sag and received cognate T-cell help. However, B cells are known to be inefficient as antigen-presenting cells for naive T cells, and it was difficult to imagine how the few infected B cells would be sufficient to trigger a potent primary immune response. In fact, priming of the naive Sag-reactive T cells appears to require the presentation of the viral Sag by DCs. As professional antigen-presenting cells, DCs are able to present the Sag much more efficiently than B cells. Sag-reactive T cells primed by DCs probably have a lower antigen recognition threshold and can recognize the Sag presented by B cells under suboptimal conditions. In addition, interaction with DCs might also be required to induce costimulatory molecules such as CD40L in Sag-reactive T cells in order to prepare them for interaction with B cells (16, 61). Indeed, mice lacking CD40L expression have been reported to be unable to sustain an MMTV-induced Sag response (10).
If the MMTV Sag is presented both by B cells and by DCs, the question arises how the DCs acquire the Sag and whether they are also infected with MMTV (62). In theory, several possibilities can be envisaged as follows. (i) DCs or DC precursors can be infected with MMTV and express the Sag classically from reverse transcribed and integrated proviral DNA. (ii) DCs can sustain the first steps of the viral life cycle, namely viral binding, entry, and reverse transcription, and the Sag is produced from incoming viral RNA or reverse transcribed but unintegrated viral DNA. (iii) DCs are not infected with MMTV, but small amounts of viral Sag are present within the viral particles and can be delivered to the DCs. (iv) The DCs are not infected, but they acquire the Sag by transfer from infected cells, e.g., B cells.
The first possibility is attractive. B cells were known to be the first targets of MMTV infection, but so far infection of DCs has not been reported. Retroviruses in general, with the exception of lentiviruses, are thought to require dividing cells to complete the proviral integration process, and DCs are known to be nondividing cells. The second possibility does not represent the classical way by which retroviral proteins are expressed, but small amounts of viral protein have been described to be produced in this way by avian retroviruses, e.g., avian myeloblastosis virus or Rous sarcoma virus (19, 28, 59). As few molecules of MMTV Sag are required to generate a potent biological effect, this possibility remains a potential explanation. The third possibility, advocating the presence of the Sag in the viral particles, would be compatible with the presence of small amounts of many viral and cellular proteins within other retroviral particles, e.g., human immunodeficiency virus (HIV). As the MMTV Sag is produced as a type II transmembrane protein, one would expect that the viral Sag is present within the viral membrane and gains access to the cell membrane upon fusion of the virus with the DC, even without further steps in the viral life cycle. Alternatively, the Sag would have to be processed to a soluble form, which could be transferred to the surface of the DC. Finally, a fourth possibility is that the Sag could be transferred from neighboring infected cells. This would require the transfer of membrane fragments of the donor cell containing the Sag or processing to a soluble form. The existence of such a processing and transfer has already been suggested by other groups with cell culture systems (12, 45, 47) as well as with earlier experiments on endogenous MMTV Sags in bone marrow chimeras (57, 58).
It is interesting to compare our observations with data from studies on HIV infection. Although CD4+ T cells are known to be the main target of HIV and to produce the largest amount of viral particles, the potential of HIV to infect DCs in vitro and in vivo has been the focus of intensive research in recent years. Conflicting reports have been generated initially as to the infectability of cultured DCs, with efficient replication reported by some groups but not by others (8, 35, 49, 66). More recently, productive HIV infection has been described in immature but not mature DCs (21). Interaction of HIV with the DCs has been shown to occur via multiple chemokine receptors (22, 53). It is now generally thought that DCs or DC precursors are an important target cell type during early HIV infection, being perhaps the first cell type to be infected within the exposed mucosa and the carrier of the virus to neighboring lymphoid tissue (17, 50, 56). In addition, DCs might play an important role as immune partners of the CD4+ T cells, by allowing intercellular transfer of HIV and favoring productive infection through activation of the CD4+ T cells (8, 65, 66). These observations have many similarities with our observations on the putative role of DCs in MMTV infection.
Finally, the cooperative involvement of B cells and DCs during MMTV infection is interesting for immunology in general, since the immune response induced by the MMTV Sag recapitulates in many aspects the response to a conventional antigen (40, 41). Our results indicate that after priming by DCs in the context of I-E, the MMTV(SIM) Sag can be recognized under limiting conditions on the surface of B cells in the context of I-A. During this process, the avidity of the Sag-reactive T cells for the Sag-MHC complex has to increase strongly. The most likely physiological relevance of our observation is to allow B cells with a low affinity for antigen to receive T-cell help in a primary immune response.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation grants 31-32271.94 to H.A.-O. and 31-46667.96 to H.D. H.A.-O. was supported by Human Frontiers grant RG-544/95 as well as the Fondation Gabriella Giorgi-Cavaglieri. Thomas Brocker is supported by the Deutsche Forschungsgemeinschaft Leibniz-Program.
REFERENCES
- 1.Andersson M, Acha-Orbea H. The primary in vivo immune response to Mls-1 (Mtv-7 sag). Route of injection determines the immune response pattern. Immunology. 1994;83:438–443. [PMC free article] [PubMed] [Google Scholar]
- 2.Ardavin C, Luthi F, Andersson M, Scarpellino L, Martin P, Diggelmann H, Acha-Orbea H. Retrovirus-induced target cell activation in the early phases of infection: the mouse mammary tumor virus model. J Virol. 1997;71:7295–7299. doi: 10.1128/jvi.71.10.7295-7299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austyn J M. Antigen uptake and presentation by dendritic leukocytes. Semin Immunol. 1992;4:227–236. [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Beutner U, Kraus E, Kitamura D, Rajewsky K, Huber B T. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med. 1994;179:1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 9.Cassell D J, Schwartz R H. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med. 1994;180:1829–1840. doi: 10.1084/jem.180.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chervonsky A V, Xu J, Barlow A K, Khery M, Flavell R A, Janeway C A., Jr Direct physical interaction involving CD40 ligand on T cells and CD40 on B cells is required to propagate MMTV. Immunity. 1995;3:139–146. doi: 10.1016/1074-7613(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 11.Chesnut R W, Grey H M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981;126:1075–1079. [PubMed] [Google Scholar]
- 12.Delcourt M, Thibodeau J, Denis F, Sekaly R P. Paracrine transfer of mouse mammary tumor virus superantigen. J Exp Med. 1997;185:471–480. doi: 10.1084/jem.185.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Brière F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–952. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eynon E E, Parker D C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelman F D, Lees A, Morris S C. Antigen presentation by B lymphocytes to CD4+ T lymphocytes in vivo: importance for B lymphocyte and T lymphocyte activation. Semin Immunol. 1992;4:247–255. [PubMed] [Google Scholar]
- 16.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 17.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E J, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 19.Gallis B M, Eisenman R N, Diggelmann H. Synthesis of the precursor to avian RNA tumor virus internal structural proteins early after infection. Virology. 1976;74:302–313. doi: 10.1016/0042-6822(76)90337-8. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert K M, Weigle W O. Tolerogenicity of resting and activated B cells. J Exp Med. 1994;179:249–258. doi: 10.1084/jem.179.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 24.Held W, Waanders G A, Acha-Orbea H, MacDonald H R. Reverse transcriptase-dependent and -independent phases of infection with mouse mammary tumor virus: implications for superantigen function. J Exp Med. 1994;180:2347–2351. doi: 10.1084/jem.180.6.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Held W, Waanders G A, Shakhov A N, Scarpellino L, Acha-Orbea H, MacDonald H R. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993;74:529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- 26.Held W, Shakhov A N, Izui S, Waanders G A, Scarpellino L, MacDonald H R, Acha-Orbea H. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med. 1993;177:359–366. doi: 10.1084/jem.177.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Held W, Shakhov A N, Waanders G, Scarpellino L, Luethy R, Kraehenbuhl J P, MacDonald H R, Acha-Orbea H. An exogenous mouse mammary tumor virus with properties of Mls-1a (Mtv-7) J Exp Med. 1992;175:1623–1633. doi: 10.1084/jem.175.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsia K J, Hara H, Izutani R, Park H T, Fujihara M, Kaji A. Infection of terminally differentiated myotubes with Rous sarcoma virus: reduced synthesis of env and v-src proteins. J Gen Virol. 1992;73:1791–1798. doi: 10.1099/0022-1317-73-7-1791. [DOI] [PubMed] [Google Scholar]
- 29.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 30.Inaba K, Metlay J P, Crowley M T, Steinman R M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inaba K, Schuler G, Witmer M D, Valinsky J, Atassi B, Steinman R M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986;164:605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingulli E, Mondino A, Khoruts A, Jenkins M K. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 34.Krummenacher C, Diggelmann H, Acha-Orbea H. In vivo effects of a recombinant vaccinia virus expressing a mouse mammary tumor virus superantigen. J Virol. 1996;70:3026–3031. doi: 10.1128/jvi.70.5.3026-3031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langhoff E, Terwilliger E F, Bos H J, Kalland K H, Poznansky M C, Bacon O M, Haseltine W A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci USA. 1991;88:7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanzavecchia A. From antigen presentation to T-cell activation. Res Immunol. 1998;149:626. doi: 10.1016/s0923-2494(99)80027-3. [DOI] [PubMed] [Google Scholar]
- 37.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 38.Le Meur M, Gerlinger P, Benoist C, Mathis D. Correcting an immune-response deficiency by creating E alpha gene transgenic mice. Nature. 1985;316:38–42. doi: 10.1038/316038a0. [DOI] [PubMed] [Google Scholar]
- 39.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 40.Luther S A, Acha-Orbea H. Mouse mammary tumor virus: immunological interplays between virus and host. Adv Immunol. 1997;65:139–243. [PubMed] [Google Scholar]
- 41.Luther S A, Gulbranson-Judge A, Acha-Orbea H, MacLennan I C M. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J Exp Med. 1997;185:551–562. doi: 10.1084/jem.185.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maillard I, Erny K, Acha-Orbea H, Diggelmann H. A V beta 4-specific superantigen encoded by a new exogenous mouse mammary tumor virus. Eur J Immunol. 1996;26:1000–1006. doi: 10.1002/eji.1830260507. [DOI] [PubMed] [Google Scholar]
- 43.Marrack P, Kushnir E, Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991;349:524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- 44.Matzinger P. Immunology. Memories are made of this? Nature. 1994;369:605–606. doi: 10.1038/369605a0. [DOI] [PubMed] [Google Scholar]
- 45.Mix D, Winslow G M. Proteolytic processing activates a viral superantigen. J Exp Med. 1996;184:1549–1554. doi: 10.1084/jem.184.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papiernik M, Pontoux C, Gisselbrecht S. Acquired Mls-1a-like clonal deletion in Mls-1b mice. J Exp Med. 1992;175:453–460. doi: 10.1084/jem.175.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park C G, Jung M Y, Choi Y, Winslow G M. Proteolytic processing is required for viral superantigen activity. J Exp Med. 1995;181:1899–1904. doi: 10.1084/jem.181.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker D C. The functions of antigen recognition in T cell-dependent B cell activation. Semin Immunol. 1993;5:413–420. doi: 10.1006/smim.1993.1047. [DOI] [PubMed] [Google Scholar]
- 49.Patterson S, Knight S C. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987;68:1177–1181. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- 50.Reece J C, Handley A J, Anstee E J, Morrison W A, Crowe S M, Cameron P U. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reis e Sousa C, Stahl P D, Austyn J M. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone A M, Fathman C G, Inaba K, Steinman R M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubbert A, Combadiere C, Ostrowski M, Arthos J, Dybul M, Machado E, Cohn M A, Hoxie J A, Murphy P M, Fauci A S, Weissman D. Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. J Immunol. 1998;160:3933–3941. [PubMed] [Google Scholar]
- 54.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuler G, Steinman R M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soto-Ramirez L E, Renjifo B, McLane M F, Marlink R, O’Hara C, Sutthent R, Wasi C, Vithayasai P, Vithayasai V, Apichartpiyakul C, Auewarakul P, Pena Cruz V, Chui D S, Osathanondh R, Mayer K, Lee T H, Essex M. HIV-1 Langerhans’ cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–1303. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 57.Speiser D E, Schneider R, Hengartner H, MacDonald H R, Zinkernagel R M. Clonal deletion of self-reactive T cells in irradiation bone marrow chimeras and neonatally tolerant mice. Evidence for intercellular transfer of Mlsa. J Exp Med. 1989;170:595–600. doi: 10.1084/jem.170.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Speiser D E, Lees R K, Hengartner H, Zinkernagel R M, MacDonald H R. Positive and negative selection of T cell receptor V beta domains controlled by distinct cell populations in the thymus. J Exp Med. 1989;170:2165–2170. doi: 10.1084/jem.170.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka A, Hara H, Park H T, Wolfert J, Fujihara M, Izutani R, Kaji A. Infection of terminally differentiated myotubes with Rous sarcoma virus (RSV): lack of DNA integration but presence of RSV mRNA. J Gen Virol. 1992;73:1781–1790. doi: 10.1099/0022-1317-73-7-1781. [DOI] [PubMed] [Google Scholar]
- 60.Tomonari K, Lovering E, Spencer S. Correlation between the V beta 4+ CD8+ T-cell population and the H-2d haplotype. Immunogenetics. 1990;31:333–339. doi: 10.1007/BF02115007. [DOI] [PubMed] [Google Scholar]
- 61.Tough D F, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vacheron S. Ph.D. thesis. Lausanne, Switzerland: University of Lausanne; 1999. [Google Scholar]
- 63.van Meerwijk J P M, Marguerat S, Lees R K, Germain R N, Fowlkes B J, MacDonald H R. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–384. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 65.Weissman D, Daucher J, Barker T, Adelsberger J, Baseler M, Fauci A S. Cytokine regulation of HIV replication induced by dendritic cell-CD4-positive T cell interactions. AIDS Res Hum Retroviruses. 1996;12:759–767. doi: 10.1089/aid.1996.12.759. [DOI] [PubMed] [Google Scholar]
- 66.Weissman D, Li Y, Orenstein J M, Fauci A S. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. J Immunol. 1995;155:4111–4117. [PubMed] [Google Scholar]