Abstract
Recently, particular cytokines have been identified to affect progression of a variety of diseases and retrovirus infections. Previously, we demonstrated that interleukin-2 (IL-2), IL-12, and gamma interferon increased in peripheral blood mononuclear cells (PBMCs) from animals with early disease and decreased in PBMCs from animals with late disease stages of bovine leukemia virus (BLV) infection. In contrast, IL-10 increased with disease progression. To examine the effects of these cytokines on BLV expression, BLV tax and pol mRNA and p24 protein were quantified by competitive PCR and immunoblotting, respectively. IL-10 inhibited BLV tax and pol mRNA levels in BLV-infected PBMCs; however, the inhibitory effect of IL-10 was prevented in PBMCs depleted of monocytes and/or macrophages (monocyte/macrophages). To determine whether these factors were secreted or monocyte/macrophage associated, monocyte/macrophage-depleted PBMCs were cultured with isolated monocyte/macrophages in transwells where contact between monocyte/macrophages and nonadherent PBMCs was blocked. BLV tax and pol mRNA levels increased in transwell cultures similar to cultures containing nonseparated cells, and IL-10 addition inhibited the increase of BLV tax and pol mRNA. These results suggest that monocyte/macrophages secrete soluble factor(s) that increases BLV mRNA levels and that secretion of these soluble factor(s) could be inhibited by IL-10. In contrast, IL-2 increased BLV tax and pol mRNA and p24 protein production. Thus, IL-10 production by BLV-infected animals with late stage disease may serve to control BLV mRNA levels, while IL-2 may increase BLV mRNA in the early disease stage. To determine a correlation between cell proliferation and BLV expression, the effect of IL-2 and IL-10 on PBMC proliferation was tested. As anticipated, IL-2 stimulated while IL-10 suppressed antigen-specific PBMC proliferation. The present study, combined with our previous findings, suggests that increased IL-10 production in late disease stages suppresses BLV mRNA levels, while IL-2-activated immune responses stimulate BLV expression by BLV-infected B cells.
Bovine leukemia virus (BLV), which is closely related to human T-cell leukemia virus type 1 (HTLV-1), is a type C retrovirus that infects bovine B cells and leads to development of enzootic bovine leukosis (13). Less than 5% of infected animals develop malignant lymphosarcoma (8), while 30% of infected animals progress to persistent lymphocytosis. In persistent lymphocytosis, nonneoplastic B cells proliferate, and leukocyte counts may exceed 10,000 cells/mm3 (16). However, most infected animals remain in the alymphocytotic (AL) stage. Despite the often long duration for disease progression, the mechanism for progression is unknown. Previously, we determined that cytokine profiles of BLV-infected animals differ depending on the stage of disease (24, 27). Interleukin-2 (IL-2), IL-12, and gamma interferon (IFN-γ) were expressed in high amounts in AL animals. In contrast, interleukin 10 (IL-10) was increased in persistently lymphocytotic (PL) animals. While IL-2, IL-12, and IFN-γ trigger cellular immune responses that activate macrophages, NK, Th1, and cytotoxic T cells to remove virus from the host, IL-10 suppresses these cytokine-activated immune responses (17). Increased IL-10 production in BLV infection could be deleterious for clearing viral infection from the host. Lundberg et al. reported that cytotoxic γδ T-cell (CTL) activity is crippled in PL animals, while γδ CTLs from AL animals efficiently lysed BLV Env and Tax presenting cells (18). Cytokine imbalance may also contribute to disease progression in human immunodeficiency virus (HIV) infection (6), autoimmune disease, and cancer (17). Alternatively, there may be beneficial effects of IL-10 for virus clearance. In HIV infection, IL-10 suppresses immune activation (20, 30), and reduced immune surveillance may permit a suitable environment for virus replication. Interestingly, whereas HIV replication was significantly reduced in experiments with macrophage cell lines and primary macrophages, this inhibitory effect was not observed in experiments with T-cell lines and primary T cells alone (29, 30, 36). These reports suggest that monocytes and/or macrophages (monocyte/macrophages) have an important role in regulating virus replication in T cells, as well as monocyte/macrophages responding to IL-10. To examine the influence of cytokines on BLV mRNA levels, BLV tax and pol mRNA were quantified from peripheral blood mononuclear cells (PBMCs) cultured with IL-2, IL-10, and IL-12. Here, we demonstrate that IL-10 inhibits detection of BLV tax and pol mRNA, while IL-2 activates BLV tax and pol mRNA and p24 protein levels. The inhibitory effect of IL-10 on BLV tax and pol mRNA was eliminated in monocyte/macrophage-depleted PBMCs.
MATERIALS AND METHODS
Animals and cell preparation.
Adult female Holstein cattle, 2 to 12 years of age, were assigned to two groups according to their disease stage. Three AL and three PL animals were used. Each experiment was performed with three different animals and at least one from each disease stage except the immunoblotting assay with two PL animals. Heparinized or EDTA-treated blood was obtained from the jugular vein, and PBMCs were isolated by density gradient centrifugation (5).
Cell cultures and monocyte/macrophage separation.
Isolated PBMCs were cultured at 5 × 106 to 10 × 106 cells/ml for BLV quantification and 5 × 105 cells/ml for cell proliferation. The cells were treated with human recombinant IL-2 (hrIL-2; 100 U/ml; PharMingen, San Diego, Calif.), hrIL-10 (10 ng/ml; R&D systems, Minneapolis, Minn.), hrIL-12 (5 ng/ml; R&D systems), anti-hrIL10 neutralizing antibody (10 μg/ml; R&D systems), concanavalin A (ConA; 10 μg/ml; Sigma, St. Louis, Mo.), and BLV (10 μg/ml). BLV was purified from the culture supernatant of the BL3 cell line (ATCC CRL-8037, Rockville, Md.) by using metrizamide (Sigma) gradient centrifugation and confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. After incubation of the PBMCs for 3 to 5 days, the cells were harvested for further experiments. In cell proliferation assays, [3H]thymidine was added 8 to 12 h before cell harvest, and the radioactivity of the harvested cells was measured with a β-scintillation counter (MATRIX 9600; Packard, Meriden, Conn.).
Monocyte/macrophages were isolated from PBMCs by exploiting their ability to adhere to plastic (34). After PBMCs were incubated for 4 to 6 h, nonadherent cells were removed and transferred to other wells. Isolated monocyte/macrophages were cultured with nonadherent cells separated by a transwell filter (0.4 μm pore size; Costar, Acton, Mass.). The purity of separated monocyte/macrophages was confirmed by flow cytometry with mouse anti-bovine CD14 immunoglobulin G (IgG; CAM36A; VMRD, Pullman, Wash.) and fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, Pa.) by using an EPICS profile analyzer. Adherent cells were also stained for esterase as previously performed (24, 27).
Primers and reverse transcriptase PCR (RT-PCR).
Based on GenBank sequence information, primers for tax and pol were designed with the Oligo 5.0 software (National Bioscience, Plymouth, Minn.). To produce plasmids containing standard tax and pol fragments, PCR products from BLV-infected PBMCs were cloned into the TA cloning vector pCR 2.1 (Invitrogen, San Diego, Calif.) as recommended by the manufacturer. Plasmids were purified from transformed Escherichia coli by using a Wizard miniprep system (Promega, Madison, Wis.) and then screened for the insert by EcoRI digestion. The amplified PCR products were assessed by agarose gel electrophoresis. Plasmid concentration was determined by spectrophotometry at 260 nm. Inserts were confirmed by automated DNA sequencing at the University of Wisconsin Biotech Center.
Cytoplasmic lysates from freshly isolated PBMCs were obtained by adding 200 μl of chloroform, and total RNA was prepared by using TRI reagent according to the manufacturer’s protocol (MRC, Cincinnati, Ohio) with DNase treatment. The concentration of purified total RNA was determined by spectrophotometry. Then, the RT reaction was performed with purified total RNA. The reaction mixture, including 400 U of Moloney murine leukemia virus (MMLV) RT, 10 mg of bovine serum albumin BSA, 40 units of RNasin, 1 μg of oligo(dT), 0.5 mM deoxynucleoside triphosphates (dNTP), and a 5× reaction buffer (250 mM Tris-HCl, pH 8.3; 375 mM KCl; 15 mM MgCl2; 50 mM dithiothreitol) was incubated with 1 to 10 μg of total RNA for 2 h at 37°C. PCR was performed in a DNA thermocycler (Perkin-Elmer, Norwalk, Conn.) for 35 cycles consisting of 1 min at 94°C for denaturation, 1 min at 62 (for pol amplification) or 65°C (for tax amplification) for annealing, and 1 min at 72°C for polymerization. Each PCR reaction contained 1.25 U of Taq polymerase, 1.5 mM MgCl2, 0.8 mM dNTP, 1 μM primers, template, and 10× thermobuffer (500 mM KCl; 100 mM Tris-HCl, pH 9.0; 1% Triton X-100). tax (5′-CAGCATTTGGGCCGCCTTTTCTAAC, 3′-ACAGCCGGAGGGGGTCCACAAGGAG, 691-bp product) and pol (5′-GCCGCCCCGCCTGAACCTGT, 3′-CCCACGCTTCGCCGAGGCATGAGTAG, 530-bp product) were used as amplification primers (Table 1). Amplified products were analyzed by 1% agarose gel electrophoresis. Samples from RT reactions without MMLV-RT and mixtures without cDNA template were used in PCR assays as controls for amplification of contaminated DNA fragments.
TABLE 1.
Primer sequences
| Primer | Sequence (5′→3′) | Standard size (bp) | Mimic size (bp) | Reference |
|---|---|---|---|---|
| BLV tax | 5′-CAGCATTTGGGCCGCCTTTTCTAAC | 691 | 450 | 25 |
| 3′-ACAGCCGGAGGGGGTCCACAAGGAG | ||||
| BLV pol | 5′-GCCGCCCCGCCTGAACCTGT | 530 | 700 | 26 |
| 3′-CCCACGCTTCGCCGAGGCATGAGTAG |
Generation of mimics and quantitative-competitive PCR (QC-PCR).
Mimics of tax and pol were generated by using PCR primers and an internal nonspecific stuffer region (24). The nonspecific fragment was produced by PCR by using a DNA thermocycler for five cycles consisting of 1 min at 94°C for denaturation, 1 min at 42°C for annealing, and 1 min at 72°C for polymerization, followed by 35 cycles consisting of 1 min at 94°C for denaturation, 1 min at 62°C or 65°C for annealing, and 1 min at 72°C for polymerization. The mimic band was excised, and the fragment was purified by using a GenElute agarose spin column (Supelco, Bellefonte, Pa.). The purified mimic fragment was amplified again by PCR with tax and pol primers, and the PCR reaction mixture was removed by using a QIAquick PCR purification kit (Qiagen, Chatsworth, Calif.). The mimic fragment was then cloned by using the TA cloning kit as described above. The concentration of tax and pol mimic-ligated plasmids were determined by spectrophotometry.
To quantify the tax and pol mRNA produced by PBMCs from BLV-infected animals, QC-PCR was performed (32). For standardization, PCR was performed by using this serially twofold-diluted standard plasmid with concentrations ranging from 8,192 to 16 fg/μl for tax and from 2,048 to 4 fg/μl for pol and a fixed amount of mimic (10 fg/μl). Synthesized cDNA from each sample and fixed amounts of mimic were added into the same tube and amplified simultaneously with tubes for standard reaction. Gel photographs were scanned, and the amplified DNA bands were analyzed by densitometry by using NIH Image program version 1.61 with standard curves constructed with Cricket Graph. Amplified products were analyzed by the methods previously described. The amount of cytokine produced was determined by comparing the density ratios of sample reactions and standard reaction.
Immunoblotting.
To detect BLV p24, PBMCs from PL animals were cultured with IL-2 and ConA in 135-cm3 flasks (Costar). The harvested cell pellet was resuspended in Triton X-100 lysis buffer (300 mM NaCl, 50 mM Tris-Cl, 0.5% Triton X-100, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml) at 108 cells/ml, kept for 30 to 45 min on ice, lightly vortexed, and centrifuged for 15 min at 12,000 × g. The supernatant was removed from the nuclear pellet. SDS-PAGE was performed with the supernatant, and the separated protein bands were transferred to nitrocellulose filter paper (Bio-Rad Laboratories, Hercules, Calif.). Immunoblotting was performed with mouse anti-BLV p24 IgG (BLV3; VMRD), anti-BLV gp51 (BLV2; VMRD), and alkaline phosphatase-conjugated anti-mouse IgG (Bio-Rad). The bands were scanned and then analyzed by densitometry as described above. The relative densities of bands from IL-2- and ConA-stimulated PBMCs were calculated and compared with those from PBMCs cultured with medium only.
RESULTS
IL-10 inhibits BLV tax and pol mRNA expression.
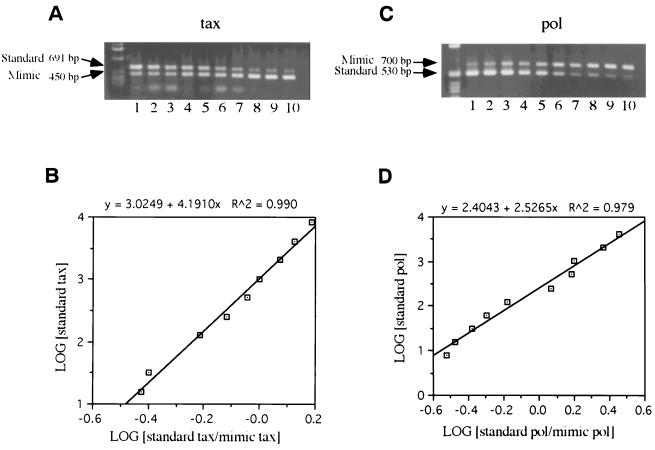
To verify differences in the level of BLV expression by PBMC cultures, tax and pol mRNA were quantified by using mimics. Tax (450 bp) and pol (700 bp) mimics were generated by nonspecific PCR amplification. According to band ratios in standard reactions, standardization graphs were drawn (Fig. 1).
FIG. 1.
Standardization curve for QC-PCR of tax (A and B) and pol (C and D) mRNA assays. Plasmids containing mimic for tax (450 bp) or pol (700 bp) were generated by using primers and an internal nonspecific stuffer region, followed by cloning. Standard fragment-ligated plasmids for tax (691 bp) and pol (539 bp) were also generated by using the primers and BLV-infected cells. Serial twofold dilution of standard plasmid and a fixed amount of mimic were amplified simultaneously in the same tube. Standard DNA (8,192 fg to 16 fg for tax [panel A, lanes 1 to 10] or 2,048 fg to 4 fg for pol [panel c, lanes 1 to 10]) was amplified with 10 fg of mimic DNA for both. The standard reactions for tax and pol are shown in panels A and C, respectively. Amplified DNA bands were analyzed by densitometry by using NIH Image program version 1.61 to generate standard curves (panels B and D). Each sample reaction was performed simultaneously with a standard reaction, and the amount of tax and pol level by PBMCs was calculated from the representative standard curve.
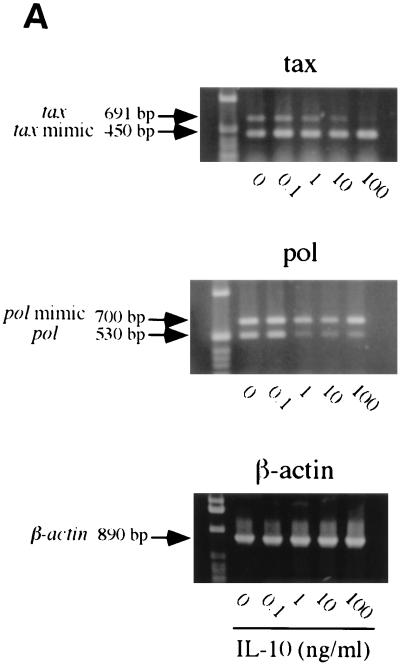
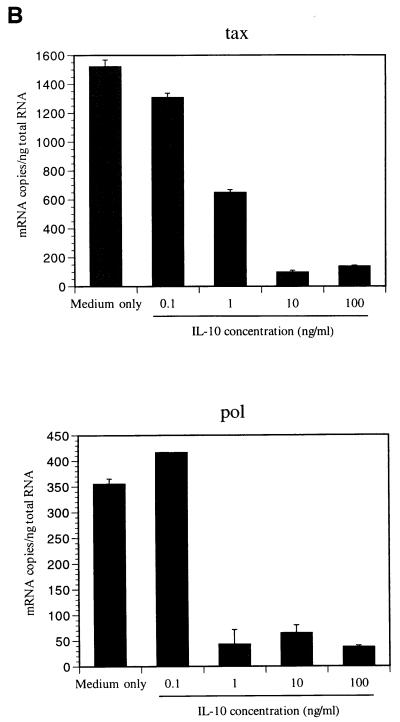
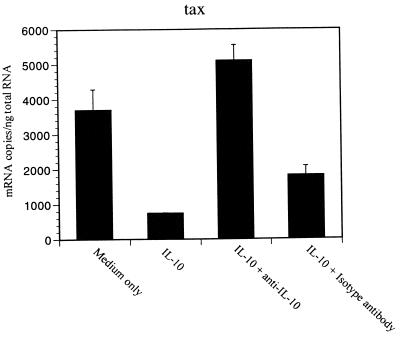
Interestingly, BLV tax and pol mRNA levels decreased in the PBMCs cultured with hrIL-10 (Fig. 2), whereas β-actin mRNA level was independent of IL-10 addition. This experiment was conducted for 1, 3, 5, 7, and 9 days, and tax mRNA level peaked in PBMCs cultured for 3 days, while pol mRNA peaked in PBMCs cultured for 5 to 7 days (data not shown). To confirm the inhibitory effect of IL-10 on BLV mRNA, anti-hrIL10 neutralizing antibody was added to the PBMCs cultured with IL-10. While hrIL-10 alone inhibited BLV tax mRNA, the addition of anti-hrIL-10 neutralizing antibody reversed BLV tax mRNA levels inhibited by IL-10 (Fig. 3). These results suggest that IL-10 decreases BLV tax and pol mRNA levels in BLV-infected PBMCs.
FIG. 2.
IL-10 inhibits BLV tax and pol mRNA levels. PBMCs from the AL animal S201 were cultured with different concentrations of human recombinant IL-10 (0.1 to 100 ng/ml) for 5 days. (A) QC-PCR analysis. The bands are as follows: 890 bp, β-actin; 691 bp, tax standard; 450 bp, tax mimic; 700 bp, pol mimic; and 530 bp, pol standard. All products were separated on a 1% agarose gel stained by ethidium bromide. (B) The bands were analyzed by densitometry in NIH image version 1.61, and the amount of BLV tax and pol mRNA is shown. The data are representative of experiments from three different animals. Standard error bars are shown from at least three experiments.
FIG. 3.
Inhibition of BLV tax mRNA level is blocked by anti-hrIL-10 neutralizing antibody. PBMCs from the AL animal S17 were cultured with hrIL-10 with or without 10 μg of anti-IL-10 neutralizing antibody per ml for 5 days. Isotype antibody was used as a negative control. The amounts of BLV tax mRNA level are shown with standard error bars from at least three different assays. The data are representative of experiments from three different animals.
Monocyte/macrophage-depleted PBMCs expressed reduced BLV tax and pol mRNA levels.
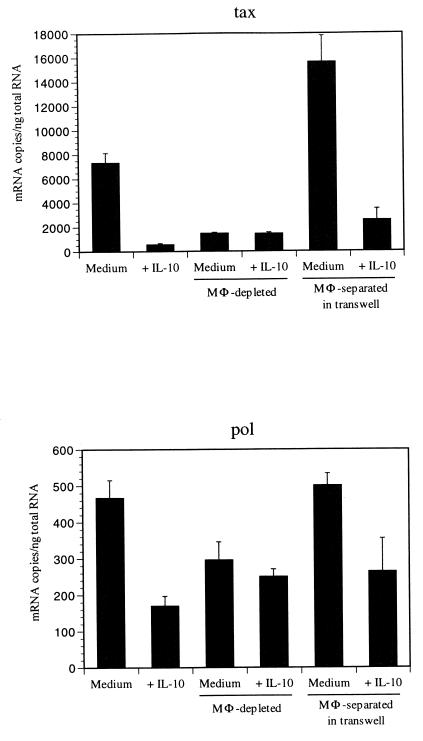
Previous reports indicate that monocyte/macrophages have a critical role in regulating retrovirus expression (29, 36). To examine whether monocyte/macrophages regulate BLV mRNA levels, monocyte/macrophages were depleted by adherence to plastic. PBMCs, 2 × 107 to 3 × 107, were used for separation, and 2 to 5 × 106 cells were isolated by adherence. Flow cytometry confirmed that more than 85% of the isolated cells were CD14+ and were more than 95% esterase positive, a result similar to our previous findings (24, 27). Monocyte/macrophage-depleted cells contained fewer than 5% CD14+ cells (data not shown). Monocyte/macrophage-depleted PBMCs had dramatically reduced BLV tax and pol mRNA compared to nonseparated PBMCs (Fig. 4). When hrIL-10 was added to monocyte/macrophage-depleted PBMCs, the inhibitory effect of IL-10 on BLV tax and pol mRNA level was not observed. To determine whether monocyte/macrophages regulate BLV levels directly or via soluble product(s), isolated monocyte/macrophages were cultured with nonadherent cells separated by a 0.4-μm (pore-size) filter in transwell plates. After culture for 3 to 5 days with or without hrIL-10, nonadherent cells were harvested, and BLV tax and pol mRNA levels were assessed. The nonadherent PBMCs, when cultured with monocyte/macrophages in transwells expressed approximately 10 times more BLV tax and twice as much pol mRNA as nonadherent PBMCs cultured alone (Fig. 4). In addition, hrIL-10 inhibited BLV tax and pol mRNA levels in nonadherent PBMCs cultured with monocyte/macrophages (Fig. 4). These results suggest that factor(s) secreted by monocyte/macrophages affect BLV mRNA levels and that IL-10 can regulate these factor(s).
FIG. 4.
The levels of BLV tax (A) and pol (B) are reduced in monocyte/macrophage-depleted PBMCs and are not affected by IL-10. Monocyte/macrophages were depleted by using plastic adherence. Nonseparated PBMCs and monocyte/macrophage-depleted PBMCs (MΦ-depleted) were cultured with or without hrIL-10. Isolated monocyte/macrophages were also cultured with monocyte/macrophage-depleted PBMCs in transwell plates (Transwell), where contact was blocked by a 0.4-μm (pore-size) membrane. After 5 days, the cells were harvested and QC-PCR was performed. Standard error bars indicate variation of at least three different assays, and the data are representative of experiments from three different animals. In panel B, the medium and medium-macrophage-depleted columns have a P ≤0.05 by using the Student’s t test.
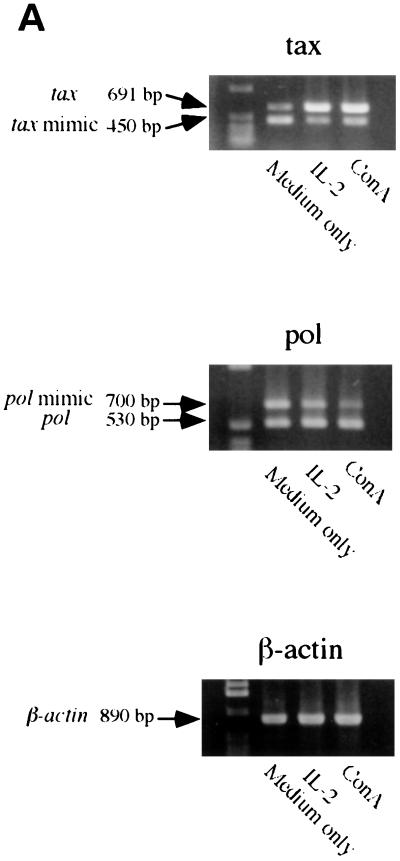
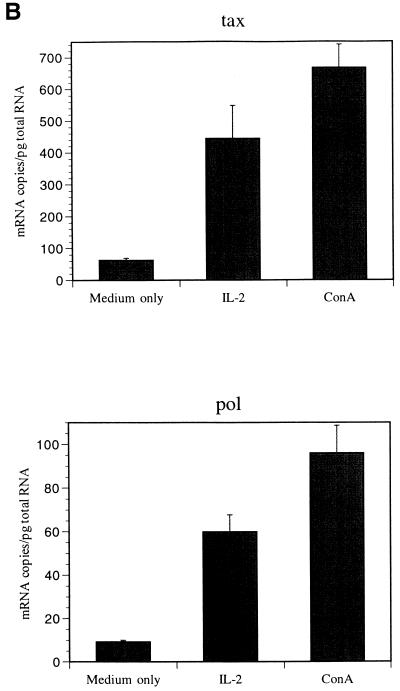
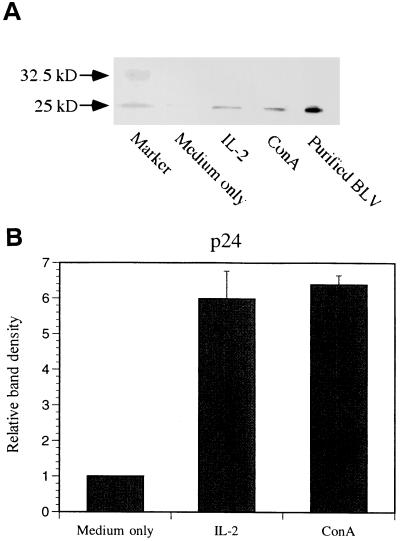
IL-2 increased BLV tax and pol mRNA levels and the level of BLV p24 protein.
To determine the effect of IL-2 and IL-12 on BLV detection, hrIL-2 and hrIL-12 were added to PBMC cultures for 3 to 5 days, and the BLV tax and pol mRNA levels were quantified by QC-PCR. BLV tax and pol mRNA levels were increased more than five times in PBMCs cultured with hrIL-2 and ConA compared to PBMCs cultured with medium only, while the β-actin level was unchanged (Fig. 5). In contrast, although the addition of hrIL-12 enhanced antigen-specific PBMC proliferation, hrIL-12 did not affect BLV tax and pol mRNA levels. To examine the effect of IL-2 on BLV translation, 1.5 × 108 PBMCs were cultured with hrIL-2 and ConA. After 3 days, cellular proteins were isolated from cultured PBMCs, and semiquantitative immunoblotting was performed. Increased BLV p24 protein production was detected by semiquantitative immunoblotting with anti-p24 antibody (Fig. 6). BLV p24 protein amount paralleled BLV tax and pol mRNA levels when PBMCs were cultured in the presence of IL-2. Purified BLV by metrizamide gradient centrifugation was used for a positive control. As predicted, p24 protein was not detected in PBMCs cultured with hrIL-10, and gp51 production was not detected in PBMCs cultured with hrIL-2 and hrIL-10 (data not shown).
FIG. 5.
IL-2 and ConA stimulate BLV tax and pol mRNA levels. PBMCs from the PL animal P191 were cultured with recombinant human IL-2 (100 U/ml) or ConA (10 μg/ml) for 5 days. QC-PCR was performed (A), and the amounts of BLV tax and pol mRNA are shown (B), with standard error bars from at least three assays, and the data are representative of experiments with three different animals. The bands are as follows: 691 bp, tax standard; 450 bp, tax mimic; 700 bp pol mimic; 530 bp pol standard; and 890 bp, β-actin. All products were analyzed on a 1% agarose gel stained with ethidium bromide.
FIG. 6.
IL-2 and ConA stimulate BLV p24 protein production. PBMCs from PL animal P49 were cultured with hrIL-2 (100 U/ml) or ConA (10 μg/ml) for 3 days. After protein purification, an equal amount of protein from each sample was analyzed by SDS–10% PAGE. (A) Immunoblotting was performed with mouse anti-BLV p24 antibody and alkaline phosphatase-conjugated goat anti-mouse IgG antibody. (B) Each band was analyzed by densitometry by using the NIH Image program version 1.61 to measure relative band density. Purified BLV was used as a positive control. The data are representative data of experiments with two different PL animals. Standard error bars are shown from at least four separate experiments.
IL-10 reduces antigen-specific PBMC proliferation, while IL-2 enhances antigen-specific PBMC proliferation.
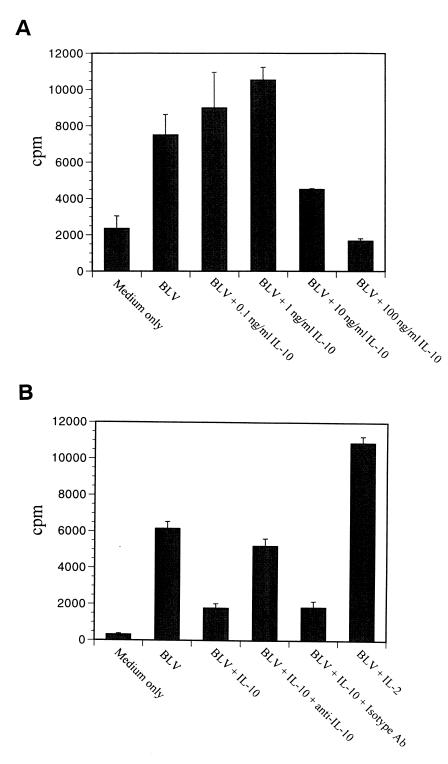
To examine whether BLV expression correlates with PBMC proliferation, increasing concentrations of hrIL-10 were added to PBMC cultures with 25% of BL3 supernatant as an antigen source (23). Although low concentrations of hrIL-10 slightly activated PBMC proliferation, 10 times more than the 50% effective dose (1 ng/ml) of hrIL-10 dramatically reduced antigen-specific PBMC proliferation (Fig. 7A). Anti-hrIL-10 neutralizing antibody restored antigen-specific PBMC proliferation, confirming that PBMC proliferation is specifically inhibited by IL-10 (Fig. 7B). As expected, hrIL-2 enhanced antigen-specific PBMC proliferation (Fig. 7B). These results indicated a correlation between BLV expression and antigen-specific PBMC proliferation and suggest that cell proliferation may provide a suitable environment for BLV expression by BLV-infected B cells.
FIG. 7.
IL-10 inhibits PBMC proliferation to BLV. PBMCs from AL animal S201 were cultured with BLV containing supernatant from the BL3* cell line or with medium alone for 5 days. Different concentrations of hrIL-10 (0.1 to 100 ng/ml) (A) or of hrIL-10 (10 ng/ml), hrIL-2 (100 U/ml), anti-IL-10 antibody (10 μg/ml), and/or isotype antibody (10 μg/ml) were added (B). Proliferation was assessed by measuring [3H]thymidine incorporation with a β-scintillation counter. The data are representative of experiments with three different animals. Standard error bars are from three assays.
DISCUSSION
The results presented here demonstrate that IL-10 inhibits BLV tax and pol mRNA levels, while IL-2 stimulates BLV tax and pol mRNA and the level of p24 protein. Interestingly, IL-10 inhibition of BLV mRNA is removed when adherent monocyte/macrophages were depleted from PBMCs. Also, the background level of tax and pol mRNA was dramatically decreased in monocyte/macrophage-depeleted PBMCs without IL-10 addition. These findings suggest that IL-2 and IL-10 regulate the amount of BLV and that monocyte/macrophages have an important role in regulating BLV expression.
Previously, we showed that IL-2, IL-12, and IFN-γ were increased in AL animals, while decreased in PL and tumor-bearing animals (24, 27). In contrast, IL-10 was increased in PL and tumor-bearing animals. These results suggest that disease progression in BLV infection is associated with specific cytokine expression. Retroviruses have a common immunosuppressive domain in their transmembrane protein. A retroviral envelope peptide, termed CKS-17, caused a shift in the cytokine balance, suppressing cell-mediated immunity by upregulating IL-10 and downregulating IL-2, IL-12, and tumor necrosis factor alpha production (10). Thus, previously we quantified IL-10 mRNA expression of PBMCs infected by tax- and env-recombinant vaccinia virus and also treated PBMCs with the comparable oligopeptide of BLV CKS-17; however, a difference in IL-10 expression was not detected (25). IL-10 can be produced by a variety of cells, including macrophages. IL-10 production also could be a result of antigen-specific T-cell apoptosis (33). T helper (Th) 1 cells express Fas and Fas ligand upon activation (33), and these cells are selectively removed by programmed cell death, while Th2 cells are not affected.
High levels of IL-10 could have deleterious or beneficial effects on the host immune defense against virus infections. Numerous studies have reported that type 2 cytokines, as well as IL-10, inhibit cell-mediated immunity and impair clearance of viral infections by cytotoxic T cells and NK cells (17, 31). In BLV infection, PL animals have impaired γδ T-cell-mediated immunity to BLV infection. Although γδ CTLs from AL animals efficiently lysed cells presenting BLV Env and Tax, CTLs isolated from PL animals could not respond to BLV Env and Tax (18). In addition, lymphocytes from PL and tumor-bearing animals failed to proliferate when cultured with BLV recombinant proteins, while lymphocytes from AL animals proliferated to BLV recombinant Gag and Env proteins (23). In addition to type 2 cytokines, IL-10 has a role in B-cell activation and proliferation. Excessive activation and proliferation may cause B-cell lymphocytosis and transformation by a chromosomal translocation of the oncogene c-myc in the immunoglobulin genes (9, 11, 12).
Alternatively, IL-10 may have a beneficial role in viral clearance. The inhibitory effect of IL-10 on virus expression observed in the present study has also been reported in HIV infection (20, 29, 30). Interestingly, IL-10 can inhibit HIV replication in the monocyte/macrophage lineage cells and in PBMCs but not in T cells (29). These results implicate that the IL-10–monocyte/macrophage interplay may have an important role in regulating HIV expression. Thus, we depleted monocyte/macrophages from PBMCs to examine whether monocyte/macrophages affect regulation of BLV expression. The inhibitory effect of IL-10 on BLV tax and pol mRNA levels was removed, and the background levels of tax and pol mRNA was dramatically decreased without addition of IL-10. When monocyte/macrophage-depleted PBMCs were cultured with isolated monocyte/macrophages in transwells, monocyte/macrophage-depleted PBMCs expressed high amounts of BLV tax and pol mRNA. The addition of IL-10 dramatically reduced this level. These results suggest that monocyte/macrophages secrete soluble factor(s) that activate BLV expression and whose action is inhibited by IL-10. Recently, we found that IL-10 inhibited and IL-2 enhanced detection of COX-2 mRNA in PBMCs (26). Also, prostaglandin E2 (PGE2) reversed IL-10 inhibition of BLV tax and pol mRNA levels. Therefore, PGE2 might be one secretory factor from monocyte/macrophages that increases BLV expression by BLV-infected B cells. Signal transduction by PGE2 receptors mediates increased cyclic AMP production (2). BLV long terminal repeats contain a cyclic AMP response element that facilitates BLV gene transcription. Although macrophages may be infected by BLV and produce low levels of BLV mRNA (37), this idea is controversial (19), and our data indicate that nonadherent cells produce significant amount of BLV mRNA that is apparently regulated by the presence of monocyte/macrophages.
IL-2 has been used therapeutically to boost the host immune response against several infectious diseases and in cancer therapy (7, 15). Although IL-2 stimulates PBMC proliferation and CTL activity in virus infections, IL-2 also activates BLV (Fig. 5, 6) and HIV expression (1, 14). In addition, opportunistic infections can stimulate HIV replication and disease progression (21, 35). Our preliminary data also show that bovine herpesvirus, one of the most prevalent opportunistic infections in cattle, increased the detection of BLV tax and pol mRNA (25). These results suggest that cellular activation may provide a suitable environment for virus replication.
To examine the level of BLV, BLV tax and pol mRNA levels were quantified in the fentagram range by QC-PCR. Anti-p24 antibody also detected expression of p24, a part of gag, in PBMCs cultured with IL-2 and ConA. However, anti-p24 antibody was not sensitive enough to detect a difference in the amount of p24 between untreated and IL-10-treated PBMCs. In addition, anti-gp51 antibody could not detect a gp51 signal from cultured cells, while gp51 was clearly detected in purified BLV. Changes in BLV tax and pol transcription paralleled the translation of BLV p24 in PBMC stimulated with IL-2 and ConA. Therefore, measuring the transcription of BLV by QC-PCR is a reliable method for examining differences in the levels of BLV. The level of pol can be detected only from genomic mRNA (28). Thus, the replication rate of BLV genomic RNA can be measured by amplification of pol mRNA. Tax is an early gene product of BLV expression and regulates both cellular and viral transcription. Although Tax protein is translated only from the shortest mRNA, all four different mRNAs expressed in BLV transcription can contain the tax sequence. Thus, amplification of the tax region can be detected in all BLV mRNAs, providing an explanation for greater tax mRNA detection than pol in the present study. Since bovine cytokines are not commercially available, hrIL-2 (22), hrIL-10 (3), and hrIL-12 (4) were used. Previous studies (3, 4, 22) and the data in the present experiments indicate that these cytokines have reactivity with bovine PBMCs.
In conclusion, the findings that IL-10 inhibits BLV tax and pol mRNA levels suggest that increased IL-10 production by monocyte/macrophages could be serve as a host immune defense mechanism during late-stage BLV infection to limit the amount of virus production. In addition, the observation that IL-2 stimulates BLV production implicates that IL-2 treatment, to boost the immune response, may contribute a deleterious effect stimulating virus multiplication in the host.
ACKNOWLEDGMENTS
This work was supported by the National Cancer Institute grant RO1 CA59127, BARD 95-34339-2556, and the College of Agricultural and Life Sciences.
REFERENCES
- 1.Al-Harthi L, Roebuck K A, Landay A. Induction of HIV-1 replication by type 1-like cytokines, interleukin (IL)-12 and IL-15: effect on viral transcriptional activation, cellular proliferation, and endogenous cytokine production. J Clin Immunol. 1998;18:124–131. doi: 10.1023/a:1023246800353. [DOI] [PubMed] [Google Scholar]
- 2.An S, Yang J, So S W, Zeng L, Goetzl E J. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33:14496–14502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- 3.Brown W C, Woods V M, Chitko-McKown C G, Hash S M, Rice-Ficht A C. Interleukin-10 is expressed by bovine type 1 helper, type 2 helper, and unrestricted parasite-specific T-cell clones and inhibits proliferation of all three subsets in an accessory-cell-dependent manner. Infect Immun. 1994;62:4697–4708. doi: 10.1128/iai.62.11.4697-4708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown W C, Davis W C, Tuo W. Human interleukin-12 upregulates proliferation and interferon-gamma production by parasite antigen-stimulated Th cell clones and gamma/delta T cells of cattle. Ann N Y Acad Sci. 1996;795:321–324. doi: 10.1111/j.1749-6632.1996.tb52682.x. [DOI] [PubMed] [Google Scholar]
- 5.Choi S H, Splitter G A. Induction of MHC-unrestricted cytolytic CD4+ T cells against virally infected target cells by cross-linking CD4 molecules. J Immunol. 1994;153:3874–3881. [PubMed] [Google Scholar]
- 6.Clerici M, Shearer G M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 7.Davey R T, Jr, Chaitt D G, Piscitelli S C, Wells M, Kovacs J A, Walker R E, Falloon J, Polis M A, Metcalf J A, Masur H, Fyfe G, Lane H C. Subcutaneous administration of interleukin-2 in human immunodeficiency virus type 1-infected persons. J Infect Dis. 1997;175:781–789. doi: 10.1086/513971. [DOI] [PubMed] [Google Scholar]
- 8.Esteban E N, Thorn R M, Ferrer J F. Characterization of the blood lymphocyte population in cattle infected with the bovine leukemia virus. Cancer Res. 1985;45:3225–3230. [PubMed] [Google Scholar]
- 9.Gupta P, Kashmiri S V, Erisman M D, Rothberg P G, Astrin S M, Ferrer J F. Enhanced expression of the c-myc gene in bovine leukemia virus-induced bovine tumors. Cancer Res. 1986;46:6295–6298. [PubMed] [Google Scholar]
- 10.Haraguchi S, Good R A, James-Yarish M, Cianciolo G J, Day N K. Differential modulation of Th1- and Th2-related cytokine mRNA expression by a synthetic peptide homologous to a conserved domain within retroviral envelope protein. Proc Natl Acad Sci USA. 1995;92:3611–3615. doi: 10.1073/pnas.92.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland G, Zlotnik A. Interleukin-10 and cancer. Cancer Invest. 1993;11:751–758. doi: 10.3109/07357909309046950. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro N, Shinagawa T, Matsui T, Shinagawa M. Putative bovine B cell lineage tumor in sporadic bovine leukosis. Vet Immunol Immunopathol. 1994;42:185–197. doi: 10.1016/0165-2427(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Kettmann R, Portetelle D, Mammerickx M, Cleuter Y, Dekegel D, Galoux M, Ghysdael J, Burny A, Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci USA. 1976;73:1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinter A L, Poli G, Fox L, Hardy L, E, Fauci A S. HIV replication in IL-2-stimulated peripheral blood mononuclear cells is driven in an autocrine/paracrine manner by endogenous cytokines. J Immunol. 1995;154:2448–2459. [PubMed] [Google Scholar]
- 15.Kovacs J A, Vogel S, Albert J M, Falloon J, Davey R T, Jr, Walker R E, Polis M A, Spooner K, Metcalf J A, Baseler M, Fyfe G, Lane H C. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency. New Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 16.Koyama H, Nakanishi H, Kajikawa O, Yoshikawa H, Tsubaki S, Yoshikawa T, Saito H. T and B lymphocytes in persistently lymphocytotic and leukemic cattle. Jpn J Vet Sci. 1983;45:471–475. doi: 10.1292/jvms1939.45.471. [DOI] [PubMed] [Google Scholar]
- 17.Lucey D R, Clerici M, Shearer G M. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundberg, P. S., and G. A. Splitter. γδ+ T-lymphocyte cytotoxicity against envelope-expressing target cells is unique to the non-progressed alymphocytotic stage of bovine leukemia virus-infection in the natural host. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 19.Mirsky M L, Olmstead C A, Da Y, Lewin H A. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J Virol. 1996;70:2178–2183. doi: 10.1128/jvi.70.4.2178-2183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naif H M, Chang J, Ho-Shon M, Li S, Cunningham A L. Inhibition of human immunodeficiency virus replication in differentiating monocytes by interleukin 10 occurs in parallel with inhibition of cellular RNA expression. AIDS Res Human Retroviruses. 1996;12:1237–1245. doi: 10.1089/aid.1996.12.1237. [DOI] [PubMed] [Google Scholar]
- 21.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 22.Orlik O, Splitter G A. Optimization of lymphocyte proliferation assay for cells with high spontaneous proliferation in vitro: CD4+ T cell proliferation in bovine leukemia virus-infected animals with persistent lymphocytosis. J Immunol Methods. 1996;199:159–165. doi: 10.1016/s0022-1759(96)00178-0. [DOI] [PubMed] [Google Scholar]
- 23.Orlik O, Splitter G A. Progression to persistent lymphocytosis and tumor development in bovine leukemia virus (BLV)-infected cattle correlates with impaired proliferation of CD4+ T cells in response to gag- and env-encoded BLV proteins. J Virol. 1996;70:7584–7593. doi: 10.1128/jvi.70.11.7584-7593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyeon D, Splitter G A. Interleukin-12 p40 mRNA expression in bovine leukemia virus-infected animals: increase in alymphocytosis but decrease in persistent lymphocytosis. J Virol. 1998;72:6917–6921. doi: 10.1128/jvi.72.8.6917-6921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyeon, D., and G. A. Splitter. Unpublished data.
- 26.Pyeon, D., F. J. Diaz, and G. A. Splitter. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA expression with cyclooxygenase-2 regulation by interleukin-2, -10, and bovine leukemia virus. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 27.Pyeon D, O’Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radke K. Bovine leukemia virus. In: Webster R G, Granoff A, editors. Encyclopedia of Virology. New York, N.Y: Academic Press, Inc.; 1994. pp. 166–175. [Google Scholar]
- 29.Saville M W, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994;83:3591–3599. [PubMed] [Google Scholar]
- 30.Schuitemaker H. IL4 and IL10 as potent inhibitors of HIV1 replication in macrophages in vitro: a role for cytokines in the in vivo virus host range? Res Immunol. 1994;145:588–592. doi: 10.1016/s0923-2494(05)80038-0. [DOI] [PubMed] [Google Scholar]
- 31.Shearer G M, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 32.Tsai S J, Wiltbank M C. Quantification of mRNA using competitive RT-PCR with standard-curve methodology. BioTechniques. 1996;21:862–866. doi: 10.2144/96215st04. [DOI] [PubMed] [Google Scholar]
- 33.Van Parijs L, Abbas A K. Homeostasis and self-tolerance in the immune system—turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 34.Wahl L M, Smith P D. Isolation of monocyte/macrophage populations. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 7.6.1–7.6.8. [Google Scholar]
- 35.Wahl S M, Orenstein J M. Immune stimulation and HIV-1 viral replication. J Leukoc Biol. 1997;62:67–71. doi: 10.1002/jlb.62.1.67. [DOI] [PubMed] [Google Scholar]
- 36.Weissman D, Poli G, Fauci A S. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Human Retroviruses. 1994;10:1199–1206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 37.Werling D, Howard C J, Niederer E, Straub O C, Saalmuller A, Langhans W. Analysis of the phenotype and phagocytic activity of monocytes/macrophages from cattle infected with the bovine leukaemia virus. Vet Immunol Immunopathol. 1998;62:185–195. doi: 10.1016/s0165-2427(98)00074-9. [DOI] [PubMed] [Google Scholar]