Abstract
Diagnosis of acute respiratory infections (ARIs) is challenging due to the broad diversity of potential microbial causes. We used metagenomic next-generation sequencing (mNGS) to analyze the nasopharyngeal virome of ARI patients, who had undergone testing with a clinical multiplex PCR panel (Amplisens ARVI-screen-FRT). We collected nasopharyngeal swabs from 49 outpatient adults, 32 of whom had ARI symptoms and were PCR-positive, and 4 asymptomatic controls in Kazakhstan during Spring 2021. We assessed the biodiversity of the mNGS-derived virome and concordance with PCR results. PCR identified common ARI viruses in 65% of the symptomatic cases. mNGS revealed viral taxa consisting of human, non-human eukaryotic and bacteriophage groups, comprising 15, 11 and 28 genera, respectively. Notable ARI-associated human viruses included rhinovirus (16.3%), betaherpesvirus 7 (14.3%) and Epstein-Barr virus (8.16%). The primary phage hosts were Streptococcus spp. (32.7%), Pseudomonas aeruginosa (24.5%) and Burkholderia spp. (20.4%). In total, 47% of ARIs were linked solely to bacterial pathogens, a third to viral-bacterial co-infections, and less than 10% to only viral infections by mNGS. PCR showed low concordance with mNGS, except for rhinovirus. These results underscore the importance of broad diagnostic methods and question the effectiveness of commonly used PCR panels in ARI diagnosis.
Keywords: acute respiratory infection, metagenomics, virome, nasopharyngeal swab
1. Introduction
Acute respiratory infections (ARI) include conditions such as the common cold, bronchitis, pharyngitis and rhinosinusitis. ARI are the leading cause of outpatient visits and antibiotic prescriptions globally [1,2]. Most ARI are considered viral, although bacterial ARI are not uncommon and can develop after viral infection [3–5]. Clinical management of uncomplicated ARI is symptomatic or empiric, especially in the limited-resource settings of developing countries [2,5,6]. Laboratory testing of ARI, typically indicated when a bacterial infection is suspected, is done using microbiologic and molecular assays targeting ‘common’ respiratory pathogens [7]. Often, the underlying pathogens remain untyped in over a third of ARI cases, and it is unclear whether the pathogens identified by targeted assays are the true cause of ARI symptoms [3,8,9].
These diagnostic gaps have stimulated studies of the respiratory virome, including not only well-known pathogenic viruses but also less-characterized viral communities that may play a role in health and disease [10,11]. The nasopharyngeal virome, in particular, is a complex and dynamic ecosystem represented by human, non-human eukaryotic host and bacteriophage sub-groups [10,12]. The metagenomic characterization of the respiratory virome holds promise both in the context of small-scale clinical settings, where it could facilitate the diagnosis of respiratory conditions [13,14], and in the context of larger scale public health surveillance, where it could provide insights into the population-level dynamics of pathogen circulation [15,16], informing the clinical guidelines [17].
Metagenomic next-generation sequencing (mNGS) is a powerful tool to explore the respiratory virome, offering an unbiased approach to detect known and novel viruses [10]. However, the concordance between mNGS and traditional diagnostic methods, like multiplex PCR, varies across different platforms and for different pathogens [8]. The contribution of bacterial-viral co-infections and the role of ‘uncommon’ viruses and bacteria in ARI patients from diverse demographic and geographical strata are also not well understood.
In our earlier work, we surveyed adult ARI outpatients in Kazakhstan during a period of low COVID-19 transmission in Spring 2021 [18,19]. One global hallmark of this period was absent/low transmission of influenza and respiratory syncitial virus (RSV), but a high incidence of human rhinovirus (HRV) [20,21]. Here, we further characterized the nasopharyngeal virome associated with ARI using mNGS as part of the national initiative to enhance the diagnostic capacity for ARI in the post-pandemic era [22]. Our specific endpoint was to compare the mNGS results to those of an ARI-targeting PCR panel used commonly by clinical laboratories in Kazakhstan and the neighbouring countries [23–25].
2. Materials and methods
2.1. Study setting
This study is a follow-up to our earlier work exploring virologic causes of ARI among outpatients of two public hospitals in Kazakhstan [18,19]. In this earlier study, 50 participants with ARI symptoms, who tested negative for SARS-CoV-2 and influenza A virus, were recruited between May 18 and June 7, 2021. The highlights of this period in Kazakhstan were the deployment of mass COVID-19 vaccination [26–28], low SARS-CoV-2 infection rates but a concomitant increase in non-COVID respiratory infections [18].
Written consent to participate was obtained from all participants and witnessed by the study coordinator. ARI was defined by the presence of the following respiratory symptoms: fever, nasal congestion with/without rhinorrhoea, cough, sore throat and lymphadenopathy. Nasopharyngeal swabs (NPS) were collected and partitioned for PCR and mNGS assays as described earlier [18]. In addition to the ARI samples, we collected NPS from four asymptomatic controls (AC). Due to limited sample availability, we excluded one ARI sample from the mNGS assays; this resulted in a total of 53 samples (49 ARI and 4 AC) processed and analysed in the current study.
2.2. mNGS
Initially, to eliminate intact host cells and ensure that the extracted nucleic acids would be mainly of viral origin, NPS were enriched using a combination of low-speed centrifugation and filtration following virome characterization protocols [18,29,30]. Specifically, NPS were centrifuged at 5 200 g for 10 min, followed by centrifugal filtration at 13 000 g for 1 min using 0.45 µm cellulose acetate filters (Spin-X Centrifuge tube filter, Corning). Free nucleic acids were removed using Pierce Universal Nuclease (Thermo Fisher Scientific). Samples were concentrated using a Pierce ™ PES protein concentrator (Thermo Fisher Scientific) at the 100 K molecular weight cutoff.
Sequencing library preparation was done as described earlier [18]. Briefly, both genomic DNA and RNA were extracted using the MagMAX Total Nucleic Acid Isolation Kit (Thermo Fisher Scientific), followed by cDNA synthesis, amplification, and primer removal using the SeqPlex RNA Amplification Kit (Sigma) yielding 150–400 nucleotide cDNA fragments. The resulting cDNA quality/purity and quantity were assessed by NanoDrop and Qubit, respectively.
Libraries were prepared using the Ion Plus Fragment Library Kit, Ion Xpress Barcode Adapters 1–96 Kit (both from Thermo Fisher Scientific), and the Agencourt AMPure XP kit (Beckman Coulter). Barcoded libraries were assembled using the Ion Plus Fragment Library Kit and Ion Xpress Barcode Adapters 1–96 Kit and quantified according to the Ion Library TaqMan Quantitation Kit's protocol. Libraries were then loaded onto Ion 530 Chips with the Ion Chef Instrument and processed on the Ion Torrent S5 System (Thermo Fisher Scientific).
2.3. Multiplex PCR panel
Real-time PCR was performed on the NPS genomic DNA and RNA using the Amplisens ARVI-screen-FRT kit (Amplisens, Interlabservis, Moscow, Russia). The PCR panel targeted both RNA (respiratory syncytial virus (RSV), metapneumovirus (MPV), human parainfluenza virus-1–4 (HPIV), coronaviruses (HCoV) ОС43/HKU-1 and NL-63/229E and rhinovirus (HRV), and DNA (adenovirus (Adv) B, C and E and bocavirus (BoV) viruses [18].
2.4. Bioinformatic analyses
Sequencing reads were assigned taxonomic information using the open-source Chan Zuckerberg ID (CZID) portal (v7.0, analysis performed in June 2022), which compares queries assembled sequences against the NCBI nucleotide and protein databases [31]. In addition, CZID v7.0 incorporates adapter trimming, low-quality read removal, low complexity read removal, external RNA controls consortium read and human genome (GRCh38) read removal [31].
Independent of the CZID analysis, we also implemented EDGE [32] to process the reads. We then used a combination of read-based taxonomic classification tools, including Genomic Origin Through Taxonomic CHAllenge (GOTTCHA2) [33], Kraken2 [34], Burrows-Wheeler Alignment tool [35], MethaPhlAn2 [36] and Diamond [37] to compare and validate the taxonomic readouts with the CZID-generated results.
The data were standardized based on unique reads mapped per million input reads at the genus tier. To eliminate background and low-frequency sequencing reads, we followed guidelines from the recent metagenomic studies [38,39] and set taxon inclusion criteria as follows: nucleotide reads per million (NT %id) of 95% or higher, the alignment length (NT L) of ≥70 base pairs, and reads per million (rpM) > 1. The recovered bacteriophage genera were grouped based on their respective bacterial hosts using the NCBI taxonomy browser (https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi).
2.5. Statistical analyses
Taxon abundance heatmaps were constructed using log-transformed normalized rpM and graphed using Morpheus (https://software.broadinstitute.org/morpheus/). Simpson's biodiversity indices were calculated on the normalized rpM data via BiodiversityR [40], using the default recommended settings, and plotted using R ggpubr [41]. Differences across the PCR and AC sub-groups were assessed using the Mann-Whitney U and Kruskal-Wallis tests. Association rule mining was performed in Orange 3.36.2 [42]. The diagnostic performance of the Amplisens PCR panel was assessed by calculating the sensitivity and specificity metrics using mNGS as the reference standard in R v. 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). Sensitivity was calculated as the proportion of positive cases identified by both PCR and mNGS (true positives) divided by the total number of cases confirmed by mNGS. Specificity was determined by dividing the number of participants who tested negative by both PCR and mNGS (true negatives) by the total number of participants who were negative for the PCR panel pathogens according to mNGS.
3. Results
3.1. Nasopharyngeal virome characteristics
The median age of ARI participants was 32 years and 73.5% of them were female; the median age of AC participants was 36 years and 50% of them were female. In 65% (32/49) of ARI participants, multiplex PCR was positive for HPIV-3 (1/49), HPIV-4 (24/49), HRV (2/49), HPIV4-HRV (4/49), HAdV (1/49) or HCoV (1/49). mNGS was performed on both ARI (n = 49) and AC (n = 4) samples, with a median of 406,954 reads obtained per sample after filtering out human reads. The initial validation and comparison of taxonomic outputs generated by the different pipelines indicated presence of similar viral taxon signatures across the different taxonomy pipelines. Therefore, we used the CZID analysis output for the rest of the analysis.
After bioinformatic processing, the viral taxa were stratified into human, non-human eukaryotic host, and bacteriophage sub-groups (figure 1), including 15, 11 and 28 genera, respectively (electronic supplementary material, tables S1 and S2). Among the human viruses, gammapapillomavirus and enterovirus were most prevalent in symptomatic participants and observed in 13/49 (26.5%) and 8/49 (16.3%) participants, respectively. No HPIV-4, the virus most prevalently detected by PCR, was detectable by mNGS. Non-human eukaryotic host viruses consisted predominantly of plant and fungal viruses, of which Tobamovirus, derived from tobacco and other Solanaceae, was most prevalent among all participants (in 23/53, 43.4%). Among the bacteriophages, Pahexavirus, a Propionibacterium (Cutibacterium) acnes bacteriophage, was most prevalent and abundant, observed in 48/53 (90.6%) participants and in both ARI and AC groups (electronic supplementary material, table S3).
Figure 1.
Nasopharyngeal virome profile of participants with acute respiratory symptoms (ARI), stratified by the multiplex PCR result, and asymptomatic controls (AC). Each row is a viral genus. Each column represents a participant. Microorganism abundance was derived by log-transformation of normalized reads per million (nrpM). Viral taxa were plotted in order of descending mNGS-derived cumulative abundance across the cohort. (a) Human viruses. (b) Non-human eukaryotic viruses. (c) Phages.
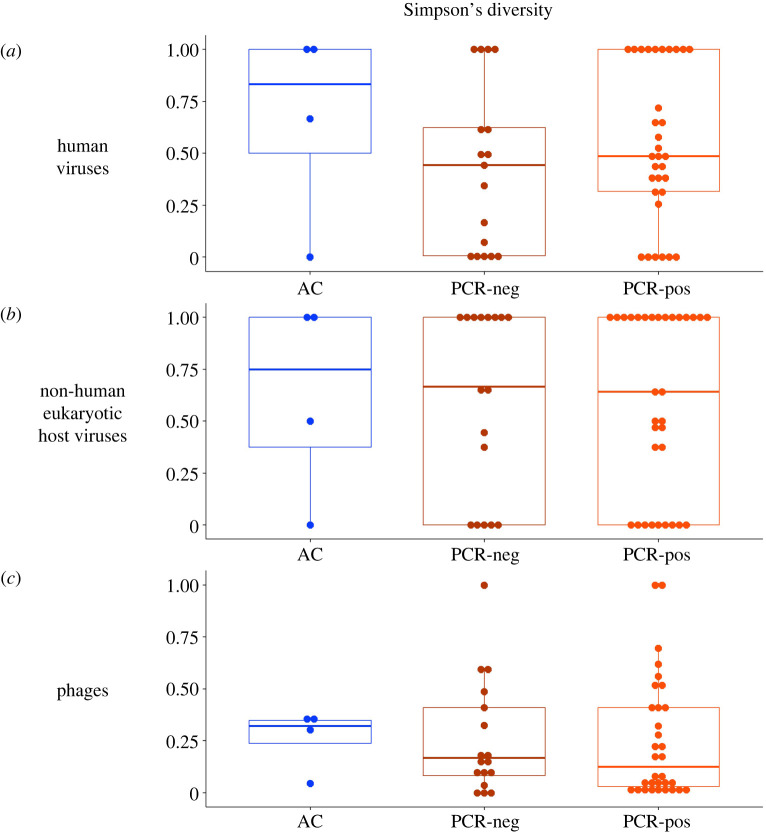
Further, we hypothesized that the nasopharyngeal virome diversity would differ between the participant sub-groups stratified by the multiplex PCR test result. To test this, we compared Simpson's alpha-diversity index across the PCR-positive, PCR-negative and AC groups (figure 2). No significant differences were observed in any of the comparisons (figure 2).
Figure 2.
Simpson's alpha biodiversity indices for each of the three major virus sub-groups: (a) Human viruses, (b) Non-human eukaryotic viruses and (c) Phages in the nasopharynx of asymptomatic controls (AC) and subjects with acute respiratory infection either with negative (PCR-neg) or positive (PCR-pos) test results on a multiplex PCR targeting common respiratory viruses (see Methods for the PCR panel description). Each box plot displays a median value for the diversity index (thick lines) and interquartile ranges (boxes).
3.2. Distribution of ARI-linked viruses and bacteria
We then focused on pathogens known for their associations with respiratory disease, narrowing our analysis to 8 human virus genera and 7 bacterial taxa representing hosts of the 16 recovered bacteriophage genera (figure 3). Here, we observed a co-detection of viral and bacterial pathogens in 16/49 (32.7%) ARI cases. Only a bacterial or viral pathogen was present in 23/49 (46.9%) and 4/49 (8.2%) ARI cases, respectively. No viral or bacterial pathogens were seen in 6/49 (12.2%) ARI cases (figure 3). The top three ARI-associated human viruses were enterovirus (16.3%, human rhinovirus, HRV-A), roseolovirus (14.3%, human betaherpesvirus 7, HBV-7) and lymphocryptovirus (8.16%, Epstein-Barr virus, EBV) (figure 3). The top three ARI-associated phage hosts were Streptococcus spp (32.7%), Pseudomonas aeruginosa (24.5%) and Burkholderia spp. (20.4%). The virome of both asymptomatic and symptomatic subjects was also abundant in the Staphylococcus (60.4%) bacteriophages. Except for mastadenovirus and Staphylococcus, all other respiratory pathogens were observed only in ARI but not in AC.
Figure 3.
Nasopharyngeal profile of ARI patients, consisting of human viruses and bacteria known to cause respiratory disease. Each row is a viral (brown) or bacterial (orange) genus. Columns represent participants grouped by their ARI/AC status and by multiplex PCR results. PCR-positive samples are further stratified by the PCR-identified virus. Metagenomically detected microorganisms are shown as present (coloured circles) or absent (no circles). Viral taxa were plotted in order of descending mNGS-derived cumulative abundance across the cohort (similar to figure 1). Bacterial genera were derived from the list of mNGS-recovered bacteriophages using phage-host classification. ARI: acute respiratory infection. AC: asymptomatic controls. HRV: human rhinovirus. HPIV: human parainfluenza. HAdV: human adenovirus. HCoV: human coronaviruses (seasonal).
HRV was detected by mNGS in all PCR + HRV cases, in addition to two PCR-negative cases and one subject with a PCR-identified HPIV-4 infection. HBV-7 was detected as a viral mono-infection in two cases, and as a co-infection in 5 cases with HRV (n = 1), EBV (n = 3) and MPV (n = 1). EBV was detected in a total of 4 samples, of which only one was a viral monoinfection. We then further explored co-infection patterns using association rule mining to describe frequently co-occurring pathogens using ‘rules’ predicting the presence of a pathogen based on the occurrence of other pathogens. Using a minimal support threshold of 6% (occurrence in >=3 participants), two associations were identified. Specifically, Staphylococcus spp. co-occurred with Enterobacter/Klebsiella, and with Mastadenovirus in 9.4% (leverage = 3.7%) and 7.5% (leverage = 3.0%) of the cases, respectively (lift = 1.66 for both, indicating strong associations). The observed positive leverage indices indicated that the pathogens co-occurred more often than would be expected based on their individual frequencies alone.
3.3. Concordance between mNGS and Amplisens PCR
The PCR and mNGS results were relatively concordant for human rhinovirus (HRV) with PCR sensitivity and specificity relative to mNGS of 62.5 (95% CI 24.5, 91.5) and 100% (95% CI 92.1, 100), respectively. The concordance between mNGS and PCR for other PCR panel targets, including human parainfluenza (HPIV), adenovirus (HAdV), bocavirus (BoV) and seasonal coronavirus (HCoV) was low (electronic supplementary material, table S4).
4. Discussion
Here we characterized the nasopharyngeal metagenome associated with ARI and assessed the concordance between mNGS and the Amplisens PCR widely used by the laboratories in the region [23–25]. In the absence of influenza and RSV [18], our findings were consistent with other recent studies, where viral and bacterial ARI were predominantly associated with rhinovirus and Streptococcus spp., respectively [3].
We observed a relatively high concordance between PCR and mNGS for HRV but low concordance for other PCR targets, such as HPIV and HAdV. To the best of our knowledge, this is the first published assessment of the Amplisens ARVI-screen-FRT PCR performance using mNGS, warranting further investigation of the clinical validity and value of using this commercial assay for ARI diagnosis. The discrepancies observed between mNGS and PCR may be attributed to the known differences between the two assays in sensitivity and specificity [8,13,14,16]. The complete lack of HPIV-4 (human orthorubulavirus 4) in the mNGS output could be due to very low nasopharyngeal HPIV-4 loads or a non-specific detection by PCR. Interestingly, our mNGS data also demonstrated a large fraction of the ARI (47%) to be associated with only respiratory bacteria— consistent with bacterial sinusitis or tonsillitis—but not viruses. Moreover, half of the mNGS-identified ‘bacterial-only’ samples were also HPIV-4 positive by PCR. This suggests that bacterial/bacteriophage load may be overwhelming and/or interfering with the molecular signal of HPIV-4 infection.
Remarkably, 33% of the ARI in our study were associated with co-detected viruses and bacteria, while viral mono-infections contributed to just a small ARI fraction (8%). These findings are in line with negative virus-to-virus interactions and positive interactions seen between viral and bacterial pathogens [3,43–45]. The co-occurrence we observed for Staphylococcus and Enterobacter/Klebsiella is consistent with a report of co-colonization by these pathogens of the respiratory tract in intensive care unit patients [46].
To the best of our knowledge, the almost ubiquitous presence in NPS samples of Pahexivirus, a Propionibacterium acnes bacteriophage, has not been previously reported. However, a recent thesis [47] described a lower relative nasopharyngeal abundance of both Pahexivirus and Propionibacterium acnes in HIV + subjects compared to HIV-negative controls from Malawi, which may reflect a commensal nature and association of Propionibacterium acnes with the systemic immune state.
The identification of lymphocryptovirus (EBV), a common cause of infectious mononucleosis, and roseolovirus (HBV-7) as top ARI-associated human viruses warrants public health consideration. Since HBV-7 primarily causes roseola infantum or sixth disease in young children, typically manifesting with a high fever and a skin rash [48], our data suggest that adults could be important HBV-7 reservoirs within communities.
Our findings should be interpreted in the light of the limitations. First, the relatively small sample size affected our ability to discern complex patterns of viral and bacterial (co-)infection patterns. The low concordance between mNGS and PCR for certain viral targets, such as HPIV and HAdV, could be due to viral variants but we were unable to address this. One potential means to increase the odds of detection by mNGS of low abundant viruses would be to sequence more reads per sample—something that we could not do due to limited study funds. The large fraction of ARIs potentially associated only with respiratory bacteria, particularly in HPIV-4+ PCR samples, suggests that further optimization of our mNGS methodology is warranted prior to deployment in clinical settings. We could not validate the identified bacterial species as other assays, such as serology, antigen detection or complement fixation, were unavailable during the study. Lastly, our analysis pipeline did not account for the presence of fungal pathogens, which could contribute to ARI pathogenesis.
Despite the limitations, our study highlights the multifaceted nature of the nasopharyngeal virome in ARI, particularly adding to other studies showing the feasibility of deploying mNGS in the limited-resource settings of developing countries [15,49]. However, the discrepancies between mNGS and multiplex PCR raise questions about the value of the Amplisens PCR panel in the clinical management of ARI and punctuate the need for the integration of complementary clinical and laboratory methods to enhance detection accuracy of ARI pathogens [50].
Acknowledgements
We thank all the study participants and the clinic staff.
Contributor Information
Nurlan Sandybayev, Email: nurlan.s@kaznaru.edu.kz.
Sergey Yegorov, Email: yegorovs@mcmaster.ca.
Ethics
All study procedures were approved by the Commission on bioethics of KazNARU (dated October 15, 2020). Written informed consent was obtained from all participants.
Data accessibility
The raw mNGS data have been deposited to NCBI under accession PRJNA904925 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA904925).
The data are provided in electronic supplementary material [51].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
N.S.: conceptualization, funding acquisition, project administration, resources, writing—original draft; V.B.: data curation, formal analysis, investigation, methodology, software; V.S.: data curation, formal analysis, investigation, methodology, visualization; M.S.: formal analysis, methodology, resources, software, validation; J.G.: data curation, investigation, methodology, resources; S.Y.: conceptualization, funding acquisition, methodology, software, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare that they have no competing interests.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP19677743).
References
- 1.Havers FP, et al. 2018. Outpatient Antibiotic Prescribing for Acute Respiratory Infections During Influenza Seasons. JAMA Netw Open 1, e180243. ( 10.1001/jamanetworkopen.2018.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen NV, et al. 2023. Outpatient antibiotic prescribing for acute respiratory infections in Vietnamese primary care settings by the WHO AWaRe (Access, Watch and Reserve) classification: an analysis using routinely collected electronic prescription data. Lancet Regional Health – Western Pacific 30, 100611. ( 10.1016/j.lanwpc.2022.100611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z-J, et al. 2021. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12, 5026. ( 10.1016/j.lanwpc.2022.100611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, et al. 2021. Outcomes of respiratory viral-bacterial co-infection in adult hospitalized patients. eClinicalMedicine 37, 100955. ( 10.1016/j.eclinm.2021.100955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi T, Arnott A, Semogas I, Falsey AR, Openshaw P, Wedzicha JA, Campbell H, Nair H. 2019. The Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 222, jiy662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feikin DR, et al. 2012. Etiology and Incidence of Viral and Bacterial Acute Respiratory Illness among Older Children and Adults in Rural Western Kenya, 2007–2010. PLoS ONE 7, e43656. ( 10.1371/journal.pone.0043656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson KE, et al. 2020. Molecular Testing for Acute Respiratory Tract Infections: Clinical and Diagnostic Recommendations From the IDSA's Diagnostics Committee. Clin. Infect. Dis. 71, 2744-2751. ( 10.1093/cid/ciaa508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlow AW, et al. 2022. Comparing variability in diagnosis of upper respiratory tract infections in patients using syndromic, next generation sequencing, and PCR-based methods. PLOS Global Public Health 2, e0000811. ( 10.1371/journal.pgph.0000811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phommasone K, et al. 2022. A case–control study of the causes of acute respiratory infection among hospitalized patients in Northeastern Laos. Sci. Rep. 12, 939. ( 10.1038/s41598-022-04816-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang G, Bushman FD. 2021. The human virome: assembly, composition and host interactions. Nat. Rev. Microbiol. 19, 514-527. ( 10.1038/s41579-021-00536-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wylie KM. 2017. The Virome of the Human Respiratory Tract. Clin. Chest Med. 38, 11-19. ( 10.1016/j.ccm.2016.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopala SV, et al. 2021. Metatranscriptomics to characterize respiratory virome, microbiome, and host response directly from clinical samples. Cell Rep Methods 1, 100091. ( 10.1016/j.crmeth.2021.100091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu CY, Miller SA. 2019. Clinical metagenomics. Nat. Rev. Genet. 20, 341-355. ( 10.1038/s41576-019-0113-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller RR, Montoya V, Gardy JL, Patrick DM, Tang P. 2013. Metagenomics for pathogen detection in public health. Genome Medicine 5, 81. ( 10.1186/gm485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oguzie JU, et al. 2023. Metagenomic surveillance uncovers diverse and novel viral taxa in febrile patients from Nigeria. Nat. Commun. 14, 4693. ( 10.1038/s41467-023-40247-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall RJ, Draper JL, Nielsen FGG, Dutilh BE. 2015. Beyond research: a primer for considerations on using viral metagenomics in the field and clinic. Front. Microbiol. 6, 224. ( 10.3389/fmicb.2015.00224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago-Rodriguez TM, Hollister EB. 2022. Unraveling the viral dark matter through viral metagenomics. Front. Immunol. 13, 1005107. ( 10.3389/fimmu.2022.1005107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandybayev N, Beloussov V, Strochkov V, Solomadin M, Granica J, Yegorov S. 2023. Characterization of viral pathogens associated with symptomatic upper respiratory tract infection in adults during a low COVID-19 transmission period. PeerJ 11, e15008. ( 10.7717/peerj.15008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strochkov V, Beloussov V, Solomadin M, Granica J, Yegorov S, Orkara S, Sandybayev N. 2023. Full genome sequence of a human rhinovirus A1B, obtained in Kazakhstan. Microbiol. Resour. Announc 12, e00749-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow EJ, Uyeki TM, Chu HY. 2022. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol 21, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen SJ. 2021. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic — United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep 70, 1013-1019. ( 10.15585/mmwr.mm7029a1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yegorov S, Goremykina M, Ivanova R, Good SV, Babenko D, Shevtsov A, MacDonald KS, Zhunussov Y. 2021. Epidemiology, clinical characteristics, and virologic features of COVID-19 patients in Kazakhstan: A nation-wide retrospective cohort study. Lancet Regional Health – Europe 4, 100096. ( 10.1016/j.lanepe.2021.100096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurskaya OG, et al. 2022. Low incidence of human coronavirus among hospitalized children in Novosibirsk city, Russia during pre-pandemic period (2013–2020). J. Microbiol. Immunol. Infect. 55, 336-340. ( 10.1016/j.jmii.2021.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurskaya OG, et al. 2023. Changes in the Etiology of Acute Respiratory Infections among Children in Novosibirsk, Russia, between 2019 and 2022: The Impact of the SARS-CoV-2 Virus. Viruses 15, 934. ( 10.3390/v15040934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sominina A, et al. 2021. Age-Specific Etiology of Severe Acute Respiratory Infections and Influenza Vaccine Effectivity in Prevention of Hospitalization in Russia, 2018-2019 Season. J. Epidemiol. Glob Health 11, 413-425. ( 10.1007/s44197-021-00009-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turmukhambetova A, et al. 2023. The impact of Gam-COVID-Vac, an Adv5/Adv26 COVID-19 vaccine, on the biomarkers of endothelial function, coagulation and platelet activation. PLoS ONE 18, e0293074. ( 10.1371/journal.pone.0293074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yegorov S, et al. 2022. Sputnik-V reactogenicity and immunogenicity in the blood and mucosa: a prospective cohort study. Sci. Rep. 12, 13207. ( 10.1038/s41598-022-17514-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadyrova I, et al. 2022. High SARS-CoV-2 seroprevalence in Karaganda, Kazakhstan before the launch of COVID-19 vaccination. PLoS ONE 17, e0272008. ( 10.1371/journal.pone.0272008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vibin J, Chamings A, Collier F, Klaassen M, Nelson TM, Alexandersen S. 2018. Metagenomics detection and characterisation of viruses in faecal samples from Australian wild birds. Sci. Rep. 8, 8686. ( 10.1038/s41598-018-26851-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conceição-Neto N, et al. 2015. Modular approach to customise sample preparation procedures for viral metagenomics: a reproducible protocol for virome analysis. Sci. Rep. 5, 16532. ( 10.1038/srep16532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalantar KL, et al. 2020. IDseq-An open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. Gigascience 9, giaa111. ( 10.1093/gigascience/giaa111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P-E, et al. 2017. Enabling the democratization of the genomics revolution with a fully integrated web-based bioinformatics platform. Nucleic Acids Res. 45, 67-80. ( 10.1093/nar/gkw1027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freitas TAK, Li P-E, Scholz MB, Chain PSG. 2015. Accurate read-based metagenome characterization using a hierarchical suite of unique signatures. Nucleic Acids Res. 43, e69. ( 10.1093/nar/gkv180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood DE, Salzberg SL. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 15, R46. ( 10.1186/gb-2014-15-3-r46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754-1760. ( 10.1093/bioinformatics/btp324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, Tett A, Huttenhower C, Segata N. 2015. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 12, 902-903. ( 10.1038/nmeth.3589) [DOI] [PubMed] [Google Scholar]
- 37.Buchfink B, Xie C, Huson DH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59-60. ( 10.1038/nmeth.3176) [DOI] [PubMed] [Google Scholar]
- 38.Roux S, Emerson JB, Eloe-Fadrosh EA, Sullivan MB. 2017. Benchmarking viromics: an in silico evaluation of metagenome-enabled estimates of viral community composition and diversity. PeerJ 5, e3817. ( 10.7717/peerj.3817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Borm S, Fu Q, Winand R, Vanneste K, Hakhverdyan M, Höper D, Vandenbussche F. 2020. Evaluation of a commercial exogenous internal process control for diagnostic RNA virus metagenomics from different animal clinical samples. J. Virol. Methods 283, 113916. ( 10.1016/j.jviromet.2020.113916) [DOI] [PubMed] [Google Scholar]
- 40.Kindt R. 2023. BiodiversityR: Package for Community Ecology and Suitability Analysis. published online Oct 22. See https://cran.r-project.org/web/packages/BiodiversityR/index.html (accessed Nov 22, 2023).
- 41.Kassambara A. 2023. ggpubr: ‘ggplot2’ Based Publication Ready Plots. published online Feb 10. See https://cran.r-project.org/web/packages/ggpubr/index.html (accessed Nov 22, 2023).
- 42.Demšar J, et al. 2013. Orange: data mining toolbox in python. J. Mach. Learn. Res. 14, 2349-2353. [Google Scholar]
- 43.Karppinen S, Terã¤Sjã¤Rvi J, Auranen K, Schuez-Havupalo L, Siira L, He Q, Waris M, Peltola V. 2017. Acquisition and Transmission of Streptococcus pneumoniae Are Facilitated during Rhinovirus Infection in Families with Children. Am. J. Respir. Crit. Care Med. 196, 1172-1180. ( 10.1164/rccm.201702-0357OC) [DOI] [PubMed] [Google Scholar]
- 44.Van Den Bergh MR, et al. 2012. Associations between Pathogens in the Upper Respiratory Tract of Young Children: Interplay between Viruses and Bacteria. PLoS ONE 7, e47711. ( 10.1371/journal.pone.0047711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacoby P, Watson K, Bowman J, Taylor A, Riley TV, Smith DW, Lehmann D. 2007. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine 25, 2458-2464. ( 10.1016/j.vaccine.2006.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thuy DB, et al. 2021. Colonization with Staphylococcus aureus and Klebsiella pneumoniae causes infections in a Vietnamese intensive care unit. Microbial Genomics 7, 000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busby J. 2022. Using shotgun metagenomics to explore the effects of HIV infection on the longitudinal nasopharyngeal microbiome of Malawian adults. See https://theses.gla.ac.uk/83430/ (accessed Aug 14, 2023).
- 48.Tesini BL, Epstein LG, Caserta MT. 2014. Clinical impact of primary infection with roseoloviruses. Curr Opin Virol 9, 91-96. ( 10.1016/j.coviro.2014.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogunbayo AE, Mogotsi MT, Sondlane H, Sabiu S, Nyaga MM. 2023. Metagenomics characterization of respiratory viral RNA pathogens in children under five years with severe acute respiratory infection in the Free State, South Africa. J. Med. Virol. 95, e28753. ( 10.1002/jmv.28753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yegorov S, et al. 2024. Application of MALDI-TOF MS and machine learning for the detection of SARS-CoV-2 and non-SARS-CoV-2 respiratory infections. Microbiology Spectrum 0, e04068-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandybayev N, Beloussov V, Strochkov V, Solomadin M, Granica J, Yegorov S. 2024. Metagenomic profiling of nasopharyngeal samples from adults with acute respiratory infection. Figshare. ( 10.6084/m9.figshare.c.7320460) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
Data Availability Statement
The raw mNGS data have been deposited to NCBI under accession PRJNA904925 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA904925).
The data are provided in electronic supplementary material [51].