Abstract
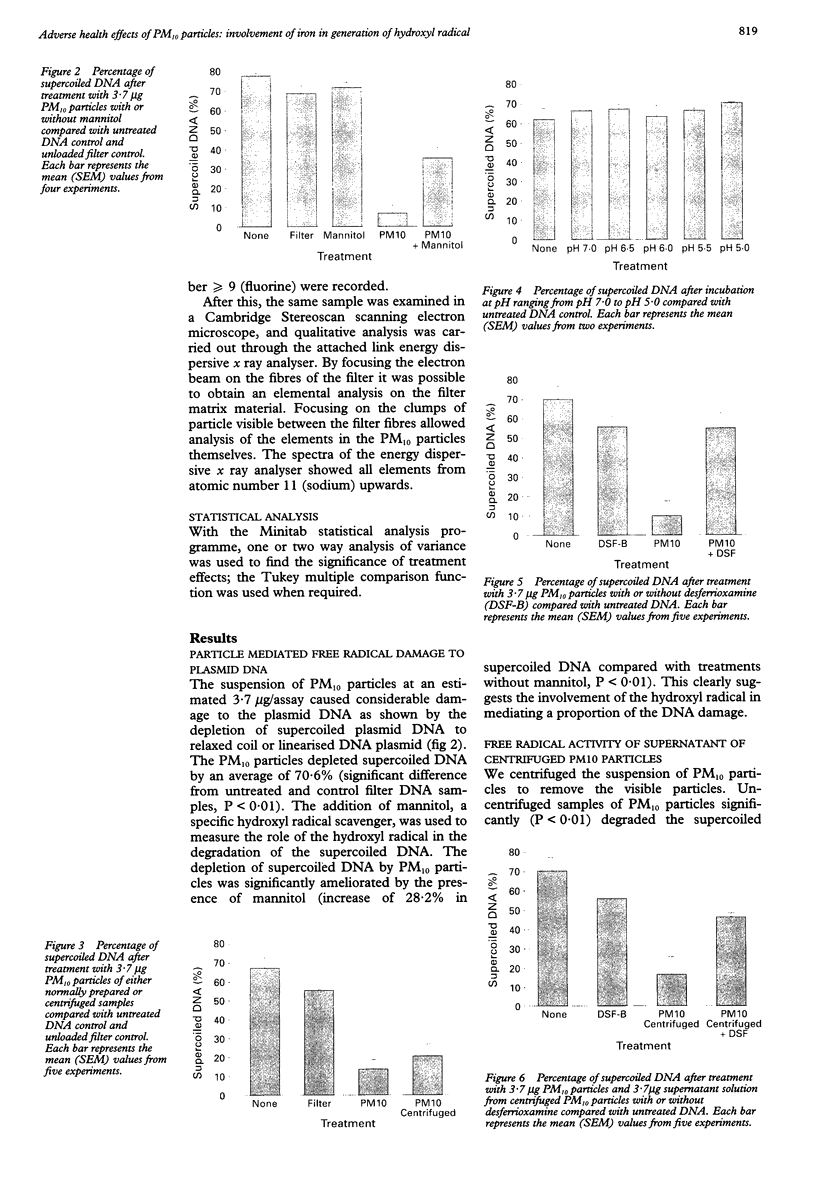
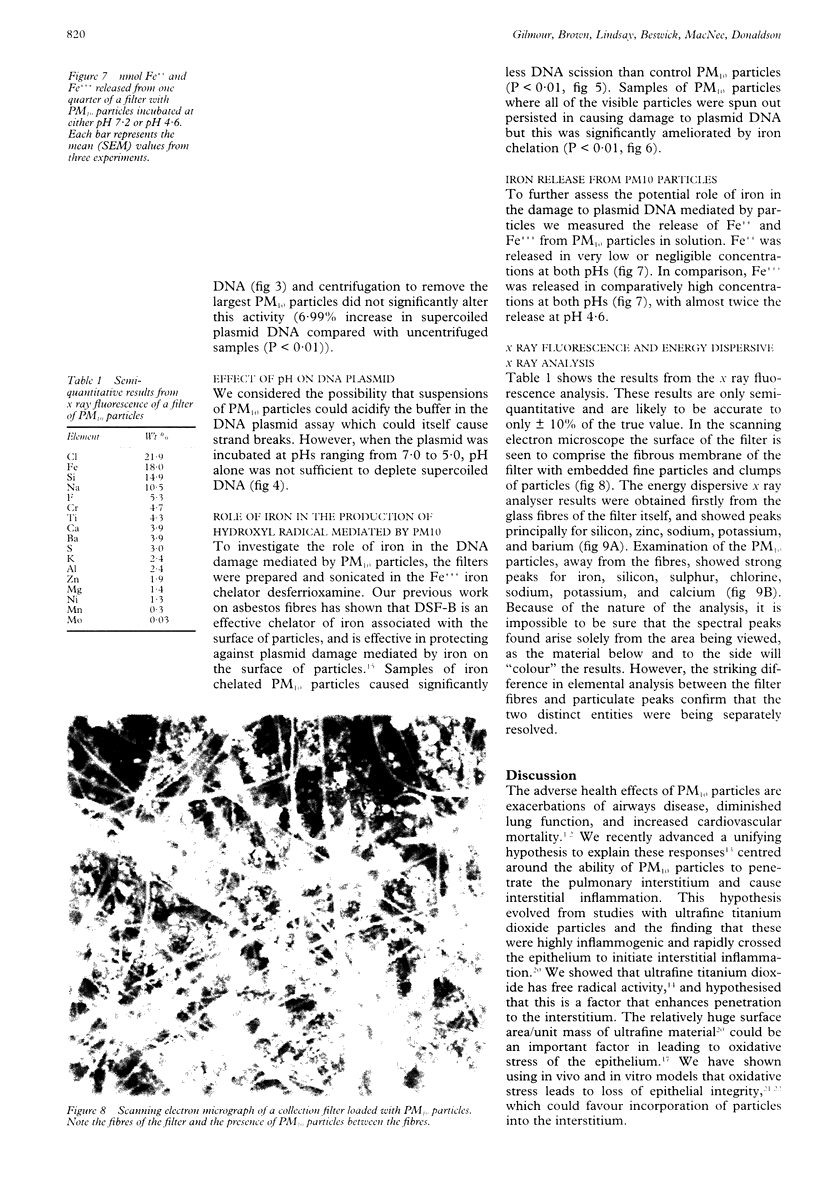
OBJECTIVES: Environmental particles < 10 microns average aerodynamic diameter (PM10) are associated with mortality, exacerbation of airways diseases, and decrement in lung function. It is hypothesised that PM10 particles, along with other pathogenic particles, generate free radicals at their surface in reactions involving iron, and that this is a factor in the pathogenicity of PM10 particles. Identification of free radical activity in PM10 and examination of the content and role of iron in this process was undertaken. METHODS: Free radical activity was detected with a supercoiled plasmid, phi X174 RF1 DNA, and measured as scission of the supercoiled DNA (mediated by free radicals) by scanning laser densitometry. The role of the hydroxyl radical was confirmed by the use of the specific scavenger mannitol, and the role of iron investigated with the iron chelator desferrioxamine-B (DSF-B). Iron released from PM10 particles at pH 7.2 and pH 4.6 (to mimic conditions on the lung surface and in macrophage phagolysosomes, respectively) was assessed spectrophotometrically with the Fe++ chelator ferrozine and the Fe+ + + chelator DSF-B. RESULTS: PM10 particles showed significant free radical activity by their ability to degrade supercoiled DNA. A substantial part of this activity was due to the generation of hydroxyl radicals, as shown by partial protection with mannitol. Similarly, DSF-B also conferred protection against the damage caused to plasmid DNA indicating the role of iron in generation of hydroxyl radicals. Negligible Fe++ was released at either pH 7.2 or pH 4.6 by contrast with Fe+ + +, which was released in substantial quantities at both pHs, although twice as much was released at pH 4.6. CONCLUSIONS: PM10 particles generate the hydroxyl radical, a highly deleterious free radical, in aqueous solution. This occurs by an iron dependent process and hydroxyl radicals could play a part in the pathogenicity of PM10 particles. Iron release was greatest at the pH of the lysosome (pH 4.6) indicating that iron may be mobilised inside macrophages after phagocytosis, leading to oxidative stress in the macrophages.
Full text
PDF

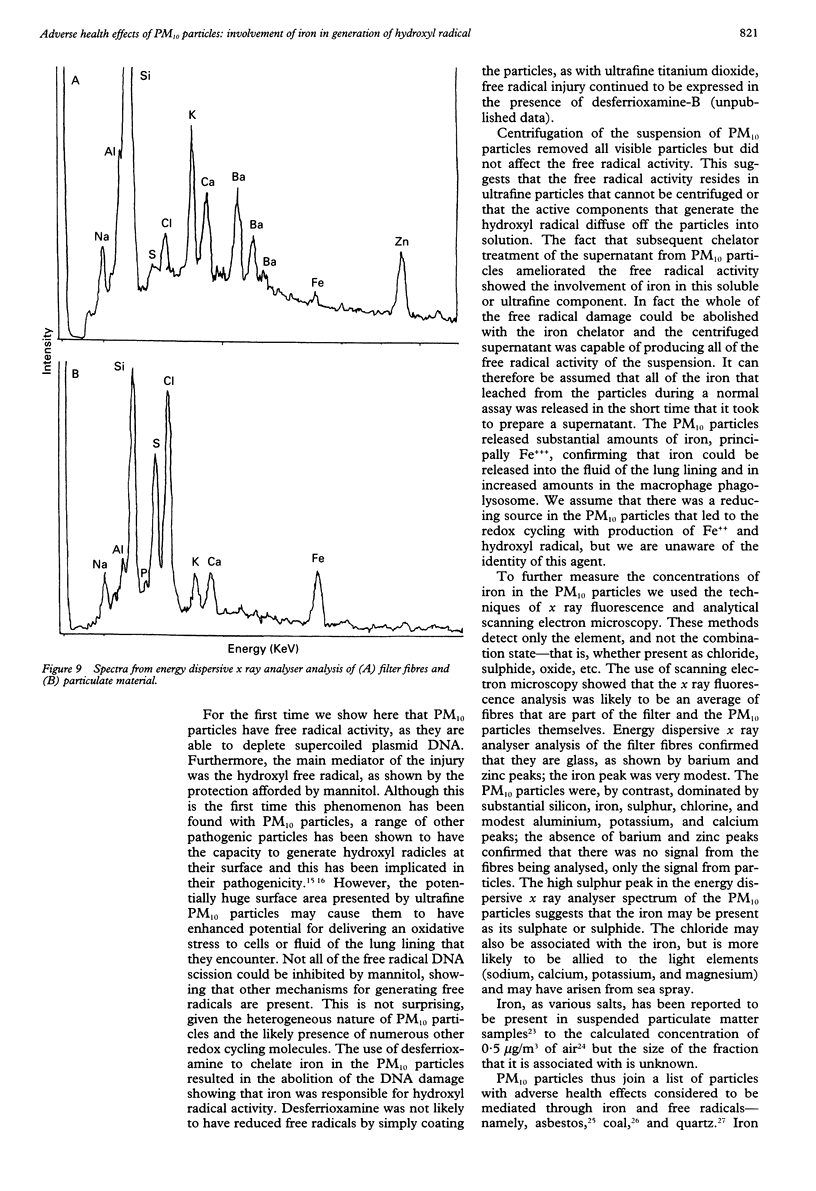

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalal N. S., Suryan M. M., Vallyathan V., Green F. H., Jafari B., Wheeler R. Detection of reactive free radicals in fresh coal mine dust and their implication for pulmonary injury. Ann Occup Hyg. 1989;33(1):79–84. doi: 10.1093/annhyg/33.1.79. [DOI] [PubMed] [Google Scholar]
- Dusseldorp A., Kruize H., Brunekreef B., Hofschreuder P., de Meer G., van Oudvorst A. B. Associations of PM10 and airborne iron with respiratory health of adults living near a steel factory. Am J Respir Crit Care Med. 1995 Dec;152(6 Pt 1):1932–1939. doi: 10.1164/ajrccm.152.6.8520758. [DOI] [PubMed] [Google Scholar]
- Fairley D. The relationship of daily mortality to suspended particulates in Santa Clara County, 1980-1986. Environ Health Perspect. 1990 Nov;89:159–168. doi: 10.1289/ehp.9089159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B., Mollo L., Giamello E. Free radical generation at the solid/liquid interface in iron containing minerals. Free Radic Res. 1995 Dec;23(6):593–614. doi: 10.3109/10715769509065280. [DOI] [PubMed] [Google Scholar]
- Ghio A. J., Kennedy T. P., Stonehuerner J. G., Crumbliss A. L., Hoidal J. R. DNA strand breaks following in vitro exposure to asbestos increase with surface-complexed [Fe3+]. Arch Biochem Biophys. 1994 May 15;311(1):13–18. doi: 10.1006/abbi.1994.1202. [DOI] [PubMed] [Google Scholar]
- Gilmour P. S., Beswick P. H., Brown D. M., Donaldson K. Detection of surface free radical activity of respirable industrial fibres using supercoiled phi X174 RF1 plasmid DNA. Carcinogenesis. 1995 Dec;16(12):2973–2979. doi: 10.1093/carcin/16.12.2973. [DOI] [PubMed] [Google Scholar]
- Janssen Y. M., Heintz N. H., Mossman B. T. Induction of c-fos and c-jun proto-oncogene expression by asbestos is ameliorated by N-acetyl-L-cysteine in mesothelial cells. Cancer Res. 1995 May 15;55(10):2085–2089. [PubMed] [Google Scholar]
- Kennedy T. P., Dodson R., Rao N. V., Ky H., Hopkins C., Baser M., Tolley E., Hoidal J. R. Dusts causing pneumoconiosis generate .OH and produce hemolysis by acting as Fenton catalysts. Arch Biochem Biophys. 1989 Feb 15;269(1):359–364. doi: 10.1016/0003-9861(89)90118-5. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Donaldson K., Brown D., MacNee W. The role of tumor necrosis factor in increased airspace epithelial permeability in acute lung inflammation. Am J Respir Cell Mol Biol. 1995 Aug;13(2):185–195. doi: 10.1165/ajrcmb.13.2.7626286. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Donaldson K., Rahman I., MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med. 1994 Jun;149(6):1518–1525. doi: 10.1164/ajrccm.149.6.8004308. [DOI] [PubMed] [Google Scholar]
- Lund L. G., Aust A. E. Iron-catalyzed reactions may be responsible for the biochemical and biological effects of asbestos. Biofactors. 1991 Jun;3(2):83–89. [PubMed] [Google Scholar]
- Lund L. G., Williams M. G., Dodson R. F., Aust A. E. Iron associated with asbestos bodies is responsible for the formation of single strand breaks in phi X174 RFI DNA. Occup Environ Med. 1994 Mar;51(3):200–204. doi: 10.1136/oem.51.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg K., Johansson U., Johansson A., Camner P. Phagolysosomal pH in alveolar macrophages. Environ Health Perspect. 1992 Jul;97:149–152. doi: 10.1289/ehp.9297149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Bates D. V., Raizenne M. E. Health effects of particulate air pollution: time for reassessment? Environ Health Perspect. 1995 May;103(5):472–480. doi: 10.1289/ehp.95103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Kanner R. E. Acute effects of PM10 pollution on pulmonary function of smokers with mild to moderate chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1336–1340. doi: 10.1164/ajrccm/147.6_Pt_1.1336. [DOI] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Schwartz J., Ransom M. R. Daily mortality and PM10 pollution in Utah Valley. Arch Environ Health. 1992 May-Jun;47(3):211–217. doi: 10.1080/00039896.1992.9938351. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Particulate air pollution and chronic respiratory disease. Environ Res. 1993 Jul;62(1):7–13. doi: 10.1006/enrs.1993.1083. [DOI] [PubMed] [Google Scholar]
- Schwartz J. What are people dying of on high air pollution days? Environ Res. 1994 Jan;64(1):26–35. doi: 10.1006/enrs.1994.1004. [DOI] [PubMed] [Google Scholar]
- Seaton A., MacNee W., Donaldson K., Godden D. Particulate air pollution and acute health effects. Lancet. 1995 Jan 21;345(8943):176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Simeonova P. P., Luster M. I. Iron and reactive oxygen species in the asbestos-induced tumor necrosis factor-alpha response from alveolar macrophages. Am J Respir Cell Mol Biol. 1995 Jun;12(6):676–683. doi: 10.1165/ajrcmb.12.6.7539275. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. L., Dalal N. S., Irr W., Castranova V. Generation of free radicals from freshly fractured silica dust. Potential role in acute silica-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1213–1219. doi: 10.1164/ajrccm/138.5.1213. [DOI] [PubMed] [Google Scholar]
- Walters S., Griffiths R. K., Ayres J. G. Temporal association between hospital admissions for asthma in Birmingham and ambient levels of sulphur dioxide and smoke. Thorax. 1994 Feb;49(2):133–140. doi: 10.1136/thx.49.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]