Abstract
Recent allostatic-interoceptive explanations using predictive coding models propose that efficient regulation of the body’s internal milieu is necessary to correctly anticipate environmental needs. We review this framework applied to understanding behavioral variant frontotemporal dementia (bvFTD) considering both allostatic overload and interoceptive deficits. First, we show how this framework could explain divergent deficits in bvFTD (cognitive impairments, behavioral maladjustment, brain atrophy, fronto-insular-temporal network atypicality, aberrant interoceptive electrophysiological activity, and autonomic disbalance). We develop a set of theory-driven predictions based on levels of allostatic interoception associated with bvFTD phenomenology and related physiopathological mechanisms. This approach may help further understand the disparate behavioral and physiopathological dysregulations of bvFTD, suggesting targeted interventions and strengthening clinical models of neurological and psychiatric disorders.
Keywords: Allostatic interoception, predictive coding, allostatic overload, interoception, frontotemporal dementia
The universe within: The body’s internal appraisal of environmental demands, and its implications for dementia
In recent years, predictive coding theories linking allostasis and interoception (see Glossary) have gained considerable attention in neuroscience[1–6]. Predictive coding refers to the assumption that the brain is actively and continuously anticipating and updating environmental (exteroception) and internal (interoception) models. One advantage of predictive coding is that it can be instantiated across several biological substrates and hierarchies (Figure 1A). Allostasis refers to a process of continuous adjustment of the organism milieu (e.g., blood pressure, temperature) to anticipate, and adapt to, environmental changes[7] and interoception to the sensing of the body signals[8]. Together, allostatic and interoceptive processes jointly contribute to meeting the upcoming internal and environmental demands through the updating of internal model predictions (e.g., increasing blood supply in a fight or flight situation, lowering heart rate when going to sleep, and reducing blood flow to skin capillaries to preserve core temperature, Figure 1B). In such models, sensory inputs are represented in low levels of the neural and computational hierarchy, while complex interpretations constitute higher levels. Based on statistical assumptions, each higher level predicts the activity in the lower level. The difference between the prediction (e.g., anticipation of feeling pain when getting vaccinated, thus tensing the arm muscles) and the actual sensory input (e.g., not even feeling the needle) generates a prediction error, which is sent back to the higher level in order to correct future predictions (e.g., relaxing arm muscles when receiving future vaccines). The predictive coding of allostasis and interoception may help understand dementia[9,10] and other neurological or psychiatric disorders[2,11].
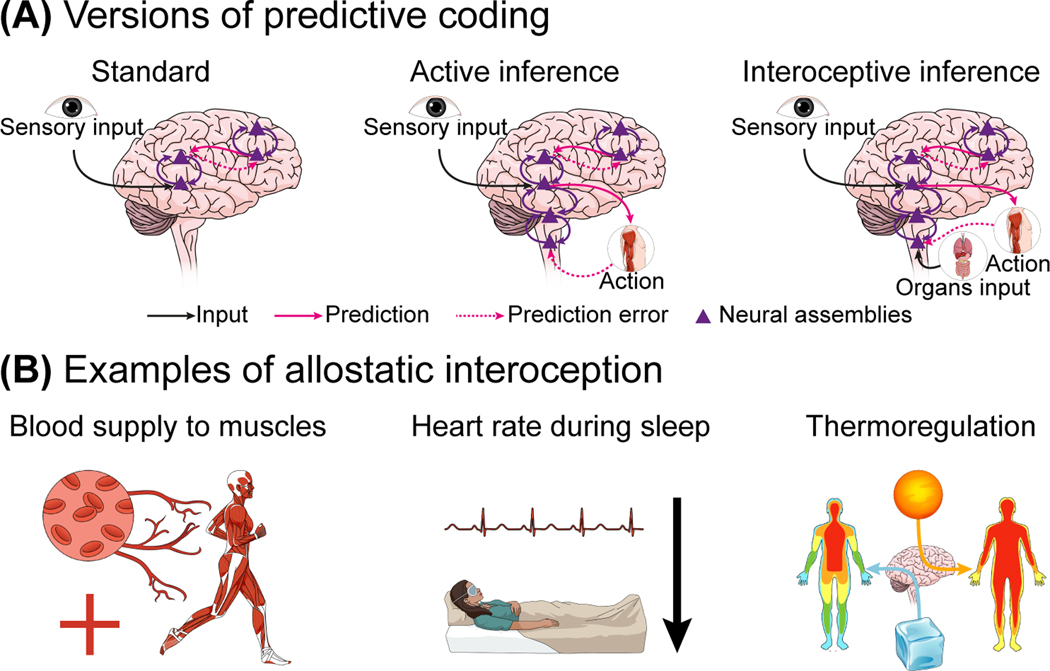
Figure 1.
Predictive coding and allostatic interoception. (A) Predictive coding is a general algorithm that can be instantiated among different biological substrates and their hierarchies [120]. It has been widely applied to cortical brain activity (i.e., standard predictive coding), brain activity and proprioception (i.e., active inference), and brain activity and interoception (i.e., interoceptive inference). Three versions of predictive coding are schematically illustrated. In the first case, the minimization of prediction error is given by the updating of predictions to accommodate unexpected sensory signals. In the second case, the minimization is through performing actions that confirm predictions about sensory inputs. Finally, in the third case, the minimization is reached by performing actions to confirm predictions of interoceptive signals. (B) Allostasis refers to a general mechanism of bodily regulation by adaptation and changes. Allostasis can be instantiated at different levels of the body substrate. Three examples of interoceptive allostasis are illustrated. Left: greater oxygen requirement in the muscles during a fight or flight situation, leading to increased blood supply to the relevant muscles and the mobilization of resources needed to perform that redistribution (e.g., increasing cardiac input); Middle: reduction of heart rate during sleep to align with the reduced metabolic needs. Right: vasoconstriction (i.e., narrowing of blood vessels) when the body is facing low temperatures to conserve core temperature by reducing the blood flow to the skin capillaries.
In this article, we review the evidence to extend these models to behavioral variant frontotemporal dementia (bvFTD), which is the most common clinical presentation of frontotemporal lobar degeneration. It is characterized by early changes in personality, social behavior, self-regulation, executive functions, motivation, and emotional regulation[12–14]. bvFTD presents a pattern of progressive neurodegeneration involving fronto-temporo-insular regions[15]. These neurocognitive early changes could be the consequence of malfunctions in the dynamics of the allostaticinteroceptive process[10]. Of note, alterations of allostasis and interoception have been observed across neurodegenerative conditions and in other variants of FTD. For instance, convergent evidence supports an allostatic overload in Alzheimer’s disease, and interoceptive deficits are observed in other neurodegenerative conditions such as Parkinson’s disease and multiple sclerosis (Box 1). Despite these related and transnosological alterations, we would argue that the proposed framework seems fairly specific to bvFTD: bvFTD’s multimodal compromise of autonomic-interoceptive pathways, the allostatic overload observed across different levels, and the abnormal responses to environmental demands distinctively fit with an allostatic-interoceptive overload account.
Box 1. Transnosological allostatic-interoceptive overload and bvFTD.
Both allostasis and interoception are dimensional processes that are compromised in many neurodegenerative conditions.
Allostatic load is closely related to Alzheimer’s disease pathophysiology, specifically regarding the bidirectional association between impaired insulin signaling and allostatic overload[120]. Lifestyle and social factors during life contribute to the allostatic load which lead to allostatic overload when these become chronically harmful. Such state may trigger pathophysiological changes in the brain, including oxidative stress and chronic inflammation, leading to insulin resistance and predisposing the organism to Alzheimer’s disease[120]. These alternative approaches to the traditional amyloid cascade hypothesis consider allostatic load as a crucial factor associated with the development and progression of Alzheimer’s disease[121].
bvFTD appears to be the only neurodegenerative disease with systematic (behavioral, peripherical, HEP, and neurofunctional) impairments of autonomic and interoceptive dimensions. Such interoceptive dysregulations, however, are also present in other neurodegenerative conditions[122]. Although not without conflicting results, individuals with Alzheimer’s disease tend to present impaired performance in interoceptive tasks[27] along with abnormal modulations of the HEP, in addition to deficits in interoceptive awareness and learning[123]. Moreover, cardiac interoception deficits are also present in Parkinson’s disease[124,125], that may help distinguish among postural instability/gait difficulty and tremor dominant variants[126]. Similarly, interoceptive deficits in multiple sclerosis[28] are linked to cardinal fatigue symptoms[127]. Thus, neurodegeneration spreading over core or bordering interoceptive hubs across neurodegenerative diseases can lead to multiple dimensional deficits.
Despite the presence of allostatic and interoceptive alterations in many neurodegenerative diseases, combined allostatic-interoceptive deficits are distinctively specific to bvFTD. The systematic affection of autonomic-interoceptive pathways combined with abnormal responses to environmental demands in bvFTD uniquely fits the allostatic-interoceptive overload account. Based on these antecedents, sui generis neurodegeneration-triggered deficits in bvFTD would influence imprecise interoceptive signals and lower precision, leading to an allostatic overload related with behavioral and neurocognitive manifestation
We first review the available evidence and propose the hypothesis that bvFTD may be characterized by an imbalance of an allostatic-interoceptive system, with manifestations across cerebral, cardiocerebral, peripheral and psychological dimensions. Then, we propose a set of theory-driven predictions in bvFTD, which are subsumed into a multidimensional framework integrating neurocognitive and physiological markers by multimodal assessments of allostatic interoceptive inference. Under such a framework, the cognitive and behavioral impairments of bvFTD are associated with (a) brain structural and connectivity deficits among critical allostatic-interoceptive brain hubs, (b) altered interoceptive electrophysiological activity, and (c) allostatic overload at biomarker levels. Such a multidimensional framework may help further understand the disparate behavioral and physiopathological dysregulations of bvFTD within a predictive coding account, suggesting targeted interventions and strengthening clinical models of neurological and psychiatric disorders.
Allostasis and allostatic overload
Allostatic-interoceptive predictive coding frameworks[1–6] can offer novel neurocognitive and physiological accounts of allostasis, integrating multimodal sources of information about the body state[16]. Importantly, such frameworks open new possibilities for assessing brain-body-environment synergetic interactions in health and disease[17], which can be integrated into a profile of allostaticinteroceptive manifestations at cerebral, cardiocerebral, peripheral and psychological levels (Figure 2).
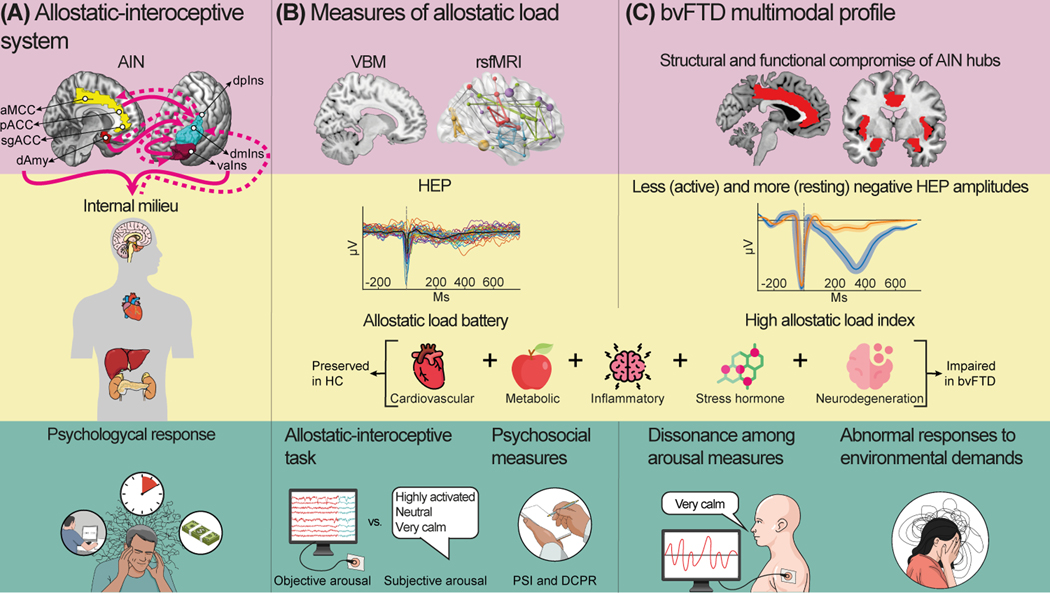
Figure 2.
Levels of the allostatic–interoceptive system and its potential characterization in behavioral variant frontotemporal dementia (bvFTD). Multimodal allostatic load measurement can be used at different levels to characterize bvFTD symptomatology and physiopathology. The allostatic–interoceptive system (A) relies on the allostatic– interoceptive network (AIN), whose main hubs include the anterior mid-cingulate cortex (aMCC), pregenual anterior cingulate cortex (pACC), subgenual anterior cingulate cortex (sgACC), dorsal amygdala (dAmy), agranular insula (vaIns), dorsal mid-insula (dmIns), and dorsal posterior insula (dpIns). Specifically, the limbic cortices send prediction signals (unbroken magenta lines) and receive prediction error signals (dashed magenta lines) from the internal milieu, evoking psychological responses. Accordingly, multimodal measures of allostatic load can be used (B). At the cerebral level, the structure of the AIN hubs can be assessed by voxelbased morphometry (VBM) technique and its functional connectivity by resting-state functional magnetic resonance imaging (rsfMRI) technique. The cardiocerebral level can be evaluated by heartbeat-evoked potential (HEP). Moreover, the peripheral level can be assessed by cardiovascular, metabolic, inflammatory, stress hormone, and neurodegenerative biomarkers, constituting an allostatic load battery. Finally, the psychological level can be evaluated by the allostatic–interoceptive task and psychosocial measures such as the psychosocial index (PSI) and the diagnostic criteria for psychosomatic research (DCPR). Some multimodal impairments are expected in bvFTD patients (C). At the cerebral level, the AIN is selectively compromised in bvFTD, along with early structural and functional compromise of core AIN hubs. At the cardiocerebral level, less negative HEP during active tasks and more negative resting-state HEP (rsHEP) in resting state and noncardiac monitoring tasks are also expected. At the peripheral level, bvFTD may present altered biomarker parameters, leading to a high allostatic load index compared with healthy controls (HC). At the psychological level, bvFTD patients will present a dissonance among the objective and subjective arousal measures and abnormal responses to environmental demands. Figures in panels (B) and (C) are illustrational examples and do not represent actual data.
The allostatic-interoceptive system
Neuroimaging techniques have identified a domain-general allostatic-interoceptive network (AIN) that integrates visceromotor and interoceptive processes into a multimodal network (Figure 2A). This network connects a wide range of cognitive domains such as memory, executive function, emotion processing and cognitive control, with allostatic load[4]. The AIN involves a large-scale brain network, the ‘neural backbone’ of the brain’s coordinated neural activity. This network is composed of specific hubs of the salience network (bilateral ventral and dorsal anterior insula, anterior cingulate cortex, ventral striatum, thalamus, central nucleus of the amygdala, hypothalamus, and brainstem) and default mode network (bilateral angular gyrus, precuneus, hippocampus, and medial prefrontal cortices).
The allostatic-interoceptive system, which is neuroanatomically defined by the AIN[4,18], seems to maintain the neurocognitive balance based on interoceptive information. Specifically, the limbic cortices project to the hypothalamus and brainstem nuclei that monitor neuroendocrine, autonomic and immune systems. These can be interpreted as anatomical paths for prediction signals from limbic cortices. Also, under a predictive coding interpretation, the hypothalamus and brainstem nuclei send signals that carry predictions of sensory outcomes of visceromotor changes to the primary interoceptive cortices. In parallel, internal sensory inputs from the body are projected through the vagus nerve to the primary interoceptive cortices, where both prediction signals and sensory inputs are contrasted resulting in an interoceptive prediction error[4] (Figure 2A).
As such, the AIN regulates cardiocerebral and peripheral activity that together have an impact on psychological and cognitive responses to the environment. For example, adults with a history of infant regulatory problems present selectively aberrant default mode and salience networks (AIN sub-networks) assessed with resting-state functional magnetic resonance imaging (rsfMRI) connectivity[19]. The AIN and internal organs of the body such as the heart and gut are integrated into a multimodal structure of interactions, based on predictive coding principles at several hierarchical levels. This complex structure of interactions is discussed in more detail in later sections.
Measurements of allostasis
Allostatic load can be understood as a cost of sustaining allostasis[3]. The saturation of the allostatic load due to the cumulative burden of chronic stress and life events is referred to as allostatic overload. In this state, the organism is exposed to repeated environmental demands evoking chronic neural and neuroendocrine responses[7]. Behavioral examples of allostatic overload include overreacting and underreacting to environmental stressors. Allostatic overload can be assessed using various measures at different levels of description (Figure 2B). These include (a) cerebral measurements employing brain structure and connectivity metrics[4]; (b) cardiocerebral estimations of the brain’s responses to sensing the heart[20]; (c) peripheral blood biomarkers[21,22]; and (d) psychological measures testing the relations between subjective and objective arousal[4], and clinical psychosocial assessments evaluating dysregulated responses to environmental demands[23,24].
At the cerebral level (Figure 2B), different measures of anatomical/structural and functional connectivity can quantify the degree of interaction between the nodes that form the AIN. For instance, the integrity of the AIN and its main hubs can be measured using methods such as voxelbased morphometry (VBM), diffusion tensor imaging (DTI) and rsfMRI[4].
The cardiocerebral level (Figure 2B) could be partially indexed by the heart-evoked potential (HEP), a marker of interoceptive and brain-body regulation processes characteristic of brain responses activated by visceral signals and regulated by the ability to feel the body[20]. Neuroimaging and electroencephalography source localization studies[25,26] associate the HEP with brain structures supporting allostatic and interoceptive processes. In addition to traditional active heartbeat detection tasks, where participants must press a key each time they feel a heartbeat[27–35], novel evidence has shown that HEP changes (commonly measured with the amplitude difference, but also with latency and power[36,37]) during non-cardiac monitoring tasks, as well as resting-state increased amplitude. Such changes have been associated with a hypervigilance to interoceptive signals, and linked to stress-related allostatic overload in both hypertensive patients and healthy controls[38]. Similarly, external demanding somatosensorial stimuli (i.e., electrical pulse) triggers increased HEP modulation[26]. In addition, the HEP involves source generators in both interoceptive and allostatic regions (e.g., the insula, anterior cingulate cortex, and amygdala), as well as associations with volume and cortical thickness of the right amygdala, bilateral insula, and bilateral anterior cingulate (key AIN regions)[10]. Similarly, a selective positive association between HEP and AIN has been observed in bvFTD, compared to other relevant resting-state networks[10]. The HEP has been evaluated among diverse populations, including people with neurodegenerative diseases[27,28,39], multiple sclerosis[29], generalized anxiety disorder[40], borderline personality disorder[41], as well as healthy participants[42]. Critically, HEP deficits are observed in cardiovascular diseases such as heart transplant and hypertension even after controlling for cardiovascular peripheral markers[44–46], including heart rate variability, heart rate fragmentation, cardiac artifact, and respiratory sinus arrhythmia. In particular, the allostatic overload canonically involves a cardiovascular response directly related to cardiovascular disease[43–45]. Given the links between allostasis, cardiovascular responses, and exacerbated HEP, the latter can be partly understood as a marker of allostatic interoceptive overload in terms of predictive coding. In this framework, the HEP can be modulated at both bottom up (i.e., triggered by cardiovascular disbalance and related error processing) and topdown pathways (i.e., impaired interoception triggering aberrant predictive inferences).
With regard to the peripheral level, allostatic overload can be triggered by several health risks, including reduced physical activity, poor sleep quality, unhealthy diet, obesity, alcohol intake, and smoking habits, among others[7]. Multiple biomarkers target physiological imbalance associated with altered allostatic load (Figure 2B, for other examples see[46–49]). This minimally invasive approach is useful in characterizing different diseases associated with allostatic overload (e.g., diabetes, musculoskeletal disorders, and cancer[7]). Due to the constant interaction of blood with all organ systems, these biomarkers have been primarily studied in blood samples[21,22]. Importantly, multiple biomarker signatures of allostatic load can be assessed through an allostatic load battery. Specifically, multisystemic biomarkers associated with allostatic overload include: (a) cardiovascular[50,51]: arterial tension and resting pulse rate; (b) metabolic[51,52]: body mass index, waist-hip ratio, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glycated hemoglobin, fasting glucose, creatinine, and albumin; (c) inflammatory[51,53–60]: tumor necrosis factor- α, tumor growth factor- β, C-reactive protein, interleukin-2, interleukin-6. (d) stress hormone[61]: cortisol; and (e) neurodegenerative parameters[54,62–67]: neurofilament light chain and progranulin. A weighted allostatic load battery based on multimodal cut-off scores being employed can lead to an integrative biomarkers score.
Finally, at the psychological level (Figure 2B), novel interoceptive-neurocognitive measures employing behavioral and electrophysiological measurements can evaluate the allostaticinteroceptive function. This is done by testing the correspondence between the subjective arousal experience (self-report) and objective sympathetic arousal (electrodermal activity) when viewing emotionally evocative images, aiming at evaluating the underlying visceromotor control and related psychological functions. The dissonance between the objective and subjective arousal measures is negatively correlated with the functional connectivity intensity of the AIN[4], and arguably positively correlated with the allostatic load. Additionally, allostatic overload is observed in a variety of mental health disorders characterized by abnormal behavioral responses to stress and environmental demands, including mood and anxiety disorders[68], affective and somatic depressive symptoms among older adults[69], post-traumatic stress disorder[46], and adulthood depression following childhood physical abuse[70]. Such allostatic overload in mental health disorders can be further assessed by psychosocial measures evaluating dysregulated responses to environmental demands, such as the diagnostic criteria for psychosomatic research[24] and the psychosocial index[23].
These cerebral, cardiocerebral, peripheral and psychological levels do not describe the same mechanisms nor quantify the same dimensions. So far, studies employing such group criteria and biomarkers are scarce but critical to provide a more holistic approach to understand allostatic overload.
Evidence of allostatic-interoceptive overload in bvFTD
Various symptoms and neurocognitive markers related to allostatic-interoceptive overload are present in bvFTD, suggesting that these processes and measurements could be integrated into a multimodal dynamic of allostatic-interoceptive processes.
At a neuroanatomical level, brain hubs mediating interoception and allostasis such as the anterior insula, anterior cingulate cortex, and amygdala are structurally and functionally compromised early in bvFTD[71–73] (Figure 2C). Usually, patients with bvFTD show aberrant connectivity[74–79] of the salience[80–82] and the default mode networks[78], which supports the involvement of the AIN[4,74]. The salience network has been related to the ongoing tracking of bodily states[81], while specific hubs of the default mode network are crucial for allostatic-interoceptive processes[4,83]. Moreover, autonomic nervous systems regulation relies on the appropriate function of insular networks, which are often compromised in bvFTD[84]. Thus, neuroanatomical evidence suggests a direct impairment of anatomical and functional AIN connectivity in bvFTD. Importantly, brain regions other than those involved in allostatic-interoceptive processes are also impaired in this disease (e.g., paracingulate gyrus)[73]. Nevertheless, allostatic-interoceptive brain hubs such as the anterior insula, anterior cingulate cortex, and amygdala seem critical for multiple manifestations in bvFTD. Specifically, what is observed is an increased impairment of AIN hubs in comparison to other brain regions. The AIN, in turn, is associated with anatomical connectivity supporting predictions and prediction errors from key nodes of the network[4]. Recent evidence has shown selective functional impairment of the AIN in bvFTD[10].
At the cardiocerebral level, atypical HEP is observed during active tasks[27,28,39] and resting in bvFTD[10] (Figure 2C). Key nodes of allostatic-interoceptive processes where the HEP is generated, such as the insula and amygdala[25,26,85,86], are structurally and functionally affected in bvFTD [27]. Moreover, contrary to healthy participants, bvFTD patients do not show increased HEP modulation during negative emotion recognition, suggesting a desynchronization and decoupling between interoceptive and emotional processing[39]. Thus, since emotional processing partly relies on the perception and integration of visceral information, the efficient coordination of those multimodal processes could prove central for successful allostasis[4,6]. In terms of predictive coding, these impairments in bvFTD may generate, or be generated by, an increase of error between predictions and interoceptive signals impacting the overall interoception of heart signals.
At the peripheral level, bvFTD patients exhibit autonomic nervous system dysregulations and imbalanced autonomic load[87], related to exacerbated behavioral responses to environmental stimuli[72,88,89], abnormal emotional reactivity and preparatory physiological response to emotional stimuli[90,91], and interoceptive dysregulations associated with socioemotional processes[27,39,92]. An additional set of evidence suggests that bvFTD patients present abnormal responses in all the forementioned measures of allostatic load, including cardiovascular, metabolic, inflammatory, stress hormone, and neurodegenerative biomarkers (Figure 2C). First, cardiovascular risk factors (e.g., elevated body mass index) could trigger a biological cascade resulting in vascular damage increasing dementia symptomatology[50]. Second, bvFTD patients show metabolic abnormalities associated with malnutrition[52]. Third, changes in inflammatory peripheral biomarkers have been reported in different frontotemporal dementia subtypes, suggesting that inflammatory factors play an important role in the pathogenesis of the disease[53,54]. Specifically, high levels of tumor necrosis factor- α and tumor growth factor- β[55–57], but low levels of interleukin-12, have been found in patients with bvFTD[58]. Also, increased interleukin-6 and C-reactive protein serum levels have been associated with cognitive decline in older adults who present metabolic syndrome[59,60]. Fourth, bvFTD patients show low cortisol levels, which would serve as a compensatory mechanism to regulate the top-down cognitive control deficits[61]. Fifth, neurodegenerative parameters have been also found dysregulated in bvFTD[54]. Neurofilament light chain is a component of the neuronal cytoskeleton[62] and its concentration levels have been shown to increase in serum and plasma of frontotemporal dementia patients, predicting disease severity and brain volume loss[63–66]. Moreover, this classical neurodegeneration marker has also been associated with body mass index and allostatic load, along with risk indicators including cardiovascular, metabolic, and inflammatory biomarkers[93]. Additionally, lower plasma levels of progranulin, a pleiotropic growth factor, have been found in FTD patients in comparison with healthy controls[67]. Importantly, all those parameters have been related to the dynamics of allostatic overload[7,93] and therefore, may play a relevant role in interoceptive changes[94].
At a psychological level, bvFTD patients commonly show cognitive deficits, including in executive function and emotion processing[12,14,91,95–97]. These impact their day-to-day functionality and behavior by increasing the misadjusted responses to environmental demands (Figure 2C)[95,96,98,99], such as underreacting to evocative emotional stimuli[90,91,97] and presenting aberrant responses to social situations[100]. Convergently, many of these processes seem to be regulated by allostatic-interoceptive mechanisms[4,92], leading to allostatic overload[101–106]. For instance, bvFTD patients exhibit impaired performance in the heartbeat detection task (i.e., less accuracy in key-pressing following the sensing of a heartbeat) followed by negative facial emotion recognition (i.e., anger, disgust, sadness, and fear). This suggests altered dynamics of interoceptive predictions and reduction in prediction errors, which may contribute to atypical emotion recognition [39]. Moreover, allostatic overload is related to abnormal stress responses, cognitive dysfunction, and behavioral disturbances, which typify the symptomatology and physiopathology of bvFTD[12]. In particular, prevalent heart rate and autonomic changes in bvFTD are associated with changes in energy expenditure[107].
To summarize, specific bvFTD symptomatology and related markers have been described at multiple levels. Beyond preliminary evidence[10], we will propose in the next section that these markers may suggest an integral allostatic-interoceptive overload in terms of predictive coding. Such approach may better characterize bvFTD and bring a more integrated vision of those deficits.
Towards an allostatic-interoceptive predictive coding model for bvFTD
The allostatic-interoceptive predictive coding model proposes that the prediction of interoceptive signals and the minimization of their prediction errors through visceromotor activity is crucial for successful allostasis[8,9,11]. This inference requires a cascade of top-down interoceptive and visceromotor predictions that are in constant evaluation to account for bottom-up interoceptive and proprioceptive prediction errors[108]. In the AIN, visceromotor predictions from agranular regions project to subcortical regions to engage homeostatic reflexes (e.g., activate sweating reflexes to cool down the body in hot environments). As such, predictions become homeostatic set-points, or attractors that guide behavioral allostatic mechanisms through interoceptive prediction errors[11] (i.e., the corrections between expectations and sensory evidence). This requires a multilevel structure described in terms of anatomical neural paths and functional neural activity. This hierarchical organization is depicted in Figure 3A (Key Figure). Top regions such as the prefrontal cortex and cingulate cortex form a layer of interactions that generate predictions of lower levels. These predictions project to relay regions such as the insula, thalamus and hypothalamus. These regions integrate top predictions and peripheral errors from organs and muscles. Pathways such as hypothalamic–pituitary–thyroid axis or hypothalamic–neurohypophysis system, also play a key role in interoceptive communication[94]. Therefore, allostatic regulation demands the integration of neural and non-neural signals through a complex network of interactions, operating at different levels and timescales.
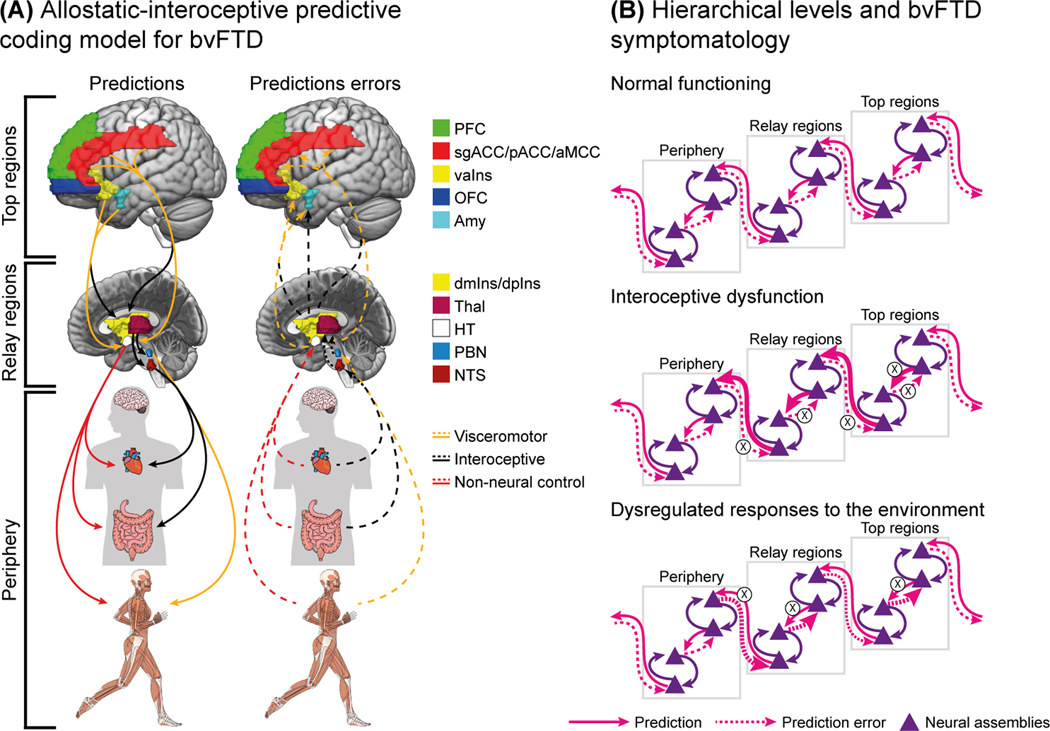
Figure 3.
The proposed model, summarized in the figure, expands previous allostatic–interoceptive models to bvFTD manifestations. (A) Prefrontal cortex (PFC), anterior mid-cingulate cortex (aMCC), pregenual cingulate cortex (pACC), subgenual anterior cingulate cortex (sgACC), agranular insula (vaIns), orbitofrontal cortex (OFC), and dorsal amygdala (Amy) are placed on top of the hierarchy. These regions are thought to modulate their activity by within-level interactions (arrows not shown) and generate predictions about the activity of other systems. In particular, they send visceromotor (yellow lines) and interoceptive predictions (dark lines) to the relay regions: the dorsal mid-insula (dmIns) and dorsal posterior insula (dpIns), thalamus (Thal), hypothalamus (HT). These relay regions integrate predictions from top regions and prediction errors from peripheral regions to generate their corresponding predictions and feedback. In this relay level, the parabrachial nucleus (PBN) and nucleus of the solitary tract (NTS) also receive predictions from dpIns, THAL, and HT. The types of predictions and errors correspond to visceromotor, neural interoceptive, and non-neural interoceptive communication. Finally, the periphery, formed by organs (heart, gut) but also autonomic, neuroendocrine, and immune systems, receives signals and communicates errors to relay regions (red lines), closing the loops through action. (B) A normal state of functioning of the organism is characterized by optimal matching among top-down predictions and sensory inputs, leading to an error minimization. In bvFTD, a predictive coding proposes a sui generis interoceptive deficits and elevated peripheral and immunological stress, leading to imprecise predictions overcharged by a feedback loop of inaccurate prediction errors, impairing error minimization. This would lead to an overconsumption of resources by the top regions to accommodate top-down predictions and sensory inputs, therefore impacting again the interoceptive system functionality. Similarly, the dysregulated responses to environmental stressors, one of the core bvFTD symptomatology, could be understood as a consequence of imprecise top-down predictions about the body’s peripheral level of energy predisposed to perform actions, therefore overreacting to seemingly inoffensive environmental stressors and underreacting to relevant ones. As actions would be inadequate, prediction errors would overcharge.
In a well-functioning system, interoceptive prediction errors can be minimized by modifying predictions and/or by the action of autonomic reflexes that make interoceptive physiological states fit predictions. Examples of the first case include the re-evaluation of predictions about the temperature of a given surface (e.g., sensory signal correcting the expectation of a hot surface). This process is associated with perception in predictive coding literature. An example of the second case is muscular activity that generates action. In this scenario, to put it different, an action generates changes to match predictions (e.g., if the system predicts a hot surface, the hand moves to avoid getting burned, even if the surface is not hot). These predictions can generate visceromotor activity only if the error signals are minimal (high precision), i.e., the system does not require to allocate resources to the reevaluation of predictions. Otherwise, the action is postponed in favor of the revision of predictions.
In other words, when predictive errors are minimal, the inferences match the causes of sensory event and therefore, plans and actions are congruent within the system. These predictions and errors come from multimodal sources. In our conceptual model, these source signals are integrated not only in the insular cortex, as commonly suggested, but also in the hypothalamus, medial nucleus of the solitary tract and the parabrachial nucleus. Collectively, they form a key relay layer between the central nervous system and peripheral systems (Figure 3A). These areas transduce both neural and nonneural interoceptive signals to generate prediction errors that will be integrated into further top layers. Top regions, such as the prefrontal and cingulate cortex, assimilate prediction errors from several modalities, including interoception. At such level, the prediction may fit both current multimodal signals as well as anticipating how these signals will change under certain actions (e.g., blood pressure). Therefore, reflexes (targeting homeostasis) and allostatic behaviors (goal-directed) interplay with each other through the constant evaluation of the precision values related to expected behaviors. A common example could be hypoglycemia, the excessive drop off of glucose in the blood. This condition will generate low-level predictions that make the autonomic reflexes store glucose through interoceptive prediction[105]. In turn, proprioceptive predictions could reduce the precision of low-level interception by way of engaging allostatic behavior and preparing the body for an eventual meal.
In the context of bvFTD, the system of predictions, errors and precision values can be understood at different explanatory levels (Box 2). On the one hand, sui generis neurodegeneration-triggered interoceptive deficits, as well as peripheral and immunological stress, may lead to imprecise interoceptive signals and lower precision. This effect overcharges higher levels in order to accommodate predictions through interoceptive priors and ensure homeostasis. In turn, the higher regions may generate inaccurate predictions, lowering, even more, the values of precision, and increasing the interoceptive system dysfunction. Both, this dysfunction and progressive neurodegeneration may reinforce the selective compromise of structural and functional organization of the AIN, as recently observed in bvFTD patients[10]. Following this model, biomarkers related to peripheral and immunological stress included in the allostatic load battery may track the dysfunction risk at this level. As discussed above, bvFTD population presents such biomarkers with abnormal values compared with healthy controls.
Box 2. Potential explanatory levels of allostatic-interoception in bvFTD.
A predictive coding approach of allostatic-interoception in bvFTD can be understood at different explanatory levels. The first level suggests that neurodegeneration triggers damage to the allostatic-interoceptive system and, as a consequence, such disturbances are related to different neurocognitive symptoms in bvFTD. The existing evidence, reviewed in the article, directly supports this claim.
The second level suggests a circular interaction between sui generis neurodegenerative processes and malfunctions in the dynamics of the allostatic-interoceptive process. Thus, early impairments triggered by neurodegeneration interact across the lifespan[120,121] with environmental demands and dysregulated behaviors, accentuating the allostatic overload and worsening the neurocognitive process. Although more speculative, this proposal is partially supported by the current evidence. For instance, neurodegeneration can be understood as a lifespan process involving both intrinsic neurodegeneration and the burden of life-long stressors[128]. Moreover, predictive coding can be understood as an increasing disbalance between an internal model of the intero-exteroceptive process and the adaptation to the environment[104]. The initial neurodegenerative changes will disrupt the allostatic-interoceptive overload, creating an inadequate response to environmental demands, resulting in increased chronic stress responses. The proposed framework detailed in Figure 3 describe these levels. Longitudinal assessment and future experiments of these complex and hypothetical interactions are required to further test this hypothesis.
Finally, a third level presumes a direct causal link between an initial malfunction of predictive coding of allostatic-interoception processes and a concomitant pathophysiology of neurodegenerative mechanisms. For instance, chronic stress plays an important role in immune regulation[129,130] that in turn impacts FTD etiology[52,53,131,132] and other neurodegenerative conditions[129]. Allostatic overload may be an important factor causing neurodegenerative disease and contributing to TDP-43 aggregation[133] associated with frontotemporal dementia[134]. Chronic stress and its associated allostatic overload influences lipoproteins, fast insulin, and glucose; and predisposes to cardiovascular disease, all associated with neurodegeneration. Also, cellular stress response and brain inflammation mechanisms in neurodegeneration are associated with allostatic load[120]. This third explanatory level, however, has not been explored and studied in detail in FTD, or in other neurodegenerative conditions, beyond this emerging evidence
On the other hand, psychological and environmental stressors may generate an adjustment of the system’s beliefs about its own capacity to regulate bodily activity. These new predictions are unable to match interoceptive prediction errors, reducing precision and generating a further loop of dysfunction. In turn, this forces the system to make prediction errors stronger in order to adjust predictions. Over time, this condition may generate overreactions to the seemingly inoffensive exterior and interoceptive stressors. Thus, in bvFTD this dysfunction would lead to a deficient inhibition and hypervigilance of interoceptive signals instantiated by stress-related allostatic overload, partially indexed by a reduced HEP during active tasks[27,39,109] and exacerbated resting-state HEP amplitudes[10].
In short, we propose that the core bvFTD psychological symptomatology are related to cerebral (AIN functional connectivity and brain volume), cardiocerebral (HEP), and peripheral (biomarkers) measures of allostatic overload.
Implications of the model
The framing of bvFTD as a condition typified by allostatic-interoceptive imbalance under the predictive coding interpretation, leads to a set of relevant implications (Figure 3B). As described in previous sections, the symptomatology of bvFTD and potential mechanisms involved are related to dysfunctions at several levels of processing. This implies that the end point described by bvFTD symptoms can be related to different cascades of mismatch error predictions generated either from top-down or bottom-up interactions. The model suggests that bvFTD corresponds to the dynamical dysfunction of these two interactive loops. Another consequence relates to the importance of nonneural interactions and their interpretation as predictions and prediction errors within the system, although at different temporal scales. A key implication of this reasoning is the role of hypothalamicperipheral axis in regulating non-neural interactions. Consequently, the model predicts a correlation between the dysfunction of this axis and the increase of peripheral markers of allostatic overload.
Another group of implications relate to early characterization and intervention. If our model proves to be useful, preventive diagnosis and early characterization is possible through the use of the battery of tests formerly outlined. This battery will generate a physiological profile quantifying the multimodal risk of bvFTD symptomatology during prodromal stages[110]. This model can be also tested on longitudinal studies. If cerebral, cardiovascular and peripheral markers prove to predict future symptomatology, this alone opens the door to early interventions. For instance, if interoceptive deficits at behavioral and cerebral levels are observed at early disease stages, intervention approaches based on meditation and body awareness impacting interoceptive process[111,112] may be helpful.
Although these comments are speculative, they offer concrete lines of research.
Concluding remarks and future perspectives
This work proposes that allostatic-interoceptive overload in bvFTD may be an underlying phenomenon across hallmark behavioral dysregulations and misadjusted physiopathological processes. This framework may offer a roadmap for future work (see Outstanding Questions).
Outstanding questions.
Can the allostatic–interoceptive framework of predictive coding bring a more integrative and multidimensional approach to psychiatric and neurological manifestations in bvFTD? How can the multimodal signatures of allostatic–interoceptive overload be simultaneously assessed at cerebral, cardiocerebral, peripheral, and psychological levels in this neurodegenerative disease? Whataretherequired steps to develop a more mechanistic and theoretically informed model of current available evidence on allostatic–interoceptive disbalance in bvFTD? Can dynamical system modeling provide a stronger approach? Whatarethemosteffectivetechniques to develop interventions aimed to modulate dysregulated allostaticinteroceptive overload in bvFTD?
bvFTD patients have been characterized by a variety of psychiatric symptomatology, such as personality and behavioral changes, often making timely diagnosis and treatment difficult[113].
Importantly, allostatic and interoceptive impairments have been more comprehensively assessed in psychiatric conditions[11,114] than in neurological conditions. Linking allostatic overload and interoceptive maladjustment with global behavioral impairments will help improve the diagnostic accuracy and diagnostic dimensionality between psychiatric and neurodegenerative conditions[98], offering novel and convergent biomarkers and clinical insights[7]. Despite the presence of disparate allostatic and interoceptive deficits across neurodegenerative conditions, these impairments seem to be selectively compromised in bvFTD, suggesting an allostatic-interoceptive overload (Box 1). The predictive coding framework of allostatic-interoceptive overload in bvFTD may also offer a transnosological account[1] towards the development of integrative clinical models across neurology and psychiatry.
The evidence reviewed here supports the position for an integrated framework that connects multiple disparate neurocognitive manifestations in bvFTD. This explanatory level does not require nor sustain single causal or mechanistic explanations of neurodegeneration, but proposes an initial sui generis neurodegenerative effect at the core of the allostatic-interoceptive overload. Other speculative, and controversial explanations that link bidirectional interactions between allostatic overload and neurodegeneration, or physiopathological causation of neurodegeneration, will require further research (Box 2).
Current evidence in bvFTD for the proposed framework is still mainly correlational, and the predictive coding approaches are not without limitation, especially when these are not instantiated by domain-specific evidence (Box 3). Independently of the mechanistic implementations, the predictive coding metaphor is useful in bringing pragmatic simulations and simpler explanations that may complement more developed mechanistic explanations (i.e., dynamical system models). In future work, the predictive coding framework of allostatic-interoceptive overload, neurocognitive markers of allostatic overload and physiological measures could be integrated into a broader multimodal dynamical structure of multilayer networks[115,116]. These multilevel layers and their corresponding measurements[16] would offer a global allostatic-interoceptive overload profile. Such assessments may bring a cohesive understanding of bvFTD and motivate a novel empirical program based on an allostatic-interoceptive dysregulation.
Box 3. Towards robust approaches to allostatic-interoception.
Finding empirical evidence that supports the main assumptions of allostatic-interoceptive overload and predictive coding models remains challenging[104,135]. Even authors supporting predictive coding are cautious about the current biological implementations[104,107]. Most evidence is correlational, and ascribes predictive mechanisms properties to the modulation of neural activity in key regions, assuming that afferent and efferent anatomical connectivity are predictions and prediction errors, respectively, by the only fact of taking one or the other direction. The role of the anterior insular cortex, for example, is assumed to be central mainly because it is an anatomical and functional hub receiving both top-down as well as bottom-up neural signals and correlating with interoceptive activity[107]. As such, it is hypothesized that the insula works as a comparator and source of anticipatory visceromotor control. Confirmatory evidence, however, does not go beyond this preliminary account[135]. Recent advanced laminar fMRI methods may bring more light regarding direct evidence of predictive coding models. Similarly, specific evidence pointing at each instantiation of the model predictions are required, including directionality, inference, errors, sui generis deficits and subsequent impairments, as well as disease stage characterization. Such domain-specific evidence is critically required to build a predictive model that surpasses basic general principles, abstract conceptualization and preliminary and indirect evidence.
Multiple divergent deficits in bvFTD patients, such as cognitive impairments[27] behavioral maladjustment[12], atrophy and impaired connectivity among fronto-insular-temporal hubs[27,39], aberrant electrophysiological activity[27,28,39], and autonomic nervous system disbalance[87] can be better explained within a predictive coding model of allostatic-interoceptive load. Within this framework, the interoceptive and exteroceptive stimuli are continuously parameterized to evaluate priorities and predict environmental changes to instantiate organism needs before incurring in errors[117,118]. These behavioral and physiological adjustments to environmental demands depend on the convergence of socioemotional stimuli with bodily signals[39], self-protection[119], and the assessment of situational context[72,88], all impaired in bvFTD patients. By integrating multimodal signatures (cerebral, cardiocerebral, peripheral and psychological markers) in a theoretical account, this framework may offer novel and relevant insights into the behavioral and physiological substrates of bvFTD.
Acknowledgments
AI is partially supported by grants from Takeda CW2680521; CONICET; ANID/FONDECYT Regular (1210195 and 1210176); FONCYT-PICT 2017–1820; ANID/FONDAP/15150012; Sistema General de Regalías (BPIN2018000100059), Universidad del Valle (CI 5316); Programa Interdisciplinario de Investigación Experimental en Comunicación y Cognición (PIIECC), Facultad de Humanidades, USACH; Alzheimer’s Association GBHI ALZ UK-20–639295; and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by National Institutes of Health, National Institutes of Aging (R01 AG057234), Alzheimer’s Association (SG-20–725707), Rainwater Charitable foundation - Tau Consortium, and Global Brain Health Institute)]. CDA is partially supported by 2018-AARG-591107, ANID/FONDEF ID20I10152, ANID/FONDECYT 1210622 and ANID/PIA/ANILLOS ACT210096.
CMS is supported by the FNRS MIS project “Evidencing sentience in low arousal states by probing brain-body interactions” (2020) and Human Brain Project task, Brain Inspired Consciousness (BRICON). OP is supported by a National Health and Medical Research Council of Australia Leadership Fellowship (GNT2008020). The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions.
Glossary
- Allostasis:
The continuous process of energy balance to anticipate and perform efficient and adaptive responses to external stressors, primarily instantiated by the brain’s predictions about the body’s needs to prepare the organism to attend to those demands before they arise. For example, before lifting a heavy object, the brain predicts the energy needed to perform that action, thus increases the blood flow to the relevant muscles to efficiently lift the target object
- Allostatic load:
Level of progressive attrition on the organism resulting from the continuous exposure to stress-inducing environmental demands evoking energy relocation processes to face those demands and balance energy levels. For instance, high allostatic load levels are commonly reported in individuals living in unsafe neighborhoods, as the organism must be constantly relocating energy levels to run from potential dangers, plan alternative routes to avoid risk exposure, and not getting quality sleep due to being vigilant at night about potential threats
- Allostatic overload:
Exacerbated allostatic load generated by poorly regulated or chronic allostatic responses, resulting in abnormal physiologic states (e.g., dysregulation of the hypothalamic– pituitary–adrenal axis) and predisposing the organism to diseases (e.g., cardiovascular diseases, diabetes, cancer, mood and anxiety disorders
- Exteroception:
Sensing or perceiving of external stimuli and environmental changes
- Interoception:
Sensing or perceiving of bodily signals. It mainly refers to the feeling of body organs’ processes, such as digestion, breath regulation, and heart rate, among others
- Interoceptive inference:
Instantiation of predictive coding algorithms that uses visceromotor actions to fit interoceptive predictions. In this case, both actions and interoception play a role in minimizing the same prediction error. Examples include how interoceptive predictions modulate physiological homeostasis through autonomic reflexes (e.g., blood pressure, glycaemia
- Precision:
The precision, or estimation of the uncertainty, corresponds to the level of confidence of predictions (i.e., signals from higher regions in the hierarchy) and prediction errors (i.e., signals from lower regions in the hierarchy). Computationally, this precision corresponds to the inverse variance, or reliability, of a signal
- Prediction:
In prediction coding literature, the prediction is understood as the activity of higher levels and their signaling to lower levels in the computational hierarchy
- Prediction error:
Under the predictive coding model, the prediction error is the mismatch between predicted and actual sensory activity. In general, it is quantified as the activity and the eventual signaling from lower levels to higher ones
Footnotes
Declaration of interests
The authors declare no competing interests.
References
- 1.Petzschner FH et al. (2021) Computational Models of Interoception and Body Regulation. Trends Neurosci. 44, 63–76. 10.1016/j.tins.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nord CL and Garfinkel SN (2022) Interoceptive pathways to understand and treat mental health conditions. Trends. Cogn. Sci. 10.1016/j.tics.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Quigley KS et al. (2021) Functions of Interoception: From Energy Regulation to Experience of the Self. Trends Neurosci. 44, 29–38. 10.1016/j.tins.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleckner IR et al. (2017) Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 1. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulkin J. and Sterling P. (2019) Allostasis: A Brain-Centered, Predictive Mode of Physiological Regulation. Trends Neurosci. 42, 740–752. 10.1016/j.tins.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 6.Sterling P. (2014) Homeostasis vs allostasis implications for brain function and mental disorders. JAMA Psychiatry 71, 1192–1193. 10.1001/jamapsychiatry.2014.1043 [DOI] [PubMed] [Google Scholar]
- 7.Guidi J. et al. (2020) Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 90, 11–27. 10.1159/000510696 [DOI] [PubMed] [Google Scholar]
- 8.Tsakiris M. and Critchley H. (2016) Interoception beyond homeostasis: affect, cognition and mental health. Philos. Trans. R. Soc. Lond., B., Biol. Sci. 371, 20160002. 10.1098/rstb.2016.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocagoncu E. et al. (2021) Evidence and implications of abnormal predictive coding in dementia. Brain 144, 3311–3321. 10.1093/brain/awab254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birba A. et al. (2022) Allostatic-Interoceptive Overload in Frontotemporal Dementia. Biol. Psychiatry 92, 54–67. 10.1016/j.biopsych.2022.02.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett LF et al. (2016) An active inference theory of allostasis and interoception in depression. Philos. Trans. R. Soc. Lond., B., Biol. Sci. 371, 20160011. 10.1098/rstb.2016.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piguet O. et al. (2011) Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol 10, 162–172. 10.1016/s1474-4422(10)70299-4 [DOI] [PubMed] [Google Scholar]
- 13.Piguet O. and Kumfor F. (2020) Frontotemporal dementias: main syndromes and underlying brain changes. Curr. Opin. Neurol. 33, 215–221. 10.1097/wco.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 14.Possin KL et al. (2013) Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology 80, 2180–2185. 10.1212/WNL.0b013e318296e940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kril JJ and Halliday GM (2004) Clinicopathological staging of frontotemporal dementia severity: Correlation with regional atrophy. Dement. Geriatr. Cogn. Disord. 17, 311–315. 10.1159/000077161 [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS et al. (2015) Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363. 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibanez A. (2022) The mind’s golden cage and cognition in the wild. Trends in Cognitve Sciences 10.1016/j.tics.2022.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS (2000) Allostasis, Allostatic Load, and the Aging Nervous System: Role of Excitatory Amino Acids and Excitotoxicity. Neurochem. Res. 25, 1219–1231. 10.1023/a1007687911139: [DOI] [PubMed] [Google Scholar]
- 19.Bäuml JG et al. (2019) The Default Mode Network Mediates the Impact of Infant Regulatory Problems on Adult Avoidant Personality Traits. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 4, 333–342. 10.1016/j.bpsc.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 20.Coll MP et al. (2021) Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neurosci. Biobehav. Rev. 122, 190–200. 10.1016/j.neubiorev.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 21.Shiels PG et al. (2017) Circulating markers of ageing and allostatic load: A slow train coming. Pract. Lab. Med. 7, 49–54. 10.1016/j.plabm.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright KD et al. (2021) Beyond Allostatic Load: Focused Biological Measures of Chronic Stress in African American Older Adults. Res. Gerontol. Nurs. 14, 222–224. 10.3928/19404921-20210825-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piolanti A. et al. (2016) Use of the psychosocial index: A sensitive tool in research and practice. Psychother. Psychosom. 85, 337–345. 10.1159/000447760 [DOI] [PubMed] [Google Scholar]
- 24.Fava GA et al. (2017) Current Psychosomatic Practice. Psychother. Psychosom. 86, 13–30. 10.1159/000448856 [DOI] [PubMed] [Google Scholar]
- 25.Pollatos O. et al. (2005) Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64. 10.1002/hbm.20121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al E. et al. (2020) Heart-brain interactions shape somatosensory perception and evoked potentials. PNAS 117, 10575–10584. 10.1073/pnas.1915629117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Cordero I. et al. (2016) Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos. Trans. R. Soc. Lond., B., Biol. Sci. 371, 20160006. 10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrevaya S. et al. (2020) At the Heart of Neurological Dimensionality: Cross-Nosological and Multimodal Cardiac Interoceptive Deficits. Psychosom. Med. 82, 850–861. 10.1097/PSY.0000000000000868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salamone PC et al. (2018) Altered neural signatures of interoception in multiple sclerosis. Hum. Brain Mapp. 39, 4743–4754. 10.1002/hbm.24319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salamone PC et al. (2020) Dynamic neurocognitive changes in interoception after heart transplant. Brain Commun. 2, fcaa095. 10.1093/braincomms/fcaa095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canales-Johnson A. et al. (2015) Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb. Cortex 25, 4490–4503. 10.1093/cercor/bhv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoris A. et al. (2017) The inner world of overactive monitoring: Neural markers of interoception in obsessive-compulsive disorder. Psychol. Med. 47, 1957–1970. 10.1017/S0033291717000368 [DOI] [PubMed] [Google Scholar]
- 33.Yoris A. et al. (2018) Multilevel convergence of interoceptive impairments in hypertension: New evidence of disrupted body–brain interactions. Hum. Brain Mapp. 39, 1563–1581. 10.1002/hbm.23933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter F. et al. (2021) Behavioral and neurophysiological signatures of interoceptive enhancements following vagus nerve stimulation. Hum. Brain Mapp. 42, 1227–1242. 10.1002/hbm.25288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter F. and Ibáñez A. (2021) Time is body: Multimodal evidence of crosstalk between interoception and time estimation. Biol. Psychol 159. 10.1016/j.biopsycho.2021.108017 [DOI] [PubMed] [Google Scholar]
- 36.Park S. et al. (2021) Evaluation of visual-induced motion sickness from head-mounted display using heartbeat evoked potential: a cognitive load-focused approach. Virtual Real. 10.1007/s10055-021-00600-8 [DOI] [Google Scholar]
- 37.Park S. et al. (2015) Evaluation of 3D cognitive fatigue using heart–brain synchronization. Int. J. Psychophysiol. 97, 120–130. 10.1016/j.ijpsycho.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Legaz A. et al. (2020) Heart–brain interactions during social and cognitive stress in hypertensive disease: A multidimensional approach. Eur. J. Neurosci. n/a. 10.1111/ejn.14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salamone PC et al. (2021) Interoception primes emotional processing: Multimodal evidence from neurodegeneration. J. Neurosci. 41, 4276–4292. 10.1523/JNEUROSCI.2578-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang J. et al. (2019) Altered Interoceptive Processing in Generalized Anxiety Disorder-A Heartbeat-Evoked Potential Research. Front. Psychiatry 10, 616-616. 10.3389/fpsyt.2019.00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flasbeck V. et al. (2020) Altered interoception in patients with borderline personality disorder: a study using heartbeat-evoked potentials. Borderline Personal. Disord. Emot. Dysregulation 7, 24. 10.1186/s40479-020-00139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couto B. et al. (2015) Heart evoked potential triggers brain responses to natural affective scenes: A preliminary study. Auton. Neurosci. 193, 132–137. 10.1016/j.autneu.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 43.Mazgelytė E. et al. (2019) Association of Hair Cortisol Concentration with Prevalence of Major Cardiovascular Risk Factors and Allostatic Load. Med. Sci. Monit. 25, 3573–3582. 10.12659/MSM.913532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logan JG and Barksdale DJ (2008) Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 17, 201–208. 10.1111/j.13652702.2008.02347.x [DOI] [PubMed] [Google Scholar]
- 45.Borrell LN et al. (2020) Racial/ethnic inequities in the associations of allostatic load with all-cause and cardiovascular-specific mortality risk in U.S. adults. PLoS One 15, e0228336. 10.1371/journal.pone.0228336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thayer Z. et al. (2017) Early life trauma, post-traumatic stress disorder, and allostatic load in a sample of American Indian adults. Am. J. Hum. Biol 29. 10.1002/ajhb.22943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillespie SL et al. (2019) Allostatic load in the association of depressive symptoms with incident coronary heart disease: The Jackson Heart Study. Psychoneuroendocrinology 109. 10.1016/j.psyneuen.2019.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepto A. et al. (2014) Disruption of multisystem responses to stress in type 2 diabetes: Investigating the dynamics of allostatic load. PNAS 111, 15693–15698. 10.1073/pnas.1410401111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hux VJ and Roberts JM (2015) A Potential Role for Allostatic Load in Preeclampsia . Matern. Child Health J. 19, 591–597. 10.1007/s10995-014-1543-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeki Al Hazzouri A. et al. (2021) Body mass index in early adulthood and dementia in late life: Findings from a pooled cohort. Alzheimers Dement. 10.1002/alz.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeman T. et al. (2008) Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994). Soc. Sci. Med. 66, 72–87. 10.1016/j.socscimed.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soysal P. et al. (2020) The Relationship Between Dementia Subtypes and Nutritional Parameters in Older Adults. J. Am. Med. .Dir Assoc. 21, 1430–1435. 10.1016/j.jamda.2020.06.051 [DOI] [PubMed] [Google Scholar]
- 53.Bright F. et al. (2019) Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 15, 540–555. 10.1038/s41582-019-0231-z [DOI] [PubMed] [Google Scholar]
- 54.Duran-Aniotz C. et al. (2021) Systematic Review: Genetic, Neuroimaging, and Fluids Biomarkers for Frontotemporal Dementia Across Latin America Countries. Front. Neurol 12. 10.3389/fneur.2021.663407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sjögren M. et al. (2004) Increased intrathecal inflammatory activity in frontotemporal dementia: Pathophysiological implications. J. Neurol. Neurosurg. Psychiatry 75, 1107–1111. 10.1136/jnnp.2003.019422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J. (2015) Mapping neuroinflammation in frontotemporal dementia with molecular PET imaging. J. Neuroinflammation 12. 10.1186/s12974-015-0236-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cagnin A. et al. (2004) In vivo detection of microglial activation in frontotemporal dementia. Ann. Neurol. 56, 894–897. 10.1002/ana.20332 [DOI] [PubMed] [Google Scholar]
- 58.Rentzos M. et al. (2006) Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J. Neurol. Sci. 249, 110–114. 10.1016/j.jns.2006.05.063 [DOI] [PubMed] [Google Scholar]
- 59.Yaffe K. et al. (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292, 2237–2242. 10.1001/jama.292.18.2237 [DOI] [PubMed] [Google Scholar]
- 60.Dik MG et al. (2007) Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care 30, 2655–2660. 10.2337/dc06-1190 [DOI] [PubMed] [Google Scholar]
- 61.Woolley JD et al. (2014) Satiety-related hormonal dysregulation in behavioral variant frontotemporal dementia. Neurology 82, 512–520. 10.1212/WNL.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan A. et al. (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 9. 10.1101/cshperspect.a018309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donker Kaat L. et al. (2018) Serum neurofilament light chain in progressive supranuclear palsy. Parkinsonism Relat. Disord. 56, 98–101. 10.1016/j.parkreldis.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 64.Rohrer JD et al. (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87, 1329–1336. 10.1212/WNL.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rojas JC et al. (2016) Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann. Clin. Transl. Neurol. 3, 216–225. 10.1002/acn3.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinacker P. et al. (2018) Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 91, E1390–E1401. 10.1212/WNL.0000000000006318 [DOI] [PubMed] [Google Scholar]
- 67.Takada LT et al. (2016) GRN and MAPT Mutations in 2 Frontotemporal Dementia Research Centers in Brazil. Alzheimer Dis. Assoc. Disord. 30, 310–317. 10.1097/WAD.0000000000000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juster R-P et al. (2018) Elevated allostatic load in individuals presenting at psychiatric emergency services. J. Psychosom. Res. 115, 101–109. 10.1016/j.jpsychores.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 69.Kobrosly RW et al. (2014) Depressive symptoms are associated with allostatic load among community-dwelling older adults. Physiol. Behav. 123, 223–230. 10.1016/j.physbeh.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheuer S. et al. (2018) Childhood abuse and depression in adulthood: The mediating role of allostatic load. Psychoneuroendocrinology 94, 134–142. 10.1016/j.psyneuen.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 71.Van den Stock J. and Kumfor F. (2019) Behavioural variant frontotemporal dementia: At the interface of interoception, emotion and social cognition? Cortex; a journal devoted to the study of the nervous system and behavior 115, 335–340. 10.1016/j.cortex.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 72.Baez S. et al. (2017) The Social Context Network Model in Psychiatric and Neurological Diseases. Curr. Top. Behav. Neurosci. 30, 379–396. 10.1007/7854_2016_443 [DOI] [PubMed] [Google Scholar]
- 73.Kamalian A. et al. (2022) Convergent regional brain abnormalities in behavioral variant frontotemporal dementia: A neuroimaging meta-analysis of 73 studies. Alzheimer’s Dement.: Diagn. Assess. Dis. Monit. 14, e12318. 10.1002/dad2.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou J. et al. (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367. 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J. and Seeley WW (2014) Network dysfunction in Alzheimer’s disease and frontotemporal dementia: implications for psychiatry. Biol. Psychiatry. 75, 565–573. 10.1016/j.biopsych.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 76.Hafkemeijer A. et al. (2017) A Longitudinal Study on Resting State Functional Connectivity in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease. J. Alzheimers. Dis. 55, 521–537. 10.3233/jad-150695 [DOI] [PubMed] [Google Scholar]
- 77.Pasquini L. et al. (2020) Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia. Cereb. Cortex 30, 5387–5399. 10.1093/cercor/bhaa119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitwell JL et al. (2011) Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology 77, 866–874. 10.1212/WNL.0b013e31822c61f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ripp I. et al. (2020) Integrity of Neurocognitive Networks in Dementing Disorders as Measured with Simultaneous PET/Functional MRI. J. Nucl. Med. 61, 1341–1347. 10.2967/jnumed.119.234930 [DOI] [PubMed] [Google Scholar]
- 80.Seeley WW et al. (2008) Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol 65, 249–255. 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seeley WW et al. (2012) Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist. 18, 373–385. 10.1177/1073858411410354 [DOI] [PubMed] [Google Scholar]
- 82.Filippi M. et al. (2013) Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex 49, 2389–2401. 10.1016/j.cortex.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 83.Ruiz-Rizzo AL et al. (2019) Decreased cingulo-opercular network functional connectivity mediates the impact of aging on visual processing speed. Neurobiol. Aging 73, 50–60. 10.1016/j.neurobiolaging.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 84.Sturm VE et al. (2018) Network Architecture Underlying Basal Autonomic Outflow: Evidence from Frontotemporal Dementia. J. Neurosci. 38, 8943–8955. 10.1523/jneurosci.0347-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azzalini D. et al. (2019) Visceral Signals Shape Brain Dynamics and Cognition. Trends. Cogn. Sci. 23, 488–509. 10.1016/j.tics.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 86.Park HD et al. (2018) Neural Sources and Underlying Mechanisms of Neural Responses to Heartbeats, and their Role in Bodily Self-consciousness: An Intracranial EEG Study. Cereb. Cortex 28, 2351–2364. 10.1093/cercor/bhx136 [DOI] [PubMed] [Google Scholar]
- 87.Ahmed RM et al. (2015) Autonomic dysregulation in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 86, 1048. 10.1136/jnnp-2014-309424 [DOI] [PubMed] [Google Scholar]
- 88.Ibañez A. and Manes F. (2012) Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 78, 1354–1362. 10.1212/WNL.0b013e3182518375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santamaría-García H. et al. (2017) A lesion model of envy and Schadenfreude: legal, deservingness and moral dimensions as revealed by neurodegeneration. Brain 140, 33573377. 10.1093/brain/awx269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen KH et al. (2022) Diminished preparatory physiological responses in frontotemporal lobar degeneration syndromes. Brain Commun. 4, fcac075. 10.1093/braincomms/fcac075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marshall CR et al. (2019) The functional neuroanatomy of emotion processing in frontotemporal dementias. Brain 142, 2873–2887. 10.1093/brain/awz204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adolfi F. et al. (2017) Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex 88, 124–142. 10.1016/j.cortex.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 93.Beydoun MA et al. (2021) BMI and Allostatic Load Are Directly Associated with Longitudinal Increase in Plasma Neurofilament Light among Urban Middle-Aged Adults. J. Nutr. 152, 535–549. 10.1093/jn/nxab381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen WG et al. (2021) The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 44, 3–16. 10.1016/j.tins.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Healey ML et al. (2015) Getting on the same page: the neural basis for social coordination deficits in behavioral variant frontotemporal degeneration. Neuropsychologia 69, 56–66. 10.1016/j.neuropsychologia.2015.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMillan CT et al. (2012) The neural basis for establishing a focal point in pure coordination games. Soc. Cogn. Affect. Neurosci. 7, 881–887. 10.1093/scan/nsr070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van den Stock J. et al. (2020) Brain-behaviour associations and neural representations of emotions in frontotemporal dementia. Brain 143, e17. 10.1093/brain/awaa005 [DOI] [PubMed] [Google Scholar]
- 98.Ibanez A. et al. (2018) Social neuroscience: undoing the schism between neurology and psychiatry. Soc. Neurosci. 13, 1–39. 10.1080/17470919.2016.1245214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsieh S. et al. (2012) Grief and joy: emotion word comprehension in the dementias. Neuropsychology 26, 624–630. 10.1037/a0029326 [DOI] [PubMed] [Google Scholar]
- 100.Van den Stock J. et al. (2021) The Interplay of Social Cognition Sub-domains in Frontotemporal Dementia. Brain Commun. 3, fcab161. 10.1093/braincomms/fcab161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ottino-González J. et al. (2019) Allostatic load and executive functions in overweight adults. Psychoneuroendocrinology 106, 165–170. 10.1016/j.psyneuen.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 102.Ruisoto P. and Contador I. (2019) The role of stress in drug addiction. An integrative review. Physiol. Behav. 202, 62–68. 10.1016/j.physbeh.2019.01.022 [DOI] [PubMed] [Google Scholar]
- 103.Evans GW et al. (2021) Early childhood poverty and adult executive functioning: Distinct, mediating pathways for different domains of executive functioning. Dev. Sci. 24, e13084. 10.1111/desc.13084 [DOI] [PubMed] [Google Scholar]
- 104.D’Amico D. et al. (2020) The association between allostatic load and cognitive function: A systematic and meta-analytic review. Psychoneuroendocrinology 121, 104849. 10.1016/j.psyneuen.2020.104849 [DOI] [PubMed] [Google Scholar]
- 105.Seth AK and Friston KJ (2016) Active interoceptive inference and the emotional brain. Philos. Trans. R. Soc. Lond., B., Biol. Sci. 371, 20160007. 10.1098/rstb.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gray JD et al. (2017) Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 13, 661–673. 10.1038/nrendo.2017.97 [DOI] [PubMed] [Google Scholar]
- 107.Ahmed RM et al. (2017) Energy expenditure in frontotemporal dementia: a behavioural and imaging study. Brain 140, 171–183. 10.1093/brain/aww263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seth AK (2013) Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci 17, 565–573. 10.1016/j.tics.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 109.Salvato G. et al. (2018) A very light lunch: Interoceptive deficits and food aversion at onset in a case of behavioral variant frontotemporal dementia. Alzheimers Dement. 10, 750–754. 10.1016/j.dadm.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barker MS et al. (2022) Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain : a journal of neurology. 10.1093/brain/awab365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laneri D. et al. (2017) Mindfulness meditation regulates anterior insula activity during empathy for social pain. Hum. Brain Mapp 38, 4034–4046. 10.1002/hbm.23646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang Y-Y et al. (2015) Short-term meditation increases blood flow in anterior cingulate cortex and insula. Front. Psychol. 6. 10.3389/fpsyg.2015.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lanata SC and Miller BL (2016) The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J. Neurol. Neurosurg. Psychiatry 87, 501–511. 10.1136/jnnp-2015-310697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Savransky A. et al. (2018) Elevated allostatic load early in the course of schizophrenia. Transl. Psychiatry 8, 246. 10.1038/s41398-018-0299-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kivelä M. et al. (2014) Multilayer networks. J. Complex. Netw. 2, 203–271. 10.1093/comnet/cnu016 [DOI] [Google Scholar]
- 116.Signorelli CM and Boils JD (2021) Multilayer networks as embodied consciousness interactions. A formal model approach. PsyArXiv. 10.31234/osf.io/3y8at [DOI] [Google Scholar]
- 117.Sterling P. (2020) What is health?: allostasis and the evolution of human design MIT Press [DOI] [PubMed] [Google Scholar]
- 118.Sterling P. (2012) Allostasis: a model of predictive regulation. Physiol. Behav. 106, 5–15. 10.1016/j.physbeh.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 119.Irish M. et al. (2012) Self-projection and the default network in frontotemporal dementia. Nat. Rev. Neurol. 8, 152–161. 10.1038/nrneurol.2012.11 [DOI] [PubMed] [Google Scholar]
- 120.Buckley CL et al. (2017) The free energy principle for action and perception: A mathematical review. J. Math. Psychol. 81, 55–79. 10.1016/j.jmp.2017.09.004 [DOI] [Google Scholar]
- 121.De Felice FG et al. (2022) Impaired insulin signalling and allostatic load in Alzheimer disease. Nat. Rev. Neurosci. 23, 215–230. 10.1038/s41583-022-00558-9 [DOI] [PubMed] [Google Scholar]
- 122.Luczkowski M. (2016) “No screams and cries will convince us that white is white and black is black”, an ode to the defenders of amyloid cascade hypothesis of Alzheimer’s disease. Coord. Chem. Rev. 327–328, 35–42. 10.1016/j.ccr.2016.03.001 [DOI] [Google Scholar]
- 123.Bonaz B. et al. (2021) Diseases, Disorders, and Comorbidities of Interoception. Trends Neurosci. 44, 39–51. 10.1016/j.tins.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Cordero I. et al. (2021) Metacognition of emotion recognition across neurodegenerative diseases. Cortex 137, 93–107. 10.1016/j.cortex.2020.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ricciardi L. et al. (2016) Know thyself: Exploring interoceptive sensitivity in Parkinson’s disease. J. Neurol. Sci. 364, 110–115. 10.1016/j.jns.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 126.Christopher L. et al. (2014) Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 137, 2143–2154. 10.1093/brain/awu084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santangelo G. et al. (2018) Interoceptive processing deficit: A behavioral marker for subtyping Parkinson’s disease. Parkinsonism Relat. Disord. 53, 64–69. 10.1016/j.parkreldis.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 128.Gonzalez Campo C. et al. (2020) Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities. Mult Scler 26, 1845–1853. 10.1177/1352458519888881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomson EM (2019) Air Pollution, Stress, and Allostatic Load: Linking Systemic and Central Nervous System Impacts. J. Alzheimers. Dis. 69, 597–614. 10.3233/jad-190015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ouanes S. and Popp J. (2019) High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 11, 43. 10.3389/fnagi.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohen S. et al. (2006) Socioeconomic status is associated with stress hormones. Psychosom. Med. 68, 414–420. 10.1097/01.psy.0000221236.37158.b9 [DOI] [PubMed] [Google Scholar]
- 132.Sirkis DW et al. (2019) Immunological signatures in frontotemporal lobar degeneration. Curr. Opin. Neurol. 32, 272–278. 10.1097/wco.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pottier C. et al. (2019) Genome-wide analyses as part of the international FTLD-TDP wholegenome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 137, 879–899. 10.1007/s00401-019-019629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cacabelos D. et al. (2016) Interplay between TDP-43 and docosahexaenoic acid-related processes in amyotrophic lateral sclerosis. Neurobiol. Dis. 88, 148–160. 10.1016/j.nbd.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 135.Armstrong R. (2020) What causes neurodegenerative disease? Folia Neuropathol. 58, 93112. 10.5114/fn.2020.96707 [DOI] [PubMed] [Google Scholar]
- 136.Clark A. (2013) Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain. Sci. 36, 181–204. 10.1017/S0140525X12000477 [DOI] [PubMed] [Google Scholar]