Abstract
Type 1 diabetes is an autoimmune disorder in which the immune system attacks and destroys insulin-producing islet cells of the pancreas. Although islet transplantation has proved to be successful for some patients with type 1 diabetes, its widespread use is limited by islet donor shortage and the requirement for lifelong immunosuppression. An encapsulation strategy that can prevent the rejection of xenogeneic islets or of stem cell-derived allogeneic islets can potentially eliminate both of these barriers. Although encapsulation technology has met several challenges, the convergence of expertise in materials, nanotechnology, stem cell biology and immunology is allowing us to get closer to the goal of encapsulated islet cell therapy for humans.
Type 1 diabetes (T1D; also known as juvenile-onset diabetes) represents 5–10% of the diagnosed cases of diabetes, corresponding to more than 1.5 million individuals in the United States and 20 million worldwide1. The disease results from the destruction of insulin-producing β-cells by the patient’s overactive immune system. Insulin injections, the most common treatment modality, do not perfectly simulate insulin secretion from β-cells; consequently, a patient’s blood glucose levels fluctuate despite close monitoring and frequent adjustments of insulin doses. Chronic hyperglycaemia leads to irreversible tissue and organ damage, and hypoglycaemia can be acutely life-threatening2.
More recently, the replacement of lost insulin-producing cells using islet transplantation has proved to be an effective therapy for some patients with T1D3,4, allowing for tighter blood glucose control. Enthusiasm for islet transplantation was initially sparked by a series of human islet transplants carried out at the University of Alberta, Canada, during which seven patients received ~800,000 human islets prepared from two or three pancreases per recipient, through portal vein injection5. This resulted in insulin independence in seven patients for an average of 1 year. Subsequent results from a worldwide, multicentre clinical trial of the Edmonton Protocol, conducted by the Immune Tolerance Network, indicated that 16 of 44 islet transplant patients (44%) became insulin-free for 1 year, with 10 patients experiencing complete graft loss6. Importantly, although the short-term survival of the grafts is up to 80%, less than 20% of the grafted patients remain insulin-independent by 5 years2.
A recent clinical trial evaluated the safety and effectiveness of a standardized human pancreatic islet product in patients in whom impaired awareness of hypoglycaemia (IAH) and severe hypoglycaemic events (SHEs) persisted despite medical treatment7. IAH and SHEs can cause substantial morbidity and mortality in patients with T1D. It was found that transplanted human pancreatic islet product provided glycaemic control, the restoration of hypoglycaemia awareness and protection from SHEs at 2 years in more than 70% of patients with previously intractable IAH and SHEs7.
Unfortunately, donor shortage and the need for lifelong immunosuppression to prevent rejection of the transplanted cells (BOX 1) limit the widespread application of islet transplantation. The required chronic systemic immunosuppression puts patients at risk of organ damage, infection and malignancies. Although two strategies have the potential to provide an unlimited supply of β-cells for transplantation — the use of xenogeneic islets and human embryonic stem cell (hESC)-derived islets8 — both have their own risks. Xenogeneic tissue induces potent rejection responses that cannot be safely and effectively controlled by anti-rejection medicine, and hESC-derived β-cells often contain undifferentiated stem cells, which may pose some regulatory concerns in terms of teratoma formation (although this has not been seen in recent studies)9. Moreover, the efficient generation of mature pancreatic β-cells with complete functional capabilities has not yet been accomplished10.
Box 1∣. Immunological challenges to islet transplantation.
The survival of transplanted islets is challenged immunologically. This challenge is a consequence of the pre-existing autoimmune disease and transplant immunity, which is of a broader magnitude than autoimmunity owing to the multitude and redundancy of pathways. Transplant recipients are frequently sensitized to alloantigens, resulting from procedures such as prior blood transfusions, which can lead to both humoral and cellular sensitization. The process of immune recognition and the immune destruction of transplanted cells has been described as following multiple steps: first, inflammation; second, maturation of dendritic cells (DCs) and migration to draining lymph nodes; third, T cell activation by DCs resulting in expansion of anti-donor T cells; and fourth, migration of T cells to the graft where they mediate cytotoxicity188. In any given donor–recipient pair situation, the primary antigen is termed the human leukocyte antigen (HLA), and the number of HLA mismatches multiplied by the number of distinct epitopes results in a large number of potentially immunogenic epitope mismatches129. Furthermore, minor histocompatibility antigens (mHAs) have been implicated in rejection, with the different types of mismatches probably eliciting immunogenicity of a wide range of strength, which may also vary based on antigen processing and presentation specific to the recipient189,190. Classical type 1 helper (TH1) CD4+ T cells and cytotoxic CD8+ T cells are considered to be mainly responsible for rejection; however, recent studies have implicated a whole range of other effector cells in this process, including TH2 cells, TH17 cells, memory CD8+ T cells, and cells of the innate immune system, such as monocytes and natural killer cells. The specific effector pathways that dominate in any given rejection process can be a function of the tissue transplanted and the host immune composition (for example, microbiota and the presence or absence of other inflammatory signals). Notably, the suppression of one pathway may induce an alternative pathway to promote rejection191.
The use of an encapsulation device, to provide a physical barrier between transplanted β-cells and their recipients, has emerged as a promising approach to overcome some of these challenges by eliminating the need for immunosuppression11-13 (FIG. 1). The key function of an encapsulation device is to create an environment that allows for normal insulin secretion in response to fluctuating blood glucose levels, while maintaining cell viability through sequestration from the immune system and effective nutrient and waste exchange. An ideal islet encapsulation device should therefore: provide ample blood supply to sustain survival and function of sufficient islet mass for the maintenance of normoglycaemia; exhibit appropriate insulin and glucose kinetics to achieve normoglycaemia; be biocompatible; serve as an immune barrier to prevent sensitization and rejection; and contain any potentially tumorigenic cells.
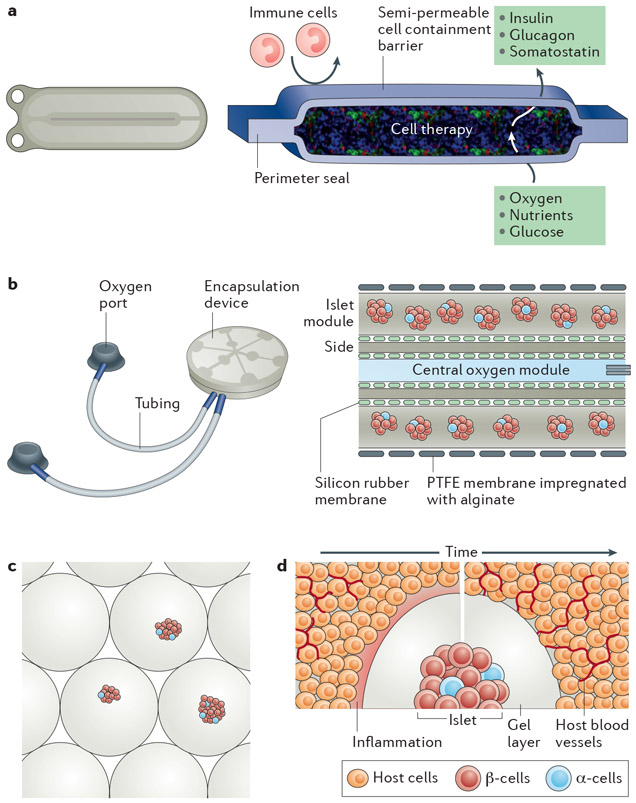
Figure 1 ∣. Islet and β-cell transplantation systems.
a ∣ Schematic of the ViaCyte device, which is a rechargeable encapsulating system about half the size of a business card in which the membrane functions to contain the cells and to limit the access of immune cells, but allows the transport of nutrients from the exterior of the device and hormones from the encapsulated cells. Patients would be implanted with 4–6 units. b ∣ Image and schematic of the Beta-O2 device demonstrating the ports for recharging oxygen and the encapsulation device. The schematic illustrates the central module that can be charged with oxygen to diffuse outwards to the islets contained within a membrane. The device is approximately 2.5 inches in diameter. The membrane allows for nutrient and hormone transport but is impregnated with alginate to restrict cell infiltration. c ∣ Islet microencapsulation with alginate hydrogels. This image shows genetically engineered pig islets entrapped within alginate hydrogels. d ∣ Host response to the transplantation of encapsulated islets. Transplantation of the capsules leads to a host response that will depend on multiple factors (for example, cells, materials, transplant site and so on). Shortly after transplantation into tissues (left-hand side), the host response to transplantation and the material can consist of an inflammatory response (pink region) with nearby blood vessels. Over time, the inflammatory response would ideally resolve without fibrosis and would allow for vascular growth adjacent to the capsule for nutrient and hormone exchange. However, shed antigens released from the islet may contribute to immune cell recruitment and activation. PTFE, polytetrafluoroethylene.
With the goal of creating immune-protected β-cells, various microencapsulating and macroencapsulating approaches have been developed over the past several decades14, each of which has its own advantages and limitations. The fundamental distinction between microdevices and macrodevices is a matter of scale: the microencapsulation approach uses many microscale capsules with each one containing a single cell or islet, which maximizes surface area to volume ratios and promotes improved nutrient exchange15. However, there is limited control of membrane thickness and pore size, and as islets are individually encapsulated, thousands of microcapsules are required for each transplant, and capsule size makes live imaging and tracking a considerable challenge. Conversely, macroencapsulation devices house many cells or islets. These larger devices allow for greater control over membrane parameters, such as pore size and porosity, but are plagued by limited nutrient and oxygen diffusion and cell response owing to the device thickness and large device reservoirs. In some cases, depending on the device design, there can be alterations in the kinetics of insulin release that might cause serious problems, such as hypoglycaemia after eating or with exercise16,17. In addition to these challenges, the chemistry and mechanical properties of materials that are typically associated with macroencapsulation devices can lead to a foreign body response and subsequent device failure from fibrotic encapsulation18.
Extensive efforts have recently focused on investigating the ideal cell encapsulation approach, including encapsulation material, site of transplantation, configuration of encapsulation device, and methods to improve vascularization and immune modulation. The key current challenges that are associated with the development of cell encapsulation technologies include biocompatibility, cell viability (as well as oxygenation and nutrient access), and immune protection or modulation. In this Review, we discuss the challenges associated with the clinical translation of cell encapsulation technologies and approaches that aim to overcome these barriers, highlighting systems that are currently in the clinic.
The emergence of islet cell encapsulation
One of the first examples of the use of encapsulation in the treatment of diabetes involved the xenotransplantation of human insulinoma tissue using membranous bags into rats in 1933 (REF. 19). However, it was not until a series of experiments in the early 1950s, which examined the survival rates of allotransplanted tissue into an extravascular space with and without a cell-impermeable encapsulating membrane, that the field of immuneisolated transplantation became established20-23. These studies demonstrated that the use of an encapsulating membrane prevented immune cell contact and the activation of the direct antigen presentation pathway, thereby prolonging the survival of the non-vascularized transplanted tissue, despite it receiving fewer nutrients.
Microencapsulation
In 1964, Chang et al.24 first described cell microencapsulation, and in 1980, Lim and Sun25 applied microcapsules to diabetes treatment, demonstrating prolonged isograft islet survival using alginate-polylysine-poly-ethyleneimine microcapsules. Post-transplantation, the encapsulated islets survived up to 3 weeks, compared with 8 days for unencapsulated islets without immunosuppression25.
The microcapsule material itself was improved in 1984 by O’Shea et al.26, who removed the polyethyleneimine component and designed alginate as the outer layer of the microcapsule. The use of alginate demonstrated substantial improvement, and in one of the five animals used, the microencapsulated islets remained viable for the duration of the 365-day experiment. An additional advantage of the new microcapsules was increased microcapsule strength. Efforts to further improve the biocompatibility of alginate microcapsules involved decreasing the impurities and increasing the guluronic acid to mannuronic acid ratio27,28.
Over the next several decades, research focused on designing microencapsulation materials with sufficient durability and biocompatibility, many of which demonstrated success in small animals26,27,29,30. For example, Wang et al.31 evaluated more than 1,000 combinations of polyanions and polycations with regards to suitability for cell encapsulation. They identified a polyelectrolyte complexation process using five different polymers, which enabled independent control over capsule size, wall thickness, mechanical strength and permeability.
Since then, many encapsulation strategies have been shown to be effective in rodents. Souza et al.32 reviewed over 60 more encapsulation studies that were carried out in rodents between 2000 and 2010 and found that the best reported survival was 100 days for alginate encapsulated islets transplanted intraperitoneally without immunosuppression, and unencapsulated islets transplanted into the liver were able to survive 164 days with immunosuppression. However, Souza et al. did not consider several studies in their analysis, such as those by Duvivier-Kali et al.33,34, which reported microencapsulation with high-mannuronic acid (high-M) alginate crosslinked with BaCl2, allowing prolonged survival of syngeneic and allogeneic transplanted islets in diabetic BALB/c and NOD mice for more than 350 days. The normalization of glycaemia in the transplanted mice was associated with normal glucose profiles in response to intravenous glucose tolerance tests.
Macroencapsulation
The earliest macroencapsulation approaches used extravascular chambers, which were developed by Algire, Prehn and Weaver in the 1950s, containing transplanted tissue21-23. During the 1970s, Millipore Corporation produced a commercially available extravascular transplantation chamber using the Algire approach14. These membranes typically had pore sizes of 450 nm, a size that was sufficiently small to prevent direct cell–cell contact and which was therefore promising for allotransplants. Studies by Algire and colleagues demonstrated improved cell viability when encapsulated in these membranes20,35,36. Although many of the initial experiments involved syngeneic cells, transplant failure occurred nonetheless, owing to fibroblastic overgrowth of the graft and chamber, highlighting the importance of biocompatibility of the chamber to transplant success14.
A series of compelling animal studies was conducted by Baxter Healthcare in the late 1990s, using a device that consisted of two membranes sealed at all sides with a loading port37,38. The membrane was designed to be robust and to encourage host vascularization, as well as allograft immune protection. Neovascularization at the membrane–tissue interface occurred in several membranes that had pore sizes large enough to allow complete penetration by host cells (0.8–8 μm pore size). When the vascularization of the membrane–tissue interface of 5 μm pore-size polytetrafluoroethylene (PTFE) membranes was compared with 0.02 μm pore-size PTFE membranes, it was found that the larger pore membranes had 80–100-fold more vascular structures. The increased vascularization was observed even though the larger pore membrane was laminated to a smaller pore inner membrane to prevent cell entry into the prototype immunoisolation device. This significantly higher level of vascularization was maintained for 1 year in the subcutaneous site in rats.
Another macroencapsulation study demonstrated that islet allografts transplanted into the epididymal fat pad of streptozotocin-induced diabetic mice could attain normoglycaemia that lasted up to 12 weeks39. However, this study raised the issue of the practicality of having diffusion-dependent macroencapsulation with large islet masses. In addition, some of the devices failed owing to a considerable amount of fibrosis around the macrocapsule, leading to numerous attempts to create more biocompatible cell encapsulation devices.
Studies have also been conducted in larger animal species, including dogs and non-human primates (NHPs). In one canine study, microencapsulated islets were transplanted intraperitoneally, and, using C-peptide analysis, the survival of grafts was found to be up to 726 days40. This long-term survival was attributed to the careful selection of high-quality capsules, ensuring that the capsules did not have macroscopic holes or cellular protrusions (FIG. 1c). Poorly encapsulated islets were rapidly rejected at a rate similar to unencapsulated islets, although the higher-quality encapsulated islets lasted 6 months41. Another study demonstrated the ability of microencapsulated islets to reduce insulin requirement42. Microcapsules placed intraperitoneally in NHPs reduced exogenous insulin requirement to 36% at 12 weeks and to 43% at 23 weeks compared with controls42.
Macroencapsulation devices have demonstrated some success in large animals, although the results have not been consistent. In NHPs, porcine islets placed within an alginate macrocapsule transplanted subcutaneously were found to provide normoglycaemia for up to 6 months, compared with 2 weeks for porcine islets within alginate microcapsules placed under the kidney capsule41. Another approach, using alginate sheets containing islets — known as the Islet Sheet Device — showed some promising results in preclinical studies but also highlighted the challenges that are associated with maintaining sheet planarity43. The overall thickness of the Islet Sheet (250 μm) was chosen to maximize nutrient diffusion. In one of their key studies, allogeneic islet equivalents in Islet Sheets were sutured to the omentum of dogs at the time of pancreatectomy. Fasting euglycaemia was maintained for 84 days and islets within alginate sheets were recovered from the interior of these capsules, suggesting that allogeneic islet tissue survived for the duration of the study and was responsible for maintaining fasting euglycaemia. This work highlighted the importance of maintaining adequate nutrient diffusion for maintaining cell viability and function.
Together, these preclinical animal studies have guided the field with regard to the optimal choice of cells, encapsulation material and useful functional measures in vivo. However, they have also highlighted several challenges that must be addressed for the successful translation of encapsulation approaches into the clinic.
Overcoming challenges to human translation
The key challenge to human translation is promoting and maintaining the survival of transplanted islets for an extended period of time14 (FIG. 1d). Islets must be delivered such that they have access to the required nutrients for survival, for which oxygen is often the limiting factor, while also achieving sufficient mass to sense blood glucose and secrete insulin that can be distributed throughout the host. In addition, T1D is an autoimmune disease, and the transplantation process and cell source can further induce immune responses that can compromise engraftment and function. Finally, the number of allogeneic donor islets available for transplantation is limited and the potential of alternative cell sources is being pursued. We discuss below strategies and approaches to overcome these key challenges, some of which have enabled encapsulation devices to enter the clinic.
Strategies to maintain cell viability
Design of encapsulation material.
The choice and design of encapsulation materials can enhance engraftment and promote islet survival post-transplantation. In contrast to unencapsulated islets44, encapsulated islets are typically delivered extrahepatically, with transplantation sites that include the intraperitoneal cavity, subcutaneous space and the omentum45-48. A challenge to engraftment is the host response to the material, which can lead to a fibrotic response that can exacerbate mass transport limitations, and the material choice or chemistry can modulate the extent of fibrosis.
Finding a material that can simultaneously achieve biocompatibility, immunoisolation and a suitable environment that minimizes stress on the islets is therefore desired. The design of microcapsules has so far focused on biocompatibility, as well as on achieving immunoisolation, while allowing sufficient nutrient availability. However, the design that optimizes these parameters may compromise the environment surrounding the cells and may negatively affect cell behaviour. In addition to biocompatibility, nutrient availability and immune protection, pancreatic β-cell function is also highly dependent on the surrounding matrix environment and organization49. In native islets, cell–cell communication is essential to provide appropriate insulin release after food intake. Even paired β-cells secrete more than twice the amount of insulin than a single cell50. Previous studies have shown that insulin production per cell increases with three-dimensional organization and optimal cluster size51,52. Thus, the inability to independently control cell environment from membrane permeability will continue to present challenges for achieving therapeutic success of microencapsulated cells.
In the search for optimal encapsulation materials, many types of natural and synthetic polymers are being explored. Although alginate has been the predominant microencapsulation material of choice owing to availability, cost and ease of production, variability in alginate production has led to inconsistencies in endotoxin content and purity, which has affected biocompatibility53. Efforts to further improve the biocompatibility of alginate microcapsules have involved decreasing impurities and increasing the guluronic acid to mannuronic acid ratio27,28. Other researchers examined the reproducibility of alginate-polylysine microcapsules and explored either their coating with a polyethylene glycol (PEG) hydrogel or manufacturing the microcapsules from a different material, such as a polyacrylate29,30 or silica54. Other natural materials, such as collagen, chitosan, gelatin and agarose, have also been investigated; however, these materials are more difficult to fabricate for optimal pore size and often have some immunogenicity.
Recently, chemically modified alginates — such as triazole–thiomorpholine dioxide (TMTD) alginate — have been identified that resist implant fibrosis in both rodents and NHPs55. These materials were shown to provide long-term glycaemic correction of a diabetic, immunocompetent animal model using human SCβ-cells for 174 days56,57. Currently, there is no consensus on the best material to use for microencapsulation, although alginate systems are predominately used owing to their in vivo biocompatibility. However, all alginate systems are not the same. One of the key considerations is whether the capsule material may be reactive, thereby triggering complement and activating leukocytes. Rokstad et al.58 showed that polycation-containing APA microcapsules (calcium beads coated with PLL and alginate) and PMCG microcapsules (formed by polyelectrolyte complexation between sodium alginate (SA)/cellulose sulfate (CS) with polycation poly(methylene–co-guanidine) hydrochloride (PMCG) and calcium cations) triggered complement and leukocyte activation, but alginate microbeads consisting of only alginate and divalent cations did not provoke complement reactions58. This demonstrates the need to closely examine all of the chemical constituents of the microcapsules.
A wider range of materials has been investigated for macroencapsulation. As these devices are typically crafted from prefabricated membranes or films, material composition has ranged from polymers, such as ePTFE38 and polycaprolactone (PCL)18, to inorganic materials such as titania and silicon59. Although the inorganic membranes (silicon and titania) have advantages in terms of their tight pore size distribution and their thinner and more precisely controllable membrane thickness, these materials are rigid and thus are limited in terms of the macrocapsule configurations that one can achieve59. In addition, increasing evidence suggests that if they are not surface modified, rigid materials are more prone to fibrotic encapsulation60. For the polymeric-based devices, the biocompatibility and ability to promote vascularization have primarily driven the choice of material. Both PTFE and PCL have been shown to induce limited fibrosis and to exhibit good vascularization, allowing for better cell viability18,38.
The volume of material and islets delivered can also affect transplanted islet survival. Indeed, large delivery volumes can lead to aggregation that further exacerbates mass transport and can result in central necrosis of islet clusters within a few days of transplantation61. Strategies are being developed to provide a thin, or conformal, coating to the islet to minimize the amount of material for transplantation and, correspondingly, the distance from the islet to the host tissue62.
With the advent of sophisticated micro-manufacturing and nano-manufacturing techniques, it is becoming increasingly possible to ‘engineer’ the membrane with precise morphologies, in order to optimize engraftment. The hypoxic environment around the device, combined with the material properties and surface topography can lead to the endogenous secretion of angiogenic factors63. Attributes such as the size, length and density of pores can now be engineered to control the diffusion and exclusion of specific molecules. These attributes are important for a membrane-based device, which seeks to allow certain molecules to pass through but to block immune components. As a point of reference, globular proteins range in diameter between 2 nm and 10 nm, whereas organic metabolites are between 0.5 nm and 1 nm in diameter64,65. Immunological cells, such as macrophages and leukocytes, are 6–10 microns in diameter so they cannot pass through membranes with submicronsized or nanometre-sized channels15. To achieve this size scale, silicon micromachining has been used to produce macrocapsules with uniform and well-controlled pore sizes, channel lengths and surface properties15. This work showed that membranes with 20 nm pore sizes could maintain cell function and reduce key immune components, and 66 nm membranes led to the loss of cell function. Controlled pore size has also been demonstrated using other inorganic materials, such as alumina66. Although control over pore size has shown differences in cell functionality in published work15, it is important to view this parameter in the context of the device composition and its overall geometric configuration. Despite many studies, the optimal pore size for a microencapsulation or macroencapsulation membrane remains unclear.
More recently, a nano-porous thin-film cell encapsulation device from PCL was developed using a nanotemplating technique18. Although it still maintained flexibility, the material was engineered to have precise nanoscale pores and showed cell viability in allogeneic mouse models for up to 90 days. The lack of foreign body response, in combination with rapid neovascularization around the device, demonstrates the promise of using this technology for cell encapsulation. Another macroencapsulation device that uses microfabrication technology is called the Nanogland. It consists of an outer membrane with parallel nanochannels (3.6–40 nm) and perpendicular microchannels (20–60 microns) surrounding islets. The nanochannels are designed to provide immunoprotection and the microchannels are thought to help with engraftment. Subcutaneous implantation of the Nanogland with human islets in mice showed the survival of implants for more than 120 days67. The defined architecture was hypothesized to improve both vascularization and immune protection in vivo; however, long-term glycaemic control has not yet been evaluated.
Facilitating nutrient and oxygen transport.
The functionality of cell-based devices has been limited by inadequate oxygen delivery owing to a lack of immediate angiogenesis after implantation57,68,69. It is well known that insufficient oxygen levels lead to cell apoptosis, particularly for highly metabolic cells such as β-cells that reduce insulin production under low oxygen tension70,71. Pancreatic islets, as highly metabolic cells, pose an especially difficult challenge for encapsulation technology. The delivery of sufficient oxygen requires diffusion from the surrounding blood vessels to the device, across the immunobarrier membrane, and then through the interior of the device to the cells themselves. The volume of cells that can occupy the device is constrained by oxygen supply limitations in the interior of the device. To enable sufficient oxygen diffusion and thus prevent cell death, the device diameter can be no more than a few hundred microns72. This limits the geometry of an encapsulation device in order to load enough cells. For example, a hollow fibre device with a diameter of 200 microns would need to be 1,700 cm long to support the viability of 250,000 IE63. Studies by the Papas group73,74 have suggested that the maximum number of cells is 1,000 IEQ for 1 cm2 of surface area, with the oxygen entering from both sides of a device. It is therefore crucial to consider oxygen requirements a priori to designing the encapsulation device and to develop ways in which to improve oxygenation.
As encapsulation devices are often implanted or injected into avascular spaces, one must try to limit the amount of hypoxia that cells experience when first introduced into the body. Designing the device with appropriate porosity and dimensions, as well as high surface to volume ratios, will help in part. However, this approach may not fulfil the oxygen needs for transplanted cells. Prevascularization of the transplant site75,76 has been encouraging for enhancing islet engraftment. A catheter was subcutaneously implanted and removed after 4 weeks to initiate and terminate a foreign body response that creates a space lined with neovessels. Transplantation of islets into the space enabled the reversal of diabetes76. The transplantation of co-encapsulated pig islets with adipose or bone marrow mesenchymal stem cells improved islet survival and function in vitro, and oxygenation and neoangiogenesis post-transplantation77.
Additional targeted methods include facilitating more rapid vascularization through the delivery of growth factors78,79, the incorporation of oxygen carriers within biomaterials80 and the in situ generation of supplemental oxygen81-84. Encapsulation devices in development by Theracyte and Sernova (see below) have a membrane or a series of rods that promote vascularization, and, upon vessel ingrowth, islets are delivered into the pouch (Theracyte) or into space cleared by the removal of the rods (Sernova). Mouse syngeneic islets and porcine autografts implanted within these devices into the subcutaneous space have been shown to induce normoglycaemia for extended periods of up to 100 days46-48. Localized delivery of angiogenic factors has been used to increase vascularization and enhance islet engraftment and function45,85,86. Alternatively, modulating the immune response at the site of implantation can promote effective vascularization87,88.
More recently, the localized generation of oxygen has been used. One approach is to insert calcium peroxide within polydimethylsiloxane disks for use as an oxygen-generating biomaterial57. By encapsulating solid peroxide within a highly hydrophobic biomaterial, a diffusional barrier is created that is capable of modulating the release of oxygen for more than 40 days. The geometry and dimensions of the disk, as well as the calcium peroxide loading, can be manipulated to achieve the desired oxygen release kinetics.
Immunomodulatory approaches
The innate immune system is the initial barrier with the potential to induce cell damage following transplantation89. Encapsulation of islets within biomaterial devices has the potential to ameliorate these responses to promote survival post-transplantation, and to thereby facilitate long-term islet function.
Immobilized ligands to enhance immunoprotection.
Several cell surface molecules have been associated with the establishment of immune privilege by manipulating T cell function at the local site90-92. These include Fas ligand (FasL), TNF-related apoptosis-inducing ligand (TRAIL) and CD200. Ligand–receptor ligation and the subsequent engagement of cell death pathways initiate activation-induced cell death (AICD), playing a pivotal part in immune homeostasis and self-tolerance92. The presentation of FasL has been most extensively studied as a means to eliminate T effector cells that would normally target the graft. The co-transplantation of FasL-overexpressing myoblasts with islets has restored euglycaemia without the need for sustained immunosuppression93. More recently, a FasL protein has been engineered that has been used to modify cells before transplantation, which, combined with short-term rapamycin treatment, yielded long-term engraftment of allogeneic and xenogeneic islets94. The use of FasL has been shown to be highly effective and potent, and represents an opportunity to investigate the complex biology that is elicited by the presentation (concentration, and immobilized versus soluble) on the relevant cell types within the various tissues being used for islet transplantation. Immobilized peptides have similarly been used to protect islets against the cytotoxic effects of diffusible factors95-98.
This approach has also been applied to encapsulation. For example, immobilization of a peptide that is inhibitory to the cell surface interleukin-1 (IL-1) receptor maintained the viability of cells that were encapsulated within PEG-based hydrogels that were exposed to combinations of cytokines, including IL-1β, tumour necrosis factor (TNF) and interferon-γ (IFNγ)95. These peptide-modified hydrogels could efficiently protect encapsulated cells against β-cell-specific T cells and supported glucose-stimulated insulin release by islets in vitro95.
Drug-releasing and cytokine-releasing scaffolds or capsules.
The material used as the vehicle for cell encapsulation and transplantation can also be engineered to release factors for dampening local inflammation and creating immune-privileged sites99. Cytokines (such as transforming growth factor-β (TGFβ) and IL-10 (REFS 100-102)), chemokines (such as CCL2 (also known as MCP1) and CXCL12 (also known as SDF1)103,104), cellular enzymes (IDO1 (REF. 105)) and prostaglandins (LTB4 and PGE2 (REFS 106-108)) are among the factors that have been locally delivered either to attenuate the local inflammatory response, through directly polarizing the cells towards an anti-inflammatory response, or to recruit suppressive cell types. The localized delivery of CXCL12 from alginate-encapsulated islets has been shown to support long-term allogeneic and xenogeneic islet transplantation without systemic immune suppression109. CXCL12 has the capacity to repel effector T cells while recruiting regulatory T cells (Treg cells) and to provide a pro-survival signal for β-cells. Similarly, islets transduced to express CCL22 induced the prolonged protection of islet allografts, maintaining euglycaemia in 75% of recipients for 80 days110. CCL22 expression was associated with an increased frequency of Treg cells, and the absence of antidonor antibodies. In addition, short-term release of TGFβ that was localized to the islet graft resulted in fewer infiltrating inflammatory immune cells and promoted the longer survival of transplant islet allografts111. Scaffolds can be engineered to release factors alone or in combination to maximally attract and/or induce suppressor cell phenotypes that can attenuate inflammatory responses.
Material chemistry and topography for immunomodulation.
The surface topography of the encapsulation device can modulate immune responses at the host–implant interface112. Porous materials promote vascularization and less fibrous tissue encapsulation relative to non-porous biomaterials. Porosity on the scale of 30–40 μm has been shown to modulate the polarization of macrophages, leading to fewer foreign body giant cells (FBGCs) and enhanced tissue repair113. Nanotopography has also been shown to modulate the immune response, with reductions in the extent of inflammatory macrophages114. Similarly, surface alignment of nanofibres can reduce the host immune reaction and can generate a thinner fibrous capsule compared with random fibres and films115. The fibre diameter also has the potential to modulate the release of pro-inflammatory cytokines116. For microencapsulation capsules, dimensions have an important role in the inflammatory response. For example, spheres with a diameter in the range of 1.5–2.5 mm had a significantly decreased foreign body response compared with smaller diameter spheres (<1 mm)117. However, the benefits of the decreased response with an increased capsule diameter must be balanced with the limitations of the volume of material that can be delivered.
Cell co-transplantation approaches.
The co-delivery of cells that are capable of modulating immune responses is being investigated as a means to provide protection both locally and potentially at distal sites. The use of mesenchymal stem cells (MSCs) is promising, based on their ability to modulate the local immune response, such as macrophage activation and T cell phenotype, in the context of alloimmune responses113,118-121.
MSCs have promoted the regeneration of pancreatic islets through an ability to restore the balance between T helper 1 (TH1) and TH2 responses122. One caveat to cell-mediated therapies is that there can be source-dependent variability in the efficacy of immunomodulatory properties. MSCs modulate myeloid leukocytes and lymphocytes that are involved in the immune response through multiple mechanisms, including direct cell–cell contact and indirect contact through cytokines and signalling molecules113,118-121,123. Relative to the drug-delivery strategies, transplantation of MSCs results in the secretion of numerous proteins that modulate a response113,119. MSCs have also been demonstrated to reduce inflammation and to confer tolerance to cell transplants119,123. Similarly, MSCs co-transplanted with allogeneic cells suppressed T cell activity and improved graft survival124. The co-encapsulation of MSCs with syngeneic islets and subsequent intraperitoneal transplantation has been shown to improve graft function in murine models relative to mice transplanted with encapsulated islets alone125. Clinical trials involving MSC delivery are currently recruiting, with the initial purpose of demonstrating safety and tolerability of autologous MSCs. Subsequently, MSCs will be infused immediately after islet autograft to determine whether glycaemic control can be improved.
Treg cells have also been co-transplanted to promote the long-term survival and function of transplanted cells without systemic immunosuppression126,127. Two types of CD4+ Treg cells, thymic-derived natural Treg cells (tTreg cells) and Treg cells induced in the periphery (pTreg cells) in response to antigen, have been reported to promote peripheral tolerance128. The innate ability of Treg cells to induce tolerance provides a viable platform on which to develop cell-based therapeutics for the treatment of autoimmune and alloimmune responses. Multiple mechanisms are used by Treg cells to reduce effector T cells (Teff cells) and dendritic cell (DC) activity, including modulating DC activity with co-stimulatory receptors, competition for antigen-presenting cells (APCs) with Teff cells, and release of cytokines129. Treg cells are reported to affect their immunosuppressive actions through the secretion of TGFβ, IL-10, IL-35 and galectin-1, and through cell–cell interactions involving glucocorticoid-induced TNFR-related protein (GITR), cytotoxic T lymphocyte-associated protein 4 (CTLA4), CD39, CD73 and lymphocyte activation gene 3 (LAG3)128. In transplanting Treg cells, the choice of polyclonal Treg cells relative to antigen-specific Treg cells, the antigen specificity, and the dosage remain open questions as Treg cell therapeutic trials are being designed130,131.
Although polyclonal Treg cells can be more readily produced132,133, preclinical data indicate that antigen specificity substantially improves suppressor function134. Furthermore, selecting the correct antigen specificity for expansion is not obvious given the complex immune setting of autoimmune T1D and allogeneic islet transplantation. Antigen-specific Treg cells co-transplanted with islets within diabetic mice prevented autoimmune rejection and allowed the restoration of normoglycaemia126. Interestingly, although the transplanted Treg cells were antigen specific, they recruited Treg cells with alternative specificities to islet grafts. Furthermore, the local delivery of Treg cells also protected cells at distal sites, indicating the potential for systemic protection with localized delivery. As with the encapsulation of MSCs, the encapsulation of Treg cells may similarly be able to modulate the local immune response to promote engraftment and long-term function.
Identifying replenishable cell sources
Given the limited number of allogeneic donor islets available for transplantation, several alternative sources of islet cells are currently being investigated. Porcine islets have the potential to provide sufficient islet numbers, and have been effective in NHP models that are provided with systemic immunosuppression135,136. Indeed, wild-type porcine islets have been isolated, transplanted intrahepatically to NHP models and shown to fully reverse diabetes137-145. However, intrahepatic transplantation of islets can induce an instant blood-mediated inflammatory reaction (IBMIR), which limits prolonged islet survival146. Encapsulated porcine islets have been shown to support graft survival for at least 6 months in NHPs41,147, and a second transplantation after the initial graft dysfunction provided glucose control for an additional 18 weeks in two recipients. These findings are consistent with independent reports from multiple groups that have transplanted non-encapsulated islets in the presence of immunosuppression in NHP models, in which long-term survival exceeding 6 months was achieved136,137,148,149. At least one group has combined porcine islet encapsulation with immunosuppressive co-stimulatory blockade in mouse models, with measurable levels of porcine C peptide and near-normal in vivo glucose tolerance tests for more than 450 days150. Porcine islets, however, pose a risk for the transmission of infections, of which porcine endogenous retroviruses are a particular concern. However, this risk is likely to be low, and emerging gene-editing technologies can further reduce the risk151. Gene editing can also be tailored to the transplantation mechanism, with intraportal islet xenografts benefiting from the expression of anticoagulant and anti-inflammatory transgenes, whereas cytoprotective transgenes are likely to be more relevant for encapsulated islets151,152. Pig islets that were genetically engineered so that they do not express the major antigens that are associated with rejection and do not secrete immunomodulatory factors, have been shown to promote islet survival and the maintenance of euglycaemia135. Neonatal porcine islets (NPIs) are also attractive given their resistance to hypoxia, human pro-inflammatory cytokines and hyperglycaemia, and their ability to differentiate and proliferate153. The transplantation of NPIs normalized blood glucose levels and provided a robust response to a glucose tolerance test.
Recent reports on the generation of insulin-producing cells from hESCs have demonstrated their potential as a cell source56,154-157. However, hESCs must be differentiated before implantation, and, so far, mature β-cells have not yet been successfully generated in vitro. However, several milestones have been achieved with the development of culture systems that enable hESCs to form definitive endoderm158, with subsequent development through pancreatic endoderm to endocrine cells that are capable of synthesizing pancreatic hormones159. These endocrine cells are able to develop into glucose-responsive insulin-secreting cells after implantation into mice160. Subsequently, the Kieffer laboratory built on these procedures to produce pancreatic progenitors in vitro that could normalize blood glucose levels in diabetic mice after approximately 120 days157. The transplantation of these cells within a Theracyte device has demonstrated the potential for survival and subsequent maturation towards a mature β-cell following transplantation161-164. The Kieffer group recently published a longer in vitro culture protocol by which hESCs develop into immature insulin-producing β-cells that responded to glucose challenge in vitro and that induced normoglycaemia within 40 days156. The Melton154 and Hebrok155 laboratories also reported a culture system for generating insulin-producing cells from hESCs that can normalize hyperglycaemia in diabetic mice. Furthermore, encapsulation of these hESC-derived immature β-cells within alginate-based hydrogels and transplantation into the intraperitoneal space in immune-competent mice rapidly established euglycaemia that persisted for 25 weeks without the use of immunosuppressive therapies56. The use of embryonic cell sources is attractive owing to their potential to create cell banks for which culture conditions can be standardized to produce a consistent cell product. Human induced pluripotent stem cells (hiPSCs) remain a compelling cell source156, as they could avoid the need for immunosuppression. However, the standardization of procedures for the generation of cells that would be required for regulatory approval may be both challenging and costly. Recently, antral stomach enteroendocrine cells were converted to insulin-positive cells that possessed molecular and functional hallmarks of pancreatic β-cells165. Bioengineered stomach spheres were able to control blood glucose levels and it was postulated that the number and size of transplanted stomach spheres could be manipulated to control β-cell numbers.
Importantly, the delivery system must be designed for the specific cell source. The immunological response to porcine islets relative to allogeneic cells will substantially differ, with the xenogeneic system potentially requiring more substantial immunomodulation locally and/or systemically135. Furthermore, the material requirements for the transplantation of adult islets may differ relative to the systems for the transplantation of neonatal or progenitor cells. The neonatal and progenitor cells are not fully mature, and the environment created by the materials will need to support their in vivo maturation to fully mature β-cells166. The potential for immature cells within the transplant may present safety concerns that should be addressed, such as through the ability to retrieve the implant should issues arise.
First-generation designs in the clinic
A small number of encapsulation systems have been applied clinically (TABLE 1), all of which have demonstrated good safety profiles, although it is too early to evaluate functional outcomes14.
Table 1∣.
Islet and β-cell encapsulation systems currently in clinical trials
| Device or method | Experimental intervention | Properties | Trial phase | Refs |
|---|---|---|---|---|
| Sernova Cell Pouch | Implantation of allogeneic islets into the Sernova Cell Pouch following pre-vascularization |
|
I/II | 192 |
| Diabecell | Laparoscopic delivery of alginate encapsulated porcine islets |
|
II | 193 |
| Monolayer alginate encapsulation | A monolayer patch of alginate encapsulated allogeneic islets |
|
I | 194 |
| Alginate encapsulation | Implantation of alginate encapsulated allogeneic islets |
|
II | 195 |
| ViaCyte Encaptra | Encaptra containing allogeneic hESC-derived pancreatic progenitors |
|
I/II | 196 |
| βAir artificial pancreas | Macroencapsulation of allogeneic islets in βAir that provides oxygen to the cells |
|
I/II | 197 |
| Thrombin plasma gel | Allogeneic islets are suspended in a gel formed from autologous plasma and recombinant thrombin |
|
I/II | 198 |
hESC, human embryonic stem cell.
Microcapsules formed from alginate and with a diameter in the range of 300–400 μm have been used to encapsulate allogeneic islets, with a modest capsule thickness to reduce mass transport limitations. These capsules were delivered intraperitoneally and have been able to reduce exogenous insulin requirements102,167-169. Clinical trials have initially focused on confirming safety with the xenotransplantation of 10,000 to 20,000 IEQ per kg body weight of alginate-encapsulated porcine islets, and have subsequently monitored HbA1C levels and determined the frequency with which patients were unaware of hypoglycaemic events. However, a challenge with intraperitoneal delivery is that oxygen levels in the peritoneum are lower than the levels necessary for maximal islet function63.
More recently, Beta-O2 developed the β Air device to provide exogenous oxygen (FIG. 1b). The disc-shaped device consists of two major components — an islet module containing islets encapsulated in an alginate hydrogel slab, which is separated from the implantation pocket, and a gas chamber, which is separated from the islet module170. The device is implanted subcutaneously, with access ports placed on the dorsal side of the animal between the scapula, connected by short polyurethane tubes, with the access ports used for daily filling with oxygen171. Islets within the central cavity receive oxygen by diffusion through gas-permeable membranes. A case report for this device in a single patient reported that islets retained function for the 10-month study duration, with a modest reduction in exogenous insulin83.
Other encapsulation devices that have reached clinical trials include the Theracyte device and the Sernova Cell Pouch14,46, which aim to pre-vascularize a subcutaneous site before the administration of the cells through a port. Enhancing microvasculature has the potential to significantly enhance the survival of encapsulated islets172. The Theracyte device is immunoisolating173-175, and is composed of a two-membrane pouch. The outer membrane has a 5 μm pore size to support cell infiltration and to promote angiogenesis throughout the device. The inner membrane has a pore size diameter of 0.4 μm for immunoisolating the islets adjacent to the vasculature. The original Theracyte device has been tested by multiple academic researchers and has evolved through multiple companies, including Living Cell Technologies, BetaLogics, and ultimately ViaCyte14 (FIG. 1a). ViaCyte has since developed a system known as Encaptra, which has a single membrane that is immunoisolating to protect the transplanted cells from direct interaction with immune cells, while allowing oxygen and nutrients to pass. ViaCyte is currently carrying out a phase I/II clinical trial using Encaptra with stem cell-derived cell sources to assess the safety and efficacy of the system176. In contrast to Encaptra, the Sernova Cell Pouch is not immunoisolating. The device is inserted under the skin for 30 days to enable vascular integration with the device. Subsequently, a series of rods are removed to expose channels that can be filled with transplanted islets. However, the 3-year phase I/II clinical study using this device recently terminated after recruiting three patients177.
A recent phase I/II pilot clinical trial has begun at the University of Miami, USA, to evaluate the safety and efficacy of transplanting allogeneic islets encapsulated within a plasmin-thrombin scaffold into the omentum using conventional immunosuppression, which will be applied with a single donor for treating people with brittle T1D178. Although this strategy does not avoid immunosuppression, success with the transplantation of islets at this extrahepatic site may provide a foundation for subsequent studies to locally and/or systemically modulate the immune response to prevent rejection.
Other applications of cell encapsulation
The technologies developed for islet transplantation may have utility in other cell transplantation strategies, and the potential use of such an approach is being investigated for various conditions. Indeed, the ability to regulate the delivery of a systemically available hormone could be applied to various diseases in addition to diabetes. This opportunity is exemplified by deficient pituitary function (hypopituitarism), which normally requires lifelong hormone replacement but which is associated with considerable side effects. The pituitary can modulate the function of the adrenal and thyroid glands, and the gonads (testes and ovaries), through the production of hormones, and can also receive signals from those tissues. Growth hormone deficiency and adrenal insufficiency can lead to developmental issues in children (such as short stature and failure to thrive), and can affect the quality of life for adults (for example, decreased muscle mass, impaired memory and fatigue). The potential of differentiating stem cells into cells of the pituitary is emerging as an approach that may provide therapies for hypopituitarism179. The hormonal communication between the pituitary and the gonads can affect fertility in adults, and can affect the ability of children to undergo puberty. Transplantation of gonadal tissue could enable young children to undergo puberty and could provide opportunities to preserve fertility in adults180. Finally, we note that dysregulated endocrine signalling has been linked to processes that are associated with age-related diseases, including cancer, cardiovascular disease, diabetes, osteoporosis and neurodegenerative diseases, all of which directly influence health in ageing181.
Furthermore, cells that intrinsically, or are genetically engineered to, secrete therapeutic proteins have been transplanted to provide sustained, and potentially localized, delivery for applications to prevent tissue degeneration, promote regeneration and as a cancer therapy. Cells engineered to secrete neurotrophic or angiogenic factors have been applied to prevent neuronal and vascular degeneration in the central nervous system (CNS) for therapies in Parkinson disease and Huntington disease182,183. MSCs have been encapsulated for transplantation after a myocardial infarction to promote cardiac repair184, and genetically engineered CHO cells that secrete angiogenic factors have been used to augment revascularization185. Similarly, the transplantation of alginate-encapsulated MSCs, which were engineered to secrete hemopexin-like protein, were able to reduce tumour growth and blood vessel formation while increasing apoptosis in a mouse model of glioblastoma186. In addition, encapsulated cells that secrete immunostimulatory monoclonal antibodies have been used to enhance tumour-specific cellular immunity187. Clearly, the development of effective cell encapsulation systems that overcome the challenges discussed above will have numerous potential applications for the treatment of various diseases.
Outlook
Recent advances in material design, nanotechnology and immunomodulation have led to promising approaches in cell-based microencapsulation and macroencapsulation. By combining our expertise across disciplines ranging from electrical engineering to immunology, we can begin to address the multiple challenges that are involved in translating encapsulated cell therapy from the laboratory to the clinic. Future success requires a willingness to collaborate, to combine new ‘device’ technologies with ‘cell’ technologies, and to understand the limitations of the biological environment in which human cell therapy must exist.
Acknowledgements
Financial support for this work was provided by R01EB009910 (L.D.S.) and JDRF (T.D. and L.D.S.).
Glossary
- Type 1 diabetes
(T1D). A chronic condition of aberrant glucose homeostasis that is characterized by a severe deficiency of insulin secretion resulting from atrophy of the islets of Langerhans.
- β-Cells
Insulin-secreting cells of the islets of Langerhans.
- Hyperglycaemia
Elevated blood glucose above normal levels.
- Hypoglycaemia
Suppressed blood glucose below normal levels.
- Immunosuppression
Suppression (such as, by drugs or disease) of the immune response.
- Xenogeneic
Derived from, originating in or being a member of another species.
- Encapsulation
To surround, encase or protect in or as if in a capsule.
- Normoglycaemia
The presence of a normal concentration of glucose in the blood.
- Vascularization
The formation of blood vessels.
- Syngeneic
Involving, derived from, or being genetically identical or similar individuals of the same species, especially with respect to antigenic interaction.
- Allogeneic
Involving, derived from or being individuals of the same species that are sufficiently genetically dissimilar to interact antigenically.
- Fibrosis
A condition marked by an increase in interstitial fibrous or scar tissue.
- Immunogenicity
The ability of a particular substance to provoke an immune response in the body of a human or an animal.
- Hypoxia
A deficiency of oxygen reaching the tissues of the body.
- Self-tolerance
The failure to mount an immune response to a person’s own proteins and other antigens.
- Cytokines
Members of a class of immunoregulatory proteins (interleukin or interferon) that are secreted by cells especially of the immune system.
- Regulatory T cells
(Treg cells). A subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens and prevent autoimmune disease.
Footnotes
Competing interests statement
The authors declare competing interests: see Web version for details.
References
- 1.Centers for Disease Control and Prevention. Diabetes report card 2014. CDC; http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf (2015). [Google Scholar]
- 2.National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014: estimates of diabetes and its burden in the United States. CDC; http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (2014). [Google Scholar]
- 3.Ryan EA et al. Five-year follow-up after clinical islet transplantation. Diabetes 54, 2060–2069 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Barton FB et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436–1445 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AM et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med 343, 230–238 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med 355, 1318–1330 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Hering BJ et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 39, 1230–1240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanekzai J, Isenovic ER & Mousa SA Treatment options for diabetes: potential role of stem cells. Diabetes Res. Clin. Pract 98, 361–368 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Hentze H et al. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2, 198–210 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Kieffer TJ Closing in on mass production of mature human beta cells. Cell Stem Cell. 18, 699–702 (2016). [DOI] [PubMed] [Google Scholar]
- 11.de Vos P, Spasojevic M & Faas MM Treatment of diabetes with encapsulated islets. Adv. Exp. Med. Biol 670, 38–53 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Vaithilingam V & Tuch BE Islet transplantation and encapsulation: an update on recent developments. Rev. Diabet. Stud 8, 51–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soon-Shiong P et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343, 950–951 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Scharp DW & Marchetti P Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev 67–68, 35–73 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Desai TA, West T, Cohen M, Boiarski T & Rampersaud A Nanoporous microsystems for islet cell replacement. Adv. Drug Deliv. Rev 56, 1661–1673 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Omer A et al. Exercise induces hypoglycemia in rats with islet transplantation. Diabetes 53, 360–365 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Trivedi N et al. Islets in alginate macrobeads reverse diabetes despite minimal acute insulin secretory responses. Transplantation 71, 203–211 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Nyitray CE et al. Polycaprolactone thin-film micro- and nanoporous cell-encapsulation devices. ACS Nano 9, 5675–5682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisceglie VV Uber die antineoplastiche Immunitat. E. Krebsforch 40, 141–158 (in German) (1933). [Google Scholar]
- 20.Algire GH & Legallais FY Recent developments in the transparent-chamber technique as adapted to the mouse. J. Natl Cancer Inst 10, 225–253 (1949). [PubMed] [Google Scholar]
- 21.Algire GH, Weaver JM & Prehn RT Growth of cells in vivo in diffusion chambers. I. Survival of homografts in immunized mice. J. Natl Cancer Inst 15, 493–507 (1954). [PubMed] [Google Scholar]
- 22.Prehn RT, Weaver JM & Algire GH The diffusion-chamber technique applied to a study of the nature of homograft resistance. J. Natl Cancer Inst 15, 509–517 (1954). [PubMed] [Google Scholar]
- 23.Weaver JM, Algire GH & Prehn RT The growth of cells in vivo in diffusion chambers. II. The role of cells in the destruction of homografts in mice. J. Natl Cancer Inst 15, 1737–1767 (1955). [PubMed] [Google Scholar]
- 24.Chang TM Semipermeable microcapsules. Science 146, 524–525 (1964). [DOI] [PubMed] [Google Scholar]
- 25.Lim F & Sun AM Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908–910 (1980). [DOI] [PubMed] [Google Scholar]
- 26.O’Shea GM, Goosen MF & Sun AM Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochim. Biophys. Acta 804, 133–136 (1984). [DOI] [PubMed] [Google Scholar]
- 27.Klock G et al. Production of purified alginates suitable for use in immunoisolated transplantation. Appl. Microbiol. Biotechnol 40, 638–643 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Otterlei M et al. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother 10, 286–291 (1991). [DOI] [PubMed] [Google Scholar]
- 29.Sawhney AS, Pathak CP & Hubbell JA Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials 14, 1008–1016 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Sefton MV & Stevenson WTK Microencapsulation of live animal cells using polyacrylates. Adv. Polym. Sci 107, 143–197 (1993). [Google Scholar]
- 31. Wang T et al. An encapsulation system for the immunoisolation of pancreatic islets. Nat. Biotechnol 15, 358–362 (1997). Examines the composition of microcapsules with regards to suitability for cell encapsulation.
- 32.Souza YE et al. Islet transplantation in rodents. Do encapsulated islets really work? Arq. Gastroenterol 48, 146–152 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Duvivier-Kali VF, Omer A, Lopez-Avalos MD, O’Neil JJ & Weir GC Survival of microencapsulated adult pig islets in mice in spite of an antibody response. Am. J. Transplant 4, 1991–2000 (2004). [DOI] [PubMed] [Google Scholar]
- 34. Duvivier-Kali VF, Omer A, Parent RJ, O’Neil JJ & Weir GC Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 50, 1698–1705 (2001). Early demonstration of immunoprotective effects of the microencapsulation approach.
- 35.Gates RJ, Hunt MI, Smith R & Lazarus NR Return to normal of blood-glucose, plasma-insulin, and weight gain in New Zealand obese mice after implantation of islets of Langerhans. Lancet 2, 567–570 (1972). [DOI] [PubMed] [Google Scholar]
- 36.Strautz RL Studies of hereditary-obese mice (obob) after implantation of pancreatic islets in Millipore filter capsules. Diabetologia 6, 306–312 (1970). [DOI] [PubMed] [Google Scholar]
- 37.Brauker J, Martinson LA, Young SK & Johnson RC Local inflammatory response around diffusion chambers containing xenografts. Nonspecific destruction of tissues and decreased local vascularization. Transplantation 61, 1671–1677 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Brauker JH et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res 29, 1517–1524 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K et al. Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation 66, 21–28 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Soon-Shiong P et al. Long-term reversal of diabetes by the injection of immunoprotected islets. Proc. Natl Acad. Sci. USA 90, 5843–5847 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dufrane D, Goebbels RM, Saliez A, Guiot Y & Gianello P Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 81, 1345–1353 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Elliott RB et al. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant. Proc 37, 3505–3508 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Storrs R, Dorian R, King SR, Lakey J & Rilo H Preclinical development of the Islet Sheet. Ann. NY Acad. Sci 944, 252–266 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Robertson RP Islet transplantation as a treatment for diabetes — a work in progress. N. Engl. J. Med 350, 694–705 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Najjar M et al. Fibrin gels engineered with proangiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol. Bioeng 112, 1916–1926 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Pepper AR et al. Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device. Transplantation 99, 2294–2300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sernova Corp. Sernova’s Cell Pouch System. Sernova http://www.sernova.com/technology (accessed 1 Nov 2016). [Google Scholar]
- 48.Gu Y et al. Development of a new method to induce angiogenesis at subcutaneous site of streptozotocin-induced diabetic rats for islet transplantation. Cell Transplant. 10, 453–457 (2001). [PubMed] [Google Scholar]
- 49. Wang RN & Rosenberg L Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet–matrix relationship. J. Endocrinol 163, 181–190 (1999). Shows the importance of the microenvironment in islet function.
- 50.Hauge-Evans AC, Squires PE, Persaud SJ & Jones PM Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 48, 1402–1408 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Chowdhury A, Dyachok O, Tengholm A, Sandler S & Bergsten P Functional differences between aggregated and dispersed insulin-producing cells. Diabetologia 56, 1557–1568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyitray CE, Chavez MG & Desai TA Compliant 3D microenvironment improves β-cell cluster insulin expression through mechanosensing and β-catenin signaling. Tissue Eng. Part A 20, 1888–1895 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dusseault J et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J. Biomed. Mater. Res. A 76, 243–251 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Peterson KP, Peterson CM & Pope EJ Silica solgel encapsulation of pancreatic islets. Proc. Soc. Exp. Biol. Med 218, 365–369 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Vegas AJ et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol 34, 345–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vegas AJ et al. Long term glycemic control using polymer-encapsulated human stem cell-derived β cells in immune-competent mice. Nat. Med 22, 306–311 (2016). Demonstration of long-term glycaemic control using a microencapsulation strategy in an immune-competent animal model.
- 57.Pedraza E, Coronel MM, Fraker CA, Ricordi C & Stabler CL Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl Acad. Sci. USA 109, 4245–4250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rokstad AM et al. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 7, 2566–2578 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Mendelsohn A & Desai T Inorganic nanoporous membranes for immunoisolated cell-based drug delivery. Adv. Exp. Med. Biol 670, 104–125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen J et al. Tunable microfibers suppress fibrotic encapsulation via inhibition of TGFβ signaling. Tissue Eng. Part A 22, 142–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitsuo M et al. Efficacy of mesh reinforced polyvinylalcohol tube as a novel device for bioartificial pancreas: a functional study of rat islets in vivo. Transplant. Proc 24, 2939–2940 (1992). [PubMed] [Google Scholar]
- 62.Tomei AA et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc. Natl Acad. Sci. USA 111, 10514–10519 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Colton CK Oxygen supply to encapsulated therapeutic cells. Adv. Drug Deliv. Rev 67–68, 93–110 (2014). Comprehensive review of the oxygen requirements of encapsulation devices.
- 64.Alberts B et al. Molecular Biology of the Cell (Garland Publishing, 1994). [Google Scholar]
- 65.Branden C & Tooze J Introduction to Protein Structure (Garland Publishing, 1991). [Google Scholar]
- 66.La Flamme KE, LaTempa TJ, Grimes CA & Desai TA The effects of cell density and device arrangement on the behavior of macroencapsulated beta-cells. Cell Transplant. 16, 765–774 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Sabek OM et al. Characterization of a nanogland for the autotransplantation of human pancreatic islets. Lab. Chip 13, 3675–3688 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Cantley J, Grey ST, Maxwell PH & Withers DJ The hypoxia response pathway and β-cell function. Diabetes Obes. Metab 12 (Suppl. 2), 159–167 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Ballian N & Brunicardi FC Islet vasculature as a regulator of endocrine pancreas function. World J. Surg 31, 705–714 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Dionne KE, Colton CK & Yarmush ML Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42, 12–21 (1993). [DOI] [PubMed] [Google Scholar]
- 71.Sato Y et al. Cellular hypoxia of pancreatic β-cells due to high levels of oxygen consumption for insulin secretion in vitro. J. Biol. Chem 286, 12524–12532 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dulong JL & Legallais C A theoretical study of oxygen transfer including cell necrosis for the design of a bioartificial pancreas. Biotechnol. Bioeng 96, 990–998 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Papas KK, Avgoustiniatos ES & Suszynski TM Effect of oxygen supply on the size of implantable islet-containing encapsulation devices. Panminerva Med. 58, 72–77 (2016). [PubMed] [Google Scholar]
- 74.Suszynski TM, Avgoustiniatos ES & Papas KK Intraportal islet oxygenation. J. Diabetes Sci. Technol 8, 575–580 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pileggi A et al. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 81, 1318–1324 (2006). [DOI] [PubMed] [Google Scholar]
- 76. Pepper AR et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol 33, 518–523 (2015). Demonstration of prevascularization of encapsulation devices as a strategy to promote cell survival.
- 77.Veriter S et al. Improvement of subcutaneous bioartificial pancreas vascularization and function by coencapsulation of pig islets and mesenchymal stem cells in primates. Cell Transplant. 23, 1349–1364 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Zisch AH et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 17, 2260–2262 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Ehrbar M et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res 94, 1124–1132 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Harrison BS, Eberli D, Lee SJ, Atala A & Yoo JJ Oxygen producing biomaterials for tissue regeneration. Biomaterials 28, 4628–4634 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Oh SH, Ward CL, Atala A, Yoo JJ & Harrison BS Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30, 757–762 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Bloch K et al. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 12, 337–344 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Ludwig B et al. Transplantation of human islets without immunosuppression. Proc. Natl Acad. Sci. USA 110, 19054–19058 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu CL, Lin LY, Yang JS, Chan MC & Hsueh CM Attenuation of lipopolysaccharide-induced acute lung injury by treatment with IL-10. Respirology 14, 511–521 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Trivedi N, Steil GM, Colton CK, Bonner-Weir S & Weir GC Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant. 9, 115–124 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Phelps EA, Templeman KL, Thule PM & Garcia AJ Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl Res 5, 125–136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krieger JR et al. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1α-releasing hydrogels enhances microvascular network remodeling. Biomaterials 77, 280–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwee BJ & Mooney DJ Manipulating the intersection of angiogenesis and inflammation. Ann. Biomed. Eng 43, 628–640 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chong AS & Alegre ML The impact of infection and tissue damage in solid-organ transplantation. Nat. Rev. Immunol 12, 459–471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falschlehner C, Schaefer U & Walczak H Following TRAIL’s path in the immune system. Immunology 127, 145–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pearl-Yafe M et al. The dual role of Fas-ligand as an injury effector and defense strategy in diabetes and islet transplantation. Bioessays 28, 211–222 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Miller SD, Turley DM & Podojil JR Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol 7, 665–677 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Lau HT, Yu M, Fontana A & Stoeckert CJ Jr. Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science 273, 109–112 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Yolcu ES et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J. Immunol 187, 5901–5909 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su J et al. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials 31, 308–314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elias D et al. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc. Natl Acad. Sci. USA 88, 3088–3091 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zanin-Zhorov A et al. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest 116, 2022–2032 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Takasaki W, Kajino Y, Kajino K, Murali R & Greene MI Structure-based design and characterization of exocyclic peptidomimetics that inhibit TNF α binding to its receptor. Nat. Biotechnol 15, 1266–1270 (1997). [DOI] [PubMed] [Google Scholar]
- 99.Dumont CM, Park J & Shea LD Controlled release strategies for modulating immune responses to promote tissue regeneration. J. Control. Release 219, 155–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuff CA, Martiney JA, Berman JW & Brosnan CF Differential effects of transforming growth factor-β1 on interleukin-1-induced cellular inflammation and vascular permeability in the rabbit retina. J. Neuroimmunol 70, 21–28 (1996). [DOI] [PubMed] [Google Scholar]
- 101.Martinez FO, Sica A, Mantovani A & Locati M Macrophage activation and polarization. Front. Biosci 13, 453–461 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Tuch BE et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32, 1887–1889 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karin N The multiple faces of CXCL12 (SDF-1α) in the regulation of immunity during health and disease. J. Leukoc. Biol 88, 463–473 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Sanchez-Martin L et al. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117, 88–97 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Chen G et al. Intragraft CD11b+ IDO+ cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am. J. Transplant 12, 2920–2929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonnamain V et al. Expression of heme oxygenase-1 in neural stem/progenitor cells as a potential mechanism to evade host immune response. Stem Cells 30, 2342–2353 (2012). [DOI] [PubMed] [Google Scholar]
- 107.Nazmi A, Mohamed Arif I, Dutta K, Kundu K & Basu A Neural stem/progenitor cells induce conversion of encephalitogenic T cells into CD4+-CD25+-FOXP3+ regulatory T cells. Viral Immunol. 27, 48–59 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Wang L et al. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp. Neurol 216, 177–183 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Chen T et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am. J. Transplant 15, 618–627 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Montane J et al. CCL22 prevents rejection of mouse islet allografts and induces donor-specific tolerance. Cell Transplant. 24, 2143–2154 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Liu JM et al. Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials 80, 11–19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Madden LR et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl Acad. Sci. USA 107, 15211–15216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan Z, Zhuansun Y, Chen R, Li J & Ran P Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp. Cell Res 324, 65–74 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Lee S et al. Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium. Acta Biomater. 7, 2337–2344 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Cao H, McHugh K, Chew SY & Anderson JM The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J. Biomed. Mater. Res. A 93, 1151–1159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saino E et al. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules 12, 1900–1911 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Veiseh O et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater 14, 643–651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barbeau DJ et al. Early growth response-2 signaling mediates immunomodulatory effects of human multipotential stromal cells. Stem Cells Dev. 23, 155–166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.English K Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol 91, 19–26 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Luz-Crawford P et al. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther 4, 65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Obermajer N et al. Conversion of Th17 into IL-17Aneg regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J. Immunol 193, 4988–4999 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Ezquer F et al. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells 30, 1664–1674 (2012). [DOI] [PubMed] [Google Scholar]
- 123.Uccelli A, Moretta L & Pistoia V Mesenchymal stem cells in health and disease. Nat. Rev. Immunol 8, 726–736 (2008). [DOI] [PubMed] [Google Scholar]
- 124.Ding Y et al. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58, 1797–1806 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kerby A, Jones ES, Jones PM & King AJ Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy 15, 192–200 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Graham JG et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng. Part A 19, 1465–1475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marek N et al. Coating human pancreatic islets with CD4+ CD25highCD127− regulatory T cells as a novel approach for the local immunoprotection. Ann. Surg 254, 512–518 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Caridade M, Graca L & Ribeiro RM Mechanisms underlying CD4+ Treg immune regulation in the adult: from experiments to models. Front. Immunol 4, 378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Luo X, Miller SD & Shea LD Immune tolerance for autoimmune disease and cell transplantation. Annu. Rev. Biomed. Eng 18, 181–205 (2016). Discussion of current immune-tolerance strategies for cell therapies.
- 130.Tang Q & Lee K Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ Transplant 17, 349–354 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Safinia N, Scotta C, Vaikunthanathan T, Lechler RI & Lombardi G Regulatory T cells: serious contenders in the promise for immunological tolerance in transplantation. Front. Immunol 6, 438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bluestone JA et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl Med 7, 315ra189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Putnam AL et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am. J. Transplant 13, 3010–3020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Veerapathran A, Pidala J, Beato F, Yu XZ & Anasetti C Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood 118, 5671–5680 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nagaraju S et al. Islet xenotransplantation from genetically engineered pigs. Curr. Opin. Organ Transplant 18, 695–702 (2013). [DOI] [PubMed] [Google Scholar]
- 136. Hering BJ et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat. Med 12, 301–303 (2006). Demonstration of successful encapsulation of xenogeneic islets in a large animal model.
- 137.Cardona K et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med 12, 304–306 (2006). [DOI] [PubMed] [Google Scholar]
- 138.Shin JS et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am. J. Transplant 15, 2837–2850 (2015). [DOI] [PubMed] [Google Scholar]
- 139.van der Windt DJ et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am. J. Transplant 9, 2716–2726 (2009). [DOI] [PubMed] [Google Scholar]
- 140.Thompson P et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am. J. Transplant 11, 2593–2602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thompson P et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am. J. Transplant 12, 1765–1775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thompson P et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am. J. Transplant 11, 947–957 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bottino R et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am. J. Transplant 14, 2275–2287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hawthorne WJ et al. Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. Am. J. Transplant 14, 1300–1309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lowe M et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am. J. Transplant 12, 2079–2087 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gibly RF et al. Advancing islet transplantation: from engraftment to the immune response. Diabetologia 54, 2494–2505 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dufrane D, Goebbels RM & Gianello P Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 90, 1054–1062 (2010). [DOI] [PubMed] [Google Scholar]
- 148.Hecht G et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc. Natl Acad. Sci. USA 106, 8659–8664 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hering BJ & Walawalkar N Pig-to-nonhuman primate islet xenotransplantation. Transpl. Immunol 21, 81–86 (2009). [DOI] [PubMed] [Google Scholar]
- 150.Cui H et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation 88, 160–169 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Cowan PJ, Ayares D, Wolf E & Cooper DK First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes — chapter 2b: genetically modified source pigs. Xenotransplantation 23, 32–37 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Cooper DK, Ekser B, Ramsoondar J, Phelps C & Ayares D The role of genetically engineered pigs in xenotransplantation research. J. Pathol 238, 288–299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ellis C, Lyon JG & Korbutt GS Optimization and scale-up isolation and culture of neonatal porcine islets: potential for clinical application. Cell Transplant. 25, 539–547 (2015). Discusses the current issues associated with the use of porcine islets in cell-based therapy.
- 154.Pagliuca FW et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Russ HA et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rezania A et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol 32, 1121–1133 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Rezania A et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 61, 2016–2029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.D’Amour KA et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol 23, 1534–1541 (2005). [DOI] [PubMed] [Google Scholar]
- 159. D’Amour KA et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol 24, 1392–1401 (2006). Development of stem cell-derived endocrine cells for hormone production.
- 160.Kroon E et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol 26, 443–452 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Bruin JE et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 56, 1987–1998 (2013). [DOI] [PubMed] [Google Scholar]
- 162.Kirk K, Hao E, Lahmy R & Itkin-Ansari P Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 12, 807–814 (2014). [DOI] [PubMed] [Google Scholar]
- 163.Motte E et al. Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am. J. Physiol. Endocrinol. Metab 307, E838–E846 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Rezania A et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31, 2432–2442 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Ariyachet C et al. Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 18, 410–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weizman A, Michael I, Wiesel-Motiuk N, Rezania A & Levenberg S The effect of endothelial cells on hESC-derived pancreatic progenitors in a 3D environment. Biomater. Sci 2, 1706–1714 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Basta G et al. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care 34, 2406–2409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Calafiore R et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care 29, 137–138 (2006). [DOI] [PubMed] [Google Scholar]
- 169.Calafiore R et al. Standard technical procedures for microencapsulation of human islets for graft into nonimmunosuppressed patients with type 1 diabetes mellitus. Transplant. Proc 38, 1156–1157 (2006). [DOI] [PubMed] [Google Scholar]
- 170.Barkai U et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 22, 1463–1476 (2013). [DOI] [PubMed] [Google Scholar]
- 171. Neufeld T et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE 8, e70150 (2013). Development of macroencapsulation device for xenotransplantation.
- 172.Thomlinson RH & Gray LH The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9, 539–549 (1955). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sorenby AK et al. Macroencapsulation protects against sensitization after allogeneic islet transplantation in rats. Transplantation 82, 393–397 (2006). [DOI] [PubMed] [Google Scholar]
- 174.Sorenby AK et al. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: studies in a rodent model. Transplantation 86, 364–366 (2008). [DOI] [PubMed] [Google Scholar]
- 175.Kumagai-Braesch M et al. The TheraCyte device protects against islet allograft rejection in immunized hosts. Cell Transplant. 22, 1137–1146 (2013). [DOI] [PubMed] [Google Scholar]
- 176.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02239354 (2015). [DOI] [PubMed]
- 177.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01652911 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Berman DM et al. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes 65, 1350–1361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Willems C & Vankelecom H Pituitary cell differentiation from stem cells and other cells: toward restorative therapy for hypopituitarism? Regen. Med 9, 513–534 (2014). [DOI] [PubMed] [Google Scholar]
- 180.Shea LD, Woodruff TK & Shikanov A Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng 16, 29–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bao Q et al. Aging and age-related diseases — from endocrine therapy to target therapy. Mol. Cell. Endocrinol 394, 115–118 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Stover NP & Watts RL Spheramine for treatment of Parkinson’s disease. Neurotherapeutics 5, 252–259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bloch J et al. Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum. Gene Ther 15, 968–975 (2004). [DOI] [PubMed] [Google Scholar]
- 184.Yu J et al. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31, 7012–7020 (2010). [DOI] [PubMed] [Google Scholar]
- 185.Zhang H, Zhu SJ, Wang W, Wei YJ & Hu SS Transplantation of microencapsulated genetically modified xenogeneic cells augments angiogenesis and improves heart function. Gene Ther. 15, 40–48 (2008). [DOI] [PubMed] [Google Scholar]
- 186.Goren A, Dahan N, Goren E, Baruch L & Machluf M Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 24, 22–31 (2010). [DOI] [PubMed] [Google Scholar]
- 187.Dubrot J et al. Delivery of immunostimulatory monoclonal antibodies by encapsulated hybridoma cells. Cancer Immunol. Immunother 59, 1621–1631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Mellor AL & Munn DH Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol 8, 74–80 (2008). Reviews strategies for local immune modulation.
- 189.Glotz D & Tambur A Stratifying patients based on epitope mismatching: ready for primetime? Am. J. Transplant 15, 2021–2022 (2015). [DOI] [PubMed] [Google Scholar]
- 190.Spierings E Minor histocompatibility antigens: past, present, and future. Tissue Antigens 84, 374–360 (2014). [DOI] [PubMed] [Google Scholar]
- 191.Yuan R et al. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS ONE 3, e1924 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01652911 (2016). [DOI] [PubMed]
- 193.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01739829 (2014). [DOI] [PubMed]
- 194.US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00790257 (2016). [DOI] [PubMed]
- 195.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01379729 (2013). [DOI] [PubMed]
- 196.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02239354 (2015). [DOI] [PubMed]
- 197.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02064309 (2016). [DOI] [PubMed]
- 198.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02213003 (2016). [DOI] [PubMed]