ABSTRACT
Extraintestinal manifestations of inflammatory bowel disease occur commonly and can lead to considerable morbidity. Pancreatic manifestations of inflammatory bowel disease have been reported to be more common in Crohn's disease (CD) than ulcerative colitis. We report a case of granulomatous inflammation in the body of the pancreas with exocrine pancreatic insufficiency, which prompted a diagnosis switch from ulcerative colitis to CD. This is of interest to readers to remind them that pancreatic manifestations can occur and are more common in CD.
KEYWORDS: Crohn's disease, pancreatitis, granulomatous inflammation, extraintestinal manifestation
INTRODUCTION
Crohn's disease (CD) is an idiopathic, inflammatory disease, which can affect the entire gastrointestinal tract. Extraintestinal manifestations of inflammatory bowel disease (IBD) occur with an estimated prevalence of 21%–41%.1 Musculoskeletal, dermatologic, and ocular manifestations are the 3 most common.1 Extraintestinal manifestations are associated with a poorer prognosis, a decreased quality of life, and generally increased morbidity and mortality.2 Associations between IBD and conditions such as pancreatitis or exocrine pancreatic insufficiency (EPI) have been described in the literature.3–6 To the best of our knowledge, this is the first case of CD-associated granulomatous inflammation in the body of pancreas with EPI, which prompted a diagnosis change from suspected ulcerative colitis (UC) to CD.
CASE REPORT
A 60-year-old man with suspected UC (diagnosed in 2013) on mesalamine therapy presented to the clinic with 2 months of loose, pasty stools, and fecal urgency. Fecal calprotectin, sedimentation rate, and C-reactive protein were checked and normal. He underwent colonoscopy with endoscopic evidence of mild inflammation only in the rectum (Mayo score 1); surprisingly, histology of colonic biopsies showed active colitis in the right and left colon. Given that there seemed to be a discrepancy between patient symptoms and degree of macroscopic inflammation, additional testing was performed. Infectious etiologies were ruled out, and fecal elastase was 224 μg/g.
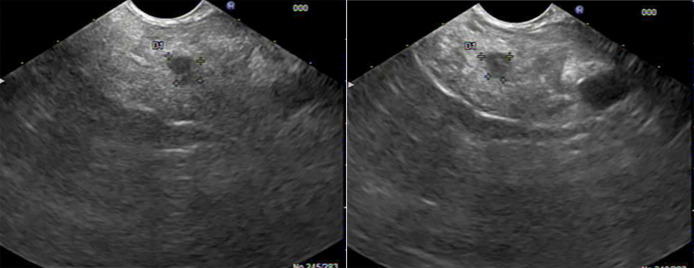
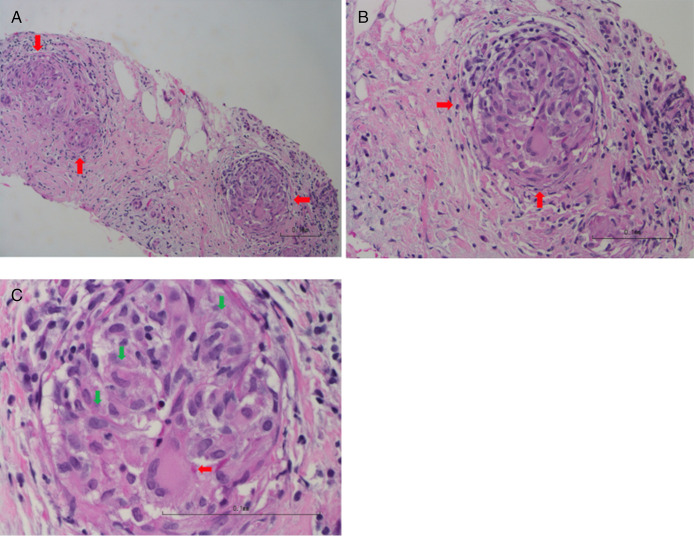
The patient was started on a trial of pancreatic enzyme supplementation with prompt resolution of loose stools, and therefore, pancreatic exocrine insufficiency was suspected. The patient underwent an upper endoscopy with endoscopic ultrasound to further assess the pancreas, which showed normal esophagus, stomach, and duodenum, and a 10-mm-by-8-mm hypoechoic lesion in the body of the pancreas with an intact interface between the lesion and the surrounding structures (Figure 1). Fine needle aspiration was performed, and histology revealed chronic, granulomatous, lymphocytic inflammation with no evidence of malignancy or infection, consistent with an extraintestinal manifestation of CD (Figure 2). The patient underwent small bowel video capsule endoscopy, which showed small ulcers scattered throughout the small bowel, which confirmed the change in the patient's diagnosis to CD from UC. The patient was started on a course of prednisone to induce remission and transitioned to infliximab monotherapy. Six months after infliximab initiation, the patient's fecal urgency resolved, and he had 1–3 formed bowel movements per day, despite stopping his pancreatic enzyme supplementation. He has since remained in steroid-free clinical remission.
Figure 1.
A 10-mm-by-8-mm hypoechoic lesion in the body of the pancreas.
Figure 2.
(A) Low power (10×) of the pancreatic fine needle aspiration cell block has denuded pancreatic acini and ducts. Three granulomas are seen (red arrows). (B) Medium power (20×) highlights lymphocytes on the right side with a granuloma (red arrows). (C) Higher power (40×) highlights 1 granuloma composed of epithelioid macrophages (green arrows) and multinucleated giant cells (red arrows).
DISCUSSION
Hyperamylasemia, hyperlipasemia, pancreatic exocrine insufficiency, and pancreatitis associated with IBD have been reported in the literature.3–6 The etiology of this association has been elusive. There have been some studies that have found increased prevalence of pancreatic autoantibodies (PABs) in patients with CD, suggesting that PABs may cause pancreatic dysfunction in CD.4,6 It is estimated that PABs are present in 20%–30% of patients with CD, 2%–9% of patients with UC, and less than 4% of patients without IBD.5 PABs are most commonly targeted against zymogen glycoprotein 2, which is found both on pancreatic cells as well as M cells, especially in the ileum.7 Therefore, it is currently hypothesized that ileal inflammation can lead to autoinflammation within the pancreas. Our patient had small bowel ulcers spread throughout the small bowel on video capsule endoscopy.
To the best of our knowledge, there have only been 2 case reports of granulomatous inflammation of the pancreas in association with CD.8,9 In both cases, the granulomatous inflammation was in the head of the pancreas, raising the possibility it may have been an extension of the luminal inflammation in the adjoining portion of the duodenum rather than a primary inflammatory process of the pancreas. Our patient's granuloma was found in the body of the pancreas, with an intact interface noted between the granuloma and surrounding pancreatic parenchyma, suggesting a primary pancreatic process.
In the 2 cases previously published, the inflammation in the head of the pancreas caused obstructive jaundice and concern for malignancy, and therefore, a Whipple procedure was performed. After surgical removal, the granulomatous inflammation was noted, and both patients were diagnosed with CD. In our patient, we were able to obtain histology of the lesion through endoscopic ultrasound–guided biopsy. The patient had an excellent clinical response to infliximab and was able to stop his pancreatic enzyme supplementation. No follow-up imaging has been performed because the patient has been doing clinically well.
EPI has been reported in the literature to be associated with CD.3 The diagnosis of EPI relies on clinical suspicion and laboratory testing. The American Gastroenterological Association recommends using fecal elastase to screen for EPI in patients at risk; however, the test is not sensitive in cases of mild EPI.10 The normal cutoff for fecal elastase is greater than 200 μg/g; however, 1 study showed that more than 70% of symptomatic patients with a fecal elastase level between 200 μg/g and 500 μg/g had improvement in symptoms after a trial of pancreatic enzyme supplementation.11 Although our patient's fecal elastase was 224 μg/g, the granulomatous inflammation of his pancreas and subsequent symptom improvement with pancreatic enzyme supplementation was consistent with mild EPI.
In conclusion, we report a case of pancreatic granulomatous inflammation with EPI in a 60-year-old male patient with CD, which improved with infliximab treatment. Previous case reports of granulomatous inflammation of the pancreas in association with CD were in the head of the pancreas, which could be related to direct luminal spread from the duodenum. This patient's granulomatous inflammation was in the body of the pancreas, and there was no inflammation noted in the duodenum. This rare presentation highlights the importance of recognizing the pancreas as a possible site of extraintestinal manifestations of CD.
DISCLOSURES
Author contributions: AA Xu identified the case, obtained relevant data and images, and drafted and edited the manuscript. V. Catania, S. Vincent, S. Ma, and N. Zarrin-Khameh were involved in literature review, writing, and editing of the manuscript. AA Xu is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Sara Vincent, Email: Sara.Vincent@bcm.edu.
Samuel Ma, Email: Samuel.Ma@bcm.edu.
Vanessa Catania, Email: Vanessa.Catania@bcm.edu.
Neda Zarrin-Khameh, Email: nzarrink@bcm.edu.
REFERENCES
- 1.Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: Do they influence treatment and outcome? World J Gastroenterol. 2011;17(22):2702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sange AH, Srinivas N, Sarnaik MK, et al. Extra-intestinal manifestations of inflammatory bowel disease. Cureus. 2021;13(8):e17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonini F, Pezzilli R, Angelelli L, Macarri G. Pancreatic disorders in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2016;7(3):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202–6. [DOI] [PubMed] [Google Scholar]
- 5.Fousekis FS, Theopistos VI, Katsanos KH, Christodoulou DK. Pancreatic involvement in inflammatory bowel disease: A review. J Clin Med Res. 2018;10(10):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebl FH, Bataille F, Huy C, Hofstädter F, Schölmerich J, Rogler G. Association of antibodies to exocrine pancreas with subtypes of Crohn's disease. Eur J Gastroenterol Hepatol. 2005;17(1):73–7. [DOI] [PubMed] [Google Scholar]
- 7.Pavlidis P, Romanidou O, Roggenbuck D, et al. Ileal inflammation may trigger the development of GP2-specific pancreatic autoantibodies in patients with Crohn's disease. Clin Dev Immunol. 2012;2012:640835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gschwantler M, Kogelbauer G, Klose W, Bibus B, Tscholakoff D, Weiss W. The pancreas as a site of granulomatous inflammation in Crohn's disease. Gastroenterology. 1995;108(4):1246–9. [DOI] [PubMed] [Google Scholar]
- 9.Reynaert H, Peters O, Van der Auwera J, Vanstapel MJ, Urbain D. Jaundice caused by a pancreatic mass—An exceptional presentation of Crohn's disease. J Clin Gastroenterol. 2001;32(3):255–8. [DOI] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Buchner AM, Forsmark CE. AGA clinical practice update on the epidemiology, evaluation, and management of exocrine pancreatic insufficiency: Expert review. Gastroenterology. 2023;165(5):1292–301. [DOI] [PubMed] [Google Scholar]
- 11.Mathew A, Fernandes D, Andreyev HJ. What is the significance of a faecal elastase-1 level between 200 and 500 μg/g? Frontline Gastroenterol. 2023;14(5):371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]