Abstract
Background:
It is hypothesized that scalp allergic contact dermatitis (ACD) in women is commonly mistaken for other disorders due to overlapping symptoms and unique clinical presentations.
Objective:
This study reviews the potential underdiagnosis and misdiagnosis of scalp ACD and explores ways to improve diagnostic accuracy.
Methods:
This study conducted an extensive literature review to identify diagnostic challenges, common misdiagnoses, and diagnostic approaches for scalp ACD, focusing on standard versus targeted patch testing techniques.
Results:
Scalp ACD, often misdiagnosed as seborrheic dermatitis due to similar symptoms, has atypical presentations such as hair thinning, hair loss, and erythematous lesions affecting neighboring regions. Trichoscopy can help distinguish scalp ACD, identifying its patchy distribution of thin white scales, in contrast to the yellow scaling of seborrheic dermatitis. Standardized patch testing further contributes to diagnostic errors, with a study reporting 83% of patients who tested negative with standardized patch tests were positive when using their personal products. Individualized patch testing is more effective in identifying causative allergens and accurately diagnosing scalp ACD.
Limitations:
It is a retrospective review.
Conclusion:
Several factors contribute to scalp ACD’s misdiagnosis for conditions such as seborrheic dermatitis. The significant discrepancy in ACD detection rates between personalized and standardized patch tests in women emphasizes the importance of using patient-specific products in diagnostic testing. Incorporating scalp ACD more readily into one’s differential, employing individualized patch testing with trichoscopy, and accounting for neighboring symptomatic areas are all crucial elements in improving diagnostic accuracy for scalp ACD in women.
Keywords: allergic contact dermatitis, scalp dermatitis, seborrheic dermatitis
Highlights
Several types of allergens can cause scalp ACD, all with varying presentations on women. Knowing what types of allergens may be in certain products and items is crucial to diagnosing ACD of the scalp.
Scalp ACD can often present differently than ACD of other anatomical regions, with findings of hair loss, hair thinning, and erythematous runoff regions from products intended for the scalp.
Testing for allergens should be carried out with women’s own products via open patch testing. Regular patch testing can identify allergens that were not reactive years before.
What is known about this subject in regard to women and their families?
Allergic contact dermatitis (ACD) of the scalp disproportionately affects women more, potentially due to increased exposure to allergenic substances in hair products, treatments, and accessories.
Scalp ACD can present as hair thinning and hair loss, which can negatively impact many women’s quality of life.
What is new from this article as messages for women and their families?
Due to the scalp’s increased thickness coupled with hair occluding scalp visibility, scalp ACD can have atypical presentations in women requiring trichoscopic examination for accurate diagnosis.
Scalp ACD is commonly overlooked and often misdiagnosed as other scalp conditions such as seborrheic dermatitis.
An increasing number of women’s personal care products contain allergens that directly interact with the scalp, contributing to the rise in scalp ACD among women.
Using patient-specific products in open patch testing as opposed to solely standardized patch testing is more sensitive in identifying the allergens causing scalp ACD.
Introduction
Allergic contact dermatitis (ACD) is an eczematous condition resulting from allergen exposure and delayed type IV hypersensitivity reaction. Women with ACD can have a diverse array of symptoms, including erythema, papules, vesicles, scaling, flaking, lichenification, edema, and pruritus. Due to diverse hair care practices, women are particularly susceptible to scalp ACD, which often presents with atypical symptoms. Despite its increasing prevalence in women, scalp ACD is not readily considered in the differential diagnosis of patients with scalp symptoms such as pruritus, rash, dandruff, or even hair loss. This review article aims to provide a comprehensive overview of scalp ACD in women, detailing the epidemiological data, common allergens, and challenges in diagnosis. It summarizes the varied clinical presentations, differential diagnoses, and both diagnostic and therapeutic approaches. The review further investigates the frequent misdiagnosis of scalp ACD and recommends strategies to improve diagnostic accuracy.
Materials and methods
This review conducted an exhaustive literature search across multiple databases, including PubMed, Web of Science, and Scopus to investigate the characteristics of scalp ACD in women. Key phrases used in the search included “allergic contact dermatitis,” “scalp dermatitis,” “scalp contact dermatitis,” “patch testing,” “hair loss,” and “scalp allergens.” These phrases were both applied individually and in combination to ensure a thorough yet focused literature review.
Inclusion criteria encompassed studies involving patients diagnosed with scalp ACD, with a focus on women, and publications from the last 40 years. Exclusion criteria involved articles published more than 40 years ago, studies focusing solely on scalp dermatitis without specifically addressing ACD of the scalp, and studies that did not involve human subjects.
The scope of this review spans publications from the last 40 years, providing a substantial timeframe to adequately capture the increasing prevalence of scalp ACD in women. Data collection focused on scalp ACD prevalence rates, most common scalp allergens, and various diagnostic tools in identifying scalp ACD.
Epidemiology and pathogenesis
Current data reveal that scalp ACD is relatively uncommon. Warshaw et al.1 found that only 4.8% of patients presenting with dermatitis had the scalp as one of their areas of concern. Ojo et al.2 and DeKoven et al.3 respectively reported similar findings, with only 7.7% and 6.7% of patients having scalp dermatitis as their symptomatic area.
The relatively infrequent occurrence of scalp ACD is influenced by several factors. The scalp’s greater skin thickness and fewer skin folds than most parts of the body contribute to increased resistance to allergen penetration and decreased allergen accumulation, reducing ACD susceptibility.4,5 However, certain demographics, particularly women and patients over 40 years old, are more susceptible to scalp dermatitis. Women are at increased risk due to more frequent use of products that directly contact the scalp, while older patients are more vulnerable due to age-related thinning of the scalp epidermis.1,6 Pediatric patients may also have thinner hair and can thus also be more sensitive to scalp allergens.7
ACD is triggered by a type IV delayed hypersensitivity reaction, in which T-cells mediate increased inflammation of the scalp and surrounding areas in response to allergens. Repeated allergen exposure can worsen inflammation, increasing pruritus and aggravation of the hair follicles with potential hair loss and hair thinning.
Common allergens
There are numerous documented allergens known to frequently cause scalp ACD. Paraphenylenediamine (PPD), an ingredient often used in hair dye, was found in multiple studies to be the most common scalp allergen.4,5 Nickel, a metal ubiquitously used in hair clasps, hairpins, and brushes, has also been marked as the number one cause of scalp ACD in other studies. Since hair dyes and hair accessories are more commonly used by women, PPD and nickel are both significant factors contributing to the rise in scalp ACD in women. Other metals (eg, cobalt), fragrances (eg, Fragrance mix I, Balsam of Peru), topical drugs, and other personal care product ingredients (eg, methylisothiazolinone) are also common allergens that can cause scalp ACD.1,8 The most clinically relevant allergens that cause scalp ACD in women can be grouped into the following: metals, hair dyes, preservatives, rubber-based items, hair hygiene and hair styling products, topical medications, and hair camouflage tools.
Metals
Nickel is a metal commonly used in many consumer hair products and devices, including hair clasps, hairpins, combs, brushes, headbands, and jewelry.4,5 This wide use of nickel in many items contributes to it being one of the most common skin allergens and one of the largest culprits of scalp ACD. Patch test studies show that 15 to 24% of patients with scalp dermatitis and 25% of patients with occupational contact dermatitis have a positive patch test for a nickel allergy.4,9,10 Another study demonstrated that 10% of women were allergic to nickel, and dimethylglyoxime testing showed that 19.3% of adult hair clasps and 79.4% of children’s hair clasps could release large amounts of nickel onto the skin.7 Since low-cost nickel-based hair clasps are widely used by many women, nickel-induced scalp ACD should always be screened for.
Cobalt is another metal shown to cause scalp ACD, especially as it is one of the common compounds added in light hair dyes. Cobalt can also be found in other hygienic products such as detergents, soaps, and antiperspirants. Studies have shown a positive patch test to cobalt in 6 to 21% of patients with scalp dermatitis, with significantly more women sensitized to it compared to men.4,9
Hair dyes
The use of hair dyes is rising exponentially, with up to 75% of women from the United States using hair-coloring agents and a growing adoption worldwide. This increased supply and demand has inadvertently led to a rapid increase in the number of hair dye ingredients causing scalp ACD. PPD, an oxidizing agent frequently used in black, brown, and some blonde hair dyes, is also one of the most common triggers of ACD involving the scalp, face, and ears. It is often detected in hair dye products through third-party testing even when not explicitly listed as an ingredient.11 It is therefore important for women allergic to PPD to consider alternatives to conventional hair dyes, even if the dyes report not having PPD. Hillen et al.9 identified hair dyes as the most common products associated with scalp ACD, with 11.8% of patients testing positive for PPD. Another study found PPD-related dermatitis prevalence to be 4% in Europe, 4.3% in Asia, and 6.2% in North America.7 Other studies have reported similar findings of 3.0 to 3.3% of their dermatitis patients testing positive for PPD sensitivity.4,12
PPD sensitivity can be a useful indicator in determining the likelihood that women may have another allergy to other para-compounds. One study revealed that 53% of patients allergic to PPD also reacted to other para-compounds, particularly p-aminobenzene and p-toluenediamine sulfate. It is important to assess patients for a delayed reaction on patch testing, as 10% of those allergic to para-compounds were negative 2 to 3 days after patch test placement but positive 6 to 7 days after.12
Many compounds in hair dyes, tints, and bleaches have been identified as potential allergens through both standardized and individualized patch testings. Some hair-coloring agents shown to cause scalp ACD are ammonium persulfate (oxidizing hair bleaching agent) and Disperse blue dye (occasionally misdiagnosed as a PPD allergy due to cross-reactivity).4 Hair perming products such as glyceryl monothioglycolate, used for permanent hair styling, are another common source of scalp ACD.5
Although standardized patch tests cover common scalp allergens such as PPD, they may miss the many other ingredients in hair products. Hillen et al.9 found that 83% of patients who reacted to their own hair tints did not react in standardized patch series. To accurately diagnose scalp ACD from hair products, it is crucial to test the specific products that women are using in personalized patch testing.
Preservatives
Preservatives are added to many consumer products to increase their shelf life. Preservatives noted to cause scalp ACD include methylisothiazolinone, methylchloroisothiazolinone, methyldibromo glutaronitrile, and formaldehyde.2,4,5
Rubber-based items
Rubber and rubber accelerants are in many women’s garments and items that are frequently in direct contact with the scalp, including headphones, masks, wetsuits, hairbrushes, and hair glues.
Most rubber first undergoes a process called vulcanization, increasing its tensile strength, elasticity, and resilience. Vulcanization typically incorporates rubber accelerators such as carba mix, a notable allergen linked to scalp ACD. It is commonly found in women’s personal care products such as shampoos, soaps, and fungicides, with Aleid et al.4 ranking it as the third most common allergen after metals and fragrances. If a rubber allergy is suspected, it is crucial to patch test other rubber accelerators such as black rubber mix, mercapto mix, and thiuram mix; individually testing for each accelerator is important in identifying the specific allergen causing scalp ACD.13
Rubber latex, a common ingredient in hair glues used for styling women’s hair weaves and extensions, can cause scalp ACD and may lead to more severe reactions such as anaphylaxis and angioedema.4,14 Women with even mild latex allergies may experience rapid escalation to severe, life-threatening conditions. It is imperative for physicians to keep in mind that, in cases where a woman presents with latex glue in her hair with symptoms, a hair bond remover soaked for 2 to 3 hours is a documented safe practice to thoroughly eliminate the adhesive.15
Hair hygiene products
Shampoos and conditioners, integral to women’s hair hygiene, now frequently include a complex array of ingredients such as surfactants, conditioners, preservatives, and other additives.16 Their limited direct contact with the scalp, however, is likely a factor contributing to the infrequent reports of hair hygiene products as triggers for scalp ACD.5
Added preservatives and fragrances, in approximately 95% of shampoos, are common culprits in hair products contributing to ACD.16 Common fragrances such as Balsam of Peru, Fragrance mix I, and Fragrance mix II are frequently identified as scalp allergens. Aleid et al.4 reported that one-third of patients had allergic reactions to at least 1 of 3 fragrances. Because of increasing ingredient complexity in hair products, standardized patch testing may not cover many potential allergens in them. For stronger diagnostic certainty for scalp ACD, it is essential to also use women’s own hair hygiene products in individualized patch testing.
Topical medications
Topical medications are applied to the scalp for a wide variety of dermatoses. Topical minoxidil, commonly used to treat hair loss in both men and women, causes scalp ACD approximately 5.6% of the time.5 Patients may also be allergic to the vehicle for minoxidil and not minoxidil itself; propylene glycol is a common minoxidil vehicle and has been identified as an allergen for 9% of patients. Many women had no allergic reactions with minoxidil formulations using butylene glycol, an alternative to propylene glycol.17 Women reacting to minoxidil that uses propylene glycol can also switch to minoxidil foam, which does not contain propylene glycol.
Other less common topical contributors of scalp ACD include benzocaine and neomycin. Hillen et al.9 reported the positive patch test rates from these substances as 2.1% and 1.1%, respectively. Multiple case reports have reported ACD reactions from topical ketoconazole, both in shampoo and cream formulations.18,19
Hair camouflage tools
Hair camouflage is a popular alternative for women experiencing hair loss who cannot undergo or do not want hair transplant surgery. Camouflaging techniques can range from temporary solutions, such as wigs, hair thickening fibers, and hair styling products, to more permanent methods, such as scalp micropigmentation.20 However, direct contact of these products with the scalp, especially in areas of hair loss or thinning, can cause scalp ACD.
Wigs are made from a variety of materials, many of which contain allergens. For example, disperse dyes used in synthetic wigs can leak from the fibers and prompt scalp ACD.5 Adhesive wig tapes containing the allergen 2-ethylhexyl acrylate can also trigger scalp ACD. Interestingly, in women with alopecia areata, both disperse dyes and adhesive tapes in wigs have incidentally improved their alopecia. This is due to the allergy-induced inflammation from the wigs paradoxically promoting hair regrowth.21,22 This inadvertent restoration of hair upon the use of wigs could act as a sign of scalp ACD.
Scalp micropigmentation, also known as “hair tattooing,” involves administering colored pigment directly into the upper dermis to mimic hair follicles, offering a lasting effect of hair camouflage that can last for several years.20 While this semipermanent method is generally considered safe and can significantly improve the aesthetic appearance of hair, the pigments used can precipitate scalp ACD in a minority of patients, underscoring careful product scrutiny for women seeking this hair camouflage method.23
Clinical presentation and differential diagnosis
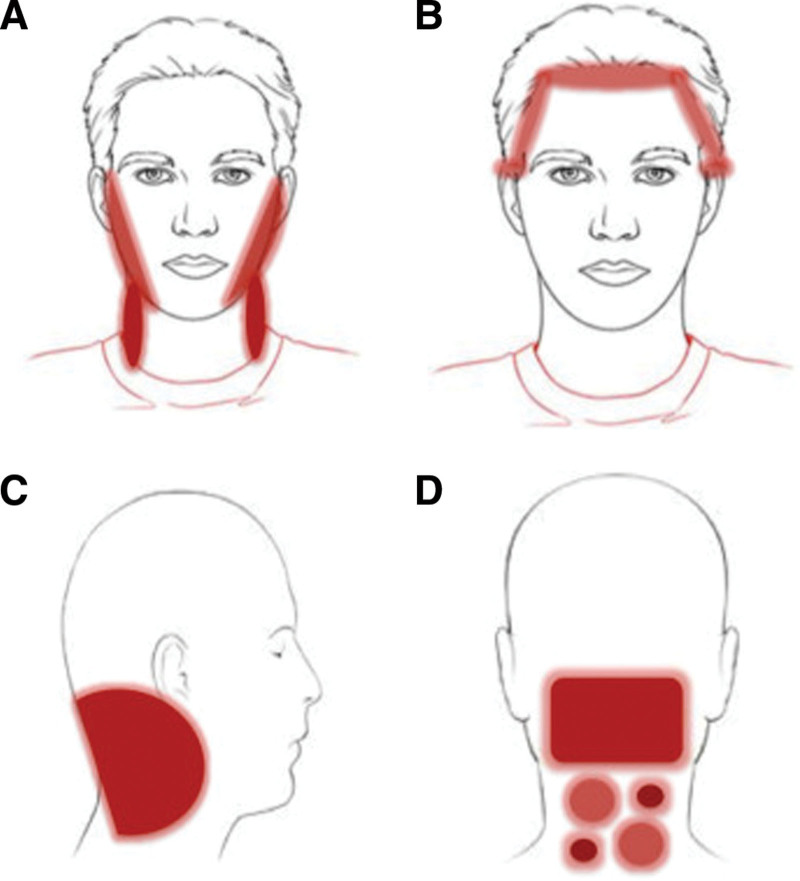
Typical ACD presentations such as erythematous papules or plaques are infrequent on the scalp due to their greater resistance to allergens. Instead, symptoms involving hair thinning, hair loss, burning sensations, and unrelenting pruritus are often associated with scalp ACD, especially in patients with repeated allergenic exposures.8,24 Although erythematous lesions are uncommon on the scalp, they may appear on anatomical regions where scalp products run off (periauricular areas, hairline, face, and neck). Figure 1 adopted from Rozas-Muñoz et al.5 highlights the clinical distribution of affected neighboring regions from scalp ACD. The face, prone to indirect contact via hands, is one of the most common sites of ACD, particularly in women.4 Allergic reactions in these scalp-adjacent areas, which are more susceptible to allergens than the scalp itself, can be strong indicators of scalp ACD (Fig. 2). Warshaw et al. found a higher incidence of ACD (59.6%) in patients with scalp dermatitis when neighboring sites were also involved compared to those with dermatitis confined to the scalp alone (38.6%) (Fig. 3). Conversely, seborrheic dermatitis was more commonly diagnosed in patients with isolated scalp dermatitis (17.2%) than in those with neighboring sites also involved (10.4%).1
Fig. 1.
Clinical patterns of allergic contact dermatitis affecting the scalp. (A) Rinse-off pattern: eczematous plaques on the sides of the face (preauricular and mandibular) and neck; (B–D) pattern along the hairline. (B) Forehead and area above the ears. (C) Occipital and retroauricular area. From Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermosifiliogr 2018;109:485–507.
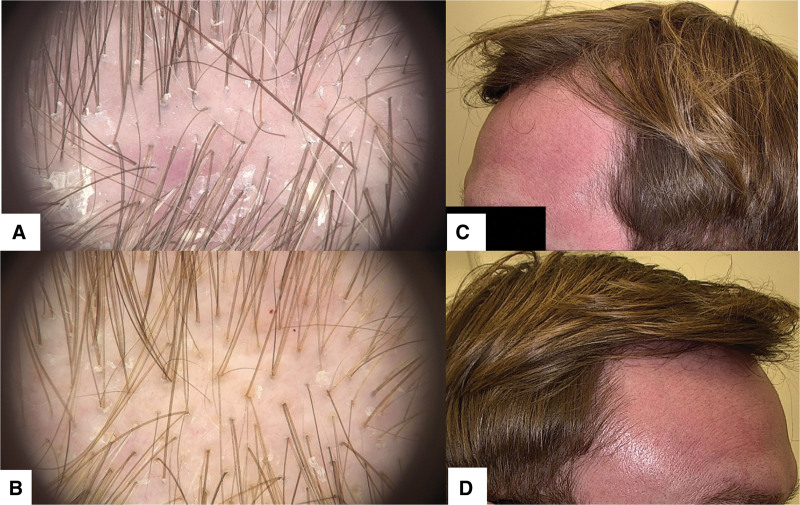
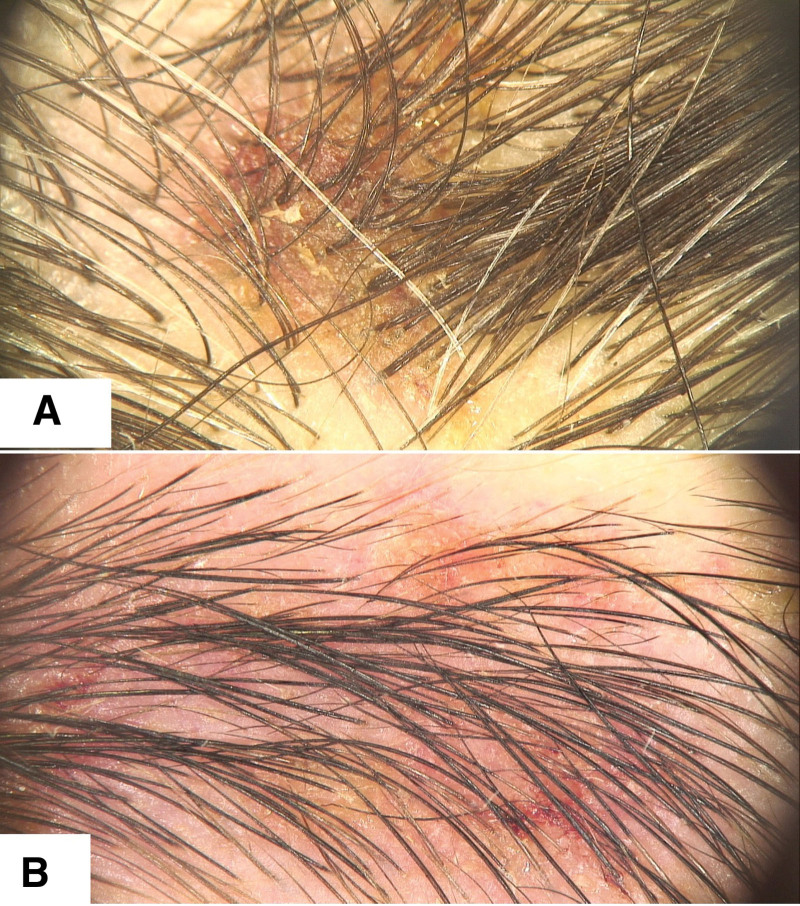
Fig. 2.
(A) Trichoscopy of scalp allergic contact dermatitis (ACD) to propylene glycol showing punctate/glomerular vessels, white scaling, and remnants of propylene glycol displayed as small white particles. (B) Another trichoscopy of scalp ACD to propylene glycol exhibiting punctate/glomerular vessels, small white scales, and extravasations, indicative of ACD due to propylene glycol exposure. (C and D) Patient with scalp ACD due to propylene glycol in minoxidil solution demonstrating erythema extending across the forehead, temples, and frontal scalp.
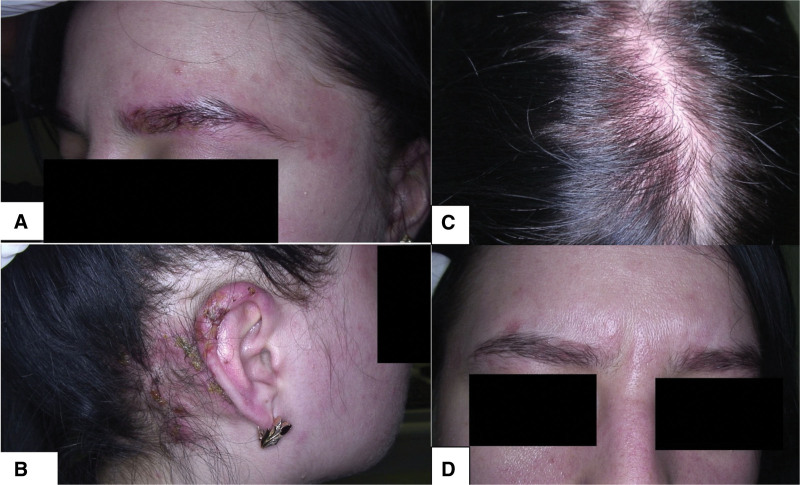
Fig. 3.
(A) Patient 1 with ACD of the scalp to PPD affecting eyebrows. (B) Patient 1 with eczematous lesions on the ears. (C) Patient 2 with diffuse scalp erythema and scaling from scalp ACD. (D) Patient 2 with an eczematous rash in the eyebrows and glabella, reflecting runoff anatomical involvement from initial scalp ACD. ACD, allergic contact dermatitis; PPD, paraphenylenediamine.
Other helpful clues in distinguishing between seborrheic dermatitis and scalp ACD are the circumstantial factors associated with the onset of pruritus. In cases of seborrheic dermatitis, pruritus typically occurs when the hair remains unwashed for an extended period, leading to sebum accumulation. In cases of scalp ACD, however, pruritus coincides with allergen contact and can occur even with regularly cleaned hair.
Scalp ACD is often accompanied by other dermatoses such as seborrheic dermatitis, alopecia, telogen effluvium, or psoriasis. This frequent comorbidity of scalp skin disease contributes to frequent misdiagnoses or underdiagnoses.1 Given the prevalence of documented cases of misdiagnosed or underdiagnosed scalp ACD, it is crucial to accurately differentiate scalp ACD from other conditions.25,26
Trichoscopy, or dermoscopy of the scalp, is often useful in helping distinguish between inflammatory scalp lesions. Scalp ACD trichoscopy can reveal dotted and comma vessels, simple loops, thin and thick arborizing vessels, white scales, simple red loops, yellow exudate, and generalized erythema.27,28 While these trichoscopic findings may be sensitive for scalp ACD, they are not highly specific. For example, scalp psoriasis can also demonstrate arborizing red lines and twisted red loops. Thin arborizing red lines are also characteristic of scalp seborrheic dermatitis, along with comma vessels and twisted red loops.29,30 Discoid lupus erythematosus also characteristically has thick arborizing lines.31
It can be difficult to distinguish seborrheic dermatitis from scalp ACD, especially since they can coexist (Fig. 4). Both conditions can present with arborizing red lines, twisted red loops, comma vessels, and scales. While scalp ACD has a patchy distribution of thin white scale, scalp seborrheic dermatitis often has yellow scale with an even distribution (Fig. 5).27,32
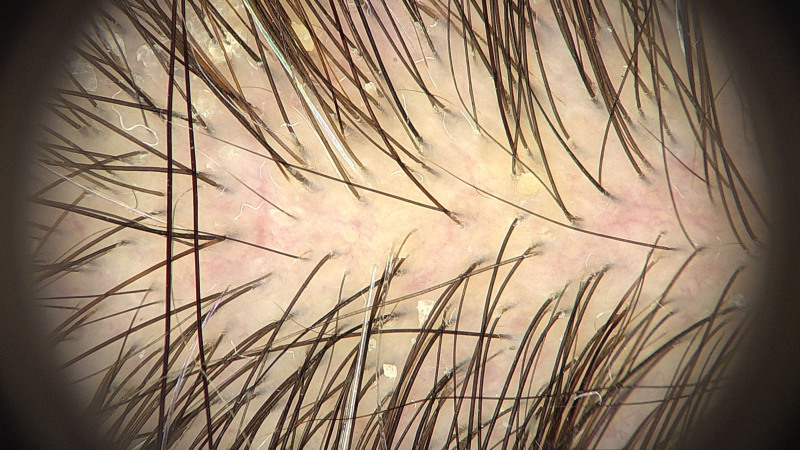
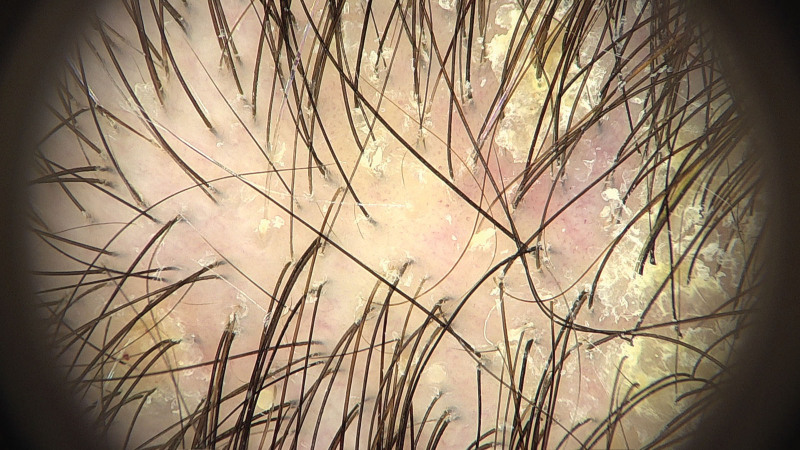
Fig. 4.
Trichoscopy demonstrating small arborizing vessels, generalized erythema, and interspersed white scales, suggesting the coexistence of scalp ACD and seborrheic dermatitis. ACD, allergic contact dermatitis.
Fig. 5.
(A) Trichoscopy of scalp ACD demonstrating patchy nummular eczematous lesion with crusts and normal air shafts. (B) Trichoscopy of ACD of the eyebrows after demonstrating eczematous changes. ACD, allergic contact dermatitis.
A comprehensive diagnosis of scalp ACD often requires integrating the clinical presentation, trichoscopic examination findings, and the patient’s symptoms. The presence of mild to severe pruritus, a history of allergen exposure, and trichoscopy findings leaning toward ACD but atypical for seborrheic dermatitis or psoriasis suggest ACD and should prompt confirmatory patch testing. ACD typically involves more severe pruritus with poor clinical and trichoscopic presentation. In contrast, psoriasis and seborrheic dermatitis often present a more pronounced clinical and trichoscopic picture but with relatively minor pruritus (Fig. 6).
Fig. 6.
Trichoscopy showing psoriasis-like glomerular vessels and yellow-white scales, features overlapping with psoriasis. However, given the concomitant presence of white scales, sparse erythema, and minimal vascular prominence, ACD is more likely. ACD, allergic contact dermatitis.
Patch testing and skin biopsies for scalp allergic contact dermatitis
Patch testing is an important tool in diagnosing ACD of the scalp (Fig. 7). Understanding what the patient’s scalp has come into contact with over the past several weeks can also allow for individualized patch testing using the patient’s own products and items. Hillen et al.9 found that many allergens causing scalp ACD were undetected through standard patch test series yet were identified by patch tests using the patients’ own products. Personalized open patch testing is preferred in testing women’s products in an individualized manner to prevent undiagnosed scalp ACD.4
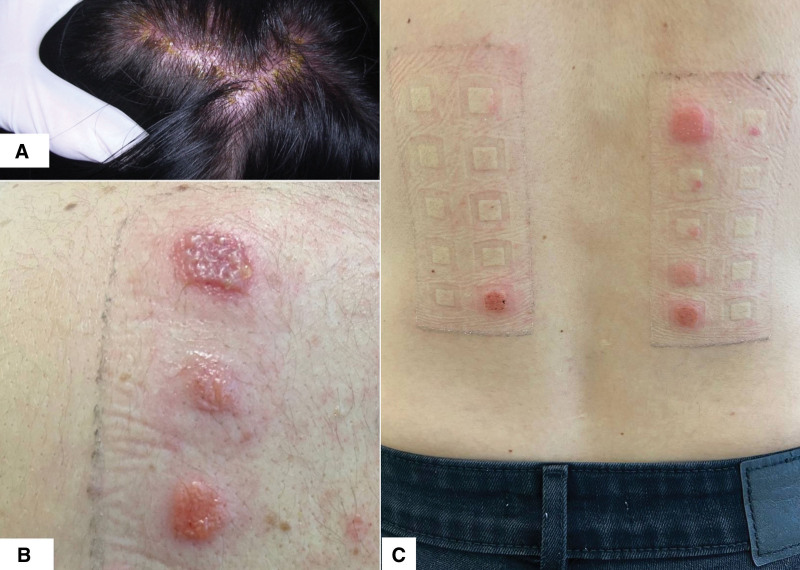
Fig. 7.
(A) An exuberant allergic contact dermatitis to PPD with severe erythema and crusting. (B) Positive patch tests to different PPD dilutions. (C) Positive patch tests for fragrance mix. PPD, paraphenylenediamine.
Skin biopsy of scalp lesions can provide additional information if patch testing is inconclusive, further distinguishing between ACD and seborrheic dermatitis. Especially if there is scale on the scalp, these areas should be biopsied and examined for distinguishing features of either scalp ACD or scalp seborrheic dermatitis. Some histopathological markers of ACD are spongiosis in the epidermis, increased frequency of eosinophils, eosinophilic exocytosis, and increased immune cell infiltrates; chronic forms of ACD can also show epidermal hyperplasia and papillary dermal fibrosis. Seborrheic dermatitis biopsies also exhibit spongiosis but can additionally display parakeratosis and follicular plugging.
Management of allergic contact dermatitis of the scalp
When managing scalp ACD in women, it is imperative for clinicians to first advise their patients to avoid any identified allergens. Topical steroids such as clobetasol solution or foam can reduce scalp ACD symptoms. For severe cases, systemic medications such as steroids or immunosuppressants may be required, although most cases can be managed with avoidance of the allergen and topical steroids for symptomatic management. It is crucial to consider the unique hair care products and practices used by women, which can significantly impact prevention and management of scalp ACD.
Conclusion and practical recommendations
In the clinical evaluation of women presenting with scaling and pruritus of the scalp, ACD should be considered within the differential diagnosis. Effective screening involves a thorough history to identify exposure to potential allergens, including metals, hair dyes, preservatives, rubber-based products, hair hygiene and hair styling products, topical medications, and hair camouflage products. Trichoscopic examination can be very useful in further distinguishing scalp ACD from other scalp dermatoses. If symptoms continue despite aggressive treatment with topical steroids, individualized patch testing should be discussed with the patient, along with a detailed evaluation of their hair products that may contain concealed allergens.
Conflicts of interest
None.
Funding
None.
Study approval
N/A
Author contributions
Each author listed has contributed significantly to the manuscript throughout the entire process. JCH performed the literature review and wrote the manuscript; CJB and VK both created the original concept and also helped to review, revise, and edit the manuscript with their clinical knowledge; KK supplied all of the figures supporting the paper. All authors have confirmed the originality of this paper and have approved of the final version of this manuscript.
Patient consent
Informed, written consent was received from all patients for whom photographs are present in the manuscript.
Footnotes
Published online 29 July 2024
References
- 1.Warshaw EM, Kullberg SA, DeKoven JG, et al. Scalp involvement in patients referred for patch testing: retrospective cross-sectional analysis of North American Contact Dermatitis Group data, 1996 to 2016. J Am Acad Dermatol 2021;84:977–88. doi: 10.1016/j.jaad.2020.08.046. [DOI] [PubMed] [Google Scholar]
- 2.Ojo EO, Gowda A, Nedorost S. Scalp dermatitis in patients sensitized to components of hair products. Dermatitis 2019;30:264–7. doi: 10.1097/DER.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 3.DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis 2018;29:297–309. doi: 10.1097/DER.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 4.Aleid NM, Fertig R, Maddy A, Tosti A. Common allergens identified based on patch test results in patients with suspected contact dermatitis of the scalp. Skin Appendage Disord 2017;3:7–14. doi: 10.1159/000453530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermosifiliogr 2018;109:485–507. doi: 10.1016/j.adengl.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Ungar OJ, Amit U, Cavel O, Oron Y, Handzel O. Age-dependent variations of scalp thickness in the area designated for a cochlear implant receiver stimulator. Laryngoscope Investig Otolaryngol 2018;3:496–9. doi: 10.1002/lio2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thyssen J, Menné T, Johansen J. Nickel release from inexpensive jewelry and hair clasps purchased in an EU country - are consumers sufficiently protected from nickel exposure? Sci Total Env 2009;407:5315–8. doi: 10.1016/j.scitotenv.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Tosti A, Piraccini BM, van Neste DJ. Telogen effluvium after allergic contact dermatitis of the scalp. Arch Dermatol 2001;137:187–90. [PubMed] [Google Scholar]
- 9.Hillen U, Grabbe S, Uter W. Patch test results in patients with scalp dermatitis: analysis of data of the Information Network of Departments of Dermatology. Contact Dermatitis 2007;56:87–93. doi: 10.1111/j.1600-0536.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 10.Wall LM, Gebauer KA. Occupational skin disease in Western Australia. Contact Dermatitis 1991;24:101–9. doi: 10.1111/j.1600-0536.1991.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Vozmediano JM, Padilla-Moreno M, Armario-Hita JC, Carranza-Romero C. [Pattern of contact sensitization to paraphenylenediamine and its detection in hair dyes]. Actas Dermosifiliogr 2011;102:206–11. doi: 10.1016/j.ad.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Koopmans A, Bruynzeel D. Is PPD a useful screening agent? Contact Dermatitis 2003;48:89–92. doi: 10.1034/j.1600-0536.2003.480207.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansen A, Brans R, Sonsmann F. Allergic contact dermatitis to rubber accelerators in protective gloves: problems, challenges, and solutions for occupational skin protection. Allergol Select 2021;5:335–44. doi: 10.5414/ALX02265E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor J, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther 2004;17:289–301. doi: 10.1111/j.1396-0296.2004.04024.x. [DOI] [PubMed] [Google Scholar]
- 15.Burla MJ, Brody AM, Welch RD, Favot MJ. Anaphylactic reaction after ongoing exposure to hair glue: a novel case report. J Emerg Med 2015;48:e5–7. doi: 10.1016/j.jemermed.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Zirwas M, Moennich J. Shampoos. Dermatitis 2009;20:106–10. [PubMed] [Google Scholar]
- 17.Friedman ES, Friedman PM, Cohen DE, Washenik K. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol 2002;46:309–12. doi: 10.1067/mjd.2002.119104. [DOI] [PubMed] [Google Scholar]
- 18.Ruggiero JL, Shaver RL, Hylwa S. Allergic contact dermatitis from stearyl alcohol in ketoconazole cream. Contact Dermatitis 2021;85:263–4. doi: 10.1111/cod.13843. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Warshaw EM. Allergic contact dermatitis from ketoconazole. Cutis 2014;94:112–4. [PubMed] [Google Scholar]
- 20.Daruwalla SB, Dhurat RS, Hamid SAT. All that a dermatotrichologist needs to know about hair camouflage: a comprehensive review. Int J Trichology 2022;14:77–83. doi: 10.4103/ijt.ijt_6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shehade SA, Beck MH. Contact dermatitis from disperse dyes in synthetic wigs. Contact Dermatitis 1990;23:124–5. doi: 10.1111/j.1600-0536.1990.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 22.Torchia D, Giorgini S, Gola M, Francalanci S. Allergic contact dermatitis from 2-ethylhexyl acrylate contained in a wig-fixing adhesive tape and its “incidental” therapeutic effect on alopecia areata. Contact Dermatitis 2008;58:170–1. doi: 10.1111/j.1600-0536.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 23.Seyhan T, Kapi E. Scalp micropigmentation procedure: a useful procedure for hair restoration. J Craniofac Surg 2021;32:1049–53. doi: 10.1097/SCS.0000000000007208. [DOI] [PubMed] [Google Scholar]
- 24.Søsted H, Agner T, Andersen KE, Menné T. 55 cases of allergic reactions to hair dye: a descriptive, consumer complaint-based study. Contact Dermatitis 2002;47:299–303. doi: 10.1034/j.1600-0536.2002.470508.x. [DOI] [PubMed] [Google Scholar]
- 25.Komamura H, Kozuka T, Ishii M, Yoshikawa K, Iyoda M. Allergic contact sensitivity to quinophthalone. Contact Dermatitis 1989;20:177–81. doi: 10.1111/j.1600-0536.1989.tb04653.x. [DOI] [PubMed] [Google Scholar]
- 26.Hughes OB, Maderal AD, Tosti A. An unusual case of contact dermatitis. Skin Appendage Disord 2017;3:163–5. doi: 10.1159/000466703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makowska K, Golińska J, Sar-Pomian M, Rudnicka L. Trichoscopy features of allergic contact dermatitis of the scalp: a prospective case series. Dermatitis 2023;34:265–8. doi: 10.1097/DER.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 28.Starace M, Bruni F, Marcondes MT, Alessandrini A, Piraccini BM. The identification of trichoscopic features of allergic scalp contact dermatitis: a pilot-study of a single center. Ital J Dermatol Venerol 2023;158:334–40. doi: 10.23736/S2784-8671.23.07578-3. [DOI] [PubMed] [Google Scholar]
- 29.Kibar M, Aktan S, Bilgin M. Dermoscopic findings in scalp psoriasis and seborrheic dermatitis; two new signs; signet ring vessel and hidden hair. Indian J Dermatol 2015;60:41–5. doi: 10.4103/0019-5154.147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Arriaga LF, Arenas R, Vega-Sánchez DC, Asz-Sigall D, Martínez-Velazco MA. Seborrheic dermatitis: three novel trichoscopic signs and its correlation to Malassezia sp. colonization. Skin Appendage Disord 2019;5:288–92. doi: 10.1159/000497782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fathy H, Ghanim BM, Refat S, Awad A. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360–6. doi: 10.25259/IJDVL_207_19. [DOI] [PubMed] [Google Scholar]
- 32.Golińska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology 2022;238:412–21. doi: 10.1159/000517516. [DOI] [PubMed] [Google Scholar]