Abstract
Background and Objective
No epidemiologic studies have formally assessed the incidence of primary progressive aphasia (PPA) and primary progressive apraxia of speech (PPAOS). Thus, we decided to assess the incidence of these disorders in Olmsted County, MN, between 2011 and 2022, and to characterize clinical, radiographic, and pathologic characteristics of these patients.
Methods
This was a retrospective examination of data from a population-based cohort of patients with PPA and PPAOS prospectively identified in Olmsted County, MN, from 2011 to 2022. The incidence of PPA among adults (older than 18 years) was calculated for Olmsted County as the number of patients per 100,000 person-years during the study period. The adult population of Olmsted County was determined by the annual catchment population reported by the Rochester Epidemiological Project for each year 2011–2022. A behavioral neurologist verified the clinical diagnoses and determined subtypes.
Results
We identified 10 patients (60% female) within the study period (median age of symptoms onset: 70 years; range: 66–73), 8 with PPA and 2 with PPAOS. Of the 8 patients with PPA (6 female patients, 2 male patients), 2 met criteria for non-fluent variant PPA (nfvPPA), 3 for logopenic variant PPA (lvPPA), and 3 for semantic variant (svPPA). Speech evaluation confirmed the clinical diagnoses in all patients and all showed typical imaging findings consistent with their respective subtype. Six patients (2 PPAOS, 2 nfvPPA, 2 lvPPA) died and 3 underwent autopsy (2 PPAOS, 1 nfvPPA), confirming the pathologic diagnosis of progressive supranuclear palsy. The incidence of PPA + PPAOS was 0.70 persons per 100,000 person-years (95% CI 0.34–1.29 persons per 100,000) during the study period. The incidence of PPAOS was 0.14 persons per 100,000 person-years (95% CI 0.02–0.55 persons per 100,000), whereas for the 8 patients with PPA, the incidence was 0.56 persons per 100,000 person-years (95% CI 0.24–1.10 cases per 100,000). The incidence of nfvPPA was 0.14 persons per 100,000 person-years (95% CI 0.02–0.55), 0.21 persons per 100,000 person-years (95% CI 0.04–0.61) for lvPPA, and 0.21 persons per 100,000 person-years (95% CI 0.04–0.61) for svPPA.
Discussion
As a group, PPA and PPAOS are a relatively rare group of diseases. PPAOS has a slightly lower incidence than PPA as a group but similar incidence to the individual PPA variants.
Introduction
Neurodegenerative speech and language disorders are a group of disorders that include primary progressive aphasia (PPA) and its variants and primary progressive apraxia of speech (PPAOS). PPA refers to a group of neurodegenerative disorders characterized by prominent impairments in language function and the absence of cognitive, motor, and behavioral symptoms, especially in the early phases of the disease.1 Three distinct variants have been recognized based on clinical presentation, radiographic characteristics, prognosis, and underlying pathology: the nonfluent/agrammatic variant (nfvPPA) and the semantic variant (svPPA) are classified as frontotemporal dementia syndromes, whereas the logopenic variant (lvPPA) can be defined as an atypical variant of Alzheimer disease.1,2 In addition, some PPA cases remain “unclassifiable,” especially early in the disease course, when the presence of mild or mixed features makes it challenging to differentiate between specific subtypes.3
Unlike PPA that affects language, PPAOS affects speech. Hence, the progressive onset of speech motor planning and programming deficits affecting speech production in the absence of aphasia, memory loss, and (extra)pyramidal features at disease onset characterizes PPAOS.4,5
No studies have formally evaluated the epidemiologic characteristics of PPA and PPAOS. Thus, we aimed to assess the incidence of these disorders in our population-based cohort study in Olmsted County, MN, from 2011 to 2022, and examined clinical, radiographic and pathologic characteristics of these patients.
Methods
Case Ascertainment
All participants had been recruited and prospectively followed by the Neurodegenerative Research Group, Mayo Clinic, Rochester Minnesota, and enlisted into NIH grant funded studies between January 1, 2011, and December 31, 2022. Only those patients who resided in Olmsted County, MN, at the time of diagnosis were included in this study. All participants presented with speech and/or language dysfunction, and they were evaluated by a board-certified Behavioral Neurologist (K.A.J.) who classified the patients with PPA into specific variants using published criteria.1
Patients were diagnosed with PPAOS if the predominant sign at onset was apraxia of speech, and there was no evidence of aphasia in verbal or written language as per criteria described elsewhere.5
All patients had an assessment of global cognitive function with the Montreal Cognitive Assessment Battery.6 Features of parkinsonism were assessed using the Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III.7
All participants underwent multimodal neuroimaging with 18F-flurodeoxyglucose PET (FDG-PET) scan, volumetric MRI brain scan, and completed a battery of additional speech and language, and neuropsychological tests, as previously reported.5,8,9 Sections below briefly summarize the relevant aspects of each test.
Speech and Language Evaluation
The Western Aphasia Battery-Revised (WAB-R) was used to assess global language ability and aphasia severity; the Northwestern Anagram Test10 was used to measure syntactic comprehension. The severity of apraxia of speech was assessed by using the Apraxia of Speech Rating Scale11 and the Motor Speech Disorder severity scale7 was used to rate the presence and degree of functional impairment. Evidence of apraxia of speech with no more than equivocal evidence of aphasia including agrammatism was required to be diagnosed with PPAOS.5 Grammar was assessed by review of conversational speech and detailed language testing, including verbal and written picture description tasks. Agrammatism was assessed in speech and writing separately, with emphasis placed on the writing samples in cases of severe apraxia of speech.
Neuropsychological Assessments
The neuropsychological examinations included the use of a bedside cognitive assessment (either the Kokmen Short Test of Mental Status -STMS- or the Montreal Cognitive Assessment -MoCA-)6,12 performed at the time of patients' initial neurologic evaluation by an experienced behavioral neurologist (K.A.J.). These were followed by a formal neuropsychological assessment which was performed by board-certified neuropsychologists with experience in treating patients with PPA and PPAOS. Memory was assessed with the administration of the Wechsler Memory Scale-III (WMS-III) Logical Memory I/II, Visual Reproduction I/II13; processing speed was assessed with the Trail Making Test (TMT) Part A14,15; executive function was assessed with the use of TMT Part B14,15; and Delis-Kaplan Executive Function CardSort16; finally, visuospatial function was assessed with the administration of the Rey-Osterreith Complex Figure Test,17 Visual Object and Space Perception Cube, and Incomplete Letters subtests.18
Neuroimaging
Details of imaging protocols were described elsewhere5,19 and will be briefly summarized below. All participants underwent a standardized MRI imaging protocol at 3.0 Tesla as previously detailed.5 All FDG-PET scans were acquired using a PET/CT scanner (GE Healthcare) operating in 3D mode. After a 30-minute uptake period, an 8-minute FDG scan was performed consisting of four 2-minute dynamic frames after a low-dose CT transmission scan. Individual frames of the FDG were realigned if motion was detected and then a mean image was created.5 Maps of hypometabolism were generated by 3-dimensional stereotactic surface projections using CortexID (GE Healthcare, Waukesha, WI). Automated average Z scores generated from Cortex ID were calculated for the following regions: lateral and medial frontal lobes, lateral and medial parietal lobes, temporal lobes, cingulate cortex, occipital lobe, and primary visual cortex. Hypometabolism was present if Z scores were greater than 2.20 Moderate hypometabolism was considered when Z scores were between 2 and 3, whereas Z scores >3 corresponded to severe hypometabolism.20 Patterns of hypometabolism were initially categorized as prerolandic or postrolandic. The prerolandic patients were then classified as widespread, focal inferior frontal, focal superior frontal, or focal inferior and superior frontal; the presence or absence of supplementary motor area hypometabolism was noted. The postrolandic patterns were further classified as anteromedial temporal or temporoparietal dominant. These classifications were based on previous studies showing different areas involved in PPA and PPAOS.21-23
Pathologic Evaluation
All participants who died and agreed to autopsy underwent brain autopsy by an experienced board-certified neuropathologist who provided pathologic diagnoses based on current diagnostic criteria.24-27
Statistical Analysis
Patients with PPA and PPAOS were prospectively identified in Olmsted County from 2011 to 2022. Continuous features were summarized with medians and interquartile ranges; categorical features were summarized with frequency counts and percentages. The incidence of PPA among adults (older than 18 years) was calculated for Olmsted County as the number of patients per 100,000 person-years during the study period. The adult population of Olmsted County was determined by the annual catchment population reported by the Rochester Epidemiological Project for each year 2011–2022. Confidence intervals were calculated using a Poisson approximation.
Standard Protocol Approvals and Patient Consents
The Mayo Clinic institutional review board approved the study, and all participants consented for enrollment into the study. STROBE cohort reporting guidelines were used for this study.28
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigators.
Results
Demographics and Incidence Analysis
We identified a total of 10 patients including 8 with PPA and 2 with PPAOS in Olmsted County, MN, from 2011 to 2022. The median age at symptoms onset was 70 years (interquartile range [IQR]: 66, 73), whereas the median age at diagnosis was 72 years (IQR: 70, 74).
Of the 8 patients with PPA (6 female patients, 2 male patients), 2 met criteria for nfvPPA, 3 for lvPPA, and 3 for svPPA. The remaining 2 patients were diagnosed with PPAOS. Additional demographic information on our patients found during the study period are listed in Table 1.
Table 1.
Patients Demographics
| PPA (N = 8) | PPAOS (N = 2) | Overall (N = 10) | |
| Sex, n (%) | |||
| Male | 2 (25) | 2 (100) | 4 (40) |
| Female | 6 (75) | 0 (0) | 6 (60) |
| Age at symptom onset, y | |||
| Median (Q1, Q3) | 69 (63, 71) | 77 (76, 78) | 70 (66, 73) |
| Age at diagnosis, y | |||
| Median (Q1, Q3) | 71 (67, 73) | 80 (79, 81) | 72 (70, 74) |
| Death at time of abstraction, n (%) | |||
| No | 4 (50) | 0 (0) | 4 (40) |
| Yes | 4 (50) | 2 (100) | 6 (60) |
| Age at death, y | |||
| Median (Q1, Q3) | 79 (76, 83) | 87 (86, 87) | 83 (78, 85) |
| PPA (N = 8) | PPAOS (N = 2) | Overall (N = 10) | |
| Sex, n (%) | |||
| Male | 2 (25) | 2 (100) | 4 (40) |
| Female | 6 (75) | 0 (0) | 6 (60) |
| Age at symptom onset, y | |||
| Median (Q1, Q3) | 70 (65, 74) | 77 (76, 78) | 70 (67, 77) |
| Age at diagnosis, y | |||
| Median (Q1, Q3) | 72 (69, 74) | 80 (79, 81) | 73 (70, 74) |
| Death at time of abstraction, n (%) | |||
| No | 4 (50) | 0 (0) | 4 (40) |
| Yes | 4 (50) | 2 (100) | 6 (60) |
| Age at death, y | |||
| Median (Q1, Q3) | 82 (76, 85) | 87 (86, 87) | 83 (79, 85) |
Abbreviations: PPA = primary progressive aphasia; PPAOS = primary progressive apraxia of speech.
Relative to the adult population in Olmsted County, the incidence of PPA and PPAOS was 0.70 persons per 100,000 person-years (95% CI 0.34–1.29 persons per 100,000) during the study period.
The incidence of PPAOS in Olmsted County from 2011 to 2022 was 0.14 persons per 100,000 person-years (95% CI 0.02–0.55 persons per 100,000). For the 8 patients with PPA, the incidence was 0.56 persons per 100,000 person-years (95% CI 0.24–1.10 persons per 100,000) (Figure 1). We then evaluated the incidence of PPA variants during the same study period; the incidence of nfvPPA was 0.14 persons per 100,000 person-years (95% CI 0.02–0.55), 0.21 persons per 100,000 person-years (95% CI 0.04–0.61) for lvPPA, and 0.21 persons per 100,000 person-years (95% CI 0.04–0.61) for svPPA.
Figure 1. Incidence of Primary Progressive Aphasia and Primary Progressive Apraxia of Speech in Olmsted County, MN, Between 2011 and 2022.
.
Neuropsychometric Testing, Speech Evaluation
All the 10 patients completed either the Short Test of Mental Status (STMS) or the MoCA at symptom/s onset (Table 2),6,12 followed by a formal neuropsychological evaluation in 5 of them. One of the 2 PPAOS patients with a STMS score of 33/38, 2 patients with lvPPA with a STMS score of, respectively, 25/38 and 30/38, as well as 2 patients with svPPA with a STMS score of, respectively, 29/38 and 36/38 declined further neuropsychological assessments. A detailed speech and language examination was performed in all the patients.
Table 2.
Summary of the Clinical Characteristics of Our Patients
| Case # | Diagnosis | Symptoms at onset | Symptoms at last follow up | Handedness | Sex | Education (y) | Bedside cognitive test score |
| #1 | nfvPPA | Single word repetition, agrammatism, apraxia of speech | Single word repetition, agrammatism, apraxia of speech | Right | F | 14 | 29/30 (MoCA) |
| #2 | nfvPPA | Agrammatism, apraxia of speech | Agrammatism, apraxia of speech, sentence comprehension | Right | M | 20 | 22/30 (MoCA) |
| #3 | lvPPA | Anomia, sentence repetition | Anomia, sentence repetition | Left | F | 16 | 25/30 (MoCA) |
| #4 | lvPPA | Anomia, sentence repetition | Anomia, sentence repetition | Right | F | 14 | 25/38 (STMS) |
| #5 | lvPPA | Anomia, sentence repetition forgetfulness | Anomia, sentence repetition, forgetfulness | Right | F | 16 | 30/38 (STMS) |
| #6 | svPPA | Anomia, loss of word knowledge | Anomia, loss of word knowledge | Right | F | 16 | 23/30 (MoCA) |
| #7 | svPPA | Anomia, loss of word knowledge | Anomia, loss of word knowledge | Right | M | 14 | 29/38 (STMS) |
| #8 | svPPA | Anomia, loss of word knowledge | Anomia, loss of word knowledge | Right | F | 20 | 36/38 (STMS) |
| #9 | PPAOS | Apraxia of speech | Apraxia of speech | Right | M | 18 | 29/30 (MoCA) |
| #10 | PPAOS | Apraxia of speech | Apraxia of speech | Right | M | 12 | 33/38 (STMS) |
Abbreviations: lvPPA = logopenic-variant primary progressive aphasia; MoCA = Montreal cognitive assessment; nfvPPA = non-fluent variant primary progressive aphasia; PPAOS = primary progressive apraxia of speech; STMS = Kokmen short test of mental status; svPPA = semantic variant primary progressive aphasia.
Formal neuropsychometric testings in those patients who agreed to them were conducted at a median of 1 year after symptom onset (range: 1–2 years), and none of them underwent repeat testing during their disease course. All patients with the exception of one with PPAOS who had a normal cognitive profile showed evidence of mild cognitive impairment with the exception of one lvPPA patient with an initial MoCA score of 25/30 (and 17/38 on the STMS) whose level of performance was consistent with a moderate-to-severe cognitive impairment.
All patients complained of “speech difficulty” as their initial symptom. Speech/language examinations were conducted in all patients at a median of 2 years after symptoms onset (range: 1–4 years); 5/10 patients (2 svPPA, 1 nfvPPA, 1 lvPPA, 1 PPAOS) underwent at least one additional formal speech and language evaluation during their disease course at a median of 4 years after the first (range: 1–5 years) (median of 6 years after symptoms onset), with expected progression of their language deficits.
Neuroimaging
All the patients had at least one MRI brain at the time of diagnosis, and only 2 patients with lvPPA did not have repeat imaging study during their disease course. The initial MRIs were performed at a median of 1 year (range: 1–2 years) after symptoms onset.
The 2 patients with nfvPPA showed mild generalized cortical atrophy at their first MRI brain after symptoms onset; however, at their last imaging follow-up, a more specific pattern was observed, with a slightly predominant involvement of the inferior aspect of the left frontal lobe. Of the 3 patients with lvPPA, one showed a rather mild generalized cortical atrophy without a specific lobar predominance throughout the disease course at both onset and follow-up; the 2 patients with lvPPA who did not have follow-up images showed evidence of a slightly predominant atrophy at the level of the left temporoparietal lobes left more than right, although mild. While mild diffuse cortical atrophy was seen in the patients with svPPA at their first MRI after disease onset, marked volume loss of the anterior temporal lobes, left more than right was seen at their last MRI brain.
Both patients with PPAOS initially showed a rather diffuse nonspecific pattern of mild cortical atrophy without any obvious specific lobar predominance. An expected progression of the initial nonspecific cortical atrophy was observed at follow-up, with predominant involvement of the precentral gyrus and supplemental motor areas.
An FDG-PET scan was performed in 8/10 patients at a median of 2 years after symptoms onset (range: 1–5 years); 2 patients (1 lvPPA, 1 svPPA) were unable to tolerate the study. The FDG-PET patterns of hypometabolism observed in our patients are shown in Table 3. A focal prerolandic pattern of hypometabolism was observed in all of our patients with the exception of the lvPPA cases who showed a postrolandic pattern, with involvement of the left lateral frontoparietal areas. More specifically, the 2 patients with nfvPPA showed hypometabolism of the dominant posterior inferior frontal lobes; the 2 patients with svPPA who underwent the initial FDG-PET evaluation showed hypometabolism of the anterior temporal poles, left more than right in one, right more than left in the other patient. Finally, the 2 patients with lvPPA showed hypometabolism of the left lateral temporoparietal lobes.
Table 3.
Timing From Disease Onset and FDG-PET Scan Findings
| Case # | Diagnosis | Time from symptom onset to FDG-PET (y) | preR Patterns | postR Patterns |
| #1 | nfvPPA | 2 | L focal superior and inferior frontal | |
| #2 | nfvPPA | 9 mo | L focal superior and inferior frontal | |
| #3 | lvPPA | 1 | L lateral temporal > posterior parietal | |
| #4 | lvPPA | 2 | L lateral temporal > posterior parietal | |
| #5 | svPPA | 1 | L anterior temporal lobe | |
| #6 | svPPA | 3 | R > L anterior temporoparietal | |
| #7 | PPAOS | 2 | L focal superior frontal and bilateral SMA (L > R) | |
| #8 | PPAOS | 5 | L focal inferior > superior frontal and L SMA |
Abbreviations: L = left; lvPPA = logopenic-variant primary progressive aphasia; nfvPPA = non-fluent variant primary progressive aphasia; postR = post-Rolandic; PPAOS = primary progressive apraxia of speech; preR = pre-Rolandic; R = right; SMA = supplementary motor cortex; svPPA = semantic variant primary progressive aphasia.
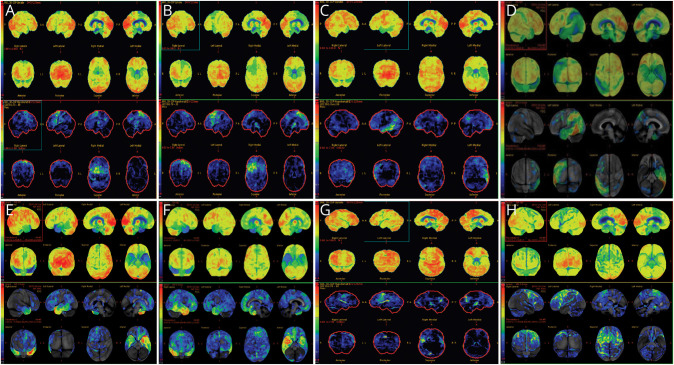
Both patients with PPAOS showed a pattern of more focal posterior superior frontal hypometabolism and supplementary motor area involvement (Figure 2, A–H).
Figure 2. FDG-PET Scan Images of 8 of Our Patients.
(A and B) FDG-PET brain scans of the 2 patients with nfvPPA showing hypometabolism of the dominant left dorsal and inferior frontal lobes. (C and D) Hypometabolism of the left lateral temporoparietal lobes in the patients with lvPPA. (E) Patient with svPPA with involvement of the anterior temporal poles, left more than right. (F) Patient with svPPA with involvement of the anterior temporal poles, right more than left. (G and H) Hypometabolism involving the premotor cortices, with a left predominance in 2 patients with PPAOS. lvPPA = logopenic-variant primary progressive aphasia; nfvPPA = non-fluent variant primary progressive aphasia; PPAOS = primary progressive apraxia of speech; svPPA = semantic variant primary progressive aphasia.
Only 2 (both with lvPPA) of 8 patients did not have at least one follow-up FDG-PET scan of the brain during their disease course. An expected progression of the previously observed baseline pattern of hypometabolism was observed in all other cases.
Disease Progression and Postmortem Examinations
Median follow-up time after their first neurologic evaluation was 5 years (range 2–8 years). All patients progressed clinically, and additional features other than language impairments emerged throughout the disease course. 4 of 10 patients (2 nfvPPA and 2 PPAOS) developed parkinsonism (median MDS-UPDRS part III score at onset: 26, range: 22–31) at a median of 3 years after symptoms onset (range: 2–11). Therapy with Carbidopa/Levodopa was tried in 2 patients after the development of Parkinsonism, but it was soon discontinued because of lack of efficacy.
No clinically significant behavioral abnormalities were noted in the patients with PPA or PPAOS, whose disease course was otherwise characterized by progression of their underlying speech and language difficulties.
The 2 patients with PPAOS who developed parkinsonism also developed vertical supranuclear palsy and experienced recurrent falls during their disease course. Ideomotor apraxia also emerged in both, and dysphagia became severe in the later stages. No patients developed features of motor neuron disease.
Six patients (2 nfvPPA, 2 lvPPA, 2 PPAOS) had died at the time of abstraction of the data at a median age of 82 years (IQR: 76, 85), and 3 of them (1 nfvPPA and 2 PPAOS) underwent brain autopsy. Pathologic evaluation showed a 4-repeat tauopathy consistent with progressive supranuclear palsy (PSP) in all of them, with low levels of Alzheimer neuropathologic changes in all but one PPAOS case. None of the autopsied patients showed evidence of alpha-synuclein or TAR DNA binding protein of 43 kDa pathologic changes. Of the 3 patients (2 lvPPA, 1nfvPPA) who had died and did not undergo autopsy, one with lvPPA had a positive amyloid PET study performed elsewhere which confirmed the presence of Alzheimer neuropathologic changes; one with nfvPPA had a negative amyloid PET study performed elsewhere; no additional pathologic or imaging data were available for the other patient with lvPPA.
Discussion
We observed an overall incidence of PPA and PPAOS in Olmsted County, MN, of 0.41 persons per 100,000 person-years during the study period. To our knowledge, this is among the first study to formally investigate the incidence of all 3 PPA variants and PPAOS because prior epidemiologic reports mainly derived their PPA prevalence from data relative to diseases for which prevalence was known2 or did not include all PPA subtypes, as in the article from Logroscino G. et al., which reported an incidence for PPA in Europe of 0.61 cases per 100.000 persons-year.29 This is the first study to assess the incidence of PPAOS.
PPA and PPAOS represent a group of relatively rare diseases, having nonetheless a great impact on the patient's ability to communicate socially and occupationally. Age of onset varies considerably, and it may differ based on the specific subtype; while most patients with PPA and PPAOS develop symptoms in their sixties around one-third/fourth of cases present in their seventies.2
Cognition in patients with PPA is relatively preserved in the early disease stages2; however, with time, neurocognitive differences beyond language impairment may become severe. Most of our patients with PPA had global cognitive scores on the MoCA that could be considered consistent with mild cognitive impairment, with the exception of one patient with lvPPA who had dementia, supporting the hypothesis that this last group of patients may suffer a relatively more widespread cognitive impairment compared with other subtypes.8
No cognitive deficits were observed in our patients with PPAOS when formally tested other than slight inefficiencies in some aspects of executive functioning. While patients with PPAOS often score well within the normal cognitive range early in the disease course,30 mild frontal lobe inefficiency as measured by the Frontal Assessment Battery may seem evident in the later stages of the disease.4 Greater involvement of other cognitive domains may suggest the presence of an additional neurodegenerative pathology.
The differences in the neurocognitive profiles likely relates to the involvement of different cortical areas relative to the specific disease and/or subtype. While it is worth noticing that it is sometimes difficult to appreciate substantial differences on imaging, particularly in patterns of atrophy on MRIs, different neuroimaging profiles differentiate PPA subtypes and characterize patients with PPAOS especially on FDG-PET. Patients with nfvPPA often show hypometabolism involving the dominant dorsal and inferior frontal lobe and at times the superior temporal gyrus, suggestive of a greater involvement of the anterior areas of the language network.2 The characteristic involvement of the left lateral temporoparietal region may be the anatomical basis for repetition deficits and phonological errors observed in patients with lvPPA.31 Although hypometabolism of the right anterior temporal pole is associated with semantic dementia,32 a greater involvement of the left anterior temporal lobe seems to be a distinctive feature of svPPA.2
The involvement of the precentral cortex and bilateral supplementary motor area in PPAOS and the relative sparing of inferior frontal language areas, at least in the early disease stages, may be the anatomical substrate for the difficulty that these patients have in planning, producing, and monitoring speech.5,22,33
Clinical progression may vary significantly depending on the disease and their subtypes, and it is believed to be a consequence of the different anatomical structures involved and patterns of disease spread,34 and although the speech and language impairment may remain the predominant feature, additional signs and symptoms may arise over time. The presence of parkinsonism is relatively common in the later stages of some patients, particularly in those with PPAOS where it can be seen in up to 40% of patients 5 years after disease onset.2 When additional features like vertical supranuclear palsy, frequent falls and limb apraxia become apparent, they may have prognostic implications because they may at times become an equally debilitating symptoms.4
None of our patients developed behavioral abnormalities which are sometimes seen in patients with PPA, particularly nfvPPA, and can at times even meet criteria for behavioral variant Frontotemporal dementia.2,34,35
Survival is associated with the specific diagnosis and seems to be shorter in individuals with nfvPPA (7 years from symptoms onset on average)2,36 compared with those with svPPA and PPAOS (around 10 years).37,38 While our relatively small sample size makes it difficult to perform a survival analysis, it is worthwhile noting that our PPAOS patients lived 5 years (median) longer than the PPA patients.
We observed similar pathologic findings in most of the PPAOS autopsied patients reported in the literature, showing underlying 4-repeat tauopathy consistent with a PSP-CBD spectrum pathology.22,39 The pathologic changes observed in patients with nfvPPA may vary40; however, 4-repeat tau pathology is commonly observed,41 as confirmed by our autopsied case showing PSP. We noted that many of our patients had low levels of Alzheimer disease neuropathologic changes which is not surprising given the average age of death of our patients.
Our study has several limitations. First, our small sample size limits the interpretation and generalizability of the data; however, it is important to note that our patients share similar clinical and paraclinical characteristics to those with similar disorders who were previously reported by our group. Second, important clinical information may be missing because patients were seen over time during our regular clinical practice rather than as part of a research protocol. This may explain why information related to follow-up neuropsychological testing or speech evaluations are limited after their initial evaluation. Third, no computerized protocol was used to assess the presence or degree of atrophy and/or hypometabolism on imaging; thus, this may lead to intervisibility when reviewing the images.
Speech and language disorders are a relatively rare group of diseases. PPAOS seems to be as common as or even more common than some of the PPA variants. Considerable advances have been made over the last decades to differentiate subtypes and analyze their clinical phenotypes and progression.
Acknowledgment
We would like to acknowledge Dr. Joseph Parisi and Mrs. Sarah Boland, Mayo Clinic, MN, whose involvement with different aspects of patient care allowed us to gather information for the study.
Glossary
- IQR
interquartile range
- lvPPA
logopenic variant PPA
- nfvPPA
non-fluent variant PPA
- PPA
primary progressive aphasia
- PPAOS
primary progressive apraxia of speech
- STMS
Short Test of Mental Status
- svPPA
semantic variant PPA
- TMT
Trail Making Test
Appendix. Authors
| Name | Location | Contribution |
| Pierpaolo Turcano, MD | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jennifer L. Whitwell, PhD | Department of Radiology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content |
| Joseph R. Duffy, PhD | Division of Speech Pathology, Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content |
| Mary M. Machulda, PhD, LP | Department of Psychiatry and Psychology (Neuropsychology), Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content |
| Aidan Mullan, MA | Department of Health Sciences Research, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Keith A. Josephs, MD, MST, MSc | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Rodolfo Savica, MD, PhD | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
The study was funded by NIH (National Institute of Deafness and Communication Disorders) grants R01 DC14942 and R01 DC12519.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botha H, Josephs KA. Primary progressive aphasias and apraxia of speech. Continuum (Minneap Minn). 2019;25(1):101-127. doi: 10.1212/CON.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82(13):1119-1126. doi: 10.1212/WNL.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain. 2014;137(Pt 10):2783-2795. doi: 10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522-1536. doi: 10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the mini-mental state examination in mild cognitive impairment. Arch Neurol. 2003;60(12):1777-1781. doi: 10.1001/archneur.60.12.1777 [DOI] [PubMed] [Google Scholar]
- 7.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 8.Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, Josephs KA. Neuropsychological profiles differ among the three variants of primary progressive aphasia. J Int Neuropsychol Soc. 2015;21(6):429-435. doi: 10.1017/S1355617715000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephs KA, Duffy JR, Clark HM, et al. A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat Commun. 2021;12(1):3452. doi: 10.1038/s41467-021-23687-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24(5):408-416. doi: 10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strand EA, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014;51:43-50. doi: 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725-728. doi: 10.1001/archneur.1991.00530190071018 [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D. Wechsler Memory Scale. Psychological Corporation; 1945. [Google Scholar]
- 14.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, Vol 4. Neuropsychology Press; 1985. [Google Scholar]
- 15.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual Mot Skills. 1958;8(7):271-276. doi: 10.2466/pms.8.7.271-276 [DOI] [Google Scholar]
- 16.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system. Assessment 2001. [DOI] [PubMed] [Google Scholar]
- 17.Osterrieth PA. Le Test de copie d'une Figure Complexe; Contribution a l'etude de la Perception et de la Memoire. Archives de psychologie; 1944. [Google Scholar]
- 18.Warrington EK. Visual Object and Space Perception Battery. Thames Valley Test Company; 1991. [Google Scholar]
- 19.Botha H, Duffy JR, Whitwell JL, et al. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex. 2015;69:220-236. doi: 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh TD, Josephs KA, Machulda MM, et al. Clinical, FDG and amyloid PET imaging in posterior cortical atrophy. J Neurol. 2015;262(6):1483-1492. doi: 10.1007/s00415-015-7732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephs KA, Duffy JR, Fossett TR, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010;67(5):596-605. doi: 10.1001/archneurol.2010.78 [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385-1398. doi: 10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335-346. doi: 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson DW. Neuropathology of Pick's disease. Neurology. 2001;56(11 suppl 4):S16-S20. doi: 10.1212/wnl.56.suppl_4.s16 [DOI] [PubMed] [Google Scholar]
- 25.Dickson DW, Bergeron C, Chin SS, et al. Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61(11):935-946. doi: 10.1093/jnen/61.11.935 [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011;45(3):384-389. doi: 10.1007/s12031-011-9589-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853-864. doi: 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 29.Logroscino G, Piccininni M, Graff C, et al. Incidence of syndromes associated with frontotemporal lobar degeneration in 9 European countries. JAMA Neurol. 2023;80(3):279-286. doi: 10.1001/jamaneurol.2022.5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polsinelli AJ, Machulda MM, Martin PR, et al. Neuropsychological profiles of patients with progressive apraxia of speech and aphasia. J Int Neuropsychol Soc. 2022;28(5):441-451. doi: 10.1017/S1355617721000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madhavan A, Whitwell JL, Weigand SD, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PLoS One. 2013;8(4):e62471. doi: 10.1371/journal.pone.0062471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landin-Romero R, Tan R, Hodges JR, Kumfor F. An update on semantic dementia: genetics, imaging, and pathology. Alzheimers Res Ther. 2016;8(1):52. doi: 10.1186/s13195-016-0219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitwell JL, Duffy JR, Strand EA, et al. Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur J Neurol. 2013;20(4):629-637. doi: 10.1111/ene.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesulam M, Coventry C, Bigio EH, et al. Nosology of primary progressive aphasia and the neuropathology of language. Adv Exp Med Biol. 2021;1281:33-49. doi: 10.1007/978-3-030-51140-1_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulugut H, Stek S, Wagemans LEE, et al. The natural history of primary progressive aphasia: beyond aphasia. J Neurol. 2022;269(3):1375-1385. doi: 10.1007/s00415-021-10689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy JR, Josephs KA. The diagnosis and understanding of apraxia of speech: why including neurodegenerative etiologies may be important. J Speech Lang Hear Res. 2012;55(5):S1518-S1522. doi: 10.1044/1092-4388(2012/11-0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133(Pt 1):300-306. doi: 10.1093/brain/awp248 [DOI] [PubMed] [Google Scholar]
- 38.Whitwell JL, Martin P, Duffy JR, et al. Survival analysis in primary progressive apraxia of speech and agrammatic aphasia. Neurol Clin Pract. 2021;11(3):249-255. doi: 10.1212/CPJ.0000000000000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11(4):283-296. doi: 10.1080/13554790590963004 [DOI] [PubMed] [Google Scholar]
- 40.Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin. 2017;35(2):339-374. doi: 10.1016/j.ncl.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris JM, Gall C, Thompson JC, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21):1832-1839. doi: 10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigators.