Abstract
Objectives:
This study aimed to investigate the impact of the COVID-19 pandemic on the examination and treatment of colorectal cancer (CRC) and on the behaviors of patients and practitioners.
Methods:
This is a retrospective analysis of the CRC patients who presented to our department between April 2019 and March 2021 and underwent surgery. Clinical presentation of CRC and time from symptom onset to medical presentation were compared between the control (April 2019 to March 2020, n=124) and COVID-19 pandemic periods (April 2020 to March 2021, n=111).
Results:
Two hundred and thirty-five patients were reviewed. The rate of positive fecal occult blood tests was significantly lower during the COVID-19 pandemic period (13.5 vs. 25.0%, P = 0.027). Among the symptomatic patients who had melena and abdominal symptoms, the time from symptom onset to medical presentation was significantly longer during the COVID-19 period (115 vs. 31 days, P < 0.001). In addition, the interval between presenting to a practitioner and being referred to our department was similar between the two periods (19 vs. 13 days, P = 0.092). There were no significant differences in the stage of cancer between the two periods. The rate of preoperative sub-obstruction was significantly higher during the COVID-19 period (41.4 vs 23.4%, P = 0.003). There was no significant difference in overall survival and recurrence-free survival between two periods.
Conclusions:
Hesitation to seek examination and treatment for CRC was observed in patients but not in practitioners during the COVID-19 pandemic period. The prognosis did not change.
Keywords: colorectal cancer, COVID-19, fecal occult blood test, delayed presentation
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has impacted many people and healthcare systems around the world. With the progression of the pandemic, health care providers had to quickly and radically rebuild their health care systems in unprecedented manners to address the spread of COVID-19. The first wave of COVID-19 occurred in Japan in March 2020. In response, the Japanese government declared a state of emergency on April 7. In consideration of the global spread of COVID-19, the Japan Surgical Society also recommended that surgical treatment should be limited to patients requiring life-threatening surgery, and that elective surgery for non-fatal or non-urgent patients should be postponed[1,2]. As a result, the number of various noncritical surgeries was significantly reduced after the first outbreak of COVID-19[3-5].
According to the American College of Surgeons COVID-19 guidelines for the triage of colorectal cancer (CRC) patients, the treatment of early CRC was recommended to be deferred for 3 months until there were fewer COVID-19 patients, hospital resources were not depleted, ICU ventilator capacity was still available, and the COVID-19 trajectory was not in a rapidly escalating stage[6]. As is the case with the recommendation in the United States, the Japanese Society of Clinical Oncology suggested that deferring surgery for 3 months was an option for early-stage CRC in which only a portion of the polyp is cancerous[7]. Previous Japanese retrospective studies have reported that the COVID-19 pandemic had reduced the number of CRC diagnoses and surgeries[3,8,9]. The COVID-19 pandemic has also affected the behavior of patients seeking medical care. We previously reported that during the COVID-19 pandemic, the time between symptom onset and consultation lengthened and the number of cases of complicated appendicitis increased[10]. However, it is unclear how the COVID-19 pandemic affected the behavior and prognosis of CRC patients. In this study, we retrospectively studied CRC patients in the year following the COVID-19 pandemic with respect to patient and practitioner behavior. We compared surgical outcomes, time from symptom onset to consultation, and duration of practitioner referral between the initial COVID-19 pandemic period and the pre-pandemic period. In addition, patient prognosis was investigated for each of these two periods.
Methods
Patients
We analyzed all the CRC patients who presented to our department at Nara Medical University Hospital between April 2019 and March 2021 and underwent surgery. Two hundred and thirty-five consecutive patients were included. Following the first surge of COVID-19, the Japanese government declared a state of emergency in April 2020, therefore, patients with CRC who presented to our department between April 2020 and March 2021 were defined as the COVID-19 pandemic group. Patients who presented to our department between April 2019 and March 2020 were defined as the control group. We retrospectively obtained the patients' data on the following clinicopathologic characteristics from medical records: age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, Charlson Comorbidity Index (CCI), C-reactive protein (CRP), albumin (Alb), Carcinoembryonic Antigen (CEA), Carbohydrate Antigen 19-9 (CA19-9), surgical approach, surgical procedure, and time from symptom onset to medical consultation or surgery. Furthermore, we investigated the duration from the onset of symptoms to the consultation with a medical institution. Surgical findings contained the procedure of surgery, operative time, blood loss, postoperative complications, and length of hospital stay. Informed consent was obtained from all patients. We also recorded the clinical presentation of CRC, including positive fecal occult blood test, melena, abdominal symptoms, anemia, and accidental detection. “Accidental detection” means that CRC is found accidentally by screening colonoscopy or follow-up after colonoscopy and polypectomy without any patients' chief complaint.
This study was approved by the Institutional Review Board at Nara Medical University (Approval number: 3453). The patients were able to opt out of the study through the hospital's website.
CRC diagnosis and perioperative factors
The depth of tumor invasion, lymph node metastasis, distant metastasis, and CRC stage were categorized in accordance with the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma[11]. We defined bowel sub-obstruction as a situation in which there were no obvious symptoms of bowel obstruction such as severe abdominal pain or vomiting, but the endoscope cannot pass through. Severe postoperative complications were defined as grade III or higher complications in accordance with the Clavien-Dindo (CD) classification[12].
Statistical analysis
Continuous variables were compared using the t-test. Categorical variables were compared using Fisher's exact test. The date of last follow-up was June 15, 2023. Overall survival (OS) was defined as the period from initial treatment to death from any cause. Recurrence-free survival (RFS) was defined as the period from surgery to first detection of recurrence or death from any cause. Kaplan-Meier survival calculations and the corresponding log-rank tests were applied to determine differences in survival and recurrence. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using the JMP software program ver. 13.2 (SAS Institute Inc. Cary, NC, USA).
Results
Patient characteristics
The number of patients presenting to our department with CRC decreased during the COVID-19 pandemic periods (124 vs. 111 cases). There were no significant differences in patient characteristics, including age, gender, BMI, ASA classification, CCI, and preoperative laboratory data, including CRP, Alb, CEA, and CA19-9 between the two periods (Table 1).
Table 1.
Patient Characteristics and Preoperative Factors.
| Control period n = 124 (%) |
COVID-19 period n = 111 (%) |
P value | |
|---|---|---|---|
| Age, years | 69 (± 11) | 69 (± 10) | 0.781 |
| Sex | 0.443 | ||
| Female | 44 (39.6%) | 43 (34.7%) | |
| Male | 67 (60.4%) | 81 (65.3%) | |
| BMI, kg/m2 | 22.7 (± 4.2) | 22.9 (± 3.8) | 0.742 |
| ASA classification | 0.070 | ||
| ≤ 2 | 103 (83.1%) | 90 (81.1%) | |
| ≥ 3 | 21 (16.9%) | 21 (18.9%) | |
| CCI | 3.0 (± 1.8) | 3.0 (± 1.9) | 0.923 |
| Laboratory data | |||
| preoperative CRP, mg/L | 0.8 (± 1.7) | 1.0 (± 2.4) | 0.454 |
| preoperative Alb, g/L | 4.1 (± 0.5) | 4.1 (± 0.5) | 0.250 |
| preoperative CEA | 71.9 (± 473.5) | 32.3 (± 150.0) | 0.383 |
| preoperative CA19-9 | 65.0 (± 256.4) | 38.2 (± 140.8) | 0.322 |
BMI Body Mass Index, ASA American Society of Anesthesiologists, CCI Charlson Comorbidity Index, CRP C-reactive protein, Alb albumin, CEA Carcinoembryonic Antigen, CA19-9 Carbohydrate Antigen 19-9
Continuous data are expressed as mean ± standard deviation.
Impact of the COVID-19 pandemic on clinical presentation of CRC
We investigated the impact of the COVID-19 pandemic on procedures for detecting CRC, because CRC screening was restricted during the pandemic period. With respect to clinical presentation of CRC, positive fecal occult blood test was significantly lower during the COVID-19 pandemic period than during the control period (13.5 vs 25.0%, P = 0.027). In contrast, the proportions of CRC cases detected by melena, abdominal symptoms, anemia, or accidentally were similar in both periods (Table 2).
Table 2.
Clinical Presentation of Colorectal Cancer and Time to Medical Presentation.
| Control period n =124 (%) |
COVID-19 period n =111 (%) |
P value | |
|---|---|---|---|
| Presentation of CRC | |||
| Positive fecal occult blood test | 31 (25.0%) | 15 (13.5%) | 0.027 |
| Melena | 35 (28.2%) | 35 (31.5%) | 0.580 |
| Abdominal symptoms | 26 (21.0%) | 27 (24.3%) | 0.539 |
| Anemia | 10 (8.1%) | 13 (11.7%) | 0.348 |
| Accidental | 24 (19.4%) | 22 (19.8%) | 0.929 |
| Patients with melena (n = 70) | n = 35 | n = 35 | |
| time from symptom to medical presentation, days | 21 (± 24) | 101 (± 20) | 0.013 |
| time from practitioner to our department, days | 16 (± 3) | 22 (± 3) | 0.178 |
| Patients with abdominal symptoms (n = 53) | n = 26 | n = 27 | |
| time from symptom to medical presentation, days | 16 (± 21) | 74 (± 20) | 0.049 |
| time from practitioner to our department, days | 8 (± 3) | 13 (± 2) | 0.187 |
Continuous data are expressed as mean ± standard error.
Patients' and practitioners' behavior
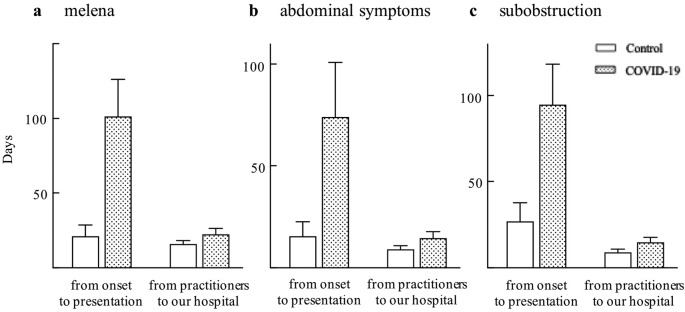
Among the patients with melena (n = 70), the time from the onset of symptoms to the consultation with a medical institution was significantly longer in the COVID-19 period than in the control period (101 vs. 21 days, P = 0.013). However, the time taken for practitioners to refer to our hospital did not change during the COVID-19 period compared with the control period (22 vs. 16 days, P = 0.178) (Table 2, Figure 1a). Among the patients with abdominal symptoms (n = 53), the time from the onset of symptoms to the consultation with a medical institution was significantly longer in the COVID-19 period than in the control period (74 vs. 16 days, P = 0.049). However, the time taken for practitioners to refer to our hospital did not change during the COVID-19 period compared with the control period (13 vs. 8 days, P = 0.187) (Table 2, Figure 1b). Among the patients who were symptomatic, including melena and abdominal symptoms (n = 123), the time from the onset of symptoms to the consultation with a medical institution was significantly longer during the COVID-19 pandemic period than during the control period (115 vs. 31 days, P < 0.001). The time taken for practitioners to refer to our hospital did not change during the COVID-19 pandemic period compared with the control period (19 vs. 13 days, P = 0.092).
Figure 1.
Time to seek medical attention in a) patients with melena, b) patients with abdominal symptoms, and c) patients who had colonic preoperative sub-obstruction.
Oncological outcome and perioperative findings
There were no significant differences in tumor size, depth of tumor invasion, lymph node metastasis, distant metastasis, CRC stage, or tumor location between the two periods (Table 3). There were no differences in the surgical approach (open or laparoscopic/robot-assisted surgery) or preoperative colonic obstruction between the two periods. The rate of preoperative colonic sub-obstruction was significantly higher in the COVID-19 pandemic period than in the control period (41.4 vs 23.4%, P = 0.003). The rates of primary tumor resection, emergency surgery, and neoadjuvant therapy were similar in the two periods. The operation time was significantly longer during the COVID-19 pandemic period than in the control period (297 min vs. 250 min, P = 0.004). Blood loss was similar between the two periods. There were no significant differences in the postoperative complications with C-D ≥ III and anastomotic leakage between the two periods. Accordingly, the length of hospital stay was significantly longer during the COVID-19 pandemic period than in the control period (7 days vs. 4 days, P = 0.045) (Table 4). Among patients who had colonic preoperative sub-obstruction (n = 75), the time from the onset of symptoms to the consultation with a medical institution was significantly longer in the COVID-19 pandemic period than in the control period (95 vs. 27 days, P = 0.034). However, the time taken for practitioners to refer to our hospital did not change during the COVID-19 pandemic period compared with the control period (15 vs. 9 days, P = 0.143) (Table 4, Figure 1c).
Table 3.
Oncological Findings.
| Control period n = 124 (%) |
COVID-19 period n = 111 (%) |
P value | |
|---|---|---|---|
| Size of tumor, mm | 40.1 (± 20.8) | 36.8 (± 17.1) | 0.179 |
| Depth of tumor invasion | 0.479 | ||
| Tis, T1 | 22 (17.7%) | 13 (11.7%) | |
| T2 | 16 (12.9%) | 14 (12.6%) | |
| T3 | 53 (42.7%) | 57 (51.4%) | |
| T4 | 33 (26.6%) | 27 (24.3%) | |
| Lymph node metastasis | 0.847 | ||
| Positive | 63 (50.8%) | 55 (49.6%) | |
| Negative | 61 (49.2%) | 56 (50.4%) | |
| Distant metastasis | 0.727 | ||
| Positive | 17 (13.7%) | 17 (15.3%) | |
| Negative | 107 (86.3%) | 94 (84.7%) | |
| Stage | 0.551 | ||
| 0 | 4 (3.2%) | 1 (0.9%) | |
| I | 25 (20.2%) | 18 (16.2%) | |
| II | 32 (25.8%) | 36 (32.4%) | |
| III | 46 (37.1%) | 39 (35.1%) | |
| IV | 175 (13.7%) | 17 (15.3%) | |
| Tumor location | 0.200 | ||
| Right side | 35 (28.2%) | 40 (36.0%) | |
| Left side | 89 (71.8%) | 71 (64.0%) |
Continuous data are expressed as mean ± standard error.
Table 4.
Perioperative Findings.
| Control period n = 124 (%) |
COVID-19 period n = 111 (%) |
P value | |
|---|---|---|---|
| Approach | 0.331 | ||
| Open | 20 (16.1%) | 13 (11.7%) | |
| Laparoscopic/ Robot-assisted | 104 (83.9%) | 98 (88.3%) | |
| Primary tumor resection | 110 (88.7%) | 105 (94.6%) | 0.107 |
| Emergency surgery | 18 (14.5%) | 19 (17.1%) | 0.585 |
| Preoperative obstruction | 11 (8.9%) | 14 (12.7%) | 0.341 |
| Preoperative sub-obstruction | 29 (23.4%) | 46 (41.4%) | 0.003 |
| Neoadjuvant therapy | 13 (10.5%) | 15 (13.5%) | 0.474 |
| Intraoperative findings | |||
| Operation time, min | 250 (± 121) | 297 (± 129) | 0.004 |
| Blood loss, ml | 46 (± 100) | 69 (± 165) | 0.211 |
| Postoperative complications | |||
| C-D classification ≥ III | 16 (12.9%) | 13 (11.7%) | 0.782 |
| Anastomotic leakage | 8 (6.5%) | 6 (5.4%) | 0.735 |
| Length of hospital stay, days | 14 (± 13) | 12 (± 10) | 0.301 |
| Patient with preoperative sub-obstraction (n = 75) | n = 29 | n = 46 | |
| time from symptom to medical presentation, days | 27 (± 24) | 95 (± 19) | 0.034 |
| time from practitioner to our department, days | 9 (± 3) | 15 (± 2) | 0.143 |
C-D Clavien-Dindo
Continuous data are expressed as mean ± standard error.
Prognosis
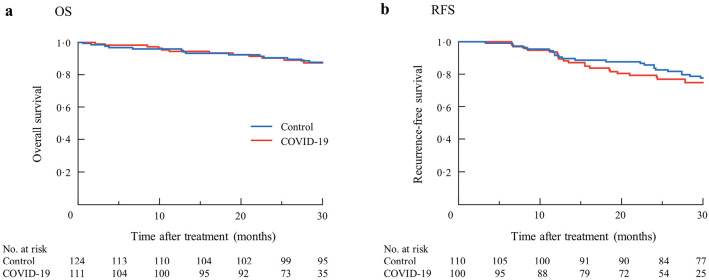
Finally, we investigated prognosis during the control period and the COVID-19 pandemic period. The mean duration of follow-up was 31.5 months. There was no significant difference in OS between two periods (P = 0.865) (Figure 2a). Two-hundred-ten patients underwent curative resection for colorectal cancer. We investigated RFS during the control period and the COVID-19 pandemic period in these patients. There was no significant difference in RFS between two periods (P = 0.434) (Figure 2b).
Figure 2.
Overall survival (a, P = 0.865) and recurrence-free survival (b, P = 0.434) during the control period and the COVID-19 pandemic period.
Discussion
The COVID-19 pandemic disrupted health care systems worldwide, resulting in fewer endoscopies being performed and therefore fewer cancers being detected[13]. Previous Japanese retrospective studies have reported that the number of early CRC surgeries and CRC diagnoses using fecal occult blood tests declined due to the COVID-19 pandemic[3]. However, to our knowledge, this is the first detailed study of the impact of the COVID-19 pandemic on the examination, treatment and prognosis of CRC with respect to specific patients' and practitioners' behaviors. Although there were no restrictions on access to medical care during the state of emergency in our country, our data highlighted that patients were hesitant to undergo surgical treatment during the early stages of the COVID-19 pandemic.
In this study, the number of CRC cases diagnosed using fecal occult blood tests declined during the COVID-19 pandemic, suggesting that the pandemic prevented the early cancer detection. Although in this study, the rates of primary tumor resections and emergency surgeries did not change during the COVID-19 pandemic, those of sub-obstructive CRC significantly increased. This may be because patients with CRC hesitated to seek medical attention during the COVID-19 pandemic. To verify this finding, we investigated the time from onset of symptoms to medical presentation, and found that this interval was increased. This indicates that the patients had been reluctant to seek medical treatment during the COVID-19 pandemic. While some studies pointed out that the incidence of obstructive CRC increased after the start of the pandemic, to our knowledge, no study has reported the actual time from symptom onset to medical consultation[14]. In contrast, the time interval between the patients' visit to the practitioner and reporting to our department was not prolonged during the COVID-19 pandemic. This indicated that the general practitioners appropriately referred the patient to the medical facility.
It is controversial whether the COVID-19 pandemic has changed the degree of cancer progression[15]. Some previous reports have shown an increase in cancer progression, while others have shown no change[9]. In the present study, cancer progression did not increase during the COVID-19 pandemic. This might be because the delay in seeing patients was insufficient to increase the cancer stage. Consequently, the prognosis including OS and RFS did not change during the COVID-19 pandemic period. Additionally, it is reported that bowel obstruction of CRC is associated with poor survival including overall and recurrence free survival[16,17]. In this study, preoperative bowel obstruction did not increase during the COVID-19 pandemic, even though sub-obstruction which we defined as a situation where the endoscope cannot pass through increased due to delayed medical presentation. It might be because OS or RFS did not worsen. This indicated that the COVID-19 pandemic might not have enough impact to change the prognosis of CRC patients. Alternatively, it may be that we have dealt with the COVID-19 pandemic appropriately. Furthermore, our hospital did not restrict admission of patients with COVID-19 or CRC during the COVID-19 pandemic period. This may not have contributed to the increase in cancer progression. However, the follow-up period in this study was short, therefore further studies are required to verify the long-term effects of COVID-19.
In the COVID-19 period postoperative serious complications did not increase compared to the control period. We showed that despite the significant increase in sub-obstruction, surgical treatment could be safely implemented. Appropriate treatment for CRC sub-obstruction prevented an increase in serious complications above C-D grade III. During the peak of a pandemic, it is important to conserve hospital resources. Preventing an increase in postoperative complications would reduce the waste of medical resources. Therefore, this study showed that hospital resources could have been saved even during a COVID-19 pandemic. Although sub-obstruction increased during the COVID-19 pandemic period, laparoscopic surgery was successful in most cases.
This study has several limitations. At first, this was a retrospective study conducted at a single institution. To minimize selection bias, all consecutive patients who underwent surgery for CRC at our institution were included. Second, the cohort in this study may have been too small to draw definitive conclusions. However, this study contained important details that were not available in large-scale studies. Third, we investigated only patients who had undergone surgery for CRC including palliative surgery as stoma construction. However, to our knowledge, this is the first detailed report in Japan that investigated the behavior and prognosis of CRC patients during the COVID-19 pandemic. Our results provide essential insights for physicians treating CRC in our health care system.
In conclusion, we highlighted the association between the delay in medical presentation and decreased positive fecal occult blood tests in the initial stages of the COVID-19 pandemic in Japan. We found hesitation in seeking examination and treatment for CRC in patients, but not in practitioners, during the COVID-19 pandemic. Appropriate care for CRC patients is a crucial challenge during the pandemic era.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
Tadataka Takagi substantially contributed to the conception of the work and acquisition of the data. Tadataka Takagi and Fumikazu Koyama drafted the work. Tadataka Takagi, Hiroyuki Kuge, Yosuke Iwasa, Takeshi Takei, Tomomi Sadamitsu, Kosuke Fujimoto, Suzuka Harada, Takashi Tamura, Goki Ejiri, Chihiro Yoshikawa, and Masayuki Sho performed the operation and final approval of the version to be published.
Approval by Institutional Review Board (IRB)
Institutional Review Board for Studies in Humans at Nara Medical University
Review board approval number: 3453
Acknowledgements
We appreciate Naoki Ozu (Nara Medical University) for his advice on statistical analysis.
References
- 1.Japan Surgical Society [Internet]. [cited 2020 Apr 29]. Available from: https://www.jssoc.or.jp/aboutus/coronavirus/info20200402. Japanese.
- 2.Mori M, Ikeda N, Taketomi A, et al. COVID-19: clinical issues from the Japan Surgical Society. Surg Today. 2020 Aug; 50(8): 794-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyo M, Hata T, Sekido Y, et al. Colorectal Surgery in the COVID-19 Pandemic Era. J Anus Rectum Colon. 2022 Jan; 6(1): 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuno T, Takada D, Shin JH, et al. Surgical volume reduction and the announcement of triage during the 1st wave of the COVID-19 pandemic in Japan: a cohort study using an interrupted time series analysis. Surg Today. 2021 Nov; 51(11): 1843-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyo M, Mizushima T, Eguchi H, et al. Impact of the COVID-19 pandemic on colorectal cancer surgery in Japan: Clinical Study Group of Osaka University―A multicenter retrospective study. Ann Gastroenterol Surg. 2022 Aug; 7(1): 121-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Surgeons. COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures [Internet]. [cited 2020 Mar 17]. Available from: https://www.facs.org/%20covid-19/clinical-guidance/triage
- 7.Japan Society of Clinical Oncology. New Coronavirus Infection (COVID-19) and Cancer Treatment [Internet]. [cited 2021 Feb 2]. Available from: http://www.jsco.or.jp/jpn/index/page/id/2333
- 8.Ikeda N, Yamamoto H, Taketomi A, et al. The impact of COVID-19 on surgical procedures in Japan: analysis of data from the National Clinical Database. Surg Today. 2022 Jan; 52(1): 22-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzuu K, Misawa N, Ashikari K, et al. Gastrointestinal Cancer Stage at Diagnosis Before and During the COVID-19 Pandemic in Japan. JAMA Netw Open. 2021 Sep; 4(9): e2126334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takagi T, Kinoshita S, Ohyama T, et al. Delayed presentation and referral time from general practitioners contributing increased complicated appendicitis during the initial COVID-19 pandemic period in Japan. J Anus Rectum Colon. 2023 Jan; 7(1): 17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japanese Society for Cancer of the C, Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon. 2019 Oct; 3(4): 175-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug; 240(2): 205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020 Aug; 3(8): e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinkwin M, Silva L, Vogel I, et al. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021 Aug; 23(8): 2014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim JH, Lee WY, Yun SH, et al. Has the COVID-19 Pandemic caused upshifting in colorectal cancer stage? Ann Coloproctol. 2021 Aug; 37(4): 253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katoh H, Yamashita K, Wang G, et al. Prognostic significance of preoperative bowel obstruction in stage III colorectal cancer. Ann Surg Oncol. 2011 Sep; 18(9): 2432-41. [DOI] [PubMed] [Google Scholar]
- 17.Yik HH, Simon KKS, Petra B, et al. The effect of obstruction and perforation on colorectal cancer disease-free survival. World J Surg. 2010 May; 34(5): 1091-101. [DOI] [PubMed] [Google Scholar]