Abstract
AIM
To describe the multimodal imaging features, treatment, and outcomes of patients diagnosed with adult-onset Coats disease.
METHODS
This retrospective study included patients first diagnosed with Coats disease at ≥18 years of age between September 2017 and September 2021. Some patients received anti-vascular endothelial growth factor (VEGF) therapy (conbercept, 0.5 mg) as the initial treatment, which was combined with laser photocoagulation as needed. All the patients underwent best corrected visual acuity (BCVA) and intraocular pressure examinations, fundus color photography, spontaneous fluorescence tests, fundus fluorescein angiography, optical coherence tomography (OCT), OCT angiography, and other examinations. BCVA alterations and multimodal image findings in the affected eyes following treatment were compared and the prognostic factors were analyzed.
RESULTS
The study included 15 patients who were aged 24-72 (57.33±12.61)y at presentation. Systemic hypertension was the most common associated systemic condition, occurring in 13 (86.7%) patients. Baseline BCVA ranged from 2.0 to 5.0 (4.0±1.1), which showed improvement following treatment (4.2±1.0). Multimodal imaging revealed retinal telangiectasis in 13 patients (86.7%), patchy hemorrhage in 5 patients (33.3%), and stage 2B disease (Shield's staging criteria) in 11 patients (73.3%). OCT revealed that the baseline central macular thickness (CMT) ranged from 129 to 964 µm (473.0±230.1 µm), with 13 patients (86.7%) exhibiting a baseline CMT exceeding 250 µm. Furthermore, 8 patients (53.3%) presented with an epiretinal membrane at baseline or during follow-up. Hyper-reflective scars were observed on OCT in five patients (33.3%) with poor visual prognosis. Vision deteriorated in one patient who did not receive treatment. Final vision was stable in three patients who received laser treatment, whereas improvement was observed in one of two patients who received anti-VEGF therapy alone. In addition, 8 of 9 patients (88.9%) who received laser treatment and conbercept exhibited stable or improved BCVA.
CONCLUSION
Multimodal imaging can help diagnose adult-onset Coats disease. Anti-VEGF treatment combined with laser therapy can be an option for improving or maintaining BCVA and resolving macular edema. The final visual outcome depends on macular involvement and the disease stage.
Keywords: adult-onset Coats disease, multimodal imaging, anti-vascular endothelial growth factor, conbercept
INTRODUCTION
External exudative retinopathy, also known as Coats disease, was first reported by George Coats in 1908. Coats disease is an idiopathic, typically unilateral retinal vasculopathy characterized by telangiectasia occurring in all the retinal vessels. In addition, capillary non-perfusion, aneurysm formation, exudation within and beneath the retina, and exudative retinal detachment can occur in patients with Coats disease. This disease frequently occurs during early childhood; however, several unequivocal cases of Coats disease have been diagnosed in older adults with no history of eye disease. Compared with typical Coats disease, the adult-onset subtype features several clinical differences; for example, the affected area is limited, the disease progresses more slowly, hemorrhage is localized near larger vascular dilatations, and the incidence of exudative retinal detachment is lower[1].
The clinical diagnosis of adult-onset Coats disease is complicated, requiring multimodal imaging examinations, with fundus fluorescein angiography (FFA) being the gold standard diagnostic technique. The typical imaging manifestations of Coats disease include telangiectasia with a typical light bulb appearance, obvious leakage during the late stage, areas of capillary nonperfusion, and obscured fluorescence with hemorrhage and exudation with or without cystic fluorescence accumulation in the macular area[2]. Optical coherence tomography (OCT) can be used to observe cystoid edema in the macular area, retinal exudation, and macular epiretinal membranes (ERM)[3]. Optical coherence tomography angiography (OCTA) can reveal superficial capillary nonperfused areas, dilated miliary aneurysms, and capillaries[4].
Although the pathogenesis underlying adult-onset Coats disease remains unclear, it is currently believed to be related to vascular factors, genes, and cytokines. First, the degradation of the blood-retinal barrier at the endothelial level causes plasma leakage into the vessel wall and thickening of parts of the vessel wall, followed by necrosis and disorganization, leading to a sausage-like shape of the vessel. Second, the presence of abnormal pericytes and endothelial cells in the retinal blood vessels, which subsequently degenerate, causes abnormal retinal vasculature and aneurysm formation alongside the closure of the vessels, resulting in ischemia[5]. Reportedly, Coats disease might be associated with mutations in norrin cysteine knot growth factor (NDP), the telomerase RNA component (TERC), mutations in the gene Crumbs homolog 1 (CRB1), frizzled class receptor 4 (FZD4), and other genes[6]–[7]. Recently, vascular endothelial growth factor (VEGF) was reported in the vessel wall and intraocular fluid of patients with Coats disease, which may be a pathogenic mechanisms underlying this disease[8].
The traditional treatment of adult-onset Coats disease includes cryotherapy and laser photocoagulation of the capillary dilated area and vitreous injection of triamcinolone acetonide for anti-inflammatory treatment. Recently, some studies have reported the effectiveness of anti-VEGF therapy in treating Coats disease[9] and the safety and efficacy of anti-VEGF therapy combined with laser treatment[10]. However, to the best of our knowledge, there is no unified clinical treatment or prognostic factor analysis for adult-onset Coats disease. Herein, we analyzed the prognostic factors of adult-onset Coats disease by observing its systemic characteristics and multimodal imaging features.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the ethics committee of Tianjin Medical University General Hospital (ethics approval No.IRB2023-WZ-143). All images used in the article were given oral permission by the patient and all study protocols adhered to the principles outlined in the Declaration of Helsinki.
Subjects
This retrospective case analysis included 15 adults (15 eyes) with Coats disease who were diagnosed in the Ophthalmology Department of Tianjin Medical University General Hospital from September 2017 to September 2021. The inclusion criteria were as follows: adult-onset Coats disease diagnosed via fundus photography, OCT, and FFA; staging according to the Shields et al[11] staging criteria; and at least 1y of follow-up. The exclusion criteria were as follows: other retinal diseases affecting visual acuity, such as trauma, inflammation, and retinal detachment; other secondary Coats-like alterations, including diabetic retinopathy and retinal vein obstruction; and combined refractive interstitial confusion and other systemic diseases preventing fundus angiography and other examinations.
Methods
All the patients underwent best corrected visual acuity (BCVA) and intraocular pressure (IOP) examinations, fundus color photography, autofluorescence tests, FFA, OCT, and OCTA. BCVA was recorded using a five-point logarithmic visual acuity chart, and manual visual acuity was recorded as 2.0. IOP was measured using a noncontact tonometer. Eyeground color photography was performed using Zeiss VISUCAM 224 camera, Zeiss, Germany. FFA and spontaneous fluorescence examinations were performed using Heidelberg Engineering GmbH 69121, Heidelberg, Germany. High-definition (HD)-OCT and OCTA were performed using Zeiss Cirrus HD-OCT 5000 system, Zeiss, Germany. Central macular thickness (CMT) was recorded via spectral domain (SD)-OCT, and OCTA was performed in a 6×6 mm2 area around the central macula. All data collected were analyzed by the software (software Zeiss Cirrus HD-OCT 9.5.2.19038, Zeiss, Germany). The changes in the pretreatment and post-treatment BCVA, multimodal imaging features, and treatment effects in the affected eyes were compared and the prognostic factors affecting the patients were analyzed. We defined that the patients' Final BCVA higher than the initial BCVA were considered improved, the Final BCVA equal to the initial BCVA were considered stable, and the Final BCVA lower than the Initial BCVA were considered worse.
Statistical Analysis
SPSS 25.0 (SPSS Inc, Chicago, IL, USA) statistical software was used for statistical analysis and processing. Measurements were expressed as mean±standard deviation. Correlation analysis was performed between the patients' final BCVA and demographic factors (age, gender) and clinical observables (general medical history, IOP, lesion extent, the lesion involved in the macula retinal hyperreflective scarring, anti-VEGF therapy, laser therapy), and P<0.05 was considered statistically significant.
RESULTS
The baseline characteristics and treatment details of the patients were summarized in Table 1. The cohort comprised 10 men (10 eyes) and 5 women (5 eyes). All the patients exhibited a unilateral presentation, with seven and eight patients presenting with the disease in the right and left eye, respectively. The age at presentation was 57.33±12.61y (24-72y). Systemic hypertension was the most common associated systemic condition, present in 13 patients (86.7%). The mean IOP was 16.58±2.19 mm Hg, and no significant abnormalities were detected on anterior segment examination. Before treatment, the patients exhibited a baseline visual acuity of 2.0-5.0 (4.0±1.1).
Table 1. Basic information and treatment of patients.
| No. | Age/sex/eye | Systemic medical history | IOP (mm Hg) | Initial BCVA | Final BCVA | Stage | Retinal condition |
CMT | Laser treatment | Anti-VEGF | ||||
| Clock bits | The lesion involved in the macula | Hemorrhage | Hyper-reflective scar | ERM | ||||||||||
| 1 | 24/M/OD | Hypertension | 17.9 | 5 | 5 | 2A | 2 | N | N | N | P | 636 | P | P |
| 2 | 72/F/OS | Hypertension | 13.5 | 5 | 5 | 3A | 10 | N | P | N | P | 392 | P | P |
| 3 | 58/F/OD | Hypertension | 15.5 | 4 | 4.2 | 2B | 6 | P | N | P | P | 782 | P | P |
| 4 | 64/M/OS | Hypertension | 17 | 4.2 | 4.8 | 2A | 9 | N | P | N | P | 513 | P | P |
| 5 | 56/M/OD | Hypertension | 14.2 | 4.2 | 5 | 2B | 7 | N | N | N | N | 291 | P | P |
| 6 | 70/M/OS | Hypertension | 20.6 | 2 | 4 | 2B | 12 | P | N | P | P | 964 | P | P |
| 7 | 60/F/OS | Hypertension | 14 | 2 | 2 | 2B | 12 | P | P | N | N | 129 | P | N |
| 8 | 66/F/OS | Hypertension | 16 | 5 | 5 | 2A | 7 | N | N | N | N | 185 | P | P |
| 9 | 48/M/OD | None | 17.8 | 5 | 5 | 2B | 12 | P | P | N | N | 385 | P | N |
| 10 | 48/M/OS | Hypertension | 15.3 | 2 | 2 | 2B | 12 | P | P | P | P | 761 | P | N |
| 11 | 66/M/OS | Hypertension | 17.2 | 4 | 4 | 2B | 6 | P | N | N | N | 482 | P | P |
| 12 | 41/M/OD | None | 19 | 4.6 | 4.8 | 2B | 12 | P | N | N | N | 353 | N | P |
| 13 | 59/M/OD | Hypertension | 14.5 | 4.8 | 4.3 | 2B | 2 | P | N | P | P | 434 | N | N |
| 14 | 63/M/OS | Hypertension | 20 | 4.8 | 4.6 | 2B | 1 | P | N | P | P | 484 | N | P |
| 15 | 65/F/OD | Hypertension | 16.2 | 4 | 4 | 2B | 2 | P | N | P | N | 304 | P | P |
OD: Right eye; OS: Left eye; N: Negative; P: Positive; CMT: Central macular thickness; IOP: Intraocular pressure; VEGF: Vascular endothelial growth factor; BCVA: Best corrected visual acuity; ERM: Epiretinal membrane.
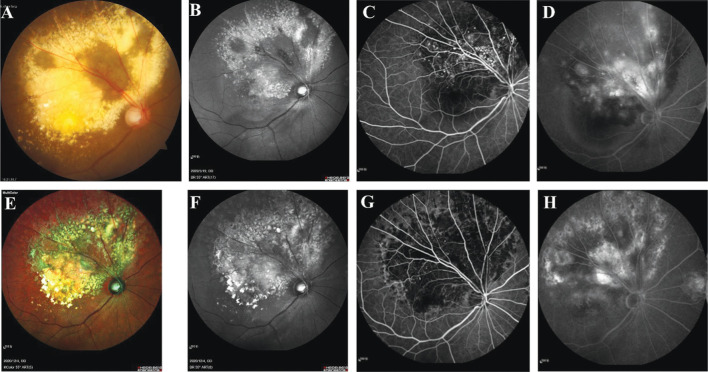
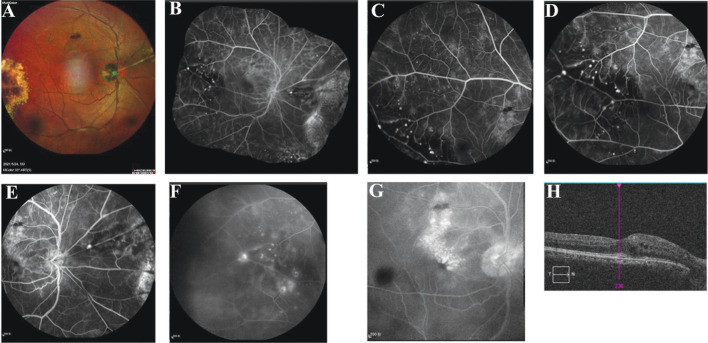
Color fundus photography and FFA revealed large amounts of yellow exudation, retinal telangiectasis and single and clusters of aneurysms in the area of the fundus lesion in 13 patients (86.7%, Figure 1A-1E), whereas two patients (13.3%) exhibited telangiectasis in the macular area. Five patients (33.3%) demonstrated patchy hemorrhage. The location of the lesion with six patients (40.0%) were less than 2 quadrant of involvement, and 9 patients (60.0%) were more than 2 quadrants of involvement. According to the Shields et al's[11] staging criteria, the disease stages were Figures 2A, 2B, and 3A in 3 (20.0%), 11 (73.3%), and 1 (6.7%) patient, respectively. Autofluorescence with hyperfluorescence in the lesion area was detected in 13 patients (86.7%), whereas the remaining two patients (13.3%) exhibited no significant abnormality. FFA uncovered telangiectasia and single and clusters of aneurysms in the lesion area in all the patients alongside significant fluorescence leakage in the late stage, peripheral nonperfused areas (Figure 2A-2F), and cystic fluorescence accumulation in the macular region of some patients in the late-stage disease (Figure 2G, 2H). OCT revealed that the baseline CMT ranged from 129 to 694 µm (473.0±230.1 µm), and 13 patients (86.7%) exhibited a baseline CMT exceeding 250 µm accompanied by a fluid capsule in the inner layer of the retina in the macular region (Figure 2H). The remaining two patients (13.3%) exhibited a normal macular region. Eight patients (53.3%, Figure 3A, 3E) presented with an ERM at baseline or during follow-up, and the ERM detached during treatment in one patient (6.7%, Figure 3B-3D, 3F-3H). Hyper-reflective scars accompanied by poor vision were observed in five patients (Figure 1F-1H). The superficial capillary plexuses of some patients were disturbed and wrinkled. The foveal avascular zone was deformed, and the blood flow density of deep capillary plexuses was reduced (Figure 3E, 3F).
Figure 1. Multimodal imaging of patient (No.3).
A-D: Baseline; E-H: Six months after anti-VEGF therapy combined with laser treatment. A: A large hard exudate was present in the superior temporal and macular regions of the retina; B: Hyperfluorescence in the corresponding area of autofluorescence; C: Capillary dilatation and multiple clusters of punctate hyperfluorescence in the corresponding area on FFA; D: Fluorescence staining in the lesion area and pool-like fluorescence accumulation in the arcuate ring area were present in the advanced stage on FFA; E: The hard exudate in the corresponding area of the right eye was reduced; F: No significant change in autofluorescence; G-H: Clusters of punctate hyperfluorescence on FFA were significantly reduced, and fluorescence staining in the advanced lesion area and pool-like fluorescence accumulation in the arcuate ring area were diminished. FFA: Fundus fluorescein angiography; VEGF: Vascular endothelial growth factor.
Figure 2. Multimodal imaging data of a patient with Coats disease (No.9).
A: Right nasal artery of the right eye featured silvery changes, aneurysmal enlargement was present on the superior nasal artery surrounded by exudation and small pieces of hemorrhage, flaky hemorrhage was present in the superior temporal part of the retina, and large yellowish-white exudation was observed in the temporal periphery; B: Puzzle of the fundus image of the right eye; C: Right temporal peripheral capillary dilatation; D: Focal tumor-like hyperfluorescence with a capillary nonperfusion area in the peripheral arteriolar end of the right temporal eye; E: Focal tumor-like hyperfluorescence on the main trunk of the lateral nasal artery of the right eye; F: Fluorescence leakage in the corresponding focal area with a prolonged contrast time; G: Cystic fluorescence accumulation in the arch ring area in the late stage; H: Cystic edema in the macular area was observed on OCT. OCT: Optical coherence tomography.
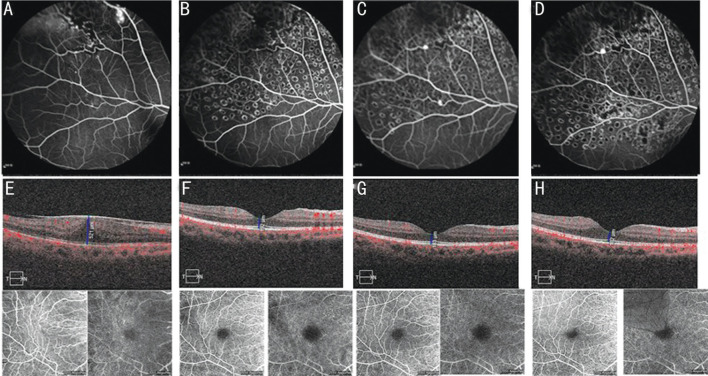
Figure 3. Fundus pictures of patient No.1 from 2019 to 2022.
Patient received four courses of anti-VEGF therapy (conbercept) and three courses of laser treatment. A: At baseline, telangiectasia was present in the temporal periphery with aneurysmal hyperfluorescence of the vascular endings and areas of capillary nonperfusion; B: After 6mo, the aneurysmal hyperfluorescence and telangiectasia in the same lesion disappeared, but small areas of capillary nonperfusion remained visible; C: After 1y; D: After 2y of follow-up, the hyperfluorescence above the nasolateral retinal vein remained, whereas the hyperfluorescence below disappeared; E-H: OCT and OCTA data of the patient at baseline and after 1, 6mo, and 1y of follow-up (blood flow maps of the SCPs and DCPs). The CMTs at these times were 521, 446, 129, and 183 µm, respectively; E: ERM is visible, SCPs were wrinkled and structurally disturbed, FAZ was missing, and the DCP blood flow density was reduced; F: The ERM was detached, the SCP was restored, part of the FAZ was visible, and DCP blood flow density was reduced; G-H: ERM was detached, macular edema had subsided, SCP was restored, normal FAZ was visible, and DCP blood flow was normal. VEGF: Vascular endothelial growth factor; OCT: Optical coherence tomography; OCTA: Optical coherence tomography angiography; CMT: Central macular thichness; SCP: Superficial capillary plexus; DCP: Deep capillary plexus; ERM: Epiretinal membrane; FAZ: Foveal avascular zone.
One patient with stage 2B disease refused treatment, and his BCVA decreased from 4.8 to 4.3 during the follow-up period (Table 2). In addition, ERM and hyper-reflective scar were visible on OCT. Three patients with stage 2B disease underwent laser treatment alone, and stable visual acuity was maintained during the follow-up period. However, an ERM was observed in one of these patients (33.3%). Two patients with stage 2B disease received anti-VEGF therapy alone. The mean initial BCVA and baseline CMT were 4.7±0.1 and 418±92.6 µm, respectively. During the follow-up period, one patient (50.0%) developed ERM, hyperreflective scarring, and vision loss. Nine patients received a combination of laser therapy and anti-VEGF treatment (conbercept), including three, five, and one patient with stage 2A, 2B, and 3A disease, respectively. These patients exhibited an initial mean BCVA of 4.2±0.9 and mean baseline CMT of 505.4±251.6 µm, whereas their mean BCVA after treatment was 4.6±0.5. The visual acuity in eight of these patients (88.9%) was improved or stable and decreased in one patient (11.1%). An ERM was observed in five patients (55.6%), and hyper-reflective scars in the macula were observed in two patients (22.2%).
Table 2. Differences in the management of adult-onset Coats disease.
| Management | Number of patients | Stage |
Initia BCVA | Final BCVA | CMT | ERM | Hyper-reflective scar | Outcome |
||||
| 2A | 2B | 3A | Improved | Stable | Worse | |||||||
| Observation | 1 | 0 | 1 | 0 | 4.8 | 4.3 | 434 | 100% | 100% | 0 | 0 | 100% |
| Laser | 3 | 0 | 3 | 0 | 3.0±1.7 | 3.0±1.7 | 425±317.9 | 33% | 67% | 0 | 100% | 0 |
| Anti-VEGF | 2 | 0 | 2 | 0 | 4.7±0.1 | 4.7±0.1 | 418.5±92.6 | 50% | 50% | 50% | 0 | 50% |
| Laser+anti-VEGF | 9 | 3 | 5 | 1 | 4.2±0.9 | 4.6±0.5 | 505.4±251.6 | 56% | 22% | 33% | 56% | 11% |
BCVA: Best corrected visual acuity; CMT: Central macular thickness; ERM: Epiretinal membrane; VEGF: Vascular endothelial growth factor.
As Table 3 showed, the patients' final BCVA were negatively correlated with the lesion involved in the macula (r=-0.525, P=-0.045), while it was positively correlated with anti-VEGF therapy (r=0.578, P=-0.024), and final BCVA was not associated with other factors such as age, sex, systemic history, IOP, CMT, hemorrhage, clock bits, stage, retinal hyperreflective scarring, and laser treatment (P>0.05).
Table 3. Correlation analysis of final BCVA with demographic factors and clinically observed indicators.
| Demographic factors | Final BCVA |
|
| r | P | |
| Age | -0.063 | -0.589 |
| sex | -0.152 | -0.824 |
| Systemic medical history | -0.134 | -0.633 |
| IOP | 0.253 | 0.363 |
| Initial BCVA | 0.856 | 0.000 |
| Stage | -0.182 | -0.517 |
| Clock bits | -0.143 | -0.611 |
| The lesion involved in the macula | -0.525 | -0.045 |
| Hemorrhage | -0.358 | -0.190 |
| Hyper-reflective scar | -0.337 | -0.219 |
| CMT | -0.111 | -0.693 |
| Laser treatment | 0.166 | 0.553 |
| Anti-VEGF | 0.578 | 0.024 |
IOP: Intraocular pressure; BCVA: Best corrected visual acuity; CMT: Central macular thickness; VEGF: Vascular endothelial growth factor.
DISCUSSION
Coats disease is an outer exudative retinal disease caused by retinal vascular abnormalities that is common in adolescent boys but rarely in adults[12]. We found that the fundus features of adult-onset Coats disease differ from those of children, including vascular abnormalities located in the temporal or nasal periphery with localized deposits of lipids, isolated large aneurysms, and peripheral rupture hemorrhages, partially accumulating in the macula. Banerjee et al[13] reported slower progression and less severe lesions in adults with Coats disease that that in children with Coats disease. Of the 15 adults with Gaucher disease in this study, 14 (93.9%) were in the second stage of the disease and the remainder (7.1%) were in the third stage of the disease. Feng et al[14] reported significantly higher VEGF factor levels in children with Coats disease than in adults with Coats disease, thus explaining the large extent of lesions and rapid disease progression in the former. In addition, BCVA significantly increased in children following anti-VEGF treatment but not in adults.
We observed that 86.7% patients with adult-onset Coats disease in this study had hypertension, similar to the findings of Rishi et al[15]. Hypertension is the most common risk factor of adult-onset Coats disease, followed by diabetes mellitus and hypercholesterolemia. Hypertension leads to irregular thickening, luminal narrowing, and decreased elasticity of the arterial wall, and when blood pressure increases, the arterial wall expands in an aneurysmal manner, bleeding from the ruptured aneurysms[16].
Although there are various options for treating Coats disease, monotherapy and combination treatment are the consensus treatments depending on the disease stage. Patients with stage 1 disease can be regularly followed up, those with stage 2 or 3 disease are chiefly treated with laser photocoagulation or cryotherapy, and those with stage 4 or 5 are treated with surgery as required[17]. Many studies have illustrated that anti-VEGF therapy can reduce macular edema and subretinal exudation and stabilize abnormal blood vessels, with it currently being the most common adjuvant therapy and primary treatment for progressive Coats disease[18]–[20]. However, Adeniran et al[21] and Bhat et al[22] reported that anti-VEGF carries risks of vitreous hyperplasia and traction retinal detachment and that they should be used with caution. Some researchers believe that combination therapy stabilizes the blood-retinal barrier and improves BCVA more effectively than anti-VEGF therapy only[23]–[24]. The 15 patients in this study exhibited stage 2-3 disease, which rendered them suitable for laser or anti-VEGF treatment, and none of the patient developed serious complications during the treatment. Anti-VEGF treatment reduced macular edema and normalized the superficial and deep retinal capillary structures observed on OCTA. The patients treated with laser photocoagulation exhibited abnormal capillaries in the lesion area with reduced leakage and their condition remained stable during follow-up. However, aneurysm recurrence was observed in two patients who required repeated photocoagulation, consistent with the results of Dave et al[25]. The final BCVA improved in >60% patients owing to timely diagnosis and macular edema was resolved by treatment with conbercept combined with laser therapy in this study, consistent with the findings of Jiang et al[26]. However, the final BCVA did not improve in six patients who exhibited poor baseline BCVA, massive hard exudation, large number of microangiomas in the macula, fluid cystic cavities in the macula, and incomplete bands of ellipsoids. Hitherto, we hypothesized that poor baseline BCVA, delayed consultation, and massive lipid exudation in the macula comprised important risk factors for poor outcomes, consistent with the findings of Shields et al[27] and Dalvin et al[28]. Furthermore, Jiang et al[29] reported that BCVA improved in 75% patients and macular edema and abnormal vessels were corrected after a year of follow-up following treatment with anti-VEGF drugs combined with laser therapy.
Herein, an ERM was present in 53.3% patients (eight cases), which we believe is associated with retinal ischaemic changes. According to Lim et al[30], the retinal ischemic alterations caused by retinal vascular diseases, such as diabetic retinopathy, are important mechanisms underlying secondary ERM formation. Moreover, the VEGF levels are elevated during tissue ischemia and hypoxia and they play a critical role in ERM formation[31]. The vascular abnormalities and nonperfused areas in patients with adult-onset Coats disease also indicate that ischemic factors are involved in ERM formation. In one patient, the ERM with vitreomacular traction detached following anti-VEGF treatment combined with laser therapy. Stalmans et al[32] reported that the mechanical effect of intravitreal drug injection induced posterior vitreous detachment. Guota et al[33] noted that a few patients presented with abnormal vitreoretinal interface abnormalities on SD-OCT, such as secondary vitreomacular traction, and some patients displayed ERM detachment on final follow-up OCT, which was suspected to be related to laser treatment. This is similar to our finding; however, more studies are warranted to demonstrate its relevance to treatment and prognosis owing to the low incidence of adult-onset Coats disease and difficulty in clinically detecting ERM.
This study provided a preliminary analysis of the multimodal imaging features, treatment, and outcome of adult-onset Coats disease, and these findings can help diagnose the disease and identify prognosis-related factors of the disease in clinical practice. However, this study had several limitations, such as a small sample size and short follow-up period. Hence, further studies involving a larger sample size and longer-term follow-up should be performed in the future.
Footnotes
Authors' contributions: Zhou W and Zhou H, Data analysis and Writing. Liu YY and Li MX, Review and editing. Wu XH and Liang J, Chart design. Hao J and Liu SN, Data analysis. Jin CJ, Formulation the research goals.
Conflicts of Interest: Zhou W, None; Zhou H, None; Liu YY, None; Li MX, None; Wu XH, None; Liang J, None; Hao J, None; Liu SN, None; Jin CJ, None.
REFERENCES
- 1.Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: an overview of classification, management and outcomes. Indian J Ophthalmol. 2019;67(6):763–771. doi: 10.4103/ijo.IJO_841_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V, Chandra P, Kumar A. Ultra-wide field imaging in the diagnosis and management of adult-onset Coats disease. Clin Exp Optom. 2017;100(1):79–82. doi: 10.1111/cxo.12418. [DOI] [PubMed] [Google Scholar]
- 3.Ong SS, Mruthyunjaya P, Stinnett S, Vajzovic L, Toth CA. Macular features on spectral-domain optical coherence tomography imaging associated with visual acuity in Coats disease. Invest Ophthalmol Vis Sci. 2018;59(7):3161–3174. doi: 10.1167/iovs.18-24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Del Rio N, García-Ibor F, Olate-Pérez Á, Duch-Samper AM. Angio-OCT findings in Coats disease. Arch Soc Esp Oftalmol. 2017;92(12):e84. doi: 10.1016/j.oftal.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Cui Y. Progress in the pathogenesis and treatment of Coats disease. Guoji Yanke Zazhi (Int Eye Sci) 2021;21(7):1183–1186. [Google Scholar]
- 6.Yang Q, Wei WB. Pathogenesis and treatment of Coats disease. International Ophthalmology Review. 2015;39(1):39–43. [Google Scholar]
- 7.Peene G, Smets E, Legius E, Cassiman C. Unilateral Coats'-like disease and an intragenic deletion in the TERC gene: a case report. Ophthalmic Genet. 2018;39(2):247–250. doi: 10.1080/13816810.2017.1401086. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Jiang C, Ruan L, Huang X. Associations of cytokine concentrations in aqueous humour with retinal vascular abnormalities and exudation in Coats disease. Acta Ophthalmol. 2019;97(3):319–324. doi: 10.1111/aos.13971. [DOI] [PubMed] [Google Scholar]
- 9.Yang XY, Wang CG, Su GF. Recent advances in the diagnosis and treatment of Coats disease. Int Ophthalmol. 2019;39(4):957–970. doi: 10.1007/s10792-019-01095-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LL, Ke YF, Wang W, Shi XY, Hei KW, Li XR. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats disease. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1339–1346. doi: 10.1007/s00417-018-3949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: the 2000 Proctor Lecture. Am J Ophthalmol. 2001;131(5):572–583. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 12.Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: epidemiology and clinical features at diagnosis. Eye (Lond) 2010;24(12):1797–1801. doi: 10.1038/eye.2010.126. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee M, Nayak S, Kumar S, Bhayana AA, Kumar V. Adult-onset Coats disease. Surv Ophthalmol. 2023;68(4):591–600. doi: 10.1016/j.survophthal.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Feng J, Zheng XX, Li B, Jiang YR. Differences in aqueous concentrations of cytokines in paediatric and adult patients with Coats disease. Acta Ophthalmol. 2017;95(6):608–612. doi: 10.1111/aos.13151. [DOI] [PubMed] [Google Scholar]
- 15.Rishi E, Rishi P, Appukuttan B, Uparkar M, Sharma T, Gopal L. Coats disease of adult-onset in 48 eyes. Indian J Ophthalmol. 2016;64(7):518–523. doi: 10.4103/0301-4738.190141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nesper PL, Simjanoski E, Mirza RG. Retinal macroaneurysm in long-standing hypertension. Ophthalmology. 2016;123(11):2327. doi: 10.1016/j.ophtha.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Paulbuddhe VS, Shukla UV, Tripathy K. 2023 Aug 25. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Exudative Retinitis (Coats Disease) [PubMed] [Google Scholar]
- 18.Mandura RA, Alqahtani AS. Coats disease diagnosed during adulthood. Cureus. 2021;13(7):e16303. doi: 10.7759/cureus.16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cennamo G, Montorio D, Comune C, Laezza MP, Fallico M, Lionetti ME, Reibaldi M. Optical coherence tomography angiography findings after intravitreal ranibizumab in patients with Coats disease. Front Med. 2020;7:615015. doi: 10.3389/fmed.2020.615015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee M, Nayak S, Kumar S, Bhayana AA, Kumar V. Adult-onset Coats disease. Surv Ophthalmol. 2023;68(4):591–600. doi: 10.1016/j.survophthal.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Adeniran JF, Duff SM, Mimouni M, Lambert N, Ramasubramanian A. Treatment of Coats disease: an analysis of pooled results. Int J Ophthalmol. 2019;12(4):668–674. doi: 10.18240/ijo.2019.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhat V, D'Souza P, Shah PK, Narendran V. Risk of tractional retinal detachment following intravitreal bevacizumab along with subretinal fluid drainage and cryotherapy for stage 3B Coats disease. Middle East Afr J Ophthalmol. 2016;23(2):208–211. doi: 10.4103/0974-9233.175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama A, Sugioka K, Kusaka S, Matsumoto C, Shimomura Y. Combined treatment for Coats disease: retinal laser photocoagulation combined with intravitreal bevacizumab injection was effective in two cases. BMC Ophthalmol. 2014;14:36. doi: 10.1186/1471-2415-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu XY, Essilfie J, Gong YY, Yu SQ, Freund KB. Resolution of foveal lipid deposition in adult-onset Coats disease with combined focal laser photocoagulation and anti-VEGF therapy. Ophthalmic Surg Lasers Imaging Retina. 2021;52(7):396–399. doi: 10.3928/23258160-20210628-07. [DOI] [PubMed] [Google Scholar]
- 25.Dave AD, Thavikulwat AT, de Silva T, Wiley HE, Keenan TDL, Wong WT, Cukras CA. Longitudinal characterization and treatment response of retinal arterial macroaneurysms in adult-onset Coats disease. Am J Ophthalmol Case Rep. 2022;27:101647. doi: 10.1016/j.ajoc.2022.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang L, Li J, Peng W. Clinical analysis of intravitreal injection of Conbercept combined with 532-laser treating Coats disease in adulthood. Guoji Yanke Zazhi (Int Eye Sci) 2017;17(7):1356–1358. [Google Scholar]
- 27.Shields CL, Udyaver S, Dalvin LA, Lim LS, Atalay HT, Khoo C, Mazloumi M, Shields JA. Visual acuity outcomes in Coats disease by classification stage in 160 patients. Br J Ophthalmol. 2020;104(3):422–431. doi: 10.1136/bjophthalmol-2019-314363. [DOI] [PubMed] [Google Scholar]
- 28.Dalvin LA, Udyaver S, Lim LS, Mazloumi M, Atalay HT, Khoo CTL, Shields CL. Coats disease: clinical features and outcomes by age category in 351 cases. J Pediatr Ophthalmol Strabismus. 2019;56(5):288–296. doi: 10.3928/01913913-20190716-01. [DOI] [PubMed] [Google Scholar]
- 29.Jiang L, Qin B, Luo XL, Cao H, Deng TM, Yang MM, Meng T, Yang HQ. Three-year follow-up of Coats disease treated with conbercept and 532-nm laser photocoagulation. World J Clin Cases. 2020;8(24):6243–6251. doi: 10.12998/wjcc.v8.i24.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim JI, Spee C, Hinton DR. A comparison of hypoxia-inducible factor-α in surgically excised neovascular membranes of patients with diabetes compared with idiopathic epiretinal membranes in nondiabetic patients. Retina (Phila) 2010;30(9):1472–1478. doi: 10.1097/IAE.0b013e3181d6df09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abu El-Asrar AA, Mohammad G, Allegaert E, Ahmad A, Siddiquei MM, Alam K, Gikandi PW, De Hertogh G, Opdenakker G. Matrix metalloproteinase-14 is a biomarker of angiogenic activity in proliferative diabetic retinopathy. Mol Vis. 2018;24:394–406. [PMC free article] [PubMed] [Google Scholar]
- 32.Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, Haller JA, MIVI-TRUST Study Group Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606–615. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 33.Gupta MP, Dow E, Jeng-Miller KW, Mukai SZ, Orlin A, Xu KY, Yonekawa Y, Paul Chan RVP. Spectral domain optical coherence tomography findings in Coats disease. Retina (Phila) 2019;39(6):1177–1185. doi: 10.1097/IAE.0000000000002120. [DOI] [PMC free article] [PubMed] [Google Scholar]