Abstract
Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is gaining attention in pharmacovigilance, but its association with antipsychotics, other than clozapine, is still unclear.
Methods
We conducted a case/non-case study with disproportionality analysis based on the World Health Organization (WHO) global spontaneous reporting database, VigiBase®. We analyzed individual case safety reports of DRESS syndrome related to antipsychotics compared to (1) all other medications in VigiBase®, (2) carbamazepine (a known positive control), and (3) within classes (typical/atypical) of antipsychotics. We calculated reporting odds ratio (ROR) and Bayesian information component (IC), with 95% confidence intervals (CIs). Disproportionate reporting was prioritized based on clinical importance, according to predefined criteria. Additionally, we compared characteristics of patients reporting with serious/non-serious reactions.
Results
A total of 1534 reports describing DRESS syndrome for 19 antipsychotics were identified. The ROR for antipsychotics as a class as compared to all other medications was 1.0 (95% CI 0.9–1.1). We found disproportionate reporting for clozapine (ROR 2.3, 95% CI 2.1–2.5; IC 1.2, 95% CI 1.1–1.3), cyamemazine (ROR 2.3, 95% CI 1.5–3.5; IC 1.2, 95% CI 0.5–1.7), and chlorpromazine (ROR 1.5, 95% CI 1.1–2.1; IC 0.6, 95% CI 0.1–1.0). We found 35.7% of cases with co-reported anticonvulsants, and 25% with multiple concurrent antipsychotics in serious compared to 8.6% in non-serious cases (p = 0.03). Fatal cases were 164 (10.7%).
Conclusions
Apart from the expected association with clozapine, chlorpromazine and cyamemazine (sharing an aromatic heteropolycyclic molecular structure) emerged with a higher-than-expected reporting of DRESS. Better knowledge of the antipsychotic-related DRESS syndrome should increase clinicians’ awareness leading to safer prescribing of antipsychotics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-024-01431-7.
Key Points
| Clozapine, chlorpromazine, and cyamemazine are associated with disproportionate reporting of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome compared to other medications. |
| Serious DRESS cases were more often associated with concurrent use of multiple antipsychotics compared to non-serious ones, while this difference was not found regarding oral/long-acting injectable formulations. |
| Fatal cases for antipsychotic-related DRESS syndrome stood at 10.7% (164 cases). |
Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, first described in 1950 [1], refers to a series of serious clinical manifestations induced by hypersensitivity reactions to pharmacotherapy [2, 3]. Although its pathogenesis is still debated [4, 5], DRESS syndrome has generally a severe and prolonged course, and up to 10% mortality rate, when misdiagnosed and untreated [6, 7]. Due to its heterogeneity of clinical manifestations (including fever, skin rash, hematological findings, internal organ involvements, and lymphadenopathy) [2, 8] and low incidence, estimated around 1/1000 to 1/10,000 antiepileptic drug exposures [9, 10], DRESS syndrome is considered a rare and unpredictable condition, also known as a designated medical event, which is generally serious and with a recognized drug-attributable component; therefore, case reports and analysis of large pharmacovigilance databases represent pivotal sources of real-world data for post-marketing monitoring and characterization of such an adverse drug reaction (ADR) [11]. DRESS syndrome is classified among severe cutaneous adverse reactions (SCARs) [12], and considering the similar clinical presentation and the probable overlapping pathogenic mechanisms with the drug-induced hypersensitivity syndrome (DiHS), it has also been proposed to refer to this condition as DiHS/DRESS syndrome [11, 13].
DRESS syndrome has been reported after exposure to several drugs [4, 14], and has been primarily associated with antiepileptics, allopurinol, sulfonamides, non-steroidal anti-inflammatory drugs (NSAIDs), and some antibiotics [14–17]. More recently, DRESS syndrome following the use of psychotropic drugs has been reported [14, 16]. Specifically, most of the reports of DRESS syndrome have been observed after the use of clozapine [17–19], describing a peculiar clinical pattern (i.e., fever, eosinophilia, and internal organ involvement) [8]. Clinical manifestations can be heterogeneous on the basis of the involved drug, thus increasing the challenge of timely DRESS syndrome identification in different contexts and in relation to previously unsuspected drugs [8, 20].
Many risk factors as well as demographic, clinical, therapeutic, and prognostic features of DRESS syndrome may have been still overlooked; in addition, a comprehensive analysis of the drugs with a strong association with DRESS syndrome, due to limited scientific evidence, is currently lacking [19]. In this regard, knowledge on antipsychotics, other than clozapine, which are potentially related to DRESS syndrome, is very scarce and limited to a few case reports or sporadic literature data [21, 22]. Thus, we need a broader analysis for all antipsychotics, as it is hypothesized that antipsychotic-related DRESS syndrome frequency may be underestimated, in line with what has been previously observed also for clozapine [17, 18].
In addition, considering the consistent worldwide growing trend in antipsychotic prescription rates since early 2000s, which further increased during the coronavirus disease 2019 (COVID-19) pandemic [23, 24], both in children/adolescents and adults [25, 26], there is a pressing imperative to gain deeper insights into whether, and to what extent, exposure to antipsychotics—both as a pharmacological class and individually—is associated with DRESS syndrome.
This study aims to analyze the reporting patterns of DRESS syndrome related to antipsychotic drugs recorded in VigiBase®, the World Health Organization (WHO) database of individual case safety reports (ICSRs) [27].
Materials and Methods
The protocol was registered in advance on OpenScienceFramework (https://osf.io/sf34j/). Using a case/non-case study design [28], we conducted a disproportionality analysis on suspected ADRs reported to the WHO global database of ICSRs, VigiBase®, which was established in 1968 and is managed by the Uppsala Monitoring Centre (UMC) [27]. With more than 30 million ICSRs from over 170 member countries, VigiBase® is the largest pharmacovigilance database worldwide [27]. Additional information about the components of ICSRs can be found on the UMC website [29].
We searched the WHO VigiBase® database using the Standardized Medical Dictionary for Regulatory Activities (MedDRA) Query (SMQ) ‘Drug reaction with eosinophilia and systemic symptoms syndrome,’ and we selected all DRESS reports in adults (≥ 18 years old) for which antipsychotic drugs were the suspected/interacting agent (cases), from inception to July 2022. To perform a case/non-case study with disproportionality analysis, we included as non-cases all the reports of other suspected ADRs in adults. We included reports involving 82 typical and atypical antipsychotic agents according to the WHO Anatomical Therapeutic Chemical (ATC) index [30]. The full list of included antipsychotic agents is reported in the Supplementary Table 1 (see the electronic supplementary material). Detailed information on the items contained in ICSRs are described on the UMC website [31]. According to WHO policy and the UMC’s guidelines, ICSRs sent from member countries to VigiBase® are anonymized.
Statistical Analysis
We summarized descriptive statistical information on demographic and clinical characteristics of cases, such as median age (and interquartile range [IQR]), sex, signs/symptoms, median (IQR) symptoms duration, outcomes, median (IQR) duration of antipsychotic treatment, median (IQR) dose of antipsychotic (expressed as a ratio between the dose in milligrams and the defined daily dose [DDD]), and comorbidities/co-medications. We used two disproportionality approaches: we estimated the reporting odds ratio (ROR) [32, 33] and the Bayesian information component (IC) [34] for all drugs with at least four reports of DRESS, with 95% confidence intervals (CIs). Traditionally used thresholds for disproportionality were adopted (i.e., lower limit of the 95% CI > 1 and > 0 for ROR and IC, respectively), and disproportionality was considered in the case of a statistically significant ROR and IC. A statistically significant disproportionality suggests the existence of a potentially causal association between drug(s) and adverse event(s) that requires further investigation.
We performed three disproportionality analyses. First, we estimated RORs and ICs of antipsychotic-related DRESS compared to all other (non-antipsychotic) drugs registered in VigiBase®. For this analysis, we provided a cumulative ROR and IC for antipsychotics altogether as a group, one for each class of antipsychotics (typical and atypical), and one for each individual antipsychotic.
Second, we calculated the so-called disproportionality by therapeutic area, namely for each individual antipsychotic compared with all other antipsychotics from the same class (e.g., olanzapine versus other atypical antipsychotics). This approach can mitigate potential bias such as confounding by indication and offers a preliminary intraclass analysis by comparing individuals sharing at least a set of common risk factors [35, 36].
Finally, we performed the so-called active-comparator disproportionality analysis by employing carbamazepine as a positive control, as carbamazepine has a well-known immunological pathogenetic potential [37] and, among psychotropic drugs, it is most frequently associated with DRESS syndrome [14–16, 38]. The use of an active comparator may limit false-positive findings and reduce channeling bias and provides a clinically relevant comparison [39].
Further, we compared age, sex, and body mass index (BMI) distribution, dose of antipsychotic (expressed as a ratio of dose to DDD), duration of antipsychotic treatment, duration of DRESS symptoms, co-medications (including anticonvulsants, antibiotics, and miscellaneous), concurrent use of multiple antipsychotics, and oral/long-acting injectable formulations between reports with serious versus non-serious of DRESS syndrome. Last, we performed a sensitivity analysis for ROR calculation including only cases with one suspected/interacting drug, reducing the potential confounding due to co-suspected drugs.
To prioritize pharmacovigilance data, we classified antipsychotics with a statistically significant disproportionality by scoring the following criteria: (1) number of cases of DRESS syndrome/total number of reports of any ADR (0–2 points); (2) number of cases of DRESS syndrome without confounders/number of all cases of DRESS (0–2 points); (3) significant ROR and IC consistent across different analyses (in the main analysis, the intraclass analysis, and with carbamazepine as a comparator) (0–2 points); and (4) magnitude of the lower limit of the 95% CI of the ROR (0–1 point). We reduced the percentage reported from the maximum of 10% to 0.4% in criterion 1 compared to previous works [40] to adapt it to the DRESS as being a rare ADR. More details about the score assigned to each criterion is reported in the Supplementary Table 2 (see the electronic supplementary material).
Results
Characteristics of the Study Sample
A total of 1534 reports involving antipsychotic-related DRESS syndrome were identified in VigiBase®, with an increasing reporting trend over the years (48.9% of cases reported in the last 10 years, 2013–2022). Of these, 164 were fatal cases (10.7%). The demographic and clinical characteristics of the patients experiencing DRESS syndrome are provided in Table 1. For cases of DRESS, the median age was 41.0 (IQR 29.0–54.0) years, with a slightly higher prevalence of males (n of males = 892, 58.1%), the median BMI was 23.9 kg/m2 (IQR 21.3–28.4), the median reported dose of antipsychotic was 0.7 DDDs (IQR 0.2–1.3), and the median duration of antipsychotic treatment was 22.0 days (IQR 12.0–52.0). The median duration of DRESS symptoms was 11.0 days (IQR 5.0–25.0). The most represented route of administration was oral (998 cases, 65.1%). Of the cases, 691 (45.0%) were concurrently prescribed another co-medication with DRESS-related potential [4, 6, 14]: 547 cases had an anticonvulsant (35.7%), 91 had an antibiotic (5.9%), while 53 presented other drugs (ibuprofen, ramipril, sulfasalazine, allopurinol) (3.5%). Reports were mostly derived from the United States (n = 391, 25.5%) and, considering the reporters, from physicians (n = 698, 45.5%) (Table 1).
Table 1.
Demographics and clinical characteristics of the antipsychotic-related DRESS syndrome cases
| Characteristics of the DRESS syndrome case | Total sample (n = 1534) |
|---|---|
| Age (years), median (Q1–Q3) | 41.0 (29.0–54.0) |
| Sex (females), n (%) | 642 (41.9) |
| BMI (kg/m2), median (Q1–Q3) | 23.9 (21.3–28.4) |
| Dose (PDD/DDD), median (Q1–Q3) | 0.7 (0.2–1.3) |
| AP treatment duration (days), median (Q1–Q3) | 22.0 (12.0–52.0) |
| DRESS symptoms duration (days), median (Q1–Q3) | 11.0 (5.0–25.0) |
| Lethal, n (%) | 164 (10.7) |
| More than one AP, n (%) | 670 (43.7) |
| Administration route, n (%) | |
| Oral | 998 (65.1) |
| Unknown | 177 (11.5) |
| Intramuscular | 29 (1.9) |
| Intravenous | 12 (0.8) |
| Other | 17 (1.1) |
| Reported co-medication with DRESS-related potential, n (%) | |
| Anticonvulsants | 547 (35.7) |
| Antibiotics | 91 (5.9) |
| Miscellaneous | 53 (3.5) |
| Country, n (%) | |
| United States | 391 (25.5) |
| United Kingdom | 214 (14.0) |
| Germany | 148 (9.6) |
| France | 143 (9.3) |
| Australia | 138 (9.0) |
| Japan | 92 (6.0) |
| Canada | 91 (5.9) |
| Other (40 countries) | 261 (17) |
| Reporter, n (%) | |
| Physician | 698 (45.5) |
| Other health professional | 170 (11.1) |
| Pharmacist | 132 (8.6) |
| Consumer/non-health professional | 39 (2.5) |
| Lawyer | 6 (0.4) |
AP antipsychotic, BMI body mass index, DDD defined daily dose, DRESS drug reaction with eosinophilia and systemic symptoms, PDD prescribed daily dose, Q1 first quartile, Q3 third quartile
Antipsychotics Versus All Other Drugs
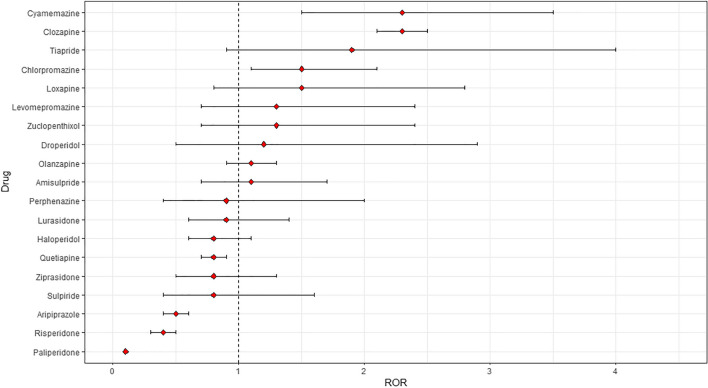
We did not find disproportionate reporting for all antipsychotics as a group, when compared to all other drugs (ROR 1.0, 95% CI 0.9–1.1; IC 0.0, 95% CI − 0.1 to 0.1). However, we found a marginal disproportionality of DRESS syndrome for atypical antipsychotics when compared to all other medications (ROR 1.1, 95% CI 1.0–1.2; IC 0.1, 95% CI 0.0–0.2), but not for typical antipsychotics (ROR 0.9, 95% CI 0.8–1.0; IC −0.2, 95% CI − 0.4 to 0.0) (Table 2). We found disproportionate reporting for clozapine (ROR 2.3, 95% CI 2.1–2.5; IC 1.2, 95% CI 1.1–1.3), cyamemazine (ROR 2.3, 95% CI 1.5–3.5; IC 1.2, 95% CI 0.5–1.7), and chlorpromazine (ROR 1.5, 95% CI 1.1–2.1; IC 0.6, 95% CI 0.1–1.0) (as illustrated in Figure 1). Detailed ROR and IC values, with their corresponding 95% CIs, for each antipsychotic, each pharmacological class of antipsychotics, and antipsychotics overall are provided in Table 2.
Table 2.
RORs and IC for DRESS syndrome by class of antipsychotic and for individual antipsychotics
| Drug | n DRESS syndrome cases | n all ADRs | ROR | Lower 95% CI | Upper 95% CI | IC | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|---|
| Antipsychotics | 1,356 | 728,105 | 1.0 | 0.9 | 1.1 | 0.0 | −0.1 | 0.1 |
| Atypical | 1,224 | 595,520 | 1.1* | 1.0 | 1.2 | 0.1* | 0.0 | 0.2 |
| Typical | 175 | 101,520 | 0.9 | 0.8 | 1.0 | −0.2 | −0.4 | 0.0 |
| Typical antipsychotics | ||||||||
| Haloperidol | 57 | 35,870 | 0.8 | 0.6 | 1.1 | −0.3 | −0.7 | 0.0 |
| Chlorpromazine | 37 | 12,636 | 1.5* | 1.1 | 2.1 | 0.6* | 0.1 | 1.0 |
| Cyamemazine | 23 | 5188 | 2.3* | 1.5 | 3.5 | 1.2* | 0.5 | 1.7 |
| Levomepromazine | 13 | 5368 | 1.3 | 0.7 | 2.4 | 0.3 | −0.6 | 1.0 |
| Loxapine | 11 | 3775 | 1.5 | 0.8 | 2.8 | 0.6 | −0.4 | 1.3 |
| Zuclopenthixol | 11 | 4359 | 1.3 | 0.7 | 2.4 | 0.4 | −0.6 | 1.1 |
| Perphenazine | 6 | 3323 | 0.9 | 0.4 | 2.0 | −0.1 | −1.5 | 0.8 |
| Droperidol | 5 | 2197 | 1.2 | 0.5 | 2.9 | 0.2 | −1.3 | 1.2 |
| Atypical antipsychotics | ||||||||
| Clozapine | 800 | 184,094 | 2.3* | 2.1 | 2.5 | 1.2* | 1.1 | 1.3 |
| Olanzapine | 152 | 71,859 | 1.1 | 0.9 | 1.3 | 0.1 | −0.1 | 0.3 |
| Quetiapine | 134 | 87,822 | 0.8 | 0.7 | 0.9 | −0.3 | −0.6 | −0.1 |
| Risperidone | 89 | 114,334 | 0.4 | 0.3 | 0.5 | −1.3 | −1.6 | −1.1 |
| Aripiprazole | 61 | 70,082 | 0.5 | 0.4 | 0.6 | −1.1 | −1.5 | −0.8 |
| Lurasidone | 23 | 13,145 | 0.9 | 0.6 | 1.4 | −0.1 | −0.8 | 0.4 |
| Ziprasidone | 23 | 14,911 | 0.8 | 0.5 | 1.3 | −0.3 | −1.0 | 0.2 |
| Amisulpride | 16 | 7725 | 1.1 | 0.7 | 1.7 | 0.1 | −0.7 | 0.7 |
| Paliperidone | 12 | 47,633 | 0.1 | 0.1 | 0.1 | −2.9 | −3.8 | −2.2 |
| Sulpiride | 7 | 4861 | 0.8 | 0.4 | 1.6 | −0.4 | −1.6 | 0.5 |
| Tiapride | 7 | 1922 | 1.9 | 0.9 | 4.0 | 0.8 | −0.4 | 1.7 |
RORs are considered statistically significant when the lower CI limit is > 1 and ICs are considered statistically significant when the lower CI limit is > 0
ADR adverse drug reaction, CI confidence interval, DRESS drug reaction with eosinophilia and systemic symptoms, IC information component, n number, ROR reporting odds ratio
*Significant
Figure 1.
Reporting odds ratios (RORs) and 95% confidence intervals for each antipsychotic (ROR > 1 indicates increased reporting of DRESS syndrome associated with antipsychotics; all other drugs were considered as a comparator). DRESS drug reaction with eosinophilia and systemic symptoms
Antipsychotics Versus Carbamazepine
DRESS syndrome for antipsychotics as a group was not disproportionately reported as compared to carbamazepine (ROR 0.13; 95% CI 0.12–0.14; IC −0.56, 95% CI − 0.61 to − 0.52). The same holds true for typical (ROR 0.04, 95% CI 0.04–0.05; IC − 3.25, 95% CI − 3.5 to − 3.07) and atypical antipsychotics (ROR 0.05, 95% CI 0.05–0.06; IC − 1.5, 95% CI − 1.59 to − 1.43), when analyzed separately. The evaluation comparing individual antipsychotic medications to carbamazepine found that for the three antipsychotics showing a DRESS safety signal in the primary analysis, there was no disproportional report of DRESS syndrome cases when compared to carbamazepine (Table 3).
Table 3.
RORs and 95% CI for each antipsychotic (ROR > 1 indicates an increased DRESS syndrome reporting associated with antipsychotics); carbamazepine was considered as a comparator
| Medication | n DRESS | n ADR | ROR | Lower 95% ROR | Higher 95% ROR | CI | Lower 95% CI | Higher 95% CI |
|---|---|---|---|---|---|---|---|---|
| Antipsychotics | 1356 | 728,105 | 0.13 | 0.12 | 0.14 | −0.56 | −0.61 | −0.52 |
| Typical | 175 | 101,520 | 0.04 | 0.04 | 0.05 | −3.25 | −3.50 | −3.07 |
| Atypical | 1224 | 595,520 | 0.05 | 0.05 | 0.06 | −1.5 | −1.59 | −1.43 |
| Clozapine | 800 | 184,094 | 0.11 | 0.10 | 0.12 | −1.65 | −1.77 | −1.57 |
| Olanzapine | 152 | 71,859 | 0.05 | 0.05 | 0.06 | −3.22 | −3.49 | −3.03 |
| Quetiapine | 134 | 87,822 | 0.04 | 0.03 | 0.05 | −3.53 | −3.82 | −3.32 |
| Risperidone | 89 | 114,334 | 0.02 | 0.02 | 0.02 | −4.25 | −4.60 | −4.00 |
| Aripiprazole | 61 | 70,082 | 0.02 | 0.02 | 0.03 | −4.46 | −4.88 | −4.15 |
| Haloperidol | 57 | 35,870 | 0.04 | 0.03 | 0.05 | −4.00 | −4.44 | −3.68 |
| Chlorpromazine | 37 | 12,636 | 0.07 | 0.05 | 0.10 | −3.45 | −4.00 | −3.06 |
| Cyamemazine | 23 | 5188 | 0.11 | 0.08 | 0.17 | −2.97 | −3.67 | −2.48 |
| Lurasidone | 23 | 13,145 | 0.04 | 0.03 | 0.07 | −4.17 | −4.87 | −3.68 |
| Ziprasidone | 23 | 14,911 | 0.04 | 0.03 | 0.06 | −4.32 | −5.02 | −3.83 |
| Amisulpride | 16 | 7725 | 0.05 | 0.03 | 0.09 | −4.01 | −4.85 | −3.43 |
| Levomepromazine | 13 | 5368 | 0.06 | 0.04 | 0.11 | −3.81 | −4.75 | −3.17 |
| Paliperidone | 12 | 47,633 | 0.01 | 0.00 | 0.01 | −6.43 | −7.41 | −5.76 |
| Loxapine | 11 | 3775 | 0.07 | 0.04 | 0.13 | −3.57 | −4.59 | −2.88 |
| Zuclopenthixol | 11 | 4359 | 0.06 | 0.04 | 0.12 | −3.76 | −4.78 | −3.07 |
| Sulpiride | 7 | 4861 | 0.04 | 0.02 | 0.08 | −4.53 | −5.83 | −3.68 |
| Tiapride | 7 | 1922 | 0.09 | 0.04 | 0.20 | −3.25 | −4.55 | −2.40 |
| Perphenazine | 6 | 3323 | 0.05 | 0.02 | 0.10 | −4.21 | −5.63 | −3.30 |
| Droperidol | 5 | 2197 | 0.06 | 0.02 | 0.14 | −3.88 | −5.44 | −2.90 |
ADR adverse drug reaction, CI confidence interval, DRESS drug reaction with eosinophilia and systemic symptoms, n number, ROR reporting odds ratio
Antipsychotics Intraclass Comparison
Disproportionate reporting of DRESS syndrome for chlorpromazine, cyamemazine, and clozapine, as compared to other antipsychotics within their respective pharmacological classes, was found. Accordingly, statistically significant disproportionality was observed for chlorpromazine (ROR 1.89, 95% CI 1.31–2.72; IC 0.92, 95% CI 0.37–1.31) and cyamemazine (ROR 2.82, 95% CI 1.82–4.37; IC 1.38, 95% CI 0.68–1.87) among the typical antipsychotics, and for clozapine (ROR 4.23, 95% CI 3.76–4.76; IC 1.47, 95% CI 1.35–1.55) among the atypical antipsychotics group (as outlined in Supplementary Table 3; see the electronic supplementary material).
Comparison of Serious Versus Non‑serious DRESS Syndrome Reports
We did not identify any difference between individuals experiencing serious (n = 1055) versus non-serious (n = 39) DRESS syndrome (Table 4). We observed a trend showing that males have a higher risk of serious DRESS syndrome compared to females (odds ratio 1.87, 95% CI 0.93–3.86; p = 0.07). Antipsychotic treatment showed a trend of a longer duration in serious versus non-serious reactions (22.00 days, quartile 1–3 [Q1–Q3] 12.00–46.00, vs. 16.00 days, Q1–Q3 4.00–23.00, p = 0.10). We did not detect differences for reported co-prescription agents, although there was a trend for more frequent co-medication with antibiotic in serious versus non-serious cases (n = 55, 7.9% vs. n = 0, 0%, p = 0.07). We found a difference between serious and non-serious DRESS syndrome cases in terms of concurrent use of multiple antipsychotics (serious [n = 245] 25% vs. non-serious [n = 3] 8.6%, p = 0.03), while this was not found regarding oral/long-acting injectable formulations (serious n = 577/18 vs. non-serious n = 16/2, p = 0.11).
Table 4.
Analysis of characteristics of serious vs. non-serious DRESS syndrome cases
| Serious DRESS syndrome cases | Non-serious DRESS syndrome cases | OR (95% CI) | P value | |
|---|---|---|---|---|
| N | 1055 | 39 | ||
| Age, median (Q1–Q3) | 43.00 (29.0–55.00) | 38.5 (27.00–52.00) | NA | 0.31 |
| Sex, female, n (%) | 435 (42.36)a | 22 (57.89)b | 1.87 (0.93–3.86) | 0.07 |
| BMI, median (Q1–Q3) | 23.88 (21.25–28.43) | 24.17 (22.76–28.52) | NA | 0.77 |
| Dose (PDD/DDD), median (Q1–Q3) | 0.67 (0.18–1.30) | 0.50 (0.21–0.67) | NA | 0.38 |
| Duration of AP treatment (days), median (Q1–Q3) | 22.00 (12.00–46.00) | 16.00 (4.00–23.00) | NA | 0.10 |
| Duration of DRESS (days), median (Q1–Q3) | 11.00 (5.00–25.00) | 12.50 (6.00–13.75) | NA | 0.57 |
| Co-medication with anticonvulsants, n (%) | 416 (39.43) | 17 (43.59) | 0.84 (0.42–1.71) | 0.62 |
| Co-medication with antibiotics, n (%) | 55 (7.87) | 0 (0) | NA | 0.07 |
| Co-medication with miscellaneous, n (%) | 51 (4.83) | 0 (0) | NA | 0.25 |
| > 2 antipsychotics, n (%) | 245 (25.0) | 3 (8.6) | 3.56 (1.10–18.3) | 0.03 |
| Oral/LAIc | 577/18 | 16/2 | 3.99 (0.41–19.16) | 0.11 |
AP antipsychotic, BMI body mass index (kg/m2), CI confidence interval, DDD defined daily dose, DRESS drug reaction with eosinophilia and systemic symptoms, n number, LAI long-acting injectable, NA not applicable, OR odds ratio, PDD prescribed daily dose, Q1 first quartile, Q3 third quartile
aData available for 1027 cases
bData available for 38 cases
cAdministration route was reported otherwise or missing in 481 cases
Ranking of Pharmacovigilance Disproportionate Reporting
We ranked the identified disproportionate reporting for clozapine, chlorpromazine, and cyamemazine, the three drugs with disproportionate reporting, using a clinical priority score. All of them were appointed with the highest clinical priority (as shown in Supplementary Table 4; see the electronic supplementary material).
Discussion
To the best of our knowledge, this is the first post-marketing investigation on DRESS syndrome related to antipsychotics, but not limited to clozapine, using the largest pharmacovigilance database. The results of our study showed notable statistical associations between DRESS syndrome and three antipsychotics of both typical (i.e., chlorpromazine and cyamemazine) and atypical (i.e., clozapine) classes. However, disproportionality did not emerge when compared to carbamazepine, a well-known DRESS syndrome-inducing drug.
These results are extremely relevant as these drugs are widely prescribed. To date, clozapine remains the only licensed antipsychotic for treatment-resistant schizophrenia [41]; chlorpromazine is one of the most commonly prescribed antipsychotics in China and African countries [42, 43], but also in some European countries such as the UK [25, 44], and it is included in the WHO online repository of essential medicines lists (EMLs) in many countries [45]; and cyamemazine is the second most frequently prescribed antipsychotic in France [46], and its use is also authorized in Portugal [47]. Thus, the heightened reporting of DRESS syndrome in patients using three specific antipsychotics highlights a potential oversight in understanding the link between drug exposure and adverse event reporting. Given the rarity and often overlooked nature of DRESS syndrome, the number of reported cases within a drug class can serve as a proxy for drug exposure in the population. This insight is valuable for estimating and comparing drug exposure using pharmacovigilance databases.
In addition, these results are highly relevant given the paucity of data reported in the literature, which has mainly focused on clozapine [17–19]. Instead, the role of chlorpromazine and cyamemazine in potentially causing DRESS is extremely limited, and has not been previously described. Cyamemazine is a typical antipsychotic with D2, 5-HT2A, 5-HT2C, and 5-HT3 receptor antagonist activity, anxiolytic properties, and a low incidence of extrapyramidal side effects [48]. Interestingly, cyamemazine shares an aromatic heteropolycyclic molecular structure with clozapine and chlorpromazine [49–51], a common structural characteristic among several other DRESS syndrome-related drugs, including phenytoin, phenobarbital, and carbamazepine [11, 52]. Although the full pathogenesis of DRESS syndrome is still unknown, many hypotheses support the role of intermediate metabolites of aromatic anticonvulsants (e.g., arene oxides) [53]. Other plausible hypotheses are delayed cell-mediated immune responses, genetic predisposition related to specific human leukocyte antigen (HLA) haplotypes with an immunological mechanism, graft-versus-host disease, and human herpes virus (HHV)-6 infection/reactivation [52, 54–59]. This would explain the disproportionate reporting found in this study for three antipsychotics with aromatic structure and with immunological effects.
There is little data on chlorpromazine and DRESS syndrome, with only three published case reports. The first one, recently described by Ghozlane and colleagues, was a suspected case of DRESS syndrome probably triggered by chlorpromazine [22]. In their literature review of antipsychotic-related DRESS syndrome, they identified only another case associated with chlorpromazine. The second chlorpromazine-related DRESS syndrome case was a female diagnosed with paranoid schizophrenia, and was effectively managed with corticosteroid therapy [60]. Finally, the third published case was a patient who developed DRESS syndrome after taking olanzapine with sodium valproate, and previous chlorpromazine-based treatment [61]. The unconfirmed hypothesis was that chlorpromazine may have sensitized the patient to the onset of DRESS syndrome. A potential explanation of the relationship between chlorpromazine and DRESS syndrome could involve a delayed hypersensitivity immune response [62], considering that prolonged use of chlorpromazine has been linked to the development of a lupus-like circulating anticoagulant and various immunological abnormalities [63]. However, this hypothesis would not explain cases of early DRESS onset, which, similar to clozapine, could be explained by a number of other factors, such as genetic predisposition, polypharmacy, and comorbidities [18].
Ultimately, clozapine has been identified as the antipsychotic most burdened by the association with DRESS syndrome, in line with previous literature [17]. To date, the most extensive pharmacovigilance analysis of the EudraVigilance identified a total of 47 cases of clozapine-related DRESS syndrome and, when added to the 27 cases previously identified from the literature review [18], raises the total to 74 clozapine-related DRESS cases [17]. The results obtained from VigiBase® confirm and extend the analysis of the EudraVigilance. The high reporting of clozapine-related DRESS could be explained both by pathogenic reasons as well as by the stringent safety monitoring dedicated to clozapine in routine care [64]. As we previously discussed, clozapine’s aromatic structure may support some hypothesis about its immunomodulating effects [65]. However, following this hypothesis, other drugs sharing similar structural characteristics (e.g., olanzapine, quetiapine) should also induce adverse events related to hypersensitivity, but current data are not consistent in this respect [16]. One hypothesis is that DRESS could be a hypersensitivity syndrome connected to a specific chemical structure such as dibenzazepine derivatives (e.g., clozapine and carbamazepine), with some of these characteristics also shared by other commonly utilized psychotropic substances, such as tricyclic antidepressants (e.g., imipramine, clomipramine, amitriptyline) or second-generation antipsychotics (e.g., quetiapine, olanzapine, clotiapine, asenapine) [66, 67]. In this regard, a connection between clozapine-induced T cell hyperstimulation has been proposed, which could lead to eosinophil activation and recruitment [6]. In essence, individuals undergoing clozapine treatment may develop a distinctive immunological profile, susceptible to DRESS syndrome together with other inflammation reactions [68]. Based on this assumption, clozapine-related DRESS syndrome would fit within the spectrum of immunological and inflammatory reactions extensively studied with clozapine [69–71]. Another partial explanation for the high number of clozapine-related DRESS syndrome cases could be the well-established safety monitoring of clozapine in routine care, which could help the timely recognition and reporting of any suspected adverse reaction, including the often underdiagnosed DRESS syndrome [72].
One final interesting result is the identification of a subgroup of patients more susceptible to severe or long-lasting DRESS syndrome. Although our analysis was not conclusive, probably due to the lack of power, we identified some trends. Males with longer duration of symptoms and co-prescribed antibiotics were more at risk of severe reactions. The median dose was lower than the DDD in both serious and non-serious cases, suggesting that DRESS may have occurred during antipsychotic titration. Additionally, literature supports the idea that the risk of antipsychotic-related DRESS syndrome may increase in patients taking multiple medications, independently from the antipsychotic dose [17]. We identified a similar trend, as serious cases had co-medications with anticonvulsants, antibiotics, and other medications, whereas non-serious cases only had co-medications in a small percentage of cases. In addition, combinations of more than two antipsychotics were more common in serious cases of DRESS than in non-serious cases. Finally, the role of synergies between antipsychotics must also be considered when assessing the risk of DRESS.
Limitations and Strengths
There are some limitations to consider when interpreting the findings of this study. Firstly, we acknowledge the well-known limitations of pharmacovigilance research [28, 73], such as the inability to establish causality and the lack of denominators to calculate incidence rates.
Indeed, under-reporting represents a well-known limitation of pharmacovigilance data. This concern would suggest that ADRs may be more frequent than what is reported in pharmacovigilance databases, and therefore the absence of signals for a particular drug should not be interpreted as an endorsement of safety. Additionally, notoriety bias cannot be ruled out, especially for clozapine [74], which can lead generally to an increase in spontaneous reports following a safety alert or when concerns are raised in the literature [75–77]. Increased reporting of known adverse reactions for a specific drug may cause the so-called competition bias by masking the identification of rare side effects for other drugs. This may explain the lack of disproportionality for different antipsychotics (e.g., olanzapine) despite the number of reported DRESS cases not being negligible. Despite these limitations, our scoring system used well-established criteria trying to overcome this limitation and trying to establish clinical relevance of safety signals [78]. Another limitation is the overlap between the nonspecific and systemic symptoms of DRESS syndrome and those of other conditions such as neuroleptic malignant syndrome or poor tolerance to the drug, making differential diagnosis challenging [79–81]. Likewise, the lack of clinical details did not allow us to fully apply RegiSCAR criteria. However, we chose the most conservative definition of DRESS syndrome, and identified cases confirmed by the VigiBase® case classification. Moreover, our analysis did not provide an intraclass comparison between chemically different substances (e.g., phenothiazines, thioxanthen derivatives, dibenzazepine derivatives) although a chemical-based approach focusing on substances with similar structurers could add some valuable insights to the etiology of antipsychotic-related DRESS, and it is desirable for future investigations. Finally, the lack of information on the modality of antipsychotic initiation makes it difficult to determine whether slower antipsychotic titration could mitigate antipsychotic-related DRESS syndrome. Despite these limitations, in the case of DRESS syndrome, pharmacovigilance databases prove to be robust tools for identifying and characterizing rare ADRs in real-world setting [11, 40, 82]. Considering that studying rare ADRs, such as DRESS syndrome, with prospective and interventional or observational studies is difficult and would require a huge number of participants [11, 83, 84], pharmacovigilance databases may be a suitable data source to provide initial ranking within a given therapeutic class [82].
Clinical Implications
In the process of weighing potential clinical advantages and disadvantages of antipsychotic use, it is crucial not to overlook DRESS syndrome, although rare. When prescribing antipsychotics, physicians should be aware of the potential onset of DRESS syndrome, which may vary across different antipsychotics. When prescribing antipsychotics with a clear potential for DRESS, such as clozapine, carbamazepine, and cyamemazine, physicians should actively monitor patients for early signs of DRESS syndrome, such as fever, eosinophilia, rash, and/or internal organ involvements. Additional monitoring may be required for certain subgroups of patients receiving the abovementioned antipsychotics. This includes men who have recently started new antipsychotic therapy with high-risk antipsychotics and with other DRESS-related drugs. It is crucial to closely monitor these patients, as they might experience more severe DRESS syndrome manifestations with potentially serious/fatal outcomes [17].
Current guidelines on the pharmacological treatment of DRESS syndrome are limited and could be expanded to include information on antipsychotic-related DRESS syndrome for clozapine, chlorpromazine, and cyamemazine [85].
Conclusion
Using the world’s largest database of spontaneous reports, clozapine, chlorpromazine, and cyamemazine, which share an aromatic heteropolycyclic molecular structure similar to other antipsychotics less associated with DRESS, were associated with higher-than-expected reports of DRESS syndrome compared with other drugs. Although we recognize that DRESS syndrome represents only a small portion of the total number of potential antipsychotic-related suspected ADR reports, it is important for healthcare providers to be aware of the potential onset of DRESS syndrome, especially for those antipsychotics. Further research is needed to strengthen these findings, investigating the biological plausibility of these results and potentially identifying antipsychotic-related DRESS syndrome risk-minimization strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We acknowledge the Uppsala Monitoring Centre (UMC), which provided and gave permission to use the data analyzed in the present study. The authors are indebted to the national centers that make up the World Health Organization (WHO) Program for International Drug Monitoring and contribute reports to VigiBase®. The information comes from a variety of sources, and the probability that the suspected adverse effect is drug related is not the same in all cases. Information and conclusion do not represent the opinion of the UMC or the WHO.
Declarations
Conflict of interest
No commercial organizations had any role in the writing of this paper for publication. In the last 3 years, RdF has received speaker fees from Janssen Pharmaceuticals and Lundbeck and travel support from Lundbeck, Janssen Pharmaceuticals, Otsuka, and ROVI Pharma Industrial Services. JMK has been a consultant and/or advisor for, or has received honoraria from, Alkermes, Allergan, LB Pharmaceuticals, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceuticals, Johnson and Johnson, Merck, Minerva, Neurocrine, Newron, Otsuka, Pierre Fabre, Reviva, Roche, Sumitomo Dainippon, Sunovion, Takeda, Teva, and UpToDate and is a shareholder in LB Pharmaceuticals and Vanguard Research Group. GT participated in advisory boards and seminars on topics not related to this presentation and sponsored by the following pharmaceutical companies in the last 2 years: Eli Lilly, Sanofi, Amgen, Novo Nordisk, Sobi, Gilead, Celgene, and Daikii Sankyo. He is also scientific coordinator of the academic spin-off INSPIRE, which carried out in the last 2 years observational studies/systematic reviews on topics not related to the content of this presentation and which were funded by PTC Pharmaceutics, Kiowa Kirin, Shonogi, Shire, Chiesi, and Daiichi Sankyo. GS has received speaker/consultation fees from Dexcel Pharma, HLS Therapeutics, Saladax, and Thermo Fisher. In the last 3 years, the remaining authors report no conflicts of interest.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Open access funding provided by Università degli Studi Magna Graecia di Catanzaro within the CRUI-CARE Agreement.
Author Contributions
All authors formulated the research question and designed the study, carrying it out. EA, UM, and GT extracted the data, based on the search strategy defined by RdF, CG, and GS. RdF, CG, and GS analyzed the data. All authors interpreted the results. RdF wrote the first draft of this article. All authors reviewed, provided modifications, and approved the final version of the study.
Data Availability
VigiBase® does not allow the distribution of the files with each case of DRESS, but in the tables and in the electronic supplementary material, all detailed information needed to perform the analyses is provided, i.e., number of cases, non-cases, number of other AEs, and total number of reports in VigiBase®. Other requests for data can be submitted to the corresponding UMC. The code will be made available upon reasonable request to the corresponding author.
Ethical Approval
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of University Hospital Mater Domini of Catanzaro (Italy) ‘Regione Calabria, sezione Area Centro’ (n. 103/April 21st, 2022). VigiBase®, the WHO global database of individual case safety reports (ICSRs), is the source of the information; the information comes from a variety of sources, and the probability that the suspected adverse effect is drug related is not the same in all cases; the information does not represent the opinion of the UMC or the WHO. According to WHO policy and UMC guidelines, reports sent from the WHO Programme for International Drug Monitoring (PIDM) member countries to VigiBase® are anonymized. Identifiable data are not published.
Consent to Participate
Patient consent was waived as VigiBase® database contains anonymized data that cannot allow patients’ identification.
Consent to Publish
Not applicable.
Code Availability
The code will be made available upon reasonable request to the corresponding author.
Footnotes
Chiara Gastaldon and Georgios Schoretsanitis have contributed equally to this work.
References
- 1.Chaiken BH, Goldberg BI, Segal JP. Dilantin sensitivity. N Engl J Med. 1950;242:897–8. 10.1056/NEJM195006082422304. 10.1056/NEJM195006082422304 [DOI] [PubMed] [Google Scholar]
- 2.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–11. [DOI] [PubMed] [Google Scholar]
- 3.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. [DOI] [PubMed] [Google Scholar]
- 4.Camous X, Calbo S, Picard D, Musette P. Drug reaction with eosinophilia and systemic symptoms: an update on pathogenesis. Curr Opin Immunol. 2012;24:730–5. [DOI] [PubMed] [Google Scholar]
- 5.Hama N, Abe R, Gibson A, Phillips EJ. Drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): clinical features and pathogenesis. J Allergy Clin Immunol Pract. 2022;10:1155-1167.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corneli HM. DRESS syndrome: drug reaction with eosinophilia and systemic symptoms. Pediatr Emerg Care. 2017;33:499–502. [DOI] [PubMed] [Google Scholar]
- 7.Wolff K, Allen R, Arturo J, Saavedra P, Roh E. Fitzpatrick’s color atlas and synopsis of clinical dermatology. In: Suurmond D (ed) 8th ed. McGraw Hill; 2018; pp. 564–5.
- 8.de Filippis R, De Fazio P, Kane JM, Schoretsanitis G. Core clinical manifestations of clozapine-related DRESS syndrome: a network analysis. Schizophr Res. 2022;243:451–3. [DOI] [PubMed] [Google Scholar]
- 9.Shiohara T, Kano Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): incidence, pathogenesis and management. Expert Opin Drug Saf. 2016. 10.1080/14740338.2017.1270940. 10.1080/14740338.2017.1270940 [DOI] [PubMed] [Google Scholar]
- 10.Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018–22. 10.1111/j.1365-2133.2003.05584.x. 10.1111/j.1365-2133.2003.05584.x [DOI] [PubMed] [Google Scholar]
- 11.de Filippis R, De Fazio P, Kane J, Schoretsanitis G. Pharmacovigilance approaches to study rare and very rare side-effects: example of clozapine-related DRESS syndrome. Expert Opin Drug Saf. 2022;21:858–587. [DOI] [PubMed] [Google Scholar]
- 12.Wilkerson RG. Drug hypersensitivity reactions. Emerg Med Clin N Am. 2022;40:39–55. [DOI] [PubMed] [Google Scholar]
- 13.Miyagawa F, Asada H. Current perspective regarding the immunopathogenesis of drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS). Int J Mol Sci. 2021;22:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–97. [DOI] [PubMed] [Google Scholar]
- 15.Mori F, Caffarelli C, Caimmi S, Bottau P, Liotti L, Franceschini F, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) in children. Acta Biomed. 2019;90:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renda F, Landoni G, Bertini Malgarini R, Assisi A, Azzolini ML, Mucchetti M, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a national analysis of data from 10-year post-marketing surveillance. Drug Saf. 2015;38:1211–8. [DOI] [PubMed] [Google Scholar]
- 17.de Filippis R, Kane JM, Kuzo N, Spina E, De Sarro G, de Leon J, et al. Screening the European pharmacovigilance database for reports of clozapine-related DRESS syndrome: 47 novel cases. Eur Neuropsychopharmacol. 2022;60:25–37. [DOI] [PubMed] [Google Scholar]
- 18.de Filippis R, Soldevila-Matías P, Guinart D, De Fazio P, Rubio JM, Kane JM, et al. Unravelling cases of clozapine-related drug reaction with eosinophilia and systemic symptoms (DRESS) in patients reported otherwise: a systematic review. J Psychopharmacol. 2021;35:1062–73. [DOI] [PubMed] [Google Scholar]
- 19.de Filippis R, Soldevila-Matías P, De Fazio P, Guinart D, Fuentes-Durá I, Rubio JM, et al. Clozapine-related drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a systematic review. Expert Rev Clin Pharmacol. 2020;13:875–83. 10.1080/17512433.2020.1787831. 10.1080/17512433.2020.1787831 [DOI] [PubMed] [Google Scholar]
- 20.Sim DW, Yu JE, Jeong J, Jung J-W, Kang H-R, Kang DY, et al. Variation of clinical manifestations according to culprit drugs in DRESS syndrome. Pharmacoepidemiol Drug Saf. 2019;28:840–8. [DOI] [PubMed] [Google Scholar]
- 21.Taleb S, Zgueb Y, Ouali U, Jomli R, Kort Y, Nacef F. Drug reaction with eosinophilia and systemic symptoms syndrome related to aripiprazole therapy. J Clin Psychopharmacol. 2019;39:691–3. 10.1097/JCP.0000000000001138. 10.1097/JCP.0000000000001138 [DOI] [PubMed] [Google Scholar]
- 22.Ghozlane L, Asma J, Ahmed Z, Ons C, Sarrah K, Riadh D, et al. Antipsychotics induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: literature review and a report of a suspected case related to chlorpromazine. Curr Drug Saf. 2023;18:571–5. [DOI] [PubMed] [Google Scholar]
- 23.Leong C, Kowalec K, Eltonsy S, Bolton JM, Enns MW, Tan Q, et al. Psychotropic medication use before and during COVID-19: a population-wide study. Front Pharmacol. 2022. 10.3389/fphar.2022.886652/full. 10.3389/fphar.2022.886652/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojeahere MI, de Filippis R, Ransing R, Karaliuniene R, Ullah I, Bytyçi DG, et al. Management of psychiatric conditions and delirium during the COVID-19 pandemic across continents: the lessons thus far. Brain, Behav Immun Health. 2020;9:100147 (Under Rev). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radojčić MR, Pierce M, Hope H, Senior M, Taxiarchi VP, Trefan L, et al. Trends in antipsychotic prescribing to children and adolescents in England: cohort study using 2000–19 primary care data. Lancet Psychiatry. 2023;10:119–28. [DOI] [PubMed] [Google Scholar]
- 26.Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, et al. International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol. 2017;27:1064–76. [DOI] [PubMed] [Google Scholar]
- 27.Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409–19. 10.1177/009286150804200501. 10.1177/009286150804200501 [DOI] [Google Scholar]
- 28.Faillie J-L. Case–non-case studies: principle, methods, bias and interpretation. Therapies. 2019;74:225–32. [DOI] [PubMed] [Google Scholar]
- 29.Uppsala Monitoring Centre. Individual case safety reports and VigiBase—the vital importance of quality. 2012. https://who-umc.org/media/163807/vigibase-the-vital-importance-of-quality-2017.pdf
- 30.World Health Organization (WHO). ATC/DDD Index 2023. 2023. https://www.whocc.no/atc_ddd_index/
- 31.World Health Organization (WHO). Uppsala Monitoring Center. https://who-umc.org/
- 32.van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10. 10.1002/pds.668. 10.1002/pds.668 [DOI] [PubMed] [Google Scholar]
- 33.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–23. 10.1002/pds.1001. 10.1002/pds.1001 [DOI] [PubMed] [Google Scholar]
- 34.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–21. 10.1007/s002280050466. 10.1007/s002280050466 [DOI] [PubMed] [Google Scholar]
- 35.Poluzzi E, Raschi E, Koci A, Moretti U, Spina E, Behr ER, et al. Antipsychotics and torsadogenic risk: signals emerging from the US FDA adverse event reporting system database. Drug Saf. 2013;36:467–79. 10.1007/s40264-013-0032-z. 10.1007/s40264-013-0032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grundmark B, Holmberg L, Garmo H, Zethelius B. Reducing the noise in signal detection of adverse drug reactions by standardizing the background: a pilot study on analyses of proportional reporting ratios-by-therapeutic area. Eur J Clin Pharmacol. 2014;70:627–35. 10.1007/s00228-014-1658-1. 10.1007/s00228-014-1658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simper GS, Hò G-GT, Celik AA, Huyton T, Kuhn J, Kunze-Schumacher H, et al. Carbamazepine-mediated adverse drug reactions: CBZ-10,11-epoxide but not carbamazepine induces the alteration of peptides presented by HLA-B. J Immunol Res. 2018;2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husain Z, Reddy BY, Schwartz RA. DRESS syndrome. J Am Acad Dermatol. 2013;68:693.e1-693.e14. [DOI] [PubMed] [Google Scholar]
- 39.Alkabbani W, Gamble J. Active-comparator restricted disproportionality analysis for pharmacovigilance signal detection studies of chronic disease medications: an example using sodium/glucose cotransporter 2 inhibitors. Br J Clin Pharmacol. 2023;89:431–9. 10.1111/bcp.15178. 10.1111/bcp.15178 [DOI] [PubMed] [Google Scholar]
- 40.Gastaldon C, Schoretsanitis G, Arzenton E, Raschi E, Papola D, Ostuzzi G, et al. Withdrawal syndrome following discontinuation of 28 antidepressants: pharmacovigilance analysis of 31,688 reports from the WHO spontaneous reporting database. Drug Saf. 2022;45:1539–49. 10.1007/s40264-022-01246-4. 10.1007/s40264-022-01246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209:385–92. [DOI] [PubMed] [Google Scholar]
- 42.Xing M, Sheng J, Cui M, Su Y, Zhang C, Chen X, et al. Differing prevalence and correlates of metabolic syndromes between chlorpromazine and clozapine: a 10-year retrospective study of a male Chinese cohort. Curr Neuropharmacol. 2022;20:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rukat A, Musisi S, Ströhle A, Mundt AP. Prescription patterns of psychotropic medications for the treatment of psychotic disorders in the largest mental health institutions of Uganda. J Clin Psychopharmacol. 2014;34:571–6. [DOI] [PubMed] [Google Scholar]
- 44.Meraya AM, Banji OJF, Khobrani MA, Alhossan A. Evaluation of psychotropic medications use among elderly with psychiatric disorders in Saudi Arabia. Saudi Pharm J. 2021;29:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todesco B, Ostuzzi G, Barbui C. Mapping the selection, availability, price and affordability of essential medicines for mental health conditions at a global level. Epidemiol Psychiatr Sci. 2022;31: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rolland B, Dalon F, Gauthier N, Nourredine M, Bérard M, Carton L, et al. Antipsychotic prescribing practices in real-life (APPREAL study): findings from the French National Healthcare System Database (2007–2017). Front Psychiatry. 2022. 10.3389/fpsyt.2022.1021780/full. 10.3389/fpsyt.2022.1021780/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovess-Masfety V, Balusson F, Leray E, Husky M, Scailteux L. Prescription patterns of first- and second-generation antipsychotic drugs in the French population. Fundam Clin Pharmacol. 2020;34:603–11. 10.1111/fcp.12553. 10.1111/fcp.12553 [DOI] [PubMed] [Google Scholar]
- 48.Benyamina A, Arbus C, Nuss P, Garay RP, Neliat G, Hameg A. Affinity of cyamemazine metabolites for serotonin, histamine and dopamine receptor subtypes. Eur J Pharmacol. 2008;578:142–7. [DOI] [PubMed] [Google Scholar]
- 49.Jafari S, Fernandez-Enright F, Huang X-F. Structural contributions of antipsychotic drugs to their therapeutic profiles and metabolic side effects. J Neurochem. 2012;120:371–84. 10.1111/j.1471-4159.2011.07590.x. 10.1111/j.1471-4159.2011.07590.x [DOI] [PubMed] [Google Scholar]
- 50.Phenothiazine MS. The parent molecule. Curr Drug Targets. 2006;7:1181–9. [DOI] [PubMed] [Google Scholar]
- 51.Fehsel K, Schwanke K, Kappel B, Fahimi E, Meisenzahl-Lechner E, Esser C, et al. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J Psychopharmacol. 2022;36:191–201. 10.1177/02698811211055811. 10.1177/02698811211055811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yun S, Lee J, Kim E, Quan G, Kim S, Won Y, et al. Drug rash with eosinophilia and systemic symptoms induced by valproate and carbamazepine: formation of circulating auto-antibody against 190-kDa antigen. Acta Derm Venereol. 2006;86:241–4. [DOI] [PubMed] [Google Scholar]
- 53.Stirton H, Shear NH, Dodiuk-Gad RP. Drug reaction with eosinophilia and systemic symptoms (DReSS)/drug-induced hypersensitivity syndrome (DiHS)—readdressing the DReSS. Biomedicines. 2022;10:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leeder JS, Riley RJ, Cook VA, Spielberg SP. Human anti-cytochrome P450 antibodies in aromatic anticonvulsant-induced hypersensitivity reactions. J Pharmacol Exp Ther. 1992;263:360–7. [PubMed] [Google Scholar]
- 55.Descamps V, Bouscarat F, Laglenne S, Aslangul E, Veber B, Descamps D, et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br J Dermatol. 1997;137:605–8. [DOI] [PubMed] [Google Scholar]
- 56.Kano Y, Inaoka M, Shiohara T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia. Arch Dermatol. 2004. 10.1001/archderm.140.2.183. 10.1001/archderm.140.2.183 [DOI] [PubMed] [Google Scholar]
- 57.Scheuerman O, Nofech-Moses Y, Rachmel A, Ashkenazi S. Successful treatment of antiepileptic drug hypersensitivity syndrome with intravenous immune globulin. Pediatrics. 2001;107:e14–e14. [DOI] [PubMed] [Google Scholar]
- 58.De A, Rajagopalan M, Sarda A, Das S, Biswas P. Drug reaction with eosinophilia and systemic symptoms: an update and review of recent literature. Indian J Dermatol. 2018;63:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhary S, McLeod M, Torchia D, Romanelli P. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Clin Aesthet Dermatol. 2013;6:31–7. [PMC free article] [PubMed] [Google Scholar]
- 60.Gowda SM, Kumar KGV, Shilpa K. Chlorpromazine-induced drug reaction with eosinophilia and systemic symptoms syndrome. Indian J Psychol Med. 2020;42:99–101. 10.4103/IJPSYM.IJPSYM_364_19. 10.4103/IJPSYM.IJPSYM_364_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penchilaiya V, Kuppili PP, Preeti K, Bharadwaj B. DRESS syndrome: addressing the drug hypersensitivity syndrome on combination of sodium valproate and olanzapine. Asian J Psychiatr. 2017;28:175–6. [DOI] [PubMed] [Google Scholar]
- 62.Kar S, Yadav S, Dhanasekaran S. Chlorpromazine-induced severe exfoliative photoallergic reaction. Int J Nutr Pharmacol Neurol Dis. 2015;5:34. [Google Scholar]
- 63.Canoso RT, Sise HS. Chlorpromazine-induced lupus anticoagulant and associated immunologic abnormalities. Am J Hematol. 1982;13:121–9. 10.1002/ajh.2830130204. 10.1002/ajh.2830130204 [DOI] [PubMed] [Google Scholar]
- 64.de Leon J, Ruan C-J, Schoretsanitis G, Rohde C, Yağcıoğlu EA, Baptista T, et al. An international guideline with six personalised titration schedules for preventing myocarditis and pneumonia associated with clozapine. Gen Psychiatry. 2022;35:e100773. 10.1136/gpsych-2022-100773. 10.1136/gpsych-2022-100773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Røge R, Møller BK, Andersen CR, Correll CU, Nielsen J. Immunomodulatory effects of clozapine and their clinical implications: What have we learned so far? Schizophr Res. 2012;140:204–13. [DOI] [PubMed] [Google Scholar]
- 66.Bruhwyler J, Liégeois JF, Lejeune C, Rogister F, Delarge J, Géczy J. New dibenzazepine derivatives with disinhibitory and/or antidepressant potential: neurochemical and behavioural study in the open-field and forced swimming tests. Behav Pharmacol. 1995;6:830–8. [PubMed] [Google Scholar]
- 67.Lawthom C, Peltola J, McMurray R, Dodd E, Villanueva V. Dibenzazepine agents in epilepsy: how does eslicarbazepine acetate differ? Neurol Ther. 2018;7:195–206. 10.1007/s40120-018-0111-2. 10.1007/s40120-018-0111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Filippis R, De las Cuevas C, Sanz EJ, Schoretsanitis G, Correll CU, de Leon J. Clozapine-associated pericarditis and pancreatitis in children and adolescents: A systematic literature review and pharmacovigilance study using the VigiBase database. Schizophr Res. 2023; https://linkinghub.elsevier.com/retrieve/pii/S0920996423003870. [DOI] [PubMed]
- 69.de Leon J. Reflections on the complex history of the concept of clozapine-induced inflammation during titration. Psychiatr Danub. 2022;34:411–21. [DOI] [PubMed] [Google Scholar]
- 70.de Leon J, Ruan C-J, Verdoux H, Wang C. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen Psychiatry. 2020;33: e100183. 10.1136/gpsych-2019-100183. 10.1136/gpsych-2019-100183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amerio A, Magnani L, Arduino G, Fesce F, de Filippis R, Parise A, et al. Immunomodulatory effects of clozapine: more than just a side effect in schizophrenia. Curr Neuropharmacol. 2023;22:1233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Correll CU, Agid O, Crespo-Facorro B, de Bartolomeis A, Fagiolini A, Seppälä N, et al. A guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs. 2022;36:659–79. 10.1007/s40263-022-00932-2. 10.1007/s40263-022-00932-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raschi E, Poluzzi E, Salvo F, Pariente A, De Ponti F, Marchesini G, et al. Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: what a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr Metab Cardiovasc Dis. 2018;28:533–42. [DOI] [PubMed] [Google Scholar]
- 74.Ak T, Erdem S, Durmus RB, Kimyon U, Engin B, Bavunoglu I. How to recognize and manage challenging <scp>DRESS</scp> cases: two case reports and a review of the literature. Dermatol Ther. 2022. 10.1111/dth.15785. 10.1111/dth.15785 [DOI] [PubMed] [Google Scholar]
- 75.Rani F, Murray ML, Byrne PJ, Wong ICK. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics. 2008;121:1002–9. [DOI] [PubMed] [Google Scholar]
- 76.Verdoux H, Tournier M, Begaud B. Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatr Scand. 2010;121:4–10. 10.1111/j.1600-0447.2009.01425.x. 10.1111/j.1600-0447.2009.01425.x [DOI] [PubMed] [Google Scholar]
- 77.Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases. Drug Saf. 2007;30:891–8. 10.2165/00002018-200730100-00007. 10.2165/00002018-200730100-00007 [DOI] [PubMed] [Google Scholar]
- 78.Gatti M, Antonazzo IC, Diemberger I, De Ponti F, Raschi E. Adverse events with sacubitril/valsartan in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur J Prev Cardiol. 2021;28:983–9. [DOI] [PubMed] [Google Scholar]
- 79.Warnock JK, Morris DW. Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol. 2002;3:629–36. 10.2165/00128071-200203090-00005. 10.2165/00128071-200203090-00005 [DOI] [PubMed] [Google Scholar]
- 80.Nurenberg JR, Schleifer SJ. Reported allergies to antipsychotic agents in a long-term psychiatric hospital. J Psychiatr Pract. 2009;15:489–92. [DOI] [PubMed] [Google Scholar]
- 81.Guinart D, Misawa F, Rubio JM, Pereira J, de Filippis R, Gastaldon C, et al. A systematic review and pooled, patient-level analysis of predictors of mortality in neuroleptic malignant syndrome. Acta Psychiatr Scand. 2021;144:329–41. 10.1111/acps.13359. 10.1111/acps.13359 [DOI] [PubMed] [Google Scholar]
- 82.Khouri C, Petit C, Tod M, Lepelley M, Revol B, Roustit M, et al. Adverse drug reaction risks obtained from meta-analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J Clin Epidemiol. 2021;134:14–21. [DOI] [PubMed] [Google Scholar]
- 83.Chan EW, Liu KQL, Chui CSL, Sing C-W, Wong LYL, Wong ICK. Adverse drug reactions—examples of detection of rare events using databases. Br J Clin Pharmacol. 2015;80:855–61. 10.1111/bcp.12474. 10.1111/bcp.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guinart D, Misawa F, Rubio JM, Pereira J, Sharma H, Schoretsanitis G, et al. Outcomes of neuroleptic malignant syndrome with depot versus oral antipsychotics. J Clin Psychiatry. 2020;82. https://www.psychiatrist.com/JCP/article/Pages/outcomes-of-nms-with-depot-versus-oral-antipsychotics.aspx. [DOI] [PubMed]
- 85.Cabañas R, Ramírez E, Sendagorta E, Alamar R, Barranco R, Blanca-López N, et al. Spanish Guidelines for Diagnosis, Management, Treatment and Prevention of DRESS syndrome. J Investig Allergol Clin Immunol. J Investig Allergol Clin Immunol; 2020. http://www.ncbi.nlm.nih.gov/pubmed/31932268. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
VigiBase® does not allow the distribution of the files with each case of DRESS, but in the tables and in the electronic supplementary material, all detailed information needed to perform the analyses is provided, i.e., number of cases, non-cases, number of other AEs, and total number of reports in VigiBase®. Other requests for data can be submitted to the corresponding UMC. The code will be made available upon reasonable request to the corresponding author.