Abstract
There is controversy as to whether the cell entry mechanism of Sindbis virus (SIN) involves direct fusion of the viral envelope with the plasma membrane at neutral pH or uptake by receptor-mediated endocytosis and subsequent low-pH-induced fusion from within acidic endosomes. Here, we studied the membrane fusion activity of SIN in a liposomal model system. Fusion was followed fluorometrically by monitoring the dilution of pyrene-labeled lipids from biosynthetically labeled virus into unlabeled liposomes or from labeled liposomes into unlabeled virus. Fusion was also assessed on the basis of degradation of the viral core protein by trypsin encapsulated in the liposomes. SIN fused efficiently with receptor-free liposomes, consisting of phospholipids and cholesterol, indicating that receptor interaction is not a mechanistic requirement for fusion of the virus. Fusion was optimal at pH 5.0, with a threshold at pH 6.0, and undetectable at neutral pH, supporting a cell entry mechanism of SIN involving fusion from within acidic endosomes. Under optimal conditions, 60 to 85% of the virus fused, depending on the assay used, corresponding to all of the virus bound to the liposomes as assessed in a direct binding assay. Preincubation of the virus alone at pH 5.0 resulted in a rapid loss of fusion capacity. Fusion of SIN required the presence of both cholesterol and sphingolipid in the target liposomes, cholesterol being primarily involved in low-pH-induced virus-liposome binding and the sphingolipid catalyzing the fusion process itself. Under low-pH conditions, the E2/E1 heterodimeric envelope glycoprotein of the virus dissociated, with formation of a trypsin-resistant E1 homotrimer, which kinetically preceded the fusion reaction, thus suggesting that the E1 trimer represents the fusion-active conformation of the viral spike.
Sindbis virus (SIN) is the prototype member of the genus Alphavirus of the family Togaviridae. Alphaviruses are structurally well-defined viruses which contain three major proteins, the capsid protein, C, and two envelope glycoproteins, E1 and E2 (27, 54). The glycoproteins are organized in 80 hetero-oligomeric spikes; a single spike consists of a trimer of E2/E1 heterodimers. In the infected cell, the spike heterodimer is assembled in the endoplasmic reticulum as a PE2/E1 heterodimer in which PE2 is the precursor of the E2. The PE2/E1 heterodimer is subsequently transported through the Golgi and the trans-Golgi network to the plasma membrane. Just before the appearance of the spike on the cell surface, the PE2 precursor is cleaved into E2 and E3 by a cellular furin-like protease, resulting in the formation of the mature E2/E1 form of the heterodimer (33). In some alphaviruses, including SIN, the E3 peptide is released from the virus particles (37), whereas in others, such as Semliki Forest virus (SFV), E3 remains associated with the E2/E1 heterodimer.
The E2/E1 heterodimer mediates the infectious entry of SIN into its host cell. The initial step in cell entry is the interaction of the virus with a cellular receptor. A high-affinity receptor for binding of SIN to rodent and monkey cells has been identified as the 67-kDa protein laminin (57). Recently, it has been shown that the widely expressed glycosaminoglycan heparan sulfate may also be involved in the binding of SIN to cells (6, 30). It is the E2 component of the alphavirus spike that is primarily involved in receptor interaction (48, 50).
Being an enveloped virus, SIN infects its host cells by a membrane fusion reaction. In principle, fusion of enveloped viruses may occur either at the plasma membrane or from within the endosomal cell compartment after internalization of the virus particles through receptor-mediated endocytosis. In the process of plasma membrane fusion, the interaction of the virus with a cellular receptor mediates the conformational changes within the viral spike protein that are required for the fusion reaction, fusion occurring at the neutral pH of the extracellular environment. In the process of virus cell entry through receptor-mediated endocytosis, it is generally the mildly acidic pH within the lumen of the endosomes that triggers the membrane fusion reaction.
There is considerable controversy with regard to the cell entry mechanism of SIN. Several lines of evidence suggest that SIN may fuse directly at the cell plasma membrane at neutral pH, mediated by interaction of the viral spike with its cellular receptor. For example, SIN has been observed to infect cells treated with weak bases, like chloroquine and ammonium chloride, which raise the pH of endosomes, as evidenced by the translation of viral RNA in the cell cytosol (7, 8). This suggests that the infection process of SIN does not involve acidic endosomes. Accordingly, SIN has been found to infect a Chinese hamster ovary (CHO) cell mutant, temperature sensitive for endosome acidification (14), although earlier observations involving similar CHO cell mutants had suggested that a lack of endosome acidification does inhibit infection (39, 47). Furthermore, Flynn et al. (17) detected conformational changes within the glycoproteins of SIN upon interaction of the virus with cells at neutral pH and suggested that the virus-receptor interaction induces the fusion-active conformation of the viral spike. Abell and Brown (1) then proposed a model for SIN entry in which the virus-receptor interaction enhances thiol-disulfide exchange reactions reorganizing the viral spike protein, allowing the virus to penetrate cells by direct fusion with the plasma membrane.
On the other hand, early (15) and quite recent evidence supports an endocytic mechanism for cell entry by SIN. In fact, while the present study was in progress, DeTulleo and Kirchhausen (12) reported that infection of cells by SIN is inhibited by dominant-negative mutant forms of dynamin, which block the budding of clathrin-coated pits, thus suggesting that SIN infects its host cells by clathrin-mediated endocytosis. Furthermore, Glomb-Reinmund and Kielian (20) published a study also providing evidence for cell entry of SIN through receptor-mediated endocytosis and fusion from within acidic endosomes. These authors made a direct comparison between SIN and SFV, because it is well established that SFV enters cells through an endocytic mechanism (21–24, 27, 35, 36). Upon exposure of SFV to low pH, the viral E2/E1 heterodimeric glycoprotein dissociates and a trypsin-resistant homotrimer of the fusion protein E1 (19, 31) is formed, which presumably represents the fusion-active conformation of the viral spike (4, 25, 29, 55, 56). SFV is also capable of fusing with liposomes in a mildly acidic environment, indicating that the sole trigger for fusion is low pH (4, 25, 42, 55, 58, 59).
Here, we studied fusion of SIN in a liposomal model system, using virus biosynthetically labeled with pyrene phospholipids (4, 42, 55, 59). It is demonstrated that the virus fuses rapidly with liposomes lacking a specific receptor, indicating that receptor binding is not essential for triggering the fusion reaction. Fusion is strictly dependent on low pH, consistent with cellular entry of SIN, like that of SFV, through acidic endosomes.
MATERIALS AND METHODS
Lipids.
Phosphatidylcholine (PC) from egg yolk, phosphatidylethanolamine (PE) prepared by transphosphatidylation of egg PC, and sphingomyelin (SPM) from egg yolk were obtained from Avanti Polar Lipids (Alabaster, Ala.). High-purity cholesterol (Chol) was a generous gift from Solvay Pharmaceuticals (Weesp, The Netherlands). The fluorescent probes 16-(1-pyrenyl)hexadecanoic acid (pyrene fatty acid) and 1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine (pyrPC) were purchased from Molecular Probes (Eugene, Oreg.).
Cells and virus.
SIN strain AR339 was a generous gift from Diane E. Griffin (Johns Hopkins University, Baltimore, Md.). The virus was propagated on baby hamster kidney cells (BHK-21). The cells were cultured in Glasgow’s modification of Eagle’s minimal essential medium (Gibco/BRL, Breda, The Netherlands), supplemented with 5% fetal calf serum, 10% tryptose phosphate broth, 200 mM glutamine, 25 mM HEPES, and 7.5% sodium bicarbonate. Pyrene-labeled SIN was isolated from the medium of infected BHK-21 cells, cultured beforehand in the presence of pyrene fatty acid, essentially as described before for SFV (4, 42, 55, 59). Briefly, BHK-21 cells, grown for 48 h in medium containing 15 μg of pyrene fatty acid per ml, were infected with SIN at a multiplicity of infection of 4. At 24 h postinfection, the pyrene-labeled SIN particles were harvested from the medium by ultracentrifugation in a Beckman type 19 rotor for 2.5 h at 100,000 × g at 4°C. The virus particles were further purified by ultracentrifugation on a 20 to 50% (wt/vol) sucrose density gradient in a Beckman SW41 rotor for 16 h at 100,000 × g at 4°C. [35S]methionine-labeled SIN and unlabeled SIN were produced in a similar fashion (4, 42, 55, 59). The purity of the SIN particles was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The viral phospholipid was determined by phoshate analysis (3). The protein concentration was determined according to Peterson (44). The infectivity of the virus preparation was determined by titration on BHK-21 cells in 96-well plates.
Liposomes.
Large unilamellar vesicles were prepared by a freeze-thaw extrusion procedure (10, 40, 42, 59). Briefly, lipid mixtures were dried from a chloroform-methanol solution under a stream of nitrogen and further dried under vacuum for at least 1 h. The lipid mixtures were hydrated in 5 mM HEPES–150 mM NaCl–0.1 mM EDTA (pH 7.4) (HNE) and subjected to five cycles of freezing and thawing. Subsequently, lipid mixtures were extruded 21 times through a Unipore polycarbonate filter with a pore size of 0.2 μm (Nuclepore, Inc., Pleasanton, Calif.) in a LiposoFast mini-extruder (Avestin, Ottawa, Canada). Smaller liposomes were prepared by extrusion an additional 81 times through two Unipore polycarbonate filters, each with a pore size of 0.05 μm. The size of the liposomes was determined by quasi-elastic light scattering analysis in a submicron particle sizer model 370 (Nicomp Particle Sizing Systems, Santa Barbara, Calif.). Liposomes consisted of PC/PE/SPM/Chol (molar ratio, 1:1:1:1.5), PC/PE/SPM (molar ratio, 1:1:1), PC/PE/Chol (molar ratio, 1:1:1), PC/PE (molar ratio, 1:1), or PC/pyrPC/PE/SPM/Chol (molar ratio, 0.85:0.15:1:1:1.5).
Trypsin-containing liposomes were also prepared by freeze-thaw extrusion, but in this case, the lipids were dispersed in HNE in the presence of 10 mg of trypsin (Boehringer, Mannheim, Germany) per ml. The liposomes were extruded 21 times through a filter with a pore size of 0.2 μm. The trypsin-containing liposomes were separated from free trypsin by gel filtration on a Sephadex G-100 column in HNE. The phospholipid concentration of liposome preparations was determined by phosphate analysis (3).
Fusion assays.
Fusion of pyrene-labeled SIN with liposomes was monitored continuously in an AB2 fluorometer (SLM/Aminco, Urbana, Ill.). Pyrene-labeled SIN (0.5 μM viral phospholipid) and liposomes (200 μM phospholipid) were mixed in a quartz cuvette of the fluorometer in a final volume of 0.665 ml in HNE, unless indicated otherwise. The contents of the cuvette were stirred magnetically and thermostated at the desired temperature. After 1 min of incubation, fusion was triggered by the addition of 35 μl of 0.1 M MES (morpholinoethanesulfonic acid)–0.2 M acetic acid, pretitrated with NaOH to achieve the final desired pH. The fusion scale was calibrated such that 0% fusion corresponded to the initial excimer fluorescence value. The 100% fusion value was obtained through the addition of 35 μl of 0.2 M octaethyleneglycol monododecyl ether (C12E8; Fluka Chemie AG, Buchs, Switzerland) to achieve an infinite dilution of the probe (4, 42, 55, 59). The initial rate of fusion was determined from the tangent to the first part of the curve. The extent of fusion was determined 60 s after acidification.
Fusion of pyrPC-labeled liposomes with SIN was measured under the same conditions, essentially as described before for SFV (32) and vesicular stomatitis virus (41). For these experiments, liposomes were prepared, with a diameter of 70 nm (see above). In the fusion reaction, liposomes (2 μM liposomal phospholipids) were mixed with SIN (10 μM viral phospholipid), unless indicated otherwise.
Transfer of the viral nucleocapsid to the liposomal lumen during SIN-liposome fusion was measured as the degradation of the viral capsid protein by trypsin, initially encapsulated in the liposomes (42, 58, 59). Briefly, a trace amount of [35S]methionine-labeled virus and unlabeled virus (final concentration of 0.5 μM viral phospholipid) were mixed with trypsin-containing liposomes (200 μM phospholipid) in the presence of 125 μg of trypsin inhibitor (Boehringer) per ml in HNE. The mixture was acidified, under continuous stirring, to the desired pH with 0.1 M MES–0.2 M acetic acid, as above. After 30 s, samples were neutralized by the addition of a pretitrated volume of NaOH. The samples were further incubated for 1 h at 37°C and subsequently analyzed by SDS-PAGE. Gels were incubated for 30 min in 1 M sodium salicylate and dried. Protein bands were visualized by autoradiography. Quantification of the capsid protein was done by phosphorimaging analysis using Image Quant 3.3 software (Molecular Dynamics, Sunnyvale, Calif.).
Virus-liposome binding assay.
Virus-liposome binding was assessed by a coflotation assay, as described before (4, 9, 40, 42, 55, 59). The reactions were carried out under the same experimental conditions as those in the fusion experiments. A trace amount of [35S]methionine-labeled virus and unlabeled virus (final concentration of 0.5 μM viral phospholipid) were mixed with liposomes (200 μM phospholipid). The mixture was acidified, under continuous stirring, to the desired pH with 0.1 M MES–0.2 M acetic acid, as above. After 60 s, samples were neutralized through the addition of a pretitrated volume of NaOH. Subsequently, 0.1 ml of the reaction mixture was added to 1.4 ml of 50% (wt/vol) sucrose in HNE. On top of this, 2.0 ml of 20% (wt/vol) sucrose and 1.0 ml of 5% (wt/vol) sucrose in HNE were layered. After centrifugation in a Beckman SW50 rotor at 150,000 × g for 2 h at 4°C, the gradient was fractionated in 10 samples, starting from the top. The distribution of the viral radioactivity was quantified by liquid scintillation analysis. The radioactivity in the top four fractions, relative to the total amount of radioactivity, was taken as a measure for SIN-liposome binding.
Analysis of the conformational changes in the viral spike protein.
The conformational changes occurring in the viral spike protein were examined under the same conditions as in the fusion and binding experiments. After low-pH treatment, samples were neutralized by addition of a pretitrated volume of NaOH, solubilized in SDS-PAGE buffer, and analyzed by SDS-PAGE. For the appearance of trypsin-resistant forms of E1, samples were incubated in the presence of 200 μg of trypsin per ml for 15 min at 37°C. Subsequently, samples were solubilized in SDS-PAGE sample buffer, heated for 4 min at 100°C, and analyzed by SDS-PAGE. Gels were further incubated for 30 min in 1 M sodium salicylate and dried. Visualization of the bands was done by autoradiography. Quantification of the trimeric form of E1 was done, using phosphorimaging analysis, as described above, by relating the intensity of the trimeric E1 to the total intensity of monomeric E1, E2, and trimeric E1, corrected for the contribution of E2 on the basis of the relative numbers of methionine residues in the E1 and E2 proteins.
RESULTS
Characterization of pyrene-labeled SIN.
In this study, we used SIN, biosynthetically labeled with the fluorescent probe pyrene. The labeling procedure involves production of the virus on BHK-21 cells cultured beforehand in the presence of pyrene fatty acid (4, 10, 42, 59). Newly formed virus particles thus carry the pyrene probe in their membrane phospholipids. In order to examine the potential effect of pyrene incorporation in the viral membrane on the infectivity of the virus, the specific infectivities of pyrene-labeled and unlabeled virus were determined. Virus was isolated and purified from the medium of infected pyrene-labeled or unlabeled BHK-21 cells, as described in Materials and Methods. Analysis by SDS-PAGE demonstrated that the virus preparations were pure, as evidenced by the presence of just the three major structural proteins, E1, E2, and C (results not shown). Subsequent determination of the viral titer and protein concentration showed that for pyrene-labeled SIN the infectious unit to particle ratio was 1/13 under the conditions of the experiment, while for unlabeled control virus the corresponding ratio was 1/12. For the calculation, a theoretical amount of 5.45 · 10−17 g of protein per virus particle was used. Since the infectious unit to particle ratios of unlabeled and pyrene-labeled SIN appeared to be very similar, indicating that pyrene labeling has no effect on the infectivity of the virus.
Low-pH-dependent fusion of pyrene-labeled SIN with liposomes.
Fusion of pyrene-labeled SIN was measured in a liposomal model system (4, 42, 55, 59). The pyrene probe forms excimers with a fluorescence emission maximum at 480 nm, about 100 nm higher than the fluorescence maximum of pyrene monomers. Pyrene excimer formation is dependent on the average distance between the probe molecules. Thus, in the virus membrane, the excimer fluorescence intensity is proportional to the surface density of pyrene-labeled phospholipid molecules (18, 43). Upon fusion of pyrene-labeled virus particles with liposomes, the pyrene phospholipids will be diluted into the liposomes, resulting in a decrease in the pyrene excimer fluorescence intensity, which can be monitored continuously. For this assay, liposomes were prepared with an average diameter of 200 nm. The diameter of the viral membrane (excluding the glycoproteins) is about 50 nm. Therefore, upon fusion of a virus particle with a liposome, the pyrene phospholipids dilute by at least an order of magnitude. In the fusion reaction, pyrene-labeled SIN (0.5 μM viral phospholipid) was mixed with an excess of liposomes (200 μM phospholipid) consisting of PC/PE/SPM/Chol with a molar ratio of 1:1:1:1.5.
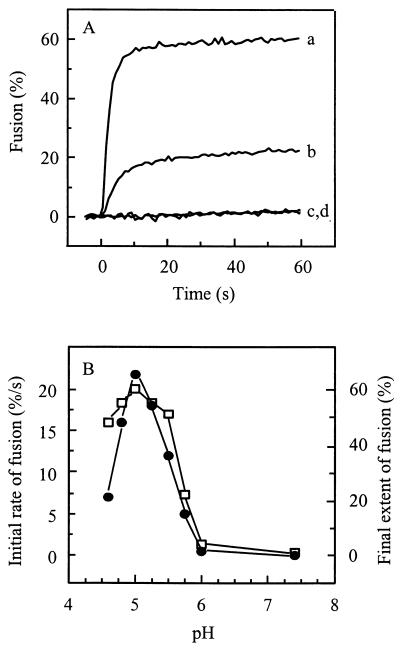
Figure 1A presents the fusion kinetics of pyrene-labeled SIN with liposomes. At pH 5.0, the virus fused rapidly and efficiently with the liposomes. By contrast, at pH 7.4, no detectable fusion occurred. Furthermore, the fluorescence intensity of pyrene-labeled SIN at low pH in the absence of target liposomes remained constant, demonstrating that the decrease in excimer fluorescence intensity observed in the presence of liposomes was due to dilution of the probe from the viral membrane into the liposomal membrane. At pH 5.75, an intermediate extent of fusion was observed with the initial rate substantially lower than that at pH 5.0.
FIG. 1.
Low-pH-dependent fusion of pyrene-labeled SIN with liposomes. Fusion was measured on-line at 37°C as a decrease of viral pyrene excimer fluorescence, as described in Materials and Methods. Final virus and liposome concentrations were 0.5 and 200 μM (membrane phospholipid), respectively. Liposomes consisted of PC/PE/SPM/Chol (molar ratio, 1:1:1:1.5). (A) Curves: a, pH 5.0; b, pH 5.75; c, pH 7.4; d, pH 5.0, without liposomes. (B) The initial rate of fusion (solid circles) was determined from the tangent to the first part of the curve. The extent of fusion (squares) was determined 60 s after acidification.
Figure 1B shows the detailed pH dependence of SIN fusion with PC/PE/SPM/Chol liposomes. Optimal fusion in terms of both initial rate and final extents was observed at pH 5.0, with a threshold at pH 6.0. Under optimal conditions, a decrease of excimer fluorescence intensity by approximately 60% was observed. This corresponds to fusion of a minimum of 60% of the virus particles, since each fusion event is expected to result in an extensive dilution of the pyrene probe (see above). However, upon fusion of a virus particle with a relatively small liposome, a residual excimer fluorescence intensity may remain. This implies that the level of 60% may represent an underestimate of the actual extent of fusion. The initial rate of fusion at pH 5.0 was very fast, corresponding to 20 to 25% of the virus particles fusing within the first second after the acidification of the virus-liposome mixture. At pH values higher or lower than the optimal pH 5.0, fusion exhibited slower kinetics and lower extents.
Fusion required an excess of liposomes, leading to an apparent saturation at 100 to 150 μM phospholipid (results not shown). In principle, it is possible that with liposome concentrations increasing beyond the saturating level, a single virus particle will fuse simultaneously with multiple liposomes. However, this is not expected to result in a significant further decrease of the fluorescence signal since, in general, a single fusion event already produces a dilution of the pyrene probe by at least an order of magnitude, as indicated above.
Fusion of unlabeled SIN with pyrene-labeled liposomes.
We also used a reverse variant of the pyrene lipid mixing assay in order to ascertain that the fusion signal seen in the experiments of Fig. 1 and 2 was not due to a peculiarity of the pyrene-labeled virus used in those experiments. In the reverse assay, an excess of unlabeled SIN was incubated with pyrPC-labeled liposomes, and dilution of the probe from the liposomal membrane into the viral membrane was assessed. For the fusion reaction, liposomes were prepared with a diameter of 70 nm. Given the diameter of the viral membrane, 50 nm, fusion of a liposome with a single virus particle thus is expected to result in a one-third enlargement of the liposomal membrane, with a concomitant decrease of the pyrene excimer fluorescence by 33%.
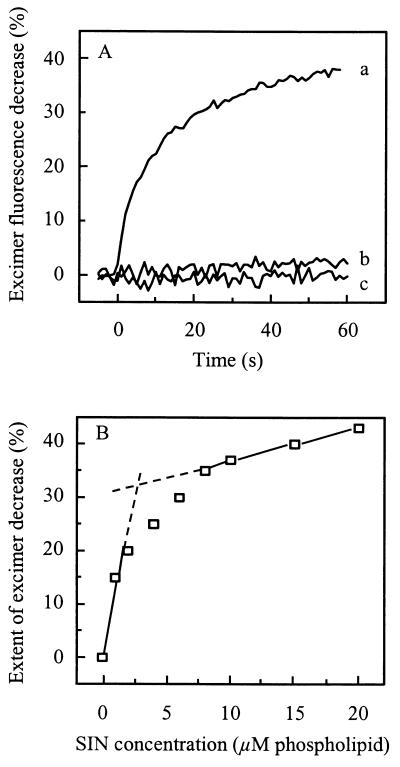
FIG. 2.
Low-pH-dependent fusion of SIN with pyrPC-labeled liposomes. Fusion was measured on-line at 37°C as a decrease of liposomal excimer fluorescence, as described in Materials and Methods. Fusion of pyrPC-labeled liposomes (2 μM liposomal phospholipid) with SIN (10 μM viral phospholipid) was measured at pH 5.0, unless indicated otherwise. Liposomes consisted of PC/pyrPC/PE/SPM/Chol (molar ratio, 0.85:0.15:1.0:1.0:1.5). (A) Curves: a, pH 5.0; b, pH 7.4; c, pH 5.0, but in the absence of SIN. (B) The final extent of excimer fluorescence decrease (squares) was determined, 60 s after acidification, as a function of the viral phospholipid concentration.
Figure 2A shows that pyrPC-labeled liposomes consisting of PC/PE/SPM/Chol under low pH conditions fused efficiently with unlabeled SIN. At pH 5.0, a decrease in pyrene excimer fluorescence by about 38% in 60 s was observed. Again, at neutral pH, there was no significant fusion. Furthermore, pyrPC-labeled liposomes in the absence of virus did not exhibit any change in fluorescence intensity upon acidification to pH 5.0.
In Fig. 2B, the extent of excimer fluorescence decrease is presented as a function of the virus concentration. When a fixed concentration of pyrPC-labeled liposomes (2 μM phospholipid, corresponding to 4 × 1010 particles per ml) was incubated with increasing virus concentrations, the extent of the decrease in excimer fluorescence intensity increased in a biphasic manner. At the extrapolated inflection point, the extent of excimer fluorescence decrease was 32%, which is very close to the theoretical value, 33%, expected for one virus particle fusing with a single liposome. The inflection occurred at a virus concentration of 2.5 μM viral phospholipid, corresponding to 1011 particles per ml. Thus, we conclude that on average each liposome fuses at least once with a single virus particle at a virus-to-liposome particle ratio of approximately 2.5. After the inflection point, the extent of excimer fluorescence decrease continued to increase, consistent with one liposome fusing simultaneously with more than one virus particle. Unlike the condition of a labeled virus particle fusing with a considerably larger liposome, fusion of a labeled 70-nm liposome with multiple smaller virus particles is expected to produce a significantly greater dilution of the probe than fusion of one such liposome with a single virus particle.
Contents mixing during SIN-liposome fusion.
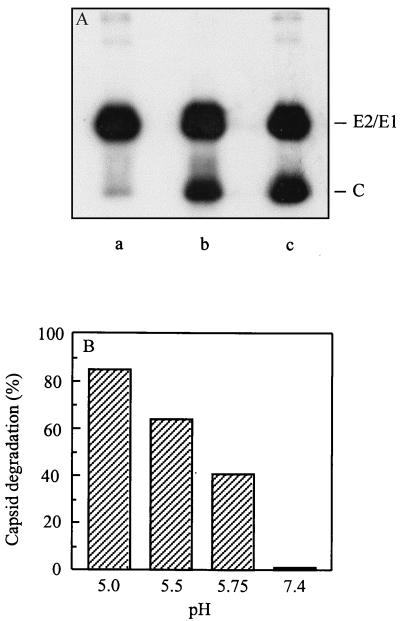
In the above experiments, the detection of SIN-liposome fusion was based on lipid mixing assays. A more stringent criterion for fusion involves the mixing of the internal contents of the virus with the liposomal lumen. To demonstrate contents mixing in the SIN-liposome system, we used an assay involving [35S]methionine-labeled virus and trypsin-containing liposomes (42, 58, 59). Fusion was assayed as the degradation of the viral capsid protein in the presence of an excess of trypsin inhibitor in the medium. As shown in Fig. 3A, incubation of SIN with an excess of trypsin-containing PC/PE/SPM/Chol liposomes at pH 5.0 resulted in the degradation of a substantial fraction of the capsid protein. At neutral pH, no degradation of the capsid protein was detected. Furthermore, incubation of virus at pH 5.0 with empty liposomes under otherwise the same conditions did not result in degradation of the capsid protein either. In these controls, the ratio of radioactivity in the capsid band relative to the total radioactivity, as determined by phosphorimaging, was close to 0.4, as expected on the basis of the number of methionine residues in the viral structural proteins (46). To exclude the possibility that the amount of trypsin was limiting under the conditions of the experiment, Triton X-100 was added to the reaction mixture in the absence of trypsin inhibitor. This resulted in complete degradation of the capsid protein, demonstrating that the amount of trypsin was not limiting in the assay (results not shown).
FIG. 3.
Transfer of the viral capsid into the liposomal lumen assayed as the degradation of the viral capsid protein. Fusion of [35S]methionine-labeled SIN (0.5 μM viral phospholipid) with trypsin-containing PC/PE/SPM/Chol liposomes (200 μM liposomal phospholipid) in the presence of trypsin inhibitor in the external medium at 37°C was determined, as described in Materials and Methods. (A) Visualization of the protein bands by autoradiography. Lanes: a, trypsin-containing liposomes at pH 5.0; b, trypsin-containing liposomes at pH 7.4; c, empty liposomes at pH 5.0. (B) Quantification of the extent of capsid protein degradation as a result of virus incubation with trypsin-containing liposomes at different pH values as determined by phosphorimaging analysis.
Figure 3B presents a quantification by phosphorimaging analysis of the extent of capsid protein degradation as a function of pH. At pH 5.0, approximately 85% of the capsid protein was degraded, while at pH 5.0 and pH 5.75 the corresponding numbers were 64 and 41%, respectively. It appears, therefore, that qualitatively the pH dependence of the SIN-liposome fusion process is the same in the contents mixing assay and the lipid mixing assay. However, the extent of fusion as assessed by the trypsin assay was consistently slightly higher than that determined by the pyrene assay. This underlines the conclusion that the pyrene assay presumably underestimates the extent of fusion, as indicated above. In addition, there may be small differences in fusion capacity between individual virus batches.
Taken together, the results obtained with the lipid mixing assays involving either pyrene-labeled SIN or pyrene-labeled liposomes, and the results of the contents mixing assay demonstrate conclusively that SIN fuses rapidly and almost quantitatively with receptor-free liposomes in a strictly low-pH-dependent manner.
Chol and SPM are required for low-pH-induced SIN-liposome fusion.
A characteristic feature of the fusion of SFV is the specific requirement of Chol and SPM in the target membrane (4, 26, 42, 45, 55, 58, 59). Because of the similarity between SFV and SIN, it was of interest to determine whether SIN exhibits the same lipid dependence. As shown in Fig. 4, liposomes of various lipid compositions were examined in order to determine the lipid dependence of SIN fusion. Under low-pH conditions, pyrene-labeled SIN fused efficiently with liposomes consisting of PE/PC/SPM/Chol. However, SIN was unable to fuse with liposomes consisting of either PE/PC/SPM or PE/PC/Chol. When either PC or PE or both were omitted from the liposomes, SPM and Chol being maintained, sustained fusion activity was observed (results not shown). This demonstrates that the presence of both Chol and SPM in the target membrane is essential for SIN-liposome fusion at low pH.
FIG. 4.
Effect of the target membrane lipid composition on fusion of pyrene-labeled SIN with liposomes. Fusion of pyrene-labeled SIN with liposomes of different lipid compositions was measured at pH 5.0 at 37°C, as described in the legend for Fig. 1. Curves: a, PC/PE/SPM/Chol (molar ratio, 1.0:1.0:1.0:1.5) liposomes; b, PC/PE/SPM (molar ratio, 1.0:1.0:1.0) liposomes; c, PC/PE/Chol (molar ratio, 1.0:1.0:1.0) liposomes.
Low-pH-dependent binding of SIN to liposomes.
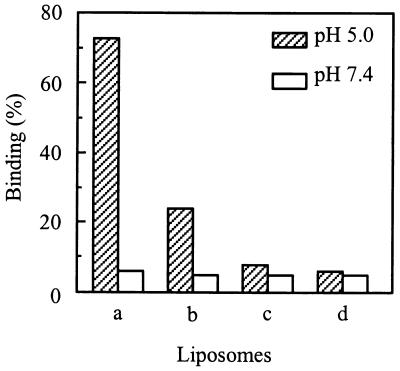
The first step in low-pH-induced SIN-liposome fusion is binding of the virus to the liposomes. Various lipid compositions in the liposomes were examined in order to determine the influence of the target membrane lipids on the binding of SIN to liposomes. Briefly, a mixture of [35S]methionine-labeled SIN, unlabeled virus, and liposomes was incubated at pH 5.0 or pH 7.4. After 60 s, the pH of the reaction mixture was neutralized by the addition of NaOH. Liposome-bound virus was separated from unbound virus by flotation on a sucrose density gradient. The results are shown in Fig. 5. Binding of SIN to liposomes was strictly dependent on acidic pH. At neutral pH, there was negligible interaction between the virus and the liposomes. At pH 5.0, we found 72% of the virus particles bound to liposomes consisting of PE/PC/SPM/Chol. By comparing the fusion data in Fig. 1 and 3 with the binding data obtained here, we conclude that all of the particles that bound to the liposomes under these conditions also fused. In the trypsin assay, we even observed a slightly higher extent of capsid protein degradation at pH 5.0 than of virus-liposome binding under the same conditions. This is presumably due to minor differences between individual virus batches. The association of SIN with PE/PC/Chol liposomes was less efficient, resulting in 25% binding. However, these virus particles only bound to the liposomes but did not fuse, fusion being undetectable with liposomes lacking SPM (Fig. 4). SIN bound very poorly to liposomes consisting of either PC/PE/SPM or PC/PE. These results indicate that cholesterol promotes binding of the virus to the liposomes but is not sufficient for extensive irreversible binding, as seen under comparable conditions with SFV (40). This indicates that in the case of SIN both Chol and SPM are required for efficient irreversible binding (and fusion) of the virus to the liposomes.
FIG. 5.
Influence of Chol and SPM on pH-dependent binding of SIN to liposomes. SIN (trace of [35S]methionine-labeled virus and unlabeled virus [0.5 μM phospholipid]) was incubated with liposomes (200 μM liposomal phospholipid) at pH 5.0 or pH 7.4 at 37°C. Binding of SIN to liposomes was determined by coflotation analysis on sucrose density gradients, as described in Materials and Methods. Bars: a, PC/PE/SPM/Chol (molar ratio, 1.0:1.0:1.0:1.5) liposomes; b, PC/PE/Chol (molar ratio, 1.0:1.0:1.0) liposomes; c, PC/PE/SPM (molar ratio, 1.0:1.0:1.0) liposomes; d, PC/PE (molar ratio, 1.0:1.0) liposomes.
Inactivation of SIN fusion capacity through preexposure of the virus alone to low pH.
It has been suggested recently that preexposure of SIN to low pH during freezing of the virus in medium or phosphate-buffered saline (PBS), which results in a transient lowering of the pH, may activate the viral fusion capacity, thus possibly explaining observations suggesting fusion of the virus under neutral pH conditions at the level of the target cell plasma membrane (12, 16). The implicit assumption underlying this suggestion is that virus activated by preexposure to low pH would remain fusion active for a significant period of time, e.g., several minutes. In this perspective, we analyzed whether preexposure to low pH of SIN alone results in sustained fusion capacity. Notably, incubation of SFV at an acidic pH in the absence of target membranes results in a very rapid loss of the fusion capacity of the virus (4).
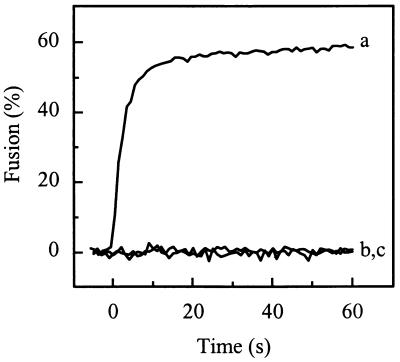
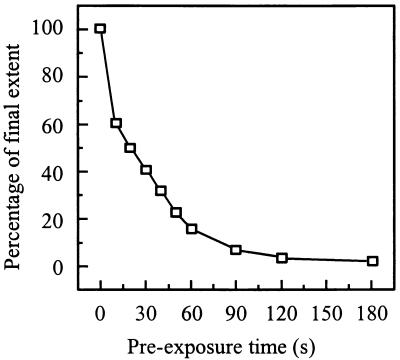
Pyrene-labeled SIN was incubated at pH 5.0 at 37°C in the absence of target liposomes for various periods of time. Subsequently, PC/PE/SPM/Chol liposomes were added and the remaining fusion activity was determined. Figure 6 shows that such a preincubation of the virus alone leads to a rapid loss of fusion activity, in agreement with earlier observations of others (13). Preincubation of SIN at pH 5.0 for 20 s resulted in a 50% reduction of fusion, while a preincubation for 2 to 3 min resulted in an essentially complete loss of fusion capacity. This indicates that fusion activation of SIN triggered by low pH is of a transient nature, resulting in a rapid subsequent irreversible loss of fusion capacity.
FIG. 6.
Inactivation of viral fusion capacity due to the preexposure of SIN alone to acidic pH. Fusion was measured on-line at 37°C as a decrease of viral pyrene excimer fluorescence, as described in the legend for Fig. 1. Pyrene-labeled SIN (0.5 μM viral phospholipid) was incubated at pH 5.0, and at the indicated time periods, liposomes (200 μM phospholipid) consisting of PC/PE/SPM/Chol (molar ratio, 1:1:1:1.5) in pH 5.0 buffer were added to the reaction mixture; subsequently, fusion was measured. The final extents of fusion, at 60 s after acidification of the liposomes, were related to the extent of fusion of an untreated control (the absolute extent for fusion of this control was 62%).
Next, we exposed SIN to low pH by slowly or rapidly freezing the virus in cell culture medium without HEPES buffer or in PBS, subsequently assessing the capacity of the virus to fuse with liposomes at neutral pH. There was no detectable fusion under these conditions (results not shown).
Conformational changes of the viral spike protein under fusion conditions.
Finally, we investigated the structural changes occurring in the SIN envelope glycoprotein occurring in the presence of target liposomes under fusion conditions, in an attempt to determine the viral spike conformational requirements for fusion. To this end, the kinetics of fusion and the kinetics of the viral spike conformational changes were determined under comparable conditions. A complicating factor in this respect relates to the extremely high rates of the fusion reaction upon acidification of a SIN-liposome mixture at 37°C to the optimal pH for fusion (pH 5.0). Therefore, for determination of the relative kinetics of virus-liposome fusion and the occurrence of spike conformational changes, we chose a pH of 5.75 and a temperature of 20°C, conditions under which the kinetics of the process are slowed down considerably. Also, the fusion process at pH 5.75 at 20°C exhibited a lag phase of approximately 9 to 10 s preceding the onset of fusion (results not shown; see Fig. 8). Furthermore, the final extent of fusion was reduced.
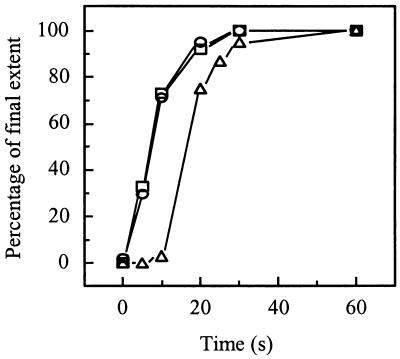
FIG. 8.
Sequence of events after acidification of a SIN-liposome mixture. The kinetics of SIN-liposome binding (squares), E1 trimerization (circles), and fusion (triangles) are shown after acidification to pH 5.75 at 20°C. To compare the kinetics of these processes, the final extents of the relative values of the three parameters were set to 100%. The absolute final extents were 30% for SIN-liposome binding, 56% for E1 trimerization, and 14% for fusion. In each case, SIN (0.5 μM viral phospholipid) was incubated with liposomes (200 μM liposomal phospholipid) consisting of PC/PE/SPM/Chol (molar ratio, 1.0:1.0:1.0:1.5) at pH 5.75 at 20°C. SIN-liposome binding was determined as described in the legend for Fig. 6. E1 trimerization was determined as described in the legend for Fig. 7. Fusion was determined as described in the legend for Fig. 1.
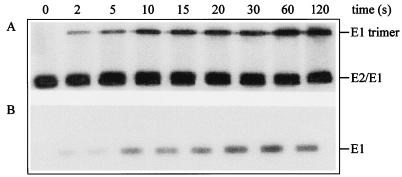
Figure 7 shows the time course of the structural changes occurring in the viral spike protein. Briefly, mixtures of [35S]methionine-labeled SIN, unlabeled SIN, and liposomes were incubated at pH 5.75 at 20°C. At the indicated time points, the mixtures were neutralized by the addition of a pretitrated volume of NaOH. Subsequently, the samples were analyzed by SDS-PAGE (Fig. 7A). Besides E1 and E2, another protein band became apparent. Determination of the size of the protein, in which a set of marker proteins was used, showed that the protein band had a molecular weight of 150, indicating that a trimer of E1 was formed. To analyze the formation of a trypsin-resistant form of the viral spike protein, we incubated the mixtures in the presence of trypsin (200 μg/ml) at 37°C. After heating of the trypsin-treated samples for 5 min at 100°C, they were analyzed by SDS-PAGE. Figure 7B shows the time course for the appearance of a trypsin-resistant phenotype of the E1 protein. Since the kinetics of the E1 trimer formation and of the appearance of the trypsin-resistant phenotype of the E1 were similar, it is likely that it is in fact the trimerization of E1 that renders it trypsin resistant.
FIG. 7.
Kinetics of the structural changes in the SIN spike protein after incubation at low pH. SIN (trace of [35S]methionine-labeled virus and unlabeled virus [0.5 μM phospholipid]) was incubated with liposomes (200 μM liposomal lipid) consisting of PC/PE/SPM/Chol (molar ratio, 1.0:1.0:1.0:1.5) at pH 5.75 at 20°C. At the indicated time points, samples were neutralized and analyzed for the appearance of E1 trimers (A) and for a trypsin-resistant form of E1 (B) by SDS-PAGE, as described in Materials and Methods.
Figure 8 presents a kinetic comparison of SIN-liposome binding, E1 trimerization, and fusion during the initial 60 s following a pH jump from 7.4 to 5.75 at 20°C. The extents of virus-liposome binding, fusion, and E1 trimerization at 60 s were 30, 14, and 56%, respectively. This indicates that not all of the virus bound to the liposomes under these suboptimal conditions also fused. In fact, a fraction of the virus initially bound to the liposomes may subsequently dissociate, possibly explaining the relatively high degree of E1 trimerization. We were not able to detect differences between the kinetics of virus-liposome binding and those of E1 trimerization. However, fusion proceeded with a significant delay after the virus-liposome binding and E1 trimerization processes.
DISCUSSION
The results of this study indicate that SIN has the capacity to fuse efficiently in a model system involving liposomes as target membranes. SIN-liposome fusion meets a stringent criterion for membrane fusion, i.e., coalescence of the internal encapsulated compartments of the interacting particles and a concomitant mixing of membrane lipids. Coalescence of the viral and liposomal internal contents was assessed on the basis of degradation of the viral core protein by trypsin initially encapsulated in the liposomes (42, 58). Membrane lipid mixing was monitored continuously by using two variants of a fluorescence assay, based on incorporation of pyrene-labeled phospholipids in either the viral or the liposomal membrane. Incorporation of the probe into the viral membrane was achieved through a biosynthetic labeling procedure, involving production of the virus from cells cultured beforehand in the presence of pyrene-labeled fatty acid. This methodology has been used before for labeling of SFV (4, 42, 55) and tick-borne encephalitis virus (11). The present results demonstrate that the procedure can also be used reliably to produce pyrene-labeled SIN, without affecting the infectivity of the virus.
SIN fuses efficiently with liposomes consisting of just phospholipids and Chol, lacking a specific protein or carbohydrate receptor for virus binding (Fig. 1 to 4). Furthermore, SIN-liposome fusion is strictly dependent on low pH, fusion being optimal at pH 5.0 and undetectable at neutral pH (Fig. 1 to 3). These characteristics of the fusion process argue strongly in favor of a cell entry mechanism of SIN, involving endocytosis of virus particles into endosomes and subsequent fusion of the viral membrane with the endosomal membrane induced by the acidic pH in the lumen of the endosomes. SIN shares the capacity to fuse to receptor-free target liposomes with a number of other enveloped viruses, such as influenza virus (51–53), SFV (4, 25, 55, 58), tick-borne encephalitis virus (11), and vesicular stomatitis virus (41). In all cases, low pH appears to be a necessary and sufficient condition for induction of the fusion process. The efficient fusion of low-pH-dependent viruses with receptor-free liposomes suggests that receptor binding is not a mechanistic requirement for expression of membrane fusion activity by these viruses. Receptor binding would appear to be primarily involved in the initial binding of the viruses to the host cell and subsequent endocytic uptake of the virus particles by the cell, although it cannot be excluded that the receptor interaction also influences the detailed characteristics of the subsequent fusion process from within the endosome. In this respect, it is interesting that, in the case of SIN, virus-receptor interaction has been found to result in conformational alterations in the viral envelope glycoprotein, detected on the basis of exposure of specific epitopes recognized by monoclonal antibodies (17, 38). These conformational changes have been suggested to be related to the viral fusion process. Clearly, our present results do not exclude that possibility. However, it would appear that low pH is the essential trigger for fusion of SIN.
The conclusion that SIN infects its host cell by entry through receptor-mediated endocytosis and fusion from within acidic endosomes is in agreement with recent observations of Glomb-Reinmund and Kielian (20). These investigators showed that the addition of weak bases during cell entry of the virus efficiently inhibits translation of viral RNA and infection. Previous studies had suggested that weak bases would not inhibit cellular infection by SIN (7, 8). Glomb-Reinmund and Kielian (20) also used balifomycin and concanamycin, two reagents that prevent endosome acidification by a mechanism different from that of weak bases, and again found that cellular infection by SIN was inhibited, in further support of the conclusion that the infection process involves acidic endosomes. Furthermore, the authors were unable to detect a specific role for reduction of disulfide bridges through thiol-disulfide exchange reactions during the SIN entry process (20). It had been suggested before that disulfide shuffling upon interaction of SIN with its cell surface receptor would reorganize the viral spike to mediate virus cell entry through fusion with the plasma membrane (1, 5). Thus, the observations of Glomb-Reinmund and Kielian (20) argue against fusion with the cell plasma membrane as the physiological infection mechanism of SIN. Recently, DeTulleo and Kirchhausen (12) also came to the conclusion that SIN does not infect cells by plasma membrane fusion but rather through entry via an endocytic pathway. Specifically, these investigators employed expression of dominant-negative mutant forms of dynamin which inhibit clathrin-dependent endocytosis and showed that these mutant dynamins also inhibit cellular infection by SIN.
It is not clear what the explanation is for the above discrepancy between the observations which suggest that SIN enters cells by plasma membrane fusion and those that argue in favor of an endocytic entry mechanism. One possibility, suggested by DeTulleo and Kirchhausen (12) and Ferlenghi et al. (16), involves an undeliberate preexposure of the virus to low pH during freezing in cell culture medium or PBS. This would induce a premature conformational change in the viral spike, allowing subsequent fusion of the virus with the cell plasma membrane at neutral pH. Indeed, DeTulleo and Kirchhausen (12) observed that SIN, frozen under such inadequate buffering conditions, has the capacity to enter cells via a clathrin-independent pathway, suggestive of fusion with the plasma membrane. We were unable to detect any fusion of SIN at neutral pH, whether or not the virus had been frozen beforehand in PBS or medium. However, this does not rule out the possibility of fusion with the plasma membrane at neutral pH of virus frozen under inadequate buffering conditions, since a low degree of fusion (on the order of 1% relative to the control) may well go unnoticed in our assay.
The characteristics of SIN fusion in the present liposomal model system in many respects resemble those of SFV-liposome fusion (4, 42, 55, 59). Both viruses fuse with liposomes in a low-pH-dependent manner, although we note that the pH optimum (pH 5.0) for fusion of the AR339 strain of SIN used here is lower by about 0.5 pH unit than that of SFV, in agreement with observations of Glomb-Reinmund and Kielian (20). Also, for the Toto 1101 infectious clone of SIN, we found a low pH optimum (pH 4.6) for fusion (49). In cell-cell fusion studies, the pH optimum for SIN AR339 has been reported to be 5.4 (2). Importantly, SIN, like SFV, requires the presence of both Chol and sphingolipid in the target membrane (Fig. 4). Recently, in an elegant study, Lu et al. (34) have shown that SIN entry into cells also requires Chol. In the liposome system, Chol is essential for the initial low-pH-dependent binding of SFV to target liposomes, while the sphingolipid appears to be involved directly in the subsequent fusion reaction (42, 59). Specifically, extensive irreversible binding of SFV occurs to liposomes consisting of PC/PE/Chol, with fusion being undetectable; on the other hand, virtually no binding (nor fusion) occurs with PC/PE/SPM liposomes (42, 59). SIN appeared to behave in essentially the same manner (Fig. 4 and 5), with virus binding to PC/PE or PC/PE/SPM liposomes being at background level, and binding to PC/PE/Chol liposomes reaching 25% (Fig. 5). On the other hand, the extent of virus binding to PC/PE/Chol liposomes was limited compared to the extent of virus binding to PC/PE/SPM/Chol liposomes (72%). One explanation would be that the interaction of the virus with PC/PE/Chol liposomes is not completely irreversible, such that in the absence of fusion part of the virus may dissociate. In the presence of Chol and SPM in the liposomes, the interaction would become irreversible as a result of fusion subsequent to binding. Indeed, all of the virus that bound to liposomes containing both Chol and SPM at pH 5.0 at 37°C appeared to be fused. Yet, even under these optimal conditions, a small fraction of the virus does not seem to interact at all with the liposomes. It is possible that, upon exposure of the virus-liposome mixture to low pH, part of the virus may become inactivated so rapidly that it does not have the opportunity to productively interact with the liposomes.
The results shown in Fig. 7 demonstrate that under fusion conditions, the E1 component of the SIN spike forms a homotrimeric structure, while at the same time E1 becomes trypsin resistant. The relative kinetics of the E1 trimer formation and the appearance of the trypsin-resistant phenotype suggest that the E1 homotrimer and the trypsin-resistant form of E1 are in fact identical structures. By analogy to the role of the E1 trimer in SFV fusion (4, 25, 55), we propose that the SIN E1 homotrimer represents the fusion-active conformation of the viral spike. The kinetics of virus-liposome binding and E1 homotrimer formation are indistinguishable (Fig. 8). For SFV, on the basis of early results, we have suggested that E1 homotrimer formation precedes virus-liposome binding (4). However, more recent observations involving selective inhibition of SFV E1 trimerization with Zn2+ ions (10) or through a mutation in the E1 protein (28) have shown that dissociation of the E2/E1 heterodimer at low pH suffices for initiation of virus-liposome binding and that E1 trimer formation occurs after the binding of the virus to the liposomes (10, 28). The results shown for SIN in Fig. 8 are in agreement with this notion. Therefore, it would appear that E1 trimer formation is facilitated by the association of the virus with the liposomes, trimerization occurring without any significant delay after the initial binding process. Under the conditions of the experiment (pH 5.75, 20°C), fusion then proceeds after a lag period. This lag presumably represents the time required for additional rearrangements within or between homotrimeric E1 spikes. We propose that the target membrane sphingolipid is critically involved in this step, leading to the actual fusion-active structure of the virus.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (grant HL 16660) and by The Netherlands Organization for Scientific Research (NWO) under the auspices of the Foundation for Chemical Research (CW).
We thank Diane E. Griffin (Johns Hopkins University) for generously providing the Sindbis virus AR339 stock.
REFERENCES
- 1.Abell B A, Brown D T. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J Virol. 1993;67:5496–5501. doi: 10.1128/jvi.67.9.5496-5501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boggs W M, Hahn C S, Strauss E G, Strauss J H, Griffin D E. Low pH-dependent Sindbis virus-induced fusion of BHK cells: differences between strains correlate with amino acid changes in the E1 glycoprotein. Virology. 1989;169:485–488. doi: 10.1016/0042-6822(89)90178-5. [DOI] [PubMed] [Google Scholar]
- 3.Böttcher C J F, van Gent C M, Fries C. A rapid and sensitive sub-micro phosphorus determination. Anal Chim Acta. 1961;24:203–204. [Google Scholar]
- 4.Bron R, Wahlberg J M, Garoff H, Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993;12:693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D T, Edwards J. Structural changes in alphaviruses accompanying the process of membrane penetration. Semin Virol. 1992;3:519–527. [Google Scholar]
- 6.Byrnes A P, Griffin D E. Binding of Sindbis virus to cell surface heparan sulfate. J Virol. 1998;72:7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassell S, Edwards J, Brown D T. Effects of lysosomotropic weak bases on infection of BHK-21 cells by Sindbis virus. J Virol. 1984;52:857–864. doi: 10.1128/jvi.52.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombs K, Mann E, Edwards J, Brown D T. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J Virol. 1981;37:1060–1065. doi: 10.1128/jvi.37.3.1060-1065.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corver J, Moesby L, Erukulla R K, Reddy K C, Bittman R, Wilschut J. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J Virol. 1995;69:3220–3223. doi: 10.1128/jvi.69.5.3220-3223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corver J, Bron R, Snippe H, Kraaijeveld C, Wilschut J. Membrane fusion activity of Semliki Forest virus in a liposomal model system: specific inhibition by Zn2+ ions. Virology. 1997;238:14–21. doi: 10.1006/viro.1997.8799. [DOI] [PubMed] [Google Scholar]
- 11.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Submitted for publication. [DOI] [PubMed]
- 12.DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. EMBO J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards J, Mann E, Brown D T. Conformational changes in Sindbis virus envelope protein accompanying exposure to low pH. J Virol. 1983;45:1090–1097. doi: 10.1128/jvi.45.3.1090-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards J, Brown D T. Sindbis virus infection of a Chinese hamster ovary cell mutant defective in the acidification of endosomes. Virology. 1991;182:28–33. doi: 10.1016/0042-6822(91)90644-q. [DOI] [PubMed] [Google Scholar]
- 15.Fan D P, Sefton B M. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978;15:985–992. doi: 10.1016/0092-8674(78)90282-9. [DOI] [PubMed] [Google Scholar]
- 16.Ferlenghi I, Gowen B, de Haas F, Mancini E J, Garoff H, Sjöberg M, Fuller S D. The first step: activation of the Semliki Forest virus spike protein precursor causes a localized conformational change in the trimeric spike. J Mol Biol. 1998;283:71–81. doi: 10.1006/jmbi.1998.2066. [DOI] [PubMed] [Google Scholar]
- 17.Flynn D C, Meyer W J, Mackenzie J M, Johnston R E. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J Virol. 1990;64:3643–3653. doi: 10.1128/jvi.64.8.3643-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galla H J, Hartmann W. Excimer forming lipids in membrane research. Chem Phys Lipids. 1974;27:199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- 19.Garoff H, Frischauf A M, Simons K, Lehrach H, Delius H. Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature. 1980;288:236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- 20.Glomb-Reinmund S, Kielian M. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology. 1998;248:372–381. doi: 10.1006/viro.1998.9275. [DOI] [PubMed] [Google Scholar]
- 21.Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki Forest virus into BHK-21 cells. J Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helenius A. Semliki Forest virus penetration from endosomes: a morphological study. Biol Cell. 1984;51:181–187. doi: 10.1111/j.1768-322x.1984.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 24.Izurun A, Nieva L, Carrasco L. Entry of Semliki Forest virus into cells: effects of concanamycin A and nigericin on viral membrane fusion and infection. Virology. 1997;227:488–492. doi: 10.1006/viro.1996.8340. [DOI] [PubMed] [Google Scholar]
- 25.Justman J, Klimjack M R, Kielian M. Role of spike protein conformational changes in fusion of Semliki Forest virus. J Virol. 1993;67:7597–7607. doi: 10.1128/jvi.67.12.7597-7607.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kielian M, Helenius A. Role of cholesterol in fusion of Semliki Forest virus with membranes. J Virol. 1984;52:281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kielian M. Membrane fusion and the alphavirus life cycle. Adv Virus Res. 1995;45:113–151. doi: 10.1016/s0065-3527(08)60059-7. [DOI] [PubMed] [Google Scholar]
- 28.Kielian M, Klimjack M R, Ghosh S, Duffus W A. Mechanisms of mutations inhibiting fusion and infection by Semliki Forest virus. J Cell Biol. 1996;134:863–872. doi: 10.1083/jcb.134.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimjack M R, Jeffrey S, Kielian M. Membrane and protein interactions of a soluble form of the Semliki Forest virus fusion protein. J Virol. 1994;68:6940–6946. doi: 10.1128/jvi.68.11.6940-6946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK-21 cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy Mintz P, Kielian M. Mutagenesis of the putative fusion domain of the Semliki Forest virus spike protein. J Virol. 1991;65:4292–4300. doi: 10.1128/jvi.65.8.4292-4300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, G., P. Schoen, J. M. Smit, L. C. Bijl, J. Corver, K. Lin, and J. Wilschut. Retention of internal aqueous contents during fusion of Semliki Forest virus with liposomes. Submitted for publication.
- 33.Lobigs M, Garoff H. Fusion function of the Semliki Forest virus spike is activated by proteolytic cleavage of the envelope glycoprotein precursor p62. J Virol. 1990;64:5214–5218. doi: 10.1128/jvi.64.3.1233-1240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y E, Cassese T, Kielian M. The cholesterol requirement for Sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol. 1999;73:4272–4278. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh M, Wellsteed J, Kern H, Harms E, Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci USA. 1982;79:5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh M, Bolzau E, Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983;32:931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- 37.Mayne J T, Rice C M, Strauss E G, Hunkapiller M W, Strauss J H. Biochemical studies of the maturation of the small Sindbis virus glycoprotein E3. Virology. 1984;134:338–357. doi: 10.1016/0042-6822(84)90302-7. [DOI] [PubMed] [Google Scholar]
- 38.Meyer W J, Gidwitz S, Ayers V K, Schoepp R J, Johnston R E. Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH. J Virol. 1992;66:3504–3513. doi: 10.1128/jvi.66.6.3504-3513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moehring J M, Moehring T J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983;41:998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moesby L, Corver J, Erukulla R K, Bittman R, Wilschut J. Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner. Biochemistry. 1995;34:10319–10324. doi: 10.1021/bi00033a001. [DOI] [PubMed] [Google Scholar]
- 41.Moor A C E, Wagenaars-van Gompel A E, Hermans R C A, van der Meulen J, Smit J M, Wilschut J, Brand A, Dubbelman T M A R, VanSteveninck J. Inhibition of various steps in the replication cycle of vesicular stomatitis virus contributes to its photoinactivation by AlPcS4 or Pc4 and red light. Photochem Photobiol. 1999;69:353–359. doi: 10.1562/0031-8655(1999)069<0353:iovsit>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Nieva J L, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pal R, Barenholz Y, Wagner R R. Pyrene phospholipid as a biological fluorescent probe for studying fusion of virus membrane with liposomes. Biochemistry. 1988;27:30–36. doi: 10.1021/bi00401a006. [DOI] [PubMed] [Google Scholar]
- 44.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 45.Phalen T, Kielian M. Cholesterol is required for infection by Semliki Forest virus. J Cell Biol. 1991;112:615–623. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice C M, Strauss J H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci USA. 1981;78:2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbins A R, Oliver C, Bateman J L, Krag S S, Galloway C J, Mellman I. A single mutation in Chinese hamster ovary cells impairs both Golgi and endosomal functions. J Cell Biol. 1984;99:1296–1308. doi: 10.1083/jcb.99.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salminen A, Wahlberg J W, Lobigs M, Liljeström P, Garoff H. Membrane fusion process of Semliki Forest virus II: cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992;116:349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smit, J. M., and J. Wilschut. Unpublished data.
- 50.Smith T J, Cheng R H, Olson N H, Peterson P, Chase E, Kuhn R J, Baker T S. Putative receptor binding sites on alphaviruses as visualized by cryoelectron microscopy. Proc Natl Acad Sci USA. 1995;92:10648–10652. doi: 10.1073/pnas.92.23.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stegmann T, Hoekstra D, Scherphof G, Wilschut J. Fusion activity of influenza virus: a comparison between biological and artificial target membrane vesicles. J Biol Chem. 1986;261:10966–10969. [PubMed] [Google Scholar]
- 52.Stegmann T, Nir S, Wilschut J. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry. 1989;28:1698–1704. doi: 10.1021/bi00430a041. [DOI] [PubMed] [Google Scholar]
- 53.Stegmann T, White J M, Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990;9:4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahlberg J M, Bron R, Wilschut J, Garoff H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J Virol. 1992;66:7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahlberg J M, Garoff H. Membrane fusion process of Semliki Forest virus. I. Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992;116:339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang K S, Kuhn R J, Strauss E G, Ou S, Strauss J H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992;66:4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White J, Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci USA. 1980;77:3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilschut J, Corver J, Nieva J L, Bron R, Moesby L, Reddy K C, Bittman R. Fusion of Semliki Forest virus with cholesterol-containing liposomes at low pH: a specific requirement for sphingolipids. Mol Membr Biol. 1995;12:143–149. doi: 10.3109/09687689509038510. [DOI] [PubMed] [Google Scholar]