Abstract
Background
Periampullary adenocarcinomas typically exhibit either intestinal or pancreatobiliary (PB) differentiation, and the type of differentiation may be prognostically more important than the anatomic site of origin. This study aimed to evaluate prognostic significance of histological type of periampullary carcinomas.
Methods
Microscopic slides from 110 consecutive pancreatoduodenectomies performed between 2010 and 2020 were reviewed and classified as intestinal or PB type. Clinicopathological factors were compared between PB-(n=93) and intestinal-type (n=17) differentiation.
Results
The intestinal type included significantly more patients with well-differentiated histology (35.3% vs. 11.8%, p=0.001) and fewer patients with perineural invasion (41.2% vs. 76.4%, p=0.029), advanced T stage (> T3; 41.2% vs.74.2%, p=0.007), and systemic recurrence (71.4% vs. 92.9%, p=0.005) than PB type. The 5-year-overall survival rate of intestinal-type was significantly higher than that of PB-type (58.8% vs. 20.4%, p=0.003). When pancreatic cancer was separately analyzed, the intestinal type showed the best 5-year-overall survival rate, with no significant difference between the PB types excluding PDAC and PDAC (39.4% vs. 19.2%, p=0.148). In multivariate analysis, curative resection (hazard ratio, 0.417; 95% CI, 0.219-0.792, p=0.008) was the only significant prognostic factor.
Conclusion
Although intestinal histologic phenotype was not an independent prognostic factor on multivariate analysis, it showed pathologic features associated with better survival, while the PB type showed more aggressive tumor biology and consequently worse survival. Further studies are needed to demonstrate the prognostic significance of histologic phenotype.
Keywords: periampullary, carcinoma, prognosis, pancreatobiliary type, intestinal type
1. Introduction
Periampullary cancer develops around the ampulla of Vater and originates anatomically from the pancreatic head, duodenum, distal bile duct, or ampulla. The World Health Organization (WHO) and American Joint Committee on Cancer (AJCC) have classified periampullary cancer into the above-mentioned four types according to the anatomical location and the tumor, node, metastasis (TNM) staging criteria are different for each origin. (1, 2), However, in the case of the duodenal, distal bile duct, and ampullary cancers, except for pancreatic head cancer which has the worst prognosis, it is often difficult to distinguish the exact site of origin owing to the destruction of normal periampullary anatomy or the presence of epithelial dysplasia even with standardized histopathologic evaluation. Furthermore, this makes it difficult to predict the prognosis and decide on adequate treatment, especially regimen of adjuvant chemotherapy, as the prognosis of patients after pancreaticoduodenectomy (PD) varies greatly even in the same anatomic location (3, 4),. Therefore, several recent studies have questioned this anatomical classification and addressed the need for a new classification (5–7).
Kimura et al. first reported dividing adenocarcinomas originating around the ampulla into intestinal and pancreaticobiliary (PB) types and demonstrated that the latter type had a worse prognosis (8). The intestinal type frequently resembles colon cancer and originates from the intestinal epithelium overlying the ampulla, evolving through an adenoma-dysplasia-adenocarcinoma sequence. In contrast, the PB type originates from the endothelium of the distal common bile duct and distal pancreatic intraepithelial neoplasia in an analogous dysplasia-adenocarcinoma sequence. Although the histologic phenotype in pancreatic ductal adenocarcinoma has not been endorsed by WHO until now, several groups have extended intestinal and pancreatobiliary concepts to the entire spectrum of periampullary adenocarcinoma including pancreatic ductal adenocarcinoma (9–13). In these studies, the intestinal type in pancreatic ductal adenocarcinoma accounted for approximately less than 10% (9–13). Histologic phenotype can be easily determined based on hematoxylin-eosin (H&E) staining. Several studies have shown that the histological type of periampullary adenocarcinomas has biological and prognostic relevance (9–15). However, some other studies have demonstrated no survival difference between the intestinal type and PB type, and the prognostic potential of the histological classification of periampullary cancer has been debated (16–18). These discrepancies between studies may be because of the low incidence of periampullary cancer excluding pancreatic cancer, and the consequent small cohorts with reliable data. Therefore, this study was designed to compare the clinicopathological characteristics and long-term outcomes between intestinal- and PB-type periampullary adenocarcinomas after curative-intent resection in a tertiary referral center to determine the potential of this histological classification as an independent prognostic factor.
2. Materials and methods
2.1. Study subjects
This study was approved by the Institutional Review Board of Chung-Ang University Hospital, Seoul, Korea. The requirement for written informed consent was waived because of the retrospective nature of the study. We retrospectively reviewed the medical records of patients who underwent curative-intended pancreatoduodenectomy for primary periampullary carcinoma from May 2010 to February 2020 at the Chung-Ang University Hospital, a tertiary referral hospital. Patients who underwent resection with macroscopic residual tumor (R2 resection) or a palliative procedure, and patients with distant metastasis and who diagnosed as other than adenocarcinoma (e.g., adenosquamous cell carcinoma, mucinous adenocarcinoma, and anaplastic type adenocarcinoma) were excluded. In addition, patients with fewer than 6 lymph nodes sampled were also excluded. Finally, a total of 110 patients were included in this study. All PDs were performed by senior pancreatic surgeons. Two expert pancreatic pathologists re-evaluated all pathological slides and determined their histological phenotypes.
2.2. Histological phenotype determination
The histological phenotype was determined as intestinal type or PB type based on the criteria first suggested by Kimura et al. and later revised by Saavedra et al. (8), (19), In brief, pancreatobiliary tumors typically feature simple or branching glands and small solid nests of cells surrounded by a desmoplastic stroma. They commonly exhibit cuboidal to low columnar epithelium arranged in a single layer without nuclear pseudostratification. The nuclei within these tumors are typically rounded, although there is notable variation in size and shape from one cell to the next. Intestinal tumors on the other hand, often resembling colon cancer, may present as solid nests with cribriform areas and are characterized by tall and often pseudostratified columnar epithelium. In these tumors, oval nuclei are typically located in the more basal aspects of the cytoplasm, and may also often contain mucin. Cases of mixed-type differentiation were classified according to the dominant pattern. In case of controversy regarding the histological phenotype, a consensus was reached by a second evaluation of the slides. Furthermore, all pathological slides were retrospectively reclassified according to the American Joint Committee on Cancer 8th edition definition.
The analyzed histopathological features included tumor size, histological grade, TNM stage, perineural invasion, and lymphovascular invasion. The tumor stage was defined according to the American Joint Committee on Cancer 8th edition definition. Perioperative medical records were reviewed for patient demographics, surgery type, follow-up, recurrence, and disease-specific survival. Overall survival was defined as the time from surgery to the last follow-up visit.
2.3. Statistical analysis
Continuous data are reported as means ± standard deviation (SDs), while numbers and percentages are used for categorical variables. Categorical variables were examined using the Chi-square test. Continuous variables such as maximum tumor diameter were compared using Mann-Whitney U test. Statistical significance was set at p < 0.05. The Kaplan–Meier method was used to calculate the curves for overall survival. Cox regression hazard models were used for multivariate analyses. The results are reported as hazard ratios (HR) with 95% confidence intervals (CI). A two-tailed p value <0.05 was considered statistically significant. All statistical analyses were performed using the SPSS version 25 for Windows (IBM Corp., Armonk, NY, USA).
3. Results
A total of 110 patients underwent curative-intended PD (R0 or R1 resection) and were pathologically diagnosed with periampullary adenocarcinoma at the Chung-Ang University Hospital between May 2010 and February 2020. The enrolled patients comprised 93 (84.5%) patients with PB type and 17 with intestinal-type differentiation. Of the total, 47 patients had extrahepatic bile duct adenocarcinoma, 40 had pancreatic adenocarcinoma, 17 had ampulla of Vater adenocarcinoma, and six had duodenal adenocarcinoma. The characteristics of each histological group are summarized in Table 1 . The intestinal type had significantly more well-differentiated histology (35.3% vs. 11.8%, p=0.001), significantly less nodal metastasis (29.4% vs.55.9%, p=0.022), perineural invasion (41.2% vs. 76.4%, p=0.029), lymphovascular invasion (23.5% vs. 36.4%, p=0.001), and advanced T stage (more than T3; 41.2% vs.74.2%, p=0.007) than the PB type. There were no significant differences in the curative resection, adjuvant treatment rates, and recurrence rates. However, systemic recurrence (92.9% vs. 66.7%, p=0.005) was more common in the PB type than in the intestinal type.
Table 1.
Clinicopathologic features according to histologic phenotype.
| Histologic Phenotype | p-value | ||
|---|---|---|---|
| Intestinal type (n=17) | Pancreatobiliary type (n=93) | ||
| Age, median, y | 64.0 ± 9.4 | 64.0 ± 9.1 | 0.984 |
| Gender (M:F) | 1.5:1 | 1.7:1 | 0.507 |
| Tumor origin | <0.001 | ||
| CBD | 8 (47.1%) | 39 (41.9%) | |
| Pancreas | 2 (11.7%) | 38 (41.2%) | |
| AoV | 6 (35.3%) | 11 (11.8%) | |
| Duodenum | 1 (5.9%) | 5 (5.4%) | |
| Tumor size (cm) | 3.7 ± 1.4 | 3.2 ± 1.7 | 0.665 |
| Elevation of preoperative serum CEA | 2 (14.3%) | 22 (27.8%) | 0.285 |
| Elevation of preoperative serum CA19-9 | 12 (70.6%) | 64 (68.8%) | 0.955 |
| Histologic grade | |||
| Well-differentiated | 6 (35.3%) | 12 (11.8%) | 0.001 |
| T stage | |||
| T1&2: T3&4 | 1:0.7 | 1:2.9 | 0.007 |
| T3&4 | 7 (41.2%) | 69 (74.2%) | 0.007 |
| Nodal status (N1&2) | 5 (29.4%) | 52 (55.9%) | 0.022 |
| PNI (Yes) | 7 (41.2%) | 76 (76.4%) | 0.029 |
| LVI (Yes) | 4 (23.5%) | 48 (36.4%) | 0.001 |
| Curative resection, R0 (Yes) | 15 (88.2%) | 78 (83.9%) | 0.514 |
| Adjuvant treatment (Yes) | 13 (76.5%) | 70 (75.3%) | 0.092 |
| Chemotherapy (Yes) | 10 (76.9%) | 39 (55.7%) | |
| Gemcitabine-based | 4 (40.0%) | 16 (41.0%) | |
| 5-FU-based | 2 (20.0%) | 18 (46.2%) | |
| Others | 4 (40.0%) | 5 (12.8%) | |
| Chemoradiotherapy (Yes) | 1 (7.7%) | 23 (32.9%) | |
| Radiotherapy (Yes) | 2 (15.4%) | 8 (11.4%) | |
| Recurrence (Yes) | 7 (41.2%) | 55 (59.1%) | 0.170 |
| Regional | 2 (28.6%) | 4 (7.1%) | 0.005 |
| Systemic | 5 (71.4%) | 51 (92.9%) | |
Comparing intestinal type, PB types excluding pancreatic ductal adenocarcinoma (PDAC) and PDAC, PDAC group had significantly more advance T stage (more than T3; 33.3% vs. 61.8% vs. 92.5%, p<0.001), nodal metastasis (33.3% vs. 41.8% vs. 72.5%, p=0.004), perineural invasion (46.7% vs.76.4% vs.85.0%, p=0.029), lymphovascular invasion (26.7% vs.36.4% vs.70.0%, p=0.001) than the other two groups. ( Table 2 ) There were no significant differences in curative resection rates, adjuvant chemotherapy and recurrence rates. For recurrence pattern, systemic recurrence was significantly more common in PB type and PDAC than intestinal type.
Table 2.
Clinicopathologic features according to histologic phenotype.
| Histologic Phenotype | p-value | |||
|---|---|---|---|---|
| Intestinal type (n=15) |
Pancreatobiliary type (n=55) |
*PDAC (n=40) |
||
| Age, median, y | 64.0 ± 9.4 | 64.0 ± 9.1 | 63.0 ± 8.3 | 0.984 |
| Gender (M:F) | 9:6 | 1:1 | 25:15 | 0.507 |
| Tumor origin | ||||
| CBD | 8 (17.0%) | 39 (83.0%) | <0.001 | |
| AoV | 6 (35.3%) | 11 (64.7%) | ||
| Duodenum | 1 (16.7%) | 5 (83.3%) | ||
| Tumor size (cm) | 3.7 ± 1.4 | 3.2 ± 1.7 | 3.1 ± 1.1 | 0.062 |
| Elevation of preoperative serum CEA | 2 (16.7%) | 9 (19.6%) | 13 (37.1%) | 0.149 |
| Elevation of preoperative serum CA19-9 | 10 (66.7%) | 32 (65.3%) | 32 (80.0%) | 0.288 |
| Histologic grade | ||||
| W/D | 5 (33.3%) | 8 (14.5%) | 4 (10.0%) | 0.005 |
| T stage | <0.001 | |||
| T1,2: T3,4 | 2:1 | 21:34 | 3:37 | |
| T3,4 | 5 (33.3%) | 34 (61.8%) | 37 (92.5%) | |
| Nodal status: N1,2 | 5 (33.3%) | 23 (41.8%) | 29 (72.5%) | 0.004 |
| PNI (Yes) | 7 (46.7%) | 42 (76.4%) | 34 (85.0%) | 0.029 |
| LVI (Yes) | 4 (26.7%) | 20 (36.4%) | 28 (70.0%) | 0.001 |
| Curative resection, R0 (Yes) | 13 (86.7%) | 43 (78.2%) | 35 (87.5%) | 0.450 |
| Adjuvant treatment (Yes) | 12 (80.0%) | 40 (72.7%) | 31 (77.5%) | 0.128 |
| Chemotherapy (Yes) | 9 (75.0%) | 21 (52.5%) | 19 (61.3%) | |
| Gemcitabine-based | 3 (33.3%) | 10 (47.6%) | 9 (47.4%) | |
| 5-FU-based | 2 (22.2%) | 7 (33.3%) | 9 (47.4%) | |
| Others | 4 (44.4%) | 4 (19.0%) | 1 (5.2%) | |
| Chemoradiotherapy (Yes) | 1 (8.3%) | 12 (30.0%) | 11 (35.5%) | |
| Radiotherapy (Yes) | 2 (16.6%) | 7 (17.5%) | 1 (3.2%) | |
| Recurrence (Yes) | 6 (40.0%) | 32 (58.2%) | 24 (60.0%) | 0.383 |
| Regional | 2 (33.3%) | 2 (6.3%) | 2 (8.0%) | 0.049 |
| Systemic | 4 (67.7%) | 30 (93.7%) | 22 (92.0%) | |
*, pancreatic ductal adenocarcinoma.
The 5-year-overall survival rate of the intestinal type was significantly better than that of the PB type (58.8% vs. 20.4%, p=0.003) ( Figure 1 ), and all patients with intestinal-type ampulla of Vater cancer (n=6) survived for more than 5 years. The 5-year relapse-free survival rate of the intestinal type was also significantly better than that of the PB type (56.3% vs. 17.4%, p=0.004). There was no significant difference in the 5-year-overall survival rate between the PB types excluding PDAC and PDAC (39.4% vs. 19.2%, p=0.148) ( Figure 2 ). There was also no significant difference in the 5-year relapse-free survival rate between the PB types excluding PDAC and PDAC (22.3% vs. 10.9%, p=0.289). For patients with systemic recurrence, adjuvant chemotherapy significantly improved survival (median survival, 25.9 mo vs. 6.6 mo, p<0.001) ( Figure 3 ). However, there was no significant difference between chemotherapy regimens. Furthermore, for patients with PB type with recurrence, adjuvant chemotherapy significantly improved patient survival (median survival, 30.9 mo vs. 6.6 mo, p<0.001) ( Figure 4 ).
Figure 1.
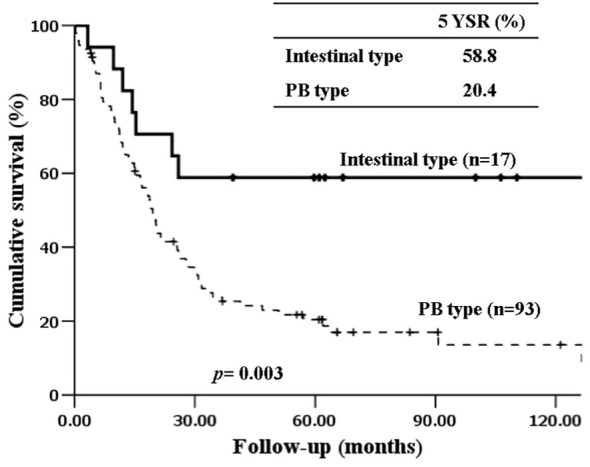
Kaplan-Meier survival curves comparing the overall survival of patients after resection of periampullary adenocarcinomas grouped by histopathologic phenotype.
Figure 2.
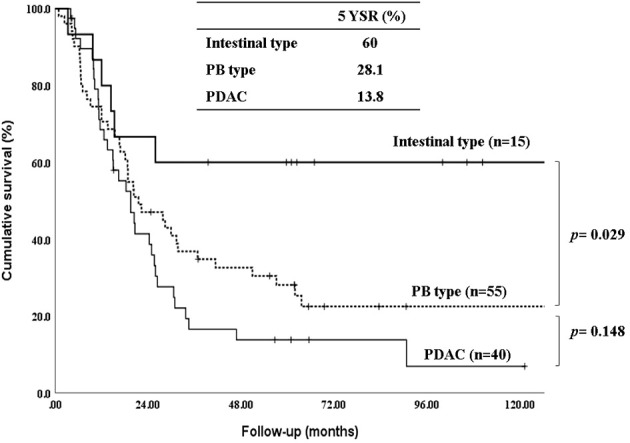
Kaplan-Meier survival curves comparing the overall survival of patients after resection of periampullary adenocarcinomas grouped by Intestinal type, PB types excluding pancreatic ductal adenocarcinoma and pancreatic ductal adenocarcinoma.
Figure 3.
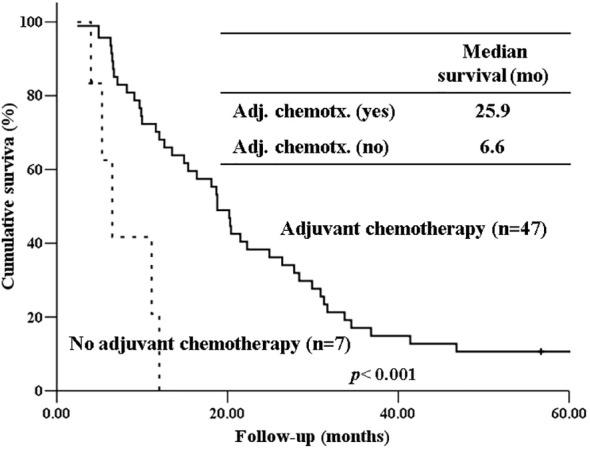
Kaplan-Meier survival curves comparing the overall survival of patients who experienced systemic recurrence depending on whether receiving adjuvant chemotherapy or not.
Figure 4.
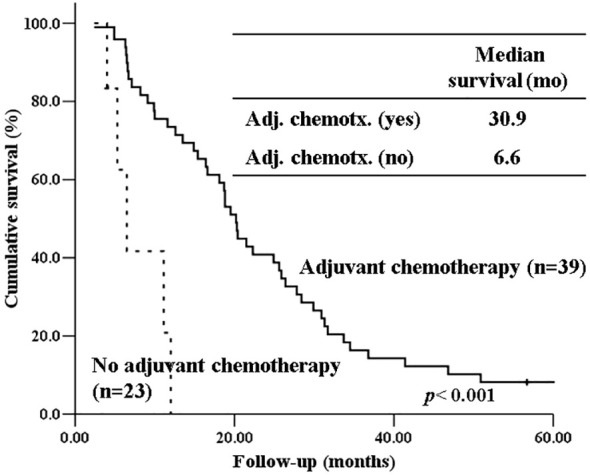
Kaplan-Meier survival curves comparing the overall survival of patients with PB type histology who experienced recurrence depending on whether receiving adjuvant chemotherapy or not.
In the univariate analysis, the intestinal type showed a longer mean survival time (MST) than that of the PB type (105.8 months vs. 41.8 months). Non-curative resection (16.8 months vs. 58.2 months, p=0.002), presence of lymph node metastasis (39.2 months vs. 64.6 months, p=0.018), perineural invasion (37.7 months vs. 97.5 months, p=0.003), and lymphovascular invasion (38.9 months vs. 63.8 months, p=0.004) showed shorter MST. However, in the multivariate analysis, only curative resection was significantly associated with survival (hazard ratio, 0.417; 95% CI, 0.219-0.792, p=0.008). Other factors, such as tumor location, histologic subtype, T stage, N stage, and lymph node metastasis were not significant. ( Table 3 ).
Table 3.
Multivariate Cox proportional hazard regression analysis for histopathologic prognostic factors.
| variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Subgroup | MST (mo) | p value | HR | 95% CI | p value | |
| Elevation of preoperative serum CEA | Yes | 21.2 | 0.024 | 1.286 | 0.585-2.828 | 0.897 |
| No | 60.0 | |||||
| Curative resection | R0 | 58.2 | 0.002 | 0.417 | 0.219-0.792 | 0.008 |
| R1,R2 | 16.8 | |||||
| Histologic type | I type | 105.8 | 0.003 | 0.402 | 0.231-1.801 | 0.333 |
| PB type | 41.8 | |||||
| Lymph node metastasis | Yes | 39.2 | 0.018 | 0.575 | 0.280-1.180 | 0.297 |
| No | 64.6 | |||||
| Perineural invasion | Yes | 37.7 | 0.003 | 0.545 | 0.197-1.508 | 0.519 |
| No | 97.5 | |||||
| Lymphovascular invasion | Yes | 38.9 | 0.004 | 0.854 | 0.425-1.715 | 0.978 |
| No | 63.8 | |||||
4. Discussion
Despite the same anatomical location, periampullary cancers show variable prognoses and responses to adjuvant therapy after tumor resection. Histologic subtyping of periampullary cancers into intestinal and PB types is emerging as an alternative to the current classification of anatomic location. This is because histologic subtyping is thought to have the potential to predict the biological characteristics and prognosis of cancer better than the conventional classification based on the anatomical origin of the tumor. Furthermore, some studies have shown that adjuvant systemic therapy improves overall survival in patients with the PB type, mimicking pancreatic ductal adenocarcinoma, whereas the intestinal type does not benefit from the same regimen (20, 21). Therefore, we hypothesized that histopathologic tumor characteristics and long-term survival after curative–intended pancreatoduodenectomies for patients with PB type cancer might be similar to pancreatic ductal adenocarcinoma and have a worse prognosis than the intestinal type.
In the present study, we found that intestinal-type cancers had lower pathological aggressiveness and better prognoses than the PB type. In contrast, the PB type showed clinicopathological features and prognosis similar to those of pancreatic ductal adenocarcinoma. The PB type had significantly more aggressive histology, nodal metastasis, perineural invasion, lymphovascular invasion, advanced T stage, and systemic metastasis than the intestinal type while the PB type and pancreatic ductal adenocarcinoma showed similar aggressive pathological features and poor overall survival. These results further strengthen the consistency of clinicopathological features of histological phenotypes and the possibility of histological classification as a prognostic factor for periampullary cancers rather than anatomic location. A meta-analysis of 37 studies on histological subtype demonstrated similar results to our study (22). PB type had significantly higher rates of advanced T stage, lymph node metastasis, poor tumor differentiation, lymphovascular invasion, perineural invasion, and worse survival than intestinal type. Although most of the studies included in this meta-analysis were focused on ampullary cancer, recently, intestinal type, not only in ampullary cancers but also in pancreatic ductal adenocarcinoma, has shown significantly better survival than PB type (9–12). This had not been demonstrated before due to the relatively rare occurrence of intestinal type in pancreatic ductal adenocarcinoma. Specifically, Westgaard, et al. demonstrated that long-term survival curves of intestinal type of four periampullary tumors, including pancreatic ductal adenocarcinoma, were very similar regardless of tumor location when adjusted for tumor diameter and nodal status (11). Therefore, in cases of periampullary cancer, we suggest determining the histological subtype routinely, in addition to the classification of anatomical location. In most cases, the histological phenotype can be determined by hematoxylin and eosin (H&E) staining alone and may provide more information about adjuvant treatment and prognosis. For morphologically challenging cases, addition of small battery of immunohistochemical markers can improve accuracy (15, 23). A recently published meta-analysis also recommended routine histopathological subtype classification, even in a clinical setting without immunohistochemistry, because the histopathological subtype is a major prognostic factor (22).
Our study also showed that systemic metastasis were more common in the PB type than in the intestinal type. For patients with recurrence, especially those with systemic recurrence, adjuvant chemotherapy significantly improved survival (median survival, 25.9 mo vs. 6.6 mo, p<0.001). Furthermore, for patients with PB type with recurrence, adjuvant chemotherapy significantly improved patient survival (median survival, 30.9 mo vs. 6.6 mo, p<0.001). Therefore, adjuvant chemotherapy should be considered more actively for PB type cancer. Due to the low number of patients per chemotherapeutic regimen in our study, we were unable to determine if there are differences in patient survival based on specific chemotherapeutic regimens. To date, only a few randomized clinical trials have been performed to demonstrate the role of adjuvant chemotherapy after the resection of periampullary adenocarcinoma, excluding pancreatic ductal adenocarcinoma, because the incidence of each tumor is still low (24, 25).. However, several recent studies have shown that gemcitabine-based adjuvant chemotherapy is associated with prolonged overall survival in the PB type of ampullary cancer but not in the intestinal type (26, 27). There has been no study yet comparing the effects of adjuvant chemotherapy between intestinal type and PB type in pancreatic ductal adenocarcinoma. Future studies should evaluate the validity of stratification by histologic type of periampullary cancers in adjuvant treatment. Therefore, when selecting an adjuvant chemotherapy regimen, the histological subtype should be considered.
An additional issue with histologic type of these cancers is effectiveness of metastatectomy in intestinal type. Given the intestinal type is associated with less aggressive histolopathologic feature, a prior study showed that resection of periampullary adenocarcinoma liver metastasis provides survival benefit only in intestinal type. Considering the benefit of hepatic metastatectomy for the colon cancer and similarity of intestinal type to colon cancer, further study on metastatectomy in intestinal type should be performed near future.
This study had some limitations. First, it was a retrospective, single-center study, leading to a possible selection bias. Second, the sample size may have been too small and unbalance of sample size between PB type and intestinal type may have been too big to show statistical significance or draw definitive conclusions. However, because periampullary cancers are uncommon malignancies and prospective studies are difficult to conduct, a well-organized retrospective study would be a reasonable alternative.
5. Conclusions
Although on multivariate analysis, histologic subtype was not an independent prognostic factor because of the small number of included patients, histologic phenotypes, including intestinal and PB types, showed the possibility of being prognostic factors in periampullary cancer. Because of the profound differences in clinical biology and prognosis, the histological classification of periampullary cancer into PB and intestinal types should be encouraged to predict prognosis and choose optimal adjuvant treatment. Further large-scale studies are required to demonstrate the prognostic significance of the histologic phenotype.
Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to database complexity and local storage guideline.
Ethics statement
The studies involving humans were approved by Chung-Ang University Hospital Institutional Review Board (IRB No. 1961-003-365). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because of the retrospective nature of the study and the patients have already passed.
Author contributions
H-SK: Conceptualization, Data curation, Formal Analysis, Writing – original draft. C-MH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. Y-SC: Methodology, Software, Validation, Writing – review & editing. S-WS: Methodology, Resources, Software, Visualization, Writing – review & editing. SL: Conceptualization, Formal Analysis, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumors of the Digestive System. 4th ed. Lyon, France: International Agency forResearch in Cancer; (2010). [Google Scholar]
- 2. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; (2010). [Google Scholar]
- 3. Frierson HF, Jr. The gross anatomy and histology of the gallbladder, extrahepatic bile ducts, Vaterian system, and minor papilla. Am J Surg Pathol. (1989) 13:146–62. doi: 10.1097/00000478-198902000-00008 [DOI] [PubMed] [Google Scholar]
- 4. Van Geenen RC, Van Gulik TM, Offerhaus GJ, De Wit LT, Busch OR, Obertop H, et al. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. (2001) 27:549–57. doi: 10.1053/ejso.2001.1162 [DOI] [PubMed] [Google Scholar]
- 5. Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. (2012) 29:127–41. doi: 10.1053/j.semdp.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 6. Adsay V, Ohike N, Tajiri T, Kim GE, Krasinskas A, Balci S, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. (2012) 36:1592–608. doi: 10.1097/PAS.0b013e31826399d8 [DOI] [PubMed] [Google Scholar]
- 7. Williams JL, Chan CK, Toste PA, Elliott IA, Vasquez CR, Sunjaya DB, et al. Association of histopathologic phenotype of periampullary adenocarcinomas with survival. JAMA Surg. (2017) 152:82–8. doi: 10.1001/jamasurg.2016.3466 [DOI] [PubMed] [Google Scholar]
- 8. Kimura W, Futakawa N, Yamagata S, Wada Y, Kuroda A, Muto T, et al. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. (1994) 85:161–6. doi: 10.1111/j.1349-7006.1994.tb02077.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albores-Saavedra J, Simpson K, Dancer YJ, Hruban R. Intestinal type adenocarcinoma: a previously unrecognized histologic variant of ductal carcinoma of the pancreas. Ann Diagn Pathol. (2007) 11:3–9. doi: 10.1016/j.anndiagpath.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 10. Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. (2008) 8:170. doi: 10.1186/1471-2407-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Westgaard A, Pomianowska E, Clausen OPF, Gladhaug IP. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary Malignancies? Ann Surg Oncol. (2013) 20:430–9. doi: 10.1245/s10434-012-2603-0 [DOI] [PubMed] [Google Scholar]
- 12. Bronsert P, Kohler I, Werner M, Makowiec F, Kuesters S, Hoeppner J, et al. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. (2013) 13:428. doi: 10.1186/1471-2407-13-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernández Moro C, Fernandez-Woodbridge A, Alistair D'souza M, Zhang Q, Bozoky B, Kandaswamy SV, et al. Immunohistochemical typing of adenocarcinomas of the pancreatobiliary system improves diagnosis and prognostic stratification. PloS One. (2016) 11:e0166067. doi: 10.1371/journal.pone.0166067. Erratum in: PLoS One. 2017 Jan 26;12(1):e0171283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. (2004) 28:875–82. doi: 10.1097/00000478-200407000-00005 [DOI] [PubMed] [Google Scholar]
- 15. Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. (2013) 31:1348–56. doi: 10.1200/JCO.2012.46.8868 [DOI] [PubMed] [Google Scholar]
- 16. De Paiva Haddad LB, Patzina RA, Penteado S, Montagnini AL, Da Cunha JE, MaChado MC, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg. (2010) 14:719–28. doi: 10.1007/s11605-010-1156-4 [DOI] [PubMed] [Google Scholar]
- 17. Jin Z, Hartgers ML, Sanhueza CT, Shubert CR, Alberts SR, Truty MJ, et al. Prognostic factors and benefits of adjuvant therapy after pancreatoduodenectomy for ampullary adenocarcinoma: Mayo Clinic experience. Eur J Surg Oncol. (2018) 44:677–83. doi: 10.1016/j.ejso.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 18. Al Abbas AI, Falvello V, Zenati M, Mani A, Hogg ME, Zeh HJ, 3rd, et al. Impact of adjuvant chemotherapy regimen on survival outcomes in immunohistochemical subtypes of ampullary carcinoma. J Surg Oncol. (2020) 121:322–9. doi: 10.1002/jso.25808 [DOI] [PubMed] [Google Scholar]
- 19. Albores-Saavedra J, Henson DE, Klimstra DS. Malignant epithelial tumors of the ampulla. In: Tumors of the gallbladder,extrahepatic bile ducts, and ampulla of Vater. Washington, DC: Armed Forces Institute of Pathology; (2000) p. 259–316. [Google Scholar]
- 20. Schiergens TS, Reu S, Neumann J, Renz BW, Niess H, Boeck S, et al. Histomorphologic and molecular phenotypes predict gemcitabine response and overall survival in adenocarcinoma of the ampulla of Vater. Surgery. (2015) 158:151–61. doi: 10.1016/j.surg.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 21. Bakshi N, Dhawan S, Nundy S, Rao S, Chopra P, Bhalla S. Role of immunohistochemistry in the subtyping of periampullary adenocarcinoma. Int J Surg Pathol. (2019) 27:598–608. doi: 10.1177/1066896919837606 [DOI] [PubMed] [Google Scholar]
- 22. Shin DW, Kim S, Jung K, Jung JH, Kim B, Ahn J, et al. Impact of histopathological type on the prognosis of ampullary carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol. (2023) 49:306–15. doi: 10.1016/j.ejso.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 23. Schueneman A, Goggins M, Ensor J, Saka B, Neishaboori N, Lee S, et al. Validation of histomolecular classification utilizing histological subtype, MUC1, and CDX2 for prognostication of resected ampullary adenocarcinoma. Br J Cancer. (2015) 113:64–8. doi: 10.1038/bjc.2015.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. (1999) 230:776–82. doi: 10.1097/00000658-199912000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. (2012) 308:147–56. doi: 10.1001/jama.2012.7352 [DOI] [PubMed] [Google Scholar]
- 26. Moekotte AL, Malleo G, van Roessel S, Bonds M, Halimi A, Zarantonello L, et al. Gemcitabine-based adjuvant chemotherapy in subtypes of ampullary adenocarcinoma: international propensity score-matched cohort study. Br J Surg. (2020) 107:1171e82.20. doi: 10.1002/bjs.11555 [DOI] [PubMed] [Google Scholar]
- 27. Bolm L, Ohrner K, Nappo G, Rückert F, Zimmermann C, Rau BM, et al. Adjuvant therapy is associated with improved overall survival in patients with pancreatobiliary or mixed subtype ampullary cancer after pancreatoduodenectomy - a multicenter cohort study. Pancreatology. (2020) 20:433e41. doi: 10.1016/j.pan.2020.01.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to database complexity and local storage guideline.


