Abstract
Modulators of cystic fibrosis transmembrane conductance regulator (CFTR) improved cystic fibrosis (CF) patients’ outcome. The elexacaftor/tezacaftor/ivacaftor (ETI) combination was safe and effective improving lung function in patients with different CFTR genotypes, including at least one F508del mutation. However, cases with liver damage were reported. We describe 105 CF patients heterozygous for F508del in trans with another CFTR mutation, treated for 1 year with ETI. We analyzed liver biochemical parameters and cholesterol metabolism, including lathosterol and phytosterols, surrogate markers of cholesterol de-novo synthesis and absorption, respectively. The treatment significantly improved sweat chloride, body mass index and forced expiratory volume in 1 s, whereas it caused a significant increase of total and conjugated bilirubin, ALT and GGT, even if no patients developed CF liver disease. Such alterations were less relevant than those previously observed in ETI-treated F508del homozygous patients. Furthermore, ETI treatment significantly increased serum cholesterol by enhancing its absorption (correlation between serum cholesterol and phytosterols). Whereas, we observed a normalization of de-novo biosynthesis (lathosterol reduction) that was not observed in homozygous patients. These data suggest that the second mutation in trans with the F508del contributes to reduce the liver cholesterol accumulation and thus, the triggering of liver inflammation. However, no differences in the alteration of biochemical indexes were observed between CF patients with and without liver steatosis, and between patients with different mutations in trans with the F508del. Such data suggest to further investigate the effects of ETI therapy on liver function indexes and new predictive biomarkers.
Keywords: Cystic fibrosis, Elexacaftor/tezacaftor/ivacaftor, Liver damage, Cholesterol metabolism
Subject terms: Biomarkers, Diseases, Medical research
Introduction
The morbidity and outcome of patients with cystic fibrosis (CF) significantly improved in the last decade also thanks to modulators which rescue the amount and the activity of cystic fibrosis transmembrane conductance regulator (CFTR) mutated protein. Various combinations of such mutation-targeted drugs can be used and in vitro and ex-vivo models like organoids1 or nasal epithelial cells2,3 help in predicting the responsivity to modulators of patients with different CFTR genotypes. Thus, the number of CF patients that may benefit from molecular drugs increased, including patients with rare4 or one unknown CFTR variants5,6, but also patients with at least one allele bearing the most frequent F508del variant, that impairs CFTR trafficking and processing7.
The combination of elexacaftor/tezacaftor/ivacaftor (ETI) corrects the CFTR misfolding and enhances its activity7,8. Such combination resulted safe9 and effective already after 24 weeks10,11 improving sweat chloride (SC) levels and lung functional and imaging parameters12,13. The treatment with ETI is effective both in CF patients homozygous14 and heterozygous15,16 for the F508del in trans with a minimal function variant16.
Previous studies described only few cases of liver disease that contraindicate the continuation of therapy11,12, but mild cytolysis17,18, hyperbilirubinemia, particularly in patients with Gilbert19, and cases of liver injury were reported20–23. Recently, we studied 64 CF patients homozygous for the F508del variant. One year of treatment with ETI caused the significant increase of serum bilirubin and alanine aminotransferase (ALT) in about 94% and 84% of patients, respectively, although none had values higher than 3 times the upper reference limit, and in none appeared clinical or instrumental evidence of liver disease24. The altered indexes of liver function may be related to the dysfunction of cholesterol metabolism in CF patients with the F508del CFTR genotype. In fact, the impaired absorption of cholesterol25,26 causes the increase of de-novo synthesis of cholesterol in the liver. Such synthesis does not correct hypocholesterolemia because the F508del mutant CFTR impairs the blood release of cholesterol causing its accumulation in the liver and the subsequent liver inflammation, as we observed in the mouse model of CF27.
Thus, we studied 105 patients with CF followed in two CF Regional Centre (i.e., Campania and Tuscany regions), all with a F508del in trans with a second different CFTR variant, treated for 1 year with ETI. The aim was to evaluate the impact of the treatment on liver biochemical parameters and on the metabolism of cholesterol, and to verify whether the effects of ETI would be related to the CFTR genotype.
Results
As shown in Table 1, the values of BMI and ppFEV1 significantly enhanced after 1 year of treatment with ETI as compared to baseline values. Serum ALT, total and conjugated bilirubin and GGT were significantly enhanced after 1 year of treatment, while serum AP levels were not significantly modified. Furthermore, serum total cholesterol (as like as LDL cholesterol) were significantly enhanced after 1 year of treatment, while HDL cholesterol as like as triglycerides were not significantly modified. Serum lathosterol was significantly reduced, while cholestanol and phytosterols serum levels were not modified; these latter values were significantly related to those of serum total cholesterol.
Table 1.
Anthropometric and biochemical parameters in 105 CF patients heterozygous for the F508del and another CFTR mutation at baseline and after 1 year of ETI therapy.
| Baseline | 1 year of ETI | p | |
|---|---|---|---|
| Body mass index (kg/m2) | 21.0 (19.9–22.6) | 23.0 (21.0–24.6) | < 0.001 |
| FEV 1 (%) | 58.5 (37.0–80.0) | 74.0 (50.3–93.0) | < 0.001 |
| Liver parameters | |||
| ALT (U/L) | 20 (12–28) | 24 (18–35) | < 0.001 |
| Total bilirubin (mg/dL) | 0.50 (0.31–0.67) | 0.74 (0.59–1.10) | < 0.001 |
| Conjugated bilirubin (mg/dL) | 0.20 (0.15–0.30) | 0.30 (0.2–0.4) | < 0.001 |
| GGT (U/L) | 15 (10–13) | 16 (13–23) | 0.028 |
| Alkaline phosphatase (U/L) | 95 (74–125) | 101 (81–126) | 0.15 |
| Lipid metabolism | |||
| Total cholesterol (mg/dL) | 140 (119–158) | 156 (138–178) | < 0.001 |
| LDL cholesterol (mg/dL) | 74 (53–90) | 88 (74–103) | < 0.001 |
| HDL cholesterol (mg/dL) | 53 (41–67) | 51 (44–65) | 0.087 |
| Triglycerides (mg/dL) | 76 (62–92) | 75 (61–103) | 0.63 |
| Cholestanol (mg/dL) | 0.33 (0.24–0.41) | 0.36 (0.28–0.42) | 0.09 |
| Phytosterols (mg/dL) | 0.40 (0.27–0.64) | 0.45 (0.32–0.59) | 0.36 |
| Lathosterol (mg/dL) | 0.37 (0.27–0.48) | 0.30 (0.22–0.41) | 0.01 |
Data are reported as median (interquartile range).
FEV forced expiratory volume, ALT alanine aminotransferase, GGT gamma-glutamyltransferase, LDL low-density lipoprotein, HDL high-density lipoprotein.
As shown in Table 2, comparing the data at baseline and after 1 year of therapy with ETI in patients which had a second variant of class I–III and those that had a second variant of class IV–VI, the values of SC at baseline were significantly higher in patients with a class I–III variant; the treatment caused a significant reduction of SC in both the subgroups with no significant difference of the percent differences (delta) between them. The values of ppFEV1 did not significantly differ between the two subgroups at baseline; the treatment caused a significant increase of ppFEV1 in both the subgroups, with a significant, more relevant increase of the delta in patients with a class I–III variant. Both serum total bilirubin and ALT did not differ between the two subgroups of CF patients at baseline; both the values significantly enhanced after the treatment with no significant differences of the delta between the two subgroups. Furthermore, the values of SC were significantly higher at baseline in patients with steatosis at baseline as compared to those free from steatosis; the treatment caused a significant reduction of SC in both the subgroups with no significant difference of the delta between them. The values of ppFEV1 did not significantly differ between the two subgroups at baseline; the treatment caused a significant increase of ppFEV1 in both the subgroups, with a significantly higher difference in patients with liver steatosis. Both serum total bilirubin and ALT did not differ between the two subgroups of CF patients at baseline; both the values significantly enhanced after the treatment with no significant differences of the delta between the two subgroups of CF patients.
Table 2.
Sweat chloride, FEV1, serum total bilirubin and ALT in subgroups of patients with CF at baseline and after 1 year treatment with ETI.
| Patient subgruops | Sweat chloride (mEq/L) | FEV1 | Total Bilirubin (mg/dL) | ALT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 1 year of ETI | p | Delta (% of baseline) | Baseline | After 1 year of ETI | p | Delta (% of baseline) | Baseline | After 1 year of ETI | p | Delta (% of baseline) | Baseline | After 1 year of ETI | p | Delta (% of baseline) | |
| F508del/class I-III mutation (n = 74) | 94 (74–111) | 48 (36–65) | < 0.001 | 54 (64–35) | 56 (36–78) | 71 (49–91) | < 0.001 | 27 (11–45) | 0.49 (0.31–0.60) | 0.70 (0.55–1.13) | < 0.001 | 50 (24–100) | 22 (13–29) | 26 (18–39) | < 0.001 | 29 (10–81) |
| F508del/class IV-VI mutation (n = 22) | 57 (30–76) | 32 (15–39) | 0.028 | 59 (67–47) | 63 (46–89) | 82 (65–96) | < 0.001 | 13 (5–22) | 0.60 (0.40–0.79) | 0.89 (0.67–1.10) | 0.008 | 42 (20–84) | 13 (11–24) | 23 (19–27) | 0.021 | 85 (14–151) |
| p | < 0.001 | 0.021 | 0.582 | 0.089 | 0.197 | 0.019 | 0.072 | 0.227 | 0.635 | 0.159 | 0.613 | 0.065 | ||||
| Liver steatosis (n = 34) | 95 (74–111) | 41 (36–54) | < 0.001 | 57 (63–45) | 60 (36–76) | 79 (59–91) | < 0.001 | 26 (15–60) | 0.50 (0.31–0.70) | 0.70 (0.57–1.10) | < 0.001 | 50 (25–90) | 24 (13–35) | 30 (20–47) | 0.005 | 32 (10–100) |
| No liver steatosis (n = 71) | 74 (64–99) | 48 (29–64) | < 0.001 | 52 (71–39) | 56 (37–81) | 72 (50–95) | < 0.001 | 17 (7–38) | 0.50 (0.30–0.62) | 0.76 (0.60–1.11) | < 0.001 | 57 (25–115) | 18 (12–26) | 23 (18–32) | < 0.001 | 33 (6–100) |
| p | 0.049 | 0.688 | 0.548 | 0.619 | 0.772 | 0.047 | 0.701 | 0.853 | 0.602 | 0.255 | 0.068 | 0.709 | ||||
Table 3 shows the number and percentage of different subgroups of CF patients heterozygous for F508del in trans with another CFTR variant, that showed an increase of serum total bilirubin and ALT after the treatment. Such data were compared with those obtained in 63 patients homozygous for F508del variant treated with ETI for 1 year, previously described by our group24. As it is shown in the Table 3, for each subgroup of patients heterozygous for the F508del variant the percentage of cases in which serum total bilirubin or ALT were increased after 1 year of treatment was significantly lower as compared to the same percentage obtained in patients homozygous for the F508del variant after 1 year of treatment24. The lone exception was the percentage of cases with increased serum ALT among patients heterozygous for the F508del and a class IV–VI variant.
Table 3.
Number and percentage of CF patients heterozygous for the F508del and another mutation which showed an increase of serum total bilirubin and ALT after 1 year of ETI treatment in comparison with patients homozygous for the F508del mutation previously studied.
| Patients | n | Increased total bilirubin | Chi square | Increased ALT | Chi square | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| All heterozygous for the F508del and another CFTR mutation | 105 | 82 | 78.1 | 0.008 | 66 | 62.9 | 0.003 |
| Heterozygous for the F508del and a class I–III CFTR mutation | 74 | 59 | 71.7 | 0.019 | 45 | 60.8 | 0.002 |
| Heterozygous for the F508del and a class IV–VI CFTR mutation | 22 | 16 | 72.7 | 0.009 | 16 | 72.7 | 0.239 |
| With liver steatosis | 34 | 27 | 79.4 | 0.035 | 19 | 55.9 | 0.002 |
| With no liver steatosis | 71 | 55 | 77.5 | 0.009 | 46 | 64.8 | 0.011 |
| F508del homozygous24 | 63 | 59 | 93.6 | – | 53 | 84.1 | – |
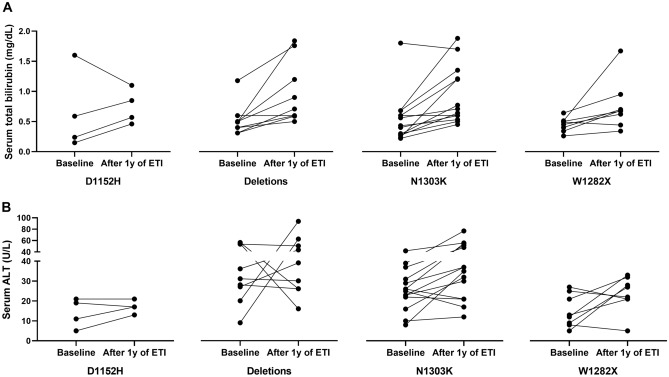
Finally, Fig. 1 shows the trend of serum values of total bilirubin (panel A) and ALT (panel B) at baseline and after 1 year of treatment with ETI in CF patients with different variants. Among the 4 patients with the D1152H variant in trans with the F508del, 3 out of 4 showed an increase of serum total bilirubin and 2 out of 4 showed an increase of ALT. Similarly, 8 out of 9 patients with a large CFTR gene deletion, showed an increase of serum total bilirubin and 4 out of 9 an increase of ALT. Such figures were 11 out of 14 for both serum total bilirubin and ALT in patients with the N1303K variant, and finally, 7 out of 8 and 5 out of 8, respectively, for patients with the W1282X variant.
Figure 1.
serum levels of total bilirubin (A) and ALT (B) in CF patients with different CFTR variants in trans with the F508del before and after 1 year of treatment with ETI.
Discussion
The data from the present study were obtained from 105 CF patients heterozygous for the F508del in trans with another CFTR variant followed in two different regional Centre, treated for 1 year with ETI. The treatment resulted safe, and no cases of interruption were recorded. Furthermore, SC was significantly reduced, while both BMI and ppFEV1 significantly improved. Such results did not differ significantly in the subgroups of patients of our study, i.e., patients with a class I–III or a class IV–VI CFTR variant in trans with the F508del and patients with and without steatosis before the treatment. On the other hand, all previous studies9–13,15, including our recent report on 63 patients homozygous for the F508del variant24 concord on the relevant clinical impact of ETI therapy on SC, BMI, and pulmonary function.
However, the ETI therapy seems to worsen the biochemical parameters of liver metabolism, although none had values higher than 3 times the upper reference limit, and none developed CFLD. In fact, 1 year of treatment with ETI caused the enhancement of both total and conjugated serum bilirubin, that depends on the bile loss at intestinal level and on the enhanced reabsorption of conjugated bilirubin typical of CF or, more likely, on the inhibition of OATP1B1 and 1B3 by ETI19. Furthermore, we observed enhanced liver cytolysis with the significant increase of ALT. While, differently from the results obtained on F508del homozygous patients24, in the present study we lack to observe the increase of serum AP and cholestanol after 1 year of ETI treatment, thus excluding the involvement of the intrahepatic biliary three.
Nevertheless, the percentage of patients with the increase of serum bilirubin or serum ALT is significantly lower as compared to that obtained among patients homozygous for the F508del variant24 and the percentage of increase of such serum parameters after the treatment is lower, suggesting a less severe impact of the therapy on liver function. We postulated that the alteration of liver biochemical parameters observed in CF patients homozygous for the F508del variant would depend on the enhanced de-novo biosynthesis24 and the subsequent intracellular accumulation of cholesterol at endosome level27, as consequence of the misassembled F508del CFTR protein28. The accumulation of liver cholesterol triggers inflammation as we demonstrated in the mice model of CF27 and may contribute to worsen liver function. The data of the present study on patients heterozygous for the F508del variant reinforce such hypothesis. In fact, we observed both the alteration of liver biochemical parameters, and the increase of serum cholesterol after ETI treatment, although less relevant of those obtained in F508del homozygous patients. Differently from patients homozygous for the F508del variant, the increase of serum cholesterol after 1 year of treatment that we observed in the present study was due to the enhanced absorption, as confirmed by the significant correlation between serum cholesterol and phytosterols, whereas de novo synthesis was reduced (as confirmed by the significant reduction of serum lathosterol after 1 year of treatment). The patients from the present study have a single F508del allele and the second variant in trans, differently from F508del, does not cause the misassembling of the CFTR protein with the consequences of intracellular trapping of cholesterol and liver inflammation and damage.
However, none of the parameters that we considered in the present study (i.e., the class of the variant in trans with the F508del, the presence of liver steatosis before the treatment and the CFTR variant in trans with the F508del) was useful to predict the patients that would develop the liver biochemical alterations after ETI treatment. Thus, the pathogenic mechanisms that cause the impairment of liver biochemical indexes must be further clarified and predictive biomarkers should be searched.
To conclude: the present study included a large number of CF patients with the F508del in trans with another CFTR variant, recruited and studied independently in two regional CF Centre. The main limit of the study is that the patients performed a single year of treatment with ETI since the use of such combination was recently approved for the treatment of CF patients with these genotypes. We observed an increase of serum bilirubin and ALT in about two thirds of patients, although none had values higher than 3 times the upper reference limit, and none developed CFLD. Although such alterations were less relevant of those previously identified in patients homozygous for the F508del variant, we suggest to further study the molecular impact of the ETI therapy on liver function indexes and to search for predictive biomarkers of such impact.
Methods
Patients
We followed the same criteria and methodology of our previous study on CF patients homozygous for the F508del variant24. The study was approved by the Ethical committee of the CF regional Centre of Tuscany (Ethics Clearance number 63/2023) and of Campania (Ethics Committee number 77/2021). Inclusion criteria included the F508del in trans with and another CFTR variant, and at least 1 year of treatment with ETI. Exclusion criteria included mechanical ventilation, advanced CF liver disease (aCFLD) according to Sellers et al.29, history of transplantation, drug or alcohol abuse in the past year, pregnancy. The study included 105 patients with CF. Of these, 59 were recruited at the regional Centre of Campania (median age 31 years, IQR: 25–42 years, 30 females) and 46 were recruited at the regional Centre of Tuscany (median age 31 years, IQR: 24–45 years, 19 females). The CFTR genotype of each patient was analyzed by CFTR gene sequencing30 and for large gene rearrangements31. Forced expiratory volume in 1 s (ppFEV1), was expressed as the percentage of predicted value for age32. Liver disease was evaluated by clinical, biochemical or ultrasonographic evaluation33 recorded in two consecutive examinations within a 3-month period, in the absence of other causes of congenital or acquired chronic liver disease. Body mass index was evaluated as previously described34.
Biochemical parameters
Serum cholesterol, HDL and LDL cholesterol, triglycerides, total and conjugated bilirubin, alkaline phosphatase (AP), ALT, and gamma-glutamyltransferase (GGT) were evaluated on serum within one hour from the blood sampling by automated analyzers using standard procedures. For all patients, the samples collected at the different times were analyzed in the same laboratory. Serum lathosterol, phytosterols and cholestanol levels were analyzed by gas-chromatography26.
Statistical analysis
Continuous data have been reported as median and interquartile range (IQR). The Shapiro–Wilk test was applied to evaluate the normality of distributions. Comparisons between two groups of independent samples were evaluated by the Mann–Whitney U test. Paired comparisons were carried out by the Wilcoxon test. Categorical data have been reported as frequence (percentage) and compared by chi-square test. Statistical analyses were performed by SPSS (version 29, IBM SPSS Statistics). Graphics were done using GraphPad Prism version 8.0 Software (GraphPad Software, San Diego, CA, USA; https://www.graphpad.com/). P values < 0.05 were considered significant.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical committee of the CF regional Centre of Tuscany (Ethics Clearance number 63/2023) and CF regional Centre of Campania (Ethics Committee number 77/2021). The participants provided their written informed consent to participate in this study.
Abbreviations
- aCFLD
Advanced CFLD
- ALT
Alanine aminostransferase
- AP
Alkaline phosphatase
- BMI
Body mass index
- CF
Cystic fibrosis
- CFLD
CF liver disease
- CFTR
Cystic fibrosis transmembrane conductance regulator
- ETI
Elexacaftor/tezacaftor/ivacaftor
- GGT
Gamma-glutamyltransferase
- IQR
Interquartile range
- ppFEV1
Forced expiratory volume in 1 s
- SC
Sweat chloride
Author contributions
Conceptualization: A.C., V.C., M.G. and V.T. Data curation: A.C., P.I., C.F., V.C., G.C., M.G. and V.T. Formal analysis: A.C., P.I., C.F., G.C. and M.G. Investigation: A.C., P.I., V.C. and V.T. Software: A.C. , P.I., S.B. and G.C. Methodology: S.B., G.C. and M.G. Visualization: A.C., P.I., S.B., C.F., G.C. and M.G. Writing – original draft: A.C., P.I., G.C. and M.G. Writing – review & editing: V.C., F.A. and V.T. Validation: F.A. Supervision: V.C. and V.T.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alice Castaldo and Paola Iacotucci.
References
- 1.Kleinfelder, K. et al. Theratyping of the rare CFTR genotype A559T in rectal organoids and nasal cells reveals a relevant response to Elexacaftor (VX-445) and Tezacaftor (VX-661) combination. Int. J. Mol. Sci.24, 10358 (2023). 10.3390/ijms241210358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato, F. et al. Two CFTR mutations within codon 970 differently impact on the chloride channel functionality. Hum. Mutat.40, 742–748 (2019). 10.1002/humu.23741 [DOI] [PubMed] [Google Scholar]
- 3.Di Lullo, A. M. et al. An “ex vivo model” contributing to the diagnosis and evaluation of new drugs in cystic fibrosis. Acta Otorhinolaryngol. Ital.37, 207–213 (2017). 10.14639/0392-100X-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terlizzi, V. et al. Ex vivo model predicted in vivo efficacy of CFTR modulator therapy in a child with rare genotype. Mol. Genet. Genomic Med.9, e1656 (2021). 10.1002/mgg3.1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comegna, M. et al. Elexacaftor–tezacaftor–ivacaftor therapy for Cystic Fibrosis patients with the F508del/unknown genotype. Antibiotics (Basel)10, 828 (2021). 10.3390/antibiotics10070828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terlizzi, V. et al. Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor therapy in three subjects with the Cystic Fibrosis genotype Phe508del/unknown and advanced lung disease. Genes (Basel)12, 1178 (2021). 10.3390/genes12081178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo, J., Garratt, A. & Hill, A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J. Cyst. Fibros.21, 456–462 (2022). 10.1016/j.jcf.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 8.Bacalhau, M. et al. Elexacaftor–tezacaftor–ivacaftor: A life-changing triple combination of CFTR modulator drugs for Cystic Fibrosis. Pharmaceuticals (Basel)16, 410 (2023). 10.3390/ph16030410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijerman, H. G. M. et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet394, 1940–1948 (2019). 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griese, M. et al. Safety and efficacy of Elexacaftor/Tezacaftor/Ivacaftor for 24 weeks or longer in people with Cystic Fibrosis and one or more F508del alleles: Interim results of an open-label phase 3 clinical trial. Am. J. Respir. Crit. Care Med.203, 381–385 (2021). 10.1164/rccm.202008-3176LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapouni, N., Moustaki, M., Douros, K. & Loukou, I. Efficacy and safety of Elexacaftor–Tezacaftor–Ivacaftor in the treatment of Cystic Fibrosis: A systematic review. Children (Basel)10, 554 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaher, A., ElSaygh, J., Elsori, D., ElSaygh, H. & Sanni, A. A review of trikafta: Triple cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy. Cureus13, e16144 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macconi, L. et al. Early effects of Elexacaftor–Tezacaftor–Ivacaftor therapy on magnetic resonance imaging in patients with Cystic Fibrosis and advanced lung disease. J. Clin. Med.11, 4277 (2022). 10.3390/jcm11154277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnovale, V. et al. Elexacaftor/Tezacaftor/Ivacaftor in patients with Cystic Fibrosis homozygous for the F508del mutation and advanced lung disease: A 48-week observational study. J. Clin. Med.11, 1021 (2022). 10.3390/jcm11041021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton, P. G. et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a single Phe508del allele. N. Engl. J. Med.381, 1809–1819 (2019). 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnovale, V. et al. Cystic Fibrosis patients with F508del/minimal function genotype: Laboratory and nutritional evaluations after one year of Elexacaftor/Tezacaftor/Ivacaftor treatment. J. Clin. Med.11, 6900 (2022). 10.3390/jcm11236900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tewkesbury, D. H., Athwal, V., Bright-Thomas, R. J., Jones, A. M. & Barry, P. J. Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on liver tests at a large single adult cystic fibrosis centre. J. Cyst. Fibros.22, 256–262 (2023). 10.1016/j.jcf.2023.01.007 [DOI] [PubMed] [Google Scholar]
- 18.Wood, M. et al. Incidence of transaminitis in adults with cystic fibrosis taking elexacaftor/tezacaftor/ivacaftor. J. Am. Pharm. Assoc.63, 920–924 (2023). 10.1016/j.japh.2023.02.015 [DOI] [PubMed] [Google Scholar]
- 19.Terlizzi, V. et al. Hyperbilirubinemia and Gilbert’s syndrome in Cystic Fibrosis patients treated with elexacaftor/tezacaftor/ivacaftor. J. Cyst. Fibros.22, 1130–1132 (2023). 10.1016/j.jcf.2023.06.013 [DOI] [PubMed] [Google Scholar]
- 20.Salehi, M., Iqbal, M., Dube, A., AlJoudeh, A. & Edenborough, F. Delayed hepatic necrosis in a cystic fibrosis patient taking Elexacaftor/Tezacaftor/Ivacaftor (Kaftrio). Respir. Med. Case Rep.34, 101553 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stylemans, D., François, S., Vincken, S., Verbanck, S. & Vanderhelst, E. A case of self-limited drug induced liver injury under treatment with elexacaftor/tezacaftor/ivacaftor: When it is worth taking the risk. J. Cyst. Fibros.20, 712–714 (2021). 10.1016/j.jcf.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 22.Lowry, S., Mogayzel, P. J., Oshima, K. & Karnsakul, W. Drug-induced liver injury from elexacaftor/ivacaftor/tezacaftor. J. Cyst. Fibros.21, e99–e101 (2022). 10.1016/j.jcf.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 23.Bower, J. K. et al. Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: Interim results of a long-term registry-based study. J. Cyst. Fibros.22, 730–737 (2023). 10.1016/j.jcf.2023.03.002 [DOI] [PubMed] [Google Scholar]
- 24.Castaldo, A. et al. One year of treatment with elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis homozygous for the F508del mutation causes a significant increase in liver biochemical indexes. Front. Mol. Biosci.10, 1327958 (2024). 10.3389/fmolb.2023.1327958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelzo, M. et al. Reduced absorption and enhanced synthesis of cholesterol in patients with cystic fibrosis: A preliminary study of plasma sterols. Clin. Chem. Lab. Med.54, 1461–1466 (2016). 10.1515/cclm-2015-1151 [DOI] [PubMed] [Google Scholar]
- 26.Gelzo, M. et al. Influence of pancreatic status on circulating plasma sterols in patients with cystic fibrosis. Clin. Chem. Lab. Med.58, 1725–1730 (2020). 10.1515/cclm-2019-1112 [DOI] [PubMed] [Google Scholar]
- 27.Amato, F. et al. Impaired cholesterol metabolism in the mouse model of cystic fibrosis. A preliminary study. PLoS One16, e0245302 (2021). 10.1371/journal.pone.0245302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentzsch, M., Choudhury, A., Chang, X. B., Pagano, R. E. & Riordan, J. R. Misassembled mutant DeltaF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J. Cell Sci.120, 447–455 (2007). 10.1242/jcs.03350 [DOI] [PubMed] [Google Scholar]
- 29.Sellers, Z. M. et al. Cystic fibrosis screening, evaluation and management of hepatobiliary disease consensus recommendations. Hepatology.10.1097/HEP.0000000000000646 (2023). 10.1097/HEP.0000000000000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergougnoux, A. et al. Multicenter validation study for the certification of a CFTR gene scanning method using next generation sequencing technology. Clin. Chem. Lab. Med.56, 1046–1053 (2018). 10.1515/cclm-2017-0553 [DOI] [PubMed] [Google Scholar]
- 31.Tomaiuolo, R. et al. Epidemiology and a novel procedure for large scale analysis of CFTR rearrangements in classic and atypical CF patients: A multicentric Italian study. J. Cyst. Fibros.7, 347–351 (2008). 10.1016/j.jcf.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Terlizzi, V. et al. Clinical expression of cystic fibrosis in a large cohort of Italian siblings. BMC Pulm. Med.18, 196 (2018). 10.1186/s12890-018-0766-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartlett, J. R. et al. Gene Modifier Study Group. Genetic modifiers of liver disease in cystic fibrosis. JAMA302, 1076–1083 (2009). 10.1001/jama.2009.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elce, A. et al. Supervised physical exercise improves clinical, anthropometric and biochemical parameters in adult cystic fibrosis patients: A 2-year evaluation. Clin. Respir. J.12, 2228–2234 (2018). 10.1111/crj.12796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.