Abstract
Preoperatively predicting extensive intraductal component in invasive breast cancer through imaging is crucial for informed decision-making, guiding surgical planning to mitigate risks of incomplete resection or re-operation for positive margins in breast-conserving surgery. This study aimed to characterize intra- and peri-tumor heterogeneity using high-spatial resolution ultrafast DCE-MRI to predict the extensive intraductal component in invasive breast cancer (IBC-EIC) preoperatively. A retrospective analysis included invasive breast cancer patients who underwent preoperative high-spatial resolution ultrafast DCE-MRI, categorized based on intraductal component status (IBC-EIC vs. IBC without EIC). Propensity score matching (PSM) was employed to balance clinicopathological covariates between the groups. Personalized kinetic intra-tumor heterogeneity (ITHkinetic) and peri-tumor heterogeneity (PTHkinetic) scores were quantified using clustered voxels with similar enhancement patterns. An image combined model, incorporating MRI features, ITHkinetic, and PTHkinetic scores, was developed and assessed. Of 368 patients, 26.4% (97/368) had IBC-EIC. PSM yielded well-matched pairs of 97 patients each. After PSM, ITHkinetic and PTHkinetic scores were significantly higher in the IBC-EIC group (ITHkinetic: 0.68 ± 0.23; PTHkinetic: 0.58 ± 0.19) compared to IBC without EIC (ITHkinetic: 0.32 ± 0.25; PTHkinetic: 0.42 ± 0.18; p < 0.001). Before PSM, ITHkinetic (0.71 ± 0.20 vs. 0.49 ± 0.28, p < 0.001) and PTHkinetic (0.61 ± 0.18 vs. 0.50 ± 0.20, p < 0.001) scores remained higher in the IBC-EIC group. The Image Combined Model demonstrated good predictive performance for IBC-EIC, with an AUC of 0.91 (95% CI 0.86–0.95) after PSM and 0.85 (95% CI 0.81–0.90) before PSM. Inclusion of ITHkinetic and PTHkinetic scores significantly improved prediction capability. ITHkinetic and PTHkinetic characterization from high-spatial resolution ultrafast DCE-MRI kinetic curves enhances preoperative prediction of IBC-EIC, offering valuable insights for personalized breast cancer management.
Keywords: Breast neoplasms, Magnetic resonance imaging, Ductal carcinoma in situ
Subject terms: Breast cancer, Cancer imaging
Introduction
Extensive ductal carcinoma in situ (DCIS) or intraductal component (IC) in invasive breast cancer (IBC) (IBC-EIC) is a commonly mentioned risk factor for incomplete resection in breast-conserving surgery (BCS)1. The rates of positive margins2, re-operation3, and local–regional recurrence4 in patients with IBC-EIC were reported to be significantly higher than those observed in IBC without EIC patients. Meanwhile, the concept of optional surgical margins has evolved. Following the recommendation of achieving “no ink on tumor” for women with IBC5 and a 2 mm margin for women with pure DCIS in BCS6, breast surgeons are adjusting their resection margins increasingly close to the presumed border between healthy tissue and the known tumor (index tumor). Therefore, preoperative prediction of IBC-EIC may help surgeons tailor surgical resection margins to avoid re-excision or re-operation for positive margins in BCS.
Compared to the conventional imaging methods (mammography and breast ultrasound), it is well-established that breast MRI enhances the accurate delineation of the true extent of the known cancer7, particularly in cases of DCIS8 and IBC-EIC9. However, routine preoperative MRI for a known breast cancer is still a controversial indication for BCS patients10,11. As indicated by published results12–14, the use of preoperative MRI has shown a controversial impact on surgical outcomes, particularly concerning reoperation rates and mastectomy rates. One possible reason is that the lack of accurate imaging biomarkers for preoperatively identifying IBC-EIC results in a consistently high rate of additional surgery4. Hence, it is necessary to develop innovative MRI sequences capable of accurately predicting IBC-EIC preoperatively14.
Ultrafast DCE-MRI is a novel approach designed to capture kinetic information of tumor with high temporal resolution while maintaining reasonable spatial resolution15. Further, a quantitative analysis of the time–intensity (kinetic) curve derived from Ultrafast DCE-MRI can elucidate the tumor’s vascularity and perfusion status, facilitating the decoding of tumoral heterogeneity in vivo. Previous research has substantiated the efficacy of this approach in offering valuable imaging insights for breast cancer screening16, diagnosis17,18, molecular subtype classification19,20, and therapeutic evaluation21,22. However, the feasibility and capability of intra- and peri-tumor heterogeneity, decoded by quantitative analysis in Ultrafast DCE-MRI, for the prediction of IBC-EIC remains unknown.
In our previous radiomic study, the combined radiomic model, incorporating intra-tumoral and peritumoral features from an early phase of high-resolution Ultrafast DCE-MRI, demonstrated a moderate ability to predict IBC-IC preoperatively, achieving an AUC of 0.8223. In this recent study, our objective is to characterize kinetic intra-tumor heterogeneity (ITHkinetic) and peritumor heterogeneity (PTHkinetic) through quantitative analysis of clustering voxels exhibiting similar enhancement patterns in high-spatial resolution ultrafast DCE-MRI, using all dynamic phase information. Our hypothesis posits that the ITHkinetic and PTHkinetic can serve as promising imaging biomarkers, enhancing the ability to preoperatively discern IBC-EIC.
Materials and methods
We confirm that the Medical Ethics Committee of the Sichuan Cancer Hospital & Institute has granted approval for this study, with the informed consent requirement waived (approval number: SCCHEC2015029). All methods in this study followed approved protocols by The Medical Ethics Committee of the Sichuan Cancer Hospital & Institute in compliance with relevant guidelines and regulations. This study complies with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD)24 and standards for reporting diagnostic accuracy (STARD)25 statements. The checklists are provided in Supplementary Materials.
Patients
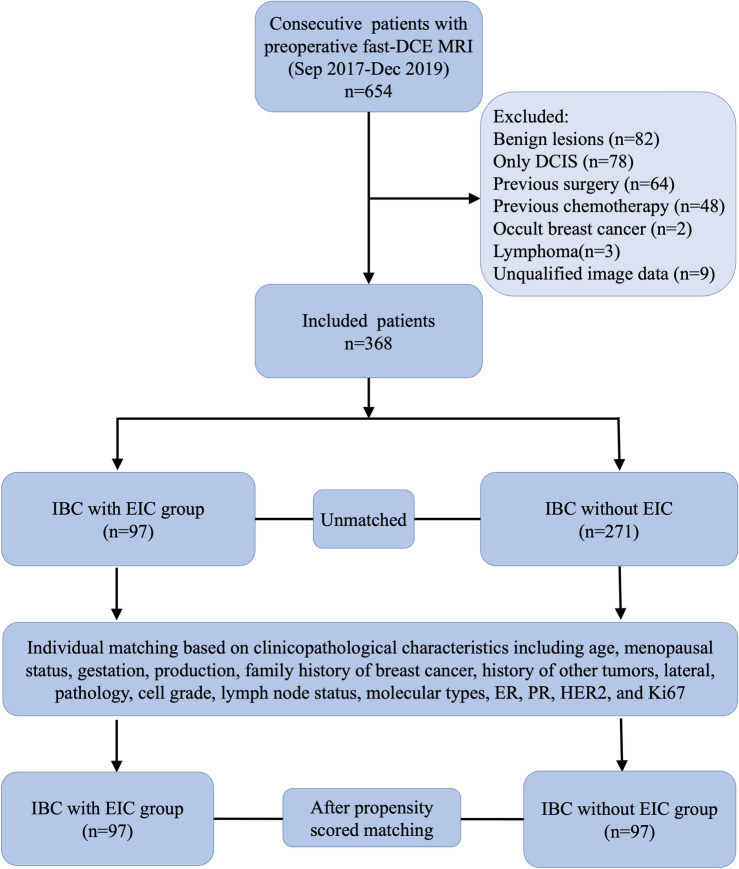
Between September 2017 and December 2019, this study retrospectively enrolled 654 consecutive female patients. The inclusion criteria comprised patients with a breast mass who underwent surgical treatment and received high spatial resolution ultrafast DCE-MRI within 1 week prior to surgery. MRI was conducted for preoperative consultation and surgical planning for these patients. Exclusion criteria included patients with benign lesions (n = 82), only ductal carcinoma in situ (DCIS) (n = 78), prior breast surgery (n = 64), previous neoadjuvant or induction chemotherapy (n = 48), occult breast cancer (n = 2), lymphoma (n = 3), and those lacking qualified image data (n = 9). Finally, 368 patients were retained for further analysis (Fig. 1). Of these, 183 patients overlapped with the sample from our prior study, which focused on radiomic research for a different research aim23.
Figure 1.
Flow diagram of patient with inclusion and exclusion criteria. DCIS ductal carcinoma in situ, IBC invasive breast cancer, EIC extensive intraductal component.
Pathologic analysis
Histopathology and immunohistochemistry data were directly extracted from the surgical pathology report. Two dedicated breast pathologists meticulously reviewed the existence and status of the intraductal component (IC). All cases underwent thorough evaluation to identify the presence of IC in hematoxylin–eosin staining sections. Additional immunohistochemical staining (cytokeratin 5/6 and p63) was performed as necessary. The status of the IC, including fraction and nuclear grade, was quantitatively measured and documented as its ratio to the entire lesion9. The nuclear grade of IC was categorized according to the modified Black nuclear grade system26. The histological grade was categorized according to the Nottingham grading system27. Estrogen receptor (ER) and progesterone receptor (PR) positivity was defined as the presence of 1% or more positively stained nuclei in 10 high-power fields. Human epidermal growth factor receptor 2 (HER2) positivity was defined as immunohistochemistry score of 3+ or 2+ with in situ hybridization amplification28.
In this study, IBC-EIC is defined as the presence of prominent IC within the confines of the invasive tumor, occupying at least 25% of the tumor, or the presence of IC in the grossly normal adjacent breast tissue. Additionally, it includes lesions predominantly composed of DCIS with one or more foci of invasive carcinoma1,4. In contrast, IBC without EIC is defined as invasive cancer lacking an EIC component.
MRI protocol
All eligible patients underwent MRI on the 3.0 T scanner (Skyra, Siemens Healthcare) with 16-channel breast coil. Axial T2WI images were acquired using turbo-inversion recovery-magnitude sequences. Gadodiamide (0.1 mmol/kg; Omniscan, GE Healthcare) was intravenously administered via a power injector at 2.5 mL/s, followed by a saline flush of 20 mL at the same rate. Axial T1-weighted DCE-MRI images were acquired through high-resolution ultrafast protocols using the CAIPIRINHA-Dixon-TWIST-VIBE sequence (Siemens Healthcare)23. The acquisition consisted of 26 phases and lasted for at least 5 min. The specific parameters of these sequences can be referenced in Supplementary Materials.
MRI features interpretation
Two radiologists, L.H.B. (15 years of experience) and C.Z. (5 years of experience), assessed DCE-MRI and T2WI features according to the breast imaging reporting and data system MRI lexicon, second edition, for inter-observer agreement. Sixty patients were randomly selected for intra-observer analysis, re-evaluated by radiologist 1 after a 1 month interval. The early DCE-MRI phase was employed to aid index tumor localization on T2WI. Both radiologists were blinded to pathological information. The detailed MRI features assessed in this study can be found in Table S1, and L.H.B.’s assessments were used for subsequent statistical analyses.
MRI model building
MRI model was constructed through univariate and multivariate analyses of MRI features. Significant predictors (p < 0.05), identified via univariate logistic regression, were integrated into a multivariate logistic regression model with backward stepwise selection, applying the likelihood ratio test with Akaike’s information criterion as the stopping rule.
Kinetic intra- and peri-tumor heterogeneity characterization
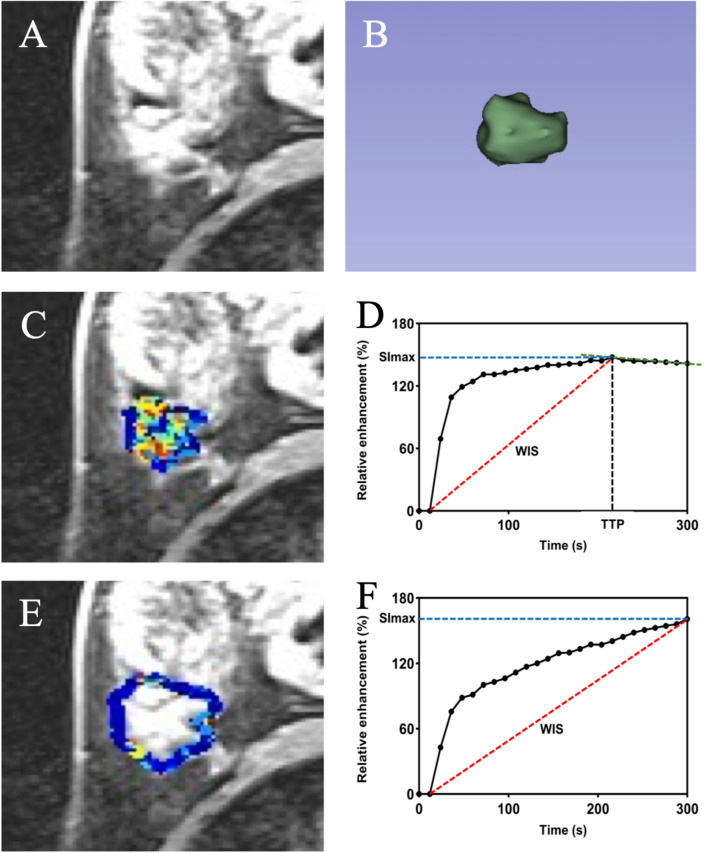
Kinetic intra- and peri-tumor heterogeneity (ITHkinetic and PTHkinetic) characterization was performed by Z.S.X. using MATLAB (R2018b, https://www.mathworks.com) following the process outlined below (Fig. 2):
Figure 2.
illustrates the process of kinetic intra and peri-tumor heterogeneity characterization. (A) Axial last-phase ultrafast T1-enhanced DCE MRI scan. (B) Tumor 3D segmentation display. (C) Intra-tumoral heterogeneity subregion. (D) High-resolution ultrafast DCE-MRI based time-signal intensity curve in the intra-tumor. (E) Peri-tumoral heterogeneity subregion. (F) High-resolution ultrafast DCE-MRI based time-signal intensity curve in the peri-tumor.
Segmentation
The intra-tumoral volume region of interest (VOI) covering the entire MRI-visible tumor (i.e., index tumor) was manually delineated slice by slice in the axial plane using ITK-SNAP (version 3.8.0, http://www.itksnap.org). The largest tumor was selected as the index tumor in cases with multiple lesions. Segmentations were performed by one radiologist (C.Z., with 5 years of experience in breast imaging). To assess the intraclass correlation coefficient, 60 randomly selected cases were segmented again by another radiologist (L.H.B., with 15 years of experience in breast imaging). The peritumoral VOI was obtained by equidistant 3-dimensional dilation of the intra-tumoral regions by 4 mm using MATLAB.
Quantitative kinetic analysis
All dynamic phases of the entire acquisition process were utilized to quantitative kinetic analysis. The signal intensity of all post-contrast phases was normalized to a percentage change relative to the pre-contrast signal intensity. Quantitative kinetic analysis was conducted using a percentage ratio to display changes throughout the entire post-contrast acquisition process in DCE-MRI, relative to the signal intensity before contrast injection. Seven quantitative parameters were derived from the tissue uptake kinetics based on the average voxels of the intra- and peri-tumoral VOIs: time to enhancement (TTE), maximum slope (MS), peak of enhancement (SImax), time to peak intensity (TTP), wash-in slope (WIS), area under the signal intensity curve (AUC), and washout rate (WR)17.
Kinetic heterogeneity characterization
We employed TTP as a criterion for homogeneous voxel clusters29, analyzing the diverse patterns of contrast agent uptake dynamics observed within the VOI. Specifically, we defined the top-K as the set of clustered voxels achieving the fastest peak signal intensity within the first K%, while the opposite was defined as the bottom-K. For both intra and peri-tumor regions, we generated 10 groups, comprising top-10, top-20, …, top-50, and bottom-10, bottom-20, …, bottom-50, respectively. Each group underwent the aforementioned quantitative analysis from intra-tumoral and peritumoral VOIs, to derive ITHkinetic and PTHkinetic parameters.
Personalized ITHkinetic and PTHkinetic score construction
ITHkinetic and PTHkinetic parameters with intraclass correlation coefficient values < 0.75 and Pearson correlation coefficient > 0.80 were excluded. The remaining parameters were standardized using z-scores and normalized via min–max scaling. The most predictive ITHkinetic parameters associated with IBC-EIC were selected using the least absolute shrinkage and selection operator (LASSO) with tenfold cross-validation. Subsequently, a logistic regression model was constructed, and individualized ITHkinetic scores were calculated for each patient based on the model coefficients. A parallel process was applied to PTHkinetic parameters, involving LASSO selection, logistic regression modeling, and computation of PTHkinetic scores for individual patients.
Imaging combined model development
The Imaging Combined model was built by integrating significant MRI features identified in univariable analysis, along with the ITHkinetic and PTHkinetic scores. Initially, our dataset was randomly split into training and validation sets in a 7:3 ratio. We employed various modeling algorithms, including logistic regression, SVM, Random Forest, KNN, Decision Tree, Neural Network, and XGBoost, LightGBM, to construct combined models. The average accuracy of each model on the training set was assessed using five-fold cross-validation. Finally, the modeling algorithm, with highest cross-validation accuracy, was employed to construct the imaging combined model in this study. Variance inflation factor (VIF) analyses were conducted to assess correlation and multicollinearity among the final predictors.
Model evaluation
Discriminatory performance of model was assessed using receiver operating characteristic (ROC) analysis, yielding metrics such as area under curve (AUC), accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) based on the Youden index-derived cut-off value. Box plots were employed to visualize the predictive performance of the ITHkinetic and PTHkinetic scores for IBC-EIC prediction. AUC comparisons between the Imaging Combined model with and without ITHkinetic and PTHkinetic score were conducted using the DeLong test. The incremental value of ITHkinetic and PTHkinetic score was complemented by net reclassification improvement (NRI) and integrated discrimination improvement (IDI) metrics. Calibration plots, Hosmer–Lemeshow test, and decision curve analysis (DCA) were used to assess the calibration and clinical utility of the models with and without the inclusion of the ITH and PTH score. Nomogram was constructed for personalized IBC-EIC prediction.
Patient selection for propensity score matching (PSM)
Patients in the IBC without EIC group were matched with those in the IBC-EIC group based on 15 covariates of clinicopathological characteristics, including age, menstruation status, gestation history, production factors, family history of breast cancer, history of other tumors, lateral involvement, pathology type, cell grade, lymph node status, molecular type, hormone receptor status (ER, PR), HER2 status, and Ki-67 expression.
Statistical analyses
Statistical analyses were performed using R software (version 4.2.3, http://www.r-project.org), Python (version 3.12.3, https://www.python.org), and the scikit-learn library (version 1.5.0). The R packages used in this study are listed in Supplementary Materials. Propensity score matching (PSM) at a 1:1 ratio was employed to address baseline and potential confounding clinicopathological differences between the IBC-EIC and IBC without EIC groups. The standardized mean difference was utilized to assess covariate balance before and after matching. The Mann–Whitney U-test or chi-square test was used to compare the differences in clinicopathological data between two groups. Cohen’s Kappa coefficient was utilized to evaluate inter- and intra-observer agreement of MRI features. A two-tailed p value less than 0.05 was considered statistically significant. The sample size was determined by the availability of patients during the retrospective study period.
Result
Clinicopathological characteristics
Finally, a total of 368 patients (mean age: 50 ± 10 [SD] years) remained, including 26.4% (97/368) IBC-EIC. Table 1 presents the clinicopathological characteristics of two groups before and after PSM. IBC-EIC displayed notable distinctions in baseline clinicopathological features, including a higher rate of family history of breast cancer (p = 0.03), a higher ratio of no special type IBC (p = 0.008), a lower ratio of high cell grade (p = 0.03), distinct molecular types (p < 0.001) and a higher rate of HER2 positivity (p < 0.001). After PSM, 97 matched pairs were successfully established, resulting in balanced clinicopathological covariates, with all standardized mean differences below 0.2, except for cell grade, which was only affected by incomplete pathological data.
Table 1.
Clinicopathologic characteristics.
| Variables | Unmatched patients | Propensity score matched patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IBC without EIC | IBC-EIC | p value | SMD | IBC without EIC | IBC-EIC | p value | SMD | ||
| (n = 271) | (n = 97) | (n = 97) | (n = 97) | ||||||
| Age (median [IQR]) | 49.0 [44.0, 56.0] | 47.0 [42.0, 54.0] | 0.080 | 0.247 | 48.0 [43.0, 53.0] | 47.0 [42.0, 54.0] | 0.665 | 0.097 | |
| Menopausal status | |||||||||
| Postmenopausal | 120 (44.3) | 40 (41.2) | 0.690 | 0.062 | 41 (42.3) | 40 (41.2) | 1.000 | 0.063 | |
| Premenopausal | 151 (55.7) | 57 (58.8) | 56 (57.7) | 57 (58.8) | |||||
| Gestation (median [IQR]) | 3.0 [2.0, 4.0] | 3.0 [2.0, 4.0] | 0.090 | 0.150 | 3.0 [2.0, 4.0] | 3.0 [2.0, 4.0] | 0.760 | 0.02 | |
| Production (median [IQR]) | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.406 | 0.107 | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.496 | 0.11 | |
| Family history of breast cancer | |||||||||
| No | 264 (97.4) | 89 (91.8) | 0.034* | 0.252 | 92 (94.8) | 89 (91.8) | 0.566 | 0.124 | |
| Yes | 7 (2.6) | 8 (8.2) | 5 (5.2) | 8 (8.2) | |||||
| Personal history of other tumors | |||||||||
| No | 239 (88.2) | 90 (92.8) | 0.285 | 0.157 | 91 (93.8) | 90 (92.8) | 1.000 | 0.04 | |
| Yes | 32 (11.8) | 7 (7.2) | 6 (6.2) | 7 (7.2) | |||||
| Lateral | |||||||||
| Left | 138 (50.9) | 47 (48.5) | 0.765 | 0.049 | 44 (45.4) | 47 (48.5) | 0.774 | 0.062 | |
| Right | 133 (49.1) | 50 (51.5) | 53 (54.6) | 50 (51.5) | |||||
| Histologic type | |||||||||
| NST-IBC | 230 (84.9) | 93 (95.9) | 0.008* | 0.380 | 93 (95.9) | 93 (95.9) | 1.000 | < 0.001 | |
| Others | 41 (15.1) | 4 (4.1) | 4 (4.1) | 4 (4.1) | |||||
| Cell grade | |||||||||
| Unknown | 29 (10.7) | 19 (19.6) | 0.03* | 0.309 | 9 (9.3) | 19 (19.6) | 0.121 | 0.297 | |
| I or II | 155 (57.2) | 57 (58.8) | 63 (64.9) | 57 (58.8) | |||||
| III | 87 (32.1) | 21 (21.6) | 25 (25.8) | 21 (21.6) | |||||
| Nuclear grade of IC | |||||||||
| NA or unknown | 217 (80.1) | 3 (3.1) | 0.116 | NA | NA | NA | NA | NA | |
| Non high | 35 (64.8) | 47 (50.0) | NA | NA | NA | NA | |||
| High | 19 (35.2) | 47 (50.0) | NA | NA | NA | NA | |||
| LN status | |||||||||
| Negative | 172 (63.5) | 60 (61.9) | 0.873 | 0.033 | 57 (58.8) | 60 (61.9) | 0.769 | 0.084 | |
| Positive | 99 (36.5) | 37 (38.1) | 40 (41.2) | 37 (38.1) | |||||
| Molecular type | |||||||||
| Luminal A | 53 (19.6) | 19 (19.6) | < 0.001* | 0.566 | 18 (18.6) | 19 (19.6) | 0.879 | 0.115 | |
| Luminal B | 130 (48.0) | 41 (42.3) | 42 (43.3) | 41 (42.3) | |||||
| HER2-enrich | 30 (11.1) | 29 (29.9) | 26 (26.8) | 29 (29.9) | |||||
| TNBC | 58 (21.4) | 8 (8.2) | 11 (11.3) | 8 (8.2) | |||||
| HER2 | |||||||||
| Negative | 212 (78.2) | 49 (50.5) | < 0.001* | 0.605 | 50 (51.5) | 49 (50.5) | 1.000 | 0.021 | |
| Positive | 59 (21.8) | 48 (49.5) | 47 (48.5) | 48 (49.5) | |||||
| ER | |||||||||
| Negative | 88 (32.5) | 37 (38.1) | 0.375 | 0.119 | 37 (38.1) | 37 (38.1) | 1.000 | < 0.001 | |
| Positive | 183 (67.5) | 60 (61.9) | 60 (61.9) | 60 (61.9) | |||||
| PR | |||||||||
| Negative | 102 (37.6) | 42 (43.3) | 0.390 | 0.116 | 43 (44.3) | 42 (43.3) | 1.000 | 0.021 | |
| Positive | 169 (62.4) | 55 (56.7) | 54 (55.7) | 55 (56.7) | |||||
| Ki67 (median [IQR]) | 0.3 [0.1, 0.5] | 0.3 [0.1, 0.4] | 0.293 | 0.161 | 0.3 [0.1, 0.4] | 0.3 [0.1, 0.4] | 0.969 | 0.002 | |
Significant values are given in bold.
Unless specified otherwise, data are presented by the numbers of patients with percentages in parentheses.
IQR interquartile range, IBC invasive breast cancer, EIC extensive intraductal component, NST no special type, LN lymph node, TNBC triple-negative breast cancer, SMD standardized mean difference.
*represent statistical significance.
MRI features differences
In univariate analysis, IBC-EIC showed higher odds of Non-Mass Enhancement (NME) (OR 15.483, CI 6.189–38.734, p < 0.001), peritumoral early NME presence (OR 4.759, CI 2.44–9.28, p < 0.001), non-circumscribed mass margins (OR 2.28, CI 1.268–4.098, p = 0.006), and increased long diameters (OR 2.08, CI 1.459–2.966, p < 0.001). Multivariate analysis identified NME (OR 9.074, CI 3.446–23.899, p < 0.001), the presence of peri-tumoral early NME (OR 3.074, CI 1.449–6.521, p = 0.003), and increased long diameters (OR 1.514, CI 1.001–2.292, p = 0.049) as significant predictors (Table 2). The Inter- and intra-observer agreement of MRI features were provided in Table S2.
Table 2.
MRI features differences in the univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| MRI features | OR | 95% CI | p value | OR | 95% CI | p value |
| BPE (marked vs non marked) | 0.648 | 0.284–1.478 | 0.302 | NA | NA | NA |
| Peritumoral edema (severe vs no or slight) | 1.543 | 0.806–2.956 | 0.191 | NA | NA | NA |
| FGT (density vs non density) | 1.276 | 0.643–2.533 | 0.486 | NA | NA | NA |
| Index tumor type (NME vs mass) | 15.483 | 6.189–38.734 | < 0.001* | 9.074 | 3.446–23.899 | < 0.001* |
| Intra-tumoral T2WI hyperintensity (presence vs absence) | 1.121 | 0.578–2.173 | 0.736 | NA | NA | NA |
| Kinetic delayed phase (III vs I & II) | 0.569 | 0.282–1.149 | 0.116 | NA | NA | NA |
| Kinetic initial phasefast (fast vs non-fast) | 1 | 0.359–2.782 | 1 | NA | NA | NA |
| Long diameter (cm) | 2.08 | 1.459–2.966 | < 0.001* | 1.514 | 1.001–2.292 | 0.049* |
| Mass margin (not circumscribed vs circumscribed) | 2.28 | 1.268–4.098 | 0.006* | 1.449 | 0.717–2.931 | 0.302 |
| Mass shape (irregular vs round or oval) | 1.138 | 0.42–3.083 | 0.8 | NA | NA | NA |
| Internal enhancement (heterogeneous vs homogeneous) | 2.09 | 0.608–7.185 | 0.242 | NA | NA | NA |
| Multi lesion (yes vs no) | 1 | 0.28–3.571 | 1 | NA | NA | NA |
| Peri-tumoral early NME (presence vs absence) | 4.759 | 2.44–9.28 | < 0.001* | 3.074 | 1.449–6.521 | 0.003* |
| Short diameter (cm) | 1.453 | 0.889–2.375 | 0.136 | NA | NA | NA |
Significant values are given in bold.
FGT fibrograndular tissue, BPE background parenchymal enhancement, NME non-mass enhancement.
Personalized ITHkinetic and PTHkinetic score for IBC-EIC prediction
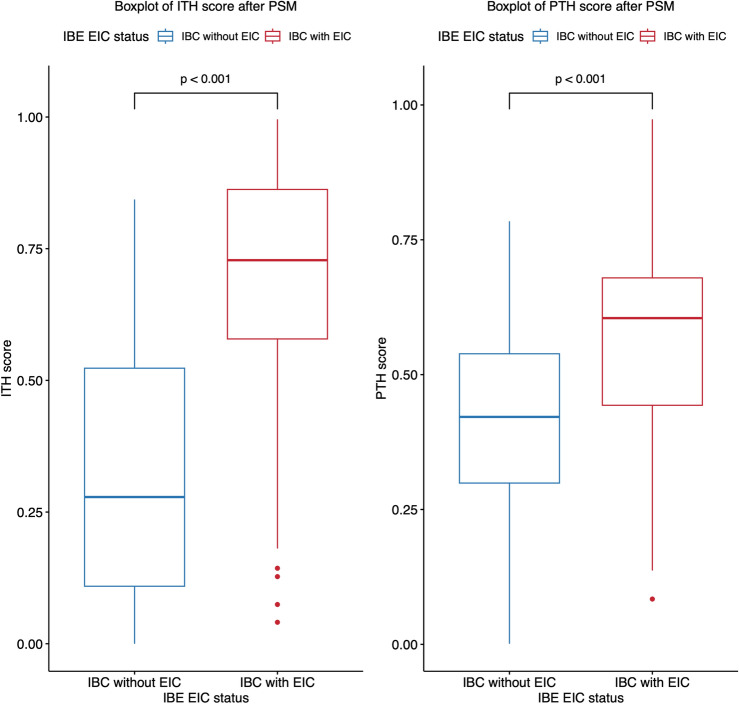
After PSM, the ITHkinetic score demonstrated a higher mean value in the IBC-EIC group (0.68 ± 0.23) compared to the IBC without EIC group (0.32 ± 0.25; p < 0.001) (Youden-cutoff value: 0.56). Similarly, the PTHkinetic score was higher in the IBC-EIC group (mean, 0.58 ± 0.19) compared to the IBC without EIC group (mean, 0.42 ± 0.18; p < 0.001) (Youden-cutoff value: 0.55) (Figs. 3, 4).
Figure 3.
Box plots depicting intra and peri-tumor heterogeneity (ITHkinetic and PTHkinetic) scores after propensity score matching (PSM), stratified by the true extensive intraductal component in invasive breast cancer (IBC-EIC) status. Mean ITHkinetic and PTHkinetic scores were significantly higher in IBC-EIC tumors (red) compared to IBC without EIC tumors (blue) after PSM (p < 0.001 for both comparisons). P values were calculated using the Mann–Whitney test. Horizontal lines in the boxes represent medians, with whiskers indicating minimum and maximum values. Filled circles denote outliers.
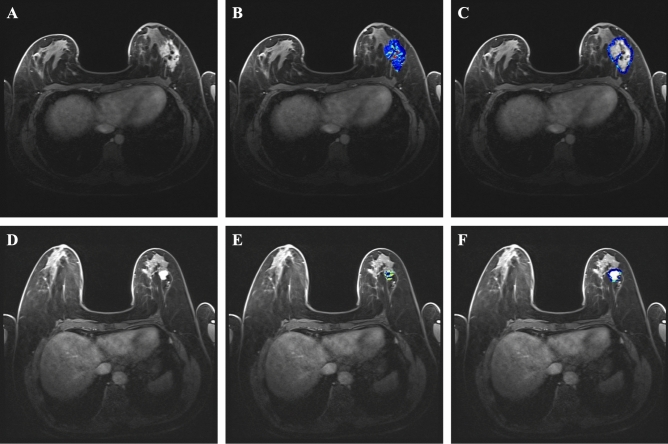
Figure 4.
Axial fat-suppressed DCE MRI and color mapping illustrating kinetic intra- and peri-tumor heterogeneity (ITHkinetic and PTHkinetic) in extensive intraductal component in invasive breast cancer (IBC-EIC) (A–C) and pure invasive breast cancer (D–F). (A–C) In a 28 year-old woman, invasive breast cancer of no special type (HER2-enriched molecular type, HER2 positive) with 50% ductal carcinoma in situ (extensive intraductal component) was confirmed by surgical pathology. (A) Axial fat-suppressed DCE-MRI revealed non-mass enhancement (NME) with a tumor measuring 5.5 cm in length. The intra-tumor (B) and peri-tumor (C) regions were segmented and characterized using color mapping in DCE-MRI. Her personalized ITHkinetic score was 0.89 (Youden-cutoff value: 0.56), and her PTHkinetic score was 0.62 (Youden-cutoff value: 0.55), accurately classifying the tumor. (D–F) In another case, a 68 year-old woman was diagnosed with invasive breast cancer, no special type (luminal B molecular type, HER2 negative), without ductal carcinoma in situ. (D) Axial fat-suppressed DCE MRI revealed a mass measuring 1.7 cm in length in the left breast. The intra-tumor (E) and peri-tumor (F) regions were segmented and characterized using color mapping in the axial DCE MRI. Her personalized ITHkinetic score was 0.12, and her PTHkinetic score was 0.37.
Before PSM, higher ITHkinetic and PTHkinetic scores were also observed in the IBC-EIC group compared to the IBC without EIC group (ITHkinetic: mean, 0.71 ± 0.20 vs. 0.49 ± 0.28, p < 0.001, Youden-cutoff value = 0.62; PTHkinetic: mean, 0.61 ± 0.18 vs. 0.50 ± 0.20, p < 0.001, Youden-cutoff value = 0.52) (Fig. S1).
Model development and evaluation
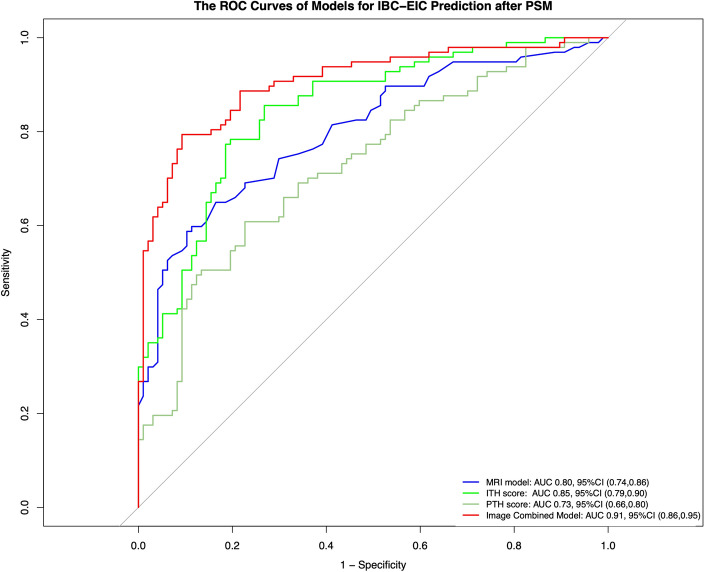
Logistic regression, with highest cross-validation accuracy (Table S3), was employed to construct the image combined model. The performance of image combined model in the training set and test set was visualized in Fig. S2. Finally, four predictive model (MRI model, ITHkinetic score, PTHkinetic score, and image combined model) were developed for IBC-EIC prediction. The performance of these models was presented in Table 3, with visualizations in Fig. 5 after PSM and Fig. S3 before PSM. The image combined models demonstrated good predictive performance for IBC-EIC prediction, achieving AUC values of 0.91 (95% CI 0.86–0.95) after PSM and 0.85 (95% CI 0.81–0.90) before PSM.
Table 3.
Diagnostic performance of models for IBC-EIC prediction in this study.
| Models | AUC (95% CI) | Accuracy | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| After PSM | MRI model | 0.80 (0.74, 0.86) | 0.74 | 0.60 | 0.89 | 0.84 | 0.69 |
| ITHkinetic score | 0.85 (0.79, 0.90) | 0.79 | 0.77 | 0.81 | 0.81 | 0.78 | |
| PTHkinetic score | 0.73 (0.66, 0.80) | 0.69 | 0.61 | 0.77 | 0.73 | 0.66 | |
| Image combined model | 0.91 (0.86, 0.95) | 0.85 | 0.80 | 0.91 | 0.90 | 0.82 | |
| Before PSM | MRI model | 0.80 (0.75, 0.85) | 0.79 | 0.60 | 0.86 | 0.61 | 0.86 |
| ITHkinetic score | 0.73 (0.68, 0.78) | 0.67 | 0.75 | 0.64 | 0.43 | 0.88 | |
| PTHkinetic score | 0.64 (0.58, 0.71) | 0.57 | 0.70 | 0.52 | 0.34 | 0.83 | |
| Image combined model | 0.85 (0.81, 0.90) | 0.83 | 0.74 | 0.86 | 0.66 | 0.90 |
IBC-EIC extensive intraductal component in invasive breast cancer, PSM propensity score matching, AUC area under the curve, PPV positive predictive value, NPV negative predictive value, CI confidence intervals, ITHkinetic kinetic intratumor heterogeneity, PTHkinetic kinetic peritumor heterogeneity.
Figure 5.
Models’ performance for preoperative prediction of extensive intraductal component in invasive breast cancer (IBC-EIC) status after propensity score matching (PSM). The ROC curves of image combined models (red) yielded an AUC of 0.91 (95% CI 0.86–0.95) for preoperative IBC-EIC prediction. The AUCs for the MRI model (blue), kinetic intra-tumor heterogeneity score (green), and kinetic peri-tumor heterogeneity score (light green) were 0.80 (95% CI 0.74–0.86), 0.85 (95% CI 0.79–0.90), and 0.73 (95% CI 0.66–0.80), respectively.
Calibration and clinical use
To facilitate clinical use, nomograms were created using predictors from the image combined model (Fig. 6, Fig. S4). The calibration curves of the nomograms yielded non-significant results (χ2 = 5.25, p = 0.73 after PSM; χ2 = 12.65, p = 0.12 before PSM) (Fig. S5). Decision curve analysis demonstrated that the combined models with ITHkinetic and PTHkinetic score provided greater net benefit in predicting IBC-EIC (Fig. S6).
Figure 6.
Nomogram based on the predictors in the image combined models after propensity score matching (PSM) for preoperative prediction of extensive intraductal component in invasive breast cancer (IBC-EIC). Each variable is assigned a specific point value indicated at the top of the graph, and the total points obtained by summing up the individual variable points are represented by a line connecting the total points to the bottom line. This line provides a preoperative estimate of the probability of IBC-EIC status.
The adding value of ITHkinetic and PTHkinetic score for IBC-EIC prediction
The inclusion of the ITHkinetic and PTHkinetic score significantly improved IBC-EIC prediction, as evidenced by the Delong test (p < 0.001, 95% CI 0.05–0.16), Categorical NRI (0.39, 95% CI 0.24–0.55; p < 0.001), and IDI (0.21, 95% CI 0.15–0.27; p < 0.001) after PSM. These findings were consistent before PSM (Delong test: p = 0.003, 95% CI 0.02–0.08, Categorical NRI: 0.16, 95% CI 0.04–0.28; p < 0.001, and IDI: 0.09, 95% CI 0.06–0.12; p < 0.001).
Discussion
IBC-EIC is a common occurrence in clinical practice, with reported incidence rates ranging from 14.7% to 35%1,30. Accurate preoperative prediction of IBC-EIC is crucial for tailoring surgical resection margins and mitigating the risk of incomplete resection in breast cancer patients undergoing breast-conserving surgery (BCS). Our study characterized the kinetic intra-tumor and peri-tumor heterogeneity (ITHkinetic and PTHkinetic) of breast cancer, through quantitative analysis of clustering voxels exhibiting similar enhancement patterns in high-resolution ultrafast DCE-MRI. To enable a more accurate comparison of ITHkinetic and PTHkinetic differences, we utilized propensity score matching (PSM) to mitigate baseline and potential confounding clinicopathological differences between IBC-EIC and IBC without EIC groups. We found that IBC-EIC demonstrated a distinctive ITHkinetic and PTHkinetic profile, characterized by higher personalized ITHkinetic and PTHkinetic scores both before and after PSM compared to IBC without EIC. Additionally, disparities in MRI features between two groups are also identified. Leveraging these image predictors, our image combined model exhibited good predictive performance, achieving AUC values of 0.91 (after PSM) and 0.85 (before PSM). Importantly, the incorporation of the ITHkinetic and PTHkinetic scores significantly improved predictive performance, as evidenced by improved AUC based on the Delong test (p < 0.05), and further confirmed by statistical significance in categorical NRI (p < 0.001) and IDI (p < 0.001) analyses.
Our findings align with prior studies that have demonstrated the detectability of IBC-EIC through contrast-enhanced MRI1,9,23,30. The preliminary study identified early and overall peritumoral enhancement, the amount of fibroglandular tissue (FGT) around the MRI-visible tumor, and HER2 positivity as predictors of IBC-EIC. The predictive model based on these factors achieved an AUC of 0.791. In our earlier radiomic study, integrating intra-tumoral and peritumoral radiomic features from an early phase of ultrafast DCE-MRI demonstrated a moderate preoperative predictive ability for IBC-IC, with an AUC of 0.8223. Our recent model outperformed the aforementioned studies, highlighting the enhanced value of intra and peri-tumor kinetic heterogeneity, which was consistent with our hypothesis. Notably, our recent predictive model, solely based on preoperative imaging features, may offer superior clinical applicability. Given that the routine utilization of preoperative MRI still shows controversial impacts on the surgical outcomes of BCS, combining these preoperative image models with intra-operative margin assessment might offer an optimal strategy to guide BCS1, potentially enhancing its surgical outcomes in the future.
Our study demonstrated significant variations in ITHkinetic and PTHkinetic between the two groups, unraveling the intricate tumor heterogeneity as potential biomarkers for predicting IBC-EIC. Notably, after PSM, ITHkinetic (AUC 0.85) and PTHkinetic (AUC 0.73) scores demonstrated good diagnostic capabilities for IBC-EIC prediction, while there was a slight decrease in performance before PSM (ITHkinetic 0.73, PTHkinetic 0.64). This might be attributed to the confounding factors, specifically the differences in molecular subtypes and HER2 status before PSM, which may influence the characteristics of tumor heterogeneity. The heterogeneity among different molecular subtypes of breast cancer is widely acknowledged31. Recent studies also revealed significant HER2 heterogeneity in breast cancer32,33. The utilization of PSM allowed us to balance potential confounding clinicopathological covariates, facilitating a more rigorous comparison and identification of imaging disparities between the two groups.
In contrast, the MRI model exhibited robust performance before and after PSM (AUC 0.80 in both instances). The MRI features, such as NME, the presence of peri-tumoral early NME, and increased long diameters, were identified as independent predictors for IBC-EIC prediction in this study. Consistent with our findings, Van Goethem et al. also observed that IBC-EIC exhibited features like ductal or linear enhancement, long spicules, a regional enhancing area, or nodules adjacent to a mass30. Additionally, they also investigated the correlation of NME surrounding index tumor on MRI with pathological examination findings. The results showed that most NME surrounding a carcinoma corresponded to in situ or invasive extension of the carcinoma34. However, relying solely on conventional morphological features on MRI poses limitations in the era of individualized medicine. Visual evaluation of these MRI features introduces potential subjective variability and may be influence by the radiologist’s experience23. The integration of conventional MRI features with personalized tumor heterogeneity scores enhances the comprehensiveness and individualized MRI-based approach for preoperative IBC-EIC prediction.
This study had some limitations. Firstly, as a retrospective study, inherent variations and selection biases may be present. Therefore, a well-designed prospective study is necessary to validate these findings. Secondly, visual evaluation of MRI features may introduce potential variability. We addressed this concern by conducting assessments of both inter- and intra-observer agreement, revealing satisfactory agreement. Lastly, the models were developed using novelty high-resolution ultrafast DCE-MRI protocols on a single MRI scanner within a single center. Consequently, the impact of different MRI scanners on the variance was not investigated. To enhance the generalizability of our findings, further studies involving diverse datasets from various scanners and centers are essential.
In conclusion, our ITHkinetic and PTHkinetic-based model offer a non-invasive and more accurate preoperative prediction of IBC-EIC, providing personalized guidance for patients undergoing BCS.
Supplementary Information
Abbreviations
- IBC-EIC
Extensive intraductal component in invasive breast cancer
- BCS
Breast-conserving surgery
- ITHkinetic
Kinetic intra-tumor heterogeneity
- PTHkinetic
Kinetic peri-tumor heterogeneity
- Ultrafast DCE-MRI
Ultrafast dynamic contrast enhanced magnetic resonance imaging
- PSM
Propensity score matching
- TRIPOD
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis statement
- STARD
Standards for reporting diagnostic accuracy
- VOI
Volume of interest
- TTE
Time to enhancement
- MS
Maximum slope
- SImax
Peak enhancement
- TTP
Time to peak intensity
- WIS
Wash-in slope
- WR
Washout rate
- AUC
Area under curve
- LASSO
Least absolute shrinkage and selection operator
- ROC
Receiver operating characteristic
- PPV
Positive predictive value
- NPV
Negative predictive value
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
- CI
Confidence interval
- OR
Odds ratio
- DCA
Decision curve analysis
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- T2WI
T2-weighted imaging
- NME
Non-mass enhancement
Author contributions
HB L has made substantial contributions to the conception, design, acquisition, analysis, and interpretation of data. He drafted the work, substantively revised it, and prepared the figures. SX Z has made substantial contributions to the design, acquisition, analysis, and interpretation of data. He also drafted part of the work, and prepared the figures. He drafted part of the Supplementary Material. WL Y has made substantial contributions to the conception, design, acquisition of data. Z C has made substantial contributions to the acquisition, analysis, and interpretation of data. YJ L has made substantial contributions to the conception, design, and acquisition of data. P Z has made substantial contributions to the conception, design, and acquisition of data. Additionally, he provided partial funding for this study. All authors read and approved the final manuscript.
Funding
This study received funding from the Joint Funds of the National Natural Science Foundation of China (Grant No. U21A20521) and the Beijing Medical Award Foundation (Grant No. YXJL-2023–0227-0066). The funding bodies played no role in the design of the study, collection, analysis, and interpretation of data, nor in the writing of the manuscript. All decisions regarding the study design, data handling, and manuscript preparation were made by the authors independently.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work: Hongbing Luo and Shixuan Zhao.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68601-6.
References
- 1.Knuttel, F. M. et al. Prediction model for extensive ductal carcinoma in situ around early-stage invasive breast cancer. Investig. Radiol.51(7), 462–468 (2016). 10.1097/RLI.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 2.van Deurzen, C. H. M. Predictors of surgical margin following breast-conserving surgery: A large population-based cohort study. Ann. Surg. Oncol.23(5), 627–633 (2016). 10.1245/s10434-016-5532-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeevan, R. et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ345, e4505. 10.1136/bmj.e4505 (2012). 10.1136/bmj.e4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnitt, S. J. & Harris, J. R. Evolution of breast-conserving therapy for localized breast cancer. J. Clin. Oncol.26(9), 1395–1396. 10.1200/JCO.2007.14.1432 (2008). 10.1200/JCO.2007.14.1432 [DOI] [PubMed] [Google Scholar]
- 5.Moran, M. S. et al. Society of surgical oncology-American society for radiation oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J. Clin. Oncol.32(14), 1507–1515. 10.1200/JCO.2013.53.3935 (2014). 10.1200/JCO.2013.53.3935 [DOI] [PubMed] [Google Scholar]
- 6.Morrow, M. et al. Society of surgical oncology–American society for radiation oncology–American society of clinical oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J. Clin. Oncol.34(33), 4040–4046. 10.1200/JCO.2016.68.3573 (2016). 10.1200/JCO.2016.68.3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg, W. A. et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology233(3), 830–849. 10.1148/radiol.2333031484 (2004). 10.1148/radiol.2333031484 [DOI] [PubMed] [Google Scholar]
- 8.Petrillo, A. et al. Added value of breast MRI for preoperative diagnosis of ductal carcinoma in situ: Diagnostic performance on 362 patients. Clin. Breast Cancer17(3), e127–e134. 10.1016/j.clbc.2016.12.007 (2017). 10.1016/j.clbc.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 9.Kuhl, C. K. et al. Impact of preoperative breast MR imaging and MR-guided surgery on diagnosis and surgical outcome of women with invasive breast cancer with and without DCIS component. Radiology284(3), 645–655. 10.1148/radiol.2017161449 (2017). 10.1148/radiol.2017161449 [DOI] [PubMed] [Google Scholar]
- 10.Mann, R. M., Cho, N. & Moy, L. Breast MRI: State of the art. Radiology292(3), 520–536. 10.1148/radiol.2019182947 (2019). 10.1148/radiol.2019182947 [DOI] [PubMed] [Google Scholar]
- 11.Kuhl, C. K., Lehman, C. & Bedrosian, I. Imaging in locoregional management of breast cancer. J. Clin. Oncol.38(20), 2351–2361. 10.1200/JCO.19.03257 (2020). 10.1200/JCO.19.03257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbull, L. et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: A randomised controlled trial. Lancet375(9714), 563–571 (2010). 10.1016/S0140-6736(09)62070-5 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez, V. et al. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: A prospective, randomized, multicenter study. World J. Surg.38(7), 1685–1693. 10.1007/s00268-014-2605-0 (2014). 10.1007/s00268-014-2605-0 [DOI] [PubMed] [Google Scholar]
- 14.Balleyguier, C. et al. Preoperative breast magnetic resonance imaging in women with local ductal carcinoma in situ to optimize surgical outcomes: Results from the randomized phase III trial IRCIS. J. Clin. Oncol.37(11), 885–892. 10.1200/JCO.18.00595 (2019). 10.1200/JCO.18.00595 [DOI] [PubMed] [Google Scholar]
- 15.Kataoka, M. et al. Ultrafast dynamic contrast-enhanced MRI of the breast: How is it used?. Magn. Reson. Med. Sci.21(1), 83–94. 10.2463/mrms.rev.2021-0157 (2022). 10.2463/mrms.rev.2021-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Zelst, J. C. M. et al. Multireader study on the diagnostic accuracy of ultrafast breast magnetic resonance imaging for breast cancer screening. Investig. Radiol.53(10), 579–586. 10.1097/RLI.0000000000000494 (2018). 10.1097/RLI.0000000000000494 [DOI] [PubMed] [Google Scholar]
- 17.Platel, B., Mus, R., Welte, T., Karssemeijer, N. & Mann, R. Automated characterization of breast lesions imaged with an ultrafast DCE-MR protocol. IEEE Trans. Med. Imaging33(2), 225–232. 10.1109/TMI.2013.2281984 (2014). 10.1109/TMI.2013.2281984 [DOI] [PubMed] [Google Scholar]
- 18.Abe, H. et al. Kinetic analysis of benign and malignant breast lesions with ultrafast dynamic contrast-enhanced MRI: Comparison with standard kinetic assessment. Am. J. Roentgenol.207(5), 1159–1166. 10.2214/AJR.15.15957 (2016). 10.2214/AJR.15.15957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi, A. et al. A multiparametric approach to predict triple-negative breast cancer including parameters derived from ultrafast dynamic contrast-enhanced MRI. Eur. Radiol.33, 8132–8141 (2023). 10.1007/s00330-023-09730-w [DOI] [PubMed] [Google Scholar]
- 20.Luo, H. B. et al. Differentiation between luminal A and B molecular subtypes of breast cancer using pharmacokinetic quantitative parameters with histogram and texture features on preoperative dynamic contrast-enhanced magnetic resonance imaging. Acad. Radiol.27(3), e35–e44. 10.1016/j.acra.2019.05.002 (2020). 10.1016/j.acra.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Ramtohul, T. et al. Prospective evaluation of ultrafast breast MRI for predicting pathologic response after neoadjuvant therapies. Radiology305(3), 565–574. 10.1148/radiol.220389 (2022). 10.1148/radiol.220389 [DOI] [PubMed] [Google Scholar]
- 22.Kataoka, M. Ultrafast DCE-MRI as a new tool for treatment response prediction in neoadjuvant chemotherapy of breast cancer. Diagn. Interv. Imaging104, 565–566 (2023). 10.1016/j.diii.2023.08.005 [DOI] [PubMed] [Google Scholar]
- 23.Xu, H. et al. Intratumoral and peritumoral radiomics based on dynamic contrast-enhanced MRI for preoperative prediction of intraductal component in invasive breast cancer. Eur. Radiol.32(7), 4845–4856. 10.1007/s00330-022-08539-3 (2022). 10.1007/s00330-022-08539-3 [DOI] [PubMed] [Google Scholar]
- 24.Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ350, g7594. 10.1136/bmj.g7594 (2015). 10.1136/bmj.g7594 [DOI] [PubMed] [Google Scholar]
- 25.Bossuyt, P. M. et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Radiology277(3), 826–832. 10.1148/radiol.2015151516 (2015). 10.1148/radiol.2015151516 [DOI] [PubMed] [Google Scholar]
- 26.Schuh, F. et al. Histopathological grading of breast ductal carcinoma in situ: Validation of a web-based survey through intra-observer reproducibility analysis. Diagn. Pathol.10, 93. 10.1186/s13000-015-0320-2 (2015). 10.1186/s13000-015-0320-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elston, C. W. & Ellis, I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology19(5), 403–410. 10.1111/j.1365-2559.1991.tb00229.x (1991). 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 28.Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J. Clin. Oncol.36(20), 2105–2122. 10.1200/JCO.2018.77.8738 (2018). 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 29.Cuenod, C. A. & Balvay, D. Perfusion and vascular permeability: Basic concepts and measurement in DCE-CT and DCE-MRI. Diagn. Interv. Imaging10.1016/j.diii.2013.10.010 (2013). 10.1016/j.diii.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 30.Van Goethem, M. et al. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur. J. Radiol.62(2), 273–282. 10.1016/j.ejrad.2006.12.004 (2007). 10.1016/j.ejrad.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 31.Turner, K. M., Yeo, S. K., Holm, T. M., Shaughnessy, E. & Guan, J. L. Heterogeneity within molecular subtypes of breast cancer. Am. J. Physiol. Cell Physiol.321(2), C343–C354. 10.1152/ajpcell.00109.2021 (2021). 10.1152/ajpcell.00109.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerriero, J. L. et al. Qualification of a multiplexed tissue imaging assay and detection of novel patterns of HER2 heterogeneity in breast cancer. npj Breast Cancer10(1), 2. 10.1038/s41523-023-00605-3 (2024). 10.1038/s41523-023-00605-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenza, C. et al. Targeting HER2 heterogeneity in breast and gastrointestinal cancers. Trends Cancer10.1016/j.trecan.2023.11.001 (2024). 10.1016/j.trecan.2023.11.001 [DOI] [PubMed] [Google Scholar]
- 34.Van Goethem, M. et al. Enhancing area surrounding breast carcinoma on MR mammography: Comparison with pathological examination. Eur. Radiol.14(8), 1363–1370. 10.1007/s00330-004-2295-3 (2004). 10.1007/s00330-004-2295-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.