Abstract
We investigated the association of CA125 response with prognosis and RECIST response/progressive disease (PD) criteria in recurrent high grade serous ovarian cancer (HGSOC) patients treated with a cell cycle checkpoint kinase 1 inhibitor (CHK1i), prexasertib. 81 patients had measurable disease per RECISTv1.1, of which 72 and 70 were measurable by Gynecologic Cancer InterGroup (GCIG) CA125 response and PD criteria, respectively. Univariate and multivariate analyses showed that GCIG CA125 response (n = 32) is associated with improved progression-free survival (PFS) and overall survival (OS) compared to no GCIG CA125 response (n = 40) (median PFS 8.0 vs. 3.5 months [HR: 0.30, 95% CI: 0.18–0.51, p < 0.0001]; median OS 19.8 vs. 10.0 months [HR: 0.38, 95% CI: 0.23–0.64, p < 0.001]) independent of BRCA mutation status, platinum-sensitivity, previous PARP inhibitor therapy, ECOG performance status, and FIGO stage. Notably, GCIG CA125 response had a high negative predictive value (NPV: 93%, 95% CI: 80–98), but poor positive predictive value (PPV: 53%, 95% CI: 35–71) in predicting RECIST response. CA125 PD criteria also showed poor concordance with RECIST PD (PPV 56%, 95% CI: 40–71; NPV 33%, 95% CI: 17–54). Therefore, serum CA125 may be useful as a highly accessible prognostic and predictive biomarker to CHK1i therapy in recurrent HGSOC.
Subject terms: Ovarian cancer, Predictive markers, Cancer, Biomarkers, Medical research, Molecular medicine, Oncology
Introduction
Ovarian cancer is rare, but represents the most lethal gynecologic malignancy in the United States1, of which high grade serous ovarian cancer (HGSOC) is the most common (~ 75%) subtype2. Approximately two-thirds of patients with advanced-stage HGSOC experience relapse despite initial responses to cytoreductive surgery and platinum-based chemotherapy3. The prognosis for patients with recurrent disease remains poor with limited treatment options or readily accessible and reliable biomarkers3. Therefore, new strategies are needed.
One novel therapeutic approach for recurrent HGSOC is targeting cell cycle signaling blockade mediated by ataxia telangiectasia mutated- and Rad3-related (ATR) and cell cycle checkpoint kinase 1 (CHK1)3,4. Several ATR inhibitors such as berzosertib and camonsertib or CHK1 inhibitors (CHK1i) remain under clinical investigation for advanced solid tumors and ovarian carcinoma (e.g. NCT02627443, NCT04497116, NCT02808650)5. Prexasertib (a.k.a. ACR-368, LY2606368), a second generation CHK1i, has previously shown clinical activity and tolerability profile in subsets of relapsed platinum-resistant recurrent HGSOC6–8. Prexasertib is currently under investigation in a multi-center, registration-intent phase II clinical trial (NCT05548296) for patients with recurrent platinum-resistant HGSOC. While ATR/CHK1 pathway targeting therapies are promising, clinical biomarkers coming from a readily attainable source such as blood or urine, are not yet available to predict response or disease progression (PD) while on prexasertib therapy.
Serum cancer antigen 125 (CA125), a secreted form of the transmembrane mucin MUC169, is a highly accessible biomarker which has shown utility for monitoring disease response and predicting long-term prognosis from initial platinum-based chemotherapy in epithelial ovarian cancer10,11. Gynecologic Cancer InterGroup CA125 criteria (GCIG CA125) have been widely used in clinical trials and practice for assessment of tumor response and progression12. However, there remains little evidence regarding its use as a biomarker for disease response and PD for small molecule inhibitors as well as for its relevance for heavily pretreated recurrent HGSOC. Hence, more studies are needed to identify the relationship of CA125 on response to targeted therapies in drug-resistant recurrent ovarian carcinoma.
Here, we performed a pooled analysis using data from 3 recurrent HGSOC cohorts of a single center phase II study of prexasertib (NCT02203513) to identify the association of CA125 response criteria with prognosis. We also compared longitudinal CA125 changes to Response Evaluation Criteria in Solid Tumors (RECIST) outcomes to elucidate the predictive value of CA125 for RECIST response and progression to prexasertib. Lastly, we examined whether there are subgroup differences according to platinum sensitivity, BRCA mutation status, prior use of poly(ADP-ribose) polymerase (PARP) inhibitors, International Federation of Gynecology and Obstetrics (FIGO) stage at diagnosis, and Eastern Cooperative Oncology Group (ECOG) performance status.
Methods
Study population
This post-hoc analysis examined data from an open-label, single-center phase II study (NCT02203513) evaluating women aged 18 years or older with measurable, recurrent high grade serous or high grade endometrioid ovarian carcinoma with no limit on the number of prior therapies. CA125 only disease without RECIST measurable tumors was not eligible. All participants provided written informed consent prior to enrollment. The study was approved by the Institutional Review Board of the Center for Cancer Research (CCR), NCI, USA. All procedures were carried out in accordance with the ethical principles founded in the Declaration of Helsinki and its amendments. The full study methodology for this trial has previously been published in detail6–8. Briefly, patients received intravenous prexasertib 105 mg/m2 once every two weeks until PD, unacceptable toxicity, or patient withdrawal of consent. Imaging for RECIST version 1.1 (v1.1) criteria was obtained at baseline and every two cycles and laboratory assessments including serum CA125 obtained at baseline and prior to administration of each cycle (every 4 weeks). The primary endpoint of the phase II study was investigator-assessed tumor response per protocol based on RECIST v1.1 in evaluable patients.
Evaluation of disease response and progression by RECIST v1.1 and GCIG CA125 criteria
Disease response was determined by investigators using RECIST v1.1, with tumors measured by computed tomography (CT) or magnetic resonance imaging (MRI). Imaging was conducted every two cycles for the first four years, and every three cycles thereafter. Progression-free survival (PFS) was defined as the time from treatment onset until the date of radiographic progression or the treatment discontinuation, whichever occurred earlier. Overall survival (OS) was defined as time from treatment onset until death or censored when data was locked on March 19, 2023.
CA125 was assessed at baseline ≤ 7 days prior to study enrollment and on day 1 of each cycle. CA125 response was defined as > 50% reduction confirmed after 28 days per the GCIG CA125 response criteria12. CA125 PD was defined as greater than two times the upper limit of normal if baseline was normal with PD confirmed after at least 7 days, in accordance with GCIG CA125 PD criteria12. If the baseline CA125 was elevated, PD was defined as greater than two times the upper limit of normal (if the CA125 nadir value was ≤ 16.2 units/mL) or doubling of nadir (if the CA125 nadir was > 16.2 units/mL).
Statistical analysis
All statistical analysis was performed using R Statistical Software v.4.3.2 (2023-10-31 urct) and figures generated in Graphpad Prism version 10.2.0 for MacOS (GraphPad Software, Boston, Massachusetts, USA). The Kaplan–Meier method was used to estimate PFS and OS. Between subgroup differences in PFS and OS were assessed using cox proportional hazards models. Variables used in the multivariate analysis included GCIG CA125 response status, included platinum-sensitivity (platinum-sensitive vs. platinum-resistant), BRCA mutation status (BRCA1/2-mutated vs. BRCA-wildtype), previous PARP inhibitor therapy (yes vs. no), FIGO stage at diagnosis (FIGO stage III vs. FIGO stage IV), and ECOG performance status at study onset (ECOG status 0 vs. 1).
To assess the relationship of GCIG CA125 response with RECIST response, contingency tables were used to calculate the positive predictive value (PPV) and negative predictive value (NPV). PPV is defined as the probability that patients with CA125 response also had RECIST response (either complete response (CR) or partial response (PR) by RECIST v1.1 criteria)13. NPV defined as the probability that patients without CA125 response also did not have RECIST response (either stable disease (SD) or PD by RECIST v1.1)13, was also computed. Differences in overall response rate (ORR) between GCIG CA125 responders and GCIG CA125 non-responders were analyzed using Fisher’s exact test.
The relationship between GCIG CA125 PD and RECIST PD was also assessed for PPV and NPV. If GCIG CA125 PD occurred, patients were assessed to determine whether RECIST PD occurred within one cycle prior to or after GCIG CA125 PD. RECIST PD was included in PPV analysis as positive only if RECIST PD occurred within one cycle prior to after to GCIG CA125 PD. All 95% confidence intervals (95% CI) for PPV and NPV were estimated using the Epi: Statistical Analysis in Epidemiology R package (v2.27.1; 2022).
Results
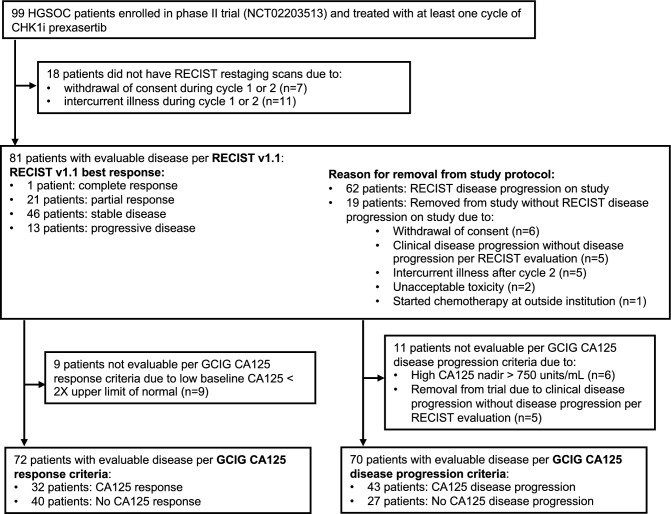
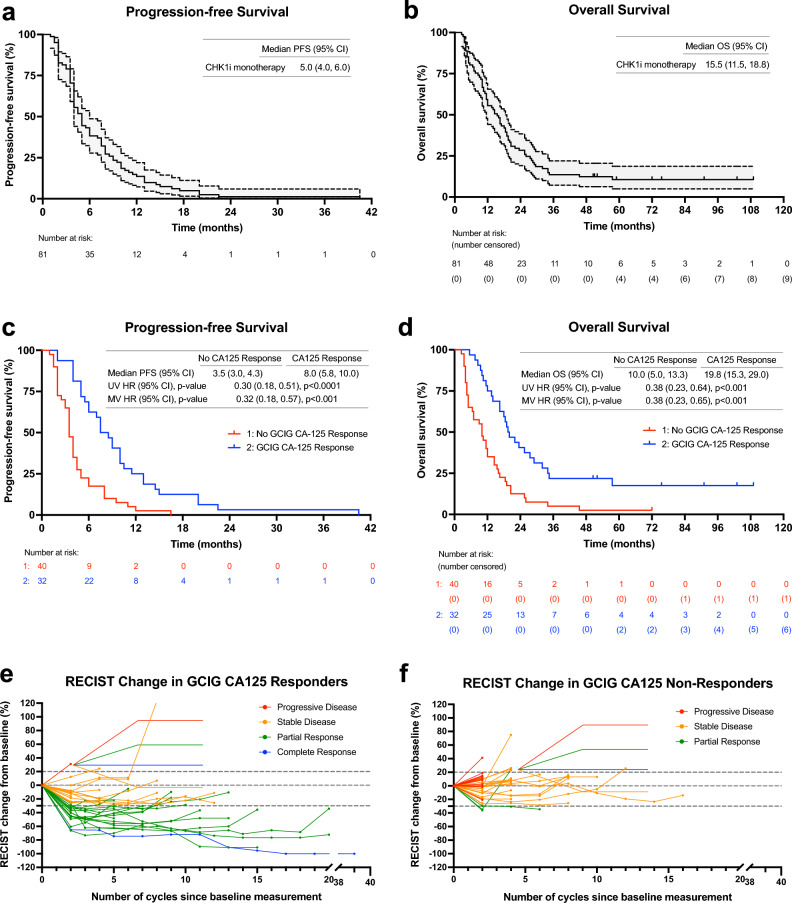
99 patients were enrolled and had baseline CA125 and CT scans. Of these, 18 patients were removed from the study prior to first restaging scans due to intercurrent illness during cycle 1 (n = 11) or withdrawal of consent during cycle 1 (n = 7). Thus, 81 patients had disease evaluable for response and PD by RECIST criteria (Fig. 1). The baseline characteristics of all 81 RECIST eligible participants are described in Table 1. Briefly, all patients were heavily pretreated with a median of 4 prior systemic therapies (interquartile range (IQR): 3 to 6). Most patients (68/81 [84.0%]) had platinum-resistant or platinum-refractory disease, and 18 (22.2%) patients had BRCA1/2 mutations. The median PFS was 5 months (95% CI: 4.0 to 6.0) (Fig. 2a), consistent with previous clinical studies of CHK1i therapy in HGSOC6,8,14. Notably, the median OS in this heavily pre-treated population was 15.5 months (95% CI: 11.5 to 18.8) (Fig. 2b). Across all patients with RECIST measurable disease, 1 patient had a CR lasting 39 months, and 21 patients had a PR, for an ORR (CR + PR) of 27.2% (22/81).
Figure 1.
Study population of HGSOC patients treated with prexasertib. Flow chart shows patients with evaluable disease by RECIST v1.1 and GCIG CA125 criteria. CA125 cancer antigen 125, CHK1i cell cycle checkpoint kinase 1 inhibitor, GCIG Gynecologic Cancer InterGroup, HGSOC high grade serous ovarian cancer, RECIST response evaluation criteria in solid tumors.
Table 1.
Patient characteristics.
| Female sex, N (%) | 81 (100%) |
| Age in years, median (IQR) | 63.2 (56.2, 68.4) |
| Prior number of systemic therapies, median (IQR) | 4 (3, 6) |
| Prior therapy exposure | |
| Prior platinum-based therapy | 81 (100%) |
| Prior PARP inhibitor | 47 (58.0%) |
| ECOG performance status at start of trial, N (%) | |
| 0 | 15 (18.5%) |
| 1 | 66 (81.5%) |
| FIGO stage at diagnosis, N (%) | |
| I or II | 3 (3.70%) |
| III | 60 (74.1%) |
| IV | 18 (22.2%) |
| Race, N (%) | |
| African American/Black | 5 (6.17%) |
| Asian | 2 (2.47%) |
| White | 73 (90.1%) |
| Unknown | 1 (1.23%) |
| BRCA mutation status, N (%) | |
| BRCA-mutant | 18 (22.2%) |
| BRCA-wildtype | 63 (77.8%) |
| Platinum sensitivity, N (%) | |
| Platinum-sensitive | 13 (16.0%) |
| Platinum-resistant | 67 (82.7%) |
| Platinum-refractory | 1 (1.23%) |
| Baseline CA125, median (IQR) | 349 (107, 1500)* |
| Baseline CA125, N (%) | |
| Normal (0–16.3 units/mL) | 3 (3.70%) |
| Abnormal (> 16.3 units/mL) | 78 (96.3%) |
ECOG Eastern Cooperative Oncology Group, FIGO International Federation of Gynecology and Obstetrics, IQR interquartile range, RECIST response evaluation criteria in solid tumors. *Values > 1500 units/mL calculated as 1500 units/mL.
Figure 2.
Kaplan–Meier curves of (a) PFS and (b) OS of all patients treated with prexasertib with evaluable disease by RECIST (95% confidence interval shaded in gray). Censored patients are denoted by tick marks along the survival curves. Kaplan–Meier curves showing (c) PFS and (d) OS in patients separated by Gynecologic Cancer Intergroup (GCIG) CA125 response or no response. Spider plot of RECIST tumor size change from baseline in (e) patients with GCIG CA125 response and (f) patients with no GCIG CA125 response. CA125 cancer antigen 125, CHK1i cell cycle checkpoint kinase 1 inhibitor, GCIG Gynecologic Cancer InterGroup, HR hazard ratio, MV multivariate, OS overall survival, PFS progression-free survival, RECIST response evaluation criteria in solid tumors, UV univariate.
Patients evaluable by both RECIST criteria and GCIG CA125 criteria were included to compare outcomes of each criteria method. For GCIG CA125 response criteria, 72 of 81 RECIST evaluable patients were eligible (8 excluded due to a low CA125 value at baseline [less than 2X the upper limit of normal]) (Fig. 1). 32 had GCIG CA125 response (n = 32/72, 44.4%) and the remaining 40 patients did not have a GCIG CA125 response (n = 40/72, 55.6%). 17 patients had both RECIST and GCIG response (n = 17/72, 23.6%).
Relationship between CA125 response criteria and clinical outcomes
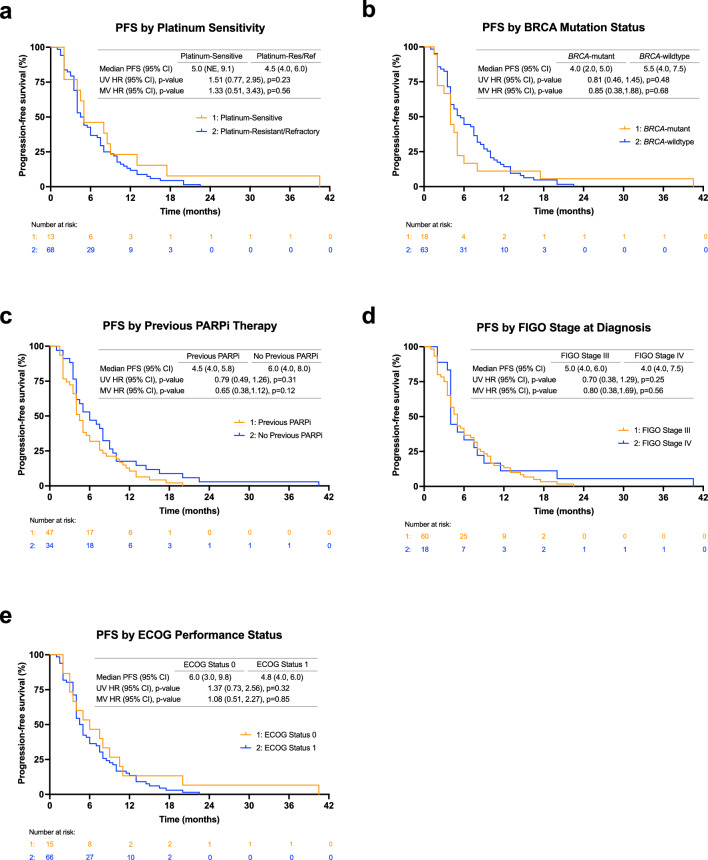
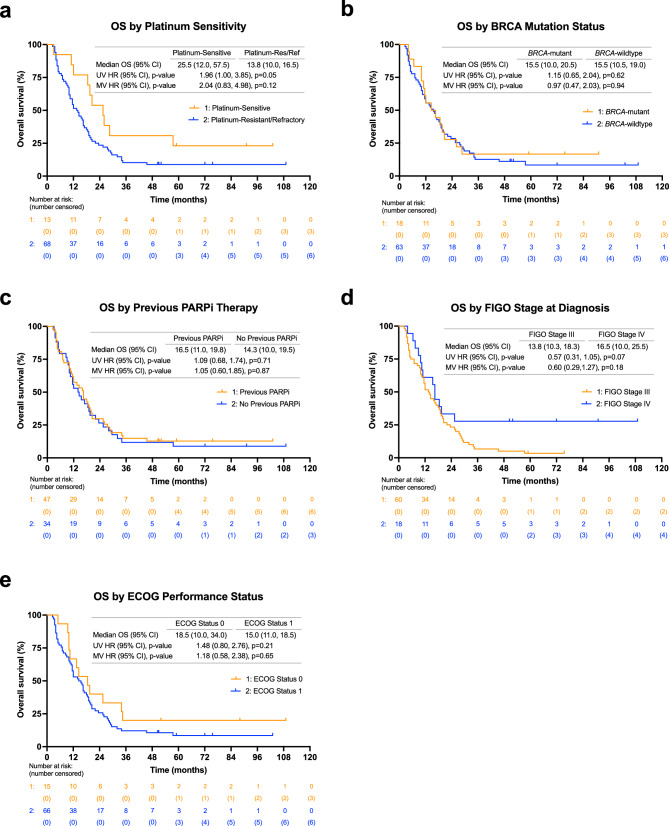
A higher ORR was associated with GCIG CA125 response (53.1%, 17/32), compared to those without GCIG CA125 response (7.5%, 3/40) (p < 0.0001). Additionally, CA125 responders were associated with a higher PFS at 8.0 months compared to CA125 non-responders at 3.5 months (hazard ratio [HR]: 0.30, 95% CI: 0.21–0.56, p < 0.0001) (Fig. 2c). Similarly, a higher OS correlated with CA125 responders at 19.8 months compared to CA125 non-responders at 10.0 months (HR: 0.38, 95% CI: 0.23–0.64, p < 0.001) (Fig. 2d). Multivariate (MV) analysis further demonstrated the relationship of CA125 response with improved PFS and OS (PFS MV HR: 0.32, 95% CI: 0.18–0.57, p < 0.001; OS MV HR: 0.38, 95% CI: 0.23–0.65, p < 0.001). In the post-hoc subgroup analysis, no significant differences were observed in PFS according to platinum-sensitivity, BRCA mutation status, previous PARP inhibitor therapy, FIGO stage at diagnosis, or ECOG performance status (Fig. 3). Likewise, no significant differences were observed in OS in these subgroups (Fig. 4), except for an increasing trend in OS of platinum-sensitive patients compared to platinum-resistant/refractory patients (median OS: 25.5 months vs. 13.8 months, univariate (UV) HR: 1.96, 95% CI: 1.00 to 3.85, p = 0.05) though this trend was not observed in the MV analysis (MV HR: 2.04, 95% CI: 0.83 to 4.98, p = 0.12). All values for UV and MV analysis are additionally shown in Supplementary Table 1.
Figure 3.
PFS by subgroups including (a) platinum sensitivity (b) BRCA mutation status, (c) previous PARP inhibitor (PARPi) therapy exposure, (d) FIGO stage at diagnosis, and (e) ECOG performance status at the start of therapy. ECOG Eastern Cooperative Oncology Group, FIGO International Federation of Gynecology and Obstetrics, HR hazard ratio, MV multivariate, NE not evaluable, PARP poly(ADP-ribose) polymerase, PFS progression-free survival, UV univariate.
Figure 4.
Overall survival by subgroups including (a) platinum sensitivity (b) BRCA mutation status, (c) previous PARP inhibitor (PARPi) therapy exposure, (d) FIGO stage at diagnosis, and (e) ECOG performance status at the start of therapy. ECOG Eastern Cooperative Oncology Group, FIGO International Federation of Gynecology and Obstetrics, HR hazard ratio, MV multivariate, NE not evaluable, PARP poly(ADP-ribose) polymerase, OS overall survival, UV univariate.
Since the scans were scheduled every 2 cycles while CA125 was collected every cycle, we hypothesized that CA125 response may predict early the tumor response measured by RECIST. In patients with CA125 response, response was recorded after 1 cycle of therapy in 27/32 patients (median time to GCIG CA125 response: 34 days, IQR: 29.8 to 39.5). In all 21 patients with RECIST response, response was observed at the first restaging scans (after two cycles of therapy).
Predictive value of CA125 response criteria for RECIST response
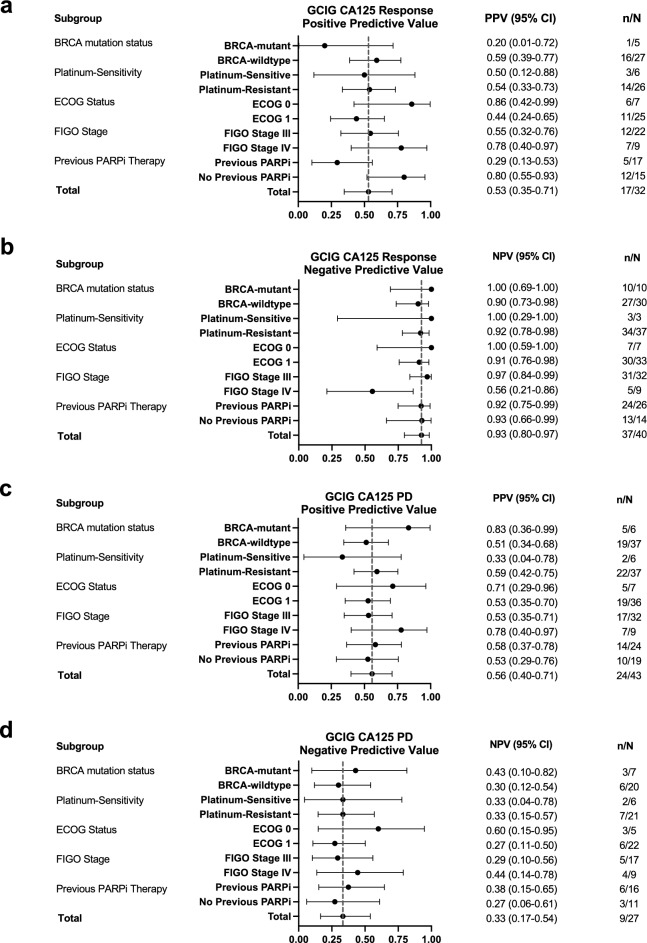
In patients evaluable by both RECIST and CA125 criteria (n = 72), approximately half of patients (n = 32) had a CA125 response. Of these, 17 also had RECIST response (Fig. 2e), resulting in a PPV of 53% (95% CI: 35 to 71%) (Table 2). Of note, while the 15 remaining patients did not meet the RECIST criteria for response, 7/15 had durable SD lasting longer than 6 months (Fig. 2e). Of those without CA125 response (n = 40), 3 had RECIST response and 37 did not (Fig. 2f), resulting in a NPV of 93% (95% CI: 80 to 98%) (Table 2). Additionally, the three patients without CA125 response, but with RECIST response did not achieve long-term responses: one patient had PR on the first staging scans at 2 months, then PD on the subsequent scans at 4 months, one patient also had PR on the first staging scans, then PD at 6 months, and one patient had PR but withdrew consent prior to cycle 4 despite improvement in tumor size (Fig. 2f). No significant differences were observed in PPV and NPV across all subgroups (Fig. 5a,b).
Table 2.
Predictive value of GCIG CA125 response/no response.
| RECIST response | No RECIST response | Total | ||
|---|---|---|---|---|
| GCIG CA125 response | 17 | 15 | 32 |
PPV: 53% 95% CI: 35 to 71 |
| No GCIG CA125 response | 3 | 37 | 40 |
NPV: 93% 95% CI: 80 to 98 |
| Total | 20 | 52 | 72 |
Positive predictive value (PPV) and negative predictive value (NPV) of GCIG CA125 response criteria to predict RECIST response (Best RECIST response of CR/PR) or no RECIST response (Best RECIST response of SD/PD). PPV: probability of patients with CA125 response that also had RECIST response, NPV: probability that those without CA125 response also did not have RECIST response.
CA125 cancer antigen 125, CI confidence interval, GCIG Gynecologic Cancer Intergroup, NPV negative predictive value, PPV positive predictive value, RECIST response evaluation criteria in solid tumors.
Significant values are in bold.
Figure 5.
Forest plots showing (a) positive predictive values (PPV) and (b) negative predictive values (NPV) of Gynecologic Cancer Intergroup (GCIG) CA125 criteria response predicting RECIST response in patient subgroups. n/N refers to the number of (CA125 response and RECIST response)/([CA125 response and RECIST response] + [CA125 response and RECIST non-response]) for PPV and (CA125 non-response and RECIST non-response)/([CA125 non-response and RECIST non-response] + [CA125 non-response and RECIST response]) for NPV. Forest plots showing (c) PPV and (d) NPV of GCIG CA125 criteria disease progression (PD) predicting RECIST PD in patient subgroups. n/N refers to the number of (CA125 PD and RECIST PD)/([CA125 PD and RECIST PD] + [CA125 PD and RECIST non-PD]) for PPV and (CA125 non-PD and RECIST non-PD)/([CA125 non-PD and RECIST non-PD] + [CA125 non-PD and RECIST PD]) for NPV.
Association between CA125 PD and RECIST PD
GCIG CA125 PD was evaluated independently of GCIG CA125 response. For GCIG CA125 PD, 70 of 81 RECIST evaluable patients were evaluable (11 were excluded due to high CA125 nadir > 750 units/mL [n = 6] or clinical PD without progression per RECIST evaluation [n = 5]) (Fig. 1). Out of 70 patients evaluable by RECIST and CA125 PD criteria, 43 patients had CA125 PD (n = 43/70, 54.8%) and 24 of those had RECIST PD occurred within a month before or after CA125 PD (n = 24/70, 38.6%), resulting in a PPV of 56% (95% CI: 40 to 71%) (Table 3). Of the 27 patients without a CA125 PD, 9 patients also did not have a RECIST PD, resulting in a NPV of 33% (95% CI: 17 to 54%) (Table 3). PPV and NPV for PD were not different across the subgroups (Fig. 5c,d).
Table 3.
Predictive value of GCIG CA125 PD/no-PD.
| RECIST PD | No RECIST PD | Total | ||
|---|---|---|---|---|
| GCIG CA125 PD | 24 | 19 | 43 |
PPV: 56% 95% CI: 40 to 71 |
| No GCIG CA125 PD | 18 | 9 | 27 |
NPV: 33% 95% CI: 17 to 54 |
| Total | 42 | 28 | 70 |
Positive predictive value (PPV) and negative predictive value (NPV) of GCIG CA125 disease progression (PD) criteria to predict RECIST PD within one cycle of CA125 PD. PPV: probability of patients with CA125 PD also had RECIST PD, NPV: probability that those without CA125 PD also did not have RECIST PD.
CA125 cancer antigen 125, CI confidence interval, GCIG Gynecologic Cancer Intergroup, NPV negative predictive value, PD disease progression, PPV positive predictive value, RECIST response evaluation criteria in solid tumors.
Significant values are in bold.
Lastly, 24 patients had PD by both CA125 PD criteria and RECIST evaluation, allowing for comparison of when PD occurred by each criteria method. The date of the initially observed CA125 PD tended to occur prior to the date of RECIST PD with a median of 28 days prior to RECIST response (IQR: 0, 87).
Discussion
The clinical application of CA125 as a readily accessible and cost effective biomarker has been studied in predicting response for the chemotherapy in the adjuvant setting9,15,16. However, it is unclear how CA125 changes are reflected in the recurrent disease setting with small molecule inhibitors. In this study, we found that CA125 response was associated with a higher ORR and improved PFS and OS, however, had poor PPV in predicting RECIST response. Indeed, CA125 has a high NPV value for predicting RECIST response, in which patients without a GCIG CA125 response after the first cycle of prexasertib therapy are unlikely to have significant tumor reduction on imaging. It is noteworthy that both CA125 response and PD typically preceded changes on imaging, in part due to the increased frequency of CA125 evaluation compared to RECIST evaluation.
There are limited reports of the predictive value of CA125 response or progression when compared with RECIST tumor changes in relapsed ovarian cancers13,17,18. For instance, CA125 exhibited poor NPV for PD (33%, 95% CI: 29 to 38) in relapsed platinum-sensitive ovarian cancer patients receiving platinum-based combination therapy13. Similarly, CA125 exhibited poor NPV for PD (53%, 95% CI: 49 to 57) in a similar population receiving PARP inhibitor maintenance therapy17. Limited knowledge of CA125 use in newer therapies in the recurrent setting exists. The relationship between CA125 and tumor response has yet to been investigated in clinical studies with cell cycle checkpoint inhibitors14,19,20, and minimal data is available for other targeted therapies. Asad et al. has reported poor concordance between CA125 and RECIST response/PD for the combination of bevacizumab and sorafenib18. Additionally, in response to immune checkpoint inhibition, Boland et al. found that CA125 did not correlate well with tumor response. In their cohort, CA125 tended to increase regardless of whether the patient achieved clinical benefit (73% [11/15] in patients with clinical benefit ≥ 24 weeks vs. 82% [36/44] in patients without clinical benefit, p = 0.48)21 Our findings align with these observations, demonstrating discordance for CA125 in predicting PD during CHK1i prexasertib therapy though CA125 still shows potential for predicting no response to CHK1i.
It is also noteworthy that in real-world clinical practice, monitoring for PD often involves regular physical exams and measurements of CA125 without routine imaging22. Imaging is conducted only in suspected clinical recurrence due to cost and overall lack of data to support routine use, unlike clinical trials that require more frequent scans (e.g. every 2 months)13,17,23. Therefore, cases of recurrent disease where CA125 does not rise in concordance with tumor growth, may result in delayed diagnosis of PD. On the other hand, it is also important to note that any benefit from early detection of PD based on CA125 changes alone is debatable. The MRC OV05/EORTC 55,955 trial investigated early vs. delayed treatment based on CA125 PD in 1442 patients with ovarian cancer in remission with unelevated CA125 values after adjuvant platinum-based chemotherapy24. Interestingly, the authors found no difference in OS or quality of life outcomes, indicating that earlier treatment based on rising CA125 alone without clinical and radiographic changes does not necessarily lead to more favorable outcomes24.
Additionally, since 2011 when the definitions for GCIG CA125 response and PD were established12, new methods of CA125 mathematical modeling have been proposed and introduced into clinical practice25,26. These models have been investigated for platinum-based chemotherapy and PARP inhibition using large datasets, such as the CALPYSO, ARIEL2, and STUDY10 trials25,27,28. Nonetheless, mathematical models of CA125 are not yet available for many small molecule inhibitors, including CHK1i. As such, current use of CA125 in clinical practice for other targeted therapies should rely on readily available measures, such as GCIG CA125 criteria.
Early changes in peripheral blood biomarkers other than CA125, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs) and immune cells, have been investigated in recurrent ovarian cancer as well, although are less accessible in clinical practice. Lee et al. reported ctDNA monitoring of minimal residual disease as a marker for predicting disease recurrence (PPV: 100%, NPV: 96.7%) in 27 patients on PARP inhibitor maintenance therapy29,30. Regarding CHK1i therapy specifically, Lampert et al. reported that an increase in the immunocompetence functional marker HLA-DR on total monocytes during the first 15 days of prexasertib treatment is associated with improved PFS (median PFS: 9.3 vs 3.5 months, p = 0.02)31. Furthermore, Giudice et al. showed that decreases in CTCs collected from peripheral blood samples expressing the epithelial markers EpCAM and MUC1 show improved PFS in recurrent HGSOC (median PFS: 7.5 vs. 4 months; HR: 0.41, 95% CI: 0.20–0.86, p = 0.02)8. However, use of these biomarkers requires further validation and they are not widely available for use in the community setting.
One major limitation of this study includes the small sample size for subgroup analysis. Specifically, low numbers in certain subgroups such as BRCA-mutated and platinum-sensitive patients (n < 20) are present due to the exploratory nature of this study and the trial design focused on heavily pretreated populations, which may have led to finding no differences between subgroups. Additionally, we were unable to account for the site of new or advancing lesions during PD. In Tjokrowidjaja et al. among participants with concordant CA125 and RECIST PD, a greater proportion had peritoneal disease as a site of recurrence (188/355, 53%) than among those with RECIST-only PD (164/454, 36%; p < 0.001)17. However, given the advanced disease in our study population, we were unable to classify patients based on site of disease during PD. A prospective study with a well-powered and more inclusive subgroup analysis would be beneficial to validate our findings.
Conclusions
GCIG CA125 response is an independent predictor of response to the CHK1i prexasertib in HGSOC prior to the first restaging scans and is associated with a higher RECIST response rate and improved PFS and OS. GCIG CA125 response has a high NPV, indicating that patients without a CA125 response after the first cycle of CHK1i therapy are unlikely to see a reduction in tumor burden, which is additionally associated with worse PFS and OS. Therefore, these patients may require a change in treatment. However, GCIG CA125 response has poor PPV for RECIST response, indicating that it is uncertain whether patients with GCIG CA125 response will have response (CR or PR) on CHK1i treatment. When CA125 is used for monitoring progression of disease on CHK1i therapy, GCIG CA125 PD has poor PPV and NPV within a one cycle window, indicating CA125 cannot reliably detect RECIST PD while on CHK1i treatment. These findings need to be validated in large prospective studies.
Supplementary Information
Acknowledgements
This research work was funded by the Intramural Research Program of the Center for CCR, NCI, NIH (grant ZIA BC011525 awarded to J-M.L.). Prexasertib was supplied to the NCI CCR by Eli Lilly under a cooperative research and development agreement. Research support was provided by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from private donors.
Author contributions
Study concept and design: K.R.I and J.-M.L. Acquisition of data: K.R.I. and J.-M.L.; analysis and interpretation of data: K.R.I., D.D, T.M., and J.-M.L.; statistical analysis: K.R.I., D.D., and T.M.; drafting of the manuscript: K.R.I. and J.-M.L.; manuscript review: all authors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
J.-M.L. has research grant funding from AstraZeneca and Acrivon Therapeutics (paid to institution) and is on the Scientific Advisory Board of Acrivon Therapeutics and Genentech (unpaid). The other authors have no competing interests to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68338-2.
References
- 1.Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74(1), 12–49. 10.3322/caac.21820 (2024). 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 2.Matulonis, U. A. et al. Ovarian cancer. Nat. Rev. Dis. Primers.2, 16061. 10.1038/nrdp.2016.61 (2016). 10.1038/nrdp.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinopoulos, P. A. & Matulonis, U. A. Clinical and translational advances in ovarian cancer therapy. Nat. Cancer.4(9), 1239–1257. 10.1038/s43018-023-00617-9 (2023). 10.1038/s43018-023-00617-9 [DOI] [PubMed] [Google Scholar]
- 4.Gupta, N., Huang, T. T., Horibata, S. & Lee, J. M. Cell cycle checkpoints and beyond: Exploiting the ATR/CHK1/WEE1 pathway for the treatment of PARP inhibitor-resistant cancer. Pharmacol. Res.178, 106162. 10.1016/j.phrs.2022.106162 (2022). 10.1016/j.phrs.2022.106162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khamidullina, A. I., Abramenko, Y. E., Bruter, A. V. & Tatarskiy, V. V. Key proteins of replication stress response and cell cycle control as cancer therapy targets. Int. J. Mol. Sci.25(2), 1263. 10.3390/ijms25021263 (2024). 10.3390/ijms25021263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, J. M. et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: A first-in-class proof-of-concept phase 2 study. Lancet Oncol.19(2), 207–215. 10.1016/s1470-2045(18)30009-3 (2018). 10.1016/s1470-2045(18)30009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta, N. et al. BLM overexpression as a predictive biomarker for CHK1 inhibitor response in PARP inhibitor-resistant BRCA-mutant ovarian cancer. Sci. Transl. Med.15(701), eadd7872. 10.1126/scitranslmed.add7872 (2023). 10.1126/scitranslmed.add7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giudice, E. et al. The CHK1 inhibitor prexasertib in BRCA wild-type platinum-resistant recurrent high-grade serous ovarian carcinoma: A phase 2 trial. Nat. Commun.15(1), 2805. 10.1038/s41467-024-47215-6 (2024). 10.1038/s41467-024-47215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charkhchi, P. et al. CA125 and ovarian cancer: A comprehensive review. Cancers (Basel).12(12), 3730. 10.3390/cancers12123730 (2020). 10.3390/cancers12123730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuxen, M. K., Sölétormos, G. & Dombernowsky, P. Serum tumour marker CA 125 in monitoring of ovarian cancer during first-line chemotherapy. Br. J. Cancer.84(10), 1301–1307. 10.1054/bjoc.2001.1787 (2001). 10.1054/bjoc.2001.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markman, M., Federico, M., Liu, P. Y., Hannigan, E. & Alberts, D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol. Oncol.103(1), 195–198. 10.1016/j.ygyno.2006.02.024 (2006). 10.1016/j.ygyno.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 12.Rustin, G. J. et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int. J. Gynecol. Cancer.21(2), 419–423. 10.1097/IGC.0b013e3182070f17 (2011). 10.1097/IGC.0b013e3182070f17 [DOI] [PubMed] [Google Scholar]
- 13.Zebic, D. S. et al. Discordance between GCIG CA-125 progression and RECIST progression in the CALYPSO trial of patients with platinum-sensitive recurrent ovarian cancer. Br. J. Cancer.130(3), 425–433. 10.1038/s41416-023-02528-z (2024). 10.1038/s41416-023-02528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantinopoulos, P. A. et al. A phase 2 study of prexasertib (LY2606368) in platinum resistant or refractory recurrent ovarian cancer. Gynecol. Oncol.167(2), 213–225. 10.1016/j.ygyno.2022.09.019 (2022). 10.1016/j.ygyno.2022.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redman, C. W. et al. Early serum CA125 response and outcome in epithelial ovarian cancer. Eur. J. Cancer.26(5), 593–596. 10.1016/0277-5379(90)90085-8 (1990). 10.1016/0277-5379(90)90085-8 [DOI] [PubMed] [Google Scholar]
- 16.Buller, R. E., Berman, M. L., Bloss, J. D., Manetta, A. & DiSaia, P. J. Serum CA125 regression in epithelial ovarian cancer: Correlation with reassessment findings and survival. Gynecol. Oncol.47(1), 87–92. 10.1016/0090-8258(92)90082-t (1992). 10.1016/0090-8258(92)90082-t [DOI] [PubMed] [Google Scholar]
- 17.Tjokrowidjaja, A. et al. Poor concordance between cancer antigen-125 and RECIST assessment for progression in patients with platinum-sensitive relapsed ovarian cancer on maintenance therapy with a poly(ADP-ribose) polymerase inhibitor. J. Clin. Oncol.42, Jco2301182. 10.1200/jco.23.01182 (2024). 10.1200/jco.23.01182 [DOI] [PubMed] [Google Scholar]
- 18.Azad, N. S. et al. Lack of reliability of CA125 response criteria with anti-VEGF molecularly targeted therapy. Cancer.112(8), 1726–1732. 10.1002/cncr.23374 (2008). 10.1002/cncr.23374 [DOI] [PubMed] [Google Scholar]
- 19.Shah, P. D. et al. Combination ATR and PARP Inhibitor (CAPRI): A phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-resistant epithelial ovarian cancer. Gynecol. Oncol.163(2), 246–253. 10.1016/j.ygyno.2021.08.024 (2021). 10.1016/j.ygyno.2021.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konstantinopoulos, P. A. et al. Randomized phase II study of gemcitabine with or without ATR inhibitor berzosertib in platinum-resistant ovarian cancer: Final overall survival and biomarker analyses. JCO Precis. Oncol.8, e2300635. 10.1200/po.23.00635 (2024). 10.1200/po.23.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boland, J. L. et al. Utility of serum CA-125 monitoring in patients with ovarian cancer undergoing immune checkpoint inhibitor therapy. Gynecol. Oncol.158(2), 303–308. 10.1016/j.ygyno.2020.04.710 (2020). 10.1016/j.ygyno.2020.04.710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombo, N. et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann. Oncol.30(5), 672–705. 10.1093/annonc/mdz062 (2019). 10.1093/annonc/mdz062 [DOI] [PubMed] [Google Scholar]
- 23.Manganaro, L. et al. Imaging strategy in recurrent ovarian cancer: A practical review. Abdom. Radiol. (NY).44(3), 1091–1102. 10.1007/s00261-018-1677-y (2019). 10.1007/s00261-018-1677-y [DOI] [PubMed] [Google Scholar]
- 24.Rustin, G. J. et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet.376(9747), 1155–1163. 10.1016/s0140-6736(10)61268-8 (2010). 10.1016/s0140-6736(10)61268-8 [DOI] [PubMed] [Google Scholar]
- 25.Lauby, A. et al. The increasing prognostic and predictive roles of the tumor primary chemosensitivity assessed by CA-125 elimination rate constant K (KELIM) in ovarian cancer: A narrative review. Cancers (Basel).14(1), 98. 10.3390/cancers14010098 (2021). 10.3390/cancers14010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledermann, J. A. et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol.35(3), 248–266. 10.1016/j.annonc.2023.11.015 (2024). 10.1016/j.annonc.2023.11.015 [DOI] [PubMed] [Google Scholar]
- 27.Colomban, O. et al. Mathematical modeling of the early modeled CA-125 longitudinal kinetics (KELIM-PARP) as a pragmatic indicator of rucaparib efficacy in patients with recurrent ovarian carcinoma in ARIEL2 & STUDY 10. EBioMedicine.89, 104477. 10.1016/j.ebiom.2023.104477 (2023). 10.1016/j.ebiom.2023.104477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You, B. et al. The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: Data from CALYPSO trial (a GINECO-GCIG study). Gynecol. Oncol.130(2), 289–294. 10.1016/j.ygyno.2013.05.013 (2013). 10.1016/j.ygyno.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Lee, D. et al. 1114 Detection of minimal residual disease using circulating tumor DNA in patients treated with long-term PARP inhibitors maintenance for epithelial ovarian cancer. Int. J. Gynecol. Cancer.34(Suppl 1), A402–A402. 10.1136/ijgc-2024-ESGO.787 (2024). 10.1136/ijgc-2024-ESGO.787 [DOI] [Google Scholar]
- 30.Lee, D., Kim, Y.-N., Won, D. & Lee, J.-Y. Monitoring minimal residual disease in ovarian cancer patients undergoing long-term treatment with PARP inhibitors using circulating tumor DNA. Presented at: 2024 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; San Diego, CA, March 16–18 (2024).
- 31.Lampert, E. J. et al. Clinical outcomes of prexasertib monotherapy in recurrent BRCA wild-type high-grade serous ovarian cancer involve innate and adaptive immune responses. J. Immunother. Cancer.8(2), e000516. 10.1136/jitc-2019-000516 (2020). 10.1136/jitc-2019-000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.