Abstract
The attachment glycoprotein G of respiratory syncytial virus (RSV) is produced as both membrane-anchored and secreted forms by infected cells. Immunization with secreted RSV G (Gs) or formalin-inactivated alumprecipitated RSV (FI-RSV) predisposes mice to immune responses involving a Th2 cell phenotype which results in more severe illness and pathology, decreased viral clearance, and increased pulmonary eosinophilia upon subsequent RSV challenge. These responses are associated with increased interleukin-4 (IL-4) production in FI-RSV-primed mice, and the responses are IL-4 dependent. RNase protection assays demonstrated that similar levels of IL-4 mRNA were induced after RSV challenge in mice primed with vaccinia virus expressing Gs (vvGs) or a construct expressing only membrane-anchored G (vvGr). However, upon RSV challenge, vvGs-primed mice produced significantly greater levels of IL-5 and IL-13 mRNA and protein than vvGr-primed mice. Administration of neutralizing anti-IL-4 antibody 11.B11 during vaccinia virus priming did not alter the levels of vvGs-induced IL-5, IL-13, pulmonary eosinophilia, illness, or RSV titers upon RSV challenge, although immunoglobulin G (IgG) isotype profiles revealed that more IgG2a was produced. vvGs-priming of IL-4-deficient mice demonstrated that G-induced airway eosinophilia was not dependent on IL-4. In contrast, airway eosinophilia induced by FI-RSV priming was significantly reduced in IL-4-deficient mice. Thus we conclude that, in contrast to FI-RSV, the secreted form of RSV G can directly induce IL-5 and IL-13, producing pulmonary eosinophilia and enhanced illness in RSV-challenged mice by an IL-4-independent mechanism.
Eosinophil recruitment and activation are promoted by a number of factors, including interleukin-5 (IL-5), IL-4, IL-8, eotaxin, RANTES, mast cell products histamine and tryptase, and leukotriene B4, with IL-5 and eotaxin being highly specific for eosinophils (10, 22, 29, 32, 46, 50, 60). Eosinophilia is generally considered to be a component of the type 2 immune response since it occurs in conjunction with IL-4-mediated events. Classically, type 1 CD4+ T cells (Th1) secrete IL-2 and gamma interferon (IFN-γ), but little IL-4 or IL-5, upon activation (18, 54). Conversely, Th2 CD4+ T cells secrete IL-4, IL-5, IL-10, and IL-13 but no IFN-γ. A similar classification system has recently been proposed for CD8+ cytotoxic T cells (Tc1 and Tc2) (63). Selective induction of either Th1 or Th2 CD4+ (or Tc1 or Tc2 CD8+) T cells has been correlated with more favorable outcomes after infection with a variety of pathogens (16, 20, 43, 53, 57), thus associating the Th1-Th2 paradigm with microbe-induced disease pathogenesis. Type 2 CD4+ and CD8+ T cells often produce both IL-4 and IL-5, suggesting coordinate regulation of these two genes (16, 18, 40, 43, 54, 80). A critical role for IL-4 in the differentiation of Th2 cells has been shown in helminth-infected mice (38, 76) and in allergen-sensitized mice (11). These data suggest a close regulation of IL-4 and IL-5, which may be explained by the presence of shared transcriptional elements in both promoters (40, 44). Thus, stimulatory signals and factors may induce transcription of both genes. However, some elements controlling transcription of IL-4 and IL-5 are distinct and, in some cases, have been shown to be selectively induced (31, 38, 45, 76).
Respiratory syncytial virus (RSV) is a major cause of respiratory disease in infants (39, 62) and the elderly (14, 17). Disease severity following RSV infection may be correlated with cytokine production by various cellular populations (23) with more severe disease resulting from induction of Th2 T-cell responses. Detailed studies in BALB/c mice have demonstrated that intranasal infection with RSV produces mild disease and mild-to-moderate pathology characterized by lymphocytic infiltrates, predominantly composed of Th1 CD4+ T cells and CD8+ cytotoxic T lymphocytes (CTLs) and without eosinophils (24, 26). However, BALB/c mice immunized with formalin-inactivated RSV (FI-RSV) develop severe disease, which is mediated by Th2 CD4+ T cells, as demonstrated by increased production of IL-4, IL-5, and IL-13 and eosinophilia upon infection with live RSV (25, 51, 73, 74, 78). IL-4 has been shown to have a crucial regulatory role in effecting these immune responses to produce enhanced disease. Neutralizing anti-IL-4 antibody administration during FI-RSV immunization results in diminished levels of illness, viral titers, and histopathology following challenge with live RSV (71). This is associated with a shift of the immune responses induced during priming from a Th2-like profile to a more Th1-like profile with decreased IL-4 mRNA (relative to IFN-γ) and increased levels of RSV-specific antibodies having an immunoglobulin G2a (IgG2a) isotype. However, the Th1-Th2 paradigm does not fully explain the pathogenesis of RSV disease profiles. Administration of recombinant IL-12 during FI-RSV priming results in decreased IL-4 production and increased titers of IgG2a RSV-specific antibodies; yet illness following RSV challenge is not reduced (72).
Two viral glycoproteins are expressed on the surface of infected cells: the fusion (F) protein and the attachment (G) protein. RSV G-induced immune responses have been proposed as an explanation for severe illness both in primary infection and in vaccine-enhanced illness based on the Th2-like phenotype of these immune responses. While T-cell clones specific for RSV F exhibit a Th1-like phenotype, producing IFN-γ and little IL-4 or IL-5, G-specific clones generally have a Th2-like phenotype (2, 4, 35, 68). Transfer of these clones into naive mice demonstrates that F-specific clones protect against infection with minimal disease, while transfer of G-specific cells results in severe disease and eosinophilia (1, 3). RSV-specific CD8+ CTLs have recently been shown to regulate induction of eosinophil-recruiting CD4+ T cells (33, 69). While RSV F and matrix (M2) proteins induce vigorous CTL responses, RSV G does not (52). However, when a defined CTL epitope of M2 is inserted into G, an M2-specific CTL response is generated (69). IL-4, IL-5, and pulmonary eosinophilia are reduced in mice immunized with the G-M2 recombinant virus and challenged with RSV, suggesting that CD8+ T cells (potentially by IFN-γ secretion) alter differentiation of G-specific CD4+ T cells to a more Th1-like phenotype. Mapping studies have demonstrated that specific epitopes of RSV G may be associated with protection against infection and with induction of eosinophilia (65, 67). These data have led to the hypothesis that the primary or secondary protein structure of RSV G is responsible for the induction of immune responses producing severe disease following subsequent live virus infection.
RSV G is produced by infected cells in both a membrane-anchored and a secreted form due to the presence of an alternative initiation codon in the transmembrane region (59). We and others have shown that it is not merely the primary or secondary structure of RSV G, but also the form of protein available for initial antigen presentation, that determines the pattern of subsequent immune response and disease expression (7, 36). Mice immunized with recombinant vaccinia viruses expressing membrane-anchored (retained) RSV G (vvGr) or secreted RSV G (vvGs) exhibit more severe disease and more extensive histopathology following RSV challenge when primed with vvGs. Since vvGs and FI-RSV immunizations result in similar disease profiles upon RSV infection, and since disease severity is reduced by anti-IL-4 treatment during FI-RSV priming, we sought to examine the role of IL-4 in regulating the induction of immune responses during priming with either vvGr or vvGs. We postulated that inhibition of IL-4 during vvGs priming would decrease the severity of illness and pathology observed following RSV challenge. Surprisingly, in this paper we show that G induces a Th2-like immune response including increased IL-5 and IL-13 production and eosinophil recruitment that is independent of IL-4.
MATERIALS AND METHODS
Mice.
Pathogen-free 8-week-old BALB/c mice were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.). IL-4-deficient mice (IL-4−/−) were generated by targeted gene disruption of the IL-4 gene as described by Kopf et al. (38) on a C57BL/6 genetic background and were a gift of O. Kanagawa (Washington University, St. Louis, Mo.). These mice have been shown to be unable to produce Th2-associated cytokines upon initial exposure to antigen and do not class switch to IgE isotype antibodies (38). As controls for experiments using IL-4−/− mice, 8-week-old pathogen-free C57BL/6 mice were purchased from Harlan Sprague Dawley, Inc. All mice were housed in a barrier facility throughout the experiment.
Cell lines, antibodies, and virus stocks.
HEp-2 and BSC40 cell lines were maintained in Eagle’s minimal essential medium (EMEM) supplemented with 10% fetal calf serum (10% EMEM). The stock cultures were screened at quarterly intervals for mycoplasma contamination by PCR analysis (American Type Culture Collection, Rockville, Md.). Neutralizing antibody 11.B11, specific for murine IL-4, was kindly provided by the Biological Response Modifiers Program, National Cancer Institute (Frederick, Md.).
A stock of RSV (A2 strain) was prepared in HEp2 cells and stored at −70°C as previously described (26). FI-RSV was prepared as described elsewhere (25). Concurrently, supernatant from uninfected HEp2 cells was similarly treated with formalin and alum precipitated to produce a mock immunization stock.
A panel of recombinant vaccinia viruses that express various RSV proteins was constructed (5, 59). The vvGwt, vvGr, and vvGs viruses (previously designated vvWT G, vvM48I, and vvM48, respectively) were kindly provided by Gail W. Wertz (University of Alabama at Birmingham, Birmingham, Ala.). The vac-lac virus (VSC8), a gift of Bernard Moss (National Institutes of Health [NIH], Bethesda, Md.), has β-galactosidase inserted into the same HindIII F site used for construction of G recombinants (9). Viral stocks were grown and purified by density gradient centrifugation on potassium tartrate gradients as previously described (36).
Mouse priming and challenge.
Mice were primed with 5 × 105 PFU of recombinant vaccinia virus by intradermal inoculation at the base of the tail and were monitored daily for lesion formation. For in vivo IL-4 depletion experiments, one-half of the mice in each priming group was injected intraperitoneally with 200 μg of 11.B11 anti-IL-4 neutralizing antibody on days −2, −1, 0, 1, 2, 7, and 14 around priming. Control mice were injected with 0.2 ml of sterile phosphate-buffered saline (PBS) on the same schedule. Six weeks after immunization, the mice were anesthetized and intranasally infected with 100 μl of a solution containing 107 PFU of RSV. We have shown that vaccinia virus replicates for more than 2 weeks after intradermal immunization (unpublished data), dictating the importance of a long interval between vaccination and challenge to allow for the resolution of innate and adaptive immune responses directly stimulated by the vaccinia virus vector. RSV-infected mice were weighed for 12 days following challenge. Illness was graded daily by a blinded observer, with clinical features of illness scored as the following: 0, no apparent illness; 1, slightly ruffled fur; 2, ruffled fur but active; 3, ruffled fur and inactive; 4, ruffled fur, inactive, hunched posture, and gaunt; and 5, dead.
To examine the effects of global IL-4 deficiency, IL-4−/− and IL-4+/+ C57BL/6 controls were immunized with 5 × 105 PFU (in 50 μl of solution) of recombinant vaccinia virus by intradermal inoculation at the base of the tail and were monitored daily for lesion formation. Two weeks later, additional groups of mice were injected intramuscularly with FI-RSV or mock preparation (100 μl). Six weeks after vaccinia virus priming and 4 weeks after FI-RSV priming, all mice were anesthetized and infected intranasally with 107 PFU of live RSV in 100 μl of solution. The mice were weighed and examined for 7 days following RSV challenge.
RSV plaque assays.
Four or six days following RSV challenge, mice were sacrificed by CO2 narcosis and cervical dislocation. The lungs were removed, placed in 10% EMEM, and quick-frozen in an alcohol-dry ice bath. RSV titers in the lungs were measured by standard plaque assays using 80% confluent HEp-2 monolayers as previously described (36). Data are represented as the geometric mean log10 PFU per gram of lung tissue at the dilution producing greater than five plaques per well.
Quantitation of IFN-γ, IL-4, IL-5, IL-13, and eotaxin in lung tissues.
Levels of IFN-γ, IL-4, and IL-5 in lung tissues of primed and challenged mice were measured with commercially available enzyme-linked immunosorbent assay (ELISA) minikits (Endogen, Woburn, Mass.) according to kit protocols, using day 4 lung supernatants. IL-13 levels were quantitated with ELISA kits from R & D Systems, Inc. (Minneapolis, Minn.). Eotaxin concentrations were measured in a sandwich ELISA as follows. Nunc Immulon MaxiSorp 96-well microtiter plates were coated overnight with 100 ng of polyclonal affinity-purified goat anti-mouse eotaxin (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) diluted in PBS. The wells were then blocked with 2% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) in PBS. After 1 h of blocking, the plates were washed in PBS containing 0.2% Tween 20 (PBS-Tween). Samples and standard dilutions were then added to each well. Serial twofold dilutions of recombinant mouse eotaxin (R & D Systems, Inc.) ranging from 2.5 to 80 μg/ml were used to generate a standard curve. The plate was covered and incubated overnight at 25°C. Bound eotaxin was detected with biotinylated polyclonal goat anti-mouse eotaxin (R & D Systems, Inc.) diluted to 0.5 μg/ml in PBS-Tween followed by horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch, West Grove, Pa.). The plate was developed with freshly prepared tetramethylbenzidene substrate solution. Color development was stopped after 30 min by the addition of 25 μl of 2.5 M H2SO4, and the plate was read at 450 nm on a Dynatech MRX microplate reader (Dynatech Laboratories, Chantilly, Va.). Concentrations of cytokines and chemokines were calculated from the standard curves by linear regression.
RPA.
Lungs from G-primed and RSV-challenged mice were harvested 4 days postchallenge, quick-frozen in liquid nitrogen, and stored at −70°C. Individual lungs were homogenized in 2 ml of RNazol (TelTest, Friendswood, Tex.), and total RNA was isolated by phenol-chloroform extraction. The aqueous phase was precipitated for 30 min at −20°C, resolubilized in diethyl pyrocarbonate-H2O, divided into two equal aliquots, and precipitated again. The RNA aliquots were stored at −70°C in isopropanol until the RNase protection assay (RPA) was performed. RPA was performed with RiboQuant kits from PharMingen (San Diego, Calif.) by using the mCK-1 cytokine and mCK-5 chemokine multiprobe template sets according to the kit protocol. RPA products were resolved by electrophoresis on a 5% polyacrylamide-8 M urea sequencing gel. After drying, autoradiography was performed on Kodak X-Omat AR film. Radiographic analysis was performed by imaging on a ScanJet 6100C/T scanner (Hewlett-Packard, Palo Alto, Calif.), and densitometry was performed with Scion Image software (NIH).
BAL of RSV-challenged mice.
Mice were sacrificed 6 days after RSV challenge. The trachea was surgically exposed, and a 19-gauge blunt-end needle was inserted into a small cut made in the trachea. Through this endotracheal tube, 0.5 ml of PBS containing 5% fetal calf serum was injected into the lungs. After washing for approximately 30 s, the fluid was withdrawn and transferred to a microcentrifuge tube. Smears were made of all bronchoalveolar lavage (BAL) samples and were air dried. The dried smears were then stained with Diff-Quick (Fisher Scientific, Pittsburgh, Pa.), and differential counts of standard cell types were performed.
Statistical analysis.
Data from individual experiments were maintained in a Paradox database. Statistical analysis was performed by transferring data from the database into SAS statistical software (Chapel Hill, N.C.) to perform analysis of variance using Kruskal-Wallis and Wilcoxon rank sum tests. Comparisons were made between individual experiments by statistical modeling and trend analysis calculated by the General Linear Model method in the SAS program. P values less than 0.05 were considered statistically significant.
RESULTS
Disease profiles of RSV-challenged vvGs- and FI-RSV-primed mice.
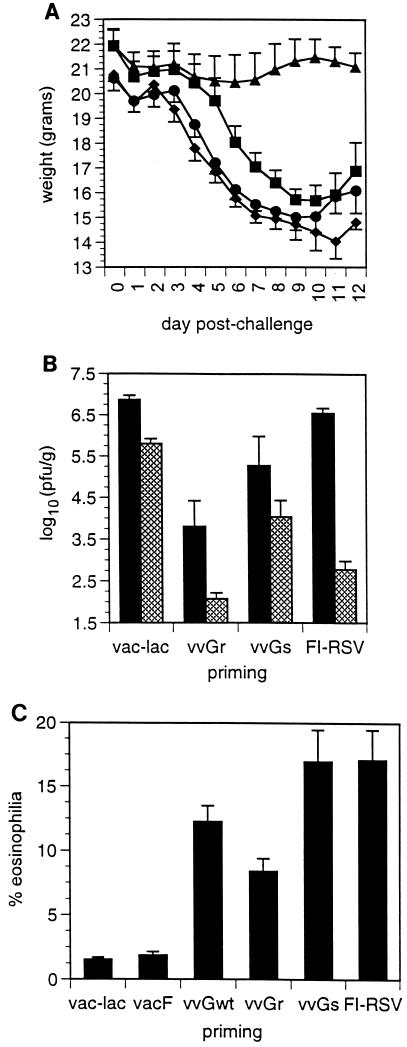
BALB/c mice were immunized with recombinant vaccinia virus expressing an RSV protein or with FI-RSV and then challenged with live RSV. Subsequent disease patterns in these primed and challenged mice were compared. Severe illness was induced by challenge of vvGs- or FI-RSV-primed mice, with no significant difference in the severity of illness observed between these groups (Fig. 1A). In contrast, immunization with vvGr protected against illness following RSV challenge. RSV titers were measured 4 and 6 days following challenge to assess the abilities of the various immunizing agents to protect against subsequent viral challenge (Fig. 1B). While vvGr- and vvGs-primed mice had significantly lower viral titers 4 days after challenge (P = 0.01), RSV titers were not significantly reduced in mice immunized with FI-RSV. Yet, some degree of protection against challenge was induced by FI-RSV, as evidenced by the more rapid clearance and significantly lower titers at day 6 (relative to control mice). No significant differences were observed in viral titers between FI-RSV- and vvGs-immunized mice at any time after challenge. Induction of pulmonary eosinophilia is a hallmark of the immune responses induced by both FI-RSV and RSV G immunizations. Therefore, BAL was performed, the cells were differentially stained, and the percentage of eosinophils was determined. Few (<1%) eosinophils were present in control or vacF-primed mice (Fig. 1C). However, significant eosinophil recruitment was induced in mice primed with FI-RSV or with any form of RSV G. Pulmonary eosinophilia was induced to similar levels in vvGs- and FI-RSV-primed mice (P > 0.05; Student’s t test). Thus, the severity of illness and the extent and cellular composition of pulmonary pathology are comparable in mice immunized with vvGs or with FI-RSV following RSV challenge, suggesting that the immune responses induced by these immunization protocols may be similar. While it has been suggested that eosinophils mediate disease during RSV infection (19, 64), the ability of vvGr immunization to protect against illness following RSV challenge while still predisposing for low levels of pulmonary eosinophilia challenges this hypothesis. These data suggest that the immune responses induced by vvGs or FI-RSV priming are distinct from those induced by vvGr immunization and that eosinophils are not sufficient to cause illness measured by weight loss in RSV-infected mice.
FIG. 1.
Comparison of disease profiles in vvGs- and FI-RSV-primed mice. Mice were primed with vaccinia virus (intradermally) or with FI-RSV (intramuscularly) and challenged with live RSV (intranasally) 4 to 6 weeks later. Panel A shows weight loss in mice primed with control vac-lac (■), vvGr (▴), vvGs (⧫), or FI-RSV (●). Panel B shows RSV titers at 4 (closed bars) and 7 (hatched bars) days after challenge. Pulmonary eosinophilia in BAL fluids is shown in panel C. Data represent the mean ± the standard error of the mean (SEM), where n = 5 to 6 mice for each priming group in one of two representative experiments.
mRNA and protein levels of cytokines and chemokines.
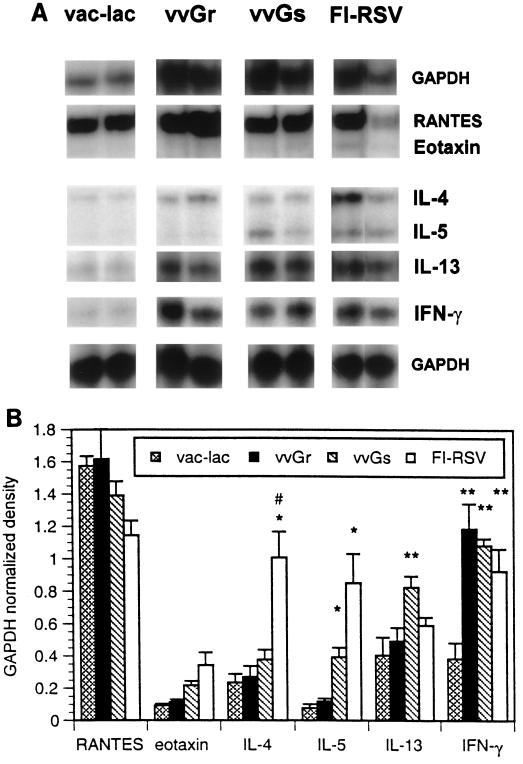
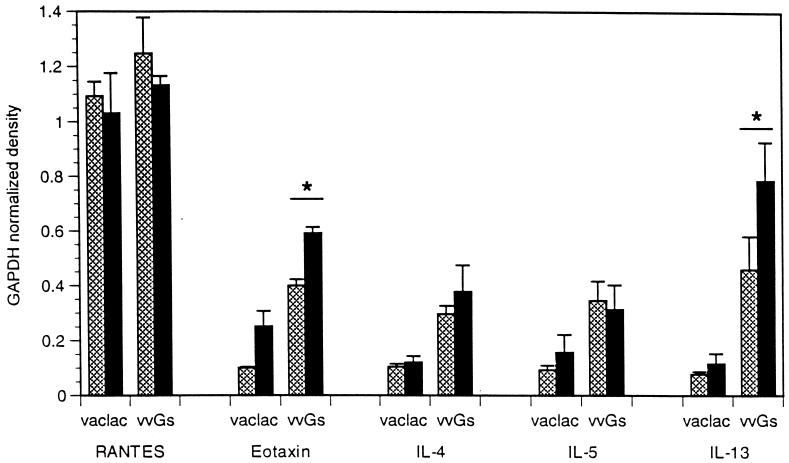
Cytokine mRNA profiles produced in lungs of vvGr-, vvGs-, and FI-RSV-primed mice following RSV challenge were examined by RPA. Similar levels of IL-4 and IFN-γ mRNA were present in lungs of vvGr- and vvGs-primed mice 4 days after challenge (Fig. 2A). These data are represented graphically in Fig. 2B, where mRNA levels for each cytokine in single lungs were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels for that sample. While not statistically significant, there was a trend toward increased IL-4 mRNA production in vvGs-primed, but not vvGr-primed, animals following challenge. In contrast, IL-4 mRNA levels in FI-RSV-primed mice were significantly greater than in vvGs-primed mice (P = 0.016), even though RSV infection of vvGs- or FI-RSV-primed BALB/c mice resulted in similar disease profiles (Fig. 1). Messenger RNA levels of IFN-γ were greater in FI-RSV- and G-primed mice than were observed in control vac-lac-primed animals (P < 0.006; Student’s t test). No significant difference in IL-13 mRNA induction occurred between vac-lac-, FI-RSV-, and vvGr-primed, RSV-infected mice. However, IL-13 mRNA levels were significantly increased in vvGs-primed mice relative to vac-lac-primed mice (P = 0.022). Also, mice immunized with vvGs had significantly greater levels of IL-13 mRNA than mice immunized with vvGr or FI-RSV (P = 0.02). The chemokine RANTES, which can induce recruitment of eosinophils to sites of inflammation (15), was produced at similar levels in all RSV-infected mice, regardless of immunization. However, mRNA expression of both IL-5 and the eosinophil-specific chemokine eotaxin were significantly greater in mice immunized with vvGs or FI-RSV relative to both vvGr- and vac-lac-primed animals (P < 0.01 and P < 0.02 for IL-5 and eotaxin, respectively; Student’s t test). IL-5 and eotaxin mRNA levels were not significantly different between vvGs- and FI-RSV-primed mice (P = 0.069 for IL-5 and P = 0.24 for eotaxin, comparing vvGs and FI-RSV groups). While levels of IL-5 and eotaxin mRNA in vvGr-primed mice were not significantly greater than in vac-lac-primed mice, there were increases in mRNA (Fig. 2B) and in protein (36). The production of IL-5 and eotaxin by vvGr-primed mice is sufficient to recruit eosinophils following RSV challenge, but the reduced IL-5 and eotaxin levels are reflected in decreased pulmonary eosinophilia relative to vvGs- and FI-RSV-primed mice. Thus, while severe disease and altered pathology correlate with increased IL-4 and IL-5 mRNA production in FI-RSV-primed mice, the illness and pulmonary eosinophilia observed in vvGs-primed mice correlate more closely with increased IL-5, IL-13, and eotaxin mRNA production.
FIG. 2.
Cytokine and chemokine mRNA after RSV infection of G-primed mice. Mice were immunized with vac-lac, vvGr, or vvGs and challenged with RSV 6 weeks later. Four days after RSV challenge, induction of cytokine and chemokine mRNA was examined by RPA using radiolabeled riboprobes. Panel A is a composite of RPA radiograms from a typical experiment where each lane represents RNA isolated from a single lung. Each RPA has been performed on at least 25 individual samples (from five separate experiments) for each group, with similar results obtained each time. Panel B represents densitometric analysis of RPA radiographs, with cytokine-chemokine levels normalized to GAPDH levels (mean ± SEM) (one of five experiments; n = 5 for each group). ∗, significant increase compared to vac-lac or vvGr (P ≤ 0.022); ∗∗, significant increase compared to vac-lac (P < 0.006); #, significant increase comparing vvGs and FI-RSV (P ≤ 0.038).
Illness and viral titers in RSV G-primed IL-4-depleted mice following RSV challenge.
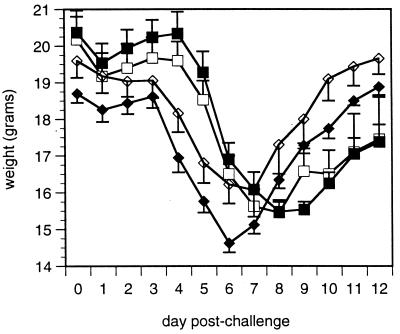
Anti-IL-4 neutralizing antibody was administered during vvGs priming to define the roles of IL-4 in disease and virus replication in G-primed mice. Weight loss and illness were monitored for 12 days following RSV challenge (Fig. 3). In contrast to the ability of vvGr immunization to protect against illness (Fig. 1A), peak weight loss was similar in vac-lac- and vvGs-primed mice. More importantly, neutralization of IL-4 during vvGs immunization did not diminish weight loss. Illness scores mirrored weight loss (data not shown). Similarly, anti-IL-4 treatment did not alter illness in vvGr-immunized mice (data not shown). Therefore, in direct contrast to the effects on FI-RSV-primed mice (71), IL-4 is not necessary for the production of immune responses associated with illness induced by vvGs immunization.
FIG. 3.
Effects of IL-4 depletion on RSV-induced illness in G-primed mice. Mice were primed with vac-lac (□, ■) or vvGs (◊, ⧫) intradermally at the base of the tail. Either PBS (open symbols) or anti-IL-4 antibody 11.B11 (closed symbols) was administered on days −2, −1, 0, 1, 2, 7, and 14 of priming. Six weeks after priming, mice were challenged intranasally with live RSV, and weight loss was monitored on days 4 to 11 following challenge. The data represent the means ± SEMs for two experiments (n = 10 for each treatment group). vvGs-primed mice exhibited greater weight loss than did vac-lac-primed mice on days 4 to 9 (P < 0.002). No significant difference was observed between PBS- and 11.B11-treated mice for a single priming vector. Weight loss mirrored illness (data not shown).
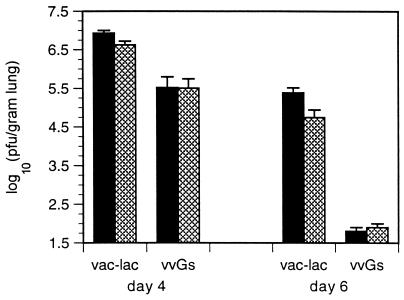
vvGs immunization provides partial protection against subsequent RSV infection. Four and six days after RSV challenge, significantly lower viral titers were measured in vvGs-primed mice relative to titers in vac-lac-primed mice (Fig. 4) (P < 0.00001 for PBS-treated controls; Student’s t test). Those memory responses mediating viral clearance induced by vvGs immunization appear to be regulated by mechanisms which do not require IL-4, since 11.B11 treatment during vvGs priming did not decrease RSV titers following challenge (Fig. 4) (P = 0.97 when comparing PBS- and 11.B11-treated vvGs-primed mice; Student’s t test). Thus, in contrast to FI-RSV immunization (71), inhibition of IL-4 during priming with vvGs or vvGr (data not shown) does not alter the anti-viral immune response or improve viral clearance.
FIG. 4.
RSV titers in lungs of G-primed mice depleted of IL-4 during priming. Mice were immunized with recombinant vaccinia virus vectors, depleted of IL-4 during priming, and challenged with RSV as described in Fig. 3. Four and six days postchallenge, viral titers were measured by standard plaque assays on HEp-2 monolayers and are represented as the mean log10 (PFU per gram of lung) ± SEM of two experiments with 10 control mice (solid bar) and 10 11.B11-treated mice (hatched bar) for each priming group. Immunization with RSV G provides partial protection against RSV challenge (P < 0.00001, comparing vac-lac-primed mice to vvGs-primed mice). 11.B11 treatment does not significantly influence viral clearance.
Composition of cells in BAL fluid.
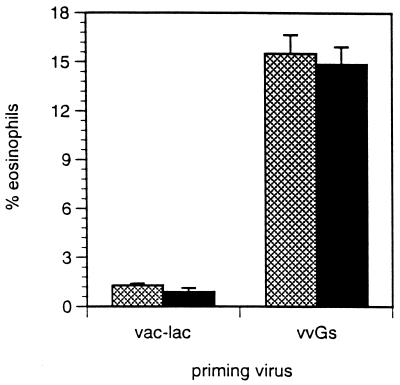
Next we sought to determine which cellular components in the RSV-infected lung correlate with severe illness. Mice were primed with vvGs and challenged with RSV, and BAL was performed 6 days after challenge. Immunization with vvGs predisposed for increased airway eosinophilia upon RSV infection (Fig. 5) (P < 0.00001, relative to vac-lac-primed mice). Administration of anti-IL-4 during immunization did not modulate induction of bronchoalveolar eosinophilia in vvGs-primed mice or in vvGr-primed mice (data not shown). Therefore, RSV G, especially in a secreted form, does not require IL-4 to induce immune responses which result in airway eosinophilia after subsequent RSV infection.
FIG. 5.
BAL and differential staining. Mice were primed, depleted of IL-4, and RSV challenged as described in Fig. 3. Six days after challenge, BAL was performed and eosinophilia was quantitated by examining Diff-Quick-stained smears of BAL cells. Significantly greater eosinophilia occurred in vvGs-primed mice than in vac-lac-primed mice (P < 0.00001), and the condition was not reduced by depletion of IL-4. The data represent the means ± SEMs for two experiments (n = 10 for each group).
Effect of IL-4 inhibition on cytokine and chemokine production.
IL-4 and IL-5 expression may be coordinately regulated (16). Thus, the ability of anti-IL-4 treatment during vvGs priming to modulate IL-5, IL-13, and eotaxin production at challenge was examined. RPA analysis was performed on total RNA isolated from individual lungs 4 days following RSV infection, and induction of cytokine and chemokine mRNA was evaluated by densitometric analysis of radiographs of RPA gels and by normalization of cytokine and chemokine mRNA levels to GAPDH mRNA levels (Fig. 6). Production of IL-4 and IL-5 mRNA at challenge was not modulated by the inhibition of IL-4 by administration of 11.B11 during priming with vvGs, as demonstrated by densitometry. However, expression of IL-13 and eotaxin mRNA was significantly increased in 11.B11-treated vvGs-immunized mice after RSV challenge (P < 0.001; Student’s t test). Similar results were observed in vvGr-primed, RSV-challenged mice (data not shown).
FIG. 6.
Cytokine and chemokine mRNA after RSV infection of G-primed and 11.B11-treated mice. Mice were immunized and infected as described in Fig. 3. Four days after RSV challenge, induction of cytokine and chemokine mRNA was examined by RPA using radiolabeled riboprobes as described in Fig. 2. Data represent the means ± SEMs of cytokine-chemokine mRNA levels normalized to GAPDH mRNA levels following densitometric analysis of RPA radiographs. Data from one of two representative experiments are shown (n = 5 for each group). ∗, significant increase comparing PBS- and 11.B11-treated vvGs-primed mice (P = 0.0007).
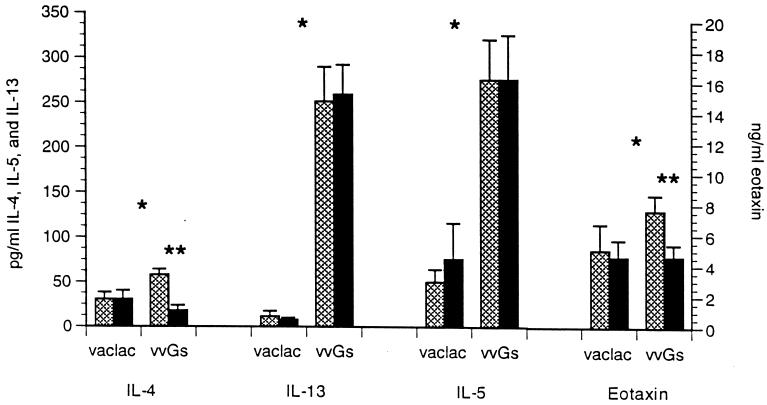
Correlation between cytokine and chemokine expression in vvGs-primed mice was further evaluated by measuring protein levels in lung supernatants. Lung supernatants were assayed by ELISA 4 days after RSV challenge to quantitate concentrations of IL-4, IL-5, IL-13, and eotaxin. All cytokines were produced at higher concentrations in the lungs of vvGs-primed mice than in vac-lac-primed mice (Fig. 7) (P < 0.05, comparing vac-lac-primed and vvGs-primed PBS-treated groups; Student’s t test). Neutralization of IL-4 by 11.B11 antibody treatment during priming did not alter the production of IL-5 or IL-13. However, anti-IL-4 treatment during vvGs immunization did modulate eotaxin protein levels with a slight but significant decrease (P = 0.041). Levels of IL-4 protein were decreased in anti-IL-4-treated mice, demonstrating that 11.B11 treatment did have an impact on the induction of immune responses (P < 0.008, for PBS- and 11.B11-treated vvGs-primed groups). Therefore, vvGs immunization induces IL-5- and IL-13-producing memory responses independently of IL-4 influences during priming. This suggests that secreted RSV G may induce early production of IL-5 or that IL-13 may compensate for the loss of IL-4 by the induction of IL-5 expression and eosinophil recruitment. In contrast, the presence of IL-4 during vvGs priming does impact subsequent eotaxin production, increasing mRNA levels while decreasing protein levels. Thus, the regulation of eotaxin expression may be influenced by other factors, including the potency of virus challenge, but the contributing factors have not been defined by these studies.
FIG. 7.
Cytokine and chemokine protein levels after RSV infection of G-primed and 11.B11-treated mice. Concentrations of IL-4, IL-5, IL-13, and eotaxin in lung supernatants were measured 4 days postchallenge by ELISA. The limits of detection are 20 pg of IL-4, IL-5, or IL-13 per ml and 40 ng of eotaxin per ml. The data represent means ± SEMs for data from two experiments (n = 10 for each group). ∗, significant increases comparing vac-lac- and vvGs-primed PBS-treated control mice; ∗∗, significant decreases comparing PBS- and 11.B11-treated vvGs primed mice.
RSV infection of FI-RSV- and G-primed IL-4−/− mice.
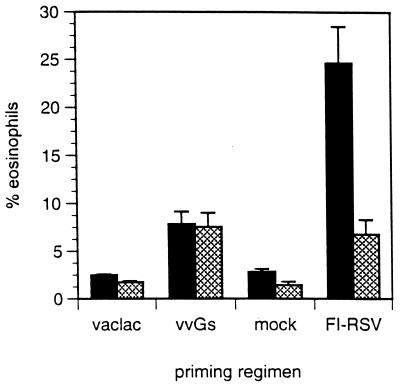
In vivo depletion of IL-4 during FI-RSV priming has been shown to reduce illness and eosinophilia upon RSV challenge (71). The contribution of IL-4 to immune regulation following vvGs and FI-RSV priming was compared by immunization and challenge of mice with targeted disruption of the IL-4 gene. Confirming data from IL-4 depletion experiments, illness and viral titers were reduced in FI-RSV-primed IL-4−/− mice but were unchanged in vvGs-immunized IL-4-deficient mice relative to IL-4+/+ controls (data not shown). The impact of systemic IL-4 deficiency on transmigration of eosinophils to the bronchial airways was examined by BAL 7 days after RSV challenge (Fig. 8). In RSV-infected parental C57BL/6 mice immunized with FI-RSV, eosinophils comprised 24.68% of BAL cells, while only 6.74% of cells were eosinophils in FI-RSV-primed IL-4−/− mice (P = 0.011 when comparing IL-4−/− and control mice). When the composition of BAL cells was examined in vvGs-primed C57BL/6 mice, 7.85% of the cells were determined to be eosinophils, while IL-4−/− mice had 7.53% eosinophils in the BAL compartment. Thus, IL-4 is not necessary for G-induced responses leading to recruitment of airway eosinophils. This is in striking contrast to the IL-4-dependent mechanisms of eosinophil recruitment induced by FI-RSV immunization. Interestingly, eosinophilia is not totally abolished in FI-RSV-primed IL-4−/− mice but is reduced to levels seen in vvGs-immunized mice. This suggests that there may be similar IL-4-independent pathways induced by both priming regimens but that an additional IL-4-dependent pathway is also utilized during FI-RSV priming to induce immune responses, resulting in more extensive eosinophilia upon subsequent RSV challenge. Additionally, these data demonstrate that IL-4 is not required at the time of either vvGs immunization or RSV challenge for induction of the type 2-associated immune responses resulting in production of IL-5 and eotaxin and in pulmonary eosinophilia.
FIG. 8.
BAL eosinophilia in FI-RSV and G-primed IL-4−/− mice. IL-4−/− mice (hatched bars) or parental C57BL/6 controls (solid bars) were immunized with vac-lac, vvGs, or FI-RSV or were mock immunized. Six weeks after vaccinia virus priming and 4 weeks after FI-RSV or mock priming, mice were infected with RSV, and 7 days later BAL was performed and smears were stained as described in Fig. 4. The percentage of airway eosinophils trafficking into the bronchial spaces was not decreased in G-primed IL-4−/− mice but was reduced in FI-RSV-primed IL-4−/− mice (P = 0.011) (n = 5 for each group).
DISCUSSION
We have shown that immunization with recombinant vaccinia virus expressing the secreted form of RSV G glycoprotein predisposes mice to immune responses which result in severe illness, recruitment of airway eosinophils, and elevated levels of IL-4, IL-5, IL-13, and eotaxin following RSV challenge (36). This pattern of disease is similar to that induced by FI-RSV priming (25, 26, 51). Depletion of IL-4 at the time of FI-RSV immunization by administration of neutralizing 11.B11 monoclonal antibody results in less severe illness, decreased RSV titers, and diminished IL-4 mRNA upon challenge (71). We thus hypothesized that 11.B11 treatment during vvGs immunization would similarly influence disease and immune responses following RSV challenge. However, depletion of IL-4 at the time of immunization with RSV G does not alter illness, viral clearance, or pathology, and IL-5 and IL-13 are not reduced by anti-IL-4 administration in vvGs-primed mice. In addition, vvGs and FI-RSV immunization of IL-4−/− mice demonstrated IL-4-dependent induction of lung eosinophilia in FI-RSV-primed mice but IL-4-independent induction of lung eosinophilia in vvGs-primed mice. We were careful in designing these experiments to allow a sufficient interval between immunization and challenge to diminish the confounding effects of the vaccinia virus vector. Previous reports suggested that RSV G-immunized C57BL/6 mice had little eosinophilia after challenge at 2 weeks (33, 34). However, we have shown that vaccinia virus replicates for more than 2 weeks after intradermal immunization (unpublished data). Therefore, in addition to immunization with control vaccinia virus vectors, RSV challenge was performed 6 weeks after vaccinia virus inoculation to minimize the impact of vaccinia virus-induced innate and adaptive immune responses on the response to RSV challenge. The data suggest that, while the vaccine-enhanced illness induced by FI-RSV utilizes primarily IL-4-mediated mechanisms, secreted RSV G induces expression of IL-5, IL-13, and eotaxin associated with enhanced disease by another mechanism(s). In addition, the data suggest that this differential requirement for IL-4 is not due to the magnitude of the immune response in FI-RSV-primed mice but rather results from the induction of distinct pathways of cytokine production leading to the common end point of eosinophilia.
Since vvGs and FI-RSV immunizations result in similar disease profiles following RSV challenge, it has been proposed that G epitopes are the component of FI-RSV which induced the immune responses resulting in vaccine-enhanced illness (2, 4, 28, 68). Identification of an immunogenic domain of RSV G directly associated with eosinophilia (65, 67) strengthened this hypothesis. However, others have demonstrated that this domain can induce both Th1 and Th2 cytokine responses (70, 75). Induction of Th1 immune responses during primary infection (1, 23, 24) or rechallenge (24, 74) with live RSV, which contains this epitope in G, is also consistent with these data. We now show that G induces eosinophilia by an IL-4-independent pathway, distinguishing it from FI-RSV-induced responses. We therefore postulate that the secreted form of the RSV G glycoprotein may be important for inducing some of the disease manifestations during primary RSV infection but that G antigenicity per se was not the key factor in the FI-RSV vaccine-enhanced illness. The dose of FI-RSV used in this study contained approximately 2 ng of G glycoprotein. Other RSV proteins, including the fusion glycoprotein and nucleoprotein, were present at 10- to 100-fold higher concentrations (27a), although the effect of formalin treatment on G antigenicity is unknown. FI-RSV immunization, therefore, resulted in the induction of Th2 CD4+ responses against multiple RSV antigens, among which G was underrepresented. The IL-4-rich microenvironment produced by memory CD4+ T cells might then alter the induction of CD8+ T cells stimulated by subsequent live RSV challenge. Delayed or diminished CTL induction has been shown to augment the immunopathology and illness induced by vvGwt priming and subsequent challenge (33, 69). Thus, we postulate that the IL-4-independent events induced by secreted G during natural infection with RSV are exacerbated in the presence of IL-4, in part because of the diminished regulatory influence of RSV-specific CD8+ cells, and that this scenario can occur following prior immunization with whole inactivated virus or with purified viral proteins or, alternatively, in the setting of allergic airway disease.
IL-4 has been demonstrated to have a regulatory role in the induction and differentiation of Th2 CD4+ and CD8+ T cells, which then secrete IL-4 and IL-5 (16, 18, 43, 54). Additionally, targeted disruption of the IL-4 gene results in reduced production of the hallmark Th2 cytokines IL-4, IL-5, IL-9, and IL-10 by in vitro-stimulated T cells or by T cells from helminth-infected mice (38, 76). This association may be extended to RSV pathogenesis, where T-cell clones specific for RSV G were shown to secrete both IL-4 and IL-5 (2, 4). These data suggest a close regulation of IL-5 by IL-4, which may in part be explained by common sequence motifs and regulatory sites in the promoters (40, 44). Cooperation between IL-4 and IL-5 may also occur at an effector level. Recruitment of eosinophils to inflammatory sites occurs in response to production of IL-5 and chemokines such as eotaxin and RANTES (32, 43, 60). Yet, administration of IL-4 can also promote migration of eosinophils to the site of injection (49), presumably through indirect stimulation of cells that secrete IL-5. Recent studies have demonstrated that at least two distinct pathways exist for the induction of IL-5 (37, 47, 48). This may account for induction of Th2-associated events, such as reported in this paper and elsewhere (30, 31), with no apparent requirement for IL-4.
This dichotomy between IL-4 and IL-5 production has also been described in vivo in models of parasitic and allergic disease. In Plasmodium chabaudi-infected IL-4-deficient mice, IL-5 levels are decreased but measurable (76). Similarly, following secondary infection of IL-4-deficient mice with Nippostrongylus brasiliensis, eosinophilia occurs (38). Cocultures of splenocytes and granuloma eosinophils from Schistosoma mansoni-infected mice secrete IL-5, but not IL-4 (45). Sensitization of mice deficient in IL-4 with ovalbumin induces IL-5 expression, eosinophilia, and airway hyperresponsiveness, demonstrating in vivo the existence of mechanisms for IL-5 induction which operate independently of IL-4 (30, 31).
Work in the fields of asthma and allergy suggests that IL-5 and the chemokine eotaxin are key regulatory factors in the induction of eosinophilia. CD4+ T cells are a major source of IL-5 in these disease states (32, 45), but CD8+ T cells (16), eosinophils (43), mast cells (43), and NK cells (79) may also produce IL-5. Eotaxin is an eosinophil-specific chemokine inducible in a variety of tissues but present at the highest levels in type 1 alveolar epithelial cells (22, 41, 60). Eotaxin functions to induce tissue eosinophilia in conjunction with IL-5, with a two-step mechanism of eosinophil activation and transmigration proposed (50, 61). Expression of eotaxin can be induced by IFN-γ, tumor necrosis factor alpha, and IL-1β (41) and appears to be independent of IL-4, since eotaxin levels are not diminished in IL-4-deficient mice (22).
IL-4 and IL-13 have similar biological functions (12), which may be due in part to shared use of a receptor subunit and subsequent activation of common STAT proteins (42), suggesting that IL-4 and IL-13 constitute a functional redundancy. This hypothesis is supported by work which demonstrates that IL-4 or IL-13 alone can produce asthma-associated pathology (27), induce tissue eosinophilia (82), and regulate cell surface protein expression (8, 77) and is important in resistance to parasitic infection (81). Yet, IL-13 secretion may be induced without a concomitant release of IL-4, suggesting multiple mechanisms of IL-13 regulation, with some distinct from IL-4 (56). However, several recent studies demonstrate that a degree of functional dichotomy also exists between IL-4 and IL-13. This is most evident in studies of parasitic infection and asthma. Bancroft et al. (6) demonstrated that mice deficient in either IL-4 or IL-13 were susceptible to infection by an intestinal nematode. However, the phenotypes of these mice differed, suggesting that IL-4 and IL-13 each play important, yet different, roles in the immune response. Wills-Karp et al. demonstrated in vivo that the use of an IL-13 receptor antagonist blocked airway hyperresponsiveness and mucus production in allergen-sensitized mice without altering eosinophil recruitment (81). Thus, IL-13 may function in the absence of IL-4 and may also mediate a distinct set of immune responses by distinct mechanisms. These IL-4-independent effects of IL-13 may have significant bearing on immune responses induced by secreted RSV G.
While the induction of differential cytokine secretion profiles has been focused at the level of the T cell, recent studies have begun to investigate the contribution of the antigen-presenting cell (APC) in this process. Chemokine production at the site of infection and selective expression of chemokine receptors by the APC result in activation, trafficking, and maturation of different subpopulations of APCs, initiating a cascade which subsequently results in differential activation of T-cell subsets (13, 21, 55, 58, 66).
The data presented in this paper demonstrate that RSV G induces immune responses resulting in the production of IL-5, IL-13, and pulmonary eosinophilia by mechanisms which operate independently of IL-4. Thus, while immunization with either vvGs or FI-RSV induces memory responses resulting in severe disease upon RSV challenge and similar profiles of cytokine induction and recruitment of eosinophils to RSV-infected lungs, these common end points are reached by distinct immune mechanisms with different requirements for IL-4 in the induction of these responses. RSV G can induce immune responses that result in eosinophil recruitment to the lung by more than one pathway. The classical Th2 response involving IL-4 has been demonstrated with G-specific T-cell clones and G-specific peptides. We now demonstrate IL-4-independent G-specific induction of IL-5, IL-13, and pulmonary eosinophilia. Moreover, such a pathway has not been described in viral pathogenesis and may be of particular importance for understanding the role of eosinophils in mediating RSV-induced airway dysfunction and wheezing. This is relevant to the problem of childhood asthma and has implications for clinical intervention strategies.
ACKNOWLEDGMENTS
This work was supported by NIH grants RO1-AI-37216 and RO1-AI-33933.
We gratefully acknowledge the technical assistance of Rauf Kuli-Zade and Robert A. Parker (Department of Medicine [Biostatistics], Harvard University) for the initial development of the SAS programs used for statistical analysis. Wyeth-Lederle-Praxis (Rochester, N.Y.) provided purified F and G glycoproteins used for ELISAs, and the Biological Response Modifiers Program (National Cancer Institute) provided 11.B11 anti-IL-4 antibody.
REFERENCES
- 1.Alwan W H, Kozlowska W J, Openshaw P J M. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W H, Openshaw P J M. Distinct patterns of T- and B-cell immunity to respiratory syncytial virus induced by individual viral proteins. Vaccine. 1993;11:431–437. doi: 10.1016/0264-410x(93)90284-5. [DOI] [PubMed] [Google Scholar]
- 3.Alwan W H, Record F M, Openshaw P J M. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus: comparison with the effects of CD8+ T cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwan W H, Record F M, Openshaw P J M. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- 5.Ball L A, Young K K Y, Anderson K, Collins P L, Wertz G W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci USA. 1986;83:246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bancroft A J, McKenzie A N J, Grencis R K. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 7.Bembridge G P, Garcia-Beato R, Lopez J A, Melero J A, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J Immunol. 1998;161:2473–2480. [PubMed] [Google Scholar]
- 8.Bochner B S, Klunk D A, Sterbinsky S A, Coffman R L, Schleimer R P. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 9.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins P D, Weg V B, Faccioli L H, Watson M L, Moqbel R, Williams T J. Eosinophil accumulation induced by human interleukin-8 in the guinea-pig in vivo. Immunology. 1993;79:312–318. [PMC free article] [PubMed] [Google Scholar]
- 11.Coyle A J, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996;183:1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries J E, Zurawski G. Immunoregulatory properties of IL-13: its potential role in atopic disease. Int Arch Allergy Immunol. 1995;106:175–179. doi: 10.1159/000236842. [DOI] [PubMed] [Google Scholar]
- 13.Dieu M-C, Vanbervliet B, Vicari A, Bridon J-M, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowell S F, Anderson L J, Gary J, Erdman D D, Plouffe J F, File J, Marston B J, Breiman R F. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 15.Ebisawa M, Yamada T, Bickel C, Klunk D, Schleimer R P. Eosinophil transendothelial migration induced by cytokines. III. Effect of the chemokine RANTES. J Immunol. 1994;153:2153–2160. [PubMed] [Google Scholar]
- 16.Erb K J, Le Gros G. The role of Th2 type CD4+ T cells and Th2 type CD8+ T cells in asthma. Immunol Cell Biol. 1996;74:206–208. doi: 10.1038/icb.1996.29. [DOI] [PubMed] [Google Scholar]
- 17.Falsey A R, Walsh E E, Betts R F. Serologic evidence of respiratory syncytial virus infection in nursing home patients. J Infect Dis. 1990;162:568–569. doi: 10.1093/infdis/162.2.568. [DOI] [PubMed] [Google Scholar]
- 18.Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garofalo R, Kimpen J L L, Welliver R C, Ogra P L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 20.Gavett S H, O’Hearn D J, Li X, Huang S-K, Finkelman F D, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalo J-A, Jia G-Q, Aguirre V, Friend D, Coyle A J, Jenkins N A, Lin G-S, Katz H, Lichtman A, Copeland N, Kopf M, Gutierrez-Ramos J-C. Mouse eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4:1–4. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]
- 23.Graham B S. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–294. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 24.Graham B S, Bunton L A, Wright P F, Karzon D T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Investig. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham B S, Henderson G S, Tang Y-W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 26.Graham B S, Perkins M D, Wright P F, Karzon D T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 27.Grunig G, Warnock M, Wakil A E, Venkayya R, Brombacher F, Rennick D M, Sheppard D, Mohrs M, Donaldson D D, Locksley R M, Corry D B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Hancock, G. E. Personal communication.
- 28.Hancock G E, Speelman D J, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Peng Q, Walls A F. Potent induction of a neutrophil- and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216–6225. [PubMed] [Google Scholar]
- 30.Hogan S P, Matthaei K I, Young J M, Koskinen A, Young I G, Foster P S. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998;161:1501–1509. [PubMed] [Google Scholar]
- 31.Hogan S P, Mould A, Kikutani H, Ramsay A J, Foster P S. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Investig. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hom J T, Estridge T. Antigen-induced recruitment of eosinophils: importance of CD4+ T cells, IL5 and mast cells. Clin Immunol Immunopathol. 1994;73:305–311. doi: 10.1006/clin.1994.1203. [DOI] [PubMed] [Google Scholar]
- 33.Hussell T, Baldwin C J, O’Garra A, Openshaw P J M. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 34.Hussell T, Georgiou A, Sparer T E, Matthews S, Pala P, Openshaw P J M. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161:6215–6222. [PubMed] [Google Scholar]
- 35.Jackson M, Scott R. Different patterns of cytokine induction in cultures of respiratory syncytial (RS) virus-specific human Th cell lines following stimulation with RS virus and RS virus proteins. J Med Virol. 1996;49:161–169. doi: 10.1002/(SICI)1096-9071(199607)49:3<161::AID-JMV2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlen S, D’Ercole M, Sanderson C J. Two pathways can activate the interleukin-5 gene and induce binding to the conserved lymphokine element 0. Blood. 1996;88:211–221. [PubMed] [Google Scholar]
- 38.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 39.La Via W V, Marks M I, Stutman H R. Respiratory syncytial virus puzzle: clinical features, pathophysiology, treatment, and prevention. J Pediatr. 1992;121:503–510. doi: 10.1016/s0022-3476(05)81135-7. [DOI] [PubMed] [Google Scholar]
- 40.Lee H J, Matsuda I, Naito Y, Yokota T, Arai N, Aria K. Signals and nuclear factors that regulate the expression of interleukin-4 and interleukin-5 genes in helper T cells. J Allergy Clin Immunol. 1994;94:594–604. doi: 10.1016/0091-6749(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 41.Lilly C M, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda E A, Rothenberg M E, Drazen J M, Luster A D. Expression of eotaxin by human lung epithelial cells. J Clin Investig. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J-X, Migone T-S, Tsang M, Friedman M, Weatherbee J A, Zhou L, Yamauchi A, Bloom E T, Mietz J, John S, Leonard W J. The role of shared receptor motifs and common stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 43.Lucey D R, Clerici M, Shearer G M. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marrugo J, Marsh D G, Ghosh B. The conserved lymphokine element-0 in the IL5 promoter binds to a high mobility group-1 protein. Mol Immunol. 1996;33:1119–1125. doi: 10.1016/s0161-5890(96)00073-9. [DOI] [PubMed] [Google Scholar]
- 45.Metwali A, Elliott D, Blum A M, Weinstock J V. Granuloma eosinophils enhance IL-5 production by lymphocytes from mice infected with Schistosoma mansoni. J Immunol. 1993;151:7048–7056. [PubMed] [Google Scholar]
- 46.Mochizuki M, Bartels J, Mallet A I, Christophers E, Schroder J M. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol. 1998;160:60–68. [PubMed] [Google Scholar]
- 47.Mori A, Kaminuma O, Suko M, Inoue S, Ohmura T, Hoshino A, Asakura Y, Miyazawa K, Yokota T, Okumura Y, Ito K, Okudaira H. Two distinct pathways of interleukin-5 synthesis in allergen-specific human T-cell clones are suppressed by glucocorticoids. Blood. 1997;89:2891–2900. [PubMed] [Google Scholar]
- 48.Mori A, Suko M, Kaminuma O, Nishizaki Y, Mikami T, Ohmura T, Hoshino A, Inoue S, Tsuruoka N, Okumura Y, Sato G, Ito K, Okudaira H. A critical role of IL-2 for the production and gene transcription of IL-5 in allergen-specific human T cell clones. Int Immunol. 1996;8:1889–1895. doi: 10.1093/intimm/8.12.1889. [DOI] [PubMed] [Google Scholar]
- 49.Moser R, Groscurth P, Carballido J M, Bruijnzeel P L B, Blaser K, Heusser C H, Fehr J. Interleukin-4 induces tissue eosinophilia in mice: correlation with its in vitro capacity to stimulate the endothelial cell-dependent selective transmigration of human eosinophils. J Lab Clin Med. 1993;122:567–575. [PubMed] [Google Scholar]
- 50.Mould A W, Matthaei K I, Young I G, Foster P S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Investig. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy B R, Sotnikov A V, Lawrence L A, Banks S M, Prince G A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 52.Nicholas J A, Rubino K L, Levely M E, Adams E G, Collins P L. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990;64:4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 54.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 55.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redrup A C, Howard B P, MacGlashan J, Kagey-Sobotka A, Lichtenstein L M, Schroeder J T. Differential regulation of IL-4 and IL-13 by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol. 1998;160:1957–1964. [PubMed] [Google Scholar]
- 57.Reiner S L, Zheng S, Wang Z E, Stowring L, Locksley R M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rissoan M-C, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu Y-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 59.Roberts S R, Lichtenstein D L, Ball L A, Wertz G W. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol. 1994;68:4538–4546. doi: 10.1128/jvi.68.7.4538-4546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothenberg M E, Luster A D, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothenberg M E, Ownbey R, Mehlhop P D, Loiselle P M, van de Rijn M, Bonventre J V, Oettgen H C, Leder P, Luster A D. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 62.Ruuskanen O, Ogra P L. Respiratory syncytial virus. Curr Probl Pediatr. 1993;23:50–79. doi: 10.1016/0045-9380(93)90003-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sad S, Mosmann T R. Interleukin (IL) 4, in the absence of antigen stimulation, induces an anergy-like state in the differentiated CD8+ TC1 cells: loss of IL-2 synthesis and autonomous proliferation but retention of cytotoxicity and synthesis of other cytokines. J Exp Med. 1995;82:1505–1515. doi: 10.1084/jem.182.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigurs N, Bjarnason R, Sigurbergsson F. Eosinophil cationic protein in nasal secretion and in serum and myeloperoxidase in serum in respiratory syncytial virus bronchiolitis: relation to asthma and atopy. Acta Paediatr. 1994;83:1151–1155. doi: 10.1111/j.1651-2227.1994.tb18269.x. [DOI] [PubMed] [Google Scholar]
- 65.Simard C, Nadon F, Seguin C, Trudel M. Evidence that the amino acid region 124-203 of glycoprotein G from the respiratory syncytial virus (RSV) constitutes a major part of the polypeptide domain that is involved in the protection against RSV infection. Antiviral Res. 1995;28:303–315. doi: 10.1016/0166-3542(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 66.Sozzani S, Allavena P, D’Amico G, Luini W, Bianchi G, Bonecchi R, Mantovani A. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 67.Sparer T E, Matthews S, Hussell T, Rae A J, Garcia-Barreno B, Melero J A, Openshaw P J M. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srikiatkhachorn A, Braciale T J. Virus-specific, CD8+ T lymphocytes down regulate Th2 type cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srikiatkhachorn A, Braciale T J. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srikiatkhachorn A, Chang W, Braciale T J. Induction of Th1 and Th2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J Virol. 1999;73:6590–6597. doi: 10.1128/jvi.73.8.6590-6597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Y-W, Graham B S. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Investig. 1994;94:1953–1958. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang Y-W, Graham B S. Interleukin-12 treatment during immunization elicits a T helper cell type 1-like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J Infect Dis. 1995;172:734–738. doi: 10.1093/infdis/172.3.734. [DOI] [PubMed] [Google Scholar]
- 73.Tang Y-W, Graham B S. T cell source of type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J Clin Investig. 1997;99:2183–2191. doi: 10.1172/JCI119391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Y-W, Neuzil K M, Fischer J E, Robinson F W, Parker R A, Graham B S. Determinants and kinetics of cytokine expression patterns in lungs of vaccinated mice challenged with respiratory syncytial virus. Vaccine. 1997;15:597–602. doi: 10.1016/s0264-410x(96)00214-9. [DOI] [PubMed] [Google Scholar]
- 75.Tebbey P W, Hagen M, Hancock G E. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von der Weid T, Kopf M, Kohler G, Langhorne J. The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Roderiquez G, Oravecz T, Norcross M A. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J Virol. 1998;72:7642–7647. doi: 10.1128/jvi.72.9.7642-7647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waris M E, Tsou C, Erdman D D, Zaki S R, Anderson L J. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warren H S, Kinnear B F, Phillips J H, Lanier L L. Production of IL-5 by human NK cells and regulation of IL-5 secretion by IL-4, IL-10, and IL-12. J Immunol. 1995;154:5144–5152. [PubMed] [Google Scholar]
- 80.Wassenaar A, Reinhardus C, Abraham-Inpijn L, Kievits F. Type-1 and type-2 CD8+ T-cell subsets isolated from chronic adult periodontitis tissue differ in surface phenotype and biological functions. Immunology. 1996;87:113–118. [PMC free article] [PubMed] [Google Scholar]
- 81.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben T Y, Karp C L, Donaldson D D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 82.Ying S, Meng Q, Barata L T, Robinson D S, Durham S R, Kay A B. Association between IL-13 and IL-4 (mRNA and protein), vascular cell adhesion molecule-1 expression, and the infiltration of eosinophils, macrophages, and T cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J Immunol. 1997;158:5050–5057. [PubMed] [Google Scholar]