Abstract
We investigated long-term human coagulation factor IX (huFIX) expression of a novel variant when delivered into mice and rhesus macaques and compared transduction efficiencies using two different adeno-associated virus (AAV) capsids. In hemophilic mice injected with KP1-packaged recombinant AAV (rAAV) expressing the hyperactive FIX variant specific activity plasma levels were 10-fold or 2-fold enhanced when compared with wild-type or Padua huFIX injected mice, respectively. In rhesus macaques AAV-LK03 capsid outperformed AAV-KP1 in terms of antigen expression and liver transduction. Two animals from each group showed sustained low-level huFIX expression at 3 months after administration, while one animal from each group lost huFIX mRNA and protein expression over time, despite comparable vector copies. We investigated whether epigenetic differences in the vector episomes could explain this loss of transcription. Cut&Tag analysis revealed lower levels of activating histone marks in the two animals that lost expression. When comparing rAAV genome associated histone modifications in rhesus macaques with those in mice injected with the same vector, the activating histone marks were starkly decreased in macaque-derived episomes. Differential epigenetic marking of AAV genomes may explain different expression profiles in mice and rhesus macaques, as well as the wide dose response variation observed in primates in both preclinical and human clinical trials.
Keywords: AAV, human coagulation factor IX, epigenetics, non-human primates, mice, histone modifications, AAV-LK03, AAV-KP1
Graphical abstract

Kay and colleagues found that transgene expression levels were positively correlated with levels of activating marks deposited on histones associated with the vector genome after dosing mice and rhesus macaques with a huFIX expressing rAAV vector.
Introduction
Hemophilia B is characterized by a deficiency in the expression of coagulation factor IX (FIX) in hepatocytes due to a variety of mutations and deletions in the coding sequence which is located on chromosome X. Hemophilia B affects one in every 30,000 births and is currently treated with standard infusions of recombinant clotting factor concentrates aiming to achieve FIX levels of more than 1% of normal to prevent bleeding into the joints.1 Gene therapy for hemophilia in which a functional gene is delivered into the target organ using a viral vector could make frequent injections of the protein obsolete. Recombinant adeno-associated virus (rAAV) containing a human (hu) FIX expression cassette has been used in many of the clinical studies to date due to the excellent safety profile and sustained expression from nuclear episomal forms of the vector (reviewed in2,3,4,5,6). Hemgenix, which had been developed by uniQure and CSL Behring, is the first hemophilia B rAAV-based gene therapy recently approved by the U.S. Food and Drug Administration.7 With dalcinonacog alfa Catalyst Biosciences has developed an engineered coagulation FIX protein with increased catalytic activity, resistance to antithrombin inhibition, and improved affinity for activated factor VIII (FVIII). Three amino acid substitutions provide this FIX variant with a 22-fold enhanced potency over wild-type (wt) FIX.8 Subcutaneously delivered dalcinonacog alfa is safe and efficacious for bleeding prophylaxis in hemophilia B patients.9 In the present study, we infused mice and rhesus macaques with single-stranded rAAV vectors expressing the hyperactive huFIX variant (termed CB2679d-GT) under the control of a strong liver-specific promoter. We used the novel chimeric KP1 capsid for studies in hemophilic mice. This capsid had been generated by directed evolution and was shown to have good transduction for human islets, as well as for human and mouse hepatocytes in a xenograft mouse model.10 We and others reported that KP1 furthermore is suitable for transduction of mouse11 and macaque12 pancreas, as well as for murine kidney13 and murine inner ear cells.14 In addition to the mouse study, we performed a direct comparison for liver transduction in rhesus macaques between KP1 and LK03, a capsid well established for rhesus and human liver transduction.15,16 Moreover, we attempted to understand the reasons for an observed loss of transgene expression, which could not be attributed to immune responses against the vector or the transgene. Previous studies had suggested that a decrease in the levels of transgene transcripts may be associated with epigenetic modifications on the rAAV episomes,17 which had been reported to be chromatinized similarly as genomic DNA.18 Our group had also recently reported that the inability of the LK03 capsid to productively transduce murine cells is associated with a lack of activating histone marks on the rAAV episome.19 The present study also confirmed previously published observations that the efficiency of transgene expression from rAAV differs by several orders of magnitude between mice and rhesus macaques, when using similar doses of identical vectors for delivery.20,21,22 We performed epigenetic profiling and found that lower levels of activating histone marks on rhesus-derived rAAV episomes were associated with the lower transgene expression when compared with mouse-derived episomes.
Results
High levels of huFIX expression in mice after delivery of an optimized rAAV vector
We used an optimized rAAV expression cassette with a strong liver-specific hu apolipoprotein E (ApoE)/C1-huSerpA chimeric promoter driving expression of mouse codon-optimized sequences for either wt, Padua, or CB 2679d-GT huFIX. A truncated version of the first Intron of huFIX was introduced between the signal peptide and the propeptide and zymogen encoding FIX sequence to enhance expression. The vectors were packaged into the chimeric KP1 capsid, as this capsid has shown strong transduction in the murine liver.10,23 Hemophilic mice were injected with a high dose (2 × 1010 vg) or a low dose (2 × 109 vg) of the three huFIX constructs and antigen levels, as well as huFIX activity levels were monitored for 18 weeks (high-dose group) or 12 weeks (low-dose group). As shown in Figure 1A, injection with the high vector dose resulted in a considerable spike in huFIX expression followed by a sharp decrease within the first 4 weeks after injection to levels of approximately 15 μg/mL, which remained stable throughout the experiment. Antigen levels were comparable between the Padua and the CB 2679d-GT injected groups, but somewhat lower for the wt injected group. This observation might be due to the different sources for the FIX standard proteins used for the ELISA. As expected, activity levels were lowest in the wt injected group and highest in the CB 2679d-GT injected group (Figure 1B). Specific activity was approximately 20- to 30-fold higher for the CB 2679d-GT injected group when compared with the wt group, whereas the improvement was only 10-fold for the Padua injected group (Figure 1C). When a 10-fold lower rAAV dose was used for injection, huFIX levels rose during the first week, followed by stable levels of huFIX antigen expression and huFIX activity (Figures 1D and 1E). Similar to the high-dose experiment, antigen levels seemed to be the lowest in the wt-injected group. Again, specific huFIX activity levels were similar to those seen with the high dose experiment group and a 2-fold increase in specific activity in the CB 2679d-GT group as compared with the Padua group was observed (Figure 1F). Genome copies as well as FIX transcript levels were analyzed in all mice at the end of the experiments, showing little to no significant difference between treatment groups (Figures 1G–1J). When compared with the previous rAAV study using CB 2679d-GT,24 we found considerably higher expression and transduction in the hemophilic mice, most likely due to the capsid and the optimized expression cassette, including the mouse codon-optimized huFIX sequence used.
Figure 1.
Expression of huFIX in hemophilic mice
(A–F) Antigen expression, (B) huFIX activity, (C) specific huFIX activity in plasma from hemophilic mice after injection of 2 × 1010 vg rAAV. (D) Antigen expression, (E) huFIX activity, (F) specific huFIX activity in plasma from hemophilic mice after injection of 2 × 109 vg. Mean values from five mice (high dose) or three mice (low dose) are shown, with error bars indicating the SD. (G and I) Vector copies and (I) huFIX transcript levels in liver of mice injected with 2 × 1010 vg rAAV.
(H and J) Vector copies and (J) huFIX transcript levels in liver of mice injected with 2 × 109 vg rAAV. Vector copies and transcript levels were normalized to mouse actin. two-way ANOVA was performed for (A–F), one-way ANOVA was performed for (G–J). Only statistically significant differences between the groups are shown. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Error bars represent standard error of the mean.
Evaluation of CpG-depleted human codon-optimized FIX constructs for expression
We next tested several CpG-depleted and human codon-optimized huFIX sequences for use in rhesus macaques, as the huFIX sequence of the vectors tested in the mouse hemophilia model was mouse codon optimized and thus contained high levels of innate immune response stimulating CpG dinucleotides. The signal sequence was reverted into the original huFIX sequence, as we reasoned that codon optimization might be detrimental for its function, as suggested previously.25 We compared the new expression constructs delivered with the AAV-KP1 capsid and injected mice with 5 × 109 vg of each vector. This medium dose was used to achieve robust huFIX protein antigen levels determined by ELISA in both the codon-optimized and non-optimized vectors. This dose mitigated the large increase and falloff to steady-state antigen levels seen with high doses of vectors. As shown in Figure S1A, substitution of the mouse codon-optimized signal sequence with the native huFIX signal sequence did not result in any considerable change of expression levels, whereas the construct that contained the three amino acid substitutions from the hyperactive CB2679d-GT variant inserted into the native huFIX sequence resulted in lower expression levels. This observation was not surprising, since the mouse codon-optimized sequence should be better expressed in mice. When three CB2679d-GT sequences depleted of CpG dinucleotides and optimized for human codon usage were used in conjunction with the native signal peptide sequence, similar expression levels as seen with the native sequence were observed (Figure S1B). Based on sequence-based expression predictions, we chose to use optimized sequence B for the rhesus macaque study. Vector copy numbers in the liver, as well as transcript levels, were determined at the end of the experiment. Copy numbers in the liver lobes were comparable between all groups (Figure S1C) and transcript levels largely correlated with antigen levels measured at the last time point (Figure S1D). It should be emphasized that only the sequence coding for the mature huFIX zymogen, but none of the other parts of the vector, such as promoter or intron, were CpG depleted.
Expression of huFIX in rhesus macaques
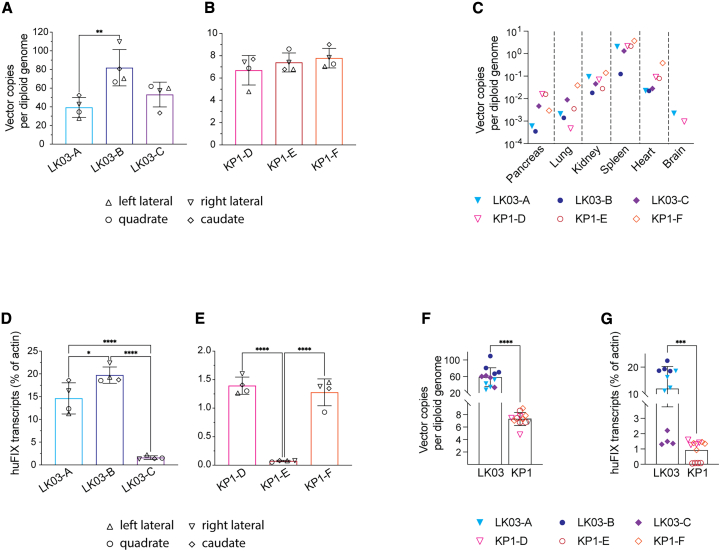
The codon-optimized and CpG-depleted CB 2679d-GT huFIX-expressing vector described above was then packaged with LK03 and KP1 capsids and infused at a dose of 3.5 × 1012 vg/kg into rhesus macaques that had been pre-screened to identify those with low or no capsid-directed neutralizing antibodies (nAbs) (see material and methods and Figure S2 for more information). As shown in Figures 2A and 2B, antigen levels spiked shortly after administration and then decreased. Two animals in the LK03 group showed very high expression levels for the first month after injection with declining levels over the next 6 weeks. One of the LK03-injected animals showed considerably lower expression levels than the other two animals; after 10 weeks, no huFIX was detected in the plasma. One of the KP1-injected animals showed a sharp increase in expression within the first 2 weeks, but levels quickly diminished to non-detectable at 6 weeks after injection. The other two animals in the KP1 cohort had lower huFIX levels just after injection, but maintained stable low-level expression throughout the study. Activity levels largely followed antigen expression data (Figures 2C and 2D), and low level expression was detected in four rhesus macaques (two for each capsid group) up to the endpoint of the study at 100 days post-dosing. The specific activity of the circulating huFIX remained relatively stable throughout the study and was similar in both the mice and macaques (Figures 2E and 2F). Plasma samples were analyzed for α-huFIX antibodies (ADA) throughout the study (Figures 2G and 2H). The timeline of antibody development correlated with loss of huFIX expression, suggesting a causal relationship between ADA and reduced expression from the rAAV vectors. We also analyzed several hematologic and clinical chemistry parameters (complete blood counts, clinical chemistry panels) throughout the course of the study. Transaminase levels were monitored to determine if liver inflammation due to a capsid-mediated cytotoxic T lymphocyte (CTL) response might have been responsible for a loss of antigen expression in animals LK03-C and KP1-E (Figure S3). While all were found primarily within normative ranges for the age group at all time points, two animals in the LK03 group had a transient mild elevation of liver enzymes above pre-injection baseline levels. LK03-A showed an increase on day 3 post-administration of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and C-reactive protein (CRP), and LK03-C an increase in ALT at weeks 4, 6, and 8, which then returned to baseline levels. Plasma samples were also analyzed for rAAV and as expected viral load was very high immediately following vector administration with a sharp decline within 2 weeks (Figure S4).
Figure 2.
Expression of huFIX and emergence of α-huFIX antibodies in rhesus macaque plasma after infusion of 3.5 × 1012 vg/kg rAAV huFIX-LK03 or rAAV huFIX-KP1
Antigen (A, B), activity (C, D), specific activity (E, F), and ADA (G, H). Animals LK03-C and KP1-E lost transgene expression after week 8 and week 4, respectively. For this reason, specific huFIX activity could not be calculated for these two animals after those timepoints.
Biodistribution and vector DNA structure
The animals were euthanized 100 days after rAAV infusion. All livers grossly looked normal at collection with weights all within the expected range for the age group. We analyzed each of the four liver lobes (quadrate, caudate, and right and left lateral) from each animal for episomal rAAV. Liver lobes of the LK03-injected animals (Figure 3A) contained up to 10-fold higher vector copy numbers than the liver lobes of the KP1-injected group (Figure 3B), indicating better transduction efficiency for rhesus macaque hepatocytes with the LK03 capsid when compared with the KP1 capsid (Figure 3F). These findings were confirmed when using Southern Blot analysis to assess vector DNA structure and copy number in the liver lobes (Figure S5A). For the LK03 group, concatemeric higher molecular weight products of approximately 20–30 kb, as well as relaxed double-stranded circular monomeric episomes at approximately 4.5 kb were found to dominate (Figure S5B). Smaller size products at approximately 2.3 kb presumably represent supercoiled double-stranded circular monomers. Other bands at 15 kb and 3 kb, as well as 4 kb were also found, but a faint band at 10 kb as well as at 3 kb was detected in the negative control. The KP1 group animals showed a main product at 4.5 kb as well as bands at 15 kb, 10 kb, 4 kb, 3 kb, and 2.5 kb. Due to the low copy numbers, concatemers could not be detected as observed for the LK03 group animals.
Figure 3.
Endpoint analysis for rAAV genomes and huFIX transcript levels in rhesus macaques
Tissues were collected at endpoint and vector copies were normalized to albumin. Vector copy numbers for different liver lobes (A), (B), and several other tissues (C) are shown. Copy numbers were assessed in select samples collected from the right lung and the right kidney. Both the left and the right ventricles of the heart were sampled to obtain vector copy numbers and average values are shown. Vector copies in the brain (right frontal lobe) were determined for one animal in each group only. Human FIX transcript levels in liver samples are shown as percentage of actin levels (D) and (E). Comparison of all animals from the LK03 and the KP1 group for vector copies (F) and transcripts (G) in the liver lobes. In (A), (B), (D), and (E) statistical analysis was performed using one-way ANOVA, in (F) and (G) the unpaired t test with Welch’s correction was used. Only statistically significant differences between the groups are shown. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Error bars represent standard error of the mean.
We also analyzed several other organs for off-target transduction (Figure 3C). When compared with the liver, vector copies in select tissues analyzed were low, particularly for the LK03 group. The spleen was the organ with the second highest rAAV copy number, followed by heart and kidney. However, no expression was detected (data not shown), likely due to the tightly controlled liver-specific expression from the vector promoter used.
It was of interest that, within both groups, the vector copy numbers were similar among all three animals, despite varied transgene expression. High levels of anti-drug antibodies (ADAs) may have been responsible for the loss of antigen expression in animals LK03-C and KP1-E, but should not affect transcript levels. However, when we determined endpoint huFIX transcript levels from the liver lobes, we found that expression in animal LK03-C was approximately 10-fold lower than in the other LK03 group animals (Figure 3D), despite comparable vector copy numbers. A similar phenomenon was observed in one of the animals (KP1-E) from the KP1 group (Figure 3E). As summarized in Figure 3G, transcript levels for the two KP1 group animals with sustained expression (KP1-D, KP1-F) were approximately 10-fold lower than for the LK03 group animals (LK03-A, LK03-B), correlating with protein expression levels during the earlier timepoints (Figures 2A and 2B).
Detection of huFIX expression in hepatocytes by in situ hybridization
To further understand the transcript profile on a cellular basis, we performed RNAScope in-situ hybridization on liver sections of the four animals that exhibited persistent antigen expression until the end of the study. Liver sections showed a normal morphology. We found abundant huFIX transcripts, visualized as small red dots, in hepatocytes of the LK03 group animals, but much less in the KP1 group macaques (Figure 4). The RNAScope data were consistent with the antigen levels (Figures 2A and 2B), as well as the bulk transcript levels as determined by qPCR (Figures 3D, 3E, and 3G). Of note, the LK03 group animals showed an uneven distribution of huFIX-expressing hepatocytes, with the majority of expressing cells located in close proximity to blood vessels. The low-magnification image for animal LK03-A shows an accumulation of huFIX-expressing hepatocytes around the portal triad, which contains the hepatic artery, portal vein, and the bile duct (Figure 4, closed-head arrow). A similar hepatic localization pattern for AAV-LK03 was recently described,26 but the mechanism remains unclear.
Figure 4.
Transcripts of rAAV expressed huFIX detected by RNAScope in situ hybridization using huFIX specific probes in rhesus macaque liver sections of two animals in the rAAV huFIX-LK03 group and two in the rAAV huFIX-KP1 group
Representative images for magnifications 10×, 20×, and 40× are shown. Also shown are representative images of the positive control and negative control (4-hydroxy-tetrahydrodipicolinate reductase [dapB]) probed liver sections. Size markers on the upper left side of each image are 200 μm for the 10x, 100 μm for the 20x, and 50 μm for the 40× magnification, respectively. The closed-head arrow in the LK03 10× magnification image points toward the portal triad. Staining of mRNA can be distinguished from background DNA hybridization by small red dots within the cytoplasm (open-head black arrows in LK03-A and KP1-D, 40× magnification images as examples), while the staining within the nucleus (gray arrow in KP1-D, 40× magnification as an example) is derived from non-specific and/or background probe binding to DNA sequences.
Epigenetic profiling of the vector and host genomes
While we had eliminated all CpG motifs from the transgene sequence for the vector used in the rhesus macaque study, the promoter sequence still contained 16 CpG motifs. We elected to investigate whether the loss of huFIX expression on the mRNA level that was observed for animals LK03-C and KP1-E was possibly due to methylation of promoter sequences in episomal rAAV genomes. Furthermore, we wanted to investigate the possibility of promoter methylation contributing to the transgene expression differences in rhesus as compared with mice. In mammals, CpG hypermethylation of promoters is known to be associated with inactivation of gene expression.27,28 Thus, we studied the methylation status of the promoter within rAAV episomes present in rhesus and mouse liver at the termination of the studies 14 and 18 weeks after vector infusion, respectively. A part of the 5′UTR of DEAD-box protein 4 (Ddx4), which has been described to be hyper-methylated,29 was used as a control. Figure 5A shows the sequence chromatograms for a 143-nt-long stretch of the Ddx4 5′UTR after bisulfite conversion of rhesus liver guide DNA (gDNA). All samples displayed the expected hyper-methylation of cytosines preceding a guanidine within this region, demonstrating that the chosen experimental conditions were suited to completely convert all non-protected cytosine residues to uracils. A few mixed peaks, most likely representing SNPs, were detected in four rhesus macaque samples (nt 32, 33). Figure 5B shows the presence of variable levels of methylated cytosine residues in the Ddx4 5′UTR amplified from bisulfite-treated mouse liver DNA. Figure 5C depicts partial sequence chromatograms for the rAAV promoter region from rhesus as well as mouse samples. No indication for CpG methylation within the depicted stretch or the remaining promoter sequence (not shown) was detected in any of the samples. These results suggest that differential methylation of the rAAV vector promoter region did not account for the loss of huFIX mRNA expression observed in two macaques, nor was it responsible for the discrepancy in transgene expression between mice and macaques after normalizing for rAAV DNA copy numbers.
Figure 5.
Analysis for rAAV promoter methylation in rhesus macaques and mice
Liver lobes were collected at endpoint after administration of 3.5 × 1012 vg/kg rAAV huFIX-LK03 or 3.5 × 1012 vg/kg rAAV huFIX-KP1 or from mice injected with 1 × 1012 vg/kg rAAV huFIX-KP1 and analyzed for methylation by targeted bisulfite sequencing. Partial sequence chromatograms for amplicons derived from bisulfite converted genomic DNA are shown. As a control for complete conversion a part of the rhesus macaque (A) and mouse (B), Ddx4 promoter sequence is shown. A stretch of the non-methylated ApoE/SerpA promoter region of the rAAV genomes in rhesus macaque and mouse liver lobes is shown in (C). The fully converted reference sequence is depicted in the line below the sequence logo. Chromatograms were viewed and aligned using Genious Pro.
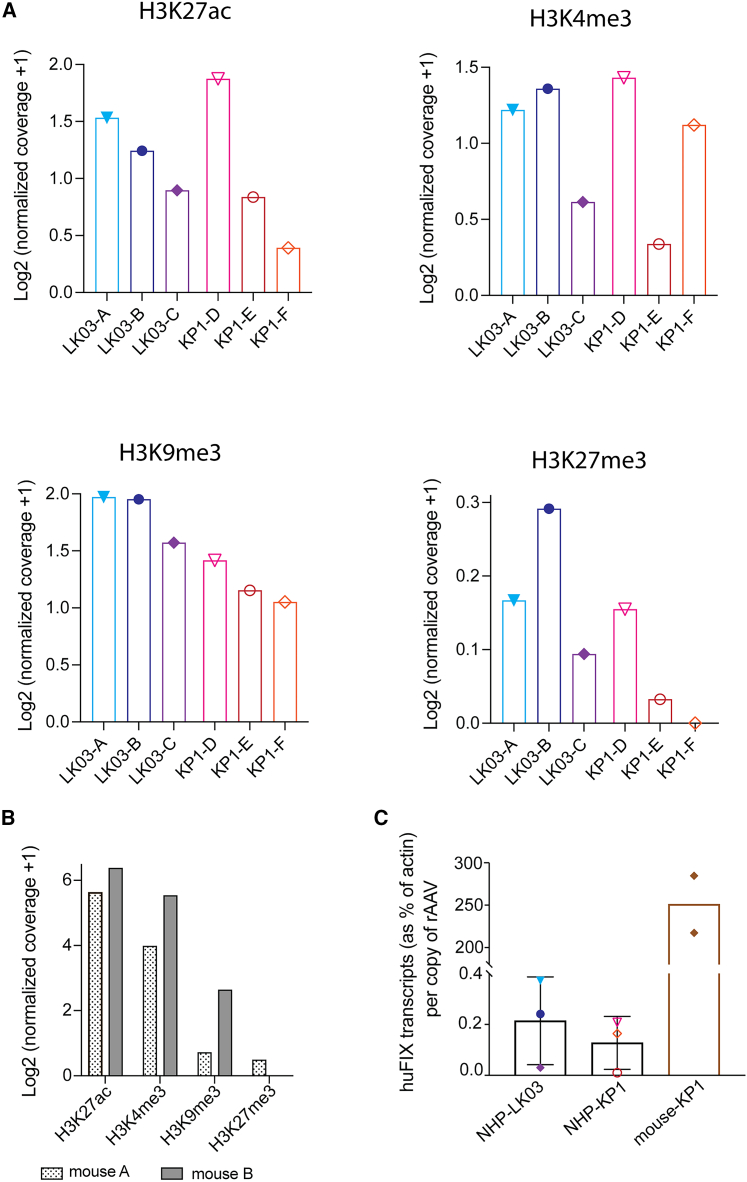
Next, we pursued analysis of histone modifications for nuclear host genomes as well as rAAV genomes by performing a Cut&Tag assay using antibodies directed against two modifications that are associated with a transcriptionally activated state (H3K4me3 and H3K27ac), as well as two antibodies that bind histone marks that are known to repress transcription (H3K9me3 and H3K27me3). As shown in Figures 6A and S6, the two animals that had lost huFIX transcript and antigen expression (LK03-C and KP1-E) had lower levels of activating H3K4me3 marks when calculating the Tn5 normalized coverage across the promoter region of the rAAV genome (see Figure S7 for normalization coverage). For animal LK03-C, H3K4me3 marks associated with the promoter region were 2.5-fold and 3-fold lower than for animal LK03-A and LK03-B, respectively; for KP1-E, the decrease in coverage was 6.4-fold and 4.4-fold when compared with KP1-D and KP1-F, respectively. The observed difference in H3K4me3 marks was not due to overall lower levels of this modification within the genome of those two animals as shown for the Albumin gene (Figure S8). Levels of histone modification associated with a repressed state were not significantly changed (Figure 6A). We also compared modifications of rAAV-associated histones between rhesus macaques and mice that had been administered the identical vector packaged with KP1 capsid and found that Tn5 normalized levels of activating mark H3K27ac within the rAAV promoter region were approximately 30-fold higher in mice (Figure 6B, mouse B, and Figure S9) than in rhesus macaques (Figure 6A, KP1-D), suggesting a causative association with higher rAAV expression observed in mice as compared with rhesus in our studies, as well as others. For activating mark H3K4me3, the difference was up to 27-fold. Levels of rAAV promoter-associated repressive histone marks, in contrast, did not differ considerably between mice and rhesus macaques.
Figure 6.
Quantification of activating and repressive histone modifications within the rAAV promoter region after administration of 3.5 × 1012 vg/kg rAAV huFIX-LK03 or 3.5 × 1012 vg/kg rAAV huFIX-KP1 to rhesus macaques (A) and 2.5 × 1011 vg/kg rAAV huFIX-KP1 into mice (B) and transcriptional efficiency of the identical rAAV huFIX vector in rhesus macaques and mice (C). Values in (A) and (B) are displayed as Log2 of Tn5 normalized coverage +1. Error bars in (C) represent standard error of the mean.
We compared the average transcriptional activity per vector genome for the same vector genome after it was delivered with macaques and mice by calculating the vector copy number and transgene transcript numbers (Figure 6C) that had been injected with KP1-packaged, CpG-depleted, and human codon-optimized sequence B (see Figure S1). The relative number of vector-derived transcripts per vector genome was approximately 1,000× higher in mice compared with the macaques. Of note, the relative number of transcripts per genome in macaques were similar when the vector was delivered with the LK03 and KP1 capsids. Mice were not treated with AAV-LK03 because, as noted, this capsid does not transduce murine cells well. These data make it clear that the identical vector is transcribed at very different levels, depending on the species. This is consistent with the higher levels of activating marks found on histones associated with AAV genomes in mice as compared with rhesus macaques.
Discussion
In the present study, we tested the in vivo expression of a hyperactive huFIX variant in hemophilic mice, as well as in rhesus macaques. As expected, specific activity was enhanced when compared with the wt as well as the Padua versions of the protein. Levels of huFIX expression in all three groups were considerably higher than those achieved in a previous study that used the DJ-8 capsid and a self-complementary hyperactive huFIX expression cassette with an hAAT promoter and MVM intron.24 In the present study, the hyperactive huFIX sequence was mouse codon optimized and contained a truncated version of the first huFIX intron (intron A), which had been shown to lead to strongly enhanced expression in vivo,30 similar to descriptions for other intronic sequences.31,32,33
For the rhesus macaque study, we used a human codon-optimized and CpG-depleted version of the hyperactive huFIX transgene to diminish the risk of a cytokine response and/or cytotoxic T cell responses, which had been associated with a loss in transgene expression.34,35,36,37 Similar to previous macaque studies,38,39,40,41 we observed a peak in transgene expression followed by a gradual decrease, concomitant with the emergence of antibodies directed against the transgene protein product. Peak huFIX activity in the KP1 group was comparable with a study described by Takeda/Baxter using a CpG-depleted Padua construct delivered by the AAV8 capsid into rhesus macaques.41 Activity levels in the LK03 group were higher than in the AAV8 study, which can also be attributed to the higher specific activity of the hyperactive FIX variant as compared with Padua.
As previously observed by others,42,43,44 we saw a sharp increase in neutralizing capsid-directed antibodies shortly after vector infusion, which remained high throughout the study. We observed that one animal from each group lost expression completely, which was not explained by an immune response against the vector or the transgene product, because transcript levels were starkly decrease despite comparable vector copy numbers in the liver. Loss of transgene expression in rhesus studies has been described previously20,41 and can often be attributed to an immune response against the vector genome,34,35,36,37 the capsid,43,45 as well as to the transgene product46,47 (see48,49,50,51,52 for reviews). In a recent study with macaques that had been infused with an AAV3B mutant packaged huFIX vector, one animal lost expression at a later time point in the study due to antibodies directed against the transgene product, while another animal showed diminished transgene expression throughout the observation period, despite a lack of immune responses.39 However, transcript levels in that animal were comparable with those from the other two macaques, suggesting an undetermined immune response as a contributing factor. For our study, we found that transduction in rhesus macaque liver was up to 10-fold higher in the LK03 group than in the KP1 group, an observation that explains the overall lower peak huFIX expression for the KP1 group. However, vector copy numbers for the two animals that lost expression were similar to those of the other macaques in the same group, suggesting that the loss in transgene protein and mRNA expression could not solely be attributed to a cytotoxic T lymphocyte (CTL) response against the rAAV capsid or innate immune responses against residual CpG motifs in the inverted terminal repeats and promoter sequences, which would have resulted in an elimination of transduced hepatocytes. In addition, while antibodies directed against huFIX were present in these two animals, it cannot explain the decrease in the mRNA transcripts. Thus, we reasoned that differences in epigenetic modifications of the vector genome may have contributed to the loss in expression. In other reports, it was recently shown that, for rAAV injected into non-human primate muscle, the episomes form chromatin-like structures that wrap around nucleosomes, similar to cellular chromatin.18 This process can stabilize episomal rAAV, but can also cause epigenetic-mediated changes in transgene expression.
DNA methylation of CpG motifs, particularly in promoter regions, often leads to stable long-term repression,27,28,53 while post-transcriptional modifications of histones are more dynamic and easily reversible.54 Cellular factors involved in both forms of modification can interact with each other and result in either an activating or repressing impact on transcription levels.55 We found no evidence of CpG methylation within the rAAV promoter in any of the animals, consistent with a previous study described in an rAAV non-human primate muscle and liver transduction study.56 These findings support that it is unlikely that DNA methylation played a role in the expression differences observed in the study described herein.
We found that permissive histone marks were decreased in the two animals with low or undectable vector transcripts, while there was no difference in the repressive histone marks. This is in contrast with a recent report that showed AAV-epigenetic silencing was mediated by the NP220/HUSH complex, and that a decrease in AAV transcript levels correlated with AAV-repressive histone marks.57 In contrast, a decreased occupancy of rAAV vector-associated histones with activating marks was recently described to be responsible for a decrease in transgene expression in mice infused with oversized (approximately 5 kb) AAV vectors.17 In addition, our group recently demonstrated that the capsid protein sequence can influence the levels of active, but not repressive, marks on rAAV genome-associated histones, correlating with transcript levels from the rAAV transgene.19 Moreover, that study revealed that those epigenetic modifications occur in a species-specific manner.19 Capsid-mediated epigenetic modifications of rAAV genome-associated histones may possibly contribute to the stable expression of huFVIII delivered with rAAV-LK03 in a human clinical trial.58
We and others have observed that transgene expression is several orders of magnitude lower in non-human primates when compared with mice after systemic infusion, with an equivalent relative dose of the same vector.20,21,22 Moreover, AAV-mediated transgene expression from nearly every single mouse hepatocyte is achievable, but this has yet to be achieved in primates. When we compared histone marks associated with active transcription between mice and rhesus macaques, we found that episomes derived from mice were associated with up to 30-fold higher levels of activating histone marks than those derived from rhesus macaques. This raises the possibility that rodents and primates have inherent differences in their ability to silence rAAV genomes by differential epigenetic mechanisms.
A recently published study analyzed human liver biopsies obtained more than 2 years after infusion with huFVIII-expressing rAAV.59 The authors found that one individual had low mRNA transcript levels and plasma huFVIII protein despite containing similar vector copy numbers as the other study participants. Moreover, histopathology did not show any evidence of liver damage due to a CTL response.59 The authors suggested that the interindividual variation in transgene expression might be attributed to differences in the expression of regulatory molecules involved in transcription, protein folding, and secretion. This finding may have been due to a similar mechanism we observed in the two rhesus that exhibited low transcript levels despite a similar number of vector genomes as the other animals from the same injection group. Together, these results support the idea that the wide differences in dose responses observed in non-human primate studies as well as in human clinical trials6,37,38,39,41,43,59,60,61,62,63,64,65,66,67 may in part be due to individual differences in vector chromatinization and vector epigenome composition. Understanding the genetic polymorphic variation between individuals may allow for better predictions on dose-response outcomes in gene therapy trials.
Material and methods
Animal and study approval
The Institutional Animal Care and Use Committee (IACUC) at Stanford University approved all mouse procedures. Mice at Stanford University received care according to the criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For the studies with rhesus macaques, all procedures conformed to the requirements of the Animal Welfare Act and the Guide, and protocols were approved prior to implementation by the UC Davis IACUC.
Vector plasmids
A single-stranded rAAV vector expressing mouse codon optimized huFIX containing a truncated version of the huFIX intron A under control of a 319-bp liver-specific ApoE hepatic control region (HCR-1) and a partial hAAT promoter (393 bp) was generated using Gibson assembly. Source of the mouse-codon optimized huFIX sequence (148T variant) as well as the ITR containing the vector backbone was a plasmid described in a previous study from our group.68 The regulatory HCR1/hAAT promoter fragment, the truncated huFIX intron A, the first 48 nt of the huFIX 3′UTR, as well as the bovine growth hormone polyA sequence were obtained from a plasmid recently generated in our laboratory.30 Sequences for those regulatory elements were identical between all vectors used in the study. To generate the hyperactive version of huFIX, the following amino acid positions (numbering according to the start of the zymogen sequence) were mutated: 318 (R->Y, aga->tac), position 338 (R->E, aga->gaa), and 343 (T->R, acc->aga). To generate the Padua version of huFIX position 338 (R->L, aga->ctg) was mutated. All mutations were introduced using the QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies, Cat #200523) and verified by Sanger sequencing.
For the rhesus macaque study, the huFIX region coding for the zymogen was optimized for human codon usage and CpG depleted using proprietary software by ATUM. The synthesized sequences were cloned into the backbone vector created previously with the difference that the signal peptide sequence was not the mouse codon optimized version, but the native one. Restriction sites BstZ17I within the huFIX intron as well as a MluI site just downstream of the polyA signal sequence were used for swapping in the new sequences. The native signal peptide sequence was obtained from a plasmid previously generated in our laboratory69,70 and was incorporated into the vector using Gibson cloning. All vectors were sequence verified prior to rAAV production and will be made available upon request.
The AAV2 rep, KP1 cap expressing rAAV packaging plasmid, the AAV2 rep, LK03 cap expressing packaging plasmid, and the CAG-FLuc rAAV vector have been described previously.10,15 All plasmids are available at Addgene (Cat #206504, 206512, 83281).
Production of rAAV
Recombinant AAV was produced by triple transfection into 293T or 293T/17 cells either by CaPO4 or PEI 25K transfection followed by two rounds of CsCl gradient purification as described previously.10 Vector genomes were purified using the QIAmp MinElute Virus Spin Kit (Qiagen, Cat #57704) and titers were determined by qPCR using a primer set specific for the respective expression cassette and a standard curve generated from serially diluted linearized plasmid DNA. For some mouse studies, rAAV purified from cell lysates with the AAVpro Purification Kit Maxi—All Serotypes (Takara, Cat #6666) was used. For the rhesus study, rAAV was manufactured under good manufacturing practice-like conditions by the Gene Therapy Center Vector Core of the University of North Carolina at Chapel Hill. The purity of vector preparations was determined by performing silver staining of preparations after polyacrylamide gel electrophoresis and by scanning electron microscopy. Vector preparations were re-titered at Stanford using the intron primer set with a serially diluted plasmid standard. Endotoxin levels were determined with the Limulus Amebocyte Lysate Endochrome-K kit from Charles River Laboratories (Thermo Fisher Scientific, Cat #NC9567565) according to the manufacturer’s instructions. Endotoxin levels of all virus preps were less than 0.2 EU/mL. Further vector testing (e.g., sterility) was performed prior to vector dosing with approximately 1 mL rAAV/kg. All conditions and assays to ensure sterility and lack of contaminants were performed as required.
Cell culture conditions
SNU-387 cells (ATCC Cat #CRL-2237) were cultured in RPMI (Thermo Fisher Scientific, Cat #A10491-01) with 10% FBS (Gemini Bioproducts, Cat #100–500), 2 mM glutamine (Thermo Fisher Scientific, Cat #25030-081), 1% antimycotic-antibiotic (Thermo Fisher Scientific, Cat #15240-062), and 1% non-essential amino acids (Thermo Fisher Scientific, Cat #11140-050).
HEK 293T cells (ATCC, Cat #CRL-3216) and 293T/17 cells (ATCC, Cat #CRL-11268) were cultured in DMEM (Thermo Fisher Scientific, Cat #15-017-CV) with 10% FBS, 2 mM glutamine, and 1% antimycotic-antibiotic. When performing CaPO4 transfection, the media were supplemented with 20 mM HEPES buffer (Thermo Fisher Scientific, Cat #15630-080).
Mouse experiments
For evaluation of mouse liver transduction efficiency, 6-week-old female hemophilic FIX knock-out mice71 (The Jackson Laboratory, strain B6.129P2-F9tm1Dws/J, Stock# 004303) or wt female C57BL/6J mice (The Jackson Laboratory, Stock# 000664) were injected with various doses of rAAV vector via normodynamic intravenous lateral tail vein injections. Mice were bled retro-orbitally at regular intervals and citrated plasma was used to perform huFIX antigen ELISA and huFIX activity assays. After 10, 12, or 18 weeks, mice were euthanized and livers were pulverized on dry ice using a mortar and pestle and stored at −80°C or below for subsequent vector copy numbers and transcript analysis.
Rhesus macaque studies
Eighteen male juvenile rhesus macaques with weights ranging from 4 to 7 kg were screened for pre-existing α-LK03 and α-KP1 capsid antibodies by an in vitro nAb assay as described below. Three macaques showed a complete absence of nAb toward the LK03 capsid, whereas four macaques were negative toward the KP1 capsid. Five animals had low nAb titers (<50% inhibition with undiluted serum) against the LK03 capsid and three of those had low titers against the KP1 capsid. Three animals were selected for each capsid group—two each with no pre-existing nAbs and one each with minimal nAb titers. nAb status was comparable at day 0 (Figure S3). Six animals were administered the vector via slow intravenous infusion (3.5 × 1012 vg/kg) with vector diluted in sterile PBS containing 0.001% Pluronic-F68 Polyol (MP Biochemicals, Cat #2750049) over 20 min and in a total volume of 20 mL. Blood was collected from a peripheral vessel at various timepoints and processed to obtain plasma and serum. Animals were euthanized approximately 3 months after vector administration. Blood and a range of tissues were collected at the endpoint. At endpoint, blood samples were collected to obtain serum and plasma and sections of select tissues were placed in sterile Sarstedt tubes then flash-frozen and stored at −80°C or below for the biodistribution study. Samples from each liver lobe were individually pulverized on dry ice to obtain a homogeneous distribution and were aliquoted and stored at −80°C, being careful to never let the tissue thaw. Those aliquots were then used to extract DNA and RNA and analyzed for vector genomes by qPCR and southern blot and transcript levels. For in situ hybridization, liver samples approximately 0.5 × 0.5 cm in size were rinsed with sterile PBS and incubated in 10% formaldehyde-PBS for 24 h prior to further processing, as described below.
Determination of huFIX antigen levels
A sandwich ELISA was used to determine antigen levels in mouse and rhesus macaque plasma samples. Briefly, ELISA plates (Maxisorp, Thermo Fisher Scientific, Cat #442404) were coated with 200 ng/well mouse monoclonal α-huFIX antibody (Haematologic Technologies, Cat #AHIX-5041) in 100 μL/well carbonate-bicarbonate buffer (Sigma, Cat #C3041) overnight at 4°C. Non-specific binding sites were blocked by a 1-h incubation with Casein-TBS (300 μL/well, Thermo Fisher Scientific, Cat #37532). After blocking, plasma samples that had been diluted in Casein-TBS were added to the plate without a prior wash step. Plates were incubated for 1 h at room temperature (RT), then washed with TBS-0.05% Tween (4 × 200 μL, Sigma, Cat #T-9039), and incubated with 100 μL 1:1,000 diluted peroxidase coupled goat α-huFIX antibody (Affinity Biologicals, Cat #GAFIX-HRP) in 100 μL/well Casein-TBS for 1 h at RT. Plates were washed again 4 times, and TMB-Ultra substrate (100 μL/well, Thermo Fisher Scientific, Cat #34028) was added and the reaction incubated for 10 min at RT before adding 100 μL/well 2 M H2SO4 to stop the reaction. Plates were read at 450 nm with 650 nm reference wavelength using a Tecan plate reader. Data analysis was performed using a four-parameter fit curve with the MPM 6 software (BioRad) on defined protein standards. Standard dilutions for wt huFIX were prepared from recombinant purified huFIX protein (Abcam, Cat #ab62544), whereas recombinant purified Padua and dalcinonacog alfa FIX protein samples were obtained from Cambridge Protein Works. When samples were diluted less than 1–30; the respective standards were spiked into Casein-TBS that contained the same amount of naive plasma. The source of naive mouse plasma was plasma from non-injected control mice, whereas citrated plasma from naive rhesus macaques was obtained from Bio IVT (Cat #NHP02PL32NCPN5). The ELISA was shown to be highly specific for huFIX and did not detect antigen in plasma from non-injected control mice or rhesus macaques.
Determination of huFIX activity levels
FIX activity was assayed at Haemtech Biopharma Services with an activated partial thromboplastin time FIX one-stage clotting assay on an ACL-TOP instrument (Instrumentation Laboratory) using the manufacturer’s recommended reagents. Calibration was performed against Instrumentation Laboratory reference plasma, which is traceable to the World Health Organization standard (09/172). Samples were analyzed using a balance assay design and each sample was run in duplicate using the required number of dilutions to obtain valid results. Control samples were included in each run.
Determination of α-huFIX antibodies
ADAs to dalcinonacog alfa were determined at Charles River Laboratories through an electrochemiluminescence immunoassay. The positive control consisted of an affinity purified sheep α-huFIX polyclonal antibody (Haematologic Technologies, Cat #PAHFIX-SAP) spiked into pooled rhesus macaque plasma at a high positive control concentration of 2,500 ng/mL and a low positive control concentration of 40 ng/mL. The negative control consisted of pooled monkey plasma to represent the background signal of the assay. Controls and samples were diluted 10-fold with 325 mM acetic acid and incubated for 30 min. Biotinylated CB2679d and sulfo-tagged CB2679d were diluted to 0.5 μg/mL each in 300 mM Tris, pH 10.0 in blocking buffer (Blocker Casein in PBS; Thermo Fisher Scientific, Cat #37528) and then incubated in a 1:1 ratio with the acid-treated controls and samples for 2 h at RT with shaking (600 RPM). An MSD gold streptavidin coated plate (MSD, Cat #L15SA) was blocked with 150 μL/per well blocking buffer for 2 h at RT with shaking (600 RPM), followed by removal of blocking buffer and the addition of 25 μL to each sample and control. The plate was incubated for 1 h at RT with shaking (600 RPM), washed three times with 0.1% Tween 20 in PBS (300 μL per well), and electrochemiluminescence was detected after addition of 4× Read Buffer T with Surfactant (MSD, Cat #R92TC) using a Meso Scale Discovery S 600 Sector plate reader. Presumptive positive samples were determined by comparing the sample’s mean enhanced chemiluminescence (ECL) response with a screening plate-specific cut point that is based on the median ECL response of the NC corrected with a screening cut point factor.
Vector copy number quantification
Genomic DNA was isolated from pulverized mouse liver tissue as well as from various pulverized rhesus macaque tissues using the GeneJET Genomic DNA Purification Kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Cat #K0721). We used 50 ng gDNA to quantify rAAV copy numbers by qPCR. For mouse livers, either huFIX specific primers F9-F (5′-ATCTACAACAACATGTTCTGCG-3′) and F9-R (5′-CTGATGATGCCGGTCAGAAA-3′) or intron A-specific primers intron-F (5′-TAGCTGACAGTACCAGGATCA-3′) and intron-R (5′-CTCCTGAAGAACAGAAGCCTAAT-3′) were used. Input DNA was normalized to mouse actin copies using primers actin-F (5′-GTCGAGTCGCGTCCACC-3′) and actin-R (5′-CAGTGAGGTACTAGCCACGAGA-3′). For the experiment shown in Figure 6C, primer pair opt2-F9-F (5′-CCTCAACAGGCCTAAGAGATAC-3′) and opt2-F9-R (5′-CACTTCTCTGGCTTCCTCAA-3′) were used instead of the intron A primer set. Genome copy number quantification in rhesus tissues was performed with opt2-F9-F and opt2-F9-R using alb-F (5′-GTTGCTGTTATCTCTTGTGGGCTGT-3′) and alb-R (5′-ACTCATGGGAGCTGCCGGTTC-3′)72 for normalization. Standard curves derived from linearized and serially diluted amplicon containing plasmids were run for each target.
Quantification of huFIX transcripts
Total RNA was isolated from pulverized mouse and macaque tissues with Trizol according to the manufacturer’s instructions (Thermo Fisher Scientific, Cat #15596026). For each reaction ,2.5 μg RNA was DNase treated using the ezDNase kit (Thermo Fisher Scientific, Cat #11766051) according to the manufacturer’s instructions. The Superscript III First strand synthesis kit (Thermo Fisher Scientific, Cat #18080-051) was used to generate cDNA employing oligodT for reverse transcription. Transcripts in mouse livers were quantified by qPCR with primers F9-F and F9-R. For the mouse study comparing different sequence optimized rAAV constructs, we quantified huFIX transcripts with primer pair signal-F (ATGCAGCGCGTGAACAT) and signal-R (ACATTCAGCACTGAGTAGATATCCTAAA). Signal-R primer was substituted with m-co-signal-R (5′-TACACTCGGCGCTCAGCA-3′) for determining transcripts from the mouse codon-optimized vector construct. Endogenous mouse actin transcript levels were used for normalization. Sequences for normalization primers were actqRT-F (5′-GTGACGTTGACATCCGTAAAGA-3′) and actqRT-R (5′-GCCGGACTCATCGTACTCC-3′). For the experiment shown in Figure 6C, primer pair opt2-F9-F and opt2-F9-R were used instead of the signal-F and signal-R primer set. huFIX transcripts in rhesus macaque tissues were quantified using primers opt2-F9-F and opt2-F9-R. Endogenous actin control primers were rh-actqRT-F (5′-GGGACCTGACTGACTACCT-3′) and rh-actqRT-R (5′-CCTTAATGTCACGCACGATTTC-3′).
RNAScope in situ hybridization
At the time of euthanasia, representative samples of each liver lobe were dissected and sectioned into approximately 2–4 cm2 cubes, rinsed in sterile PBS several times, and immediately placed in 10% neutral buffered formalin (Thermo Fisher Scientific) at 4°C with gentle agitation. Tissue was rinsed with PBS and deionized H2024 h later , then sequentially placed for 24 h each in 10%, 20%, and 30% sucrose solutions (Sigma Aldrich). Tissues were then embedded in Optimal Cutting Temperature media (Sakura Finetek) and frozen using 2-methyl butane (Sigma Aldrich) with liquid nitrogen. Frozen blocks were placed at −20°C or less for 1 h until final storage at −80°C or below. Tissue was sliced into 9-μm sections, placed on slides, and stored desiccated at −80°C or below until use. These sections were used for RNAScope hybridization performed according to the manufacturer’s instructions (RNAscope 2.5 HD Detection Reagent – RED User Manual, Advanced Cell Diagnostics) using a series of 18- to 25-nt anti-sense probes (approximately 20 oligonucleotides) tiled across a 1.0-kb region of target DNA. A custom probe was designed to detect codon-optimized human factor 9 mRNA with minimal binding to Macaca mulatta clotting FIX. Control probes consisted of an M. mulatta peptidylprolyl isomerase B (positive control for RNA quality) or bacterial 4-hydroxy-tetrahydrodipicolinate reductase (negative control). RNA specificity was confirmed using RNase digestion of control tissue sections (example shown in Figure S10) and slides were counterstained with 50% hematoxylin (Thermo Fisher Scientific). Imaging was performed using a Leica DM2000 brightfield microscope.
Neutralization assay
Rhesus macaque sera were analyzed for sensitivity to nAbs against LK03 and KP1 capsids. Neutralization assays were essentially performed as described.73 Briefly, rhesus sera were serially diluted (nine 3-fold dilutions, starting at undiluted sera) in complement-inactivated FBS and 25 μL of each dilution as well as a no-rhesus serum control were combined with 1.67 × 107 vector copies of Firefly luciferase expressing rAAVs10 diluted in 25 μL RPMI media without FBS. Virus-serum mixtures were incubated for 1 h at 37°C. SNU-387 cells that had been seeded on 96-well plates the night before (8 × 103 per well) were transduced with the virus-serum mixtures in triplicate (7.5 μL each well, corresponding with a multiplicity of infection of approximately 200) and luciferase activity in the cells was determined 24 h later by adding Bright-Glo substrate (100 μL/well, Promega, Cat #E2620) and measuring luminescence with a Veritas luminometer using the GloMax-96 software (Promega). A standard curve using serial dilutions of recombinant luciferase protein (QuantiLum, Promega, Cat #E1701) was included in each assay to ensure the luminescence readings fell within the linear range of the assay. For quality control purposes, a neutralization assay using a previously determined concentration range10 of human pooled immunoglobulin (Baxter, Cat #LE1500190) was run with each assay. Some rhesus sera, particularly the later time points, had to be repeated with more dilutions to determine the 50% neutralizing titer.
Southern blot
Standard methods for southern blot with genomic DNA were applied. Briefly, for estimating vector copies, 10 μg of each liver gDNA sample consisting of a pool of gDNA isolated from the four liver lobes (left lateral, right lateral, caudate, and quadrate) was subjected to restriction digest with 200 U PacI, 200 U SpeI, and 200 U Sac I overnight at 37°C in a volume of 600 μL. Pac I and Spe I cut in the vector close to the ITRs and thus yield a 4,037-nt vector fragment, while Spe I and Sac I cut within the rhesus macaque albumin locus yielding a 2,538-nt-long fragment. For analysis of the different forms of episomal rAAV 10 μg liver gDNA were digested with 200 U Nco I, 100 units of Btg I, and 100 U Blp I, none of which cuts in the vector genome. Naive rhesus macaque gDNA (Zyagen) was loaded as a negative control. Vector copy standards were generated by spiking 100, 10, and 1 genome equivalents of the rAAV vector plasmid into 10 μg negative rhesus macaque gDNA and digesting with Pac I, Spe I, and Sac I. For preparation of size standards, 1 genome equivalent of a 7.2-kb plasmid that contained the huFIX rAAV vector sequence was spiked into 2.5 μg Swiss-Webster albino mouse gDNA (Promega) and digested in a volume of 200 μL with various combinations of enzymes to yield the desired fragment sizes of 7.1 kb, 3.6 kb, and 1.6 kb. All enzymes were purchased from NEB. Digests were pooled only after addition of phenol-chloroform-isoamyl alcohol (Invitrogen). Fragments were phenol-chloroform purified following the overnight restriction digest and precipitated using one volume 2-propanol after the addition of 1/10 volume of 3 M sodium acetate and 5 μL GlycoBlue (15 mg/mL, Invitrogen). After an overnight incubation −20°C or below, fragments were pelleted by centrifugation at 4°C, washed with 70% ethanol, air dried, and resuspended in 40 μL Tris-EDTA buffer with gel loading buffer (Blue Juice, Invitrogen). Samples were loaded onto a 10 × 6-inch ethidiumbromide containing 0.8% agarose gel using Tris-Acetate-EDTA as running buffer alongside a 1kbPlus size marker (Thermo Fisher Scientific). The gel was run at 40 V overnight, incubated with denaturating buffer (3 M NaCl, 0.4 M NaOH) twice for 30 min with agitation, and incubated in transfer buffer (3 M NaCl, 8 mM EDTA) for 15 min with agitation. The gel was then blotted overnight onto a positively charged nylon membrane (Roche) using a piece of Whatman paper serving as a wick to transfer the buffer and thus the DNA from the gel onto the membrane by the means of capillary action. After crosslinking (GS GeneLinker, BioRad) the membrane was pre-hybridized with 10 μg/mL salmon sperm DNA in PerfectHyb Plus Hybridization buffer (Sigma, Cat #H7033) for a minimum of 2 h at 60°C with rotation. The probe against the rAAV vector sequence was generated by amplification of a 431-nt-long intron A fragment from the rAAV vector plasmid using primers intr-F (5′-TAGCTGACAGTACCAGGATCA-3′) and intr-R (5′-AGGGCATGGAGCCAAAATC-3′). The probe against rhesus macaque albumin consisted of a 475-nt-long sequence encompassing rhesus macaque albumin exon 12 and part of the adjacent intron. The genomic fragment was amplified from rhesus macaque gDNA using primers rh-alb-F (5′-CCGGTTATGTGTGTTGCATG-3′) and rh-alb-R (5′-TCTACTGAACATGGCCTAAGATG-3′), cloned into a ZeroBlut TOPO vector and sequence verified. The amplicons were gel purified and 10 ng were labeled with [˂-32P] dCTP using the BcaBEST Labeling Kit (Takara) according to the manufacturer’s instructions. Unincorporated nucleotides were removed with an Illustra Microspin G-25 column and the probe was added to the pre-hybridized membrane. Only the intron A probe was added to the blot derived from the non-cutter gel. Hybridization was allowed to occur for 2–3 days at 60°C with rotation. The membrane was washed twice under low-stringent condition (2× SSC, 20 min at RT), followed by one wash under high-stringent conditions (2× SSC with 0.1% SDS, 30 min at 60°C). The membrane was exposed onto a phosphoimager screen and visualized using the Personal Molecular Imager (BioRad). Image analysis was performed using QuantityOne software (BioRad). Prior to the transfer the agarose gels were imaged using a ChemDoc instrument (BioRad) and the molecular weight marker lanes were aligned with the blot after imaging.
Targeted bisulfite sequencing
We analyzed liver samples from each rhesus macaque as well as two mice that had received the huFIX expressing rAAV. For the mouse, liver samples from the group that was injected with 2 × 1010 vg (approximately 1 × 1012 vg/kg) of the mouse codon-optimized CB 2679d-GT construct due to the low vector copy numbers in the mice that had been injected with the human codon-optimized CpG-depleted construct (injected with approximately 2.5 × 1011 vg/kg), which might hamper analysis. Bisulfite conversion was performed using the EpiTect Bisulfite kit (Qiagen) according to the manufacturer’s instructions with the exception that an additional 5 min 95°C, 2 h 60°C step was added to the recommended cycling protocol. A 532-nt stretch of the promoter region containing 16 CG dinucleotides was amplified from converted DNA in two overlapping parts using primers conv-Prom-F1 (5′-TGGGGTAGAGGTTAGAGATTTTTTT-3′) and conv-Prom-R1 (5′-ATCATTATACCTAACTCAAAAACCACAA-3′), as well as primers conv-Prom-F2 (5′-GGATTTTGTAGTGAGAGTAGAGGGTT-3′) and conv-Prom-R2 (5′-AAAATCATTCACTATCCCAAATCAA-3′) using a previously published protocol,53 with the exception that the number of PCR cycles was reduced to 30 or 40 cycles, depending on the rAAV vector copy number in the sample. As a control, we queried the methylation status of the promoter containing the 5′UTR of the Ddx4, a region within a CpG island that has been described to be differentially methylated in mouse tissue.29 The hyper-methylated state in mouse liver is associated with promoter shut-off, while its hypo-methylated state in testis is associated with high expression. A 354-bp (macaque) or 310-bp (mouse) long fragment was amplified in a 40-cycle PCR using primers rhDdx-F (5′- TTTTTAGAATAAGGATTTTATTAGAGAA-3′) and rhDdx-R (5′- AAAAAAACAACCCACTCACCTC-3′) for the rhesus macaque samples or mDdx-F (5′- TTTTATAGGTTATGGAGTTAAGAGGTTTTT-3′) and mDdx-R (5′-ACCAAAACCAAACTTAAAAAACAAA-3′) for the mouse samples. All primers were designed using the MethPrimer software74 (available at http://www.urogene.org/methprimer/index.html). An aliquot of the amplification reaction was loaded on an agarose gel for quality control and a pool consisting of at least two independent reactions was subjected to ExoSAP-IT clean-up according to the manufacturer’s instructions (Thermo Fisher Scientific). Each amplicon was Sanger sequenced with the primers used for amplification, and chromatograms aligned to the fully converted reference sequence were visualized using Genious Prime 2023.0.4. It should be noted that very low levels of CpG methylation may not be detected using amplicon sequencing, as it was employed for this study.
Cut&Tag and Tn5 normalization
Analysis of epigenetic modifications of histone proteins from mouse liver was performed as described previously.19 The protocol of nuclei isolation from rhesus macaque samples was slightly modified from the protocol described for mouse liver. Briefly, equal amounts of each of the four pulverized liver lobe samples were pooled (approximately 100 mg total weight) and homogenized in RINO 1.5 mL Screw-Cap tubes filled with stainless steel beads and 1 mL NE buffer using a bead homogenizer (Next Advance Bullet Blender Storm - BBY24M) at speed 8 for 6 min, and fibrous tissue was removed using a 40-μm cell strainer. Sequencing was performed on Illumina HiSeq 4000 and Illumina NovaSeq 6000 instruments by the Genomics Core at Stanford University and by Novogene. The GEO accession files can be accessed at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE261047.
Data processing and analysis
Reads obtained from Cut&Tag as well as from Tn5 normalization high-throughput sequencing were processed as previously described.19 Mapping was performed using the Mmul_1 and mm10 build of the rhesus macaque and mouse genome respectively, Host genomes were indexed with the rAAV genome sequence prior to analysis.
Statistics
Statistical analyses were conducted with GraphPad Prism 9 (Version 9.3.1) software applying the statistical methods indicated in the figure legends. Error bars represent standard error of the mean. Experimental values were assessed via one-way or two-way ANOVA using Tukey’s multiple comparisons test. p values of less than 0.05 were considered statistically significant.
Data and code availability
All raw and processed sequence data have been deposited in GEO (Gene Expression Omnibus) and are available under accession number GSE261047.
Acknowledgments
We thank Francesco Puzzo (Stanford University) for guidance on statistics, Yuqing Jing (Stanford University) for suggestions regarding targeted bisulfite sequencing, and Hagoon Jang (Stanford University) for help with Adobe Illustrator for figure generation and for help with bioinformatics. We also wish to acknowledge Addie von Eynern (Haematologic Technologies, Essex Junction, VT) for performing huFIX activity assays, and Valerie Leesch (Charles River, Skokie, IL) for α-huFIX assays. Natacha LeMoan and Lauren Kelly (Catalyst Biosciences) helped with data compilation during the rhesus study. We also thank Claes Gustafsson and Jeremy Minshull (ATUM) for helpful advice and discussions regarding sequence optimization for the rhesus study. Support for this study was provided by NIH R01HL064274 (MAK), NIH R01AI116698 (MAK), CatalystBio 150627, and the Primate Center base operating grant P51-OD011107. Next-generation sequencing was provided by the Genomics Core at Stanford University using instrumentation that had been funded with NIH awards S10OD025212 and S10OD021763. The graphical abstract was generated using BioRender and a publication license was obtained. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the various funding bodies or universities involved.
Author contributions
K.P., C.J.S., and M.A.K. designed the experiments. K.P., C.J.S., F.Z., A.G-S., A.G., G.B., and M.A.K. generated reagents, protocols, performed experiments and analyzed data. A.F.T. performed the rhesus study, performed analysis of blood parameters, and distributed samples for further analysis. K.P and M.A.K. wrote the manuscript and generated the figures. All authors reviewed, edited, and commented on the manuscript.
Declaration of interests
K.P., G.B., and M.A.K. are inventors on patents used in this paper. G.B., L.K., and N.L.M. have commercial affiliations. G.B. and M.A.K. have stock and/or equity in companies with technology broadly related to this paper.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2024.05.005.
Supplemental information
References
- 1.Ljung R.C. Can haemophilic arthropathy be prevented? Br. J. Haematol. 1998;101:215–219. doi: 10.1046/j.1365-2141.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- 2.Batty P., Lillicrap D. Hemophilia Gene Therapy: Approaching the First Licensed Product. Hemasphere. 2021;5:e540. doi: 10.1097/HS9.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George L.A. Hemophilia gene therapy: ushering in a new treatment paradigm? Hematol. Am. Soc. Hematol. Educ. Program. 2021;2021:226–233. doi: 10.1182/hematology.2021000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani A.C., McIntosh J., Sheridan R. Liver Gene Therapy. Hum. Gene Ther. 2022;33:879–888. doi: 10.1089/hum.2022.169. [DOI] [PubMed] [Google Scholar]
- 5.Samelson-Jones B.J., George L.A. Adeno-Associated Virus Gene Therapy for Hemophilia. Annu. Rev. Med. 2023;74:231–247. doi: 10.1146/annurev-med-043021-033013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soroka A.B., Feoktistova S.G., Mityaeva O.N., Volchkov P.Y. Gene Therapy Approaches for the Treatment of Hemophilia B. Int. J. Mol. Sci. 2023;24:10766. doi: 10.3390/ijms241310766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullard A. FDA approves first haemophilia B gene therapy. Nat. Rev. Drug Discov. 2023;22:7. doi: 10.1038/d41573-022-00199-8. [DOI] [PubMed] [Google Scholar]
- 8.Nichols T.C., Levy H., Merricks E.P., Raymer R.A., Lee M.L. Preclinical evaluation of a next-generation, subcutaneously administered, coagulation factor IX variant, dalcinonacog alfa. PLoS One. 2020;15:e0240896. doi: 10.1371/journal.pone.0240896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahlangu J., Levy H., Lee M., Del Greco F. Efficacy and safety of subcutaneous prophylaxis with dalcinonacog alfa in adults with haemophilia B. Haemophilia. 2021;27:574–580. doi: 10.1111/hae.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pekrun K., De Alencastro G., Luo Q.J., Liu J., Kim Y., Nygaard S., Galivo F., Zhang F., Song R., Tiffany M.R., et al. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. JCI Insight. 2019;4:e131610. doi: 10.1172/jci.insight.131610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms E.J., Berry M.W., Chaw R.C., DuFort C.C., Sun D., Onate M.K., Oon C., Bhattacharyya S., Sanford-Crane H., Horton W., et al. Mesenchymal Lineage Heterogeneity Underlies Nonredundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2022;12:484–501. doi: 10.1158/2159-8290.CD-21-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi K., Horikawa M., Dorrell D., Baggett H., Dissen G., Hobbs T., Kievit P., Brissova M., Powers A., Roberts C., et al. A high-throughput barcode screening identifies AAV-KP1 as a capsid that efficiently transduced pancreatic islets in hon-human primates following retrograde pancreatic duct injection. Mol. Ther. 2022;30:76. [Google Scholar]
- 13.Furusho T., Adachi K., Galbraith-Liss M., Sairavi A., Das R., Nakai H. Enhancing gene transfer to renal tubules and podocytes by context-dependent selection of AAV capsids. bioRxiv. 2023 doi: 10.1101/2023.07.28.548760. Preprint at. [DOI] [Google Scholar]
- 14.Aaron K.A., Pekrun K., Atkinson P.J., Billings S.E., Abitbol J.M., Lee I.A., Eltawil Y., Chen Y.S., Dong W., Nelson R.F., et al. Selection of viral capsids and promoters affects the efficacy of rescue of Tmprss3-deficient cochlea. Mol. Ther. Methods Clin. Dev. 2023;30:413–428. doi: 10.1016/j.omtm.2023.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisowski L., Dane A.P., Chu K., Zhang Y., Cunningham S.C., Wilson E.M., Nygaard S., Grompe M., Alexander I.E., Kay M.A. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George L.A., Monahan P.E., Eyster M.E., Sullivan S.K., Ragni M.V., Croteau S.E., Rasko J.E.J., Recht M., Samelson-Jones B.J., MacDougall A., et al. Multiyear Factor VIII Expression after AAV Gene Transfer for Hemophilia A. N. Engl. J. Med. 2021;385:1961–1973. doi: 10.1056/NEJMoa2104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handyside B., Ismail A.M., Zhang L., Yates B., Xie L., Sihn C.R., Murphy R., Bouwman T., Kim C.K., De Angelis R., et al. Vector genome loss and epigenetic modifications mediate decline in transgene expression of AAV5 vectors produced in mammalian and insect cells. Mol. Ther. 2022;30:3570–3586. doi: 10.1016/j.ymthe.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penaud-Budloo M., Le Guiner C., Nowrouzi A., Toromanoff A., Chérel Y., Chenuaud P., Schmidt M., von Kalle C., Rolling F., Moullier P., Snyder R.O. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Sandoval A., Pekrun K., Tsuji S., Zhang F., Hung K.L., Chang H.Y., Kay M.A. The AAV capsid can influence the epigenetic marking of rAAV delivered episomal genomes in a species dependent manner. Nat. Commun. 2023;14:2448. doi: 10.1038/s41467-023-38106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greig J.A., Breton C., Martins K.M., Zhu Y., He Z., White J., Bell P., Wang L., Wilson J.M. Loss of transgene expression limits liver gene therapy in primates. bioRxiv. 2022 doi: 10.1101/2022.03.24.485675. Preprint at. [DOI] [Google Scholar]
- 21.Hurlbut G.D., Ziegler R.J., Nietupski J.B., Foley J.W., Woodworth L.A., Meyers E., Bercury S.D., Pande N.N., Souza D.W., Bree M.P., et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol. Ther. 2010;18:1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nietupski J.B., Hurlbut G.D., Ziegler R.J., Chu Q., Hodges B.L., Ashe K.M., Bree M., Cheng S.H., Gregory R.J., Marshall J., Scheule R.K. Systemic administration of AAV8-alpha-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol. Ther. 2011;19:1999–2011. doi: 10.1038/mt.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabanes-Creus M., Navarro R.G., Liao S.H.Y., Baltazar G., Drouyer M., Zhu E., Scott S., Luong C., Wilson L.O.W., Alexander I.E., Lisowski L. Single amino acid insertion allows functional transduction of murine hepatocytes with human liver tropic AAV capsids. Mol. Ther. Methods Clin. Dev. 2021;21:607–620. doi: 10.1016/j.omtm.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair N., De Wolf D., Nguyen P.A., Pham Q.H., Samara-Kuko E., Landau J., Blouse G.E., Chuah M.K., VandenDriessche T. Gene therapy for hemophilia B using CB 2679d-GT: a novel factor IX variant with higher potency than factor IX Padua. Blood. 2021;137:2902–2906. doi: 10.1182/blood.2020006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahlab S., Linial M. Speed controls in translating secretory proteins in eukaryotes--an evolutionary perspective. Plos Comput. Biol. 2014;10:e1003294. doi: 10.1371/journal.pcbi.1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabanes-Creus M., Navarro R.G., Liao S.H.Y., Scott S., Carlessi R., Roca-Pinilla R., Knight M., Baltazar G., Zhu E., Jones M., et al. Characterization of the humanized FRG mouse model and development of an AAV-LK03 variant with improved liver lobular biodistribution. Mol. Ther. Methods Clin. Dev. 2023;28:220–237. doi: 10.1016/j.omtm.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda T.B., Jones P.A. DNA methylation: the nuts and bolts of repression. J. Cell. Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 28.Klose R.J., Bird A.P. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Song F., Smith J.F., Kimura M.T., Morrow A.D., Matsuyama T., Nagase H., Held W.A. Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression. Proc. Natl. Acad. Sci. USA. 2005;102:3336–3341. doi: 10.1073/pnas.0408436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao C.H., Ohashi K., Patijn G.A., Meuse L., Ye X., Thompson A.R., Kay M.A. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- 31.Brinster R.L., Allen J.M., Behringer R.R., Gelinas R.E., Palmiter R.D. Introns increase transcriptional efficiency in transgenic mice. Proc. Natl. Acad. Sci. USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmiter R.D., Sandgren E.P., Avarbock M.R., Allen D.D., Brinster R.L. Heterologous introns can enhance expression of transgenes in mice. Proc. Natl. Acad. Sci. USA. 1991;88:478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K., Sandgren E.P., Palmiter R.D., Stein A. Rat growth hormone gene introns stimulate nucleosome alignment in vitro and in transgenic mice. Proc. Natl. Acad. Sci. USA. 1995;92:7724–7728. doi: 10.1073/pnas.92.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright J.F. Quantification of CpG Motifs in rAAV Genomes: Avoiding the Toll. Mol. Ther. 2020;28:1756–1758. doi: 10.1016/j.ymthe.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertolini T.B., Shirley J.L., Zolotukhin I., Li X., Kaisho T., Xiao W., Kumar S.R.P., Herzog R.W. Effect of CpG Depletion of Vector Genome on CD8(+) T Cell Responses in AAV Gene Therapy. Front. Immunol. 2021;12:672449. doi: 10.3389/fimmu.2021.672449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright J.F. Codon Modification and PAMPs in Clinical AAV Vectors: The Tortoise or the Hare? Mol. Ther. 2020;28:701–703. doi: 10.1016/j.ymthe.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konkle B.A., Walsh C.E., Escobar M.A., Josephson N.C., Young G., von Drygalski A., McPhee S.W.J., Samulski R.J., Bilic I., de la Rosa M., et al. BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood. 2021;137:763–774. doi: 10.1182/blood.2019004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spronck E.A., Liu Y.P., Lubelski J., Ehlert E., Gielen S., Montenegro-Miranda P., de Haan M., Nijmeijer B., Ferreira V., Petry H., van Deventer S.J. Enhanced Factor IX Activity following Administration of AAV5-R338L "Padua" Factor IX versus AAV5 WT Human Factor IX in NHPs. Mol. Ther. Methods Clin. Dev. 2019;15:221–231. doi: 10.1016/j.omtm.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S.R.P., Xie J., Hu S., Ko J., Huang Q., Brown H.C., Srivastava A., Markusic D.M., Doering C.B., Spencer H.T., et al. Coagulation factor IX gene transfer to non-human primates using engineered AAV3 capsid and hepatic optimized expression cassette. Mol. Ther. Methods Clin. Dev. 2021;23:98–107. doi: 10.1016/j.omtm.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majowicz A., Nijmeijer B., Lampen M.H., Spronck L., de Haan M., Petry H., van Deventer S.J., Meyer C., Tangelder M., Ferreira V. Therapeutic hFIX Activity Achieved after Single AAV5-hFIX Treatment in Hemophilia B Patients and NHPs with Pre-existing Anti-AAV5 NABs. Mol. Ther. Methods Clin. Dev. 2019;14:27–36. doi: 10.1016/j.omtm.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiller M., Wang H., Coulibaly S., Schuster M., Rottensteiner H., Sun K., Chuah M.K., Vandendriessche T., Scheiflinger F., Höllriegl W. Evaluation of the Human Factor IX Gene Therapy Vector TAK-748 in Hemophilia: Results from Non-Clinical Studies in Factor IX Knockout Mice and Rhesus Monkeys. Blood. 2019;134:4633. [Google Scholar]
- 42.Leborgne C., Barbon E., Alexander J.M., Hanby H., Delignat S., Cohen D.M., Collaud F., Muraleetharan S., Lupo D., Silverberg J., et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 2020;26:1096–1101. doi: 10.1038/s41591-020-0911-7. [DOI] [PubMed] [Google Scholar]
- 43.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 44.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E.J., Ragni M.V., Manno C.S., Sommer J., Jiang H., et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 46.Nayak S., Sarkar D., Perrin G.Q., Moghimi B., Hoffman B.E., Zhou S., Byrne B.J., Herzog R.W. Prevention and Reversal of Antibody Responses Against Factor IX in Gene Therapy for Hemophilia B. Front. Microbiol. 2011;2:244. doi: 10.3389/fmicb.2011.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundgren T.S., Denning G., Stowell S.R., Spencer H.T., Doering C.B. Pharmacokinetic analysis identifies a factor VIII immunogenicity threshold after AAV gene therapy in hemophilia A mice. Blood Adv. 2022;6:2628–2645. doi: 10.1182/bloodadvances.2021006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colella P., Ronzitti G., Mingozzi F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2018;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monahan P.E., Negrier C., Tarantino M., Valentino L.A., Mingozzi F. Emerging Immunogenicity and Genotoxicity Considerations of Adeno-Associated Virus Vector Gene Therapy for Hemophilia. J. Clin. Med. 2021;10:2471. doi: 10.3390/jcm10112471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingozzi F., High K.A. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 51.Ertl H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022;13:975803. doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhuri M., Levy D.I., Schulz M., McCarty D., Gao G. Durability of transgene expression after rAAV gene therapy. Mol. Ther. 2022;30:1364–1380. doi: 10.1016/j.ymthe.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parrish R.R., Day J.J., Lubin F.D. Direct bisulfite sequencing for examination of DNA methylation with gene and nucleotide resolution from brain tissues. Curr. Protoc. Neurosci. 2012;7 doi: 10.1002/0471142301.ns0724s60. Unit 7 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li B., Carey M., Workman J.L. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Cedar H., Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 56.Leger A., Le Guiner C., Nickerson M.L., McGee Im K., Ferry N., Moullier P., Snyder R.O., Penaud-Budloo M. Adeno-associated viral vector-mediated transgene expression is independent of DNA methylation in primate liver and skeletal muscle. PLoS One. 2011;6:e20881. doi: 10.1371/journal.pone.0020881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das A., Vijayan M., Walton E.M., Stafford V.G., Fiflis D.N., Asokan A. Epigenetic Silencing of Recombinant Adeno-associated Virus Genomes by NP220 and the HUSH Complex. J. Virol. 2022;96:e0203921. doi: 10.1128/jvi.02039-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elkouby L., Armour S.M., Toso R., DiPietro M., Davidson R.J., Nguyen G.N., Willet M., Kutza S., Silverberg J., Frick J., et al. Preclinical assessment of an optimized AAV-FVIII vector in mice and non-human primates for the treatment of hemophilia A. Mol. Ther. Methods Clin. Dev. 2022;24:20–29. doi: 10.1016/j.omtm.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fong S., Yates B., Sihn C.R., Mattis A.N., Mitchell N., Liu S., Russell C.B., Kim B., Lawal A., Rangarajan S., et al. Interindividual variability in transgene mRNA and protein production following adeno-associated virus gene therapy for hemophilia A. Nat. Med. 2022;28:789–797. doi: 10.1038/s41591-022-01751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chowdary P., Shapiro S., Makris M., Evans G., Boyce S., Talks K., Dolan G., Reiss U., Phillips M., Riddell A., et al. Phase 1-2 Trial of AAVS3 Gene Therapy in Patients with Hemophilia B. N. Engl. J. Med. 2022;387:237–247. doi: 10.1056/NEJMoa2119913. [DOI] [PubMed] [Google Scholar]
- 61.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D., et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nathwani A.C., Rosales C., McIntosh J., Rastegarlari G., Nathwani D., Raj D., Nawathe S., Waddington S.N., Bronson R., Jackson S., et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C., et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pipe S.W., Leebeek F.W.G., Recht M., Key N.S., Castaman G., Miesbach W., Lattimore S., Peerlinck K., Van der Valk P., Coppens M., et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N. Engl. J. Med. 2023;388:706–718. doi: 10.1056/NEJMoa2211644. [DOI] [PubMed] [Google Scholar]
- 65.Von Drygalski A., Giermasz A., Castaman G., Key N.S., Lattimore S., Leebeek F.W.G., Miesbach W., Recht M., Long A., Gut R., et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 2019;3:3241–3247. doi: 10.1182/bloodadvances.2019000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Drygalski A., Gomez E., Giermasz A., Castaman G., Key N.S., Lattimore S.U., Leebeek F.W.G., Miesbach W.A., Recht M., Gut R., et al. Stable and durable factor IX levels in patients with hemophilia B over 3 years after etranacogene dezaparvovec gene therapy. Blood Adv. 2023;7:5671–5679. doi: 10.1182/bloodadvances.2022008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J., et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barzel A., Paulk N.K., Shi Y., Huang Y., Chu K., Zhang F., Valdmanis P.N., Spector L.P., Porteus M.H., Gaensler K.M., Kay M.A. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature. 2015;517:360–364. doi: 10.1038/nature13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu J., Zhang F., Xu S., Fire A.Z., Kay M.A. The extragenic spacer length between the 5' and 3' ends of the transgene expression cassette affects transgene silencing from plasmid-based vectors. Mol. Ther. 2012;20:2111–2119. doi: 10.1038/mt.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z.Y., He C.Y., Meuse L., Kay M.A. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- 71.Lin H.F., Maeda N., Smithies O., Straight D.L., Stafford D.W. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]
- 72.Meliani A., Boisgerault F., Hardet R., Marmier S., Collaud F., Ronzitti G., Leborgne C., Costa Verdera H., Simon Sola M., Charles S., et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 2018;9:4098. doi: 10.1038/s41467-018-06621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meliani A., Leborgne C., Triffault S., Jeanson-Leh L., Veron P., Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li L.C., Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw and processed sequence data have been deposited in GEO (Gene Expression Omnibus) and are available under accession number GSE261047.