Abstract
Different delivery methods can cause variations in the composition and structure of intestinal microbiota in neonates. However, the impact of the microecological environment on host immune function requires further investigation. In this study, 75 healthy neonates were divided into two groups: vaginal delivery group (n = 36) and cesarean section group (n = 39). Fecal and peripheral blood samples were collected from the 7th to the 10th day. 16S rRNA sequencing technique was performed to investigate the gut microbiota on fecal samples. Levels of immunoglobulins and Th1 and Th2 cells in the peripheral blood of neonates were measured. The abundance of Escherichia, Bifidobacterium, and Bacteroides in neonates in the cesarean section group was significantly lower than that in the vaginal delivery group. Metabolic pathway analysis showed three significantly up-regulated metabolic pathways in the intestinal microbiota of neonates in the cesarean section group. The levels of serum IgG and IL-12p70 in the cesarean section group were lower than those in the vaginal delivery group, and the proportion of IFN-γ/IL-4 was significantly lower in the cesarean section group compared to the vaginal delivery group. The mode of delivery has potential impact on the intestinal microbiota and immune functions of neonates, potentially leading to an imbalance of Th1/Th2 cells in neonates delivered by cesarean section.
Subject terms: Immunology, Microbiology
Introduction
The neonate period is crucial for the initial colonization of normal intestinal microbiota and is the most important stage for the formation and establishment of intestinal microbiota for the future. The presence and activity of a “symbiotic” intestinal microbita in neonates plays a crucial role in maintaining intestinal barrier function, immune response, immune system maturation, and neurodevelopment1. Many diseases, including allergic diseases and asthma related to the immune system, are associated with disorders of intestinal microbiota2. Therefore, bacterial colonization in the intestine significantly influences health throughout the entire life cycle3. The colonization of intestinal microbiota is a complex process during infancy and is pivotal for the formation of intestinal microbiota. The establishment of intestinal microbiota during this stage not only affects the health of infants and young children but also influences future growth and health status. The intestinal microbiota of infants undergoes dynamic changes, with significant individual differences, and is easily influenced by various external factors4. Intestinal microbial colonization in neonates during the first few months is often linked to pathogen resistance and immune developmental disorders. Besides, some metabolites from intestinal microorganisms have immunomodulatory effects on the host5,6. Therefore, studying the differences in the impact of intestinal microecological environment on the mechanism of immune function is of great significance.
The mode of delivery is widely acknowledged as a crucial determinant in the early acquisition and development of intestinal microbiota, particularly during the first year of life7. In neonates born through vaginal delivery, the early intestinal microbiota largely originates from the maternal intestine, vagina, skin, and other sources. However, infants delivered via cesarean section lack direct contact with the mother’s digestive tract and birth canal, hindering their ability to acquire microbiota essential for early colonization8. Cesarean section, while developed to mitigate risks for high-risk women and fetus, is increasingly used without medical necessity. Currently, approximately 21.1% of women worldwide undergo cesarean sections, with an expected increase to 28.5% by 20309. Numerous epidemiological and cohort studies have indicated that newborns delivered via cesarean section are more prone to immune and metabolic disorders compared to those born vaginally10,11. For instance, cesarean-born children have a heightened risk of immune function-related disorders such as asthma, idiopathic arthritis, and gastroenteritis, primarily affecting mucosal immune system functions12. The procedure bypasses the natural transmission of microorganisms from the birth canal and maternal intestinal microbiota to the fetus, resulting in disturbances in the infant’s intestinal microbiota. Consequently, there is weaker activation of intestinal immune cell receptors regulating intrinsic and adaptive immunity, thereby increasing the risk of asthma in school-age children13. Therefore, explaining the mechanism of immune development disorders in cesarean-born neonates from the perspective of intestinal microbiota alterations is of paramount importance. Understanding the intestinal ecosystem and its impact on immune function in neonates can offer new insights and methods to promote immune development in cesarean-born infants. Therefore, we hypothesized that neonates delivered by cesarean section may experience dysbiosis in their intestinal microbiota as a result of reduced microbial diversity, leading to impaired the maturation of immune system.
In this study, structural characteristics of the neonates’ intestinal microbiota were obtained by the 16S rRNA sequencing technique, and, meanwhile, the levels of immune factors in neonates in terms of humoral and cellular immunity functions were measured. We demonstrated that different delivery modes led to differences in the intestinal microbiota structure of neonates, which could further regulate neonatal immune function, resulting in Th1/Th2 cell imbalance in postnatal neonates. This study confirms that perturbations to the neonatal intestinal microbiota have a significant impact on immune system development, highlighting the need for personalized interventions aimed at restoring a “healthy” microecological environment in cesarean section neonates and reducing the incidence of immune-related disorders.
Results
Clinical characteristics of enrolled subjects
To investigate the effects of different delivery modes on the intestinal microbiota and immune function of neonate, subjects were selected based on the inclusion and exclusion criteria between March and September 2022. Ultimately, we successfully enrolled 75 neonates after accounting for missing data or subject withdrawal. Thirty-nine neonates were delivered with cesarean section (Cesarean), while thirty-six were delivered vaginally (Vaginal). All enrolled neonates were breastfed as required. Analysis of the data presented in Table 1 indicates no significant differences between the two groups regarding general clinical data such as gender, age, gestational week, birth weight, and Apgar score, etc. Therefore, interference from relevant factors can be excluded. In addition, blood cell test results revealed significantly higher peripheral blood leukocyte and neutrophil counts in neonates from the vaginal delivery group compared to those from the cesarean section group (Table 1).
Table 1.
Hematologic parameters of the neonates.
| Cesarean group (n=39) | Vaginal group (n=36) | Statistic value | p-value | |
|---|---|---|---|---|
| ± SD | ± SD | |||
| White blood cell (×109/L) | 11.15 ± 3.17 | 14.67 ± 4.54 | t = − 3.912 | < 0.001 |
| Neutrophil granulocyte (×109/L) | 5.97 ± 2.84 | 8.23 ± 4.68 | t = − 2.479 | 0.015 |
| Monocyte (×109/L) | 0.98 ± 0.52 | 1.10 ± 0.54 | t = − 0.929 | 0.356 |
| Immature granulocyte (×109/L) | 0.33 ± 0.54 | 0.35 ± 0.47 | t = − 0.166 | 0.869 |
| Red Blood Cell (×1012/L) | 4.73 ± 0.76 | 4.94 ± 0.80 | t = − 1.161 | 0.250 |
| Hemoglobin (g/L) | 159.82 ± 24.45 | 166.44 ± 20.60 | t = − 1.263 | 0.210 |
| Hematokrit | 49.73 ± 7.73 | 46.44 ± 8.24 | t = 1.787 | 0.078 |
Composition of intestinal microbiota in neonates with different delivery methods
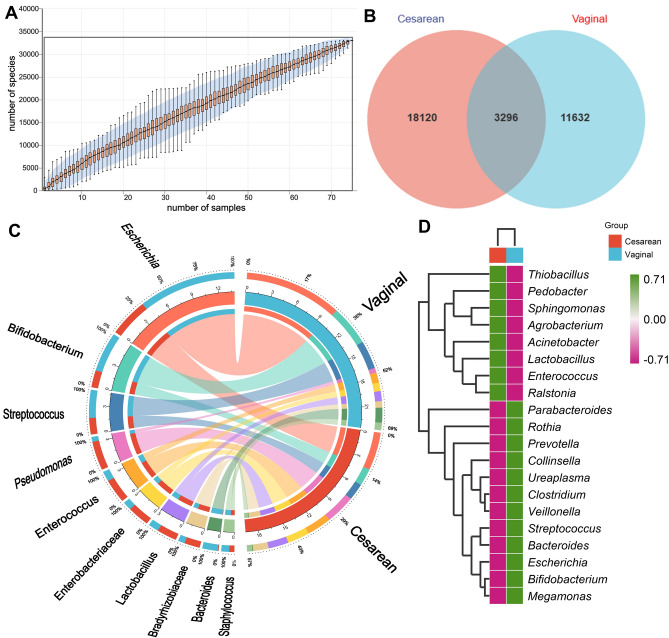
The species accumulation curves obtained from sequencing indicated a plateau, suggesting adequate sample size for estimation (Fig. 1A). To assess shared and unique microbiota between groups, Venn diagrams were used for community analysis, revealing 3296 shared ASVs between groups ((Fig. 1B). Results from the phylogenetic tree displayed the position of each ASV in the evolutionary along with the evolutionary distances between them. The abundance distribution of bacterial species represented by different ASV sequences in the two groups was illustrated. Specifically, the abundance of Escherichia represented by ASV_37570 sequences was higher in neonates from the vaginal delivery group compared to those from the cesarean section group, with statistically significant differences (Fig. S1). Moreover, to further explore the compositional differences between the two groups of neonate intestinal microbiota at the genus level and to visualize the species abundance distribution trend of the samples, species relationship Circos maps and heat maps were employed for composition analysis. The results revealed higher abundances of Escherichia, Bifidobacterium, and Bacteroides in the vaginal delivery group compared to the cesarean section group. Conversely, the abundance of Pseudomonas and Enterococcus was higher in the cesarean section group than in the vaginal delivery group (Fig. 1C and D).
Figure 1.
General description of sequencing. (A) Species accumulation curve. (B) The Venn diagram. (C) Circo graph. The figure is effective in visualizing relationships between the microbiota and each group. (D) The heat map of bacterial composition at the genus level.
Analysis of composition differences in intestinal microbiota
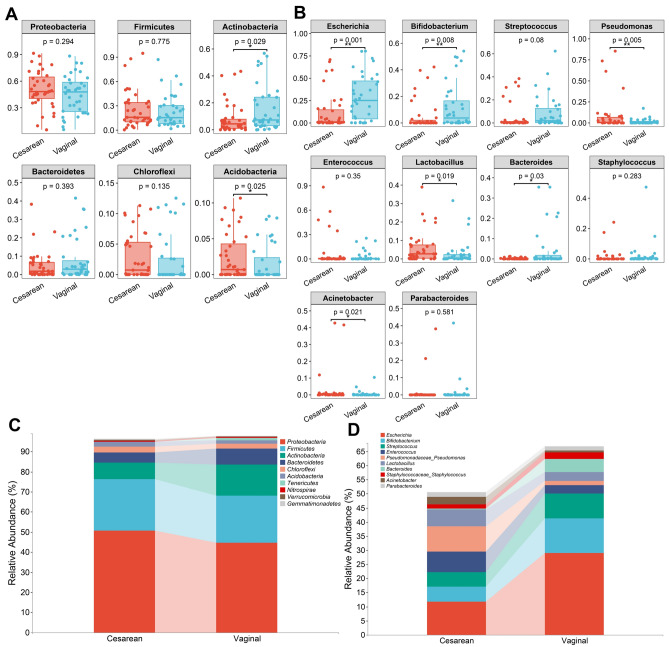
Further comparison of the intestinal microbiota levels between the two groups of neonates revealed significant differences regarding phylum and genus. The abundance of Actinobacteria in the neonatal intestinal microbiota of the vaginal delivery group was higher than that in the cesarean section group in terms of phylum, while the abundance of Acidobacteria in the neonatal intestinal microbiota of the cesarean section group was also higher than that in the vaginal delivery group, with statistically significant differences (Fig. 2A). The results showed that neonates in the vaginal delivery group exhibited higher abundances of Escherichia, Bifidobacterium, and Bacteroides compared to neonates in the cesarean section group. Conversely, neonates in the cesarean section group had higher abundances of Pseudomonas, Lactobacillus, and Acinetobacter compared to neonates in the vaginal delivery group, with statistically significant differences (Fig. 2B). Figure 2C and D illustrate the composition at the family and genus levels, respectively, of the two groups.
Figure 2.
Microbiota composition in each group. (A) Differences in relative abundance at phylum levels between the two groups. (B) Differences in relative abundance at genus levels between the two groups. (C) The bar chart representing data at the phylum level. (D) The bar chart representing data at the genus level. *p < 0.05, **p < 0.01.
Microbial diversity analysis and group differences
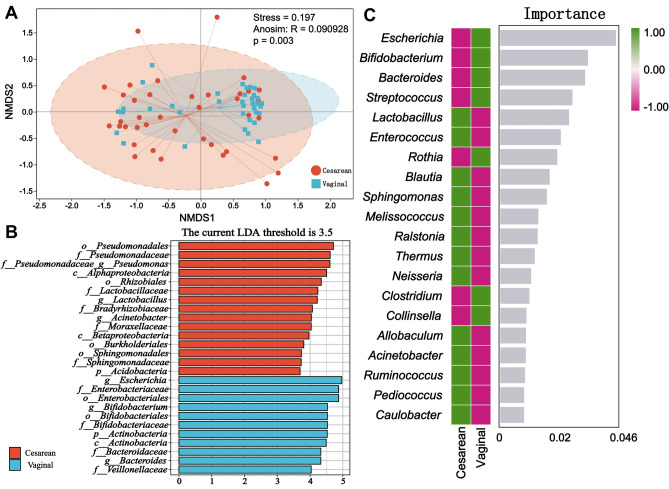
To evaluate the richness, diversity, and evenness of intestinal microbiota in the two groups of neonates, we employed the Alpha diversity index (including Chao 1, Faith_pd, Observed_species, Pielou_e, Shannon, and Simpson) to comprehensively assess the microbial communities in their intestines. Alpha diversity measurement results revealed no statistical difference between the two groups (Fig. S2). Beta diversity measurement via NMDS exhibited differences in neonatal intestinal microbiota between the vaginal delivery and cesarean section groups (Fig. 3A). Further exploration of differences in the composition of intestinal microbiota led us to employ LEfSe analysis to identify biomarkers between the two groups, with an LDA score threshold of 3.5. The histogram of LDA score demonstrated enrichment of 15 bacteria in the intestines of neonates in the cesarean section group, while 11 bacteria were enriched in the intestines of neonates in the vaginal delivery group (Fig. 3B). Higher abundance was observed in the cesarean section group included Pseudomonadales, Pseudomonadaceae, Pseudomonadaceae_Pseudomonas, Alphaproteobacteria, Rhizobiales, Lactobacillaceae, Lactobacillus, Bradyrhizobiaceae, Acinetobacter, Moraxellaceae, Betaproteobacteria, Burkholderiales, Sphingomonadales, Sphingomonadaceae, and Acidobacteria. In contrast, higher abundance was observed in the vaginal delivery group included Escherichia, Enterobacteriaceae, Enterobacteriales, Bifidobacterium, Bifidobacteriales, Bifdobacteriaceae, Actinobacteria, Bacteroidaceae, Bacteroides, and Veillonellaceae. The Cladogram illustrates the taxonomic hierarchical distribution of intestinal marker bacteria in both groups of neonates (Fig. S3). Additionally, random forests analysis revealed the importance ranking of marker bacteria (Fig. 3C).
Figure 3.
Differences in microbiota composition between the two groups. (A) Nonmetric multidimensional scaling analysis (NMDS) of the two groups. (B) Histogram of LDA scores, showing significant differences in microbe type and abundance between the two groups. The letters p, c, o, f, and g represent the taxonomic ranks of phylum, class, order, family, and genus level respectively. (C) The analysis of random forests at the genus level.
Differential analysis of intestinal microbiota signaling pathways
In addition to analyzing the composition and differences in neonatal intestinal microbiota between the two groups, we also focused on the interrelationships of the microbiota and their functions. Network analysis based on microbial interactions revealed a predominantly positive correlation trend between the intestinal microbiota of the two groups of neonates, with Proteobacteria and Firmicutes showing closer connections with other phyla, suggesting the presence of keystone within these phyla (Fig. 4A). We further predicted the functional abundance of gene families in the intestinal microbiota of the two groups using the PICRUSt2. Analysis using the R language based on the MetaCyc database classified metabolic processes, showing that the intestinal microbiota of the two groups of mainly participate in metabolic processes such as Biosynthesis, Degradation/Utilization/Assimilation, and Generation of Precursor Metabolite and Energy (Fig. S4). PCoA results based on MetaCyc metabolic pathways showed differences in metabolic pathways between the two groups, with statistically significant difference in results (Fig. 4B). The intestinal microbiota in neonates in the cesarean section group showed three significantly upregulated signaling pathways, namely PWY-6071, PWY0-321, and PWY-6562 (Fig. 4C).
Figure 4.
The network analysis and function prediction. (A) The result of network analysis. (B) The principal co-ordinates analysis (PCoA) based on MetaCyc pathways of the two groups. (C) Differential analysis of metabolic pathways. (The analysis was conducted based on the up-regulated expression of cesarean section group).
Correlation between intestinal microbiota and immune function
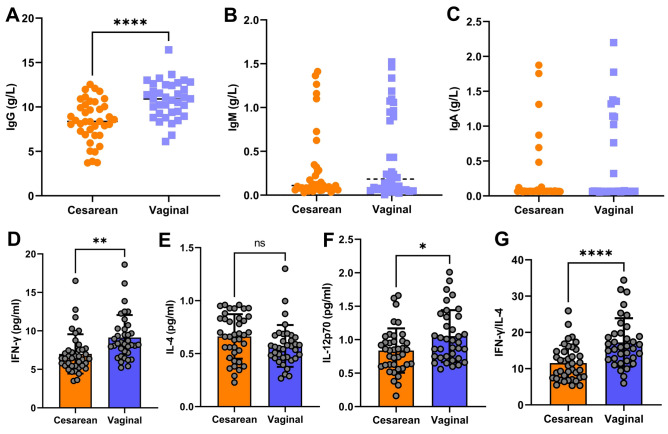
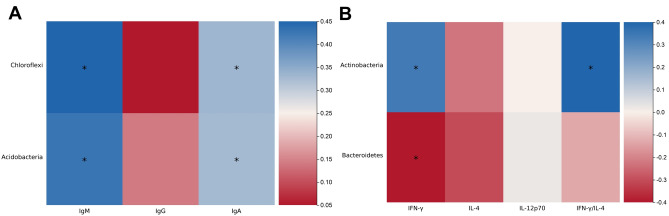
To investigate the impact of differences in intestinal microbiota on the immune function of neonates, we measured the levels of IgG, IgM, IgA, Th1 cells, and Th2 cells in the blood of the participants of the two groups. The results showed that the IgG levels in the cesarean section group of neonates were significantly lower than those in the vaginal delivery group, with statistically significant differences (Fig. 5A). There were no statistically significant differences in the levels of IgM and IgA between the two groups (Fig. 5B and C). We measured IFN-γ and IL-4 levels, secreted predominantly by Th1 and Th2 cells respectively, to assess their relative abundance. In addition, we also measured the level of a key cytokine, IL-12p70, that promotes the differentiation of Th1 cells. The results revealed that the levels of IFN-γ and IL-12p70 in the vaginal delivery group of neonates were significantly higher than those in the cesarean section group (Fig. 5D and F), while there were no statistically significant differences in the levels of IL-4 between the two groups (Fig. 5E). The ratio of IFN-γ/IL-4 in the vaginal delivery group of neonates was significantly higher than that in the cesarean section group (Fig. 5G). Spearman analysis was also conducted to evaluate the correlation between intestinal microbiota and immune function. It was found that the Chloroflexi and Acidobacteria in the intestine of the neonates in the vaginal delivery group were positively correlated with the levels of IgM and IgA in the serum, while the level of Actinobacteria showed a positive correlation with the levels of IFN-γ in the serum and the IFN-γ/IL-4 ratio, with statistically significant results (Fig. 6). However, in the cesarean section group of neonates, we did not find microbiota which was closely related to immune function.
Figure 5.
The results of blood indicators in each group. (A) Serum IgG levels in each group. (B) Serum IgM levels in each group. (C) Serum IgA levels in each group. (D) Serum IFN-γ levels in each group. (E) Serum IL-4 levels in each group. (F) Serum IL-12p70 levels in each group. (G) The ratio of IFN-γ/IL-4 in each group. *p < 0.05, **p < 0.01, ****p < 0.0001.
Figure 6.
The correlation between intestinal microbiota at phylum level and blood indicators in vaginal delivery group. The blue color on the heat map indicates a positive correlation, while red color indicates a negative correlation. The darker the color, the larger the correlation coefficient. *p < 0.05, **p < 0.01.
Discussion
In this study, we compared the differences in intestinal microbiota and immune function between 39 cesarean section and 36 healthy neonates who had a natural birth. The results of Alpha diversity showed that there was no difference in the abundance and diversity of intestinal microbiota between the two groups. However, the results of Beta diversity indicated significant differences in the composition and structure of intestinal microbiota between the two groups. At the phylum level, we observed that the abundance of intestinal Actinobacteria was lower in the cesarean section group compared to the vaginal delivery group, while the abundance of Acidobacteria was higher in the vaginal delivery group. At the genus level, the abundance of Escherichia, Bifidobacterium, and Bacteroides in the intestinal tract of neonates in the cesarean section group was lower than that in the vaginal delivery group. Conversely, the abundance of Pseudomonas, Lactobacillus, and Acinetobacter was higher in the cesarean section group. The MetaCyc metabolic pathways of the intestinal microbiota differed between the two groups. There were three significantly up-regulated signaling pathways in the cesarean section group. Additionally, we observed a decrease in Th1/Th2-cell imbalance in neonates in the cesarean section group, presumably associated with a decrease in the abundance of Actinobacteria.
Delivery mode is recognized as an important influential factor in determining intestinal microbiota colonization in early neonates14. The meta-analysis conducted by Wang et al. elucidated that the influence of delivery mode on the composition of neonatal gut microbiome reaches its pinnacle approximately one week postpartum and gradually diminishes with advancing age, utilizing a comprehensive dataset of shotgun metagenomic sequencing data7. It has been reported that the intestinal microbiota of neonates delivered by cesarean section exhibit reduced diversity and altered composition15. In this study, there was no variance in Alpha diversity between the two groups of neonatal intestinal microbiota, while the results of Beta diversity analysis revealed significant differences in the structure of intestinal microbiota between the two groups. The findings of our study are consistent with the results reported in the current research16,17, indicating that there are no significant alterations in intestinal microbial diversity induced by the mode of delivery. However, the mode of delivery is associated with variations in the structure of the intestinal microbiota. Our study further substantiates the significance of delivery mode in determining early neonatal intestinal flora colonization, highlighting that cesarean section disrupts the vertical transmission of maternal microbiota to neonates. LEfSe analysis in this study indicated that Pseudomonas, Lactobacillus, and Acinetobacter were abundant in the intestines of neonates in the cesarean section group, while Escherichia, Bifidobacterium, and Bacteroides were abundant in the intestines of neonates in the vaginal delivery group. This is consistent with recent studies in other cohorts where cesarean section delayed the colonization of Bacteroides and Bifidobacterium18,19. Bifidobacterium and Bacteroides are major components of early infantile intestinal microbiota, and the colonization of early infantile bifidobacteria is crucial for future health20,21. Bifidobacterium exerts various physiological functions on the host, such as serving as a biological barrier, providing nutrition, enhancing immunity, exhibiting anti-tumor effects, and improving metabolism22,23. Reduced abundance of Bacteroides due to cesarean section has been associated with an increased risk of opportunistic pathogen colonization in the intestine14,24. Bacteroides can activate the newborn immune system, participate in regulating immune system development, and play a key role in infant health25. Therefore, the colonization of these two beneficial bacteria in the intestine can resist the colonization and invasion of exogenous pathogens, protecting the host from infection and positively impacting the host. In this study, the results of the MetaCyc database-based metabolic function prediction revealed differences in MetaCyc metabolic pathways between the two groups of neonate intestinal microbiota. There were three significantly up-regulated signaling pathways in the cesarean section group: PWY-6071 (superpathway of phenylethylamine degradation), PWY0-321 (phenylacetate degradation I [aerobic]), and PWY-6562 (norspermidine biosynthesis). These pathways are involved in the degradation of phenylethylamine and phenylacetate in vivo and the synthesis of norspermidine. It has been reported that D-LANA-14, a lysine-based membrane-active small molecule conjugated with an aliphatic norspermidine analogue bearing a tetradecanoyl chain prepared using norspermidine, has a positive effect on multidrug-resistant Acinetobacter26. We speculate that the body may have initiated a self-protection mechanism against the colonization of Acinetobacter, but further research is needed to fully understand this mechanism.
The intestine serves not only as the digestive organ but also as the largest immune organ in the body. It comprises non-hematopoietic cells (epithelial cells, Paneth cells, goblet cells) and hematopoietic cells (macrophages, dendritic cells, T cells) and serves as the habitat for trillions of microorganisms27. The intestinal microbiota represents the primary source of microbial stimulation and is a fundamental factor driving postnatal immune system maturation and inducing immune response balance21. The impact of intestinal microbiota on the immune system is multifaceted, influencing intrinsic and adaptive immune responses, as well as both mucosal and systemic immune systems28. In this study, we observed significantly higher levels of serum IgG, peripheral blood leukocytes, and neutrophils in neonates delivered vaginally compared to those delivered via cesarean section, consistent with findings in some literature reports29,30. We hypothesize that during vaginal delivery, uterine contractions exert pressure on the placenta, increasing pressure on the placental vasculature (feto-placental vasculature), which allows for infiltration of IgG from maternal blood into the umbilical cord blood. Besides, passage through the birth canal may lead to increased white blood cells and neutrophils. While levels of IgA and IgM did not differ between the two groups, we observed a positive correlation between serum IgA and IgM levels and intestinal Actinobacteria in vaginal delivery group. Actinobacteria primarily consist of Bifidobacterium and Micrococcus. Studies have demonstrated that earlier colonization of beneficial intestinal microbiota, such as Bacteroides and Bifidobacterium, corresponds to earlier detection of IgA-secreting cells in peripheral blood31. Furthermore, diversity within the genus Bifidobacterium promotes maturation of the mucosal secretory IgA (SIgA) system32. Thus, colonization by beneficial microbiota, particularly Bifidobacterium, plays a crucial role in the humoral immune development of the host.
Intestinal microbiota not only plays an essential role in the development and activation of the intestinal mucosal immune system but also regulates the host’s immune response. Numerous studies have demonstrated that the bacterial environment plays a crucial role in the balance of Th1/Th2 cells through various mechanisms, with cytokines synthesized by intrinsic immune cells, especially IL-12 and IFN-γ, exerting a decisive influence33–36. IL-12 is a major cytokine that promotes the differentiation of naive T cells into Th1 cells and is primarily produced by activated dendritic cells. Th1 cells primarily participate in cellular immune responses, and their overactive potentially leading to delayed-type hypersensitivity, inflammatory bowel disease, and autoimmune disorders. Conversely, Th2 cells induce B cells to produce large quantities of isotype antibodies and their subclasses, and an overactive Th2 response can result in allergic reactions. The activation of Th1 and Th2 cells involves mutual inhibition, with factors secreted by each cell type stimulating their own differentiation and development while inhibiting the differentiation and development of the other. That is, Th1 cells promote the proliferation of themselves and the production of Th1 cell-like cytokines through the secretion of IFN-γ, thereby downregulating the immune response of Th2 cells. Conversely, IL-4 and IL-10 produced by Th2 cells inhibit the production of cytokines by Th1 cells. This mutual inhibition maintains the dynamic immune balance of Th1/Th2 cells, ensuring the normal immune response of the body37–39. We therefore assessed the levels of Th1 and Th2 cells by detecting the cytokine content of IFN-γ, IL-4, and IL-12p70. In this study, the levels of serum IL-12p70, IFN-γ, and the proportion of IFN-γ/IL-4 decreased in the cesarean section group, indicating the presence of Th1/Th2 cell imbalance. Our study’s findings provided more evidence that a neonate delivered via cesarean section may not develop their immune system normally due to an imbalance in their intestinal microbiota. Within a neonate’s first five days of life, variations in the intestinal microbiota’s immunostimulatory capacity based on the route of delivery can be seen11. Our study’s findings provided more evidence that a neonate delivered via cesarean section may not develop their immune system normally due to an imbalance in their intestinal microbiota. Within a neonate’s first five days of life, variations in the intestinal microbiota’s immunostimulatory capacity based on the route of delivery can be seen. We speculate that a lack of certain intestinal bacteria that can effectively regulate Th-cell development could be the cause of the Th1/Th2 cell imbalance seen in neonates delivered via cesarean section. We also carried out a correlation analysis between the two groups of neonates’ intestinal microbiota makeup and immune function indicators. The results showed that the content of IFN-γ and the proportion of IFN-γ/IL-4 were positively correlated with intestinal Actinobacteria. As mentioned earlier, the main representative bacteria of the Actinobacteria comprise the genus Bifidobacterium. Bifidobacterium regulates Th cell responses and maintains the balance between Th1 and Th2 cells40. Unfortunately, no significant correlation was observed between the immune function indicators of neonates in the cesarean section group and their intestinal microbiota. In conclusion, the delivery mode of cesarean section attenuates the cell-mediated immune response in neonates, leading to an imbalance of Th1/Th2 cells. This imbalance is not conducive to neonates’ adaptation to their new environment and poses risks to those with incomplete active immunity. In the case of neonates delivered by cesarean section with compromised immune function, the clinician may consider addressing the dysbiosis of intestinal microbiota or implementing early intervention, which has the potential to impede or decelerate the onset of a spectrum of diseases associated with impaired immune function.
Our study has several limitations. The colonization of intestinal microbiota in neonates is a dynamic process influenced by various factors at different times, including feeding patterns, supplementation, antibiotic use, and household hygiene. (a). Our study did not allow for dynamic observation and comprehensive assessment at multiple time points. (b). In addition, we were unable to directly demonstrate that Th1/Th2-cell imbalances in neonates born by cesarean section are correlated with reduced abundance of specific microbiota. (c). It is unclear whether the Th1/Th2 cell imbalance in cesarean section newborns is short-term or long-term. In future studies, we aim to address these limitations by employing macro-genome sequencing and macro-metabolite analysis to further investigate the community functions and metabolites of intestinal microbiota. This approach will enable us to delve deeper into the functions of the microbiota and explore how the metabolites of intestinal microbiota regulate the immune function of the host.
Conclusion
The abundance of Bifidobacterium and Bacteroides in neonates in the cesarean section group decreased significantly. This reduction in abundance may be related to the decrease in blood IgG content and the imbalance of Th1/Th2 cells.
Materials and methods
Participants and sample collection
All subjects participating in the study obtained consent from the parents or guardians of the neonates and signed the informed consent form. Recruitment of subjects took place from March to September 2022, resulting in the enrollment of 75 neonates, with 39 delivered via elective cesarean section and 36 delivered vaginally. Inclusion criteria were as follows: (1) singleton term neonates; (2) birth weight exceeding 2500 g; (3) no antibiotic usage post-birth; (4) absence of infection or asphyxia history post-birth; and (5) all neonates were breastfeeding. Exclusion criteria included: (1) contamination of amniotic fluid or feces, asphyxia, or Apgar score below seven; (2) occurrence of pregnancy-related complications; and (3) prolonged use of probiotics or antibiotics by the mother during pregnancy or lactation. Additionally, clinical data on neonates, such as gender, age, ethnicity, birth weight, and gestational age, were collected (Table 2). Neonatal stool samples were collected using sterile collection tubes and stored in a -80°C refrigerator until processing. Simultaneously, peripheral blood from the neonates was collected in non-anticoagulant sampling tubes. After coagulation of the blood (approximately 0.5–1 h), it was centrifuged at 2000 g at 4°C for 10 min. The upper serum was then collected and stored in a refrigerator at -80°C for subsequent detection of immunoglobulins and cytokines. None of the neonates had been given any antibiotics or probiotics prior to sample collection. The protocol underwent review and approval by the Medical Ethics Committee of The Second Nanning People’s Hospital (ID: Y2021001).
Table 2.
Clinical characteristics of the neonates.
| Characteristics | Cesarean group | Vaginal group | p-value |
|---|---|---|---|
| n | 39 | 36 | |
| Sex | 0.281 | ||
| Male | 19 | 22 | |
| Female | 20 | 14 | |
| Nation | 0.355 | ||
| Han zu | 21 | 19 | |
| Zhuang zu | 17 | 15 | |
| Other ethnic minorities | 1 | 2 | |
| Age, days, ± SD | 9.26 ± 2.65 | 9.53 ± 2.44 | 0.646 |
| Gestational age, weeks, ± SD | 38.82 ± 1.07 | 39.06 ±1.04 | 0.339 |
| Birth weight, g, ± SD | 3200.26 ± 387.07 | 3173.33 ± 262.81 | 0.728 |
| Apgar 1 min, ± SD | 9.54 ± 0.82 | 9.72 ± 0.57 | 0.261 |
| Apgar 5 min, ± SD | 9.85 ± 0.43 | 9.94 ± 0.23 | 0.220 |
| Apgar 10 min, ± SD | 9.97 ± 0.16 | 9.94 ± 0.23 | 0.515 |
Sequencing experiment
The total genomic DNAs of the intestinal microbiota were extracted from fecal samples following the instructions provided in the OMEGA Soil DNA Kit (M5635-02, Omega Bio-Tek, Norcross, GA, USA). Subsequently, the concentrations of the DNAs were adjusted accordingly and stored in a refrigerator at − 20 °C for future use. The DN solution was amplified by polymerase chain reaction. Primers (Personal Biotechnology Co., Ltd., Shanghai, China) were specifically designed for the V3-V4 variable region of the microbial 16S rRNA gene. The primer sequences were as follows: Forward (F): ACTCCTACGGGAGGCAGCA, Reverse (R): GGACTACHVGGGTWTCTAAT. The amplification products were purified and quantified using a BioTek Flx 800 microplate reader with the Quant-iT PicoGreen dsDNA Assay Kit (P7589, Invitrogen, Carlsbad, CA, USA). The Illumina TruSeq DNA library preparation experimental process was subsequently employed to construct the required onboard library. Finally, sequencing analysis was conducted using the Illumina NovaSeq 6000 platform. The sequencing data has been deposited in the sequencing reading file of the NCBI, accessible via the login number PRJNA1103762.
Basic analysis for sequencing results
To enhance analysis accuracy, the original sequencing data underwent several steps, including primer removal, joining, quality filtration, splicing, and dechimerization by using DADA2. This process resulted in deduplicated sequences known as amplicon sequence variants (ASVs). Subsequently, these sequences were systematically annotated utilizing the Greengenes database within QIIME2’s 2019.4. Phylogenetic trees were constructed using the qiime phylogeny align-to-tree-mafft-fasttree analysis process to elucidate genetic distances or relationships between sequences.
Composition analysis of intestinal microbiota
The R programming language (v3.2.0) was used to create the species accumulation curve, which shows the total number of ASVs for each sample. To create Venn diagrams, use the R programming language and the VennDiagram package. The PhyloTree was built using the R programming language, the ggtree package, and the phyloseq package. We created a Circos graph using the R programming language and the Circlize package to show the composition of the intestinal microbiota in two groups. The whole composition of the intestinal microbiota in the two groups was mapped using the R programming language and the pheatmap package. Using QIIME2 and Perl, the makeup of the intestinal microbiota in two groups was examined at the phylum and genus levels.
Analysis of microbial diversity and group differences
Alpha diversity analysis was conducted using QIIME2, ggplot2 package, and the Kruskal–Wallis test to validate the significance of differences between groups. Beta diversity was assessed through non-metric multidimensional scaling (NMDS) by using R programming language, the vegan package, and the Bray–Curtis distance matrix algorithm. Anosim was employed to evaluate significant differences between groups. Furthermore, linear discriminant analysis effect size (LEfSe) analysis was executed using various analysis software, including the Python LEfSe package and the ggtree package. Perform a random forest analysis using the QIIME2.
Predictive analysis of intestinal microbiota function
Constructing a correlation network using R programming language, SparCC, igraph package, and RMThreshold package. Functional prediction analysis of MetaCyc metabolic pathways in intestinal microbiota was conducted using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2). Differences in functional units were further explored using principal coordinate analysis (PCoA), and the permanova test was utilized to ascertain statistically significant differences. Differences in metabolic pathways were analyzed using R language and the metagenomeSeq package. Finally, Spearman’s test was employed to investigate the correlation between intestinal microbiota and immune function.
Detection of immunoglobulin and cytokines
Serum samples stored in the refrigerator at − 80 °C were thawed at room temperature for 30 min prior to analysis. Immunoglobulin levels (IgA, IgM, and IgG) in the neonates’ serum were measured using an immunoglobulin test kit (Chongqing BioStec Biotechnology Co., Ltd., China) on an automated specific protein analyzer (BA400, Chongqing BioStec Biotechnology Co., Ltd.). Control materials are tested together with these samples to ensure the accuracy of the results. The experiment was conducted strictly following the instructions provided with the kit. Cytokines IFN-γ, IL-4, and IL-12p70 were detected using the QuantiCyto® Human IFN-γ ELISA kit, QuantiCyto® Human IL-4 ELISA kit, and QuantiCyto® Human IL-12p70 ELISA kit, respectively (Neobioscience, Shenzhen, China). Measurements were taken at a wavelength of 630 nm using a microplate reader at an automated ELISA work station (ADC ELISA 400, Yantai Adcom Biological Technology Co., LTD).
Statistical analysis methods for general data
General data in this study mainly included hematologic parameters, sex, nation, age, gestational age, birth weight and Apgar score. The results and clinical data of all subjects in this study should be completed. If the data is lost, the data will be excluded. There were no missing data exists in this study. The data was analyzed using SPSS 26.0. Measurement data from the two groups were presented as mean ± standard deviation (± s). Normal distribution data was analyzed using the t-test (assuming equal variances) or Welch’s t-test (assuming unequal variances), while skewed distribution data were analyzed using the rank sum test. Counting data were assessed using Chi-square test. Differences with small sample sizes were compared using Fisher’s exact test. A P-value of less than 0.05 was set to determine statistical significance.
Ethics approval and consent to participate
The Second Nanning People’s Hospital Ethics Committee approved this study (No. Y2021001). The study was conducted in compliance with the ethical guidelines of the Declaration of Helsinki. Informed consent and signatures were obtained from the families of the neonates.
Supplementary Information
Acknowledgements
Sequencing services were provided by Personal Biotechnology Co., Ltd. (Shanghai, China). The data were analysed by using the free online platform Personalbio GenesCloud.
Author contributions
Chunhui Lai and Li Huang performed the data analyses and wrote the original draft. Chunhui Lai and Jianghui Zeng contributed to the conception of the study and helped perform the analysis with constructive discussions. Chaosheng Huang, Yibing Luo and Xuemei Qin performed the experiment. Yijin Wang collected the clinical information and performed the data analyses. All the authors read and commented on the manuscript.
Funding
This work was supported by the Scientific Research Project of Guangxi Zhuang Autonomous Region Health Commission (Z20210019), the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2024GXNSFBA010112).
Data availability
Raw sequence data are available in the write full meaning of SRA database under accession number PRJNA1103762 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1103762).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chunhui Lai and Li Huang.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68599-x.
References
- 1.Grech, A. et al. Maternal exposures and the infant gut microbiome: A systematic review with meta-analysis. Gut Microbes13, 1–30 (2021). 10.1080/19490976.2021.1897210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronan, V., Yeasin, R. & Claud, E. C. Childhood development and the microbiome-the intestinal microbiota in maintenance of health and development of disease during childhood development. Gastroenterology160, 495–506 (2021). 10.1053/j.gastro.2020.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanachi, M. et al. Longitudinal and comparative analysis of gut microbiota of Tunisian Newborns according to delivery mode. Front. Microbiol.13, 780568 (2022). 10.3389/fmicb.2022.780568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quin, C. & Gibson, D. L. Human behavior, not race or geography, is the strongest predictor of microbial succession in the gut bacteriome of infants. Gut Microbes11, 1143–1171 (2020). 10.1080/19490976.2020.1736973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokholm, J. et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med.12, eaax9929 (2020). 10.1126/scitranslmed.aax9929 [DOI] [PubMed] [Google Scholar]
- 6.Patrick, D. M. et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir. Med.8, 1094–1105 (2020). 10.1016/S2213-2600(20)30052-7 [DOI] [PubMed] [Google Scholar]
- 7.Wang, S. et al. Metagenomic analysis of mother-infant gut microbiome reveals global distinct and shared microbial signatures. Gut Microbes13, 1–24 (2021). 10.1080/19490976.2021.1900996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferretti, P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe24, 133-145.e5 (2018). 10.1016/j.chom.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betran, A. P., Ye, J., Moller, A. B., Souza, J. P. & Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Glob. Health6, e005671 (2021). 10.1136/bmjgh-2021-005671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darmasseelane, K., Hyde, M. J., Santhakumaran, S., Gale, C. & Modi, N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: A systematic review and meta-analysis. PLoS One9, e87896 (2014). 10.1371/journal.pone.0087896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wampach, L. et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun.9, 5091 (2018). 10.1038/s41467-018-07631-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensen, K. & Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol.137, 587–590 (2016). 10.1016/j.jaci.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 13.Gürdeniz, G. et al. Neonatal metabolome of caesarean section and risk of childhood asthma. Eur. Respir. J.59, 2102406 (2022). 10.1183/13993003.02406-2021 [DOI] [PubMed] [Google Scholar]
- 14.Shao, Y. et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature574, 117–121 (2019). 10.1038/s41586-019-1560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi, Y. C. et al. Initial meconium microbiome in Chinese neonates delivered naturally or by cesarean section. Sci. Rep.8, 3255 (2018). 10.1038/s41598-018-21657-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, N. et al. Distinct gut microbiota and metabolite profiles induced by delivery mode in healthy Chinese infants. J. Proteomics232, 104071 (2021). 10.1016/j.jprot.2020.104071 [DOI] [PubMed] [Google Scholar]
- 17.Liu, D. et al. Bacterial community structure associated with elective cesarean section versus vaginal delivery in Chinese newborns. J. Pediatr. Gastroenterol. Nutr.60, 240–246 (2015). 10.1097/MPG.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 18.Dominguez-Bello, M. G. et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med.22, 250–253 (2016). 10.1038/nm.4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adlerberth, I. et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol.120, 343–350 (2007). 10.1016/j.jaci.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 20.Makino, H. et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS One8, e78331 (2013). 10.1371/journal.pone.0078331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matharu, D. et al. Bacteroides abundance drives birth mode dependent infant gut microbiota developmental trajectories. Front. Microbiol.13, 953475 (2022). 10.3389/fmicb.2022.953475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu, J. et al. Prophylactic treatment with Bacteroides uniformis and Bifidobacterium bifidum counteracts hepatic NK cell immune tolerance in nonalcoholic steatohepatitis induced by high fat diet. Gut Microbes16, 2302065 (2024). 10.1080/19490976.2024.2302065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chichlowski, M., Shah, N., Wampler, J. L., Wu, S. S. & Vanderhoof, J. A. Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current state of knowledge. Nutrients12, 1581 (2020). 10.3390/nu12061581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell165, 1551 (2016). 10.1016/j.cell.2016.05.056 [DOI] [PubMed] [Google Scholar]
- 25.Reyman, M. et al. Author correction: Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun.10, 5352 (2019). 10.1038/s41467-019-13373-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konai, M. M. & Haldar, J. Lysine-based small molecule sensitizes rifampicin and tetracycline against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. ACS Infect. Dis.6, 91–99 (2020). 10.1021/acsinfecdis.9b00221 [DOI] [PubMed] [Google Scholar]
- 27.Yang, Z., Liu, X., Wu, Y., Peng, J. & Wei, H. Effect of the microbiome on intestinal innate immune development in early life and the potential strategy of early intervention. Front. Immunol.13, 936300 (2022). 10.3389/fimmu.2022.936300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, B. et al. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front. Immunol.11, 575 (2020). 10.3389/fimmu.2020.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adeyeye, T. E. et al. Effects on neonatal immunoglobulin concentrations by infant mode of delivery in the upstate KIDS study (2008–2010). Am. J. Reprod. Immunol.89, e13688 (2023). 10.1111/aji.13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werlang, I. et al. Associations of birth mode with cord blood cytokines, white blood cells, and newborn intestinal Bifidobacteria. PLoS One13, e0205962 (2018). 10.1371/journal.pone.0205962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grönlund, M. M., Arvilommi, H., Kero, P., Lehtonen, O. P. & Isolauri, E. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: A prospective follow up study of healthy infants aged 0–6 months. Arch. Dis. Child. Fetal Neonatal Ed.83, F186-192 (2000). 10.1136/fn.83.3.F186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjögren, Y. M. et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy39, 1842–1851 (2009). 10.1111/j.1365-2222.2009.03326.x [DOI] [PubMed] [Google Scholar]
- 33.Wu, B. et al. Correlation between the intestinal microflora and peripheral blood Th1/Th2 balance in hypothyroidism during the first half of pregnancy. Front. Cell Infect. Microbiol.13, 1159238 (2023). 10.3389/fcimb.2023.1159238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramakrishna, C. et al.Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun.10, 2153 (2019). 10.1038/s41467-019-09884-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kageyama, T., Matsuo, T., Kurakake, R. & Sano, T. Relationship between T cells and microbiota in health and disease. Prog. Mol. Biol. Transl. Sci.171, 95–129 (2020). 10.1016/bs.pmbts.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 36.Wang, Q. et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med.203, 2853–2863 (2006). 10.1084/jem.20062008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bousso, P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat. Rev. Immunol.8, 675–684 (2008). 10.1038/nri2379 [DOI] [PubMed] [Google Scholar]
- 38.Zhu, X. & Zhu, J. CD4 T helper cell subsets and related human immunological disorders. Int. J. Mol. Sci.21, 8011 (2020). 10.3390/ijms21218011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butcher, M. J. & Zhu, J. Recent advances in understanding the Th1/Th2 effector choice. Fac. Rev.10, 30 (2021). 10.12703/r/10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science331, 337–341 (2011). 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence data are available in the write full meaning of SRA database under accession number PRJNA1103762 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1103762).