Abstract
Introduction:
This study used different irrigation techniques to compare the levels of apical bacterial extrusion during the preparation of root canals with a reciprocating instrument widely used in endodontics, the Reciproc files 25/0.08 and 40/0.06.
Materials and Methods:
The irrigation techniques employed were conventional syringe irrigation and passive ultrasonic irrigation (PUI); the latter, with one or two activation cycles. Seventy extracted mandibular human premolars were contaminated with Enterococcus faecalis for 5 days and were distributed into 6 experimental groups (n=10), and the remaining specimens were used as positive and negative control groups (n=5). Group 1: instrumentation performed with Reciproc 25/0.08 and conventional syringe irrigation; Group 2: instrumentation performed with Reciproc 25/0.08 along with PUI for one minute after instrumentation (PUI-1); and Group 3: instrumentation performed with Reciproc 25/0.08 along with PUI for one minute before and after instrumentation (PUI-2). Groups 4, 5 and 6 were instrumented with Reciproc 40/0.06, and irrigation was performed similar to the previous groups, in the aforementioned order. Each root canal was irrigated with saline solution. Extruded debris was collected in microtubes. The contents of the microtubes were homogenized, diluted, and spread on Brain Heart Infusion agar. After 48 hours, the number of colony-forming units was determined for each sample. For statistical analysis, the Kruskal-Wallis test followed by the Dunn’s tests were used (α=0.05).
Results:
The CFU/mL count indicated that the instrumentation with Reciproc 25/0.08 was associated with the highest bacterial extrusion, mainly when PUI was performed (P<0.05).
Conclusion:
All the instrumentation techniques caused bacterial extrusion through the apical foramen; however, the largest file size of the Reciproc 40/0.06 groups was associated with less apical bacterial extrusion.
Key Words: Endodontics, Enterococcus faecalis, Instrumentation, Irrigation, Ultrasound
Introduction
During chemomechanical preparation of the root canal system, instruments and irrigation solutions are used with the aim of eliminating pulp tissue, microorganisms, as well as removing debris [1, 2]. In this way, infection control and prevention of apical bacterial extrusion are achieved, as they are part of endodontic treatment goals [3]. However, the extrusion of intracanal bacteria beyond the apical foramen and to the periradicular tissues can occur during the instrumentation of root canals, regardless of the preparation technique and the irrigation protocol employed [1-4]. Clinically, these factors promote complications such as postoperative pain, inflammation or infection, and flare-ups, and possibly delay the healing process [5].
The extent of bacterial debris extrusion may vary based on the instrument type, design, instrumentation technique, number of files, size of apical preparation, irrigation solution and technique, kinematics, as well as different root canal anatomies [6-9]. Up until now, laboratory studies have shown that all instrumentation systems commercially available can extrude bacteria beyond the apical foramen [4, 6-11]. Among these, the Reciproc (VDW, Munich, Germany) is a widely used single-file system in endodontics. It is made from a nickel-titanium alloy called M-Wire and has previously been suggested that its apical preparation sizes did not have a significant effect on bacterial extrusion [11]. Despite that, the literature is limited on the correlation between different irrigation techniques as well as the Reciproc system and its apical diameter; considering the possible effect of irrigation technique as an influential factor on the extrusion of bacterial debris [9].
The Reciproc single-file system manufacturer recommends its use in three steps, with irrigation performed alternately to the use of the instrument. Then, irrigation can possibly be considered insufficient in this treatment protocol. The conventional syringe irrigation (CSI) uses a needle attached to a disposable plastic syringe and has been performed routinely; nevertheless, this technique has several limitations [2, 9, 12]. To compensate these limitations, in addition to mechanical systems, irrigating solution agitation has been suggested for increasing cleanliness. The passive ultrasonic irrigation (PUI) is one indicated method, wherein an ultrasonic tip is activated inside the root canal along the working length (WL), moved passively in up-and-down motions to induce cavitation and acoustic streaming [12], and facilitates the removal of debris and remnants of pulp tissue [12-14]. Recently, PUI was associated with greater debris and bacterial extrusion [9]. However, it is necessary to investigate the variety of file systems and irrigation protocols that could be employed in endodontic therapy to provide consistent scientific evidence. Additionally, we can hypothesize that the number of activation cycles performed could have some influence on bacterial extrusion.
Therefore, the present study aimed for a comparative evaluation of the apical bacterial extrusion during instrumentation of teeth contaminated with Enterococcus (E.) faecalis, using Reciproc files with two distinct sizes and tapers and varying number of ultrasonic activation cycles. The null hypothesis tested was that no differences in the extent of bacterial extrusion exist between the different Reciproc diameters associated with PUI technique employed.
Material and Methods
Specimen selection
This study was approved by the Research Ethics Committee of the University of São Paulo, Bauru, São Paulo, Brazil (Number: 941.422). A calculation was performed using G*Power 3.1 software (Heinrich Heine University, Dusseldorf, Germany) based on a previous study [9]. The calculation indicated that the sample size for each group should be a minimum of 10 teeth, using an effect size of 0.63, α error probability of 0.05, and a power of 0.95. So, 10 specimens were assigned to each experimental group (n=10).
To select only teeth with single oval-shaped root canals, root curvature degree less than 10°, and an initial apical diameter corresponding to a size 15 K-file, micro-computed tomography (micro-CT) was performed for each tooth in a micro-CT system (SkyScan 1174v2; Bruker-microCT, Kontich, Belgium) using 50 kV, 800 mA, and an isotropic resolution of 19.7 μm. The scanned images were reconstructed, and the volume (mm3) of the root canals were measured with CTan software (CTan v1.11.10.0, Bruker SkyScan, Kontich, Belgium). The Kruskal-Wallis test followed by the Dunn’s tests were performed to confirm the uniform root canal volume distribution between the groups (P>0.05). This selection process resulted in seventy extracted human mandibular premolars that met all the aforementioned criteria. Teeth that presented immature apices, root caries, root fractures, cracks, lacerations, sharp curvatures, canal calcifications or previous endodontic treatment were excluded. These features were identified with the aid of a stereomicroscope (SMX800, Nikon Co., NY, USA) under 20× magnification.
After immersion in a 0.1% thymol solution, endodontic access cavities were prepared (EndoAccess Bur; Dentsply Maillefer, Ballaigues, Switzerland) with a high-speed handpiece. The pulp chambers were accessed, and the crown was maintained to create a reservoir for the irrigant solution during the chemomechanical preparation, with the aim of better simulating the clinical conditions [10]. The canals were explored with #10 and #15 K-files (Dentsply Maillefer, Ballaigues, Switzerland) until the tip of the instrument was observed at the apical foramen. The WL was determined by subtracting 1 mm from the measured length, establishing the zero position. The cusps were flattened to standardize root specimen length at 20±1 mm. Then, the root canals were instrumented to standardize the initial diameters with a #20 K-file (Dentsply Maillefer, Ballaigues, Switzerland) 1 mm short of the apical foramen and irrigated with 5 mL of saline solution. Next, three ultrasonic baths were performed with 1% sodium hypochlorite, 17% ethylenediaminetetraacetic acid and phosphate-buffered saline solution, for 10 min each, followed by distilled water to eliminate ethylenediaminetetraacetic acid and sodium hypochlorite residues and open up the dentinal tubules [15].
Contamination of the specimens
The bacterial strain Enterococcus faecalis ATCC 29212 (American Type Culture Collection) was reactivated. The purity was confirmed by colonial morphology and Gram staining (Oxoid, Basingstone, UK). The culture was adjusted according to the McFarland standard #1 (3×108 CFU/mL) using an SF325NM spectrophotometer (Bel Photonics do Brasil Ltda., Osasco, SP, Brazil), and the suspension was kept at 37°C for 7 h to reach exponential bacterial growth. Two coats of red nail varnish (Colorama, Rio de Janeiro, RJ, Brazil) were applied to the external surface of all roots to prevent bacterial microleakage through lateral canals, and as a manner to ensure that the microorganisms would only penetrate via the root canal. The contamination of the specimens lasted for 5 days at 37°C, according to the Ma et al. [16] sequence of centrifugations and the Andrade et al. [17] protocol previously reported [9, 18]. On the fifth day, the samples were removed from the microtubes used for contamination. All experiments were performed under aseptic conditions inside a laminar flow hood.
Root canal instrumentation
A single operator performed the instrumentation of the root canals. First, the external surfaces of roots were profusely disinfected by gauze strips immersed in 5% sodium hypochlorite followed by gauze strips immersed in phosphate-buffered saline solution. Then, for the experimental procedures, the specimens were transferred to a previously sterilized, closed system experimental model modified with microtubes (2 mL) [10] (Figure 1). The model system was vented with a 27-gauge Endo-Eze (Ultradent, South Jordan, UT, USA) needle to equalize the internal and external air pressure. The apical part of the root was suspended within the microtube, which acted as a collecting container for any apical material extruded through the foramen of the root. The files were activated in a VDW Silver electric motor (VDW GmbH, Munich, Germany) using the Reciproc mode and were introduced into the canal with a slow in-and-out pecking motion, without pulling the instruments completely out of the canal, according to the manufacturer’s instructions. The amplitude of the in-and-out movements did not exceed 3–4 mm. Each third of the root length was instrumented with 3 in-and-out pecking movements until it reached the WL [10]. After instrumentation, the files were sterilized for another use. Each instrument was only used to prepare three canals before being discarded. The specimens were randomly distributed.
Figure 1.
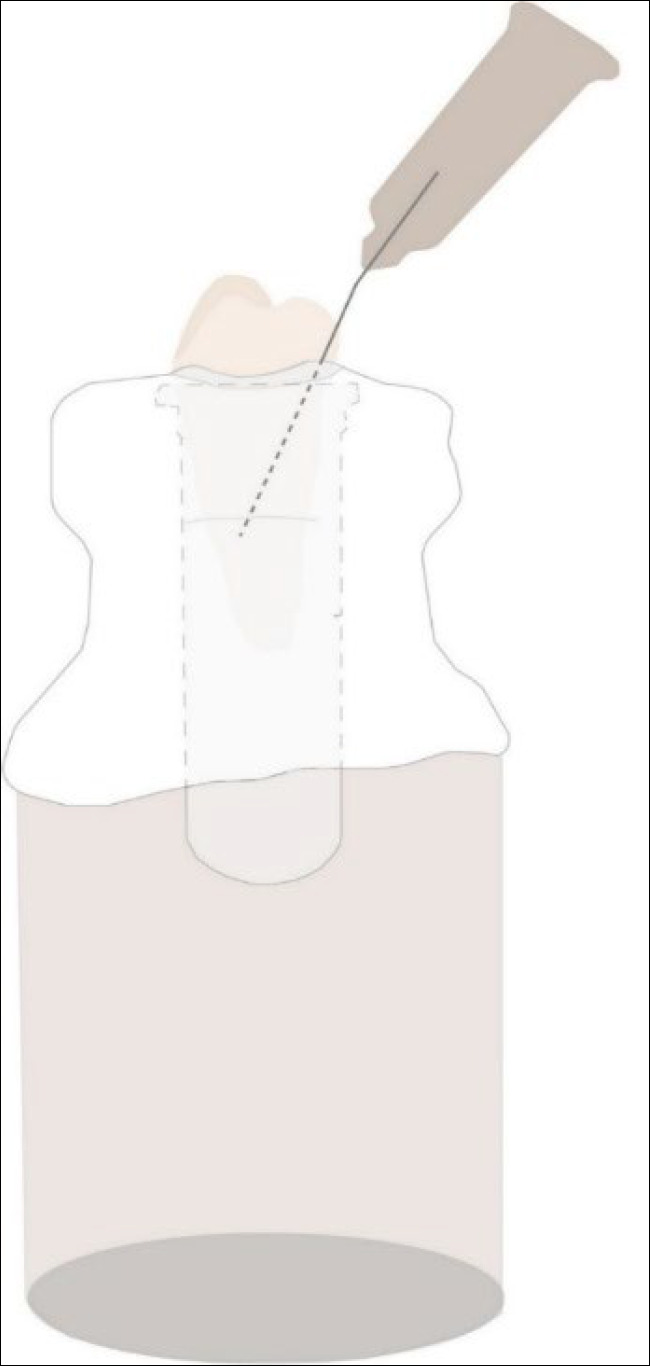
The experimental model system
Table 1 describes the six experimental groups evaluated (n=10), based on the single-file and irrigation technique performed.
Table 1.
Distribution of groups according to root canal treatment performed (n=10)
| Groups | File | Irrigation protocol |
|---|---|---|
| G1 | Reciproc R25.08 | CSI before and after file use |
| G2 | Reciproc R25.08 | PUI-1: One-time PUI-activation cycle |
| G3 | Reciproc R25.08 | PUI-2: Two-times PUI-activation cycles |
| G4 | Reciproc R40.06 | CSI before and after file use |
| G5 | Reciproc R40.06 | PUI-1: One-time PUI-activation cycle |
| G6 | Reciproc R40.06 | PUI-2: Two-times PUI-activation cycles |
CSI: Conventional Syringe Irrigation; PUI: Passive Ultrasonic Irrigation
Groups 1 and 4
Reciproc R25 file (25/0.08) or Reciproc R40 file (40/0.06) was introduced into the canal associated with CSI: the specimens were irrigated with 6 mL of sterilized saline solution before and after root canal preparation. Irrigation was performed with a disposable plastic syringe with a 27-gauge Endo-Eze needle (Ultradent, South Jordan, UT, USA), positioned 3 mm short of the apical foramen. The irrigant was aspirated to simulate a clinical situation with a portable surgical aspirator (Nevoni -5005BRST, Barueri, SP, Brazil).
Groups 2 and 5
Reciproc R25 file (25/0.08) or Reciproc R40 file (40/0.06) was used along with PUI performed in one-time activation cycle (PUI-1): specimens were irrigated with 6 mL of saline solution before and after root canal preparation, using a conventional syringe positioned 3 mm short of the apical foramen, but after preparation, the saline solution was activated. For PUI, a piezoelectric device was used at a frequency of 30,000 Hz (Emissonic MMO Jardim São Carlos, São Carlos, SP, Brazil), along with an Irrisonic E1 tip (Helse, Santa Rosa de Viterbo, São Paulo, Brazil) for 1 min (30 sec in the mesiodistal direction and 30 sec in the buccolingual direction), inserted at 3 mm short of the WL, in the “endo mode” (10% power).
Groups 3 and 6
Reciproc R25 file (25.08) or Reciproc R40 file (40.06) was used associated with PUI in two-times activation cycles (PUI-2): specimens were irrigated, but the saline solution was activated for one min before and after complete root canal preparation, similar to Groups 2 and 5.
Control groups
Five mandibular pre-molars, which had been previously infected, were used as positive controls (C+) to confirm intratubular contamination. The negative control group (C-) consisted of five uninfected pre-molars to confirm sterility. The specimens were sectioned longitudinally in an Isomet machine (Buehler Ltd, Lake Bluff, IL, USA) with a diamond disk under constant irrigation with sterile saline solution. The smear layer resulting from the cut was removed by immersing the specimens in 17% ethylenediaminetetraacetic acid for 5 min and washing them with saline solution, as previously reported in the literature [9, 17]. According to these reported studies, the ethylenediaminetetraacetic acid wash after the cut showed no effect on bacterial viability.
Root halves were stained with 30 μL of dye from a LIVE/DEAD BacLight bacterial viability kit (Invitrogen Molecular Probes, Eugene, OR, USA). This kit contains the green dye SYTO 9, which stains viable bacteria, and the red dye propidium iodide, which stains dead bacteria. After 20 min of contact with the dye, each sample was gently washed with a phosphate-buffered saline to remove the residual dye. The specimens were placed on a glass slide with immersion oil and observed by a Leica TCS-SPE confocal microscope (Leica Microsystems GmbH, Mannheim, Germany) at 40× magnification.
Bacterial debris assessment
Three absorbent paper points (#20) were inserted into the microtubes with the contaminated debris and irrigant, and stirred for 1 min. Next, the paper points were transferred to a microtube with 1 mL of Brain Heart Infusion broth (Difco, Detroit, MI, USA).
Dilutions were made with 100 µL of the content of each tube and transferred to other microtubes, until it reached the 10-4 concentration. One hundred microliter of the dilutions was seeded in Petri dishes with BHI-agar broth. The dishes were stored in a bacteriological incubator at 37°C for 48 h, for later counting of the colony-forming units per mL (CFU/mL).
Statistical analysis
All data were initially analyzed using the Shapiro-Wilk test to verify the normality. The Kruskal-Wallis and Dunn’s post-hoc tests were performed to analyze the data of bacterial debris extrusion. The CFU counts were log-transformed before the statistical tests. The GraphPad Prism 8.0 software (GraphPad San Diego, CA, USA) was the analytical tool used (α=0.05).
Results
No bacterial growth was observed in the negative control group. All positive controls demonstrated a higher proportion of viable bacteria inside the dentinal tubules, confirming the efficacy of the contamination protocol (Figure 2). All the instruments tested caused bacterial extrusion through the apical foramen. The instrumentation with Reciproc Primary 25/0.08 files resulted in higher CFU values compared to the Reciproc 40/0.06 files (P<0.05). There were no statistically significant differences in the amount of bacterial extrusion between the groups instrumented with the same files but different irrigation techniques (P>0.05) (Figure 3).
Figure 2.
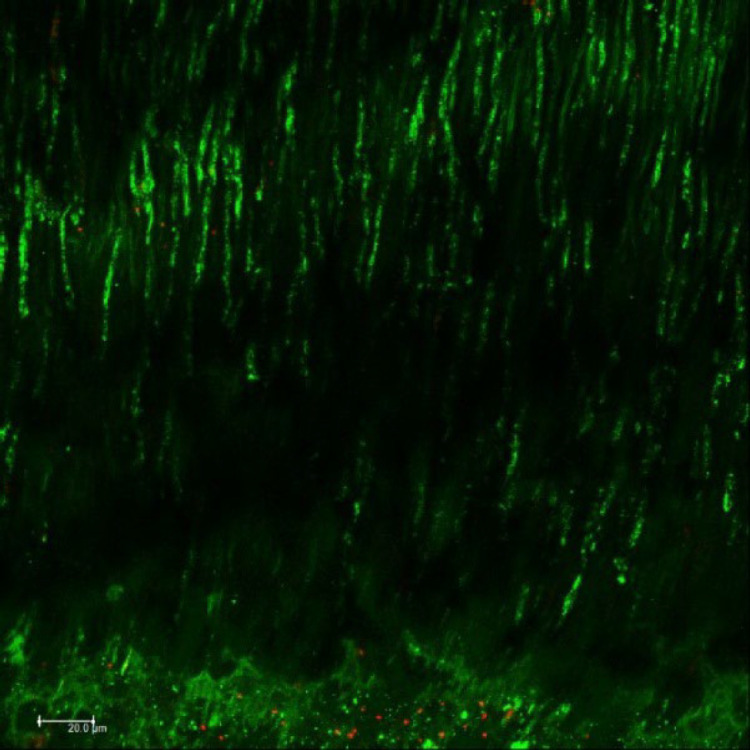
Confocal laser scanning microscopy image of the positive control group after the intratubular contamination protocol. Viable bacteria are indicated in green, and nonviable bacteria are indicated in red; magnification: 40×
Figure 3.
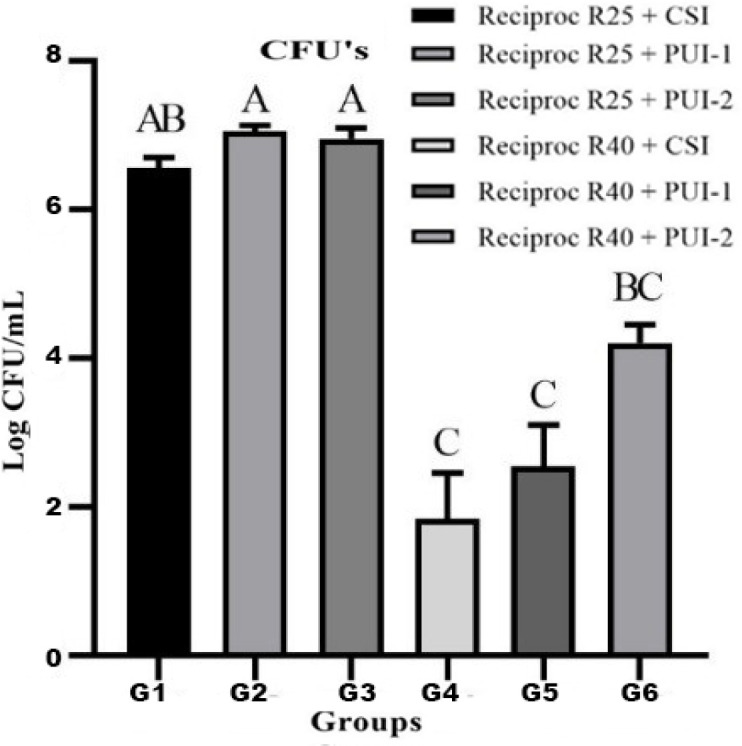
Data of apical extrusion of Enterococcus faecalis after instrumentation with Reciproc files, comparisons were done by the Kruskal-Wallis and Dunn’s post hoc tests; different letters represent significant differences between the groups (P<0.05)
Discussion
The present study highlights the role of the root canal preparation with different irrigation techniques, conventional or PUI, performed on the amount of apically extruded bacteria. The Reciproc files were chosen because there is a lack of information regarding their effects on bacterial extrusion during root canal instrumentation when associated with commonly performed irrigation techniques. The main result of this study revealed that Reciproc file 40/0.06 associated with irrigation techniques extruded fewer bacteria apically than the Reciproc file 25/0.08. Therefore, the null hypothesis was rejected.
Considering that the bacteria extruded is the main pathological component of debris [4], E. faecalis, a commonly found Gram-positive facultative anaerobe in teeth with failed endodontic treatment was selected for the contamination of root canals [4, 5, 9]. In order to evaluate the extent of bacterial extrusion, a 5-day protocol for contaminating intratubular dentin with E. faecalis was performed, wherein specimens were subjected to centrifugation cycles with inoculum addition at each cycle. After centrifugation, sterilized BHI broth was added and incubated at 37 °C for 24 hours. Centrifugation steps were performed until the fifth day, when contamination was verified using CLSM, revealing a mature bacterial biofilm with a complex structure that is more difficult to eliminate [17]. To minimize variations between different groups and produce a reliable and comparable anatomic baseline, single-rooted, single-canal mandibular pre-molars instrumented to the initial diameters with a #20 K-file were selected for the current study. In addition, according Siqueira et al. [19] the root canals of premolars presented approximately 35% of unprepared wall area with Reciproc R40, containing remnants of pulp tissue, bacteria, and dentin chips [19], which makes the assessment of the amount of bacterial extrusion important.
It is important to point out that caution is required to infer clinical significance about the present results due to the in vitro nature of this study. Saline solution was used as an irrigant because it is innocuous, since our objective was to evaluate the bacterial extrusion promoted by instrumentation techniques and not the antimicrobial efficacy of the irrigant. Extrusion of a solution with antimicrobial effect, such as sodium hypochlorite, could produce false negative results and prevent the detection and differentiation of the experimental groups. This approach is commonly performed in studies to facilitate quantification of apically extruded bacteria and avoid their destruction [4, 9-11]. To circumvent the limitations of debris extrusion methods, no device was used that could simulate the periodontal ligament. Although periapical tissues may act as a natural barrier in preventing debris and bacterial extrusion, studies reported that extrusion accidents generally occur in teeth with necessary to consider the existence of an interplay between the periapical pathology that lack such a barrier [9, 10]. In addition, it is number of bacteria and bacterial virulence as a correlated factor to acute inflammatory response of the periradicular tissues [9, 10]. In the presented study, reciprocating files with two different file diameters and tapers (Reciproc 25/0.08 or 40/0.06) were used with rrigation techniques (CSI, PUI-1, or PUI-2) to simulate the root i canal preparation. All instrumentation protocols resulted in apical extrusion of bacteria and these findings are in agreement with previous reports [4, 10, 20].
The influence of apical size preparation has been discussed in literature [19, 21, 22]. Although no specific guidelines are available, minimal shaping often combines a smaller apical diameter with increased taper [23-25]. On the other hand, apical sizes beyond #30 or #35 have been suggested [9, 23], as well as increased root canal enlargements by three instruments larger than the anatomic diameter [9, 19]. Likewise, the literature is controversial in regards to the apical limit [26], but the adoption of the zero position technique has been shown to enhance apical repairs [27]. In the present study, the file diameter proved to be important for the results found. A possible explanation for this is that an increase from 25 to 40 file diameter could result in a greater removal of cut dentin chips and an improved reflux toward the direction of the crown, making it easier to remove debris by suction flow of the irrigant. The aspiration of solution was performed to simulate a clinical situation with the intention of reducing the excess debris inside the root canals [11]. These findings could be related to previous studies wherein an irrigant flow in a minimally tapered root canal with a large apical preparation size also improved irrigant replacement and wall shear stress, and reduced the risk for irrigant extrusion, compared to the tapered root canals with a smaller apical preparation size [24, 25]. Therefore, the hypothesis is that root canals instrumented with greater apical diameters (sizes) such as Reciproc 40/0.06, can produce an extra space for debris to flow back toward the pulpal chamber, and consequently, decrease the bacterial extrusion to the periapical region, regardless of the irrigation technique performed.
A reduction in debris extrusion is desirable to help reduce postoperative pain after root canal treatment [5]. The results indicate that apical bacterial extrusion occurred in all the single-file systems tested with the distinct irrigation techniques evaluated. However, apical bacterial extrusion occurred mainly when PUI activation was performed. In a recent study, Cuellar et al. [9] evaluated the apical bacterial extrusion from root canals instrumented with rotary and reciprocating systems (ProDesign Logic or ProDesign R), with different file diameters, and using CSI or PUI. As a result, PUI was associated with a greater extrusion of contaminated dentinal debris [9] and exhibited congruence with the present findings, wherein the ultrasonic agitation of saline solution in the apical third of the root canal may force this solution towards the apical foramen, which could consequently cause the largest extrusion of bacteria, although not statistically significant.
The agitation of solution by PUI is important and strongly recommended for improving the antiseptic effects of the irrigants in the root canal system and eliminating microorganisms, including areas of difficult access such as the isthmi, ramifications, and dentinal tubules, where E. faecalis and other species can penetrate and remain viable as in persistent infections [5, 7]. It should therefore be considered that there is a possible amount of bacterial debris produced during the treatments. Passive ultrasonic irrigation removes debris from the dentinal walls of the root canal, but without due care, the debris can be extruded through the foramen and can cause tissue inflammation, even though painful symptoms may not be present. In relation to different agitation times (PUI-1 and PUI-2), it is noteworthy that the Reciproc 40+PUI-2 group presented an intermediate value, same as the Reciproc 25+CSI group. The agitation of solution performed in two different intervals, before and after preparation, may have some influence on the amount of bacterial debris extrusion; although the size and taper of the files have shown greater importance, mainly when root canals are instrumented with files larger than size 25 [23].
In the present study, the Irrisonic E1 tip was used for PUI-activation. This insert has a diameter equivalent to a #20 K-file and 0.01 taper. It was operated at 10% power according to the manufacturer’s recommendation and used in in-and-out motions to prevent it from touching the root canal walls [28]. The use of ultrasonic agitation may be suggested only after the chemomechanical decontamination procedures have been performed, as well as an instrumentation by files with larger tips whenever possible, along with considering the clinical conditions, to avoid greater bacterial extrusion. Another important consideration is that the insert should be placed 2 mm short of the WL, as ultrasonic movements of the irrigant solution can reach 2-3 mm ahead. With that in mind, all apparatus, like ultrasonic activation, should be used correctly.
Another topic to discuss is the bacterial quantification method performed in this study. It can be argued that polymerase chain reaction (PCR), a molecular technique based on DNA extraction, used for identifying difficult-to-grow endodontic pathogens could have been used [28]. However, we performed the CFU/mL counting analysis to quantify only the viable bacteria. The PCR technique can account for non-viable bacteria, that is, cells that may exert some virulence due to their components. However, viable bacteria could be more harmful to periapical tissues. Therefore, the CFU/mL counting analysis was applied for being a more realistic quantification method in this situation.
Conclusion
Considering the limitations of this in vitro study, it can be concluded that Reciproc #40/0.06 are associated with less bacterial extrusion compared with Reciproc #25/0.08 size/taper preparations. The agitation of solution performed in two different intervals, before and after preparation, did not exhibit a significant effect on the amount of apically extruded bacteria; however, the influence of PUI agitation should not be discarded since the extruded bacterial debris can cause tissue inflammation with or without painful symptoms. Moreover, the correct manner of using the ultrasonic agitation (2-3 mm short of radicular apex) should be emphasized for all clinicians, to avoid the extrusion of contaminated debris to the apical periodontal space.
Acknowledgements
The authors thank Coordination of Higher Education and Post-Graduation (CAPES, National Council of Scientific and Technological Development (CNPq) and São Paulo Research Foundation (FAPESP-process number 2010/20186-3) for supporting this study.
Conflict of interest
None.
Funding support
None.
Author contributions
Cuellar MRC: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing-review & editing. Pereira TC: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing-original draft, Writing-review & editing. Vasconcelos LRSM: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization. Pedrinha VF: Data curation, Investigation, Methodology, Validation, Visualization, Writing-original draft, Writing-review & editing. Vivan RR: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation. Duarte MAH: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Visualization, Writing-review & editing. Andrade FB: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing-review & editing.
References
- 1.Desai P, Himel V. Comparative safety of various intracanal irrigation systems. J Endod. 2009;35(4):545–9. doi: 10.1016/j.joen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa-Ribeiro M, Arruda-Vasconcelos R, Fabretti FL, Silva E, De-Deus G, Gomes B. Evaluation of Apically Extruded Debris Using Positive and Negative Pressure Irrigation Systems in Association with Different Irrigants. Braz Dent J. 2018;29(2):184–8. doi: 10.1590/0103-6440201801750. [DOI] [PubMed] [Google Scholar]
- 3.Alves FRF, Paiva PL, Marceliano-Alves MF, Cabreira LJ, Lima KC, Siqueira JF Jr, Rôças IN, Provenzano JC. Bacteria and Hard Tissue Debris Extrusion and Intracanal Bacterial Reduction Promoted by XP-endo Shaper and Reciproc Instruments. J Endod. 2018;44(7):1173–8. doi: 10.1016/j.joen.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Aksel H, Küçükkaya Eren S, Çakar A, Serper A, Özkuyumcu C, Azim AA. Effect of Instrumentation Techniques and Preparation Taper on Apical Extrusion of Bacteria. J Endod. 2017;43(6):1008–10. doi: 10.1016/j.joen.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Siqueira JF Jr. Microbial causes of endodontic flare-ups. Int Endod J. 2003;36(7):453–63. doi: 10.1046/j.1365-2591.2003.00671.x. [DOI] [PubMed] [Google Scholar]
- 6.Er K, Sümer Z, Akpinar KE. Apical extrusion of intracanal bacteria following use of two engine-driven instrumentation techniques. Int Endod J. 2005;38(12):871–6. doi: 10.1111/j.1365-2591.2005.01029.x. [DOI] [PubMed] [Google Scholar]
- 7.Türker SA, Uzunoğlu E, Aslan MH. Evaluation of apically extruded bacteria associated with different nickel-titanium systems. J Endod. 2015;41(6):953–5. doi: 10.1016/j.joen.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Cabreira LJ, Gominho LF, Rôças IN, Dessaune-Neto N, Siqueira JF Jr, Alves FR. Quantitative analysis of apically extruded bacteria following preparation of curved canals with three systems. Aust Endod J. 2019;45(1):79–85. doi: 10.1111/aej.12287. [DOI] [PubMed] [Google Scholar]
- 9.Cuellar MRC, Velásquez-Espedilla EG, Pedrinha VF, Vivan RR, Duarte MAH, Andrade FB. Can kinematics, file diameter, and PUI influence the intracanal decontamination and apical bacterial extrusion? Braz Oral Res. 2020;35:e003. doi: 10.1590/1807-3107bor-2021.vol35.0003. [DOI] [PubMed] [Google Scholar]
- 10.Tinoco JM, De-Deus G, Tinoco EM, Saavedra F, Fidel RA, Sassone LM. Apical extrusion of bacteria when using reciprocating single-file and rotary multifile instrumentation systems. Int Endod J. 2014;47(6):560–6. doi: 10.1111/iej.12187. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira JM, Cunha FM, Jesus RO, Silva EJ, Fidel SR, Sassone LM. Influence of working length and apical preparation size on apical bacterial extrusion during reciprocating instrumentation. Int Endod J. 2015;48(7):648–53. doi: 10.1111/iej.12357. [DOI] [PubMed] [Google Scholar]
- 12.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40(6):415–26. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 13.Pacheco-Yanes J, Provenzano JC, Marceliano-Alves MF, Gazzaneo I, Pérez AR, Gonçalves LS, Siqueira JF Jr. Distribution of sodium hypochlorite throughout the mesial root canal system of mandibular molars after adjunctive irrigant activation procedures: a micro-computed tomographic study. Clin Oral Investig. 2020;24(2):907–14. doi: 10.1007/s00784-019-02970-5. [DOI] [PubMed] [Google Scholar]
- 14.Di Fiore PM, Genov KA, Komaroff E, Li Y, Lin L. Nickel-titanium rotary instrument fracture: a clinical practice assessment. Int Endod J. 2006;39(9):700–8. doi: 10.1111/j.1365-2591.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 15.Marinho AC, Martinho FC, Gonçalves LM, Rabang HR, Gomes BP. Does the Reciproc file remove root canal bacteria and endotoxins as effectively as multifile rotary systems? Int Endod J. 2015;48(6):542–8. doi: 10.1111/iej.12346. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37(10):1380–5. doi: 10.1016/j.joen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Andrade FB, Arias MP, Maliza AG, Duarte MA, Graeff MS, Amoroso-Silva PA, Midena RZ, Moraes IG. A new improved protocol for in vitro intratubular dentinal bacterial contamination for antimicrobial endodontic tests: standardization and validation by confocal laser scanning microscopy. J Appl Oral Sci. 2015;23(6):591–8. doi: 10.1590/1678-775720140261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu Hasna A, Khoury RD, Toia CC, Gonçalves GB, de Andrade FB, Talge Carvalho CA, Ribeiro Camargo CH, Carneiro Valera M. In vitro Evaluation of the Antimicrobial Effect of N-acetylcysteine and Photodynamic Therapy on Root Canals Infected with Enterococcus faecalis. Iran Endod J. 2020;15(4):236–45. doi: 10.22037/iej.v15i4.26865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siqueira JF Jr, Pérez AR, Marceliano-Alves MF, Provenzano JC, Silva SG, Pires FR, Vieira GCS, Rôças IN, Alves FRF. What happens to unprepared root canal walls: a correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int Endod J. 2018;51(5):501–8. doi: 10.1111/iej.12753. [DOI] [PubMed] [Google Scholar]
- 20.Kuştarci A, Akpinar KE, Sümer Z, Er K, Bek B. Apical extrusion of intracanal bacteria following use of various instrumentation techniques. Int Endod J. 2008;41(12):1066–71. doi: 10.1111/j.1365-2591.2008.01470.x. [DOI] [PubMed] [Google Scholar]
- 21.Brunson M, Heilborn C, Johnson DJ, Cohenca N. Effect of apical preparation size and preparation taper on irrigant volume delivered by using negative pressure irrigation system. J Endod. 2010;36(4):721–4. doi: 10.1016/j.joen.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 22.de Gregorio C, Arias A, Navarrete N, Del Rio V, Oltra E, Cohenca N. Effect of apical size and taper on volume of irrigant delivered at working length with apical negative pressure at different root curvatures. J Endod. 2013;39(1):119–24. doi: 10.1016/j.joen.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira JF Jr, Rôças IN, Riche FN, Provenzano JC. Clinical outcome of the endodontic treatment of teeth with apical periodontitis using an antimicrobial protocol. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(5):757–62. doi: 10.1016/j.tripleo.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Boutsioukis C, Gogos C, Verhaagen B, Versluis M, Kastrinakis E, Van der Sluis LW. The effect of apical preparation size on irrigant flow in root canals evaluated using an unsteady Computational Fluid Dynamics model. Int Endod J. 2010;43(10):874–81. doi: 10.1111/j.1365-2591.2010.01761.x. [DOI] [PubMed] [Google Scholar]
- 25.Boutsioukis C, Gogos C, Verhaagen B, Versluis M, Kastrinakis E, Van der Sluis LW. The effect of root canal taper on the irrigant flow: evaluation using an unsteady Computational Fluid Dynamics model. Int Endod J. 2010;43(10):909–16. doi: 10.1111/j.1365-2591.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 26.Ricucci D. Apical limit of root canal instrumentation and obturation, part 1 Literature review. Int Endod J. 1998;31(6):384–93. doi: 10.1046/j.1365-2591.1998.00184.x. [DOI] [PubMed] [Google Scholar]
- 27.Ricucci D, Langeland K. Apical limit of root canal instrumentation and obturation, part 2 A histological study. Int Endod J. 1998;31(6):394–409. doi: 10.1046/j.1365-2591.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- 28.Aveiro E, Chiarelli-Neto VM, de-Jesus-Soares A, Zaia AA, Ferraz CCR, Almeida JFA, Marciano MA, Feres M, Gomes B. Efficacy of reciprocating and ultrasonic activation of 6% sodium hypochlorite in the reduction of microbial content and virulence factors in teeth with primary endodontic infection. Int Endod J. 2020;53(5):604–18. doi: 10.1111/iej.13261. [DOI] [PubMed] [Google Scholar]


