Abstract
Introduction
This study aimed to compare the effectiveness of two endodontic cleaning techniques, passive ultrasonic irrigation (PUI) and the XP-endo Finisher R (XPR) system, in removing residual filling material during endodontic retreatment procedures.
Materials and Methods:
Forty mandibular premolars with oval canals were divided into four groups based on the sealer used (AH-Plus or Bio-C Sealer) and the cleaning technique employed (PUI or XPR). To ensure uniformity of canal volume among groups, initial micro-CT scans were conducted. The canals were instrumented, filled, and then re-instrumented before undergoing either PUI or XPR cleaning techniques. Residual filling material volumes were assessed through micro-CT scans, and statistical analysis was performed using the Kruskal-Wallis and Mann-Whitney U tests.
Results:
Following instrumentation, there was no significant difference in residual filling material volumes between AH-Plus and Bio-C Sealer groups (1.35 mm3and 1.02 mm3, respectively; P>0.05). However, after supplementary cleaning techniques, XPR-cleaned specimens exhibited significantly less residual material compared to PUI-cleaned specimens (0.01 mm3 and 0.29 mm3 for Bio-C Sealer, and 0.07 mm3 and. 0.30 mm3 for AH-Plus, P<0.05).
Conclusion:
The XPR system was found to be more effective than PUI in removing residual filling material from Bio-C Sealer-filled root canals. This highlights its potential as a useful supplementary cleaning technique in endodontic retreatment procedures.
Key Words: Endodontic Retreatment, Micro-CT, Premolar Tooth, Root Canal Obturation
Introduction
Endodontic treatment is recognized for producing predictable outcomes and is associated with high success rates [1]. Nevertheless, treatment failures can occur in certain cases due to the presence of microorganisms within the root canal system (RCS), despite the proper execution of all therapeutic procedures [2, 3].
In cases of failure, non-surgical retreatment is the initial choice [4], as it provides access to the RCS and facilitates bacterial decontamination [5]. However, complete removal of contaminated filling material from dentinal walls is often unattainable through conventional techniques and instruments [6]. Moreover, the intricate anatomy of the RCS, encompassing isthmuses, apical deltas, and lateral canals exacerbate the challenge of achieving thorough cleaning. Residual filling material within the RCS can impede the irrigation in the regions harboring microorganisms, potentially compromising the success of retreatment [4, 7, 8].
The epoxy resin–based sealer most frequently referenced in the literature is AH-Plus (Dentsply De Trey, Konstanz, Germany) [9-11]. This hydrophilic sealer exhibits excellent properties of intratubular penetration and sealing capacity [12]. In contrast, the bioceramic Bio-C Sealer (Angelus, Londrina, PR, Brazil) is a relatively novel material, composed of calcium silicate, monobasic calcium phosphate, zirconia oxide, and calcium hydroxide [8, 11, 13]. Notably, it has the ability to form hydroxyapatite through chemical interaction with dentin [14].
Mechanized systems have been developed to facilitate retreatment procedures, and supplementary sonic and ultrasonic cleaning techniques have been tested to enhance the removal of filling material [2, 15]. Among them, passive ultrasonic irrigation (PUI) is the most commonly cited technique in the literature. The ultrasonic insert typically employed in dental practice operates at a 30 kHz frequency [16, 17], and the effectiveness of this technique relies on the cavitation phenomena and acoustic microstreaming.
Another supplementary cleaning technique described in the literature involves the XP-endo Finisher R system (XPR; FKG Dentaire AS, La Chaux-de-Fonds, Switzerland). This system was introduced with the aim of reaching areas within the RCS that were previously inaccessible [18], especially in morphologically intricate canals [19]. Its action is reliant on the transformation of the final 3 mm of the instrument’s tip from a straight to a semi-circular shape upon exposure to body temperature within the root canal. Recent investigations have provided evidenced for its efficacy in removing filling material subsequent to endodontic retreatment procedures [20, 21].
Numerous studies [2, 4, 22, 23] have employed microcomputed tomography (micro-CT) to quantify the residual filling material within root canals, following retreatment procedures. This method enables non-destructive analysis of specimens [8], offers three-dimensional visualization of the internal anatomy of the RCS [10, 24], and facilitates automated quantification of the RCS area and/or volume [10].
Only a limited number of studies have compared the efficacy of supplementary cleaning techniques in oval-shaped canals filled with bioceramic versus resinous sealers, particularly in endodontic retreatment procedures and in areas left untouched by endodontic instruments. Given the potential for chemical interaction with dentin and high penetration capacity into dentinal tubules associated with bioceramic sealers, there is controversy in the literature regarding the challenges encountered in removing these sealers compared to their resinous counterparts. Hence, it is imperative to investigate the effectiveness of available supplementary cleaning techniques in removing these sealers from the RCS [25-27], considering the potential impacts of residual filling material on the persistence of pathogens within the root canal and, ultimately, on the success of endodontic retreatment procedures.
This study aimed to conduct a micro-CT assessment of the residual filling material within the RCS subsequent to endodontic filling removal procedures, utilizing either PUI or XPR, in oval-shaped canals filled with AH-Plus resin-based sealer or bioceramic Bio-C Sealer. The null hypothesis was that there would be no significant differences between the two supplementary cleaning techniques investigated regarding their efficacy in removing either gutta-percha along with AH-Plus sealer or gutta-percha along with Bio-C Sealer.
Materials and Methods
The manuscript of this laboratory study is written according to the Preferred Reporting Items for Laboratory studies in Endodontology (PRILE) 2021 guidelines [28] and received approval from the local research ethics committee (Approval No. 4.721.275). The 40 specimens used in the study were teeth indicated for extraction due to orthodontic reasons and were expressly donated by the patients. Based on a study conducted by Volponi et al. [29], sample size calculation was performed using the analysis of variance test. Considering a minimum difference between treatment means of 0.087, a standard deviation of error of 0.055, four treatments, a test power of 0.80, and an alpha value of 0.05, it was calculated that 10 specimens per group would suffice (n=10). The calculation was performed using G*Power v. 3.1.9.4 software (Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany).
Specimen selection
Prior periapical radiographic examinations were conducted in both buccolingual and mesiodistal directions to screen for eligible teeth meeting the following inclusion criteria: absence of prior endodontic treatment, presence of a single oval-shaped root canal (with a buccolingual diameter twice that of the mesiodistal diameter, measured at 5 mm from the root apex), absence of internal or external calcifications or resorptions, straight root morphology [30], and fully formed apex. Then, an initial scan of all specimens was carried out using a microtomography system (Skyscan High Energy model 1173; Bruker microCT, Kontich, Belgium), configured to operate at 70 kV, 114 mA, 1.0 mm Al filter, 16.5 μm pixel size, and a 360° rotation angle with 0.5° steps. The acquired images were reconstructed into cross-sections using NRecon v. 1.7.0.4 software (Bruker microCT). The CTAn v. 1.16.4.1 software (Bruker microCT) was used to select the region of interest, as well as image binarization and segmentation for subsequent analysis, while Ctvox v. 3.2.0.0 software (Bruker microCT) was employed for viewing and analyzing both 2D and 3D images. Analysis included the entire length of the root canal, with the observed volumes (in mm3) tabulated to match study groups based on the initial root canal volume of the specimens [31]. The 40 paired specimens were then randomly assigned (by www.random.org) to four study groups, according to the sealer and supplementary cleaning technique employed (n=10): Group AH-PUI, AH-Plus and PUI; Group AH-XPR, AH-Plus and XPR; Group BC-PUI, Bio-C Sealer and PUI; and Group BC-XPR, Bio-C Sealer and XPR.
All teeth underwent calculus removal and cleaning using an ultrasonic periodontal insert (G1; Gnatus, Ribeirão Preto, SP, Brazil) coupled to an ultrasound device (Jetsonic; Gnatus). Then, the teeth were stored for two months in a 0.1% thymol and distilled water solution (Siafarma, Campinas, SP, Brazil). Prior to utilization in the study, the specimens were thoroughly rinsed with saline solution.
Specimen preparation
The coronal portion of each tooth was sectioned at the cement enamel junction using a diamond disk operating at low speed and under refrigeration (Isomet 100; Buehler, Lake Bluff, IL, USA). Each root was abraded in the cervical-apical direction until achieving a standardized length of 16 mm, which was measured using a digital caliper (500 DIN 862 Series; Mitutoyo, São Paulo, SP, Brazil). The working length was established by introducing a #10 K-file (Dentsply, Maillefer, Ballaigues, Switzerland) into the root canal until its tip was observed exiting the apical foramen, thus confirming foraminal patency, and then subtracting 1 mm. All procedures were conducted under 13× magnification with an operating microscope (Zeiss OPMI Pico; Carl Zeiss, Oberkochen, Germany) and performed by a single endodontics specialist who had undergone prior training in all the instrumentation and cleaning systems applied in this study.
The root canals were prepared using the ProTaper Next system (Dentsply Maillefer) up to the X3 file, employing an X-Smart Plus motor (Dentsply Maillefer) configured to operate in “ProTaper Next” mode at a speed of 300 rpm and a torque setting of 2.0 Ncm, in accordance with the manufacturer’s instructions. Instrumentation proceeded in the crown-to-apex direction by introducing the file into the canal with three in-and-out movements and applying a brushing action on the withdrawal stroke. This process was repeated until files X1, X2, and X3 reached the working length. Upon each instrument change, a #15 K-file (Dentsply Maillefer) was inserted, reaching up to 1 mm beyond the working length to ensure foraminal patency. Subsequently, 2 mL of a 2.5% sodium hypochlorite solution (Fórmula & Ação, São Paulo, SP, Brazil) was delivered into the root canal by a disposable syringe (BD, São Paulo, SP, Brazil) with a 30-G NaviTip needle (Ultradent, South Jordan, UT, USA), positioned 2 mm short of the working length, totaling 20 mL of solution per specimen. Each set of files was used on a single specimen and then discarded.
Following instrumentation, PUI was conducted to remove the smear layer, using 2 mL of 17% ethylenediaminetetraacetic acid solution (Formula & Ação) and an Irrisonic 20/.01 ultrasonic insert (Helse, Santa Rosa do Viterbo, SP, Brazil) coupled to an ultrasound device (Jetsonic; Gnatus) set to operate at 20% power. The insert was introduced up to 2 mm short of the working length and activated in three cycles of 20 sec each, with the irrigating solution being renewed after each cycle. Subsequently, another round of PUI was performed using 2.5% sodium hypochlorite solution, following the same protocol performed with the 17% ethylenediaminetetraacetic acid solution. In the AH-PUI and AH-XPR groups, final aspiration was performed by a capillary tip (Ultradent), followed by drying of the canals using absorbent paper points (Dentsply Maillefer). Conversely, in the BC-PUI and BC-XPR groups, aspiration was omitted, and only excess moisture was removed from the canals by two sterile absorbent paper points (Dentsply Maillefer). The goal in these groups was to maintain the dentin moisture required for enabling the bioactivity of the Bio-C Sealer. Subsequently, a second micro-CT scan was conducted post-instrumentation to determine the final volumes of the instrumented root canals.
Root canal obturation
All root canals were filled with X3 gutta-percha cones (Dentsply Maillefer). In the AH-PUI and AH-XPR groups, sealer manipulation was conducted at a 1:1 ratio. Following manipulation, 1 mL of sealer was introduced into the RCS with a 2-mL plastic syringe (BD, Curitiba, PR, Brazil) and the dispensing tip provided with the bioceramic Bio-C Sealer. The gutta-percha cone was tested and adjusted to the working length prior to insertion of the sealer. Subsequently, the gutta-percha was trimmed at the canal orifice using a heated Paiva plugger (Golgran, São Caetano do Sul, SP, Brazil) and subjected to vertical compaction. The pulp cavity was cleaned using cotton pellets moistened with alcohol and sealed with a provisional restorative cement (Coltosol; Coltene, Altstätten, Switzerland). In the BC-PUI and BC-XPR groups, 1 mL of pre-manipulated Bio-C Sealer was introduced into the RCS also using a 2-mL plastic syringe (BD, Curitiba, PR, Brazil) and the dispensing tip provided with the sealer. Subsequently, a gutta-percha cone was immediately inserted into the canal up to the working length, employing the single-cone technique. Excess filling material was removed, and the pulp chamber was sealed, following the same procedure as in the AH-Plus-filled specimens.
Because the pilot study showed that there could be artifacts in the micro-CT scans following obturation, periapical radiographs were obtained in both buccolingual and mesiodistal directions to assess the quality of obturation. Specimens presenting bubbles or gaps in the obturation were replaced. Subsequently, all specimens were incubated for four weeks at 100% humidity and 37 °C to allow complete setting of the sealer [29].
All root canals underwent re-instrumentation utilizing Reciproc R40 files (VDW, Munich, Germany) coupled to an X-Smart Plus motor (Dentsply Maillefer) configured to operate in “Reciproc” mode. Each instrument of the system was inserted into the canal utilizing in-and-out movements with an amplitude of 3 mm. Following each cycle of three movements, the instrument was withdrawn from the canal and cleaned with a gauze soaked in alcohol, while the canal was irrigated with 2 mL of 2.5% sodium hypochlorite, totaling 20 mL per specimen. This process was repeated until the R40 file reached the working length, and no remnants of filling material were detected on the instrument. Subsequently, a #15 K-type file (Dentsply Maillefer) was inserted up to 1 mm beyond the apical foramen to confirm foraminal patency. A new file from the Reciproc system was employed for each specimen and then discarded, following the manufacturer’s recommendation. Retreatment was deemed complete when no filling material was observed under the operating microscope (Zeiss Opmi Pico; Carl Zeiss, Oberkochen, Germany) at 13× magnification, either on the instrument or suspended in the irrigating solution. Subsequently, the canals were dried using sterile absorbent paper points (Dentsply Maillefer), and a third micro-CT scan was conducted to assess the amount of residual filling material.
Supplementary cleaning techniques
In the AH-PUI and BC-PUI groups, the root canals were irrigated with 2 mL of a 17% ethylenediaminetetraacetic acid solution. An E1 Irrisonic 20/0.1 insert (Helse) coupled to an ultrasound unit (NSK, Tokyo, Japan) was configured to operate at 20% power. The insert was positioned 2 mm short of the working length, and the solution was activated in three cycles of 20 sec each [32], with renewal of the solution after each cycle. Subsequently, the canal was aspirated and irrigated with 2 mL of a 2.5% sodium hypochlorite solution, employing the same activation protocol as used for the ethylenediaminetetraacetic acid.
In the AH-XPR and BC-XPR groups, an XP-endo Finisher R instrument (size 30/.00) was coupled to an X-Smart Plus motor (Dentsply Maillefer) and operated at a speed of 800 rpm with a torque setting of 1 Ncm. The instrument was applied using slow in-and-out movements with an amplitude of 7 to 8 mm, until reaching the working length. The irrigation protocol employed in these groups was consistent with that used in the other groups.
All supplementary cleaning techniques were performed within a container maintained at a thermostat-controlled (Plas-Labs, Lansing, MI, USA) temperature of 37 °C, emulating body temperature conditions. This ensured the appropriate environment for the XP-endo Finisher R phase change to occur. Lastly, all specimens were dried with sterile absorbent paper tips (Dentsply Maillefer), after which a final micro-CT scan was performed. The images acquired before and after application of the supplementary cleaning techniques were visualized and superimposed using DataViewer v. 1.5.2.4 software (Bruker microCT).
Statistical analysis
The normality of data distribution was assessed utilizing the Shapiro-Wilk test (P<0.05), indicating a non-normal distribution of the data. Therefore, comparisons among the study groups were conducted by the Kruskal-Wallis non-parametric test, followed by the Mann-Whitney U post-test for pairwise comparisons. All statistical analyses were carried out using IBM SPSS software (version 26.0, IBM Corporation, Armonk, NY, USA), with a significance level set at 5%.
Results
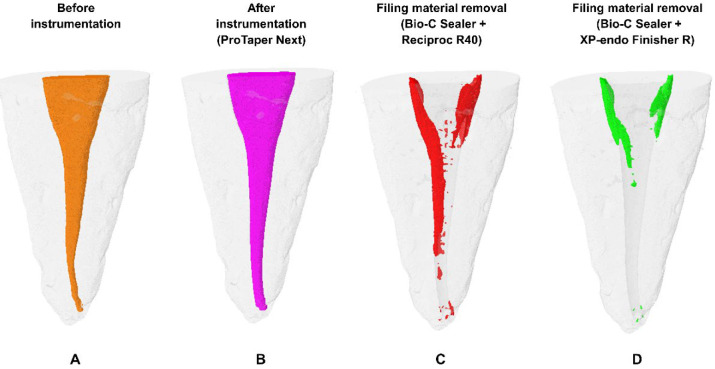
Figure 1 depicts the four time points of the micro-CT assessment conducted in the study. No significant differences were observed among the specimens regarding their initial root canal volumes, final root canal volumes post-instrumentation with the ProTaper Next system, or volumes of residual filling material subsequent to filling removal conducted with the Reciproc R40 instrument (P>0.05, Table 1).
Figure 1.
Illustration of the four time points in which specimens from the study groups were evaluated using micro-CT; A) Initial volume assessment of the root canal; B) Assessment of root canal volume after instrumentation with the ProTaper Next system; C) Assessment of volume of residual filling material after filling removal with the Reciproc R40 instrument; D) Assessment of volume of residual filling material after utilizing one of the supplementary cleaning techniques tested
Table 1.
Median, 25th percentile, and 75th percentile of initial canal volume (mm3), canal volume after instrumentation performed with the ProTaper Next system, and volume of residual material after filling removal performed with the Reciproc R40 instrument
| Groups | Initial volume of the root canal | Root canal volume after instrumentation with ProTaper Next | Volume of residual material after filling removal with Reciproc R40 |
|---|---|---|---|
| Median (p25–p75) | Median (p25–p75) | Median (p25–p75) | |
| AH-PUI and AH-XPR | 7.51 (6.10–11.68) A | 14.65 (11.80–16.95) A | 1.35 (0.42–4.01) A |
| BC-PUI and BC-XPR | 8.64 (5.84–10.48) A | 13.92 (12.27–15.24) A | 1.02 (0.19–1.97) A |
| P-value | 0.935 | 0.607 | 0.213 |
AH-PUI, AH Plus sealer and passive ultrasonic irrigation (PUI); AH-XPR, AH-Plus sealer and XP-endo Finisher R instrument (XPR); BC-PUI, Bio-C Sealer and PUI; and BC-XPR, Bio-C Sealer and XPR. Different letters within the column indicate a statistically significant difference (Mann-Whitney U test, P<0.05)
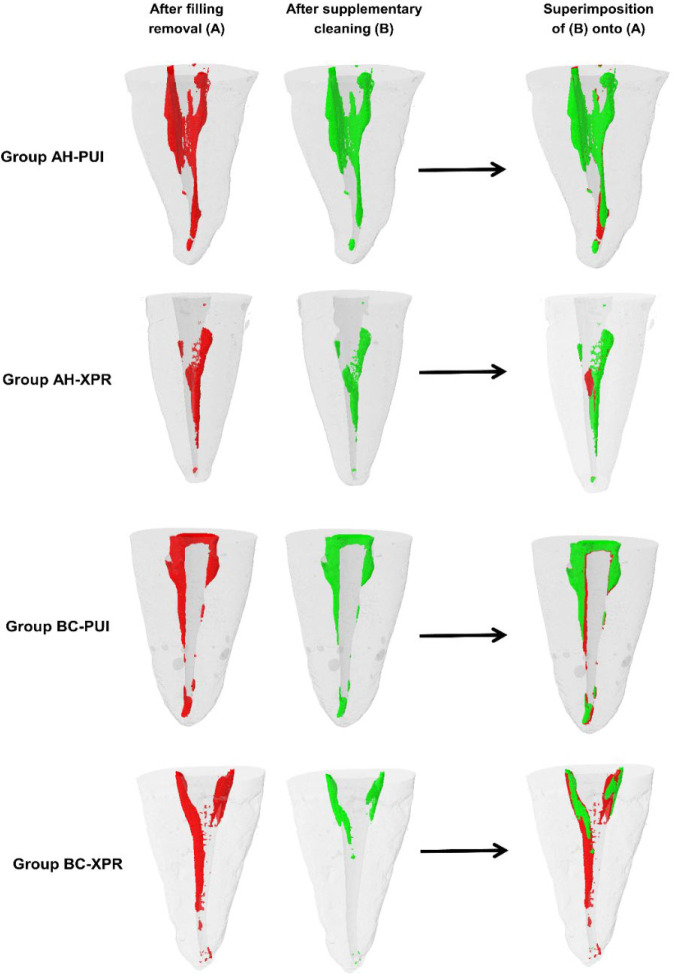
Figure 2 presents superimposed images acquired before and after the application of the supplementary cleaning techniques, illustrating the additional reductions in residual filling material. The specimens filled with Bio-C Sealer and subjected to supplementary cleaning with XPR exhibited a significantly lower volume of residual filling material compared with those treated with PUI (P<0.05). Conversely, no significant difference was observed between the PUI and XPR methods in specimens filled with AH-Plus sealer (P>0.05, Table 2).
Figure 2.
Superimpositions of images acquired before and after application of the supplementary cleaning techniques tested, illustrating the additional reductions in residual filling material promoted by them
Table 2.
Median, 25th percentile, and 75th percentile of the volume (mm3) of residual material after filling removed with the Reciproc R40 instrument, and after using either passive ultrasonic irrigation or the XP-endo Finisher R system
| Groups | Volume of residual material after filling removal with Reciproc R40 | Volume of residual material after supplementary cleaning technique |
|---|---|---|
| Median (p25–p75) | Median (p25 – p75) | |
| AH-PUI | 1.02 (0.49–3.44) A | 0.30 (0.09–0.46) A |
| AH-XPR | 1.18 (0.16–4.61) A | 0.07 (0.02–0.28) AB |
| BC-PUI | 1.13 (0.31–2.09) A | 0.29 (0.08–0.51) A |
| BC-XPR | 0.50 (0.08–1.02) A | 0.01 (0.00–0.08) B |
| P-value | 0.285 | 0.016 |
AH-PUI, AH-Plus sealer and passive ultrasonic irrigation (PUI); AH-XPR, AH-Plus sealer and XP-endo Finisher R instrument (XPR); BC-PUI, Bio-C Sealer and PUI; and BC-XPR, Bio-C Sealer and XPR. Different letters within the column indicate a statistically significant difference (Kruskal-Wallis test and pairwise comparison Mann-Whitney U test, P<0.05)
Discussion
The results indicate that both supplementary cleaning techniques tested removed filling material from the root canals; however, the XPR instrument demonstrated greater efficacy compared with PUI in root canals filled with Bio-C sealer. Therefore, the null hypothesis was rejected.
Both supplementary cleaning techniques were performed in a controlled environment maintained at 37 °C to standardize the experimental conditions, thus meeting the requirements of the XPR system. The choice of oval-shaped canals of mandibular premolars was deliberate due to their tendency to possess numerous regions with anatomical irregularities, which are prone to remain untouched by endodontic instruments [2, 29]. Removal of filling material from these canals can pose a challenge, particularly when relying solely on conventional instruments.
Micro-CT is widely regarded as the gold standard for in vitro endodontic research due to its non-destructive nature [2]. This method enables precise assessment of the volume of residual filling material within the same specimen at different stages of the experiment. Consequently, it mitigates the risks associated with interpretation bias that may arise when relying solely on direct observation methods.
The results of the present study underscored the persistence of residual filling material, even subsequent to the application of the Reciproc R40 instrument, thereby aligning with previous findings reported by Martins et al. [2], Crozeta et al. [33], and Volponi et al. [29]. Notably, no discernible difference was found between the two tested sealers concerning the removal of filling material following the application of the Reciproc R40 instrument. This finding contrasts the results reported by Rajda et al. [27], who observed that a reciprocating instrument exhibited superior efficacy in removing the combination of gutta-percha and a bioceramic sealer (TotalFill BC; FKG Dentaire, La-Chaux-de-fonds, Switzerland) from root canals compared with the combination of gutta-percha and an epoxy resin–based sealer (AH-Plus).
Bioceramic sealers have garnered attention due to their recognized biocompatibility and bioactivity attributes [34, 35]. Notably, the XPR supplementary cleaning technique exhibited superior performance compared with PUI in canals filled with Bio-C Sealer. This sealer possesses bioactive properties that enable it to interact with phosphate ions in dentin, forming a new layer of hydroxyapatite. However, Bio-C Sealer was observed to be more readily removed than AH-Plus in the present investigation, implying that its interaction with dentin may not promote extensive chemical bonding. In contrast, AH- Plus sealer demonstrated greater resistance to removal only when the PUI supplementary cleaning technique was employed, potentially due to the covalent bonds established between the dentinal amino group and the epoxy resin ring [36], thereby creating a micromechanical bond between the material and the root canal wall [37]. AH-Plus sealer is renowned for its exceptional properties of intratubular penetration and sealing capacity [38]. Furthermore, it has been documented to exhibit chemical interaction with dentin, even within isthmuses and oval-shaped root canals, thus rendering it challenging to be removed from these regions [15].
The superior performance of XPR observed in the present study may be attributed to the phase change of this instrument when exposed to temperatures exceeding 35°C, leading to the transformation of the last millimeters of its active tip into a semi-circumferential shape [39]. This alteration facilitates enhanced contact between the instrument and the irregularities present within the root canal, thereby promoting mechanical displacement of filling material.
Passive ultrasonic irrigation is another technique assessed in the present study, and has been widely established in the literature. While PUI did prove capable of removing filling material, it was significantly less effective than XPR in canals filled with Bio-C Sealer. Passive ultrasonic irrigation operates based on the cavitation phenomena and acoustic microstreaming [32]. However, to facilitate these effects, the ultrasonic insert must not make contact with the root canal walls and should remain positioned 2 mm short of the working length. This prerequisite allows for proper circulation of the irrigating solution, thereby ensuring maximal efficiency of the technique. Consequently, PUI is most effective when the instrument operates within the central portion of the canal. Initially developed to address the smear layer-a layer of debris adhering to the canal walls post-instrumentation-PUI's efficacy in displacing this material may not translate equivalently to removing more compact filling material in retreatment cases. Thus, while cavitation plays a role, its capacity to displace firmly adhered filling material may be insufficient, because the technique does not aim to engage with the root canal walls directly.
Studies conducted by De-Deus et al. [21], Volponi et al. [29], and Ferreira et al. [40] provide supporting evidence for the efficacy of the XPR system, aligning with the findings of the present study. However, it is noteworthy that the methodologies employed by De-Deus et al. (21) and Ferreira et al. (40) differed. Specifically, De-Deus et al. (21) evaluated the efficiency of XPR in mandibular incisors filled with AH-Plus, while Ferreira et al. (40) assessed the efficiency of XPR in mandibular incisors with the assistance of a solvent.
Martins et al. [2] observed that neither PUI nor EndoActivator had a discernible positive impact on filling material removal. However, the authors used a zinc oxide and eugenol–based sealer, which typically exhibits greater susceptibility to removal by mechanized instrumentation compared with bioceramic sealers during the primary step of filling removal [15]. Consequently, the potential contribution of supplementary techniques may be less pronounced in such scenarios.
Some authors have concluded that bioceramic sealers present greater challenges for removal [8, 15], whereas others have reported divergent findings [9, 38]. These discrepancies could potentially stem from variations in methodology, such as the inclusion of solvents, as well as anatomical disparities among the root types under investigation.
Given the limited availability of studies comparing the sealers and supplementary cleaning techniques assessed in the present study, it is recommended that future research be conducted to elucidate the underlying factors contributing to these outcome disparities. Specifically, studies exploring other brands of bioceramic sealers, utilizing diverse obturation techniques, and employing confocal microscopy are warranted to illuminate the adhesion mechanisms of bioceramic sealers to dentin. Additionally, investigations examining the potential correlation between micro-CT analyses (regarded as the gold standard for endodontic assessments) and molecular biology techniques (e.g., polymerase chain reaction) are necessary. Such research endeavors would provide the scientific foundation necessary for the development of techniques capable of facilitating more effective removal of various types of filling materials utilized in endodontic re-intervention procedures. It is essential to recognize that the outcomes of the present ex vivo study cannot be directly extrapolated to the clinical effectiveness of the evaluated supplementary cleaning systems in achieving favorable retreatment outcomes. Consequently, clinical studies are imperative to explore this potential association.
Conclusions
The supplementary cleaning technique performed with the XPR system in canals filled with Bio-C Sealer demonstrated a significantly greater removal of residual filling material compared with that performed with PUI. However, it is noteworthy that none of the techniques tested achieved complete removal of the residual filling material.
Acknowledgments
The authors thank the laboratory staff of the Federal University of Rio de Janeiro (UFRJ) for their support and assistance in performing the micro-CT scans. The authors deny any conflicts of interest related to this study.
Conflict of interest
None.
Funding support
No funding was received for this study.
Author contributions
Conceptualization: Seckler INB, Pelegrine RA, Stringheta CP, Lopes RT, Silva ASS, Bueno CES. Data curation: Seckler INB, Pelegrine RA, Bueno CES. Formal Analysis: Seckler INB, Bueno CES. Investigation: Seckler INB, Pelegrine RA, Stringheta CP. Methodology: Seckler INB, Lopes RT, Silva ASS, Bueno CES. Project administration: Seckler INB. Supervision: Bueno CES. Validation: Lopes RT, Silva ASS, Bueno CES. Writing original draft: Seckler INB, Pelegrine RA, Stringheta CP. Writing review & editing: Seckler INB, Pelegrine RA, Stringheta CP, Lopes RT, Silva ASS, Bueno CES. All the authors gave their final approval of the version to be published, and agreed to be accountable for all aspects of the study.
References
- 1.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment: part 2: tooth survival. Int Endod J. 2011;44(7):610–25. doi: 10.1111/j.1365-2591.2011.01873.x. [DOI] [PubMed] [Google Scholar]
- 2.Martins MP, Duarte MA, Cavenago BC, Kato AS, da Silveira Bueno CE. Effectiveness of the ProTaper Next and Reciproc Systems in Removing Root Canal Filling Material with Sonic or Ultrasonic Irrigation: A Micro-computed Tomographic Study. J Endod. 2017;43(3):467–71. doi: 10.1016/j.joen.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Alakabani TF, Faus-Llácer V, Faus-Matoses I, Ruiz-Sánchez C, Zubizarreta-Macho Á, Sauro S, Faus-Matoses V. The Efficacy of Rotary, Reciprocating, and Combined Non-Surgical Endodontic Retreatment Techniques in Removing a Carrier-Based Root Canal Filling Material from Straight Root Canal Systems: A Micro-Computed Tomography Analysis. J Clin Med. 2020;9(6) doi: 10.3390/jcm9061989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K, Kim DV, Kim SY, Yang S. A micro-computed tomographic study of remaining filling materials of two bioceramic sealers and epoxy resin sealer after retreatment. Restor Dent Endod. 2019;44(2):e18. doi: 10.5395/rde.2019.44.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair PN. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39(4):249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 6.Fruchi Lde C, Ordinola-Zapata R, Cavenago BC, Hungaro Duarte MA, Bueno CE, De Martin AS. Efficacy of reciprocating instruments for removing filling material in curved canals obturated with a single-cone technique: a micro-computed tomographic analysis. J Endod. 2014;40(7):1000–4. doi: 10.1016/j.joen.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Kok D, Rosa RA, Barreto MS, Busanello FH, Santini MF, Pereira JR, Só MV. Penetrability of AH plus and MTA fillapex after endodontic treatment and retreatment: a confocal laser scanning microscopy study. Microsc Res Tech. 2014;77(6):467–71. doi: 10.1002/jemt.22371. [DOI] [PubMed] [Google Scholar]
- 8.Oltra E, Cox TC, LaCourse MR, Johnson JD, Paranjpe A. Retreatability of two endodontic sealers, EndoSequence BC Sealer and AH Plus: a micro-computed tomographic comparison. Restor Dent Endod. 2017;42(1):19–26. doi: 10.5395/rde.2017.42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnermeyer D, Bunne C, Schäfer E, Dammaschke T. Retreatability of three calcium silicate-containing sealers and one epoxy resin-based root canal sealer with four different root canal instruments. Clin Oral Investig. 2018;22(2):811–7. doi: 10.1007/s00784-017-2156-5. [DOI] [PubMed] [Google Scholar]
- 10.Alberto Rubino G, de Miranda Candeiro GT, Gonzales Freire L, Faga Iglecias E, de Mello Lemos É, Luiz Caldeira C, Gavini G. Micro-CT Evaluation of Gutta-Percha Removal by Two Retreatment Systems. Iran Endod J. 2018;13(2):221–7. doi: 10.22037/iej.v13i2.18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Liu S, Dong Y. In vitro study of dentinal tubule penetration and filling quality of bioceramic sealer. PLoS One. 2018;13(2):e0192248. doi: 10.1371/journal.pone.0192248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furtado TC, de Bem IA, Machado LS, Pereira JR, Só MVR, da Rosa RA. Intratubular penetration of endodontic sealers depends on the fluorophore used for CLSM assessment. Microsc Res Tech. 2021;84(2):305–12. doi: 10.1002/jemt.23589. [DOI] [PubMed] [Google Scholar]
- 13.Lee BN, Hong JU, Kim SM, Jang JH, Chang HS, Hwang YC, Hwang IN, Oh WM. Anti-inflammatory and Osteogenic Effects of Calcium Silicate-based Root Canal Sealers. J Endod. 2019;45(1):73–8. doi: 10.1016/j.joen.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Vilas-Boas DA, Grazziotin-Soares R, Ardenghi DM, Bauer J, de Souza PO, de Miranda Candeiro GT, Maia-Filho EM, Carvalho CN. Effect of different endodontic sealers and time of cementation on push-out bond strength of fiber posts. Clin Oral Investig. 2018;22(3):1403–9. doi: 10.1007/s00784-017-2230-z. [DOI] [PubMed] [Google Scholar]
- 15.de Siqueira Zuolo A, Zuolo ML, da Silveira Bueno CE, Chu R, Cunha RS. Evaluation of the Efficacy of TRUShape and Reciproc File Systems in the Removal of Root Filling Material: An Ex Vivo Micro-Computed Tomographic Study. J Endod. 2016;42(2):315–9. doi: 10.1016/j.joen.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad M, Pitt Ford TR, Crum LA, Walton AJ. Ultrasonic debridement of root canals: acoustic cavitation and its relevance. J Endod. 1988;14(10):486–93. doi: 10.1016/S0099-2399(88)80105-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Wu MK, Wesselink PR. The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from different-sized simulated plastic root canals. Int Endod J. 2004;37(9):607–12. doi: 10.1111/j.1365-2591.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 18.Karamifar K, Mehrasa N, Pardis P, Saghiri MA. Cleanliness of Canal Walls following Gutta-Percha Removal with Hand Files, RaCe and RaCe plus XP-Endo Finisher Instruments: A Photographic in Vitro Analysis. Iran Endod J. 2017;12(2):242–7. doi: 10.22037/iej.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denna J, Shafie LA, Alsofi L, Al-Habib M, AlShwaimi E. Efficacy of the Rotary Instrument XP-Endo Finisher in the Removal of Calcium Hydroxide Intracanal Medicament in Combination with Different Irrigation Techniques: A Microtomographic Study. Materials (Basel) 2020;13(10) doi: 10.3390/ma13102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campello AF, Almeida BM, Franzoni MA, Alves FRF, Marceliano-Alves MF, Rôças IN, Siqueira JF Jr, Provenzano JC. Influence of solvent and a supplementary step with a finishing instrument on filling material removal from canals connected by an isthmus. Int Endod J. 2019;52(5):716–24. doi: 10.1111/iej.13047. [DOI] [PubMed] [Google Scholar]
- 21.De-Deus G, Belladonna FG, Zuolo AS, Cavalcante DM, Carvalhal JCA, Simões-Carvalho M, Souza EM, Lopes RT, Silva E. XP-endo Finisher R instrument optimizes the removal of root filling remnants in oval-shaped canals. Int Endod J. 2019;52(6):899–907. doi: 10.1111/iej.13077. [DOI] [PubMed] [Google Scholar]
- 22.Machado AG, Guilherme BPS, Provenzano JC, Marceliano-Alves MF, Gonçalves LS, Siqueira JF Jr, Neves MAS. Effects of preparation with the Self-Adjusting File, TRUShape and XP-endo Shaper systems, and a supplementary step with XP-endo Finisher R on filling material removal during retreatment of mandibular molar canals. Int Endod J. 2019;52(5):709–15. doi: 10.1111/iej.13039. [DOI] [PubMed] [Google Scholar]
- 23.Kaloustian MK, Nehme W, El Hachem C, Zogheib C, Ghosn N, Michetti J, Naaman A, Diemer F. Evaluation of Two Shaping Systems and Two Ultrasonic Irrigation Devices in Removing Root Canal Filling Material from Mesial Roots of Mandibular Molars: A Micro CT Study. Dent J (Basel) 2019;7(1) doi: 10.3390/dj7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida A, Romeiro K, Cassimiro M, Gominho L, Dantas E, Silva S, Albuquerque D. Micro-CT analysis of dentinal microcracks on root canals filled with a bioceramic sealer and retreated with reciprocating instruments. Sci Rep. 2020;10(1):15264. doi: 10.1038/s41598-020-71989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arul B, Varghese A, Mishra A, Elango S, Padmanaban S, Natanasabapathy V. Retrievability of bioceramic-based sealers in comparison with epoxy resin-based sealer assessed using microcomputed tomography: A systematic review of laboratory-based studies. J Conserv Dent. 2021;24(5):421–34. doi: 10.4103/jcd.jcd_376_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baranwal HC, Mittal N, Garg R, Yadav J, Rani P. Comparative evaluation of retreatability of bioceramic sealer (BioRoot RCS) and epoxy resin (AH Plus) sealer with two different retreatment files: An in vitro study. J Conserv Dent. 2021;24(1):88–93. doi: 10.4103/jcd.jcd_657_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajda M, Miletić I, Baršić G, Krmek SJ, Šnjarić D, Baraba A. Efficacy of Reciprocating Instruments in the Removal of Bioceramic and Epoxy Resin-Based Sealers: Micro-CT Analysis. Materials (Basel) 2021;14:21. doi: 10.3390/ma14216670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagendrababu V, Murray PE, Ordinola-Zapata R, Peters OA, Rôças IN, Siqueira JF Jr, Priya E, Jayaraman J, S JP, Camilleri J, Boutsioukis C, Rossi-Fedele G, Dummer PMH. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: A consensus-based development. Int Endod J. 2021;54(9):1482–90. doi: 10.1111/iej.13542. [DOI] [PubMed] [Google Scholar]
- 29.Volponi A, Pelegrine RA, Kato AS, Stringheta CP, Lopes RT, Silva ASS, Bueno C. Micro-computed Tomographic Assessment of Supplementary Cleaning Techniques for Removing Bioceramic Sealer and Gutta-percha in Oval Canals. J Endod. 2020;46(12):1901–6. doi: 10.1016/j.joen.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32(2):271–5. doi: 10.1016/0030-4220(71)90230-1. [DOI] [PubMed] [Google Scholar]
- 31.De-Deus G, Simões-Carvalho M, Belladonna FG, Versiani MA, Silva E, Cavalcante DM, Souza EM, Johnsen GF, Haugen HJ, Paciornik S. Creation of well-balanced experimental groups for comparative endodontic laboratory studies: a new proposal based on micro-CT and in silico methods. Int Endod J. 2020;53(7):974–85. doi: 10.1111/iej.13288. [DOI] [PubMed] [Google Scholar]
- 32.van der Sluis LW, Vogels MP, Verhaagen B, Macedo R, Wesselink PR. Study on the influence of refreshment/activation cycles and irrigants on mechanical cleaning efficiency during ultrasonic activation of the irrigant. J Endod. 2010;36(4):737–40. doi: 10.1016/j.joen.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Crozeta BM, Chaves de Souza L, Correa Silva-Sousa YT, Sousa-Neto MD, Jaramillo DE, Silva RM. Evaluation of Passive Ultrasonic Irrigation and GentleWave System as Adjuvants in Endodontic Retreatment. J Endod. 2020;46(9):1279–85. doi: 10.1016/j.joen.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Li Z, Peng B. Effects of iRoot SP on mineralization-related genes expression in MG63 cells. J Endod. 2010;36(12):1978–82. doi: 10.1016/j.joen.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CE, Pécora JD. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45(5):419–28. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- 36.Camargo RV, Silva-Sousa YTC, Rosa R, Mazzi-Chaves JF, Lopes FC, Steier L, Sousa-Neto MD. Evaluation of the physicochemical properties of silicone- and epoxy resin-based root canal sealers. Braz Oral Res. 2017;31:e72. doi: 10.1590/1807-3107BOR-2017.vol31.0072. [DOI] [PubMed] [Google Scholar]
- 37.Polineni S, Bolla N, Mandava P, Vemuri S, Mallela M, Gandham VM. Marginal adaptation of newer root canal sealers to dentin: A SEM study. J Conserv Dent. 2016;19(4):360–3. doi: 10.4103/0972-0707.186453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romeiro K, de Almeida A, Cassimiro M, Gominho L, Dantas E, Chagas N, Velozo C, Freire L, Albuquerque D. Reciproc and Reciproc Blue in the removal of bioceramic and resin-based sealers in retreatment procedures. Clin Oral Investig. 2020;24(1):405–16. doi: 10.1007/s00784-019-02956-3. [DOI] [PubMed] [Google Scholar]
- 39.Silva E, Belladonna FG, Zuolo AS, Rodrigues E, Ehrhardt IC, Souza EM, De-Deus G. Effectiveness of XP-endo Finisher and XP-endo Finisher R in removing root filling remnants: a micro-CT study. Int Endod J. 2018;51(1):86–91. doi: 10.1111/iej.12788. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira I, Babo PS, Braga AC, Lopes MA, Gomes ME, Pina-Vaz I. Supplementary solvent irrigation efficacy on filling remnants removal comparing XP-endo Finisher R vs IrriSafe. Sci Rep. 2021;11(1):12659. doi: 10.1038/s41598-021-92175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]