Abstract
Abstract
Objective
Adopting a physically active lifestyle and maintaining a diet rich in antioxidants can reduce the risk of vascular diseases. Arterial stiffness is an early marker for cardiovascular diseases, indicating vascular damage. This study investigates the relationship between physical activity (PA), sedentary behaviour (SB), dietary antioxidant, trace elements intake and vascular health in men and women, with a focus on pulse wave velocity (PWV), the gold standard for assessing arterial stiffness.
Design
This is a nationwide population-based cross-sectional study (Observation of Cardiovascular Risk Factors in Luxembourg 2 (ORISCAV-LUX 2)).
Setting
The study was conducted in Luxembourg, between November 2016 and January 2018.
Participants
In total, 988 participants from the ORISCAV-LUX 2 study, who were Luxembourg residents, aged 25–79 years, underwent the required physical examination, agreed to wear an accelerometer for 1 week and presented no personal history of myocardial infarction or stroke, were included in the analysis.
Primary outcome measure
PWV was assessed with the validated Complior instrument. Elastic-net models were used to investigate the associations of dietary intake (antioxidant and trace elements) and movement behaviours (PA and SB) with PWV in men and women.
Results
The findings reveal diverse associations between PA, SB, dietary intake and PWV, with distinct patterns observed in men and women. In women, a longer median moderate-to-vigorous PA bout length (mean coefficient (β)=−0.039), a higher long-range temporal correlation (higher scaling exponent alpha) at larger time scales (>120 min; β=−1.247) and an increased intake of vitamin C (β=−1.987) and selenium (β=−0.008) were associated with lower PWV. In men, a shorter median SB bout length (β=0.019) and a lower proportion of SB time accumulated in bouts longer than 60 min (β=1.321) were associated with lower PWV. Moreover, a higher daily intake of polyphenols (β=−0.113) and selenium (β=−0.004) was associated with lower PWV in men.
Conclusion
This study underscores the multifaceted nature of the associations between movement behaviours and dietary intake with PWV, as well as sex differences. These findings highlight the significance of considering both movement behaviours and dietary antioxidant intake in cardiovascular health assessments.
Keywords: NUTRITION & DIETETICS, Cardiovascular Disease, Behavior, SPORTS MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study used a validated Food Frequency Questionnaire that enabled a good overview of the participants’ dietary intake, as well as wearable-specific indicator of physical activity behaviours that enabled the assessment of the complex movement behaviour in an all-encompassing manner.
Pulse wave velocity was used to assess arterial stiffness, which has been proven to be among the most important predictors of future cardiovascular diseases.
The Elastic-net variable selection approach was used as it is expected to deal well with situations where predictors are highly correlated.
Limitations include the absence of a posture allocation algorithm to mitigate the misclassification of movement behaviours, such as standing as sedentary time, which is common with wrist-worn accelerometers.
Additionally, our study participants were generally healthier than the general population.
Introduction
Cardiovascular diseases (CVDs), particularly ischaemic heart disease (49% of CVD deaths in 2019) and stroke (18% of CVD deaths in 2019), are among the leading causes of death and disability worldwide.1 2 A primary concern regarding vascular health is arterial stiffness, one of the earliest manifestations of vascular damage.3 Arterial stiffness describes the inflexibility and rigidity of the arterial wall4 and is a significant contributor to systolic hypertension.5 The gold standard for assessing arterial stiffness is pulse wave velocity (PWV), a powerful blood pressure-related indicator of vascular status and cardiovascular risk.6 7 Measuring PWV between the carotid and femoral arteries is the most clinically relevant method, with higher PWV values representing higher arterial stiffness.8 9 A review and meta-analysis found that a 1 m/s increase in carotid-femoral PWV was associated with a 15% increased cardiovascular mortality risk.10
It is well established that lifestyle factors, including physical activity (PA) and a healthy diet, play a crucial role in reducing the risk of CVDs.11 12 Recent findings have evidenced the role of dietary antioxidants in mitigating oxidative stress and inflammation, contributing to the prevention of cardiovascular and metabolic diseases.11 13 These antioxidants encompass vitamins C and E, polyphenols and carotenoids, which can be found in fruits, vegetables, coffee, tea, red wine, cereals and chocolate.12 13 Additionally, trace elements like zinc and selenium, found in nuts, vegetables, cereal grains, meat and fish, are essential for the proper functioning of the body and deficiencies of these elements can lead to adverse health effects.14 15
Regular PA and a reduced sedentary behaviour (SB) are associated with decreased cardiovascular mortality11 and with a lower risk of atherosclerosis and CVDs such as myocardial infarction and stroke.16 17 The literature suggests a dose-dependent relationship between exercise intensity and arterial stiffness improvement, with higher intensities enhancing the benefits of aerobic exercise on arterial stiffness.18 However, to the best of our knowledge, no research has investigated movement behaviour indicators that encompass the full spectrum of intensities and behaviours during waking hours and their relationship to cardiovascular health. These indicators may provide more informative insights than focusing solely on moderate-to-vigorous PA (MVPA), which has been found to constitute only 6% of daily time in a study with the same population.19 Therefore, wearable-specific indicators of PA behaviour (WIPAB), recently defined as advanced analytical methods and their corresponding summary variables that aim to capture the complex nature of the movement behaviour by extending beyond the classical frequency, intensity, time and type framework,20 are needed to study their association with health outcomes.
Previous research has already provided evidence of sex-related differences in markers of arterial stiffness,21,23 movement behaviours,19 dietary patterns24 and their associations with cardiovascular risk factors.25 26 These findings underscore the significance of considering sex-specific factors in cardiovascular health assessment.
Several studies have examined the role of dietary patterns11 27 28 or exercise and MVPA separately on vascular health.11 16 29 30 However, there is limited research on the complementary roles of a diet’s antioxidant potential and an active lifestyle assessed using a more holistic approach, on vascular health. Therefore, the present study aimed to investigate how intake of dietary antioxidants and trace elements (ie, beta-carotene, vitamins A, C, E, polyphenols, selenium and zinc) and a physically active lifestyle (assessed using WIPAB) are related to arterial stiffness, with mutual adjustments for each other. In addition, the study explored whether these associations varied by sex.
Methods
Study population
The present study is based on the second wave of the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX 2), a cross-sectional, nationwide survey monitoring the prevalence of cardiovascular risk factors in 1558 Luxembourg residents between November 2016 and January 2018.31 Population coverage and sample representativeness have been analysed and it has been concluded that the ORISCAV-LUX dataset represents a valid tool for epidemiological studies in Luxembourg.31 The present analysis includes participants aged between 25 and 79 years, who underwent the required physical examination, agreed to wear an accelerometer for 1 week and presented no personal history of myocardial infarction or stroke (n=367 excluded). Participants with missing personal characteristics, anthropometric, dietary intake, PWV or PA data as well as those with erroneous acceleration files were excluded (n=125). In addition, participants with extreme dietary intake (<800 kcal/day or >6000 kcal/day), outlying PWV values (>40 m/s), calibration errors in the acceleration signal (>10 mg) and insufficient accelerometer data (<4 days including at least 1 weekend day) as well as outliers for each variable located above or below 5-SD were excluded (n=78). In total, 988 participants remained for this complete-case analysis (63.4% of the initial sample). A flow chart of the participants’ inclusion process can be found in the supplementary material (online supplemental figure S1).
Vascular health
The validated Complior instrument (ALAM medical, Vincennes, France) was used to assess PWV (m/s).32 Measurements were performed following 10 min of rest in a supine position. The distance between sites was calculated as a direct line (measured with a Seca 207 rod) adjusted by a scaling factor of 0.8.33 PWV was calculated by dividing the carotid-femoral distance by the transit time of the forward-travelling pulse between the carotid and femoral arteries. In case of difficulties finding the pulse during clinical examination, multiple measurements per person were taken. For participants with two measurements that differed <0.5 m/s, the average value of these two measures was used for the analysis. In case of a difference >0.5 m/s between the last two PWV measurements, another assessment was performed and the median of the last three measures was included in the analysis.
Movement behaviours
Participants were asked to continuously wear an accelerometer on the wrist of the non-dominant hand for 7 consecutive days, except when showering and during water activities (eg, swimming). The accelerometer (ActiGraphTM GT3X+, Pensacola, Florida, USA) collected triaxial acceleration data with a sampling frequency of 30 Hz and a dynamic range of ±8 g. Raw acceleration data were extracted using the manufacturer’s software (ActiLife V.6.13.3) and calibrated using the open-source R package GGIR (V.2.2-0).34 Acceleration values were averaged over 5-s epochs and expressed relative to gravity (g units: 1 g=9.81 m/s2). Acceleration data between the first and last midnight were used. Validated thresholds were used to estimate the average daily awake time spent in sedentary (<44.8 mg) and MVPA (>100.6 mg).35
The data were used to derive WIPAB measures describing the distribution of PA intensities, activity accumulation and the temporal correlation and regularity of movement behaviour.20 Average daily acceleration (mg) quantifies the average volume of the entire movement behaviour.36 The intensity gradient represents the activity intensity distribution over the entire measurement period by describing the negative curvilinear relationship between activity intensities and the accumulated time at these intensities.37 The M“X” metric represents the average acceleration above which the most active “X” minutes are accumulated.36 In the present study, “X” was set to 30 min (M0.5).36 38 Additionally, we computed the number of prolonged sedentary episodes of a duration higher than 30 min (SB bouts >30 min),39 the median duration of sedentary episodes (median SB bout length) and the median MVPA bout length, which describe the activity accumulation at specific intensities. The proportion of the total SB time accumulated in bouts longer than 60 min was also computed. The autocorrelation at lag 24 hours quantifies the regularity and consistency of activity patterns that are 24 hours apart.40 41 Another indicator of the regularity of a time series is the sample entropy, which analyses the presence of similar subpatterns in the time series. Higher (less negative) sample entropy values represent a more complex, irregular and unpredictable time series.42 43 Three additional metrics, the scaling exponent alpha at small (<90 min) and large time scales (>120 min)44 as well as the Lempel-Ziv complexity,45 were computed to detect temporal correlations and quantify the presence of subpatterns, respectively. More detailed information on these WIPAB measures has been provided previously.20
Dietary intake
Dietary intake was determined based on the quantity and frequency of food consumption reported in the validated 174-item Food Frequency Questionnaire (FFQ).46 Food quantity was estimated using portion-size images. Food frequency consumption was categorised as never/rarely, one to three times per month, one to two times per week, three to five times per week, once a day and twice or more a day.47
Based on the ANSES-CIQUAL French Food Composition Table Database, daily food consumption was converted into an amount of macronutrient and micronutrient intake.47 48 Polyphenols have been extracted using the Phenol-Explorer.49 50 In the present study, we focused on dietary antioxidants and trace elements, namely beta-carotene (μg/day), vitamin A (retinol, μg/day), vitamin C (ascorbic acid, mg/day), vitamin E (tocopherols, mg/day), polyphenols (mg/day), zinc (mg/day) and selenium (μg/day).
Covariates
Participants self-reported their age and sex as well as their marital, smoking and employment status which were categorised as married/living with partner or single/divorced/separated/widowed; current smoker, former smoker or non-smoker; and employed or unemployed/at home/retired/unable to work, respectively. In addition, the use of any medication for hypertension, diabetes or dyslipidaemia (yes/no) as well as their family history of CVD or diabetes (yes/no/unknown) were assessed by a questionnaire. Total sleep period (min) was calculated based on the acceleration data.51 Daily estimates of total energy intake (kcal/day) were calculated based on reported food consumption in the FFQ.
Statistical analysis
Participants were stratified by sex (women, n=530; men, n=458). To compare participant characteristics among these two groups, a Pearson’s χ2 test for categorical variables and a Kruskal-Wallis test for not normally distributed continuous variables were employed. Spearman correlations were used to analyse correlations among the movement behaviour variables and the dietary pattern variables, separately. This analysis was performed independently for both women and men, and the results were visually represented by a correlation matrix.
For women and men, separate generalised linear regression models with embedded Elastic-net variable selection approach were built to explore how movement behaviour and dietary patterns related to PWV. Elastic-net analysis has been shown to yield superior results, particularly in situations where predictors are highly correlated, as compared with LASSO and ridge regression.52 53 All covariates, excluding sex, were integrated into these elastic-net models. The elastic-net modelling procedure was iterated 1000 times, each time incorporating internal 10-fold cross validation to tune the hyperparameters alpha (mixing term, ranges between 0 and 1) and lambda (shrinkage term). Lambda.min, which represents the value of Lambda that gives minimum mean cross-validated error, was used to identify the best prediction model.53 Mean coefficients and percentages of appearance were computed across these 1000 iterations. Variables were considered as robust if they were selected in at least 70% of all iterations. To visualise the outcomes, bar plots were generated with separate plots for women and men, respectively. The elastic-net analysis was implemented using the R package glmnet.
All statistical analyses were conducted using R (V.4.3.1) with RStudio (V.2023.06.01 Build 524). Statistical significance was defined as p values below 0.05.
Patient and public involvement
None.
Results
Participant characteristics
A description of the study participants stratified by sex is presented in table 1. The average age across all participants was 50.4 years, with women comprising 53.6% of the 988 individuals included in the analysis. Women had a longer total sleep period, a lower daily energy intake and a lower PWV compared with men. Additionally, women presented a higher average 24 hours acceleration, a lower (steeper) intensity gradient, shorter median SB bout lengths, a lower number of prolonged SB bouts, a lower proportion of SB bouts lasting at least 60 min, a higher scaling exponent alpha at larger time scales (>120 min), a higher autocorrelation at lag 24 hours as well as a more complex PA behaviour (Lempel-Ziv complexity) compared with men. Women also reported lower daily intakes of vitamins A and E, zinc and selenium compared with men.
Table 1. Participant characteristics overall and stratified by sex.
| Total population (n=988) | Women (n=530) | Men (n=458) | P value | |
| MED (IQR) or n (%) | MED (IQR) or n (%) | MED (IQR) or n (%) | ||
| Age (years) | 50.4 (41.4–59.5) | 51.2 (42.1–59.3) | 49.9 (41.0–59.8) | 0.437 |
| Marital status | <0.001 | |||
| Married/living with partner | 740 (74.9) | 374 (70.6) | 366 (79.9) | |
| Single/divorced/separated/widowed | 248 (25.1) | 156 (29.4) | 92 (20.1) | |
| Employment status | 0.060 | |||
| Employed | 657 (66.5) | 338 (63.8) | 319 (69.7) | |
| Unemployed/at home/retired/unable to work | 331 (33.5) | 192 (36.2) | 139 (30.3) | |
| Family history of CVD or diabetes | <0.001 | |||
| No | 178 (18.0) | 84 (15.8) | 94 (20.5) | |
| Yes | 671 (67.9) | 394 (74.3) | 277 (60.5) | |
| Unknown | 139 (14.1) | 52 (9.8) | 87 (19.0) | |
| Use of antihypertensive, diabetes and/or lipid-lowering medication | 0.060 | |||
| No | 724 (73.3) | 402 (75.8) | 322 (70.3) | |
| Yes | 264 (26.7) | 128 (24.2) | 136 (29.7) | |
| Smoking status | <0.01 | |||
| Current smoker | 121 (12.2) | 58 (10.9) | 63 (13.8) | |
| Former smoker | 275 (27.8) | 130 (24.5) | 145 (31.7) | |
| Non-smoker | 592 (59.9) | 342 (64.5) | 250 (54.6) | |
| Total sleep period (min) | 410.9 (373.3–444.8) | 420.1 (390.5–451.7) | 391.7 (360.2–431.7) | <0.001 |
| Energy intake (kcal/day) | 2359 (1858–2965) | 2131 (1734–2633) | 2653 (2119–3264) | <0.001 |
| PWV (m/s) | 7.75 (6.85–9.00) | 7.53 (6.75–8.70) | 7.95 (7.05–9.25) | <0.001 |
| Average 24 hours acceleration (mg) | 25.53 (21.26–30.40) | 26.25 (22.21–30.84) | 24.77 (20.50–29.64) | <0.001 |
| Intensity gradient | −2.64 (−2.76–−2.51) | −2.66 (−2.78–−2.52) | −2.61 (−2.74–−2.47) | <0.001 |
| M0.5 (g) | 105.75 (85.06–141.95) | 107.36 (85.67–141.30) | 104.19 (84.83–142.54) | 0.761 |
| Median SB bout length (s) | 1900 (1700–2100) | 1850 (1700–2050) | 1900 (1700–2150) | <0.05 |
| Median MVPA bout length (s) | 300 (200–450) | 300 (200–400) | 300 (200–450) | 0.370 |
| Number of SB bouts >30 min | 5.67 (4.67–6.67) | 5.50 (4.33–6.33) | 6.00 (5.00–7.00) | <0.001 |
| Proportion SB >60 min (%) | 25.59 (18.69–33.49) | 24.46 (16.67–32.33) | 27.14 (20.34–34.88) | <0.001 |
| Scaling exponent alpha (<90 min) | 0.96 (0.91–1.02) | 0.96 (0.92–1.02) | 0.96 (0.90–1.02) | 0.164 |
| Scaling exponent alpha (>120 min) | 0.98 (0.87–1.07) | 0.99 (0.89–1.09) | 0.96 (0.84–1.06) | <0.001 |
| Autocorrelation at lag 24 hours | 0.13 (0.10–0.17) | 0.14 (0.11–0.18) | 0.12 (0.09–0.15) | <0.001 |
| Lempel-Ziv complexity | 0.17 (0.15–0.20) | 0.18 (0.16–0.21) | 0.16 (0.13–0.19) | <0.001 |
| Sample entropy | −0.39 (−0.64–0.15) | −0.38 (−0.65–0.14) | −0.42 (−0.64–0.16) | 0.604 |
| Beta-carotene (μg/day) | 4947 (3328–7271) | 5052 (3444–7534) | 4675 (3222–7000) | 0.061 |
| Vitamin A (retinol, μg/day) | 465.8 (329.1–640.1) | 419.0 (304.6–572.5) | 530.5 (366.9–722.8) | <0.001 |
| Vitamin C (ascorbic acid, mg/day) | 145.2 (100.2–201.9) | 150.4 (103.0–206.9) | 140.6 (97.8–199.1) | 0.095 |
| Vitamin E (tocopherols, mg/day) | 18.19 (13.59–25.15) | 16.78 (13.03–22.51) | 19.93 (14.40–28.29) | <0.001 |
| Polyphenols (mg/day) | 2484 (1749–3379) | 2490 (1755–3331) | 2483 (1745–3433) | 0.766 |
| Zinc (mg/day) | 12.20 (9.47–15.66) | 11.13 (8.90–13.63) | 13.91 (10.59–17.18) | <0.001 |
| Selenium (μg/day) | 91.57 (71.63–111.92) | 83.97 (67.87–103.11) | 100.0 (79.01–122.0) | <0.001 |
Data are presented as n (%) for categorical variables and median (IQR) for non-normally distributed continuous variables. P- values represent a test of group differences by sex performed using χ2chi-square tests or Kruskal-Wallis tests as appropriate. Bold font signifies statistically significant difference between women and men (pp<0.05).disease; M0.5 acceleration above which the most activemin of the day were accumulated;vigorous ; Proportionof total SB time accumulated in bouts longer thanmin; ; .
CVDcardiovascular diseaseM0.5average acceleration above which the most active 30 min of the day were accumulatedMVPAmoderate-to-vigorous physical activityProportion SBproportion of total SB time accumulated in bouts longer than 60 minPWVpulse wave velocitySBsedentary behaviour
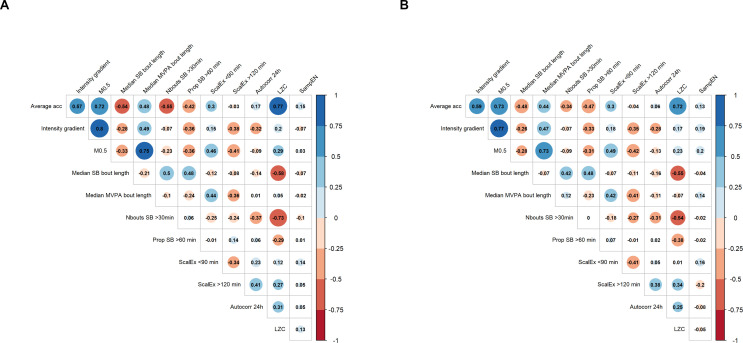
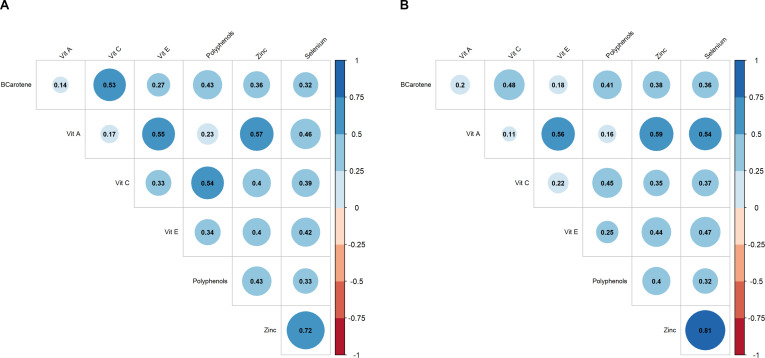
Figure 1 shows high correlations between the average acceleration and Lempel-Ziv complexity as well as between the average acceleration and M0.5, for both women (r=0.77 and r=0.72, respectively) and men (r=0.72 and r=0.73, respectively). Moreover, the intensity gradient and median MVPA bout length presented high correlations with M0.5 (r=0.80 and r=0.75 for women and r=0.77 and r=0.73 for men, respectively). Additionally, a high negative correlation could be observed between the number of prolonged sedentary bouts (>30 min) and Lempel-Ziv complexity in women (r=−0.73). Low-to-high positive correlations were found between each of the dietary intake components; zinc and selenium presented the highest correlation (r=0.72 and r=0.81 for women and men, respectively figure 2). Overall, correlations among all the exposures were similar across men and women.
Figure 1. Correlation matrix of the movement behaviour variables for (A) women and (B) men. The size of the circle is proportional to the correlation strength. Red and blue colours represent negative and positive correlations, respectively. Autocorr 24 hours, autocorrelation at lag 24 hours; Average acc, average acceleration; LZC, Lempel Ziv complexity; M0.5, average acceleration above which the most active 30 min of the day were accumulated; MVPA, moderate-to-vigorous physical activity; Nbouts SB>30 min, number of prolonged sedentary bouts (>30 min); Prop SB>60 min, proportion of total SB time accumulated in bouts longer than 60 min; SampEn, sample entropy; SB, sedentary behaviour; ScalEx <90 min, scaling exponent alpha at small time scales (<90 min); ScalEx>120 min, scaling exponent alpha at large time scales (>120 min).
Figure 2. Correlation matrix of the dietary intake variables for (A) women and (B) men. The size of the circle is proportional to the correlation strength. Red and blue colours represent negative and positive correlations, respectively. BCarotene, beta-carotene; Vit A, vitamin A; Vit C, vitamin C; Vit E, vitamin E.
Association of PA and dietary intake with vascular health
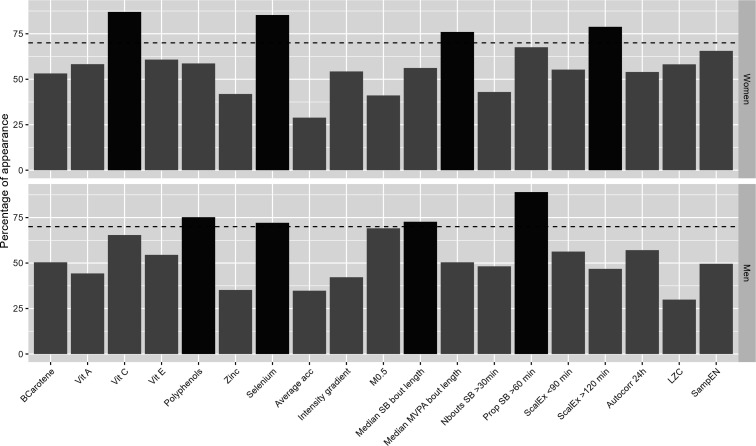
Figure 3 visually presents the frequency of appearance of each predictor in the elastic-net models, calculated over 1000 iterations.
Figure 3. Percentages of appearance of each predictor included in the elastic-net models, calculated over 1000 iterations. Horizontal dashed line represents percentage of appearance of 70%. Percentages above this line are highlighted in dark grey. All models were adjusted for age, marital status, employment status, family history of cardiovascular diseases or diabetes, use of medication, smoking status, total sleep period and daily energy intake.
Table 2 presents the mean coefficients and percentages of appearance of the predictors that remained in the elastic-net models in at least 70% of cases. In women, a longer median MVPA bout length, a higher scaling exponent alpha at larger time scales (>120 min) as well as a higher intake of vitamin C and selenium were associated with lower PWV. In men, a longer median SB bout length and a greater proportion of SB time accumulated in bouts longer than 60 min were associated with higher PWV, whereas a higher daily intake of polyphenols and selenium were associated with lower PWV.
Table 2. Elastic-net regression models for PWV, stratified by sex.
| PWV (m/s) | ||||
| Women (n=530) | Men (n=458) | |||
| Mean coefficient | % of appearance | Mean coefficient | % of appearance | |
| Average 24 hours acceleration (mg) | −0.007 | 29 | −0.004 | 35 |
| Intensity gradient | −0.081 | 54 | −0.121 | 42 |
| M0.5 (g) | −0.001 | 41 | −0.002 | 69 |
| Median SB bout length (min)* | 0.011 | 56 | 0.019 | 73 |
| Median MVPA bout length (min)* | −0.039 | 76 | −0.022 | 50 |
| Number of SB bouts >30 min | −0.021 | 43 | 0.039 | 48 |
| Proportion SB >60 min (%) | 0.825 | 68 | 1.321 | 89 |
| Scaling exponent alpha (<90 min) | −0.791 | 55 | −0.610 | 56 |
| Scaling exponent alpha (>120 min) | −1.247 | 79 | −0.542 | 47 |
| Autocorrelation at lag 24 hours | 1.247 | 54 | −1.188 | 57 |
| Lempel-Ziv complexity | 4.342 | 58 | 1.185 | 30 |
| Sample entropy | 0.084 | 66 | 0.012 | 50 |
| Beta-carotene (mg/day)† | −0.008 | 53 | 0.005 | 50 |
| Vitamin A (mg/day)† | −0.307 | 58 | 0.100 | 44 |
| Vitamin C (g/day)† | −1.987 | 87 | 1.273 | 65 |
| Vitamin E (mg/day) | 0.008 | 61 | 0.008 | 55 |
| Polyphenols (g/day)† | −0.040 | 59 | −0.113 | 75 |
| Zinc (mg/day) | 0.023 | 42 | −0.004 | 35 |
| Selenium (μg/day) | −0.008 | 85 | −0.004 | 72 |
Values are presented as mean coefficients averaged over all bootstrapped samples (nn=1000). Bold font signifies a percentage of appearance of at least 70%. All models were adjusted for age, marital status, employment status, family history of CVD or diabetes, use of medication, smoking status, total sleep period and daily energy intake. Abbreviations:disease; M0.5 acceleration above which the most activemin of the day were accumulated;vigorous ; Proportionof total SB time accumulated in bouts longer thanmin; ; .
Variables scaled by dividing by 60; .
Variables scaled by dividing by 1000.
CVDcardiovascular diseaseM0.5average acceleration above which the most active 30 min of the day were accumulatedMVPAmoderate-to-vigorous physical activityProportion SBproportion of total SB time accumulated in bouts longer than 60 minPWVpulse wave velocitySBsedentary behaviour
Discussion
The primary aim of this study was to investigate the associations of movement behaviours and dietary intake with vascular health, measured using an indicator of arterial stiffness (PWV), in an adult population of Luxembourg. Elastic-net models indicated that distinct predictors were associated with PWV across different sex groups. Presently, research examining the effect of complex pattern of movement behaviour on vascular health, independent of dietary intake and vice versa, is limited. Moreover, many studies have overlooked stratification by sex, despite evidence revealing sex-dependent patterns of association between PA and dietary intake with vascular health.19 21 25 26
A recent review examined the role of oxidative stress and endothelial dysfunction in the development of CVD. The review highlighted the potential for lifestyle modifications, specifically focusing on diet and exercise, to enhance vascular health.11 The findings of the review indicated that regular PA has a positive impact on glutathione levels in body fluids and promotes the activities of antioxidant enzymes such as superoxidase dismutase and glutathione peroxidase, which are dependent on essential minerals like zinc and selenium, respectively.11 In the present study, the relationship between the intake of antioxidant-rich nutrients and arterial stiffness, independent of an active lifestyle, could also be demonstrated. In particular, the important role of selenium in combination with polyphenols and vitamin C could be outlined, with distinct patterns observed in men and women. In women, higher daily intakes of vitamin C and selenium were associated with lower PWV, while in men, higher intakes of polyphenols and selenium were associated with lower PWV. Dietary polyphenols offer a range of activities that can influence various pathways related to vascular health, inflammation, oxidative stress, autophagy and glycation.54 These nutrients have shown promising results in controlling or preventing age-related vascular stiffening. Several studies have observed an inverse association between higher polyphenol intake and arterial stiffness.55 56 A review examining dietary interventions found that plant-derived natural products, including polyphenols, were associated with significant improvements in arterial stiffness among both healthy individuals and those with a slightly elevated CVD risk.57 Another review summarised evidence from interventional and epidemiological studies, highlighting the direct or indirect effects of polyphenols by their metabolites, derived from microbial or host metabolic processes, on vascular stiffness.54 Similarly, studies have suggested a potential protective effect of selenium intake against arterial stiffness and vascular diseases.58 A population-based cohort study conducted using NHANES data concluded that individuals with higher selenium intake had significantly lower odds of having a high vascular risk status compared with those with lower selenium intake.59 The crucial role of vitamin C in the autonomic nervous regulation of blood pressure has also been highlighted in a review,60 emphasising its significance. It has been proposed that vitamin C restores baroreflex dysfunction induced by oxidative stress, mitigating baroreflex sensitivity and sustaining a hypertensive state.61 Another review suggested that the combined intake of vitamin C and E enhances endothelial function and demonstrated beneficial effects on lipid and glucose metabolism, blood pressure and arterial plasticity in individuals with cardiovascular risk factors.11 Nevertheless, caution is warranted regarding long-term and high-dose usage of these vitamins, as they could have adverse effects on cardiovascular outcomes.11 62 In conclusion, further research, including randomised controlled trials and larger scale studies, is needed to establish a cause-and-effect relationship between dietary intake and their impact on arterial stiffness and vascular risk. Despite this, the available evidence suggests that incorporating polyphenol-rich foods and dietary sources of vitamin C and selenium into a balanced diet may hold benefits for maintaining cardiovascular health.11 14 60
Independent of dietary intake, the median MVPA bout length and the scaling exponent alpha at time scales above 2 hours demonstrated a favourable association with arterial stiffness in women. This implies that a longer duration at higher activity intensities (eg, brisk walking, jogging, lawn mowing or washing windows) as well as a more pronounced long-range temporal correlation in activity fluctuations (ie, regular exercise routine with repetitive patterns over time) may have a beneficial impact on arterial health in women. In men, an accumulation pattern of SB time characterised by longer bouts was found to be associated with higher PWV. Previous studies have already demonstrated the beneficial impact of breaking up prolonged SB periods on cardiometabolic health.63 64 However, in the context of arterial health, recent research using compositional data analysis,30 a meta-analysis65 and a study on older British men66 have found that there is no influence of interrupting prolonged sitting on arterial stiffness, suggesting that shorter SB bouts alone might not provide benefit to the central vasculature.30 It is important to note that these studies focused more on the number of SB breaks rather than the accumulation pattern of SB, and this difference may contribute to the apparent inconsistencies. Nevertheless, previous work on long-term effects of PA and SB on changes in arterial stiffness have demonstrated that spending more time engaged in PA and minimising SB are associated with a slower age-related progression of arterial stiffness.67 Additionally, substituting SB time with LPA or MVPA has shown beneficial associations with a wide range of cardiometabolic risk factors.39 Indeed, the positive effect of engaging in PA on a regular basis, independent of the specific daily timing, has already been noted in previous research. It has been emphasised that frequent PA positively influences chronic functional adaptation and promotes arterial structural remodelling, which leads to reduced arterial stiffness and a decreased risk of CVD.16 Moreover, frequent PA has the potential to reduce chronic inflammation by releasing muscle-derived myokines, which promote an anti-inflammatory environment and help protect arteries from CVD progression.68 Conversely, prolonged sitting has been found to impair overall vasodilatory function.69 These findings highlight the significance of engaging in PA on a regular basis and minimising SB for the maintenance and promotion of vascular health. Specifically, our results indicate that, for women, engaging in regular PA with longer durations at higher activities is beneficial, while, for men, reducing SB is crucial for vascular health. Therefore, activity accumulation patterns and regularity appear to be key factors. Furthermore, it is important to note that, for men, the average acceleration above which the most active 30 min were accumulated (M0.5) had a percentage of appearance of 69%, just below the inclusion threshold. Consequently, the importance of PA intensity to men’s vascular health should not be overlooked. There is merit in exploring a combination of information related to the amount, timing and way of accumulation of PA. Profiling individuals’ activity patterns might reveal additional, in-depth insights.
The relationship between movement behaviour, dietary intake and vascular health varied between women and men. Existing research has underscored sex disparities in movement behaviours,19 markers of arterial stiffness21,23 and their associations with cardiovascular risk factors.25 26 DuPont et al22 highlight the differences between women and men concerning the age-related progression of arterial stiffness and the associated risk of CVD. Prior studies have demonstrated the impact of sex hormones, such as oestrogens and testosterone, on arterial wall behaviour.21,2370 Fluctuations in oestrogen levels seem to influence the impact of cardiovascular risk factors on PWV both across hormonal phases in women and in comparison to men.22 23 25 A recent study identified a clinically significant acceleration in arterial stiffness during the late post-menopause phase (8+ years), probably related to a long-term exposition to the withdrawal of oestrogen, and potentially increasing the risk of CVD during this life stage.71 Moreover, testosterone deficiency has been linked to increased PWV, indicative of early vascular ageing.22 The substantial impact of sex hormones on arterial stiffness underscores the importance of considering sex-specific factors in the assessment of cardiovascular health.
Strengths and limitations
One of the strengths of the ORISCAV-LUX 2 study is the use of a validated FFQ, which has been linked to a European food composition database. This approach enabled a good overview of the participants’ dietary intake. Moreover, WIPAB measures were used, which enable the assessment of the complex movement behaviour in an all-encompassing manner. Another strength consists in the use of PWV to assess arterial stiffness, which has been proven to be among the most important predictors of future CVDs.6 7 This study also has some limitations. The declared nutrient intakes exceeded common recommendations, which may have altered the findings. Furthermore, it’s worth noting that our study participants were generally healthier than the general population, as observed in previous studies.26 31 Nevertheless, this does not necessarily compromise the validity or applicability of the study findings. In fact, considering the total population of Luxembourg, the present study had a high sample size and thus provides a comprehensive overview of the Luxembourgish adult population. Another limitation of this study is the absence of a posture allocation algorithm to mitigate the misclassification of movement behaviours, such as standing as sedentary time, which is common with wrist-worn accelerometers. Additionally, it’s crucial to recognise that there is potential for residual confounding or mediating effects resulting from imperfectly measured or unspecified covariates. Furthermore, given the observational and cross-sectional study design, drawing definitive conclusions about causality is not possible. To establish a firmer basis for these findings, longitudinal studies are warranted for result replication and a more comprehensive understanding.
Conclusions
This study highlights the importance of engaging in regular PA, reducing SB and incorporating dietary sources rich in vitamin C, polyphenols and selenium into a well-balanced diet for the promotion of cardiovascular health in adults. Notably, the associations between movement behaviours, dietary intake and vascular health displayed variations between women and men, emphasising the need to account for sex-specific factors in cardiovascular health assessment and that caution is needed when considering these variables as predictors of cardiovascular health. Nevertheless, further research using a longitudinal design is necessary to establish causality and comprehensively explore the effects of dietary components and movement behaviours on cardiovascular health.
supplementary material
Acknowledgements
The authors thank the nurses and participants from the ORISCAV-LUX 2 study. Additionally, the authors thank the ORISCAV-LUX study group for their contribution to the study. Furthermore, we express our gratitude to the Department of Public Health at the University of Liège (Belgium) for granting us permission to utilise their Food Frequency Questionnaire (https://www.dssp-uliege.be/FFQ).
Footnotes
Funding: The ORISCAV-LUX 2 study was funded by the Ministry of Higher Education and Research of Luxembourg.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084933).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: The National Research Ethics Committee (CNER, No 201.505/12) and the National Commission for Private Data Protection (CNPD) approved the ORISCAV-LUX 2 study. Participants gave informed consent to participate in the study before taking part.
Data availability free text: Deidentified participant data might be available after the consent of all authors and the ORISCAV study group. Requests to access the dataset should be directed to the corresponding author, laurent.malisoux@lih.lu.
Collaborators: Ala’a Alkerwi, Stephanie Noppe, Charles Delagardelle, Jean Beissel, Anna Chioti, Saverio Stranges, Jean-Claude Schmit, Marie-Lise Lair, Marylène D’Incau, Jessica Pastore, Gloria A Aguayo, Brice Appenzeller, Sophie Couffignal, Manon Gantenbein, Yvan Devaux, Michel Vaillant, Laetitia Huiart, Dritan Bejko, Magali Perquin, Maria Ruiz, Isabelle Ernens, Guy Fagherazzi.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Anne Backes, Email: anne.backes@lih.lu.
Paul J Collings, Email: pjcollings88@gmail.com.
Berta Portugal, Email: Berta.MartinsPortugal@lih.lu.
Lilly Carina Quintero, Email: lilly.benedikt@gmail.com.
Farhad Vahid, Email: farhad.vahid@lih.lu.
Gwenaëlle Le Coroller, Email: Gwenaelle.LeCoroller@lih.lu.
Laurent Malisoux, Email: laurent.malisoux@lih.lu.
Data availability statement
Data are available upon reasonable request.
References
- 1.World Health Organization Cardiovascular diseases (CVDs) [11-Jun-2021]. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Available. Accessed.
- 2.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H-L, Kim S-H. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41. doi: 10.3389/fcvm.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung Y-F. In: Paediatric cardiology. 3rd. Anderson RH, Baker EJ, Penny D, et al., editors. Philadelphia: Churchill Livingstone Elsevier; 2010. Systemic circulation; pp. 91–116. edn. [Google Scholar]
- 5.Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol. 2006;22 Suppl B:72B–80B. doi: 10.1016/s0828-282x(06)70990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 7.Zhong Q, Hu M-J, Cui Y-J, et al. Carotid-femoral pulse wave velocity in the prediction of cardiovascular events and mortality: an updated systematic review and meta-analysis. Angiol Open Access. 2018;69:617–29. doi: 10.1177/0003319717742544. [DOI] [PubMed] [Google Scholar]
- 8.Avolio AP, Kuznetsova T, Heyndrickx GR, et al. Arterial flow, pulse pressure and pulse wave velocity in men and women at various ages. Adv Exp Med Biol . 2018;1065:153–68. doi: 10.1007/978-3-319-77932-4_10. [DOI] [PubMed] [Google Scholar]
- 9.Jannasz I, Sondej T, Targowski T, et al. Pulse wave velocity - a useful tool in assessing the stiffness of the arteries. Pol Merkur Lekarski. 2019;46:257–62. [PubMed] [Google Scholar]
- 10.Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, et al. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6:99–109. doi: 10.1161/CIRCOUTCOMES.112.966903. [DOI] [PubMed] [Google Scholar]
- 11.Man AWC, Li H, Xia N. Impact of lifestyles (diet and exercise) on vascular health: oxidative stress and endothelial function. Oxid Med Cell Longev. 2020;2020:1496462. doi: 10.1155/2020/1496462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. 2007;73:326–40. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Ha K, Liao LM, Sinha R, et al. Dietary total antioxidant capacity, a diet quality index predicting mortality risk in US adults: evidence from the NIH-AARP diet and health study. Antioxidants (Basel) 2023;12:1086. doi: 10.3390/antiox12051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gać P, Czerwińska K, Macek P, et al. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ Toxicol Pharmacol. 2021;82:103553. doi: 10.1016/j.etap.2020.103553. [DOI] [PubMed] [Google Scholar]
- 15.Kuria A, Tian H, Li M, et al. Selenium status in the body and cardiovascular disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021;61:3616–25. doi: 10.1080/10408398.2020.1803200. [DOI] [PubMed] [Google Scholar]
- 16.Green DJ, Smith KJ. Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harb Perspect Med. 2018;8:a029819. doi: 10.1101/cshperspect.a029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavie CJ, Ozemek C, Carbone S, et al. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124:799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 18.Ashor AW, Lara J, Siervo M, et al. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9:e110034. doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collings PJ, Backes A, Aguayo GA, et al. Device-measured physical activity and sedentary time in a national sample of Luxembourg residents: the ORISCAV-LUX 2 study. Int J Behav Nutr Phys Act. 2022;19:161. doi: 10.1186/s12966-022-01380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backes A, Gupta T, Schmitz S, et al. Advanced analytical methods to assess physical activity behavior using accelerometer time series: a scoping review. Scand J Med Sci Sports. 2022;32:18–44. doi: 10.1111/sms.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J-Y, Park JB, Kim DS, et al. Gender difference in arterial stiffness in a multicenter cross-sectional study: the Korean Arterial Aging Study (KAAS) Pulse (Basel) 2015;2:11–7. doi: 10.1159/000365267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DuPont JJ, Kenney RM, Patel AR, et al. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol. 2019;176:4208–25. doi: 10.1111/bph.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Kiechl SJ, Wang J, et al. Global distributions of age- and sex-related arterial stiffness: systematic review and meta-analysis of 167 studies with 509,743 participants. EBioMedicine. 2023;92:104619. doi: 10.1016/j.ebiom.2023.104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tindall AM, Stallings VA. Sex differences in cardiovascular risk may be related to sex differences in diet patterns: a narrative review. Ann Hum Biol. 2021;48:517–24. doi: 10.1080/03014460.2021.1998621. [DOI] [PubMed] [Google Scholar]
- 25.Zemtsovskaja G, Abina J, Meigas K, et al. Pulse wave velocity and its gender-related associations with cardiovascular risk factors in a high cardiovascular risk population. Arch Med Sci Atheroscler Dis. 2018;3:e99–105. doi: 10.5114/amsad.2018.76919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backes A, Aguayo GA, Collings PJ, et al. Associations between wearable-specific indicators of physical activity behaviour and insulin sensitivity and glycated haemoglobin in the general population: results from the ORISCAV-LUX 2 study. Sports Med Open. 2022;8:146. doi: 10.1186/s40798-022-00541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casas R, Castro-Barquero S, Estruch R, et al. Nutrition and cardiovascular health. Int J Mol Sci. 2018;19:3988. doi: 10.3390/ijms19123988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callejo M, Barberá JA, Duarte J, et al. Impact of nutrition on pulmonary arterial hypertension. Nutrients. 2020;12:169. doi: 10.3390/nu12010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Gao F. Exercise improves vascular health: role of mitochondria. Free Radic Biol Med. 2021;177:347–59. doi: 10.1016/j.freeradbiomed.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Collings PJ, Backes A, Malisoux L, et al. Arterial stiffness and the reallocation of time between device-measured 24-hour movement behaviours: a compositional data analysis. Atherosclerosis. 2023;379:117185. doi: 10.1016/j.atherosclerosis.2023.117185. [DOI] [PubMed] [Google Scholar]
- 31.Alkerwi A, Pastore J, Sauvageot N, et al. Challenges and benefits of integrating diverse sampling strategies in the observation of cardiovascular risk factors (ORISCAV-LUX 2) study. BMC Med Res Methodol. 2019;19:27. doi: 10.1186/s12874-019-0669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies JM, Bailey MA, Griffin KJ, et al. Pulse wave velocity and the non-invasive methods used to assess it: Complior, SphygmoCor, Arteriograph and Vicorder. Vascular. 2012;20:342–9. doi: 10.1258/vasc.2011.ra0054. [DOI] [PubMed] [Google Scholar]
- 33.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Migueles JH, Rowlands AV, Huber F, et al. GGIR: a research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav. 2019;2:188–96. doi: 10.1123/jmpb.2018-0063. [DOI] [Google Scholar]
- 35.Hildebrand M, Hansen BH, van Hees VT, et al. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand J Med Sci Sports. 2017;27:1814–23. doi: 10.1111/sms.12795. [DOI] [PubMed] [Google Scholar]
- 36.Rowlands AV, Dawkins NP, Maylor B, et al. Enhancing the value of accelerometer-assessed physical activity: meaningful visual comparisons of data-driven translational accelerometer metrics. Sports Med Open. 2019;5:47. doi: 10.1186/s40798-019-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowlands AV, Edwardson CL, Davies MJ, et al. Beyond cut points: accelerometer metrics that capture the physical activity profile. Med Sci Sports Exerc. 2018;50:1323–32. doi: 10.1249/MSS.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 38.Rowlands AV, Sherar LB, Fairclough SJ, et al. A data-driven, meaningful, easy to interpret, standardised accelerometer outcome variable for global surveillance. J Sci Med Sport. 2019;22:1132–8. doi: 10.1016/j.jsams.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Collings PJ, Backes A, Aguayo GA, et al. Substituting device-measured sedentary time with alternative 24-hour movement behaviours: compositional associations with adiposity and cardiometabolic risk in the ORISCAV-LUX 2 study. Diabetol Metab Syndr. 2023;15:70. doi: 10.1186/s13098-023-01040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen HM, Wu YC, Tsai CM, et al. Relationships of circadian rhythms and physical activity with objective sleep parameters in lung cancer patients. Cancer Nurs. 2015;38:215–23. doi: 10.1097/NCC.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 41.Taibi DM, Price C, Voss J. A pilot study of sleep quality and rest–activity patterns in persons living with HIV. J Assoc Nurses AIDS Care. 2013;24:411–21. doi: 10.1016/j.jana.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delgado-Bonal A, Marshak A. Approximate entropy and sample entropy: a comprehensive tutorial. Entropy (Basel) 2019;21:541. doi: 10.3390/e21060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott J, Vaaler AE, Fasmer OB, et al. A pilot study to determine whether combinations of objectively measured activity parameters can be used to differentiate between mixed states, mania, and bipolar depression. Int J Bipolar Disord. 2017;5 doi: 10.1186/s40345-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu K, Van Someren EJW, Shea SA, et al. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: involvement of the circadian pacemaker. Proc Natl Acad Sci USA. 2009;106:2490–4. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paraschiv-Ionescu A, Büla CJ, Major K, et al. Concern about falling and complexity of free-living physical activity patterns in well-functioning older adults. Gerontology . 2018;64:603–11. doi: 10.1159/000490310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauvageot N, Alkerwi A, Albert A, et al. Use of food frequency questionnaire to assess relationships between dietary habits and cardiovascular risk factors in NESCAV study: validation with biomarkers. Nutr J. 2013;12:143. doi: 10.1186/1475-2891-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkerwi A, Vernier C, Crichton GE, et al. Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the observation of cardiovascular risk factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr. 2015;113:259–69. doi: 10.1017/S0007114514003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.French Agency for Food Environmental and Occupational Health & Safety ANSES-CIQUAL: French food composition table for nutritional intake. 2020. https://ciqual.anses.fr/ Available.
- 49.Neveu V, Perez-Jiménez J, Vos F, et al. Phenol-explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothwell JA, Perez-Jimenez J, Neveu V, et al. Phenol-explorer 3.0: a major update of the phenol-explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013;2013:bat070. doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Hees VT, Sabia S, Jones SE, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8:12975. doi: 10.1038/s41598-018-31266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han H, Dawson KJ. Applying elastic-net regression to identify the best models predicting changes in civic purpose during the emerging adulthood. J Adolesc. 2021;93:20–7. doi: 10.1016/j.adolescence.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Hastie T, Qian J, Tay K. An introduction to glmnet (glmnet vignette) [27-Mar-2023]. https://glmnet.stanford.edu/articles/glmnet.html#introduction Available. Accessed.
- 54.De Bruyne T, Steenput B, Roth L, et al. Dietary polyphenols targeting arterial stiffness: interplay of contributing mechanisms and gut microbiome-related metabolism. Nutrients. 2019;11:578. doi: 10.3390/nu11030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Del Giorno R, Scanzio S, De Napoli E, et al. Habitual coffee and caffeinated beverages consumption is inversely associated with arterial stiffness and central and peripheral blood pressure. Int J Food Sci Nutr. 2022;73:106–15. doi: 10.1080/09637486.2021.1926935. [DOI] [PubMed] [Google Scholar]
- 56.Lilamand M, Kelaiditi E, Guyonnet S, et al. Flavonoids and arterial stiffness: promising perspectives. Nutr Metab Cardiovasc Dis. 2014;24:698–704. doi: 10.1016/j.numecd.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 57.Alidadi M, Jamialahmadi T, Cicero AFG, et al. The potential role of plant-derived natural products in improving arterial stiffness: a review of dietary intervention studies. Trends Food Sci Technol. 2020;99:426–40. doi: 10.1016/j.tifs.2020.03.026. [DOI] [Google Scholar]
- 58.Doğan MF. In: Selenium and human health. Volkan G, Adem K, Abdulsamed K, editors. Rijeka: IntechOpen; 2023. Vascular system: role of selenium in vascular diseases. [Google Scholar]
- 59.Zhu D, Zhong Q, Lin T, et al. Higher serum selenium concentration is associated with lower risk of all-cause and cardiovascular mortality among individuals with chronic kidney disease: a population-based cohort study of NHANES. Front Nutr. 2023;10:1127188. doi: 10.3389/fnut.2023.1127188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (A review) Vasc Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Botelho-Ono MS, Pina HV, Sousa KHF, et al. Acute superoxide scavenging restores depressed baroreflex sensitivity in renovascular hypertensive rats. Auton Neurosci. 2011;159:38–44. doi: 10.1016/j.autneu.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 62.Shah S, Shiekh Y, Lawrence JA, et al. A systematic review of effects of vitamin E on the cardiovascular system. Cureus. 2021;13 doi: 10.7759/cureus.15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saunders TJ, McIsaac T, Douillette K, et al. Sedentary behaviour and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45:S197–217. doi: 10.1139/apnm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 64.Chastin SFM, Egerton T, Leask C, et al. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity (Silver Spring) 2015;23:1800–10. doi: 10.1002/oby.21180. [DOI] [PubMed] [Google Scholar]
- 65.Zheng C, Zhang X, Sheridan S, et al. Effect of sedentary behavior interventions on vascular function in adults: a systematic review and meta-analysis. Scand J Med Sci Sports. 2021;31:1395–410. doi: 10.1111/sms.13947. [DOI] [PubMed] [Google Scholar]
- 66.Parsons TJ, Sartini C, Ellins EA, et al. Objectively measured physical activity, sedentary time and subclinical vascular disease: cross-sectional study in older British men. Prev Med. 2016;89:194–9. doi: 10.1016/j.ypmed.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmadi-Abhari S, Sabia S, Shipley MJ, et al. Physical activity, sedentary behavior, and long-term changes in aortic stiffness: the Whitehall II study. J Am Heart Assoc. 2017;6:e005974. doi: 10.1161/JAHA.117.005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15:731–43. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 69.Credeur DP, Miller SM, Jones R, et al. Impact of prolonged sitting on peripheral and central vascular health. Am J Cardiol. 2019;123:260–6. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010;55:9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464. [DOI] [PubMed] [Google Scholar]
- 71.O’Neill SM, Travers CM, Otahal P, et al. Menopause and accelerated aortic stiffness. Maturitas. 2024;180:107900. doi: 10.1016/j.maturitas.2023.107900. [DOI] [PubMed] [Google Scholar]