Abstract
Objective
Psychological stress can be a common risk factor for the development of oral and systemic disease; therefore, analysis of a pathophysiologic mechanisms that may explain this association may be significant in planning preventive strategies. The aim of this study was to investigate the association amongst academic stress, periodontal health, and salivary cortisol and nitrite and nitrate levels in a sample of university students.
Methods
Participants (N = 14) were classified into 2 groups according to their exposure to academic stress due to periods of university exams (n = 6 and n = 8, respectively). All participants were subjected evlauted for their behavioural, psychological, and anthropometric parameters, as well as an oral health examination. A real-time polymerase chain reaction analysis in samples of saliva and plaque was used to detect Prevotella intermedia and Veillonella dispar as well as the total bacterial count. Nitrite/nitrate ratio (NR ratio) and cortisol in saliva were evaluated by enzyme-linked immunosorbent assay.
Results
Full Mouth Bleeding Score, Full Mouth Plaque Score, and Gingival Index were significantly higher in the group exposed to academic stress. Nitrite was directly related to the presence of V dispar (coefficient, 0.13; P = .00; CI, 0.07 to 0.19) and inversely related to total bacterial count (coefficient, −0.07; P = .012; CI, −0.13 to 0.02). NR ratio was directly related to V dispar (coefficient, 4.35; P = .010; 95% CI, 1.35 to 7.36) and inversely related to total bacterial count (coefficient, −4.05; P = .018; 95% CI, −7.32 to 0.86).
Conclusions
These results confirm the importance of stress on periodontal health and salivary nitrite concentration and highlight a potential differential role of specific bacteria on nitrite concentration in saliva.
Key words: Periodontal, Gingival, Oral health, Psychological stress
Introduction
Psychological stress has been related to different conditions, including gum tissue inflammation and periodontal disease.1, 2, 3 The results of a recent review show that periodontal diseases are associated with different systemic pathologies, including rheumatoid arthritis, cardiovascular pathologies, and neurodegenerative pathologies.4 In this context, the specific relation of oral microbiology and serum reactive oxygen metabolite levels, such as nitric oxide (NO), has been studied.5, 6, 7 Previous research has shown the role of stress hormones on growth of selected periodontitis-related bacteria,6 and evidence shows that NO deficiency at the endothelial level may be associated with the development of hypertension and other forms of cardiovascular disease.7 Moreover, periodontal and cardiovascular diseases have been linked,8, 9, 10, 11 which preempts a hypothesis of a linkage amongst stress, periodontal disease, oral microbiota, and systemic homeostasis. According to different authors, academic stress is an important psychosocial challenge for both students and undergraduates,12 and the role of academic stress has already been studied with regards to oral NO levels.5,13 Moreover, the observation of salivary nitrite/nitrate modifications in healthy participants exposed to acute stress may be a useful model to study the linkage amongst stress, periodontal health, and nitrite/nitrate levels. Since the definition of a possible pathophysiologic mechanism linking periodontal disease and stress may be significant in planning preventive strategies targeting different diseases, this relationship has been an intensive area of research.
The aim of this study was to evaluate the correlation between academic stress, periodontal health, gum inflammation (measured via Full Mouth Bleeding Score [FMBS], Full Mouth Plaque Score [FMPS], and Gingival Index [GI]), nutritional habits, salivary cortisol, and nitrite/nitrate levels in a sample of university students. Periodontal health status was evaluated by investigating the load of Veillonella dispar, which is associated with oral health, and Prevotella interemedia, implicated in gingivitis and periodontitis, as according to some studies these species are linked to the concentration of NO.14
Materials and methods
Study population and methodology
A cross-sectional study was carried out in 2016 amongst students attending their third year of different degree programmes in the Faculty of Medicine and Surgery at the Università Politecnica delle Marche, Ancona, Italy. A total of 14 participants, all aged 22 years, were recruited. Participants were classified into 2 groups according to exposure to academic stress: one group was about to face an exam session and the other group was not. Students with diabetes or mental health conditions, pregnant students, and those taking selected drugs (calcium channel blockers; anticonvulsant, anti-inflammatory, immunosuppressive, and antihypertensive agents; narcotic substances; and antibiotics during the 6 weeks before enrollment) were excluded from the study.15 Moreover, other exclusion criteria included having tooth decay or periodontal probing ≥4 mm; having had periodontal treatment during the 6 months before the study; and having had an oral hygiene session performed in the last 3 months.
All participants were evaluated for distribution of sociodemographic, behavioural, psychological, and anthropometric parameters; oral health examination; and assessment of selected components of the oral microbiota, salivary cortisol, salivary nitrate/nitrite levels, and pH.
Behavioural, psychological, and anthropometric parameters included sex, body mass index (BMI; classified as underweight, BMI < 18.4 kg/m2; normal weight, BMI = 18.5–24.9 kg/m2; and overweight, BMI > 25 kg/m2), smoking habits, alcohol consumption as evaluated by the 4-item Alcohol Use Disorders Identification Test,16 physical activity as evaluated by the International Physical Activity Questionnaire (IPAQ) 27-item questionnaire.17 Self-perceived stress was evaluated with the Perceived Stress Scale Questionnaire (PSS-10), a 10-item instrument18 measuring the degree to which one's life situations are appraised as stressful using a 5-point Likert scale, and a Visual Analogue Scale (VAS; ranging from 0 to 10).
Nutritional habits were examined using a food frequency questionnaire, including the evaluation of consumption of coffee (never, rarely, several times a week, every day, several times a day), magnesium-rich foods (nuts and seeds, dark chocolate, bananas, avocado, soybeans), green leafy vegetables, and fish as well as pro- and anti-inflammatory foods such as ginger, garlic, and curcuma.19 Food frequency consumption options ranged from several times a day to daily, weekly, and monthly. Students underwent a clinical examination of the oral cavity with evaluation of the dental formula and plaque index (FMPS) and the dental gingival bleeding index (FMBS). Presence of any dental caries and the state of the restorations were evaluated by means of an oral and physical examination an assisted interview. Periodontal probing was performed to evaluate the presence of periodontal pockets ≥4 mm (see exclusion criteria). GI was assessed evaluating 6 sites in each student. GI ranged from 0 to 3 (0 = normal gum; 1 = medium inflammation, slight change of colour, no bleeding on probing; 2 = moderate inflammation, redness and oedema, bleeding on probing; 3 = severe inflammation, marked redness and oedema, ulceration with a tendency for spontaneous bleeding), and an accurate anamnesis was carried out.
This study was approved by Ethics Committee of the Polytechnic University of Marche (Prot.n. 0000197). All enrolled participants provided written informed consent. The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Laboratory evaluation
Sample collection
Plaque samples was collected during a dental visit, and salivary samples were collected on awakening (between 7:00 and 8:30 am, t1) and then after 15 (t2) and 30 minutes (t3). Participants refrained from eating, drinking, smoking, and brushing teeth from awakening upto the saliva and plaque collection time point. At least 1 mL of saliva was collected in a Salivette device (Sarsted Aktiengesellschaft & Co.), and the procedures were conducted as previously described.20 Saliva samples were centrifuged at 1000 rpm for 2 minutes, and the supernatant was collected and stored at −20 °C. Supragingival and subgingival plaque sampling was performed using sterile paper points (ISO 30, Dentsply, GmbH). Supragingival plaque samples from the mesial-buccal surface of the first molar (second molar if the first molar was missing) in each quadrant were collected. The pooled plaque from each participant was placed in a microcentrifuge tube containing 1.5 mL sterile 1X Tris-EDTA buffer. Subgingival plaque was sampled from the site of the deepest pocket of the same tooth where the supragingival plaque was collected. Collections were stored at −20 °C until real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis.
Oral microbiology evaluation
Genomic DNA extraction from the specimens was performed using a PureLink Genomic DNA Mini Kit (Invitrogen) according to the manufacturer's instructions with some modifications. DNA concentrations were determined spectrophotometrically using Qubit 2.0 Fluorometer (Invitrogen).
Bacteria-specific TaqMan probe and primer sets were designed from the species-specific region on the 16S rRNA and were used to identify Prevotella intermedia and Veillonella dispar.21, 22, 23 In addition, a universal primer was used to quantify the total amount of bacterial species in the oral plaque.20 Real-time polymerase chain reaction (PCR) was carried out using a Rotor-Gene 3000 (Corbett-Research); all samples were run in duplicate. Each PCR was performed in a total volume of 20 µL containing 4 µL of 5X Takara Buffer (Takara Bio Inc), 0.2 µL each of forward and reverse primers (final concentration, 500 nM each), an appropriate dose of TaqMan Probe (final concentration, 200 nM), an appropriate amount of MgCl2 solution (final concentration, 1.5–6 mM) and dNTP mixture (final concentration, 0.2 mM), 1 U Taq DNA Polymerase, 2 µL of template DNA solution, and an appropriate dose of sterilised UltraPure DNase/RNase-Free Distilled Water (Invitrogen). Amplification conditions are shown in Table 3. The bacterial DNA level was quantified by qRT-PCR and transformed to theoretical cell numbers as previously described.22 In the present study, the total bacterial load in the specimens was calculated with the assumption that the 16s rRNA gene copy numbers of the eubacterial species were not significantly different from each other.21
Table 3.
Results of multiple linear regression modeling for factors related to NO2 salivary levels.
| Organism | Coefficient | P value | 95% CI |
|---|---|---|---|
| Veilonella dispar | 0.13 | <.001 | 0.07 to 0.19 |
| Total bacterial count | −0.07 | .012 | −0.12 to 0.02 |
Salivary cortisol detection
Procedures were conducted as previously described.24 At least 1 mL of saliva was collected in Salivette devices (Sarsted Aktiengesellschaft & Co.). Saliva samples were centrifuged at 1000 rpm for 2 minutes, and supernatant was collected and stored at −20 °C. A commercial enzyme immunoassay kit was used to determine salivary cortisol (DiaMetra) according to the manufacturer's instructions. Cortisol concentration was expressed as nmol/L. The lower limit of detection for the assay was 0.5 nmol/L, and the upper limit of the standard curve was 1750 nmol/L. To investigate the circadian pattern of basal cortisol secretion, 3 salivary samples were collected: at wake-up (between 7:00 and 8:30 am, t1) and then after 15 (t2) and 30 minutes (t3).
NO determination
An enzyme immunoassay kit (R&D Systems, Inc.) to determine NO2− and NO3− in saliva was used according to the manufacturer's instruction. The assay determines nitric oxide concentration based on the enzymatic conversion of nitrate to nitrite through the activity of nitrate reductase. The reaction is followed by a colorimetric detection of nitrite as an azo dye product of the Griess reaction. The Griess reaction is based on the 2-step diazotisation reaction in which acidified NO2− produces a nitrosating agent, which reacts with sulfonic acid to produce the diazonium ion. This ion is then coupled to N-(1-naphthyl) ethylenediamine to form the chromophoric azo-derivative which absorbs light at 540 nm and is then read by the enzyme-linked immunosorbent assay reader. For the determination of NO, the saliva sample upon awakening (between 7:00 and 8:30 am, t1) was used.
Salivary pH and flow measurement
For measurement of unstimulated flow, patients were asked not to swallow for 1 minute, and at the end of that time the salivary sample was collected using a 2.5-Cl syringe in a glass and measured. Then, salivation was stimulated by an acid liquid, such as lemon juice, inserted into the oral cavity of the patient and removed after 10 seconds; then, stimulated salivary flow was measured. Salivary pH was measured using unstimulated saliva.
Statistical analysis
Normal distribution for raw cortisol values, NO2− and NO3− concentration, and bacterial count was checked with Shapiro–Wilk test; if the test rejected normality, data were log-transformed. NR ratio was calculated as follows: 100 * [nitrite] / ([nitrate] + [nitrite]). Bivariate analyses were performed to analyse the association of stress and periodontal health variables in the 2 groups of students, using Chi-square and Student t test as appropriate. Multiple linear regression models were developed to evaluate factors independently associated with salivary nitrite, nitrate, and NR capacity levels. FMPS was categorised into 2 levels: 0% to 20% and 50% to 100% considering the percentage of sites harbouring plaque <20% as an accepted standard and as a tolerable level of oral hygiene amongst the general population, and FMBS was categorised into 2 levels: 0% to 20% and 50% to 100% according to the cutoffs for localised and generalised gingival inflammation. The significance level for variables to enter the multiple logistic regression models was set at ≤0.2, and for removing them from the model at ≤0.4. Analyses were performed with STATA, version 15 (Stata Corp.). The level of significance was set at 0.05.
The comparison of cortisol levels between the 2 groups was performed using the ggstatsplot v.0.8.0 package.25 The analysis was carried out in RStudio v. 4.2.1.26
To better understand the complexity and interrelationships amongst the variables, a principal component analysis (PCA) was performed and the exposed and the unexposed groups were analysed using the ggplot2 package. The analysis was carried out in RStudio v. 4.2.1.26
Results
Fourteen students were enrolled in this study; 71.4% (n = 10) were female (Table 1). Bivariate analysis revealed no statistical differences between groups regarding sociodemographic variables and dietary and habits sych as smoking alcohol consumption and physical activity. No differences were detected in the PSS-10 score, but the VAS score was significantly higher in the group exposed to academic stress (P = .04; Table 1). Selected nutritional habits did not show any significant effect in the present sample.
Table 1.
Distribution of sociodemographic, behavioural, psychological, and anthropometric parameters in participants exposed and not exposed to academic stress.
| Academic stress |
|||
|---|---|---|---|
| Not exposed | Exposed | P value | |
| Sex | No. (%) | No. (%) | |
| Female | 7 (87.50) | 3 (50.00) | .12* |
| Male | 1 (12.50) | 3 (50.00) | |
| Smoking | |||
| Yes | 3 (37.50) | 5 (83.33) | .39* |
| No | 5 (62.50) | 1 (16.67) | |
| BMI | |||
| Underweight | 1 (12.50) | 0 (0.00) | .42* |
| Normal | 6 (75.00) | 6 (100.00) | |
| Overweight | 1 (12.50) | 0 (0.00) | |
| Physical excercise | |||
| Yes | 4 (50.00) | 5 (83.33) | .21* |
| No | 4 (50.00) | 1 (16.67) | |
| Alcohol consumption | |||
| Never | 4 (50.00) | 4 (66.67) | .53* |
| Rarely | 4 (50.00) | 2 (33.33) | |
| Fish consumption | |||
| Rarely | 4 (50.00) | 3 (50.00) | 1.00* |
| Several times a week | 4 (50.00) | 3 (50.00) | |
| Fruit and vegetable consumption | |||
| Every day | 5 (62.50) | 2 (33.33) | .28* |
| Several times a day | 3 (37.50) | 4 (66.67) | |
| Spinach consumption | |||
| Never/rarely | 6 (75.00) | 3 (50.00) | .33* |
| Several times a week | 2 (25.00) | 3 (50.00) | |
| Coffee consumption | |||
| Never | 1 (12.50) | 0 (0.00) | .10* |
| Rarely | 0 (0.00) | 1 (16.67) | |
| Several times a week | 3 (37.50) | 1 (16.67) | |
| Every day | 1 (12.50) | 2 (33.33) | |
| Several times a day | 3 (37.50) | 0 (0.00) | |
| Consumption of high-magnesium food | .63* | ||
| Rarely | 4 (50.00) | 2 (33.33) | |
| Several times a week | 3 (37.50) | 2 (33.33) | |
| Every day | 1 (12.50) | 2 (33.33) | |
| Turmeric | .72* | ||
| Yes | 1 (12.50) | 1 (12.50) | |
| No | 7 (87.50) | 4 (66.67) | |
| Saffron | 1.00* | ||
| Yes | 4 (50.00) | 3 (50.00) | |
| No | 4 (50.00) | 3 (50.00) | |
| Ginger | |||
| Yes | 7 (87.50) | 5 (83.33) | .825* |
| No | 1 (12.50) | 1 (16.67) | |
| FMPS | |||
| 0%–20% | 8 (100.00) | 1 (16.67) | .001* |
| 50%–100% | 0 (0.00) | 5 (83.33) | |
| FMBS | |||
| 0%–20% | 8 (100.00) | 1 (16.67) | .001* |
| 50%–100% | 0 (0.00) | 5 (83.33) | |
| GI | |||
| Normal | 8 (100.00) | 2 (33.33) | .024* |
| Mild inflammation | 0 (0.00) | 3 (21.43) | |
| Moderate inflammation | 0 (0.00) | 1 (16.67) | |
| Mean (SEM) | Mean (SEM) | ||
| PSS-10 score | 21.50 (1.09) | 21.83 (1.49) | .86† |
| VAS | 3.50 (0.78) | 6.00 (0.68) | .04† |
| Salivary cortisol | |||
| At awakening | 27.31 (14.16) | 68.61 (9.09) | .02† |
| 15 min after awakening | 52.92 (17.39) | 87.59 (21.78) | .12† |
| 30 min after awakening | 31.02 (10.84) | 113.29 (38.17) | .04† |
| Bacterial count | |||
| Total bacterial count | 1.66E+04 (1.20E+04) | 1.67E+08 (1.67E+08) | .76† |
| Prevotella intermedia | 287.50 (156.34) | 1700.00 (1660.12) | .83† |
| Veillonella dispar | 165.14 (120.42) | 1.67E+05 (1.67E+05) | .35† |
| NO (µmol/L) | 61.41 (17.35) | 57.07 (16.57) | .86† |
| pH | 6.38 (0.18) | 6.50 (0.22) | .67† |
Chi-square test.
Student t test.
BMI, body mass index; FMBS, Full Mouth Bleeding Score; FMPS, Full Mouth Plaque Score; GI, gingival index; NO, nitric oxide; PSS-10, Perceived Stress Scale Questionnaire; VAS, Visual Analogue Scale
FMBS, FMPS, and GI were significantly higher in the group exposed to academic stress, and basal levels of salivary cortisol on awakening (t1) and after 30 minutes (t3; Table 2) were also significantly higher.
Table 2.
Results of multiple linear regression modeling for factors related to NO3 salivary levels.
| Nitrate | Coefficient | P value | 95% CI |
|---|---|---|---|
| Nitrite | 0.27 | .43 | −0.46 to 1.01 |
| Salivary cortisol t3 | −0.3 | .008 | −0.50 to 0.92 |
| Sex | −1.02 | .012 | −1.76 to −0.27 |
The linear regression model showed that nitrate concentration was inversely related to female sex (coefficient, −1.02; P = .012; 95% CI, −1.76 to 0.27) and to concentration of salivary cortisol at t3 (coefficient, −0.3; P = .008; 95% CI, −0.50 to 0.92; Table 2). Nitrite was directly related to the presence of V dispar (coefficient, 0.13; P = 0.00; 95% CI, 0.07 to 0.19) and inversely related to total bacterial count (coefficient, −0.07; P = .012; 95% CI, −0.12 to 0.02; Table 3). Moreover, despite being significant (P = .0267), the only factors independently related to NR ratio were the count of V dispar (coefficient, 4.35; P = .010; 95% CI, 1.35 to 7.36) and the total bacterial count (−4.05; P = .018; 95% CI, −7.32 to 0.86). However, the significance of the model explaining the NR ratio variability was improved by the inclusion of increasing FMBS (coefficient, 8.71; P = .051; 95% CI, −0.03 to 17.44), reduction of alcohol intake (coefficient, −3.58; P = .075; 95% CI, −7.61 to 0.44), reduction of PSS-10 scores (coefficient, −1.19; P = .081; 95% CI, −4.15 to 0.29), and smoking habit (coefficient, −9.91; P = .127; 95% CI, −23.23 to 3.41; see Table 4).
Table 4.
Results of multiple linear regression modeling for factors related to nitrite/nitrate ratio (NR).
| NR | Coefficient | P value | 95% CI |
|---|---|---|---|
| Veilonella dispar | 4.35 | .010 | 1.35 to 7.36 |
| Total bacterial count | −4.05 | .018 | −7.23 to 0.86 |
| FMBS | 8.71 | .051 | −0.03 to 17.44 |
| Alcohol intake | −3.58 | .075 | −7.61 to 0.44 |
| PSS-10 score | −1.19 | .081 | −4.15 to 0.29 |
| Smoking habit | −9.91 | .127 | −23.23 to 3.41 |
FMBS, Full Mouth Bleeding Score; PSS-10, PSS-10, Perceived Stress Scale Questionnaire.
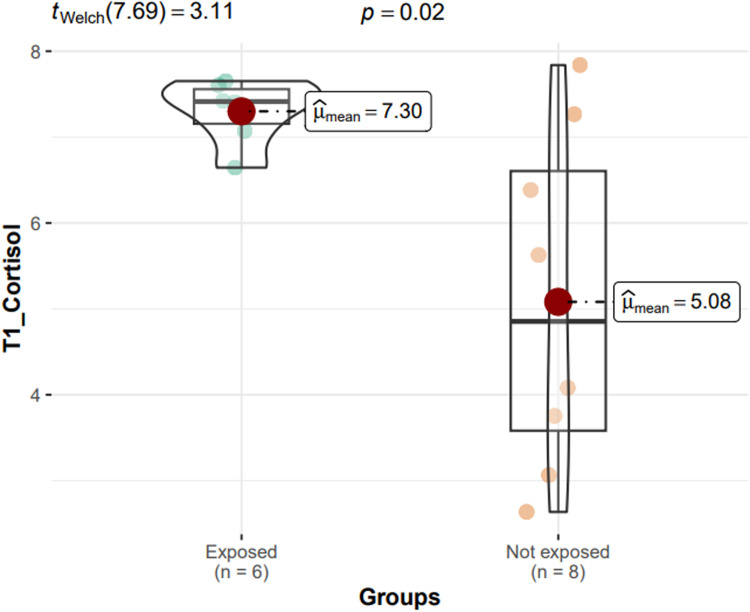
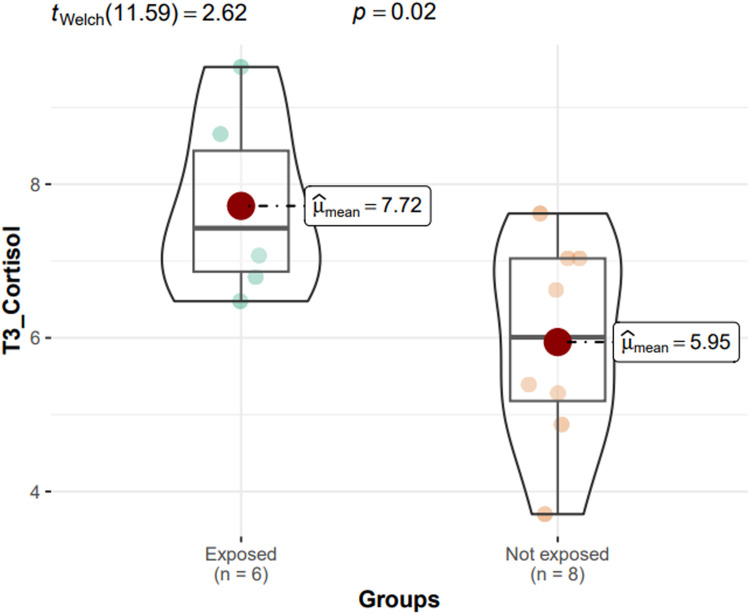
The ggstatsplot showed that in these 2 groups the cortisol levels measured upon awakening (tWelch = 3.11; P = .02) and after 30 minutes (tWelch = 2.62; P = .02) were higher in exposed participants than in those not exposed to stress (Fig. 1, Fig. 2).
Fig. 1.
ggstatsplot showing that cortisol levels upon awakening (t1) are higher in participants exposed to stress than in those not exposed to stress.
Fig. 2.
ggstatsplot showing that cortisol levels after 30 minutes (t3) are higher in participants exposed to stress than in those not exposed to stress.
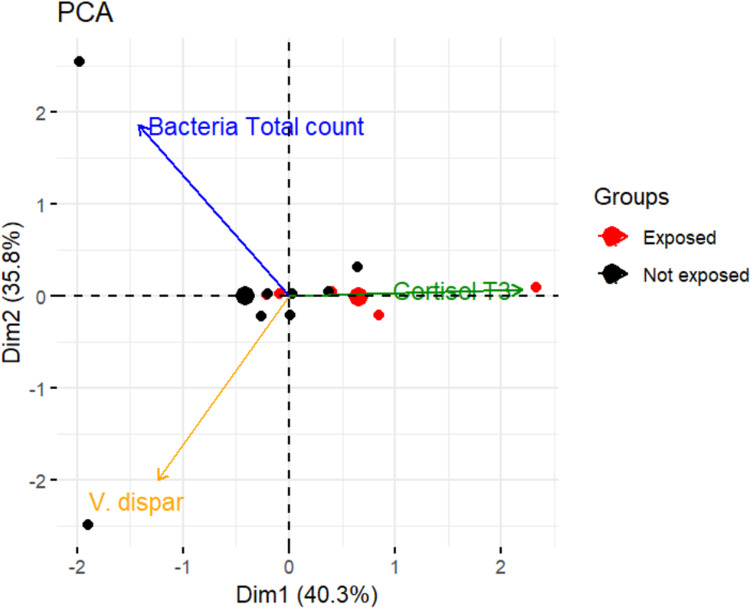
Figure 3 shows the relationships amongst 3 variables simultaneously (total bacterial count, cortisol t3, and V dispar). These 3 variables were selected after calculating the correlation coefficient through the cor() function. Variables that share more than 80% of the covariance were selected.
Fig. 3.
Principal component analysis score relates the variables bacterial total count, cortisol t3, and Veillonella dispar in participants exposed and not exposed to stress.
Discussion
According to other studies evaluating the association of stress with increased plaque index, our work has highlighted significant periodontal modifications in participants undergoing stress. In fact, our results agree with those of Deinzer et al,3 since 83.3% of participants exposed to academic stress had an FMPS level between 50% and 100%, which was significantly higher than controls. Moreover, the proportion of participants with bleeding gums was as high as 83.3%, significantly greater than that of students not exposed to academic stressors (P < .01).
In the context of this association, at awakening (t1) and after 30 minutes (t3), the academic stress group had a greater concentration of salivary cortisol than the control group, which is in agreement with observations by Johannsen et al27 in 2010, which revealed that the increase of bacterial plaque in students experiencing academic stress was related to elevated levels of salivary cortisol.
PCA showed that variables that provide similar information are grouped together, showing a possible relationship (Figure 3). Total bacterial count and V dispar are examples of 2 positively related variables. When the numerical value of one variable increases or decreases, the numerical value of the other variable tends to change in the same way. This is because total bacterial count comprises both bacteria associated with good (ie, V dispar) and poor oral hygiene. When variables are negatively ("inversely") correlated, they are placed on opposite sides of the origin of the chart. For example, the variables cortisol t3 and V dispar are inversely related, which means that when cortisol levels increase, V dispar decreases. In addition, the distance from the origin of the axes also communicates information. The farther a variable is from the origin of the diagram, the stronger the impact is that the variable has on the model. Figure 3 shows how the impact of the variable cortisol t3 is stronger in participants exposed to stress (red dots) than in those not exposed (black dots). Again, for example, there were higher levels of V dispar in participants not exposed to stress than those exposed to stress. Multiple linear regression modelling showed a reduced NO3− concentration in female participants and increased salivary cortisol at t3. Reduced levels of NO3− could be explained by the interaction of many factors; it is known that increases in the adrenal–cortisol axis may decrease the production of NO.28 Moreover, despite the role of estrogens in female participants in increasing NO levels,29 this upregulation could be attenuated by their cortisol-induced downregulation in those exposed to chronic stress. Similar results have been recently reported in participants of the National Health and Nutrition Examination Survey where exhaled NO was greater in men and obese individuals.30 In our sample, BMI was not related to NO; however, the adjustment for weight was significant in the whole model.
Following the results by Ozer et al,31 the amount of salivary NO in students exposed to academic stress was lower than that in individuals not exposed, suggesting that the production of NO may be suppressed in individuals with periodontitis. However, contrasting findings were reported by Reher et al32 and Parwani et al.33
On the other hand, despite our small sample size, a significant variation in clinical data testifies to a clinically important correlation amongst stress and oral microbiota (ie, V dispar) and oral NO2−, confirming previous results showing that the effects of nitrate reduction in the oral cavity was linked to the presence of commensal bacteria34,35 and specifying the potentially different role of total bacterial count (reducing nitrite levels) with respect to other species (ie, V dispar). Moreover, the toll of Veillonella spp. as a nitrate reducer is confirmed in the present study, in agreement with results of previous works.36
Our study has some important limitations: We had a small sample size in terms of recruited participants, and we have been able to study only 2 bacterial species whilst a deepened analysis between oral and general health status should include searching of more bacteria species and their relative prevalence. For these reasons, our results are extremely preliminary in the context of a pilot study and future work is needed to better investigate this scenario.
Conclusions
This study demonstrated that academic stress was associated with gum inflammation and periodontal health risk in a cohort of healthy young participants. From a clinical point of view, patients should be advised on stress as a risk factor for the homeostais of periodontal health. Professional help should be provided for patients who are unable to maintain appropriate oral hygiene to obtain a more intensive follow-up during psychological stress periods. Despite the lack of statistical significance as an independent variable regarding periodontal levels, results suggested the importance of oral microbiota in cardiovascular disease and blood pressure control.37, 38, 39
These results point out the necessity to plan appropriate strategies to improve health education, promoting good oral health habits particularly in university students exposed to high academic stress. Moreover, awareness must be increased about stress as an important risk factor for gingival inflammation and periodontal disease.
Conflict of interest
None disclosed.
Acknowledgments
Acknowledgements
Sources of funding for this work came from the Università Politecnica delle Marche (to PB and MMD). This research received no external funding.
Author contributions
PB and EP conceived the study and contributed to data acquisition; PB and EP prepared the original draft; SS provided assistance with study design; and JD provided assistance in preparing the manuscript. Laboratory analysis was done by EP and GF. Statistical analyses were done by PB, and MMD supervised the project. All authors read and agreed to the published version of the manuscript.
REFERENCES
- 1.Peruzzo DC, Benatti BB, Ambrosano GMB, et al. A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78:1491–1504. doi: 10.1902/jop.2007.060371. [DOI] [PubMed] [Google Scholar]
- 2.Ravishankar TL, Ain TS, Gowhar O. Effect of academic stress on plaque and gingival health among dental students of Moradabad, India. J Int Acad Periodontol. 2014;16:115–120. [PubMed] [Google Scholar]
- 3.Deinzer R, Granrath N, Spahl M, Linz S, Waschul B, Herforth A. Stress, oral health behaviour and clinical outcome. Br J Health Psychol. 2005;10:269–283. doi: 10.1348/135910705x26858. [DOI] [PubMed] [Google Scholar]
- 4.Fiorillo L, Cervino G, Laino L, et al. Porphyromonas gingivalis, periodontal and systemic implications: a systematic review. Dent J (Basel) 2019;11:114. doi: 10.3390/dj7040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paizan M, Vilela-Martin J. Is there an association between periodontitis and hypertension? Curr Cardiol Rev. 2014;10:355–361. doi: 10.2174/1573403x10666140416094901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol. 2017;8:693. doi: 10.3389/2Ffphys.2017.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jentsch HFR, März D, Krüger M. The effects of stress hormones on growth of selected periodontitis related bacteria. Anaerobe. 2013;24:49–54. doi: 10.1016/j.anaerobe.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Oh JY, Youk TM, Jeong SN, Kim YT, Choi SO. Association between periodontal disease and non-communicable diseases: a 12-year longitudinal health-examinee cohort study in South Korea. Med. 2017;96:e7398. doi: 10.1097/md.0000000000007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Dyke TE, Starr JR. Unraveling the link between periodontitis and cardiovascular disease. J Am Heart Assoc. 2013;2 doi: 10.1161/jaha.113.000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung TJT, Nijland N, Gerdes VEA, Loos BG. Prevalence of periodontal disease among patients at the outpatient clinic of internal medicine in an academic hospital in the Netherlands: a cross-sectional pilot study. J Clin Med. 2022;11:6018. doi: 10.3390/jcm11206018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preuß D, Schoofs D, Schlotz W, Wolf OT. The stressed student: influence of written examinations and oral presentations on salivary cortisol concentrations in university students. Stress. 2010;3:221–229. doi: 10.3109/10253890903277579. [DOI] [PubMed] [Google Scholar]
- 13.Rosier BT, Takahashi N, Zaura E, et al. The importance of nitrate reduction for oral health. J Dent Res. 2022;101:887–897. doi: 10.1177/00220345221080982. [DOI] [PubMed] [Google Scholar]
- 14.Ritz T, Trueba AF, Liu J, Auchus RJ, Rosenfield D. Exhaled nitric oxide decreases during academic examination stress in asthma. Ann Am Thorac Soc. 2015;12:1638–1645. doi: 10.1513/annalsats.201504-213oc. [DOI] [PubMed] [Google Scholar]
- 15.Waschul B, Herforth A, Stiller-Winkler R, Idel H, Granrath N, Deinzer R. Effects of plaque, psychological stress and gender on crevicular II-1 beta and II-1ra secretion. J Clin Periodontol. 2003;30:238–248. doi: 10.1034/j.1600-051x.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 16.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 17.Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.mss.0000078924.61453.fb. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Kamarck T, Mermelstein RA. Global Measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 19.Shin D, Won Lee K, Brann L, Shivappa N, Hébert JR. Dietary inflammatory index is positively associated with serum high-sensitivity C-reactive protein in a Korean adult population. Nutrition. 2019;(63–64):155–161. doi: 10.1016/j.nut.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Lucertini F, Ponzio E, Di Palma M, et al. High cardiorespiratory fitness is negatively associated with daily cortisol output in healthy aging men. PLoS One. 2015;10 doi: 10.1371/2Fjournal.pone.0141970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 22.Kuboniwa M, Amano A, Kimura KR, et al. Quantitative detection of periodontal pathogens using real-time polymerase chain reaction with TaqMan probes. Oral Microbiol Immunol. 2004;19:168–176. doi: 10.1111/j.0902-0055.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 23.Ciric L, Pratten J, Wilson M, Spratt D. Development of a novel multi-triplex qPCR method for the assessment of bacterial community structure in oral populations. Environ Microbiol Rep. 2010;2:770–774. doi: 10.1111/j.1758-2229.2010.00183.x. [DOI] [PubMed] [Google Scholar]
- 24.Barbadoro P, Ponzio E, Coccia E, et al. Association between hypertension, oral microbiome and salivary nitric oxide: a case-control study. Nitric Oxide. 2021;106:66–71. doi: 10.1016/j.niox.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Patil I. Visualizations with statistical details: the ‘ggstatsplot’ approach. J Open Source Softw. 2021;6(61):3167. [Google Scholar]
- 26.R.C.R. Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. A language and environment for statistical computing. [Google Scholar]
- 27.Johannsen A, Bjurshammar N, Gustafsson A. The influence of academic stress on gingival inflammation. Int J Dent Hyg. 2010;8:22–27. doi: 10.1111/j.1601-5037.2009.00397.x. [DOI] [PubMed] [Google Scholar]
- 28.Sigola LB, Zinyama RB. Adrenaline inhibits macrophage nitric oxide production through β1 and β2 adrenergic receptors. Immunology. 2000;100:359–363. doi: 10.1046/2Fj.1365-2567.2000.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castardo-De-Paula JC, De Campos BH, Amorim EDT. Cardiovascular risk and the effect of nitric oxide synthase inhibition in female rats: the role of estrogen. Exp Gerontol. 2017;97:38–48. doi: 10.1016/j.exger.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Uppalapati A, Gogineni S, Espiritu JR. Association between body mass index (BMI) and fraction of exhaled nitric oxide (FeNO) levels in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Obes Res Clin Pract. 2016;10:652–658. doi: 10.1016/j.orcp.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Ozer L, Elgun S, Ozdemir B, Pervane B, Ozmeric N. Arginine-nitric oxide-polyamine metabolism in periodontal disease. J Periodontol. 2011;82:320–328. doi: 10.1902/jop.2010.100199. [DOI] [PubMed] [Google Scholar]
- 32.Reher VGS, Zenóbio EG, Costa FO, Reher P, Soares RV. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J Oral Sci. 2007;49:271–276. doi: 10.2334/josnusd.49.271. [DOI] [PubMed] [Google Scholar]
- 33.Parwani SR, Chitnis PJ, Parwani RN. Salivary nitric oxide levels in inflammatory periodontal disease - a case-control and interventional study. Int J Dent Hyg. 2012;10:67–73. doi: 10.1111/j.1601-5037.2011.00508.x. [DOI] [PubMed] [Google Scholar]
- 34.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Woessner M, Smoliga JM, Tarzia B, et al. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide. 2016;54:1–7. doi: 10.1016/j.niox.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 37.Raizada MK, Joe B, Bryan NS, et al. Report of the National Heart, Lung, and Blood Institute Working Group on the Role of Microbiota in Blood Pressure Regulation: current status and future directions. Hypertension. 2017;70:479–485. doi: 10.1161/2FHYPERTENSIONAHA.117.09699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med. 2013;55:93–100. doi: 10.1016/2Fj.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed KA, Nichols AL, Honavar J, Dransfield MT, Matalon S, Patel RP. Measuring nitrate reductase activity from human and rodent tongues. Nitric Oxide. 2017;66:62–70. doi: 10.1016/j.niox.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]