Abstract
Background
Work participation of patients with inflammatory arthritis (IA) is important not only economically but also for physical and psychological health. There is no Cochrane Review to date on studies of non‐pharmacological interventions specifically aimed at preventing job loss in people with IA.
Objectives
To assess the effects of non‐pharmacological interventions that aim to prevent job loss, work absenteeism or improve work functioning for employees with IA (rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), other spondylarthritis (SpA) or IA associated with connective tissue diseases, such as Systemic Lupus Erythematosus (SLE)).
Search methods
We searched the following databases from inception up to 30 April 2014; The Cochrane Library (including Cochrane Central Register of Controlled Trials, i.e. CENTRAL and DARE), MEDLINE (PubMed), EMBASE (Embase.com), CINAHL (EbSCOhost), ClinicalTrials.gov and PsycINFO (ProQuest). We did not impose language restrictions in the search.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated interventions aimed at preventing job loss in adults of working age (18 to 65 years) diagnosed with IA, including RA, AS, PsA, SpA or other types of IA. Primary outcomes were job loss and sickness absenteeism and the secondary outcome was work functioning.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data and assessed risk of bias in the included RCTs.
Main results
We included three RCTs with a total of 414 participants at risk of job loss. The majority of participants had IA, most with RA and to a lesser degree AS. The interventions aimed to prevent job loss and improve work functioning in several ways: firstly by evaluating work changes or adaptations and secondly by providing any person‐directed interventions including vocational counselling, advice or education. Interventions directly targeted at the work environment were minimal and included workplace visits (one trial) or any actions by an occupational physician (one trial). The duration or dose of the interventions varied from two 1.5‐hour sessions (one RCT) over five months, two consultation and multidisciplinary treatments during three months (one RCT), to six to eight individual or group sessions over six months (also one RCT). All participants were recruited through rheumatology clinics, both in or outside hospitals. Included trials investigated job loss (n = two RCTs; 382 participants), work absenteeism and work functioning (n = one RCT; 32 participants). Overall, we evaluated the two smaller trials as having a high risk of bias and the large trial as having a low risk of bias. Trials showed marked differences in how they performed on risk of bias items, particularly on performance bias.
We assessed the quality of the evidence using the GRADE approach and judged there to be very low quality evidence across the three reported outcomes. Of the two RCTs investigating job loss, the larger one (n = 242 participants) reported a large statistically significant reduction in job loss (relative risk (RR) = 0.35, 95% confidence interval (CI) 0.18 to 0.68) and the other RCT (n = 140) reported similar effects in both groups, although the CI was very wide (RR = 1.05, 95% CI 0.53 to 2.06). The latter one probably suffered from performance bias and we judged it to have a high risk of bias. The one small trial investigating sickness absenteeism found uncertain results at six months' follow‐up (MD = ‐2.42 days, 95% CI ‐5.03 to 0.19). Finally, in the same small trial investigating work functioning using the Rheumatoid Arthritis‐Work Instability Scale (RA‐WIS), there was a moderate improvement of intermediate term work functioning (six months; scale range 0 to 23; mean improvement ‐4.67 points, 95% CI ‐8.43 to ‐0.91). We identified no adverse effects in the publications of the three trials.
Authors' conclusions
This Cochrane review of three RCTs found very low quality evidence overall for job loss prevention interventions having an effect on job loss, work absenteeism and work functioning in workers with inflammatory arthritis. While this review highlights that further high quality RCTs are required, the results suggest that these strategies have potential to be effective.
Keywords: Adult; Humans; Middle Aged; Employment; Vocational Guidance; Absenteeism; Arthritis; Arthritis/therapy; Arthritis, Psoriatic; Arthritis, Psoriatic/therapy; Arthritis, Rheumatoid; Arthritis, Rheumatoid/therapy; Efficiency; Randomized Controlled Trials as Topic; Spondylarthritis; Spondylarthritis/therapy; Spondylitis, Ankylosing; Spondylitis, Ankylosing/therapy
Plain language summary
Non‐drug interventions for helping workers with inflammatory arthritis stay at work
Background
Inflammatory arthritis (IA), also called rheumatism, is a group of diseases that cause long‐lasting pain, stiffness and swelling in the joints. These symptoms make it difficult to move and make you feel tired, which in turn can make it difficult to work. The most common types of IA are: rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Worldwide about 3% of people have IA. The disease usually begins when people are between 30 to 40 years old, at a time when they still have many years of working life left. Therefore, it is important to know if there are effective ways in which we can help people with IA stay at work. This Cochrane Review focuses on non‐drug interventions.
Trials we found
We searched the available literature up to 30 April 2014. We included three randomised controlled trials with 414 participants who had IA and who were at risk of losing their jobs. These trials first evaluated how the work environment could be adapted and then provided counselling, advice or education for work problems. One trial gave two 1.5‐hour sessions over five months. Another trial gave two consultation and multidisciplinary treatments during three months. The third trial gave six to eight individual or group sessions over six months. The included trials compared the effects of interventions to usual care (two trials) or to written information only (one trial). Two of the included trials measured the effect of the intervention on job loss (382 participants) when the third measured effect on work absenteeism and work functioning (32 participants).
What the research says
When considered together, the evidence from the three trials was of very low quality. Two trials found different results on job loss measured at two years' follow‐up: one trial on job counselling found a large reduction in people who lost their job and the other trial found similar effects in both groups. Another trial did not find a considerable effect on absenteeism at six months' follow‐up but found a moderate improvement in work functioning.
Conclusions
Because of positive results from one RCT with long term follow‐up, we see potential for job loss prevention interventions in helping workers with inflammatory arthritis to stay at work. The certainty of these results is limited by the very low quality evidence of the three RCTs overall.
Summary of findings
Summary of findings for the main comparison. Job loss prevention compared to control intervention in people with inflammatory arthritis.
| Job loss prevention compared to control intervention in people with IA | ||||||
| Patient or population: people with IA Settings: Intervention: job loss prevention Comparison: control intervention consisting of usual care or information | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control intervention 7 | Job loss prevention | |||||
| Job loss Self report by questionnaire Follow‐up: mean 24 months | See comment | See comment | Not estimable | 340 (2 trials) | ⊕⊝⊝⊝ very low1,2,3,4 | Due to inconsistency in the trials we did not pool these two trials |
| Sickness absenteeism Self reported no workdays missed in past month. Scale from: 0 to 22. Follow‐up: mean 6 months | The mean sickness absenteeism in the control groups was 2.75 days absent | The mean sickness absenteeism in the intervention groups was 2.42 lower (5.03 lower to 0.19 higher) | ‐ | 32 (1 study) | ⊕⊝⊝⊝ very low5,6 | ‐ |
| Work functioning Rheumatoid Arthritis Work Instability Scale. Scale from: 0 to 23 (higher score = worse work functioning) Follow‐up: mean 6 months | The mean work functioning in the control groups was 13.67 score points | The mean work functioning in the intervention groups was 4.67 lower (8.43 to 0.91 lower) | ‐ | 32 (1 study) | ⊕⊝⊝⊝ very low5,6 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 We judged the RCTs by de Buck 2005 and Macedo 2009 to have an overall high risk of bias and the RCT by Allaire 2003 to have an overall low risk of bias. Overall, we judged serious risk of bias to be present and therefore we downgraded the quality of the evidence by one level (‐1). 2 The magnitude of the effect on job loss for Allaire 2003 and de Buck 2005 show different relative risks. Allaire 2003 cites a highly significant and large beneficial effect (24 months: RR 0.35, 95% CI 0.18 to 0.68) whereas de Buck 2005 shows no effect (RR 1.05, 95% CI 0.53 to 2.06). The CIs hardly overlap. In addition, considerable statistical heterogeneity is present (I2 > 80%). Hence we did not pool the results. As we failed to identify a plausible explanation for the inconsistency, we downgraded the quality of the evidence by two levels (‐2). For the outcomes sickness absenteeism and work functioning, we only had one included RCT. Hence we judged inconsistency as not serious. 3 The number of job loss events was less than 300. The 'threshold rule of thumb value' states that there should be at least 300 events for job loss (dichotomous outcome), and for continuous outcomes there should be at least 400 participants (Higgins 2011). None of the trials fulfilled this criterion, nor did any of the comparisons for any of the outcomes. As the two RCTs reporting on job loss (Allaire 2003; de Buck 2005) were considerably larger than the one very small study (Macedo 2009) we judged the two to have serious imprecision, and so we downgraded the quality of the evidence by just one level (‐1). 4 We included both trials with smaller (N = 32 in Macedo 2009) and larger (N = 140 in de Buck 2005, N = 242 in Allaire 2003) sample sizes and both trials with positive (Allaire 2003 ; Macedo 2009) as well as non‐significant results (de Buck 2005). Given the low number of trials we were unable to further analyse publication bias with funnel plots or with statistical tests. Consequently we did not downgrade the quality of the evidence. 5 Risk of bias in the Macedo 2009 RCT related in particular to an absence of prognostic comparability and blinding of outcome assessment, which we judged to be sources for serious risk of bias. Consequently we downgraded the quality of the evidence by one level (‐1). 6Macedo 2009 included only 32 participants in total. We considered it to have very serious imprecision for the outcomes sickness absenteeism and work functioning. Thus we downgraded the quality of the evidence by two levels (‐2).
7 All control groups received either usual care or a minimal intervention such as written information in Allaire 2003.
Background
For any working age adult, the ability to participate in working life is important not only economically but also for physical and psychological health (Fifield 1991; Hoving 2013; MacKinnon 1992; Mehnert 1990). This is equally true for people with inflammatory arthritis (IA) who, in qualitative studies, have identified employment as an important problem and lack of employment services as a significant unmet need (Hoving 2009; Lacaille 2007; Mancuso 2000; Nilsson 2007). Although this importance has been recognised, the topic receives little attention in most clinical guidelines and evidence summaries. However, over the past decade work participation has received more attention, as shown by the growing number of both prognostic and intervention employment studies in rheumatic diseases (Lacaille 2005). Although work participation as an outcome has been included in recent reviews of pharmacological interventions (ter Wee 2012), to date no Cochrane Reviews of rheumatic diseases have reviewed studies of non‐pharmacological interventions specifically aimed at improving work participation. A systematic review of non‐randomised studies on this topic dates back to 2002 (de Buck 2002) and anon‐systematic overview was published in 2009 (Vliet Vlieland 2009). The overview published by Vliet Vlieland 2009 on vocational rehabilitation concluded that the effects of vocational rehabilitation interventions in people with chronic arthritis is "scanty" and work disability among individuals with chronic arthritis is substantial. In this Cochrane Review we study participation in paid work, and review the impact of job loss prevention interventions encompassing not only employment status (i.e. existence or degree of work disability) but also absences from paid work, and work functioning or presenteeism as per Outcome Measures in Rheumatology Clinical Trials (OMERACT) recommendations (Escorpizo 2007).
Description of the condition
IA, including its most common types: rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS) and undifferentiated spondyloarthritis (SpA), place a person at increased risk of work disability. IA is characterized by its chronic, progressive nature. Inflammation causes pain, stiffness and swelling in the joints, leading to restricted range of motion, decreased strength and reduced physical function, as well as general fatigue. Worldwide, IA prevalence is estimated to be around 3%, including a prevalence of 1% for RA (Gabriel 2001) and 1.9% for SpA (including AS and PsA) (Braun 1998). IA typically begins at a younger age, often in people aged between 30 and 40 years, at a time when they still have many years of potential active workforce participation. In the past, people who stopped working due to IA were not likely to return to work or gain employment elsewhere. This is not surprising, as IA is progressively disabling, with potential long‐term complications and it is difficult for people to return to active employment after prolonged absences from work. However, it seems that the prognosis for people with certain types of IA is improving (ter Wee 2012). The emergence of new disease‐modifying antirheumatic drugs (DMARDs) has renewed research interest in the relationship between IA and work functioning. DMARDs are now provided at an earlier stage of the disease to eradicate inflammation and thereby prevent radiological damage. It has been suggested that new biological agents, such as tumour necrosis factor (TNF) inhibitors, also have a beneficial effect on work participation and work functioning, although the magnitude of the effect is unclear and warrants further research (Allaire 2008; Hoving 2009; Verstappen 2007; Yelin 2003).
Description of the intervention
In this Cochrane Review, we are interested in interventions primarily targeted towards the consequences of IA on work participation. Earlier studies aimed at improving employment outcomes for people with IA have attempted to help people disabled by IA return to work. However, in general, once people are unable to work they are unlikely to re‐enter the workforce. For example, data from the USA show that people with arthritis infrequently utilized vocational rehabilitation services aiming to return them to work (Straaton 1997), and success was generally low. Recently, emphasis has shifted towards tertiary prevention, which helps people cope with impairments in their work, and primary prevention of work disability. Thus the focus is shifting from return to work towards job retention.
This review focuses on non‐pharmacological interventions aimed directly at addressing work participation in one or more ways. Firstly, there should be an analysis of a person's work activities, work functioning, ergonomic needs or communication at work to identify those features of working life that are placing the person at risk of having to stop working. Secondly, interventions should include some form of consultation, such as advice on job accommodations, vocational counselling or work rehabilitation strategies to deal with challenges in relation to work. Both components include the context of work directly. To our knowledge, a few intervention studies conducted in the past decade have looked at ways to implement and evaluate interventions directed at improving work participation for people with inflammatory rheumatic diseases (de Buck 2002; Vliet Vlieland 2009).
Other types of interventions not included in this review can also have an impact on work participation in people with IA. Pharmacological interventions for IA aiming to decrease the inflammatory process may have an impact on work participation. This may be through their effect on disease activity, pain or physical function, which are all known predictors of work disability. In this category we also place interventions that aim to improve the delivery of medical services, such as starting pharmacological treatment early or multidisciplinary interventions for the treatment of arthritis not including specific work‐related interventions. Both pharmacological interventions and interventions that promote the delivery of medical services are excluded from this review and have already been addressed in several Cochrane Reviews (Colebatch 2011; Radner 2012; Ramiro 2011).
There are also interventions, such as physical therapy or exercise therapy on the one hand, and psychological interventions or behavioral counselling on the other, that try respectively to improve physical functioning and fatigue, or coping and problem‐solving skills, for example, but weren't designed to change work participation. This enables people with IA to have more energy, have better joint function and strength, or be better able to cope with everyday stressors. These interventions do not target the disease but rather the disease symptoms and consequences. A benefit of these physical and psychosocial therapies, sometimes offered as part of a multi‐disciplinary rehabilitation program, may be that they also have an effect on work participation, through physical, physiological or psychological pathways. These therapies are not as commonly prescribed as medical (pharmacological) treatment, but are available to many people with IA, either by referral or direct access. We have also excluded these types of interventions from this Cochrane Review because they do not specifically target employment. They have also been addressed in previous reviews including several Cochrane Reviews (Dagfinrud 2008; Hurkmans 2009; Riemsma 2003; Silva 2010; Steultjens 2004).
How the intervention might work
As shown in several studies (de Croon 2004; de Croon 2005; Hoving 2013; Lacaille 2007; Van der Meer 2011), people with arthritis struggle to find a balance between work and home demands, medical appointments, work issues, communication with co‐workers and transportation, while coping with decreasing energy levels and pain. Work disability in people with RA has also been described as a: "...biopsychosocially determined misfit between individual capability and work demands" (de Croon 2004). This relationship is influenced by contextual factors. Interventions that target an individual's capability for work, or that target work demands by changing work routines or provide accommodations, enable people with IA to have fewer difficulties in functioning at work and thereby improves work participation.
Why it is important to do this review
Work participation is important to people with IA. Although medical treatment is improving, many continue to experience challenges in maintaining employment over time (Hoving 2009; Hoving 2013). The effectiveness of mono‐therapies and multidisciplinary programmes in people with IA has been evaluated, though not on work participation, in several Cochrane Reviews including physiotherapy (Dagfinrud 2008), dynamic exercise therapy (Hurkmans 2009), occupational therapy (Steultjens 2004), patient education (Riemsma 2003) and balance training (Silva 2010). Other reviews on interventions for people with rheumatic diseases that focus on work participation have either not included RCTs (de Buck 2002; Mahalik 2006) or have used a non‐systematic review process (Mahalik 2006; Vliet Vlieland 2009). For example, a systematic review on vocational rehabilitation in people with chronic rheumatic diseases reported only five studies published up to May 2001, and none were controlled (de Buck 2002). In their review of work site interventions for people with RA and osteoarthritis (OA), Mahalik 2006 indicated a need for "randomised, comparative studies, measuring multiple outcome measures". On the other hand, Vliet Vlieland 2009 looked at vocational rehabilitation programs for people with chronic arthritis and identified only two RCTs on vocational rehabilitation up to 2005. In addition, the importance of work participation as a topic is clear from the increasing number of Cochrane Reviews on other chronic diseases such as cancer (de Boer 2011) and multiple sclerosis (Khan 2009). All these reviews suggest that more research is warranted to study the effects of interventions that promote work participation for people with IA.
Objectives
To assess the effectiveness of non‐pharmacological interventions that aim to prevent job loss for employees with IA.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
We included trials in which the population was limited to adults of working age (18 to 65 years) of which at least half of all workers (>50%) had been diagnosed with IA, including RA, AS, PsA or SpA, or IA associated with connective tissue diseases such as Systemic Lupus Erythematosus (SLE). We included trials conducted with participants from hospital settings, occupational settings, primary care or community settings, or outpatient care settings.
Types of interventions
We included intervention where the focus was on job loss prevention or improving work function. To fulfil this criteria, we included interventions that fulfilled at least two of the following three components:
a) An evaluation of the work challenges or work adaptations as a step in the main intervention of the study;
b) Interventions directed at the person, meaning:
job coaching, job or vocational training, vocational counselling; or
empowerment for work; or
self‐management, including support or information regarding work or both;
c) Interventions directed at the work environment, meaning:
work adaptations, ergonomic measures, job accommodations; or
interventions targeted directly at the employer, supervisor or co‐workers.
We have named interventions that fulfil the above criteria job loss prevention interventions in this systematic review. The above includes interventions such as vocational rehabilitation, occupational rehabilitation or ergonomic interventions, but are not exclusive to these terms. These interventions include some kind of information or communication about work participation as part of the intervention directed to the worker or to the work environment. Included in the above are also multi‐disciplinary interventions as long as they include, or are part of, a), b) or c).
Types of outcome measures
Primary outcomes
As interventions should aim to prevent job loss, we judged the following outcomes to be important:
1. Job loss measured as:
the number of people that become unemployed at some stage following diagnosis, regardless of disability pension status; or
the time to job loss.
2. Sickness absenteeism measured as:
time lost from work (number of work days or hours missed at work due to sick leave, or absenteeism); or
time to return to work; or
the proportion of workers on sick leave at a certain moment of follow‐up.
Secondary outcomes
Work functioning measured using any at‐work productivity, work functioning or presenteeism questionnaire: including but not limited to the work limitations questionnaire, the work instability scale or the work role functioning questionnaire.
We categorised trials according to these follow‐up times:
short term (more than one day, up to three months);
intermediate term (more than three and less than 12 months); or
long term (one year or longer).
Search methods for identification of studies
We have listed the search strategies we used to search the different medical databases in Appendix 1, Appendix 2 , Appendix 3, Appendix 4 and Appendix 5. We devised and conducted the systematic search in collaboration with Leena Isotalo, the Trials Search Coordinator of the Cochrane Occupational Safety and Health Review Group.
Electronic searches
We searched the following electronic databases from inception up to 30 April 2014:
The Cochrane Library (including Cochrane Central Register of Controlled Trials, i.e. CENTRAL and the Database of Reviews of Effectiveness, i.e. DARE);
MEDLINE (PubMed);
EMBASE (Embase.com);
CINAHL (EbSCOhost);
ClinicalTrials.gov:
PsycINFO (ProQuest).
We ran the searches without language restrictions, using the Cochrane Collaboration highly‐sensitive search strategy to identify all RCTs (Lefebre 2011). We used specific MeSH headings and additional keywords to identify all RCTs on rheumatic diseases and different types of arthritis. The strategy combines disease terms as listed above and a controlled vocabulary for describing interventions to prevent job loss and improve work participation, including search terms such as work participation, work rehabilitation and vocational rehabilitation, for which we included search terms from a search string for identifying studies on work‐related outcomes and interventions (Haafkens 2006; Verbeek 2005).
Searching other resources
We retrieved additional references in several ways. We screened the reference lists of relevant systematic reviews from the Cochrane Database of Systematic Reviews (CDSR, The Cochrane Library). We also screened references from included RCTs and other reviews on work participation rehabilitation in rheumatic diseases. Finally, we asked experts in the field to identify any published or unpublished studies that may be relevant to this Cochrane Review.
Data collection and analysis
Selection of studies
We divided the identified trials between pairs of review authors (including JH in each pair) in order to examine each reference twice. To determine whether a study should be included, we read the title and abstract of all identified hits from the electronic database searches. We then decided independently of each other the article eligibility according to the predetermined selection criteria (see Criteria for considering studies for this review). If there was any doubt, the review authors retrieved and read the full text article. We resolved any disagreements about the eligibility of a trial in a consensus meeting or by email.
Data extraction and management
JH, and MF or JS independently extracted information regarding study design, interventions, patient population, outcome measures and timing of outcome assessment, co‐interventions, adverse effects, loss to follow‐up and results data for outcomes of interest using a standardised data extraction form.
Assessment of risk of bias in included studies
We evaluated the validity of the RCTs using the Cochrane Collaboration’s tool for assessing risk of bias as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed each study for risk of bias in each of the following seven domains, with ratings of low risk of bias, high risk of bias or unclear risk of bias:
Random sequence generation;
Allocation concealment;
Blinding of participants and personnel;
Blinding of outcome assessment (per outcome); and attrition
Incomplete outcome data;
Selective reporting;
Other sources of bias including prognostic comparability at baseline, and compliance.
JH, and MF or JS independently assessed the risk of bias of each included trial. To determine the risk of bias of a trial, each criterion was evaluated for the presence of sufficient information and the likelihood of potential bias. We discussed any disagreements between the review authors and resolved them in a consensus meeting. We sought expert advice from the Cochrane OSH Review Group (Jos Verbeek) in a number of cases to facilitate decision making. Given the low number of included trials, we did not calculate agreement statistics as proposed by Brennan 1992 and Cohen 1960.
Measures of treatment effect
We expressed the results of each RCT as RRs with corresponding 95% confidence intervals (CI) for dichotomous data (job loss), and mean differences (MDs) or standardised mean differences (SMDs) for continuous data (sickness absenteeism and work functioning), depending on the similarity of outcome measurement scale (i.e. MDs were used when all trials used the same outcome measurement scale and SMDs when trials used different outcome measurement scales). RR smaller than 1.0 indicates a beneficial effect of the experimental intervention i.e. fewer job losses or days lost from work (Deeks 2008). RRs are considered clinically relevant if the RR is smaller than 0.7 or larger than 1.5, in favour of the intervention or control group, respectively.
Unit of analysis issues
We searched for all RCTs but did not find any trials that employed a cluster‐randomised design. Therefore we did not have to make an allowance for the design effect by assuming intra‐cluster correlations following methods stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If when we update this review we should identify studies that contain more than two intervention groups, we will divide the number of participants in the control group with the number of interventions so as not to count the same control participants several times in the meta‐analysis.
Dealing with missing data
We contacted one trial author (Allaire 2003) to clarify data reported in their publication, which we could possibly use for meta‐analysis, but used no other unpublished data. In the absence of missing data, we had no need of calculating them from other available statistics such as P values according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We assessed clinical homogeneity based on similarity of population, intervention, outcome and follow‐up. We considered populations as similar when they fell into the specified diagnosis types. We considered interventions as similar when they fell into the pre‐defined interventions (see Types of interventions). We considered the work participation outcome categories similar within each of the three categories. We regarded follow‐up times of less than three months, three months to one year and more than one year as different. We tested for statistical heterogeneity using the I2 statistic (Higgins 2011), using the following as a rough guide for interpretation: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity. In the absence of homogeneous comparisons we did not further explore heterogeneity (defined as I2 ≥ 75%) or include subgroup analyses.
Assessment of reporting biases
We reduced the effect of reporting bias by including trials rather than publications in order to avoid the introduction of duplicate data, as was present in two trials. We extracted data only once. We prevented location bias by searching across multiple databases. Language bias was prevented by not excluding any article based on language. Given the low number of included trials, we did not further explore publication bias using a funnel plot.
Data synthesis
If more than one trial reported on the same outcome, we planned to calculate the SMD and corresponding 95% confidence interval (CI). However, as we did not identify multiple trials per outcome, we reported the original scale scores at follow‐up, the mean difference and its 95% CI. In future review updates with more included trials, we plan to use the following formula to calculate SMD = (Or‐Ot/ PSD) where Or is mean improvement in the reference group, Ot is mean improvement in the treatment group and PSD is the pooled standard deviation (Deeks 2008). Interpretation of the SMD as described by Cohen 1988 is as follows: for example, an SMD of 0.2 is considered to indicate a small beneficial effect, 0.5 a medium effect, and 0.8 a large effect. The SMD is considered to indicate a clinically relevant effect when it is larger than 0.5. The calculation of SMDs is only useful if the outcome measures use different scales but measure the same concept.
In the current review we identified no more than two intervention groups (job loss prevention versus control intervention consisting of usual care or advice or both), enabling just one pair‐wise comparison. For future updates, we plan to include multiple pair‐wise comparisons between all possible pairs of intervention groups, but using the same group of participants only once in the meta‐analysis.
Similarly, in the absence of homogeneous comparisons we did not perform meta‐analyses in this review. If future updates can incorporate meta‐analyses and the results show statistically significant overall estimates we will transform these back (pooled estimate of RR, MD or SMD) into measures that are clinically useful in daily practice, such as the number needed to treat (NNT) and the absolute or relative improvement, or both, on the original units to express the final results of the review. In this case we will only pool data from trials judged to be clinically homogeneous using Review Manager 5.2 software (RevMan 2011). Likewise, if future review updates include trials that are statistically heterogeneous, we will use a random‐effects model; otherwise we will use a fixed‐effect model.
Regarding the primary outcome, we combined the following outcomes:
Number of people employed before and after intervention, number of people or percentages, either defined as job loss or otherwise;
Time lost from work and time to return to work were combined if necessary and analysed as continuous outcomes. We used the mean number of time lost from work to calculate the effect size using days on sick leave with the following formula: mean change difference over standard deviation (SD);
Time to job loss was analysed as a continuous outcome.
We used the GRADE approach as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and as implemented in the GRADEPro 3.2 software (GRADE 2008) to present the quality of evidence and 'Summary of Findings' tables.
The downgrading of the quality of a body of evidence for a specific outcome was based on five factors:
Limitations in the design and implementation of available studies suggesting high likelihood of bias;
Indirectness of evidence (indirect population, intervention, control, outcomes);
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
Imprecision of results (wide CIs);
High probability of publication bias.
The GRADE approach specifies four levels of quality (high, moderate, low and very low).
Subgroup analysis and investigation of heterogeneity
We intended to do subgroup analyses based on the most prevalent diagnosis categories, including RA, AS and remaining categories (other arthritis types). Similarly, we planned this in case job loss prevention (person‐directed or work environment‐directed) was given exclusively or as part of a multidisciplinary intervention that includes other interventions. As we didn't find a sufficient number of trials that met our inclusion criteria, we may plan to do these in future review updates.
Sensitivity analysis
We planned to conduct a sensitivity analysis to test the robustness of our meta‐analysis results by omitting trials we judged to have a high risk of bias. Given the low number of included trials we may do so in future updates. In that case we will use a random‐effects model to combine data and perform a sensitivity analysis by using the fixed‐effect model to reveal differences in results.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
We conducted electronic and manual literature searches on April 30th 2014 and identified 5436 titles. This number was reduced to 4291 titles and abstracts after duplicate removal. We identified five additional studies through additional handsearches. Of these 3553 studies, 55 passed the title and abstract screening and were selected for further full text review (see Figure 1). From these, we identified 11 trials that dealt with the topic of this review. Of these, three original RCTs fulfilled all inclusion criteria (Allaire 2003; de Buck 2005; Macedo 2009). We also included multiple publications of the same research groups on the same trials (Allaire 2005; de Buck 2004).
1.
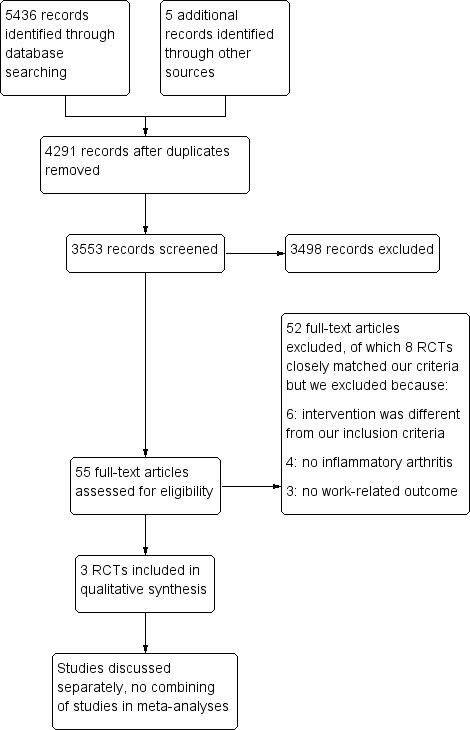
Study flow diagram.
Included studies
see Characteristics of included studies for details of all three RCTs.
Characteristics of included studies and participants
The trials by Allaire 2003 (USA), de Buck 2005 (Netherlands) and Macedo 2009 (UK) included a total of 414 participants, with 242, 140 and 32 participants, respectively. Participants in all three RCTs were recruited from rheumatology practices, inside or outside the hospital. The majority of participants had IA but the case mix varied: Allaire 2003 included participants with IA (RA, AS, PsA, SLE) but also a proportion with OA (36%), de Buck 2005 included participants with a mix of IA (RA, AS, PA or reactive arthritis) and Macedo 2009 included participants with RA. In all three RCTs, participants had a paid job and were considered at risk of losing their job or of work disability; in the de Buck 2005 trial some participants were already on extended sick leave (between 3% to 8% had sick leave> 40 weeks) at the start of the trial. Sick leave occurred before the start of the trial or at baseline, as in Macedo 2009 and de Buck 2005. de Buck 2005 reported between 39% to 42% of participants having more than six weeks of sick leave, and participants in Macedo 2009 had missed between two to three days in the past month. Data from the Allaire 2003 trial shows that all participants were 'at work' at baseline.
Intervention characteristics
The interventions reported in the three included RCTs aimed to prevent job loss and improve work functioning in several ways: firstly by a systematic assessment of work challenges or work adaptations, or both; and secondly by providing several person‐directed interventions including vocational counselling, advice or education. Interventions directly targeted at the work environment were minimal, and included workplace visits (Macedo 2009) or any actions by an occupational physician (de Buck 2005). The duration or dose of the interventions varied from two 1.5‐hour sessions over five months (Allaire 2003), two consultation and multidisciplinary treatments during three months (de Buck 2005), to six to eight individual or group sessions for six months (Macedo 2009).
In Allaire 2003, experimental group participants received a job retention vocational rehabilitation intervention designed for the trial; its focus was on job accomodation, vocational counselling, education and self‐advocacy. It was provided by one of two rehabilitation counsellors hired for the study in a community setting. Training on how to provide the intervention was provided to the counsellors. The experimental intervention was provided in two 1.5‐hour sessions over a five‐month period.This intervention may have had some similarity to services that could be provided to employed persons through the US public vocational rehabilitation program; the self‐advocacy component in particular was used (on disability rights etc.). Control group participants were sent printed information about disability‐related employment issues and resources in the postal mail; this was considered a minimal intervention, but more than the employment‐related care usually provided.
The multi‐disciplinary intervention described by de Buck 2005 was conducted in a hospital setting and care was provided by several hospital professionals (rheumatologist, social worker, physical therapy, occupational therapist and psychologist) with consultation by an occupational physician. de Buck 2005 provided multi‐disciplinary treatment over four to 12 weeks, including a discussion between the patient and a team coordinator and a report to the occupational physician. The control group were treated and referred to other health professionals in relation to their working problem if regarded necessary by their rheumatologist. Participants in both groups received written information about the Dutch social security system regarding sick leave and work disability.
The intervention by Macedo 2009 was provided by an occupational therapist experienced in vocational rehabilitation and rheumatology. The number of sessions varied. Macedo provided six to eight group sessions and individual sessions by the occupational therapist over six months lasting between 30 minutes and two hours, including a workplace visit for seven participants. The control group received usual care, which involved routine reviews by the rheumatologist, but no occupational therapy involvement.
In all interventions the start point included an assessment of workplace barriers, job or task evaluation, assessment of work problems or work challenges, along with subsequent analyses and treatment strategy to overcome these. For the assessment, Allaire 2003 used a screening tool called the augmented Work Experience Survey tool, and Macedo 2009 used a biopsychosocial model stemming from the Canadian Occupational Performance Measure (COPM). In de Buck 2005, a rheumatologist and a coordinator performed a systematic assessment, of medical and work‐related issues, using a list of potential challenges and psychosocial situations.
In all three RCTs, participant were recruited from rheumatology practices. Only de Buck 2005 clearly indicated that medical care was included in the intervention. Macedo 2009 stated that medical management and rheumatology clinic visit schedules were not changed from normal practice for either group and the participating clinics focused on early aggressive medical management with a goal of achieving remission. In contrast, standard medical treatment in Allaire 2003 was assumed as participants were recruited from rheumatologist's practices.
Outcome characteristics
Job loss was the only outcome reported in the RCTs by Allaire 2003 and de Buck 2005, and measured in various ways and at different time points. Both trials measured job loss counts (job losses were described as events in Allaire's trial). Allaire also makes a distinction between temporary and permanent job loss events. The outcomes sickness absenteeism and work functioning were only measured in the RCT by Macedo 2009.
Follow‐up times also varied: Macedo 2009 had a follow‐up of six months (i.e. intermediate term outcome), data on sickness absenteeism (cumulative work days missed) and work functioning (RA WIS: RA work instability scale), whereas both de Buck 2005 and Allaire 2003 reported job loss at six‐month intervals up to 24 and 48 months, respectively. In other words, de Buck 2005 and Allaire 2003 employed both intermediate (3‐12 months) and long term (>12 months) follow‐up. The Allaire 2003 trial originally had a follow‐up of 36 months with 80% of participants, but follow‐up was continued for a smaller proportion of participants (66%) up to 48 months. In addition, recruitment was staggered over time so that during the trial period not all participants completed the 36‐ or 48‐month follow‐up. Mainly because of this reason, the Allaire 2003 job loss counts were based on 12 and 24 months' follow‐up data.
Ongoing studies
We have identified three ongoing RCTs, which are listed in the Ongoing studies section. We obtained these through electronic database searching, i.e. published protocols (van Vilsteren 2012 and Carruthers 2014), or through our rheumatology experts on the review team (Keysor 2013). Updates of the current review should provide more evidence in coming years (see Characteristics of ongoing studies).
Excluded studies
Of the 11 studies that dealt with the topic of this review, we excluded eight studies for the following reasons (multiple reasons apply); 1) Intervention did not fulfill all criteria (six studies); 2) not sufficient workers with IA (four studies); and no work‐related outcome as specified in protocol (three studies). See Characteristics of excluded studies.
Risk of bias in included studies
For results of risk of bias assessment of RCTs, see the Characteristics of included studies section. We summarised the results in the risk of bias graph in Figure 2, which is an overview of the review authors' judgements about each risk of bias item presented as percentages across all included trials. Figure 3 shows the risk of bias summary of each risk of bias item for each included trial.
2.
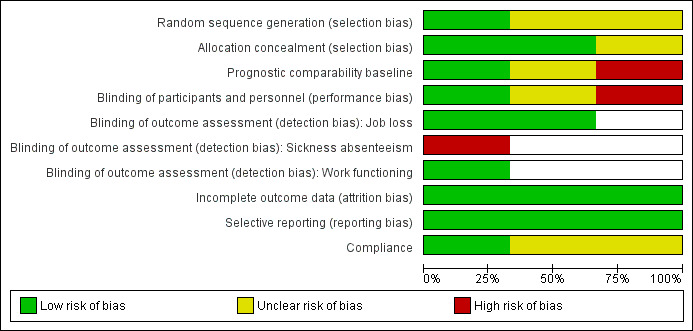
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.
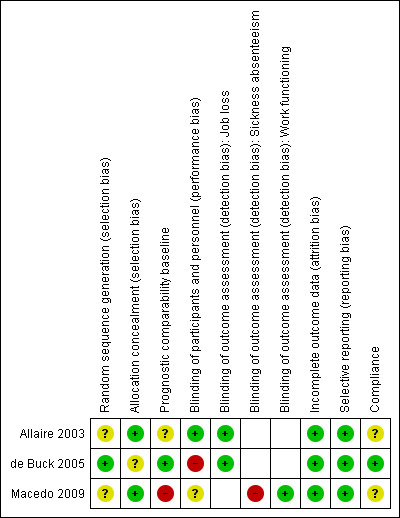
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Allaire 2003 and Macedo 2009 provided insufficient information about sequence generation to permit judgement. Only de Buck 2005 adequately described sequence generation, using a randomisation list created by a random digit generator. de Buck 2005 provided insufficient information about allocation concealment. Allaire 2003 and Macedo 2009 used a statistician or independent research nurse to respectively create an access database requiring every participant to be entered before the randomisation code was provided, or perform sealed envelop randomisation respectively. We judged the risk of bias to be low for both RCTs.
Blinding
None of the three RCTs provided information about whether workers or the people performing the intervention were blinded. However, given the nature of the intervention provided (assessment, information, advice or counselling), it would have been impossible to blind the workers or at least some people performing the intervention to the allocation. de Buck 2005 asked workers not to inform the principal investigator or the research nurse about the type of care they received. All three outcomes in this review (job loss, sickness absenteeism or work functioning) were assessed through participant self‐report.
Performance bias (blinding of participants and intervention providers)
We judged the risk of bias to be unclear for performance bias (blinding participants and personnel) in the trial of Macedo 2009. Potentially personnel, the participants or the participants work colleagues were more aware or willing to support or influence the participants' work status, as a consequence of belonging to the experimental group or control group but for this trial we have no information that this happened. The extent of this effect remains unclear.
Allaire 2003 reported that participants were not told whether they had received either the experimental or control intervention. When enrolling subjects, participants were told they were testing two different ways of helping people stay employed. The counsellors provided intervention to experimental group participants only and did not know or have any contact with control group participants. Whether participants told their work colleagues about their trial participation is doubtful. Employers were not involved in any way in Allaire 2003. The researcher was blinded as to which participants were in each group (she had little contact with any participants – only brief contact with a few who called with a question when the research assistant was away). For this reason we judged the risk of bias to be low.
In the case of de Buck 2005, there are indications that participants and rheumatologists took extra effort providing care to the control group, as discussed by the trial authors. The experimental intervention consisted of at least two visits: a systematic (vocational) assessment and discussion of a multi‐disciplinary (vocational) report/plan. In addition, referral to education, counselling and different therapies (occupational therapist, physical therapist, exercise therapist, psychologist, rheumatology nurse, social worker) was provided based on the specific problems that were encountered in the (vocational) systematic assessment. It seems likely that participants and hospital personnel in the control group put extra effort into getting similar treatments as the experimental group. The number of referrals to the rheumatologist rose during the intervention period, and the control group received an equivalent number of therapies which were part of the experimental group intervention. As the control group received similar therapies, this affected the contrast between the experimental and control intervention. For this reason the RCT by de Buck 2005 was considered to have high risk of bias.
Detection bias (blinding of outcome assessors)
We considered the influence of detection bias to be potentially different across the three pre‐specified outcome measures of job loss (Allaire 2003; de Buck 2005), sickness absenteeism (Macedo 2009) and work functioning (Macedo 2009). We therefore evaluated the presence of detection bias separately for each outcome.
Job loss: in an older validation study, it was shown that self‐reported unemployment was in close agreement with the company administration of unemployment (Duncan 1985). We judged job loss a relative robust and objective measure given this information, even when it was self assessed as in Allaire 2003 and de Buck 2005. We therefore judged the risk of bias to be low.
Sickness absenteeism: we considered the self report data as reported by Macedo 2009 to have a high risk of bias, and not an objective measure. Participants had to remember how many days they missed from work and whether this was due to RA. The intervention was quite intensive with six months of comprehensive OT including workplace visits which may have influenced participants recall at the six month follow‐up. Certainly there would be more emphasis on the importance of job loss or taking sick leave, although it is not clear how big this effect would be in either group.
Job functioning: Macedo 2009 used the Rheumatoid Arthritis‐Work Instability Scale (RA‐WIS) which is a validated questionnaire with discrete items, judged to have a low risk of bias.
Incomplete outcome data
Allaire 2003 and de de Buck 2005 reported reasons for drop‐out of participants and had incomplete outcome data addressed. Macedo 2009 reported the same number of participants at baseline and six months, and stated no job loss during the six month follow‐up. Loss to follow‐up accounted for (total 25/140: 13/74 in I and 12/66 in C) 18% at 24 months for de Buck 2005 and was quite evenly distributed between experimental and control group, and baseline characteristics distribution of non‐completers were similar. Allaire 2003 originally had a 36‐month follow‐up, which was extended with an additional year of follow‐up. Recruitment was staggered and as a consequence not all participants were able to complete the 36‐month or 48‐month follow‐up. During the original 36‐month follow‐up, four participants dropped out of the intervention group and six from the control group, and one participant died in each group. The total reported attrition rate one year later, at 48 months, by Allaire 2003 was 18% in the intervention and 20% in the control. Allaire 2003 reported that most of the drop‐outs were participants who chose not to participate in the additional year of follow‐up (up to 48 months). For all three trials, the risk of bias was considered to be low.
Selective reporting
All included RCTs were judged to be free of selective reporting of the outcomes because all outcomes described in the methods were reported.
Other potential sources of bias
Prognostic comparability
For two out of the three included RCTs we raised some questions about baseline comparability. Allaire 2003 showed that baseline factors were comparable between groups on all variables presented, but did not assess the extent of previous work absenteeism, job or disease duration. For de Buck 2005, the effect of some baseline differences is unclear: there was a difference in the number of participants with a sick leave of > 40 weeks (8% in the intervention group and 3% in the control group). Though not statistically significant, sick leave is likely to influence the likelihood of job loss, as it is more difficult to return to work after prolonged sick leave.
As Macedo 2009 only had 32 participants in their RCT, any differences in prognostic factors will have a larger impact on the results. We judged the risk of bias higher in this trial for several reasons. Baseline imbalances, with reference to the experimental group, included longer disease duration, more workers with full time employment, more university postgraduates and younger people. All these factors favour a better prognosis with regard to the outcome 'work days missed' in the experimental group.
Compliance
We judged compliance in de Buck 2005 trial at low risk of bias even though few participants were seen by their companies' occupational physician and did not receive additional guidance. We judged unclear risk of bias due to the absence of information in Macedo 2009 and Allaire 2003.
Overall judgement of risk of bias
We judged overall risk of bias for each study. We judged Allaire 2003 to have an overall low risk of bias, de Buck 2005 trial to have an overall high risk of bias due to performance bias and Macedo 2009 to have an overall high risk of bias due to detection bias and baseline imbalances.
Effects of interventions
See: Table 1
All three included RCTs evaluated the effects of job loss prevention (see Types of interventions) in workers at risk of job loss with IA compared to a control intervention consisting of usual care or information, or both. To establish the effectiveness of job loss prevention versus control intervention we looked at three outcomes: job loss and sickness absenteeism as primary outcomes, and work functioning as a secondary outcome. See Table 1 for details.
1. Job loss prevention versus control
1.1 Job loss
1.1.1 to 1.1.4 Long term effects
A priori we defined the number of participants with a job loss as the most relevant job loss outcome, based on the goal of the intervention (i.e. job retention). The number of participants with a job loss (Analysis 1.1) was the only outcome reported by more than one trial (Allaire 2003; de Buck 2005). We retrieved data from two RCT publications by Allaire (Allaire 2003; Allaire 2005) and only one for De Buck (de Buck 2005). Short term data were not provided by both RCTs, unsurprisingly as the intervention period took between three and five months to complete on average, and job loss would take time to occur. Therefore, we only present long term data of one year or more. The main comparison for job loss reported by both Allaire 2003 and de Buck 2005 was long term job loss measured at 12 and 24 months, which were considered relevant and reliable. Allaire 2003 reports a long term statistically significant reduction in the number of participants with a job loss in the experimental group compared to the control group, represented by a 12‐month relative risk of 0.32 (95% CI 0.11 to 0.97) and a 24‐month relative risk of 0.35 (95% CI 0.18 to 0.68) (see Analysis 1.1). This represents an absolute difference of ‐7.0% (95% CI 13.2% to ‐0.6%) at 12 months and a ‐16.0% (95% CI ‐25.4% to ‐6.6%) absolute difference at 24 months. Similarly, the number needed to treat was 14.4 at 12 months and 6.2 at 24 months in favour of the job loss prevention intervention. Though the magnitude of this effects is quite large, it is only reported in one RCT (Allaire 2003).
1.1. Analysis.
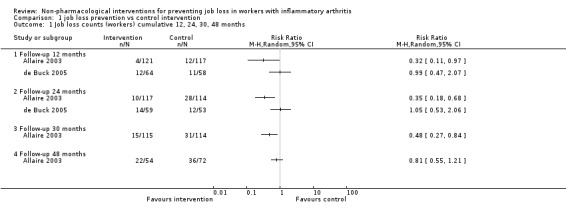
Comparison 1 job loss prevention vs control intervention, Outcome 1 Job loss counts (workers) cumulative 12, 24, 30, 48 months.
In contrast, de Buck 2005 reported no difference in job losses in favour of any group, neither at 12 months or at 24 months, as represented by a RR of 0.99 (95% CI 0.47 to 2.07) at 12 months and a RR of 1.05 (95% CI 0.53 to 2.06) at 24 months. Absolute differences are respectively ‐0.2% (95% CI ‐14.1% to 13.7%) at 12 months and 1.1% (95% CI ‐14.6% to 16.7%) at 24 months.
We did not pool the data for job loss reported above as statistical heterogeneity (I² > 80%) was considerable and would lead to non‐informative pooled estimates (see Analysis 1.1). Follow‐up times in the Allaire 2003 extended beyond 24 months up to 48 months but as stated previously, recruitment was staggered in Allaire 2003 and follow‐up data after 30 months were considered unreliable when reporting count data in calculation of RRs. Using a pooled logistic model, analyses reported by Allaire 2003 for time to any type of job loss over 48 months, adjusting for confounders, showed an overall odds ratio of 0.58 (95% CI 0.34 to 0.99), in line with individual previous results at 12, 24 and 30 months.
1.2 Sickness absenteeism
Macedo 2009 only reported sickness absenteeism at six months (Analysis 1.2), which is an intermediate term follow‐up. The results show a reduction of 2.4 sick days in the experimental group (MD ‐2.42, 95% CI ‐5.03 to 0.19) compared to the control group, measured over the past month but the CI around the estimated reduction is wide and also includes a possible small increase.
1.2. Analysis.
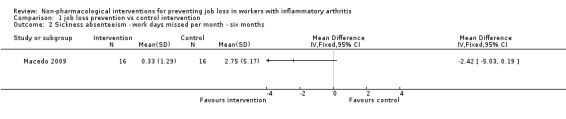
Comparison 1 job loss prevention vs control intervention, Outcome 2 Sickness absenteeism ‐ work days missed per month ‐ six months.
1.3 Work functioning
Macedo 2009 reported work functioning at six months using the RA‐WIS which ranges from 0 to 28 points, a higher score reflecting more risk of work disability. At six months the mean difference between the RA‐WIS score in the control and the RA‐WIS score in the experimental group was ‐4.67 points (‐8.43 to ‐0.91), in favour of the experimental group (Analysis 1.3).
1.3. Analysis.
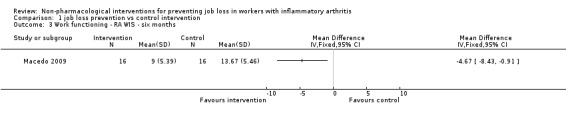
Comparison 1 job loss prevention vs control intervention, Outcome 3 Work functioning ‐ RA WIS ‐ six months.
Adverse effects
None of the included trials reported any adverse effects as a result of the intervention.
Other outcome data
We present effects of other supporting job loss outcome data that were reported in the trials including data on job loss events over 48 months, combined job loss or increase of disability pension data, or sickness absenteeism defined as workdays missed as proportion of days worked for informative purposes in Table 2. These results (Analysis 1.4; Analysis 1.5; Analysis 1.6) do not contribute to the conclusions.
1. Other outcome data reported in the RCTs.
| Outcome measures | Follow‐up times | Trials | Extra analyses |
| Combined job loss or increase of disability pension | 24 months | de Buck 2005 | Analysis 1.4 |
| Job loss events | 48 months | Allaire 2003 | Analysis 1.5 |
| Sickness absenteeism defined as workdays missed in proportion to days worked | Macedo 2009 | Analysis 1.6 |
In this table we present other supporting job loss outcome data reported in the trials for informative purposes only. These data do not contribute to our conclusions.
1.4. Analysis.
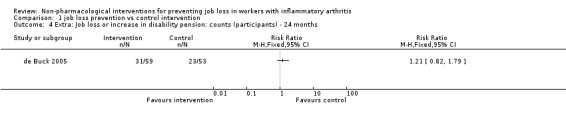
Comparison 1 job loss prevention vs control intervention, Outcome 4 Extra: Job loss or increase in disability pension: counts (participants) ‐ 24 months.
1.5. Analysis.
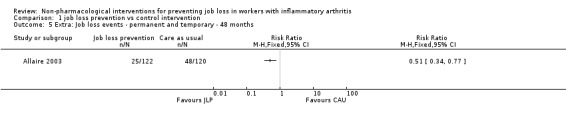
Comparison 1 job loss prevention vs control intervention, Outcome 5 Extra: Job loss events ‐ permanent and temporary ‐ 48 months.
1.6. Analysis.
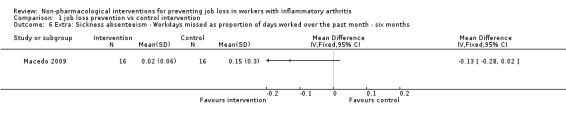
Comparison 1 job loss prevention vs control intervention, Outcome 6 Extra: Sickness absenteeism ‐ Workdays missed as proportion of days worked over the past month ‐ six months.
Sensitivity analyses
We did not perform sensitivity analyses by omitting trials judged to be at high risk of bias, or using a random‐effects model for pooling data due to a lack of data in any one category and the absence of pooling.
Subgroup analyses and analyses of heterogeneity
Although we had planned subgroup analyses to assess the influence of the most prevalent diagnosis categories, the inclusion of vocational intervention with or without additional (multi‐disciplinary) interventions, these were not possible.
Discussion
Summary of main results
Overall we found very low quality evidence for a reduction of job loss in the long term in participants with IA for job loss prevention compared to a control intervention consisting of usual care or information. We downgraded the quality of evidence because of differences in findings between two trials: one large RCT (Allaire 2003) reported a large statistically significant reduction in job loss (RR 0.35, 95% CI 0.18 to 0.68) while the other (de Buck 2005) reported no effect (RR 1.05, 95% CI 0.53 to 2.06). We judged the first RCT (Allaire 2003) on vocational counselling as having an overall low risk of bias. We evaluated the second RCT (de Buck 2005) that used a mix of multi‐disciplinary and vocational elements to have an overall high risk of bias, and it probably suffered substantially from performance bias.
We also found very low quality evidence for a small non‐significant reduction (MD ‐2.42, 95% CI ‐5.03 to 0.19) of intermediate term sickness absenteeism (six months) in participants with IA, reported by one small underpowered RCT (Macedo 2009). This trial provided occupational therapy in group and individual sessions with vocational elements and was evaluated as having an overall high risk of bias. In addition, there was very low quality evidence for a moderate improvement of intermediate term work functioning (six months) ‐4.67 points (‐8.43 to ‐0.91) in participants with RA, reported by Macedo 2009.
Overall completeness and applicability of evidence
As presented in the Characteristics of included studies, one could question whether the three included RCTs were similar enough to be combined. A priori, the selection criteria for the interventions and outcomes in this review were considered quite strict and excluded a number of RCTs. In this sense, we combined specific types of interventions that primarily aim to reduce sickness absenteeism and prevent job loss, referred to by us as 'job loss prevention'. Nonetheless, the three trial interventions show differences regarding the duration of the intervention period and number of contact moments, the professionals involved, the setting or in the way the content of the job loss prevention components were delivered (see Types of interventions). We found that the trial authors provided little information on the hypothesized working mechanism of the interventions, making it harder to base the combining of studies on any solid theory (Verbeek 2012) through the descriptions of the interventions. Likewise, it was unclear how the optimal dosage or delivery (sessions, duration, etc.) was determined. It is clear that all three trial interventions focus around coaching, advising and the provision of information to the participant. With the exception of some workplace visits (Macedo 2009) and a few contacts with the participants' occupational physicians (de Buck 2005), the workplace was not directly involved.
We acknowledge that variation in effectiveness may exist between these trial interventions which should be investigated if more trials allow for subgroup analyses in future updates of this review. Control interventions included medical treatment but also included other co‐interventions described in a superficial manner in Allaire 2003, de Buck 2005 and Macedo 2009. It could be argued that given the available resources in the community, health care and increasingly on the internet, people can manage their work‐related challenges in many ways (Hoving 2013). This may require more understanding of how we interpret control interventions labelled as 'care‐as‐usual' or 'provision of pamphlets', and allow for better descriptions of these control interventions.
In the US trial, Allaire 2003 shows that more than one out of six job losses (risk difference 16%) can be prevented using vocational counselling in contrast to the absence of any job loss reduction for multi‐disciplinary vocational rehabilitation in the Dutch trial by de Buck 2005. The marked differences in effect on the outcome job loss begs the question if other variables than intervention characteristics could explain this difference. Over half of all trial participants in de Buck 2005 were on sick leave at baseline, and about 40% for longer than six weeks. Return to work after long sick leave may require more motivation and effort compared to keeping one's job while working. Given this long‐term sick leave, workers in de Buck 2005 possibly had more severe limitations in work capacity making reintegration more difficult and permanent work disability more unavoidable compared to the workers in Allaire 2003.
Socioeconomic differences are also believed to explain differences in effect of job loss prevention efforts between countries (Anema 2009). Paid sick leave for up to one year, possibly followed by long term disability benefits was customary for Dutch employees in 2001 during the trial period of de Buck 2005. Permanent work disability thus may have been easier to accept by Dutch trial participants. Whereas incentives in the US were different, with workers having a greater at risk of income loss if they choose to stop work permanently, and having less compensation for the initial days of sickness absence, and having a greater risk of dismissal after long‐term incapacitation (Anema 2009). However, the extent to which these differences in incentives explain the differences in effect between de Buck 2005 and Allaire 2003 remains uncertain.
All three trials included participants with IA, but Allaire 2003 also included participants with OA and Macedo 2009 only included participants with RA. We consider the interventions provided to be broadly applicable in the sense that they may be applied to many persons with progressive joint disorders who are at risk of job loss, but more trials are needed. The interventions in our review target the consequences of the disease and we believe that the intervention components in the review are potentially beneficial across many types of IA. However, the low number of included trials precludes any subgroup analyses at this point. Adverse effects were not reported in any of the RCTs. Given our extensive search and the low number of studies, we believe we have included all RCTs.
Quality of the evidence
Following the results for the GRADE assessment (see Table 1) we found the following results for (1) risk of bias, (2) inconsistency, (3) indirectness, (4) imprecision and (5) publication bias.
Risk of bias: we judged this as serious (‐1) for each of the three outcomes, based on the assessment of the risk of bias across all RCTs where several items scored unclear or high risk of bias. Therefore we downgraded the quality of the evidence by one level (‐1).
Inconsistency: we considered inconsistency to be very serious for the outcome job loss, suggesting true differences in underlying treatment effect, with almost no overlap in the CIs of the relative risks of two RCTs. As we failed to identify a plausible explanation for the inconsistency, we downgraded the quality of the evidence by two levels (‐2). For the outcomes sickness absenteeism and work functioning we only had one RCT, hence we judged inconsistency to be not serious and thus we did not downgrade the quality of the evidence.
Indirectness: we judged it to be a minor issue in all comparisons as we could not detect any. We did not downgrade the quality of the evidence for this reason.
Imprecision: results are imprecise when studies include relatively few participants or events and thus have wide CIs around the estimate of effect (RR or MD). For job loss we included two RCTs but the heterogeneity prevented us from pooling these trials. Hence we looked at individual RCTs. The 'threshold rule of thumb value' states there should be at least 300 events for job loss (dichotomous outcome), and for the continuous outcomes there should be at least 400 participants (Higgins 2011). None of the included trials fulfilled this criterion, and neither did any of the comparisons, for any of the outcomes. Macedo 2009 had only 32 participants and we considered it to have very serious imprecision for the outcomes sickness absenteeism and work functioning. Thus, we downgraded the quality of the evidence by two levels (‐2). The other two RCTs reporting on job loss were larger and we judged them to have serious imprecision, and we downgraded the quality of the evidence by just one level (‐1).
Publication bias: given the low number of included trials we were not able to analyse publication bias with funnel plots or tests.
The quality of the evidence was variable across the three included RCTs. On the level of outcomes, the investigation of sickness absenteeism could be improved by using sickness registries or employer databases, and as such use more objective outcomes. However, confidentiality issues and availability of such registries might limit the feasibility of using such data sources.
As in the de Buck 2005 trial, contrast between interventions was a major issue and this may have been present in the other studies, especially in the Macedo 2009 trial where there were few participants. This may be particularly problematic if participants perceive that they have received the 'non‐effective' treatment, which can create biased outcome assessments and bias whereby workers assigned to the control group might seek other interventions (see de Buck 2005). Allaire 2003 provided written information in the control group (and experimental group) but this may not have been perceived as sufficiently valuable to prevent participants in the control group from seeking additional resources. Providing participants with a reasonable alternative intervention may therefore be considered if blinding cannot be guaranteed, as is the case in these types of interventions.
Potential biases in the review process
The primary investigator of the Allaire 2003 trial, Saralynn Allaire , helped us with study screening and by commenting drafts of text. However, she did not participate in data extraction, GRADE assessment or risk of bias assessment of any of the included studies, including Allaire 2003. Similarly we strived to minimise the possible effects of our personal views by having every single step in the review process performed independently by at least two review authors.
We identified some publications in languages other than English during study screening stage but none of them fulfilled our inclusion criteria.
We included only three trials, of which two were essentially positive and the other showed no difference. Although we had an extensive search strategy in several electronic databases we cannot exclude the possibility of publication bias, i.e. that we missed negative trials that did not reach the published literature. For this reason we welcome contact from any readers who are aware of any important trials that would meet the inclusion criteria of this review but are not included so far.
Agreements and disagreements with other studies or reviews
We only know of two other systematic reviews, one by de Buck 2002 and the other by Vliet Vlieland 2009. The review by de Buck 2002 was based on six non‐controlled studies and reported that five of six studied vocational rehabilitation programs consisted of multidisciplinary intervention and 15% to 69% of the participants successfully returned to work. Vliet Vlieland 2009 performed a narrative review of a range of vocational rehabilitation studies on chronic arthritis including the studies by de Buck 2005 and Allaire 2003. Vliet Vlieland 2009 also included studies that did not fulfil our selection criteria regarding disease types, interventions or outcomes. We present our reasons for excluding all these studies in the Characteristics of excluded studies. The conclusion by Vliet Vlieland 2009 was that "results of studies on interventions employed in the early phases of sick leave are promising". Their findings stress the need for early identification of persons at risk for work disability in stages when an intervention would be most effective. We agree with this, but we think this is not enough. We think additional efforts should be taken to come up with a sound theory or working mechanism for interventions that try to prevent job loss, i.e. what works for whom and for what reason?
Authors' conclusions
Implications for practice.
It is possible that job loss prevention interventions can produce beneficial effects on job loss, work absenteeism and work functioning in people with IA. We did not find evidence of adverse effects.
Job loss prevention interventions included multiple components including an assessment of work related challenges, vocational counselling, advice and education or self‐advocacy. Interventions directed at the work environment included workplace visits and consultation with the participants' company occupational physician. In addition, the duration and intensity of these job loss prevention interventions varied. To date, it is unclear what components constitute the most effective strategy for what population or problem and in what stage of the disease or working life.
The job loss prevention interventions described in this review include participants with different types of IA and OA. To date there are no indications that certain arthritis types would benefit less or more from job loss prevention strategies.
Implications for research.
While this Cochrane Review highlights that more high quality trials are required, the results suggest that job loss prevention strategies have potential for effectiveness. It is unclear however through what mechanisms the interventions are effective, or what components of these multifaceted interventions are responsible for the effect. We need more innovative and theory‐based research to support the choice of interventions that target job loss prevention in workers with IA.
Two trials in this review included work visits but this was only performed for a few trial participants. Trials should investigate the benefit of interventions directed at the work environment in combination with, or compared to, interventions targeted at the individual worker. However, interventions directed at the workplace may be limited by reluctance of workers to disclose their disease status at work for fear of job loss, especially in North American work contexts.
Given the positive results found in the RCT that provided vocational counselling by a single skilled professional, future trials should use this intervention as an active control in studies evaluating a range of vocational rehabilitation interventions provided by different professionals.
Future trials should take care in preventing bias. Co‐interventions should be avoided or at least measured, reported and compared across the two groups or acceptable control interventions should be provided. In addition, more attention should be given to clearly reporting methodological key points addressed in this review, including performance bias and detection bias.
Future trials should address not only the effectiveness, but also cost effectiveness of job loss prevention interventions. Losing one's job due to IA takes time and depends on many personal and environmental factors as described in the International Classification of Functioning, Disability and Health (ICF) (WHO 2001).
We propose including a uniform assessment of job loss, job absenteeism and work functioning across trials including a long term follow‐up of at least two years but preferably longer.
Further research to evaluate job loss prevention programs is required in different countries, welfare systems and settings so that the effectiveness of job loss prevention can be evaluated in light of different socioeconomic contexts and settings.
Acknowledgements
We thank Saralynn Allaire for participating in study selection and commenting on drafts of the review text. We thank Jani Ruotsalainen and Jos Verbeek for providing expert advice on Cochrane procedures during all phases of the Cochrane Review, for writing the plain language summary and for editing the text. We thank Leena Isotalo for designing and performing the electronic database searches and Deirdre Walshe and Jani Ruotsalainen for copy editing the text.
Appendices
Appendix 1. Cochrane Library search strategy (including CENTRAL and DARE)
#1 MeSH descriptor: [Arthritis] explode all trees )
#2 MeSH descriptor: [Rheumatic Diseases] explode all trees
#3 arthritis or ankylosing next spondyl* or rheumatic next disease* or rheumatic next disorder* or rheumatic next condition* or bechterew*:ti,ab,kw (Word variations have been searched)
#4 #1 OR #2 OR #3
#5 MeSH descriptor: [Rehabilitation, Vocational] explode all trees
#6 MeSH descriptor: [Occupational Health] explode all trees
#7 MeSH descriptor: [Occupational Health Services] explode all trees
#8 MeSH descriptor: [Occupational Health Nursing] explode all trees
#9 MeSH descriptor: [Workers’ Compensation] explode all trees
#10 MeSH descriptor: [Social Participation] explode all trees
#11 MeSH descriptor: [Occupational Medicine] explode all trees
#12 MeSH descriptor: [Employment] explode all trees
#13 MeSH descriptor: [Disability Evaluation] explode all trees
#14 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
#15 work* or “sick leave” or absenteeism or presenteeism or retirement or employment or “sickness absence” or “disabled persons” or “social participation” or job or ergonomic or vocational or occupation* or employee or employer or unemployment or “disability evaluation”:ti,ab,kw (Word variations have been searched)
#16 #14 OR #15
#17 #4 AND #16
Appendix 2. MEDLINE (PubMed) search strategy
#1 “rheumatic diseases”[MeSH Terms] OR “Arthritis”[Mesh]
#2 arthritis[tw] OR ankylosing spondyl*[tw] OR rheumatic disease*[tw] OR rheumatic disorder*[tw] OR rheumatic condition*[tw] OR bechterew*[tw]
#3 #1 OR #2
#4 “Rehabilitation, Vocational”[Mesh] OR “Occupational Health”[Mesh] OR “Occupational Health Services”[Mesh] OR “workers' compensation”[MeSH] OR “Social Participation”[Mesh] OR “Occupational medicine” [MESH] OR employment[MESH] OR unemployment[MESH] OR “disability evaluation”[MeSH]
#5 “return to work”[tw] OR “sick leave”[tw] OR absenteeism[tw] OR presenteeism[tw] OR retirement[tw] OR employment[tw] OR “sickness absence”[tw] OR “disabled persons”[tw] OR “social participation”[tw] OR job[tw] OR work[tw] OR works*[tw] OR worka*[tw] OR worke*[tw] OR workg*[tw] OR worki*[tw] OR workl*[tw] OR workp*[tw] OR ergonomic[tw] OR vocational[tw] OR occupation*[tw] OR employee[tw] OR employer[tw] OR unemployment[tw] OR “disability evaluation”[tw]
#6 #4 OR #5
#7 randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR clinical trials as topic[mesh:noexp] OR randomly[tiab] OR trial[ti] NOT (animals[mh] NOT humans [mh])
#8 #3 AND #6 AND #7
Appendix 3. EMBASE (embase.com) search strategy
(((arthritis:de,ab,ti OR 'ankylosing spondylitis':de,ab,ti OR (rheumatic NEXT/1 disease*):de,ab,ti OR (rheumatic NEXT/1 disorder*):de,ab,ti OR (rheumatic NEXT/1 condition*):ab,ti OR bechterew*:de,ab,ti) OR ('arthritis'/exp OR 'rheumatic disease'/exp)) AND (('return to work':ab,ti OR employment:ab,ti OR unemployment:ab,ti OR unemployed:ab,ti OR retirement:ab,ti OR 'sick leave':ab,ti OR 'sickness absence':ab,ti OR absenteeism:ab,ti OR presenteeism:ab,ti OR vocational*:ab,ti OR 'work ability':ab,ti OR 'work capacity':ab,ti OR 'work activity':ab,ti OR 'work disability':ab,ti OR 'work rehabilitation':ab,ti OR 'work status':ab,ti OR workability:ab,ti OR employability:ab,ti OR employable:ab,ti OR employee*:ab,ti OR occupation*:ab,ti OR 'job satisfaction':ab,ti OR 'job performance':ab,ti OR 'disability evaluation':ab,ti OR 'disability management':ab,ti OR disabled:ab,ti OR ergonomic:ab,ti OR worker*:ab,ti OR (loss OR remain* OR return* OR retention OR participation*) NEAR/2 (work OR job)) OR ('occupational health'/exp OR 'occupational medicine'/exp OR 'employment'/exp OR 'disability'/exp OR 'work'/exp OR 'vocational rehabilitation':de OR 'workman compensation':de OR 'social participation':de OR unemployment:de OR 'medical leave':de OR retirement:de)) AND (('crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de) OR (random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR (cross NEXT/1 over*):de,ab,ti OR placebo*:de,ab,ti OR (doubl* NEAR/1 blind*):de,ab,ti OR (singl* NEAR/1 blind*):de,ab,ti OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti))) AND [embase]/lim
Appendix 4. CINAHL (EbSCOhost) search strategy
S28 S25 AND S26 AND S27
S27 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S26 (TX allocat* random*) OR (MH "Quantitative Studies") OR (MH "Placebos") OR (TX placebo* ) OR (TX random* allocat*) OR (MH "Random Assignment") OR (TX randomi* control* trial*) OR ( (singl* n1 blind*) or (singl* n1 mask*) ) OR TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) OR TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) OR TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) OR (TX clinic* n1 trial*) OR (PT Clinical trial) OR (MH "Clinical Trials+")
S25 S20 OR S21 OR S22 OR S23 OR S24
S24 (MH "Spondylitis, Ankylosing") OR "ankylosing spondylitis"
S23 "rheumatic conditions"
S22 "rheumatic disorders"
S21 (MH "Rheumatic Diseases+") OR "rheumatic diseases"
S20 (MH "Arthritis+") OR "arthritis" OR (MH "Spondylarthritis+") OR (MH "Arthritis, Rheumatoid+")
S19 "employer"
S18 "Occupational"
S17 "occupation"
S16 "work*"
S15 (MH "Ergonomics+") OR "ergonomic"
S14 (MH "Employee, Disabled+") OR "Employee"
S13 (MH "Job Accommodation") OR "job"
S12 (MH "Work") OR "work"
S11 "disabled persons"
S10 "sickness absence"
S9 "presenteeism"
S8 (MH "Absenteeism") OR "Absenteeism"
S7 (MH "Sick Leave") OR "sick leave"
S6 (MH "Job Re‐Entry") OR "Return to work"
S5 (MH "Unemployment") OR "Unemployment"
S4 (MH "Disability Evaluation+") OR "disability evaluation" OR (MH "Employee, Disabled+") OR (MH "Employment of Disabled+") OR (MM "Disability Management") OR (MM "Sick Leave")
S3 (MH "Employment+") OR "employment" OR (MM "Employment Status") OR (MH "Employment of Disabled+")
S2 (MH "Occupational Health+") OR (MH "Occupational Health Services+")
S1 (MH "Rehabilitation, Vocational+") OR "Vocational rehabilitation" OR (MM "Vocational Guidance") OR (MM "Job Change")
Appendix 5. PsychINFO (ProQuest) search strategy
((SU(Arthritis) OR (ab(arthritis) OR ti(arthritis) OR all("rheumatic disease*") OR all("rheumatic disorder*") OR all("rheumatic condition*") OR all(bechterew*) OR all(ankylosing spondyl*))) AND ((SU.EXACT.EXPLODE("Vocational Rehabilitation") OR SU("occupational health") OR SU("occupational therapy") OR SU("Employability") OR SU("Human Factors Engineering") OR SU("Workers' Compensation Insurance") OR SU.EXACT.EXPLODE("Interpersonal Interaction") OR SU("Occupational Adjustment") OR SU("Occupational Aspirations") OR SU.EXACT.EXPLODE("Employment Status") OR SU("Disabled Personnel") OR SU("Disability Evaluation") OR SU("Occupational Attitudes") OR SU("Work (Attitudes Toward)") OR SU("Occupational Choice") OR SU("Job Involvement") OR SU("Job Satisfaction") OR SU("Employee Absenteeism") OR SU("Reemployment") OR SU("Employee Leave Benefits") OR SU.EXACT("rehabilitation") OR SU.EXACT.EXPLODE("retirement") OR SU.EXACT("Working Conditions")) OR (ab("return to work") OR ab("sick leave") OR ab(absenteeism) OR ab(presenteeism) OR ab(retirement) OR ab(employment) OR ab("sickness absence") OR ab("disabled persons") OR ab("social participation") OR ab(job) OR ab(work*) OR ab(ergonomic) OR ab(vocational) OR ab(occupation*) OR ab(employee) OR ab(employer) OR ab(unemployment) OR ab("disability evaluation") OR ab(employability) OR ab(employable) OR ti("return to work") OR ti("sick leave") OR ti(absenteeism) OR ti(presenteeism) OR ti(retirement) OR ti(employment) OR ti("sickness absence") OR ti("disabled persons") OR ti("social participation") OR ti(job) OR ti(work*) OR ti(ergonomic) OR ti(vocational) OR ti(occupation*) OR ti(employee) OR ti(employer) OR ti(unemployment) OR ti("disability evaluation") OR ti(employability) OR ti(employable)))) AND ((all(random*) OR all(control*) OR SU.EXACT.EXPLODE(treatment) OR SU.EXACT("Treatment Effectiveness Evaluation") OR SU.EXACT.EXPLODE("Treatment Outcomes")) OR (SU.EXACT.EXPLODE(Intervention) OR SU(intervention)))
Data and analyses
Comparison 1. job loss prevention vs control intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Job loss counts (workers) cumulative 12, 24, 30, 48 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Follow‐up 12 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up 24 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow‐up 30 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Follow‐up 48 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Sickness absenteeism ‐ work days missed per month ‐ six months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Work functioning ‐ RA WIS ‐ six months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Extra: Job loss or increase in disability pension: counts (participants) ‐ 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extra: Job loss events ‐ permanent and temporary ‐ 48 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Extra: Sickness absenteeism ‐ Workdays missed as proportion of days worked over the past month ‐ six months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allaire 2003.
| Methods | RCT | |
| Participants | Participants were employed people with a rheumatic disease who were at risk for job loss and resided in eastern Massachusetts. Participants were recruited through letters from rheumatologists. Randomized: 242 participants, 122 in experimental group (I) and 120 in control group (C). Baseline characteristics: female 81.2% vs 81.7%, overall 81%; age 50 (9.4) vs 49 (9.8), overall 49 (range 24 to 66); education beyond high school 39.2% vs 31.2%, overall 35% (n = 85); work type professional/managerial 35.2% vs 30.8%, overall 33% (n = 80); HAQ score functional limitation, mean 0.51(0.4) vs 0.57 (0.4), overall 0.54 (range 0‐1.7) judged as 'mild limitation' in patients with RA; IA 63.9% vs 62.5%. Type of rheumatic disease not provided, overall (in experimental and control group): RA (n = 142), OA (n = 53), SLE (n = 36), AS (n = 8), PsA (n = 3). Duration arthritis: unknown. All participants were either full time or part time employed and at 'work' at baseline, i.e. none were on either short term or long term disability leave (personal communication with first author). Previous job loss: unknown; previous sick leave: unknown. |
|
| Interventions |
Intervention group: Job retention vocational rehabilitation provided by one of two rehabilitation counsellors during two 1.5‐hour sessions (or more) over a five‐month period. Intervention development was based on previous research findings and experience from rehabilitation counsellors. The intervention consisted of three components: (1) job accommodation, (2) vocational counselling and guidance, and (3) education and self‐advocacy.
The counsellors also gave the participants in the experimental group copies of pamphlets and flyers about how to manage health‐related employment problems and about other available resources. Training professionals: The job retention vocational rehabilitation intervention was developed and delivered by one of two experienced rehabilitation counsellors employed by the study; both counsellors had several years of experience, but no particular expertise in job retention vocational rehabilitation. Control group: Written information: Participants were given copies of the same pamphlets and flyers about how to manage health related employment problems and available resources that the experimental group participants received. These materials were mailed to the home addresses of the control group participants within one month after randomisation. |
|
| Outcomes | 1) Job loss: follow‐up at 6 months intervals up to 48 months:
Job loss could be due to all health or arthritis related reasons. 2) Sickness absenteeism: not reported 3) Work functioning: not reported |
|
| Notes | This study was run in a community setting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Strata allocation lists were generated by the statistician and included in an Access database in such a way that treatment assignment was revealed only after a participant's characteristics were entered into the database." No further information regarding random sequence generation. |
| Allocation concealment (selection bias) | Low risk | See above: Using Access database, treatment assignment was revealed only after participants characteristics were entered in database. |
| Prognostic comparability baseline | Unclear risk | Baseline differences were minimal, though baseline information on sickness absenteeism was absent. HAQ scores were in same range (Table 2, Allaire 2003). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personal communication with first author: 16 August2013: "Patients were not told whether they had received either the experimental or control intervention; in enrolling subjects, patients were told we were testing two different ways of helping people stay employed. The counsellors provided intervention to experimental group subjects only and did not know or have any contact with control group subjects. Whether subjects told their work colleagues about their study participation remains doubtful." Employers were not involved in any way in Allaire's trial. |
| Blinding of outcome assessment (detection bias) Job loss | Low risk | Personal communication with first author: 16 August 2013: "The researcher was blinded as to which subjects were in each group (she had little contact with any subjects – only brief contact with a few who called with a question when the research assistant was away)". Allaire 2005: "A professional data collector hired for the study collected the data by telephone." Overall the assessment of job loss was assessed by patients self report and judged low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Allaire originally had a 36‐month follow‐up which was extended with an additional year of follow‐up. The main findings of this review are based on the outcomes of 12 and 24 months: in this time frame attrition was low. Recruitment was staggered and as a consequence not all participants were able to complete the 36 or 48 month follow‐up. During the original 36 months follow‐up 4 participants dropped out of the intervention group and 6 from the control group, and one patient died in each group. The total reported attrition rate one year later, at 48 months, by Allaire was 18% in the intervention and 20% in the control groups. Allaire reported that most of the drop outs were participants who choose not to participate in the additional year of follow‐up (up to 48 months). |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available but given target of intervention ('job retention') and the primary outcome 'job loss' we consider reporting bias not likely. |
| Compliance | Unclear risk | Not documented in the trial text. |
de Buck 2005.
| Methods | RCT | |
| Participants | Participants were people with a paid job (full time or part time, on sick leave, or with partial disability pension) with chronic rheumatic disease (RA, AS, PA, other) with a self‐perceived disease‐related work problem threatening their ability to work. Participants were recruited at 11 hospital outpatient rheumatology departments near Leiden, The Netherlands. Randomized: 140 participants, 74 in experimental group (I) and 66 in control group (C). Baseline characteristics were: female 41 (55%) vs 38 (58%), age 43 (21 to 57) vs 44 (24 to 58); educational level High 15 (20%) Medium 37 (50%) Low 22 (30%) vs 10 (15%), 39 (59%) and 17 (26%); work type ‐ occupational category ‐ mental demands 20 (28%), mixed mental/physical demands 15 (20%), light physical demands 20 (27%), heavy physical demands 19 (25%) vs 24 (36%), 13 (20%), 19 (29%), 10 (15%); HAQ score (0‐3): 0.76 (0.50) vs 0.83 (0.55); type of rheumatic disease, RA 34 (46%) vs 36 (55%), AS PA or reactive arthritis 17(23) vs 12 (18), SLE or scleroderma 23 (31%) vs 18 (27%). Duration disease: median (range) 11.0(0‐158) vs 19.5(0‐174). Full time or part time employment: unknown; previous job loss: unknown; sick leave: 42(57%) vs 35 (53%), duration sick leave median weeks (range) 16 (1‐52) vs 18 (3‐48); partial work disability benefit 12 (16%) vs 11 (17%). Adaptation at work due to rheumatic disease 22(29%) vs 15 (23%). |
|
| Interventions |
Experimental group: Hospital‐based multidisciplinary vocational rehabilitation programme ran by a multi‐disciplinary team (rheumatologist, social worker, physical therapist, occupational therapist and psychologist) during 4 to 12 weeks. During the intervention period participants visited Leiden University Medical Center, the Netherlands at least twice in connection with the rehabilitation program, which always consisted of a systematic assessment and a discussion with the team coordinator:
The final report was then sent to the rheumatologist who referred the patient and was sent to the participants' (company) occupational physician, but only if the patient had given written informed consent. Control group: Usual outpatient care: participants were treated and referred to other health professionals in relation to their working problem if regarded necessary by their rheumatologist. In addition, all participants received the same written information about the Dutch social security system regarding sick leave and work disability as participants in the experimental group. In both experimental and control groups, physicians had free choice with respect to their medical prescriptions and other treatment strategies. All medical treatment and the use of health services during the intervention period and two‐year follow‐up were recorded in both groups. |
|
| Outcomes | 1) Job loss:
2) Sickness absenteeism: not reported 3) Work functioning: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done with stratification for center (academic hospital versus nonacademic hospital) and 3 diagnosis groups (RA; AS, psoriatic arthritis, or reactive arthritis; and SLE or scleroderma), according to a randomisation list that was created by a random digit generator." |
| Allocation concealment (selection bias) | Unclear risk | Not clearly documented in the text of the study. |
| Prognostic comparability baseline | Low risk | Some imbalance in number of participants with long sick leave > 40 weeks: n = 6 (8%) in the intervention group, and n = 2 (3%) in the control group (difference p = 0.17). Other sick leave variables measured at baseline were similar. Overall we judged differences to have a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "To maintain allocation concealment, patients were instructed not to inform the principal investigator or the research nurse about the type of care they received." However, participants received many interventions in the control group as prescribed by the participating rheumatologists who were not blinded. de Buck 2005: "Moreover, the rheumatologist was informed about the treatment allocation in a later stage, another factor that could have induced enhanced treatment or referrals in connection with the work problem in the usual care (UC) group. Indeed, participants in the UC group initially paid more visits to the rheumatologist than participants in the Vocational Rehabilitation (VR) group. The participants' participation in the trial could have made rheumatologists aware of their participants' problems at work, and if a patient was allocated to the UC group rheumatologists might have thought they needed to act on account of their patients. In addition, it is possible that participants who were allocated to the control group made an extra appointment with their rheumatologist to discuss their working problem and potential solutions." Considering the high referral rate in the control group we think the contrast between treatment groups diminished (towards no effect), hence we judged high risk of bias. |
| Blinding of outcome assessment (detection bias) Job loss | Low risk | See above. The assessment of job loss was assessed by participants' self report and judged low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up accounted for (total 25/140: 13/74 in intervention group and 12/66 in control group) 18% at 24 months and was fairly evenly distributed between experimental and control group. In addition, baseline characteristics distribution of non‐completers were similar. |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available but given target of intervention ('vocational rehabilitation'), primary outcome 'job loss' and the non‐significant results we consider reporting bias not likely. |
| Compliance | Low risk | We judged compliance as low risk of bias even though few participants were seen by their companies' occupational physician and did not receive additional guidance. |
Macedo 2009.
| Methods | RCT | |
| Participants | Participants were employed patients with RA and medium to high work disability risk (assessed by RA WIS) who were recruited from RA Centre clinics in London, UK. Randomized: 32 participants, 16 in experimental group (I) and 16 in control group (C). Baseline characteristics: female 15 vs 15; age 49 (12) vs 53 (8); educational level college/university 10 vs 8, postgraduate 2 vs 0; work type: occupational category ‐ managers and professionals 11 vs 10, administrative and skilled trades 5 vs 4, customer service and sales 0 vs 2. Full time work 15 (94%) vs 9 (56%); Job duration < 1 year 3 vs 2, 1 to 3 years 4 vs 1, 3 to 5 years 1 vs 1, > 5 years 8 vs 12; HAQ DI score 1.36 (0.84) vs 1.39 (0.46); Duration disease: median (range) 11.6 (9.9) vs 8.4(6.2). Previous job loss: unknown; days (in month) missed from work, 3.3 (6.2) vs 2.1 (5.0). Biologic agents 2 vs 3 (all other on DMARDs). |
|
| Interventions |
Experimental group: Comprehensive occupational therapy provided by one occupational therapist (OT) specialized in rheumatology and vocational rehabilitation for a maximum duration of 6 months for 6 to 8 individualized treatment sessions lasting 30 minutes to 2 hours either in groups or individually, including work visits. Usual rheumatology care was provided as well. The case management role of the OT incorporated a biopsychosocial model stemming from the Canadian Occupational Performance Measure (COPM).
Control group: Usual care in the RA Centre clinics involved routine reviews by the rheumatologist. The focus of treatment was on early, aggressive medical management with a goal of achievement of remission (DAS28 score 2.6). There was no OT involvement with these participants. Medical management and rheumatology clinic visit schedules were not changed from normal practice for either group. |
|
| Outcomes | 1) Job loss: None reported 2) Sickness absenteeism due to illness/RA:
The instrument was a self‐developed Modified Health Economics Questionnaire combined measures of presenteeism and absenteeism. This is a written self‐report questionnaire that includes the number of days/hours spent at work per week, the number of days missed from work in the past month due to RA. 3) Work functioning:
|
|
| Notes | See text publication: under "occupational therapy received" page 1525: the COPM was used as an outcome measure but its outcomes were also used to direct the intervention. It's unclear whether this could bias participants in the intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation. |
| Allocation concealment (selection bias) | Low risk | "Patients were stratified into medium‐risk (RA WIS score 10 and 17) or high‐risk (RA WIS score 17) groups. Then within these strata, patients were randomly allocated in equal numbers to the occupational therapy or usual care group. The allocation procedure was completed in a blinded manner by an independent research nurse, using a sealed envelope method." |
| Prognostic comparability baseline | High risk | Some questions can be made about the prognostic comparability of both groups. As the group size is small with only 16 participants per group, little differences in prognosis can have substantial influence on outcome. Differences that may count are: longer disease duration in experimental group; more workers with full time employment in the experimental group; more university postgraduates in experimental group; younger people in experimental group. Regardless of whether these differences are significant or not, these factors could have a relationship with both the intervention and the outcome 'work days missed'. The analyses do not account for differences in prognostic imbalances (other outcomes did, but not workdays missed): independent samples T‐tests have been done for the work related outcomes. In addition, using T‐tests for skewed data like work absenteeism (workdays missed) is probably not adequate as well, especially if sample size is small. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Potentially personnel, the participants or the participants work colleagues were more aware or willing to support or influence the participants' work status, as a consequence of belonging to the experimental group or control group but for this trial we have no information that this happened. The extent of this effect remains unclear. |
| Blinding of outcome assessment (detection bias) Sickness absenteeism | High risk | "self‐report questionnaire that includes the number of days/hours spent at work per week, the number of days missed from work in the past month due to RA." We consider these self report data to have a high risk of bias, and not be an objective measure. The intervention was quite intensive with 6 months of comprehensive OT including workplace visits which may have influenced patients recall at the six‐month follow‐up. Certainly there would be more emphasis on the importance of job loss or taking sick leave, although it is not clear how big this effect would be in either group. |
| Blinding of outcome assessment (detection bias) Work functioning | Low risk | The outcome used, the RA WIS is a "self‐administered, written, validated questionnaire used to screen for work disability" and was assessed by patients' self report and judged low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Macedo reports the same number of participants at baseline and 6 months, and reports no job loss during the 6 months follow‐up. |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available but given target of intervention ('targeted comprehensive Occupational Therapy" and participants with "with perceived work disability risk" ) and the primary outcome work productivity we consider reporting bias not likely. |
| Compliance | Unclear risk | No information is provided on the control group. Little information is provided for experimental group on the number of sessions of OT sessions. Not all attended group sessions or received work visits, and the type of interventions was highly variable. We considered the risk (and direction) of bias unclear. Macedo 2009: "half had a work visit completed and recommendations provided to their employer" and "Most patients (n=15) had approximately 6–8 occupational therapy sessions (30–120 minutes) to review work, home, and social needs on a one‐to‐one basis or in a group format. The remaining patient had a single consultation session due to poor attendance. Six patients attended the group education program run by the OT." |
RA: rheumatoid arthritis, AS: ankylosing spondylitis, OA: osteoarthritis, PsA: psoriatic arthritis, SLE: systemic lupus erythematosus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abásolo 2007 | Not sufficient participants with IA. Intervention does not fulfill all criteria. |
| Fleten 2006 | Not sufficient participants with IA. |
| Hammond 2004 | Intervention does not fulfill all criteria. No work‐related outcome. Sub‐population not working (some retired). |
| Ince 2006 | Intervention does not fulfill all criteria. No work‐related outcome. |
| Leon 2009 | Not sufficient participants with IA. Intervention does not fulfill all criteria. |
| Masiero 2007 | Intervention does not fulfill all criteria. No work‐related outcome. |
| Schlademan 2007 | Intervention does not fulfill all criteria. |
| Varekamp 2011 | Not sufficient participants with IA. |
Characteristics of ongoing studies [ordered by study ID]
Carruthers 2014.
| Trial name or title | “Employment and arthritis: making it work”‐ a randomized controlled trial evaluating an online program to help people with inflammatory arthritis maintain employment |
| Methods | RCT of the making it work program compared to usual care and receiving printed information about arthritis and employment |
| Participants | Employed people, aged 18‐59, with inflammatory arthritis (RA, AS,PsA or SLE with arthritis) , at risk of job loss |
| Interventions | The intervention consists of a) 5 online group sessions; b) 5 web‐based e‐learning modules; c) consultations with an occupational therapist for an ergonomic assessment of their work and with a vocational rehabilitation counselor for job retention vocational counselling. |
| Outcomes | Primary outcomes include at‐work productivity (presenteeism)and work cessation (define as stopping work for > 6 months for any reason). Secondary outcomes include temporary work cessation, number of days missed from work per year, reduction in hours worked per week. A cost‐utility analysis of the intervention will also be performed, from a societal perspective |
| Starting date | September 2013 |
| Contact information | Diane Lacaille: dlacaille@arthritisresearch.ca |
| Notes | Trial registration NCT01852851 |
Keysor 2013.
| Trial name or title | Efficacy of a Modified Vocational Rehabilitation Intervention for Work Disability |
| Methods | RCT |
| Participants | Employed persons with rheumatoid and other forms of arthritis, as well as other rheumatic conditions |
| Interventions | Job retention delivered by physical and occupational therapists. Intervention has similarity to that in Allaire 2003. Control group: printed materials concerning working with a disability and relevant resources. |
| Outcomes | Ability to work, satisfaction with ability to work, job loss. |
| Starting date | Ongoing |
| Contact information | Julie Keysor: jkeysor@bu.edu |
| Notes | US |
van Vilsteren 2012.
| Trial name or title | "Care for Work" study |
| Methods | RCT |
| Participants | The population consists of RA patients (18 to 64 years of age) who have visited a rheumatologist of one of the participating hospitals during the last year. Eligible patients are diagnosed with RA, and have a paid job (paid‐employment or self‐employed) for at least 8 hours per week. Furthermore, eligible patients experience difficulties in functioning at work. |
| Interventions | The intervention program consists of two components which complement each other; integrated care including a participatory workplace intervention. |
| Outcomes | The primary outcome measure is work productivity, measured by hours lost from work due to presenteeism. Secondary outcome measures include sick leave, quality of life, pain and fatigue. Cost‐effectiveness of the intervention program will be evaluated from the societal perspective. |
| Starting date | 2012, first results 2015 |
| Contact information | Myrthe van Vilsteren: m.vanvilsteren@vumc.nl |
| Notes | Trial registration NTR2886 |
RA: rheumatoid arthritis, AS: ankylosing spondilitis, OA: osteoarthritis, PsA: psoriatic arthritis, SLE: systemic lupus erythematosus
Differences between protocol and review
We changed the review title from "Non‐pharmacological interventions for improving work participation in patients with inflammatory arthritis" to "Non‐pharmacological interventions for preventing job loss in workers with inflammatory arthritis" as the latter title better represents what the review is about. We did not report funding sources of studies. During the study selection phase we specified that at least half of all participants had to have IA.
Contributions of authors
In pairs, JH performed study selection with another review author (DL, DU, TH, JS and MF). Similarly, JH and either JS or MF performed the risk of bias assessment and data extraction. JH performed data‐analysis and the GRADE assessment following instructions from Jos Verbeek. JH wrote the draft of the review. Comments and suggestions on draft and final versions of the review were provided, in order of contribution, by DL, DU, TH, JS and MF. JH is the guarantor of this review.
Sources of support
Internal sources
Academic Medical Center, Coronel Institute of Occupational Health, Amsterdam, Netherlands.
External sources
-
Instituut GAK, Netherlands.
Salary support for this review (JH, MF) was made possible by a grant from Instituut Gak and is part of the research program "Pathways to work" (www.verbeteronderzoek.nl). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations of interest
Jan Hoving: None known
Diane Lacaille: Prof Lacaille has been awarded a grant by the Canadian Institute of Health Research (CIHR) to evaluate an intervention to improve at work productivity and prevent work disability in people with inflammatory arthritis.
Donna Urquhart: None known.
Timo Hannu: Dr. Timo Hannu owns 150 shares (share class B) of Orion Corporation, a European pharmaceuticals and diagnostics company. In 2011, he received an honorarium of GBP 200 for writing a review titled "Reactive arthritis", which was published in the journal Best Practice & Research Clinical Rheumatology.
Judith Sluiter: None known.
Monique Frings‐Dresen: None known.
New
References
References to studies included in this review
Allaire 2003 {published data only}
- Allaire SH, Li W, LaValley MP. Reduction of job loss in persons with rheumatic diseases receiving vocational rehabilitation: a randomized controlled trial. Arthritis and Rheumatism 2003;48(11):3212‐8. [DOI] [PubMed] [Google Scholar]
de Buck 2005 {published data only}
- Buck PD, Cessie S, Hout WB, Peeters AJ, Ronday HK, Westedt ML, et al. Randomized comparison of a multidisciplinary job‐retention vocational rehabilitation program with usual outpatient care in patients with chronic arthritis at risk for job loss. Arthritis and Rheumatism 2005;53(5):682‐90. [DOI] [PubMed] [Google Scholar]
Macedo 2009 {published data only}
- Macedo AM, Oakley SP, Panayi GS, Kirkham BW. Functional and work outcomes improve in patients with rheumatoid arthritis who receive targeted, comprehensive occupational therapy. Arthritis and Rheumatism 2009;61(11):1522‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abásolo 2007 {published data only}
- Abásolo L, Carmona L, Hernández‐García C, Lajas C, Loza E, Blanco M, et al. Musculoskeletal work disability for clinicians: time course and effectiveness of a specialized intervention program by diagnosis. Arthritis and Rheumatism 2007;57(2):335‐42. [DOI] [PubMed] [Google Scholar]
Fleten 2006 {published data only}
- Fleten N, Johnsen R. Reducing sick leave by minimal postal intervention: a randomised, controlled intervention study. Occupational and Environmental Medicine 2006;63(10):676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammond 2004 {published data only}
- Hammond A, Young A, Kidao R. A randomised controlled trial of occupational therapy for people with early rheumatoid arthritis. Annals of the Rheumatic Diseases 2004;63(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ince 2006 {published data only}
- Ince G, Sarpel T, Durgun B, Erdogan S. Effects of a multimodal exercise program for people with ankylosing spondylitis. Physical Therapy 2006;86(7):924‐35. [PubMed] [Google Scholar]
Leon 2009 {published data only}
- Leon L, Jover JA, Candelas G, Lajas C, Vadillo C, Blanco M, et al. Effectiveness of an early cognitive‐behavioral treatment in patients with work disability due to musculoskeletal disorders. Arthritis and Rheumatism 2009;61(7):996‐1003. [DOI] [PubMed] [Google Scholar]
Masiero 2007 {published data only}
- Masiero S, Boniolo A, Wassermann L, Machiedo H, Volante D, Punzi L. Effects of an educational‐behavioral joint protection program on people with moderate to severe rheumatoid arthritis: a randomized controlled trial. Clinical Rheumatology 2007;26(12):2043‐50. [DOI] [PubMed] [Google Scholar]
Schlademan 2007 {published data only}
- Schlademann S, Hüppe A, Raspe H. Results of a randomised controlled trial on the acceptance and the outcomes of a counselling on medical inpatient rehabilitation in gainfully employed members of statutory health insurances with rheumatoid arthritis. Gesundheitswesen 2007;69(6):6. [clinicaltrials.gov identifier NCT00229541] [DOI] [PubMed] [Google Scholar]
Varekamp 2011 {published data only}
- Varekamp I, Verbeek JH, Boer A, Dijk FJ. Effect of job maintenance training program for employees with chronic disease ‐ a randomized controlled trial on self‐efficacy, job satisfaction, and fatigue. Scandinavian Journal of Work Environment and Health 2011;37(4):288‐97. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Carruthers 2014 {published and unpublished data}
- Carruthers EC, Rogers P, Backman CL, Goldsmith CH, Gignac MA, Marra C, Village J, Li LC, Esdaile JM, Lacaille D. "Employment and arthritis: making it work" a randomized controlled trial evaluating an online program to help people with inflammatory arthritis maintain employment (study protocol).. BMC Med Inform Decis Mak 2014;14:59. [DOI: 10.1186/1472-6947-14-59.; PUBMED: 25043631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keysor 2013 {unpublished data only}
- Efficacy of a Modified Vocational Rehabilitation Intervention for Work Disability. Ongoing study Ongoing.
van Vilsteren 2012 {published data only}
- Vilsteren M, Boot CR, Steenbeek R, Schaardenburg D, Voskuyl AE, Anema JR. An intervention program with the aim to improve and maintain work productivity for workers with rheumatoid arthritis: design of a randomized controlled trial and cost‐effectiveness study. BMC Public Health 2012;12:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allaire 2005
- Allaire SH, Niu J, LaValley MP. Employment and satisfaction utcomes from a job retention intervention delivered to persons with chronic diseases. Rehabilitation Counselling Bulletin 2005;48(2):100‐9. [Google Scholar]
Allaire 2008
- Allaire S, Wolfe F, Niu J, Zhang Y, Zhang B, LaValley M. Evaluation of the effect of anti‐tumor necrosis factor agent use on rheumatoid arthritis work disability: the jury is still out. Arthritis and Rheumatism 2008;59(8):1082‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anema 2009
- Anema JR, Schellart AJ, Cassidy JD, Loisel P, Veerman TJ, Beek AJ. Can cross country differences in return‐to‐work after chronic occupational back pain be explained? An exploratory analysis on disability policies in a six country cohort study. Journal of Occupational Rehabilitation 2009;19(4):419‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 1998
- Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, et al. Prevalence of spondyloarthropathies in HLA‐B27 positive and negative blood donors. Arthritis and Rheumatism 1998;41(1):58‐67. [DOI] [PubMed] [Google Scholar]
Brennan 1992
- Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ 1992;304(6840):1491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Journal of Educational and Psychological Measurement 1960;20(1):37‐46. [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, New Jersey: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Colebatch 2011
- Colebatch AN, Marks JL, Edwards CJ. Safety of non‐steroidal anti‐inflammatory drugs, including aspirin and paracetamol (acetaminophen) in people receiving methotrexate for inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis). Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD008872.pub2] [DOI] [PubMed] [Google Scholar]
Dagfinrud 2008
- Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002822.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Boer 2011
- Boer AG, Taskila T, Tamminga SJ, Frings‐Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return‐to‐work for cancer patients. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD007569] [DOI] [PubMed] [Google Scholar]
de Buck 2002
- Buck PD, Schoones JW, Allaire SH, Vliet Vlieland TP. Vocational rehabilitation in patients with chronic rheumatic diseases: a systematic literature review. Seminars in Arthritis and Rheumatism 2002;32(3):196‐203. [DOI] [PubMed] [Google Scholar]
de Buck 2004
- Buck PD, Breedveld J, Giesen FJ, Vliet Vlieland TP. A multidisciplinary job retention vocational rehabilitation programme for patients with chronic rheumatic diseases: patients' and occupational physicians' satisfaction. Annals of the Rheumatic Diseases 2004;63(5):562‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Croon 2004
- Croon EM, Sluiter JK, Nijssen TF, Dijkmans BA, Lankhorst GJ, Frings‐Dresen MH. Predictive factors of work disability in rheumatoid arthritis: a systematic literature review. Annals of the Rheumatic Diseases 2004;63(11):1362‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Croon 2005
- Croon EM, Sluiter JK, Nijssen TF, Kammeijer M, Dijkmans BA, Lankhorst GJ, et al. Work ability of Dutch employees with rheumatoid arthritis. Scandinavian Journal of Rheumatology 2005;34(4):277‐83. [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2008:285‐312. [Google Scholar]
Duncan 1985
- Duncan J, Hill DH. An investigation of the extent and consequences of measurement error in labor‐economic survey data. Journal of Labor Economics 1985;3(4):508‐32. [Google Scholar]
Escorpizo 2007
- Escorpizo R, Bombardier C, Boonen A, Hazes JMW, Lacaille D, Strand V, et al. Worker productivity outcome measures in arthritis. Journal of Rheumatology 2007;34(6):1372‐80. [PubMed] [Google Scholar]
Fifield 1991
- Fifield J, Reisine ST, Grady K. Work disability and the experience of pain and depression in rheumatoid arthritis. Social Science & Medicine 1991;33(5):579‐85. [DOI] [PubMed] [Google Scholar]
Gabriel 2001
- Gabriel SE. The epidemiology of rheumatoid arthritis. Rheumatic Disease Clinics of North America 2001;27(2):269‐81. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE working group, 2008.
Haafkens 2006
- Haafkens J, Moerman C, Schuring M, Dijk F. Searching bibliographic databases for literature on chronic disease and work participation. Occupational Medicine (Oxford, England) 2006;56(1):39‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoving 2009
- Hoving JL, Bartelds GM, Sluiter JK, Sadiraj K, Groot I, Lems WF, et al. Perceived work ability, quality of life, and fatigue in patients with rheumatoid arthritis after a 6‐month course of TNF inhibitors: prospective intervention study and partial economic evaluation. Scandinavian Journal of Rheumatology 2009;38(4):246‐50. [DOI] [PubMed] [Google Scholar]
Hoving 2013
- Hoving JL, Zwieten MC, Meer M, Sluiter JK, Frings‐Dresen MH. Work participation and arthritis: a systematic overview of challenges, adaptations and opportunities for interventions. Rheumatology (Oxford) 2013;52(7):1254‐64. [DOI] [PubMed] [Google Scholar]
Hurkmans 2009
- Hurkmans E, Giesen FJ, Vliet Vlieland TP, Schoones J, Ende EC. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006853.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2009
- Khan F, Ng L, Turner‐Stokes L. Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007256.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacaille 2005
- Lacaille D. Arthritis and employment research: where are we? Where do we need to go?. Journal of Rheumatology. Supplement 2005;72:42‐5. [PubMed] [Google Scholar]
Lacaille 2007
- Lacaille D, White MA, Backman CL, Gignac MA. Problems faced at work due to inflammatory arthritis: New insights gained from understanding patients' perspectives. Arthritis and Rheumatism 2007;57(7):1269‐79. [DOI] [PubMed] [Google Scholar]
Lefebre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, Available from www.cochrane‐handbook.org., 2011.
MacKinnon 1992
- MacKinnon JR. Occupational profiles: individuals with rheumatoid arthritis and a matched comparison sample. Work 1992;2:39‐49. [Google Scholar]
Mahalik 2006
- Mahalik J, Shigaki CL, Baldwin D, Johnstone B. A review of employability and work site interventions for persons with rheumatoid arthritis and osteoarthritis. Work 2006;26(3):303‐11. [PubMed] [Google Scholar]
Mancuso 2000
- Mancuso CA, Paget SA, Charlson ME. Adaptations made by rheumatoid arthritis patients to continue working: A pilot study of workplace challenges and successful adaptations. Arthritis Care and Research 2000;13(2):89‐99. [PubMed] [Google Scholar]
Mehnert 1990
- Mehnert T, Krauss HH, Nadler R, Boyd M. Correlates of life satisfaction in those with disabling conditions. Rehabilitation Psychology 1990;35:3‐17. [Google Scholar]
Nilsson 2007
- Nilsson I, Fitinghoff H, Lilja M. Continuing to work after the onset of rheumatoid arthritis. Work 2007;28:335‐42. [PubMed] [Google Scholar]
Radner 2012
- Radner H, Ramiro S, Buchbinder R, Landewé RBM, Heijde D, Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD008951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramiro 2011
- Ramiro S, Radner H, Heijde D, Tubergen A, Buchbinder R, Aletaha D, et al. Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis). Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008886.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2011.
Riemsma 2003
- Riemsma RP, Kirwan JR, Taal E, Rasker HJJ. Patient education for adults with rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003688] [DOI] [PubMed] [Google Scholar]
Silva 2010
- Silva KNG, Mizusaki Imoto A, Almeida GJM, Atallah ÁN, Peccin MS, Fernandes Moça Trevisani V. Balance training (proprioceptive training) for patients with rheumatoid arthritis. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007648.pub2] [DOI] [PubMed] [Google Scholar]
Steultjens 2004
- Steultjens EEMJ, Dekker JJ, Bouter LM, Schaardenburg DD, Kuyk MAMAH, Ende ECHM. Occupational therapy for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003114.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straaton 1997
- Straaton KV, Fine PR. Addressing work disability through vocational rehabilitation services. Bulletin on the Rheumatic Diseases 1997;46(3):1‐3. [PubMed] [Google Scholar]
ter Wee 2012
- ter Wee MM, Lems WF, Usan H, Gulpen A, Boonen A. The effect of biological agents on work participation in rheumatoid arthritis patients: a systematic review. Annals of the Rheumatic Diseases 2012;71(2):161‐71. [DOI] [PubMed] [Google Scholar]
Van der Meer 2011
- Meer M, Hoving JL, Vermeulen MI, Herenius MM, Tak PP, Sluiter JK, et al. Experiences and needs for work participation in employees with rheumatoid arthritis treated with anti‐tumour necrosis factor therapy. Disability and Rehabilitation 2011;33(25‐26):2587‐95. [DOI] [PubMed] [Google Scholar]
Verbeek 2005
- Verbeek J, Salmi J, Pasternack I, Jauhiainen M, Laamanen I, Schaafsma F, et al. A search strategy for occupational health intervention studies. Occupational and Environmental Medicine 2005;62(10):682‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2012
- Verbeek J, Ruotsalainen J, Hoving JL. Synthesizing study results in a systematic review. Scandinavian Journal of Work Environment and Health 2012;38(3):282‐90. [DOI] [PubMed] [Google Scholar]
Verstappen 2007
- Verstappen SM, Jacobs JW, Hyrich KL. Effect of anti‐tumor necrosis factor on work disability. Journal of Rheumatology 2007;34(11):2126‐8. [PubMed] [Google Scholar]
Vliet Vlieland 2009
- Vliet Vlieland TP, Buck PD, Hout WB. Vocational rehabilitation programs for individuals with chronic arthritis. Current Opinion in Rheumatology 2009;21(2):183‐8. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. International classification of functioning, disability and health: ICF. World Health Organization, 2001. [Google Scholar]
Yelin 2003
- Yelin E, Trupin L, Katz P, Lubeck D, Rush S, Wanke L. Association between etanercept use and employment outcomes among patients with rheumatoid arthritis. Arthritis and Rheumatism 2003;48(11):3046‐54. [DOI] [PubMed] [Google Scholar]


