Abstract
The human immunodeficiency virus type 1 (HIV-1) Gag polyprotein directs the formation of virions from productively infected cells. Many gag mutations disrupt virion assembly, but little is known about the biochemical effects of many of these mutations. Protein-protein interactions among Gag monomers are believed to be necessary for virion assembly, and data suggest that RNA may modify protein-protein interactions or even serve as a bridge linking Gag polyprotein monomers. To evaluate the primary sequence requirements for HIV-1 Gag homomeric interactions, a panel of HIV-1 Gag deletion mutants was expressed in bacteria and evaluated for the ability to associate with full-length Gag in vitro. The nucleocapsid protein, the major RNA-binding domain of Gag, exhibited activity comparable to that of the complete polyprotein. In the absence of the nucleocapsid protein, relatively weak activity was observed that was dependent upon both the capsid-dimer interface and basic residues within the matrix domain. The relevance of the in vitro findings was confirmed with an assay in which nonmyristylated mutant Gags were assessed for the ability to be incorporated into virions produced by wild-type Gag expressed in trans. Evidence of the importance of RNA for Gag-Gag interaction was provided by the demonstration that RNase impairs the Gag-Gag interaction and that HIV-1 Gag interacts efficiently with Gags encoded by distantly related retroviruses and with structurally unrelated RNA-binding proteins. These results are consistent with models in which Gag multimerization involves indirect contacts via an RNA bridge as well as direct protein-protein interactions.
The major gag product of human immunodeficiency virus type 1 (HIV-1) and related retroviruses is a cytoplasmic polyprotein necessary and sufficient for the assembly, budding, and release of virions—albeit noninfectious particles—from expressing cells (for reviews, see references 12, 28, and 30). Concurrent with virion assembly, the Gag polyprotein incorporates several viral elements, including viral genomic RNA, the Env glycoprotein, and the pol-encoded enzymes, into nascent virions. As virions are released from the cell surface, the Gag polyprotein is cleaved by the viral protease to form the matrix protein (MA), which lines the virion envelope, the capsid protein (CA), which forms the outer shell of the virion core, and the centrally located nucleocapsid protein (NC), which coats the genomic RNA.
It is generally believed that HIV-1 virion assembly requires noncovalent interactions among Gag polyprotein monomers. Retroviral Gag polyprotein monomers form homomultimers (7, 12, 32, 35, 37, 51, 55), but little is known about the stoichiometry of the resulting complex or the forces that drive its formation. Purified HIV-1 CA forms dimers (23), higher-order oligomers (18), and, under certain conditions, lattices of hexamers and trimers (2). Similar information is not available for the Gag polyprotein.
The CA dimer interface has been pinpointed (23), but the identity of amino acid residues required for Gag polyprotein multimerization is unknown. Many gag mutations disrupt virion assembly (9, 16, 22, 28, 29, 48, 57–59), but it has not been directly demonstrated that these mutations disrupt Gag multimerization. The primary effect of these mutations could equally well be the disruption of other processes, such as the interaction of Gag with an unknown cellular factor required for targeting the plasma membrane or the release of virions with proper density (3). Therefore, it has not been conclusively demonstrated that virion assembly requires protein-protein interactions between Gag monomers. Some data even suggest that heterologous bridging molecules such as nucleic acids or ubiquitous RNA-binding proteins may be required for Gag-Gag interaction (7, 38, 64).
We previously reported attempts to map the primary sequence requirements for HIV-1 Gag polyprotein multimerization using the two-hybrid system (21). A gag fragment retaining coding sequences for the major homology region and extending through the complete NC domain was sufficient for full activity in this assay (21). Unfortunately, assessment of the activity of larger deletion mutations was not possible due to technical limitations. Therefore, definition of the minimal domain required for activity was not possible with the two-hybrid system.
In the experiments presented here, a large panel of HIV-1 Gag deletion mutants was evaluated in a more direct assay of HIV-1 Gag-Gag interaction using recombinant protein in solution. NC, the major RNA-binding domain of Gag, exhibited activity almost equivalent to that of the complete polyprotein. In the absence of the NC domain, relatively weak activity was observed that was dependent on residues forming the CA-dimer interface and upon basic residues within the MA domain. Additional experiments demonstrated that RNase disrupts the Gag-Gag interaction and that Gag interacts with heterologous RNA-binding proteins. Finally, an in vivo assay in which nonmyristylated mutant Gags are tested for the ability to be incorporated into wild-type virions expressed in trans was used to confirm the relevance of the in vitro findings. The results presented here are consistent with models in which Gag multimerization requires RNA (perhaps as a bridge between Gag monomers) as well as direct protein-protein interactions.
MATERIALS AND METHODS
Bacteria, yeast, and transformations.
All plasmid DNAs were propagated in Escherichia coli DH5α. Glutathione S-transferase (GST) fusion proteins and HIV-1 Gag protein derivatives were expressed in E. coli BL21(DE3)pLysS (Novagen Inc., Madison, Wis.); this strain contains the λDE3 lysogen which expresses the T7 polymerase from the lacUV5 promoter, as well as pLysS, a plasmid expressing low levels of T7 lysozyme (45).
Cloned DNAs and plasmids.
Nucleotide positions in gag are relative to the 5′ edge of the 5′ long terminal repeat (LTR) in the HIV-1 proviral clone HXB2C; gag-encoded proteins were either expressed as GST fusion proteins or with the HA1 epitope (amino acid residues YPYDVPDYA) from the hemagglutinin (HA) protein of influenza virus (47) appended to the amino terminus.
The construction of pGST-Gag containing coding sequences for the HIV-1HXB2C Gag polyprotein has been described (5). pGST-NC was constructed by PCR amplification of NC-encoding sequences from HIV-1HXB2C with the oligonucleotides 5′-CGCGGATCCATGCAGAGAGGCAATTTTAGGAAC-3′ and 5′-CGCGTCGACTTAATTAGCCTGTCTCTCAGTACAATC-3′; the product was sequenced by standard methods and cloned into a modified pGEX vector (54).
HA-fusion proteins were expressed either from the trc promoter in pSE420 (Invitrogen, Carlsbad, Calif.) or from the T7 promoter in standard pET vectors (Novagen). HA-fusion protein expression constructs were engineered by using a 45-bp synthetic oligonucleotide duplex (5′-CTAGTGCCACCATGGGTTACCCATACGATGTTCCAGATTACGCTG-3′ hybridized to 5′-GATCCAGCGTAATCTGGAACATCGTATGGGTAACCCATGGTGGCA-3′) encoding a methionine initiation codon plus the HA1 epitope. The oligonucleotide duplex was fused to the 5′ end of each gag mutant, as was previously described in detail (12).
Mutations 5′Δ831, 5′Δ1632, and 5′Δ1712 were created by deleting nucleotides at the 5′ end of the gag coding sequence by exploiting unique ClaI, BsmI, or HindIII sites, respectively. Mutations 5′Δ906 and 5′Δ1509 were created similarly by using previously described XhoI sites that had been engineered at the indicated positions (40). The 5′Δ1184 mutation was subcloned from a CA expression plasmid that was previously described (38). Details of the isolation of deletion mutation 5′Δ1320 in a genetic screen were described previously (12). 5′Δ1920, 5′Δ1962, and 5′Δ2004 were generated by PCR using oligonucleotide 5′-GCAACGACCCCTCGTCACAATAAGAATTCGCGC-3′ in combination, respectively, with the following oligonucleotides: 5′-CGCGGATCCATGCAGAGAGGCAATTTTAGGAAC-3′, 5′-CGCGGATCCTGTTTCAATTGTGGCAAAGAAGGG-3′, or 5′-CGCGGATCCAGGGCCCCTAGGAAAAAGGGC-3′.
Mutations 3′Δ2093, 3′Δ2007, 3′Δ1906, and 3′Δ1787 were previously described (21), and all were constructed by the insertion of an Xba linker into restriction endonuclease recognition sites at the indicated nucleotide positions. This linker contains nonsense codons in all three reading frames (linker number 1062; New England Biolabs, Inc., Beverly, Mass.). Engineering of the 3′Δ1681 mutation was described previously (12). The termination codon of the CA coding sequence in 3′Δ1878 was constructed by PCR amplification of gag sequences using the oligonucleotides 5′-CGCGCCATGGGTGCGAGAGCGTCAG-3′ and 5′-GCGCGGATTCTTACAAAACTCTTGCCTTATGGCCGGG-3′. The termination codon of the MA coding sequence in 3′Δ1184 was constructed by PCR of gag sequences using the oligonucleotides 5′-CGCGCCATGGGTGCGAGAGCGTCAG-3′ and 5′-CGCGAATTCTTAGTAATTTTGGCTGACCTG-3′. The 3′Δ1715 and 3′Δ1757 mutations were engineered by PCR using 5′-CCAGTGCATGCAGGGCCTATTGC-3′ and either 5′-GCGCCTCGAGCTAAGCTTGCTCAGCTCTTAGAGTTTTATAG-3′ or 5′-GCGCCTCGAGCTAGACCAACAAGGTTTCTGTCATCC-3′, respectively. The 3′Δ1862 mutation was engineered by PCR using oligonucleotides 5′-GCGCGGATCCATAAGACAAGGACCAAAGGAGCCC-3′ and 5′-GCCGCTCGAGTTAATGGCCGGGTCCTCCTACTCC-3′.
Double mutants 5′Δ831-3′Δ2093, 5′Δ831-3′Δ2007, 5′Δ906-3′Δ2093, 5′Δ906-3′Δ2007, 5′Δ1320-3′Δ2093, and 5′Δ1320-3′Δ2007 were constructed by combining single mutations by standard cloning methods. To construct the HA-NC fusion protein, NC coding sequences were amplified with the oligonucleotides 5′-CGCGGATCCATGCAGAGAGGCAATTTTAGGAAC-3′ and 5′-CGCGTCGACTTAATTAGCCTGTCTCTCAGTACAATC-3′.
Mutant 5′Δ850-884, previously called dB5 (63), was a generous gift of Max Essex. This mutant deletes 11 amino acids encompassing the basic region of MA. Mutants W184A and M185A (23) were generous gifts from Uta VonSchwedler and Wesley Sundquist; the numbering of these two mutants is with respect to the amino terminus of CA as previously described (23).
Gag polyprotein coding sequences from the simian immunodeficiency virus strain MAC239 (SIVMAC239) and the feline immunodeficiency virus strain Petaluma (FIVPETALUMA) were subcloned into a modified pGEX vector (54) from previously described plasmids (21) to generate GST-fusion protein expression vectors. A plasmid for bacterial expression of GST-Rous sarcoma virus (RSV) Gag (34) obtained from the pATV8 clone was a gift from Stephen Goff. To engineer a GST-visna virus Gag fusion protein expression plasmid, Gag coding sequences (GenBank accession no. L06906) were PCR amplified from a plasmid kindly provided by Janice Clements by using oligonucleotides 5′-CGCCCATGGCGAAGCAAGGCTCAAAGG-3′ and 5′-GCGAGATCTTTACAACATAGGGGGCGCGGACGG-3′. The product was subcloned into a pGEX vector. The human foamy virus (HFV) Gag polyprotein coding sequences (GenBank accession no. U21247) were PCR amplified with oligonucleotides 5′-CGCCCCGGGGGATCCATGGCTTCAGGAAGTAATGTTGAAG-3′ and 5′-GCGGAATTCTTACAATTTGTATACTGGCTTTGCC-3′ using pHSRV13 as template (obtained from Stephen Goff). The product was subcloned into a pGEX vector. The HFV NC domain (62) was subcloned by using an ApoI restriction fragment encompassing nucleotides 1394 to 2436 (numbering with respect to the 5′ end of gag).
The HIV-1 Rev cDNA was subcloned into a modified pGEX vector (54) from pT7Rev, a generous gift of Martin Andreansky and Eric Hunter. The cDNA encoding the human ribosomal L8 protein (GenBank accession no. Z28407) was subcloned from a two-hybrid library vector (38) to generate a GST-fusion protein expression plasmid. The cDNA encoding the human autoantigen small nuclear ribonucleoprotein Sm-D (GenBank accession no. J03798) was PCR amplified from a two-hybrid library plasmid (38) by using the oligonucleotides 5′-GCGGGATCCATGAAGCTCGTGAGATTTTTGATG-3′ and 5′-GCGGAATTCTTATCGCCTAGGACCCCCTCTTCC-3′ to generate a GST-fusion protein expression construct.
Constructs for HIV-1 gag expression in mammalian cells.
To express the HIV-1 gag cDNA in mammalian cells in the absence of viral regulatory proteins, we used gag sequences provided by George Pavlakis (National Cancer Institute, Frederick, Md.). These sequences generate wild-type protein but contain multiple, conservative mutations that act to render the mRNA Rev independent (53). To express the Rev-independent gag such that the C terminus of Gag is fused with the Myc-epitope tag (EQKLISEEDL), the cDNA was amplified by PCR by using the oligonucleotides 5′-CGCGCCATGGGTGCGAGAGCGTCAG-3′ and 5′-GCGCGAATTCGAACCGGTCTACATAGTCTC-3′. The product was subcloned into pBluescript (Stratagene), and its identity was confirmed by dideoxy-sequencing. The product was then cloned into the unique NcoI and XhoI sites of pEF/myc/cyto (Invitrogen) to generate pGag-myc. This permitted expression of the Rev-independent gag as a C-terminal fusion with the Myc-epitope tag from the EF-1α promoter. Similar Rev-independent expression plasmids were generated with cDNAs for wild-type HA-Gag, HA-Gag-3′Δ1878, HA-Gag-W184A, HA-Gag-3′Δ1878/W184A, and HA-NC, except that these contained the normal gag stop codon and so were not fused to the Myc-epitope.
In vitro binding experiments.
Bacterial lysates containing recombinant proteins were prepared as described previously (38). All binding steps were performed in a 200-μl reaction volume with TK buffer (20 mM Tris-HCl [pH 7.5], 100 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 5 mM dithiothreitol, 0.5% Nonidet P-40, 0.5 mM phenylmethylsulfonyl fluoride, and 5% glycerol). Twenty microliters of a 50% (vol/vol) slurry of glutathione-agarose beads (Sigma), prepared as described (38), was added to each reaction mixture, as described below.
Bacterial lysates containing equivalent amounts of GST-fusion protein (as normalized by staining polyacrylamide gels with Coomassie blue) were adsorbed to glutathione-agarose beads at 4°C for 30 min. Beads were pelleted by a 5-s spin in a microcentrifuge, and unbound protein was removed by washing with TK buffer. The washed beads containing the preloaded GST proteins were then resuspended in TK buffer containing the indicated HA-Gag fusion proteins and were incubated for 1 h at 4°C on a nutator (Becton-Dickinson, Parsippany, N.J.). Beads were again pelleted and then washed with TK buffer three times. Beads were resuspended in 35 μl of 2× sodium dodecyl sulfate (SDS) sample buffer (52), boiled for 5 min, and pelleted. Aliquots (8 μl) of supernatant were then subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were either stained with Coomassie blue or processed for Western blot analysis.
Western blot analysis.
A murine monoclonal antibody (12CA5) (19) raised against the 9-amino-acid HA1 epitope from the influenza virus HA protein was purchased from Berkeley Antibody Company, Berkeley, Calif. A murine monoclonal antibody directed against the 10-amino-acid Myc-epitope tag (EQKLISEEDL) was purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif. A rabbit anti-RNase A antibody was purchased from Cortex Biochem, San Leandro, Calif. Western blot analysis was performed as described (38).
RNase A treatment.
To examine the effect of RNase A treatment on the in vitro binding reaction, a slightly modified protocol was developed. GST-fusion proteins were bound to glutathione-agarose beads as described above and then washed once with TEK buffer (20 mM Tris-HCl [pH 7.5], 100 mM KCl, 5 mM EDTA, 5 mM dithiothreitol, 0.5% Nonidet P-40, 0.5 mM phenylmethylsulfonyl fluoride, and 5% glycerol). The beads were resuspended in TEK buffer containing 100 ng of RNase A per ml and 1 mg of bovine serum albumin per ml and then incubated for 1 h at 37°C. Simultaneously, bacterial lysate containing HA-Gag protein was diluted at a ratio of 1:100 TEK buffer containing 100 ng of RNase A and 1 mg of bovine serum albumin (1:100) per ml, and the mixture was incubated for 1 h at 37°C.
At the conclusion of the 1-h incubation at 37°C, the glutathione-agarose beads with bound GST-fusion protein were pelleted, and the buffer was removed. The beads were resuspended in the buffer containing RNase-treated HA-Gag protein and were incubated at 4°C for 1 h. At the conclusion of the 1-h binding reaction, the supernatant was removed and processed in two ways. Fifty microliters of the supernatant was mixed with 1 ml of 10% trichloroacetic acid, and the mixture was incubated at 4°C for 30 min and accelerated in a microcentrifuge for 30 min at 4°C to precipitate any proteins that had failed to bind to the beads. The pellet was boiled in SDS and processed for Western blotting. As a rough guide to the effectiveness of the RNase treatment, 100 μl of the supernatant was extracted with phenol-chloroform and extracted with chloroform, and then nucleic acids were precipitated with ethanol. The pellet was electrophoresed on a 2% agarose gel, and the nucleic acid was visualized with ethidium bromide. After removal of the supernatant, the glutathione-agarose beads were washed three times with TEK buffer. Beads were resuspended in 35 μl of 2× SDS sample buffer (52), boiled for 5 min, and pelleted. Aliquots (8 μl) of supernatant were then subjected to SDS-PAGE. Gels were either stained with Coomassie blue or processed for Western blot analysis as described above.
Production of HIV-1 Gag virions.
Human fibroblast 293T cells were maintained in Dulbecco modified Eagle medium-F12 (1:1) supplemented with 10% fetal calf serum. HIV-1 Gag proteins were expressed transiently by calcium phosphate transfection of 10 μg of supercoiled pGag-myc with 10 μg of carrier DNA into 293T cells using the Mammalian Cell Transfection Kit (Specialty Media, Lavallette, N.J.). Cotransfections of pGag-myc with the HA-Gag expression plasmids contained 10 μg of each plasmid.
Forty-eight hours posttransfection, 8 ml of supernatant was collected from the transfected 293T cells. The cells were placed on ice, washed with phosphate-buffered saline, and lysed in radioimmunoprecipitation assay buffer. The supernatant was centrifuged at 1,000 rpm for 5 min and passed through a 0.45-μm-pore-size filter to remove cellular debris. The filtrate was layered onto 2 ml of 25% sucrose in TNE (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, and 1 mM EDTA) and centrifuged at 80,000 × g for 2 h in a Beckman SW41 rotor. The pellet was resuspended in 30 μl of 2× SDS sample buffer and was processed for Western blot analysis.
RESULTS
Establishment of an in vitro assay for Gag-Gag interaction.
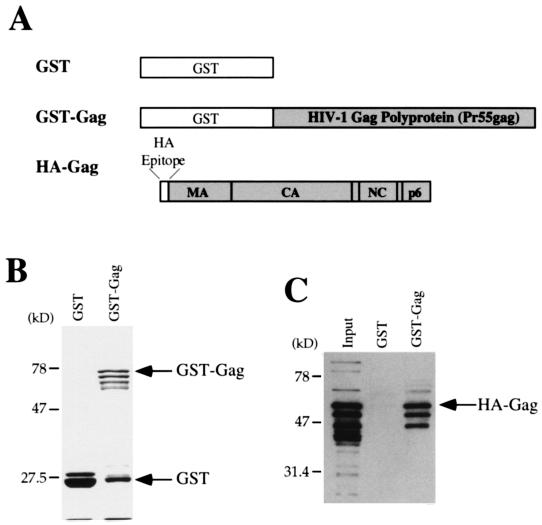
To map primary sequence requirements for Gag-Gag interaction, we developed an in vitro assay by using recombinant proteins expressed in bacteria. The HIV-1 Gag polyprotein was first expressed as a GST-fusion protein in E. coli (Fig. 1A) and was purified from total bacterial lysate in a single step with glutathione-agarose beads as previously described (12, 38). As a negative control for the binding experiment, GST was expressed without Gag residues fused to it (Fig. 1A).
FIG. 1.
In vitro assay for HIV-1 Gag polyprotein multimerization. (A) Schematic diagram of the three proteins that were expressed in bacteria. (B and C) The results of a typical binding experiment in which GST or GST-Gag was purified from bacterial lysates by incubation with glutathione-agarose beads. Unbound proteins were removed by washing. The beads with bound proteins were then incubated with bacterial lysate containing HA-Gag. Beads were washed three more times, and proteins that remained bound were eluted by boiling in SDS and then were analyzed by SDS-PAGE. Proteins that remained associated with the glutathione beads were visualized by staining with Coomassie blue (B) or by Western blotting with anti-HA antibody (C). The input lane shows 10% of the HA-Gag lysate added to the binding reaction. The positions of migration of GST, GST-Gag, and HA-Gag are indicated with arrows.
The glutathione-agarose beads, with associated GST or GST-Gag proteins, were washed three times with TK buffer to remove unbound proteins and were then resuspended and incubated for 1 h at 4°C in a solution containing lysate from bacteria expressing HA-Gag. The latter protein is the HIV-1 Gag polyprotein with the influenza virus HA amino acid residues YPYDVPDYA fused to the amino terminus (Fig. 1A). The glutathione-agarose beads were again washed three times with TK buffer, and proteins that remained associated with the beads were processed by SDS-PAGE.
Recovery on the beads of the GST and GST-Gag fusion proteins was monitored by staining the gel with Coomassie blue. The full-length HIV-1 Gag polyprotein fused to GST was clearly visible (Fig. 1B). Two equimolar, faster-migrating products were also routinely observed. These truncated proteins had previously been shown to lack carboxyl-terminal residues (39). In all binding experiments reported here, the quantities of GST and GST-Gag proteins were normalized to each other with Coomassie blue-stained gels, and enough GST protein was added to each control binding reaction to surpass the sum quantity of the three GST-Gag products.
HA-Gag fusion protein that remained associated with the glutathione-agarose beads was visualized by Western blotting using anti-HA antibody (19). The HA epitope permitted us to unambiguously distinguish HA-Gag from the GST-Gag fusion protein in the Western blot and to demonstrate that these two proteins associated with each other (Fig. 1C). In contrast to its ability to bind to GST-Gag, no HA-Gag was detected in association with GST (Fig. 1C), indicating that the interaction between HA-Gag and GST-Gag was specific. By loading serial dilutions of bacterial lysate containing HA-Gag protein onto the SDS-PAGE gel, it was determined that 10% of the HA-Gag protein added to the reaction was recovered by GST-Gag under our standard binding conditions. In all subsequent Western blots shown in the figures for this paper, 10% of the HA-fusion protein added to the binding reaction is shown in the lanes labeled Input.
Construction of HA-tagged Gag deletion mutants.
To determine the primary sequence requirements for Gag-Gag interaction in vitro, a panel of mutations, each encoding a different, truncated Gag protein, was constructed. Mutants were named for the gag nucleotide where the deletion or disruption of coding sequence begins. Nucleotides were numbered with respect to the 5′ end of the 5′ LTR of HIV-1HXB2 (20). For example, 5′Δ1184 deletes all coding sequences 5′ of nucleotide 1184, and 3′Δ1184 deletes all coding sequences 3′ of nucleotide 1184.
The construction of some of the mutations was described previously (12, 21). Additional mutations were constructed by the same methods or by PCR amplification of gag sequences using mutagenic oligonucleotides. Each mutation was expressed in bacteria as an HA-fusion protein, and the mutant protein was tested for the ability to associate with GST-Gag. Except where specifically indicated, all HA-Gag mutants assessed here for the ability to bind GST-Gag were expressed at levels comparable to that of wild-type Gag. As shown below, there was no detectable binding of any of the mutants to GST alone, indicating that, if the mutant associated with GST-Gag, the interaction was specific for the Gag residues.
Effect of amino-terminal truncations on HA-Gag interaction with GST-Gag.
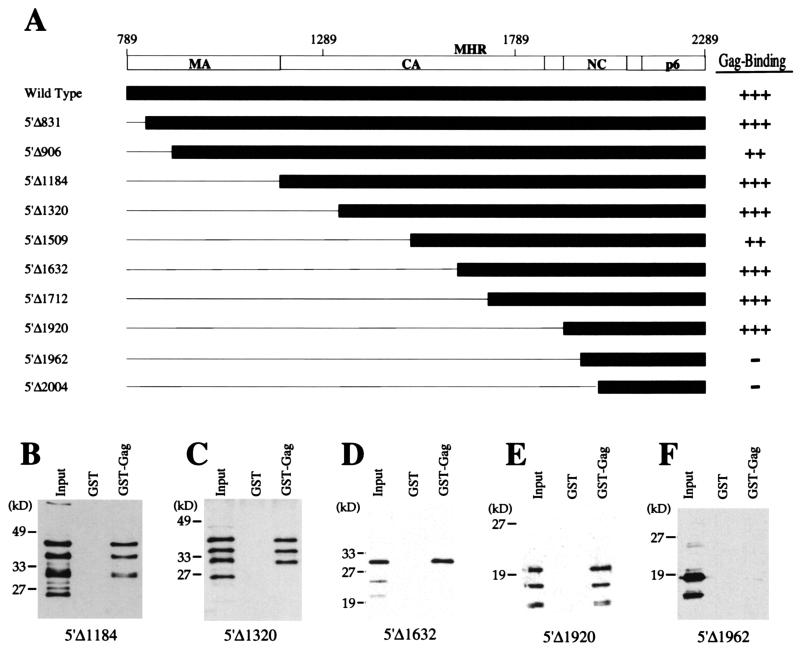
The first set of deletion mutations to be tested encoded Gag polyproteins with truncated amino termini (Fig. 2). The coding sequences retained or deleted by the amino-terminal truncation mutants are shown schematically in Fig. 2A. Binding strength of individual mutant Gags was estimated in Western blots by first normalizing the quantity of the mutant protein added to the binding reaction with the input for the wild-type protein. Then, the signal intensity of the mutant Gag bound to GST-Gag was compared with serial dilutions of the wild-type Gag bound to GST-Gag as has been reported previously to quantitate signals on Western blots (6, 61). Figure 2B to F shows Western blots obtained from binding experiments with critical mutant proteins; primary data for the remaining mutants is not shown due to lack of space.
FIG. 2.
Interaction of HIV-1 HA-Gag amino-terminal deletion mutants with the full-length HIV-1 Gag polyprotein. (A) The primary structure of the HIV-1 Gag polyprotein is depicted schematically at the top. Numbers refer to the position of encoding nucleotides with respect to the 5′ end of the 5′ LTR. Vertical bars indicate the location of viral protease recognition sites. The major proteolytic products are labeled using standard nomenclature: MA indicates matrix protein, CA indicates capsid protein, and NC indicates nucleocapsid protein. MHR, major homology region. Shaded horizontal bars indicate the residues expressed by the mutants, and horizontal lines indicate residues that were deleted. Gag-binding activity of each mutant, quantitated as described in the text, is presented at the right of the figure: +++ indicates activity 50 to 100% of wild type, ++ indicates activity 20 to 50% of wild type, and − indicates activity less than 5% of wild type. (B to F) Immunoblots showing the HIV-1 Gag polyprotein-binding activity of select amino-terminal deletion mutants. Primary data for the remaining mutants is not shown due to lack of space. GST or GST-Gag was purified from bacterial lysates by incubation with glutathione-agarose beads. Beads with associated proteins were washed and then incubated with lysate from bacteria expressing the indicated HA-Gag mutant proteins: 5′Δ1184 (B), 5′Δ1320 (C), 5′Δ1632 (D), 5′Δ1920 (E), or 5′Δ1962 (F). After extensive washing, bound proteins were subjected to SDS-PAGE and were visualized by Western blotting with anti-HA antibody. In each case the input lane shows 10% of the HA-mutant fusion protein lysate added to the binding reaction.
Deletion of gag coding sequences from the 5′ end up to nucleotide 1920 had no significant effect on the ability of the encoded, mutant HA-Gag proteins to associate with GST-Gag (Fig. 2, mutants 5′Δ831, 5′Δ906, 5′Δ1184, 5′Δ1320, 5′Δ1509, 5′Δ1632, 5′Δ1712, and 5′Δ1920). In other words, in our semiquantitative assay, these proteins bound to GST-Gag with strength comparable to that of the wild type. These results indicate that the MA, CA, and p2 domains of HIV-1 Gag are not required for association with GST-Gag in vitro.
When 5′ deletion mutations extended into sequences encoding the NC domain, interaction with GST-Gag was attenuated. Mutant 5′Δ1962 deletes nucleotides encoding NC residues up to the amino-terminal zinc finger; this mutant protein possessed binding activity barely detectable above the background (Fig. 2F). Mutant 5′Δ2004 extends further into NC, deleting all of the amino-terminal zinc finger and possessing no detectable binding activity (Fig. 2A). These results suggest that the NC domain is critical for interaction with the Gag polyprotein in vitro.
Effect of carboxyl-terminal truncations on HA-Gag interaction with GST-Gag.
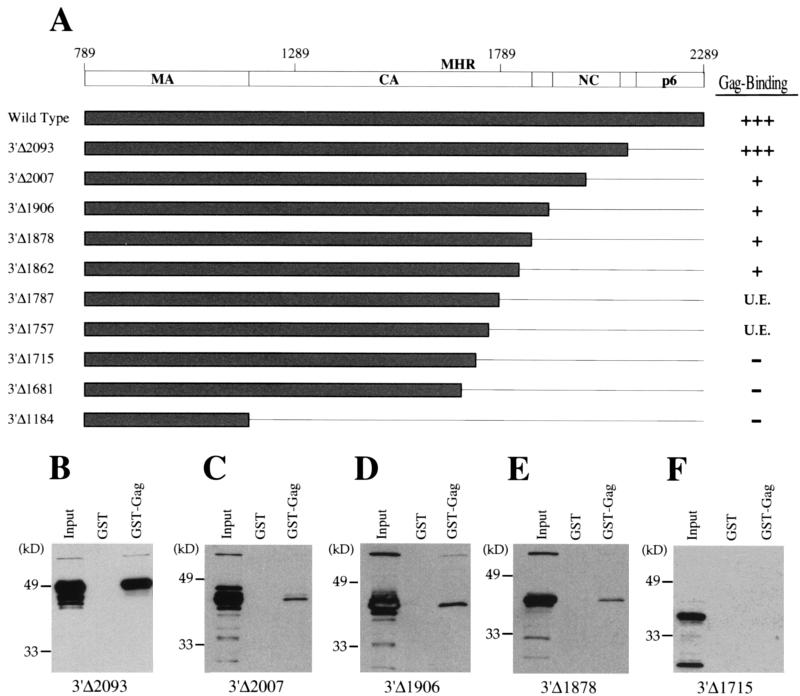
The identity of the carboxyl-terminal truncation mutants tested here is shown schematically in Fig. 3A. Mutant 3′Δ2093 prematurely terminates translation before the beginning of the p6 domain and just after the end of the NC domain. This protein bound to GST-Gag with strength comparable to that of the wild type (Fig. 3B). This result demonstrates that the p6 domain is dispensable for interaction with the Gag polyprotein in vitro.
FIG. 3.
Interaction of HIV-1 Gag polyprotein carboxyl-terminal deletion mutants with full-length Gag polyprotein. (A) Schematic diagram as in Fig. 2 depicting the primary structure of the carboxyl-terminal mutants. Gag polyprotein-binding activity is indicated at the right of the figure: +++ indicates activity 50 to 100% of wild type, + indicates activity 5 to 10% of wild type, − indicates activity less than 5% of wild type; U.E. indicates that expression of the mutant protein was unstable. (B to F) Immunoblots presented as in Fig. 2 showing the HIV-1 Gag polyprotein-binding activity of select carboxyl-terminal deletion mutants 3′Δ2093 (B), 3′Δ2007 (C), 3′Δ1906 (D), 3′Δ1878 (E), and 3′Δ1715 (F). After extensive washing, bound proteins were subjected to SDS-PAGE and were visualized by Western blotting with anti-HA antibody. In each case, the input lane shows 10% of the HA-mutant fusion protein lysate added to the binding reaction.
When the carboxyl-terminal zinc finger of NC was removed by further truncation of the Gag polyprotein (mutant 3′Δ2007 in Fig. 3C), a significant reduction in binding activity was observed. Compared with the wild-type protein, or the protein encoded by 3′Δ2093, the binding strength of this protein was 10- to 20-fold less, as determined by comparing the signal of 3′Δ2007 bound to GST-Gag with serial dilutions of the sample containing 3′Δ2093 bound to GST-Gag (data not shown). With further truncation of carboxyl-terminal residues by mutations 3′Δ1906, 3′Δ1878, and 3′Δ1862, weak binding activity comparable to that of 3′Δ2007 was detected (Fig. 3). 3′Δ1878 expresses a protein with the authentic CA carboxyl terminus that results from viral protease cleavage of the Gag polyprotein.
Further truncation revealed a free carboxyl terminus that was not stably expressed in bacteria (3′Δ1787 and 3′Δ1757). With truncations that extended beyond this point (3′Δ1715, 3′Δ1681, and 3′Δ1184), stable proteins were expressed, but no binding activity was detected (Fig. 3F). 3′Δ1184 was constructed to encode a protein with the same carboxyl terminus as the MA viral protease cleavage product. The results with the carboxyl-terminal deletion mutants support the contention that NC is the major Gag-interacting domain. The fact that mutants 3′Δ2007, 3′Δ1906, 3′Δ1878, and 3′Δ1862 exhibit weak binding activity suggests that a contribution to the interaction is also made by carboxyl-terminal CA residues encoded between nucleotides 1715 and 1862.
The CA-dimer interface contributes to Gag polyprotein multimerization in vitro.
To better pinpoint the residues at the carboxyl terminus of CA that contribute to Gag multimerization, we have attempted to generate several mutations with stop codons between nucleotides 1715 and 1862. Unfortunately, these expression constructs fail to produce stable protein products in bacteria that can be reasonably normalized to the full-length polyprotein (for example, mutants 3′Δ1757 and 3′Δ1787 in Fig. 3).
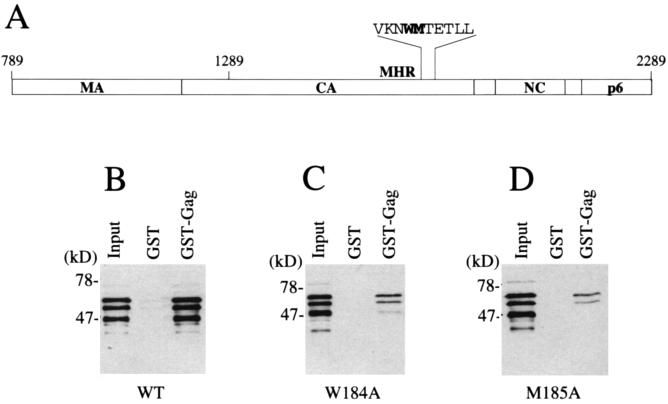
Nucleotides 1715 to 1862 encode amino acids spanning the hydrophobic, dimer interface (Fig. 4A) that was identified in the crystal structure of the carboxyl-terminal domain of HIV-1 CA (23). Mutation of critical residues at the interface disrupts CA dimerization in vitro (23). In the context of the complete provirus, these mutations reduce virion release from cells two- to fourfold, consistent with a possible effect on Gag polyprotein multimerization (23), though the effect of these mutations on Gag polyprotein multimerization was not directly examined. The same authors have proposed that cleavage of the Gag polyprotein by the viral protease induces a conformational change that creates a new dimer interface located at the amino terminus of CA (26). Similarly, the C-terminal dimer interface of the mature CA protein might not be functional within the context of the Gag polyprotein.
FIG. 4.
Carboxyl-terminal CA residues contribute to Gag polyprotein multimerization. (A) Schematic diagram showing the location and hydrophobic residues constituting the CA-dimer interface (23). Amino acids W184 and M185 are shown in bold. In vitro binding experiments were performed as described for Fig. 1 with wild-type HA-Gag (B) or with HA-Gag possessing the W184A (C) or M185A (D) mutation. The inputs of the three HA-Gag proteins were normalized to each other by serial dilution (data not shown). The amino acid numbering is with respect to the amino-terminal residue of CA.
To determine if the CA-dimer interface contributes to Gag polyprotein multimerization, two mutants that had been shown to disrupt the CA-dimer interface were subcloned into the HA-Gag bacterial expression vector so that each mutant was expressed within the context of the full Gag polyprotein. These two mutants were W184A and M185A (23) (amino acid numbering with respect to the amino-terminal residue of CA). Both mutants were found to be expressed at levels comparable to that of the wild-type protein (compare input lanes in Fig. 4B to D). Each mutant was found to bind to GST-Gag, though with less strength than the wild type (Fig. 4C and D). The magnitude of the reduction with either mutant was four- to fivefold, as determined by comparing the signal of the mutant proteins bound to GST-Gag with serial dilutions of the sample containing the wild-type protein bound to GST-Gag (data not shown). These results indicate that, though it is not essential, the CA-dimer interface contributes to Gag multimerization in vitro.
Contribution of MA basic residues to Gag multimerization.
Mutant 3′Δ2093 bound to GST-Gag with wild-type activity (Fig. 3B). Though significantly decreased with respect to 3′Δ2093, mutant 3′Δ2007 retained modest, though clearly detectable, Gag-binding activity (Fig. 3C). When these mutants were expressed in cis with mutants that deleted residues from the amino terminus, a different result was observed: mutant 5′Δ1320/3′Δ2093 exhibited binding activity comparable to that of the wild type, but mutant 5′Δ1320/3′Δ2007 exhibited no detectable activity (Fig. 5B and C). These results suggested that, in the absence of NC, sequences at the amino terminus of the Gag polyprotein are required to detect GST-Gag binding activity.
FIG. 5.
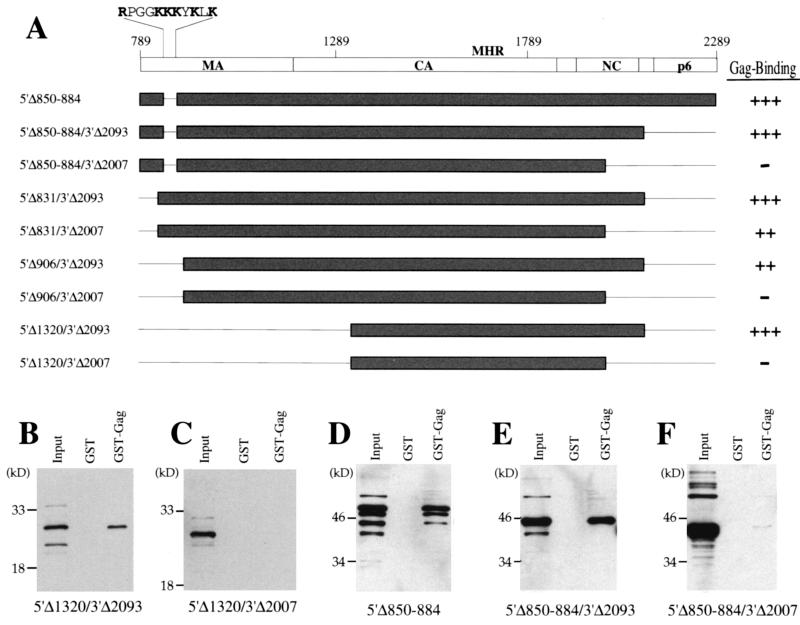
Basic residues in matrix contribute to the multimerization of Gag mutants in which NC is deleted. (A) Schematic diagram as in Fig. 2 depicting the primary structure of the mutants tested here. The 11 amino acid residues deleted from mutant 5′Δ850-884 are shown in blow-up at the top left of the schematic, and basic residues are indicated with bold letters. Gag-binding activity of each mutant is presented at the right of the figure: +++ indicates activity 50 to 100% of wild type, ++ indicates activity 20 to 50% of wild type, − indicates activity less than 5% of wild type. (B to F) Immunoblots presented as in Fig. 2 showing the HIV-1 Gag polyprotein-binding activity of mutants 5′Δ1320/3′Δ2093 (B), 5′Δ1320/3′Δ2007 (C), 5′Δ850-884 (D), 5′Δ850-884/3′Δ2093 (E), and 5′Δ850-884/3′Δ2007 (F). In each case the input lane shows 10% of the HA-mutant fusion protein lysate added to the binding reaction.
To better map the location of the amino-terminal residues that contribute to the stabilization of Gag binding, the effect of expression in cis of additional amino-terminal deletions on the activity of carboxyl-terminal deletions 3′Δ2093 and 3′Δ2007 was examined. Mutants 5′Δ831/3′Δ2093 and 5′Δ831/3′Δ2007 retained binding activity, demonstrating that coding sequences 5′ to nucleotide 831 do not encode residues that contribute significantly to the stabilization of binding (Fig. 5A). In contrast, when the 5′ deletion was extended to nucleotide 906 (5′Δ906/3′Δ2007), no binding activity was detected (Fig. 5A), indicating that the stabilizing activity was encoded by nucleotides located between nucleotides 831 and 906.
The amino acids encoded by nucleotides 831 to 906 include a cluster of basic amino acids (Fig. 5A). A previously characterized mutation (63) that deletes 11 amino acids encompassing the basic cluster (mutant 5′Δ850-884 in Fig. 5A) was tested next, and mutants 5′Δ850-884 and 5′Δ850-884/3′Δ2093 retained full Gag-binding activity (Fig. 5D and E). Mutant 5′Δ850-884/3′Δ2007 had no detectable binding activity (Fig. 5F), indicating that the basic residues in MA contribute to the stabilization of Gag binding when NC residues are deleted.
The role of NC in Gag-Gag interaction.
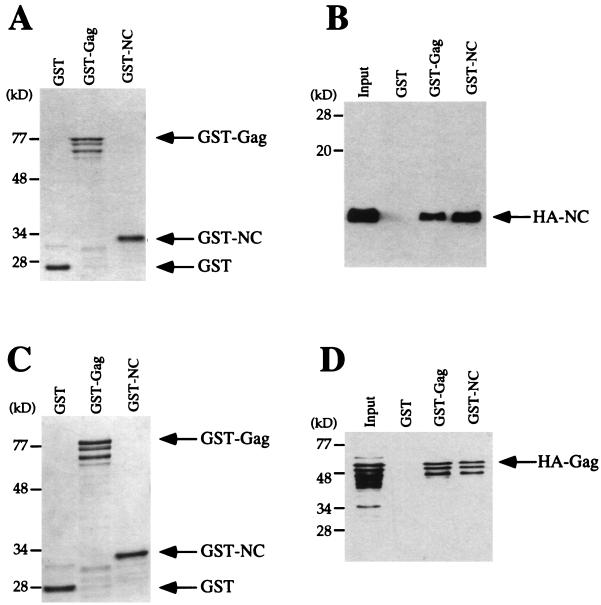
The deletion analysis presented in Fig. 2 and 3 indicates that NC is the major domain contributing to Gag multimerization. To determine if the isolated NC domain is able to associate with Gag, the 55-amino-acid NC protein was expressed in bacteria as an HA-fusion protein and as a GST-fusion protein. The ability of HA-NC (Fig. 6A and B) or HA-Gag (Fig. 6C and D) to bind to GST, GST-Gag, or GST-NC was then compared directly. The quantity of the GST-fusion proteins loaded on the beads was normalized by measuring signal intensity on Coomassie blue-stained SDS-PAGE gels (Fig. 6A and C). Comparable amounts of HA-NC were recovered by GST-Gag or GST-NC (Fig. 6B). Similarly, comparable amounts of HA-Gag were associated with either GST-Gag or GST-NC (Fig. 6D). These results confirm the conclusion from the deletion analysis that the NC domain is sufficient to account for the majority of Gag multimerization activity in vitro.
FIG. 6.
NC associates with the Gag polyprotein. GST, GST-Gag, or GST-NC was purified from bacterial lysates by incubation with glutathione-agarose beads. Beads with associated proteins were washed and then incubated with lysate from bacteria expressing HA-NC (A and B) or HA-Gag (C and D). After extensive washing, bound proteins were subjected to SDS-PAGE and were visualized with Coomassie blue (A and C) or by Western blotting with anti-HA antibody (B and D). Input lanes show 10% of the HA-fusion protein lysate added to the binding reaction. The positions of the migrations of GST, GST-Gag, GST-NC, HA-NC, and HA-Gag are indicated by arrows.
Establishment of an in vivo assay for Gag-Gag interaction.
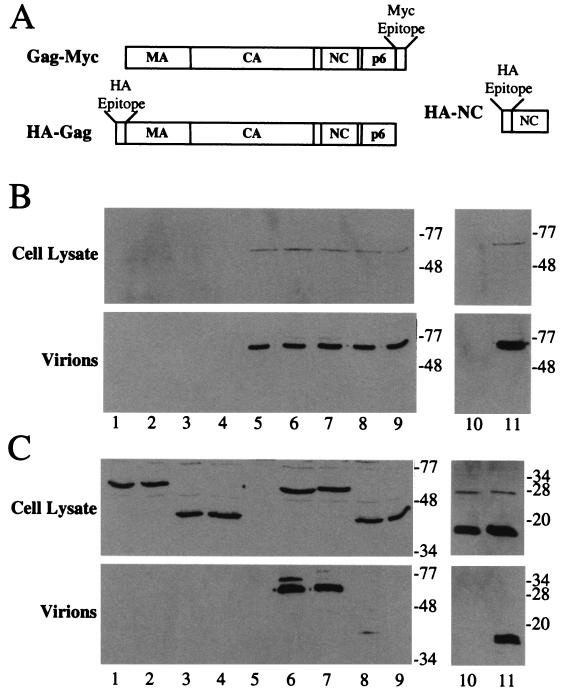
To test the significance of the in vitro mapping results, an assay for HIV-1 Gag-Gag interaction was established in human fibroblasts. To express HIV-1 gag in human cells in the absence of other viral components, we used a construct provided to us by George Pavlakis that contains multiple, conservative mutations that render HIV-1 gag mRNA Rev independent without changing the primary amino sequence (53). This construct was used to generate plasmids for the expression of two Gag-fusion proteins, Gag-Myc and HA-Gag, shown schematically in Fig. 7A. Gag-Myc is the complete HIV-1 Gag polyprotein with a Myc-epitope tag fused at the carboxyl terminus so that it can be distinguished from other Gag proteins (e.g., HA-Gag) expressed in the same cells. Gag-Myc was well expressed in transfected 293 cells and produced extracellular virions that pellet through 25% sucrose (Fig. 7B, lane 5). HA-Gag has an HA-epitope tag fused at the amino terminus, precluding recognition by the host N-myristyl transferase; though produced in significant quantity in the cytoplasm of transfected cells, HA-Gag was found to be incapable of directing the formation of extracellular virions (Fig. 7C, lane 1).
FIG. 7.
In vivo assay for HIV-1 Gag-Gag interaction. The Gag fusion proteins expressed in this assay are shown schematically in panel A. Gag-Myc is an HIV-1 Gag polyprotein with a Myc-epitope tag fused at the carboxyl terminus. HA-Gag, either wild type or bearing one of the mutations listed below, and HA-NC have an HA-epitope tag fused at the amino terminus so that neither protein is myristylated when expressed in eukaryotic cells. 293T cells were transfected with plasmids expressing wild-type HA-Gag (lane 1) or HA-Gag-W184A (lane 2), HA-Gag-3′Δ1878 (lane 3), HA-Gag-3′Δ1878/W184A (lane 4), or HA-NC (lane 10) mutants. The Gag-Myc expression plasmid was transfected alone (lane 5) or in combination with expression plasmids for wild-type HA-Gag (lane 6) or HA-Gag-W184A (lane 7), HA-Gag-3′Δ1878 (lane 8), HA-Gag-3′Δ1878/W184A (lane 9), or HA-NC (lane 11) mutants. Cell lysates (upper panels in B and C) and purified virions (lower panels in B and C) were processed for Western blotting and probed with either primary, anti-Myc antibody (B) or primary, anti-HA antibody (C). Positions of migration of molecular mass markers in kilodaltons are indicated on the right.
293 cells were then cotransfected with plasmids encoding Gag-Myc and HA-Gag. Both proteins were expressed in the cytoplasm at levels comparable to that in the cells transfected with a single plasmid (Fig. 7A and B, compare lanes 1, 5, and 6). Virion-associated Gag-Myc was also produced at levels comparable to the singly transfected cells. HA-Gag was now virion associated, indicating that Gag-Myc expression in trans rescued incorporation of HA-Gag into virions, presumably via Gag-Gag interactions.
Effect of Gag mutants on Gag-Gag interaction in vivo.
With the establishment of an in vivo assay for Gag-Gag interaction, mutations exhibiting significant phenotypes in the vitro assay were subcloned into the HA-Gag expression vector. The mutant proteins were then tested for the ability to be incorporated into virions when Gag-Myc was expressed in trans. HA-Gag-W184A was tested first. As with HA-Gag, when expressed by itself, this protein was unable to direct the assembly of virions (Fig. 7C, lane 2). When Gag-Myc was expressed in trans, HA-Gag-W184A was incorporated into virions (lane 7). Compared with HA-Gag, there was a slight reduction in incorporation efficiency. HA-Gag-3′Δ1878 was also well expressed but unable to produce virions when expressed by itself (lane 3). This mutant exhibited a significant reduction in the ability to be incorporated in trans into Gag-Myc virions (lane 8), consistent with its effect in the in vitro assay. No detectable incorporation in trans into Gag-Myc virions was observed with the double mutant HA-Gag-3′Δ1878/W184A (lane 9), consistent with the complete disruption of Gag-Gag interaction by this mutant. In the next experiment, an HA-NC expression construct was shown to be expressed in the cytoplasm, but the protein was not released from the cell (lane 10). When Gag-Myc was expressed in the same cells, HA-NC was efficiently incorporated into virions (lane 11). The results with the in vivo assay correspond to those obtained in vitro: NC is the major Gag polyprotein domain required for Gag-Gag interaction, and, to a lesser extent, the CA-dimer interface contributes to the interaction.
RNA is required for Gag polyprotein multimerization.
The NC domain of Gag possesses a high percentage of basic residues and two zinc fingers and has been shown to bind to RNA (for review see reference 4). The basic residues in MA that contribute to Gag multimerization in the context of an NC deletion (Fig. 5A) also have the potential to interact with RNA (36). In response to the results of the deletion analysis presented above, experiments were initiated to test the hypothesis that Gag multimerization requires RNA-protein interactions as well as protein-protein interactions.
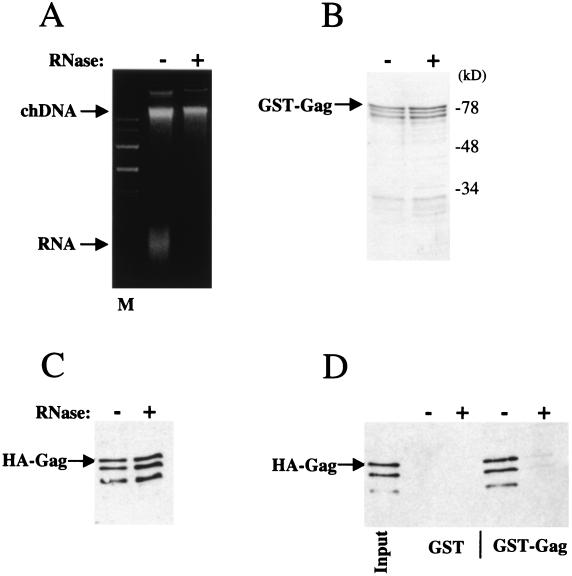
A modified binding assay was established in which the two recombinant proteins were treated with RNase A prior to mixing them together. GST-Gag was bound to glutathione-agarose beads. The beads were washed three times before they were resuspended and incubated in a buffer containing RNase A for 1 h at 37°C. After the RNase treatment, the beads were again washed three times. Simultaneously, bacterial lysate containing HA-Gag protein was added to a buffer containing RNase A, and this mixture was also incubated for 1 h at 37°C. The RNase-treated HA-Gag lysate was then added to the RNase-treated GST-Gag that was bound to glutathione-agarose beads and was incubated at 4°C for 1 h. The postbinding supernatant was saved, and the proteins that remained associated with the beads after another three washes were processed by SDS-PAGE. Recovery on the beads of GST-Gag was monitored by staining the gel with Coomassie blue; recovery of HA-Gag was monitored by Western blot with an anti-HA antibody.
As a gross measure of RNase activity, an aliquot of the RNase-treated, HA-Gag-containing bacterial lysate was run on an agarose gel which was then stained with ethidium bromide. It was discovered that the majority of the RNA had been degraded, while the bacterial chromosomal DNA had remained intact (Fig. 8A). The Coomassie-stained gel of the products of the final binding reaction indicated that the GST-Gag remained intact through all the incubation steps (Fig. 8B). A Western blot of the postbinding supernatant indicated that the HA-Gag protein had also remained intact through all of the incubation steps (Fig. 8C). Upon examination of the proteins that remained associated with the glutathione-agarose beads at the end of the binding experiment, it was evident that RNase A had significantly impaired the interaction of HA-Gag with GST-Gag (Fig. 8D). The products of the binding reaction were probed in a Western blot with an RNase A antibody. With the concentration of RNase A used here, no RNase A was found associated with GST-Gag (data not shown), indicating that RNase A was not disrupting the interaction by competing for binding to GST-Gag. These results demonstrate that Gag multimerization in vitro is dependent upon RNA.
FIG. 8.
RNase A inhibits HIV-1 Gag polyprotein multimerization. Bacterial lysate containing HA-Gag was incubated for 1 h at 37°C with (+) or without (−) RNase A. (A) Ethidium-stained agarose gel of the HA-Gag-containing bacterial lysates. M indicates the marker lane. The positions of migration of bacterial chromosomal DNA (chDNA) and bacterial RNA (RNA) are shown. GST or GST-Gag was purified from bacterial lysates by incubation with glutathione-agarose beads. Unbound proteins were removed by washing. Beads with bound proteins were incubated for 1 h at 37°C with (+) or without (−) RNase A. Beads were washed three times and then used in binding experiments with the bacterial lysates shown in panel A as described in Fig. 1. Beads were washed three more times, and proteins that remained bound were eluted by boiling in SDS and then analyzed by SDS-PAGE. GST-Gag that remained associated with the glutathione beads was visualized by staining with Coomassie blue (B). The stability of HA-Gag to RNase treatment is monitored in panel C, which shows a Western blot with anti-HA antibody of the protein that failed to associate with GST-Gag bound to glutathione-agarose beads. (D) Western blot with anti-HA antibody showing the HA-Gag protein that remained associated with the glutathione-agarose beads at the end of the binding reaction. The input lane shows 10% of the RNase-treated HA-Gag fusion protein lysate added to the binding reaction.
The HIV-1 Gag polyprotein interacts in vitro with the Gag polyproteins encoded by other retroviruses.
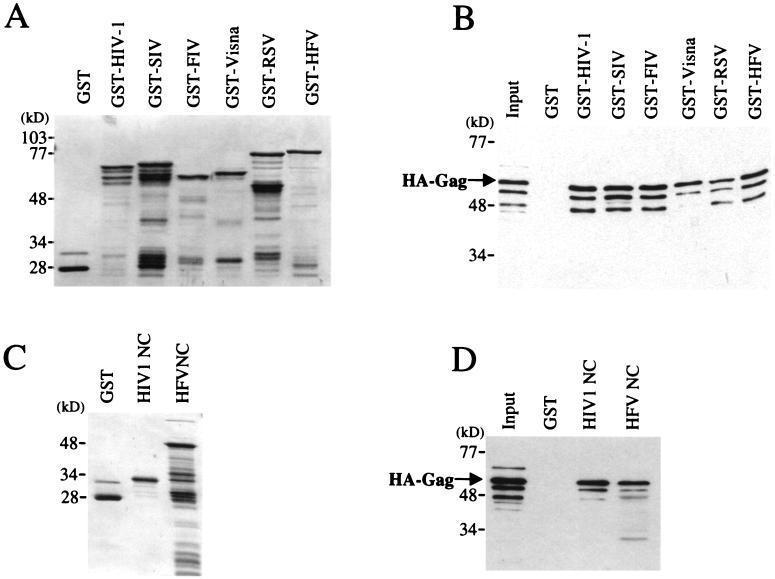
It had been previously shown with the yeast two-hybrid system that HIV-1 Gag interacts with the Gag polyproteins encoded by two SIV isolates and FIV (21). These results were confirmed here with the in vitro binding assay: HA–HIV-1 Gag associates with GST fusions to the Gag polyproteins encoded by SIVMAC239 and FIV (Fig. 9A and B). HIV-1 Gag was also shown to interact with the Gag polyproteins of the visna lentivirus, RSV, and even HFV (Fig. 9A and B).
FIG. 9.
HIV-1 Gag interacts in vitro with Gag polyproteins encoded by other retroviruses. In vitro binding experiments were performed as described for Fig. 1. (A and B) The ability of wild-type HIV-1 HA-Gag protein to bind to GST–HIV-1 Gag was compared with its ability to bind to GST fusions with the Gag polyproteins encoded by SIVMAC239, FIVPETALUMA, visna virus, RSV, or HFV, as indicated above the lanes. Proteins that remained associated with the glutathione beads were visualized by staining with Coomassie blue (A) or by Western blotting with anti-HA antibody (B). The gels in panels C and D, Coomassie blue stained and Western blot, respectively, show a similar comparison between GST fusion proteins with HIV-1 NC and GST fusion protein with HFV NC (62). The input lane shows 10% of the HA-Gag lysate added to the binding reaction. The position of migration of HA-Gag is indicated.
Unlike the Gags of other retroviruses, HFV Gag does not possess zinc fingers. Rather, it is thought to package genomic RNA via glycine-arginine-rich motifs in the NC domain (62). The NC domain of HFV retained the ability to interact with HA–HIV-1 Gag, consistent with the hypothesis that the basic residues at the carboxyl terminus are required for interaction with the HIV-1 Gag polyprotein (Fig. 9C and D). This result, in combination with the fact that HIV-1 and HFV Gags have little primary sequence in common, suggests that these two proteins interact with each other via an RNA bridge.
The HIV-1 Gag polyprotein interacts in vitro with heterologous RNA-binding proteins.
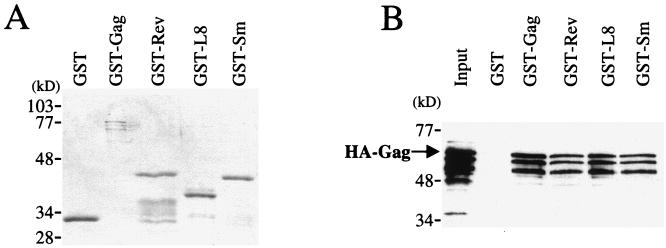
Since HIV-1 Gag interacts with HFV Gag, a collection of heterologous RNA-binding proteins was expressed as GST fusions to determine if they also interact with HIV-1 Gag. The first of the heterologous RNA-binding proteins to be tested was HIV-1 Rev, and it was found to associate with HIV-1 Gag in our assay (Fig. 10).
FIG. 10.
HIV-1 Gag interacts in vitro with heterologous RNA-binding proteins. In vitro binding experiments were performed as described for Fig. 1. The ability of wild-type HIV-1 HA-Gag protein to bind to GST–HIV-1 Gag was compared with its ability to bind to GST fusions with HIV-1 Rev (Rev), human ribosomal protein L8 (L8), or human autoantigen small nuclear ribonucleoprotein Sm-D (Sm), as indicated above the lanes. Proteins that remained associated with the glutathione beads were visualized by staining with Coomassie blue (A) or by Western blotting with anti-HA antibody (B). The input lane shows 10% of the HA-Gag lysate added to the binding reaction. The position of migration of HA-Gag is indicated.
We previously reported the results of a two-hybrid screen of a human cDNA library for encoded proteins that interact with the HIV-1 Gag polyprotein (38). In addition to two members of the cyclophilin family of proteins, seven RNA-binding proteins were identified in this screen. Two of these RNA-binding proteins, ribosomal protein L8 and the human autoantigen small nuclear ribonucleoprotein Sm-D, were expressed as GST fusions and were tested for the ability to interact with HIV-1 Gag in vitro. Like HIV-1 Rev, both of these heterologous proteins were able to interact with HIV-1 Gag (Fig. 10), providing further evidence that Gag-Gag interaction may involve bridging by RNA.
DISCUSSION
Numerous reports in the literature have shown that the NC domain plays an essential role in retroviral assembly. Studies with RSV and HIV-1 have identified three discrete domains essential for virion assembly (13), and one of these assembly domains overlaps NC. A number of groups have shown that the deletion of nucleotides encoding NC disrupts normal particle production (8, 14, 25, 31), as does the mutation of basic residues in NC (10, 14) or the simultaneous mutation of homologous residues in both zinc fingers (17).
In the experiments presented here, any deletion that encroached upon sequences encoding NC residues resulted in a significant decrease in the ability to interact with the Gag polyprotein. In addition, the Gag-binding activity of isolated NC was comparable to that of the full Gag polyprotein. These studies greatly refine previous deletion mapping studies reported with the two-hybrid system (21). NC-NC interaction has been observed in the two-hybrid system, though the activity of the isolated NC domain was not compared with that of the full Gag polyprotein or of other Gag fragments (56). Also, the major activity in a ligand affinity blot examining HIV-1 Gag-Gag interaction was found to disappear when sequences encoding NC were deleted (8).
Our observation that NC is incorporated into HIV-1 virions in trans is consistent with a number of published observations suggesting that one of the major roles of NC in virion assembly is to promote intermolecular interactions among Gag polyprotein monomers. Budding deficient HIV-1 gag mutants can be efficiently rescued in trans by wild-type gag as long as they carry NC sequences (3, 60). Cysteines within the NC domain of the Gag polyprotein can be crosslinked to form dimers (43, 49, 50).
Though NC appears to be the major domain driving Gag-Gag association, other domains appear to contribute to the interaction. Analysis of the three-dimensional structure of a protein fragment encompassing the carboxyl-terminal third of HIV-1 CA identified a hydrophobic dimer interface (23). In the context of an otherwise wild-type provirus, the dimer interface mutants are associated with a fourfold reduction in virion assembly (23); this result correlates with the magnitude of the reduction of Gag-Gag interactions that we observed, suggesting that these mutants disrupt Gag-Gag interaction as well as CA dimer formation. On the other hand, the affinity of the dimer interface is not very high (23), and the activity of the dimer interface that we observed in our assay for Gag-Gag interaction was relatively weak when compared with that of NC. In fact, in the absence of the basic region of MA, we are unable to detect an interaction due to the CA-dimer interface in our assay.
Deletion of MA caused no detectable reduction in Gag-binding activity, and the isolated MA domain was unable to associate detectably with GST-Gag. Though MA trimers have been observed in solution (46), our results are in agreement with reports from other groups that MA is primarily monomeric in solution, even at millimolar concentrations (41, 42). Binding activity associated with the patch of basic residues in MA was revealed in our assay when NC was deleted. Deletion of these exact MA residues prevents viral assembly (63), presumably by disrupting plasma membrane association (65) or, perhaps, because they make a contribution to the Gag-Gag interaction.
NC binds specifically to HIV-1 RNA in vitro and is required for the encapsidation of viral genomic RNA into virions (for a review, see reference 4). The basic residues in MA that contribute to Gag multimerization in the context of an NC deletion (Fig. 5A) also have the potential to interact with RNA (36). These facts, along with the observations that RNase disrupts Gag-Gag interaction and that Gag interacts with heterologous RNA-binding proteins, suggest that RNA plays an important role in virion assembly.
A Gag fragment consisting of CA and NC assembles structures in vitro, in an RNA-dependent manner (7, 24). Some researchers have reported that NC mutations which disrupt packaging of viral RNA attenuate virion assembly (14, 17), but others disagree (1, 27). These conflicting results might be explained by the fact that none of these groups examined the effect of the mutations on the packaging of the heterologous RNAs that might substitute for viral genomic RNA in the assembly function. Nonspecific RNA can substitute for the assembly function in vitro (7), and substitution of NC and p6 by the Bacillus subtilis MtrB tryptophan leader RNA-binding protein domain released particles efficiently (64). Also, specific NC mutants which are defective in the packaging of viral genomic RNA but which package increased amounts of heterologous cellular RNAs have been described (44).
Perhaps interaction with RNA alters the structure of Gag to a form that is permissive for multimerization. RNA might promote protein-protein interactions among Gag polyproteins by neutralizing charge repulsions between the basic residues. This latter possibility is supported by the observation that very high concentrations of salt will substitute for RNA in the in vitro assembly of an HIV-1 CA-NC fragment into virion-type structures (24). The finding that purified NC in complex with the HIV-1 SL3 RNA stem-loop is a monomer in solution (15) suggests that RNA does not promote protein-protein interactions among NC monomers. Rather, RNA may serve as a thread on which NC monomers are strung, thereby promoting protein-protein interactions involving other domains of the Gag polyprotein.
In a previously reported two-hybrid screen of a cDNA library for encoded proteins that interact with HIV-1 Gag, we isolated a large number of RNA-binding proteins (38). At the time we proposed that RNA might serve as a bridge, in effect linking Gag polyprotein molecules to these heterologous RNA-binding proteins. The fact that Gag interacts with such a structurally diverse group of RNA-binding proteins suggests that the interactions among Gag monomers may similarly occur via an RNA bridge, with a smaller contribution from protein-protein interactions. In support of this hypothesis, and indicating that these results are not simply artifacts of an in vitro system, it has been observed that two cellular RNA-binding proteins, elongation factor 1-alpha (11) and histidyl-tRNA synthetase (33), each interact with Gag in vitro, and both proteins are incorporated into HIV-1 virions.
ACKNOWLEDGMENTS
We thank Martin Andreansky, Janice Clements, Max Essex, Eric Hunter, George Pavlakis, Wesley Sundquist, and Uta VonSchwedler for plasmid DNAs and Thomas Bertsch, Philippe El-Helou, and Julie Harris for technical assistance.
This work was supported by grant AI 41857 (J.L.) and by shared core facilities of the Columbia-Rockefeller Center for AIDS Research (P30 AI42848), both from the National Institutes of Health.
REFERENCES
- 1.Aldovini A, Young R. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barklis E, McDermott J, Wilkens S, Fuller S, Thompson D. Organization of HIV-1 capsid proteins on a lipid monolayer. J Biol Chem. 1998;273:7177–7180. doi: 10.1074/jbc.273.13.7177. [DOI] [PubMed] [Google Scholar]
- 3.Bennet R P, Nelle T D, Wills J W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1993;67:6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz R, Luban J, Goff S P. Specific binding of human immunodeficiency virus type 1 gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J Virol. 1993;67:7190–7200. doi: 10.1128/jvi.67.12.7190-7200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braaten D, Franke E K, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell S, Vogt V M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carriere C, Gay B, Chazal N, Morin N, Boulanger P. Sequence requirements for encapsidation of deletion mutants and chimeras of human immunodeficiency virus type 1 Gag precursor into retrovirus-like particles. J Virol. 1995;69:2366–2377. doi: 10.1128/jvi.69.4.2366-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chazal N, Carriere C, Gay B, Boulanger P. Phenotypic characterization of insertion mutants of the human immunodeficiency virus type 1 Gag precursor into retrovirus-like particles. J Virol. 1994;68:111–122. doi: 10.1128/jvi.68.1.111-122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimarelli A, Luban J. Basic residues in HIV-1 nucleocapsid are required for virion assembly. 1999. Unpublished data. [Google Scholar]
- 11.Cimarelli A, Luban J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J Virol. 1999;73:5388–5401. doi: 10.1128/jvi.73.7.5388-5401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colgan J, Yuan H E H, Franke E K, Luban J. Binding of the human immunodeficiency virus type 1 Gag polyprotein to cyclophilin A is mediated by the central region of capsid and requires Gag dimerization. J Virol. 1996;70:4299–4310. doi: 10.1128/jvi.70.7.4299-4310.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craven R C, Parent L J. Dynamic interactions of the Gag polyprotein. Curr Top Microbiol Immunol. 1996;214:65–94. doi: 10.1007/978-3-642-80145-7_3. [DOI] [PubMed] [Google Scholar]
- 14.Dawson L, Yu X F. The role of nucleocapsid of HIV-1 in virus assembly. Virology. 1998;251:141–157. doi: 10.1006/viro.1998.9374. [DOI] [PubMed] [Google Scholar]
- 15.De Guzman R N, Wu Z R, Stalling C C, Pappalardo L, Borer P N, Summers M F. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science. 1998;279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 16.Dorfman T, Bukovsky A, Ohagen A, Hoglund S, Göttlinger H G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J Virol. 1994;68:8180–8187. doi: 10.1128/jvi.68.12.8180-8187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorfman T, Luban J, Goff S P, Haseltine W A, Göttlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrlich L S, Agresta B E, Carter C A. Assembly of recombinant human immunodeficiency virus type 1 capsid protein in vitro. J Virol. 1992;66:4874–4883. doi: 10.1128/jvi.66.8.4874-4883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field J, Nikawa J-I, Broek D, MacDonald B, Rodgers L, Wilson I A, Lerner R A, Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher A G, Collalti E, Ratner L, Gallo R C, Wong-Staal F. A molecular clone of HTLV-III with biological activity. Nature. 1985;316:262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- 21.Franke E K, Yuan H E H, Bossolt K L, Goff S P, Luban J. Specificity and sequence requirements for interactions between various retroviral Gag proteins. J Virol. 1994;68:5300–5305. doi: 10.1128/jvi.68.8.5300-5305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke E K, Yuan H E H, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 23.Gamble T R, Yoo S, Vajdos F F, von Schwedler U K, Worthylake D K, Wang H, McCutcheon J P, Sundquist W I, Hill C P. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science. 1997;278:849–853. doi: 10.1126/science.278.5339.849. [DOI] [PubMed] [Google Scholar]
- 24.Ganser B K, Li S, Klishko V Y, Finch J T, Sundquist W I. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. doi: 10.1126/science.283.5398.80. [DOI] [PubMed] [Google Scholar]
- 25.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, DeWilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 26.Gitti R K, Lee B M, Walker J, Summers M F, Yoo S, Sundquist W I. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science. 1996;273:231–235. doi: 10.1126/science.273.5272.231. [DOI] [PubMed] [Google Scholar]
- 27.Gorelick R J, Nigida S M, Bess J R, Arthur L O, Henderson L E, Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990;64:3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Göttlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong S S, Boulanger P. Assembly-defective point mutations of the human immunodeficiency virus type 1 Gag precursor phenotypically expressed in recombinant baculovirus-infected cells. J Virol. 1993;67:2787–2798. doi: 10.1128/jvi.67.5.2787-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 31.Jowett J B M, Hockley D J, Nermut M V, Jones I M. Distinct signals in human immunodeficiency virus type 1 Pr55 necessary for RNA binding and particle formation. J Gen Virol. 1992;73:3079–3086. doi: 10.1099/0022-1317-73-12-3079. [DOI] [PubMed] [Google Scholar]
- 32.Klikova M, Rhee S S, Hunter E, Ruml T. Efficient in vivo and in vitro assembly of retroviral capsids from Gag precursor proteins expressed in bacteria. J Virol. 1995;69:1093–1098. doi: 10.1128/jvi.69.2.1093-1098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lama J, Trono D. Human immunodeficiency virus type 1 matrix protein interacts with cellular protein HO3. J Virol. 1998;72:1671–1676. doi: 10.1128/jvi.72.2.1671-1676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Yuan B, Goff S P. Genetic analysis of interactions between Gag proteins of Rous sarcoma virus. J Virol. 1997;71:5624–5630. doi: 10.1128/jvi.71.7.5624-5630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lingappa J R, Hill R L, Wong M L, Hegde R S. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J Cell Biol. 1997;136:567–581. doi: 10.1083/jcb.136.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lochrie M A, Waugh S, Pratt D G, Jr, Clever J, Parslow T G, Polisky B. In vitro selection of RNAs that bind to the human immunodeficiency virus type-1 gag polyprotein. Nucleic Acids Res. 1997;25:2902–2910. doi: 10.1093/nar/25.14.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luban J, Alin K B, Bossolt K L, Humaran T, Goff S P. Genetic assay for multimerization of retroviral gag polyproteins. J Virol. 1992;66:5157–5160. doi: 10.1128/jvi.66.8.5157-5160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luban J, Bossolt K A, Franke E K, Kalpana G V, Goff S P. Human immunodeficiency virus type 1 gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 39.Luban J, Goff S. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 gag polyprotein. J Virol. 1991;65:3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luban J, Lee C, Goff S P. Effect of linker insertion mutations in the human immunodeficiency virus type 1 gag gene on activation of viral protease expressed in bacteria. J Virol. 1993;67:3630–3634. doi: 10.1128/jvi.67.6.3630-3634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massiah M A, Starich M R, Paschall C, Summers M F, Christensen A M, Sundquist W I. Three-dimensional structure of the human immunodeficiency virus type 1 matrix protein. J Mol Biol. 1994;244:198–223. doi: 10.1006/jmbi.1994.1719. [DOI] [PubMed] [Google Scholar]
- 42.Matthews S, Barlow P, Boyd J, Barton G, Russell R, Mills H, Cunningham M, Meyers N, Burns N, Clark N, Kingsman S, Kingsman A, Campbell I. Structural similarity between the p17 matrix protein of HIV-1 and interferon-γ. Nature. 1994;370:666–668. doi: 10.1038/370666a0. [DOI] [PubMed] [Google Scholar]
- 43.McDermott J, Farrell L, Ross R, Barklis E. Structural analysis of human immunodeficiency virus type 1 Gag protein interactions, using cysteine-specific reagents. J Virol. 1996;70:5106–5114. doi: 10.1128/jvi.70.8.5106-5114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meric C, Goff S P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989;63:1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffatt B A, Studier F W. T7 lysozyme inhibits transcription by T7 RNA polymerase. Cell. 1987;49:221–227. doi: 10.1016/0092-8674(87)90563-0. [DOI] [PubMed] [Google Scholar]
- 46.Morikawa Y, Zhang W H, Hockley D J, Nermut M V, Jones I M. Detection of a trimeric human immunodeficiency virus type 1 Gag intermediate is dependent on sequences in the matrix protein, p17. J Virol. 1998;72:7659–7663. doi: 10.1128/jvi.72.9.7659-7663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niman H L, Houghten R A, Walker L E, Reisfeld R A, Wilson I A, Hogle J M, Lerner R A. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci USA. 1983;80:4949–4953. doi: 10.1073/pnas.80.16.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reicin A, Paik S, Berkowitz R D, Luban J, Lowy I, Goff S P. Linker insertion mutations in the human immunodeficiency virus type 1 gag gene: effects on virion particle assembly, release, and infectivity. J Virol. 1995;69:642–650. doi: 10.1128/jvi.69.2.642-650.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rein A, Ott D, Mirro J, Arthur L, Rice W, Henderson L. Inactivation of murine leukemia virus by compounds that react with the zinc finger in the viral nucleocapsid protein. J Virol. 1996;70:4966–4972. doi: 10.1128/jvi.70.8.4966-4972.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice W G, Supko J G, Malspeis L, Buckheit R W, Jr, Clanton D, Bu M, Graham L, Schaeffer C A, Turpin J A, Domagala J, et al. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science. 1995;270:1194–1197. doi: 10.1126/science.270.5239.1194. [DOI] [PubMed] [Google Scholar]
- 51.Sakalian M, Parker S D, Weldon R A, Jr, Hunter E. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J Virol. 1996;70:3706–3715. doi: 10.1128/jvi.70.6.3706-3715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber B K, Pavlakis G N. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992;66:7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 55.Spearman P, Ratner L. Human immunodeficiency virus type 1 capsid formation in reticulocyte lysates. J Virol. 1996;70:8187–8194. doi: 10.1128/jvi.70.11.8187-8194.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanchou V, Gabus C, Rogemond V, Darlix J-L. Formation of stable and functional HIV-1 nucleoprotein complexes in vitro. J Mol Biol. 1995;252:563–571. doi: 10.1006/jmbi.1995.0520. [DOI] [PubMed] [Google Scholar]
- 57.Trono D, Feinberg M, Baltimore D. HIV-1 gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989;59:113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- 58.von Poblotzki A, Wagner R, Niedrig M, Wanner G, Wolf H, Modrow S. Identification of a region in the Pr55gag-polyprotein essential for HIV-1 particle formation. Virology. 1993;193:981–985. doi: 10.1006/viro.1993.1210. [DOI] [PubMed] [Google Scholar]
- 59.Wang C-T, Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 gag mutants. J Virol. 1993;67:4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wills J W, Craven R C. Form, function, and use of retroviral Gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Yin L, Braaten D, Luban J. Human immunodeficiency virus type 1 replication is modulated by host cyclophilin A expression levels. J Virol. 1998;72:6430–6436. doi: 10.1128/jvi.72.8.6430-6436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu S F, Edelmann K, Strong R K, Moebes A, Rethwilm A, Linial M L. The carboxyl terminus of the human foamy virus Gag protein contains separable nucleic acid binding and nuclear transport domains. J Virol. 1996;70:8255–8262. doi: 10.1128/jvi.70.12.8255-8262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan X, Xiaofang Y, Lee T-H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Barklis E. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J Virol. 1997;71:6765–6776. doi: 10.1128/jvi.71.9.6765-6776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of the human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]