Abstract
Aims
Fractional excretion of urea nitrogen (FEUN), used to differentiate the cause of acute kidney injury, has emerged as a useful fluid index in patients with heart failure (HF). We hypothesized that FEUN could be useful in identifying worsening renal function (WRF) associated with poor outcomes in patients with acute HF (AHF).
Methods and results
Overall, 1103 patients with AHF (median age, 78 years; male proportion, 60%) were categorized into six groups according to the presence of WRF and FEUN values (low, ≤32.1%; medium, >32.1% and ≤38.0%; and high, >38.0%) at discharge. WRF was defined as an increase of ≥0.3 mg/dL in the serum creatinine level from admission to discharge. FEUN was calculated by the following formula: (urinary urea × serum creatinine) × 100/(serum urea × urinary creatinine). The cut‐off values for low, medium, and high FEUN were based on a previous study. The primary outcome of this study was HF readmission after hospital discharge. During the 1 year follow‐up, 170 HF readmissions occurred. Kaplan–Meier analysis revealed significantly higher HF readmission rates in patients with WRF than in those without WRF (log‐rank test, P < 0.001). Additionally, among patients with WRF, HF readmission rates were lowest in those with medium FEUN values, followed by those with low FEUN values and those with high FEUN values. On multivariable analysis, the presence of WRF with low or high FEUN values was independently associated with increased HF readmission, as compared with the absence of WRF with medium FEUN values. Notably, no association was noted between WRF with medium FEUN values and HF readmission.
Conclusions
The prognostic impact of WRF was significantly mediated by the FEUN values and was associated with worse outcomes only when the FEUN values were either low or high. Our study suggests that FEUN can identify prognostically relevant WRF in patients with AHF.
Keywords: Acute decompensated heart failure, Cardiorenal syndrome, Renal dysfunction, Biomarker
Introduction
During aggressive decongestion in acute heart failure (AHF), an increase in the serum creatinine level, known as ‘worsening renal function’ (WRF), often occurs. 1 Although the pathogenesis of WRF is not fully understood, renal hypoperfusion and venous congestion appear to be key factors. 2 , 3 WRF has traditionally been associated with a worse prognosis 4 ; however, recent studies show that its prognostic impact may vary depending on the clinical scenario. 5 , 6 Interestingly, WRF does not necessarily contribute to a poor outcome when accompanied by haemoconcentration or reduced brain natriuretic peptide (BNP) levels, which are factors suggestive of adequate decongestion. 5 , 7 , 8 Conversely, WRF has been associated with adverse outcomes in the presence of residual congestion. 5 , 6 , 9 Thus, a novel concept has been proposed, distinguishing between ‘true WRF’, which is associated with a worse prognosis, and ‘pseudo WRF’, which is not. 10 Because WRF may lead to inappropriate treatment modifications, such as reducing or discontinuing the use of diuretics or renin–angiotensin system inhibitors, the identification of ‘true WRF’ is crucial for therapeutic decision making. Thus, clinicians have attempted to distinguish ‘true’ from ‘pseudo’ WRF by evaluating fluid status and congestion based on biomarkers and echocardiography. 5 , 6 , 7 , 8 , 11
Fractional excretion of urea nitrogen (FEUN) is a useful index of volume status in patients with acute kidney injury (AKI) receiving diuretic therapy. 12 FEUN reflects renal blood flow and helps differentiate the causes of renal dysfunction, with low values indicating pre‐renal failure and high values indicating other causes. Recently, FEUN has emerged as a surrogate marker of volume status in patients with heart failure (HF). 13 , 14 In a previous study, we investigated the clinical significance of FEUN in patients with AHF and renal dysfunction. 14 We observed a significant positive correlation between the FEUN values and right atrial pressure. Furthermore, patients with low (FEUN ≤ 32.1%) or high (FEUN > 38.0%) values at discharge had significantly higher rates of HF readmissions than those with medium (32.1% < FEUN ≤ 38.0%) values. These findings suggest that low FEUN values may represent renal hypoperfusion, whereas high FEUN values may reflect intravascular congestion, both of which lead to poor outcomes. Thus, FEUN may be a useful marker to determine volume status in patients with HF and renal dysfunction.
Given that the pathophysiology of WRF involves renal hypoperfusion and intravascular congestion, FEUN may assist in differentiating the underlying causes of WRF. Additionally, FEUN reflects fluid status, which may be useful in identifying true and pseudo WRFs. This study aimed to investigate the prognostic relevance of WRF and FEUN values at discharge in patients with AHF.
Methods
Study design
This was a single‐centre observational study based on the inpatient database of the Nippon Medical School Hospital. This database includes the following data: patient age and sex, height and body weight, main diagnoses and comorbidities, therapeutic procedures, medications, discharge status, and post‐discharge outcomes. The definition of main diagnoses and comorbidities was based on the International Classification of Diseases 10th Revision (ICD‐10) codes (Supporting Information, Table S1 ). Patients with a treatment history of percutaneous coronary intervention or coronary artery bypass graft surgery at our hospital were included in the definition of ischaemic heart disease, regardless of the applicable ICD‐10 codes. Clinical laboratory data and echocardiographic parameters were collected electronically from medical records. For loop diuretics other than furosemide, we converted to the equivalent dose of furosemide with reference to previous studies; 30 mg of azosemide and 4 mg of torasemide were considered equivalent to 20 mg of furosemide. 15 , 16
This study was approved by the Institutional Review Board of Nippon Medical School (Reference No. B‐2021‐433) and conducted in accordance with the revised Declaration of Helsinki. The requirement for obtaining written informed consent was waived because of the observational nature of the study, and an opt‐out method was used for participant recruitment.
Patient selection and evaluation
From the database, patients with HF as the main diagnosis, who were admitted emergently to the Department of Cardiovascular Medicine/Cardiovascular Intensive Care Unit at our hospital between April 2014 and December 2022, were selected. The exclusion criteria were as follows: in‐hospital death, haemodialysis during hospitalization, and a lack of FEUN data within a week before discharge. Patients were divided into a no‐WRF (nWRF) and a WRF group according to the occurrence of WRF at discharge. WRF was defined as an increase of ≥0.3 mg/dL in the serum creatinine level from admission to discharge. 1 In addition, they were classified into six groups according to the occurrence of WRF and FEUN values at discharge: nWRF with low FEUN (nWRF/lFEUN), nWRF with medium FEUN (nWRF/mFEUN), nWRF with high FEUN (nWRF/hFEUN), WRF with low FEUN (WRF/lFEUN), WRF with medium FEUN (WRF/mFEUN), and WRF with high FEUN (WRF/hFEUN). FEUN was calculated by the following formula: FEUN = (urinary urea × serum creatinine) × 100/(serum urea × urinary creatinine). 12 , 17 The cut‐off values for low (≤32.1%), medium (>32.1% and ≤38.0%), and high (>38.0%) FEUN were based on our previous study. 14 Patient characteristics and clinical outcomes were compared between the groups.
Outcomes
The main outcome of this study was post‐discharge HF readmission, which was defined as readmission with the main diagnosis of HF.
Statistical analyses
Categorical variables are presented as numbers and percentages and were compared using the χ 2 exact test. Continuous variables are presented as medians with inter‐quartile ranges (IQRs) and were tested using the Kruskal–Wallis test.
For survival analysis, the Kaplan–Meier method was used to estimate the cumulative incidences of outcomes, and differences were compared using the log‐rank test between the groups. We performed Cox regression analysis to determine the prognostic relevance of WRF and FEUN values. Variables for multivariable analysis included age, male sex, atrial fibrillation, ischaemic heart disease, haemoglobin level, albumin level, blood urea nitrogen (BUN) level, estimated glomerular filtration rate (eGFR), log N‐terminal pro‐BNP (NT‐proBNP) level, beta‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), aldosterone antagonists, and oral loop diuretics. Moreover, we assessed the relationship between FEUN and HF readmission using a restricted cubic spline model with five knots, adjusting for the same variables in multivariable analysis. As a sensitivity analysis, we conducted Cox regression analysis using different definitions of WRF: an increase of ≥0.3 mg/dL and >25% in the serum creatinine level from admission to discharge and a decrease of ≥20% in the eGFR from admission to discharge. 1
Two‐sided P values of <0.05 were considered statistically significant. Statistical analyses were performed using SPSS Version 28 (IBM Corp., Armonk, NY, USA) and R software Version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
The patient flowchart is shown in Figure 1 . A total of 2118 patients with HF were included, and after applying the exclusion criteria, 1103 were analysed. The eligible patients were divided into two groups according to the presence/absence of WRF at discharge: nWRF (n = 964) and WRF (n = 139). In addition, they were classified into six groups according to WRF and FEUN values at discharge: nWRF/lFEUN (n = 277), nWRF/mFEUN (n = 243), nWRF/hFEUN (n = 444), WRF/lFEUN (n = 47), WRF/mFEUN (n = 34), and WRF/hFEUN (n = 58). In the study population, the median age was 78 (IQR, 68–85) years, and 60% were male individuals. On admission, the median serum creatinine and eGFR levels were 1.11 mg/dL (IQR, 0.82–1.49) and 47 mL/min/1.73 m2 (IQR, 31–62), respectively. At discharge, the median FEUN value was 36.8% (IQR, 30.6–42.7). The median interval between the discharge date and time point of the FEUN measurement was 2 (IQR, 1–4) days.
Figure 1.
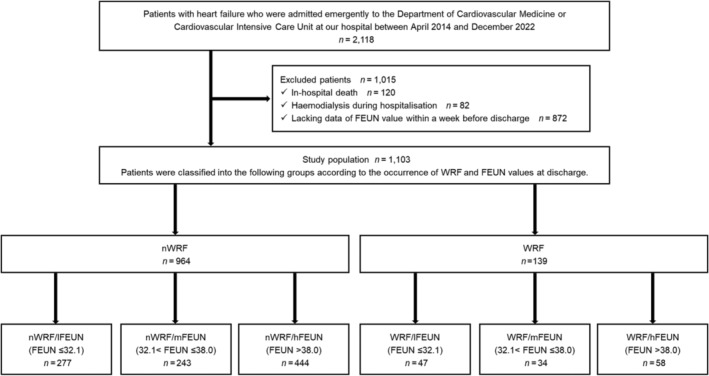
Patient flow chart. A total of 2118 patients with heart failure were included, and after applying the exclusion criteria, 1103 were analysed. Eligible patients were classified into six groups according to the occurrence of worsening renal function (WRF) and fractional excretion of urea nitrogen (FEUN) values at discharge. hFEUN, high FEUN; lFEUN, low FEUN; mFEUN, medium FEUN; nWRF, no WRF; WRF, worsening renal function.
Clinical characteristics
Table 1 shows the comparison of clinical characteristics between the two groups, stratified by the occurrence of WRF at discharge. The WRF group had a higher prevalence of diabetes mellitus and a lower prevalence of atrial fibrillation. Laboratory data on admission showed that the WRF group had lower levels of haemoglobin and albumin, lower BUN/creatinine ratio, poorer renal function, and higher NT‐proBNP levels. Laboratory data at discharge showed that the WRF group had lower haemoglobin and serum sodium levels, as well as poorer renal function. The dosage of intravenous loop diuretics during hospitalization was higher in the WRF group. At discharge, the WRF group had a higher use rate of loop diuretics and tolvaptan.
Table 1.
Patient characteristics stratified by WRF at discharge
| Variables | All patients (n = 1103) | nWRF (n = 964) | WRF (n = 139) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 78 (68–85) | 78 (68–85) | 80 (72–86) | 0.151 |
| Male sex | 658 (60%) | 573 (59%) | 85 (61%) | 0.701 |
| Body mass index (kg/m2) | 23.1 (20.6–26.0) | 23.1 (20.6–25.9) | 23.1 (20.4–26.2) | 0.602 |
| Systolic blood pressure (mmHg) | 131 (115–150) | 130 (114–148) | 136 (122–159) | 0.003 |
| Diastolic blood pressure (mmHg) | 77 (64–90) | 77 (64–90) | 78 (66–91) | 0.913 |
| Heart rate (b.p.m.) | 89 (73–108) | 89 (73–108) | 86 (74–106) | 0.404 |
| Comorbidities | ||||
| Hypertension | 826 (75%) | 714 (74%) | 112 (81%) | 0.098 |
| Dyslipidaemia | 324 (29%) | 280 (29%) | 44 (32%) | 0.528 |
| Diabetes mellitus | 381 (35%) | 320 (33%) | 61 (44%) | 0.013 |
| Atrial fibrillation | 500 (45%) | 457 (47%) | 43 (31%) | <0.001 |
| Ischaemic heart disease | 312 (28%) | 272 (28%) | 40 (29%) | 0.891 |
| Echocardiography | ||||
| LVEF (%) | 45 (29–62) | 45 (29–62) | 45 (33–60) | 0.848 |
| LVEF ≤ 40% | 491 (45%) | 437 (45%) | 54 (39%) | 0.150 |
| Body weight | ||||
| At admission (kg) | 58.3 (49.8–69.3) | 58.3 (49.7–69.6) | 58.4 (50.7–67.9) | 0.962 |
| At discharge (kg) | 53.2 (45.2–63.4) | 53.1 (45.3–63.6) | 54.3 (43.9–62.0) | 0.934 |
| Reduction during hospitalization (kg) | 4.4 (2.1–7.4) | 4.2 (2.0–7.4) | 5.1 (2.8–7.2) | 0.084 |
| Reduction rate (%) | 7.4 (3.7–12.0) | 7.2 (3.6–11.9) | 8.3 (5.1–12.5) | 0.067 |
| Laboratory data at admission | ||||
| Haemoglobin (g/dL) | 12.0 (10.3–13.9) | 12.1 (10.4–14.0) | 11.3 (9.9–12.8) | <0.001 |
| Albumin (g/dL) | 3.6 (3.2–3.9) | 3.6 (3.2–3.9) | 3.5 (3.1–3.9) | 0.047 |
| BUN (mg/dL) | 22.3 (16.8–31.8) | 22.3 (16.9–31.7) | 22.3 (15.7–32.6) | 0.800 |
| Creatinine (mg/dL) | 1.11 (0.82–1.49) | 1.10 (0.82–1.45) | 1.18 (0.86–1.80) | 0.022 |
| BUN/creatinine ratio | 20.4 (16.2–26.0) | 20.7 (16.4–26.5) | 18.8 (14.9–22.7) | <0.001 |
| eGFR (mL/min/1.73 m2) | 47 (31–62) | 48 (32–62) | 41 (28–61) | 0.011 |
| Serum sodium (mEq/L) | 140 (137–142) | 140 (137–142) | 140 (138–142) | 0.426 |
| NT‐proBNP (pg/mL) | 4683 (2318–9751) | 4546 (2291–9318) | 5632 (2563–11 707) | 0.045 |
| Laboratory data at discharge | ||||
| Haemoglobin (g/dL) | 11.7 (10.2–13.6) | 11.9 (10.4–13.8) | 11.1 (9.8–12.5) | <0.001 |
| Albumin (g/dL) | 3.4 (3.1–3.8) | 3.4 (3.1–3.8) | 3.4 (3.0–3.8) | 0.197 |
| BUN (mg/dL) | 21.4 (16.1–29.7) | 20.1 (15.7–27.9) | 31.6 (24.6–44.3) | <0.001 |
| Creatinine (mg/dL) | 1.08 (0.84–1.44) | 1.02 (0.81–1.33) | 1.69 (1.28–2.30) | <0.001 |
| BUN/creatinine ratio | 19.8 (15.6–25.1) | 19.9 (15.7–25.1) | 19.3 (14.5–24.6) | 0.095 |
| eGFR (mL/min/1.73 m2) | 46 (32–61) | 49 (36–64) | 29 (21–38) | <0.001 |
| Serum sodium (mEq/L) | 140 (137–142) | 140 (137–142) | 139 (137–141) | 0.044 |
| NT‐proBNP (pg/mL) | 2074 (907–4321) | 1994 (907–4214) | 2683 (922–5409) | 0.052 |
| In‐hospital treatment | ||||
| Intravenous nitrate | 392 (36%) | 323 (34%) | 69 (50%) | <0.001 |
| Intravenous loop diuretic | 883 (80%) | 761 (79%) | 122 (88%) | 0.015 |
| Intravenous loop diuretic dosage (mg) | 140 (30–280) | 140 (20–280) | 180 (80–410) | <0.001 |
| Medications at discharge | ||||
| Beta‐blocker | 832 (75%) | 730 (76%) | 102 (73%) | 0.548 |
| ACE inhibitor/ARB | 772 (70%) | 673 (70%) | 99 (71%) | 0.735 |
| ARNi | 57 (5.2%) | 51 (5.3%) | 6 (4.3%) | 0.628 |
| Aldosterone antagonist | 595 (54%) | 526 (55%) | 69 (50%) | 0.276 |
| SGLT2 inhibitor | 114 (10%) | 100 (10%) | 14 (10%) | 0.913 |
| Oral loop diuretic | 810 (73%) | 695 (72%) | 115 (83%) | 0.008 |
| Oral loop diuretic dose (mg) |
20 (0–30) 20 ± 19 |
20 (0–30) 20 ± 19 |
20 (10–30) 23 ± 20 |
0.029 |
| Tolvaptan | 250 (23%) | 206 (21%) | 44 (32%) | 0.007 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor/neprilysin inhibitor; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; nWRF, no worsening renal function; SGLT2, sodium–glucose cotransporter 2; WRF, worsening renal function.
Continuous data are presented as median values (inter‐quartile ranges). Categorical data are presented as n (%). For oral loop diuretic doses, the means and standard deviations are also listed.
Table 2 shows the clinical characteristics of the six groups, stratified by WRF presence and FEUN values at discharge. Among patients without WRF, the nWRF/hFEUN group had the highest median age, and the nWRF/lFEUN group showed the most significant loss of body weight during hospitalization. Regarding laboratory data, the nWRF/lFEUN group had the highest BUN/creatinine ratio, whereas the nWRF/hFEUN group had the poorest renal function. At discharge, the nWRF/lFEUN group had the highest usage rates of ACE inhibitors/ARBs, aldosterone antagonists, and oral loop diuretics. For patients with WRF, no significant differences were observed in age and sex. Laboratory data on admission revealed that the WRF/hFEUN group had the highest BUN levels and poorest renal function. At discharge, the WRF/lFEUN group had the highest BUN/creatinine ratio.
Table 2.
Patient characteristics stratified by WRF and the FEUN values at discharge
| Variables | nWRF | WRF | ||||||
|---|---|---|---|---|---|---|---|---|
| nWRF/lFEUN (n = 277) | nWRF/mFEUN (n = 243) | nWRF/hFEUN (n = 444) | P value a | WRF/lFEUN (n = 47) | WRF/mFEUN (n = 34) | WRF/hFEUN (n = 58) | P value b | |
| Demographics | ||||||||
| Age (years) | 77 (65–84) | 77 (67–85) | 80 (69–87) | 0.005 | 77 (71–84) | 80 (72–84) | 82 (73–88) | 0.670 |
| Male sex | 159 (57%) | 143 (59%) | 271 (61%) | 0.612 | 29 (62%) | 23 (68%) | 33 (57%) | 0.591 |
| Body mass index (kg/m2) | 23.4 (20.5–26.7) | 23.2 (21.1–25.5) | 22.9 (20.3–25.5) | 0.135 | 22.8 (20.4–26.1) | 24.5 (20.2–26.8) | 23.1 (20.6–25.1) | 0.491 |
| Systolic blood pressure (mmHg) | 129 (114–148) | 130 (117–147) | 132 (115–150) | 0.441 | 129 (119–141) | 142 (120–162) | 141 (124–160) | 0.073 |
| Diastolic blood pressure (mmHg) | 77 (65–90) | 76 (64–90) | 77 (64–90) | 0.973 | 75 (66–87) | 79 (64–99) | 78 (66–93) | 0.832 |
| Heart rate (b.p.m.) | 92 (78–108) | 88 (72–105) | 88 (71–108) | 0.165 | 87 (76–108) | 88 (75–105) | 83 (70–103) | 0.351 |
| Comorbidities | ||||||||
| Hypertension | 207 (75%) | 172 (71%) | 335 (76%) | 0.392 | 38 (81%) | 30 (88%) | 44 (76%) | 0.350 |
| Dyslipidaemia | 82 (30%) | 62 (26%) | 136 (31%) | 0.358 | 12 (26%) | 15 (44%) | 17 (29%) | 0.182 |
| Diabetes mellitus | 83 (30%) | 88 (36%) | 149 (34%) | 0.312 | 25 (53%) | 14 (41%) | 22 (38%) | 0.274 |
| Atrial fibrillation | 137 (50%) | 105 (43%) | 215 (48%) | 0.306 | 17 (36%) | 9 (27%) | 17 (29%) | 0.609 |
| Ischaemic heart disease | 82 (30%) | 69 (28%) | 121 (27%) | 0.790 | 14 (30%) | 12 (35%) | 14 (24%) | 0.512 |
| Echocardiography | ||||||||
| LVEF (%) | 47 (29–63) | 43 (30–60) | 45 (29–62) | 0.956 | 42 (29–58) | 45 (35–60) | 49 (37–60) | 0.225 |
| LVEF ≤ 40% | 123 (44%) | 113 (47%) | 201 (45%) | 0.891 | 23 (49%) | 14 (41%) | 17 (29%) | 0.116 |
| Body weight | ||||||||
| At admission (kg) | 58.3 (48.8–73.3) | 58.3 (50.6–68.4) | 58.3 (49.6–68.4) | 0.600 | 56.4 (51.4–67.8) | 59.6 (51.0–71.1) | 57.9 (51.2–63.4) | 0.454 |
| At discharge (kg) | 52.6 (44.5–66.3) | 54.5 (45.8–63.3) | 53.0 (45.5–62.6) | 0.821 | 53.8 (43.9–62.0) | 56.4 (46.2–64.7) | 54.1 (43.1–58.6) | 0.320 |
| Reduction during hospitalization (kg) | 5.0 (2.4–8.3) | 4.2 (2.3–6.7) | 3.8 (1.7–6.9) | 0.006 | 5.1 (3.4–8.1) | 5.9 (3.2–6.6) | 4.7 (1.9–7.2) | 0.769 |
| Reduction rate (%) | 7.9 (4.1–13.5) | 7.3 (4.0–11.7) | 6.5 (3.2–11.0) | 0.014 | 8.8 (5.3–12.7) | 8.8 (6.0–12.1) | 7.8 (4.2–12.3) | 0.708 |
| Laboratory data at admission | ||||||||
| Haemoglobin (g/dL) | 12.2 (10.3–14.2) | 12.2 (10.7–14.0) | 12.1 (10.4–13.7) | 0.213 | 11.4 (9.9–13.4) | 11.5 (9.7–13.3) | 11.1 (10.0–12.4) | 0.682 |
| Albumin (g/dL) | 3.6 (3.2–3.9) | 3.6 (3.2–3.9) | 3.6 (3.2–3.9) | 0.992 | 3.5 (2.8–3.9) | 3.6 (3.2–3.9) | 3.4 (3.1–3.7) | 0.461 |
| BUN (mg/dL) | 22.7 (16.9–32.0) | 21.1 (16.1–30.1) | 22.5 (17.7–31.8) | 0.177 | 18.2 (13.1–27.3) | 21.9 (17.1–31.7) | 25.3 (17.8–40.2) | 0.026 |
| Creatinine (mg/dL) | 0.98 (0.76–1.31) | 1.07 (0.81–1.41) | 1.16 (0.87–1.54) | <0.001 | 1.11 (0.80–1.39) | 1.13 (0.80–1.79) | 1.36 (0.95–2.30) | 0.029 |
| BUN/creatinine ratio | 22.6 (18.0–28.4) | 20.6 (16.3–26.4) | 19.5 (15.8–25.2) | <0.001 | 18.8 (15.5–23.0) | 19.6 (15.1–23.1) | 17.9 (14.3–22.0) | 0.666 |
| eGFR (mL/min/1.73 m2) | 54 (34–68) | 48 (34–63) | 44 (31–58) | <0.001 | 45 (34–64) | 42 (29–65) | 34 (18–47) | 0.006 |
| Serum sodium (mEq/L) | 140 (137–142) | 140 (138–143) | 140 (137–143) | 0.384 | 140 (138–142) | 140 (138–142) | 139 (137–142) | 0.546 |
| NT‐proBNP (pg/mL) |
4219 (1971–9043) |
4688 (2469–8857) |
4686 (2493–9990) |
0.211 |
4038 (2235–10 749) |
4818 (3067–14 543) |
6232 (2675–11 989) |
0.579 |
| Laboratory data at discharge | ||||||||
| Haemoglobin (g/dL) | 12.1 (10.2–14.1) | 12.1 (10.8–14.0) | 11.7 (10.3–13.5) | 0.093 | 11.0 (9.7–12.7) | 11.5 (10.0–13.5) | 10.8 (9.8–11.6) | 0.313 |
| Albumin (g/dL) | 3.5 (3.1–3.7) | 3.5 (3.1–3.8) | 3.4 (3.1–3.8) | 0.704 | 3.7 (3.0–3.9) | 3.4 (3.1–3.8) | 3.3 (2.8–3.5) | 0.033 |
| BUN (mg/dL) | 22.3 (16.3–29.8) | 19.2 (15.7–28.0) | 19.4 (15.5–25.9) | 0.008 | 31.2 (25.1–41.9) | 32.2 (24.5–51.4) | 29.9 (23.0–45.5) | 0.883 |
| Creatinine (mg/dL) | 0.94 (0.76–1.26) | 0.99 (0.78–1.29) | 1.08 (0.86–1.38) | <0.001 | 1.54 (1.23–1.94) | 1.67 (1.20–2.33) | 1.88 (1.35–2.79) | 0.032 |
| BUN/creatinine ratio | 22.7 (17.8–28.3) | 20.1 (16.1–25.9) | 18.4 (14.7–22.7) | <0.001 | 21.8 (17.7–25.3) | 20.3 (16.3–24.6) | 16.6 (11.5–20.8) | <0.001 |
| eGFR (mL/min/1.73 m2) | 55 (38–70) | 49 (38–65) | 47 (34–58) | <0.001 | 31 (27–41) | 29 (21–37) | 24 (15–33) | 0.005 |
| Serum sodium (mEq/L) | 139 (137–141) | 140 (138–141) | 140 (137–142) | 0.121 | 138 (136–141) | 140 (137–142) | 139 (137–141) | 0.374 |
| NT‐proBNP (pg/mL) |
1982 (848–4199) |
1715 (803–3720) |
2120 (1056–4282) |
0.109 |
2231 (867–5366) |
2891 (549–5078) |
3106 (1174–5721) |
0.606 |
| In‐hospital treatment | ||||||||
| Intravenous nitrate | 99 (36%) | 94 (39%) | 130 (29%) | 0.029 | 20 (43%) | 19 (56%) | 30 (52%) | 0.455 |
| Intravenous loop diuretic | 227 (82%) | 187 (77%) | 347 (78%) | 0.325 | 39 (83%) | 33 (97%) | 50 (86%) | 0.144 |
| Intravenous loop diuretic dosage (mg) | 140 (40–280) | 120 (20–290) | 120 (20–260) | 0.426 | 160 (70–510) | 180 (100–280) | 180 (60–440) | 0.997 |
| Medications at discharge | ||||||||
| Beta‐blocker | 200 (72%) | 182 (75%) | 348 (78%) | 0.160 | 34 (72%) | 29 (85%) | 39 (67%) | 0.164 |
| ACE inhibitor/ARB | 210 (76%) | 170 (70%) | 293 (66%) | 0.020 | 35 (75%) | 27 (79%) | 37 (64%) | 0.233 |
| ARNi | 15 (5.4%) | 12 (4.9%) | 24 (5.4%) | 0.961 | 1 (2.1%) | 2 (5.9%) | 3 (5.2%) | 0.654 |
| Aldosterone antagonist | 178 (64%) | 139 (57%) | 209 (47%) | <0.001 | 24 (51%) | 19 (56%) | 26 (45%) | 0.575 |
| SGLT2 inhibitor | 32 (12%) | 19 (7.8%) | 49 (11%) | 0.312 | 5 (11%) | 5 (15%) | 4 (6.9%) | 0.480 |
| Oral loop diuretic | 218 (79%) | 166 (68%) | 311 (70%) | 0.013 | 37 (79%) | 31 (91%) | 47 (81%) | 0.310 |
| Oral loop diuretic dose (mg) | 20 (10–40) | 20 (0–20) | 20 (0–30) | 0.006 | 20 (10–30) | 20 (20–40) | 20 (10–40) | 0.587 |
| Oral loop diuretic dose (mg) | 23 ± 21 | 17 ± 16 | 19 ± 19 | 0.006 | 24 ± 24 | 26 ± 21 | 21 ± 15 | 0.587 |
| Tolvaptan | 60 (22%) | 41 (17%) | 105 (24%) | 0.116 | 13 (28%) | 11 (32%) | 20 (35%) | 0.752 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor/neprilysin inhibitor; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; FEUN, fractional excretion of urea nitrogen; hFEUN, high fractional excretion of urea nitrogen; lFEUN, low fractional excretion of urea nitrogen; LVEF, left ventricular ejection fraction; mFEUN, medium fractional excretion of urea nitrogen; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; nWRF, no worsening renal function; SGLT2, sodium–glucose cotransporter 2; WRF, worsening renal function.
Continuous data are presented as median values (inter‐quartile ranges). Categorical data are presented as n (%). For oral loop diuretic doses, the means and standard deviations are also listed.
P values calculated from statistical tests among the three nWRF groups are shown.
P values calculated from statistical tests among the three WRF groups are shown.
Post‐discharge HF readmission
The median follow‐up was 301 days. During the 1 year follow‐up, 170 HF readmissions occurred. The Kaplan–Meier analysis showed that the HF readmission rate was significantly higher in the WRF group than in the nWRF group (log‐rank test, P < 0.001) (Figure 2 ). In the six groups stratified by the presence of WRF and FEUN values at discharge, HF readmission rates differed significantly, with the lowest rates observed in the following order: nWRF/mFEUN, nWRF/lFEUN, nWRF/hFEUN, WRF/mFEUN, WRF/lFEUN, and WRF/hFEUN (log‐rank test, P < 0.001) (Figure 3 ).
Figure 2.
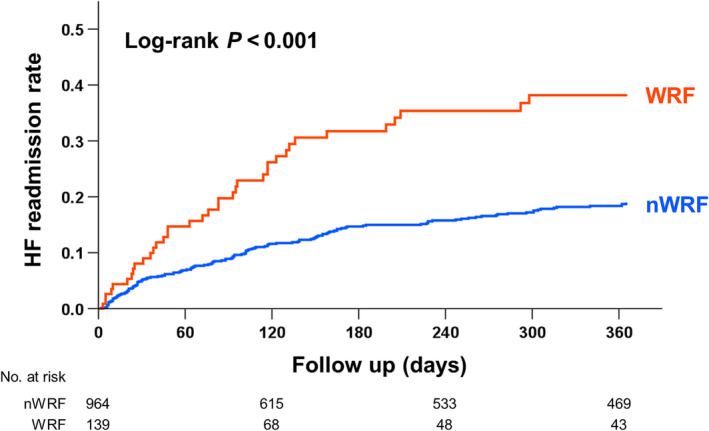
Kaplan–Meier curves in the two groups stratified by the occurrence of worsening renal function (WRF) at discharge. The heart failure (HF) readmission rate was significantly higher in the WRF group than in the no‐WRF (nWRF) group (log‐rank test, P < 0.001).
Figure 3.
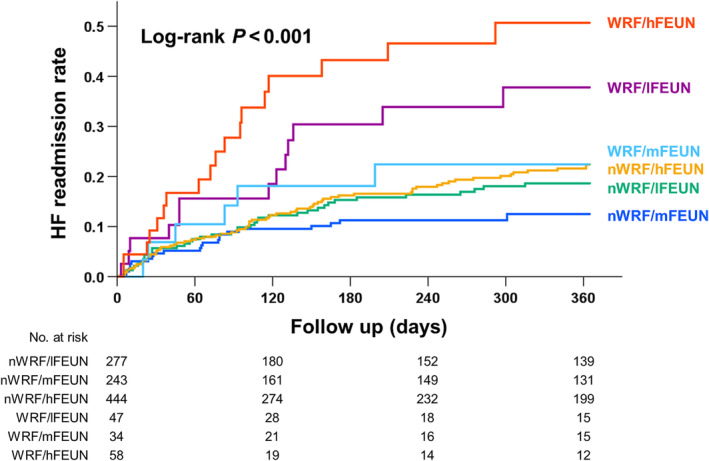
Kaplan–Meier curves in the six groups stratified by the occurrence of worsening renal function (WRF) and fractional excretion of urea nitrogen (FEUN) values at discharge. The heart failure (HF) readmission rates differed significantly, with the lowest rates observed in the following order: no WRF (nWRF)/medium FEUN (mFEUN), nWRF/low FEUN (lFEUN), nWRF/high FEUN (hFEUN), WRF/mFEUN, WRF/lFEUN, and WRF/hFEUN (log‐rank test, P < 0.001).
Table 3 shows the results of the Cox regression analysis for HF readmission. In the univariable analysis, the nWRF/hFEUN, WRF/lFEUN, and WRF/hFEUN groups were associated with increased HF readmission, compared with the nWRF/mFEUN group. The multivariable Cox regression analysis revealed the WRF/lFEUN and WRF/hFEUN groups as independent factors associated with increased HF readmission, after adjusting for confounders.
Table 3.
Cox regression analysis for heart failure readmission
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| nWRF/lFEUN | 1.506 | 0.900–2.522 | 0.119 | 1.333 | 0.788–2.255 | 0.284 |
| nWRF/mFEUN | 1.000 (ref) | 1.000 (ref) | ||||
| nWRF/hFEUN | 1.780 | 1.112–2.850 | 0.016 | 1.449 | 0.900–2.333 | 0.127 |
| WRF/lFEUN | 3.420 | 1.732–6.754 | <0.001 | 2.795 | 1.378–5.671 | 0.004 |
| WRF/mFEUN | 1.939 | 0.790–4.763 | 0.149 | 1.158 | 0.452–2.965 | 0.760 |
| WRF/hFEUN | 5.070 | 2.731–9.411 | <0.001 | 2.788 | 1.446–5.372 | 0.002 |
| Other factors | ||||||
| Age (years) | 1.040 | 1.025–1.054 | <0.001 | 1.018 | 1.001–1.035 | 0.037 |
| Male sex | 0.993 | 0.729–1.353 | 0.965 | 1.343 | 0.954–1.890 | 0.091 |
| Atrial fibrillation | 1.317 | 0.974–1.780 | 0.073 | 1.709 | 1.233–2.368 | 0.001 |
| Ischaemic heart disease | 1.515 | 1.106–2.075 | 0.010 | 1.639 | 1.165–2.307 | 0.005 |
| Haemoglobin (g/dL) | 0.745 | 0.691–0.804 | <0.001 | 0.822 | 0.748–0.903 | <0.001 |
| Albumin (g/dL) | 0.485 | 0.358–0.658 | <0.001 | 0.954 | 0.657–1.385 | 0.805 |
| BUN (mg/dL) | 1.030 | 1.022–1.039 | <0.001 | 1.011 | 0.997–1.025 | 0.131 |
| eGFR (mL/min/1.73 m2) | 0.975 | 0.967–0.983 | <0.001 | 0.999 | 0.989–1.010 | 0.908 |
| Log NT‐proBNP | 2.399 | 1.789–3.217 | <0.001 | 1.653 | 1.180–2.317 | 0.003 |
| Beta‐blocker | 0.809 | 0.567–1.153 | 0.240 | 0.903 | 0.623–1.308 | 0.588 |
| ACE inhibitor/ARB | 0.691 | 0.502–0.952 | 0.024 | 0.833 | 0.592–1.173 | 0.296 |
| Aldosterone antagonist | 0.588 | 0.435–0.796 | <0.001 | 0.821 | 0.595–1.133 | 0.231 |
| Oral loop diuretic | 1.039 | 0.727–1.485 | 0.834 | 0.753 | 0.518–1.095 | 0.138 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; CI, confidence interval; eGFR, estimated glomerular filtration rate; hFEUN, high fractional excretion of urea nitrogen; HR, hazard ratio; lFEUN, low fractional excretion of urea nitrogen; mFEUN, medium fractional excretion of urea nitrogen; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; nWRF, no worsening renal function; WRF, worsening renal function.
The restricted cubic spline curve demonstrates the relationship between FEUN values and adjusted hazard ratios (HRs) for HF readmission rates (Figure 4 ). The lowest adjusted HR was observed at an FEUN value of 36.1%, which was within the medium range in this study, whereas FEUN values below or above this threshold were associated with higher HR.
Figure 4.
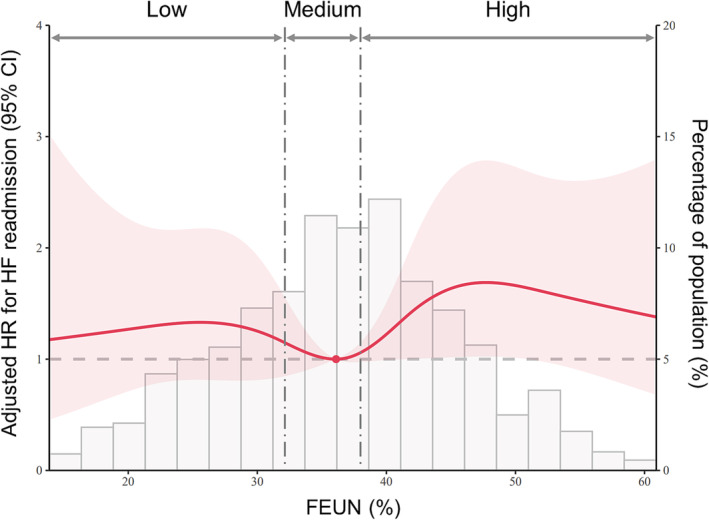
The relationship between fractional excretion of urea nitrogen (FEUN) values and adjusted hazard ratio (HR) for heart failure (HF) readmission rates. The lowest adjusted HR was observed at an FEUN value of 36.1%, which was within the medium range in this study, whereas values below or above this threshold were associated with higher HR. CI, confidence interval.
Sensitivity analysis
Supporting Information, Tables S2 and S3 show the results of the sensitivity analysis based on various WRF definitions. Consistent with our main findings, multivariable analysis demonstrated that the WRF/lFEUN and WRF/hFEUN groups were independently associated with HF readmission, whereas the WRF/mFEUN group was not.
Discussion
To the best of our knowledge, this is the first study to elucidate the prognostic relevance of WRF and FEUN values at discharge in patients with AHF. In this study, WRF, with both low and high FEUN values, was an independent factor associated with increased HF readmission, whereas WRF with medium FEUN values was not. Therefore, the prognostic impact of WRF was significantly mediated by the FEUN values. These findings suggest that FEUN can assist in identifying prognostically relevant WRF in patients with AHF.
Clinical significance of fractional excretion of urea nitrogen
FEUN is a useful index of volume status in patients with AKI. 12 Pre‐renal failure, such as intravascular dehydration, leads to the release of vasopressin, which promotes BUN reabsorption through urea‐transporter proteins in the intramedullary collecting ducts, resulting in a decrease in the FEUN value. 18 , 19 , 20 An FEUN value of <35% indicates pre‐renal failure with a high sensitivity (85%) and specificity (92%). Unlike the commonly used indicator, fractional excretion of sodium (FENa), which is significantly affected by diuretics and compromises its diagnostic accuracy, FEUN is less influenced by diuretic use, making it applicable even in the presence of diuretic therapy. 21 Thus, in patients undergoing diuretic therapy, FEUN has been established as a useful tool for the differential diagnosis of AKI and has played an important role as a fluid index.
Recently, FEUN has emerged as a surrogate marker of volume status in patients with HF. 13 , 14 , 22 In a single‐centre observational study among patients with AHF, those with low FEUN values at discharge showed a higher BUN/creatinine ratio and a lower BNP level than other patients, indicating that a low FEUN value reflects intravascular dehydration. 13 Whereas a high FEUN value may indicate intravascular volume overload. In our previous research involving patients with HF and renal dysfunction (eGFR < 60 mL/min/1.73 m2), we demonstrated a significant positive correlation between the FEUN values and right atrial pressure obtained through pulmonary artery catheterization (R = 0.243, P = 0.005). 14 Furthermore, we found that the use of loop diuretics during hospitalization was associated with lower FEUN values at discharge, in a dose‐dependent manner. These findings suggest that FEUN may serve as an indicator of intravascular fluid status in patients with HF.
Furthermore, FEUN has prognostic value in patients with HF. A low FEUN value (<35%), indicating pre‐renal failure, has been reported as a poor prognostic factor. 13 This could be attributed to neurohormonal activation caused by decreased renal perfusion. 13 , 23 Additionally, a high FEUN value (>38.0%) has been identified as an adverse prognostic factor. 14 In our previous study, patients with both high and low FEUN values experienced worse clinical outcomes than those with medium FEUN values. A high FEUN value may indicate residual intravascular congestion, contributing to a poor outcome, 24 whereas a medium FEUN value was associated with a favourable outcome and considered indicative of euvolaemic status. Thus, this study builds on these previous reports and extends them by demonstrating the prognostic relevance of WRF and FEUN values at discharge in patients with AHF.
The cut‐off values for FEUN used in this study were derived from the quartiles of FEUN values among eligible patients in our previous study. 14 Thus, the optimal FEUN value for risk stratification remained unclear. To address this, we performed a restricted cubic spline analysis to examine the relationship between FEUN values and outcomes. This analysis indicated that an FEUN value of 36.1%, which was within the medium FEUN range (>32.1% and ≤38.0%), was associated with the lowest HR. Notably, FEUN values below or above this threshold were associated with higher HR results, suggesting a U‐shaped‐like relationship between FEUN values and outcomes. This relationship aligns with the established role of hypovolaemia and hypervolaemia in contributing to poor outcomes in patients with HF. 25 Similarly, plasma volume status, a common fluid index in HF, has been demonstrated to have a U‐shaped (or J‐shaped) relationship with prognosis. 26 This observation was explained by the fact that both myocardial hypoperfusion due to dehydration and myocardial oedema caused by congestion can worsen the failing myocardium. 27 As such, euvolaemia is crucial for optimized organ perfusion without organ congestion.
Fractional excretion of urea nitrogen in worsening renal function
Our study suggests that the occurrence of WRF during treatment for AHF does not lead to worse outcomes if the FEUN values are within the medium range. Although the underlying mechanism of this result remains unclear, it is possible that a medium FEUN value, which may indicate euvolaemia based on our previous study, 14 could attenuate the negative impact of WRF on clinical outcomes. Furthermore, our findings are consistent with those of previous reports, showing that WRF in the context of successful fluid management is not linked to adverse outcomes. 5 , 6
Patients with WRF and high FEUN values presented with the worst outcomes in this study. Because these patients exhibited higher NT‐proBNP levels at discharge and lesser weight loss during hospitalization, residual congestion was the likely factor associating WRF with poor outcomes. 5 , 6 , 9 Furthermore, patients in this group showed severe clinical profiles, particularly on kidney function, suggesting that underlying differences in baseline characteristics may have contributed to worse outcomes.
On the other hand, patients with WRF and low FEUN values exhibited lower NT‐proBNP levels at discharge and greater weight loss during hospitalization, which suggests adequate decongestion. However, adverse outcomes were still observed, and this group showed the highest BUN/creatinine ratio at discharge. Generally, a high BUN/creatinine ratio may reflect sympathetic and neurohormonal hyperactivity and is associated with poor outcomes in HF. 28 Interestingly, increased BUN during hospitalization has been associated with poor outcomes even when effective decongestion was achieved. 29 Furthermore, WRF was associated with adverse outcomes in cases of increased BUN or persistent clinical congestion. 29 Thus, a low FEUN and high BUN/creatinine ratio with the occurrence of WRF may reflect the neurohormonal activation that is associated with poor outcomes.
Clinical implications and future perspective
FEUN may be useful in the fluid management of patients with HF and WRF. In cases of WRF with a low FEUN value, it may be beneficial to consider de‐escalation of the diuretic dose for maintaining renal perfusion. Conversely, in cases of WRF with a high FEUN value, escalation of the diuretic dose may be necessary to relieve congestion. Additionally, in cases of WRF with a medium FEUN value, the current diuretic dose would be considered appropriate. This FEUN‐guided diuretic strategy may lead to the optimization of diuretic dosing for cardiorenal protection. Additionally, FEUN assessment is a non‐invasive, quantitative, and inexpensive method; this parameter can be repeatedly measured, making it suitable for assessing volume status in AHF. Our study highlights that FEUN may be a useful marker to guide decongestive therapy in the management of cardiorenal syndrome.
Although our studies have demonstrated the prognostic value of FEUN in patients with HF and WRF, its clinical significance has not been fully clarified. To understand how FEUN behaves in conditions of renal hypoperfusion and congestion, a direct assessment of the relationship between FEUN value and renal haemodynamics is required. Additionally, despite having a lower impact on FEUN compared with FENa, the role of diuretics in altering FEUN cannot be ignored. 30 Further research is warranted to clarify the underlying mechanisms behind the association between FEUN values and clinical outcomes.
Limitations
This study had some limitations that should be acknowledged. First, this was a single‐centre study. Second, the inclusion criteria for HF were based on the ICD‐10 codes selected at the discretion of the cardiologist. However, previous studies have validated the accuracy of the ICD‐10 codes with high specificity and sensitivity. 31 , 32 Third, we were unable to evaluate the temporal changes in the FEUN values during hospitalization because the FEUN values were deficient at admission. Therefore, it is unclear how the FEUN values change during decongestive treatment. Fourth, selection bias may be possible due to the exclusion of patients with missing FEUN data. Because FEUN is usually used in patients with renal dysfunction under diuretic therapy, the study findings may not be generalizable in other clinical situations. Fifth, the impact of WRF and FEUN values on death rates is undetermined because mortality was not included as an endpoint in this study. Finally, the cut‐off values for low, medium, and high FEUN were based on our previous study. These values should be externally validated in other cohorts. Further large‐scale prospective studies are needed to determine the optimal cut‐off values of FEUN for risk stratification in patients with HF.
Conclusions
In this study, we demonstrated that the prognostic impact of WRF was significantly mediated by the FEUN values in patients with AHF. Patients with WRF and a low or high FEUN value had worse clinical outcomes than those without WRF, whereas patients with WRF and a medium FEUN value had favourable clinical outcomes. On multivariable analysis, the presence of WRF with low or high FEUN values was independently associated with increased HF readmission, as compared with the absence of WRF with medium FEUN values. Notably, WRF with medium FEUN values was not a significant factor for HF readmission. Our study suggests that FEUN can identify ‘true WRF’ associated with a poor outcome and is useful to guide fluid management in patients with AHF.
Conflict of interest
None declared.
Funding
None.
Supporting information
Table S1. International Classification of Diseases 10th Revision (ICD‐10) codes.
Table S2. Sensitivity analysis (0.3 mg/dL and 25% increase in creatinine): Cox regression analysis for heart failure readmission.
Table S3. Sensitivity analysis (20% decrease in eGFR): Cox regression analysis for heart failure readmission.
Acknowledgements
The authors extend their sincere thanks to all the people who have been involved in patient care, including emergency staff, technicians, medical engineers, nurses, pharmacists, physicians, and surgeons at Nippon Medical School Hospital.
Watanabe, Y. , Kubota, Y. , Nishino, T. , Tara, S. , Kato, K. , Hayashi, D. , Matsuda, J. , Miyachi, H. , Tokita, Y. , Iwasaki, Y. , and Asai, K. (2024) Fractional excretion of urea nitrogen can identify true worsening renal function in patients with heart failure. ESC Heart Failure, 11: 2043–2054. 10.1002/ehf2.14755.
References
- 1. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020;22:584‐603. doi: 10.1002/ejhf.1697 [DOI] [PubMed] [Google Scholar]
- 2. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009;53:589‐596. doi: 10.1016/j.jacc.2008.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boorsma EM, Ter Maaten JM, Voors AA, van Veldhuisen DJ. Renal compression in heart failure: The renal tamponade hypothesis. JACC Heart Fail 2022;10:175‐183. doi: 10.1016/j.jchf.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 4. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004;43:61‐67. doi: 10.1016/j.jacc.2003.07.031 [DOI] [PubMed] [Google Scholar]
- 5. Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, et al. B‐type natriuretic peptide trend predicts clinical significance of worsening renal function in acute heart failure. Eur. J. Heart Fail. 2019;21:1553‐1560. doi: 10.1002/ejhf.1627 [DOI] [PubMed] [Google Scholar]
- 6. McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, et al. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail 2020;8:537‐547. doi: 10.1016/j.jchf.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, et al. Haemoconcentration, renal function, and post‐discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Eur. J. Heart Fail. 2013;15:1401‐1411. doi: 10.1093/eurjhf/hft110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, et al. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur. J. Heart Fail. 2017;19:226‐236. doi: 10.1002/ejhf.667 [DOI] [PubMed] [Google Scholar]
- 9. Metra M, Cotter G, Senger S, Edwards C, Cleland JG, Ponikowski P, et al. Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: A post hoc analysis of the PROTECT data. Circ. Heart Fail. 2018;11:e004644. doi: 10.1161/CIRCHEARTFAILURE.117.004644 [DOI] [PubMed] [Google Scholar]
- 10. Damman K, Testani JM. The kidney in heart failure: An update. Eur. Heart J. 2015;36:1437‐1444. doi: 10.1093/eurheartj/ehv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayasaka K, Matsue Y, Kitai T, Okumura T, Kida K, Oishi S, et al. Tricuspid regurgitation pressure gradient identifies prognostically relevant worsening renal function in acute heart failure. Eur. Heart J. Cardiovasc. Imaging 2021;22:203‐209. doi: 10.1093/ehjci/jeaa035 [DOI] [PubMed] [Google Scholar]
- 12. Gotfried J, Wiesen J, Raina R, Nally JV Jr. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve. Clin. J. Med. 2012;79:121‐126. doi: 10.3949/ccjm.79a.11030 [DOI] [PubMed] [Google Scholar]
- 13. Nogi K, Kawakami R, Ueda T, Nogi M, Ishihara S, Nakada Y, et al. Prognostic value of fractional excretion of urea nitrogen at discharge in acute decompensated heart failure. J. Am. Heart Assoc. 2021;10:e020480. doi: 10.1161/JAHA.120.020480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe Y, Kubota Y, Nishino T, Tara S, Kato K, Hayashi D, et al. Utility of fractional excretion of urea nitrogen in heart failure patients with chronic kidney disease. ESC Heart Fail 2023;10:1706‐1716. doi: 10.1002/ehf2.14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krück F, Bablok W, Besenfelder E, Betzien G, Kaufmann B. Clinical and pharmacological investigations of the new saluretic azosemid. Eur. J. Clin. Pharmacol. 1978;14:153‐161. doi: 10.1007/BF02089953 [DOI] [PubMed] [Google Scholar]
- 16. Díez J, Coca A, de Teresa E, Anguita M, Castro‐Beiras A, Conthe P, et al. TORAFIC study protocol: Torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Rev. Cardiovasc. Ther. 2009;7:897‐904. doi: 10.1586/erc.09.74 [DOI] [PubMed] [Google Scholar]
- 17. Carvounis CP, Nisar S, Guro‐Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223‐2229. doi: 10.1046/j.1523-1755.2002.00683.x [DOI] [PubMed] [Google Scholar]
- 18. Sands JM, Layton HE. The physiology of urinary concentration: An update. Semin. Nephrol. 2009;29:178‐195. doi: 10.1016/j.semnephrol.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sands JM. Renal urea transporters. Curr. Opin. Nephrol. Hypertens. 2004;13:525‐532. doi: 10.1097/00041552-200409000-00008 [DOI] [PubMed] [Google Scholar]
- 20. Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am. J. Physiol. 1987;253:F823‐F832. doi: 10.1152/ajprenal.1987.253.5.F823 [DOI] [PubMed] [Google Scholar]
- 21. Lima C, Macedo E. Urinary biochemistry in the diagnosis of acute kidney injury. Dis. Markers 2018;2018:4907024. doi: 10.1155/2018/4907024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nogi K, Ueda T, Nakamura T, Nogi M, Ishihara S, Nakada Y, et al. New classification for the combined assessment of the fractional excretion of urea nitrogen and estimated plasma volume status in acute heart failure. J. Am. Heart Assoc. 2023;12:e025596. doi: 10.1161/JAHA.122.025596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016;12:610‐623. doi: 10.1038/nrneph.2016.113 [DOI] [PubMed] [Google Scholar]
- 24. Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ. Heart Fail. 2013;6:240‐245. doi: 10.1161/CIRCHEARTFAILURE.112.969246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapelios CJ, Canepa M, Savarese G, Lund LH. Use of loop diuretics in chronic heart failure: Do we adhere to the Hippocratian principle ‘do no harm’? Eur. J. Heart Fail. 2021;23:1068‐1075. doi: 10.1002/ejhf.2214 [DOI] [PubMed] [Google Scholar]
- 26. Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur. J. Heart Fail. 2015;17:35‐43. doi: 10.1002/ejhf.193 [DOI] [PubMed] [Google Scholar]
- 27. Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J. Am. Coll. Cardiol. 2002;39:1901‐1908. doi: 10.1016/s0735-1097(02)01903-4 [DOI] [PubMed] [Google Scholar]
- 28. Testani JM, Coca SG, Shannon RP, Kimmel SE, Cappola TP. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: Analysis of three randomized controlled trials. Eur. J. Heart Fail. 2011;13:1224‐1230. doi: 10.1093/eurjhf/hfr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palazzuoli A, Ruocco G, Pellicori P, Incampo E, di Tommaso C, Favilli R, et al. The prognostic role of different renal function phenotypes in patients with acute heart failure. Int. J. Cardiol. 2019;276:198‐203. doi: 10.1016/j.ijcard.2018.11.108 [DOI] [PubMed] [Google Scholar]
- 30. Cox ZL, Sury K, Rao VS, Ivey‐Miranda JB, Griffin M, Mahoney D, et al. Effect of loop diuretics on the fractional excretion of urea in decompensated heart failure. J. Card. Fail. 2020;26:402‐409. doi: 10.1016/j.cardfail.2020.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med. Care 2005;43:1130‐1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 32. Nakai M, Iwanaga Y, Sumita Y, Kanaoka K, Kawakami R, Ishii M, et al. Validation of acute myocardial infarction and heart failure diagnoses in hospitalized patients with the nationwide claim‐based JROAD‐DPC database. Circ Rep 2021;3:131‐136. doi: 10.1253/circrep.CR-21-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases 10th Revision (ICD‐10) codes.
Table S2. Sensitivity analysis (0.3 mg/dL and 25% increase in creatinine): Cox regression analysis for heart failure readmission.
Table S3. Sensitivity analysis (20% decrease in eGFR): Cox regression analysis for heart failure readmission.


