Abstract
We report the case of a 36‐year‐old woman who presented to the emergency department complaining of palpitations and asthenia. Investigations showed frequent ventricular ectopy and severe left ventricular ejection fraction impairment. She was diagnosed with a peculiar condition defined multifocal ectopic premature Purkinje‐related contractions syndrome, which in some cases can be associated with a dilated cardiomyopathy phenotype. Genetic testing showed a novel mutation in the SCN5A gene (c.673C > G). In the context of acute left ventricular dysfunction in a young patient, we discuss the clinical presentation of this rare condition and its clinical management, as well as its genetic substrate.
Keywords: Acute heart failure, Dilated cardiomyopathy, Genetic disorders, Multifocal ectopic premature Purkinje‐related contractions syndrome, SCN5A gene
Introduction
Multifocal ectopic premature Purkinje‐related contractions (MEPPC) syndrome is characterized by a high burden of premature ventricular complexes (PVC) originating from the fascicular‐Purkinje fibres, which can lead to an important impairment of the left ventricular ejection fraction (LVEF), sometimes in the context of a dilated cardiomyopathy (DCM) phenotype. 1 This condition is related to mutations in the SCN5A gene and it often shows an excellent clinical response to therapy with flecainide, quinidine, hydroquinidine, or amiodarone. 2 However, up to now very few mutations have been associated with this phenotype. 3 , 4 , 5 , 6 , 7 We describe a case of MEPPC syndrome in a patient carrying a novel mutation in the SCN5A gene (c.673C > G, pArg225Gly).
Case Report
Initial presentation
A 36‐year‐old woman (Caucasian) presented to the emergency department complaining of palpitations and asthenia lasting for 2 days. She conveyed the absence of chest pain, fever, confusion, or recent syncopal episodes. On admission, she was apyretic, her blood pressure was 127/69 mmHg, pulse was 112 bpm, and respiratory rate was 13 breaths/min. Oxygen saturation was 96% on room air. Physical examination did not reveal any particular finding except for a fast heart rate with an irregular rhythm.
The patient had no previous cardiovascular events. Her past medical history included a microprolactinoma treated with cabergoline 18 years earlier and allergy to magnetic resonance contrast medium. There was no family history of sudden cardiac death, but her mother suffered from frequent ventricular ectopy (maximum 21% PVCs/d).
Investigations
Owing to the clinical presentation, the patient underwent a broad diagnostic workup. Laboratory findings revealed hyperthyroidism [TSH (thyroid stimulating hormone) = 10.674 μUI/mL, reference 0.55–4.78 μUI/mL; fT3 = 6.5 pg/mL, reference 2.3–4.2 pg/mL; fT4 = 2.96 ng/dL, reference 0.89–1.76 ng/dL; negative anti‐TSH receptor Abs, anti‐thyroid peroxidase Abs and anti‐thyroglobulin Abs], modest increases in white blood cell count (13 700/mm3, reference 3900–10 500/mm3), neutrophils (9060/mm3, reference 1800–7700/mm3), brain natriuretic peptide (202 pg/mL, reference <100 pg/mL), and troponin I (346 ng/L, reference <38.64 ng/L), but no anaemia or renal insufficiency. Prolactine was normal. Infectious disease [IgM anti‐Toxoplasma, IgM anti‐EBV (Epstein Barr virus), IgM anti‐CMV (Citomegalovirus)] and autoimmune screenings were negative. Chest X‐rays were unremarkable. The electrocardiogram (ECG) showed sinus tachycardia interrupted by frequent PVCs (5 PVCs/tracing). Bedside echocardiography revealed diffuse hypokinesis leading to severe left ventricular dysfunction (LVEF = 28%), increased left ventricular end‐diastolic volume index (LVEDVi = 65 mL/m2), functional moderate mitral regurgitation, normal tricuspid annular plane systolic excursion (TAPSE = 20 mm), no other significant valve diseases, no pericardial effusion (Figure 1 ).
Figure 1.
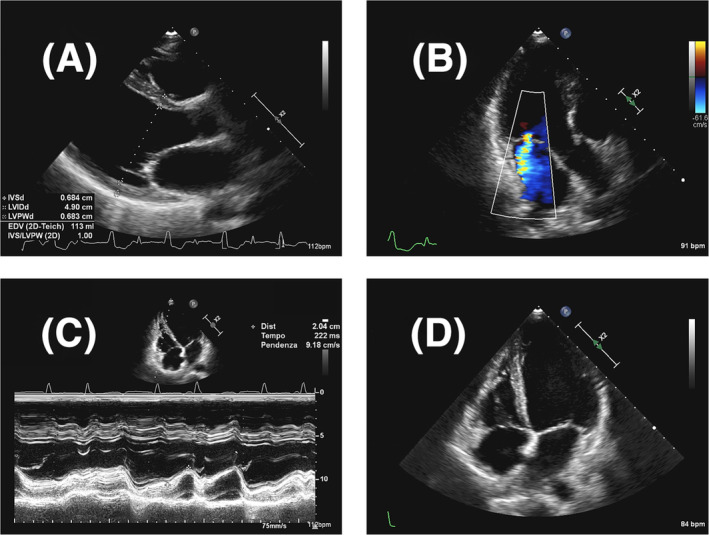
Initial Transthoracic Echocardiogram Findings. Transthoracic echocardiography showing dilated left ventricle (A), functional moderate mitral regurgitation (B), normal longitudinal systolic function of the right ventricle (C), and no pericardial effusion (D).
Considered the first finding of left ventricular dysfunction, the patient underwent a coronary angiography which excluded the presence of significant coronary artery disease (stenosis >50%).
An electrophysiological study was then performed: in basal conditions it documented the presence of accelerated idioventricular rhythm in polymorphic pairs (Figure 2 ) with decremental retroconduction; with stimulation at high frequency (above 130 b.p.m.) atrial capture with normal atrioventricular and intraventricular conduction was obtained, with inhibition of PVCs. Electroanatomical mapping of the left ventricle localized the focus of prevalent PVCs at the level of the left anterior fascicle. With radiofrequency application at this site acute suppression of the ventricular ectopy was obtained. However, it was followed by the onset of different PVCs morphologies [some with narrow QRS and normal axis, some with RBBB (right bundle branch block) morphology and superior axis, others with wide QRS and complete LBBB (left bundle branch block) or RBBB patterns], suggesting the presence of multiple foci originating from the fascicular‐Purkinje system, and it was therefore decided to avoid further ablation attempts. At the end of the procedure an endomyocardial biopsy from the interventricular septum was performed, which showed nonspecific fibrous replacement. Molecular analysis found no viral nucleic acids (Adenovirus, CMV, CMV mRNA, EBV, Enterovirus/Rhynovirus, Human Herpes virus 6, Herpes Simplex virus, Influenza virus A and B, Parvovirus B‐19). The subsequent genetic analysis revealed a heterozygous mutation in the SCN5A gene (c.673C > G, pArg225Gly). The same mutation was then demonstrated to be carried also by the mother (Figure 3 ).
Figure 2.
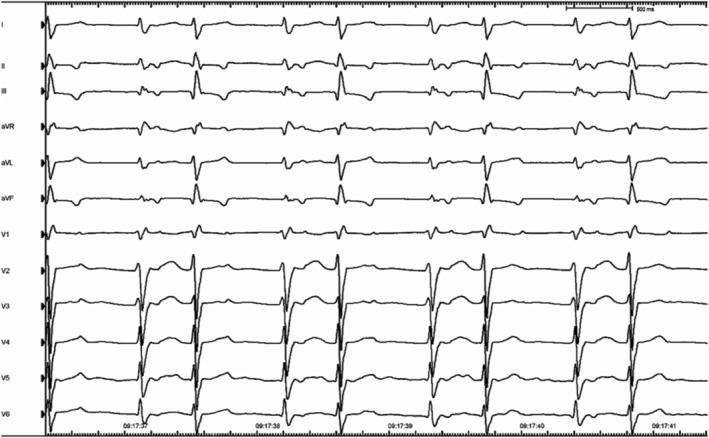
Baseline electrocardiogram during electrophysiological study. Electrocardiogram showing an accelerated idioventricular rhythm in polymorphic pairs. The origin of ventricular ectopy is compatible with the left anterior fascicle.
Figure 3.
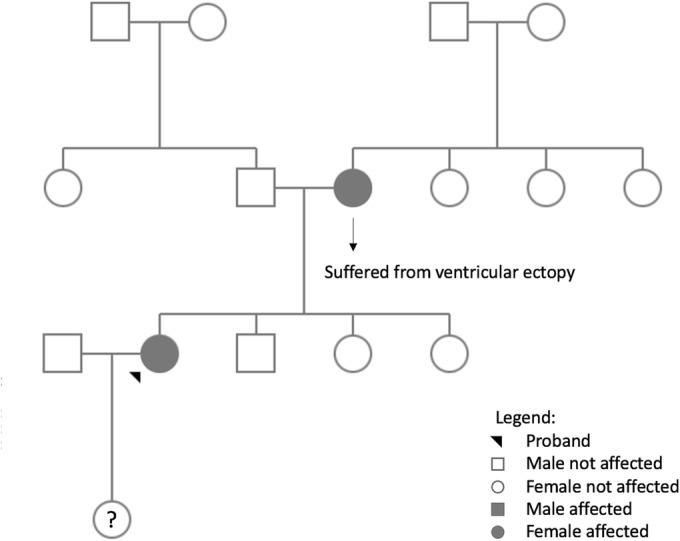
Pedigree chart. Pedigree chart illustrating that no member of the proband's family developed MEPPC syndrome; however, the mother is a carrier of the same mutation. MEPPC, multifocal ectopic premature Purkinje‐related contractions.
A cardiac magnetic resonance (CMR) was later performed (no contrast media was administered because of the allergy of the patient), showing a modest reduction in left ventricular systolic function (LVEF = 37%), increased left ventricular end‐systolic volume (LVESV = 98 mL, LVESVi = 68 mL/m2), modest reduction in right ventricular ejection fraction (RVEF = 42%), normal native T1 and T2 mapping values, and no pathological findings on T2‐weighted sequences (Figure 4 ). Before discharge, a subcutaneous loop recorder device was implanted.
Figure 4.
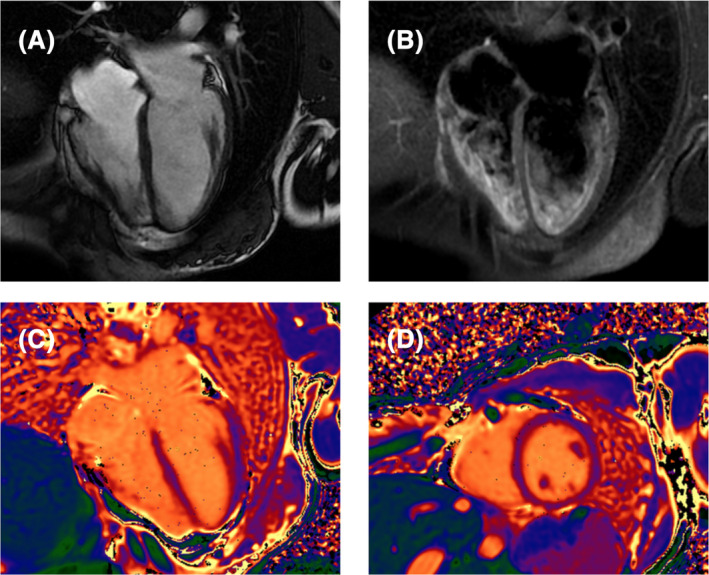
Initial cardiac magnetic resonance findings. Cardiac magnetic resonance demonstrating the absence of pericardial effusion (A), no pathological findings on T2‐weighted sequences (B) and normal native T1 mapping (C, D).
Initial therapy and short‐term course
Heart failure medications (bisoprolol, sacubitril/valsartan, aldactone) were administered throughout the hospitalization. Propylthiouracil was administered for 1 week until normalization of thyroid function tests. Based on the clinical presentation of acute severe left ventricular dysfunction (probably triggered by hyperthyroidism), the persistence of ventricular ectopy despite radiofrequency ablation, the high variability of extrasystoles morphology and the normal tissue characterization on CMR, the diagnosis of MEPPC syndrome was considered to be the most likely. Hence, flecainide (100 mg bid) was introduced, achieving good control of the arrhythmic burden (87 PVCs/d) and restoration of sinus rhythm. Patients was discharged in good haemodynamic compensation after 20 days of hospitalization.
Long‐term course
After discharge the patient did not report any symptom and periodic echocardiographic assessments demonstrated a progressive recovery of the left ventricular systolic function and a complete restoration 6 months after the acute event (LVEF = 62%). A CMR performed 18 months after the index event showed a normalization of ventricular volume (LVESV = 51 mL, LVESVi = 34 mL/m2), besides a normal left ventricular ejection function (LVEF = 56%). Serial Holter ECG evaluations documented a low arrhythmic burden (maximum 109 PVCs/d). No malignant arrhythmias were detected since the loop recorder implantation. In order to prevent arrhythmic recurrences and avoid further episodes of acute decompensation, the patient was kept on therapy with flecainide (100 mg bid). Figure 5 summarizes the patient's entire clinical course.
Figure 5.
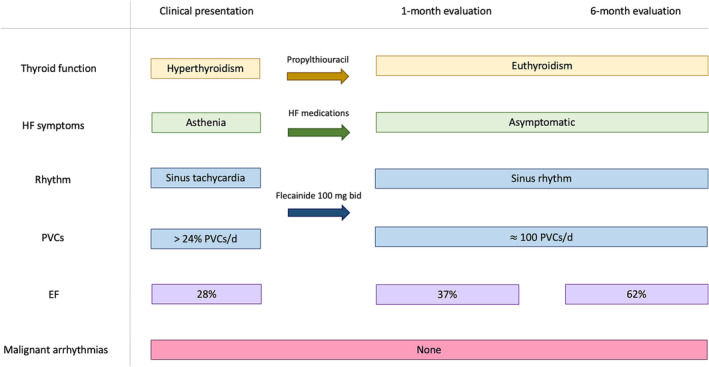
Clinical course. Timeline summarizing the clinical presentation, therapeutic management besides the clinical evaluation at 1 and 6 months since the acute event. HF symptoms include breathlessness, ankle swelling, fatigue, or asthenia. Malignant arrhythmias are defined as sustained VT, polymorphic VT or VF. EF, ejection fraction; HF, heart failure; PVC, premature ventricular contraction; VF, ventricular fibrillation; VT, ventricular tachycardia.
Given the uncertain clinical significance of the mutation and the absence of malignant arrhythmias, genetic counselling did not deem it necessary to extend genetic screening to other family members.
Discussion
SCN5A gene encodes the pore‐forming ion‐conducting α‐subunit of the cardiac sodium channel (Nav1.5), which is responsible for the initiation and propagation of action potentials, playing a major role in cardiac excitability and conduction of electrical impulses. 8 Mutations in the SCN5A gene are often associated with channelopathies, such as long QT syndrome (usually gain of function mutations) and Brugada syndrome (usually loss of function mutations). Nevertheless, more than 15 articles have described families or sporadic cases of dilated cardiomyopathy associated with mutations in the SCN5A gene. 1
One hypothesis that has been proposed to justify this correlation suggests that modifications within the sodium channel may hold a structural significance in the genesis of DCM: the Nav1.5 is part of a macromolecular complex situated at the intercalated disc, and perturbations in sodium homeostasis precipitate fluctuations in intracellular pH and calcium concentrations, thereby inciting cellular remodelling processes. 9 , 10 , 11 However, within the context of MEPPC syndrome, it is noteworthy that a remarkably favourable response to anti‐arrhythmic therapy has been documented, suggesting that a preeminent factor may indeed be represented by the burden of arrhythmias. 10 Moreover, PVCs might trigger the activation of baroreceptors and the sympathetic nervous system, thus inducing myocardial stress. 11
Gain of function mutations in the SCN5A gene determine an increased activity of sodium channels leading to the onset of action potentials during the repolarization phase of Purkinje cells. 1
MEPPC patients usually present with severe ventricular dysfunction associated to frequent ventricular ectopy (≥24% PVCs/d) 12 arising from the conduction system. In a significant proportion of patients, the restoration of sinus rhythm is followed by the normalization of the LVEF. 10 In this specific phenotype, in which the origin of the PVCs is multifocal as it extends to the Purkinje fibres, evidences suggest that medical therapy with class IC anti‐arrhythmic drugs such as flecainide or quinidine is more effective than transcatheter ablation. 13 , 14 , 15 In some cases, an important reduction of the PVCs burden has also been obtained with amiodarone. 3 , 4 Moreover, as described in a case report, it is possible that atrial overdrive pacing may be effective in suppressing PVCs for patients refractory to medical therapy. 6
Nevertheless, it is crucial to underscore that MEPPC syndrome represents a relatively recent clinical entity and that there exists a dearth of knowledge regarding the most effective strategies for its long‐term management. There is a possibility that these patients could derive lasting benefits from a regimen of chronic treatment involving class IC anti‐arrhythmic agents, aimed at forestalling relapses. 3 A longitudinal investigation into the extended clinical trajectories experienced by affected individuals is needed.
Up to now very few mutations in the SCN5A gene (R222Q, R225P, G213D, R814W, and A204E) have been associated with this clinical condition. 3 , 4 , 5 , 6 , 7 To the best of our knowledge, this is the first case of MEPPC syndrome described in a patient carrying the R225G (c.673C > G, pArg225Gly) mutation in the SCN5A gene.
Conclusions
The literature on MEPPC syndrome is lacking and less than 20 cases have been described so far. However, this clinical condition should be considered in young patients presenting with newly detected systolic dysfunction associated with frequent ventricular ectopy arising from the conduction system. The diagnostic workup should include advanced imaging, endomyocardial biopsy besides genetic testing. Given the reversible nature of this phenotype, reaching a prompt diagnosis is of mainstay importance, as it can guide the treatment and affect prognosis.
Conflict of interest
None declared.
Funding
This research was supported by the Italian Ministry of Health‐Ricerca Corrente to Centro Cardiologico Monzino IRCCS.
Acknowledgements
Open access funding provided by BIBLIOSAN. [Correction added on 4 April 2024, after first online publication: BIBLIOSAN funding statement has been added.]
Open access funding provided by BIBLIOSAN.
Ventrella, N. , Bianchini, L. , Riva, S. , Pizzamiglio, F. , Dessanai, M. A. , Tundo, F. , Sattin, T. , De Lio, F. , Cellucci, S. , and Tondo, C. (2024) Novel SCN5A gene mutation in a patient affected by multifocal ectopic premature Purkinje‐related contractions syndrome. ESC Heart Failure, 11: 2399–2404. 10.1002/ehf2.14677.
References
- 1. Wilde AAM, Amin AS. Clinical spectrum of SCN5A mutations: Long QT syndrome, Brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol 2018;4:569‐579. doi: 10.1016/j.jacep.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 2. Laurent G, Saal S, Amarouch MY, Béziau DM, Marsman RFJ, Faivre L, et al. Multifocal ectopic Purkinje‐related premature contractions: A new SCN5A‐related cardiac channelopathy. J Am Coll Cardiol 2012;60:144‐p156. doi: 10.1016/j.jacc.2012.02.052 [DOI] [PubMed] [Google Scholar]
- 3. Mann SA, Castro ML, Ohanian M, Guo G, Zodgekar P, Sheu A, et al. R222Q SCN5A mutation is associated with reversible ventricular ectopy and dilated cardiomyopathy. J Am Coll Cardiol 2012;60:1566‐1573. doi: 10.1016/j.jacc.2012.05.050 [DOI] [PubMed] [Google Scholar]
- 4. Beckermann TM, McLeod K, Murday V, Potet F, George AL. Novel SCN5A mutation in amiodarone‐responsive multifocal ventricular ectopy‐associated cardiomyopathy. Heart Rhythm 2014;11:p1446‐p1453. doi: 10.1016/j.hrthm.2014.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calloe K, Broendberg AK, Christensen AH, Pedersen LN, Olesen MS, de los Angeles Tejada M, et al. Multifocal atrial and ventricular premature contractions with an increased risk of dilated cardiomyopathy caused by a Nav1.5 gain‐of‐function mutation (G213D). Int J Cardiol 2018;257:160‐167. doi: 10.1016/j.ijcard.2017.11.095 [DOI] [PubMed] [Google Scholar]
- 6. Zakrzewska‐Koperska J, Bilińska ZT, Truszkowska GT, Franaszczyk M, Elikowski W, Warmiński G, et al. A combination of quinidine/mexiletine reduces arrhythmia in dilated cardiomyopathy in two patients with R814W SCN5A mutation. ESC Heart Fail 2020;7:4326‐4335. doi: 10.1002/ehf2.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doisne N, Waldmann V, Redheuil A, Waintraub X, Fressart V, Ader F, et al. A novel gain‐of‐function mutation in SCN5A responsible for multifocal ectopic Purkinje‐related premature contractions. Hum Mutat 2020;41:850‐859. doi: 10.1002/humu.23981 [DOI] [PubMed] [Google Scholar]
- 8. Veerman CC, Wilde AAM, Lodder EM. The cardiac sodium channel gene SCN5A and its gene product NaV1.5: Role in physiology and pathophysiology. Gene 573:177‐187. doi: 10.1016/j.gene.2015.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amin AS. SCN5A‐related dilated cardiomyopathy: What do we know? Heart Rhythm 2014;11:1454‐1455. doi: 10.1016/j.hrthm.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 10. Peters S, Thompson BA, Perrin M, James P, Zentner D, Kalman JM, et al. Arrhythmic phenotypes are a defining feature of dilated cardiomyopathy‐associated SCN5A variants: A systematic review. Circ Genom Precis Med 2022;15:E003432. doi: 10.1161/CIRCGEN.121.003432 [DOI] [PubMed] [Google Scholar]
- 11. Calloe K, Magnusson HBD, Lildballe DL, Christiansen MK, Jensen HK. Multifocal ectopic purkinje‐related premature contractions and related cardiomyopathy. Front Cardiovasc Med 2023;10:1‐9. doi: 10.3389/fcvm.2023.1179018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865‐869. doi: 10.1016/j.hrthm.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Liu J, Dai D, Jiefu Yang HS. An unusual incessant narrow‐wide complex polymorphic tachycardia: Electrophysiological phenotype due to an allelic variant. JACC Clin Electrophysiol 2022; doi: 10.1016/j.jacep.2022.06.005 [DOI] [PubMed] [Google Scholar]
- 14. Leventopoulos G, Perperis A, Karelas D, Almpanis G. You cannot ablate the Lernaean hydra: SCN5A mutation in a patient with multifocal ectopic Purkinje‐related premature contractions syndrome treated with flecainide and an implant of a subcutaneous defibrillator‐A case report. Eur Heart J Case Rep 2021;5. doi: 10.1093/ehjcr/ytab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young ML, Kilinc O, Benhayon D. Multifocal ectopic premature Purkinje‐related complexes syndrome in children. HeartRhythm Case Rep 2023;9:545‐550. doi: 10.1016/j.hrcr.2023.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


