Abstract
Islet transplantation is a potential cure for diabetic patients, however this procedure is not widely adopted due to the high rate of graft failure. Islet encapsulation within hydrogels is employed to provide a three-dimensional microenvironment conducive to survival of transplanted islets to extend graft function. Herein, we present a novel macroencapsulation device, composed of PEG hydrogel, that combines encapsulation with lithography techniques to generate polydimethylsiloxane (PDMS) molds. PEG solutions are mixed with islets, which are then cast into PDMS molds for subsequent crosslinking. The molds can also be employed to provide complex architectures, such as microchannels that may allow vascular ingrowth through pre-defined regions of the hydrogel. PDMS molds allowed for the formation of stable gels with encapsulation of islets, and in complex architectures. Hydrogel devices with a thickness of 600 μm containing 500 islets promoted normoglycemia within 12 days following transplantation into the epididymal fat pad, which was sustained over the two-month period of study until removal of the device. The inclusion of microchannels, which had a similar minimum distance between islets and the hydrogel surface, similarly promoted normoglycemia. A glucose challenge test indicated hydrogel devices achieved normoglycemia 90 min post-dextrose injections, similar to control mice with native pancreata. Histochemical staining revealed that transplanted islets, identified as insulin positive, were viable and isolated from host tissue at 8 weeks post-transplantation, yet devices with microchannels had tissue and vascular ingrowth within the channels. Taken together, these results demonstrate a system for creating non-degradable hydrogels with complex geometries for encapsulating islets capable of restoring normoglycemia, which may expand islet transplantation as a treatment option for diabetic patients.
Keywords: polyethylene glycol (PEG), hydrogel, encapsulation, macroencapsulation device, polydimethylsiloxane (PDMS), microchannels
Introduction
Islet transplantation is a potential cure for type 1 diabetes (T1D) and, relative to exogenous insulin delivery, may provide better control over blood glucose levels. Although insulin injections promote normoglycemia in patients with T1D, significant morbidity and complications persist such as heart disease, kidney failure, and blindness despite advances in insulin delivery technologies (Gibly et al., 2011; Gruessner and Gruessner, 2013). Islet transplantation has emerged as an experimental therapy in which islets are delivered into the hepatic sinusoids. This strategy has enabled insulin independence for some patients (Ryan et al., 2005), and has the potential to avoid the complications associated with insulin therapy. However, insulin independence is transient and this procedure is reserved for a subset of T1D patients, particularly those with severe glycemic variability and recurrent hypoglycemia, due to factors such as a limited islet supply, poor engraftment post-transplantation, and the host immune response (Gibly et al., 2011).
Islets have been encapsulated within biomaterials as a means to protect cells from the challenges associated with transplantation (Gibly et al., 2011). Biomaterials are used at extrahepatic sites, which avoid the negative effects of the instant blood-mediated inflammatory response (IBMIR) that is associated with hepatic transplantation. Encapsulation of islets within biomaterials has been investigated to protect the islets from direct contact with immune cells, with the goal of reducing or eliminating the use of immunosuppressive drugs. While encapsulation aims to prevent contact with host cells, they also impose mass transport limitations that can influence the exchange of necessary factors such as glucose, insulin, oxygen, and other nutrients (Beck et al., 2007; O’Sullivan et al., 2011; Scharp and Marchetti, 2013; Vaithilingam and Tuch, 2011). The encapsulating materials have commonly been formulated as microcapsules that are delivered into the peritoneal cavity, with exposure to oxygen levels that are typically less than in the vasculature (Colton, 2014). Microcapsules formed from alginate have been widely used for islet encapsulation with efficacy demonstrated in rodent and non-human primate models (Buder et al., 2013). However, alginate hydrogels and their various modifications, such as poly-L-lysine to control permeability, have the potential for fibrotic overgrowth, which can impose additional mass transport limitations that can limit islet function over time following transplantation (Scharp and Marchetti, 2013). Recent studies with polyethylene glycol (PEG)-based hydrogels or coatings for cell transplantation, and particularly islet transplantation, have minimal foreign body response and a demonstrated ability to support islet engraftment and function (Jeong et al., 2013; Kizilel et al., 2010; Liu et al., 2015; Park et al., 2015; Phelps et al., 2013; Rengifo et al., 2014).
Macroencapsulation devices are also being developed that minimize contact with the host cells, yet provide the opportunity to better control the site at which the islets are transplanted. Macroencapsulation systems have been created in various forms, such as preformed polymer membranes or hollow fibers (Buder et al., 2013; Colton, 2014; Song and Roy, 2015). These devices are often loaded with a high density of cells, which can impose mass transport challenges. To address the mass transport limitations, these devices may have complex geometries that allow for vascular growth near the islets (O’Sullivan et al., 2011; Scharp and Marchetti, 2013). These systems have often been pre-formed and islets are subsequently loaded into the devices, which can be retrievable.
In this report, we investigated the feasibility of using PEG hydrogels to incorporate islets at the time that the macro-encapsulating device is formed. A non-degradable hydrogel was employed to encapsulate islets and isolate them from the host cells. PDMS molds are formed by photolithography, and the multi-arm PEG/islet mixture is cast into the mold and subsequently crosslinked. Molds were employed to create PEG hydrogel slabs, or slabs with microchannels that can support tissue ingrowth in defined regions among the islets. Adhesion ligands were also incorporated to support the survival of encapsulated islets. (Papavasiliou et al., 2012; Pinkse et al., 2006; Weber et al., 2007; Weber and Anseth, 2008). Devices were transplanted into the epididymal fat, an established site for islet transplantation, of diabetic mice. These studies investigated islet engraftment and function using a syngeneic islet transplant model, in order to demonstrate the feasibility of the approach and provide a foundation for future studies with allogeneic and xenogeneic islets. Taken together, these studies determine the feasibility of this encapsulation device as a means to efficiently encapsulate islets and isolate them from host tissue.
Materials and Methods
Master Mold Fabrication
Silicon wafers (3-inch, WRS Materials, San Jose, CA) were cleaned and coated evenly with 3.25 g of SU8-100 photoresist (MicroChem, Westborough, MA). Wafers were then pre-baked on a hot plate overnight. A photomask (CAD Art Services, Bandon, OR) was aligned with the photoresist-coated wafer using the Q4000 mask aligner (Quintel, NU Materials Processing and Microfabrication Facility). UVexposure of 1,400 s was then applied to the wafer using the mask aligner to imprint photomask features on the photoresist. Wafers were then post-baked overnight and residual photoresist was removed, or “developed,” using 300 mL of polyethylene monomethyl ether acetate (Sigma, St. Louis, MO) on a laboratory shaker for 1 h. An additional 30-min wash with fresh polyethylene monomethyl ether acetate was used to ensure all residual photoresist was removed from the resulting master mold.
PDMS Mold Fabrication
A Sylgard 184 Silicone Elastomer Kit (Dow Corning, Midland, MI) was used in conjunction with the master mold to form a PDMS mold. Briefly, 25 g of silicone elastomer base from the kit was mixed with 2.5 g of curing agent for 5 min (10:1 ratio of elastomer base/curing agent). The master mold was then placed in a petri dish (150 × 15 mm) and the elastomer base/curing agent solution was poured over the master mold. The petri dish was then covered and placed in a vacuum for 2 h to remove air bubbles from the elastomer base/curing agent solution. The dish was then transferred to a 60°C oven overnight. The PDMS mold was carefully removed from the master mold and used to form hydrogels with or without microchannels. PDMS mold outer dimensions were 7.7 × 7.7 mm2 with a 1 × 1 mm2 border. For molds to create microchannels, the PDMS inner pattern structure contained ~200 × 200-μm2 posts spaced 500 μm apart.
Hydrogel Preparation and Macroenapsulation Devices Formed Within PDMS Molds
PEG-maleimide (4-arm, 10 kDa MW, JenKem Technology, Plano, TX) was suspended in HEPES Buffer (pH 7.2) and functionalized with 2.5 mM CGRGDS (CelTek Peptides, Franklin, TN) via Michael-Type addition for 30 min at 37°C. The concentration of adhesion peptide (2.5 mM) was chosen such that the number of cysteines corresponds to 6.25% of the number of maleimide groups on PEG. Following addition of functionalized PEG precursor solution to the PDMS mold, approximately 10 μL of media containing islets was transferred to the PDMS mold using a glass transfer pipette. Islets were then mixed into the PEG precursor solution to ensure an even distribution within the mold. Next, a YKNR non-degradable crosslinker solution, GCYKNRGCYKNRCG (custom synthesis and purification by CelTek Peptides), which contained tyrosine (Y) and asparagine (N) amino acids in the D-configuration, was added in the PDMS mold at a 1:1.1 ratio (remaining mol maleimide/ mol cysteine) to initiate gel formation. Specifically, the 3-cysteine crosslinking peptide (GCYKNRCGYKNRCG) was added at a concentration of 14 mM to crosslink the PEG through the unreacted maleimide groups. The crosslinker was added dropwise at multiple locations in the PDMS mold. The components (islets, PEG-functionalized with CGRGDS, and crosslinker) had to be added in this manner to allow casting within the PDMS, as attempts to mix all components in one solution and subsequently deposit within the mold were unsuccessful due to the rapid gelation of the PEG. The specific configuration of the Y and N amino acids renders the crosslinker peptide as non-degradable because it is not cleavable by plasmin. The ability of this tri-cysteine peptide crosslinker, versus a di-cysteine peptide crosslinker, to rapidly and efficiently crosslink 4-arm PEG was confirmed in a study by Shikanov et al. (2011). However, other crosslinkers such as DTT (Hudalla et al., 2008) or thiolated agents (Kharkar et al., 2015) can be utilized to initiate gel formation and control degradation. Gels were then incubated at 37°C for 5 min to facilitate crosslinking via Michael-Type addition. Molds containing crosslinked gels were immediately submersed in media supplemented with 10% fetal bovine serum for 3–5 min prior to transplantation. The resulting macroencapsulation devices were carefully removed from the mold using a spatula. Final gels were ~30 μL in volume and 10% PEG wt%. Gels without mirochannels were formed in a similar manner, in a PDMS mold.
Hydrogel Swelling and Mesh Size Experiments
After hydrogel formation, samples were immediately weighed to obtain the mass prior to swelling. Gels were then swelled overnight in PBS and weighed to determine the mass after swelling. Samples were then rinsed in DI water for 4 h to remove excess salts and lyophilized to determine the dry mass. The Flory–Rehner model was then used to calculate the hydrogel mesh size (Zustiak et al., 2010).
Viability Assessment
A Live/Dead assay (ThermoFischer Scientific, Waltham, MA), based on membrane integrity, was used to assess islet viability. In brief, 20 μL of 2 mM ethidium homodimer-1 and 5 μL of 4 mM calcein AM were added to 10 mL of sterile PBS to make a Live/Dead reagent stock. The stock was then vortexed to ensure proper mixing. A sample of 50 freshly isolated islets was either placed in a 48-culture well (unencapsulated or “free” islets) with HBSS 1X media (supplemented with 10% FBS) or encapsulated in a 30 μL hydrogel using the gelation conditions specified, and subsequently submersed in media in the well plate. After removal of media in the well, 500 μL of the Live/Dead stock solution was added to the samples and allowed to incubate for 30 min at 37°C prior to imaging.
Islet Isolation and Transplantation Into Diabetic Mice
Islets were isolated from healthy 10–12 week old male C57BL/6J (Jackson Laboratories, Bar Harbor, ME) following standard islet isolation procedures. Male C57BL/6J recipient mice were between 16–18 weeks of age. Four days prior to islet transplantation, recipient mice were injected with 220 mg/kg of streptozotocin (Sigma, St. Louis, MO) to chemically induce irreversible diabetes. Nonfasting blood glucose levels were taken using a OneTouch Basic Glucose Monitor (Aviva) and only those mice with a measurement of 300 mg/dL or greater on consecutive days (day before and day of transplant) were used as recipients. Macroencapsulation hydrogel devices were formed in PDMS molds with approximately 1,000 islets in each gel. Upon removal from the mold, the hydrogel device was cut into four equal quadrants with each quadrant containing ~250 islets. Each mouse received one gel quadrant per fat pad (left and right fat pad), a total of 500 islets/mouse. The hydrogel device was transplanted into the fat pad using the same procedure as reported previously for scaffold implantation (Blomeier et al., 2006). The fat pad transplantation site allows for a minimally invasive surgery and access to vasculature to support islet engraftment as demonstrated in previous studies from our lab (Blomeier et al., 2006; Gibly et al., 2011; Salvay et al., 2008). Thus, this site is a feasible site for clinical translation and is analogous to the human omentum. All studies were approved by the Northwestern University Animal Care and Use Committee.
Intraperitoneal Glucose Tolerance Test
Intraperitoneal glucose tolerance tests (IPGTTs) were performed at 8 weeks post-transplantation to assess the ability of the hydrogel device to respond to glucose challenges. After a 3 h fast period, 2 g/kg of 50% dextrose (Abbott Labs, Chicago, IL) was injected intraperitoneally. Blood glucose levels were measured at baseline (before injection), 15, 30, 60, 90, 120, and 150 min after the dextrose injection. Statistical testing for area under the curve was performed with a one-way ANOVA and Bonferroni’s Multiple Comparison Post-Hoc Test using Prism software.
Immunohistochemistry
Histological sections were stained with primary antibodies guinea pig polyclonal anti-swine insulin (Jackson Labs, West Grove, PA), CD31 (Life Technologies, Carlsbad, CA), and Hoechst (Invitrogen) at dilutions 1:250, 1:500, 1:2,000, respectively. Secondary antibodies included Dylight donkey anti-guinea pig 488 (Jackson Labs, West Grove, PA) and AlexaFluor 555 goat anti-rat (Invitrogen, Waltham, MA) at a dilution of 1:400 and 1:500, respectively.
Histology
Upon device removal, fat pad samples containing hydrogel devices were placed in 4% PFA overnight. Samples were then submersed in sucrose/PBS solutions and sucrose concentration was progressively increased over a 2-day period. Devices were then embedded in OCT containing 30% sucrose and stored in −80°C until sectioning. Sections were stained with hematoxylin and eosin (H&E) to assess cellular ingrowth into microchannels of the hydrogel device. A picrosirius red stain kit (Abcam, Cambridge, MA), a connective tissue stain, was used according to manufacturer instructions to evaluate fibrosis at the hydrogel–adipose tissue interface.
Results
Macroencapsulating PEG Hydrogels With Microchannels
A non-degradable, PEG hydrogel macroencapsulation device with microchannels was developed using microfabrication techniques. A 4-arm PEG macromer was initially functionalized with 2.5 mM CGRGDS in order to provide sites for cell adhesion, and this functionalized PEG was crosslinked using a non-degradable, three-cysteine-containing peptide (GCYKNRGCYKNRCG). This peptide is non-degradable as the tyrosine (Y) and asparagine (N) amino acids were in the D-form. Hydrogel devices were composed of a final PEG content of 10% by weight. This percentage was the lowest concentration that formed stable hydrogels after 5 min of crosslinking. Note that 5% PEG hydrogels could readily be formed through bulk mixing (i.e., no PDMS molds). Casting of the PEG/islet solution within PDMS affects the ability to efficiently mix the crosslinking reagents, which necessitated the use of greater PEG concentrations. The microchannel structure was consistently maintained upon removal of 10% PEG gels from PDMS molds. The components of this device and their concentration are summarized in Table I.
Table I.
Concentration of PEG macromer, maleimide, adhesion peptide, and crosslinker peptide in hydrogel devices.
| PEG (mM) 10 wt% | Mal (mM) | Adhesion peptide | Crosslinker (GCYKNRCGYKNRCG) |
|---|---|---|---|
| 10 | 40 | 2.5 mM (cys: 2.5 mM) | 14 mM (cys: 42 mM) |
Cysteine (cys) concentration in either the adhesion peptide or crosslinker peptide is indicated in parentheses.
Microchannels throughout defined regions of the hydrogel were subsequently incorporated to allow for cell and blood vessel ingrowth that would minimize diffusion distances. Hydrogels with microchannels were formed by casting the functionalized PEG and peptide crosslinker solution inside a PDMS mold. Hydrogels without microchannels (Fig. 1A) were also formed in a PDMS mold. The PDMS mold was created using a photomask and standard photolithography techniques (Fig. 1B). The mold created hydrogels that were 7.7 × 7.7 mm2 (length × width), and a thickness of approximately 600 μm. Microchannel diameters of ~200 μm were readily observed within the hydrogel device (Fig. 1C and D) and the spacing between the edges of the microchannels was 500 μm (800 μm from center to center of microchannel). For the slab devices and devices with microchannels, the maximum distance to the edge of the hydrogel was approximately 300 μm. This spacing was maintained between both hydrogel forms in order to isolate the impact of more complex molds on the hydrogel properties and their ability to support islet function. For hydrogels with microchannels, islets were observed to be 204 ± 21 μm (±SEM, n = 17) from a pore edge on average, a distance that is consistent with the effective diffusion of oxygen and nutrients from capillaries to neighboring cells (Wilson and Chaikof, 2009). Furthermore, swelling experiments were performed on 10% hydrogels with microchannels to determine mesh size. A mesh size of 9.3 ± 0.3 nm (±SEM, n = 3) was calculated using the Flory–Rehner model (Zustiak et al., 2010), which is sufficient for transport of insulin and nutrients and consistent with reports of other encapsulation systems (Desai et al., 2004; Song et al., 2016; Weber et al., 2009).
Figure 1.
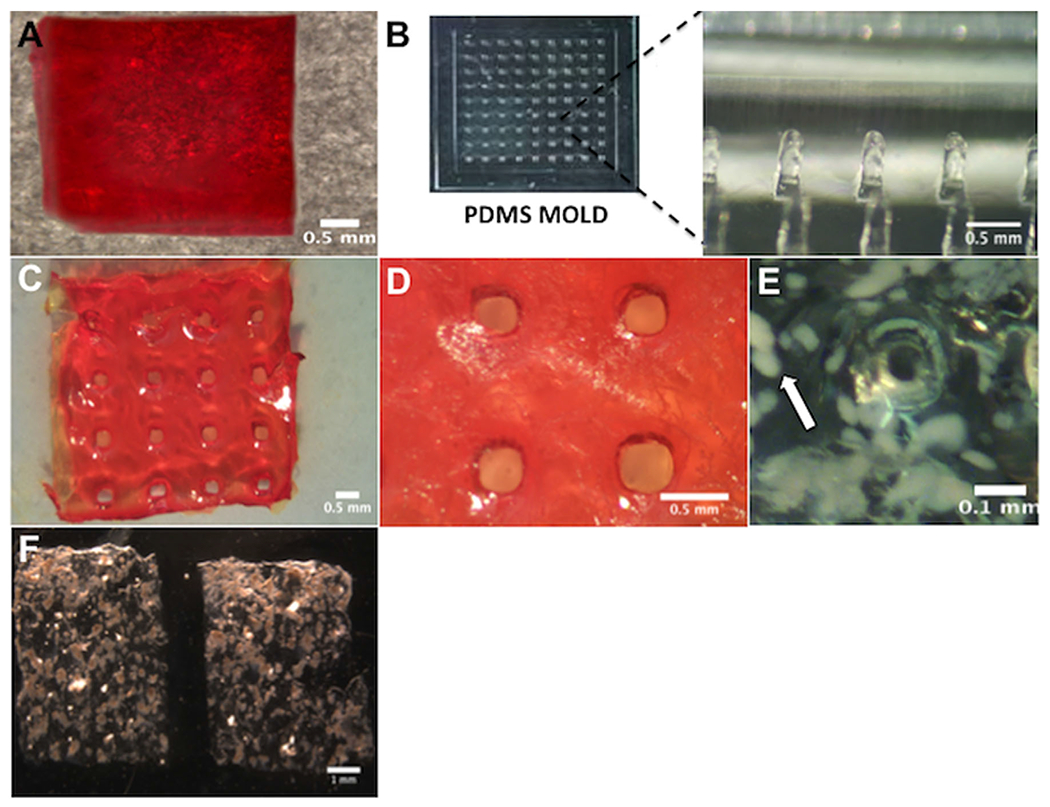
Macroencapsulating PEG hydrogel devices without or with microchannels. (A) Macroview of a hydrogel without microchannels. (B) A PDMS mold that contained column-like features was employed to form hydrogels with microchannels. (C) Macroview of gel device with microchannels. (D) Close-up view of micropores that form microchannels. Microchannels are ~200 μm in diameter with 500 μm spacing between pore edges. Gels were stained with sirius red for visualization. (E) Encapsulated islets surrounding a microchannel in a hydrogel. Islets appear opaque and a white arrow indicates a representative islet. (F) Representative images of hydrogel devices with encapsulated islets.
Islets could be readily encapsulated within the hydrogel. Islets were suspended within the RGD-functionalized PEG solution, mixed thoroughly, and then laid into the PDMS mold. The YKNR crosslinker solution was then added dropwise at multiple locations in the mold, and the mold was then placed in an incubator (37°C) for 5 min for gel crosslinking. After incubation, islets were identified throughout the hydrogel device and fully encapsulated with minimal to no protrusion from the hydrogel (Fig. 1E). The distribution of islets between the quadrants was investigated through counting of multiple quadrants, which confirmed similar islet numbers per quadrant despite some groups of aggregated islets in the gel (Fig. 1F). Prior to transplantation, islet viability was confirmed using the specified gelation conditions. Encapsulated islet viability within the bulk hydrogels post-gelation was assessed using a live/dead stain. Encapsulated islets remained viable after hydrogel formation, with viability similar to that observed with islets cultured on tissue culture polystyrene (Fig. 2). Taken together, this encapsulation approach resulted in an even distribution of islets among the hydrogels, with retention of viability.
Figure 2.
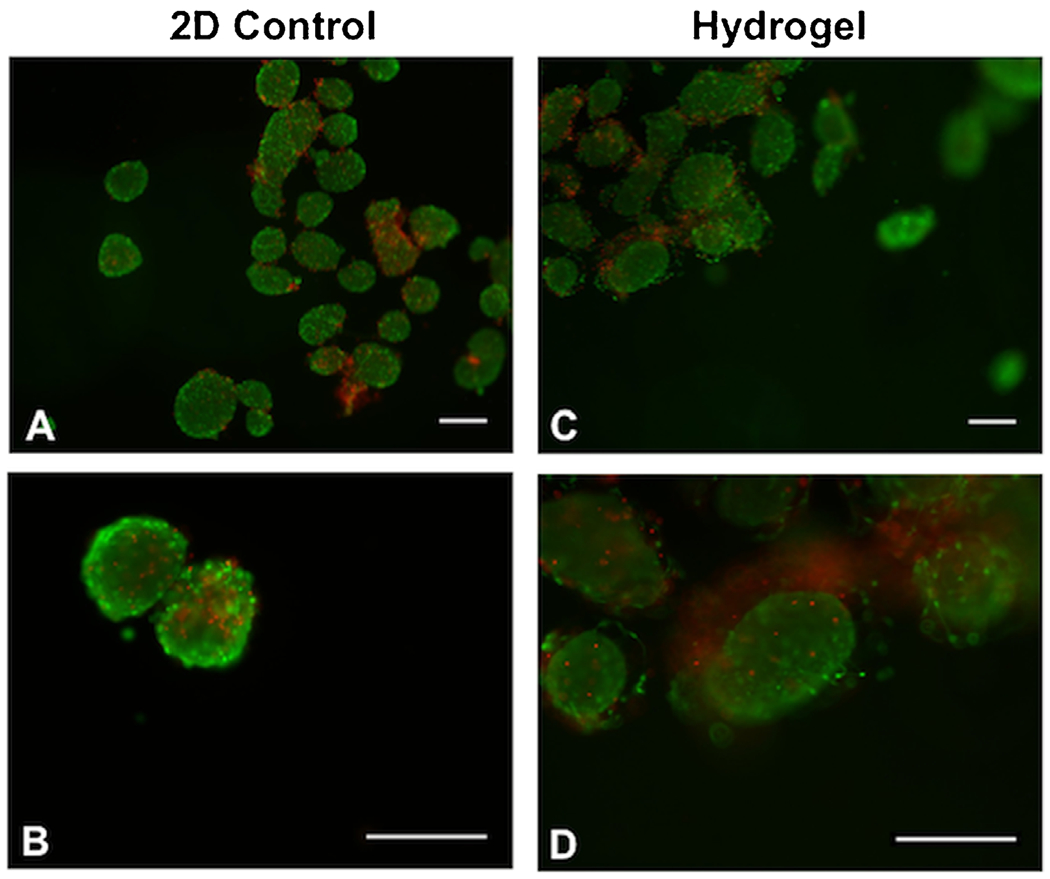
Islet viability confirmed in vitro prior to transplantation. After islet isolation, samples of 50 islets were transferred to a 48-well plate (2D control, unencapsulated or “free” islets) or encapsulated in 30 μL bulk PEG hydrogels (10% wt/vol). Islet viability was assessed using a live (green)/dead (red) stain. Islets remained viable post-isolation in the 2D control (A,B) and hydrogel condition with a 5 min incubation period (C,D). Scale bar: 200 μm.
Islet Transplantation Into Diabetic Mice
The engraftment and function of encapsulated islets was investigated by transplanting hydrogel-encapsulated islets into mice that were made diabetic by injection of streptozotocin (STZ). To accommodate the size of the vascularized fat pad transplant site (Fig. 3A), gel devices were sectioned into quadrants (~4×4 mm2) prior to implantation and each fat pad received one-hydrogel quadrant (Fig. 3B). Devices remained intact after 2 months post-transplant and were easily identified in the fat pad (Fig. 3C).
Figure 3.
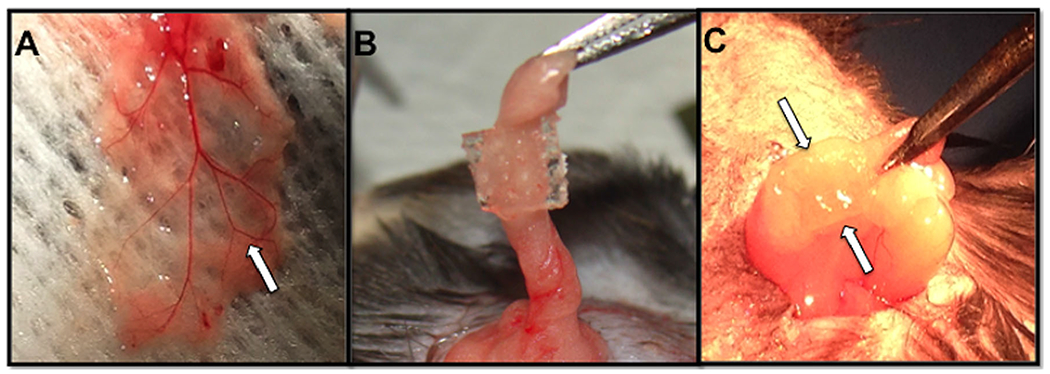
Hydrogel macroencapsulation device in vascularized fat pad transplantation site. (A) Gross vascularization of the native fat pad in a diabetic recipient mouse. White arrow indicates a blood vessel. (B) Hydrogel devices, with or without microchannels, were transplanted into the intraperitoneal fat pad of diabetic mice. This image depicts a hydrogel with microchannels wrapped into the fat pad. (C) The hydrogel device remains intact and undegraded upon removal after 2 months post-transplantation. Arrows indicate gel location.
Mice transplanted with hydrogel slabs (without microchannels) containing 500 islets achieved consistent normal blood glucose levels (<200 mg/dL) within 2 weeks post-transplantation, as early as Day 11 post-transplant (188 ± 58 mg/dL) (Fig. 4A). Mice transplanted with hydrogels with microchannels achieved stable normoglycemia at Day 12 post-transplant (192 ± 28 mg/dL) (Fig. 4B). Both hydrogel groups maintained normoglycemia over the 2-month period of the study. Upon graft removal at Day 60, all mice quickly reverted to a diabetic state within 2–4 days, which confirmed maintenance of blood glucose levels was due to the hydrogel graft and not remaining endogenous islets (Fig. 4A and B). Transplantation of hydrogels containing 300 islets resulted in euglycemia for 25% of the mice, suggesting that 300 islets is an insufficient mass for this system (Fig. 4C).
Figure 4.
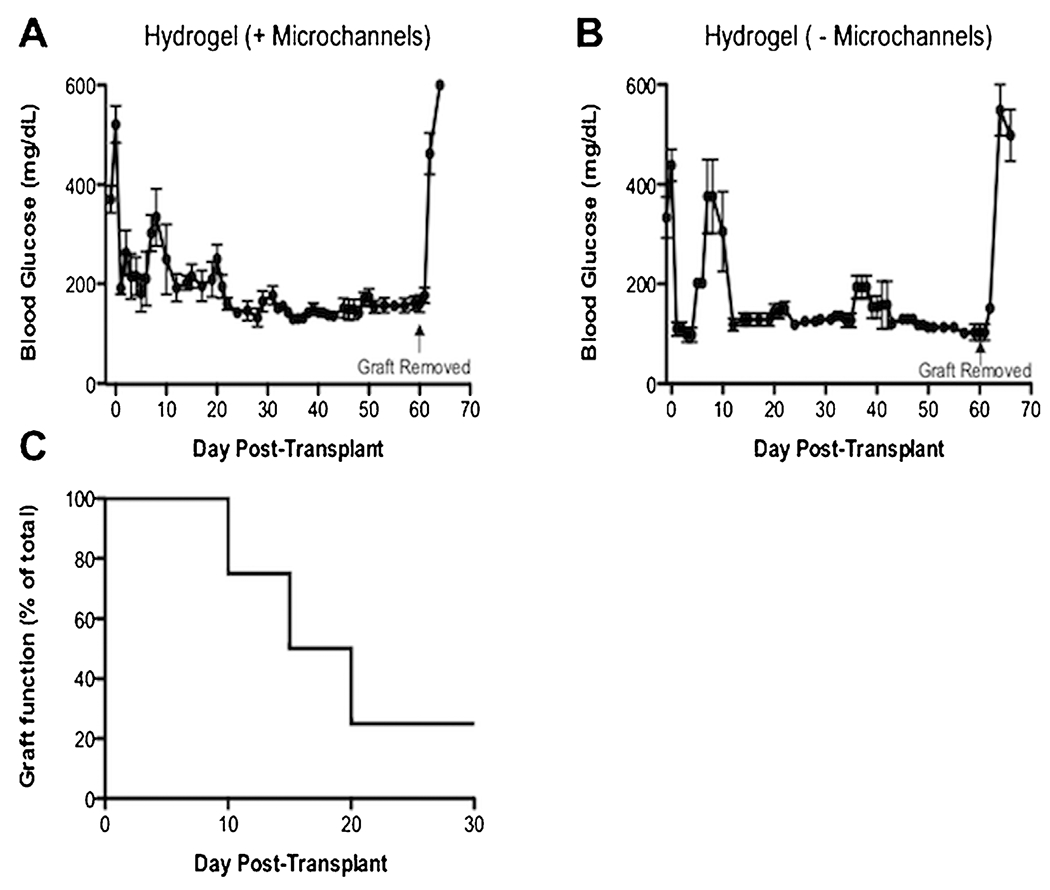
Device function monitored in diabetic mice. (A) Hydrogel devices with 500 islets with microchannels (±SEM, n = 6), or (B) without micro channels (±SEM, n = 3), reversed diabetes in all recipient mice. Normoglycemic levels (<200 mg/dL) were achieved within 2 weeks post-transplantation and maintained over a 2 month period in both groups. Upon removal of hydrogel devices between Day 60–61 (indicated by an arrow), all mice reverted to a diabetic state (>300 mg/dL) within 2 days. Statistical differences were not seen in engraftment between these groups (P = 0.10) according to an unpaired t-test. (B) Devices with microchannels with 300 islets only reversed diabetes in 25% of recipient mice (n = 4), which indicates 300 islets is an insufficient number to reverse diabetes with this device.
An intraperitoneal glucose tolerance test (IPGTT) was performed on mice receiving 500 islets to investigate glucose responsiveness of encapsulated islets in the PEG hydrogels, with or without microchannels (Fig. 5A). The blood glucose levels of both experimental groups and control mice peaked after 15 min post-injection. At 30 and 60 min time points, blood glucose levels mice continued to decrease toward normoglyemia. At 90 min, all groups achieved normoglycemic levels (~200 mg/dL) and their blood glucose continued to decrease for the remainder of timepoints. Area under the curve (AUC) analysis indicated that mice which received hydrogels with encapsulated islets, with or without microchannels, were not statistically different compared to control mice with native pancreata (P = 0.12, Fig. 5B).
Figure 5.
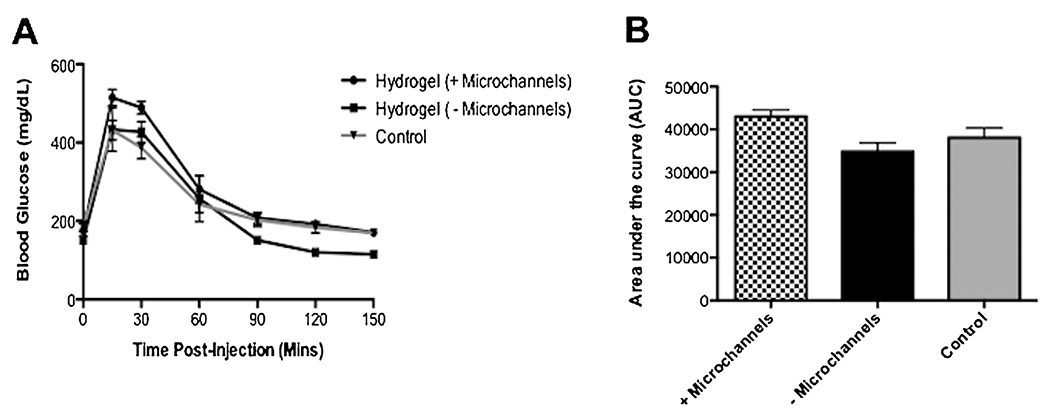
Glucose responsiveness of hydrogel devices. (A) An intraperitoneal glucose tolerance test (IPGTT) confirms both experimental hydrogel groups (with and without microchannels) and control mice achieved normoglycemia 90 min post-dextrose injection. (B) Area under the curve (AUC) indicates the mice with hydrogel devices, with or without microchannels, are not statistically significant compared to control mice according to a one-way ANOVA (±SEM, P = 0.12; n, +Microchannels (n = 6), −Microchannels (n = 3), Control (n = 9). Mice in all groups achieved normoglycemic levels (~200 mg/dL) 90 min post-injection of dextrose.
Histological Analysis of Hydrogel Implants
Hydrogel devices removed at 8-weeks post-transplant were sectioned and stained with insulin and a Hoechst nuclear counterstain to confirm their presence and functionality post-encapsulation in vivo. Encapsulated islets stained positive for insulin and were clearly identified across graft samples (Fig. 6A–D). Islets were observed to be surrounded by the hydrogel and were not in contact with the host tissue. These results indicate encapsulated islets maintained their morphology, viability, and function in the non-degradable hydrogels.
Figure 6.
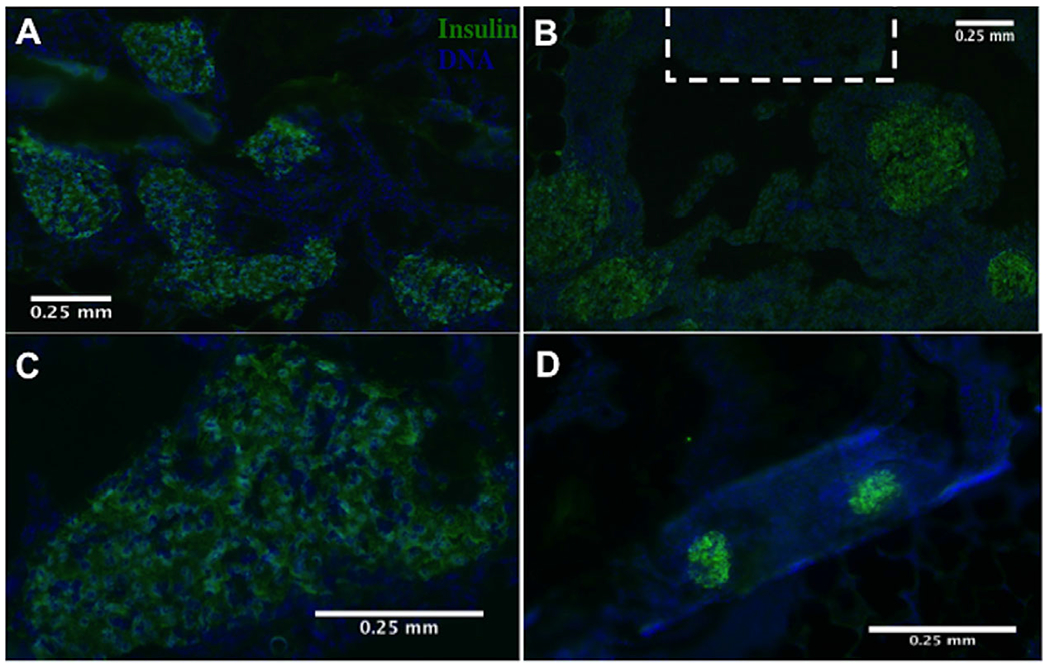
Hydrogel devices explanted day 60 post-transplantation retain islet morphology and viability. (A) Insulin-positive islets were identified throughout the devices with microchannels and (B) near microchannels (indicated with a dashed white line) (10× magnification). (C) Higher magnification images confirmed islet morphology was maintained (20× magnification) in microchanneled devices. (D) Presence of insulin-positive islets was also confirmed in hydrogel devices without microchannels.
Explanted hydrogels, with and without microchannels, were analyzed histologically for cellular infiltration around the implant and vascularization within the microchannel regions of the hydrogel. H&E staining confirmed cellular growth was confined to the perimeter of hydrogel devices without microchannels (Fig. 7A), and islets were identified in both gel groups (Fig. 7A and B). For hydrogels with microchannels, the cell distribution around the hydrogel exterior was similar to the hydrogels without microchannels, yet cellular infiltration was observed in the microchannels (Fig. 7C). Histological staining identified CD31-positive cells within the microchannels, consistent with the opportunity of directing vascular growth through defined regions of the hydrogel (Fig. 7B and C). Collectively, histological results suggest this microchannel architecture can direct vascular growth among the transplanted islets and may be a parameter to further investigate to reduce the number of encapsulated islets needed to achieve normoglycemia. Furthermore, picrosirius red staining indicated no significant fibrotic overgrowth as confirmed by thin layers of connective tissue at the gel-adipose tissue interface in both experimental groups (Fig. 8).
Figure 7.
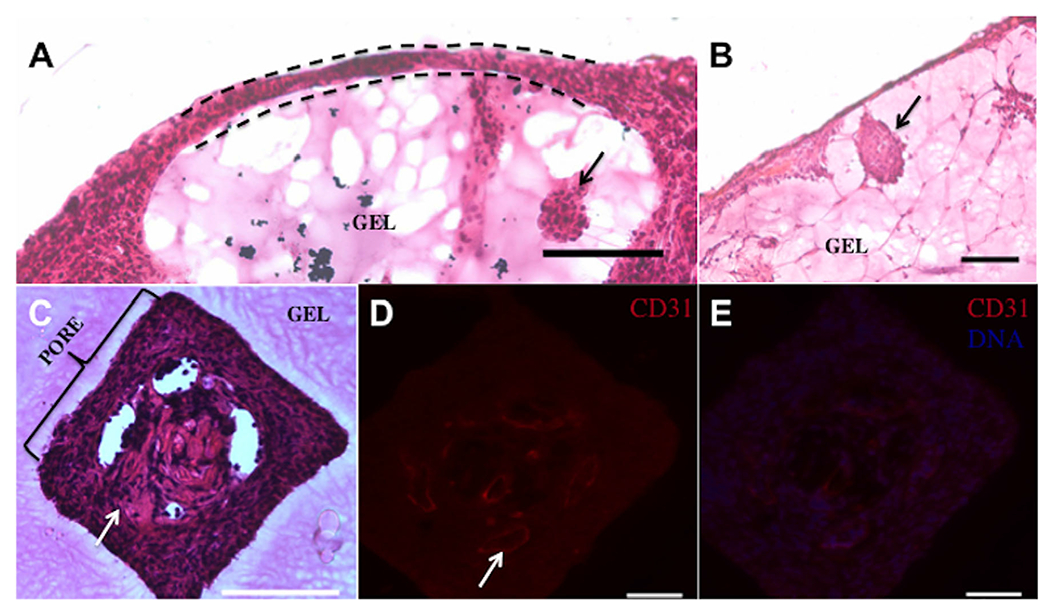
Cellular growth and vessel infiltration of hydrogel devices at day 60 post-transplantation. (A) Cellular growth (indicated by dashed line) was confined to the perimeter of hydrogel devices without microchannels. Encapsulated islets (denoted by a black arrow) were identified in (−) microchannels and (B) (+) microchannel devices. (C) Cellular ingrowth (indicated by a white arrow) occurred into the specific microchannel regions of hydrogels relative to the surrounding hydrogel. (D) CD31-positive cells are identified in the microchannels of the gel which demonstrates vessels (indicated by a white arrow) are available to nearby encapsulated islets. (E) CD31-positive cells are present in the microchannels with a nuclear counterstain. Scale bar: 100 μm.
Figure 8.
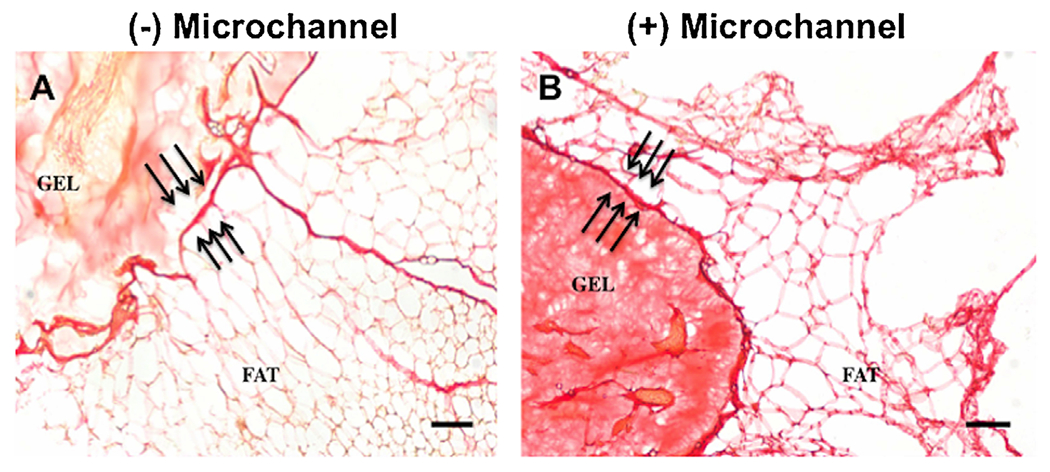
Absence of fibrosis around hydrogel devices confirmed at hydrogel-adipose tissue interface. Thin layers of connective tissue at the hydrogel–adipose tissue interface (indicated by triple arrows) demonstrates no foreign body response to the hydrogel material, either without (A) or with microchannels (B). Scale bar: 100 μm.
Discussion
In this report, we demonstrated the feasibility of non-degradable PEG hydrogels as a macroencapsulation device to encapsulate 500 pancreatic islets and restore normoglycemia in diabetic mice over a 2-month period using the peritoneal fat transplantation site. Rodent studies with unencapsulated murine islets in other transplantation sites, such as the liver and the renal subcapsule, have achieved euglyecmia with lower islet numbers (~200–300 islets). Additional sites such as subcutaneous and the intraperitoneum have used comparable numbers of encapsulated islets to achieve normoglycemia, 500–800 and 750–800 islets, respectively (Merani et al., 2008). The encapsulation system provides the opportunity to isolate the transplanted islets from direct contact with the host tissue, and can allow for retrieval of the transplanted islets. The approach investigated herein employs hydrogel encapsulation that has been implemented with numerous microencapsulation strategies, yet also creates a 3D implantable structure that aims to create a defined site in vivo for cell delivery. Among the hydrogels employed for encapsulation, alginate microcapsules are the most common, which have demonstrated engraftment and function in rodent models with transplantation of islets able to maintain euglycemia for times typically on the order of 75 days, though some reports have demonstrated function for much longer times (de Souza et al., 2011). However, alginate microcapsules have had limited efficacy in larger animal models (Buder et al., 2013; de Souza et al., 2011). Approaches to minimize mass transport limitations have included coating of islets with non-adhesive polymers, or islet-loaded microcapsules with a minimal volume of alginate and procedures are continuing to be refined to provide stable coatings and to prevent a fibrotic overgrowth with the capsule (Jang et al., 2004; Safley et al., 2008). Microcapsules have most commonly been delivered into the peritoneal cavity, which can be challenging due to the relatively low oxygen concentrations relative to that observed in the pancreas. To avoid oxygen deprivation and limited access to nutrients, the hydrogel devices utilized in this study were transplanted into the fat pad, a highly vascularized site, to promote survival of encapsulated islets.
In contrast to microencapsulation, a macroencapsulation device is implanted to a defined site with the objective of modulating the environment to enhance islet survival and function. Numerous in vitro-based studies have demonstrated that PEG hydrogels can provide a controllable 3D environment that supports islet survival and function. (Lin et al., 2009; Su et al., 2010; Weber et al., 2007; Weber and Anseth, 2008). A degradable PEG hydrogel (5%w/v), in conjunction with localized delivery of vascular endothelial growth factor (VEGF), has been reported to support the in vivo survival and function of transplanted islets following implantation into the mesentery, with normalization of blood glucose levels by day 24 (Liao et al., 2013; Phelps et al., 2013). Herein, we report on the use of non-degradable PEG hydrogels (10% w/v) for cell transplantation, and demonstrate normalization of blood glucose levels by day 12 with transplantation into the epididymal fat pad. A 10% PEG hydrogel was employed, as a lower percentage of PEG would not form well within the PDMS mold, though gels could be formed with lower PEG percentages if formed outside of the mold. The epididymal fat pad implantation site, relative to the mesentery, may improve islet survival following transplantation, as the delivery of angiogenic factors were not employed for these studies. Glucose tolerance tests demonstrated a return to normoglycemia within 90 min, which is consistent with or superior to many encapsulating hydrogels (Dang et al., 2013; de Souza et al., 2011; Yun et al., 2007). The use of non-degradable gels was aimed at future studies with allogeneic transplantation, in which the adaptive immune cells can be excluded from contacting the islets. The penetration of inflammatory cytokines into the gel will ultimately need to be addressed, for which multiple reports have indicated the potential for modifying PEG with peptides or antibodies against key inflammatory cytokines, such as TNF-α (Lin et al., 2009; Su et al., 2010).
Many macroencapsulation devices have complex architectures to support vascular growth among the transplanted islets, while excluding immune cells. Devices, such as Theracyte, have been implanted to promote vascularization of the device, with islets loaded through a port at later times (Kumagai-Braesch et al., 2013; Qi, 2014). Similarly, the Sernova pouch is pre-vascularized prior to delivery of islets through channels that are opened in the device (Qi, 2014). Herein, we demonstrated that PDMS casting can be employed to create devices with regularly spaced channels. A previous study reported that similarly sized micropores permitted mature vascularized tissue formation throughout a porous PEG hydrogel (Chiu et al., 2011). The microchanneled hydrogels reported herein demonstrated vessel growth through the channels that are near the islets, with the transplanted islets able to restore euglycemia. These studies herein focused on the casting approach and its impact on islet survival and function, thus the slab and microchanneled hydrogels had a similar minimum diffusion distance, and differences in function were not expected or observed between the gel designs. Ongoing studies are focusing on parameters such as channel size and spacing for their impact on islet survival and function, which may be important for delivering the relatively large mass of islets that are needed clinically. These parameters are of particular importance if thicker devices are needed to accomodate the islet mass delivered as they can reduce mass transport limitations. Furthermore, a channeled architecture may be combined with the delivery of angiogenic factors to promote a robust vascular network.
This hydrogel platform had one main mechanism to promote adhesion for islets, however bioactive coatings with extracellular matrix proteins can be considered to facilitate integration with the host tissue. Extracellular matrix proteins provide structural support and bind cell surface integrins that mediate adhesion and activate intracellular signaling pathways that promote islet survival. PEG hydrogel devices in this study were modified with RGD peptide, which has been reported to reduce islet apoptosis and support islet function (Weber et al., 2007; Weber and Anseth, 2008). The PEG-maleimide used herein had greater incorporation efficiencies of RGD and faster gelation kinetics relative to other PEG chemistries such as PEG-vinyl sulfone or PEG-acrylate (Phelps et al., 2012). RGD modification may also help reduce fibrosis around the hydrogel graft, as PEG modified with RGD has been reported to limit the development of fibrotic overgrowth due to activated macrophages (Jang et al., 2004). Transplantation of the devices into the peritoneal fat can avoid IBMIR that is associated with hepatic delivery, yet the devices can be apposed to the blood vessels that are presented throughout the fat pad. Micro- and macroencapsulation systems have typically required large masses of islets for transplantation due to poor engraftment, (Phelps et al., 2013) and the ability to create architectures that can define vascular ingrowth may ultimately provide an opportunity to support this relatively large islet mass.
Conclusion
We present a non-degradable hydrogel-based device and demonstrate the feasibility of this approach for long-term function of encapsulated islets in vivo. The microchannel regions of the gel permit vascular ingrowth near the islets, however a functional difference was not observed in mice that received hydrogel implants with microchannels. Islet engraftment and long-term restoration of normoglycemia were observed for all groups following transplantation. These studies with a syngeneic transplantation model provide a foundation for future studies with allogeneic and xenogeneic islets. This design can be refined to maximally protect the islets and minimize mass transport limitations, and additional modifications are possible to modulate the host response at the site of transplantation, which are the focus of ongoing studies with allogeneic islet transplantation.
Acknowledgments
This work was funded by the Juvenile Diabetes Research Foundation (JDRF). Peter Rios performed this work under funding support from the National Science Foundation (NSF).
Footnotes
Conflicts of interest: The authors of this manuscript have no disclosures or conflicts of interest to report. No competing financial interests exists.
References
- Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. 2007. Islet encapsulation: Strategies to enhance islet cell functions. Tissue Eng 13:589–599. http://www.ncbi.nlm.nih.gov/pubmed/17518605 [DOI] [PubMed] [Google Scholar]
- Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL. 2006. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation 82:452–459. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2648394&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder B, Alexander M, Krishnan R, Chapman DW, Lakey JR. 2013. Encapsulated islet transplantation: Strategies and clinical trials. Immune Netw 13:235–239. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3875781&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, Brey EM. 2011. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 32:6045–6051. http://www.ncbi.nlm.nih.gov/pubmed/21663958 [DOI] [PubMed] [Google Scholar]
- Colton CK. 2014. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev 68:93–110. [DOI] [PubMed] [Google Scholar]
- Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, Ma M, Hollister-Lock J, Tang KM, Gu Z, Cheng H, Weir GC, Langer R, Anderson DG. 2013. Enhanced function of immuno-isolated islets in diabetes therapy bycoencapsulation with an anti-inflammatory drug. Biomaterials 34:5792–5801. 10.1016/j.biomaterials.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TA, West T, Cohen M, Boiarski T, Rampersaud A. 2004. Nanoporous microsystems for islet cell replacement. Adv Drug Deliv Rev 56:1661–1673. http://www.sciencedirect.com/science/article/pii/S0169409x04001462 [DOI] [PubMed] [Google Scholar]
- de Souza YEDM, Chaib E, de Lacerda De PG, Crescenzi A, Bernal-Filho A, D’Albuquerque LAC. 2011. Islet transplantation in rodents. Do encapsulated islets really work? Arq Gastroenterol 48:146–152. [DOI] [PubMed] [Google Scholar]
- Gibly RF, Graham JG, Luo X, Lowe WL, Hering BJ, Shea LD. 2011. Advancing islet transplantation: From engraftment to the immune response.Diabetologia 54:2494–2505. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3193607&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruessner RWG, Gruessner AC. 2013. The current state of pancreas transplantation. Nat Rev Endocrinol 9:555–562. http://www.ncbi.nlm.nih.gov/pubmed/23897173 [DOI] [PubMed] [Google Scholar]
- Hudalla GA, Eng TS, Murphy WL. 2008. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules 9:842–849. 10.1021/bm701179s [DOI] [PubMed] [Google Scholar]
- Jang JY, Lee DY, Park SJ, Byun Y. 2004. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials 25:3663–3669. http://www.ncbi.nlm.nih.gov/pubmed/15020141 [DOI] [PubMed] [Google Scholar]
- Jeong J-H, Yook S, Hwang JW, Jung MJ, Moon HT, Lee DY, Byun Y. 2013. Synergistic effect of surface modification with poly(ethylene glycol) and immunosuppressants on repetitive pancreatic islet transplantation into antecedently sensitized rat. Transplant Proc 45:585–590. http://www.sciencedirect.com/science/article/pii/S0041134512006768 [DOI] [PubMed] [Google Scholar]
- Kharkar PM, Kiick KL, Kloxin AM. 2015. Design of thiol- and light-sensitive degradable hydrogels using michael-type addition reactions. Polym Chem 6:5565–5574. http://pubs.rsc.org/en/content/articlehtml/2015/py/c5py00750j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizilel S, Scavone A, Liu X, Nothias J-M, Ostrega D, Witkowski P, Millis M. 2010. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng Part A 16:2217–2228. http://online.liebertpub.com/doi/abs/10.1089/ten.TEA.2009.0640?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.orgrfr_dat=cr_pub%3Dpubmed [DOI] [PubMed] [Google Scholar]
- Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodström-Tullberg M, Tibell A. 2013. The theracyte™ device protects against islet allograft rejection in immunized hosts. Cell Transplant 22:1137–1146. [DOI] [PubMed] [Google Scholar]
- Liao SW, Rawson J, Omori K, Ishiyama K, Mozhdehi D, Oancea AR, Ito T, Guan Z, Mullen Y. 2013. Maintaining functional islets through encapsulation in an injectable saccharide-peptide hydrogel. Biomaterials 34:3984–3991. http://www.ncbi.nlm.nih.gov/pubmed/23465491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-C, Metters AT, Anseth KS. 2009. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials 30:4907–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Nothias J-M, Scavone A, Garfinkel M, Millis JM. 2015. Biocompatibility investigation of polyethylene glycol and alginate-poly-L-lysine for islet encapsulation. ASAIO J 56:241–245. http://www.ncbi.nlm.nih.gov/pubmed/20400892 [DOI] [PubMed] [Google Scholar]
- Merani S, Toso C, Emamaullee J, Shapiro AMJ. 2008. Optimal implantation site for pancreatic islet transplantation. Br J Surg 95:1449–1461. http://www.ncbi.nlm.nih.gov/pubmed/18991254 [DOI] [PubMed] [Google Scholar]
- O’Sullivan ES, Vegas A, Anderson DG, Weir GC. 2011. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr Rev 32:827–844. http://www.ncbi.nlm.nih.gov/pubmed/21951347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou G, Sokic S, Turturro M. 2012. Synthetic PEG hydrogels as extracellular matrix mimics for tissue engineering applications. In: Sammour R, editor. Biotechnology—Molecular studies and novel applications for improved quality of human life. ISBN: 978-953-51-0151-2, InTech, Available from: http://www.intechopen.com/books/biotechnology-molecular-studies-and-novel-applications-for-improved-quality-of-human-life/synthetic-peg-hydrogels-as-extracellular-matrix-mimics-for-tissue-engineering-applicationsInTech [Google Scholar]
- Park H-S, Kim J-W, Lee S-H, Yang HK, Ham D-S, Sun C-L, Hong TH, Khang G, Park C-G, Yoon K-H. 2015. Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation. J Tissue Eng Regen Med 1–11. http://www.ncbi.nlm.nih.gov/pubmed/26043934 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, García AJ. 2012. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater 24:64–70, 2. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3517145&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Headen DM, Taylor WR, Thulé PM, García AJ. 2013. Vasculogenic biosynthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials 34:4602–4611. http://www.ncbi.nlm.nih.gov/pubmed/23541111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkse GGM, Bouwman WP, Jiawan-lalai R, Terpstra OT, Bruijn JA, Heer EDe. 2006. Integrin signaling via RGD peptides and anti-B1 Abs confers resistance to apoptosis in islets of Langerhans. Diabetes 55:1–6. [DOI] [PubMed] [Google Scholar]
- Qi M. 2014. Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 Diabetes Mellitus. Adv Med 2014:1–15. http://www.hindawi.com/journals/amed/2014/429710/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengifo HR, Giraldo JA, Labrada I, Stabler CL. 2014. Long-term survival of allograft murine islets coated via covalently stabilized polymers. Adv Healthc Mater 3:1061–1070. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4107175&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JRT, Shapiro AMJ. 2005. Five-year follow-up after clinical islet transplantation. Diabetes 54:2060–2069. http://www.ncbi.nlm.nih.gov/pubmed/15983207 [DOI] [PubMed] [Google Scholar]
- Safley SA, Cui H, Cauffiel S, Tucker-Burden C, Weber CJ. 2008. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine. J Diabetes Sci Technol 2:760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, Shea LD. 2008. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation 85:1456–1464. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2597660&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharp DW, Marchetti P. 2013. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 67–68:35–73. http://www.ncbi.nlm.nih.gov/pubmed/23916992 [DOI] [PubMed] [Google Scholar]
- Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. 2011. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 32:2524–2531. http://www.sciencedirect.com/science/article/pii/S0142961210015875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, Tang Q, Roy S. 2016. Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport. Sci Rep 6:23679. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4806308&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Roy S. 2015. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices. Biotechnol Bioeng 9999:1–22. http://www.readcube.com/articles/10.1002%2Fbit.25895?r3_referer=wol&trackingaction=preview_click&show_checkout=1&purchase_referrer=onlinelibrary.wiley.com&purchase_site_license=LICENSE_DENIED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Hu B-H, Lowe WL, Kaufman DB, Messersmith PB. 2010. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials 31:308–314. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2784009&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaithilingam V, Tuch BE. 2011. Islet transplantation and encapsulation: An update on recent developments. Rev Diabet Stud 8:51–67. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3143677&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LM, Anseth KS. 2008. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol 27:667–673. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2631362&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Haskins K, Anseth KS. 2007. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 28:3004–3011. http://www.ncbi.nlm.nih.gov/pubmed/17391752 [DOI] [PubMed] [Google Scholar]
- Weber LM, Lopez CG, Anseth KS. 2009. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J Biomed Mater Res A 90:720–729. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2913724&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JT, Chaikof EL. 2009. Challenges in immunoisolation. Adv Drug Deliv Rev 60:124–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Lee D, Hee Nam J, Byun Y. 2007. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials 28:1957–1966. [DOI] [PubMed] [Google Scholar]
- Zustiak SP, Boukari H, Leach JB. 2010. Solute diffusion and interactions in cross-linked poly(ethylene glycol) hydrogels studied by fluorescence correlation spectroscopy Soft Matter 6:3609. http://xlink.rsc.org/?DOI=c0sm00111b [DOI] [PMC free article] [PubMed] [Google Scholar]


