Abstract
The in vitro culture of ovarian follicles has provided critical insight into the biology of the follicle and its enclosed oocyte and the physical interaction and communication between the theca and granulosa cells and the oocyte that is necessary to produce meiotically competent oocytes. Various two-dimensional (2D) and three-dimensional (3D) culture systems have been developed to evaluate the effect of growth factors, hormones, extracellular matrix components and culture conditions on follicle development and oocyte growth and maturation. Among these culture systems, 3D systems make it possible to maintain follicle structure and support communication between the various cell compartments within the follicle. In this review article, we will discuss the three main approaches to ovarian follicle culture: 2D attachment systems, 3D floating systems and 3D encapsulated systems. We will specifically emphasise the development of and advances in alginate-based encapsulated systems for in vitro follicle culture.
Keywords: cell culture, cytoskeleton, folliculogenesis, growth
Introduction
In vitro follicle culture is an important tool used to investigate events that occur during folliculogenesis to produce meiotically competent oocytes (Picton et al. 2008) by creating a microenvironment similar to that found in the ovaries (Hartshorne 1997; West et al. 2007a). This technique may also have clinical applications for women who want to preserve their fertility by cryopreserving ovarian tissue before undergoing cancer therapy; at a later time, thawed follicles could be cultured to produce mature oocytes for in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI). This approach would provide an alternative to ovarian tissue transplantation (Hovatta 2004).
Follicles may be cultured in either two-dimensional (2D, or non-spherical) systems or three-dimensional (3D, or spherical) systems. In 2D ‘attachment’ systems, only part of the follicle surface makes contact with the culture substrate on a flat tissue-culture plate. As granulosa cells attach, the follicle flattens and expands outward. To maintain the spherical structure of the follicle, 3D culture systems were developed in which the follicle ‘floats’ in rotating tubes or inverted microdrops, or is encapsulated in a culture matrix, such as alginate. Although major advances in our understanding of follicle biology have been achieved with 2D attachment culture systems (Eppig and O’Brien 1996; O’Brien et al. 2003; Lenie et al. 2004), recent studies have shown that 3D culture systems more closely mimic the physiological environment of the ovary, preserving follicular architecture and the interaction between somatic and germ cells (Benton et al. 2009).
Three-dimensional follicle culture is performed using various methods, including suspension in inverted drops, within rotating vessels or in round-bottomed ultra-low-attachment plates (Boland et al. 1993; O’Brien et al. 2003; Rowghani et al. 2004; Wycherley et al. 2004; Heo et al. 2010; Sánchez et al. 2012). Encapsulation methods using hydrogels such as alginate have been applied to the in vitro culture of various tissues (Butcher and Nerem 2004; Azab et al. 2006; Eyrich et al.2007), including ovarian follicles (Pangas et al. 2003; Kreeger et al. 2005; Xu et al. 2006b). Alginate is a natural biopolymer extracted from brown algae and its absence of bioactivity, intrinsic biocompatibility and capacity for forming a gel with well-defined features have made it particularly useful for in vitro follicle culture (Heise et al. 2005).
In this paper, we will first discuss the importance of preserving follicle structure and cellular communication between follicle cells in culture. We will then review the different in vitro ovarian follicle culture methods, with emphasis on the use of alginate-based matrices.
Follicle structure
Cell morphology
The follicle is composed of an oocyte surrounded by somatic granulosa and theca cells and represents the morpho-functional unit of the ovary. Folliculogenesis is an extremely complex process, in which various endocrine, autocrine and paracrine factors act in a coordinated manner to direct the proliferation, growth and differentiation of granulosa and theca cells, as well as oocyte growth and maturation. Follicles can be classified according to their developmental stage. Preantral follicles (primordial, primary and secondary) represent 90% of the ovarian follicular population and form the pool of female gametes available throughout a woman’s lifetime, also called the ovarian reserve (Liu et al. 2001). Antral follicles (tertiary and preovulatory) are large follicles that contain a fluid-filled antrum and, in humans, only one of these large follicles is selected to undergo ovulation per month (Silva 2005). With each cycle, a cohort of preantral follicles is activated and begins to develop, with oocyte growth, granulosa cell proliferation and a shift in the morphology of granulosa cells from squamous to cubic. As follicles develop, the somatic cells surrounding the oocyte proliferate and differentiate and the oocyte grows in preparation for maturation, ovulation and fertilisation (van den Hurk and Zhao 2005).
Follicular development is highly dependent on the morphology and arrangement of the cells that compose the follicle, which are essential to preserve its function and support oocyte growth and maturation (Hay 1984). Researchers have demonstrated that several cellular processes are affected by cell morphology, such as histone acetylation (Le Beyec et al. 2007) and gene expression (Birgersdotter et al. 2005), in addition to apoptosis, cellular proliferation and differentiation and steroidogenesis (Chen et al. 1997; Birgersdotter et al. 2005).
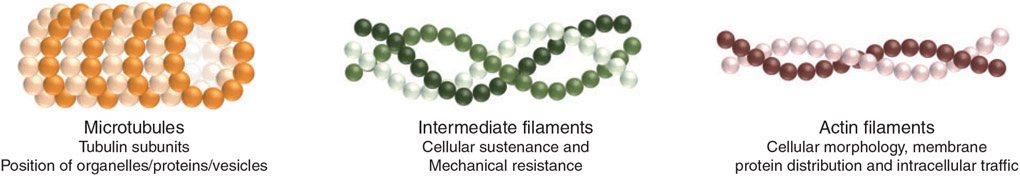
The structural integrity of the cells is determined by the cytoskeleton, a network of filaments that acts as a mechanical support system, maintaining the shape of cells and influencing the arrangement of intracellular components. The cytoskeleton is composed of simple protein subunits that polymerise to form filaments. The three main types of filaments are: (1) microtubules, which are composed of tubulin subunits and determine the position of organelles, proteins and vesicles, (2) intermediate filaments that provide cellular sustenance and mechanical resistance to tension and traction and (3) actin filaments, which are responsible for the control of cellular morphology, membrane protein distribution and intracellular traffic (Eitzen 2003; Fig. 1). These filaments are dynamic and adaptable structures that reorganise themselves according to cellular requirements (Ferreira et al. 2009).
Fig. 1.
The cytoskeleton is composed of three types of filaments: microtubules, intermediate filaments and actin filaments.
Activities performed by the cytoskeleton are dependent on proteins that associate with the filaments and interconnect the filaments to form networks, as well as interacting with the plasma and organelle membranes. According to Ben-Ze’ev and Amsterdam (1986), changes in the expression of genes encoding cytoskeleton proteins negatively affects granulosa cell differentiation and, consequently, steroidogenesis, as well as the formation of cellular junctions and actin cytoskeleton remodelling (Sechi and Wehland 2000).
The cytoskeleton also plays an important role in the organisation of the extracellular matrix, which is composed of several proteins (collagen, elastin, fibronectin and laminin) and polysaccharides (glycosaminoglycan and proteoglycan) that are either secreted locally or remain associated with the cell membrane. The extracellular matrix acts as a structural support within follicles by associating with receptors, the integrins, which are present on the surface of follicle cells.
Researchers have verified that the cytoskeletal arrangement and extracellular matrix orientation influence one another and this interaction is crucial to the preservation of cellular architecture. Indeed, intracellular actin filaments interact with extracellular fibronectin molecules through cell-surface integrins, creating a tension that exposes additional fibronectin binding sites that connect with other proteins and integrins (Alberts et al. 2002). In turn, the extracellular matrix influences cytoskeletal organisation that can stimulate changes in cellular behaviour, such as proliferation (Lochter and Bissel 1995). For example, fibronectin deficiency had been linked to the abnormal morphology of cancer cells (Alberts et al. 2002). Proteins associated with actin filaments also interact with integrins on the internal surface of the plasma membrane (Luna 1991; Adams and Watt 1993) allowing a bidirectional transfer of information between the extracellular matrix and cellular cytoplasm (Cukierman et al. 2002). Signals transmitted from outside the cell via integrins can reach the nucleus to regulate gene expression (Adams and Watt 1993; Wang et al. 1993).
The extracellular matrix also regulates critical events in follicle development and functionality, including cell migration, growth, proliferation and survival, as well as cellular shape and organisation within the follicle (Cukierman et al. 2002; Woodruff and Shea 2007).
Cell–cell connections and communication
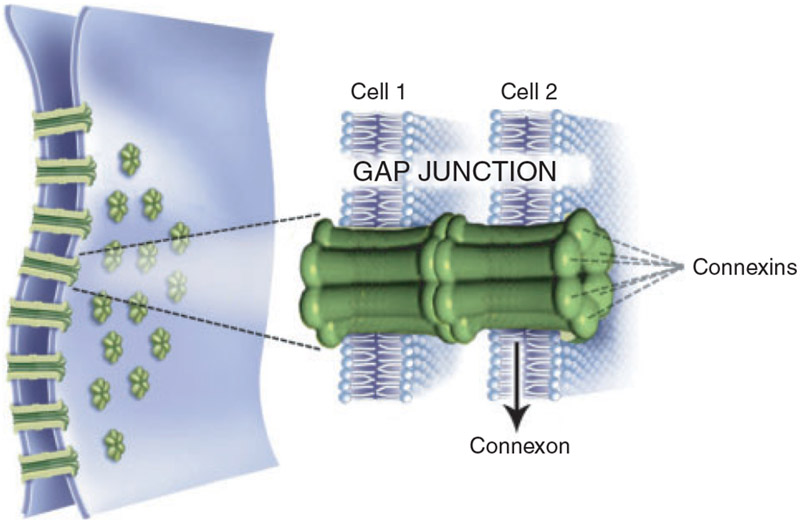
Maintenance of cellular morphology is not only essential to preserve the interaction of cells with the extracellular matrix, but also to maintain physical contact between neighbouring cells, which occurs through various cell–cell junctions. Among these, gap junctions transfer ions and molecules of low molecular weight between cells (Eppig 1991). These junctions are specialised cell-surface channels composed of a hexagonal arrangement of proteins called connexins (Cxs), which form connexons by interacting with the Cx proteins of neighbouring cells (Granot and Dekel 2002; Fig. 2).
Fig. 2.
A gap-junction channel is composed of two connexons present in the membranes of adjacent cells. Each connexon is formed of a hexagonal arrangement of proteins called connexins.
In various mammalian species, several types of connexin have been identified in ovarian tissue, including Cx26, Cx30.3, Cx32, Cx37, Cx40, Cx43 and Cx45 (Grazul-Bilska et al. 1997). Cx43 is the primary type expressed in ovarian follicles (Risek et al. 1990). Simon et al. (1997) reported that Cx43 expression is restricted to granulosa cells, whereas oocytes produce only Cx37. Other studies have found Cx43 mRNA and protein in the oocytes of the rat (Granot and Dekel 2002) and cow (Sutovsky 1993). Folliculogenesis is disrupted in mice null for Cx37 (Simon et al. 1997) or Cx43 (Ackert et al. 2001), with no follicles containing multiple layers of granulosa cells observed in the ovary, supporting a crucial role of these proteins in follicle development.
Indeed, gap junctions are essential to maintain bidirectional communication between different follicular compartments (oocyte, granulosa cells and theca cells), ensuring the delivery of nutrients, growth factors and hormones required for follicle growth and oocyte development. Evidence suggests that the oocyte does not contain certain amino acids and is unable to perform glycolysis and cholesterol biosynthesis without the help of growth factors provided by neighbouring granulosa cells (Eppig 1991).
Conversely, proliferation and some metabolic processes of the granulosa cell are controlled by factors derived from oocytes (Eppig et al. 2005; Su et al. 2009). During follicle development, the granulosa cell compartment is not vascularised and requires contact with neighbouring cells through gap junctions to exchange gases and metabolic nutrients and waste products. Moreover, ovarian follicles fail to develop beyond the primary stage in mice that do not express growth differentiation factor-9 (GDF-9), a member of the transforming growth factor-β (TGF-β) family expressed only in oocytes (McGrath et al. 1995). Oocytes secrete paracrine signals that enable cumulus cells to produce hyaluronic acid and undergo cumulus expansion in response to FSH stimulation (Buccione et al. 1990; Salustri et al. 1990) and suppress progesterone production by granulosa cells (Vanderhyden and Tonary 1995).
Contact between oocytes and granulosa cells is also required to regulate the expression of genes involved in follicular development. This is evidenced by studies that show that oocytes influence different patterns of gene expression in granulosa cells, such as luteinizing hormone receptor (LHR), Kit Ligand (KL) and anti-Müllerian hormone (AMH) mRNA (Eppig et al. 1997; Joyce et al. 1999; Salmon et al. 2004). Luciano et al. (2011) suggest that gap junction-mediated communication between germinal and somatic compartments plays a fundamental role in the regulation of chromatin remodelling and transcription, which, in turn, are related to meiotic competence acquisition. This study showed that gap-junction coupling during oocyte culture before in vitro maturation enhanced the ability of early antral oocytes to undergo meiosis and early embryonic development. On the other hand, when the gap junction was opened, cyclic guanosine monophosphate (cGMP) entered the oocyte from the granulosa cells and, as cGMP inhibits phosphodiesterase 3A in the oocyte, cAMP levels were kept high and meiotic arrest maintained (Norris et al. 2009; Vaccari et al. 2009).
In vitro follicle culture
Media components and conditions
Over the last few decades several culture systems have been developed to support ovarian follicle development in vitro. As with the culture of other tissues, follicle growth in culture is dependent on various components within the culture medium, including nutrients, electrolytes, antioxidants, amino acids, energy substrates and vitamins (Eppig and Schroeder 1989; Boland et al. 1994; Fortune 2003; Picton et al. 2008). Ascorbic acid, an important antioxidant, supports folliculogenesis by inhibiting apoptosis in rat preantral follicles (Murray et al. 2001) and enhancing the viability of goat follicles (Rossetto et al. 2009; Silva et al. 2011) after long-term culture. Moreover, the addition of pyruvate, glutamine, hypoxanthine, transferrin and selenium to the culture medium can promote follicle growth and increase the percentage of morphologically normal follicles (Silva et al. 2004; Demeestere et al. 2005). Another component routinely added to the culture medium of cells and tissues is insulin, a hormone that acts as an important mediator of follicular development, steroidogenesis, oocyte maturation and subsequent development of the embryo (Yaseen et al. 2001). However, at high concentrations, insulin appears to have a toxic effect on follicle cells. This hypothesis was corroborated by recent studies showing that basic culture medium containing 10 μg mL−1 insulin was not able to maintain the viability of caprine preantral follicles (Chaves et al. 2010; Faustino et al. 2011). Furthermore, when high insulin exposure occurs during the period of oocyte growth, impaired chromatin remodelling and condensation during meiosis have been reported (Acevedo et al. 2007). On the other hand, the exposure of follicles to low (physiological) insulin concentrations will positively influence oocyte gene expression, allowing in vitro-produced oocytes to express similar levels to those produced in vivo (Sánchez et al. 2011).
In addition, in vivo and in vitro studies have established the importance of gonadotrophins and growth factors in folliculogenesis, and the supplementation of the culture medium with these factors is necessary for efficient and complete follicle development and oocyte maturation in vitro. Luteinising hormone (LH) and follicle-stimulating hormone (FSH) act in a coordinated manner to direct gonadal development and, in the adult mammal, control follicle cell growth and differentiation, steroidogenesis and oocyte development and maturation (Kumar and Matzuk 2000). LH stimulates p450 aromatase activity in theca cells required for steroidogenesis, promotes ovulation and stimulates luteinisation of granulosa cells and theca cells of post-ovulatory follicles (Richards et al. 2002). FSH is the essential driver of antral follicle growth in vivo, stimulating the proliferation and differentiation of granulosa cells and inducing antrum formation. In preantral follicles, it may play an indirect effect via factors released by larger follicles or ovarian stromal cells (Matos et al. 2007).
Studies have investigated the possible role of FSH in earlier follicle development using different culture systems. In alginate-based 3D systems, FSH is essential for the in vitro survival of primate encapsulated preantral follicles (Xu et al. 2010) and promotes the growth of rodent (Kreeger et al. 2005; Xu et al. 2006a), non-human primate (Xu et al. 2010, 2011) and human (Xu et al. 2009a) follicles. The delivery of FSH for preantral follicles cultured in alginate-based 3D systems was also investigated. Heise et al. (2005) verified that the encapsulation may limit access to hormones in the medium resulting in altered development compared with unencapsulated follicles, while the inclusion of FSH in the alginate bead restores follicle growth in response to FSH. Sánchez et al. (2012) used round-bottomed ultra-low-attachment plates to prohibit attachment and flattening out of the early preantral follicles and verified that this new non-attachment condition in combination with decreased FSH concentrations during the antral growth stages positively affected cumulus cells, Lhcgr expression and led to an improvement in oocyte developmental potential.
In addition to gonadotrophins, several growth factors produced by the cells of the follicle act locally to direct various stages of follicle development. Kit ligand (KL), leukaemia inhibitory factor (LIF) and bone morphogenetic protein-4 and −7 (BMP-4 and BMP-7) stimulate the transition from primordial to primary follicles (Nilsson et al. 2002; Nilsson and Skinner 2003, 2004). Growth and differentiation factor-9 (GDF-9) and BMP-15, both secreted by the oocyte, promote proliferation of granulosa cells and the recruitment of theca cells, events that are required for the transition of follicles from the primary to the secondary stage (Knight and Glister 2006). In many species of rodents and domestic animals, factors produced by secondary follicles, including vascular endothelial growth factor (VEGF), transforming growth factor (TGF), insulin-like growth factor (IGF), fibroblast growth factor-2 and −7 (FGF-2 and FGF-7), BMPs and activin are necessary for survival and further development, and removal of these factors (null animals (in vivo) or culture medium (in vitro)) promotes apoptosis.
At the antral stage, locally synthesised peptides play a key role in the regulation of follicular development, through endocrine and paracrine mechanisms (Webb et al. 2003; Fortune et al. 2004). Among these peptides, those of the IGF system, including IGF-1, IGF-2 and the IGF binding proteins (IGFBPs) and some members of the FGF family, such as FGF-2, FGF-7 (or KGF), FGF-8 and FGF-10 (Buratini et al. 2005; Fortune et al. 2004; Buratini 2007), appear to be critical for late-stage follicle development.
Beyond the composition of the culture medium, other factors, such as pH, temperature and oxygen tension affect follicle development in vitro and must mimic the conditions of the ovarian environment (Ye et al. 2007). Changes in pH may compromise cellular function and viability (Kapus et al. 1994), as well as impair the development of preimplantation embryos (rats, Lane et al. 1998; hamsters, Zhao et al. 1995). With regard to temperature, in the transport of ovaries, generally lower temperatures (4 to 20°C) are used to reduce cellular metabolism and, consequently, minimise energy spending and decrease tissue autolysis levels (Salehi et al. 2004; Chaves et al. 2008).
The ideal oxygen concentration for in vitro follicle culture remains somewhat controversial. Some studies suggest that a 5% oxygen concentration is ideal to stimulate follicular growth. In ovine preantral follicle culture, the low O2 concentration (5%) stimulated follicle growth with a high proportion of follicles developing an antral cavity and a healthy cumulus–oocyte complex (Cecconi et al. 1999). Xu et al. (2011) observed that there was an increase in follicular diameter and more healthy oocytes were obtained when the follicles were cultured at 5% O2 compared with those in 20% O2. Similarly, Eppig and Wigglesworth (1995) concluded that concentrations of O2 above 5% have a deleterious effect on oocyte development. On the other hand, different teams demonstrated that the concentration of 20% O2 was more efficient in promoting follicular growth, survival, cellular differentiation, oocyte meiosis resumption and blastocyst formation from preantral follicles grown in vitro (mouse, Hu et al. 2001; pig, Park et al. 2005; cow, Gigli et al. 2006; goat, Silva et al. 2010).
Culture substrate
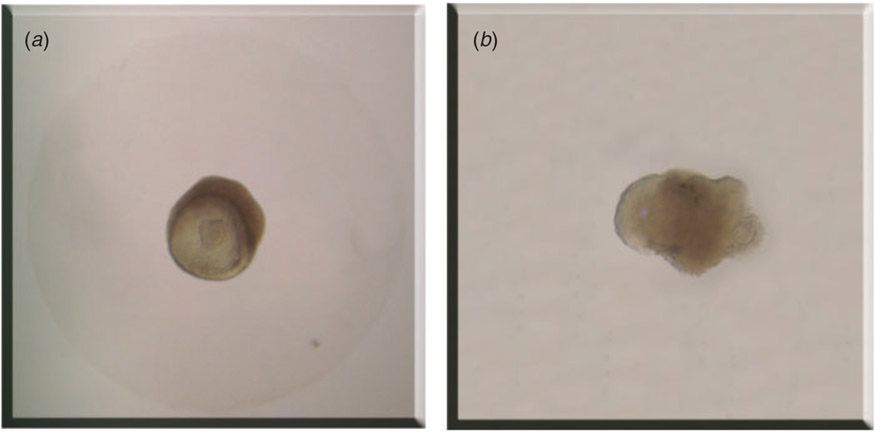
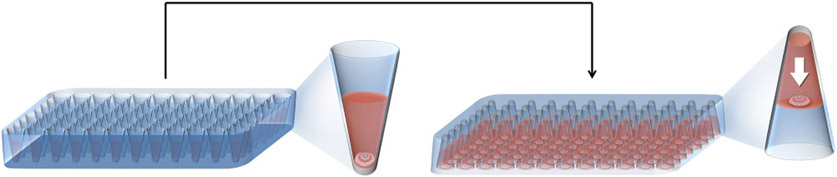
In vitro follicle culture is carried out in various supporting substrates and systems. In 2D systems, follicles are cultured on top of a substrate, such that only a portion of the follicle is in direct contact with the culture surface and thus follicle structure is only partly supported. The culture surface may be plastic or a deposited matrix, such as collagen or agar. In 3D culture systems, follicles are either suspended or encapsulated in the culture substrate, which permits contact with the entire follicle and supports the follicle structure in vitro (Fig. 3a). In these systems, follicles not only maintain their architecture but also phenocopy in vivo growth patterns.
Fig. 3.
Follicles cultured in vitro in different systems. (a) Follicle encapsulated in the culture substrate with its architecture intact. (b) Follicle cultured in 2D system with basal membrane rupture and oocyte extrusion.
During in vitro culture of ovarian follicles in a 2D system, the follicle cells attach to the substrate and flatten out (Zhang et al. 2005). According to Lenie et al. (2004), while the follicle structure is remodelled after attachment to a 2D surface, the interactions among the three different cell types (theca cells, granulosa cells and oocyte) are preserved. Thus, it has been suggested that this remodelling allows better oxygenation and access to nutrition and hormonal support by the innermost cells of the follicle. On the other hand, culture of follicles on the flat and rigid 2D culture surface disrupts gap junctions between follicle cells (Desai et al. 2010) and affects communication between the cells and the extracellular matrix via integrins (Cukierman et al. 2002). The 2D system may also cause basal membrane rupture and spreading of somatic cells away from the oocyte, resulting in a diffuse follicular morphology (Cortvrindt et al. 1996; Fig. 3b).
By contrast, in vitro culture of ovarian follicles in 3D systems maintains the follicle structure and the normal cell–cell and cell–extracellular matrix interactions between the theca and granulosa cells and the oocyte (Xu et al. 2009c). In this system, oocyte growth and granulosa cell proliferation occur radially, starting from the centre of the follicle, similar to what occurs in the ovary (West et al. 2007a). Factors secreted by the granulosa cells and by the oocyte remain within the follicle and act locally to support follicle development and the formation of gap junctions (Sutton et al. 2003).
Studies with different cellular types have shown that 3D culture systems modulate the survival and communication between cells (fibroblasts, Lee et al. 2008), as well as cellular proliferation (smooth muscle cell, Mann and West 2002) and differentiation (mesenchymal stem cells, Salinas and Anseth 2008). Hwa et al. (2007) also verified that the gene expression profile of endothelial cells cultured in a 3D system is more similar to that observed in vivo, when compared with cells cultured on a 2D substrate. For the in vitro culture of ovarian follicles, the use of 3D substrates promotes a higher rate of survival (Oktem and Oktay 2007) and greater follicular growth than in 2D culture systems (Abir et al. 2001; Loret de Mola et al. 2004) and stimulates antrum formation, steroid secretion and DNA synthesis (Roy and Greenwald 1989, 1996). In addition, the culture of mouse preantral follicles under non-attachment conditions, which maintains the three-dimensional relationships between the different cell types, positively influences cumulus cell gene expression (Sánchez et al. 2012).
The in vitro culture of ovarian follicles in 2D and 3D systems has significantly increased our understanding of follicle biology and folliculogenesis and has resulted in the production of fertilisation competent, mature oocytes and the birth of live offspring (Eppig and O’Brien 1996; O’Brien et al. 2003; Xu et al. 2006a). Thereby, the choice of culture system is still controversial. Although numerous studies have optimised the use of 2D systems for a range of different animal models, the good results of 3D systems have shown that they are effective vehicles to support three-dimensional follicular growth (Xu et al. 2006a, 2009a).
3D in vitro follicle culture
To address the limitations of 2D culture systems and better recapitulate the in vivo environment by maintaining the spatial relationships between cells within the follicle, several 3D culture methods have been developed and tested in various mammalian species. These 3D culture systems fall into two general categories: floating systems and encapsulation systems.
Floating system: rotating vessels
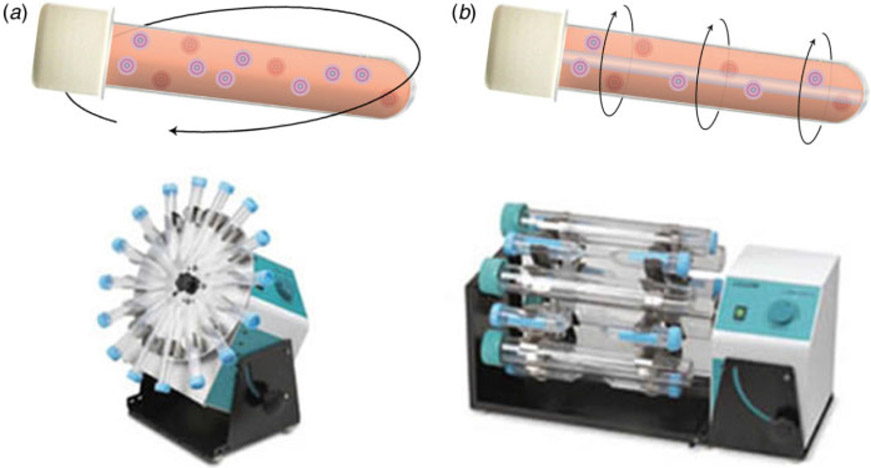
Rotating follicle culture techniques, in which follicle suspensions are inserted into test tubes and subjected to gentle orbital movement (Fig. 4a), have been used by various researchers to prevent follicle adhesion to the substrate surface (Rowghani et al. 2004; Heise et al. 2005, 2009). Rowghani et al. (2004) described the culture of follicles in a suspension within rotating wall vessels, cylindrical chambers that rotate the medium axially (Fig. 4b). Both test tube and rotating wall vessel techniques are able to maintain follicular three-dimensional architecture; however, Rowghani et al. (2004) found that the rate of rotation needed to prevent follicle attachment to the cylinder surface also caused follicle degeneration.
Fig. 4.
Rotating follicle culture techniques. (a) Follicle suspensions are cultured in test tubes subjected to gentle orbital movement. (b) Follicles in a suspension are cultured within rotating wall vessels.
Floating system: microdrops
Different methods for follicular in vitro culture in a 3D system using microdrops have been developed. Sánchez et al. (2012) used hydrogel-coated round-bottomed plates to inhibit cell attachment. These non-attachment conditions influence follicle steroid secretory capacity and a high two-cell rate and total blastocyst yield were obtained.
Boland et al. (1993) used a modified conventional microdrop method, in which follicles were transferred, every 24 h, to new wells containing a microdrop of medium under mineral oil, to prevent the adhesion of the cells to the plate and, consequently, the loss of follicular morphology. This system supported mouse preantral follicle growth, providing oocytes able to develop to blastocyst stage after fertilisation (Bishonga et al. 2001).
Inverted microdrops were developed to achieve a more homogeneous exposure of follicles to nutrients and oxygen, and to maintain 3D follicular structure (Nation and Selwood 2009). In this system, drops of medium containing a single follicle are placed on a tissue culture plate, which is then inverted (Fig. 5). Tension in the liquid surface of the medium maintains its attachment to the plate surface while the follicle sinks downward within the drop. The inverted microdrop system promoted significantly faster follicle growth and increased oestradiol production compared with a conventional microdrop system, in which the culture plate is not inverted and a mineral oil overlay may or may not be used (Wycherley et al. 2004).
Fig. 5.
In the inverted microdrop system, the culture plate is inverted and the follicle moves down to the extremity of the medium.
Floating system: membranes
Three-dimensional cellular culture can be performed using membranes that contain micropores inserted into well plates. This system exposes the entire follicular surface to the medium and, consequently, a metabolic process similar to what occurs in vivo. By using this system, O’Brien et al. (2003) performed in vitro culture of the whole ovary of mice followed by culture of oocyte–granulosa cell complexes, achieving the birth of 59 viable offspring.
Encapsulation systems: hydrogels
Hydrogels are natural or synthetic polymers that are capable of absorbing large amounts of water. Structurally, they are composed of one or more polymeric networks of macromolecular chains interconnected by covalent binding, physical interactions such as ionic or hydrophobic interactions, or hydrogen bridges (Oviedo et al. 2008). Because of their reticulated structure, hydrogels are able to transport oxygen, nutrients and metabolic wastes (Nguyen and West 2002). Synthetic hydrogels can be designed to mimic the physical properties of native extracellular matrix and provide controlled reproducible cellular environments for encapsulation and 3D culture of cells and tissues (Lutolf and Hubbell 2005). Polyethylene glycol (PEG; Sawhney et al. 1993), polyglycolic acid (PGA; Harris et al. 1998; Thomson et al. 1999) and polyvinyl alcohol (PVA; Martens and Anseth 2000) are the most commonly used synthetic polymers for in vitro culture. Although these synthetic polymers have excellent mechanical and hydrophilic properties (Kiritoshi and Ishihara 2004), the hydrogels they form do not have bioactive properties, and specific ligands or molecules must be added to make them functional. Furthermore, some synthetic hydrogels are not biodegradable and are unable to support dynamic remodelling by growing cells. This limitation may elicit negative cellular responses and so their application is restricted depending on the polymer type and cells used (Lee and Mooney 2001).
By contrast, hydrogels of natural polymers contain proteins and extracellular matrix components, such as collagen (Butcher and Nerem 2004), fibrin (Eyrich et al. 2007), hyaluronic acid (Masters et al. 2004) or matrigel, as well as polysaccharides obtained from other biological sources, such as agarose, alginate and chitosan (Azab et al. 2006). Because these hydrogels are derived from natural sources, they exhibit an inherent biocompatibility and, depending on the origin of the gel, they have bioactive properties (Dawson et al. 2008) that can influence cellular processes such as viability and proliferation. While this inherent bioactivity may make it difficult to determine exactly what cellular activities are affected by the hydrogel (Cushing and Anseth 2007), natural alginate hydrogels have been used extensively for 3D cell culture. Alginate hydrogels are physicochemically well defined and provide a stable and reproducible culture system that does not interfere with cellular functions (Heise et al. 2005).
Natural hydrogels, such as alginate, have been used successfully to encapsulate and serve as a 3D support structure for the culture of ovarian follicles (Shikanov et al. 2011a) and immature mouse ovarian follicles cultured in alginate have yielded live, fertile offspring (Xu et al. 2006a). However, it was found that the alginate does not degrade at the same rate that follicles grow (Boontheekul et al. 2005), which restricts follicle expansion (Xu et al. 2006b). In an attempt to avoid this problem, Shikanov et al. (2009) developed a new hydrogel composed of an interpenetrating network of fibrin–alginate (FA-IPN), which is a more ‘soft’ hydrogel. This hydrogel provides a permissive growth environment relative to more rigid hydrogels, improving the rate of meiotically competent oocytes when compared with alginate alone (Shikanov et al. 2009). This same team has recently developed a new biomaterial based on the synthetic hydrogel polyethylene glycol (PEG), which strengthens the network and improves cross-linking conditions to better maintain cell viability during encapsulation. Like FA-IPN, the PEG hydrogel also accommodates the significant increase in follicle volume while preserving the follicle’s 3D architecture (Shikanov et al. 2011a). Recently, a novel tyramine-based hyaluronan (HA) hydrogel was tested for its biocompatibility with ovarian follicles. In this study, the HA hydrogel supported follicle growth, oestradiol secretion and resumption of meiosis (Desai et al. 2012). The glycosaminoglycan HA is primarily found in the connective tissue matrix and plays an important role in a variety of biological processes, such as maintenance of the matrix structure, homeostasis of the extracellular space and interactions with other components of the matrix, thus forming a network of macromolecules that interacts with the surrounding cells (Laurent and Fraser 1992). The HA hydrogel has been tested over the years for numerous applications (Gojgini et al. 2011; Xu et al. 2012) and its characteristics of plasticity and viscosity, together with its biocompatibility, make HA a good candidate as a biomaterial for the in vitro culture of many cell types and tissues (Belli et al. 2012).
Alginate characteristics
Alginate is an anionic polymer that is produced from alginic acid that is present in the cellular wall and intercellular spaces of brown algae, particularly those of the genus Laminaria. This acid is found in a gel form and there is some evidence that its main function is the construction of a sustaining skeleton that confers resistance and flexibility to the algae tissue (Thu et al. 1996).
Alginic acid is composed of two types of uronic acids: β-d-mannuronic acid and α-l-guluronic acid. Because the solubilisation of alginic acid in water occurs only at high temperatures, it is increasingly common to use uronic acid salts to produce alginate polymer. Through a neutralisation process, uronic acids are converted to mannuronate and guluronate salts, which then form alginate.
Strength
Mannuronate (M) and guluronate (G) salts combine to form two types of homopolymeric blocks (MMMMMM or GGGGGG) or a heteropolymeric block (GMGMGMGM). The proportion, distribution and length of these blocks determine the physical and chemical properties of the alginate gel, mainly gel strength and gelation capacity. A greater proportion of G salts in the polymer contributes to greater gel strength, stability and porosity, as G salts have greater interaction with bivalent cations, resulting in the formation of a stronger structure and more rigid gel (Remminghorst and Rehm 2006). On the other hand, a greater proportion of M salts produces a more elastic gel; however, these gels are also more susceptible to wrinkling and have fewer pores (Zimmermann 1999; Yang and Wright 1999). Thus, the rigidity of alginate gel increases in the following order: MG < MM < GG (Thu et al. 1996).
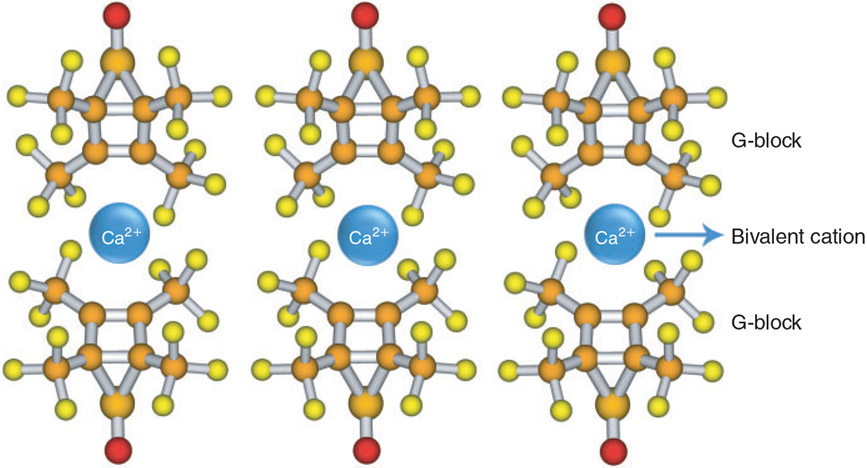
It is believed that only alginate G-blocks form intermolecular connections with bivalent cations to form a structure known as an ‘egg box’ (George and Abraham 2006; Fig. 6). One study suggests that MG-blocks are also involved in gel formation (Donati et al. 2005). In general, each cation interacts with two adjacent G salts and with two G salts of the opposite chain, thereby inducing the formation of junction zones. The affinity of G residues to cations also correlates with gel strength, which increases in the following order: Mg2+ < Mn2+ < Ca2+ < Sr2+ < Ba2+ < Cu2+ < Pb2+ (Haug and Smidsrod 1970). Studies have shown that the minimum length of G-blocks required to form ion connections decreases as the affinity of the G salts for the cation increases; in the case of Ca2+, 8–20 adjacent G salts are required to form stable junctions (Kohn and Luknar 1977; Donati et al. 2006).
Fig. 6.
The ‘egg box’ structure formed by alginate G-blocks and bivalent cations (e.g. Ca2+).
Moreover, alginate has a high affinity for water and readily forms gels in the presence of calcium ions at room temperature (Martinsen et al. 1989; Martinsen 1992; Smidsrød and Skjåk-Bræk 1990; Amsden and Turner 1999; Dulieu et al. 1999). The stability of the alginate gels when exposed to heat is also important for three-dimensional cell culture applications.
Porosity
Porosity is a feature of extreme relevance to producing gels for use in tissue and cell culture because it directly affects the cellular morphology of the encapsulated tissue and the diffusion of molecules through the gel. A smaller pore size may limit the transport of large molecules (such as nutrients) or cause the retention of substances eliminated by cells, resulting in a decrease in cell growth and viability (Gautier et al. 2011). Several factors can influence gel porosity, including gel composition; as the G-salt content increases, porosity increases (Smidsrød 1974). This may be due to the fact that gels with higher proportions of G salts are more rigid and therefore exhibit less shrinkage. Increasing the final concentration of alginate can reduce the porosity of the gel.
The capacity for medium diffusion through the gel is also affected by the methods used for gel formation. When the gel is formed in the presence of inorganic salts, such as NaCl, it tends to be more homogeneous, i.e. there is an isotropic distribution of the molecules that form alginate, promoting faster diffusion of the solute (Yang and Wright 1999).
Studies have demonstrated that alginate has an elevated porosity, allowing substrates with molecular weights lower than 2 × 104, such as glucose, l-tryptophan and α-lactalbumin, to be diffused at the same speed as water. While proteins with high molecular weights, like albumin (MW = 6.9 × 104), can be diffused easily through gels with a higher proportion of G salts, the diffusion rate is lower than that of water (Tanaka et al. 1984). This same study concluded that the diffusion of substrates with high molecular weights was predominantly limited by the higher alginate concentration and not by the higher concentration of CaCl2 used in gel preparation. In contrast, Martinsen et al. (1989) showed that two factors impact the reduction of the alginate drop volume (and therefore the reduction in pore size) after the gelation process: (1) CaCl2 concentration (elevated concentration reduces drop volume) and (2) gelation time (increased incubation time corresponds with a decrease in drop volume).
Biocompatibility
The primary goal in the development of new biomaterials is to mimic the function of the extracellular matrix of different animal tissues. Thus, biomaterials of natural origin have been prominent in cell-culture applications because of their inherent biocompatibility. Alginate is one of the natural biopolymers often used in in vitro culture because of its similarity to the extracellular matrix of the cultured tissues. This feature allows the alginate to modulate and support several biological processes, including the transport of bioactive agents, such as growth factors and hormones (Lee and Mooney 2012).
Another fundamental feature of biocompatibility is gel purity. After extraction from algae, alginate contains various contaminants, such as proteins, carbohydrates, fatty acids, phospholipids, lipopolysaccharides, toxins and polyphenols (Skjåk-Bræk et al. 1989), which can interact with encapsulated cells and lead to unwanted cell responses. Therefore, an effective purification process that does not compromise the properties of the gel is crucial for its use in cell culture.
Furthermore, the chemical composition of alginate, which we know affects key gel properties, ranges across algae species and even within different parts of the same plant. Soon-Shiong et al. (1991, 1992) and Otterlei et al. (1991) described that M salts present in alginate stimulate the production of tumour necrosis factor (TNF) and interleukin 1 (IL-1) by animal tissues. Gels with a lower M content minimally stimulate these cytokines and, therefore, have higher biocompatibility.
An alternative approach to obtaining high-purity gels and a better-defined polymeric structure is the biosynthesis of alginate by bacteria, such as Azotobacter or Pseudomonas (Lee and Mooney 2012). Chemically, this gel differs from alginic acid present in algae only in its higher level of acetylation. In addition, extraction of the alginate from bacteria allows the careful selection of strains that are free from seasonal and geographic variations in order to consistently derive gels with specific properties (Clementi et al. 1999). However, this approach to hydrogel production is currently limited by low yields.
Degradation
Alginate can be degraded by a specific enzyme, alginate lyase, which cleaves the polymeric chains of the gel. Other methods of alginate gel degradation include binding to ethylene glycol tetraacetic acid (EGTA), a Ca2+ chelator (West et al. 2007a). In addition, alginate gels can be ionically dissolved through the release of bivalent ions (Ca2+) from the gel to the medium in an exchange reaction with monovalent cations, such as Na+ (Lee and Mooney 2012). Alginate is also susceptible to oxidation by free radicals, as well as to hydrolysis or degradation in acid or alkaline medium, especially at high temperatures for prolonged periods of time (Thu et al. 1996). Skjåk-Bræk et al. (1989) verified that significant degradation occurs when pH is near neutral. Alginate degradation occurs naturally in brown algae due to the action of varying amounts of phenolic compounds that promote depolymerisation; these compounds are extracted with alginate, but can be removed in the alginate purification process (Skjåk-Bræk et al. 1989).
The molecular weight of alginate in solution can be significantly and rapidly reduced in conditions that favour degradation, a fact that will directly affect the physical properties of the gel that depend on its molecular weight (Thu et al. 1996). For the application of alginate gels to cell culture, it is therefore important to consider the factors that determine and limit alginate gel stability and the chemical reactions that are responsible for its degradation.
Application of alginate hydrogels to in vitro follicle culture
The physical and chemical properties of alginate produced by brown algae were found to be suitable for the encapsulation and culture of ovarian follicles (Pangas et al. 2003; Kreeger et al. 2005; Xu et al. 2006a, 2006b, 2009a, 2009b, 2009c; West et al. 2007b; Amorim et al. 2009). Pangas et al. (2003) used alginate for the first time for the in vitro culture of oocyte–granulosa cell complexes isolated from mouse preantral follicles. In this study, the authors verified that encapsulation in alginate maintained the communication between cellular compartments, promoted granulosa cell proliferation and spread and increased oocyte volume.
Although studies have shown limitations in the diffusion of large molecules through alginate pores, which have diameters between 5 and 200 nm (Gombotz and Wee 1998), encapsulated secondary follicles were able to respond to FSH, a molecule with high molecular weight (MW = 3 × 104), when added to the culture medium (Kreeger et al. 2005). FSH treatment led to dose-dependent increases in survival, follicular growth and steroid secretion and oocyte development. Heise et al. (2005) reported an increase in follicular diameter when FSH was added to the alginate gel before encapsulation and to the culture medium. FSH treatment stimulated follicle expression of Cx43 to levels comparable to those seen in vivo.
Several works have shown that the physical environment regulates follicle function (West et al. 2007b; West-Farrell et al. 2009; Xu et al. 2006b). This evidence is supported by histological analyses, which found that the majority of immature ovarian follicles are present within the ovarian cortex, a collagen-rich zone of the ovary (Edson et al. 2009; Tingen et al. 2009). During follicle growth, follicles move to the perimedullar zone of the ovary, which has an extracellular matrix (ECM) density lower than that of the cortex, and then complete their development before ovulation at the ovarian surface (Woodruff and Shea 2011). Indeed, Hornick et al. (2012) found that isolated primordial follicles require a higher alginate concentration (2%) to survive and grow in culture. These findings are also consistent with another study that reported that isolated human primordial follicles remain viable during short-term culture in 1% alginate (Amorim et al. 2009). Using the theory that early follicles need a more rigid environment, primordial follicles included in human ovarian cortical slices were cultured in alginate scaffold, which supported the survival and development of these follicles (Kedem et al. 2011).
On the other hand, some authors have reported that in vitro culture of secondary follicles in low alginate concentrations (0.25% to 0.5%) promotes greater follicle survival, antrum formation, follicular growth, steroid secretion and resumption of meiosis (mice, Xu et al. 2006b; West et al. 2007b; West-Farrell et al. 2009; macaques, Xu et al. 2009c; Songsasen et al. 2011; Tagler et al. 2012). Moreover, secondary follicles cultured in 0.25% alginate were able to produce mature oocytes that could be fertilised in vitro to create embryos that developed to blastocyst stage (Xu et al. 2006b). However, unlike the above studies, secondary follicle culture in a high concentration of alginate (1.5%), produced oocytes that were able to resume meiosis and be fertilised, resulting in the birth of viable and fertile offspring (Xu et al. 2006a).
The physical environment also is responsible for inducing changes in the gene-expression profile of cells (Birgersdotter et al. 2005). Parrish et al. (2011) investigated the dynamic expression of key developmental genes during folliculogenesis in vivo and during in vitro culture in a 3D alginate-hydrogel system. The results showed that the 3D alginate culture system allows follicular gene expression similar, in part, to that of follicles grown in vivo. Moreover, Mainigi et al. (2011) showed that the transcriptome of oocytes that develop in vitro using the alginate system is very similar (~99.5%) to the transcriptome of oocytes that develop in vivo. Nevertheless, in this study the developmental competence of the oocytes was compromised by the high incidence of abnormal spindle formation and chromosome alignment. Variations in rigidity of the hydrogel also can affect the gene-expression pattern, as shown by Jiao and Woodruff (2013). Oocyte-specific gene-transcript levels in cultured oocytes and polar bodies were decreased in oocytes cultured in 1.5% alginate compared with 0.25% (Jiao and Woodruff 2013).
Although studies have analysed alterations that occur in gene expression during in vitro culture in a 3D system relative to folliculogenesis in vivo, a detailed analysis of the critical molecular events involved in folliculogenesis is necessary in order that these similarities between in vitro and in vivo validate the use of the 3D culture system as a convergent tool for investigating ovarian follicular development and modelling follicle biology.
Gels derived from alginate
Although alginate has been widely used as a substrate for 3D cell culture, the absence of alginate receptors on mammalian cells prevents their adhesion to the gel, which is important to promote and regulate cellular interactions and processes in vitro. However, this feature of alginate, which is associated with low protein adsorption, allows the incorporation of synthetic polymers into the hydrogel that specifically interact with adhesion receptors on the cell membrane. Gel synthesis can be modified to incorporate natural polymers or mixtures of synthetic and natural polymers. This strategy has allowed greater control over the phenotypic characteristics ofthe cultured cells based on their interaction with the modified hydrogel culture substrate.
Peptides containing the sequence aspartic acid–glycine–glutamic acid–alanine (DGEA; Alsberg et al. 2001) and tyrosine–isoleucine–glycine–serine–arginine (YIGSR; Dhoot et al. 2004) have been incorporated into alginate hydrogels to increase their capacity for adhesion with various cell types. For follicle culture, peptides including the sequence arginine–glycine–aspartic acid (RGD) have been used extensively as adhesion ligands within hydrogels, due to the presence of receptors for these peptides in a large variety of cells (Lehenkari and Horton 1999; Koo et al. 2002). Alginate containing the RGD sequence interacted with murine granulosa cells cultured in vitro, affecting their morphology and progesterone and oestradiol secretion (Kreeger et al. 2003). In mice, in vitro culture of secondary follicles in alginate modified with RGD peptides promoted follicle growth and produced meiotically competent oocytes (Kreeger et al. 2006).
Alginate can also be modified with the addition of extracellular matrix components. This approach was used by Kreeger et al. (2006) for follicle culture, in which collagen I and IV, fibronectin and laminin were incorporated into the alginate gel. Gels modified with various types of extracellular matrix proteins supported somatic-cell growth and differentiation, as well as the meiotic competence of the oocytes, with responses varying based on the proteins used. Recently, Vanacker et al. (2012) developed a biodegradable artificial ovary using alginate-matrigel matrix to graft isolated follicles and ovarian cells. After transplantation, this matrix was able to degrade, allowed vascularisation and elicited a low inflammatory response demonstrating that an alginate-based matrix is a promising candidate for grafting of isolated ovarian cells.
Other studies have described the combination of alginate and fibrin, a protein involved in blood coagulation, to produce an innovative biomaterial (Shikanov et al. 2009, 2011b, 2011c). When associated with alginate, fibrin interacts with alginate G salts to form a dynamic interpenetrating network (FA-IPN) in which the fibrin is degraded as the follicle grows outward. Follicles cultured in FA-IPN maintained follicle morphological features and had positive effects on follicle growth, antrum formation and oocyte maturation in mice (Shikanov et al. 2009). Similar results were obtained by Xu et al. (2010), who cultured preantral follicles from macaques using a matrix constituted by the association of alginate, fibrin and matrigel. In other work, secondary follicles of mice recovered from cultured ovaries were cultured in FA-IPN beads and produced a very high rate of oocyte progression to metaphase II and formation of two-cell embryos (Jin et al. 2010).
Final considerations
Currently, technologies using cells, biological materials and molecular signals to repair, maintain or improve the functionality of different cell types and tissues are improving our understanding of the effects of artificial environments on cell functions. In this context, a wide variety of natural and synthetic polymers has been produced or modified, forming gels with different physical characteristics that are able to positively affect the development and function of encapsulated cells and tissues. With regard to the in vitro culture of ovarian follicles, the development of 3D microenvironments using hydrogels that support follicular growth and oocyte maturation remains an area of active research. Most of the studies conducted on in vitro follicle culture have investigated the use of alginate-based gels and modifications of these gels in which synthetic peptides or extracellular matrix components have been incorporated to improve gel function and bioactivity.
While research related to the chemistry of polymers is advancing and producing increasingly sophisticated gels, the complex interactions between natural or synthetic materials and follicles during 3D culture have not been completely characterised. The factors that are essential for proper growth and maturation of follicles in vitro, such as the availability of oxygen, nutrients, growth factors and hormones, and their diffusion and distribution properties, must be defined. The culture conditions in these hydrogels will depend on their effects on various cellular processes and the physical properties of the gel that impact its function, such as the formation of gradients or the presence of structural defects.
The establishment of a system for 3D in vitro culture of ovarian follicles that provides a physical environment capable of supporting the development of follicles and successfully producing fertilisable oocytes is extremely important. Such a system will not only provide crucial information regarding follicle biology and the events of folliculogenesis, but also open up the possibility for clinical applications in assisted reproductive technologies.
References
- Abir R, Fisch B, Nitke S, Okon E, Raz A, and Ben Rafael Z (2001). Morphological study of fully and partially isolated early human follicles. Fertil. Steril 75, 141–146. doi: 10.1016/S0015-0282(00)01668-X [DOI] [PubMed] [Google Scholar]
- Acevedo N, Ding J, and Smith GD (2007). Insulin signalling in mouse oocytes. Biol. Reprod 77, 872–879. doi: 10.1095/BIOLREPROD.107.060152 [DOI] [PubMed] [Google Scholar]
- Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, and Kidder GM (2001). Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol 233, 258–270. doi: 10.1006/DBIO.2001.0216 [DOI] [PubMed] [Google Scholar]
- Adams JC, and Watt FM (1993). Regulation of development and differentiation by extracellular matrix. Development 117, 1183–1198. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, and Lewis J (2002). ‘Molecular Biology of the Cell’. 4th edn. (Garland Science: New York.) [Google Scholar]
- Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, and Mooney DJ (2001). Cell-interactive alginate hydrogels for bone-tissue engineering. J. Dent. Res 80, 2025–2029. doi: 10.1177/00220345010800111501 [DOI] [PubMed] [Google Scholar]
- Amorim CA, Van Langendonckt A, David A, Dolmans MM, and Donnez J (2009). Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum. Reprod 24, 92–99. doi: 10.1093/HUMREP/DEN343 [DOI] [PubMed] [Google Scholar]
- Amsden B, and Turner N (1999). Diffusion characteristics of calcium alginate gels. Biotechnol. Bioeng 65, 605–610. doi: [DOI] [PubMed] [Google Scholar]
- Azab AK, Orkin B, Doviner V, Nissan A, Klein M, Srebnik M, and Rubinstein A (2006). Cross-linked chitosan implants as potential degradable devices for brachytherapy, in vitro and in vivo analysis. J. Control. Release 111, 281–289. doi: 10.1016/J.JCONREL.2005.12.014 [DOI] [PubMed] [Google Scholar]
- Belli M, Vigone G, Merico V, Redi CA, Zuccotti M, and Garagna S (2012). Towards a 3D culture of mouse ovarian follicles. Int. J. Dev. Biol 56, 931–937. doi: 10.1387/IJDB.120175MZ [DOI] [PubMed] [Google Scholar]
- Benton G, George J, Kleinman HK, and Arnaoutova IP (2009). Advancing science and technology via 3D culture on basement membrane matrix. J. Cell. Physiol 221, 18–25. doi: 10.1002/JCP.21832 [DOI] [PubMed] [Google Scholar]
- Ben-Ze’ev A, and Amsterdam A (1986). Regulation of cytoskeletal proteins involved in cell contact formation during differentiation of granulosa cells on extracellular matrix. Proc. Natl. Acad. Sci. USA 83, 2894–2898. doi: 10.1073/PNAS.83.9.2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgersdotter A, Sandberg R, and Ernberg I (2005). Gene expression perturbation in vitro – a growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol 15, 405–412. doi: 10.1016/J.SEMCANCER.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Bishonga C, Takahashi Y, Katagiri S, Nagano M, and Ishikawa A (2001). In vitro growth of mouse ovarian preantral follicles and the capacity of their oocytes to develop to the blastocyst stages. J. Vet. Med. Sci 63, 619–624. doi: 10.1292/JVMS.63.619 [DOI] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, and Gosden RG (1993). Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol. Reprod 48, 798–806. doi: 10.1095/BIOLREPROD48.4.798 [DOI] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, and Gosden RG (1994). Characterization of follicular energy metabolism. Hum. Reprod 9, 604–609. [DOI] [PubMed] [Google Scholar]
- Boontheekul T, Kong H, and Mooney D (2005). Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26, 2455–2465. doi: 10.1016/J.BIOMATERIALS.2004.06.044 [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, and Eppig JJ (1990). FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev. Biol 138, 16–25. doi: 10.1016/0012-1606(90)90172-F [DOI] [PubMed] [Google Scholar]
- Buratini J Jr (2007). Controle endócrino e local da foliculogênese em bovinos. Rev Bras Reprod An 31, 190–196. [Google Scholar]
- Buratini J Jr, Teixeira AB, Costa IB, Glapinski VF, Pinto MGL, Giometti IC, Barros CM, Cao M, Nicola ES, and Price CA (2005). Expression of fibroblast growth factor-8 and regulation of cognate receptors, fibroblast growth factor receptor-3c and −4, in bovine antral follicles. Reproduction 130, 343–350. doi: 10.1530/REP.1.00642 [DOI] [PubMed] [Google Scholar]
- Butcher JT, and Nerem RM (2004). Porcine aorticvalve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J. Heart Valve Dis 13, 478–485. [PubMed] [Google Scholar]
- Cecconi S, Barboni B, Coccia M, and Mattioli M (1999). In vitro development of sheep preantral follicles. Biol. Reprod 60, 594–601. doi: 10.1095/BIOLREPROD60.3.594 [DOI] [PubMed] [Google Scholar]
- Chaves RN, Martins FS, Saraiva MVA, Celestino JJH, Lopes CAP, Correia JC, Lima-Verde IB, Matos MHT, Báo SN, Name KPO, Campello CC, Silva JRV, and Figueiredo JR (2008). Chilling ovarian fragments during transportation improves viability and growth of goat preantral follicles cultured in vitro. Reprod. Fertil. Dev 20, 640–647. doi: 10.1071/RD07195 [DOI] [PubMed] [Google Scholar]
- Chaves RN,Alves AMCV, Duarte ABG,Araújo VR, Celestino JJH, Matos MHT, Lopes CAP, Campello CC, Name KPO, Báo SN, and Figueiredo JR (2010). Nerve growth factor promotes the survival of goat preantral follicles cultured in vitro. Cells Tissues Organs 192, 272–282. doi: 10.1159/000317133 [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, and Ingber DE (1997). Geometric control of cell life and death. Science 276, 1425–1428. doi: 10.1126/SCIENCE.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- Clementi F, Crudele MA, Parente E, Mancini M, and Moresi M (1999). Production and characterization of alginate from Azotobacter vinelandii. J. Sci. Food Agric 79, 602–610. doi: [DOI] [Google Scholar]
- Cortvrindt R, Smitz J, and Van Steirteghem AC (1996). In vitro maturation, fertilisation and embryo development of immature oocytes from early preantral follicles from pre-pubertal mice in a simplified culture system. Hum. Reprod 11, 2656–2666. doi: 10.1093/OXFORDJOURNALS.HUMREP.A019188 [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, and Yamada KM (2002). Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol 14, 633–640. doi: 10.1016/S0955-0674(02)00364-2 [DOI] [PubMed] [Google Scholar]
- Cushing MC, and Anseth KS (2007). Hydrogel cell cultures. Science 316, 1133–1134. doi: 10.1126/SCIENCE.1140171 [DOI] [PubMed] [Google Scholar]
- Dawson E, Mapili G, Erickson K, Taqvi S, and Roy K (2008). Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev 60, 215–228. doi: 10.1016/J.ADDR.2007.08.037 [DOI] [PubMed] [Google Scholar]
- Demeestere I, Centner J, Gervy Y, and Delbaere A (2005). Impact of various endocrine and paracrine factors on culture of preantral follicles in rodents. Reproduction 130, 147–156. doi: 10.1530/REP.1.00648 [DOI] [PubMed] [Google Scholar]
- Desai N, Alex A, Abdel Hafez F, Calabro A, Goldfarb J, Fleischman A, and Falcone T (2010). Three-dimensional in vitro follicle growth, overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol 8, 119. doi: 10.1186/1477-7827-8-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Abdelhafez F, Calabro A, and Falcone T (2012). Three-dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod. Biol. Endocrinol 10, 29–40. doi: 10.1186/1477-7827-10-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhoot NO,Tobias CA, Fischer I, and Wheatley MA (2004). Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res 71A, 191–200. doi: 10.1002/JBM.A.30103 [DOI] [PubMed] [Google Scholar]
- Donati I, Holtan S, Morch YA, Borgogna M, Dentini M, and Skjåk-Bræk G (2005). New hypothesis on the role of alternating sequences in calcium–alginate gels. Biomacromolecules 6, 1031–1040. doi: 10.1021/BM049306E [DOI] [PubMed] [Google Scholar]
- Donati I, Benegas JC, and Paoletti S (2006). Polyelectrolyte study of the calcium-induced chain association of pectate. Biomacromolecules 7, 3439–3447. doi: 10.1021/BM060164T [DOI] [PubMed] [Google Scholar]
- Dulieu C, Poncelet D, and Neufeld R (1999). Encapsulation and immobilization techniques. In ‘Cell Encapsulation Technology and Therapeutics’. (Eds Kuhtreiber WM, Lanza RP and Chick WL.) pp. 3–17. (Birkhauser: Boston.) [Google Scholar]
- Edson MA, Nagaraja AK, and Matzuk MM (2009). The mammalian ovary from genesis to revelation. Endocr. Rev 30, 624–712. doi: 10.1210/ER.2009-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G. (2003). Actinremodellingto facilitate membrane fusion. Biochim. Biophys. Acta 1641, 175–181. doi: 10.1016/S0167-4889(03)00087-9 [DOI] [PubMed] [Google Scholar]
- Eppig JJ (1991). Intercommunication between mammalian oocytes and companion somatic cells. Bioessays 13, 569–574. doi: 10.1002/BIES.950131105 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, and O’Brien MJ (1996). Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod 54, 197–207. doi: 10.1095/BIOLREPROD54.1.197 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, and Schroeder AC (1989). Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation and fertilisation in vitro. Biol. Reprod 41, 268–276. doi: 10.1095/BIOLREPROD41.2.268 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, and Wigglesworth K (1995). Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol. Reprod. Dev 42, 447–456. doi: 10.1002/MRD.1080420412 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL, and Hirao Y(1997). Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol. Reprod 56, 976–984. doi: 10.1095/BIOLREPROD56.4.976 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, and Pendola JK (2005). Mouse oocytes regulate metabolic co-operativity between granulosa cells and oocytes: amino acid transport. Biol. Reprod 73, 351–357. doi: 10.1095/BIOLREPROD.105.041798 [DOI] [PubMed] [Google Scholar]
- Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, Staudenmaier R, Goepferich A, and Blunk T (2007). Long-term stable fibrin gels for cartilage engineering. Biomaterials 28, 55–65. doi: 10.1016/J.BIOMATERIALS.2006.08.027 [DOI] [PubMed] [Google Scholar]
- Faustino LR, Rossetto R, Lima IM, Silva CM, Saraiva MV, Lima LF, Silva AW, Donato MA, Campello CC, Peixoto CA, Figueiredo JR, and Rodrigues APR (2011). Expression of keratinocyte growth factor in goat ovaries and its effects on preantral follicles within cultured ovarian cortex. Reprod. Sci 18, 1222–1229. doi: 10.1177/1933719111410709 [DOI] [PubMed] [Google Scholar]
- Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, and Navarro PAAS (2009). Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71, 836–848. doi: 10.1016/J.THERIOGENOLOGY.2008.10.023 [DOI] [PubMed] [Google Scholar]
- Fortune JE (2003). The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim. Reprod. Sci 78, 135–163. doi: 10.1016/S0378-4320(03)00088-5 [DOI] [PubMed] [Google Scholar]
- Fortune JE, Rivera GM, and Yang MY (2004). Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim. Reprod. Sci 82—83, 109–126. doi: 10.1016/J.ANIREPROSCI.2004.04.031 [DOI] [PubMed] [Google Scholar]
- Gautier A, Carpentier B, Dufresne M, Vu Dinh Q, Paullier P, and Legallais C (2011). Impact of alginate type and bead diameter on mass transfers and the metabolic activities of encapsulated c3a cells in bioartificial liver applications. Eur. Cell. Mater 21, 94–106. [DOI] [PubMed] [Google Scholar]
- George M, and Abraham TE (2006). Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan – a review. J. Control. Release 114, 1–14. doi: 10.1016/J.JCONREL.2006.04.017 [DOI] [PubMed] [Google Scholar]
- Gigli I,Byrd DD, and Fortune JE (2006). Effects of oxygen tension and supplements to the culture medium on activation and development of bovine follicles. Theriogenology 66, 344–353. doi: 10.1016/J.THERIOGENOLOGY.2005.11.021 [DOI] [PubMed] [Google Scholar]
- Gojgini S, Tokatlian T, and Segura T (2011). Utilising cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels. Mol. Pharm 8, 1582–1591. doi: 10.1021/MP200171D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombotz WR, and Wee S (1998). Protein release from alginate matrices. Adv. Drug Deliv. Rev 31(3), 267–285. doi: 10.1016/S0169-409X(97)00124-5 [DOI] [PubMed] [Google Scholar]
- Granot I, and Dekel N (2002). Connexin43 in rat oocytes: developmental modulation of its phosphorylation. Biol. Reprod 66, 568–573. doi: 10.1095/BIOLREPROD66.3.568 [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Reynolds LP, and Redmer DA (1997). Gap junctions in the ovaries. Biol. Reprod 57, 947–957. doi: 10.1095/BIOLREPROD57.5.947 [DOI] [PubMed] [Google Scholar]
- Harris LD, Kim BS, and Mooney DJ (1998). Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res 42,396–402. doi: [DOI] [PubMed] [Google Scholar]
- Hartshorne GM (1997). In vitro culture of ovarian follicles. Rev. Reprod 2, 94–104. doi: 10.1530/ROR.0.002009 [DOI] [PubMed] [Google Scholar]
- Haug A, and Smidsrød O (1970). Selectivity of some anionic polymers for divalent metal ions. Acta Chem. Scand 24, 843–854. doi: 10.3891/ACTA.CHEM.SCAND.24-0843 [DOI] [Google Scholar]
- Hay E. (1984). ‘The Role of Extracellular Matrix in Development’. (Liss: New York.) [Google Scholar]
- Heise M, Koepsel R, Russell AJ, and McGee EA (2005). Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod. Biol. Endocrinol 3, 47. doi: 10.1186/1477-7827-3-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise MK,Koepsel R,McGee EA,and Russell AJ (2009). Dynamic oxygen enhances oocyte maturation in long-term follicle culture. Tissue Eng. Part C Methods 15, 323–332. doi: 10.1089/TEN.TEC.2007.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, and Smith GD (2010). Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum. Reprod 25, 613–622. doi: 10.1093/HUMREP/DEP449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, and Woodruff TK (2012). Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod 27, 1801–1810. doi: 10.1093/HUMREP/DER468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O. (2004). Cryopreservation and culture of human ovarian–cortical tissue containing early follicles. Eur. J. Obstet. Gynecol. Reprod. Biol 113(Suppl. 1), S50–S54. doi: 10.1016/J.EJOGRB.2003.11.01 [DOI] [PubMed] [Google Scholar]
- Hu Y, Betzendahl I, Cortvrindt R, Smitz J, and Eichenlaub-Ritter U (2001). Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Hum. Reprod 16, 737–748. doi: 10.1093/HUMREP/16.4.737 [DOI] [PubMed] [Google Scholar]
- Hwa AJ, Fry RC, Sivaraman A, So PT, Samson LD, Stolz DB, and Griffith LG (2007). Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 21, 2564–2579. doi: 10.1096/FJ.06-7473COM [DOI] [PubMed] [Google Scholar]
- Jiao ZX, and Woodruff TK (2013). Follicle microenvironment-associated alterations in gene expression in the mouse oocyte and its polar body. Fertil. Steril, In press. doi: 10.1016/J.FERTNSTERT.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, and Woodruff TK (2010). A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil. Steril 93, 2633–2639. doi: 10.1016/J.FERTNSTERT.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce IM, Pendola FL, Wigglesworth K, and Eppig JJ (1999). Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev. Biol 214, 342–353. doi: 10.1006/DBIO.1999.9437 [DOI] [PubMed] [Google Scholar]
- Kapus A, Grinstein S,Wasan S, Kandasamy R, and Orlowski J (1994). Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells: ATP dependence, osmotic sensitivity and role in cell proliferation. J. Biol. Chem 269, 23 544–23 552. [PubMed] [Google Scholar]
- Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S, Ben-Haroush A, Dor J,Freud E, Felz C, and Abir R (2011). Alginate scaffold for organ culture of cryopreserved–thawed human ovarian cortical follicles. J. Assist. Reprod. Genet 28, 761–769. doi: 10.1007/S10815-011-9605-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritoshi Y, and Ishihara K (2004). Synthesis of hydrophilic cross-linker having phosphorylcholine-like linkage for improvement of hydrogel properties. Polymer 45, 7499–7504. doi: 10.1016/J.POLYMER.2004.09.014 [DOI] [Google Scholar]
- Knight PG, and Glister C (2006). TGF-β superfamily members and ovarian follicle development. Reproduction 132,191–206. doi: 10.1530/REP.1.01074 [DOI] [PubMed] [Google Scholar]
- Kohn R, and Luknar O (1977). Intermolecular calcium ion binding on polyuronates – polygalacturonate and polyguluronate. Collect. Czech. Chem. Commun 42, 731–744. doi: 10.1135/CCCC19770731 [DOI] [Google Scholar]
- Koo LY, Irvine DJ, Mayes AM, Lauffenburger DA, and Griffith LG (2002). Co-regulation of cell adhesion by nanoscale RGD organisation and mechanical stimulus. J. Cell Sci 115, 1423–1433. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Woodruff TK, and Shea LD (2003). Murine granulosa cell morphology and function are regulated by a synthetic Arg/Gly/Asp matrix. Mol. Cell. Endocrinol 205, 1–10. doi: 10.1016/S0303-7207(03)00209-0 [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, and Shea LD (2005). Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol. Reprod 73, 942–950. doi: 10.1095/BIOLREPROD.105.042390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, and Shea LD (2006). The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 27, 714–723. doi: 10.1016/J.BIOMATERIALS.2005.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, and Matzuk MM (2000). Gene knockout models to study the hypothalamus–pituitary–gonadal axis. In ‘Gene Engineering and Molecular Models in Endocrinology’. Vol. 22. (Ed. Shupnik MA.) pp. 167–216. (The Human Press: Totowa, NJ.) [Google Scholar]
- Lane M, Baltz JM, and Bavister BD(1998). Regulation of intracellular pH in hamster preimplantation embryos by the sodium–hydrogen (Na+/H+) antiporter. Biol. Reprod 59, 1483–1490. doi: 10.1095/BIOLREPROD59.6.1483 [DOI] [PubMed] [Google Scholar]
- Laurent TC, and Fraser JR (1992). Hyaluronan. FASEB J. 6, 2397–2404. [PubMed] [Google Scholar]
- Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, and Bissell MJ (2007). Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp. Cell Res 313, 3066–3075. doi: 10.1016/J.YEXCR.2007.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, and Mooney DJ (2001). Hydrogels for tissue engineering. Chem. Rev 101, 1869–1880. doi: 10.1021/CR000108X [DOI] [PubMed] [Google Scholar]
- Lee KY, and Mooney DJ (2012). Alginate: properties and biomedical applications. Prog. Polym. Sci 37, 106–126. doi: 10.1016/J.PROGPOLYMSCI.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Moon JJ, and West JL (2008). Three-dimensional micro-patterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials 29, 2962–2968. doi: 10.1016/J.BIOMATERIALS.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehenkari PP, and Horton MA (1999). Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem. Biophys. Res. Commun 259, 645–650. doi: 10.1006/BBRC.1999.0827 [DOI] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Adriaenssens T, and Smitz J (2004). A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol. Reprod 71, 1730–1738. doi: 10.1095/BIOLREPROD.104.028415 [DOI] [PubMed] [Google Scholar]
- Liu HC, He Z, and Rosenwaks Z (2001). Application of complementary DNA microarray (DNA chip) technology in the study of gene-expression profiles during folliculogenesis. Fertil. Steril 75, 947–955. doi: 10.1016/S0015-0282(01)01706-X [DOI] [PubMed] [Google Scholar]
- Lochter A, and Bissel M (1995). Involvement of extracellular matrix constituents in breast cancer. Semin. Cancer Biol 6, 165–173. doi: 10.1006/SCBI.1995.0017 [DOI] [PubMed] [Google Scholar]
- Loret de Mola JR, Barnhart K, Kopf GS, Heyner S, Garside W, and Coutifaris CB (2004). Comparison of two culture systems for the in vitro growth and maturation of mouse preantral follicles. Clin. Exp. Obstet. Gynecol 31, 15–19. [PubMed] [Google Scholar]
- Luciano AM, Franciosi F, Modina SC, and Lodde V (2011). Gap junction-mediated communications regulate chromatin remodelling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s). Biol. Reprod 85, 1252–1259. doi: 10.1095/BIOLREPROD.111.092858 [DOI] [PubMed] [Google Scholar]
- Luna EJ (1991). Molecular links between the cytoskeleton and membranes. Curr. Opin. Cell Biol 3, 120–126. doi: 10.1016/0955-0674(91)90174-W [DOI] [PubMed] [Google Scholar]
- Lutolf MP,and Hubbell JA (2005). Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 23, 47–55. doi: 10.1038/NBT1055 [DOI] [PubMed] [Google Scholar]
- Mainigi MA, Ord T, and Schultz RM (2011). Meiotic and developmental competence in mice are compromised following follicle development in vitro using an alginate-based culture system. Biol. Reprod 85, 269–276. doi: 10.1095/BIOLREPROD.111.091124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BK, and West JL (2002). Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration and matrix protein synthesis on modified surfaces and in polymer scaffolds. J. Biomed. Mater. Res 60, 86–93. doi: 10.1002/JBM.10042 [DOI] [PubMed] [Google Scholar]
- Martens P, and Anseth KS (2000). Characterization of hydrogels formed from acrylate-modified poly(vinyl alcohol) macromers. Polymer 41, 7715–7722. doi: 10.1016/S0032-3861(00)00123-3 [DOI] [Google Scholar]
- Martinsen A. (1992). Alginate as immobilization material: III. Diffusional properties. Biotechnol. Bioeng 39, 186–194. doi: 10.1002/BIT.260390210 [DOI] [PubMed] [Google Scholar]
- Martinsen A, Skjåk-Bræk G, and Smidsrød O (1989). Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng 33, 79–89. doi: 10.1002/BIT.260330111 [DOI] [PubMed] [Google Scholar]
- Masters KS, Shah DN, Walker G, Leinwand LA, andAnseth KS (2004). Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J. Biomed. Mater. Res 71A,172–180. doi: 10.1002/JBM.A.30149 [DOI] [PubMed] [Google Scholar]
- Matos MHT, Lima-Verde IB, Luque MCA, Maia JE Jr, Silva JRV, Celestino JJH, Martins FS, Bao SN, Lucci CM, and Figueiredo JR (2007). Essential role of follicle-stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote 15,173–182. doi: 10.1017/S0967199407004169 [DOI] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, and Lee SJ (1995). Oocyte-specific expression of growth differentiation factor-9. Mol. Endocrinol 9, 131–136. doi: 10.1210/ME.9.1.131 [DOI] [PubMed] [Google Scholar]
- Murray AA, Molinek MD, Baker SJ, Kojima FN, Smith MF, Hillier SG, and Spears N (2001). Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles in vitro. Reproduction 121, 89–96. doi: 10.1530/REP.0.1210089 [DOI] [PubMed] [Google Scholar]
- Nation A, and Selwood L (2009). The production of mature oocytes from adult ovaries following primary follicle culture in a marsupial. Reproduction 138, 247–255. doi: 10.1530/REP-09-0028 [DOI] [PubMed] [Google Scholar]
- Nguyen KT, and West JL (2002). Photopolymerisable hydrogels for tissue engineering applications. Biomaterials 23, 4307–4314. doi: 10.1016/S0142-9612(02)00175-8 [DOI] [PubMed] [Google Scholar]
- Nilsson EE, and Skinner MK (2003). Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol. Reprod 69, 1265–1272. doi: 10.1095/BIOLREPROD.103.018671 [DOI] [PubMed] [Google Scholar]
- Nilsson EE, and Skinner MK (2004). Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol. Cell. Endocrinol 214, 19–25. doi: 10.1016/J.MCE.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Kezele P, and Skinner MK (2002). Leukaemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol. Cell. Endocrinol 188, 65–73. doi: 10.1016/S0303-7207(01)00746-8 [DOI] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, and Jaffe LA (2009). Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136, 1869–1878. doi: 10.1242/DEV.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MJ, Pendola JK, andEppig JJ (2003).A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod 68, 1682–1686. doi: 10.1095/BIOLREPROD.102.013029 [DOI] [PubMed] [Google Scholar]
- Oktem O, and Oktay K (2007). The role of extracellular matrix and activin-A in in vitro growth and survival of murine preantral follicles. Reprod. Sci 14, 358–366. doi: 10.1177/1933719107303397 [DOI] [PubMed] [Google Scholar]
- Otterlei M, Ostgaard K, Skjåk-Bræk G, and Smidsrod G (1991). Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother 10, 286–291. doi: 10.1097/00002371-199108000-00007 [DOI] [PubMed] [Google Scholar]
- Oviedo IR, Mendez NAN, Gomez MPG, Rodriguez HC, and Martinez AR (2008). Design of a physical and nontoxic cross-linked poly(vinyl alcohol) hydrogel. Int. J. Polym. Mater 57, 1095–1103. doi: 10.1080/00914030802341661 [DOI] [Google Scholar]
- Pangas SA, Saudye H, Shea LD, and Woodruff TK (2003). Novel approach for the three-dimensional culture of granulosa cell–oocyte complexes. Tissue Eng. 9, 1013–1021. doi: 10.1089/107632703322495655 [DOI] [PubMed] [Google Scholar]
- Park JI, Hong JY,Yong HY, Hwang WS, Lim JM, and Lee ES (2005). High oxygen tension during oocyte maturation improves development of porcine oocytes after fertilization. Anim. Reprod. Sci 87, 133–141. doi: 10.1016/J.ANIREPROSCI.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Parrish EM, Siletz A, Xu M, Woodruff TK, and Shea LD (2011). Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction 142, 309–318. doi: 10.1530/REP-10-0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton HM, Harris SE, Muruvi W, and Chambers EL (2008). The in vitro growth and maturation of follicles. Reproduction 136, 703–715. doi: 10.1530/REP-08-0290 [DOI] [PubMed] [Google Scholar]
- Remminghorst U, and Rehm BHA (2006). Bacterial alginates: from biosynthesis to applications. Biotechnol. Lett 28, 1701–1712. doi: 10.1007/S10529-006-9156-X [DOI] [PubMed] [Google Scholar]
- Richards J, Russel D, Ochsner S, Hsieh M, Doyle K, Falender A, Lo Y, and Sharma S (2002). Novel signalling pathways that control ovarian follicular development, ovulation and luteinisation. Recent Prog. Horm. Res 57, 195–220. doi: 10.1210/RP.57.1.195 [DOI] [PubMed] [Google Scholar]
- Risek B, Guthrie S, Kumar N, and Gilula NB (1990). Modulation of gap junction transcript and protein expression during pregnancy in the rat. J. Cell Biol 110, 269–282. doi: 10.1083/JCB.110.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto R, Lima-Verde IB, Matos MH, Saraiva MVA, Martins FS, Faustino LR, Araújo VR, Silva CM,Name KP, Campello CC, Figueiredo JR, and Blume H (2009). Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domest. Anim. Endocrinol 37, 112–123. doi: 10.1016/J.DOMANIEND.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Rowghani NM, Heise MK, McKeel D, McGee EA, Koepsel RR, and Russell AJ (2004). Maintenance of morphology and growth of ovarian follicles in suspension culture. Tissue Eng. 10, 545–552. doi: 10.1089/107632704323061906 [DOI] [PubMed] [Google Scholar]
- Roy SK, and Greenwald GS (1989). Hormonal requirements for the growth and differentiation of hamster preantral follicles in long-term culture. J. Reprod. Fertil 87, 103–114. doi: 10.1530/JRF.0.0870103 [DOI] [PubMed] [Google Scholar]
- Roy SK, and Greenwald GS (1996). Methods of separation and in vitro culture of preantral follicles from mammalian ovaries. Hum. Reprod. Update 2, 236–245. doi: 10.1093/HUMUPD/2.3.236 [DOI] [PubMed] [Google Scholar]
- Salehi P, Spratlin J, Chong TF, and Churchill TA (2004). Beneficial effects of supplemental buffer and substrate on energy metabolism during small bowel storage. Cryobiology 48, 245–253. doi: 10.1016/J.CRYOBIOL.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Salinas CN, and Anseth KS (2008). The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29, 2370–2377. doi: 10.1016/J.BIOMATERIALS.2008.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon NA, Handyside AH, and Joyce IM (2004). Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev. Biol 266, 201–208. doi: 10.1016/J.YDBIO.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Salustri A, Yanagishita M, and Hascall VC (1990). Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev. Biol 138, 26–32. doi: 10.1016/0012-1606(90)90173-G [DOI] [PubMed] [Google Scholar]
- Sánchez F, Romero S, and Smitz J (2011). Oocyte and cumulus cell transcripts from cultured mouse follicles are induced to deviate from normal in vivo condition by combinations of insulin, follicle-stimulating hormone and human chorionic gonadotrophin. Biol. Reprod 85, 565–574. doi: 10.1095/BIOLREPROD.111.091744 [DOI] [PubMed] [Google Scholar]
- Sánchez F, Romero S, Albuz FK, and Smitz J (2012). In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J. Assist. Reprod. Genet 29, 141–152. doi: 10.1007/S10815-011-9690-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney AS, Pathak CP, and Hubbell JA (1993). Bio-erodible hydrogels based on photopolymerised poly(ethylene glycol)-co-poly(a-hydroxy acid) diacrylate macromers. Macromolecules 26, 581–587. doi: 10.1021/MA00056A005 [DOI] [Google Scholar]
- Sechi AS, and Wehland J (2000). The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J. Cell Sci 113, 3685–3695. [DOI] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, and Shea LD (2009). Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 30, 5476–5485. doi: 10.1016/J.BIOMATERIALS.2009.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Smith RM, Shikanov A, Xu M, Woodruff TK, and Shea LD (2011a). Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 32, 2524–2531. doi: 10.1016/J.BIOMATERIALS.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, and Shea LD (2011b). A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3-dimensional system. J. Vis. Exp 15, pii2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, and Shea LD (2011c). Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. Part A 17, 3095–3104. doi: 10.1089/TEN.TEA.2011.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JRV (2005). Growth factors in goat ovaries and the role of activina-A in the development of early-staged follicles. PhD Thesis, Utrecht University, The Netherlands. [Google Scholar]
- Silva JRV, van denHurk R, Costa SHF, Andrade ER, Nunes APA, Ferreira FVA, Lobo RNB, and Figueiredo JR (2004). Survival and growth of goat primordial follicles after in vitro culture of ovarian cortical slices in media containing coconut water. Anim. Reprod. Sci 81, 273–286. doi: 10.1016/J.ANIREPROSCI.2003.09.006 [DOI] [PubMed] [Google Scholar]
- Silva CMG, Matos MHT, Rodrigues GQ, Faustino LR, Pinto LC, Chaves RN, Araújo VR, Campello CC, and Figueiredo JR (2010). In vitro survival and development of goat preantral follicles in two different oxygen tensions. Anim. Reprod. Sci 117, 83–89. doi: 10.1016/J.ANIREPROSCI.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Silva GM, Araujo VR, Duarte ABG, Chaves RN, Silva CMG, Lobo CH, Almeida AP, Matos MHT, Tavares LMT, Campelo CC, and Figueiredo JR (2011). Ascorbic acid improves the survival and in vitro growth of isolated caprine preantral follicles. Anim Reprod 8, 14–24. [Google Scholar]
- Simon AM, Goodenough DA, Li E, and Paul DL (1997). Female infertility in mice lacking connexin 37. Nature 385, 525–529. doi: 10.1038/385525A0 [DOI] [PubMed] [Google Scholar]
- Skjåk-Bræk G, Murano E, and Paoletti S (1989). Alginate as immobilization material. II: Determination of polyphenol contaminants by fluorescence spectroscopy and evaluation of methods for their removal. Biotechnol. Bioeng 33, 90–94. doi: 10.1002/BIT.260330112 [DOI] [PubMed] [Google Scholar]
- Smidsrod O. (1974). Molecular basis of some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc 57, 263–274. doi: 10.1039/DC9745700263 [DOI] [Google Scholar]
- Smidsrod O, and Skjåk-Bræk G (1990). Alginate as immobilization matrix for cells. Trends Biotechnol. 8, 71–78. doi: 10.1016/0167-7799(90)90139-0 [DOI] [PubMed] [Google Scholar]
- Songsasen N, Woodruff TK, and Wildt DE (2011). In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction 142, 113–122. doi: 10.1530/REP-10-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon-Shiong P, Otterlie M, Skjåk-Bræk G, Smidsrød O, Heintz R, Lanza RP, and Espevik T (1991). An immunological basis for the fibrotic reaction to implanted microcapsules. Transplant. Proc 23, 758–759. [PubMed] [Google Scholar]
- Soon-Shiong P, Feldman E, Nelson R, Komtebedde J, Smidsrød O, Skjåk-Bræk G, Espevik T, Heintz R, and Lee M (1992). Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation 54, 769–774. doi: 10.1097/00007890-199211000-00001 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, and Eppig JJ (2009). Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med 27, 32–42. doi: 10.1055/S-0028-1108008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P. (1993). Dynamic changes of gap junctions and cytoskeleton during in vitro culture of cattle oocyte cumulus complexes. Biol. Reprod 49, 1277–1287. doi: 10.1095/BIOLREPROD49.6.1277 [DOI] [PubMed] [Google Scholar]
- Sutton ML, Gilchrist RB, and Thompson JG (2003). Effects of in vivo and in vitro environments on the metabolism of the cumulus–oocyte complex and its influence on oocyte developmental capacity. Hum. Reprod. Update 9, 35–48. doi: 10.1093/HUMUPD/DMG009 [DOI] [PubMed] [Google Scholar]
- Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, and Shea LD (2012). Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng. Part A 18, 1229–1238. doi: 10.1089/TEN.TEA.2011.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Matsumura M, and Veliky IA (1984). Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol. Bioeng 26, 53–58. doi: 10.1002/BIT.260260111 [DOI] [PubMed] [Google Scholar]
- Thomson RC, Mikos AG, Beahm E, Lemon JC, Satterfield WC, Aufdemorte TB, and Miller MJ (1999). Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials 20, 2007–2018. doi: 10.1016/S0142-9612(99)00103-9 [DOI] [PubMed] [Google Scholar]
- Thu B, Smidsrod O, and Skjåk-Bræk G (1996). Alginate gels – some structure–function correlations relevant to their use as immobilization matrix for cells. In ‘Immobilized Cells – Basics and Applications. Vol. 11. (Eds Wijffels RH, Buitelaar RM, Bucke C and Tramper J.) pp. 19–30. (Elsevier Science: Amsterdam, NL.) [Google Scholar]
- Tingen C, Kim A, and Woodruff TK (2009). The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol. Hum. Reprod 15, 795–803. doi: 10.1093/MOLEHR/GAP073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL, Hsieh M, Menniti FS, and Conti M (2009). Cyclic GMP signalling is involved in the LH-dependent meiotic maturation of mouse oocytes. Biol. Reprod 81, 595–604. doi: 10.1095/BIOLREPROD.109.077768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A, Donnez J, and Amorim CA (2012). Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 33, 6079–6085. doi: 10.1016/J.BIOMATERIALS.2012.05.015 [DOI] [PubMed] [Google Scholar]
- van den Hurk R, and Zhao J (2005). Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 63, 1717–1751. doi: 10.1016/J.THERIOGENOLOGY.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, and Tonary AM (1995). Differential regulation of progesterone and oestradiol production by mouse cumulus and mural granulosa cells by a factor(s) secreted by the oocyte. Biol. Reprod 53, 1243–1250. doi: 10.1095/BIOLREPROD53.6.1243 [DOI] [PubMed] [Google Scholar]
- Wang NC, Butler JP, and Ingber DE (1993). Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127. doi: 10.1126/SCIENCE.7684161 [DOI] [PubMed] [Google Scholar]
- Webb R, Nicholas B, Gong JG, Campbell BK, Gutierrez CG, Garverick HA, and Armstrong DG (2003). Mechanisms regulating follicular development and selection of the dominant follicle. Reprod. Suppl 61, 71–90. [PubMed] [Google Scholar]
- West ER, Shea LD, and Woodruff TK (2007a). Engineering the follicle microenvironment. Semin. Reprod. Med 25, 287–299. doi: 10.1055/S-2007-980222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, and Shea LD (2007b). Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 28, 4439–4448. doi: 10.1016/J.BIOMATERIALS.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, and Shea LD (2009). The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol. Reprod 80, 432–439. doi: 10.1095/BIOLREPROD.108.071142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, and Shea LD (2007). The role of the extracellular matrix in ovarian follicle development. Reprod. Sci 14, 6–10. doi: 10.1177/1933719107309818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, and Shea LD (2011). A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J. Assist. Reprod. Genet 28, 3–6. doi: 10.1007/S10815-010-9478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycherley G, Downey D, Kane MT, and Hynes AC (2004). A novel follicle culture system markedly increases follicle volume, cell number and oestradiol secretion. Reproduction 127,669–677. doi: 10.1530/REP.1.00040 [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, and Woodruff TK (2006a). Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 12, 2739–2746. doi: 10.1089/TEN.2006.12.2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, and Woodruff TK (2006b). Identification of a stage-specific permissive in vitro-culture environment for follicle growth and oocyte development. Biol. Reprod 75, 916–923. doi: 10.1095/BIOLREPROD.106.054833 [DOI] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, and Shea LD (2009a). Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng 103, 378–386. doi: 10.1002/BIT.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewette SE, Shea LD, and Woodruff TK (2009b). In vitro-grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod 24, 2531–2540. doi: 10.1093/HUMREP/DEP228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, and Zelinski MB (2009c). Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol. Reprod 81, 587–594. doi: 10.1095/BIOLREPROD.108.074732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, and Stouffer RL (2010). Survival, growth and maturation of secondary follicles from pre-pubertal, young and older adult rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotrophins and insulin. Reproduction 140, 685–697. doi: 10.1530/REP-10-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, and Stouffer RL (2011). Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum. Reprod 26, 1061–1072. doi: 10.1093/HUMREP/DER049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Jha AK, Harrington DA, Farach-Carson MC, and Jia X (2012). Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter 8, 3280–3294. doi: 10.1039/C2SM06463D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, and Wright JR (1999). Calcium alginate. In ‘Cell Encapsulation Technology and Therapeutics’. (Eds Kuhtreiber WM, Lanza RP and Chick WL.) pp. 79–89. (Birkhauser: Boston.) [Google Scholar]
- Yaseen MA, Wrenzycki C, Herrmann D, Carnwath JW, and Niemann H (2001). Changes in the relative abundance of mRNA transcripts for insulin-like growth factor (IGF-I and IGF-II) ligands and their receptors (IGF-IR/IGFIIR) in preimplantation bovine embryos derived from different in vitro systems. Reproduction 122, 601–610. doi: 10.1530/REP.0.1220601 [DOI] [PubMed] [Google Scholar]
- Ye J, Coleman J, Hunter MG, Craigon J, Campbell KH, and Luck MR (2007). Physiological temperature variants and culture media modify meiotic progression and developmental potential of pig oocytes in vitro. Reproduction 133, 877–886. doi: 10.1530/REP-06-0318 [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhao X, and Spirio L (2005) Pura Matrix: self-assembling peptide nanofiber scaffolds. In ‘Scaffolding in Tissue Engineering’. (Eds Ma PX and Elisseeff J.) pp. 217–238. (CRC Press: Boca Raton, FL, USA.) [Google Scholar]
- Zhao Y, Chauvet PJ-P, Alper SL, and Baltz JM (1995). Expression and function of bicarbonate/chloride exchangers in the preimplantation mouse embryo. J. Biol. Chem 270, 24428–24434. doi: 10.1074/JBC.270.41.24428 [DOI] [PubMed] [Google Scholar]
- Zimmermann U. (1999). Biocompatible encapsulation materials: fundamentals and application. In ‘Cell Encapsulation Technology and Therapeutics’. (Eds Kuhtreiber WM, Lanza RP and Chick WL.) pp. 40–52. (Birkhauser: Boston.) [Google Scholar]