Key Points
Question
Does obesity have an association with early-onset breast cancer and diagnosis of specific molecular subtypes?
Findings
In this cohort study of 1085 patients with breast cancer, those with obesity and of Black race were diagnosed more often with breast cancer than their White counterparts. Furthermore, Black women with obesity exhibited a significantly higher risk of early onset and diagnosis of luminal A and triple-negative breast cancer subtypes.
Meaning
Findings suggest that obesity is a significant predisposing health condition associated with observed disparities in age of breast cancer onset and is also a risk factor for diagnosis of luminal A and triple-negative breast cancer molecular subtypes.
This cohort study of patients with breast cancer examines whether a race-specific association exists between obesity and early-onset breast cancer or the diagnosis of specific molecular subtypes.
Abstract
Importance
Epidemiologic data suggest an association of obesity with breast cancer (BC); however, obesity’s contribution to early onset and risk of diagnosis with specific molecular subtypes by race is uncertain.
Objective
To examine the race-specific association of body mass index with early onset and diagnosis of specific molecular subtypes.
Design, Setting, and Participants
This retrospective cohort study included patients with BC diagnosed between October 1, 2017, and March 31, 2022, at 3 University of South Alabama Mitchell Cancer Institute clinics. Participants were also prospectively enrolled for serum leptin measurement.
Main Outcomes and Measures
The primary outcome was age at BC onset and specific subtype diagnosis. The secondary outcome was race-specific differences. Odds ratios (ORs) for associations of body mass index with age at onset and subtype were estimated using the Fisher exact test. Race was self-reported.
Results
Of the 1085 study patients, 332 (30.6%) were Black with a median age of 58 (IQR, 50-66) years, and 753 (69.4%) were White with a median age of 63 (IQR, 53-71) years. A total of 499 patients (46.0%) had obesity, with Black women with obesity receiving more frequent BC diagnosis than their White counterparts (OR, 2.40; 95% CI, 1.87-3.15; P < .001). In addition, Black women had a significantly higher incidence of early-onset disease (OR, 1.95; 95% CI, 1.33-2.86; P = .001) than White women, and obesity increased this risk significantly in Black women (OR, 2.92; 95% CI, 1.35-6.22; P = .006). Black women with obesity also had a significantly higher risk of luminal A BC (OR, 2.53; 95% CI, 1.81-3.56; P < .001) and triple-negative BC (TNBC) (OR, 2.48; 95% CI, 1.43-4.22; P = .002) diagnosis than White counterparts. Black women, with or without BC, had significantly higher serum leptin levels (median [IQR], 55.3 [40.3-66.2] ng/mL and 29.1 [21.1-46.5] ng/mL, respectively, P < .001) than White women (median [IQR], 33.4 [18.9-47.7] ng/mL and 16.5 [10.0-22.9] ng/mL, respectively), which was associated with higher odds of luminal A disease (OR, 5.25; 95% CI, 1.69-14.32, P = .003). Higher odds of early-onset disease (OR, 3.50; 95% CI, 0.43-23.15; P = .33 for trend), and TNBC diagnosis (OR, 6.00; 95% CI, 0.83-37.27; P = .14 for trend) were also seen, although these outcomes were not statistically significant.
Conclusions and Relevance
In this cohort study of patients with BC, obesity and high serum leptin levels were associated with an enhanced risk of early-onset BC and diagnosis of luminal A and TNBC subtypes in Black women. These findings should help in developing strategies to narrow the existing disparity gaps.
Introduction
Obesity, defined as the excessive accumulation of fat, is a concerning disorder that increases the risk of various other health problems. Black women in the US tend to have more obesity based on body mass index (BMI) than their non-Hispanic White counterparts.1,2 Social, economic, psychological, and neighborhood factors likely contribute to this racial disparity, along with increasing the risk of several other diseases, including cancer.1,3,4 Breast cancer (BC) is the most commonly diagnosed cancer and the second leading cause of cancer-related death among US women. It also exhibits significant race-associated health disparities.5,6 Although both Black and White women have nearly equal rates of BC diagnosis, the incidence of BC diagnosis in individuals younger than 45 years is higher among Black women.7,8 Black women are also more likely to be diagnosed with an aggressive molecular subtype, triple-negative BC (TNBC or basal-like), compared with their White counterparts.9,10 In addition, Black women with hormone receptor–positive, ERBB2 (OMIM 164870) (previously HER2/neu)–negative BC (luminal A subtype) experience a high disparity in survival, contributing to their overall higher mortality.11
Leptin is an obesity-associated molecule functioning both as a hormone and a cytokine. It is known to have antiapoptotic, mitotic, and proangiogenic roles through direct actions via its receptor (Ob-R) and its crosstalk with other oncogenic signaling pathways.12,13,14,15,16 Thus, leptin could be an important part of the nexus of social experiences, obesity, and race-associated BC disparities. Several race-associated differences in BC biology have been reported. SOS1 (OMIM 182530), a key regulator of Ras signaling, is overexpressed in BC in Black women compared with White women. It is shown to promote metastasis through the obesity-activated hepatocyte growth factor receptor pathway.17 Likewise, a role of cancer disparity-linked genes, CRYBB2 and CRYBB2P1 (OMIM 123620), has been reported in BC.18 Other genetic differences, specifically those pertaining to immune responses, have also been reported and suggested to have an association with higher rates of TNBC in Black women.19
In this exploratory cohort study, we sought to identify whether obesity has an association with early-onset BC in Black women and favors diagnosis with a specific molecular subtype, such as TNBC. We also analyzed whether levels of leptin in a patient’s serum vary by race and exhibit a similar association to suggest its potential as a biological risk factor and mediator of pathobiological association.
Methods
Study Population
For the current retrospective cohort study, clinical records of patients between October 1, 2017, and March 31, 2022, were pulled from 3 University of South Alabama Mitchell Cancer Institute (MCI) clinics (MCI Mobile–Children’s and Women’s Hospital, MCI Fairhope–University Hospital, and MCI Springhill–University Hospital) with geographic bounds concentrated in a single metropolitan area. Details of the number of patients with their counties and states are given in eTable 1 in Supplement 1. Women who self-reported as Black or White and had confirmed BC diagnosis with no prior history of any other cancer and who were older than 18 years at the time of diagnosis were included. Other inclusion criteria were available information on BMI and BC molecular subtypes. Black and White women with or without BC diagnosis were also prospectively recruited for blood biomarker analysis. Written informed consent was obtained from all the volunteers, and the collected data were deidentified. The study protocol was reviewed and approved by the USA Institutional Review Board, and all the relevant regulations and guidelines were followed. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.20
Definition of Obesity, Early Onset, and BC Molecular Subtypes
The World Health Organization defines obesity as excessive fat accumulation posing a risk to health. A person with a BMI (calculated as weight in kilograms divided by height in meters squared) of 30 or higher is considered to have obesity; between 25 and 30, overweight; and less than 25, normal weight. Body mass index was recorded at patients’ visits to the clinic before any surgery. Early-onset BC is defined as a diagnosis made before age 45 years.21 For association analysis, we divided the patients into 3 age categories (<45, 45-65, and >65 years). On the basis of the expression of hormone receptors (HRs), which included estrogen receptors (ERs) and progesterone receptors (PRs) and ERBB2 receptor, BC cases were classified into 4 molecular subtypes: luminal A (HR positive and ERBB2 negative), luminal B (HR positive and ERBB2 positive), ERBB2 enriched (HR negative and ERBB2 positive), and basal BC or TNBC (HR negative and ERBB2 negative). Patients with HR-positive BC were positive for ER, PR, or both.
Leptin: An Adiposity Biomarker
Leptin is a hormone predominantly produced by adipocytes and is regarded as a biomarker of fat mass.22 Its primary role is to regulate appetite and energy balance, but it can also influence several other biological processes, including carcinogenesis.23,24 Serum leptin levels were measured in prospectively enrolled patients with BC or non-BC diagnosis using a commercial enzyme-linked immunosorbent assay kit (R&D Systems) as per the manufacturer’s instructions. On the basis of the median of leptin levels, participants were divided into 3 categories: low, when levels were lower than the median level (<21.9 ng/mL) in women without BC; high, when above the median level (>44.1 ng/mL) in women with BC; and moderate, when the levels were in between (21.9-44.1 ng/mL).
Statistical Analysis
Patients were compared based on their BMI, age at BC onset, and molecular subtypes. The descriptive statistics were computed to calculate the mean (SD) and median (IQR). The Fisher exact test was used to calculate the odds ratio (ORs) and 95% CIs for Black vs White women with respect to categorized age, BMI, and BC subtypes. Serum leptin levels were measured in triplicates, and the 2-tailed, unpaired t test was performed to compare the mean levels in BC and non-BC (control) groups. Pearson correlation analysis was performed to study the linear association between BMI and leptin levels. Results were considered significant with a 2-sided P < .05. All statistical calculations were performed using GraphPad Prism, version 8 (GraphPad Software Inc).
Results
We included a cohort of 1085 women with a confirmed BC diagnosis. Among them, 332 (30.6%) were Black and 753 (69.4%) were White based on self-reporting. Median (IQR) age at diagnosis was 58 (50-66) in the Black women and 63 (53-71) years in the White women. The median (IQR) BMI was 32.5 (27.5-38.0) in the Black women and 28.2 (24.0-32.7) in the White women (Table). Among the 3 categories of BMI, the highest percentage of women with BC fell in the obesity category (499 [46.0%]), with nearly equal distribution in the normal weight and overweight groups (Figure 1A). However, Black women with obesity were at a significantly higher risk of being diagnosed with BC than White women (OR, 2.40; 95% CI, 1.87-3.15; P < .001) (Figure 1B). Significant differences in the age distribution of patients with BC were also reported, with Black women exhibiting a higher incidence of early-onset BC (age <45 years) compared with White women. Black women were 1.95 times more likely to be diagnosed with BC than White women at an early age (95% CI, 1.33-2.86; P = .001) (Figure 1C). Notably, Black women also exhibited a significantly positive association of obesity with early-onset BC (OR, 2.92; 95% CI, 1.35-6.22; P = .006) (Figure 1D).
Table. Baseline Characteristics of the Retrospective Cohort (2017-2022)a.
| Characteristic | Black | White |
|---|---|---|
| Total patients | 332 (30.6) | 753 (69.4) |
| Age, median (IQR), y | 58 (50-66) | 63 (53-71) |
| BMI, median (IQR) | 32.5 (27.5-38.0) | 28.2 (24.0-32.7) |
| BMI categories | ||
| Normal weight | 60 (18.1) | 230 (30.5) |
| Overweight | 69 (20.8) | 227 (30.2) |
| Obesity | 203 (61.1) | 296 (39.3) |
| Subtype | ||
| Luminal A | 191 (57.5) | 547 (72.6) |
| Luminal B | 25 (7.5) | 67 (8.9) |
| ERBB2 enriched | 10 (3.0) | 34 (4.5) |
| TNBC | 106 (32.0) | 105 (14.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); TNBC, triple-negative breast cancer.
Data are presented as number (percentage) of patients unless otherwise indicated.
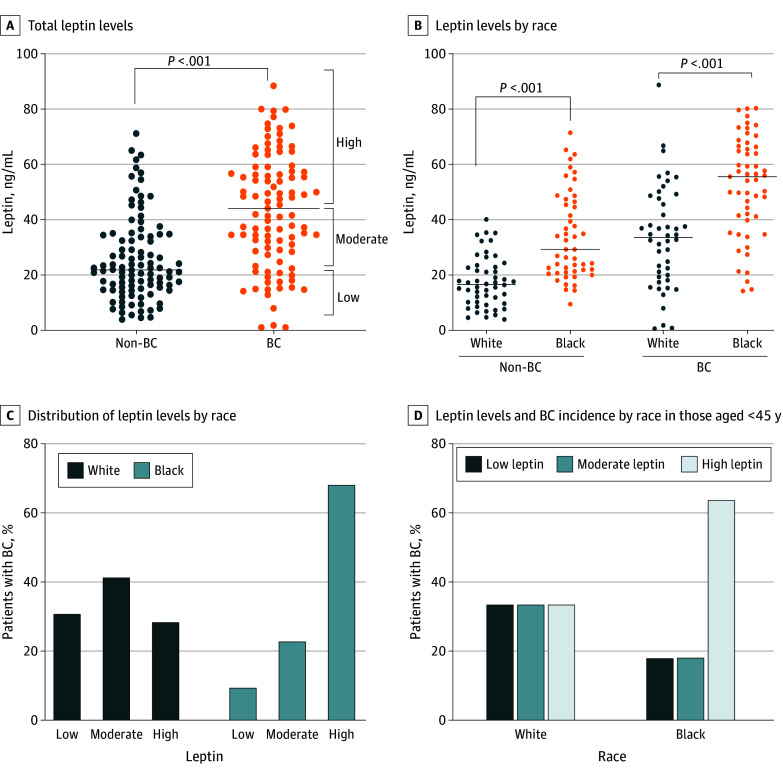
Figure 1. Association of Breast Cancer (BC) With Body Mass Index (BMI) and Age.
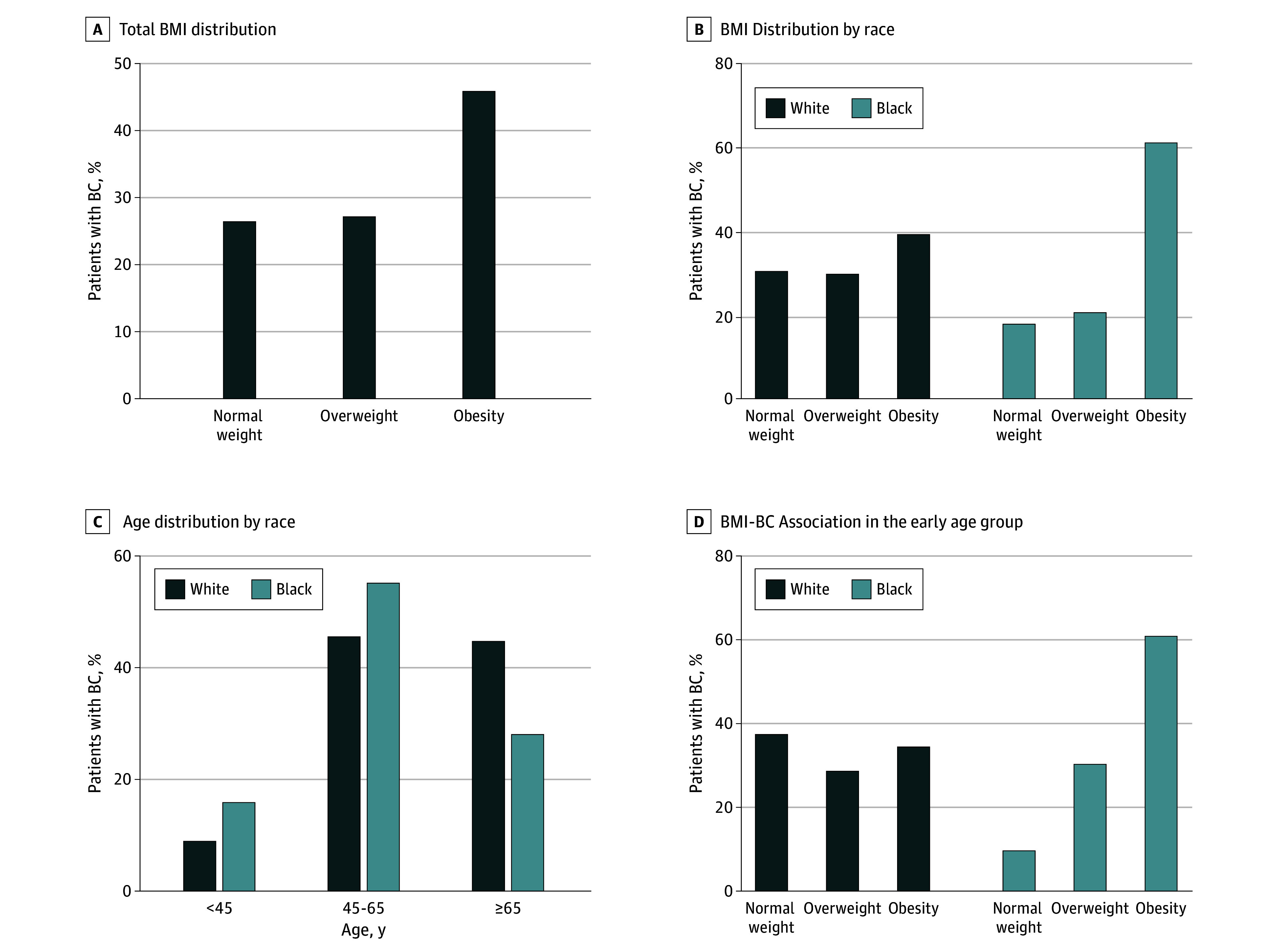
A, Distribution of BMI (calculated as weight in kilograms divided by height in meters squared) in patients with BC (n = 1085). All patients were divided into 3 BMI categories: less than 24.9 (normal weight), 25.0 to 29.9 (overweight), and greater than 30.0 (obesity). B, Distribution of BMI in patients with BC by race. C, Distribution of age between Black and White women. Total patients were divided into 3 age categories: younger than 45, 45 to 65, and older than 65 years. D, Association of BMI with BC among the early age group (aged <45 years) between Black and White women.
All patients were most frequently diagnosed with the luminal A subtype (Black, 191 [57.5%]; White, 547 [72.6%]), followed by TNBC (Black, 106 [32.0]%; White, 105 [14.0%]), luminal B (Black, 25 [7.5%]; White, 67 [8.9%]), and ERBB2-enriched (Black, 10 [3.0%]; White, 34 [4.5%]) subtypes. Black women were more likely to receive a TNBC diagnosis than White women (OR, 2.90; 95% CI, 2.12-3.96; P < .001), whereas White women were more likely to be diagnosed with the luminal A subtype compared with Black women (OR, 1.96; 95% CI, 1.50-2.56; P < .001) (Figure 2A). A positive association of obesity with the diagnosis of TNBC subtype (OR, 1.36; 95% CI, 1.01-1.83; P = .05 for trend) was observed. Higher odds of diagnosis of luminal A subtype were also observed, although this finding was not statistically significant (OR, 1.17; 95% CI, 0.90-1.57; P = .26 for trend) (Figure 2B). Moreover, the odds for Black women with obesity were 153% and 148% higher compared with White women with obesity for the diagnosis of luminal A BC (OR, 2.53; 95% CI, 1.81-3.56; P < .001) and TNBC (OR, 2.48; 95% CI, 1.43-4.22; P = .002), respectively (Figure 2C and D).
Figure 2. Prevalence of Breast Cancer (BC) Subtypes in Association With Body Mass Index (BMI).
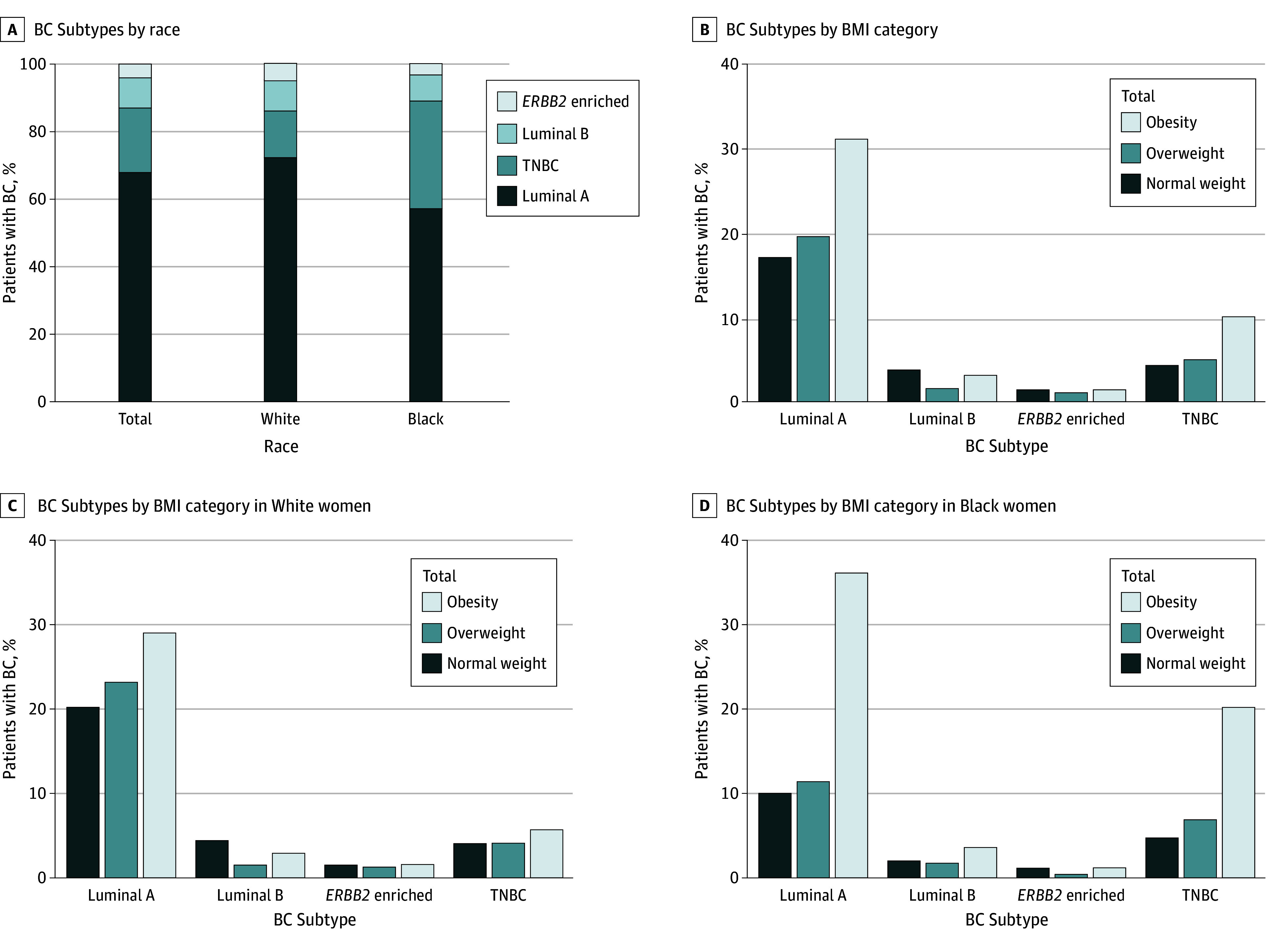
A, Percentage distribution of BC subtypes from a retrospective cohort among total, Black, and White women. B, Percentage distribution of BC subtypes under 3 BMI (calculated as weight in kilograms divided by height in meters squared) groups in the total cohort of patients. C and D, Percentage distribution of BC subtypes under 3 BMI groups between White (C) and Black (D) women. TNBC indicates triple-negative BC.
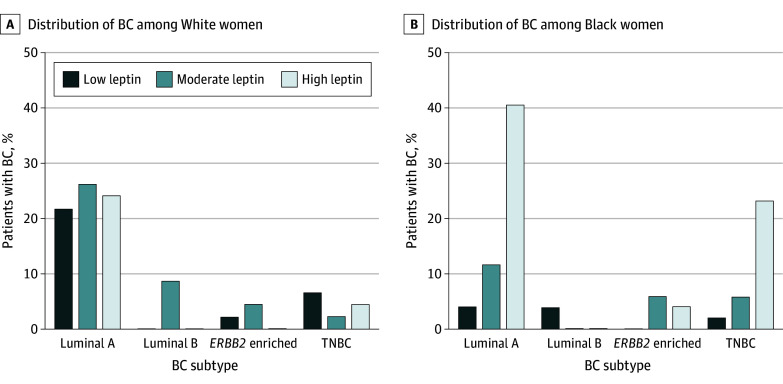
To observe whether leptin, being a bioactive signaling molecule, could mediate the risk association of BMI with early-onset BC and luminal A and TNBC diagnosis, we prospectively enrolled 99 patients with BC (53 Black and 46 White) along with 100 controls without BC (50 each from self-reported Black and White racial groups) (eTable 2 in Supplement 1). We observed significantly elevated serum leptin levels in women with a BC diagnosis (median [IQR], 44.1 [28.6-57.4] ng/mL) compared with the control group (median [IQR], 21.9 [15.2-34.3] ng/mL) (P < .001) (Figure 3A). Notably, Black women with or without BC exhibited significantly higher leptin levels (median [IQR], 55.3 [40.3-66.2] ng/mL and 29.1 [21.1-46.5] ng/mL, respectively) than White women (median [IQR], 33.4 [18.9-47.7] ng/mL and 16.5 [10.0-22.9] ng/mL, respectively) (P < .001) (Figure 3B; eTable 3 in Supplement 1). Leptin levels were positively correlated with BMI in both patients with BC (r = 0.52, P < .001) and control patients (r = 0.55, P < .001) in both racial groups; however, a slightly stronger association was observed in Black patients with BC (r = 0.48, P < .001) or Black controls without BC (r = 0.56, P < .001) compared with their White counterparts (r = 0.46, P = .001 for both groups) (eFigure in Supplement 1). Black women with high leptin levels were more likely to be diagnosed with BC than White women (OR, 5.38; 95% CI, 2.18-12.35; P < .001) (Figure 3C). Furthermore, compared with White women, we observed higher odds of early-onset BC in Black women, although this finding was not statistically significant (OR, 3.50; 95% CI, 0.43-23.15; P = .33 for trend) (Figure 3D). In addition, higher leptin levels were associated with increased odds of being diagnosed with luminal A (OR, 5.25; 95% CI, 1.69-14.32; P = .003) in Black women compared with White women. However, there was no statistically significant difference for TNBC diagnosis (OR, 6.00; 95% CI, 0.83-37.27; P = .14 for trend) (Figure 4).
Figure 3. Levels of Serum Leptin and Association With Early-Onset Breast Cancer (BC).
A, Serum levels of leptin in patients without BC (n = 100) and patients with BC (n = 99). B, Serum levels of leptin in Black and White women (n = 50 each for non-BC group and n = 53 and 46 for the BC groups, respectively. C, Distribution of leptin levels between Black and White women in patients with BC. D, Association of leptin levels with BC incidence among the early age group (aged <45 years) between Black and White women.
Figure 4. Association of Serum Leptin Levels With Breast Cancer (BC) Subtype Diagnosis.
Percentage distribution of BC subtypes among 3 leptin groups between White and Black women. TNBC indicates triple-negative BC.
Discussion
Obesity plays a significant role in the development of cancer. Data presented in this study provide novel evidence suggesting an association of obesity with early-onset BC and the risk of diagnosis with luminal A and TNBC molecular subtypes, with a higher risk in Black women. The global epidemic of obesity is continuously increasing; however, a disparate prevalence of obesity exists among different racial groups. The prevalence of obesity in Black women is 56.9% compared with 39.8% in White women in the US.25 Furthermore, Black women, although exhibiting a similar incidence rate for BC diagnosis as their White counterparts, are more often diagnosed at a younger age. Black women also present with more aggressive disease and have a poorer response to existing therapies, which contributes to their overall higher mortality.10,26
While analyzing the retrospective cohort dataset, we found that most women with BC had a BMI that fell in the obesity category. Furthermore, in a race-wise comparison, we observed that the percentage of Black women with obesity was significantly higher than that of White women of a similar BMI. As previously reported,8 we also observed a higher incidence of early-onset BC in Black women in our cohort. Interestingly, when we compared BMI distribution in early onset of BC, we found that Black women with obesity were at a significantly higher risk compared with White women. These observations suggest that although increased BMI may be a predominant risk factor associated with the diagnosis of BC at a younger age in Black women, early onset of BC in White women likely results from genetic predisposition or other risk behaviors. Among the genetic risk factors, BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185) are most commonly (>50%) associated with early-onset BC.21 Some race-associated differences in the frequency of these mutations are observed, with Black women having a lower frequency of BRCA1 and a relatively higher frequency of BRCA2 than White women.21
In our study, we also found that a higher percentage of patients with increasing BMI were diagnosed with luminal A and TNBC subtypes in both Black and White women; however, the risk was significantly higher in Black women for both the molecular subtypes. A previous study found that higher adiposity was associated with a risk of BC development in postmenopausal women, especially the risk of being diagnosed with HR-positive but not HR-negative BC.27 In addition, women with obesity are more likely to have lymph node metastasis associated with poorer survival.28,29 Furthermore, obesity can impact the treatment response, including endocrine therapy primarily administered to patients with HR-positive BC.30 A previous study also found that obesity increased the risk of recurrence among patients with HR-positive BC treated with aromatase inhibitors.28 Thus, a higher rate of obesity in Black women with HR-positive BC could be associated with disparate high mortality rates for this subtype. Triple-negative BC is a highly aggressive molecular subtype that is diagnosed more frequently in Black women and has limited available therapeutic options.31,32 Patients with TNBC often have their disease diagnosed at higher stages and grades and often have large, fast-growing tumors. Earlier studies have demonstrated that obesity is positively associated with increasing stage and grade of tumor in patients with TNBC.33,34,35 Thus, it appears that obesity and race should be evaluated as a significant risk factor for the diagnosis of aggressive disease molecular subtypes as well as the disease outcomes.
Leptin is a signaling hormone produced by adipose cells and has been shown to have a causative link with BC.36 It not only enhances the progression of HR-positive BC also promotes chemoresistance in TNBC cells.37,38 We found that leptin levels were increased in the serum of patients with BC. Furthermore, Black women exhibited higher serum levels of leptin than White women regardless of their BC diagnosis. Furthermore, leptin levels were positively associated with the patient’s BMI and exhibited a similar association with age at disease onset and molecular subtype diagnosis. These data suggest that leptin could be a biological intermediary for the observed association of obesity with early onset of BC and specific subtype diagnosis. However, because we observed a significantly stronger association in Black patients, other race-associated risk factor(s) should be analyzed that likely work in concert with leptin at the signaling level to support early development of BC and favor the growth of luminal A and TNBC molecular subtypes. A previous report highlighted the differences in the African ancestry–associated genes in patients with TNBC that impact tumor biology and clinical outcomes.39 Thus, it will be interesting to see whether leptin signaling has a molecular crosstalk with these African ancestry–associated genes and whether that contributes to observed disparities in age at onset and more frequent diagnosis of luminal A and TNBC subtypes in Black women with obesity.
Limitations
This study has several limitations. First, this is a single-institution study enrolling patients who visited clinics during 5 years. Information on BC subtype for all patients was difficult to access, especially those who visited clinics before 2017, due to changes in the electronic medical record management server that resulted in a small sample size. In addition, despite our best efforts, it was not possible to enroll a large cohort of patients in the prospective study. Second, racial grouping was solely based on self-reporting; thus, there is a risk of misrepresentation.
Conclusions
Our findings suggest that obesity is associated with an early onset of BC and a higher risk of diagnosis with luminal A or TNBC subtypes, especially in Black women. In addition, higher levels of serum leptin appear to be an important biological intermediary in these risk associations. Additional race-associated risk factors should be explored, such as social exposures and economic and psychological stressors that promote obesity rates in Black women and may have a broader effect on human biology. These factors may create further hormonal imbalance or inflammation that may lead to aggressive progression of breast tumors in Black women, leading to its presentation at an early age. Future research along these lines could increase our understanding of the underlying causes of BC racial disparities and help shape community-based approaches to diminish the existing disparity gaps.
eFigure. Correlation Between Serum Leptin Levels and BMI
eTable 1. Details of Counties and States of Retrospective Cohort (2017-2022)
eTable 2. Characteristics of Prospectively Enrolled Patients
eTable 3. Serum Levels of Leptin in BC and Non-BC Patients
Data Sharing Statement
References
- 1.Agyemang P, Powell-Wiley TM. Obesity and Black women: special considerations related to genesis and therapeutic approaches. Curr Cardiovasc Risk Rep. 2013;7(5):378-386. doi: 10.1007/s12170-013-0328-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of Minority Health. Obesity and African Americans. Accessed January 18, 2024. https://minorityhealth.hhs.gov/obesity-and-african-americans
- 3.Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105(2):260-275. doi: 10.1037/0033-2909.105.2.260 [DOI] [PubMed] [Google Scholar]
- 4.Dean LT, Gehlert S, Neuhouser ML, et al. Social factors matter in cancer risk and survivorship. Cancer Causes Control. 2018;29(7):611-618. doi: 10.1007/s10552-018-1043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 6.Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality—United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. doi: 10.15585/mmwr.mm6540a1 [DOI] [PubMed] [Google Scholar]
- 7.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357-370. doi: 10.1007/s10549-008-9926-3 [DOI] [PubMed] [Google Scholar]
- 8.Baquet CR, Mishra SI, Commiskey P, Ellison GL, DeShields M. Breast cancer epidemiology in blacks and whites: disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc. 2008;100(5):480-488. doi: 10.1016/S0027-9684(15)31294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer. 2014;14:62. doi: 10.1186/1471-2407-14-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results Cancer of the Breast (Female)—Cancer Stat Facts. Accessed January 18, 2024. https://seer.cancer.gov/statfacts/html/breast.html
- 11.Hoskins KF, Calip GS, Huang HC, Ibraheem A, Danciu OC, Rauscher GH. Association of social determinants and tumor biology with racial disparity in survival from early-stage, hormone-dependent breast cancer. JAMA Oncol. 2023;9(4):536-545. doi: 10.1001/jamaoncol.2022.7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan L, Lu Y, Xie W, et al. Leptin promotes bone metastasis of breast cancer by activating the SDF-1/CXCR4 axis. Aging (Albany NY). 2020;12(16):16172-16182. doi: 10.18632/aging.103599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu CJ, Dong LL, Kang XL, Li ZM, Zhang HY. Leptin promotes proliferation and inhibits apoptosis of prostate cancer cells by regulating ERK1/2 signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(16):8341-8348. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Tan M, Tian X, et al. Leptin receptor mediates the proliferation and glucose metabolism of pancreatic cancer cells via AKT pathway activation. Mol Med Rep. 2020;21(2):945-952. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42(3):1113-1119. doi: 10.3892/ijo.2013.1789 [DOI] [PubMed] [Google Scholar]
- 16.Ribatti D, Belloni AS, Nico B, Di Comite M, Crivellato E, Vacca A. Leptin-leptin receptors are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29(9):1596-1602. doi: 10.1016/j.peptides.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 17.Xing F, Zhao D, Wu SY, et al. Epigenetic and posttranscriptional modulation of SOS1 can promote breast cancer metastasis through obesity-activated c-Met signaling in African-American women. Cancer Res. 2021;81(11):3008-3021. doi: 10.1158/0008-5472.CAN-19-4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrow MA, Martin ME, Coffey A, et al. A functional role for the cancer disparity-linked genes, CRYβB2 and CRYβB2P1, in the promotion of breast cancer. Breast Cancer Res. 2019;21(1):105. doi: 10.1186/s13058-019-1191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogony JW, Radisky DC, Ruddy KJ, et al. Immune responses and risk of triple-negative breast cancer: implications for higher rates among African American women. Cancer Prev Res (Phila). 2020;13(11):901-910. doi: 10.1158/1940-6207.CAPR-19-0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Chelmow D, Pearlman MD, Young A, et al. Executive summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135(6):1457-1478. doi: 10.1097/AOG.0000000000003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benbaibeche H, Bounihi A, Koceir EA. Leptin level as a biomarker of uncontrolled eating in obesity and overweight. Ir J Med Sci. 2021;190(1):155-161. doi: 10.1007/s11845-020-02316-1 [DOI] [PubMed] [Google Scholar]
- 23.Zimmet P, Hodge A, Nicolson M, et al. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. BMJ. 1996;313(7063):965-969. doi: 10.1136/bmj.313.7063.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589-593. doi: 10.1038/nm0596-589 [DOI] [PubMed] [Google Scholar]
- 25.National Institute of Diabetes and Digestive and Kidney Diseases. Overweight & Obesity Statistics. Accessed January 18, 2024. https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity
- 26.Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200(1):75-83. doi: 10.1007/s10549-023-06943-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: Continuous Update Project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183-1200. doi: 10.1007/s10552-019-01223-w [DOI] [PubMed] [Google Scholar]
- 28.Harborg S, Feldt M, Cronin-Fenton D, et al. Obesity and breast cancer prognosis: pre-diagnostic anthropometric measures in relation to patient, tumor, and treatment characteristics. Cancer Metab. 2023;11(1):8. doi: 10.1186/s40170-023-00308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Cai Y, Yu F, Ping Z, Liu L. Body mass index increases the lymph node metastasis risk of breast cancer: a dose-response meta-analysis with 52904 subjects from 20 cohort studies. BMC Cancer. 2020;20(1):601. doi: 10.1186/s12885-020-07064-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirdarreh M, Pezo RC. Impact of obesity on clinical outcomes in hormone receptor-positive breast cancer: a systematic review. Breast Cancer. 2021;28(3):755-764. doi: 10.1007/s12282-020-01213-w [DOI] [PubMed] [Google Scholar]
- 31.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15(4):248-254. doi: 10.1038/nrc3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obidiro O, Battogtokh G, Akala EO. Triple negative breast cancer treatment options and limitations: future outlook. Pharmaceutics. 2023;15(7):1796. doi: 10.3390/pharmaceutics15071796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117(18):4132-4140. doi: 10.1002/cncr.26019 [DOI] [PubMed] [Google Scholar]
- 34.Mowad R, Chu QD, Li BDL, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res. 2013;184(1):253-259. doi: 10.1016/j.jss.2013.05.037 [DOI] [PubMed] [Google Scholar]
- 35.Hao S, Liu Y, Yu KD, Chen S, Yang WT, Shao ZM. Overweight as a prognostic factor for triple-negative breast cancers in Chinese women. PLoS One. 2015;10(6):e0129741. doi: 10.1371/journal.pone.0129741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atoum MF, Alzoughool F, Al-Hourani H. Linkage between obesity leptin and breast cancer. Breast Cancer (Auckl). PUblished online January 10, 2020. doi: 10.1177/1178223419898458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raut PK, Choi DY, Kim SH, et al. Estrogen receptor signaling mediates leptin-induced growth of breast cancer cells via autophagy induction. Oncotarget. 2017;8(65):109417-109435. doi: 10.18632/oncotarget.22684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiagarajan PS, Zheng Q, Bhagrath M, et al. STAT3 activation by leptin receptor is essential for TNBC stem cell maintenance. Endocr Relat Cancer. 2017;24(8):415-426. doi: 10.1530/ERC-16-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martini R, Delpe P, Chu TR, et al. African ancestry–associated gene expression profiles in triple-negative breast cancer underlie altered tumor biology and clinical outcome in women of African descent. Cancer Discov. 2022;12(11):2530-2551. doi: 10.1158/2159-8290.CD-22-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Correlation Between Serum Leptin Levels and BMI
eTable 1. Details of Counties and States of Retrospective Cohort (2017-2022)
eTable 2. Characteristics of Prospectively Enrolled Patients
eTable 3. Serum Levels of Leptin in BC and Non-BC Patients
Data Sharing Statement