This cohort study evaluates associations between losing a loved one and accelerated biological aging.
Key Points
Question
Is the experience of losing a loved one associated with accelerated biological aging?
Findings
In a cohort study of 3963 participants from the National Longitudinal Study of Adolescent to Adult Health, nearly 40% experienced the loss of a close relation by adulthood. Participants who had experienced a greater number of losses exhibited significantly older biological ages compared with those who had not experienced such losses.
Meaning
These findings suggest that loss can accelerate biological aging even before midlife and that frequency of losses may compound this, potentially leading to earlier chronic diseases and mortality.
Abstract
Importance
The link between familial loss of a loved one and long-term health decline is complex and not fully understood.
Objective
To test associations of losing a parent, sibling, child, or partner or spouse with accelerated biological aging.
Design, Setting, and Participants
Data from the National Longitudinal Study of Adolescent to Adult Health, a US population-based longitudinal cohort study, were analyzed. Participants were enrolled from 1994 to 1995 for wave 1, while in grades 7 to 12, and followed up through wave 5 in 2018. The study analyzed participant reports of loss collected at each wave from 1 to 5 over 24 years and used a banked wave 5 blood sample for subsequent DNA methylation testing and epigenetic clock calculation from 2018 to 2024. Data were analyzed from January 2022 to July 2024.
Exposure
Loss of biological parents or parental figures, partners or spouses, siblings, or children at waves 1 to 3 or during childhood, adolescence (aged <18 years), or adulthood at wave 4 to wave 5 (aged 18-43 years).
Main Outcomes and Measures
Biological aging assessed from blood DNA methylation using the Horvath, PhenoAge, GrimAge, and DunedinPACE epigenetic clocks at wave 5.
Results
Data from 3963 participants were analyzed, with a weighted mean (range) age of 38.36 (36.78-39.78) years at wave 5; 2370 (50.3%) were male, 720 (15.97%) were Black, 400 (8.18%) were Hispanic, and 2642 (72.53%) were White. Nearly 40% of participants experienced loss by wave 5 when they were aged 33 to 43 years, and participants who were Black (379 participants [56.67%]), Hispanic (152 participants [41.38%]), and American Indian (18 participants [56.08%]) experienced a greater proportion of losses compared with White participants (884 participants [34.09%]). Those who experienced 2 or more losses tended to have older biological ages for several of the clocks (PhenoAge β = 0.15; 95% CI, 0.02 to 0.28; GrimAge β = 0.27; 95% CI, 0.09 to 0.45; DunedinPACE β = 0.22; 95% CI, 0.10 to 0.34) compared with those with no losses. In contrast, there were no associations with 2 or more losses for the Horvath clock (β = −0.08; 95% CI, −0.23 to 0.06).
Conclusions and Relevance
This study reveals associations between various measures of loss experienced from childhood to adulthood and biological aging in a diverse sample of the US population. These findings underscore the potentially enduring impact of loss on biological aging even before middle age and may contribute to understanding racial and ethnic disparities in health and mortality.
Introduction
The relationship between bereavement and health across the life course is well-established and enduring.1 However, there are indications that certain life stages may be more susceptible to the health and mortality risks associated with loss. For instance, the loss of a parent or sibling in early life is notably traumatic and linked to poor mental health, cognitive impairment, increased cardiometabolic risk, and higher mortality risk in later life.2,3,4,5 Nonetheless, the death of a close family member at any age poses health risks, such as heightened cardiovascular, mortality, and dementia risks in adulthood.1,6,7,8 Repeated family losses over one’s life further compound these health risks.6 The impacts of loss may persist or become apparent long after the event.5,6,8,9
The mechanisms connecting loss to poor health and mortality remain unclear. There are at least 2 pathways, each with intermediate processes. One pathway begins with deficits in material and social resources formerly provided by the person who dies. Another begins with the psychological distress of bereavement. Both can ultimately result in dysregulation of biological systems due to changes in health behaviors related to bereavement-related distress and stress from economic constraints. One framework that integrates the impacts of these diverse pathways and intermediate processes on human health is emerging research on biological aging.10
Biological aging refers to the progressive loss of integrity and resilience capacity in our cells, tissues, and organs as we grow older.11 While there are no universally accepted methods to measure biological aging in humans, the best-validated of these methods are a family of DNA-methylation algorithms known as epigenetic clocks, including several that show robust evidence for prediction of future morbidity and mortality.10,12,13 However, few studies have empirically explored the association between loss across developmental periods and DNA methylation markers of aging, and rarely have these investigations been conducted in racially and ethnically diverse population-based studies.
To address existing gaps, we investigated if familial loss and quantity of loss, occurring either in early life or adulthood, were associated with biological aging in a diverse, population-based sample. Acknowledging prior research on racial disparities in loss experiences and variability in exposure histories,6,14 we also examined the interaction between loss and race on biological aging. We analyzed survey responses from waves 1 to 5 of the National Longitudinal Study of Adolescent to Adult Health (Add Health), coupled with new epigenetic data from wave 5.
Methods
We used data from wave 1 to wave 5 of Add Health, which has been previously described15 and is a US nationally representative cohort, following up participants since the 1994 to 1995 school year. Wave 5 took place between 2016 and 2018 and completed interviews with 12 300 participants. During wave 5, participants were invited for an additional home examination where a venous blood sample was gathered. Of the 7995 individuals who agreed to the home examination, 5381 were successfully visited, and 4940 provided a blood sample. We used data from 3963 participants who had a blood sample, reported losses at each wave, and reported data on covariates. We used weighting to ensure representation of the Add Health cohort since wave 1.15 The weights account for the original sampling design, attrition to the current wave 5, and differential consent to the in-home blood collection.16
Participants and their parents or caregiver in childhood provided written consent at waves 1 through 2; at age 18 years, only the participant’s consent was obtained (waves 3-5). The study was approved by the institutional review boards of Columbia University and the University of North Carolina, Chapel Hill. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.17
Epigenetic Clocks
From 2018 to 2024, we conducted epigenome-wide profiling and construction of clocks, using whole-blood DNA for 4700 participants at wave 5 using the Infinium MethylationEPIC BeadChip array (Illumina, Inc), as previously described.18 After standard quality control, we calculated 4 biological clocks, including GrimAge, PhenoAge, and Horvath, to assess epigenetic age acceleration,19,20,21,22 and the DunedinPACE to assess pace of biological aging.23,24 For analysis, Horvath, GrimAge, and PhenoAge were first residualized on chronological age and residuals were z-transformed to allow comparability across measures. DunedinPACE is a measure of the pace of aging—how rapidly or slowly a person is aging.24 It is a rate, not an age, and so no residualization was conducted, but values were z-transformed for comparability.
Familial Loss
Familial loss and the timing of loss variables were derived from waves 1 to 5. We included the following from each wave: (1) parental death (biological and parent figures), (2) death of a sibling, (3) death of a spouse or partner, and (4) death of a child. We calculated the total number of losses by pooling across 5 waves, using only new reports of loss. Number of losses were coded as 0, 1, or 2 or more losses.
Parental Loss Across Waves 1 to 5
Given research suggesting a strong association between parental loss, in particular, and the health of surviving children,25,26,27 in addition to the fact that it was the most common type of loss in Add Health, we assessed parental loss at any time point across waves 1 to 5. The parental loss variable included the death of parents and/or parent figures at any wave.
Developmental Period of Loss
To classify loss at varying developmental periods, we used survey reports of parental and sibling loss reported on surveys from wave 1 to 2 by identifying whether the participant was aged less than 18 years at the time of the loss. To classify loss among participants aged less than 18 years, we used the month and year of parental and sibling deaths reported at wave 3 or 4 or as reported in the last 12 months on wave 5. We also used reports of death of a partner or spouse and child from waves 3 to 5.
We developed variables for any loss in childhood or adolescence and any loss in adulthood based on reported loss timing. Any loss in childhood or adolescence includes deaths of parents (biological and parental figures) and siblings before age 18 years. Any loss in adulthood encompasses deaths of parents, siblings, spouses or partners, and children when the participant was 18 or older. Additionally, we created variables for parental loss in childhood or adolescence (death of a parent or parental figure before age 18 years) and parental loss in adulthood (death of a parent or parental figure at age 18 years or older).
Covariates
Several covariates were included in the analytic models based on known or potential confounders and directed acyclic graphs.10,13,28,29,30 Chronological age at blood draw was included as a continuous variable. Participant self-identified race and ethnicity was based on the race or ethnicity that the participant indicated that they most strongly identified with at the wave 5 survey, including Asian, Black, Hispanic, American Indian, other, Pacific Islander, or White. Participants that responded as other did not identify with any race or ethnic categories or identified as multiracial only. In a small proportion of cases (9 participants) where wave 5 information was missing or unavailable, wave 1 was used to identify self-reported race and ethnicity. We present loss for all racial and ethnic groups where sample sizes permit. In our regression models, we categorized race as Black, Hispanic, and a combined category for all other racial groups due to limited sample sizes. Respondent’s report on sex assigned at birth at wave 5 was used to categorize participants as female or male. To account for environmental or neighborhood influences on epigenetic aging in early life, we included a variable that represented the proportion of households with an income below the poverty line in 1989 based on participant addresses at wave 1. We also adjusted for the number of household members in wave 1, to account for the increased likelihood of loss among those with greater members. We also included parental educational attainment based on the highest attainment of either parent at wave 1 (high school degree or less, some college, and college or higher). Smoking was based on parent’s self-report of smoking at wave 1. We adjusted for epigenetic assay batch (batch 1, 41.34%; batch 2, 56.51%; batch 3, 1.15%). We also conducted sensitivity analyses by adjusting for cell counts as described in eTable 1, eTable 2, eTable 3, and eTable 4, and for duration since loss in eTable 5, eTable 6, eTable 7, and eTable 8 in Supplement 1.
Statistical Analysis
We used survey linear regression models for assessing the associations between each number of losses and each of the biological aging measures, adjusting for all covariates described previously. Models were estimated and graphs created using the survey,31,32,33,34 jtools,35 and ggplot236,37 packages in R version 4.2.1 (R Project for Statistical Analysis).35,36,37,38,39
In models investigating timing of loss (any loss or parental loss), we included a variable indicating loss during childhood or adolescence and a variable indicating loss during adulthood so that we could assess the association of childhood or adolescence and adulthood loss independently of one another. Following Add Health guidelines,16,38 analyses accounted for complex sampling features (ie, probability weights, nesting of individuals in primary sampling units, no siblings, and stratification) so that results represent the population of individuals who were enrolled in grades 7 to 12 in the US in 1994 to 1995.16 We used 95% CIs to evaluate statistical significance.
Racial and ethnic disparities exist in the frequency and nature of losing loved ones, often due to racism and a history of disadvantage.3,6,40,41,42,43 Race is not a biological factor and while race itself is not racism, it is a sociopolitical and contextual construct linked to greater exposure to adverse environments and health disadvantages from a history of oppression.44,45,46,47,48 Furthermore, clocks, mainly developed in predominantly White samples, show varying sensitivities to social and environmental exposures across different races.49 Thus, we presented exposure to loss by race and included an interaction term between loss and race in our biological aging models to explore effect modification by race.44,47,48 Interaction analyses focused on Black (and White participants due to the smaller number of other racial and ethnic categories) (see Table). We applied the same models as in the overall sample, adding an interaction term between loss and race, considering interaction terms significant at a 2-sided P < .10. Data were analyzed from Janaury 2022 to July 2024.
Table. Population Descriptive Statistics (Weighted Proportions and Unweighted Ns).
| Characteristic | Participants (N = 3963), No. (weighted %) |
|---|---|
| Age at wave 5, median (weighted IQR) | 38.36 (36.78-39.78) |
| Sex assigned at birth | |
| Female | 2370 (49.97) |
| Male | 1593 (50.03) |
| Race and ethnicity | |
| Asian | 145 (1.89) |
| Black | 720 (15.97) |
| Hispanic | 400 (8.18) |
| American Indiana | 28 (0.89) |
| Othera,b | 11 (0.34) |
| Pacific Islanderb | 17 (0.21) |
| White | 2642 (72.53) |
| Parental education, wave 1 | |
| High school | 1261 (35.15) |
| Some college | 1196 (30.72) |
| College or higher | 1506 (34.14) |
| No. of household members, wave 1, median (weighted IQR) | 3.00 (2.00-4.00) |
| Proportion of households under poverty, wave 1, mean (SE) | 0.14 (0.01) |
| Caregiver smoking, wave 1 | 1034 (27.94) |
| Any loss, waves 1-5 | 1491 (38.49) |
| No. of losses, waves 1-5 | |
| 1 | 1066 (28.84) |
| ≥2 | 425 (9.65) |
| Types of loss, wave 1-5 | |
| Any loss in childhood | 351 (7.97) |
| Any loss in adulthood | 1095 (28.59) |
| Parental loss | 1245 (32.37) |
| Parental loss in childhood | 263 (6.31) |
| Parental loss in adulthood | 1019 (26.73) |
| Any loss by sex | |
| Female | 911 (39.12) |
| Male | 580 (37.87) |
| Parental loss by sex | |
| Female | 755 (32.81) |
| Male | 490 (31.92) |
| Any loss by race and ethnicity | |
| Asian | 41 (28.83) |
| Black | 379 (56.67) |
| Hispanic | 152 (41.38) |
| American Indianb | 18 (56.08) |
| Otherc | NA |
| Pacific Islanderc | NA |
| White | 884 (34.09) |
| Parental loss by race and ethnicity | |
| Asian | 34 (25.03) |
| Black | 323 (48.72) |
| Hispanic | 127 (31.70) |
| American Indianb | 14 (41.08) |
| Otherc | NA |
| Pacific Islanderc | NA |
| White | 732 (28.78) |
| Biological aging clock raw scores, wave 5, mean (SE) | |
| Horvath | 39.16 (0.17) |
| PhenoAge | 30.02 (0.19 |
| GrimAge | 52.50 (0.17) |
| DunedinPACE | 0.99 (0.00) |
| Biological aging clock residuals, wave 5, mean (SE) | |
| Horvath | 0.14 (0.09) |
| PhenoAge | 0.07 (0.13) |
| GrimAge | 0.31 (0.14) |
| Biological aging clock z scores, wave 5, mean (SE) | |
| Horvath | 0.03 (0.02) |
| PhenoAge | 0.01 (0.02) |
| GrimAge | 0.08 (0.03) |
| DunedinPACE | −0.02 (0.04) |
Abbreviation: NA, not available.
Estimate based on small sample size (eg, <30).
Participants who self-identified as other did not identify with any other race or ethnic category or indicated multiracial only.
Estimate cannot be presented because of deductive disclosure risks based on Add Health restricted data use policy.
Results
The analytical sample included 3963 Add Health participants who had data on all variables in the analysis (Table). The weighted sample mean (range) age was 38.36 (36.78-39.78) years at wave 5; 2370 (50.3%) were male, 720 (15.97%) were Black, 400 (8.18%) were Hispanic, and 2642 (72.53%) were White. Almost 40% of participants experienced 1 or more losses (Table). Most losses occurred in adulthood and parental loss was more common in adulthood vs in childhood or adolescence (26.73% vs 6.31%) (Table). Loss was most frequently reported by Black (379 participants [56.67%]), and American Indian (18 participants [56.08%]) participants, followed by Hispanic (152 participants [41.38%]), White (884 participants [34.09%]), and Asian (41 participants [28.83%]) participants.
Number of Losses
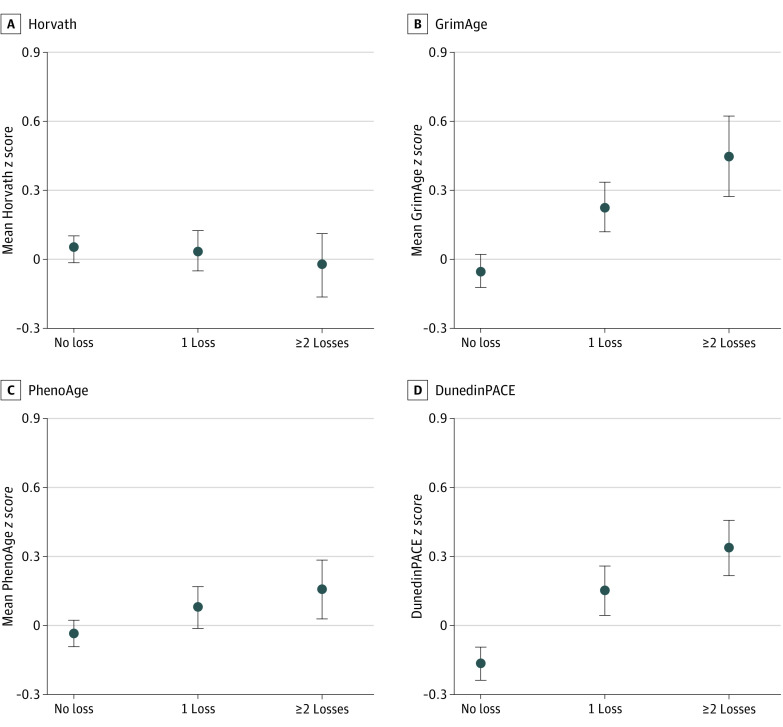
The mean z score and SEs for each biological clock by number of losses are presented in Figure 1. As the number of losses increased, GrimAge, PhenoAge, and DunedinPACE increased. There were no discernible differences in the Horvath clock by number of losses (β = −0.08; 95% CI, −0.23 to 0.06).
Figure 1. Weighted Means for Biological Aging Clocks (z Scored) by Number of Losses.
Dots indicate means and bars indicate SEs. Values are weighted means.
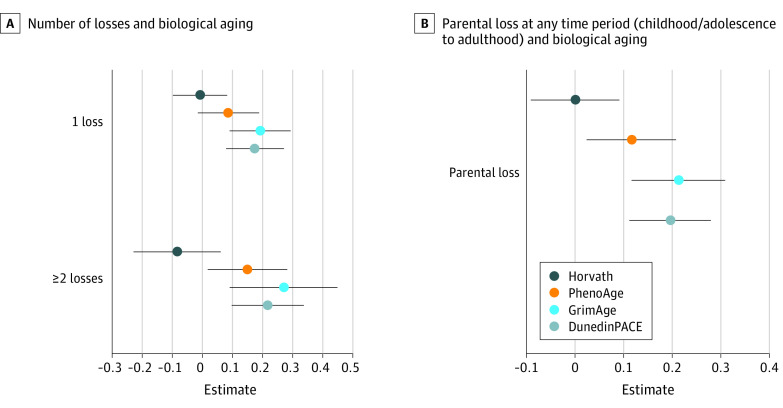
In adjusted models, participants who experienced only 1 loss vs no loss had older biological ages for GrimAge (β = 0.19; 95% CI, 0.09 to 0.29) and DunedinPACE (β = 0.17; 95% CI, 0.08 to 0.27), but not for PhenoAge or Horvath clocks (See Figure 2A; eTable 9 in Supplement 1). Participants with 2 or more losses tended to have significantly older biological ages for several of the biological clocks (PhenoAge β = 0.15; 95% CI, 0.02 to 0.28; GrimAge β = 0.27; 95% CI, 0.09 to 0.45; DunedinPACE β = 0.22; 95% CI, 0.10 to 0.34). Two or more losses, however, were not associated with biological aging as measured by the Horvath clock (β = −0.14; 95% CI, −0.40 to 0.12). All results are shown in eTable 9 in Supplement 1.
Figure 2. Number of Losses and Biological Aging and Parental Loss at Any Time Period (Childhood/Adolescence to Adulthood) and Biological Aging.
Clock values are z scored. Models adjusted for age, race and ethnicity, parental education, gender, proportion of households in poverty, number of household members, caregiver smoking, and epigenetic assay batch. See eTable 1 in Supplement 1 for full model specifications and estimates.
Parental Loss From Childhood/Adolescence to Adulthood
Participants who lost a parent in either childhood or adolescence or adulthood tended to have an older PhenoAge (β = 0.12; 95% CI, 0.02-0.21), GrimAge (β = 0.21; 95% CI, 0.12-0.31), and DunedinPACE (β = 0.19; 95% CI, 0.11-0.28) than participants who had no losses. The Horvath clock was not associated with parental loss. Results are shown in Figure 2B and eTable 10 in Supplement 1.
Timing of Loss
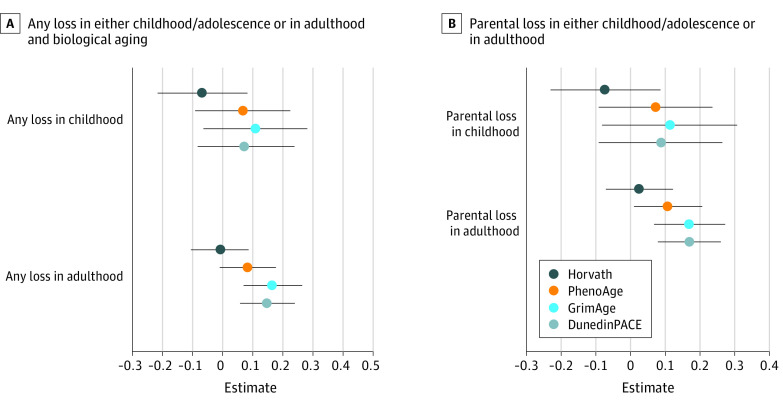
Participants who experienced any losses during childhood or adolescence (adjusting for later life loss) exhibited similar biological aging to those who experienced no losses during childhood (Horvath β = −0.07; 95% CI, −0.22 to 0.08; PhenoAge β = 0.07; 95% CI, −0.09 to 0.23; GrimAge β = 0.11; 95% CI, −0.06 to 0.28; DunedinPACE β = 0.08; 95% CI, −0.08 to 0.24). Results are shown in Figure 3A and eTable 11 in Supplement 1.
Figure 3. Any Loss in Either Childhood/Adolescence or in Adulthood and Biological Aging and Parental Loss in Either Childhood/Adolescence or in Adulthood and Biological Aging.
Clock values are z scored. Models adjusted for age, race and ethnicity, parental education, gender, proportion of households in poverty, number of household members, caregiver smoking, and epigenetic assay batch. See eTable 1 in Supplement 1 for full model specifications and estimates.
In contrast, participants who experienced any loss during adulthood, adjusted for childhood or adolescent losses, exhibited older biological ages as compared with peers with no adulthood loss for GrimAge (β = 0.16; 95% CI, 0.07-0.26) and DunedinPACE (β = 0.15; 95% CI, 0.06-0.24), but not for PhenoAge or Horvath clocks. Results are shown in Figure 3A and eTable 11 in Supplement 1.
Participants who had lost a parent during childhood did not differ in their biological aging from participants who had not, after adjusting for loss of a parent in adulthood (Figure 3B; eTable 12 in Supplement 1). In contrast, participants who lost a parent during adulthood, adjusting for loss of a parent in childhood, exhibited older biological ages for several clocks (PhenoAge β = 0.10; 95% CI, 0.01 to 0.20; GrimAge β = 0.17; 95% CI, 0.07 to 0.27; DunedinPACE β = 0.17; 95% CI, 0.08 to 0.26), but not the Horvath Clock (β = 0.02; 95% CI, −0.07 to 0.12). Results are shown in Figure 3B and eTable 12 in Supplement 1.
Sensitivity Analyses
Controlling for cell counts was not associated with overall effect sizes (see eTable 1, eTable 2, eTable 3, and eTable 4 in Supplement 1). Similarly, inclusion of time since parental loss showed very little difference in estimates overall (see eTable 5, eTable 6, eTable 7, and eTable 8 in Supplement 1). We also examined the impact of loss by parental gender (maternal versus paternal) and found no significant differences (see eTable 17 in Supplement 1), and there were no significant interactions between loss in childhood and adulthood on epigenetic aging (see eTable 18 and eTable 19 in Supplement 1)
Interactions With Race
Race by loss interaction model results are reported in eTable 13, eTable 14, eTable 15, and eTable 16 in Supplement 1. There were no statistically significant interactions between race and loss and biological clocks at the α = .10 level.
Discussion
Our findings suggest that accelerated biological aging may represent a key mechanism associating exposure to death of family members with later life risk of morbidity and mortality. Among US adults aged 33 to 44 years, experiences of loss were consistently associated with older biological age as measured by PhenoAge and GrimAge clocks and faster pace of aging as measured by the DunedinPACE, with a higher burden of loss exhibiting a dose-response association with the extent of accelerated biological aging. In contrast, there was no association between loss and biological aging as measured by the Horvath clock. Losses experienced during adulthood showed greater associations with biological aging as compared with losses experienced during childhood or adolescence. Findings suggest that experiences of loss may contribute to trajectories of biological aging even before the onset of midlife, thus helping to set the course for earlier risk of chronic disease and mortality.
There is an increasing amount of research indicating that prolonged stress and related traumas may cause accelerated biological aging.10,13,28,29 However, studies that directly link loss specifically with epigenetic-clock measures of aging are scarce. Most research has focused on early-life adversity or adverse childhood experiences based on review of existing research,10,13,28 with only 2 prior studies50,51 specifically examining loss of a parent or loved one. Of these, 1 considered only first-generation epigenetic clocks and found no association with loss, consistent with our analysis of the Horvath clock.50 While highly precise in predicting chronological age, the Horvath clock and other first-generation clocks tend to show lesser and inconsistent associations with morbidity and mortality and low sensitivity to stressors.22,24,28,52,53 The other available study concerned a small sample of older adults in Ireland.51 Our study therefore contributes evidence from a large, US population-based sample observed relatively early in the life course when experiences of loss may contribute to an acceleration of biological processes of aging.
We found that adult losses showed greater associations with biological aging markers than childhood or adolescence losses, a pattern also seen in a smaller sample of older adults in the Ireland study.51 This may be due to stronger adult reactions to traumatic events, impacting physiological risk, or greater epigenetic recovery from losses that occurred in the more distant past. Understanding the distinct impacts of loss at different life stages requires studies with repeated measures of loss and DNA methylation over time.
Several studies have highlighted significant disparities in loss among Black and Hispanic populations compared with White populations in the US.3,6,14,40,41,42,43 Consistent with recent work by Donnelly et al,14 we noted that Hispanic participants experienced a higher number of losses than White non-Hispanic participants. American Indian and Pacific Islanders also experienced greater loss, but the sample size for these estimates were small. Our interaction analysis suggests that there were no significant differences between loss and epigenetic aging across Black and White participants. A previous study noted higher instances and impacts of loss on GrimAge among Black participants.54 We were unable to stably test for effect modification across the smaller samples of racial and ethnic groups. Future research should examine these associations among American Indian, Hispanic, and Pacific Islanders, as well as individuals of multiracial and other identities.
Limitations
We acknowledge limitations. We had access to only 1 time point of epigenetic data, leaving open questions as to why childhood or adolescent losses exhibited lesser associations with epigenetic clock measures as compared with losses occurring during adulthood, closer to the time of DNA collection. It is also possible that individuals with parents who die prematurely may inherit familial health conditions that impact both parental loss and biological aging. However, we adjusted for time since loss and did not observe significant differences in model estimates. This study may not have had sufficient power to detect minor interaction effects by race. Given the significant differences in exposure to loss across racial and ethnic groups, future research will require larger and more diverse population samples. The consistency of our results across 3 different epigenetic clocks and multiple model specifications in a large, diverse, prospective, national cohort contribute important evidence that experiences of loss represent a marker of risk for accelerated biological aging. Future research should aim to replicate our innovative findings related to the quantity of loss and its variability by type.
Conclusions
In conclusion, our study sheds light on how loss may affect biological aging and ultimately health and mortality. We found that adults with a history of loss had higher biological ages than those without such experiences. More losses were associated with older biological age. These findings suggest that loss can accelerate biological aging even before midlife and frequency of losses may compound this, potentially leading to earlier chronic diseases and mortality. Future research should focus on identifying coping strategies and social support to lessen the negative effects of loss on aging, aiding clinical and public health approaches.
eTable 1. Number of Losses and Biological Aging
eTable 2. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 3. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 4. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 5. Count of Losses and Biological Aging
eTable 6. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 7. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 8. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 9. Number of Losses and Biological Aging
eTable 10. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 11. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 12. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 13. Number of Losses and Biological Aging
eTable 14. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 15. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 16. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 17. Parental Loss by Gender of Parent at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 18. Interaction Between Any Loss in Childhood and in Adulthood and its Association With Biological
eTable 19. Interaction Between Parental Loss in Childhood and in Adulthood and its Association With Biological Aging
Data Sharing Statement
References
- 1.Stroebe M, Stroebe W, Schut H, Boerner K. Grief is not a disease but bereavement merits medical awareness. Lancet. 2017;389(10067):347-349. doi: 10.1016/S0140-6736(17)30189-7 [DOI] [PubMed] [Google Scholar]
- 2.Conde-Sala JL, Garre-Olmo J. Early parental death and psychosocial risk factors for dementia: a case-control study in Europe. Int J Geriatr Psychiatry. 2020;35(9):1051-1059. doi: 10.1002/gps.5328 [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Lin Z, Umberson D.. Parental death and cognitive impairment: an examination by gender and race–ethnicity. J Gerontol Psychol Sci Soc Sci. 2022;77(6):1164-1176. doi: 10.1093/geronb/gbab125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rostila M, Saarela J, Kawachi I. The forgotten griever: a nationwide follow-up study of mortality subsequent to the death of a sibling. Am J Epidemiol. 2012;176(4):338-346. doi: 10.1093/aje/kws163 [DOI] [PubMed] [Google Scholar]
- 5.Smith KR, Hanson HA, Norton MC, Hollingshaus MS, Mineau GP. Survival of offspring who experience early parental death: early life conditions and later-life mortality. Soc Sci Med. 2014;119:180-190. doi: 10.1016/j.socscimed.2013.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly R, Cha H, Umberson D. Multiple family member deaths and cardiometabolic health among Black and White older adults. J Health Soc Behav. 2022;63(4):610-625. doi: 10.1177/00221465221114485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Umberson D, Xu M. Widowhood and mortality: gender, race/ethnicity, and the role of economic resources. Ann Epidemiol. 2020;45:69-75.e1. doi: 10.1016/j.annepidem.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umberson D, Donnelly R, Xu M, Farina M, Garcia MA. Death of a child prior to midlife, dementia risk, and racial disparities. J Gerontol Psychol Sci Soc Sci. 2020;75(9):1983-1995. doi: 10.1093/geronb/gbz154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly R, Umberson D, Hummer RA, Garcia MA. Race, death of a child, and mortality risk among aging parents in the United States. Soc Sci Med. 2020;249:112853. doi: 10.1016/j.socscimed.2020.112853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belsky DW, Baccarelli AA. To promote healthy aging, focus on the environment. Nat Aging. 2023;3(11):1334-1344. doi: 10.1038/s43587-023-00518-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243-278. doi: 10.1016/j.cell.2022.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Moqri M, Herzog C, Poganik JR, et al. ; Biomarkers of Aging Consortium . Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023;186(18):3758-3775. doi: 10.1016/j.cell.2023.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oblak L, van der Zaag J, Higgins-Chen AT, Levine ME, Boks MP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348. doi: 10.1016/j.arr.2021.101348 [DOI] [PubMed] [Google Scholar]
- 14.Donnelly R, Garcia MA, Cha H, Hummer RA, Umberson D. Exposure to family member deaths across the life course for Hispanic individuals. Demography. 2023;60(2):539-562. doi: 10.1215/00703370-10604036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris KM, Halpern CT, Whitsel EA, et al. Cohort profile: The National Longitudinal Study of Adolescent to Adult Health (Add Health). Int J Epidemiol. 2019;48(5):1415-1415k. doi: 10.1093/ije/dyz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen P, Harris KM. Construction of Wave V Biomarker Weight. 2020. Accessed July 9, 2024. https://addhealth.cpc.unc.edu/wp-content/uploads/docs/user_guides/WaveVBiomarkerSampleWeightUserGuide.pdf
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 18.Harris KM, Levitt B, Gaydosh L, et al. The Sociodemographic and Lifestyle Correlates of Epigenetic Aging in a Nationally Representative U.S. Study of Young Adults. bioRxiv. Preprint posted online March 28, 2024. doi: 10.1101/2024.03.21.585983 [DOI]
- 19.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371-384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 21.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;5(9):e54870. doi: 10.7554/eLife.54870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife. 2022;14(11):e73420. doi: 10.7554/eLife.73420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sood AB, Razdan A, Weller EB, Weller RA. Children’s reactions to parental and sibling death. Curr Psychiatry Rep. 2006;8(2):115-120. doi: 10.1007/s11920-006-0008-0 [DOI] [PubMed] [Google Scholar]
- 26.Burrell LV, Mehlum L, Qin P. Parental death by external causes during childhood and risk of psychiatric disorders in bereaved offspring. Child Adolesc Ment Health. 2022;27(2):122-130. doi: 10.1111/camh.12470 [DOI] [PubMed] [Google Scholar]
- 27.Kailaheimo-Lonnqvist S, Kotimaki S. Cause of parental death and child’s health and education: the role of parental resources. SSM Popul Health. 2020;11:100632. doi: 10.1016/j.ssmph.2020.100632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffington L, Belsky DW. Integrating DNA methylation measures of biological aging into social determinants of health research. Curr Environ Health Rep. 2022;9(2):196-210. doi: 10.1007/s40572-022-00338-8 [DOI] [PubMed] [Google Scholar]
- 29.Musci RJ, Kush JM, Masyn KE, et al. ; Conduct Problems Prevention Research Group6 . Psychosis symptom trajectories across childhood and adolescence in three longitudinal studies: an integrative data analysis with mixture modeling. Prev Sci. 2023;24(8):1636-1647. doi: 10.1007/s11121-023-01581-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169(10):1182-1190. doi: 10.1093/aje/kwp035 [DOI] [PubMed] [Google Scholar]
- 31.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1-19. doi: 10.18637/jss.v009.i08 [DOI] [Google Scholar]
- 32.Lumley T. Complex surveys: a guide to analysis using R. John Wiley & Sons; 2011. [Google Scholar]
- 33.Lumley T. Package ‘survey’. 2020. Accessed June 26, 2024. https://cran.r-project.org
- 34.Lumley T, Huang X. Linear mixed models for complex survey data: implementing and evaluating pairwise likelihood. arXiv. Preprint posted online July 11, 2023. doi: 10.48550/arXiv.2307.04944 [DOI]
- 35.Long JA. jtools: Analysis and presentation of social scientific data. R package version 2.2.0. Accessed June 26, 2024. https://cran.r-project.org/package=jtools. 2022
- 36.Wickham H, Chang W, Wickham MH. Package ‘ggplot2’. 2016. Accessed June 26, 2024. https://ggplot2.tidyverse.org/reference/ggplot2-package.html
- 37.Wickham H. Getting started with ggplot2. ggplot2: Elegant graphics for data analysis. 2016. Accessed June 26, 2024. https://ggplot2-book.org/
- 38.Binder DA. On the variances of asymptotically normal estimators from complex surveys. Int Statist Rev. 1983;51(3):279-292. doi: 10.2307/1402588 [DOI] [Google Scholar]
- 39.R Core Team . R: a language and environment for statistical computing. Accessed July 9, 2024. https://www.R-project.org/
- 40.Donnelly R, Lin Z, Umberson D. Parental death across the life course, social isolation, and health in later life: racial/ethnic disadvantage in the U.S. Soc Forces. 2023;102(2):586-608. doi: 10.1093/sf/soad027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umberson D, Donnelly R. The death of a child and parents’ psychological distress in mid to later life: racial/ethnic differences in exposure and vulnerability. J Gerontol B Psychol Sci Soc Sci. 2022;77(8):1561-1570. doi: 10.1093/geronb/gbab206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cha H, Thomas PA, Umberson D. Sibling deaths, racial/ethnic disadvantage, and dementia in later life. J Gerontol B Psychol Sci Soc Sci. 2022;77(8):1539-1549. doi: 10.1093/geronb/gbab202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YK, Kim K, Fingerman KL, Umberson DJ. Racial differences in early parental death, midlife life problems, and relationship strain with adult children. J Gerontol B Psychol Sci Soc Sci. 2021;76(8):1617-1628. doi: 10.1093/geronb/gbaa232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones CP. Invited commentary: “race,” racism, and the practice of epidemiology. Am J Epidemiol. 2001;154(4):299-304. doi: 10.1093/aje/154.4.299 [DOI] [PubMed] [Google Scholar]
- 45.Adkins-Jackson PB, Chantarat T, Bailey ZD, Ponce NA. Measuring structural racism: a guide for epidemiologists and other health researchers. Am J Epidemiol. 2022;191(4):539-547. doi: 10.1093/aje/kwab239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Ann Rev Sociology. 2015;41:311-330. doi: 10.1146/annurev-soc-073014-112305 [DOI] [Google Scholar]
- 47.Martinez RAM, Andrabi N, Goodwin AN, Wilbur RE, Smith NR, Zivich PN. Conceptualization, operationalization, and utilization of race and ethnicity in major epidemiology journals, 1995-2018: a systematic review. Am J Epidemiol. 2023;192(3):483-496. doi: 10.1093/aje/kwac146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swilley-Martinez ME, Coles SA, Miller VE, et al. “We adjusted for race”: now what? A systematic review of utilization and reporting of race in American Journal of Epidemiology and Epidemiology, 2020-2021. Epidemiol Rev. 2023;45(1):15-31. doi: 10.1093/epirev/mxad010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkins SH, Testa C, Simpkin AJ, et al. An epigenome-wide analysis of DNA methylation, racialized and economic inequities, and air pollution. bioRxiv. Preprint posted online December 8, 2023. doi: 10.1101/2023.12.07.570610 [DOI]
- 50.Lawn RB, Anderson EL, Suderman M, et al. Psychosocial adversity and socioeconomic position during childhood and epigenetic age: analysis of two prospective cohort studies. Hum Mol Genet. 2018;27(7):1301-1308. doi: 10.1093/hmg/ddy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCrory C, Fiorito G, O’Halloran AM, Polidoro S, Vineis P, Kenny RA. Early life adversity and age acceleration at mid-life and older ages indexed using the next-generation GrimAge and Pace of Aging epigenetic clocks. Psychoneuroendocrinology. 2022;137:105643. doi: 10.1016/j.psyneuen.2021.105643 [DOI] [PubMed] [Google Scholar]
- 52.Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8(9):1844-1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graf GH, Crowe CL, Kothari M, et al. Testing Black-White disparities in biological aging among older adults in the United States: analysis of DNA-methylation and blood-chemistry methods. Am J Epidemiol. 2022;191(4):613-625. doi: 10.1093/aje/kwab281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holloway TD, Harvanek ZM, Xu K, Gordon DM, Sinha R. Greater stress and trauma mediate race-related differences in epigenetic age between Black and White young adults in a community sample. Neurobiol Stress. 2023;26:100557. doi: 10.1016/j.ynstr.2023.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number of Losses and Biological Aging
eTable 2. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 3. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 4. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 5. Count of Losses and Biological Aging
eTable 6. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 7. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 8. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 9. Number of Losses and Biological Aging
eTable 10. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 11. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 12. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 13. Number of Losses and Biological Aging
eTable 14. Parental Loss at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 15. Any Loss in Childhood and in Adulthood and Biological Aging
eTable 16. Parental Loss in Childhood and in Adulthood and Biological Aging
eTable 17. Parental Loss by Gender of Parent at Any Time Period (Childhood to Adulthood) and Biological Aging
eTable 18. Interaction Between Any Loss in Childhood and in Adulthood and its Association With Biological
eTable 19. Interaction Between Parental Loss in Childhood and in Adulthood and its Association With Biological Aging
Data Sharing Statement