Abstract
Macrophages comprise the major population of cells infiltrating pancreatic islets during the early stages of infection in DBA/2 mice by the D variant of encephalomyocarditis virus (EMC-D virus). Inactivation of macrophages prior to viral infection almost completely prevents EMC-D virus-induced diabetes. This investigation was initiated to determine whether a tyrosine kinase signalling pathway might be involved in the activation of macrophages by EMC-D virus infection and whether tyrosine kinase inhibitors might, therefore, abrogate EMC-D virus-induced diabetes in vivo. When isolated macrophages were infected with EMC-D virus, inducible nitric oxide synthase mRNA was expressed and nitric oxide was subsequently produced. Treatment of macrophages with the tyrosine kinase inhibitor tyrphostin AG126, but not tyrphostin AG556, prior to EMC-D virus infection blocked the production of nitric oxide. The infection of macrophages with EMC-D virus also resulted in the activation of the mitogen-activated protein kinases (MAPKs) p42MAPK/ERK2/p44MAPK/ERK1, p38MAPK, and p46/p54JNK. In accord with the greater potency of AG126 than of AG556 in blocking EMC-D virus-mediated macrophage activation, the incidence of diabetes in EMC-D virus-infected mice treated with AG126 (25%) was much lower than that in AG556-treated (75%) or vehicle-treated (88%) control mice. We conclude that EMC-D virus-induced activation of macrophages resulting in macrophage-mediated β-cell destruction can be prevented by the inhibition of a tyrosine kinase signalling pathway involved in macrophage activation.
Insulin-dependent diabetes mellitus (IDDM), also known as type 1 diabetes, results from the destruction of pancreatic β cells (27, 31). Genetic and environmental factors are believed to be involved in the pathogenesis of IDDM (24, 25, 30). Viral infection is one environmental factor considered to play a role in this disease. Among the viruses implicated in the development of IDDM, the most clear and unequivocal evidence that a virus induces the disease comes from studies on the D variant of encephalomyocarditis (EMC) virus (EMC-D virus) in mice (7, 34) and Kilham rat virus in rats (6, 11). In genetically susceptible strains of mice, EMC-D virus causes diabetes by the selective destruction of β cells.
The molecular identification of the EMC virus genes responsible for the induction of diabetes (16, 17, 29) and the genetic factors of the host (19, 32) have been extensively studied. However, the molecular immune mechanisms involved in the pathogenesis of diabetes in EMC virus-infected mice remain to be determined. Earlier studies showed that the selective infection of pancreatic β cells with EMC-D virus leads to the recruitment of macrophages into the islets followed by infiltration of T lymphocytes (1). Depletion of macrophages prior to infection of mice with a low dose of EMC-D virus resulted in the prevention of diabetes (2, 14). In contrast, the incidence of diabetes increased when macrophages were activated prior to viral infection (2). The depletion of T lymphocytes failed to alter the incidence of diabetes in EMC-D virus-infected mice (33). These results indicate that macrophages play a primary role in the destruction of β cells in mice infected with a low dose of EMC-D virus.
Our recent study showed that macrophages activated by EMC-D virus in vivo produce the soluble mediators interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and inducible nitric oxide synthase (iNOS), which play an important role in the destruction of β cells (12). However, the mechanisms that activate macrophages are not known. This investigation was initiated to determine whether a tyrosine kinase signal pathway might be involved in the EMC-D virus-induced activation of macrophages in vitro and, if so, whether the administration of a tyrosine kinase inhibitor in vivo might protect against EMC-D virus-induced diabetes. For our study, we focused on the tyrosine kinase inhibitors tyrphostin AG126 and tyrphostin AG556 (hereafter referred to simply as AG126 and AG556), which have been shown to prevent lipopolysaccharide (LPS)-induced lethal toxicity either in mice (23) or dogs (28), respectively. We now report that AG126 prevents EMC-D virus-mediated macrophage activation in vitro. Further, when administered in vivo prior to infection of DBA/2 mice with EMC-D virus, AG126 led to a reduction of β-cell destruction and a reduction in the incidence of diabetes. This result suggests that a tyrosine kinase signalling pathway involved in the EMC-D virus-induced activation of macrophages plays a role in macrophage-dependent β-cell destruction, leading to the development of diabetes in mice.
MATERIALS AND METHODS
Virus.
The source and preparation of EMC virus have been described elsewhere (13, 35, 36). The virus was purified by CsCl2 gradient centrifugation from a supernatant of L929 cell culture infected with plaque-purified EMC-D virus.
Mice.
DBA/2 mice were obtained from Jackson Laboratory (Bar Harbor, Maine). The animals were housed in an animal facility at the Health Science Centre, University of Calgary, Calgary, Alberta, Canada. Eight-week-old male mice were used for in vitro and in vivo experiments.
Reagents.
The tyrosine kinase inhibitors AG126, AG556, herbimycin A, and genistein were purchased from Calbiochem Inc. (La Jolla, Calif.). LPS from Escherichia coli O26:86 was purchased from Sigma Chemical Co. (St. Louis, Mo.).
Macrophage preparation and infection.
Peritoneal macrophages were harvested from DBA/2 mice 4 days after intraperitoneal injection of 2 ml of 3% thioglycolate (Difco Laboratories, Detroit, Mich.). Cells were washed twice and resuspended in RPMI 1640 medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Cells were plated at 2 × 106 cells per well in 24-well plates (for analysis of nitric oxide [NO] release and virus replication) or at 107 cells in 60-mm dishes, (or reverse transcriptase-PCR [RT-PCR] and Western blot analyses). Cells were cultured for 2 h at 37°C in 5% CO2 and then washed three times to remove nonadherent cells. More than 95% of the adherent cells were determined to be macrophages on the basis of morphologic criteria. Cells were infected with EMC virus at a multiplicity of infection (MOI) of 5 or stimulated with 1 μg of LPS per ml. For experiments with tyrosine kinase inhibitors, cells were pretreated with the inhibitors for 2 h and then washed three times before EMC virus infection.
RT-PCR.
The total RNA was extracted from macrophages or pancreatic cells from DBA/2 mice with Trizol reagent (Gibco BRL Life Technologies Inc., Gaithersburg, Md.). The cDNA was synthesized with 4 μg of RNA in 20 μl of reaction mixture containing 50 pmol of oligo(dT)12–18 primer, 10 mM dithiothreitol, 75 mM KCl, 50 mM Tris-HCl (pH 8.3), 5 mM MgCl2, 15 U of RNase inhibitor, 0.2 mM each deoxynucleoside triphosphate, and 20 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL). PCR was performed with 1 μl of cDNA with pairs of oligonucleotide primers corresponding to the cDNA sequences. The following oligonucleotide sequences were used: for β-actin, GTTACCAACTGGGACGACA and TTCGAGCAGGAGATGGCCA; for IL-1β, GGAATGACCTGTTCTTTGAAGTT and GGCTCCGAGATGAACAACAAAA; for TNF-α, CTTAGACTTTGCGGAGTCCG and GGGACAGTGACCTGGACTGT; for iNOS, GCATGGACCAGTATAAGGCAAGAC and TTGCTCATGACATCGACCAGAAGC; and for EMC virus VP1, GGAGTTGAGAATGCTGAGAGAGGGGTT and GGAATTCATTCCAGCATAAGGACTCCAGCTCTCTCGG (25 cycles). PCR amplification was carried out in 50 μl of the reaction mixture containing 50 pmol of sense and antisense primer, 0.2 mM deoxynucleoside triphosphate, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, and 0.1% Triton X-100, with denaturation at 94°C for 1 min, annealing at 60°C (IL-1β, TNF-α, iNOS, and β-actin) or 55°C (EMC virus VP1), and extension at 72°C, using a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). The products were separated by electrophoresis on a 1.5% agarose gel and detected by ethidium bromide staining.
NO assay.
NO formation was measured as the stable end product nitrite (NO2−) in culture supernatants with the Griess reagent (10). Briefly, 100 μl of culture supernatant was added to each well of 96-well plates and mixed with the same volume of Griess reagent [0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in H2O, 1% sulfanilamide in 5% H3PO4], and the optical density at 540 nm was read with a Biokinetics reader (Mandel Scientific Co. Ltd., Guelph, Ontario, Canada).
Measurement of virus replication.
The virus concentrations of the culture supernatants and the pancreatic tissues from EMC virus-infected mice were determined by plaque assay using L929 cells as described previously (13, 36).
Western blot analysis.
Macrophages were lysed in lysis buffer (50 mM Tris [pH 7.6], 1% Nonidet P-40, 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 1 mM phenylmethlsulfonyl fluoride, 10 μg of aprotinin per ml) at 4°C for 30 min. Lysates were cleared of debris by centrifugation at 12,000 × g for 20 min. Samples were analyzed by electrophoresis using 10% sodium dodecyl sulfate-polyacrylamide gels followed by transfer to nitrocellulose membranes (Amersham Life Science Inc., Oakville, Ontario, Canada). The membrane was blocked with 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 and then incubated with antiphosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnology, Lake Placid, N.Y.), anti-p38 mitogen-activated protein kinase (MAPK) phosphospecific antibody, anti-SAPK/JNK phosphospecific antibody, anti-extracellular signal-regulated kinase 1/2 (ERK1/2) phosphospecific antibody (Calbiochem, La Jolla, Calif.), anti-ERK2 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.), or polyclonal anti-EMC-D virus antibodies obtained from EMC-D virus-infected mice. After washing, the membrane was incubated with peroxidase-conjugated goat anti-mouse or anti-rabbit antibody, and specific bands were detected with an enhanced chemiluminescence detection system (Amersham).
Treatment of EMC virus-infected DBA/2 mice with tyrosine kinase inhibitors.
Male DBA/2 mice infected with EMC virus (50 PFU/mouse) were injected intraperitoneally with 400 μg of tyrosine kinase inhibitor (AG126 or AG556) in 100 μl of 10% dimethyl sulfoxide (DMSO)–phosphate-buffered saline (PBS). As a control, 100 μl of 10% DMSO–PBS was injected into male DBA/2 mice. Daily administration of tyrosine kinase inhibitor or 10% DMSO–PBS was initiated on the same day as EMC virus infection and continued for 9 days. Blood glucose was measured at 3, 5, 7 and 9 days postinfection. RT-PCR analyses of cytokines and iNOS in the pancreas were performed 5 days postinfection, and viral titers in the pancreatic cells were determined 4 and 5 days postinfection. Histological examination of the pancreatic cells was performed 12 days postinfection.
Measurement of blood glucose.
Blood glucose levels of nonfasting mice were measured with a one-touch Basic glucometer (Lifescan, Burnaby, British Columbia, Canada). The mean blood glucose level of 43 uninfected DBA/2 male mice was 132 ± 15 mg/dl (mean ± standard deviation [SD]). In this experiment, nonfasting animals with blood glucose levels greater than 177 mg/dl (3 SD above the mean) were scored as diabetic.
Histological examination.
Eight mice per group were sacrificed at 12 days postinfection, and each pancreas was fixed in 10% buffered neutral formalin. Paraffin-embedded sections were stained with hematoxylin and eosin and examined. Histological changes of the pancreatic islets were classified as peri-islet infiltration, mild to moderate insulitis, severe insulitis, and atrophied morphology. The islets with peri-islet infiltration had infiltrating mononuclear cells around them. The architecture of islets having mild to moderate insulitis was well preserved, but 1 to 49% of these islets exhibited lymphocytic infiltration within the islets. Severe insulitis was characterized by morphological damage to the pancreatic β cells, with 50 to 100% of these islets exhibiting lymphocytic infiltration. Islets with atrophied morphology were small and retracted, exhibiting severe β-cell necrosis with or without residual lymphocytic infiltration.
Statistical analysis.
Statistical analysis was conducted by Student’s t test or Kruskal-Wallis one-way analysis of variance on ranks.
RESULTS
EMC-D virus-induced activation of macrophages, cytokine and iNOS gene expression, and production of NO.
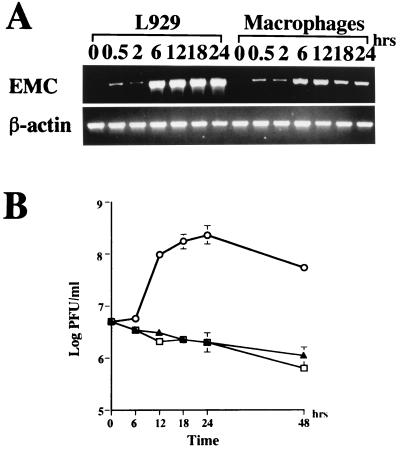
To determine whether EMC-D virus can infect macrophages, we isolated peritoneal macrophages from DBA/2 mice, inoculated the macrophages with EMC-D virus, and examined the EMC-D viral RNA in the macrophages, using RT-PCR at different times after inoculation. We found that viral RNA was detectable in macrophages within 30 min after infection. At 6 and 12 h after infection, there was a marked increase in viral RNA, indicating new RNA synthesis (Fig. 1A). As controls, we inoculated L929 cells with EMC-D virus and examined the expression of EMC-D viral RNA. The amounts of EMC-D viral RNA seen between 6 and 24 h after infection were much higher than those detected in EMC-D virus-infected macrophages (Fig. 1A).
FIG. 1.
(A) RT-PCR analysis of EMC virus RNA and β-actin mRNA in L929 cells and macrophages; (B) EMC-D virus replication in macrophages and L929 cells. (A) RNA was isolated from EMC-D virus-infected L929 cells and macrophages at 0, 0.5, 2, 6, 12, 18 and 24 h post-EMC-D virus infection and analyzed by RT-PCR. (B) Triplicate culture supernatants of macrophages (▴) and L929 cells (○) infected with EMC-D virus at an MOI of 5, and culture medium containing the same amount of virus without cells (□), were harvested at 6, 12, 18, 24 and 48 h postinfection. Virus concentration was determined with a plaque assay on L929 cells. Bars represent SD.
To determine whether infection of macrophages by EMC-D virus results in the production of progeny virus, we measured the amount of progeny virus in the macrophage culture medium at various times after infection. Viral progeny were hardly detected in the culture medium, and the infectious virus titer was not increased from the time of the inoculation (0 h) to the end of the experiment (48 h). In contrast, viral titers from the EMC-D virus-infected L929 cell culture supernatant continuously increased up to 24 h after infection. (Fig. 1B).
To determine whether the failure of progeny viral production in EMC-D virus-infected macrophages was due to a defect in the synthesis of viral proteins, we measured the viral capsid proteins in the lysate of the infected macrophages by Western blot analysis using antibodies against EMC-D virus. Viral capsid proteins were hardly detected, whereas significant amounts of EMC viral capsid proteins were detected in the lysate of the infected L929 cells (data not shown). These results indicate that EMC-D virus can infect macrophages and synthesize viral RNA but cannot produce progeny virus, probably due to a defect in viral protein synthesis. Thus, EMC-D virus does not lyse macrophages but may activate them.
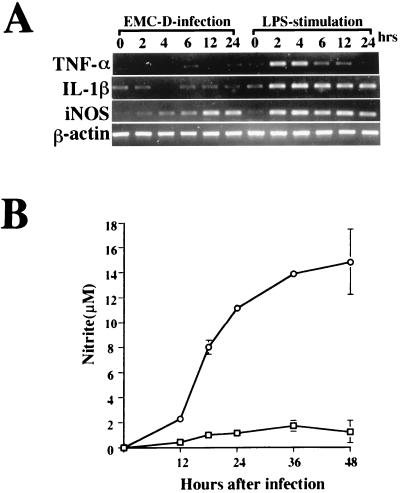
To determine whether the activation of macrophages by EMC-D virus results in macrophage-derived cytokine or iNOS gene expression, we measured levels of IL-1β, TNF-α, and iNOS mRNAs in EMC-D virus-infected macrophages at various times after infection. As controls, we measured expression of the same genes in LPS-stimulated macrophages. We found that IL-1β, TNF-α, and iNOS mRNAs were clearly detected in the LPS-treated macrophages from 2 to 6 h after stimulation (Fig. 2A). TNF-α mRNA was not detected thereafter, but IL-1β and iNOS mRNAs remained at the same level at the termination of the experiment at 24 h (Fig. 2A). In contrast, EMC-D virus-infected macrophages produced undetectable amounts of TNF-α and IL-1β mRNAs, but iNOS mRNA was clearly expressed from 4 h after infection to the end of the experiment at 24 h (Fig. 2A). This result showed that EMC-D virus can activate macrophages directly and induce the expression of iNOS, but not TNF-α or IL-1β, mRNA.
FIG. 2.
(A) RT-PCR analysis of TNF-α, IL-1β, iNOS, and β-actin mRNAs in macrophages infected with EMC-D virus (MOI = 5) or stimulated with LPS (1 mg/ml). RNA was isolated from the cells at 0, 2, 4, 6, 12, and 24 h after EMC-D virus infection or LPS stimulation and analyzed by RT-PCR. (B) Kinetics of NO production in uninfected macrophages (□) and macrophages infected with EMC-D virus (○). At the indicated time intervals, triplicate samples were removed for nitrite determination by Griess assay. Bars represent SD.
To determine whether the expression of iNOS mRNA in EMC-D virus-infected macrophages results in the production of NO, we measured the production of NO at various times after infection with EMC-D virus. Increased levels of NO production were observed, with a clear elevation at 12 h and a marked elevation at 48 h (Fig. 2B).
Involvement of tyrosine kinase signalling pathways in the production of NO in EMC-D virus-infected macrophages.
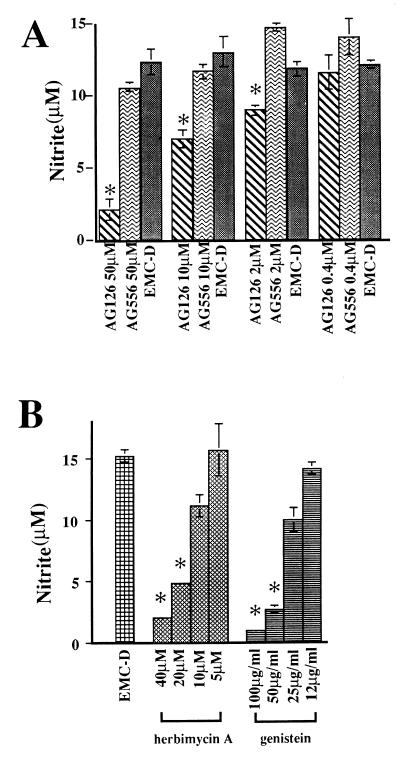
To determine whether tyrosine kinase signalling pathways might be involved in the production of NO in EMC-D virus-activated macrophages, we examined the effects of tyrosine kinase inhibitors AG126 and AG556 on the production of NO in macrophages infected with EMC-D virus. We found that the treatment with AG126 resulted in a significant reduction of NO production, whereas treatment with AG556 had little effect (Fig. 3A). In contrast, NO production was inhibited in an LPS-stimulated macrophage cell line (RAW246) treated with AG556 (data not shown). To determine whether other tyrosine kinase inhibitors also inhibit NO production in isolated macrophages, we measured NO production in EMC-D virus-activated macrophages that were treated with herbimycin A or genistein. We found that NO production in these macrophages was clearly inhibited in a concentration-dependent manner by these inhibitors (Fig. 3B).
FIG. 3.
Effects of tyrosine kinase inhibitors on NO production of macrophages infected with EMC-D virus. Peritoneal macrophages were infected with EMC-D virus (MOI = 5) in the presence of AG126 or AG556 (A) herbimycin A or genistein (B) with 2 h of preincubation. DMSO was added as a control. At 24 h postinfection, NO production was determined from triplicate samples. Bars represent SD. ∗, P < 0.01 by Student’s t test.
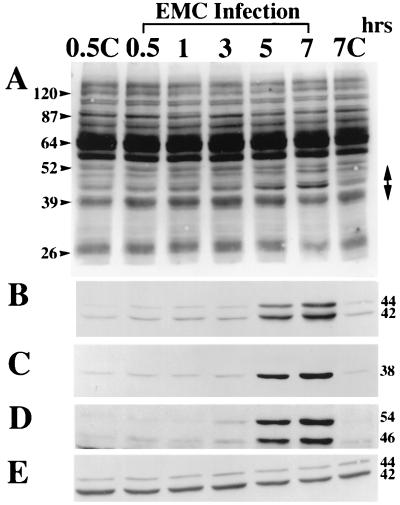
Since the tyrosine kinase inhibitors suppressed the EMC-D virus-mediated production of NO in the activated macrophages, we hypothesized that EMC-D virus may induce tyrosine phosphorylation of host proteins in the infected macrophages. To determine whether infection of macrophages by EMC-D virus might result in the induction of tyrosine phosphorylation, we examined the tyrosine-phosphorylated proteins in EMC-D virus-infected macrophages by Western blot analysis using an antiphosphotyrosine antibody. We found that the phosphorylation of 44- and 42-kDa proteins was significantly increased from 5 to 7 h after EMC-D virus infection (Fig. 4A). Because MAPKs (or ERKs) can become phosphorylated upon cell activation and since p42MAPK/ERK2 and p44MAPK/ERK1 have molecular masses of 42 and 44 kDa, respectively, we performed Western blot analysis with anti-phosphospecific ERK1/2 antibody to determine whether the 42- and 44-kDa phosphorylated might actually be ERKs. We found that the phosphorylation and activation of p42MAPK/ERK2 and p44MAPK/ERK1 were clearly increased in the macrophages at 5 h after EMC-D virus infection, indicating that the 42- and 44-kDa tyrosine-phosphorylated proteins (Fig. 4A) were p42MAPK/ERK2 and p44MAPK/ERK1, respectively (Fig. 4B). In addition, we examined other members of the MAPK family. We found that p38MAPK and p46/p54JNK were also activated at 5 h after EMC-D virus infection (Fig. 4C and D, respectively). Western blot analysis using anti-total ERK2 antibody revealed that all samples contained comparable amounts of protein (Fig. 4E).
FIG. 4.
Western blot analysis of protein tyrosine phosphorylation (A), phosphorylated p42MAPK/ERK2 and p44MAPK/ERK1 (B), phosphorylated p38MAPK (C), phosphorylated p46/p54JNK (D), and total ERK (E) of macrophages during EMC-D virus infection. Cell lysates were blotted with (A) antiphosphotyrosine monoclonal antibody 4G10 (A), antiphosphospecific ERK1/2 antibody (B), anti-phosphospecific p38 antibody (C), anti-phosphospecific SAPK/JNK antibody (D), and anti-ERK2 antibody (E). Extracts were prepared from uninfected cells at 0.5 (0.5C) and 7 (7C) h and from EMC-D virus-infected cells at 0.5, 1, 3, 5, and 7 h after infection. The double-headed arrow indicates the region of phosphorylated proteins. Positions of size markers and proteins are indicated in kilodaltons on the left and right, respectively.
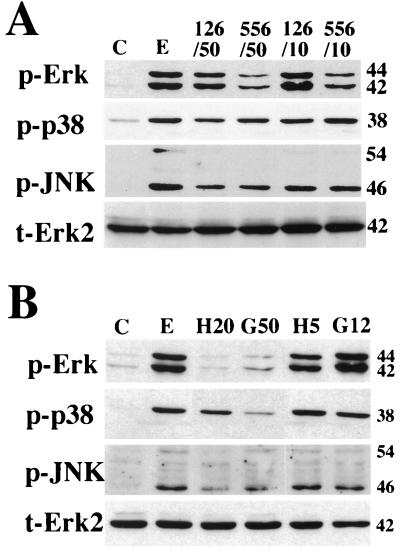
To determine whether AG126 and AG556 suppress the activation of MAPKs, the phosphorylation of p42MAPK/ERK2, p44MAPK/ERK1, p38MAPK, and p46/p54JNK was examined in EMC-D virus-infected macrophages treated with AG126 or AG556. We found that AG556 was more effective than AG126 in inhibiting the phosphorylation of p42MAPK/ERK2 and p44MAPK/ERK1, whereas AG126 was slightly more effective than AG556 in inhibiting the phosphorylation of p38MAPK. The two drugs appeared equally effective in reducing the phosphorylation of p46/p54JNK (Fig. 5A). We also found that herbimycin and genistein were effective in inhibiting the phosphorylation of p42MAPK/ERK2, p44MAPK/ERK1, p38MAPK, and p46/p54JNK at the higher of the two doses used (Fig. 5B).
FIG. 5.
Effects of AG126 and AG556 (A) and herbimycin A and genistein (B) on the phosphorylation of MAPKs in macrophages infected with EMC-D virus. Extracts were prepared from uninfected cells (lanes C), infected cells (lanes E), and infected cells treated with 10 (126/10) or 50 (126/50) μM AG126, 10 (556/10) or 50 (556/50) μM AG556, 5 (H5) or 20 (H20) μM herbimycin A, or 5 (G5) or 12 (G12) μg of genistein per ml. Western blot analyses were performed with phosphospecific antibodies against ERK1/2 (p-Erk), p38 (p-p38), and SAPK/JNK (p-JNK) and antibody against ERK2 (t-Erk2). Sizes are indicated kilodaltons on the right.
Prevention of EMC-D virus-induced diabetes in DBA/2 mice by AG126.
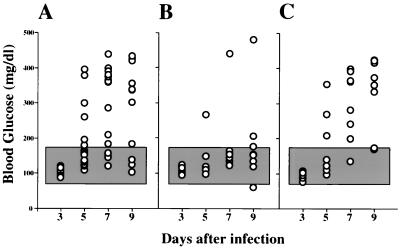
Since the tyrosine kinase inhibitor AG126 blocked macrophage activation in vitro and since macrophages play a critical role in the EMC-D virus-mediated destruction of pancreatic β cells in vivo, we examined whether the in vivo administration of AG126 might prevent EMC-D virus-induced diabetes in DBA/2 mice. We found that the incidence of diabetes was significantly decreased in mice treated with AG126. Twenty-five percent of the mice treated with AG126 (Fig. 6B) and approximately 88% of mice treated with 10% DMSO–PBS (vehicle) (Fig. 6A) developed diabetes 9 days after EMC-D virus infection. In contrast with AG126, AG556 did not suppress the production of NO in the EMC-D virus-infected macrophages in vitro (Fig. 3A). Thus, we were interested in determining the effect of AG556 on the EMC-D virus-induced diabetes in DBA/2 mice. We found that AG556 treatment failed to prevent EMC-D virus-induced diabetes (Fig. 6C). There was no significant difference in the incidence of diabetes between the AG556-treated group (75%) and the vehicle-treated control group (88%).
FIG. 6.
Effects of tyrosine kinase inhibitors AG126 and AG556 on the development of EMC-D virus-induced diabetes in DBA/2 mice. Mice were treated with 10% DMSO–PBS (A) or 400 μg of AG126 (B) or AG556 (C) every day. Each circle represents an individual animal. The shaded area shows the mean blood glucose level ± 3 SD of the value for uninfected control mice (P < 0.05 compared with 10% DMSO–PBS- or AG556-treated mice at 5, 7, and 9 days after infection, using Kruskal-Wallis one-way analysis of variance on ranks).
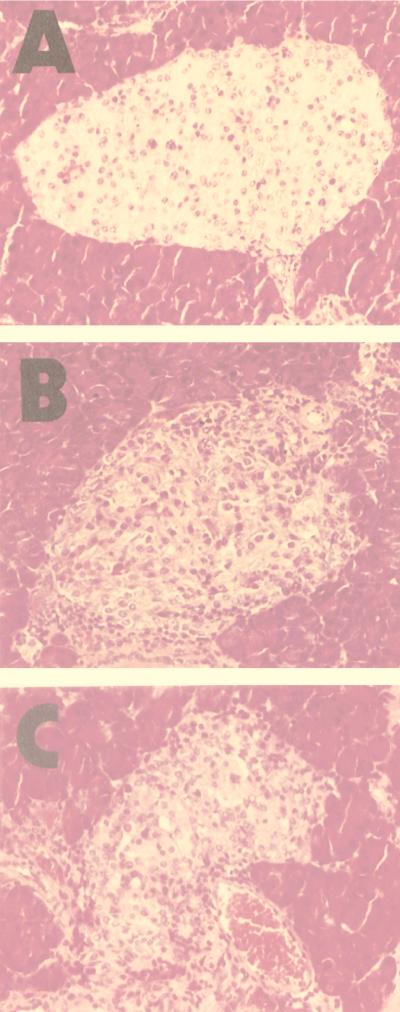
Examination of pancreatic islet architecture revealed a significant reduction in β-cell destruction and mononuclear cell infiltration when mice were treated with AG126. The majority (75%) of examined islets from AG126-treated mice showed only mild to moderate insulitis with peri-islet infiltration, while 23% showed severe insulitis and 2% showed an atrophied morphology. In contrast, only 16% of examined islets from vehicle-treated control mice showed mild to moderate insulitis, while 49% showed severe insulitis and 35% showed an atrophied morphology. The islet histopathology seen in AG556-treated mice was similar to that seen in the vehicle-treated control mice (Fig. 7; Table 1). This result indicated that the tyrosine kinase inhibitor AG126 substantially prevented the destruction of β cells, resulting in the prevention of diabetes, while AG556 did not confer this preventative effect.
FIG. 7.
Pancreatic islets of EMC-D virus-infected mice treated with AG126 (A), AG556 (B), or 10% DMSO–PBS (C) at 12 days postinfection (hematoxylin and eosin staining).
TABLE 1.
Histological changes in the pancreatic islets of EMC-D virus-infected mice treated with AG126 and AG556
| Treatment | No. of islets examineda | % of isletsb
|
|||
|---|---|---|---|---|---|
| P | M | S | A | ||
| AG126 | 190 | 7 | 68 | 23 | 2 |
| AG556 | 207 | 0 | 26 | 43 | 31 |
| DMSO | 198 | 1 | 15 | 49 | 35 |
Combined data from diabetic and nondiabetic mice (20 to 30 islets per mouse) 12 days after infection with EMC virus. Each group contains eight mice.
P, peri-islet infiltration; M, mild to moderate insulitis; S, severe insulitis; A, atrophied morphology (see text for details).
To determine whether there was any difference in viral replication in pancreatic islets between AG126- and vehicle-treated mice, we measured infectious virus titers in the pancreatic tissues of AG126- and vehicle-treated mice at 4 and 5 days after infection. We found that there was no significant difference in the viral concentration between AG126- and vehicle-treated mice at day 4 (7.06 ± 0.07 and 7.49 ± 0.23 log10 PFU/g of tissue [mean ± SD], respectively) or day 5 (6.47 ± 0.34 and 6.47 ± 0.26 log10 PFU/g of tissue, respectively) after infection. These results indicate that the prevention of diabetes in AG126-treated mice is not due to the inhibition of viral replication.
Effect of AG126 on expression of IL-1β, TNF-α and iNOS mRNAs in pancreatic tissue infected with EMC-D virus.
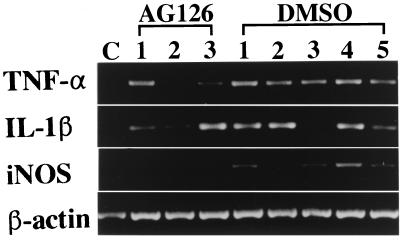
To determine whether treatment with AG126 might affect the expression of macrophage-derived cytokines and iNOS in pancreatic islets from EMC-D virus-infected mice, we analyzed the expression of IL-1β, TNF-α, and iNOS in the pancreatic tissue of 10% DMSO-PBS- and AG126-treated, EMC-D virus-infected mice at 5 days postinfection by RT-PCR. We found that the expression of iNOS mRNA was clearly suppressed in the pancreatic tissue of AG-126-treated mice compared with that in the pancreatic tissue of vehicle-treated mice; the expression of IL-1β and TNF-α also appeared to be reduced (Fig. 8). This result indicates that AG126 not only suppressed the expression of iNOS but also attenuated the induction of mRNA for cytokines such as IL-1β and TNF-α in the pancreatic islet infiltrates of mice infected with EMC-D virus.
FIG. 8.
RT-PCR analysis of IL-β, TNF-α, iNOS, and β-actin mRNA expression in the pancreas of mice infected with EMC-D virus at 5 days postinfection. RNA was isolated from the pancreatic tissue of an uninfected mouse (lane C), nondiabetic EMC-D virus-infected, AG126-treated mice (lanes 1 to 3), nondiabetic EMC-D virus-infected, 10% DMSO–PBS-treated mice (lanes 1, 2, 3, and 5), and diabetic EMC-D virus-infected, 10% DMSO–PBS-treated mice (lane 4).
DISCUSSION
Infection of DBA/2 mice with a high dose (5 × 105 PFU/mouse) of the diabetogenic EMC-D virus results in the rapid destruction of pancreatic β cells and the development of diabetes within 4 days (34). However, infection of mice with a lower dose (102 PFU) of EMC-D virus results in a significant decrease in the incidence of diabetes and delay in the onset of the disease (1, 2). In mice infected with the lower dose of EMC-D virus, macrophages play a critical role in the destruction of pancreatic β cells, as activation of macrophages prior to viral infection results in a significant increase in the incidence of diabetes and inactivation of macrophages prior to viral infection almost completely prevents EMC-D virus-induced diabetes (2, 14). Our additional studies showed that the selective EMC-D viral infection of pancreatic β cells results in an initial recruitment of macrophages into the islets, followed by infiltration of other immunocytes including T cells, NK cells, and B cells (1). In the pancreatic islets containing activated macrophages, there is production of soluble mediators such as NO, IL-1β, and TNF-α that contribute to the destruction of pancreatic β cells, resulting in the development of diabetes in mice infected with a low dose of EMC-D virus. However, it was not known whether macrophages are directly activated by the virus and produce such soluble mediators when infected with EMC-D virus in vitro. Thus, we infected isolated peritoneal macrophages with EMC-D virus in vitro and examined the expression of IL-1β, TNF-α, and iNOS mRNAs. We found that iNOS mRNA expression and NO production were induced, whereas the expression of TNF-α and IL-1β mRNA was undetectable. This result differed from our previous observation that the expression of TNF-α and IL-1β as well as iNOS was clearly increased in macrophages that infiltrated the pancreatic islets in vivo. There may be differences in the induction of cytokines from macrophages between in vitro (isolated, infected with EMC-D virus in vitro) and in vivo (macrophages present in the target tissue along with other immunocytes) conditions. Nevertheless, NO was a consistent product of infected macrophages in both in vivo and in vitro conditions.
NO is known to play an important role in the progression of inflammation in the pancreatic islets and the destruction of β cells, resulting in the development of diabetes in mice (8, 26). Exogenous and endogenous NO has been shown to induce apoptosis in isolated rat pancreatic islet cells as well as in the HIT pancreatic β cell line (18). In addition, the NO-mediated upregulation of fas in pancreatic β cells significantly contributes to their destruction. In animal models of spontaneous IDDM, BioBreeding (BB) rats and nonobese diabetic mice, NO has been shown to contribute to pancreatic β-cell destruction. In BB rats, iNOS mRNA is highly expressed in inflammatory cells in the pancreatic islets (20). Treatment of BB rats with N-nitro-l-arginine methyl ester, an NOS inhibitor, results in a significant decrease in the incidence of diabetes (21). Treatment of nonobese diabetic mice with the iNOS inhibitor aminoguanidine caused a delay in the onset of diabetes in adoptive-transfer models (3). Our recent study also showed that treatment of DBA/2 mice with the iNOS inhibitor aminoguanidine resulted in a significant decrease in the incidence of EMC-D virus-induced diabetes (12). In addition to contributing to β-cell destruction, NO can enhance the activity of cyclooxygenases 1 and 2 so as to augment the production of prostaglandins and thromboxanes, resulting in an acceleration of the inflammatory response (21).
Recent studies have reported that the induction of tyrosine phosphorylation by viral infection may play a role in the generation of inflammatory mediators by immune cells. For instance, astrocytes stimulated with Newcastle disease virus produce TNF-α via a tyrosine kinase signalling pathway (9), and adenovirus infection stimulates the Raf-MAPK signalling pathway and induces the expression of IL-8 in HeLa cells (4). Thus, we examined whether inhibition of tyrosine phosphorylation would suppress the production of NO in EMC-D virus-infected macrophages in vitro. We found that the treatment of isolated macrophages with the tyrosine kinase inhibitors AG126, herbimycin A, or genistein prior to EMC-D virus infection resulted in the suppression of NO production. This result indicated that EMC virus infection activates a tyrosine kinase signalling pathway involved in iNOS mRNA expression and NO production in macrophages. However, AG556, which is more lipophilic than AG126, failed to inhibit the production of NO in EMC-D virus-infected macrophages although AG556 blocked the NO production by LPS-stimulated macrophages. The differential effects of AG126 and AG556 in blocking iNOS induction in LPS- and virus-stimulated cells suggest that different tyrosine kinase signalling pathways may be activated by EMC-D virus and LPS.
We next examined the tyrosine-phosphorylated proteins in EMC-D virus-infected macrophages by Western blot analysis using antiphosphotyrosine antibodies. We found that proteins with molecular masses of 38 to 46 kDa were tyrosine phosphorylated. We went on to show that viral infection caused the activation of the MAPK family members; p42MAPK/ERK2, p44MAPK/ERK1, p38MAPK, and p46/p54JNK. These results indicate that the EMC-D virus can activate MAPK signal pathways kinases, including those which may be involved in the induction of iNOS in macrophages. A causative role for p46/p54JNK in the induction of iNOS in TNF-α and gamma interferon-stimulated macrophages has recently been established (5). It was also reported that echovirus 1 can induce the phosphorylation of p42MAPK/ERK2 and p44MAPK/ERK1 as well as p38MAPK (15). In our study, we found a differential action between AG126 and AG556 on the phosphorylation of MAPKs. AG556 was more effective than AG126 in inhibiting the phosphorylation of p42MAPK/ERK2 and p44MAPK/ERK1, whereas AG126 was slightly more effective than AG556 in inhibiting the phosphorylation of p38MAPK. We do not know whether these differences are sufficient to account for our observation that AG126 could inhibit NO production in EMC-D-infected macrophages whereas AG556 could not, as there may be other differential effects of AG126 and AG556 on other tyrosine kinase signalling pathways not measured in these experiments. This possibility is presently under investigation.
Given the substantial evidence linking NO production to β-cell destruction, we hypothesized that the suppression of NO production in macrophages by a tyrosine kinase inhibitor might prevent the development of EMC-D virus-induced diabetes in vivo. Thus, we tested the effect of AG126 on the development of diabetes in DBA/2 mice infected with a low dose of EMC-D virus. We found that the incidence of diabetes dramatically decreased in mice treated with AG126. Twenty-five percent of AG126-treated mice and 88% of the vehicle-treated controls became diabetic. In contrast, a related tyrosine kinase inhibitor, AG556, which failed to suppress the production of NO in infected macrophages in vitro, failed to prevent diabetes when given to EMC-D virus-infected DBA/2 mice. The level of iNOS mRNA in AG126-treated mice that developed diabetes at 7 days after infection was similar to that in 10% DMSO–PBS-treated diabetic mice (data not shown). Thus, the suppression of macrophage-derived NO in vitro and the prevention of diabetes are strongly correlated.
In our previous study, we showed that macrophages infiltrating the pancreatic islets express IL-1β, TNF-α, and iNOS mRNAs and that mice treated with antibodies against IL-1β or TNF-α or with the iNOS inhibitor aminoguanidine exhibited a significant decrease in the incidence of diabetes (12). Furthermore, mice treated with a combination of anti-IL-1β antibody, anti-TNF-α antibody, and aminoguanidine showed a lower incidence of diabetes than mice treated with any of these agents alone (12). Thus, we tested whether the expression of IL-1β, TNF-α, and iNOS mRNAs in the pancreatic islets was also suppressed in the EMC-D virus-infected mice treated with AG126. We found that the expression of these cytokine genes (IL-1β and TNF-α) and iNOS mRNA was clearly suppressed compared to vehicle-treated controls. In our present study, iNOS mRNA (but not IL-1β or TNF-α mRNA) was expressed in isolated macrophages infected with EMC-D virus in vitro. However, IL-1β and TNF-α mRNAs as well as iNOS mRNA was expressed in the pancreas after infection in vivo, suggesting that the induction of cytokine gene expression in infected macrophages may depend on the immune environment. Thus, the local suppression of NO production in macrophage-infiltrated pancreatic islets by treatment of mice with a tyrosine kinase inhibitor may result in the reduction of mononuclear cell infiltration of the islets, leading to the suppression of production of cytokines such as IL-1β and TNF-α by macrophages in the inhibitor-treated mice.
On the basis of these observations, we suggest that the infection of macrophages in vivo with EMC-D virus results in a cascade of signalling pathway kinase activation, induction of iNOS expression, NO production, NO-mediated upregulation of fas, and fas-mediated apoptosis of pancreatic β cells, resulting in the development of diabetes in DBA/2 mice infected with a low dose of EMC-D virus. Treatment of EMC-D virus-infected mice with tyrosine kinase inhibitors results in an inhibition of the tyrosine kinase signalling pathway, suppression of NO production, and prevention of macrophage-mediated β-cell destruction, leading to the prevention of EMC-D virus-induced diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council of Canada to J.W.Y. and M.D.H. and the Canadian Diabetes Association and the Korea Science and Engineering Foundation (97-0403-0101-3) to J.W.Y., fellowships from the Japan Society for the Promotion of Science for Japanese Junior Scientist to K.H., and studentships from the Alberta Heritage Foundation for Medical Research to H.S.H. J.W.Y. is a Heritage Medical Scientist awardee of the Alberta Heritage Foundation for Medical Research. K.H. is a postdoctoral research fellow.
We are grateful to Alex Levitzki and Aviv Gazit for help in obtaining AG126 and AG556. We gratefully acknowledge the editorial assistance of A. L. Kyle and Karen L. Clarke and the technical assistance of Joy M. Goldberg and Lori D. Zbytnuik.
REFERENCES
- 1.Baek H S, Yoon J W. Role of macrophages in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 1990;64:5708–5715. doi: 10.1128/jvi.64.12.5708-5715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek H S, Yoon J W. Direct involvement of macrophages in the destruction of beta cells leading to development of diabetes in virus-infected mice. Diabetes. 1991;40:1586–1596. doi: 10.2337/diab.40.12.1586. [DOI] [PubMed] [Google Scholar]
- 3.Bowman M, Simell O, Look Z, Luchetta R, Atkinson M. Pharmacokinetic and therapeutic analysis of aminoguanidine. Diabetes. 1994;43(Suppl. 1):235A. [Google Scholar]
- 4.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signalling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan E D, Winston B W, Uh S T, Wynes M W, Rose D M, Riches W. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-gamma and TNF-alpha in mouse macrophages. J Immunol. 1999;162:415–422. [PubMed] [Google Scholar]
- 6.Chung Y H, Jun H S, Hirasawa K, Lee B R, van Rooijen N, Yoon J W. Role of macrophages and macrophage-derived cytokines in the pathogenesis of Kilham rat virus-induced autoimmune diabetes in diabetes-resistant BB rats. J Immunol. 1997;159:446–471. [PubMed] [Google Scholar]
- 7.Craighead J E, McLane M F. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968;162:913–915. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- 8.Faust A, Kleemann R, Rothe H, Kolb H. Role of macrophages and cytokines in beta cell death. In: Shafrir E, editor. Lessons from animal diabetes VI. Cambridge, Mass: Birkhauser Boston; 1996. pp. 47–56. [Google Scholar]
- 9.Fischer S N, Kim Y U, Shin M L. Tyrosine kinase activation by Newcastle disease virus is required for TNF-α gene induction in astrocytes. J Immunol. 1994;153:3210–3217. [PubMed] [Google Scholar]
- 10.Green L C, Wagner D A, Goglowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 11.Guberski D L, Thomas V A, Shek W R, Like A A, Handler E S, Rossini A A, Wallace J E, Welsh R M. Induction of type I diabetes by Kilham’s rat virus in diabetes-resistant BB/Wor rats. Science. 1991;254:1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa K, Jun H S, Maeda K, Kawaguchi Y, Itagaki S, Mikami T, Baek H S, Doi K, Yoon J W. Possible role of macrophage-derived soluble mediators in the pathogenesis of encephalomyocarditis virus-induced diabetes. J Virol. 1997;71:4024–4031. doi: 10.1128/jvi.71.5.4024-4031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirasawa K, Takeda M, Itagaki S, Doi K. Involvement of macrophages in the development of encephalomyocarditis virus (EMC)-induced diabetes. Exp Anim. 1996;45:77–80. doi: 10.1538/expanim.45.77. [DOI] [PubMed] [Google Scholar]
- 14.Hirasawa K, Tsutsui S, Takeda M, Mizutani M, Itagaki S, Doi K. Depletion of Mac-1-positive macrophages protects DBA/2 mice from encephalomyocarditis virus-induced myocarditis and diabetes. J Gen Virol. 1996;77:737–741. doi: 10.1099/0022-1317-77-4-737. [DOI] [PubMed] [Google Scholar]
- 15.Huttunen P, Hyypia T, Vihnen P, Nissinen L, Heino J. Echovirus 1 infection induces both stress and growth-activated mitogen activated protein kinase pathways and regulates the transcription of cellular immediate early genes. Virology. 1998;250:85–93. doi: 10.1006/viro.1998.9343. [DOI] [PubMed] [Google Scholar]
- 16.Jun H S, Kang Y, Notkins A L, Yoon J W. Gain or loss of diabetogenicity resulting from a single point mutation in recombinant encephalomyocarditis virus. J Virol. 1997;71:9782–9785. doi: 10.1128/jvi.71.12.9782-9785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun H S, Kang Y, Yoon H S, Kim K H, Notkins A L, Yoon J W. Determination of encephalomyocarditis viral diabetogenicity by a putative binding site of the viral capsid protein. Diabetes. 1998;47:576–582. doi: 10.2337/diabetes.47.4.576. [DOI] [PubMed] [Google Scholar]
- 18.Kaneto H, Fujii J, Seo H S, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes. 1995;44:733–738. doi: 10.2337/diab.44.7.733. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Yoon J W. A genetically determined host factor controlling susceptibility to encephalomyocarditis virus-induced diabetes in mice. J Gen Virol. 1993;74:1207–1213. doi: 10.1099/0022-1317-74-6-1207. [DOI] [PubMed] [Google Scholar]
- 20.Kleemann R, Rothe H, Kolb-Bachofen V, Xie Q W, Nathan C, Martin S, Kolb H. Transcription and translation of inducible nitric oxide synthase in the pancreas of prediabetic BB rats. FEBS Lett. 1993;328:9–12. doi: 10.1016/0014-5793(93)80954-s. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay R M, Smith W, Rossiter S P, McIntyre M A, Williams B C, Baird J D. Nw-nitro-l-arginine methyl ester reduces the incidence of IDDM in BB/E rats. Diabetes. 1995;44:365–368. doi: 10.2337/diab.44.3.365. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel M L, Kwon G, Hill J R, Marshall C A, Corbett J A. Cytokine and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med. 1996;211:24–32. doi: 10.3181/00379727-211-43950d. [DOI] [PubMed] [Google Scholar]
- 23.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- 24.Rossini A A, Greiner D L, Friedman H P, Mordes J P. Immunopathogenesis of diabetes mellitus. Diabetes Rev. 1993;1:43–75. [Google Scholar]
- 25.She J X. Susceptible to type 1 diabetes: HLA-DQ and DR revisited. Immunol Today. 1996;17:323–329. doi: 10.1016/0167-5699(96)10014-1. [DOI] [PubMed] [Google Scholar]
- 26.Stassi G, De Maria R, Trucco G, Rudert W, Tesiti R, Galluzzo A, Giordano C, Trucco M. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med. 1997;186:1193–1200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 28.Vanichkin A, Patya M, Gazit A, Levitzki A, Novogrodsky N. Late administration of a lipophilic tyrosine kinase inhibitor prevents lipopolysaccharide and Escherichia coli-induced lethal toxicity. J Infect Dis. 1996;173:927–933. doi: 10.1093/infdis/173.4.927. [DOI] [PubMed] [Google Scholar]
- 29.Yoon J W. A new look at viruses in type 1 diabetes. Diabetes Metab Rev. 1995;11:83–107. doi: 10.1002/dmr.5610110202. [DOI] [PubMed] [Google Scholar]
- 30.Yoon J W. Pathogenesis of IDDM: environmental factors. In: Pickup J, Williams G, editors. Textbook of diabetes. London, England: Blackwell Scientific Publications; 1997. pp. 1–14. [Google Scholar]
- 31.Yoon J W, Jun H S. Insulin-dependent diabetes mellitus. In: Roitt I M, Delves P J, editors. Encyclopedia of immunology. 2nd ed. London, England: Academic Press; 1998. pp. 1390–1398. [Google Scholar]
- 32.Yoon J W, Lesniak M A, Fussganger R, Notkins A L. Genetic differences in susceptibility of pancreatic β-cells to virus-induced diabetes mellitus. Nature. 1976;264:178–180. doi: 10.1038/264178a0. [DOI] [PubMed] [Google Scholar]
- 33.Yoon J W, McClintock P R, Bachurski C J, Longstreth J D, Notkins A L. Virus-induced diabetes mellitus: no evidence for immune mechanisms in the destruction of β-cells by the D-variant encephalomyocarditis virus. Diabetes. 1985;34:922–925. doi: 10.2337/diab.34.9.922. [DOI] [PubMed] [Google Scholar]
- 34.Yoon J W, McClintock P R, Onodera T, Notkins A L. Virus-induced diabetes mellitus. XVIII. Inhibition by a non-diabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980;152:878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon J W, Rodrigues M M, Currier C, Notkins A L. Longterm complications of virus-induced diabetes in mice. Nature. 1982;296:567–569. doi: 10.1038/296566a0. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J W, Wong A K C, Bae Y S, Eun H M. An apparent deletion of an oligonucleotide detected by RNA fingerprint in the nondiabetogenic B variant of encephalomyocarditis virus is caused by a point mutation. J Virol. 1988;62:637–640. doi: 10.1128/jvi.62.2.637-640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]